Abstract
Adolescence is critical period of neurocognitive development as well as increased prevalence of mood pathology. This cross-sectional study replicated developmental patterns of neurocognition and tested whether mood symptoms moderated developmental effects. Participants were 419 adolescents (n=246 with current mood disorders) who completed reward learning and executive functioning tasks, and reported on age, puberty, and mood symptoms. Structural equation modeling revealed a quadratic relationship between puberty and reward learning performance that was moderated by symptom severity: in early puberty, adolescents reporting higher manic symptoms exhibited heightened reward learning performance (better maximizing of rewards on learning tasks), whereas adolescents reporting elevated anhedonia showed blunted reward learning performance. Models also showed a linear relationship between age and executive functioning that was moderated by manic symptoms: adolescents reporting higher mania showed poorer executive functioning at older ages. Findings suggest neurocognitive development is altered in adolescents with mood pathology and suggest directions for longitudinal studies.
Keywords: reward sensitivity, executive functioning, age, puberty, mania, anhedonia
Adolescence is a critical period for the onset of mood disorders (Paus et al., 2008). The majority of first-episodes of mania and depression occur in adolescence (Kessler et al., 2005) and mood symptoms in adolescence precede and are associated with adult mood disorders (Bertha & Balazs, 2013). Adolescence is also characterized by remarkable changes in neurocognitive functioning, and specifically in neurocognitive domains in which abnormalities have been observed in mood disorders (Nielson et al., 2020; Nusslock & Alloy, 2017; Zald & Treadway, 2017). These converging patterns suggest that abnormal development of neurocognition may be a marker for bipolar and unipolar mood disorders (Alloy & Nusslock, 2019; Kujawa et al., 2020). In light of the translational implications of such work (Gaffrey et al., 2013; Van Rheenen et al., 2021), exploration of abnormal neurocognitive development in mood pathology is needed.
Two areas of neurocognitive functioning are especially relevant to adolescent development and mood pathology: reward sensitivity, defined as an individual’s behavioral, physiological, or emotional responsiveness to rewards (Berridge, 2018), and executive functioning, which includes higher-order cognitive abilities that support goal-directed behavior and adaptation (Friedman & Miyake, 2017; Miyake et al., 2000). Both reward sensitivity and executive functioning undergo changes during adolescence, but prior research indicates that developmental timing differs between these neurocognitive domains. Reward sensitivity follows a curvilinear pattern, increasing steeply in early adolescence, and peaking in mid-adolescence before declining or reaching asymptote in late adolescence into young adulthood (Casey et al., 2008; Luna & Wright, 2016; Shulman et al., 2016). In contrast, executive functioning shows linear improvement across adolescence into young adulthood (Luna et al., 2015; Luna & Wright, 2016), with peak levels of executive functioning not achieved until mid-twenties or later (Best & Miller, 2010). These differences in trajectory are paralleled by differences in the developmental factors believed to contribute to changes in each domain: Puberty and pubertal hormones are related to reward-approach behavior (although the nature of such associations may depend on sex (Braams et al., 2015; Harden et al., 2018)), whereas age is associated with executive functioning (Best & Miller, 2010) (also see (Blakemore et al., 2010; Forbes & Dahl, 2010). Together, this research has motivated a set of dual-systems models (Geier & Luna, 2009; Shulman et al., 2016; Steinberg et al., 2008) proposing that adolescent behavior is shaped by the distinct developmental trajectories of reward sensitivity and executive functioning, with the former more strongly related to pubertal changes and the latter driven by factors related to aging (e.g., maturation of prefrontal cortical regions) (Casey et al., 2008).
Reward sensitivity and executive functioning are also key domains of neurocognitive dysfunction in mood disorders. Reward sensitivity is conceptually related to symptom dimensions of mood pathology, including depressive anhedonia (loss of interest and pleasure) and mania/hypomania. Indeed, blunted reward sensitivity has been associated with anhedonia across mood diagnoses (Forbes & Dahl, 2012; Huys et al., 2013; Pizzagalli, 2014; Pizzagalli et al., 2008; Zald & Treadway, 2017). In contrast, abnormalities in reward sensitivity have been observed in bipolar disorders and at higher levels of manic symptom severity (Alloy & Nusslock, 2019), including increased corticostriatal reward response (Nusslock et al., 2012; Phillips & Swartz, 2014). However, evidence is mixed regarding the direction of reward processing abnormalities in bipolar disorders. For example, some research has indicated reward hyposensitivity in the form of blunted reward response bias (Pizzagalli et al., 2008) or lower self-reported reward response (Biuckians et al., 2007). Other studies provide evidence that reward anomalies in bipolar or highly manic youth may depend on sex, developmental processes, or co-recruitment of other cognitive abilities (Maresh et al., 2019; Urošević, Luciana, et al., 2016; Urošević, Youngstrom, et al., 2016). Therefore, the nature of reward abnormalities associated with mood disorders and symptoms is complex and may depend on the form of reward sensitivity that is targeted.
Meanwhile, global and overlapping deficits in executive functioning have been observed across unipolar and bipolar mood disorders (Lee et al., 2014; Rock et al., 2014; Snyder, 2013) including adolescent samples (Horn et al., 2011; Joseph et al., 2008; Wagner et al., 2015), and the severity of such deficits is shown to scale with severity of illness (Dixon et al., 2004; Scult et al., 2017). Executive deficits may contribute to cognitive symptoms of mood pathology (e.g., problems concentrating, racing thoughts), but also to difficulties regulating affective processes that characterize mood disorders (Lima et al., 2018). Together, this research suggests that overlapping executive functioning deficits but (possibly) distinctive reward processing anomalies chatacterize mood symptom dimensions of anhedonia and mania/hypomania (Nusslock & Alloy, 2017).
The convergence of dual systems changes and heightened mood symptoms in adolescence has led to integrative models that aim to describe how mood pathology shapes –– or is shaped by ––developmental factors (Alloy et al., 2016; Alloy & Nusslock, 2019; Forbes et al., 2021; Forbes & Dahl, 2012). However, there are several key gaps in empirical work into such models. First, prior work on mood pathology in adolescence has typically explored executive functioning and reward processing separately, limiting our ability to compare across neurocognitive domains or link results to dual systems models informed by developmental theory. Second and relatedly, although these separate lines of research into executive functioning and reward processing have revealed abnormalities in adolescents with mood disorders (Horn et al., 2011; Wagner et al., 2015), mixed evidence regarding the presence or magnitude of such effects (Joseph et al., 2008; Reyes et al., 2017) makes their reliability unclear. One source of unreliability in these studies may be heterogeneity in the tasks used to evaluate reward sensitivity or executive functioning. Given their unique characteristics, any individual task paradigm is contaminated by perceptual or other task-specific factors (Friedman et al., 2008) and neuropsychological tasks often draw upon multiple cognitive functions (Snyder et al., 2015). Research that adopts a multi-measure, latent variable approach to estimate reward response and executive functioning in clinical samples may provide more reliable evaluation of these constructs.
Third, the majority of research examining adolescent mood pathology and neurocognitive functioning has taken a categorical, diagnostic approach. This approach has limitations, given that within both unipolar and bipolar diagnostic categories there is significant heterogeneity in phenotype (Cuthbert & Insel, 2010; McIntyre et al., 2015) and neurocognitive functioning (Van Rheenen et al., 2020). In turn, symptom dimensions of anhedonia and mania may be as or more strongly related to neurocognitive indices than diagnoses, and subclinical symptoms have been related to functionally significant impairments (Ayuso-Mateos et al., 2010; Copeland et al., 2015; Vrieze et al., 2013). Investigation of symptom dimensions in transdiagnostic samples can provide important insight that complements information from diagnoses.
Fourth, few studies have incorporated developmental differences into the investigation of mood pathology and neurocognitive functioning (Gaffrey et al., 2013). For example, although pubertal status has been linked to reward processing (Harden et al., 2018) and puberty onset is associated with both depression and mania (Mendle, 2014; Yazici et al., 2013), these factors have not been well integrated. Prior clinical research has viewed puberty and adolescence as a window of risk, but there is a lack of conclusive evidence for whether or how developmental differences in neurocognitive functioning are altered in individuals with mood pathology.
The present study aimed to address the above gaps by investigating reward sensitivity and executive functioning in relation to developmental variables and mood symptom dimensions in a large sample of adolescents. This study was a cross-sectional investigation, an approach that can test developmental differences in neurocognitive functioning but cannot answer questions about within-person development over time. Neurocognitive functioning was measured with a methodologically robust multi-task design. For the present study, we chose to operationalize reward sensitivity as performance across a set of widely used reward learning tasks based on prior research indicating that this dimension of reward sensitivity is altered in mood disorders (Zald & Treadway, 2017) and undergoes adolescent development (Nussenbaum & Hartley, 2019). In turn, we chose to operationalize executive functioning in the form of performance across gold-standard executive functioning tasks based on evidence that common executive functions are broadly disrupted in psychopathology (Friedman et al., 2018) and improve over development (Friedman et al., 2016).
Structural equation modeling (SEM) tested the following hypotheses in a single model. First, we predicted that there would be a significant curvilinear association between pubertal stage and a latent reward learning performance factor. Second, we predicted that the association between pubertal stage and reward learning performance would be moderated by manic symptom severity and by anhedonia severity, and that these patterns of moderation would differ from one another. We did not have specific predictions for the directions of moderation, but we predicted that adolescents reporting high severity of manic symptoms would show elevations in reward learning performance that varied by pubertal stage, whereas adolescents reporting high severity of anhedonic symptoms would show reductions in reward learning performance that varied by pubertal stage. Third, we predicted a significant linear association between age and a latent executive functioning factor. Fourth, we predicted that the association between age and executive functioning would be moderated by manic and anhedonic symptom severity, but we predicted an overlapping pattern, in which age-related increases in executive functioning would be weaker at (any) higher symptom severity. Together, the SEM was designed to replicate developmental dual-systems research in a large sample with a robust methodological approach, and extend this research to address the question of how dual-systems developmental differences are altered in adolescents reporting disturbances in specific mood dimensions. Below and in Supplement, we report how we determined our sample size, all data exclusions, all manipulations, and all measures in the study.
Method
Participants
We recruited 419 participants to research testing at two sites (University of Colorado Boulder, University of California Los Angeles) (Tables 1–2; also see Supplement and Table S1). To be eligible for these studies, participants were fluent in English or native English speakers, had normal or corrected-to-normal vision, and reported no history of head injury, neurological conditions, or other conditions that would interfere with cognitive testing. Recruitment was aimed at individuals in the developmental period of adolescence, which can be defined as beginning when an individual enters puberty, and ending when the individual transitions to independence in social-emotional functioning (Forbes & Dahl, 2010). In practice, recruitment across studies was anchored to age (ages 13 to 24) and social role (e.g., middle, high-school, or college students who had not yet entered the workforce). Recruitment was also aimed at high variance in clinical mood symptoms, including those with bipolar or unipolar mood disorders (in final sample, n=34 with full or subthreshold bipolar disorders, n=184 with full or subthreshold unipolar disorders, n=28 with schizoaffective or other mood disorders, n=173 with no mood disorders; Table 2). To achieve this sample, recruitment included distribution of research advertisements and outreach in community, school, healthcare, and outpatient psychiatric settings in the Boulder and Los Angeles metropolitan areas. Research protocols for each study were approved by the Institutional Review Boards at each site. Consent was obtained from legal adults (ages 18 and older) and parental consent and child assent was obtained from legal minors (ages 17 and younger) participating in the study.
Table 1.
Demographics
| CU Boulder | UCLA | All | |
|---|---|---|---|
| n = 264 | n = 155 | n = 419 | |
| M (SD) | M (SD) | M (SD) | |
| Age | 17.37 (2.23) | 20.34 (2.21) | 18.46 (2.65) |
| PDS* | 3.56 (0.49) | 3.82 (0.24) | 3.66 (0.44) |
| GBI-MH | 4.00 (4.22) | 4.48 (5.90) | 4.16 (4.85) |
| MASQ-AD | 16.60 (6.65) | 18.99 (8.63) | 17.49 (7.53) |
| % | % | % | |
| Gender | |||
| Cisgender Female | 54.75% | 69.03% | 60.03% |
| Cisgender Male | 38.78% | 29.03% | 35.17% |
| Other | 6.46% | 1.94% | 4.79% |
| Ethnicity | |||
| Hispanic or Latinx | 11.85% | 19.75% | 14.77% |
| Non-Hispanic and Non-Latinx | 84.44% | 75.93% | 81.29% |
| Other | 3.70% | 4.32% | 3.93% |
| Race | |||
| Asian | 4.43% | 19.14% | 9.87% |
| Biracial or More than One Race | 9.96% | 14.81% | 11.75% |
| Black or African American | 1.84% | 3.10% | 2.31% |
| Native Hawaiian | 0.00% | 0.00% | 0.00% |
| Native American | 0.00% | 0.00% | 0.00% |
| White | 80.07% | 49.38% | 68.72% |
| Other | 3.69% | 13.58% | 7.35% |
| Parent Education (Highest Completed) | |||
| 8th Grade or Less | 0.00% | 6.17% | 2.28% |
| Partial High School | 2.95% | 3.09% | 3.00% |
| High School/GED | 8.49% | 10.49% | 9.23% |
| Vocational/Trade | 1.85% | 0.62% | 1.39% |
| Partial College or 2-year Degree | 12.55% | 17.90% | 14.53% |
| College or 4-year Degree | 30.63% | 35.80% | 32.54% |
| Graduate Degree | 39.48% | 21.60% | 32.87% |
| Not reported | 4.06% | 3.10% | 3.70% |
| Family Income (yr) | |||
| <10,000 | 5.54% | 10.49% | 7.37% |
| ~10,000–25,000 | 8.12% | 9.26% | 8.54% |
| ~25,000–50,000 | 12.18% | 12.96% | 12.47% |
| ~50,000–75,000 | 17.34% | 15.43% | 16.63% |
| ~75,000–100,000 | 18.82% | 14.20% | 17.11% |
| >100,000 | 34.32% | 33.33% | 33.95% |
| Not reported | 3.69% | 4.32% | 3.92% |
Note: Age = age in years, CU = University of Colorado Boulder, GBI-MH = General Behavior Inventory, Mania/Hypomania subscale, MASQ-AD = Mood and Anxiety Symptom Questionnaire, Anhedonic Loss of Interest subscale, PDS = Pubertal Development Scale
on n=402, UCLA = University of California Los Angeles
Table 2.
Clinical Characteristics
| CU Boulder | UCLA | All | |
|---|---|---|---|
| n = 264 | n = 155 | n = 419 | |
| % | % | % | |
| Mood Disorders | |||
| Unipolar Disorders | |||
| Major Depressive Disorder | 21.40% | 23.46% | 22.16% |
| Persistent Depressive Disorder | 5.90% | 22.84% | 12.17% |
| Subclinical Depressive Disorder | 12.55% | 4.32% | 9.51% |
| Bipolar Disorders | |||
| Bipolar I Disorder | 1.85% | 4.32% | 2.76% |
| Bipolar II Disorder | 2.58% | 3.09% | 2.77% |
| Bipolar Disorder Not Otherwise Specified | 1.11% | 1.85% | 1.38% |
| Subthreshold Bipolar Disorder | 1.48% | 0.62% | 1.16% |
| Other Mood Disorder Not Otherwise Specified | 5.90% | 6.79% | 6.23% |
| Schizoaffective Disorder | 0.00% | 1.23% | 0.46% |
| No Mood Disorders | 47.23% | 31.48% | 41.40% |
| Secondary Disorders | |||
| Anxiety Disorders | 14.77% | 27.10% | 19.33% |
| Substance Use Disorders | |||
| Mild | 5.30% | 12.26% | 7.87% |
| Moderate or Severe | 1.14% | 8.39% | 3.82% |
| Eating Disorders | 1.51% | 5.16% | 2.86% |
| Trauma and Stress-Related Disorders (Lifetime) | 6.43% | 14.84% | 9.54% |
| Attention Deficit Hyperactivity Disorder | 8.33% | 14.19% | 10.50% |
| Unknown | 3.03% | 7.10% | 4.54% |
| Medications | |||
| Anticonvulsant | 1.14% | 8.39% | 3.82% |
| Anxiolytic (not within 48 hours of session) | 0.76% | 1.93% | 1.19% |
| Atypical antipsychotic | 0.38% | 5.16% | 2.15% |
| Benzodiazepine (not within 48 hours of session) | 0.76% | 3.22% | 1.67% |
| Lithium | 0.00% | 1.93% | 0.71% |
| Beta-blockers (not within 48 hours of session) | 1.51% | 1.93% | 1.67% |
| Norepinephrine-dopamine reuptake inhibitor | 0.38% | 7.74% | 3.10% |
| Selective serotonin-norepinephrine reuptake inhibitor | 0.76% | 3.87% | 1.91% |
| Serotonin antagonist and reuptake inhibitor | 1.14% | 2.58% | 1.67% |
| Selective serotonin reuptake inhibitor | 8.71% | 17.42% | 11.93% |
| Stimulant (not within 48 hours of session) | 4.54% | 5.16% | 4.77% |
| Tetracyclics | 0.00% | 0.38% | 0.14% |
| Any psychoactive medication | 20.07% | 32.26% | 24.58% |
Note. Reported are lifetime mood diagnoses. Current mood symptom severity is reflected in primary dimensional measures reported in main text. Comorbid psychiatric disorders secondary to current mood disorders were permitted in this study and are reported above, based on standard DSM5 criteria and with extended reporting on Substance Use Disorders and Eating Disorders (i.e., remitted but recent diagnoses – past one year for SUDs, past two years for EDs – are reported for those categories) and Trauma and Stress Related Disorders (lifetime history). Current use of psychoactive medications is reported, and eligibility criteria required that participants were on a stable medication and dose (no changes in past six weeks) and abstained from use of stimulant, beta-blocker, anxiolytic, or benzodiazepine medication for 48+ hours prior to the research session. Analyses were repeated excluding individuals with secondary disorders or for whom secondary disorder data was incomplete/unknown, or covarying use of psychoactive medications, but these exclusions did not influence the nature or significance of effects therefore the full sample was retained for analyses.
Procedures
Participants were recruited for an in-person research session that included a series of behavioral tests and surveys. Participants were informed that they would receive a monetary bonus based on their task performance and winnings to enhance motivation. Participants did not know the specific bonus amount and did not receive the bonus until experimental procedures were completed. After behavioral testing, clinical evaluation was performed (along with procedures that will be reported elsewhere, see Supplement).
Measures
Neurocognitive Functioning
To evaluate neurocognitive functioning, six behavioral tasks were electronically administered with a research staff member present in the room to assist in instruction and data collection (Figure S1). Tasks varied between 7–12 minutes per task, and administration of all six tasks (including instructions and breaks between tasks) took approximately one hour. Task administration was conducted using a Mac Mini, a 27’ high-resolution monitor, and a button box with millisecond accuracy to record responses and reaction time (RT). Verbal responses (to the antisaccade task) were recorded by the research assistant. Tasks were programmed in PsychoPy version 1.85.1. Stimuli within tasks were counterbalanced and randomized, and the order of stimuli within each task was standardized across participants. Each task included practice trials or description of stimuli at the beginning, to confirm that participants understood instructions and could perform the task. The order of task administration was standardized to be one of two sequences, and participants were randomly assigned to a sequence (there were no significant differences in performance between task sequences).
Reward Learning Tasks.
The learning tasks used to index reward sensitivity were selected because they are widely-used and well-validated in developmental and clinical samples (Andre Der-Avakian et al., 2016; Lawlor et al., 2020; Master et al., 2020; Nussenbaum & Hartley, 2019; Pizzagalli et al., 2005). Reward tasks featured overlapping instrumental learning demands and monetary rewards, but differed in perceptual characteristics and task structure, making this set of tasks eligible for latent variable analysis in which the shared process across tasks was behavioral ability to maximize reward by learning stimulus-response associations.
Two-armed Bandit Task.
The two-armed bandit is a probability learning task. In each trial the participant was presented with a pair of stimuli (symbols) and instructed to make a choice that elicited a desired outcome, using a button to select either the top or the bottom stimulus. For one stimulus pair the desired outcome was to gain monetary reward, for a second stimulus pair the desired outcome was to avoid monetary loss, and for the third stimulus pair the desired outcome was to view a special graphic. Unbeknownst to the participant, within each stimulus pair, one stimulus had an 80% probability of a desired outcome, and the other stimulus had a 20% probability of a desired outcome. Participants had up to 4 seconds to respond for each stimulus pair; after making a choice, the outcome (reward, loss, special neutral outcome, or null outcome) was displayed for 2 seconds. The task consisted of 72 trials, with 24 trials for each condition.
Probabilistic Reward Task.
The probabilistic reward task (Pizzagalli et al., 2005) is a probability learning task that takes a signal detection approach in which participants are instructed to discriminate between two closely-matched stimuli (McCarthy & Davison, 1979). For each trial, the participant was presented (for 500ms) with a cartoon face, and either a short or long mouth stimulus (for 100ms) appeared on the face. The participant was asked to indicate which stimulus was displayed as quickly as possible by pressing a button. Correct responses either resulted in reward feedback (“Correct! You win $0.05!) or null feedback (blank screen). The reward reinforcement schedule was asymmetrical: One “rich” stimulus was rewarded for correct responses at a rate that was three times higher than the reward rate for the “lean” stimulus (the mouth stimulus that was “rich”, long vs. short, was counterbalanced across participants). The task included two blocks of 100 trials each, and in each block 30 of the 50 rich trials (60%) and 10 of the 50 lean trials (20%) was followed by reward feedback.
Instrumental Learning Task.
This instrumental learning task (modeled on (Collins & Frank, 2012)) is a stimulus-response learning task in which all correct responses earn rewards. Participants were instructed to learn which of three response buttons corresponded with each stimulus in a set of sequentially-presented neutral images. Before each block, participants were shown the set of images that would be used in that block. During the task, each stimulus image was presented for up to 2 seconds, and after responding, feedback on accuracy was displayed (“Correct” or “Try Again!”). Participants were instructed that a monetary bonus would be calculated based on accuracy. Stimuli were blocked, and in each block the participant had to learn stimulus-response mappings for two, three, four, or five images. Blocks consist of 11 trials per image and there were two blocks per type (two blocks each of 22, 33, 44, and 55 trials).
Executive Functioning Tasks.
Executive functioning was measured using a set of well-validated tasks that have been administered in developmental and clinical samples, and used in prior latent variable analysis (Friedman et al., 2008, 2016). The tasks featured overlapping demands for general executive control but differed in perceptual characteristics and specific task demands.
Antisaccade task.
The antisaccade task is a measure of response inhibition. For each trial, the participant viewed a fixation point presented at the center of the monitor. After a variable amount of time (delay ranging from 1500 to 3500ms, at increments of 250ms) a visual distractor was shown on one side of the screen for 150ms, after which a target stimulus appeared on the opposite side of the screen (target displayed for 250, 233, or 200 ms, then masked). The visual distractor was a small black square (1/8 inch) and the target stimulus was a number symbol (between 1 and 9). The participant was instructed to keep their eyes on the fixation point and not saccade to the visual distractor, instead saccading to the target stimulus in time to see it before it was masked, and then report the numeric value out loud to the research assistant. The participant completed 36 trials in which the distractor and stimulus were on the same side (target displayed for 200 ms), and 12 practice trials in which the distractor and stimulus were on opposite sides (target displayed for 250 ms). Then, the task consisted of three blocks (24 blocked trials of each target duration in ascending difficulty) of antisaccade trials in which the distractor and stimulus were on opposite sides.
Spatial N-Back Task.
The spatial n-back is a test of working memory updating ability. In each block of this task, the participant was presented with a display of twelve open squares distributed across the screen. For each trial, one square in the display turned black (“flashed”) for 500ms, followed by a 1500ms inter-trial interval. The participant was instructed to respond by pressing a button to indicate whether the square that flashed for that trial was the same spatial location as the square that flashed two trials earlier. Each block consisted of 24 trials, and within each trial there were six match trials (on which the correct answer was “yes”) and 18 reject trials (on which the correct answer was “no”; among these reject trials, there were five lures on which the same spatial location was flashed three trials earlier). Participants completed a practice block of 20 trials. The task consisted of four blocks (24 trials each).
Color-Shape Task.
The color-shape task is a test of mental set shifting. In this task, for each trial, a shape stimulus (red or blue, circle or triangle) appeared in the center of the screen accompanied by a cue letter (C or S) to indicate whether the participant should categorize the stimulus based on color or shape. The cue appeared 350 ms before the target and remained on the screen until the participant responded using one of two buttons (left for red or circle, right for blue or triangle). The next cue appeared 350 ms after the end of the trial. The participant started with 12 trials practicing identification of color, 12 trials practicing identification of shape, and then 24 trials practicing a mixed sequence in which a trial of one cue type could be preceded by the same (“repeat” trials) or the other (“switch” trials) cue type. The task consisted of two blocks (52 trials each) of mixed trials. The first four trials of each block were discarded, yielding 48 trials (half repeat, half switch trials) for each of the two task blocks for analysis.
Developmental Differences
Self-report measures were administered using either Qualtrics or REDCap software. A research staff member was available for questions outside the experiment room.
Age.
Participants reported age in years.
Pubertal Development Scale
(PDS, (Petersen et al., 1988)). The PDS is a self-report measure of physical pubertal changes which shows good reliability and correlates well with hormone measures (Shirtcliff et al., 2009). Individual items were summed and averaged to yield a PDS score that ranged from 1 to 4.
Gender.
Participants reported on sex and gender according to a two-step procedure that asks about current gender identity and sex assigned at birth. Gender was contrast-coded as cisgender male (−1), cisgender female (+1), or other, including transgender, non-binary, or gender-fluid (0).
Mood Pathology
See Supplement for exploratory analyses that considered diagnostic variables.
Mood and Anxiety Symptom Questionnaire- Anhedonia
(MASQ-AD, (Watson, Clark, Weber, & Assenheimer, 1995; Watson, Clark, Weber, Assenheimer, et al., 1995)). The 8-item MASQ-AD loss of interest scale was administered to evaluate anhedonic depression in the past week. Each item was rated on a scale of 1 (not at all) to 5 (extremely). The summed score provided a measure of anhedonic symptom severity.
General Behavior Inventory- Mania/Hypomania
(GBI-MH, (Depue et al., 1989)). The 10-item GBI-MH scale was administered to measure symptoms of mania or hypomania in the past week1. This measure has been used to assess current mania severity (Moriarity et al., 2020) in clinical and community samples (e.g., in the Adolescent Brain and Cognitive Development Study, (Barch et al., 2018)). Each item was rated on a scale of 0 (never or hardly ever) to 3 (very often or constantly). The summed score indexed severity of manic symptoms.
Analyses
Neurocognitive Functioning
The reward learning and executive functioning performance parameters selected for this study are widely used and well-validated (Friedman et al., 2016; Pizzagalli et al., 2005). These parameters were chosen based on evidence that these measures are sensitive to individual differences or development (Friedman et al., 2016; Master et al., 2020; Nussenbaum & Hartley, 2019) and to symptoms of mood pathology (Lawlor et al., 2020; Robinson & Chase, 2017; Zald & Treadway, 2017). The executive function performance parameters are the same as those used in prior latent variable research (Friedman & Miyake, 2017).
Performance Parameters.
Performance parameters from each task are described below with additional details in Supplement (Tables S2–3).
Two-armed Bandit Task.
Performance on the reward condition was modeled with a prediction error computational model (Daw, 2011; Frank et al., 2007; Rescorla & Wagner, 1972), programmed in R. See (Frank et al., 2007) for formulas and modeling approach. This model fits the participant’s trial-by-trial sequence of responses to the pair of stimuli that can elicit reward. The expected value of selecting one of the stimuli i (here, either the stimulus with an 80% probability of reward, or the stimulus with a 20% probability of reward) was estimated using the following equation for each participant:
Where t is trial number, and r(t) is the reward value for that trial (either 1=received reward, or 0=did not receive reward). (Note that r(t) - Qi(t) reflects the prediction error for that trial). Q is initialized at 0 at the beginning of the model. The learning rate parameter α that best fits each participant’s sequence of responses over time is selected to reflect the degree to which prior reinforcement outcomes affect subsequent Q values. In addition, the probability of choosing one stimulus (choosing A instead of B) was calculated using this equation for each participant:
Where β is the inverse temperature parameter reflecting the participant’s tendency to randomly choose a stimulus (explore) or to choose the stimulus with the currently-highest Q value (exploit). The model was fit to each participant’s sequence of responses, searching through the parameter space (1000 values and 200 random starts for each parameter). Model fit was estimated for each participant according to the log likelihood estimate fit of the model to each participant’s choices over time:
Guided by prior clinical and developmental research (Nussenbaum & Hartley, 2019; Zald & Treadway, 2017), we selected learning rate (α) as the primary performance parameter, and the quality of the model was assessed with the fit parameter (see below). Learning rate can be interpreted as the speed at which the highest reward association is learned for Q.
Probabilistic Reward Task.
Performance measures for this task (Pizzagalli et al., 2005) were computed using Python. The primary performance parameter was discriminability, a measure of the participant’s ability to accurately discriminate between the two stimuli (long and short mouths). The formula for computing discriminability is described in (Pizzagalli et al., 2005). Discriminability was calculated as:
Discriminability reflects the extent to which the participant is able to accurately discriminate between stimuli overall, reaping the maximum possible rewards for both trial types. We also computed a second parameter (response bias) that reflects the extent to which the participant develops an accuracy bias to respond more accurately to “rich” compared with “lean” stimuli over time (Supplement). Of note, both of these performance parameters have been associated with anhedonic depression (Lawlor et al., 2020; Pizzagalli et al., 2005). However, discriminability was selected as the primary performance parameter for this study because of superior covariance with reward learning parameters from other tasks (Supplement).
Instrumental Learning Task.
Performance on this task was operationalized as accuracy across trial blocks, calculated by estimating proportion accuracy for each block and averaging across blocks for a global measure.
Antisaccade Task.
The performance measure for the antisaccade task was proportion of correct responses across the antisaccade blocks (Friedman et al., 2016).
Spatial N-Back Task.
The performance measure for the spatial n-back task was proportion of correct responses across the task (Friedman et al., 2016).
Color-Shape Task.
The performance measure for the color-shape task was switch cost, or the difference between average reaction time on switch trials and average reaction time on repeat trials for each participant (Friedman et al., 2016). Reaction times for incorrect responses, for trials immediately following incorrect responses, below 200ms, and that were >3.32 times the median absolute deviation in each condition were removed before calculating condition averages (Wilcox & Keselman, 2003).
Data Quality Assurance and Checks.
Data quality was checked for each task and measure. Participants who failed quality assurance steps were removed from analyses involving that parameter (Table S2) (Friedman et al., 2016; Pizzagalli et al., 2005). For the probabilistic reward task, participants were removed who failed to discriminate above-chance level between stimulus types (<55% accuracy), who failed to achieve an overall reinforcement schedule of approximately 3:1 (reward ratio of rich:lean), or who showed high frequency of reaction times indicating invalid responses or outlier responses (>20% trials with RT<150ms or RT>2500ms). These performance checks are standard for this task (Pizzagalli et al. 2005). For the two-armed bandit task, participants were removed on the basis of poor fit for the prediction error model (fit <2). For other tasks, participants were removed who showed chance-level accuracy: instrumental learning task (<32% accuracy), antisaccade task (<20% accuracy), spatial n-back (<60% accuracy), and color-shape task (<60% accuracy). The distributions of all measures were evaluated (Table S2); all performance, developmental, and mood measures showed acceptable normality (skew <2, kurtosis<6) (Hair, 2010; Kline, 2011). Outlier checks failed to reveal measurements more than +/−3 standard deviations from mean scores.
Site Checks
There were no significant differences between sites in experimental variables or symptom severity, ps>0.13. There were significant differences between sites in average age and pubertal stage, ps<0.05, which was expected given the younger ages eligible for recruitment at the CU Boulder site (Table 1, Supplement). Supplementary analyses were performed to further evaluate potential site differences, including testing invariance in the factor structures across sites and repeating models with site as a covariate or moderator. There were no significant differences in factor structure or experimental effects between sites. (Supplement).
Modeling
All continuous variables were standardized for analyses. Task performance parameters that loaded negatively on a factor were reversed for SEM and Figures to enhance interpretability.
We conducted modeling in Mplus (Muthen & Muthen, 2017) using full information maximum likelihood estimation (i.e., including individuals missing subsets of data) with robust standard error estimates (robust maximum likelihood estimator; MLR). Neurocognitive factors were estimated with confirmatory factor analyses across performance parameters from reward processing tasks (reward learning performance factor), and across performance parameters from the executive function tasks (executive functioning factor). The structural equation model (SEM) tested a priori hypotheses regarding developmental patterns in neurocognitive factors and moderation of these developmental patterns by mood symptoms. The following predicted the reward learning performance factor: linear and quadratic effects of puberty, linear effects of anhedonic and manic symptom severity, and interactions between symptom measures and puberty variables (covariates were the linear and quadratic effects of age). In the same model, the following predicted the executive functioning factor: linear and quadratic effects of age, linear effects of anhedonic and manic symptom severity, and interactions between symptom measures and age variables (covariates were the linear and quadratic effects of puberty). Model fit was evaluated on the basis of confirmatory fit index (CFI) > 0.9 and root-mean-square error of approximation (RMSEA) < 0.06, (Hair, 2010). Model comparison was performed using the chi-squared difference test using the Satorra-Bentler scaled chi-square (Satorra, 2000). Associations between developmental or clinical variables and neurocognitive factors are reported as standardized estimates, with confidence intervals and (two-tailed) significance reported. To ensure the interaction effects were appropriately standardized, we standardized the components of the interactions prior to multiplying them, then used the STDY estimates from Mplus, which standardize the estimates with respect to the dependent variables. Thus, the products were not standardized and the interaction effects (e.g., of X1*X2) are interpretable as the change in X1’s standardized effect on Y with one standard deviation change in X2.
Results
Confirmatory Factor Analysis
Confirmatory factor analysis modeling the reward learning performance and executive functioning factors is reported in the Supplement (Figure S2). Significant indicator loadings supported the latent variable approach for evaluating individual differences in reward learning performance and executive functioning. Of note, reward learning performance and executive functioning were correlated.
Structural Equation Model
The SEM evaluated direct and moderated effects of puberty and mood symptom severity on the reward learning performance factor, and direct and moderated effects of age and mood symptom severity on the executive functioning factor (Figure 1). The model showed adequate fit, χ2 (72) = 89.87, p>0.05, RMSEA = 0.03 [90% CI 0.00 to 0.04], CFI = 0.92. The chi-square difference test showed this model was superior to the constrained model in which paths were fixed at zero, χ2 diff(20) = 81.40, p<0.05.
Figure 1. Structural Equation Model.
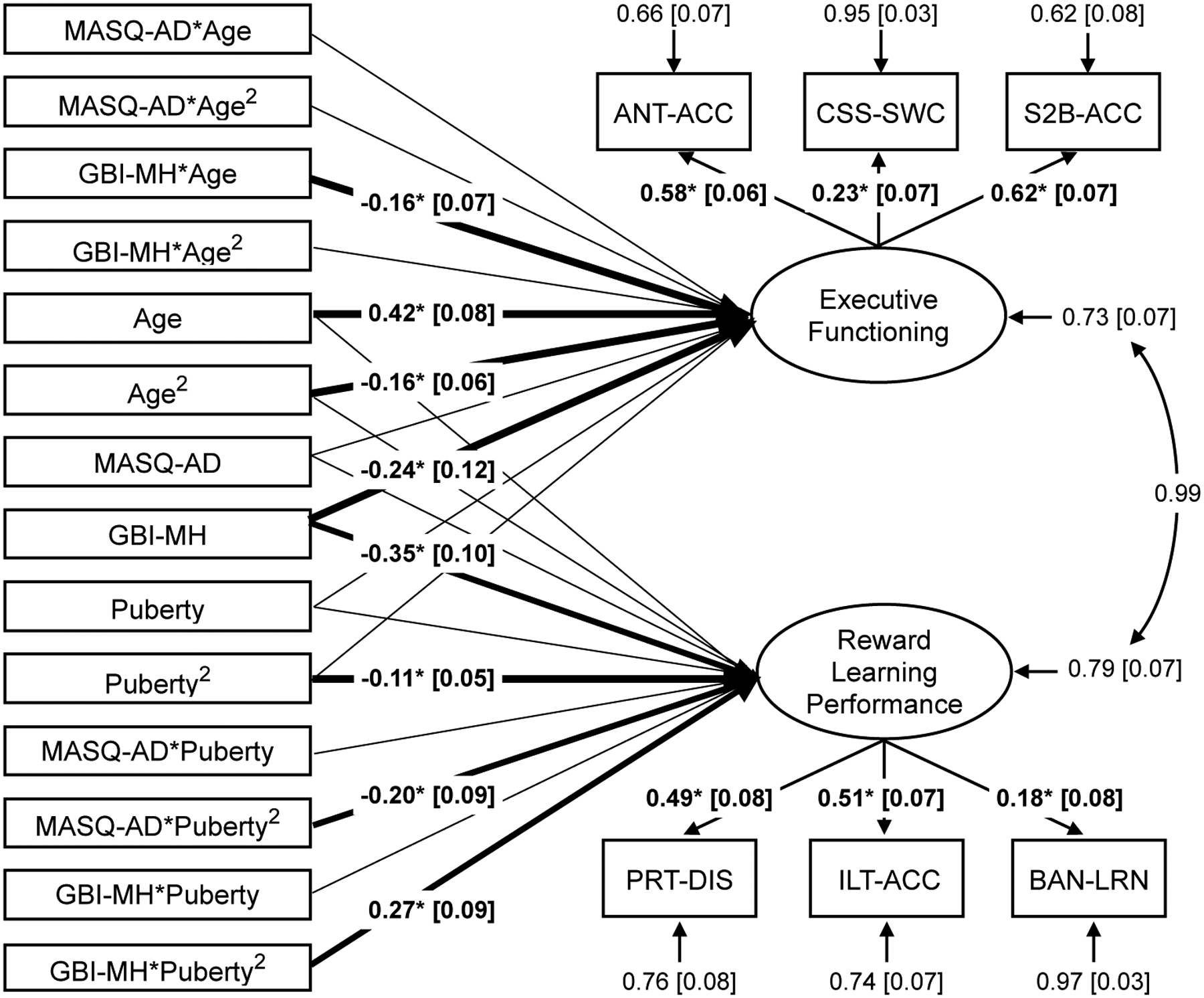
A structural equation model evaluated developmental differences in reward learning performance and executive functioning, and moderation of developmental differences by mood symptom severity. The reward learning performance factor was estimated across probabilistic reward task discriminability (PRT-DIS), instrumental learning task accuracy (ILT-ACC), and bandit task learning rate (BAN-LRN). The executive functioning factor was estimated across antisaccade accuracy (ANT-ACC), color-shape task reaction time switch cost (reversed for display, CSS-SWC), and spatial 2-back accuracy (S2B-ACC). The model tested the predictive linear and quadratic effects of pubertal stage (Pubertal Development Scale scores, PDS) and age on reward learning performance, and moderation of pubertal effects on reward learning performance by severity of manic symptoms (General Behavior Inventory-Mania/Hypomania scale, GBI-MH) and severity of anhedonic symptoms (Mood and Anxiety Symptom Questionnaire- Anhedonic Loss of Interest subscale, MASQ-AD). The model also tested predictive linear and quadratic effects of pubertal stage and age on executive functioning, and moderation of age effects on executive functioning by severity of manic or anhedonic symptoms. Note: Heavy lines with standardized estimates indicate significant path effects at *p<0.05.
There was a significant quadratic effect of pubertal status on reward learning performance, (standardized) estimate = −0.11 [90% CI −0.20 to −0.02], p=0.037, in which participants at early or late pubertal stages exhibited relatively lower reward learning performance than participants mid-puberty. The quadratic effect of pubertal status on reward learning performance was moderated by both anhedonic symptoms, (standardized) estimate = −0.20 [90% CI −0.35 to −0.05], p=0.027, and manic symptoms (standardized) estimate = 0.27 [90% CI 0.13 to 0.42], p=0.002. Adolescents who were more severely anhedonic exhibited lower reward learning performance especially at early pubertal stages, whereas adolescents who were more severely manic exhibited higher reward learning performance at early pubertal stages that declined at mid-to-late pubertal stages.
The linear and quadratic effects of age on executive functioning were significant, (standardized) linear estimate = 0.42 [90% CI 0.30 to 0.55], p<0.001, and (standardized) quadratic estimate = −0.16 [90% CI −0.25 to −0.06], p=0.008. Adolescents at older ages showed better executive functioning, and age-related improvements leveled off in the oldest adolescents. Linear age differences in executive functioning were moderated by manic symptom severity, (standardized) estimate = −0.16 [90% CI −0.28 to −0.05], p=0.022. Adolescents reporting higher mania showed worse executive functioning, especially at older ages. Anhedonia did not significantly moderate age differences in executive functioning.
Although we had no a priori hypotheses regarding gender, we repeated the above model including self-reported gender as a predictor (Supplement). Developmental and psychopathology effects were not significantly altered by the addition of gender (changes in standardized estimates <0.06, changes in ps<0.02).
Together, results showed pubertal differences in reward learning performance that were moderated by anhedonic or manic symptom severity, and age differences in executive functioning that were moderated by manic symptom severity (Figure 2).
Figure 2. Mood symptom severity moderates developmental differences in reward learning performance and executive functioning.
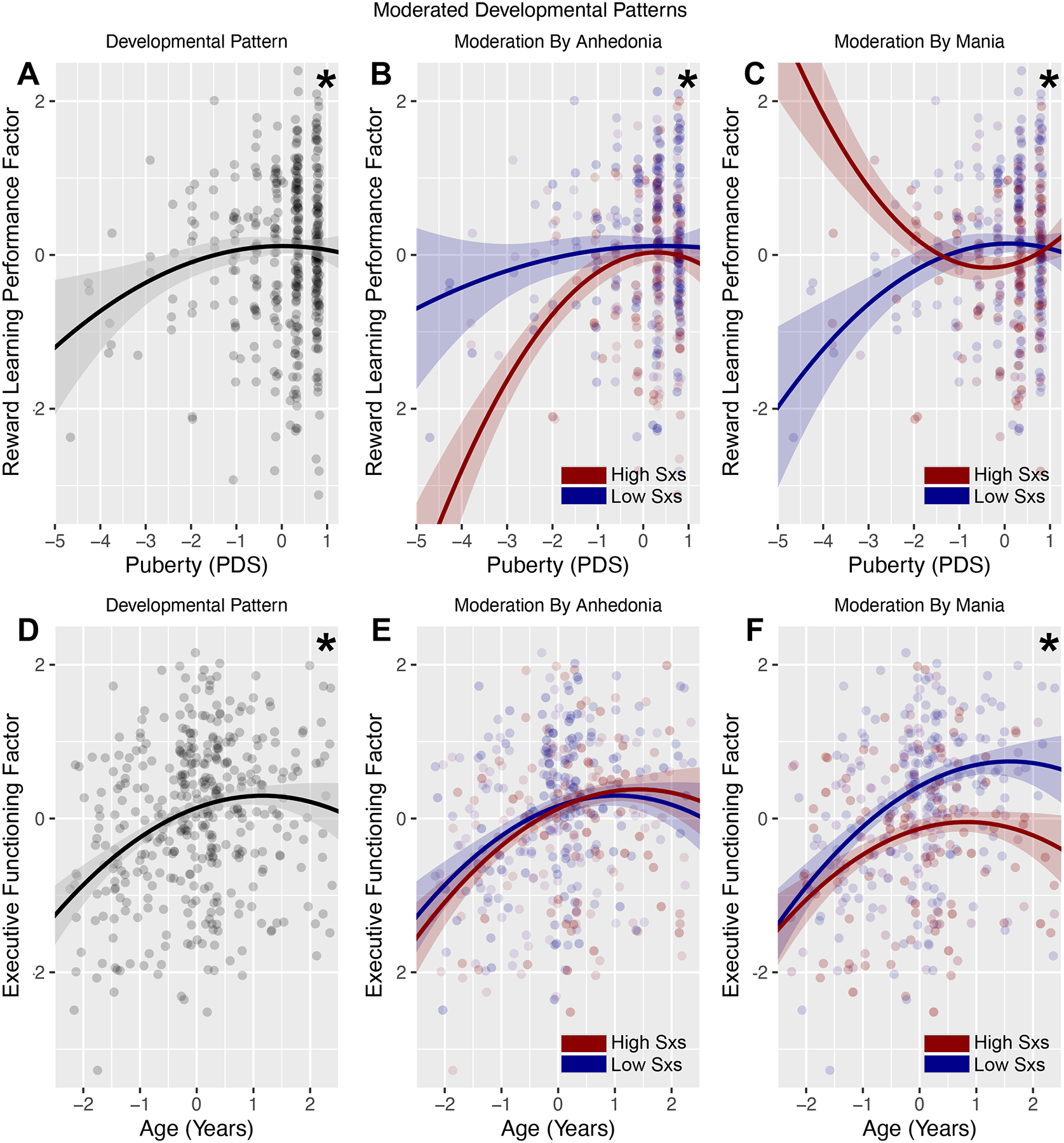
(A) Quadratic association between pubertal stage and reward learning performance factor scores. (B) Higher severity of anhedonic depression was associated with stronger quadratic pubertal differences in reward learning performance, including lower reward learning performance at earlier pubertal stages. (C) Higher severity of mania was associated with weaker or reversed quadratic pubertal differences in reward learning performance, including higher reward learning performance at earlier pubertal stages. (D) Curvilinear (linear and quadratic effects) association between age and executive functioning factor scores. (E) Severity of anhedonic depression did not significantly moderate age differences in executive functioning. (F) Higher severity of mania moderated linear age differences in executive functioning, in the form of poorer executive functioning especially at older ages. Note: Plots show extracted factor scores on the y axes for ease of presentation, but statistical associations among variables were estimated using structural equation models in which reward learning performance and executive functioning were modeled as latent variables. Displayed are lines fitted to models at low (−1.5 SD, blue) or high (+1.5 SD, red) levels of symptom severity, and point color is scaled to low (blue) to high (red) symptom severity. *p<0.05 developmental (A, D) or moderated (B-C, E-F) effects.
Discussion
The goals of this study were to replicate developmental differences in reward sensitivity and executive functioning, and test whether such developmental differences followed distinctive patterns in adolescents with high levels of anhedonic or manic symptoms. Results support a model in which dual-systems developmental differences emerge differently for adolescents with mood problems, but the nature of the developmental abnormalities depended on the nature of mood symptoms. Higher levels of manic symptoms were associated with higher reward learning performance at early pubertal stages but declining reward learning performance later in puberty, and poorer executive functioning especially at older ages. In contrast, higher levels of anhedonic symptoms were associated with lower reward learning performance at early pubertal stages. These findings inform models in which abnormal developmental trajectories correspond with specific dimensions of mood pathology, and motivate future longitudinal research to evaluate developmental and clinical changes over time.
In this cross-sectional adolescent sample, we replicated previous research demonstrating puberty-related curvilinear differences in reward sensitivity, and age-related linear (and curvilinear) differences in executive functioning (Geier & Luna, 2009; Shulman et al., 2016). Consistent with prior work, in this study mid-pubertal adolescents showed the highest reward learning performance (ability to maximize rewards across learning tasks), but reward learning performance was lower at early or late pubertal stages (Braams et al., 2015; Davidow et al., 2016). In contrast, adolescents at older ages showed better executive functioning (Steinberg et al., 2008). In the present study, reward learning performance and executive functioning were evaluated using a robust latent variable approach. Performance indexed by a single task parameter derives from a number of sources (Collins & Frank, 2012) and may reflect a mixture of neurocognitive abilities, contributing to mixed findings in developmental or clinical samples (Snyder et al., 2015). Latent variable approaches take advantage of shared processes across a set of conceptually-related tasks to derive a more reliable estimate of a common dimension (Friedman et al., 2016; Harden et al., 2018), and the present study leveraged this approach for replication.
The main contribution of this study is to show that developmental differences in reward sensitivity and executive functioning are altered in adolescents with currently elevated mood symptoms. These findings have implications for conceptual models of mood pathology and clinical application. First, results highlight the complexity of neurocognitive abnormalities as they manifest over development. Here, reward learning performance was elevated in more highly manic, early-puberty adolescents, but decreased over pubertal development as a function of symptom severity. This may reflect a developmental shift from reward hypersensitivity to hyposensitivity in this particular domain of reward processing, or changes in learning and performance ability that cause mania-related reward abnormalities to manifest differently over development. To explore these interpretations, it will be valuable to test how reward response changes over puberty or the course of illness. Second, these results point to developmental periods within adolescence when neurocognitive functioning may be altered in mood pathology, and therefore periods in which it is especially valuable to identify neurocognitive abnormalities that may inform risk prediction and intervention (Kujawa et al., 2020). Third, these results point to distinctive patterns of neurocognitive abnormality that may inform differential diagnosis in adolescence. Because early-pubertal reward hypersensitivity was related to manic symptom severity over and above elevated anhedonia (and even in the absence of bipolar diagnoses), reward processing abnormalities may be risk markers for bipolar illness (Alloy & Nusslock, 2019; Nusslock & Alloy, 2017). In light of the serious challenges distinguishing between unipolar and bipolar disorders in adolescence (Birmaher & Axelson, 2006), differential neurocognitive markers of illness are a needed advancement for clinical treatment.
These findings point to several important next steps in this research. One direction for this work will be to evaluate neurobiological and physiological mechanisms of mood-related neurocognitive anomalies (Galvan, 2010). It may be that among adolescents with mood pathology, abnormal reward learning performance in early puberty is driven by emerging abnormalities in pubertal hormone functioning: for example, elevated testosterone and lower estradiol in adolescents are associated with higher levels of reward sensitivity (Harden et al., 2018) and maturation of dopaminergic projections to medial prefrontal systems (Delevich et al., 2021). Individual differences in sex hormones have been linked with mania and depression, although effects are mixed across studies and commonly examined in adults (Johnson et al., 2013; Lombardo et al., 2021). Research that evaluates how changes in pubertal hormones track with striatal development and emerging mood symptoms may address these ideas. In turn, given evidence that adolescent improvements in executive functioning are related to maturation of prefrontal systems and synchrony among frontoparietal brain networks (Luna et al., 2015), research that explores differences in structural and functional connectivity of prefrontal regions may clarify and extend these results. For example, in the present study (and contrary to our hypotheses) anhedonia did not significantly moderate developmental patterns of executive functioning; neuroimaging may identify subtle differences in prefrontal development that do not emerge behaviorally.
A second important direction for this work is to extend to longitudinal research designs. The key limitation of the present study is that, in this cross-sectional design, it was only possible to test for differences related to developmental variables, but not to test developmental processes that unfold over time. Longitudinal evaluation of neurocognitive functioning is needed to characterize the temporal associations between, e.g., pubertal development and reward processing (Van Leijenhorst et al., 2010). Relatedly, longitudinal assessment would clarify the extent to which neurocognitive developmental trajectories correspond with onset of or fluctuations in mood pathology within a person. Cross-sectional mood symptoms reflect both state-dependent information about a given episode and trait-like information about overall illness severity (Sarapas et al., 2012). Only repeated measurement of mood over time can disentangle state- versus trait-like features, and address key questions about the timing of neurocognitive anomalies as related to the course of mood pathology. Finally, longitudinal assessment is a superior approach for disentangling neurocognitive changes associated with age versus puberty, as these variables tend to be correlated in cross-sectional research (Braams et al., 2015). In the present study, age and pubertal stage were correlated (r=0.52), but neurocognitive differences related to age versus puberty were significant when controlling for the other developmental variable. However, longitudinal studies can provide nuanced investigation e.g., of puberty-related changes in neurocognition that occur within age groups.
Several other limitations and future directions are noted. First, we chose to focus on common dimensions of reward learning performance and executive functioning, but these are non-unitary constructs that can also be decomposed into subdimensions (e.g., within reward learning performance, exploration). Second, there are other domains of reward processing and executive function that are not captured in the present study (e.g., reward liking, decision making, selective attention). Future research that expands to capture additional domains of reward and higher-order cognition, or that targets subdimensions of these neurocognitive domains, may provide complementary insight. Third, we note that although reward learning performance and executive functioning had different associations with developmental and symptom variables, these dimensions were highly correlated at the factor and indicator levels (Table S3). These patterns indicate that latent variables may have captured both independent processes unique to reward learning performance versus executive functioning, and shared processes that influence behavior across neurocognitive domains (e.g., motivation). Confirmatory factor analyses should be replicated. Fourth, this study relied on self-report measurement of puberty, and did not include pre-pubertal participants or the transition to puberty. Future research in younger samples and that captures other (e.g., hormone) measures of puberty is needed. Together, next steps for this work include replication and extension of the present findings in large-scale and well-powered longitudinal developmental samples.
In conclusion, this study used a latent-variable approach to evaluate age- or puberty-related differences in neurocognitive functioning in adolescence, and showed that youths with elevated symptoms of mania or anhedonia exhibited altered developmental patterns. Our findings replicate and extend research on reward and executive functioning in adolescent mood disorders, and suggest that mood-related abnormalities in neurocognition may manifest differently over development. More broadly, this work lies at the intersection of clinical, developmental and neurocognitive science, and highlights the value of interdisciplinary models to inform efforts to understand — and intervene in — adolescent mood disorders. Future longitudinal investigation will take the important next step of evaluating trajectories of neurocognitive development in adolescents with mood pathology.
Supplementary Material
Acknowledgements
With sincere thanks to Daniel Dillon for sharing a Python-based analytic pipeline for scoring the probabilistic reward task. Research supported by NARSAD Young Investigator Award (24879) and NIMH grant MH117131 (R.H.K.).
Footnotes
Declaration of Conflicting Interests
The authors declared no conflicts of interest with respect to the authorship or the publication of this article.
We note that due to researcher error, the instructional text preceding GBI items was inconsistent across sites in reminding the participant to consider the past-week timeframe when reporting on symptoms of mania. Sensitivity analyses were performed to ensure that this error did not engender site differences that influenced results, including testing site as a moderator of effects. No site-related differences were observed.
References
- Alloy LB, & Nusslock R (2019). Future Directions for Understanding Adolescent Bipolar Spectrum Disorders: A Reward Hypersensitivity Perspective. Journal of Clinical Child & Adolescent Psychology, 48(4), 669–683. 10.1080/15374416.2019.1567347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alloy LB, Olino T, Freed RD, & Nusslock R (2016). Role of Reward Sensitivity and Processing in Major Depressive and Bipolar Spectrum Disorders. Behavior Therapy, 47(5), 600–621. 10.1016/j.beth.2016.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andre Der-Avakian, Barnes Samuel A., Markou Athina, & Pizzagalli Diego A.. (2016). Translational Assessment of Reward and Motivational Deficits in Psychiatric Disorders. In Translational Neuropsychopharmacology (Vol. 28). Springer International Publishing. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayuso-Mateos JL, Nuevo R, Verdes E, Naidoo N, & Chatterji S (2010). From depressive symptoms to depressive disorders: The relevance of thresholds. British Journal of Psychiatry, 196(5), 365–371. 10.1192/bjp.bp.109.071191 [DOI] [PubMed] [Google Scholar]
- Barch DM, Albaugh MD, Avenevoli S, Chang L, Clark DB, Glantz MD, Hudziak JJ, Jernigan TL, Tapert SF, Yurgelun-Todd D, Alia-Klein N, Potter AS, Paulus MP, Prouty D, Zucker RA, & Sher KJ (2018). Demographic, physical and mental health assessments in the adolescent brain and cognitive development study: Rationale and description. Developmental Cognitive Neuroscience, 32, 55–66. 10.1016/j.dcn.2017.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC (2018). Evolving Concepts of Emotion and Motivation. Frontiers in Psychology, 9, 1647. 10.3389/fpsyg.2018.01647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertha EA, & Balazs J (2013). Subthreshold depression in adolescence: A systematic review. European Child & Adolescent Psychiatry, 22(10), 589–603. 10.1007/s00787-013-0411-0 [DOI] [PubMed] [Google Scholar]
- Best JR, & Miller PH (2010). A Developmental Perspective on Executive Function. Child Development, 81(6), 1641–1660. 10.1111/j.1467-8624.2010.01499.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birmaher B, & Axelson D (2006). Course and outcome of bipolar spectrum disorder in children and adolescents: A review of the existing literature. Development and Psychopathology, 18(04). 10.1017/S0954579406060500 [DOI] [PubMed] [Google Scholar]
- Biuckians A, Miklowitz DJ, & Kim EY (2007). Behavioral activation, inhibition and mood symptoms in early-onset bipolar disorder. Journal of Affective Disorders, 97(1–3), 71–76. 10.1016/j.jad.2006.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore S-J, Burnett S, & Dahl RE (2010). The role of puberty in the developing adolescent brain. Human Brain Mapping, 31(6), 926–933. 10.1002/hbm.21052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braams BR, van Duijvenvoorde ACK, Peper JS, & Crone EA (2015). Longitudinal Changes in Adolescent Risk-Taking: A Comprehensive Study of Neural Responses to Rewards, Pubertal Development, and Risk-Taking Behavior. Journal of Neuroscience, 35(18), 7226–7238. 10.1523/JNEUROSCI.4764-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Jones RM, & Hare TA (2008). The Adolescent Brain. Annals of the New York Academy of Sciences, 1124, 111–126. 10.1196/annals.1440.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins AGE, & Frank MJ (2012). How much of reinforcement learning is working memory, not reinforcement learning? A behavioral, computational, and neurogenetic analysis: Working memory in reinforcement learning. European Journal of Neuroscience, 35(7), 1024–1035. 10.1111/j.1460-9568.2011.07980.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland WE, Wolke D, Shanahan L, & Costello EJ (2015). Adult Functional Outcomes of Common Childhood Psychiatric Problems: A Prospective, Longitudinal Study. JAMA Psychiatry, 72(9), 892. 10.1001/jamapsychiatry.2015.0730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert B, & Insel T (2010). The Data of Diagnosis: New Approaches to Psychiatric Classification. Psychiatry, 5. [DOI] [PubMed] [Google Scholar]
- Davidow JY, Foerde K, Galván A, & Shohamy D (2016). An Upside to Reward Sensitivity: The Hippocampus Supports Enhanced Reinforcement Learning in Adolescence. Neuron, 92(1), 93–99. 10.1016/j.neuron.2016.08.031 [DOI] [PubMed] [Google Scholar]
- Daw N (2011). Trial-by-trial data analysis using computational modelx. In Decision Making, Affect, and Learning: Attention and Performance XXIII (pp. 3–37). Oxford University Press. [Google Scholar]
- Delevich K, Klinger M, Okada NJ, & Wilbrecht L (2021). Coming of age in the frontal cortex: The role of puberty in cortical maturation. Seminars in Cell & Developmental Biology, 118, 64–72. 10.1016/j.semcdb.2021.04.021 [DOI] [PubMed] [Google Scholar]
- Depue RA, Krauss S, Spoont MR, & Arbisi P (1989). General Behavior Inventory Identification of Unipolar and Bipolar Affective Conditions in a Nonclinical University Population. Journal of Abnormal Psychology, 98(2), 117–126. [DOI] [PubMed] [Google Scholar]
- Dixon T, Kravariti E, Frith C, Murray RM, & McGUIRE PK (2004). Effect of symptoms on executive function in bipolar illness. Psychological Medicine, 34(5), 811–821. 10.1017/S0033291703001570 [DOI] [PubMed] [Google Scholar]
- Forbes EE, & Dahl RE (2010). Pubertal development and behavior: Hormonal activation of social and motivational tendencies. Brain and Cognition, 72(1), 66–72. 10.1016/j.bandc.2009.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, & Dahl RE (2012). Research Review: Altered reward function in adolescent depression: what, when and how?: Reward function and adolescent depression. Journal of Child Psychology and Psychiatry, 53(1), 3–15. 10.1111/j.1469-7610.2011.02477.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Eckstrand KL, Rofey DL, & Silk JS (2021). A Social Affective Neuroscience Model of Risk and Resilience in Adolescent Depression: Preliminary Evidence and Application to Sexual and Gender Minority Adolescents. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 6(2), 188–199. 10.1016/j.bpsc.2020.07.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MJ, Moustafa AA, Haughey HM, Curran T, & Hutchison KE (2007). Genetic triple dissociation reveals multiple roles for dopamine in reinforcement learning. Proceedings of the National Academy of Sciences, 104(41), 16311–16316. 10.1073/pnas.0706111104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman N, & Miyake A (2017). Unity and diversity of executive functions: Individual differences as a window on cognitive structure. Cortex, 86, 186–204. 10.1016/j.cortex.2016.04.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman NP, du Pont A, Corley RP, & Hewitt JK (2018). Longitudinal Relations Between Depressive Symptoms and Executive Functions From Adolescence to Early Adulthood: A Twin Study. Clinical Psychological Science, 6(4), 543–560. 10.1177/2167702618766360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman NP, Miyake A, Altamirano LJ, Corley RP, Young SE, Rhea SA, & Hewitt JK (2016). Stability and change in executive function abilities from late adolescence to early adulthood: A longitudinal twin study. Developmental Psychology, 52(2), 326–340. 10.1037/dev0000075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman NP, Miyake A, Young SE, DeFries JC, Corley RP, & Hewitt JK (2008). Individual differences in executive functions are almost entirely genetic in origin. Journal of Experimental Psychology-General, 137(2), 201–225. 10.1037/0096-3445.137.2.201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffrey MS, Luby JL, & Barch DM (2013). Towards the study of functional brain development in depression: An Interactive Specialization approach. Neurobiology of Disease, 52, 38–48. 10.1016/j.nbd.2012.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan. (2010). Adolescent development of the reward system. Frontiers in Human Neuroscience. 10.3389/neuro.09.006.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geier C, & Luna B (2009). The maturation of incentive processing and cognitive control. Pharmacology, Biochemistry, and Behavior, 93(3), 212–221. 10.1016/j.pbb.2009.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hair JF (Ed.). (2010). Multivariate data analysis (7th ed). Prentice Hall. [Google Scholar]
- Harden KP, Mann FD, Grotzinger AD, Patterson MW, Steinberg L, Tackett JL, & Tucker-Drob EM (2018). Developmental differences in reward sensitivity and sensation seeking in adolescence: Testing sex-specific associations with gonadal hormones and pubertal development. Journal of Personality and Social Psychology, 115(1), 161–178. 10.1037/pspp0000172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn K, Roessner V, & Holtmann M (2011). Neurocognitive performance in children and adolescents with bipolar disorder: A review. European Child & Adolescent Psychiatry, 20(9), 433–450. 10.1007/s00787-011-0209-x [DOI] [PubMed] [Google Scholar]
- Huys QJ, Pizzagalli DA, Bogdan R, & Dayan P (2013). Mapping anhedonia onto reinforcement learning: A behavioural meta-analysis. Biology of Mood & Anxiety Disorders, 3(1), 12. 10.1186/2045-5380-3-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JM, Nachtigall LB, & Stern TA (2013). The Effect of Testosterone Levels on Mood in Men: A Review. Psychosomatics, 54(6), 509–514. 10.1016/j.psym.2013.06.018 [DOI] [PubMed] [Google Scholar]
- Joseph MF, Frazier TW, Youngstrom EA, & Soares JC (2008). A Quantitative and Qualitative Review of Neurocognitive Performance in Pediatric Bipolar Disorder. Journal of Child and Adolescent Psychopharmacology, 18(6), 595–605. 10.1089/cap.2008.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, & Walters EE (2005). Lifetime Prevalence and Age-of-Onset Distributions of DSM-IV Disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry, 62(6), 593. 10.1001/archpsyc.62.6.593 [DOI] [PubMed] [Google Scholar]
- Kline RB (2011). Principles and practice of structural equation modeling (3rd ed). Guilford Press. [Google Scholar]
- Kujawa A, Klein DN, Pegg S, & Weinberg A (2020). Developmental trajectories to reduced activation of positive valence systems: A review of biological and environmental contributions. Developmental Cognitive Neuroscience, 43, 100791. 10.1016/j.dcn.2020.100791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawlor VM, Webb CA, Wiecki TV, Frank MJ, Trivedi M, Pizzagalli DA, & Dillon DG (2020). Dissecting the impact of depression on decision-making. Psychological Medicine, 50(10), 1613–1622. 10.1017/S0033291719001570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RSC, Hermens DF, Scott J, Redoblado-Hodge MA, Naismith SL, Lagopoulos J, Griffiths KR, Porter MA, & Hickie IB (2014). A meta-analysis of neuropsychological functioning in first-episode bipolar disorders. Journal of Psychiatric Research, 57, 1–11. 10.1016/j.jpsychires.2014.06.019 [DOI] [PubMed] [Google Scholar]
- Lima IMM, Peckham AD, & Johnson SL (2018). Cognitive deficits in bipolar disorders: Implications for emotion. Clinical Psychology Review, 59, 126–136. 10.1016/j.cpr.2017.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardo G, Mondelli V, Dazzan P, & Pariante CM (2021). Sex hormones and immune system: A possible interplay in affective disorders? A systematic review. Journal of Affective Disorders, 290, 1–14. 10.1016/j.jad.2021.04.035 [DOI] [PubMed] [Google Scholar]
- Luna B, Marek S, Larsen B, Tervo-Clemmens B, & Chahal R (2015). An Integrative Model of the Maturation of Cognitive Control. Annual Review of Neuroscience, 38(1), 151–170. 10.1146/annurev-neuro-071714-034054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna B, & Wright C (2016). Adolescent brain development: Implications for the juvenile criminal justice system. In APA handbook of psychology and juvenile justice (pp. 91–116). American Psychological Association. 10.1037/14643-005 [DOI] [Google Scholar]
- Maresh EL, Stim JJ, Van Voorhis AC, Kang SS, Luciana M, Sponheim SR, & Urošević S (2019). Neurophysiological correlates of cognitive control and approach motivation abnormalities in adolescent bipolar disorders. Cognitive, Affective, & Behavioral Neuroscience, 19(3), 677–691. 10.3758/s13415-019-00719-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Master SL, Eckstein MK, Gotlieb N, Dahl R, Wilbrecht L, & Collins AGE (2020). Distentangling the systems contributing to changes in learning during adolescence. Developmental Cognitive Neuroscience, 41, 100732. 10.1016/j.dcn.2019.100732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy D, & Davison M (1979). Signal probability, reinforcement, and signal detection. Journal of the Experimental Analysis of Behavior, 32, 373–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre RS, Soczynska JK, Cha DS, Woldeyohannes HO, Dale RS, Alsuwaidan MT, Gallaugher LA, Mansur RB, Muzina DJ, Carvalho A, & Kennedy SH (2015). The prevalence and illness characteristics of DSM-5-defined “mixed feature specifier” in adults with major depressive disorder and bipolar disorder: Results from the International Mood Disorders Collaborative Project. Journal of Affective Disorders, 172, 259–264. 10.1016/j.jad.2014.09.026 [DOI] [PubMed] [Google Scholar]
- Mendle J (2014). Why Puberty Matters for Psychopathology. Child Development Perspectives, 8(4), 218–222. 10.1111/cdep.12092 [DOI] [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, & Wager TD (2000). The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: A latent variable analysis. Cognitive Psychology, 41(1), 49–100. 10.1006/cogp.1999.0734 [DOI] [PubMed] [Google Scholar]
- Moriarity DP, Ng T, Titone MK, Chat IK-Y, Nusslock R, Miller GE, & Alloy LB (2020). Reward Responsiveness and Ruminative Styles Interact to Predict Inflammation and Mood Symptomatology. Behavior Therapy, 51(5), 829–842. 10.1016/j.beth.2019.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthen LK, & Muthen BO (2017). Mplus user’s guide (8th ed.). Muthen & Muthen. [Google Scholar]
- Nielson DM, Keren H, O’Callaghan G, Jackson SM, Douka I, Zheng CY, Vidal-Ribas P, Pornpattananangkul N, Camp CC, Gorham LS, Wei C, Kirwan S, & Stringaris A (2020). Great Expectations: A Critical Review of and Recommendations for the study of Reward Processing as a Cause and Predictor of Depression. Biological Psychiatry, S0006322320317005. 10.1016/j.biopsych.2020.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nussenbaum K, & Hartley CA (2019). Reinforcement learning across development: What insights can we draw from a decade of research? Developmental Cognitive Neuroscience, 40, 100733. 10.1016/j.dcn.2019.100733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusslock R, & Alloy LB (2017). Reward processing and mood-related symptoms: An RDoC and translational neuroscience perspective. Journal of Affective Disorders, 216, 3–16. 10.1016/j.jad.2017.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusslock R, Almeida JR, Forbes EE, Versace A, Frank E, LaBarbara EJ, Klein CR, & Phillips ML (2012). Waiting to win: Elevated striatal and orbitofrontal cortical activity during reward anticipation in euthymic bipolar disorder adults. Bipolar Disorders, 14(3). 10.1111/j.1399-5618.2012.01012.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T, Keshavan M, & Giedd JN (2008). Why do many psychiatric disorders emerge during adolescence? Nature Reviews Neuroscience, 9(12), 947–957. 10.1038/nrn2513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen AC, Crockett L, Richards M, & Boxer A (1988). A self-report measure of pubertal status: Reliability, validity, and initial norms. Journal of Youth and Adolescence, 17(2), 117–133. 10.1007/BF01537962 [DOI] [PubMed] [Google Scholar]
- Phillips ML, & Swartz HA (2014). A Critical Appraisal of Neuroimaging Studies of Bipolar Disorder: Toward a New Conceptualization of Underlying Neural Circuitry and a Road Map for Future Research. American Journal of Psychiatry, 171(8), 829–843. 10.1176/appi.ajp.2014.13081008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA (2014). Depression, Stress, and Anhedonia: Toward a Synthesis and Integrated Model. Annual Review of Clinical Psychology, Vol 10, 10, 393–423. 10.1146/annurev-clinpsy-050212-185606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA, Goetz E, Ostacher M, Iosifescu DV, & Perlis RH (2008). Euthymic Patients with Bipolar Disorder Show Decreased Reward Learning in a Probabilistic Reward Task. Biological Psychiatry, 64(2), 162–168. 10.1016/j.biopsych.2007.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA, Jahn AL, & O’Shea JP (2005). Toward an objective characterization of an anhedonic phenotype: A signal detection approach. Biological Psychiatry, 57(4), 319–327. 10.1016/j.biopsych.2004.11.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes AN, Cardoso TA, Jansen K, Mondin TC, Souza LDM, Magalhães PVS, Kapczinski F, & Silva RA (2017). Functional impairment and cognitive performance in mood disorders: A community sample of young adults. Psychiatry Research, 251, 85–89. 10.1016/j.psychres.2017.01.069 [DOI] [PubMed] [Google Scholar]
- Rescorla Robert & Wagner Allan. (1972). A theory of Pavlovian conditioning: Variations in the effectiveness of reinforcement and nonreinforcement. In Classical conditioning: Current research and theory (pp. 64–99). Appleton-Century-Crofts. [Google Scholar]
- Robinson O, & Chase H (2017). Learning and Choice in Mood Disorders: Searching for the Computational Parameters of Anhedonia. Computational Psychiatry, 1, 208–233. 10.1162/CPSY_a_00009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock PL, Roiser JP, Riedel WJ, & Blackwell AD (2014). Cognitive impairment in depression: A systematic review and meta-analysis. Psychological Medicine, 44(10), 2029–2040. 10.1017/S0033291713002535 [DOI] [PubMed] [Google Scholar]
- Sarapas C, Shankman SA, Harrow M, & Goldberg JF (2012). Parsing trait and state effects of depression severity on neurocognition: Evidence from a 26-year longitudinal study. Journal of Abnormal Psychology, 121(4), 830–837. 10.1037/a0028141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satorra A (2000). Scaled and adjusted restricted tests in multi-sample analysis of moment structures. In Innovations in multivariate statistical analysis. A Festschrift for Heinz Neudecker (pp. 223–247). Kluwer Academic Publishers. [Google Scholar]
- Scult MA, Paulli AR, Mazure ES, Moffitt TE, Hariri AR, & Strauman TJ (2017). The association between cognitive function and subsequent depression: A systematic review and meta-analysis. Psychological Medicine, 47(1), 1–17. 10.1017/S0033291716002075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirtcliff EA, Dahl RE, & Pollak SD (2009). Pubertal Development: Correspondence Between Hormonal and Physical Development: Hormonal Correlates of Pubertal Stage. Child Development, 80(2), 327–337. 10.1111/j.1467-8624.2009.01263.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman EP, Smith AR, Silva K, Icenogle G, Duell N, Chein J, & Steinberg L (2016). The dual systems model: Review, reappraisal, and reaffirmation. Developmental Cognitive Neuroscience, 17, 103–117. 10.1016/j.dcn.2015.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder HR (2013). Major Depressive Disorder is associated with broad impairments on neuropsychological measures of executive function: A meta-analysis and review. Psychological Bulletin, 139(1), 81–132. 10.1037/a0028727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder HR, Miyake A, & Hankin BL (2015). Advancing understanding of executive function impairments and psychopathology: Bridging the gap between clinical and cognitive approaches. Frontiers in Psychology, 6, 24. 10.3389/fpsyg.2015.00728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L, Albert D, Cauffman E, Banich M, Graham S, & Woolard J (2008). Age differences in sensation seeking and impulsivity as indexed by behavior and self-report: Evidence for a dual systems model. Developmental Psychology, 44(6), 1764–1778. 10.1037/a0012955 [DOI] [PubMed] [Google Scholar]
- Urošević S, Luciana M, Jensen JB, Youngstrom EA, & Thomas KM (2016). Age associations with neural processing of reward anticipation in adolescents with bipolar disorders. NeuroImage: Clinical, 11, 476–485. 10.1016/j.nicl.2016.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urošević S, Youngstrom EA, Collins P, Jensen JB, & Luciana M (2016). Associations of age with reward delay discounting and response inhibition in adolescents with bipolar disorders. Journal of Affective Disorders, 190, 649–656. 10.1016/j.jad.2015.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Leijenhorst L, Zanolie K, Van Meel CS, Westenberg PM, Rombouts SARB, & Crone EA (2010). What Motivates the Adolescent? Brain Regions Mediating Reward Sensitivity across Adolescence. Cerebral Cortex, 20(1), 61–69. 10.1093/cercor/bhp078 [DOI] [PubMed] [Google Scholar]
- Van Rheenen TE, Lewandowski KE, Bauer IE, Kapczinski F, Miskowiak K, Burdick KE, & Balanzá-Martínez V (2020). Current understandings of the trajectory and emerging correlates of cognitive impairment in bipolar disorder: An overview of evidence. Bipolar Disorders, 22(1), 13–27. 10.1111/bdi.12821 [DOI] [PubMed] [Google Scholar]
- Van Rheenen TE, Miskowiak K, & Burdick KE (2021). Recognising the relevance of cognitive dysfunction in the clinical management of bipolar disorder. Bipolar Disorders, bdi.13063. 10.1111/bdi.13063 [DOI] [PubMed] [Google Scholar]
- Vrieze E, Pizzagalli DA, Demyttenaere K, Hompes T, Sienaert P, de Boer P, Schmidt M, & Claes S (2013). Reduced Reward Learning Predicts Outcome in Major Depressive Disorder. Biological Psychiatry, 73(7), 639–645. 10.1016/j.biopsych.2012.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner S, Müller C, Helmreich I, Huss M, & Tadić A (2015). A meta-analysis of cognitive functions in children and adolescents with major depressive disorder. European Child & Adolescent Psychiatry, 24(1), 5–19. 10.1007/s00787-014-0559-2 [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Weber K, & Assenheimer JS (1995). Testing a tripartite model II: Exploring the symptom structure of anxiety and depression in student, adult, and patient samples. Journal of Abnormal Psychology, 104(1), 15–25. 10.1037/0021-843x.104.1.15 [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Weber K, Assenheimer JS, Strauss ME, & McCormick RA (1995). Testing a tripartite model I: Evaluating the convergent and discriminant validity of anxiety and depression symptom scales. Journal of Abnormal Psychology, 104(1), 3–14. 10.1037/0021-843x.104.1.3 [DOI] [PubMed] [Google Scholar]
- Yazici E, Bursalioglu FS, Aydin N, & Yazici AB (2013). Menarche, puberty and psychiatric disorders. Gynecological Endocrinology, 29(12), 1055–1058. 10.3109/09513590.2013.829447 [DOI] [PubMed] [Google Scholar]
- Zald DH, & Treadway MT (2017). Reward Processing, Neuroeconomics, and Psychopathology. Annual Review of Clinical Psychology, 13(1), 471–495. 10.1146/annurev-clinpsy-032816-044957 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


