Abstract
Background and aims
Compulsive sexual behavior disorder (CSBD) has been included as an impulse control disorder in the International Classification of Diseases (ICD-11). However, the neurobiological mechanisms underlying CSBD remain largely unknown, and given previous indications of addiction-like mechanisms at play, the aim of the present study was to investigate if CSBD is associated with structural brain differences in regions involved in reward processing.
Methods
We analyzed structural MRI data of 22 male CSBD patients (mean = 38.7 years, SD = 11.7) and 20 matched healthy controls (HC; mean = 37.6 years, SD = 8.5). Main outcome measures were regional cortical thickness and surface area. We also tested for case-control differences in subcortical structures and the effects of demographic and clinical variables, such as CSBD symptom severity, on neuroimaging outcomes. Moreover, we explored case-control differences in regions outside our hypothesis including white matter.
Results
CSBD patients had significantly lower cortical surface area in right posterior cingulate cortex than HC. We found negative correlations between right posterior cingulate area and CSBD symptoms scores. There were no group differences in subcortical volume.
Conclusions
Our findings suggest that CSBD is associated with structural brain differences, which contributes to a better understanding of CSBD and encourages further clarifications of the neurobiological mechanisms underlying the disorder.
Keywords: compulsive sexual behavior, hypersexual disorder, neuroimaging, surface area, reward, brain structure
Introduction
According to the International Statistical Classification of Diseases and Related Health Problems (ICD-11) (World Health Organization, [WHO], 2022), compulsive sexual behavior disorder (CSBD) is characterized by a persistent pattern of failure to control intense sexual impulses or urges often resulting in repetitive sexual behavior. In CSBD, out-of-control sexual behaviors are pursued regardless of adverse consequences. Approximately 3–5% of the general population suffer from CSBD, rendering it a significant health burden (Bőthe et al., 2020; Briken et al., 2022). While promising treatment options are already been offered, their effectiveness can potentially be improved (Antons et al., 2022; Borgogna, Garos, Meyer, Trussell, & Kraus, 2022; Briken, 2020; Turner et al., 2022).
Despite CSBD being classified as an impulse control disorder in ICD-11, the underlying neurobiological mechanisms remain substantially unknown (Derbyshire & Grant, 2015). While similar mechanisms to those playing a role in obsessive compulsive and substance use disorders may be involved in CSBD (Coleman, 1991; Gola et al., 2017; Voon et al., 2014), impairments of brain regions regulating sexual arousal and desire have also been proposed (Blum, Badgaiyan, & Gold, 2015; Carnes et al., 2012; Derbyshire & Grant, 2015; Estellon & Mouras, 2012; Kingston & Bradford, 2013; Kor, Fogel, Reid, & Potenza, 2013; Kraus, Voon, & Potenza, 2016; Kuhn & Gallinat, 2016; Weinstein, Katz, Eberhardt, Cohen, & Lejoyeux, 2015). Neuroimaging studies suggested that processing of sexual stimuli is altered in CSBD patients (Stark, Klucken, Potenza, Brand, & Strahler, 2018) and that CSBD is associated with altered functioning in brain areas involved in sensitization, habituation, impulse control, and importantly reward processing (Kowalewska et al., 2018), e.g., fronto-temporal cortices, amygdala, and ventral striatum (Gola & Draps, 2018; Kowalewska et al., 2018; Voon et al., 2014). In summary, previous studies strongly indicate that the brain reward system plays a crucial role in CSBD (Kowalewska et al., 2018; Schmidt et al., 2017; Voon et al., 2014), and that the underlying key mechanisms are similar to those found in substance or behavioral addictions (Gola & Draps, 2018; Kowalewska et al., 2018; Mechelmans et al., 2014). However, structural MRI studies in CSBD are scarce and the brain structural correlates of CSBD remain largely unknown (Balodis & Potenza, 2015).
The aim of the present study was to test for differences in brain structure between CSBD patients and healthy controls, after correcting for age. We focused on predefined regions of interest known to be involved in reward processing. We analyzed structural MRI data from 22 men with CSBD and 20 healthy controls (HC). Main outcome measures were cortical thickness and surface area. We also tested for subcortical differences and correlations between MRI-derived phenotypes and CSBD symptoms. We also explored case-control differences in cortical regions outside our hypothesis and white matter underlying all cortical regions investigated. Moreover, we conducted sensitivity analyses testing for the effects of demographic and clinical variables on our outcomes, such as medication use and other clinical characteristics.
Methods
Participants
The study was conducted at ANOVA, a multidisciplinary clinic for research, assessment, and treatment in the fields of andrology, sexual and transgender medicine at the Karolinska University Hospital, and at Karolinska Institutet, Stockholm, Sweden, Patients seeking treatment for CSBD at ANOVA were recruited through the phone helpline PrevenTell, designed to prevent sexual offences by targeting persons at risk or with unwanted sexual behaviors. Details on recruitment, eligibility criteria, and procedures can be found in the Supplemental Material and elsewhere (Hallberg et al., 2020; Liberg et al., 2022; Savard et al., 2020). In brief, psychiatric diagnoses were established through interviews with clinical psychologists and psychiatrists, including the Mini-International Neuropsychiatric Interview (M.I.N.I. v7.0) (Sheehan et al., 1998), and a semi-structured clinical interview focusing on compulsive sexual behavior. Male participants who met criteria for Hypersexual Disorder (HD) as proposed for DSM-5 (Kafka, 2010) and for CSBD according to ICD-11 were included in the present study. Age-matched controls were recruited through online and public advertisements. Controls were physically and psychologically healthy and screened negative for CSBD, e.g. a score below 20 points on the Hypersexual Disorder Screening Inventory (HDSI) (Parsons et al., 2013), and below 53 points on the Hypersexual Behavior Inventory (HBI) (Reid, Garos, & Carpenter, 2011). Patients had not started any psychological treatment.
General inclusion criteria were being male, at least 18 years of age, and fluent in the Swedish language. Detailed exclusion criteria are listed in the Supplemental Material. In short, for both patients and controls, we excluded for MRI contraindications, severe neurological/psychiatric diseases or conditions, current alcohol or substance dependence, pathological gambling or other behavioral addictions, untreated endocrine diseases, and patients using specific medications. Patients with paraphilias (except for pedophilic interests) were included.
We included 23 CSBD males, of which 22 underwent an MRI scan, and 20 HC. While we previously reported functional MRI findings including the same study cohort (Liberg et al., 2022), this study focusses on structural MRI outcomes.
Measures
Clinical characteristics and questionnaires
Clinical characteristics were also assessed through questionnaires; e.g. symptoms of compulsive sexual behavior and neurodevelopmental disorders, depression, drug- and alcohol use and impulsivity. In brief, CSBD related symptoms were assessed using the Hypersexual Behavior Inventory (HBI), a 19-item scale constructed to reflect the proposed criteria of hypersexual disorder (Reid et al., 2011), the Sexual Compulsivity Scale (SCS), a 10-item scale that assesses compulsive sexual behaviors and urges (Kalichman & Rompa, 1995), and the Hypersexual Disorder Screening Inventory (HDSI), a screening questionnaire according to the DSM-5 criteria (Parsons et al., 2013).
We further assessed sexual desire using the Sexual Desire Inventory (SDI, 14 items) (Spector, Carey, & Steinberg, 1996) and severity of depression using the Montgomery Åsberg Depression Rating Scale (MADRS-S, 9 items) (Svanborg & Asberg, 2001). The full list of questionnaires used in this study can be found in Supplemental Material. Finally, we assessed the frequency of sexual encounters within the last 6 months, as well as weekly pornography consumption.
Magnetic resonance imaging
A 3T GE MRI scanner (Discovery MR750) equipped with an eight-channel head coil was used. Sagittal T1-weighted images were acquired using a 3D-BRAVO sequence (TR = 6.40 ms, TE = 2.81 ms, FOV = 24.0 cm, flip angle = 12°, TI = 450 ms, acquisition matrix 240 × 240 × 180, voxel size 1 × 1 × 1 mm3).
Procedure
Image processing and region of interest selection
Measures for regional cortical thickness and surface area of 34 regions per hemisphere (Desikan atlas (Desikan et al., 2006)) were obtained from structural T1-weighted images using the semi-automated cortical surface reconstruction and parcellation methods provided by FreeSurfer (Dale, Fischl, & Sereno, 1999; Fischl & Dale, 2000; Fischl, Salat, et al., 2004; Fischl, Sereno, & Dale, 1999; Fischl, van der Kouwe, et al., 2004). The primary analysis was a hypothesis-driven region of interest (ROI) approach and focused on cortical brain areas involved in reward processes. The main selection was done using neurosynth.org (keyword “reward”) which resulted in the selection of following seven cortical structures: medial and lateral orbitofrontal cortex, rostral and caudal cingulate cortex, posterior cingulate cortex, anterior insula, and superior frontal cortex. Three subcortical structures were investigated: nucleus accumbens, amygdala, and caudate. Considering left and right hemispheres, this yielded 14 cortical and six subcortical ROIs. The regions selected align with those reported to be involved in reward-related processes according to recent review articles (Oldham et al., 2018). In addition, the selected ROIs largely overlap with regions related to the processing of visual sexual stimuli and arousal (Georgiadis & Kringelbach, 2012), which is of further interest for CSBD. Regions outside of the main hypothesis were analyzed in exploratory whole brain analyses as well as volume of white matter underlying these regions. Volumetric measures were also obtained using FreeSurfer.
Statistical analyses
Group characteristics (demographic and clinical data)
Demographic and clinical variables were compared using t-tests or Fisher's exact Chi2.
Differences in brain structure between CSBD patients and healthy controls
In main analyses, we tested for the effect of group (CSBD vs HC; independent variable of interest) on cortical thickness (and area), as well as subcortical volumes. Age was used as covariate to account for potential age-related variance in the MRI data given that cortical changes occur along with age. For each imaging phenotype (thickness, area, subcortical volumes) the tests were separately conducted in SPSS v26 using multiple univariate analyses of covariance (ANCOVAs), where brain imaging phenotypes were set as dependent variables, group was entered as fixed factor and age was set as covariate. Since cortical volume is a function of the two genetically and phenotypically independent measures cortical thickness and surface area, these measures were analyzed separately, and cortical volume analyses were omitted (Winkler et al., 2010). We further treated cortical and subcortical analyses as independent studies, which are often reported separately (Hibar et al., 2016, 2018). However, we report cortical and subcortical findings in the same manuscript. Although we did not have lateral hypotheses, we did not combine measures from left and right hemisphere to total measures. While this increased the number of tests conducted for each phenotype, this approach was chosen to provide lateral information. Hence, within each imaging phenotype, we corrected for multiple testing using Bonferroni's Dubey Armitage-Parmar/Sidak's adjustment of α-level, which considers the number of regions (N = 14 for cortical regions, and N = 6 for the subcortical regions) performed and their inter-correlation (rthickness = 0.41, rarea = 0.41, rsubcortical = 0.37; leading to adjusted α-levels of αthickness = αarea = 0.013, αsubcortical = 0.016) (Sankoh, Huque, & Dubey, 1997).
Distributions of dependent variables were tested for normality using the one-sample Kolmogorov-Smirnov test. Mean differences between HC and CSBD subjects were quantified with Cohen's d. Results were visualized with ENIGMA Viewer v.2016.07.08, where p-values and effect size (Cohen's d) for each brain regions were color-graded and mapped to into the brain space.
Correlations between structural brain phenotypes and CSBD symptom scores (exploratory)
The primary findings were further complemented with regression analyses testing for correlations between brain regions that showed group differences and CSBD symptoms scores. These analyses were performed in the combined sample of patients and controls to a) widen the range of symptoms and b) increase statistical power. Another rationale was to test if a relationship between symptom scores and structural brain variation can be detected regardless of a categorical diagnostic label. Brain measures were dependent variables, symptoms scores and age were independent variables. We tested for correlations with the following symptoms scores (one at a time): HDSI, HBI, SDI and SCS. Analyses were also run separately within patients and controls, and in the combined cohort while controlling for case-control status, to test if observed correlations were driven by case-control differences. Note, that a correlation driven by case-control differences can still reflect a genuine relationship between CSBD and brain structure, and results obtained when controlling for case-control status should be interpreted with caution.
Sensitivity analyses
In sensitivity analyses testing for potential confounders, the main analysis was repeated while adding potential confound variables as covariates. These included demographic or clinical variables (e.g., comorbidities, medication use) as listed in Table 1. The full list of variables tested for in sensitivity analyses can be found the Supplemental Material (Table S1a). Such variables were entered one at a time in the ANCOVA models. In additional tests, when fewer than ten participants had a specific comorbidity or used a specific medication, the sensitivity analyses were repeated one at a time after excluding those individuals. CSBD symptom scores were not controlled for, as they relate to the phenotype of interest and controlling for these was assumed to remove variance related to the effects of interest (Hyatt et al., 2020).
Table 1.
Demographic and clinical characteristics
| Measure | HC (n = 20) | CSBD (n = 23) | HC vs. CSBD (p-value) |
| Age, mean (SD) | 37.6 (8.5) | 38.7 (11.7) | 0.741 |
| BMI, mean (SD) | 23.1 (2.8) | 25.8 (4.5) | 0.026 |
| Nicotine use (yes/no/sometimes), n | |||
| Moist snuff | 3/16/0* | 7/13/0* | 0.157 |
| Smoking | 0/16/4 | 0/21/0* | 0.048 |
| Handedness (R/L/M), n | 16/4/0 | 16/1/1* | 0.822 |
| Sexual orientation | |||
| Self-identified homosexual, n | 1 | 1 | 0.919 |
| Kinsey scale, mean (SD) | 0.6 (1.1) | 0.71 (1.3) | 0.778 |
| HDSI, mean (SD) | 1.9 (2.2) | 20.2 (3.8) | <0.001 |
| HBI, mean (SD) | 22.5 (4.1) | 69.4 (13.4) | <0.001 |
| SDI, mean (SD) | 55.2 (12.6) | 80.6 (17.1) | <0.001 |
| SCS, mean (SD) | 11.2 (0.9) | 29.4 (6.3) | <0.001 |
| Pornography consumption | |||
| times per week, mean (SD) | 2.2 (2.3) | 13.0 (20.7) | 0.033 |
| hours per week, mean (SD) | 0.7 (0.7) | 9.2 (8.0) | <0.001 |
| age first consumption, mean (SD) | 14.2 (3.4) | 13.2 (4.9) | 0.424 |
| MADRS-S, mean (SD) | 3.9 (4.9) | 18.3 (7.8) | <0.001 |
| AUDIT, mean (SD) | 4.1 (3.8) | 6.3 (3.8) | 0.059 |
| DUDIT, mean (SD) | 2.7 (4.5) | 2.1 (3.0) | 0.582 |
| RAADS, mean (SD) | 6.1 (6.0) | 11.1 (7.7) | 0.025 |
| ASRS, mean (SD) | 14.7 (10.6) | 34.2 (11.7) | <0.001 |
| BIS-11, mean (SD) | 53.1 (7.3) | 66.7 (10.8) | <0.001 |
| BIS/BAS | |||
| BAS drive, mean (SD) | 7.4 (2.3) | 9.0 (2.7) | 0.048 |
| BAS fun seeking, mean (SD) | 10.5 (2.5) | 11.9 (1.7) | 0.037 |
| BAS reward response, mean (SD) | 16.3 (2.1) | 16.5 (1.6) | 0.726 |
| BIS, mean (SD) | 17.9 (5.1) | 20.7 (3.1) | 0.033 |
| STAI-S, mean (SD) | 9.3 (2.0) | 12.6 (2.5) | <0.001 |
Notes: Demographic and clinical characteristics mean (SD) or number of participants (n) of healthy controls (HC) and males with Compulsive Sexual Behavior Disorder (CSBD) are provided. Results (p-values) of group comparisons are presented. Sexual orientation was measured through a 7-point Kinsey scale. Data reported for all patients enrolled. * indicates variables with missing data. Abbreviations: Hypersexual Disorder Screening Inventory (HDSI), Hypersexual Behavior Inventory (HBI), Sexual Desire Inventory (SDI), Sexual Compulsivity Scale (SCS), Montgomery Asberg Depression Rating Scale (MADRS-S), Alcohol Use Disorders Identification Test (AUDIT), Drug Use Disorders Identification Test (DUDIT), Ritvo Autism Asperger Diagnostic Scale (RAADS), Adult ADHD Self-Report Scale (ASRS), Barratt Impulsiveness Scale (BIS-11), Behavioral Inhibition/Activation System (BIS/BAS), State-Trait Anxiety Inventory - State (STAI-S).
Ethics
The study was carried out in accordance with the Declaration of Helsinki. All participants were informed about the procedures and provided informed consent. The study was approved by the regional Ethical Review Board in Stockholm, Sweden and was preregistered at clinicaltrials.gov (NCT03495414).
Results
Participants
Patient characteristics are shown Table 1. Note that these have previously been reported as part of our functional MRI study (Liberg et al., 2022). CSBD and HC males matched on age, sexual orientation and handedness. HC had lower BMI than CSBD males. With the exception of four occasional smokers in the HC group, both groups contained only non-smoking individuals. While HC had no psychiatric comorbidities, one patient reported panic disorder, and two patients reported depression, generalized anxiety disorder (GAD), and attention-deficit/hyperactivity disorder (ADHD), respectively (Table S1b). In line with this, two patients used stimulants, and four patients used antidepressants (Table S1b). However, groups did not significantly differ in psychiatric comorbidity or medication use. While age was controlled for in main analyses, we conducted further sensitivity tests to test for any potential confounds by demographic and clinical variables (see below).
CSBD patients scored significantly higher than HC on all questionnaires, except on a reward response subscale (BAS), where no group differences were found. CSBD males used pornography more frequently and for longer periods than HC. There were no group differences in age of onset of pornography use, nor for drug and alcohol consumption. We did not find group differences with respect to the frequency of sexual encounters or the number of sexual partners within the last six months (Table S2).
Main analysis
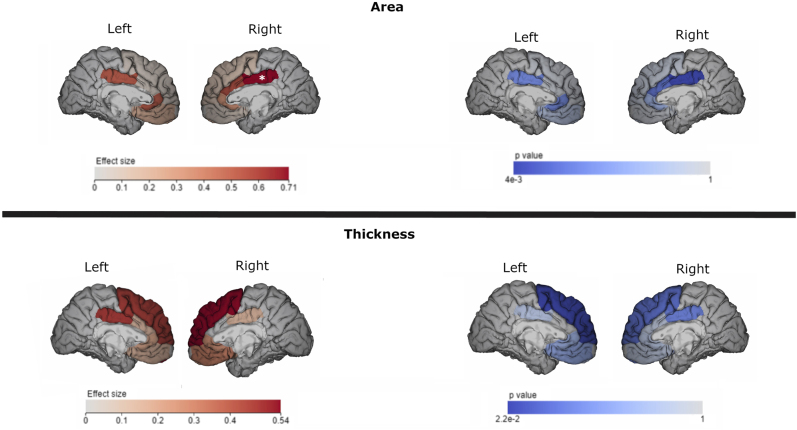
After multiple comparison correction, we found a significant difference between HC and CSBD patients in right posterior cingulate surface area (p = 0.004, Cohen's d = 0.95, Table 2), with CSBD patients showing lower surface area than the control group. Differences in left posterior cingulate and right caudal anterior cingulate (cACC) cortical surface area were statistically significant on the p < 0.05 level but did not survive multiple comparison corrections (Table 2). Similarly, left superior frontal thickness differed between HC and CSBD patients (p = 0.022, Cohen's d = 0.58, Table 3) but did not withstand multiple comparison corrections. Effect sizes were moderate to large (Tables 2 and 3). Findings are displayed in Fig. 1 (and Figure S1). There were no group differences in subcortical volume (Table S3).
Table 2.
Group differences in cortical surface area
| HC | CSBD | ||||||
| Brain region | Mean | SD | Mean | SD | F (1, 39) | p | Cohens d |
| Lh lateral orbito frontal | 2,861 | 276 | 2780 | 291 | 0.846 | 0.363 | 0.29 |
| Lh medial orbito frontal | 2,098 | 262 | 2,034 | 195 | 0.834 | 0.367 | 0.28 |
| Lh caudal anterior cingulate | 666 | 141 | 677 | 111 | 0.069 | 0.795 | −0.08 |
| Lh rostral anterior cingulate | 917 | 144 | 876 | 166 | 0.688 | 0.412 | 0.26 |
| Lh posterior cingulate | 1,148 | 119 | 1,260 | 187 | 5.086 | 0.030* | −0.71 |
| Lh insula | 2,501 | 181 | 2,486 | 247 | 0.052 | 0.822 | 0.07 |
| Lh superior frontal | 7,805 | 719 | 7,748 | 665 | 0.07 | 0.793 | 0.08 |
| Rh lateral orbito frontal | 2,969 | 336 | 2,891 | 325 | 0.583 | 0.450 | 0.24 |
| Rh medial orbito frontal | 2,106 | 171 | 2,092 | 170 | 0.079 | 0.780 | 0.09 |
| Rh caudal anterior cingulate | 764 | 148 | 664 | 111 | 6.166 | 0.017* | 0.76 |
| Rh rostral anterior cingulate | 655 | 111 | 609 | 92 | 2.082 | 0.157 | 0.45 |
| Rh posterior cingulate | 1,302 | 181 | 1,157 | 117 | 9.422 | 0.004** | 0.95 |
| Rh insula | 2,422 | 218 | 2,474 | 284 | 0.429 | 0.516 | −0.21 |
| Rh superior frontal | 7,583 | 674 | 7,480 | 739 | 0.215 | 0.645 | 0.15 |
Lh indicates left hemisphere, rh indicates right hemisphere. *p < 0.05 ** after multiple comparisons (p < 0.011). Surface area measures are given in mm2. Results were mapped into brain space shown in Fig. 1.
Table 3.
Group differences in cortical thickness
| HC | CSBD | ||||||
| Brain region | Mean | SD | Mean | SD | F (1, 39) | p | Cohens d |
| Lh lateral orbito frontal | 2.847 | 0.137 | 2.876 | 0.106 | 0.605 | 0.441 | 0.24 |
| Lh medial orbito frontal | 2.644 | 0.138 | 2.686 | 0.116 | 1.639 | 0.208 | 0.33 |
| Lh caudal anterior cingulate | 2.691 | 0.273 | 2.690 | 0.226 | 0.001 | 0.981 | 0.00 |
| Lh posterior cingulate | 2.580 | 0.154 | 2.606 | 0.169 | 0.453 | 0.505 | 0.16 |
| Lh rostral anterior cingulate | 2.944 | 0.208 | 2.999 | 0.177 | 1.22 | 0.276 | 0.28 |
| Lh insula | 3.082 | 0.149 | 3.111 | 0.143 | 0.471 | 0.497 | 0.20 |
| Lh superior frontal | 2.803 | 0.154 | 2.89 | 0.148 | 5.726 | 0.022* | 0.58 |
| Rh caudal anterior cingulate | 2.524 | 0.231 | 2.558 | 0.255 | 0.272 | 0.605 | 0.14 |
| Rh lateral orbito frontal | 2.717 | 0.113 | 2.781 | 0.125 | 3.344 | 0.075 | 0.54 |
| Rh medial orbito frontal | 2.637 | 0.122 | 2.658 | 0.161 | 0.306 | 0.583 | 0.15 |
| Rh posterior cingulate | 2.580 | 0.127 | 2.647 | 0.154 | 3.363 | 0.074 | 0.48 |
| Rh rostral anterior cingulate | 2.944 | 0.184 | 2.989 | 0.264 | 0.457 | 0.503 | 0.20 |
| Rh insula | 3.065 | 0.134 | 3.09 | 0.196 | 0.278 | 0.601 | 0.15 |
| Rh superior frontal | 2.774 | 0.135 | 2.839 | 0.141 | 3.986 | 0.053 | 0.47 |
Lh indicates left hemisphere, rh indicates right hemisphere. *p < 0.05. Thickness measures are given in mm. Results were mapped into brain space shown in Fig. 1.
Fig. 1.
Case-control differences in cortical thickness and surface area. Results (p-values) and effect sizes (Cohen's d) are indicated (color bars). Numerical results can be found in Tables 2–3 for transparency, significance is thresholded at p = 0.05. The brain area in which significant case-control differences were observed after multiple comparison correction (right posterior cingulate) is indicated with * (shown isolated in Figure S1)
Sensitivity and secondary analyses
Group difference in right posterior cingulate surface area remained statistically significant when correcting for any additional covariate tested, including total brain volume, with one exception: when correcting for depression symptom levels (MADRS-S) scores, the group difference in posterior cingulate surface area was no longer statistically significant (p = 0.286, Table S1a).
In the combined cohort, we observed a negative correlation between right posterior cingulate area and scales assessing hypersexuality symptoms and sexual compulsivity (HDSI, HBI, SCS). There was no significant correlation with the sexual desire (SDI) score (p = 0.061, Table S4). These correlations were not significant when correcting for group status or when repeating the correlation analysis within each group separately (CSBD p = 0.812─0.989; HC p = 0.396─0.972, see Table S4 for details).
The exploratory whole brain analysis was indicative for group differences in brain areas not included in the main analysis. These included thickness of the left frontal pole, right precuneus, and the left superior frontal cortex. Volume of white matter underlying the right posterior cingulate cortex was lower in the patient group than in controls. The full results, including means and SD for each group can be found in the Supplemental material, Tables S5–8.
Discussion
The neurobiological mechanisms underlying CSBD are unknown, and structural MRI studies that can help provide valuable insights into the neurobiology of CBSD are scarce. This study investigated structural brain differences in reward-related brain areas between 22 males diagnosed with CSBD and 20 healthy controls. Further, we tested for correlation between CSBD symptom severity and the MRI-derived phenotypes.
As expected, CSBD patients scored significantly higher on CSBD-related symptom measures than HC. The main neuroimaging finding was that, compared to healthy controls, the CSBD group had significantly lower cortical surface area in right posterior cingulate cortex, indicating that CSBD is associated with cortical brain alterations. The results remained robust when controlling for several potential confounding variables.
Intriguingly, in the combined cohort, there were negative correlations between right posterior cingulate surface area and CSBD symptoms scores, indicating that CSBD symptoms were more severe in individuals displaying more pronounced cortical variations. This also suggests that the brain metrics investigated may have functional relevance in CSBD. Furthermore, our results were supported by exploratory analyses which revealed group differences in white matter volume solely in the region underlying the posterior cingulate cortex (see Table S8).
The body of structural neuroimaging studies conducted in compulsive sexual behavior have been increased in the last years, however, is still sparse. For example, Schmidt et al. (Schmidt et al., 2017) reported greater left amygdala gray-matter volume in males with CSBD than males without CSBD. Seok & Sohn (Seok & Sohn, 2018) found lower volume of the left superior and right middle temporal gyrus in males with as compared with males without CSBD. Volume of the left superior temporal gyrus was negatively correlated with the severity of CSBD, suggesting relationships between structural brain phenotypes and CSBD symptoms, as observed in the present study.
However, most previous studies used region of interest approaches that did not include the posterior cingulate cortex and/or have used different cortical metrics (predominantly volume), which does not allow the independent investigation of cortical thickness and surface area. Hence, differences in cortical surface area, as found in the present study, could have been present but remained undetected in previous studies. Moreover, previous studies varied in cohort definition, image processing methodology, and/or MR modality.
The posterior cingulate cortex is highly connected with other brain areas, yet there is no consensus about its function. In a review, Leech et al. (Leech & Sharp, 2014) state that this region is likely to function as an integrative hub, with the ventral part to be involved in memory retrieval and planning, whilst the dorsal part with its prominent connections to the frontal lobes is suggested to be involved in control of attention. Further, it is suggested that the posterior cingulate is a key structure in the network responsible for environmental change detection and provides a signal for behavioral changes when actions result in suboptimal consequences (Pearson, Heilbronner, Barack, Hayden, & Platt, 2011). Traumatic brain injury affecting the posterior cingulate have been reported to result in difficulty with sustained attention and switching from automatic to controlled responses leading to perseverative behavior (Leech & Sharp, 2014). Altered attention has been reported in CSBD (Savard et al., 2021) and one could speculate that impaired modification in behavioral changes in responses to environmental demands contribute to sexual behaviors being pursued regardless of adverse consequences. Importantly, however, the posterior cingulate cortex is implicated in reward processing (Oldham et al., 2018), which aligns with the theory that reward-related processes are impacted in CSBD (Liberg et al., 2022; Schmidt et al., 2017; Voon et al., 2014). While the exact role of the posterior cingulate in CSBD remains to be clarified, results from previous studies and the present study are in line with the notion that CSBD is associated with brain alterations in areas implicated in sensitization, habituation, impulse control, and reward processing.
Limitations
The cross-sectional nature of the study does not allow for inference regarding causality. Although structural abnormalities are commonly associated with functional impairments (Burzynska et al., 2012; Joshi et al., 2016), functional MRI studies need to be conducted to enable conclusions about the direct functional involvement of implicated brain areas and their association with CSBD. In addition, in order to detect focal and smaller effects, e.g., in cortical thickness differences, replication in larger samples is needed. Furthermore, the cohort consisted solely of self-referred males. Although female patients seek treatment for CSBD, they are the minority in these patients groups, and future studies should focus on the recruitment of females.
The result that group differences are not significant after correcting for depression symptom levels needs to be interpreted with caution, as depression is highly correlated to CSBD (Antons & Brand, 2021); compulsive sexual behavior has been suggested to be the result of coping mechanism compensating for negative affective states, but distress and depressive symptoms can potentially also be caused by out-of-control sexual fantasies and behaviors (Briken, 2020). While both mechanisms may contribute, they cannot be separated in this study. In addition, we did not evaluate the prevalence of comorbid paraphilic disorder in this study. Although our previous study in an independent sample of CSBD patients, notably from the same catchment area, indicated a paraphilic disorder prevalence of only 8.3% (Hallberg et al., 2020), future studies should investigate the impact of paraphilic disorder, as well as other comorbidities, such as mood disorders, ADHD, and autism, on MRI-based outcomes. Finally, several participants have been recruited during the COVID-19 pandemic. Although studies report no significant changes in pornography use during the COVID-19 pandemic (Grubbs, Perry, Grant Weinandy, & Kraus, 2022; Koós, Demetrovics, Griffiths, & Bőthe, 2022), it remains unknown if sexual behavior changed in our sample, e.g. increased pornography use and reduced number of sexual partners. Thus, it remains to be investigated whether our results are more generalizable to CSBD subgroups with high-frequency pornography use (Antons & Brand, 2021). Nevertheless, our study cohort represented an ecologically valid clinical sample of patients with CSBD.
Conclusions
Our findings suggest that CSBD is associated with structural brain differences. This study provides valuable insights into a largely unexplored field of clinical relevance and encourages further clarifications of the neurobiological mechanisms underlying CSBD, which is a prerequisite for improving future treatment outcomes. The findings may also contribute to ongoing discussion around whether the current classification of CSBD as an impulse-control disorder is reasonable.
Funding sources
This work was supported by Karolinska Institutet's Research Foundation Grants (2016 and 2017; CA), the Swedish Research Council (2020-01183; JJ, CA), the Swedish Prison and Probation System, and through a regional agreement between Umeå University and Region Västerbotten (ALF) (RV-941086).
Authors’ contribution
CA was principal investigator and designed the study. CA collected MRI and behavioral data. PG performed the majority of analyses and wrote the first draft of the manuscript. KJÖ, SA, CD, and MI contributed to study design and with clinical advice. KJÖ, JJ, and JS provided important intellectual input and contributed to manuscript writing. JS recruited and screened patients for eligibility and contributed to data collection. All authors had full access to all data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. All authors have reviewed the manuscript, provided intellectual input, and approved the submission of the manuscript.
Conflict of interest
CA is employed by Quantify Research (consultancy work unrelated to the present work). The authors report no financial or other relationship relevant to the subject of this article.
Acknowledgements
We thank the study nurses, medical, and administrative staff at ANOVA for their support in data collection and study organization, Christoffer Rahm for discussions during the study design phase, and Christian Mannfolk for his help in the recruitment of HC participants.
Supplementary material
Contributor Information
Per Görts, Email: per.gorts@gmail.com.
Josephine Savard, Email: josephine.savard@umu.se.
Katarina Görts-Öberg, Email: katarina.gorts-oberg@regionstockholm.se.
Cecilia Dhejne, Email: cecilia.dhejne@regionstockholm.se.
Stefan Arver, Email: stefan.arver@ki.se.
Jussi Jokinen, Email: jussi.jokinen@ki.se.
Martin Ingvar, Email: martin.ingvar@ki.se.
Christoph Abé, Email: christoph.abe@ki.se.
References
- Antons, S., & Brand, M. (2021). Diagnostic and classification considerations related to compulsive sexual behavior disorder and problematic pornography use. Current Addiction Reports, 8(3), 452–457. 10.1007/s40429-021-00383-7. [DOI] [Google Scholar]
- Antons, S., Engel, J., Briken, P., Krüger, T. H. C., Brand, M., & Stark, R. (2022). Treatments and interventions for compulsive sexual behavior disorder with a focus on problematic pornography use: A preregistered systematic review. Journal of Behavioral Addictions, 11(3), 643–666. 10.1556/2006.2022.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balodis, I. M., & Potenza, M. N. (2015). Anticipatory reward processing in addicted populations: A focus on the monetary incentive delay task. Biological Psychiatry, 77(5), 434–444. 10.1016/j.biopsych.2014.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum, K., Badgaiyan, R. D., & Gold, M. S. (2015). Hypersexuality addiction and withdrawal: Phenomenology, neurogenetics and epigenetics. Cureus, 7(10), e348. 10.7759/cureus.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgogna, N. C., Garos, S., Meyer, C. L., Trussell, M. R., & Kraus, S. W. (2022). A review of behavioral interventions for compulsive sexual behavior disorder. Current Addiction Reports, 9(3), 99–108. 10.1007/s40429-022-00422-x. [DOI] [Google Scholar]
- Bőthe, B., Potenza, M. N., Griffiths, M. D., Kraus, S. W., Klein, V., Fuss, J., & Demetrovics, Z. (2020). The development of the Compulsive Sexual Behavior Disorder Scale (CSBD-19): An ICD-11 based screening measure across three languages. Journal of Behavioral Addictions, 9(2), 247–258. 10.1556/2006.2020.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briken, P. (2020). An integrated model to assess and treat compulsive sexual behaviour disorder. Nature Reviews. Urology, 17(7), 391–406. 10.1038/s41585-020-0343-7. [DOI] [PubMed] [Google Scholar]
- Briken, P., Wiessner, C., Štulhofer, A., Klein, V., Fuß, J., Reed, G. M., & Dekker, A. (2022). Who feels affected by “out of control” sexual behavior? Prevalence and correlates of indicators for ICD-11 compulsive sexual behavior disorder in the German health and sexuality survey (GeSiD). Journal of Behavioral Addictions, 11(3), 900–911. 10.1556/2006.2022.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burzynska, A. Z., Nagel, I. E., Preuschhof, C., Gluth, S., Bäckman, L., Li, S. C., … Heekeren, H. R. (2012). Cortical thickness is linked to executive functioning in adulthood and aging. Human Brain Mapping, 33(7), 1607–1620. 10.1002/hbm.21311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnes, P. J., Green, B. A., Merlo, L. J., Polles, A., Carnes, S., & Gold, M. S. (2012). Pathos: A brief screening application for assessing sexual addiction. Journal of Addiction Medicine, 6(1), 29–34. 10.1097/ADM.0b013e3182251a28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman, E. (1991). Compulsive sexual behavior. Journal of Psychology & Human Sexuality, 4(2), 37–52. 10.1300/J056v04n02_04. [DOI] [Google Scholar]
- Dale, A. M., Fischl, B., & Sereno, M. I. (1999). Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage, 9(2), 179–194. 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Derbyshire, K. L., & Grant, J. E. (2015). Compulsive sexual behavior: A review of the literature. Journal of Behavioral Addictions, 4(2), 37–43. 10.1556/2006.4.2015.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan, R. S., Segonne, F., Fischl, B., Quinn, B. T., Dickerson, B. C., Blacker, D., … Killiany, R. J. (2006). An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage, 31(3), 968–980. 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Estellon, V., & Mouras, H. (2012). Sexual addiction: Insights from psychoanalysis and functional neuroimaging. Socioaffective Neuroscience & Psychology (SNP), 2, 11814. 10.3402/snp.v2i0.11814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl, B., & Dale, A. M. (2000). Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proceedings of the National Academy of Sciences of the United States of America, 97(20), 11050–11055. 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl, B., Salat, D. H., van der Kouwe, A. J., Makris, N., Segonne, F., Quinn, B. T., & Dale, A. M. (2004). Sequence-independent segmentation of magnetic resonance images. Neuroimage, 23(Suppl 1), S69–S84. 10.1016/j.neuroimage.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Fischl, B., Sereno, M. I., & Dale, A. M. (1999). Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage, 9(2), 195–207. 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Fischl, B., van der Kouwe, A., Destrieux, C., Halgren, E., Segonne, F., Salat, D. H., … Dale, A. M. (2004). Automatically parcellating the human cerebral cortex. Cerebral Cortex, 14(1), 11–22. [DOI] [PubMed] [Google Scholar]
- Georgiadis, J. R., & Kringelbach, M. L. (2012). The human sexual response cycle: Brain imaging evidence linking sex to other pleasures. Progress in Neurobiology, 98(1), 49–81. 10.1016/j.pneurobio.2012.05.004. [DOI] [PubMed] [Google Scholar]
- Gola, M., & Draps, M. (2018). Ventral striatal reactivity in compulsive sexual behaviors. Frontiers in Psychiatry, 9, 546. 10.3389/fpsyt.2018.00546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gola, M., Wordecha, M., Sescousse, G., Lew-Starowicz, M., Kossowski, B., Wypych, M., … Marchewka, A. (2017). Can pornography be addictive? An fMRI study of men seeking treatment for problematic pornography use. Neuropsychopharmacology, 42(10), 2021–2031. 10.1038/npp.2017.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubbs, J. B., Perry, S. L., Grant Weinandy, J. T., & Kraus, S. W. (2022). Porndemic? A longitudinal study of pornography use before and during the COVID-19 pandemic in a nationally representative sample of Americans. Archives of Sexual Behavior, 51(1), 123–137. 10.1007/s10508-021-02077-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallberg, J., Kaldo, V., Arver, S., Dhejne, C., Piwowar, M., Jokinen, J., & Öberg, K. G. (2020). Internet-administered cognitive behavioral therapy for hypersexual disorder, with or without paraphilia(s) or paraphilic disorder(s) in men: A pilot study. Journal of Sexual Medicine, 17(10), 2039–2054. 10.1016/j.jsxm.2020.07.018. [DOI] [PubMed] [Google Scholar]
- Hibar, D. P., Westlye, L. T., Doan, N. T., Jahanshad, N., Cheung, J. W., Ching, C. R. K., … Andreassen, O. A. (2018). Cortical abnormalities in bipolar disorder: An MRI analysis of 6503 individuals from the ENIGMA bipolar disorder working group. Molecular Psychiatry, 23(4), 932–942. 10.1038/mp.2017.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibar, D. P., Westlye, L. T., van Erp, T. G., Rasmussen, J., Leonardo, C. D., Faskowitz, J., … Andreassen, O. A. (2016). Subcortical volumetric abnormalities in bipolar disorder. Molecular Psychiatry, 21(12), 1710–1716. 10.1038/mp.2015.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyatt, C. S., Owens, M. M., Crowe, M. L., Carter, N. T., Lynam, D. R., & Miller, J. D. (2020). The quandary of covarying: A brief review and empirical examination of covariate use in structural neuroimaging studies on psychological variables. Neuroimage, 205, 116225. 10.1016/j.neuroimage.2019.116225. [DOI] [PubMed] [Google Scholar]
- Joshi, S. H., Vizueta, N., Foland-Ross, L., Townsend, J. D., Bookheimer, S. Y., Thompson, P. M., … Altshuler, L. L. (2016). Relationships between altered functional magnetic resonance imaging activation and cortical thickness in patients with euthymic bipolar I disorder. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 1(6), 507–517. 10.1016/j.bpsc.2016.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kafka, M. P. (2010). Hypersexual disorder: A proposed diagnosis for DSM-V. Archives of Sexual Behavior, 39(2), 377–400. 10.1007/s10508-009-9574-7. [DOI] [PubMed] [Google Scholar]
- Kalichman, S. C., & Rompa, D. (1995). Sexual sensation seeking and sexual compulsivity scales: Reliability, validity, and predicting HIV risk behavior. Journal of Personality Assessment, 65(3), 586–601. 10.1207/s15327752jpa6503_16. [DOI] [PubMed] [Google Scholar]
- Kingston, D. A., & Bradford, J. M. (2013). Hypersexuality and recidivism among sexual offenders. Sexual Addiction & Compulsivity, 20(1–2), 91–105. 10.1080/10720162.2013.768131. [DOI] [Google Scholar]
- Koós, M., Demetrovics, Z., Griffiths, M. D., & Bőthe, B. (2022). No significant changes in addictive and problematic behaviors during the COVID-19 pandemic and related lockdowns: A three-wave longitudinal study. Frontiers in Psychology, 13, 837315. 10.3389/fpsyg.2022.837315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kor, A., Fogel, Y., Reid, R. C., & Potenza, M. N. (2013). Should hypersexual disorder be classified as an addiction? Sexual Addiction & Compulsivity, 20(1–2). 10.1080/10720162.2013.768132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalewska, E., Grubbs, J., Potenza, M., Gola, M., Draps, M., & Kraus, S. (2018). Neurocognitive mechanisms in compulsive sexual behavior disorder. Current Sexual Health Reports. 10.1007/s11930-018-0176-z. [DOI] [Google Scholar]
- Kraus, S. W., Voon, V., & Potenza, M. N. (2016). Neurobiology of compulsive sexual behavior: Emerging science. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 41(1), 385–386. 10.1038/npp.2015.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn, S., & Gallinat, J. (2016). Neurobiological basis of hypersexuality. International Review of Neurobiology, 129, 67–83. 10.1016/bs.irn.2016.04.002. [DOI] [PubMed] [Google Scholar]
- Leech, R., & Sharp, D. J. (2014). The role of the posterior cingulate cortex in cognition and disease. Brain, 137(Pt 1), 12–32. 10.1093/brain/awt162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberg, B., Görts-Öberg, K., Jokinen, J., Savard, J., Dhejne, C., Arver, S., … Abé, C. (2022). Neural and behavioral correlates of sexual stimuli anticipation point to addiction-like mechanisms in compulsive sexual behavior disorder. Journal of Behavioral Addictions, 11(2), 520–532. 10.1556/2006.2022.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechelmans, D. J., Irvine, M., Banca, P., Porter, L., Mitchell, S., Mole, T. B., … Voon, V. (2014). Enhanced attentional bias towards sexually explicit cues in individuals with and without compulsive sexual behaviours. PLoS One, 9(8), e105476. 10.1371/journal.pone.0105476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldham, S., Murawski, C., Fornito, A., Youssef, G., Yücel, M., & Lorenzetti, V. (2018). The anticipation and outcome phases of reward and loss processing: A neuroimaging meta-analysis of the monetary incentive delay task. Human Brain Mapping, 39(8), 3398–3418. 10.1002/hbm.24184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons, J. T., Rendina, H. J., Ventuneac, A., Cook, K. F., Grov, C., & Mustanski, B. (2013). A psychometric investigation of the hypersexual disorder screening inventory among highly sexually active gay and bisexual men: An item response theory analysis. The Journal of Sexual Medicine, 10(12), 3088–3101. 10.1111/jsm.12117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson, J. M., Heilbronner, S. R., Barack, D. L., Hayden, B. Y., & Platt, M. L. (2011). Posterior cingulate cortex: Adapting behavior to a changing world. Trends in Cognitive Sciences, 15(4), 143–151. 10.1016/j.tics.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid, R. C., Garos, S., & Carpenter, B. N. (2011). Reliability, validity, and psychometric development of the hypersexual behavior inventory in an outpatient sample of men. Sexual Addiction & Compulsivity, 18(1), 30–51. 10.1080/10720162.2011.555709. [DOI] [Google Scholar]
- Sankoh, A. J., Huque, M. F., & Dubey, S. D. (1997). Some comments on frequently used multiple endpoint adjustment methods in clinical trials. Statistics in Medicine, 16(22), 2529–2542. [DOI] [PubMed] [Google Scholar]
- Savard, J., Hirvikoski, T., Görts Öberg, K., Dhejne, C., Rahm, C., & Jokinen, J. (2021). Impulsivity in compulsive sexual behavior disorder and pedophilic disorder. Journal of Behavioral Addictions, 10(3), 839–847. 10.1556/2006.2021.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savard, J., Öberg, K. G., Chatzittofis, A., Dhejne, C., Arver, S., & Jokinen, J. (2020). Naltrexone in compulsive sexual behavior disorder: A feasibility study of twenty men. The Journal of Sexual Medicine, 17(8), 1544–1552. 10.1016/j.jsxm.2020.04.318. [DOI] [PubMed] [Google Scholar]
- Schmidt, C., Morris, L. S., Kvamme, T. L., Hall, P., Birchard, T., & Voon, V. (2017). Compulsive sexual behavior: Prefrontal and limbic volume and interactions. Human Brain Mapping, 38(3), 1182–1190. 10.1002/hbm.23447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seok, J. W., & Sohn, J. H. (2018). Gray matter deficits and altered resting-state connectivity in the superior temporal gyrus among individuals with problematic hypersexual behavior. Brain Research, 1684, 30–39. 10.1016/j.brainres.2018.01.035. [DOI] [PubMed] [Google Scholar]
- Sheehan, D. V., Lecrubier, Y., Sheehan, K. H., Amorim, P., Janavs, J., Weiller, E., & Dunbar, G. C. (1998). The mini-international neuropsychiatric interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. Journal of Clinical Psychiatry, 59(Suppl 20), 22–33 quiz 34–57. [PubMed] [Google Scholar]
- Spector, I. P., Carey, M. P., & Steinberg, L. (1996). The sexual desire inventory: Development, factor structure, and evidence of reliability. Journal of Sex & Marital Therapy, 22(3), 175–190. 10.1080/00926239608414655. [DOI] [PubMed] [Google Scholar]
- Stark, R., Klucken, T., Potenza, M. N., Brand, M., & Strahler, J. (2018). A current understanding of the behavioral neuroscience of compulsive sexual behavior disorder and problematic pornography use. Current Behavioral Neuroscience Reports, 5(4), 218–231. 10.1007/s40473-018-0162-9. [DOI] [Google Scholar]
- Svanborg, P., & Asberg, M. (2001). A comparison between the beck depression inventory (BDI) and the self-rating version of the Montgomery Asberg depression rating scale (MADRS). Journal of Affective Disorders, 64(2–3), 203–216. 10.1016/s0165-0327(00)00242-1. [DOI] [PubMed] [Google Scholar]
- Turner, D., Briken, P., Grubbs, J., Malandain, L., Mestre-Bach, G., Potenza, M. N., & Thibaut, F. (2022). The World Federation of Societies of Biological Psychiatry guidelines on the assessment and pharmacological treatment of compulsive sexual behaviour disorder. Dialogues in Clinical Neuroscience, 24(1), 10–69. 10.1080/19585969.2022.2134739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voon, V., Mole, T. B., Banca, P., Porter, L., Morris, L., Mitchell, S., … Irvine, M. (2014). Neural correlates of sexual cue reactivity in individuals with and without compulsive sexual behaviours. PLoS One, 9(7), e102419. 10.1371/journal.pone.0102419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein, A., Katz, L., Eberhardt, H., Cohen, K., & Lejoyeux, M. (2015). Sexual compulsion--relationship with sex, attachment and sexual orientation. Journal of Behavioral Addictions, 4(1), 22–26. 10.1556/jba.4.2015.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler, A. M., Kochunov, P., Blangero, J., Almasy, L., Zilles, K., Fox, P. T., … Glahn, D. C. (2010). Cortical thickness or grey matter volume? The importance of selecting the phenotype for imaging genetics studies. Neuroimage, 53(3), 1135–1146. 10.1016/j.neuroimage.2009.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (February 2022). ICD-11 for mortality and morbidity statistics. https://icd.who.int/browse11/l-m/en#/http://id.who.int/icd/entity/1630268048. [Google Scholar]