Abstract
Background and aims
Problem gambling and tobacco use are highly comorbid among adults. However, there are few treatment frameworks that target both gambling and tobacco use simultaneously (i.e., an integrated approach), while also being accessible and evidence-based. The aim of this two-arm open label RCT was to examine the efficacy of an integrated online treatment for problem gambling and tobacco use.
Methods
A sample of 209 participants (Mage = 37.66, SD = 13.81; 62.2% female) from North America were randomized into one of two treatment conditions (integrated [n = 91] or gambling only [n = 118]) that lasted for eight weeks and consisted of seven online modules. Participants completed assessments at baseline, after treatment completion, and at 24-week follow-up.
Results
While a priori planned generalized linear mixed models showed no condition differences on primary (gambling days, money spent, time spent) and secondary outcomes, both conditions did appear to significantly reduce problem gambling and smoking behaviours over time. Post hoc analyses showed that reductions in smoking and gambling craving were correlated with reductions in days spent gambling, as well as with gambling disorder symptoms. Relatively high (versus low) nicotine replacement therapy use was associated with greater reductions in gambling behaviours in the integrated treatment condition.
Discussion and conclusions
While our open label RCT does not support a clear benefit of integrated treatment, findings suggest that changes in smoking and gambling were correlated over time, regardless of treatment condition, suggesting that more research on mechanisms of smoking outcomes in the context of gambling treatment may be relevant.
Keywords: problem gambling, tobacco smoking, integrated treatment, cognitive behavioural therapy, motivational interviewing, online, self-help
Introduction
Problem gambling and tobacco use are highly comorbid in North America (Grant, Hasin, Chou, Stinson, & Dawson, 2004; McGrath & Barrett, 2009; Welte, Barnes, Tidwell, Hoffman, & Wieczorek, 2015; Wood, Williams, Wood, & Williams, 2008). Studies show that 41%–60% of individuals with problem gambling also have a tobacco use disorder (Dowling et al., 2015; Grant, Desai, & Potenza, 2009; Lorains, Cowlishaw, & Thomas, 2011; McGrath, Barrett, Stewart, & McGrath, 2012; Smart & Ferris, 1996). Additionally, research has shown that comorbid smoking compounds gambling-related harms, such that individuals with problem gambling who smoke have more severe gambling disorder symptoms (Grant, Kim, Odlaug, & Potenza, 2008), report stronger gambling cravings (Grant & Potenza, 2005), are more likely to have other mental disorders (Grant et al., 2008), spend more money and time on gambling activities (Petry & Oncken, 2002), and have greater debt (Potenza et al., 2004). It has been suggested that untreated daily smoking may undermine the treatment of problem gambling, given the salient associations between the two behaviours (McGrath & Barrett, 2009). In fact, one study reported that daily smoking predicted higher post-treatment relapse rates among people treated for gambling problems (Grant, Donahue, Odlaug, & Kim, 2011). As such, there is a need to integrate smoking cessation into evidence-based interventions for problem gambling.
Evidence for the association between problem gambling and tobacco smoking
Based on the epidemiological literature showing high prevalence rates of tobacco smoking among people with gambling problems, research has begun to examine the associations between these behaviours. Two distinct theories have emerged; First, neurobiological studies suggest that tobacco use and gambling are both mediated by similar reward circuits in the brain (Grant et al., 2004). Research has demonstrated increased dopamine transmission in the mesocorticolimbic regions of the brain following the use of nicotine (di Chiara & Imperato, 1988; Pontieri, Tanda, Orzi, & Chiara, 1996) and research has also found that when individuals receive variable monetary rewards (i.e., unpredictable amounts, similar to gambling), they demonstrate increased dopaminergic activity in the very same neural regions that are associated with, and activated by, nicotine (Barrett, Boileau, Okker, Pihl, & Dagher, 2004). Tobacco use and problem gambling may thus act on similar neural reward pathways, thereby reinforcing the relations between these addictive behaviours.
Second, behavioural research has demonstrated that nicotine may alter the reward-related cognitive processes that increase an individual's risk for problem gambling (McGrath & Barrett, 2009), such that when these behaviours are paired together, nicotine may enhance the salience of short-term rewards while distracting an individual from gambling's longer-term negative outcomes. Indeed, studies have shown that heavy smokers often select riskier options (e.g., short-term rewards at the expense of long-term loses) on gambling tasks (Businelle et al., 2009) and demonstrate a pattern of steep discounting of future rewards (MacKillop et al., 2011; Syan, González-Roz, Amlung, Sweet, & MacKillop, 2021) (i.e., an impulsive preference for immediate rewards). Further, cross-cue reactivity studies have demonstrated that tobacco use and problem gambling may in fact become reciprocal triggers for each behaviour (Wulfert, Harris, & Broussard, 2016). For example, a recent study found that gamblers who smoke had greater cross-cue reactivity (compared to gambling and smoking only control groups) (Wulfert et al., 2016), and that smoking gamblers had increased physiological arousal and greater subjective desire to smoke, irrespective of whether cues were smoking- or gambling-related (Wulfert et al., 2016).
In sum, if tobacco use potentiates gambling – and vice versa – it suggests that attempting to reduce either behaviour in isolation would be challenging and could also impact the other behaviour. Integrated treatments that target both behaviours simultaneously could be an effective method of reducing this comorbidity. Additionally, one gold standard care guideline for smoking cessation interventions is the combined use of nicotine replacement therapy (NRT) and psychosocial support (CBT/MI) (Abrams et al., 2003; Kim et al., 2021).
Existing evidence-based treatments for problem gambling
Existing treatments for problem gambling tend to integrate CBT and MI (Gooding & Tarrier, 2009; Hodgins, Currie, & el-Guebaly, 2001). Evidence demonstrates that combined CBT and MI has synergistic, positive effects on gambling and smoking behaviours (Cowlishaw et al., 2012; Gooding & Tarrier, 2009; Hodgins et al., 2001; Perkins, Conklin, & Levine, 2013; Yakovenko, Quigley, Hemmelgarn, Hodgins, & Ronksley, 2015). By increasing motivation for change using MI, people with problem gambling may be more willing to complete the effortful activities of CBT (e.g., homework), which in turn, promote better coping skills. Integrated MI may also help individuals with problem gambling to clarify their core values in CBT by creating discrepancy between their current and desired behaviour. Supporting this, meta-analytic findings showed that CBT/MI treatments reduce problem gambling behaviours with medium effect sizes (Gooding & Tarrier, 2009), and that online CBT/MI treatments reduce gambling activity engagement, and cravings (Casey et al., 2017).
However, research on CBT/MI treatment approaches has also reported several concerns. This literature demonstrates medium to large effect sizes for short- and long-term gambling outcomes (Gooding & Tarrier, 2009). As well, treatment trials for problem gambling often report substantial drop out rates (Battersby & Tolchard, 2013; Melville, Casey, & Kavanagh, 2007), marked problems with treatment adherence (Petry et al., 2006), and considerable rates of relapse (Smith et al., 2015). These issues clearly indicate the need for additional research examining effective ways to augment CBT/MI interventions for problem gambling to improve clinical outcomes. To date, very few studies have examined smoking status and its relation to gambling treatment outcomes (Merkouris, Thomas, Browning, & Dowling, 2016) even though (as discussed previously), comorbid tobacco use is a factor that might maintain and reinforce problem gambling behaviours (Wulfert et al., 2016). Thus, a promising augmentation to CBT/MI treatments for problem gambling may be to include content to address co-occurring tobacco use.
Integrated treatment
Traditionally, comorbid addictive disorders or mental illnesses have been treated using a sequential or parallel intervention framework (Barrett, Darredeau, & Pihl, 2006). During a sequential approach, clinicians treat the “primary” addiction/disorder first, followed by the treatment of the comorbid condition; treatment is provided for one disorder at a time. In contrast, the parallel model attempts to treat comorbid disorders at the same time, but through separate clinicians — each of whom have an expertise with one of the comorbid disorders (Mueser, Noordsy, Drake, & Lindy, 2003). To illustrate, a person who suffers from comorbid tobacco use and problem gambling, may work with their family doctor to reduce their tobacco use while also working with a psychologist to manage their gambling problems. As such, in the parallel model, a person receives support for both issues at once, but from different professionals. While sequential and parallel approaches are widely used, there are limited intervention models for comorbid addictions (Mueser et al., 2003). The sequential treatment framework does not consider the interconnectedness of addictions and leaves one disorder temporarily untreated, while the parallel treatment framework, engages multiple professionals and thus can be inefficient in terms of healthcare costs and can also result in miscommunication, conflicting advice, differing treatment recommendations (Mueser et al., 2003). Overall, attesting to these limitations, sequential and parallel approaches have been shown to result in poor treatment outcomes in those struggling with comorbid addictive behaviours (Mueser et al., 2003); hence the utility of the integrated treatment framework.
The integrated approach recognizes the common etiological mechanisms that underlie co-occurring addictive behaviours (Bickel & Mueller, 2009). As such, individuals receive treatment (in the same program) for more than one addictive behaviour, allowing them to make notable improvements on interconnected addiction problems. Given the emphasis that CBT and MI place on coping skill development, as well as the effectiveness for CBT/MI therapies with respect to problem gambling, tobacco use, and addictive behaviours more broadly (Abrams et al., 2003; Gooding & Tarrier, 2009) it suggests that a CBT/MI framework would be ideal for an integrated intervention for co-occurring problem gambling and tobacco use.
The current study
We conducted a two-arm open label RCT testing a novel online integrated treatment for problem gambling and comorbid tobacco use in a sample of North Americans with problem gambling. Our aims were twofold; our first aim was to examine if concurrently treating smoking within an integrated intervention would improve gambling outcomes relative to a gambling only treatment. We hypothesized that participants in the integrated treatment condition would demonstrate larger reductions in problem gambling than participants in the gambling-only intervention. Our second aim was to examine whether tobacco use would explain, or mediate, the effects of the integrated gambling treatment. We hypothesized that reductions in tobacco use would mediate the beneficial effects of integrated treatment on gambling outcomes.
Methods
Design
A pre-registered, open label, two-arm RCT was conducted to test our hypotheses (Clinicaltrials.gov ID NCT03614884; See Bilevicius et al., 2020 for the full study protocol). We randomized participants into either: (1) an 8-week online integrated intervention for problem gambling and smoking or (2) an 8-week gambling only (control) intervention. Participants completed online assessments before randomization (T1; baseline), at 8-weeks since baseline (T2; treatment end), and at 24-weeks since baseline (T3; follow up). We used simple randomization (via the intervention website) on a 1:1 allocation ratio. Participants were compensated with $20 Amazon.com gift cards per assessment; bonuses were also available for a maximum of $100 per participant. Ethical approval for this study was granted from the University of Manitoba and York University.
Procedure
Participants were recruited across Canada and the United States. Given that the lead researchers are based in Canada, we used a mixture of local (e.g., posting flyers near casinos and/or gambling help organizations) and online advertisements (e.g., Google Ads, Facebook Ads, Kijiji etc.). For American recruitment, we used online advertisements exclusively. Prior to participation, interested individuals read the consent form and provided informed consent (for full information about the informed consent process, please see Bilevicius et al., 2020). After consenting, participants self-registered on the intervention website and completed the baseline eligibility survey. Eligibility criteria included: 1) ages 19+; 2) score of >3 on the Problem Gambling Severity Index (PGSI; Ferris & Wynne, 2001); 3) current daily smoker of regular cigarettes (>1 cigarette per day with any level of nicotine dependence, but no primary use of vapes or chewing tobacco); 4) English fluency; and 5) consistent access to internet. A PGSI cut-off of >3 was used in the current study because we wanted to be inclusive of a wider range of gambling severity and this cut-off has also been used in previous online gambling treatment studies evaluating similar CBT/MI programs (see Cunningham et al., 2019; Cunningham et al., 2020). Participants were considered ineligible if they were actively participating in other psychosocial treatments for smoking or gambling, if they reported current psychosis, mania, or moderate-to-severe substance use disorder (scoring ≥20 on the Alcohol Use Disorder Identification Test [AUDIT; Saunders, Aasland, Babor, de La Fuente, & Grant, 1993] or >5 on the Drug Abuse Screening Test-10 [DAST-10; Skinner, 1982], if they endorsed greater than minimal risk with respect to suicidality. Participants, as well as an undergraduate-level research assistant, were aware of respective participant treatment conditions (i.e., unblinded), however principal investigators were blind to condition assignment. In particular, the lead PI of the study (MTK) analyzed the data prior to knowing the group variable coding or the participant composition of the groups. Once data analysis was completed, the main research assistant shared this information. Given the open-label nature of our study, this was our best attempt to reduce bias in data reporting for this RCT.
Participants
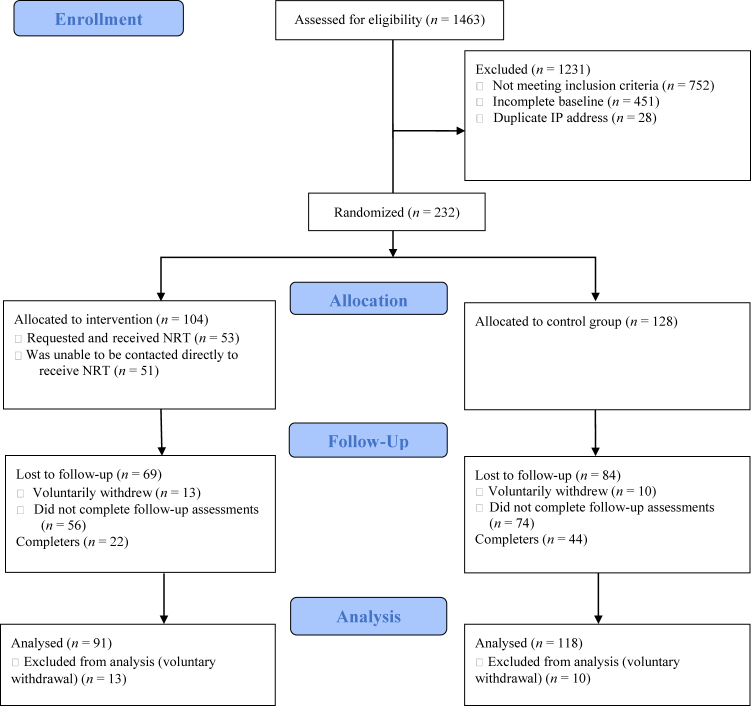
A total of 1,463 participants completed the baseline survey (see Fig. 1 for the CONSORT trial flow diagram). Of these, 232 were eligible and were randomized into the two online treatment conditions. A small subset of participants (n = 23) voluntarily withdrew from the study, and as per the host institution REB guidelines, data for these participants could not be included in the final analyses. The final sample consisted of 209 participants (Mage = 37.66, SD = 13.81), with 91 participants in the integrated treatment condition and 118 in the control condition. Full participant characteristics are presented in Table 1. Participants reported on their motivation to reduce gambling behaviours at the outset of treatment (0 = “not motivated at all” to 10 = “very motivated”) and the mean scores suggested moderate-to-high initial motivation to change (M = 7.81, SD = 2.32).
Fig. 1.
CONSORT trial flow
Table 1.
Sample Demographics
| Variable | % (n) |
| Sex | |
| Female | 62.2 (130) |
| Male | 37.8 (79) |
| Ethnicity | |
| Caucasian/White | 60.8 (127) |
| Indigenous | 11.5 (24) |
| Black | 10.0 (21) |
| East Asian/South-East Asian/Pacific Islander | 7.7 (16) |
| South Asian | 3.8 (8) |
| Middle Eastern/North African/Central Asian | 2.4 (5) |
| Hispanic/Latino | 2.4 (5) |
| Other | 1.4 (3) |
| Geographic location | |
| Canada | 51.1 (107) |
| Manitoba | 16.3 (34) |
| Ontario | 23.9 (50) |
| Alberta | 5.3 (11) |
| Québec | 3.8 (8) |
| British Columbia | 0.5 (1) |
| New Brunswick | 0.5 (1) |
| Prince Edward Island | 0.5 (1) |
| Saskatchewan | 0.5 (1) |
| United States | 48.9 (102) |
| Nevada | 18.2 (38) |
| Pennsylvania | 5.3 (11) |
| Michigan | 4.8 (10) |
| Missouri | 4.3 (9) |
| Oklahoma | 4.3 (9) |
| Illinois | 2.4 (5) |
| Louisiana | 1.9 (4) |
| Indiana | 1.9 (4) |
| Montana | 1.4 (3) |
| South Dakota | 1.4 (3) |
| Iowa | 1.0 (2) |
| Nebraska | 0.5 (1) |
| New Jersey | 0.5 (1) |
| New York | 0.5 (1) |
| Washington | 0.5 (1) |
| Household Income | |
| Less than $15,000 | 18.7 (39) |
| $15,000–$29,999 | 14.8 (31) |
| $30,000–$49,999 | 21.1 (44) |
| $50,000–$100,000 | 31.1 (65) |
| Over $100,000 | 14.4 (30) |
| Enrolled in trial before COVID-19 | 55.5 (116) |
| Diagnosed with mental health condition | 34.9 (73) |
| Received previous treatment for mental health | 29.2 (61) |
Treatment conditions
Integrated treatment (experimental condition)
Participants had access to seven treatment modules over eight weeks. The gambling content came from a self-directed treatment validated in previous trials (Hodgins, Currie, Currie, & Fick, 2009; Hodgins et al., 2001), and has been successfully adapted for online delivery (Abrams et al., 2003; Bilevicius et al., 2020). Smoking content was derived from previously published CBT/MI protocols and best practice guidelines (Abrams et al., 2003; Kim et al., 2021). In the first module, participants in this treatment arm were provided with an NRT use fact sheet, which summarized the strong evidence for its effectiveness in combination with psychosocial treatment. Participants were: (1) encouraged to use NRT patches for the 8-week active treatment period, (2) advised that NRT patches are commonly available “over the counter” at local pharmacies, (3) shipped NRT patches during the active treatment phase, and (4) advised to speak with a family physician regarding any medical questions about NRT use. As noted in best practice guidelines (Fiore, Jorenby, Baker, & Kenford, 1992; Fiore et al., 2008), the dosage schedule of NRT patches was: 24 mg for four weeks then 14 mg for two weeks and finally 7 mg for remaining two weeks. While we observed a wide range of baseline nicotine dependence severity in our sample, most of our participants were in the mild-to-moderate symptom range. According to the latest best practice guidelines, NRT is a viable treatment for light-to-moderate smoking, as well (U.S. Department of Health and Human Services, 2020; Rigotti, 2022). Therefore, use of NRT in our trial was empirically informed. We shipped NRT to participants (via Amazon) at registration, so that they would have it for the duration of the study. We tracked self-reported weekly NRT use at each assessment point.
With respect to the website, participants had immediate access to all modules, were able to complete and revisit the modules multiple times, were able to track their gambling and smoking goal progress using a diary feature and were able to see a graph depicting their individualized treatment gains. Participants were able to track their way through each module using a progress bar at the bottom of the treatment page. Once participants finished each module, the progress bar would turn from red to green and would be logged as completed in their data profile on the website. Incomplete modules were also logged in the data as a percentage of the content completed. The website was also fully adapted for use on smartphones and tablets.
Gambling only treatment (control condition)
The control group received a similar 8-week online intervention for gambling only. The gambling content was the same as in the integrated treatment condition, but there was no content about smoking (see our published protocol for full intervention content; Bilevicius et al., 2020) (Abrams et al., 2003; Gooding & Tarrier, 2009).
Measures
Demographics
A demographic questionnaire was given to participants at T1 (baseline) to characterize the sample and determine eligibility. Participants were asked questions regarding their age, biological sex, ethnicity, household income, and mental health treatment history.
Primary outcomes
Three self-reported primary outcomes were used to capture change in gambling behaviour during the trial. Using the Timeline Followback (TLFB) method (Rueger, Trela, Palmeri, & King, 2012; Sobell & Sobell, 1992), participants indicated past-30-day gambling frequency (number of days gambled), money spent on gambling activities (dollars), and time spent engaged in gambling activities (minutes). These primary outcomes were selected because they were common outcomes in previous studies evaluating CBT/MI interventions for gambling problems (Gooding & Tarrier, 2009; Hodgins et al., 2009). Web-based TLFB measures have been shown to produce reliable and valid estimates of addictive behaviours (Rueger et al., 2012).
Secondary outcomes
Gambling Symptom Assessment Scale (G-SAS): The 12-item G-SAS was created to specifically capture self-reported changes in gambling symptoms during the intervention (Suck Won Kim, Grant, Potenza, Blanco, & Hollander, 2009). The G-SAS is unidimensional and has been shown to have good reliability and convergence with clinician-rated measures of change during gambling interventions (Kim et al., 2009). Internal consistencies of the total G-SAS in our sample were excellent: α = 0.93–0.96.
Problem Gambling Severity Index (PGSI): The well-validated 9-item PGSI was used to capture self-reported changes in gambling problems following the program (Ferris & Wynne, 2001). Internal consistencies of the PGSI total scores in our sample were excellent: α = 0.90–0.93.
Cigarette Use: The self-reported TLFB method was also used to capture past-30-day cigarette use. A sum score was calculated to reflect the total number of cigarettes smoked in the 30-days prior to each assessment. This secondary outcome was used to evaluate changes in smoking during the intervention. Recent data show that an online self-report TLFB provides reliable and valid estimates of cigarette smoking (Rueger et al., 2012).
Nicotine Dependence Symptoms: The 6-item Fagerstrom Test of Nicotine Dependence (FTND) was used to evaluate self-reported changes in nicotine dependence symptoms during the intervention. The Fagerstrom has been shown to have good reliability and validity (Heatherton, Kozlowski, Frecker, & Fagerstrom, 1991) and is sensitive to intervention-related change (Rohsenow, Martin, Tidey, Monti, & Colby, 2013). The internal consistencies of the Fagerstrom total scores in our sample were adequate: α = 0.70.
Gambling Craving: The 9-item Gambling Craving Scale (GCS) was used to assess intervention-related subjective changes in gambling urges. Previous work supports the reliability and validity of the GCS total score (Young & Wohl, 2009). The internal consistencies of the GCS total scores were good at all timepoints: α = 0.86–0.88.
Data analytic plan
Prior to evaluating intervention effects on primary and secondary outcomes, preliminary analyses were conducted, which included data screening (i.e., winsorizing outliers to ±3.29 SDs (Tabachnick & Fidell, 2013); verifying assumptions of multiple regression), and a missing data analysis. For the missing data analysis, we compared participants with missing data to those with complete data on various baseline measures using t-tests. Second, as outlined in our published protocol (Bilevicius et al., 2020), we used generalized linear mixed models (GLMMs) to evaluate hypothesized treatment condition differences on primary and secondary outcomes. We added several planned covariates to all primary and secondary mixed models, including sex, age, and mental health treatment history. We further added one unplanned covariate to all models. The COVID-19 pandemic resulted in widespread (but regionally varying) closures of casinos and other venues related to gambling during the trial, and for this reason a binary variable reflecting enrolment in the trial pre-(=0) and post-(=1) COVID-19 was added. We used an intent-to-treat framework, with full information maximum likelihood (FIML) as the estimator. All GLMMs were run specifying random intercepts and slopes.
Following the main (a priori) planned analyses, we conducted two sets of exploratory (post hoc) mixed models. The second objective of our study was to examine if reductions in smoking mediated the association between intervention condition (IV) and changes in gambling outcomes (DVs). However, as noted below (see Results for more detail), no significant condition differences were found; therefore, we instead conducted posthoc GLMMs that collapsed across treatment condition and examined whether overall changes in smoking were associated with overall changes in gambling behaviours and disordered symptoms during and after the trial. In addition, we included a measure of gambling craving in these models as a competing correlate (relative to changes in smoking) of gambling behaviour reductions. Including a known correlate of change in gambling interventions allowed us to examine the impact of smoking reduction (uniquely) during our online intervention. In the second set of exploratory analyses, the moderating role of NRT use on gambling outcomes was examined in the integrated treatment condition only. This allowed us to examine the incremental effects of NRT use, above and beyond the psychosocial smoking treatment content included in the integrated arm of the trial.
A priori power analysis
As described in our published protocol (Bilevicius et al., 2020), we needed a sample size of N = 214 to achieve sufficient power to detect a small effect size (also assuming a 30% attrition rate).
Ethics
The study procedures were carried out in accordance with the Declaration of Helsinki and the Tri-Council Policy Statement. The Institutional Review Board of the University of Manitoba and York University approved the study. All subjects were informed about the study and all provided informed consent.
Results
Descriptive statistics and missing data analysis
See Table 2 for full descriptive statistics by condition. Regarding data screening, we observed that less than 2% of the primary and secondary outcomes were outliers and all variables showed acceptable skew (<3.0) and kurtosis (<8.0) values, indicating univariate normality (Kline, 2015). We also did not observe any multivariate outliers and other statistical assumptions were verified. Participants with complete data at all three time points (n = 81) did not significantly differ at baseline from participants with missing data (n = 128) with regard to past 30-day gambling days (t(207) = −0.68, P = 0.50, d = −0.10), minutes spent gambling (t(207) = 1.45, P = 0.15, d = 0.18), and cigarettes smoked (t(207) = −0.11, P = 0.91, d = −0.02). Further, there were no differences on the G-SAS (t(207) = −0.53, P = 0.60, d = −0.08), PGSI (t(207) = −0.43, P = 0.68, d = −0.06), or FTND (t(207) = −0.48, P = 0.63, d = −0.07). Participants with missing data reported spending more money on gambling at baseline (t(207) = 2.42, P = 0.02, d = 0.31), but this did not pose biases in the GLMMs because baseline values were included.
Table 2.
Descriptive statistics of study variables by condition
| Integrated Condition (n = 91) | Gambling-Only Condition (n = 118) | |||
| Variable | M (SD) | Range | M (SD) | Range |
| TLFB gambling days (past 30 days) | ||||
| Baseline (n = 209) | 12.81 (9.35) | 0–30 | 14.36 (9.38) | 0–30 |
| 8 weeks (n = 69) | 8.53 (7.94) | 0–23 | 7.08 (7.75) | 0–28 |
| 24 weeks (n = 84) | 4.14 (5.29) | 0–25 | 5.32 (7.05) | 0–27 |
| TLFB money spent gambling (past 30 days) | ||||
| Baseline (n = 209) | $2291.16 (3542.94) | $0–20,005 | $3464.44 (6074.82) | $0–30,000 |
| 8 weeks (n = 69) | $472.79 (700.37) | $0–2,864 | $364.05 (524.49) | $0–2,863 |
| 24 weeks (n = 84) | $234.64 (473.87) | $0–1,861 | $196.01 (388.46) | $0–1,861 |
| TLFB minutes spent gambling (past 30 days) | ||||
| Baseline (n = 209) | 472.24 (719.96) | 0–5,016 | 579.15 (906.17) | 0–5,016 |
| 8 weeks (n = 69) | 34.86 (72.15) | 0–284 | 18.20 (47.32) | 0–284 |
| 24 weeks (n = 84) | 33.67 (127.34) | 0–834 | 25.48 (84.78) | 0–530 |
| TLFB cigarettes smoked (past 30 days) | ||||
| Baseline (n = 209) | 83.82 (68.32) | 7–319 | 91.36 (67.99) | 10–319 |
| 8 weeks (n = 69) | 16.72 (31.44) | 0–132 | 16.63 (34.73) | 0–132 |
| 24 weeks (n = 84) | 13.57 (27.25) | 0–120 | 15.71 (31.97) | 0–120 |
| FTND | ||||
| Baseline (n = 209) | 3.97 (2.79) | 0–10 | 4.50 (2.74) | 0–10 |
| 8 weeks (n = 54) | 3.92 (2.41) | 0–8 | 3.20 (2.41) | 0–8 |
| 24 weeks (n = 65) | 2.97 (3.01) | 0–10 | 3.44 (2.72) | 0–8 |
| GACS | ||||
| Baseline (n = 209) | 33.77 (12.74) | 9–61 | 33.07 (13.32) | 11–63 |
| 8 weeks (n = 55) | 24.42 (9.58) | 9–52 | 24.69 (13.92) | 9–55 |
| 24 weeks (n = 66) | 21.83 (10.50) | 10–46 | 21.32 (10.38) | 9–45 |
| G-SAS | ||||
| Baseline (n = 209) | 27.79 (11.28) | 0–48 | 30.03 (10.73) | 0–48 |
| 8 weeks (n = 55) | 17.15 (8.23) | 0–33 | 19.48 (9.38) | 0–37 |
| 24 weeks (n = 66) | 14.93 (9.15) | 0–32 | 14.82 (11.57) | 0–38 |
| PGSI (n = 209) | ||||
| Baseline (n = 209) | 13.81 (6.95) | 3–27 | 15.80 (7.63) | 3–27 |
| 8 weeks (n = 66) | 11.50 (5.35) | 2–22 | 12.89 (6.60) | 1–27 |
| 24 weeks (n = 81) | 8.83 (6.64) | 0–29 | 10.65 (6.89) | 0–27 |
Note. TLFB = Timeline Follow-Back, FTND = Fagerström Test of Nicotine Dependence, GACS = Gambling Craving Scale, G-SAS = Gambling Symptom.
Assessment Scale, PGSI = Problem Gambling Severity Index.
While both conditions experienced reductions in gambling behaviours at the end of treatment and at follow up, mean PGSI scores remained in the “problem gambler” interpretive category (>7). Changes in mean G-SAS scores indicate that both groups began with moderate severity gambling symptoms but fell into the mild severity category (8–20) at the end of treatment and at follow-up. Reductions in mean FTND scores indicate that both groups fell into the very low-to-low range (<4) in terms of nicotine dependence symptoms at the end of treatment and at follow-up. The average number of modules completed was relatively similar in both conditions (integrated: M = 4.75, SD = 1.52; gambling-only: M = 4.90, SD = 1.30). Finally, we tracked participant use of the daily diary feature of the website over the course of the intervention. On average, participants completed just over M = 16.10 diary entries (SD = 3.57) while in active treatment, translating to approximately two entries per week.
Main trial analyses (A priori planned)
Primary outcomes
There was no significant condition by time interaction effect predicting past-month number of gambling days (see Table 3). However, the significant main effect of time indicated that both groups reported similar reductions in days spent gambling over the course of the trial (integrated: b = −4.56, SE = 0.57, P <0.001; gambling only: b = −4.33, SE = 0.50, P <0.001; see Fig. 2a). Likewise, no significant interaction was found between condition and time on money spent on gambling in the past 30 days, but a significant main effect of time was found, again suggesting similar reductions in both conditions (integrated: b = −354.55, SE = 125.32, P <0.01; gambling only: b = −404.96, SE = 110.14, P <0.001; see Fig. 2b). There was no significant condition by time interaction nor main effects of time for minutes spent gambling in the past 30 days (ps > 0.05; see Fig. 2c).
Table 3.
Mixed models for primary outcomes (past 30-days gambling)
| Parameter | B | SE | t | Sig. |
| Gambling Days | ||||
| Intercept | 12.86 | 0.96 | 13.43 | <0.001 |
| Age - Cov | 0.07 | 0.04 | 1.67 | 0.097 |
| Sex - Cov | 0.07 | 1.10 | 0.07 | 0.949 |
| Mental Health Treatment - Cov | 0.44 | 1.23 | 0.36 | 0.723 |
| COVID - Cov | 0.20 | 1.06 | 0.19 | 0.852 |
| Intervention | 0.96 | 1.28 | 0.75 | 0.453 |
| Time | −4.56 | 0.57 | −8.06 | <0.001 |
| Intervention × Time | 0.24 | 0.76 | 0.31 | 0.754 |
| Money Spent Gambling | ||||
| Intercept | 932.19 | 221.14 | 4.21 | <0.001 |
| Age - Cov | 2.22 | 3.16 | 0.70 | 0.484 |
| Sex - Cov | −113.03 | 85.94 | −1.32 | 0.193 |
| Mental Health Treatment - Cov | 57.47 | 102.95 | 0.56 | 0.578 |
| COVID - Cov | −22.11 | 82.94 | −0.27 | 0.791 |
| Intervention | 26.74 | 293.74 | 0.09 | 0.928 |
| Time | −354.55 | 125.32 | −2.83 | 0.006 |
| Intervention × Time | −50.41 | 166.75 | −0.30 | 0.763 |
| Minutes Spent Gambling | ||||
| Intercept | 43.90 | 14.57 | 3.01 | 0.003 |
| Age - Cov | 0.23 | 0.30 | 0.78 | 0.435 |
| Sex - Cov | −14.41 | 8.49 | −1.70 | 0.091 |
| Mental Health Treatment - Cov | −9.09 | 9.36 | −0.97 | 0.332 |
| COVID - Cov | 4.09 | 8.13 | 0.50 | 0.616 |
| Intervention | −22.38 | 19.38 | −1.16 | 0.250 |
| Time | −7.32 | 11.22 | −0.65 | 0.515 |
| Intervention × Time | 6.53 | 14.93 | 0.44 | 0.662 |
Note. Outcome variables were assessed with a Timeline Follow-Back procedure. “Cov” denotes the inclusion of a relevant covariate.
Fig. 2.
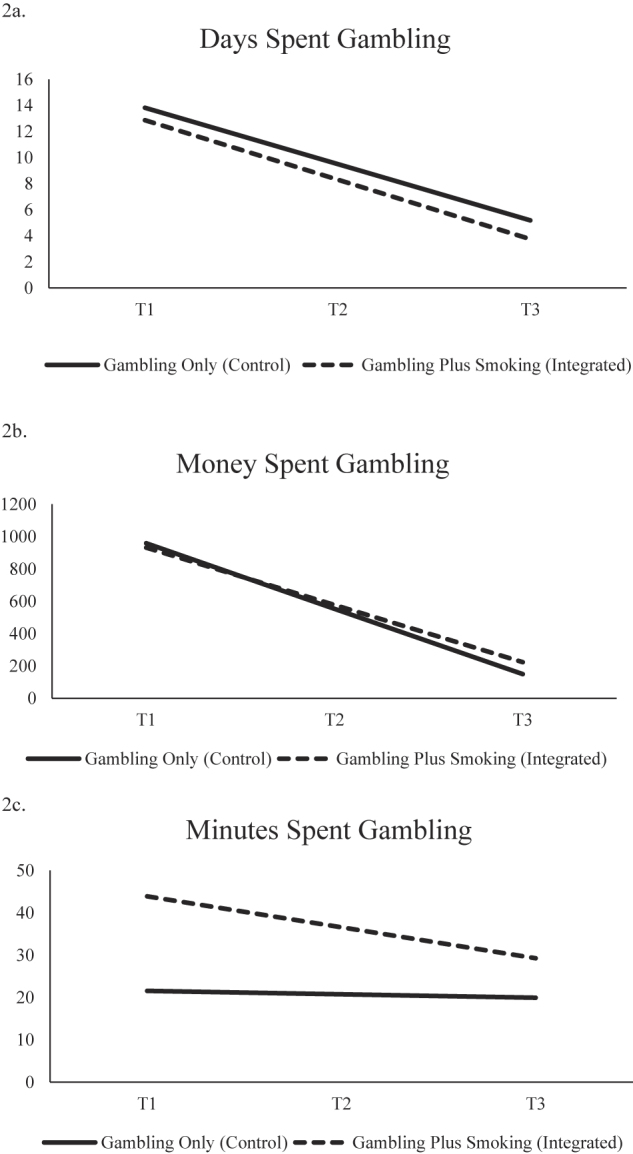
Condition simple slopes for primary outcomes
Secondary outcomes
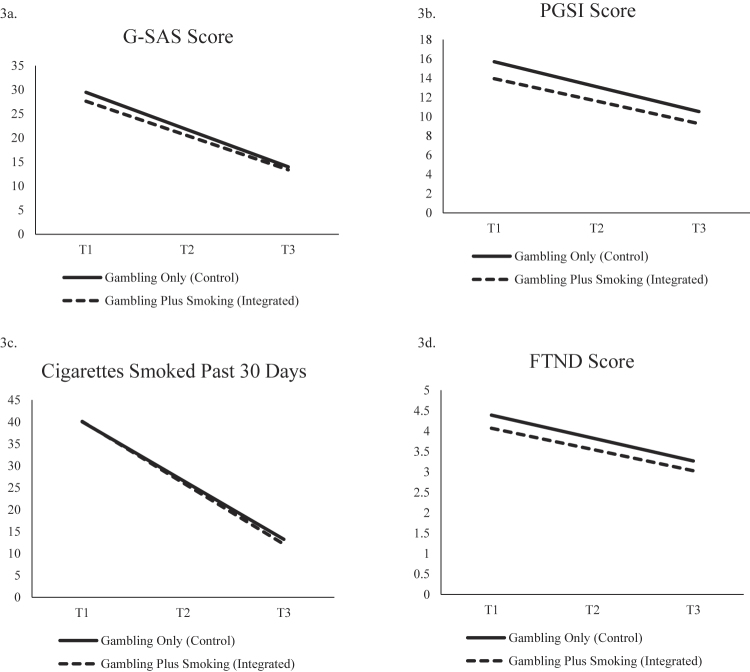
Gambling Disorder Symptoms. The condition by time interactions were not significant for either G-SAS or PGSI scores (see Table 4). However, both conditions reduced similarly in their overall G-SAS symptom scores (integrated: b = −7.11, SE = 0.90, P < 0.001; gambling-only: b = −7.74, SE = 0.79, P < 0.001; see Fig. 3a) and PGSI scores (integrated: b = −2.34, SE = 0.59, P < 0.001; gambling-only: b = −2.59, SE = 0.52, P < 0.001; see Fig. 3b) over time.
Table 4.
Mixed models for secondary outcomes
| Parameter | B | SE | t | Sig. |
| G-SAS | ||||
| Intercept | 27.60 | 1.05 | 26.13 | <0.001 |
| Age - Cov | 0.27 | 0.05 | 5.49 | <0.001 |
| Sex - Cov | 0.67 | 1.36 | 0.49 | 0.623 |
| Mental Health Treatment - Cov | 1.53 | 1.53 | 1.00 | 0.318 |
| COVID - Cov | 0.90 | 1.32 | 0.67 | 0.493 |
| Intervention | 1.87 | 1.41 | 1.33 | 0.186 |
| Time | −7.11 | 0.90 | −7.89 | <0.001 |
| Intervention × Time | −0.63 | 1.20 | −0.53 | 0.600 |
| PGSI | ||||
| Intercept | 13.95 | 0.71 | 19.74 | <0.001 |
| Age - Cov | 0.17 | 0.03 | 5.33 | <0.001 |
| Sex - Cov | 0.23 | 0.88 | 0.26 | 0.792 |
| Mental Health Treatment - Cov | 0.91 | 0.98 | 0.92 | 0.357 |
| COVID - Cov | 0.01 | 0.84 | 0.01 | 0.990 |
| Intervention | 1.75 | 0.94 | 1.85 | 0.066 |
| Time | −2.34 | 0.59 | −3.95 | <0.001 |
| Intervention × Time | −0.25 | 0.79 | −0.32 | 0.751 |
| Cigarettes Smoked Past 30 Days | ||||
| Intercept | 40.11 | 5.33 | 7.51 | <0.001 |
| Age - Cov | 0.33 | 0.15 | 2.26 | 0.025 |
| Sex - Cov | −3.42 | 4.14 | −0.83 | 0.410 |
| Mental Health Treatment - Cov | 6.17 | 4.52 | 1.37 | 0.173 |
| COVID - Cov | −4.26 | 4.00 | −1.07 | 0.288 |
| Intervention | −0.06 | 7.11 | −0.01 | 0.993 |
| Time | −13.99 | 2.69 | −5.21 | <0.001 |
| Intervention × Time | 0.56 | 3.58 | 0.16 | 0.875 |
| FTND | ||||
| Intercept | 4.07 | 0.26 | 15.13 | <0.001 |
| Age - Cov | 0.05 | 0.01 | 4.14 | <0.001 |
| Sex - Cov | −0.15 | 0.36 | −0.42 | 0.675 |
| Mental Health Treatment - Cov | 1.11 | 0.40 | 2.80 | 0.006 |
| COVID - Cov | −0.89 | 0.34 | −2.58 | 0.011 |
| Intervention | 0.33 | 0.36 | 0.91 | 0.364 |
| Time | −0.52 | 0.21 | −2.41 | 0.018 |
| Intervention × Time | −0.05 | 0.29 | −0.17 | 0.866 |
Note. G-SAS = Gambling Symptom Assessment Scale, PGSI = Problem Gambling Severity Index, FTND = Fagerström Test of Nicotine Dependence. “Cov” denotes the inclusion of a relevant covariate.
Fig. 3.
Condition simple slopes for secondary outcomes
Smoking. There was no statistically significant interaction effect between condition and time on past 30-day cigarette use or FTND scores (See Table 4). Both groups experienced reductions in both past 30-day cigarette use (integrated: b = −13.99, SE = 2.69, P < 0.001; gambling-only: b = −13.43, SE = 2.36, P < 0.001; see Fig. 3c) and FTND scores (integrated: b = −0.52, SE = 0.21, P < 0.05; gambling only: b = −0.56, SE = 0.19, P < 0.01; see Fig. 3d) during the trial.
Exploratory analyses (post hoc)
Changes in smoking and gambling craving as correlates of intervention change
After controlling for condition and time, reductions in both gambling craving and smoking during the trial were associated with a reduction in number of gambling days (see Table 5). Further, reductions in gambling craving (but not smoking) predicted reductions in both money and time spent gambling. Regarding G-SAS score, reductions in both gambling craving and smoking predicted reductions in problem gambling symptoms.
Table 5.
Mixed models examining reductions in smoking and gambling craving as predictors of gambling treatment outcomes
| Parameter | B | SE | t | Sig. |
| Gambling Days | ||||
| Intercept | 2.82 | 1.52 | 1.82 | 0.064 |
| Age - Cov | 0.05 | 0.04 | 1.27 | 0.207 |
| Sex - Cov | 0.38 | 1.00 | 0.38 | 0.706 |
| Mental Health Treatment - Cov | −1.06 | 1.17 | −0.91 | 0.367 |
| COVID - Cov | 1.20 | 0.97 | 1.24 | 0.219 |
| Intervention | 1.08 | 0.94 | 1.15 | 0.253 |
| Time | −2.32 | 0.45 | −5.19 | <0.001 |
| Gambling Craving (Time Varying) | 0.25 | 0.04 | 7.14 | <0.001 |
| TLFB Smoking (Time Varying) | 0.02 | 0.01 | 2.57 | 0.011 |
| Money Spent Gambling | ||||
| Intercept | 755.48 | 233.26 | 3.24 | 0.002 |
| Age - Cov | 4.03 | 4.60 | 0.88 | 0.384 |
| Sex - Cov | −112.05 | 104.55 | −1.07 | 0.289 |
| Mental Health Treatment - Cov | 41.38 | 144.35 | 0.29 | 0.775 |
| COVID - Cov | 107.05 | 102.92 | 1.04 | 0.303 |
| Intervention | −38.01 | 98.76 | −0.39 | 0.702 |
| Time | −418.66 | 98.94 | −4.23 | <0.001 |
| Gambling Craving (Time Varying) | 15.01 | 4.91 | 3.06 | 0.003 |
| TLFB Smoking (Time Varying) | 0.44 | 1.47 | 0.30 | 0.768 |
| Minutes Spent Gambling | ||||
| Intercept | 84.65 | 43.40 | 1.95 | 0.056 |
| Age - Cov | 0.57 | 1.05 | 0.54 | 0.591 |
| Sex - Cov | −10.28 | 24.35 | −0.42 | 0.675 |
| Mental Health Treatment - Cov | −6.04 | 33.80 | −0.18 | 0.859 |
| COVID - Cov | 11.08 | 23.17 | 0.48 | 0.635 |
| Intervention | −41.31 | 22.42 | −1.84 | 0.074 |
| Time | −40.44 | 20.26 | −2.00 | 0.050 |
| Gambling Craving (Time Varying) | 3.52 | 1.02 | 3.45 | 0.001 |
| TLFB Smoking (Time Varying) | −0.08 | 0.31 | −0.25 | 0.800 |
| G-SAS | ||||
| Intercept | 12.62 | 1.66 | 7.58 | <0.001 |
| Age - Cov | 0.19 | 0.04 | 4.35 | <0.001 |
| Sex - Cov | 1.09 | 1.11 | 0.98 | 0.327 |
| Mental Health Treatment - Cov | −0.79 | 1.28 | −0.62 | 0.538 |
| COVID - Cov | 2.14 | 1.07 | 2.00 | 0.048 |
| Intervention | 1.44 | 1.04 | 1.38 | 0.171 |
| Time | −4.10 | 0.63 | −6.51 | <0.001 |
| Gambling Craving (Time Varying) | 0.36 | 0.04 | 9.08 | <0.001 |
| TLFB Smoking (Time Varying) | 0.04 | 0.01 | 4.09 | <0.001 |
Note. Gambling days, money spent gambling, and minutes spent gambling were assessed with a Timeline Follow-Back procedure. TLFB = Timeline Follow-Back, G-SAS = Gambling Symptom Assessment Scale. “Cov” denotes the inclusion of a relevant covariate.
Moderation by NRT use in the integrated treatment condition
Approximately 51% of participants in the integrated treatment condition agreed to receive NRT and reported using it during the trial. Participants were asked to indicate discrete episodes of use in the past 30-days prior to the end of treatment and follow-up assessments. On average, participants who received and used NRT reported approximately 16.65 days of NRT prior to the T1 survey and 10.55 days prior to the longer-term T2 follow-up survey.
Gambling Behaviour: Regarding the number of gambling days, there was a significant NRT use by time interaction effect (see Table 6). Specifically, at high levels of NRT use, participants experienced greater reductions in gambling days over the course of the trial (b = −6.20, SE = 0.99, P < 0.001) relative to participants with low levels of NRT use (b = −2.31, SE = 0.99, P = 0.024). A marginally significant NRT use by time interaction effect was found for money spent on gambling activities, with greater reductions in money spent among participants with high (b = −921.72, SE = 225.51, P < 0.001) versus low NRT use (b = −269.81, SE = 209.10, P = 0.201). A marginally significant interaction between NRT use and time was also found for minutes spent gambling (b = 2.76, SE = 1.51, P = 0.073). At high levels of NRT use, participants experienced significant reductions in time spent gambling across the trial (b = −143.79, SE = 41.12, P < 0.001), but this effect was not found at low levels of NRT use (b = −32.70, SE = 33.16, P = 0.331).
Table 6.
Mixed models examining NRT use as a moderator of gambling treatment outcomes
| Parameter | B | SE | t | Sig. |
| Gambling Days | ||||
| Intercept | 13.55 | 0.99 | 13.71 | <0.001 |
| Age - Cov | 0.09 | 0.07 | 1.31 | 0.194 |
| Sex - Cov | −1.21 | 1.62 | −0.75 | 0.458 |
| Mental Health Treatment - Cov | 0.88 | 2.25 | 0.39 | 0.695 |
| COVID - Cov | −0.17 | 1.56 | −0.11 | 0.915 |
| Time | −5.09 | 0.68 | −7.53 | <0.001 |
| NRT Use (Time Varying) | −0.09 | 0.04 | −2.00 | 0.048 |
| NRT Use × Time | 0.10 | 0.04 | 2.43 | 0.018 |
| Money Spent Gambling | ||||
| Intercept | 1666.47 | 273.47 | 6.10 | <0.001 |
| Age - Cov | 10.81 | 9.19 | 1.18 | 0.245 |
| Sex - Cov | −355.95 | 172.90 | −2.06 | 0.045 |
| Mental Health Treatment - Cov | −229.84 | 314.77 | −0.73 | 0.468 |
| COVID - Cov | 184.79 | 166.62 | 1.11 | 0.273 |
| Time | −736.32 | 163.52 | −4.50 | <0.001 |
| NRT Use (Time Varying) | −28.96 | 11.93 | −2.43 | 0.017 |
| NRT Use × Time | 16.20 | 8.18 | 1.98 | 0.051 |
| Minutes Spent Gambling | ||||
| Intercept | 338.34 | 54.54 | 6.20 | <0.001 |
| Age - Cov | 6.84 | 3.31 | 2.06 | 0.044 |
| Sex - Cov | 14.26 | 71.96 | 0.20 | 0.844 |
| Mental Health Treatment - Cov | −127.00 | 111.16 | −1.14 | 0.258 |
| COVID - Cov | 21.18 | 69.14 | 0.31 | 0.761 |
| Time | −112.20 | 27.73 | −4.05 | <0.001 |
| NRT Use (Time Varying) | −5.18 | 2.11 | −2.45 | 0.018 |
| NRT Use × Time | 2.76 | 1.51 | 1.83 | 0.073 |
| G-SAS | ||||
| Intercept | 27.94 | 1.22 | 22.78 | <0.001 |
| Age - Cov | 0.23 | 0.09 | 2.58 | 0.012 |
| Sex - Cov | 0.46 | 2.16 | 0.21 | 0.833 |
| Mental Health Treatment - Cov | 1.69 | 2.90 | 0.58 | 0.561 |
| COVID - Cov | −0.61 | 2.07 | −0.29 | 0.770 |
| Time | −7.13 | 0.90 | −7.89 | <0.001 |
| NRT Use (Time Varying) | −0.13 | 0.05 | −2.42 | 0.017 |
| NRT Use × Time | 0.10 | 0.05 | 1.86 | 0.069 |
Note. Gambling days, money spent gambling, and minutes spent gambling were assessed with a Timeline Follow-Back procedure. NRT = Nicotine Replacement Therapy, G-SAS = Gambling Symptom Assessment Scale. “Cov” denotes the inclusion of a relevant covariate.
Gambling Disorder Symptoms: A marginally significant NRT use by time interaction was found for G-SAS score (see Table 6). Probing this interaction revealed that, at high levels of NRT use, participants experienced significant reductions in G-SAS score over the trial (b = −8.23, SE = 1.30, P < 0.001). While still statistically significant, this effect was attenuated at low levels of NRT use (b = −4.33, SE = 1.32, P = 0.002).
Discussion and conclusions
The primary aim of the current study was to examine if treatment of smoking within an online gambling intervention led to improved gambling outcomes. We designed an integrated treatment for gambling and tobacco smoking and compared clinical outcomes to a gambling-only control treatment condition. Broadly speaking, we found that participants in both the integrated and gambling-only treatment conditions reduced their gambling activity, showed less severe gambling disorder symptoms, and reduced their smoking. The lack of condition differences prevented us from formally examining the mediating role of smoking; however, post hoc exploratory analyses showed evidence for correlated change. Overall reductions in gambling craving and smoking were associated with fewer gambling days and less severe gambling problems during the trial. Interestingly, NRT usage moderated these relations. Participants in the integrated group who used NRT relatively more frequently saw greater reductions in gambling behaviours across time (versus those with low NRT use). Taken together, these results suggest that a gambling-only intervention was approximately as effective as an integrated gambling and smoking intervention at reducing both gambling and smoking behaviours.
Initially, it was surprising that participants in the gambling-only condition spontaneously reduced their rates of smoking (without any psychosocial or pharmacotherapeutic support). However, recent literature offers several explanations for this finding. First, there is a growing literature on concurrent recovery (Kim et al., 2021) which refers to simultaneous recovery from at least two addictive behaviours. Kim et al. (2021) recently published a review on addiction substitution in recovery and found that 53% of studies supported concurrent recovery. This meant that over half of the studies included showed that recovering from one addictive disorder increases the likelihood of recovering from another (concurrent) addictive disorder. While the review found lower rates of concurrent recovery among those with a primary gambling disorder, our findings lend support for concurrently changing smoking behaviours in a gambling treatment. Furthermore, research in behavioural medicine shows that when people are successful in changing one harmful behaviour, their self-efficacy for reducing other negative health behaviours increases overall (Prochaska, Spring, & Nigg, 2008). Our participants had moderate-to-high motivation to change gambling at the outset of treatment; and this may have translated into meaningful behavioural change in both conditions.
While correlational in nature, our exploratory models suggest that smoking may be an important clinical factor to consider in gambling treatments to enhance outcomes. We found that reduced smoking was a unique predictor of reduced days spent gambling, as well as lower disordered gambling symptoms during the trial. This effect was observed even after controlling for changes in gambling craving, which is a documented mechanism of gambling treatment improvements (Young & Wohl, 2009). Thus, findings suggest that smoking may be a relevant concurrent target in clinical interventions for gambling. Further, we observed that the benefits of reducing smoking for gambling behaviours is strongest when individuals use relatively more frequent NRT during treatment. In fact, 52% of participants in the integrated condition reported using NRT during the trial. Our study indicates that a significant proportion of people with problem gambling are open to using pharmacological and psychosocial methods for reducing their smoking. Nevertheless, our study should be considered an initial proof-of-concept study regarding the benefit of targeting smoking in a gambling treatment.
Several limitations of this trial should be noted. First and foremost, retention in this trial was just under 39%, indicating significant attrition over time. Retention in the integrated arm was also much lower relative to the control arm. High rates of attrition in longitudinal studies are a concern and is not uncommon in other online gambling intervention trials (Cunningham et al., 2019), as well as online treatments for addictive behaviours more broadly (Murray et al., 2013). As noted earlier, however, participants who dropped out of our study did not differ systematically on key variables from participants who were retained. Our intervention framework was largely self-guided in nature with minimal support, which may account for the high rate of attrition. This may have been especially relevant to the integrated treatment arm, since we were asking participants to change two addictive behaviours rather than just one, largely on their own. Some participants may have felt overwhelmed, and thus may have dropped out early from the study. Future studies in this area should examine ways to enhance participant engagement. Notably, research shows that even small amounts of expert therapist guidance improve engagement and reduces attrition rates in online treatments for mental health and addiction (Hadjistavropoulos, Mehta, Wilhelms, Keough, & Sundström, 2020). A large-scale follow up RCT to the current study is needed, where integrated treatment is compared with gambling only treatment, under the guidance of a therapist following a standardized protocol.
A second limitation is that we were unable to verify self-reported NRT use or reductions in smoking using biochemical verification (the gold standard). In addition to the suggestions above, future research should also incorporate biochemical verification to validate self-reported reductions in smoking. Relatedly, our inclusion criterion for daily smoking was quite minimal, and accordingly, we observed that our participants were low-to-moderate in their initial overall nicotine dependence symptoms. This may explain why many of our participants were able to reduce their smoking effectively during the trial. Considering this, our findings may not generalize to individuals who gamble and who are also severely nicotine dependent. Third, we did not collect fulsome information about mental health diagnosis, treatment history, and other potentially relevant sociodemographic factors. Fourth, our final sample size was slightly smaller than expected and we did not have a no-treatment control condition. These limitations may have reduced our power to find condition differences and examine other potential moderators of treatment effects and impeded our ability to conclude that it was the treatment itself that was responsible for the changes we observed. Finally, our online treatment platform only tracked percentage of module completion and daily diary entries as metrics of treatment material engagement. Future work using this platform should incorporate additional metrics (i.e., time spent on each module, number of modules completed more than once etc.).
Our trial is a proof-of-concept RCT study. Based on both the empirical literature and theories of addiction co-morbidity, we designed an online integrated treatment to target concurrent smoking within a gambling intervention. We did not find evidence for a clear benefit of integrated (verses gambling only) treatment in this trial, as both interventions led to comparable reductions in primary and secondary gambling and smoking outcomes. As noted above, to draw firm conclusions about the efficacy of integrated gambling-smoking treatment, we need future large-scale RCTs in the area that address some of the key methodological limitations of our trial. Nevertheless, our study shows that people who enroll in a gambling treatment may be inspired to reduce their concurrent smoking behaviours, which in turn, could promote better outcomes.
Funding sources
This research was funded by the Manitoba Gambling Research Program of Manitoba Liquor & Lotteries; however, the findings and conclusions of this paper are those solely of the author(s) and do not necessarily represent the views of Manitoba Liquor & Lotteries. Sponsoring organization: University of Manitoba.
Authors’ contribution
VB: wrote sections of the manuscript; contributed substantially to trial flow and data collection. CB: created the intervention website; contributed to treatment development and trial flow; provided feedback on the manuscript. EB: contributed substantially to trial flow and data collection; provided feedback on the manuscript. AS: wrote sections of the final manuscript; contributed substantially to trial flow and data collection. LV: conducted analyses and wrote results section; provided feedback on the manuscript. VM: provided feedback on the manuscript. TK: wrote sections of the manuscript; contributed to data collection; provided feedback on the manuscript. MPS, JM, DH, RO: helped to secure funding for the project; contributed to treatment development and trial flow; provided feedback on the manuscript. SHS, JDW, JR, HDH, CS, SD, AHK: contributed to treatment development and trial flow; provided feedback on the manuscript. MTK: project lead; secured funding for the project; contributed to treatment development and trial flow; provided feedback on the manuscript. All authors had full access to all data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Conflict of interest
The authors declare no conflict of interest.
Contributor Information
Van Bui, Email: van.bui@uwaterloo.ca.
Christian Baumgartner, Email: christian.baumgartner@isgf.uzh.ch.
Elena Bilevicius, Email: bilevice@myumanitoba.ca.
Alanna Single, Email: singlea@myumanitoba.ca.
Lana Vedelago, Email: lvedelag@yorku.ca.
Vanessa Morris, Email: v.morris@unb.ca.
Tyler Kempe, Email: kempet@myumanitoba.ca.
Michael P. Schaub, Email: michael.schaub@isgf.uzh.ch.
Sherry H. Stewart, Email: sherry.h.stewart@gmail.com.
James MacKillop, Email: jmackill@mcmaster.ca.
David C. Hodgins, Email: dhodgins@ucalgary.ca.
Jeffrey D. Wardell, Email: jwardell@yorku.ca.
Rosin O’Connor, Email: Roisin.Oconnor@concordia.ca.
Jennifer Read, Email: jpread@buffalo.edu.
Heather D. Hadjistavropoulos, Email: Heather.Hadjistavropoulos@uregina.ca.
Christopher Sundström, Email: christopher.sundstrom@ki.se.
Sarah Dermody, Email: ssdermody@ryerson.ca.
Andrew H. Kim, Email: andrewhs.kim@ryerson.ca.
Matthew T. Keough, Email: keoughmt@yorku.ca.
References
- Abrams, D., Niaura, R., Brown, R., Emmons, K., Goldstein, M., & Monti, P. (2003). The tobacco dependence treatment handbook: A guide to best practices. New York: Guilford Press. [Google Scholar]
- Barrett, S. P., Boileau, I., Okker, J., Pihl, R. O., & Dagher, A. (2004). The hedonic response to cigarette smoking is proportional to dopamine release in the human striatum as measured by positron emission tomography and [11C]raclopride. Synapse, 54(2), 65–71. 10.1002/syn.20066. [DOI] [PubMed] [Google Scholar]
- Barrett, S. P., Darredeau, C., & Pihl, R. O. (2006). Patterns of simultaneous polysubstance use in drug using university students. Human Psychopharmacology: Clinical and Experimental, 21(4), 255–263. 10.1002/hup.766. [DOI] [PubMed] [Google Scholar]
- Battersby, M., & Tolchard, B. (2013). Treatment completion in a cognitive behaviour therapy service for problem gamblers: Clinical outcome study. Journal of Addiction Research & Therapy, 4(5). 10.4172/2155-6105.1000165. [DOI] [Google Scholar]
- Bickel, W. K., & Mueller, E. T. (2009). Toward the study of trans-disease processes: A novel approach with special reference to the study of Co-morbidity. Journal of Dual Diagnosis, 5(2), 131–138. 10.1080/15504260902869147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilevicius, E., Single, A., Baumgartner, C., Bui, V., Kempe, T., Schaub, M. P., … Keough, M. T. (2020). Developing and testing the effectiveness of a novel online integrated treatment for problem gambling and tobacco smoking: A protocol for an open-label randomized controlled trial. Trials, 21(1), 937. 10.1186/s13063-020-04867-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Businelle, M. S., Kendzor, D. E., Rash, C. J., Patterson, S. M., Coffey, S. F., & Copeland, A. L. (2009). Heavy smokers perform more poorly than nonsmokers on a simulated task of gambling. Substance Use & Misuse, 44(7), 905–914. 10.1080/10826080802484173. [DOI] [PubMed] [Google Scholar]
- Casey, L. M., Oei, T. P. S., Raylu, N., Horrigan, K., Day, J., Ireland, M., & Clough, B. A. (2017). Internet-based delivery of cognitive behaviour therapy compared to monitoring, feedback and support for problem gambling: A randomised controlled trial. Journal of Gambling Studies, 33(3), 993–1010. 10.1007/s10899-016-9666-y. [DOI] [PubMed] [Google Scholar]
- di Chiara, G., & Imperato, A. (1988). Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proceedings of the National Academy of Sciences, 85(14), 5274–5278. 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowlishaw, S., Merkouris, S., Dowling, N., Anderson, C., Jackson, A., & Thomas, S. (2012). Psychological therapies for pathological and problem gambling. Cochrane Database of Systematic Reviews. 10.1002/14651858.CD008937.pub2. [DOI] [PubMed] [Google Scholar]
- Cunningham, J., Hodgins, D. C., Keough, M. T., Hendershot, C. S., Schell, C., & Godinho, A. (2020). Online interventions for problem gamblers with and without co-occurring unhealthy alcohol use: Randomized controlled trial. Internet Interventions, 19, 100307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham, J. A., Hodgins, D. C., Mackenzie, C. S., Godinho, A., Schell, C., Kushnir, V., & Hendershot, C. S. (2019). Randomized controlled trial of an Internet intervention for problem gambling provided with or without access to an Internet intervention for co-occurring mental health distress. Internet Interventions, 17, 100239. 10.1016/j.invent.2019.100239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling, N. A., Cowlishaw, S., Jackson, A. C., Merkouris, S. S., Francis, K. L., & Christensen, D. R. (2015). Prevalence of psychiatric co-morbidity in treatment-seeking problem gamblers: A systematic review and meta-analysis. Australian & New Zealand Journal of Psychiatry, 49(6), 519–539. 10.1177/0004867415575774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris, J., & Wynne, H. (2001). The Canadian problem gambling Index: Final report. Ottawa. [Google Scholar]
- Fiore, M. C., Jaén, C. R., Baker, T. B., Bailey, W. C., Benowitz, N. L., Curry, S. J, …, et al. (2008). Treating tobacco use and dependence: 2008 update. U.S. Public health service clinical practice guideline. Rockville (MD): U.S. Department of Health and Human Services. [Google Scholar]
- Fiore, M. C., Jorenby, D. E., Baker, T. B., & Kenford, S. L. (1992). Tobacco dependence and the nicotine patch: Clinical guidelines for effective use. JAMA, 268(19), 2687–2694. [PubMed] [Google Scholar]
- Gooding, P., & Tarrier, N. (2009). A systematic review and meta-analysis of cognitive-behavioural interventions to reduce problem gambling: Hedging our bets? Behaviour Research and Therapy, 47(7), 592–607. 10.1016/j.brat.2009.04.002. [DOI] [PubMed] [Google Scholar]
- Grant, J. E., Desai, R. A., & Potenza, M. N. (2009). Relationship of nicotine dependence, subsyndromal and pathological gambling, and other psychiatric disorders. The Journal of Clinical Psychiatry, 70(3), 334–343. 10.4088/JCP.08m04211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant, J. E., Donahue, C. B., Odlaug, B. L., & Kim, S. W. (2011). A 6-month follow-up of imaginal desensitization plus motivational interviewing in the treatment of pathological gambling. Annals of Clinical Psychiatry, 23(1), 3–10. [PMC free article] [PubMed] [Google Scholar]
- Grant, B. F., Hasin, D. S., Chou, S. P., Stinson, F. S., & Dawson, D. A. (2004). Nicotine dependence and psychiatric disorders in the United States. Archives of General Psychiatry, 61(11), 1107. 10.1001/archpsyc.61.11.1107. [DOI] [PubMed] [Google Scholar]
- Grant, J. E., Kim, S. W., Odlaug, B. L., & Potenza, M. N. (2008). Daily tobacco smoking in treatment-seeking pathological gamblers: Clinical correlates and Co-occurring psychiatric disorders. Journal of Addiction Medicine, 2(4), 178–184. 10.1097/ADM.0b013e3181878673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant, J. E., & Potenza, M. N. (2005). Tobacco use and pathological gambling. Annals of Clinical Psychiatry, 17(4), 237–241. 10.1080/10401230500295370. [DOI] [PubMed] [Google Scholar]
- Hadjistavropoulos, H. D., Mehta, S., Wilhelms, A., Keough, M. T., & Sundström, C. (2020). A systematic review of internet-delivered cognitive behavior therapy for alcohol misuse: Study characteristics, program content and outcomes. Cognitive Behaviour Therapy, 49(4), 327–346. 10.1080/16506073.2019.1663258. [DOI] [PubMed] [Google Scholar]
- Heatherton, T. F., Kozlowski, L. T., Frecker, R. C., & Fagerstrom, K.-O. (1991). The Fagerstrom test for nicotine dependence: A revision of the Fagerstrom tolerance questionnaire. Addiction, 86(9), 1119–1127. 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hodgins, D. C., Currie, S. R., Currie, G., & Fick, G. H. (2009). Randomized trial of brief motivational treatments for pathological gamblers: More is not necessarily better. Journal of Consulting and Clinical Psychology, 77(5), 950–960. 10.1037/a0016318. [DOI] [PubMed] [Google Scholar]
- Hodgins, D. C., Currie, S. R., & el-Guebaly, N. (2001). Motivational enhancement and self-help treatments for problem gambling. Journal of Consulting and Clinical Psychology, 69(1), 50–57. 10.1037/0022-006X.69.1.50. [DOI] [PubMed] [Google Scholar]
- Kim, S. W., Grant, J. E., Potenza, M. N., Blanco, C., & Hollander, E. (2009). The gambling symptom assessment scale (G-SAS): A reliability and validity study. Psychiatry Research, 166(1), 76–84. 10.1016/j.psychres.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, H. S., Hodgins, D. C., Garcia, X., Ritchie, E. v., Musani, I., McGrath, D. S., & von Ranson, K. M. (2021). A systematic review of addiction substitution in recovery: Clinical lore or empirically-based? Clinical Psychology Review, 89, 102083. 10.1016/j.cpr.2021.102083. [DOI] [PubMed] [Google Scholar]
- Kline, R. (2015). Principles and practice of structural equation modeling (4th ed.). Guilford Press. [Google Scholar]
- Lorains, F. K., Cowlishaw, S., & Thomas, S. A. (2011). Prevalence of comorbid disorders in problem and pathological gambling: Systematic review and meta-analysis of population surveys. Addiction, 106(3), 490–498. 10.1111/j.1360-0443.2010.03300.x. [DOI] [PubMed] [Google Scholar]
- MacKillop, J., Amlung, M. T., Few, L. R., Ray, L. A., Sweet, L. H., & Munafò, M. R. (2011). Delayed reward discounting and addictive behavior: A meta-analysis. Psychopharmacology, 216(3), 305–321. 10.1007/s00213-011-2229-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath, D. S., & Barrett, S. P. (2009). The comorbidity of tobacco smoking and gambling: A review of the literature. Drug and Alcohol Review, 28(6), 676–681. 10.1111/j.1465-3362.2009.00097.x. [DOI] [PubMed] [Google Scholar]
- McGrath, D. S., Barrett, S. P., Stewart, S. H., & McGrath, P. R. (2012). A comparison of gambling behavior, problem gambling indices, and reasons for gambling among smokers and nonsmokers who gamble: Evidence from a provincial gambling prevalence study. Nicotine & Tobacco Research, 14(7), 833–839. 10.1093/ntr/ntr294. [DOI] [PubMed] [Google Scholar]
- Melville, K. M., Casey, L. M., & Kavanagh, D. J. (2007). Psychological treatment dropout among pathological gamblers. Clinical Psychology Review, 27(8), 944–958. 10.1016/j.cpr.2007.02.004. [DOI] [PubMed] [Google Scholar]
- Merkouris, S. S., Thomas, S. A., Browning, C. J., & Dowling, N. A. (2016). Predictors of outcomes of psychological treatments for disordered gambling: A systematic review. Clinical Psychology Review, 48, 7–31. 10.1016/j.cpr.2016.06.004. [DOI] [PubMed] [Google Scholar]
- Mueser, K., Noordsy, D., Drake, R., & Lindy, S. (2003). Integrated treatment for dual disorders: A guide to effective practice. Guilford Press. [Google Scholar]
- Murray, E., White, I. R., Varagunam, M., Godfrey, C., Khadjesari, Z., & McCambridge, J. (2013). Attrition revisited: Adherence and retention in a web-based alcohol trial. Journal of Medical Internet Research, 15(8), e162. 10.2196/jmir.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins, K. A., Conklin, C. A., & Levine, M. D. (2013). Cognitive-behavioral therapy for smoking cessation. Routledge. 10.4324/9780203844533. [DOI] [Google Scholar]
- Petry, N. M., Ammerman, Y., Bohl, J., Doersch, A., Gay, H., Kadden, R., … Steinberg, K. (2006). Cognitive-behavioral therapy for pathological gamblers. Journal of Consulting and Clinical Psychology, 74(3), 555–567. 10.1037/0022-006X.74.3.555. [DOI] [PubMed] [Google Scholar]
- Petry, N. M., & Oncken, C. (2002). Cigarette smoking is associated with increased severity of gambling problems in treatment-seeking gamblers. Addiction, 97(6), 745–753. 10.1046/j.1360-0443.2002.00163.x. [DOI] [PubMed] [Google Scholar]
- Pontieri, F. E., Tanda, G., Orzi, F., & Chiara, G. di. (1996). Effects of nicotine on the nucleus accumbens and similarity to those of addictive drugs. Nature, 382(6588), 255–257. 10.1038/382255a0. [DOI] [PubMed] [Google Scholar]
- Potenza, M. N., Steinberg, M. A., McLaughlin, S. D., Wu, R., Rounsaville, B. J., Krishnan-Sarin, S., … O’Malley, S. S. (2004). Characteristics of tobacco-smoking problem gamblers calling a gambling helpline. American Journal on Addictions, 13(5), 471–493. 10.1080/10550490490483044. [DOI] [PubMed] [Google Scholar]
- Prochaska, J. J., Spring, B., & Nigg, C. R. (2008). Multiple health behavior change research: An introduction and overview. Preventive Medicine, 46(3), 181–188. 10.1016/j.ypmed.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigotti, N. A. (2022). Pharmacotherapy for smoking cessation in adults. In Aronson M. D., & Kathuria H. (Eds.), UpToDate. from https://www.uptodate.com/contents/pharmacotherapy-for-smoking-cessation-in-adults#H3481789469 [Retrieved 17 October 2022]. [Google Scholar]
- Rohsenow, D. J., Martin, R. A., Tidey, J. W., Monti, P. M., & Colby, S. M. (2013). Comparison of the Cigarette Dependence Scale with four other measures of nicotine involvement: Correlations with smoking history and smoking treatment outcome in smokers with substance use disorders. Addictive Behaviors, 38(8), 2409–2413. 10.1016/j.addbeh.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueger, S. Y., Trela, C. J., Palmeri, M., & King, A. C. (2012). Self-administered web-based timeline Followback procedure for drinking and smoking behaviors in young adults. Journal of Studies on Alcohol and Drugs, 73(5), 829–833. 10.15288/jsad.2012.73.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders, J. B., Aasland, O. G., Babor, T. F., de La Fuente, J. R., & Grant, M. (1993). Development of the alcohol use disorders identification test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption-II. Addiction, 88(6), 791–804. 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- Skinner, H. A. (1982). The drug abuse screening test. Addictive Behaviors, 7(4), 363–371. 10.1016/0306-4603(82)90005-3. [DOI] [PubMed] [Google Scholar]
- Smart, R. G., & Ferris, J. (1996). Alcohol, drugs and gambling in the ontario adult population, 1994. The Canadian Journal of Psychiatry, 41(1), 36–45. 10.1177/070674379604100109. [DOI] [PubMed] [Google Scholar]
- Smith, D. P., Battersby, M. W., Pols, R. G., Harvey, P. W., Oakes, J. E., & Baigent, M. F. (2015). Predictors of relapse in problem gambling: A prospective cohort study. Journal of Gambling Studies, 31(1), 299–313. 10.1007/s10899-013-9408-3. [DOI] [PubMed] [Google Scholar]
- Sobell, L. C., & Sobell, M. B. (1992). Timeline follow-back. Measuring alcohol consumption (pp. 41–72). Totowa, NJ: Humana Press. 10.1007/978-1-4612-0357-5_3. [DOI] [Google Scholar]
- Syan, S. K., González-Roz, A., Amlung, M., Sweet, L. H., & MacKillop, J. (2021). Delayed reward discounting as a prognostic factor for smoking cessation treatment outcome: A systematic review. Nicotine & Tobacco Research, 23(10), 1636–1645. 10.1093/ntr/ntab052. [DOI] [PubMed] [Google Scholar]
- Tabachnick, B., & Fidell, L. (2013). Using multivariate statistics (7th ed.). Boston: Pearson. [Google Scholar]
- United States. Public Health Service. Office of the Surgeon General (2020). Smoking cessation: A report of the surgeon general. U.S. Department of Health and Human Services, Public Health Service, Office of the Surgeon General. [PubMed] [Google Scholar]
- Welte, J. W., Barnes, G. M., Tidwell, M. C. O., Hoffman, J. H., & Wieczorek, W. F. (2015). Gambling and problem gambling in the United States: Changes between 1999 and 2013. Journal of Gambling Studies, 31(3), 695–715. 10.1007/s10899-014-9471-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood, R. T., Williams, R. J., Wood, R., & Williams, R. (2008). Internet gambling: Prevalence, patterns, problems, and policy options. [Google Scholar]
- Wulfert, E., Harris, K., & Broussard, J. (2016). The role of cross-cue reactivity in coexisting smoking and gambling habits. Journal of Gambling Issues, 32, 28. 10.4309/jgi.2016.32.3. [DOI] [Google Scholar]
- Yakovenko, I., Quigley, L., Hemmelgarn, B. R., Hodgins, D. C., & Ronksley, P. (2015). The efficacy of motivational interviewing for disordered gambling: Systematic review and meta-analysis. Addictive Behaviors, 43, 72–82. 10.1016/j.addbeh.2014.12.011. [DOI] [PubMed] [Google Scholar]
- Young, M. M., & Wohl, M. J. A. (2009). The gambling craving scale: Psychometric validation and behavioral outcomes. Psychology of Addictive Behaviors, 23(3), 512–522. 10.1037/a0015043. [DOI] [PubMed] [Google Scholar]