Abstract
The impact of the COVID-19 pandemic on emerging adults is of global concern. We examine changes in depressive symptoms, physical symptoms, and sleep-wake problems from before to during the pandemic among college students, and examine inequalities by gender, socioeconomic status (SES), and race (N=263, 52% Black, 48% White, 53% female). As compared to pre-pandemic levels, increases were evident in depressive symptoms, physical symptoms, and sleep problems. Females had greater increases than males in depressive symptoms, sleep problems, and physical symptoms. Students from disadvantaged SES backgrounds had greater increases in physical symptoms. Among White students, those from disadvantaged backgrounds also had greater increases in sleep problems. Lastly, daytime sleepiness increased more among Black male than White male students. Overall, findings suggest notable shifts in sleep and health during the early phase of the pandemic among emerging adults, and that attention to inequality by gender, SES, and race is warranted.
Keywords: COVID-19 pandemic, psychological stress, sleep-wake problems, physical symptoms, emerging adult development, sex differences, socioeconomic status inequality, health disparities
The pandemic has had a profound impact on the daily lives of most people. It has changed the way we work and learn, how we socialize, the places we spend our time, and the amount of contact that we have with others. In the United States, the first wave of COVID-19 cases began in mid-March, 2020, and were followed by national and local social distancing and stay-at-home orders (Hsiang et al., 2020). The first 1.5 months of the shutdown had the broadest compliance with social distancing guidelines, followed by a gradual return to some activities and more variability in compliance (Andersen, 2020; Lecocq et al., 2020). Colleges mostly shuttered their doors, sending students home with little advanced notice; milestones such as graduation ceremonies were delayed, social networks disrupted, and students were required to quickly adjust to an online learning environment (Beaney et al., 2020). While young adults have been largely spared from the harshest physical consequences of the virus, disruptions to their lives, as well as distress associated with perceived and actual health risks to self and loved ones, have been substantial (Pfefferbaum & North, 2020). With documented disparities in the broader impact of the pandemic (Clouston et al., 2021; Rossen et al., 2020; Sehra et al., 2020), variability across demographic groups is also highly likely. Understanding the magnitude and course of changes in psychological distress, physical symptoms, and sleep-wake problems among emerging adult college students, and inequities in this impact by race/ethnicity, socioeconomic status, and gender, are therefore of high significance in efforts to mitigate and offset the adverse public health consequences of the pandemic.
Early research examining the impact of the pandemic provides an important initial knowledgebase. An early cross sectional survey conducted approximately 1 week into the lockdown in China found that that the majority of adults reported a moderate to severe psychological impact of the pandemic (Wang, Pan, Wan, Tan, Xu, Ho, et al., 2020) and that psychological distress was still present in a follow-up survey conducted 1 month later (Wang, Pan, Wan, Tan, Xu, McIntyre, et al., 2020). A study conducted in the UK with a similar design (surveys administered approximately 1 and 5 weeks into the shutdown) found that suicidal thoughts increased among young adults across this period (O’Connor et al., 2020). Repeated cross-sectional studies, although not following the same individuals over time, also suggest changes in the population prevalence of psychological distress. For example, a study of adults in the United States found higher levels of depressive symptoms 1 month into the pandemic than in a previous survey conducted in 2017-2018 (Ettman et al., 2020). A study of adults in Japan also found higher psychological distress 1 month into the shutdown, as compared to immediately prior (Kikuchi et al., 2020). One longitudinal study conducted in the UK found that psychological distress, assessed 5 weeks into the initial shutdown, had increased from 2018-2019 levels, and that these changes were largest among young adults (Pierce et al., 2020). A similarly designed study of adults in the United States documented increases in psychological distress five weeks into the pandemic, as compared to 2019 levels (Wanberg et al., 2020). Although few studies have used a longitudinal design with a pre-pandemic baseline to assess changes, taken as a whole, initial evidence suggests likely increases in psychological distress during the pandemic shutdown (Xiong et al., 2020).
The current study examines longitudinal changes in depressive symptoms, sleep-wake problems, and physical symptoms from before to during the pandemic in a sample of emerging adult college students in the United States. Our focus on an emerging adult college student sample is important for several reasons. First, preliminary evidence suggests larger psychosocial effects of the pandemic in this age group (O’Connor et al., 2020; Pierce et al., 2020). Second, emerging adult college students in particular are known to have experienced substantial life changes during the pandemic such as to their living situation, educational context, and family and peer environment. Such marked changes occurring in unison over a relatively short period of time are likely to induce substantial psychological stress with mental and physical health consequences (Carney et al., 2006; B. P. Dohrenwend, 2006; B. S. Dohrenwend & Dohrenwend, 1984; Holmes & Rahe, 1967). Lastly, emerging adulthood is a pivotal time in the life span in which students are facing many possibilities, experiencing instability, and attempting to establish career and relational identities which may often set the course of subsequent development and health (Arnett, 2007; Arnett et al., 2014; Schwartz, 2016). Disruptions within this population due to the COVID-19 pandemic are therefore essential to elucidate and address. We expect to replicate recent studies showing increases in depressive symptoms during the pandemic (Ettman et al., 2020; Pierce et al., 2020), and extend this work to the context of emerging adult college students.
Importantly, this study also adds to prior research by examining effects of the pandemic on two additional outcomes—sleep-wake problems and physical symptoms. These outcomes were selected because of their known links to the stress response (Juster & Lupien, 2012; Juster & McEwen, 2015). For example, prior research indicates that major life events and chronic stressors are associated with increases in physical symptoms and sleep-wake problems (Carney et al., 2006; S. Cohen & Rodriquez, 1995; Yap et al., 2020). Physical symptoms or subjective health complains are defined in this study as minor aches and pains such as back pain, headaches, nausea, or stomach pain (S. Cohen & Hoberman, 1983). Such symptoms, which do not necessarily meet criteria for specific illness, can nonetheless have a profound impact on productivity and quality of life (Allen et al., 2017), and have been linked to subsequent morbidity and mortality (Sha et al., 2005; Zhu et al., 2007).
Sleep-wake problems were defined as problems relating to sleep quality, latency, duration, efficiency, or daytime dysfunction (e.g., daytime sleepiness). A global index of sleep-wake problems and specific dimensions relating to duration, efficiency, and daytime sleepiness were considered (Buysse et al., 1989; Johns, 1992). Sleep-wake problems have also been linked to productivity (Rosekind et al., 2010), quality of life (Baum et al., 2014), and subsequent morbidity and mortality (Kim et al., 2020; Magee & Hale, 2012; Vgontzas et al., 2004).
Although very few studies have considered population changes in these measures during the pandemic, a handful of studies examining sleep-wake problems among adults have found conflicting results. One small longitudinal study found no changes and even slight improvements in sleep 1-2 weeks into the shutdown among adults (Gao & Scullin, 2020), while other cross-sectional surveys relying on retrospective reports of sleep before the pandemic found some preliminary evidence of increases in sleep problems (Gupta et al., 2020; Mandelkorn et al., 2020; Robillard et al., 2020). With few longitudinal studies available, and fewer (if any) to our knowledge focusing on an emerging adulthood population, more work is needed to determine the impact of the pandemic on depressive symptoms, physical symptoms, and sleep-wake problems among young adults. We hypothesize increases in all three of these outcomes during the pandemic.
A second key focus of this study was to examine differences by socioeconomic status (SES), race, and gender. Stress process theories suggest that stigmatized or disadvantaged groups may be more likely to be exposed to psychosocial stress and have fewer available resources to cope with this stress when it occurs (Meyer et al., 2008; Pearlin, 1989; Turner, 2013). Furthermore, in times of stress, such as pandemics, stigma and bias against marginalized groups may increase (Bodenhausen, 1993; Yu, 2016). Based on these perspectives, alongside a larger impact of the virus in disadvantaged and minority communities (Abedi et al., 2020; Wright et al., 2020), we expect that Black and lower SES students will have greater increases in depressive symptoms, physical symptoms, and sleep-wake problems than White and higher SES students. Although race and SES differences in rates of COVID-19 infection and mortality have been well documented (Clouston et al., 2021; Rossen et al., 2020; Sehra et al., 2020), relatively few studies have considered such group differences in the broader psychosocial impacts of the pandemic and even fewer have considered these questions within emerging adult samples (Thombs et al., 2020). Furthermore, studies that have been conducted on this topic suggest mixed results (Breslau et al., 2021; Wanberg et al., 2020). The current study will add importantly to this area of research by considering the race and background socioeconomic status of our college student sample as predictors of changes in outcomes of interest from before to during the pandemic.
Gender differences in the impact of the pandemic are an additional important consideration. Although mortality rates from COVID-19 infection have proved to be higher among males than females (Peckham et al., 2020), gender differences in the broader psychosocial impact of the pandemic are not yet well understood. Related prior work suggests that women may be more impacted by disruptions in social networks (Shye et al., 1995). Furthermore, strain on romantic relationships during the pandemic may also have had a greater detrimental effect on women (Boserup et al., 2020; Bradbury-Jones & Isham, 2020). Based on these perspectives, we expect greater increases in depressive symptoms, physical symptoms, and sleep problems among female than male students.
Methods
Design and Participants
Participants were 263 undergraduate college students (53% female; Mage = 19.21 years, SD = 1.01) at a four-year university in the Southeastern United States. The student body was 86% White, 4% Black, 3% Hispanic, 2% Asian, and 5% other or multiracial at the time of recruitment. Using student records to identify demographic characteristics, equal numbers of first and second year African American (Black) and European American (White) students were recruited during the academic year, between September, 2018 and April, 2019 (T1). This approach allowed for adequate sample sizes to examine differences between Black (N = 137, 58% female) and White (N = 127, 48% female) students. Inclusion criteria were (1) being an undergraduate student aged 18 to 25, and (2) having a race of Black/African American or White in student records. Exclusion criteria were (1) reporting a diagnosed sleep disorder, (2) screening above threshold values on sleep disorder screening measures (e.g., apnea, restless leg syndrome), or (3) having a serious medical condition that would substantially influence sleep. Additional information about the design of the parent study and sleep disorder screening measures is provided elsewhere (Fuller-Rowell et al., 2021). With respect to socioeconomic background, 33% of participants were first generation (neither parent had a four year college degree), 20% grew up in families that did not own their own home for most of their childhood, and 8% had household incomes less than 150% of the federal poverty line. Participants were compensated $75 for the initial assessment during which a variety of general health and sleep measures were administered.
A follow-up study was conducted 1.27 years later (SD = 0.18), between April 27, 2020 and June 12, 2020, approximately 1.5 months into the initial wave of COVID-19 cases in the United States (T2). Participants were emailed a Qualtrics survey and compensated $25 for their participation. Of the 263 participants in the T1 sample, 76% (N = 200) participated in the T2 survey (56% female, 49% Black). T2 participants were slightly more likely to be White (p = .074) and female (p = .058) than non-responders but showed no other differences on study variables. Primary analyses focus on the full sample (N = 263) of T1 participants (see analysis plan for missing data methods). Sensitivity analyses focusing only on the participants with complete data at both time points (i.e., listwise deletion) yielded no differences in the pattern of findings reported (results available in Electronic Supplementary Materials, Tables S1-S4).
Measures
Depressive Symptoms
The Beck Depressive Inventory-II (BDI) was used to measure depressive symptoms (Beck et al., 1996). BDI is composed of 21-items that asks participants to rate the severity of depressive symptoms experienced on a four-point scale in the last two weeks. Responses are summed with possible scores ranging from 0 to 63 such that higher scores reflect more severe depressive symptoms. BDI has been previously used with college samples and shown strong validity in both community and clinical settings (Coelho et al., 2002; Moo-Estrella et al., 2005). Cronbach’s alpha was .85 for T1 and T2.
Physical Symptoms
The Cohen-Hoberman Inventory of Physical Symptoms (CHIPS) was used to measure physical symptoms (S. Cohen & Hoberman, 1983; Hall et al., 2013). CHIPS is composed of 33 physical symptoms (e.g., back pain, headache), rated on a five-point scale by how much each symptom has bothered the participant in the last two weeks. Responses were summed with possible scores ranging from 0 to 132 so that higher scores reflect more difficulty with physical symptoms. Cronbach’s alphas for T1 and T2 were .84 and .93.
Sleep-Wake Problems
The Pittsburgh Sleep Quality Index (PSQI) was used to measure sleep-wake problems (Buysse et al., 1989). The PSQI is an established measure of sleep quality and disturbance, and has been frequently used in college student samples (Lund et al., 2010; Orzech et al., 2011). The index is composed of 19 items grouped into seven components (subjective sleep quality, sleep latency, sleep duration, sleep efficiency, sleep disturbances, uses of sleep medication and daytime dysfunction), each scored on a 0-3 scale and then summed to create the global score. Possible scores for the full measure range from 0 to 21 with higher scores reflecting worse sleep problems. Internal consistency across the 7 components was .59 at T1 and .71 at T2.
Three PSQI components – sleep duration, efficiency, and disturbances – were also examined separately. Sleep efficiency was calculated as a percentage from the number of hours slept divided by the number of hours in bed. Sleep disturbance was measured as a sum score of 9 items on how often they had trouble sleeping on a scale from (0) not in the past month to (3) 3 or more times per week. Sleep duration was measured from a single item as the number of hours of actual sleep per night. The global sleep problems scale and the aforementioned three specific sleep parameters were each examined separately in analyses.
The Epworth Sleepiness Scale (ESS) was used to measure daytime sleepiness (Johns, 1992). ESS is a self-report 8-item scale that measures the likelihood of dozing off during daily activities (e.g., eating, driving). Responses were summed with a possible range from 0-24 such that higher scores indicate greater daytime sleepiness. ESS has been validated in prior studies with clinical and community samples (Buysse et al., 2008; Smith et al., 2008), including college students (Lund et al., 2010; Moo-Estrella et al., 2005). Cronbach’s alpha was .77 for T1, and .76 for T2.
Demographic Measures
Race and gender were coded from student records obtained from the university (White=0, Black=1; Female = 0, Male = 1) and confirmed via self-reports. Age at time of T1 data collection was coded in years. An aggregate index of socioeconomic status was derived from four established socioeconomic indicators: (a) having at least one parent with a college degree, (b) household income above 150% of poverty line, (c) home ownership (>50% of childhood), and (d) low family financial strain (Galobardes et al., 2006; Szanton et al., 2010). A sum score was created from the four dichotomous variables (range: 0-4) with higher scores indicating higher SES. Combined indexes of socioeconomic status are frequently used and are optimal when attempting to capture overall disadvantage within a single variable (Galobardes et al., 2006; Gruenewald et al., 2012). Participants were asked to report each parent’s highest level of education on a seven-point scale ranging from 8th grade or less (coded as 1) to graduate degree (coded as 7). Household size and participant report of parents’ estimated combined household income, measured on a scale with 30 possible categories (1 = <$5,000 to 30 = >$500,000), was used to create a dichotomous variable to indicate family incomes above 150% of the federal poverty line (Olson et al., 2004). Participant reports of the number of years during childhood that their primary residence was owned or rented by primary caregivers was used to create a dichotomous variable indicating participants who spent >50% of the years prior to 18 in an owned home, which is a reliable indicator of assets/wealth (S. Cohen et al., 2004). Lastly, family financial strain during childhood was assessed using the question, “How well off was your family when you were growing up?” with responses including “not getting by”, “barely getting by”, “doing okay”, “doing well”, and “very well off” (Szanton et al., 2010). A dichotomous variable, defined as “doing okay” to “very well off” was used to designate those at high levels of SES (coded as 1), and “not getting by” or “barely getting by” to designate low SES (coded as 0).
Analysis Plan
For each outcome variable, a series of longitudinal lagged regression models were estimated in Mplus Version 8.4 to examine predictors of change (Muthen, L.K. & Muthen, B.O., 1998; Newsom et al., 2013). To allow for model parameter estimates to be interpreted with respect to changes over time in T1 SD units, T2 outcomes were standardized (z-scored) using T1 values by subtracting the T1 mean and dividing by the T1 SD (J. Cohen et al., 2003). Model 1 included only the T1 autoregressive control which was z-scored. In these models the intercept can be interpreted as the magnitude of change between the two assessments in T1 SD units. Model 2 added age, race, gender, and SES to test whether change varied across demographic groups. Age and SES were z-scored for clarity of interpretation. Model 3 added two-way interactions between the three demographic variables to test for subgroup differences. Statistically significant interactions were plotted to display the magnitude of change for each relevant demographic group (e.g., Black student with high and low SES). Unstandardized parameter estimates, SEs, and 95% CIs are reported in tables; and effect sizes, SEs, and exact p-values are reported in the text. Missing data were dealt with using full-information maximum likelihood estimation (FIML). This approach allows for the full sample (N = 263) to be analyzed, and ensures a consistent sample across models (Enders, 2013). Of the full baseline sample, 76% participated in the T2 assessment (N = 200). Missing data on individual measures within each time point of data collection was ≤ 1% T1 and ≤ 2% at T2. Levels of missingness over time and within each time point are acceptable for FIML estimation (Enders, 2010) and thus models reported in the main manuscript use the full baseline sample (N = 263). Missing data sensitivity analyses are described in participants section above.
Results
Descriptive statistics for study variables at T1 and T2 assessments are shown in Table 1. Participants reported significantly more depressive symptoms (d = 0.52, p < 0.001), physical symptoms (d = 0.56, p < 0.001), and global sleep problems (d = 0.35, p < 0.001) during the COVID-19 assessment than the baseline assessment. With respect to the sleep-wake problem subdomains, participants also reported worse sleep efficiency (d = 0.23, p = 0.017), more sleep disturbances (d = 0.57, p < 0.001), and longer sleep durations (d = 0.45, p < 0.001). No change was found for daytime sleepiness in the full sample (d = 0.06, p = 0.409). Correlations between study variables are shown in Electronic Supplementary Material (Table S5). Depressive symptoms, physical symptoms, and global sleep problems were moderately correlated between .47 and .56 at T1 and between .46 and .59 at T2.
Table 1.
Descriptive Statistics for baseline (2018-2019) and COVID-19 (2020) collection periods
| Baseline (T1) |
COVID-19 (T2) |
||||
|---|---|---|---|---|---|
| Variables | M ± SD | % | M ± SD | % | p |
| Race (Black) | 52.1 | 49.0 | .074 | ||
| Gender (Male) | 46.8 | 43.5 | .058 | ||
| SES Composite | 3.34 ± .90 | 3.35 ± .92 | .685 | ||
| College Degree | 65.8 | 65.0 | .740 | ||
| Household Income | 87.5 | 88.0 | .973 | ||
| Home Ownership | 78.3 | 79.0 | .406 | ||
| Low Financial Strain | 93.9 | 93.5 | .756 | ||
| Depressive Symptoms | 6.50 ± 7.25 | 10.75 ± 8.95 | <.001 | ||
| Physical Symptoms | 10.61 ± 9.29 | 18.07 ± 16.35 | <.001 | ||
| Sleep Problems | 5.09 ± 2.33 | 6.11 ± 3.44 | <.001 | ||
| Sleep Efficiency | 89.39 ± 10.71 | 86.53 ± 13.55 | .017 | ||
| Sleep Disturbance | 4.21 ± 3.15 | 6.49 ± 4.70 | <.001 | ||
| Sleep Duration | 408.10 ± 67.13 | 443.69 ± 89.97 | <.001 | ||
| Daytime Sleepiness | 6.17 ± 4.04 | 6.39 ± 3.89 | .409 | ||
Note. Paired sample t-tests and Pearson chi-square tests were used to test differences between T1 and T2.
Gender differences in study variables at T1 and T2 are shown in the Electronic Supplementary Material (Table S6). No gender differences were found at T1. However, several gender differences were evident at T2; females had higher levels of depressive symptoms (d = 0.48, p = 0.001), physical symptoms (d = 0.47, p = 0.001), and sleep problems (d = 0.40, p = 0.007). Of the sleep-wake problem subdomains, females also reported more sleep disturbances (d = 0.46, p = 0.002), and shorter sleep duration (d = 0.29, p = 0.041) than males. No gender difference was found in sleep efficiency (d = 0.20, p = 0.166), or daytimes sleepiness (d = 0.15, p = 0.291).
Depressive Symptoms
Results from models examining predictors of change in depressive symptoms (BDI) are shown in Table 2. Model 1 estimates showed an increase in depressive symptoms between T1 and T2 of 0.61 SD units (SE = 0.09, p < 0.001). Model 2 indicated a main effect of gender, such that females had 0.49 SD unit greater increase in depressive symptoms than males (SE = 0.17, p = 0.004) but no differences by race (p = 0.998) or SES (p = 0.253). Overall, Model 2 explained 18.3% of the variance in T2 depressive symptoms, an increase of 4.4% from Model 1. Model 3 interactions were not significant.
Table 2.
Results from regression models examining predictors of change in depressive symptoms and physical symptoms
| Model 1 |
Model 2 |
Model 3 |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Variables | B | SE | 95% CI | B | SE | 95% CI | B | SE | 95% CI |
| T2 Depressive Symptoms (BDI) | |||||||||
| Intercept | 0.61 | 0.09 | (0.44-0.78) | 0.83 | 0.15 | (0.55-1.11) | 0.86 | 0.16 | (0.54-1.18) |
| T1 BDI | 0.48 | 0.09 | (0.32-0.64) | 0.46 | 0.08 | (0.30-0.63) | 0.46 | 0.08 | (0.30-0.62) |
| Race (Black) | 0.00 | 0.17 | (−0.34-0.34) | −0.02 | 0.22 | (−0.46-0.42) | |||
| Gender (Male) | −0.49 | 0.17 | (−0.83--0.16) | −0.46 | 0.23 | (−0.92--0.01) | |||
| Age | −0.02 | 0.09 | (−0.19-0.15) | −0.03 | 0.09 | (−0.20-0.14) | |||
| SES | −0.10 | 0.09 | (−0.27-0.07) | −0.20 | 0.15 | (−0.49-0.08) | |||
| Race x Gender | −0.01 | 0.34 | (−0.67-0.66) | ||||||
| Gender x SES | −0.21 | 0.18 | (−0.56-0.15) | ||||||
| Race x SES | 0.30 | 0.17 | (−0.04-0.63) | ||||||
| R2 | 13.9% | 18.3 % | 19.6% | ||||||
| T2 Physical Symptoms (CHIPS) | |||||||||
| Intercept | 0.79 | 0.12 | (0.56-1.03) | 1.12 | 0.20 | (0.73-1.51) | 1.16 | 0.23 | (0.72-1.60) |
| T1 CHIPS | 0.50 | 0.12 | (0.26-0.73) | 0.46 | 0.12 | (0.23-0.68) | 0.47 | 0.12 | (0.24-0.69) |
| Race (Black) | −0.24 | 0.23 | (−0.48-0.43) | −0.06 | 0.31 | (−0.66-0.54) | |||
| Gender (Male) | −0.73 | 0.24 | (−1.19--0.26) | −0.74 | 0.32 | (−1.38--0.11) | |||
| Age | 0.14 | 0.12 | (−0.10-0.37) | 0.14 | 0.12 | (−0.10-0.37) | |||
| SES | −0.26 | 0.12 | (−0.48--0.03) | −0.52 | 0.20 | (−0.91--0.12) | |||
| Race x Gender | 0.07 | 0.47 | (−0.86-0.99) | ||||||
| Gender x SES | 0.09 | 0.25 | (−0.39-0.57) | ||||||
| Race x SES | 0.38 | 0.23 | (−0.08-0.84) | ||||||
| R2 | 8.0% | 14.7% | 15.5% | ||||||
Note. All coefficients are unstandardized. Statistically significant parameter estimates (p < .05) are shown in bold font. T1 = 2018-2019 collection, T2 = April-June, 2020 collection. SES = socioeconomic status. N = 263.
Physical Symptoms
A similar pattern of findings was evident for change in physical symptoms (Table 2). Model 1 estimates showed a significant increase in physical symptoms between T1 and T2 of 0.79 SD units (SE = 0.12, p < 0.001). Model 2 indicated a main effect of both gender and SES. Females had a 0.73 SD unit larger increase in physical symptoms than males (SE = 0.24, p = 0.002). And with respect to SES, each SD unit increase was associated with 0.26 SD unit smaller increase in physical symptoms (SE = 0.12, p = 0.025). Overall, Model 2 explained 14.7% of the variance in T2 physical symptoms, an increase of 6.7% from Model 1. No race differences were found in the magnitude of increases in physical symptoms (p = .917). Model 3 showed no significant interactions.
Sleep-Wake Problems
Global Sleep Problems
Model 1 estimates showed a significant increase in global sleep problems (PSQI) between T1 and T2 of .35 SD units (SE = 0.09, p < 0.001). Model 2 indicated a main effect of gender, such that females had a 0.48 SD unit greater increase in global sleep problems than males (SE = 0.18, p = 0.008). Model 3 showed an additional main effect of SES, such that each SD unit increase in SES was associated with a 0.47 SD unit smaller increase in global sleep problems (SE = 0.15, p = 0.002), and a significant interaction between race and SES (β = 0.49, SE = 0.18, p = 0.006). Figure 1 shows the interaction effect. The effect of SES on global sleep problems was strongest among White students. Specifically, global sleep problems increased by 0.58 SD units for Black students with high SES (SE = 0.17, p = 0.001), by 0.44 SD units for Black students with low SES (SE = 0.17, p = 0.010), by 0.35 SD units for White students with high SES (SE = 0.17, p = .043), and by 1.04 SD units for White students with low SES (SE = 0.21, p < 0.001). Low SES is defined as 1 SD below the mean, equivalent to a score of 2.4 on the 0 to 4 SES composite scale; high SES is defined as .73 SD units above the mean, equivalent to a score of 4 out of 4 (highest possible value). Model 3 explained 17.6% of the variance in T2 global sleep problems, compared to 14.3% in Model 2, and 10.8% in Model 1. Full model results for global sleep problems are shown in Table 3.
Figure 1.
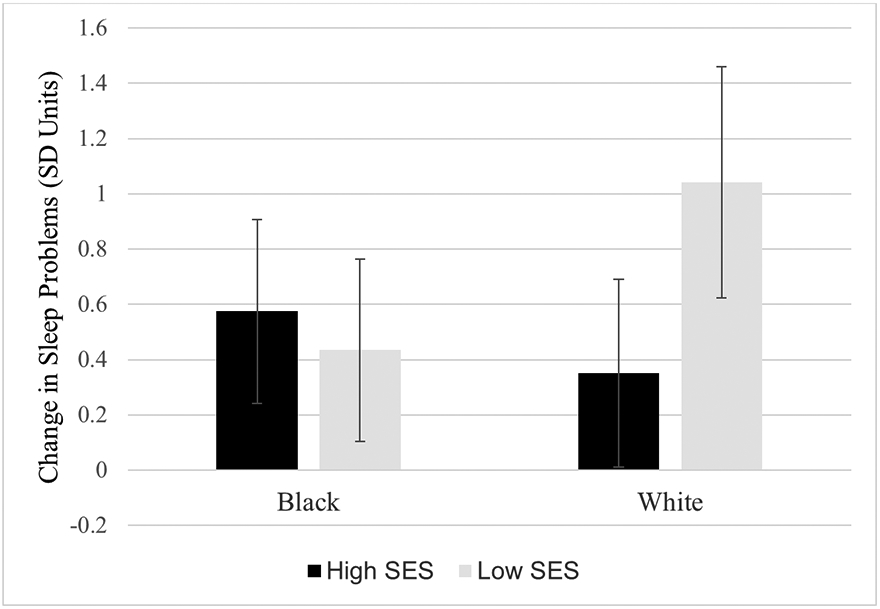
Fitted interaction plot showing the magnitude of change in sleep problems (PSQI global score) by race and socioeconomic status (SES). Values are derived from Model 3 with non-significant interaction effects removed. Error bars reflect 95% confidence intervals. Low SES is defined as 1 SD below the mean, equivalent to a score of 2.4 on the 0 to 4 scale. High SES is defined as .73 SD units above the mean, equivalent to a score of 4 (highest possible value).
Table 3.
Results from regression models examining predictors of change in sleep problems and daytime sleepiness
| Model 1 |
Model 2 |
Model 3 |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Variables | B | SE | 95% CI | B | SE | 95% CI | B | SE | 95% CI |
| T2 Sleep Problems (PSQI) | |||||||||
| Intercept | 0.35 | 0.09 | (0.17-0.52) | 0.61 | 0.15 | (0.31-0.91) | 0.72 | 0.17 | (0.39-1.06) |
| T1 PSQI | 0.44 | 0.10 | (0.25-0.64) | 0.43 | 0.10 | (0.24-0.62) | 0.45 | 0.10 | (0.26-0.63) |
| Race (Black) | −0.11 | 0.18 | (−0.46-0.24) | −0.28 | 0.23 | (−0.73-0.17) | |||
| Gender (Male) | −0.48 | 0.18 | (−0.84--0.13) | −0.65 | 0.25 | (−1.13--0.17) | |||
| Age | 0.04 | 0.09 | (−0.14-0.22) | 0.03 | 0.09 | (−0.15-0.21) | |||
| SES | −0.11 | 0.09 | (−0.28-0.62) | −0.47 | 0.15 | (−0.77--0.17) | |||
| Race x Gender | 0.39 | 0.36 | (−0.31-1.09) | ||||||
| Gender x SES | 0.22 | 0.19 | (−0.14-0.59) | ||||||
| Race x SES | 0.49 | 0.18 | (0.14-0.84) | ||||||
| R2 | 10.8% | 14.3% | 17.6% | ||||||
| T2 Daytime Sleepiness (ESS) | |||||||||
| Intercept | 0.03 | 0.06 | (−0.09-0.14) | −0.09 | 0.10 | (−0.29-0.10) | 0.07 | 0.11 | (−0.15-0.28) |
| T1 ESS | 0.53 | 0.06 | (0.41-0.64) | 0.51 | 0.06 | (0.40-0.62) | 0.51 | 0.06 | (0.40-0.62) |
| Race (Black) | 0.28 | 0.12 | (0.05-0.51) | 0.01 | 0.15 | (−0.28-0.30) | |||
| Gender (Male) | −0.06 | 0.12 | (−0.29 -0.18) | −0.33 | 0.16 | (−0.64--0.02) | |||
| Age | 0.10 | 0.06 | (−0.02-0.21) | 0.09 | 0.06 | (−0.03-0.20) | |||
| SES | 0.06 | 0.06 | (−0.06-0.17) | −0.05 | 0.10 | (−0.24-0.14) | |||
| Race x Gender | 0.61 | 0.23 | (0.16-1.06) | ||||||
| Gender x SES | −0.07 | 0.12 | (−0.31-0.17) | ||||||
| Race x SES | 0.21 | 0.11 | (−0.01-0.43) | ||||||
| R2 | 28.9% | 32.9% | 37.4% | ||||||
Note. All coefficients are unstandardized. Statistically significant parameter estimates (p < .05) are shown in bold font. T1 = 2018-2019 collection, T2 = April-June, 2020 collection. SES = socioeconomic status. N = 263.
Sleep Problem Subdomains
The pattern of findings and inference for sleep efficiency and sleep disturbance were consistent with the findings for global sleep problems (see Electronic Supplementary Material for full model results). However, Model 1 estimates showed a significant increase in sleep duration between T1 and T2 of 0.59 SD units (SE = 0.09, p < 0.001), equivalent to 39.9 minutes per night (SE = 6.0). Model 2 results indicated no significant differences across demographic groups and Model 3 indicated no significant interactions.
Daytime Sleepiness
Although Model 1 estimates showed no significant difference in daytime sleepiness between T1 and T2 (p = 0.854), Model 2 indicated the magnitude of change was different for Black and White students. Specifically, Black students had 0.28 SD unit greater increase in daytime sleepiness than White students (SE = 0.12, p = 0.018). Model 3 also indicated a significant interaction between race and gender (β = 0.61, SE = 0.23, p = 0.008). Figure 2 shows the interaction effect. Daytime sleepiness increased by 0.36 SD units for Black males (SE = 0.14, p = .008), and decreased by 0.30 units for White males (SE = 0.11, p = .007). No change in daytime sleepiness was found for Black females (p = 0.536) or White females (p = 0.593). Model 3 explained 37.4% of the variance in T2 daytime sleepiness, compared to 32.9% in Model 2 and 28.9% in Model 1. Full model results for daytime sleepiness are shown in Table 3.
Figure 2.
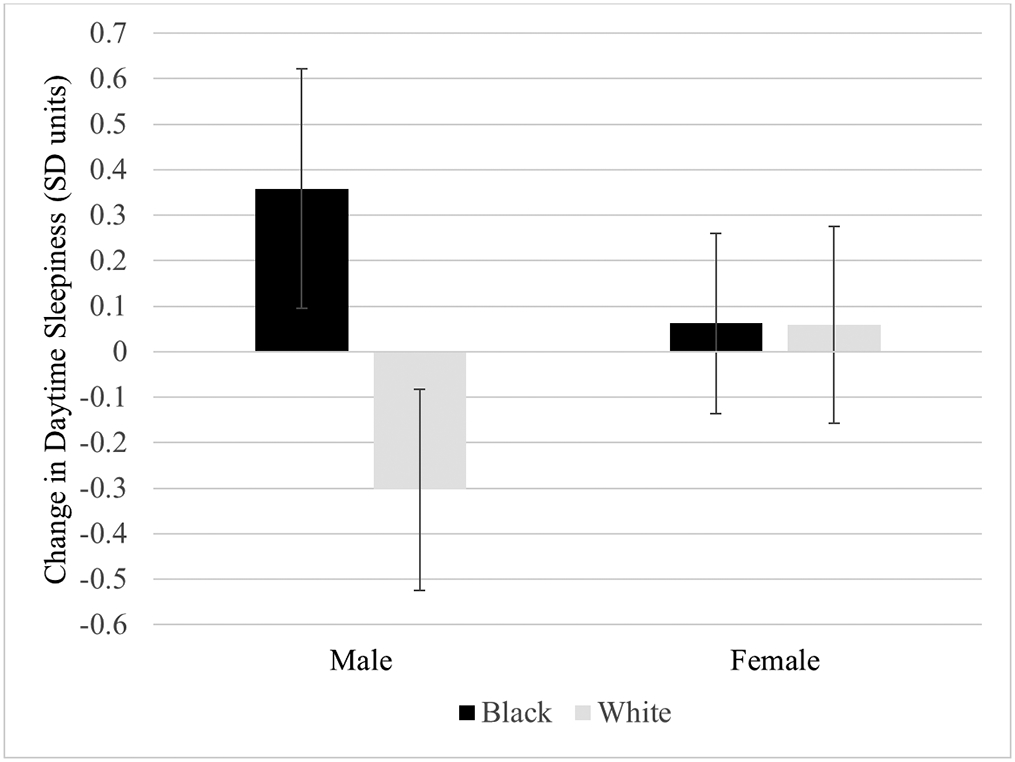
Fitted interaction plot showing the magnitude of change in daytime sleepiness by gender and race. Coefficients are from Model 3 with non-significant interaction effects removed. Error bars reflect 95% confidence intervals.
Discussion
Understanding the impact of the pandemic on emerging adults—and disparities in this impact across demographic groups—is essential to inform an efficient and effective pandemic response. While much has already been written about the pandemic, few studies have used longitudinal data with a pre-pandemic baseline assessment; considered disparities by race, gender, and socioeconomic position; or focused on emerging adult college students who experienced particularly abrupt changes to their lives and living arrangements (Salari et al., 2020; Thombs et al., 2020). Using longitudinal data, the current study examined changes in depressive symptoms, physical symptoms, and sleep-wake problems from before to 1.5 months into the pandemic in this population, and considered differences in the magnitude of these changes across demographic groups. Results indicated substantial increases in depressive symptoms, physical symptoms, and sleep-wake problems in the full sample. The magnitude of the increase was largest for physical symptoms (0.79 SDs), followed by depressive symptoms (0.61 SDs) and global sleep problems (0.35 SDs). Two subdomains of sleep problems (efficiency and disturbance) also showed a similar shift toward more sleep problems. Interestingly however, sleep duration increased and the percentage of individuals sleeping less than seven hours decreased (from 44% to 27%).
These results are consistent with recent work showing shifts in psychological distress during the pandemic (Xiong et al., 2020). It also extends this work by showing (1) that these changes are evident among college students, and (2) that, in addition to increases in depressive symptoms, increases in physical symptoms and sleep problems also appear to be substantial. It should be noted that at the time of our T2 assessment, a very small percentage of the population had been infected with COVID-19. Increases in physical symptoms are therefore interpreted as likely resulting from psychosocial stresses relating to the pandemic rather than from viral symptoms. Chronic or severe stress exposures are known to elicit a cascade of psychological and physiological processes that are linked to immune suppression, delayed wound healing, and multi-system physiological dysregulation or allostatic load (Christian et al., 2006; Juster et al., 2010). Evidence also shows that these physiologic changes relating to chronic stress are reflected in a variety of physical symptoms including increased joint and muscle pain, headaches, stomach pain, congestion, digestive problems, and fatigue (Goertzel et al., 2006; Juster & Lupien, 2012). The results of our study suggest that, among college students, psychosocial stresses during the pandemic have elicited an increase in reports of physical symptoms or subjective health complaints.
Results relating to changes in sleep help to resolve discrepancies in prior work (Gao & Scullin, 2020; Mandelkorn et al., 2020) and suggest that although sleep duration and time in bed have increased during the pandemic (presumably due to the schedule flexibility afforded by virtual activities and stay-at-home recommendations), sleep problems, including those relating to efficiency and disturbance, have also increased. Although troubling, the substantial increases in depressive symptoms, physical symptoms and sleep problems reported are not surprising given the considerable life changes that have occurred during the pandemic within this population.
Consistent with stress process theory (Meyer et al., 2008; Turner, 2013), some increases were larger among women, Black students, and those from less advantaged socioeconomic backgrounds. Females had greater increases in sleep problems, depressive symptoms, and physical symptoms. This findings builds on prior work which has shown sizable effects of the pandemic on indicators of mental health problems among females (Iob et al., 2020; Losada-Baltar et al., 2020; Özdin & Bayrak Özdin, 2020). One plausible albeit tentative explanation for the observed gender difference is that social network changes may have more of an impact on females than males (Shye et al., 1995). Additional research will be necessary to test this possibility.
Results also showed that students from less advantaged SES backgrounds had greater increases in physical symptoms (in the full sample) and sleep problems (among White students). This finding is consistent with the documented greater impact of the virus in less advantaged communities, which has likely led to greater psychosocial disruptions in these communities (Singu et al., 2020). With a focus on background socioeconomic position among college students, results also add to prior research which has focused on the role of SES within broader population samples of adults (Breslau et al., 2021; Wanberg et al., 2020). This research had shown mixed results with one study showing greater increases in psychological distress among those with higher levels of education (Wanberg et al., 2020), and another finding smaller increases in psychological distress among those with higher incomes (Breslau et al., 2021). Our results provide evidence that socioeconomic disadvantage is a risk factor for some specific outcomes and population subgroups among college students. More work will be needed to further explicate the roles of SES during the pandemic.
Another important finding relating to SES was related to the differential association between SES and increases in sleep problems for Black and White students. This result is consistent with recent research showing that the health benefits of socioeconomic attainment are less evident among Black Americans (Assari, 2018; Fuller-Rowell et al., 2015). Reasons for this are not clearly understood but may relate to differences in neighborhood contexts, which are evident even after adjusting for family SES (Fuller-Rowell et al., 2016); or experiences of discrimination, which for Black Americans are most prevalent in advantaged socioeconomic contexts (Fuller-Rowell et al., 2017).
Among males, race differences were evident in daytime sleepiness, with Black males showing a significant increase in sleepiness while White males enjoyed a decrease. Consistent with data suggesting a substantially larger impact of the virus in Black communities, these differences persisted after adjusting for family SES (Tai et al., 2020). Just as mortality rates from COVID-19 are higher in Black than White samples (Rogers et al., 2020), our results suggest that with respect to daytime sleepiness, the psychosocial effects of the pandemic may also be greater within Black communities.
Some limitations should be noted. First, self-report measures were used to assess sleep and health at each time point. Although examining change over time helps to address the limitations of self-report measures, additional research using objective assessments will be useful to clarify effect sizes. Some prior research suggests that the magnitude of Black-White differences may be underestimated (Grandner et al., 2016; Petrov & Lichstein, 2016) or overestimated (Jackson et al., 2018) by self-report measures, and thus further consideration of race differences using objective measures is warranted. Second, as noted above, all individuals reporting a diagnosed sleep disorder or screening above threshold values for a sleep disorder at T1 were excluded from participation in the study. Thus, results are of most relevance to non-clinical populations and should interpreted as such. Third, specific reasons for changes over time were not tested. Our overarching interpretation is that changes to daily life that necessarily occurred during the initial months of the pandemic, and related psychosocial stress, is responsible for the reported results. However, because no control group was possible, developmental change or other factors unrelated to the pandemic cannot be ruled out. Additional research examining specific mechanisms for changes, including those directly tied to the pandemic, will be useful in this regard. Studies examining effectiveness of specific responses to the pandemic at various levels (e.g., national or local) will also be highly informative. Last, our focus on a cohort of college students in the United States limits generalizability beyond this context. Although this study suggests substantial changes during the pandemic, additional research will be useful to compare these change to those of other demographic groups.
Overall, results suggest notable shifts in sleep and health among emerging adults during the initial wave of the COVID-19 pandemic in the United States. The findings also suggest that attention to inequalities in the effects of the pandemic by race, gender, and SES—and efforts to mitigate these inequities—are warranted. Determining the degree to which the reported changes are enduring and thus of longer-term developmental significance within emerging adults populations will be important next step to inform the continuing pandemic response.
Supplementary Material
Acknowledgments
This work was supported by the National Heart, Lung, and Blood Institute (grant number 1R15HL140504-01A1); and the USDA National Institute of Food and Agriculture (Hatch project 1003947). The funding sources had no role in the study design or in the collection, analysis, interpretation, or write up of the data. The authors declare that they have no conflict of interest.
References
- Abedi V, Olulana O, Avula V, Chaudhary D, Khan A, Shahjouei S, Li J, & Zand R (2020). Racial, Economic, and Health Inequality and COVID-19 Infection in the United States. Journal of Racial and Ethnic Health Disparities. 10.1007/s40615-020-00833-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen SF, Wetherell MA, & Smith MA (2017). The Cohen–Hoberman inventory of physical symptoms: Factor structure, and preliminary tests of reliability and validity in the general population. Psychology & Health, 32(5), 567–587. 10.1080/08870446.2017.1290237 [DOI] [PubMed] [Google Scholar]
- Andersen M (2020). Early Evidence on Social Distancing in Response to COVID-19 in the United States. SSRN, 1–35. 10.2139/ssrn.3569368 [DOI] [Google Scholar]
- Arnett JJ (2007). Emerging Adulthood: What Is It, and What Is It Good For? Child Development Perspectives, 1(2), 68–73. 10.1111/j.1750-8606.2007.00016.x [DOI] [Google Scholar]
- Arnett JJ, Žukauskienė R, & Sugimura K (2014). The new life stage of emerging adulthood at ages 18–29 years: Implications for mental health. The Lancet Psychiatry, 1(7), 569–576. 10.1016/S2215-0366(14)00080-7 [DOI] [PubMed] [Google Scholar]
- Assari S (2018). Health Disparities due to Diminished Return among Black Americans: Public Policy Solutions. Social Issues and Policy Review, 12(1), 112–145. 10.1111/sipr.12042 [DOI] [Google Scholar]
- Baum KT, Desai A, Field J, Miller LE, Rausch J, & Beebe DW (2014). Sleep restriction worsens mood and emotion regulation in adolescents. Journal of Child Psychology and Psychiatry, 55(2), 180–190. 10.1111/jcpp.12125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaney T, Clarke JM, Jain V, Golestaneh AK, Lyons G, Salman D, & Majeed A (2020). Excess mortality: The gold standard in measuring the impact of COVID-19 worldwide? Journal of the Royal Society of Medicine, 113(9), 329–334. 10.1177/0141076820956802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steer RA, & Brown GK (1996). Manual for the Beck Depression Inventory-II. 1996. Psychological Corporation. [Google Scholar]
- Bodenhausen GV (1993). Chapter 2 - Emotions, Arousal, and Stereotypic Judgments: A Heuristic Model of Affect and Stereotyping. In Mackie DM & Hamilton DL (Eds.), Affect, Cognition and Stereotyping (pp. 13–37). Academic Press. 10.1016/B978-0-08-088579-7.50006-5 [DOI] [Google Scholar]
- Boserup B, McKenney M, & Elkbuli A (2020). Alarming trends in US domestic violence during the COVID-19 pandemic. The American Journal of Emergency Medicine, 0(0). 10.1016/j.ajem.2020.04.077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury-Jones C, & Isham L (2020). The pandemic paradox: The consequences of COVID-19 on domestic violence. Journal of Clinical Nursing, 29(13–14), 2047–2049. 10.1111/jocn.15296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslau J, Finucane ML, Locker AR, Baird MD, Roth EA, & Collins RL (2021). A longitudinal study of psychological distress in the United States before and during the COVID-19 pandemic. Preventive Medicine, 143, 106362. 10.1016/j.ypmed.2020.106362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse DJ, Hall ML, Strollo PJ, Kamarck TW, Owens J, Lee L, Reis SE, & Matthews KA (2008). Relationships Between the Pittsburgh Sleep Quality Index (PSQI), Epworth Sleepiness Scale (ESS), and Clinical/Polysomnographic Measures in a Community Sample. Journal of Clinical Sleep Medicine : JCSM : Official Publication of the American Academy of Sleep Medicine, 4(6), 563–571. [PMC free article] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, Monk TH, Berman SR, & Kupfer DJ (1989). The Pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Research, 28(2), 193–213. 10.1016/0165-1781(89)90047-4 [DOI] [PubMed] [Google Scholar]
- Carney CE, Edinger JD, Meyer B, Lindman L, & Istre T (2006). Daily activities and sleep quality in college students. Chronobiology International, 23(3), 623–637. 10.1080/07420520600650695 [DOI] [PubMed] [Google Scholar]
- Christian LM, Graham JE, Padgett DA, Glaser R, & Kiecolt-Glaser JK (2006). Stress and Wound Healing. Neuroimmunomodulation, 13(5–6), 337–346. 10.1159/000104862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clouston SAP, Natale G, & Link BG (2021). Socioeconomic inequalities in the spread of coronavirus-19 in the United States: A examination of the emergence of social inequalities. Social Science & Medicine, 268, 113554. 10.1016/j.socscimed.2020.113554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelho R, Martins A, & Barros H (2002). Clinical profiles relating gender and depressive symptoms among adolescents ascertained by the Beck Depression Inventory II. European Psychiatry, 17(4), 222–226. 10.1016/S0924-9338(02)00663-6 [DOI] [PubMed] [Google Scholar]
- Cohen J, Cohen P, Aiken LS, & West SG (2003). Applied multiple regression/correlation analysis for the behavioral sciences (Third Edition). Lawrence Erlbaum Associates, Inc. [Google Scholar]
- Cohen S, Doyle WJ, Turner RB, Alper CM, & Skoner DP (2004). Childhood socioeconomic status and host resistance to infectious illness in adulthood. Psychosomatic Medicine, 66(4), 553–558. 10.1097/01.psy.0000126200.05189.d3 [DOI] [PubMed] [Google Scholar]
- Cohen S, & Hoberman HM (1983). Positive Events and Social Supports as Buffers of Life Change Stress. Journal of Applied Social Psychology, 13(2), 99–125. 10.1111/j.1559-1816.1983.tb02325.x [DOI] [Google Scholar]
- Cohen S, & Rodriquez MS (1995). Pathways linking affective disturbances and physical disorders. Health Psychology: Official Journal of the Division of Health Psychology, American Psychological Association, 14(5), 374–380. 10.1037//0278-6133.14.5.374 [DOI] [PubMed] [Google Scholar]
- Dohrenwend BP (2006). Inventorying Stressful Life Events as Risk Factors for Psychopathology: Toward Resolution of the Problem of Intracategory Variability. Psychological Bulletin, 132(3), 477–495. 10.1037/0033-2909.132.3.477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohrenwend BS, & Dohrenwend BP (Eds.). (1984). Stressful life events & their contexts. Rutgers University Press. [Google Scholar]
- Enders CK (2010). Applied Missing Data Analysis. The Guilford Press. [Google Scholar]
- Enders CK (2013). Dealing with missing data in developmental research. Child Development Perspectives, 7(1), 27–31. 10.1111/cdep.12008 [DOI] [Google Scholar]
- Ettman CK, Abdalla SM, Cohen GH, Sampson L, Vivier PM, & Galea S (2020). Prevalence of Depression Symptoms in US Adults Before and During the COVID-19 Pandemic. JAMA Network Open, 3(9). 10.1001/jamanetworkopen.2020.19686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller-Rowell TE, Curtis DS, Doan SN, & Coe CL (2015). Racial disparities in the health benefits of educational attainment: A study of inflammatory trajectories among African American and white adults. Psychosomatic Medicine, 77(1), 33–40. 10.1097/PSY.0000000000000128 [DOI] [PubMed] [Google Scholar]
- Fuller-Rowell TE, Curtis DS, El-Sheikh M, Chae DH, Boylan JM, & Ryff CD (2016). Racial disparities in sleep: The role of neighborhood disadvantage. Sleep Medicine, 27–28(Supplement C), 1–8. 10.1016/j.sleep.2016.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller-Rowell TE, Curtis DS, El-Sheikh M, Duke AM, Ryff CD, & Zgierska AE (2017). Racial discrimination mediates race differences in sleep problems: A longitudinal analysis. Cultural Diversity & Ethnic Minority Psychology, 23(2), 165–173. 10.1037/cdp0000104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller-Rowell TE, Nichols OI, Robinson AT, Boylan JM, Chae DH, & El-Sheikh M (2021). Racial disparities in sleep health between Black and White young adults: The role of neighborhood safety in childhood. Sleep Medicine, 81, 341–349. 10.1016/j.sleep.2021.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galobardes B, Shaw M, Lawlor DA, Lynch JW, & Smith GD (2006). Indicators of socioeconomic position. Journal of Epidemiology and Community Health (1979-), 60(1), 7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao C, & Scullin MK (2020). Sleep health early in the coronavirus disease 2019 (COVID-19) outbreak in the United States: Integrating longitudinal, cross-sectional, and retrospective recall data. Sleep Medicine, 73, 1–10. 10.1016/j.sleep.2020.06.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goertzel BN, Pennachin C, de Souza Coelho L, Maloney EM, Jones JF, & Gurbaxani B (2006). Allostatic load is associated with symptoms in chronic fatigue syndrome patients. Pharmacogenomics, 7(3), 485–494. 10.2217/14622416.7.3.485 [DOI] [PubMed] [Google Scholar]
- Grandner MA, Williams NJ, Knutson KL, Roberts D, & Jean-Louis G (2016). Sleep disparity, race/ethnicity, and socioeconomic position. Sleep Medicine, 18, 7–18. 10.1016/j.sleep.2015.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenewald TL, Karlamangla AS, Hu P, Stein-Merkin S, Crandall C, Koretz B, & Seeman TE (2012). History of socioeconomic disadvantage and allostatic load in later life. Soc Sci Med, 74(1), 75–83. 10.1016/j.socscimed.2011.09.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R, Grover S, Basu A, Krishnan V, Tripathi A, Subramanyam A, Nischal A, Hussain A, Mehra A, Ambekar A, Saha G, Mishra KK, Bathla M, Jagiwala M, Manjunatha N, Nebhinani N, Gaur N, Kumar N, Dalal PK, … Avasthi A (2020). Changes in sleep pattern and sleep quality during COVID-19 lockdown. Indian Journal of Psychiatry, 62(4), 370–378. 10.4103/psychiatry.IndianJPsychiatry_523_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall CW, Row KA, Wuensch KL, & Godley KR (2013). The Role of Self-Compassion in Physical and Psychological Well-Being. The Journal of Psychology, 147(4), 311–323. 10.1080/00223980.2012.693138 [DOI] [PubMed] [Google Scholar]
- Holmes TH, & Rahe RH (1967). The Social Readjustment Rating Scale. Journal of Psychosomatic Research, 11(2), 213–218. [DOI] [PubMed] [Google Scholar]
- Hsiang S, Allen D, Annan-Phan S, Bell K, Bolliger I, Chong T, Druckenmiller H, Huang LY, Hultgren A, Krasovich E, Lau P, Lee J, Rolf E, Tseng J, & Wu T (2020). The effect of large-scale anti-contagion policies on the COVID-19 pandemic. Nature, 584(7820), Article 7820. 10.1038/s41586-020-2404-8 [DOI] [PubMed] [Google Scholar]
- Iob E, Steptoe A, & Fancourt D (2020). Abuse, self-harm and suicidal ideation in the UK during the COVID-19 pandemic. The British Journal of Psychiatry, 217(4), 543–546. 10.1192/bjp.2020.130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson CL, Patel SR, Jackson WB, Lutsey PL, & Redline S (2018). Agreement between self-reported and objectively measured sleep duration among white, black, Hispanic, and Chinese adults in the United States: Multi-Ethnic Study of Atherosclerosis. Sleep, 41(6), 1–12. 10.1093/sleep/zsy057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns MW (1992). Reliability and Factor Analysis of the Epworth Sleepiness Scale. Sleep, 15(4), 376–381. 10.1093/sleep/15.4.376 [DOI] [PubMed] [Google Scholar]
- Juster R-P, & Lupien S (2012). A Sex- and Gender-Based Analysis of Allostatic Load and Physical Complaints. Gender Medicine, 9(6), 511–523. 10.1016/j.genm.2012.10.008 [DOI] [PubMed] [Google Scholar]
- Juster R-P, & McEwen BS (2015). Sleep and chronic stress: New directions for allostatic load research. Sleep Medicine, 16(1), 7–8. 10.1016/j.sleep.2014.07.029 [DOI] [PubMed] [Google Scholar]
- Juster R-P, McEwen BS, & Lupien SJ (2010). Allostatic load biomarkers of chronic stress and impact on health and cognition. Neuroscience & Biobehavioral Reviews, 35(1), 2–16. 10.1016/j.neubiorev.2009.10.002 [DOI] [PubMed] [Google Scholar]
- Kikuchi H, Machida M, Nakamura I, Saito R, Odagiri Y, Kojima T, Watanabe H, Fukui K, & Inoue S (2020). Changes in Psychological Distress During the COVID-19 Pandemic in Japan: A Longitudinal Study. Journal of Epidemiology, 30(11), 522–528. 10.2188/jea.JE20200271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J-W, Stewart R, Lee H-J, Kang H-J, Kim S-W, Shin I-S, Kim M-C, Hong Y-J, Ahn Y-K, Jeong M-H, Yoon J-S, & Kim J-M (2020). Sleep problems associated with long-term mortality in acute coronary syndrome: Effects of depression comorbidity and treatment. General Hospital Psychiatry, 66, 125–132. 10.1016/j.genhosppsych.2020.08.004 [DOI] [PubMed] [Google Scholar]
- Lecocq T, Hicks SP, Noten KV, van Wijk K, Koelemeijer P, Plaen RSMD, Massin F, Hillers G, Anthony RE, Apoloner M-T, Arroyo-Solórzano M, Assink JD, Büyükakpınar P, Cannata A, Cannavo F, Carrasco S, Caudron C, Chaves EJ, Cornwell DG, … Xiao H (2020). Global quieting of high-frequency seismic noise due to COVID-19 pandemic lockdown measures. Science, 369(6509), 1338–1343. 10.1126/science.abd2438 [DOI] [PubMed] [Google Scholar]
- Losada-Baltar A, Jiménez-Gonzalo L, Gallego-Alberto L, Pedroso-Chaparro M. del S., Fernandes-Pires J, & Márquez-González M (2020). “We Are Staying at Home.” Association of Self-perceptions of Aging, Personal and Family Resources, and Loneliness With Psychological Distress During the Lock-Down Period of COVID-19. The Journals of Gerontology: Series B, gbaa048. 10.1093/geronb/gbaa048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund HG, Reider BD, Whiting AB, & Prichard JR (2010). Sleep Patterns and Predictors of Disturbed Sleep in a Large Population of College Students. Journal of Adolescent Health, 46(2), 124–132. 10.1016/j.jadohealth.2009.06.016 [DOI] [PubMed] [Google Scholar]
- Magee L, & Hale L (2012). Longitudinal associations between sleep duration and subsequent weight gain: A systematic review. Sleep Medicine Reviews, 16(3), 231–241. 10.1016/j.smrv.2011.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandelkorn U, Genzer S, Choshen-Hillel S, Reiter J, Meira E Cruz M, Hochner H, Kheirandish-Gozal L, Gozal D, & Gileles-Hillel A (2020). Escalation of sleep disturbances amid the COVID-19 pandemic: A cross-sectional international study. Journal of Clinical Sleep Medicine: JCSM: Official Publication of the American Academy of Sleep Medicine. 10.5664/jcsm.8800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer IH, Schwartz S, & Frost DM (2008). Social patterning of stress and coping: Does disadvantaged social statuses confer more stress and fewer coping resources? Social Science & Medicine, 67(3), 368–379. 10.1016/j.socscimed.2008.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moo-Estrella J, Pérez-Benítez H, Solís-Rodríguez F, & Arankowsky-Sandoval G (2005). Evaluation of Depressive Symptoms and Sleep Alterations in College Students. Archives of Medical Research, 36(4), 393–398. 10.1016/j.arcmed.2005.03.018 [DOI] [PubMed] [Google Scholar]
- Muthen LK & Muthen BO (1998). Mplus User’s Guide: Seventh Edition. Muthen & Muthen. [Google Scholar]
- Newsom J, Jones RN, & Hofer SM (2013). Longitudinal Data Analysis: A Practical Guide for Researchers in Aging, Health, and Social Sciences. Routledge. [Google Scholar]
- O’Connor RC, Wetherall K, Cleare S, McClelland H, Melson AJ, Niedzwiedz CL, O’Carroll RE, O’Connor DB, Platt S, Scowcroft E, Watson B, Zortea T, Ferguson E, & Robb KA (2020). Mental health and wellbeing during the COVID-19 pandemic: Longitudinal analyses of adults in the UK COVID-19 Mental Health & Wellbeing study. The British Journal of Psychiatry, 1–17. 10.1192/bjp.2020.212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson CM, Strawderman MS, & Reed RG (2004). Efficacy of an intervention to prevent excessive gestational weight gain. American Journal of Obstetrics and Gynecology, 191(2), 530–536. 10.1016/j.ajog.2004.01.027 [DOI] [PubMed] [Google Scholar]
- Orzech KM, Salafsky DB, & Hamilton LA (2011). The state of sleep among college students at a large public university. Journal of American College Health: J of ACH, 59(7), 612–619. 10.1080/07448481.2010.520051 [DOI] [PubMed] [Google Scholar]
- Özdin S, & Bayrak Özdin Ş (2020). Levels and predictors of anxiety, depression and health anxiety during COVID-19 pandemic in Turkish society: The importance of gender. International Journal of Social Psychiatry, 66(5), 504–511. 10.1177/0020764020927051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearlin LI (1989). The Sociological Study of Stress. Journal of Health and Social Behavior, 30(3), 241–256. 10.2307/2136956 [DOI] [PubMed] [Google Scholar]
- Peckham H, de Gruijter NM, Raine C, Radziszewska A, Ciurtin C, Wedderburn LR, Rosser EC, Webb K, & Deakin CT (2020). Male sex identified by global COVID-19 meta-analysis as a risk factor for death and ITU admission. Nature Communications, 11(1), Article 1. 10.1038/s41467-020-19741-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrov ME, & Lichstein KL (2016). Differences in sleep between black and white adults: An update and future directions. Sleep Medicine, 18, 74–81. 10.1016/j.sleep.2015.01.011 [DOI] [PubMed] [Google Scholar]
- Pfefferbaum B, & North CS (2020). Mental Health and the Covid-19 Pandemic. New England Journal of Medicine, 383(6), 510–512. 10.1056/NEJMp2008017 [DOI] [PubMed] [Google Scholar]
- Pierce M, Hope H, Ford T, Hatch S, Hotopf M, John A, Kontopantelis E, Webb R, Wessely S, McManus S, & Abel KM (2020). Mental health before and during the COVID-19 pandemic: A longitudinal probability sample survey of the UK population. The Lancet Psychiatry, 7(10), 883–892. 10.1016/S2215-0366(20)30308-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robillard R, Dion K, Pennestri M-H, Solomonova E, Lee E, Saad M, Murkar A, Godbout R, Edwards JD, Quilty L, Daros AR, Bhatla R, & Kendzerska T (2020). Profiles of sleep changes during the COVID-19 pandemic: Demographic, behavioural and psychological factors. Journal of Sleep Research, n/a(n/a), e13231. 10.1111/jsr.13231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers TN, Rogers CR, VanSant-Webb E, Gu LY, Yan B, & Qeadan F (2020). Racial Disparities in COVID-19 Mortality Among Essential Workers in the United States. World Medical & Health Policy, 12(3), 311–327. 10.1002/wmh3.358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosekind MR, Gregory KB, Mallis MM, Brandt SL, Seal B, & Lerner D (2010). The Cost of Poor Sleep: Workplace Productivity Loss and Associated Costs. Journal of Occupational and Environmental Medicine, 52(1), 91–98. 10.1097/JOM.0b013e3181c78c30 [DOI] [PubMed] [Google Scholar]
- Rossen LM, Branum AM, Ahmad FB, Sutton P, & Anderson RN (2020). Excess Deaths Associated with COVID-19, by Age and Race and Ethnicity—United States, January 26–October 3, 2020. Morbidity and Mortality Weekly Report, 69(42), 1522. 10.15585/mmwr.mm6942e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salari N, Hosseinian-Far A, Jalali R, Vaisi-Raygani A, Rasoulpoor S, Mohammadi M, Rasoulpoor S, & Khaledi-Paveh B (2020). Prevalence of stress, anxiety, depression among the general population during the COVID-19 pandemic: A systematic review and meta-analysis. Globalization and Health, 16(1), 57. 10.1186/s12992-020-00589-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz SJ (2016). Turning Point for a Turning Point: Advancing Emerging Adulthood Theory and Research. Emerging Adulthood, 4(5), 307–317. 10.1177/2167696815624640 [DOI] [Google Scholar]
- Sehra ST, Fundin S, Lavery C, & Baker JF (2020). Differences in race and other state-level characteristics and associations with mortality from COVID-19 infection. Journal of Medical Virology, 92(11), 2406–2408. 10.1002/jmv.26095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sha MC, Callahan CM, Counsell SR, Westmoreland GR, Stump TE, & Kroenke K (2005). Physical symptoms as a predictor of health care use and mortality among older adults. The American Journal of Medicine, 118(3), 301–306. 10.1016/j.amjmed.2004.12.015 [DOI] [PubMed] [Google Scholar]
- Shye D, Mullooly JP, Freeborn DK, & Pope CR (1995). Gender differences in the relationship between social network support and mortality: A longitudinal study of an elderly cohort. Social Science & Medicine, 41(7), 935–947. 10.1016/0277-9536(94)00404-H [DOI] [PubMed] [Google Scholar]
- Singu S, Acharya A, Challagundla K, & Byrareddy SN (2020). Impact of Social Determinants of Health on the Emerging COVID-19 Pandemic in the United States. Frontiers in Public Health, 8. 10.3389/fpubh.2020.00406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SS, Oei TPS, Douglas JA, Brown I, Jorgensen G, & Andrews J (2008). Confirmatory factor analysis of the Epworth Sleepiness Scale (ESS) in patients with obstructive sleep apnoea. Sleep Medicine, 9(7), 739–744. 10.1016/j.sleep.2007.08.004 [DOI] [PubMed] [Google Scholar]
- Szanton SL, Thorpe RJ, & Whitfield K (2010). Life-course financial strain and health in African–Americans. Social Science & Medicine, 71(2), 259–265. 10.1016/j.socscimed.2010.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai DBG, Shah A, Doubeni CA, Sia IG, & Wieland ML (2020). The Disproportionate Impact of COVID-19 on Racial and Ethnic Minorities in the United States. Clinical Infectious Diseases. 10.1093/cid/ciaa815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thombs BD, Bonardi O, Rice DB, Boruff JT, Azar M, He C, Markham S, Sun Y, Wu Y, Krishnan A, Thombs-Vite I, & Benedetti A (2020). Curating evidence on mental health during COVID-19: A living systematic review. Journal of Psychosomatic Research, 133, 110113. 10.1016/j.jpsychores.2020.110113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner RJ (2013). Understanding Health Disparities: The Relevance of the Stress Process Model. Society and Mental Health, 3(3), 170–186. 10.1177/2156869313488121 [DOI] [Google Scholar]
- Vgontzas AN, Zoumakis E, Bixler EO, Lin H-M, Follett H, Kales A, & Chrousos GP (2004). Adverse effects of modest sleep restriction on sleepiness, performance, and inflammatory cytokines. The Journal of Clinical Endocrinology & Metabolism, 89(5), 2119–2126. 10.1210/jc.2003-031562 [DOI] [PubMed] [Google Scholar]
- Wanberg CR, Csillag B, Douglass RP, Zhou L, & Pollard MS (2020). Socioeconomic status and well-being during COVID-19: A resource-based examination. The Journal of Applied Psychology. 10.1037/apl0000831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Pan R, Wan X, Tan Y, Xu L, Ho CS, & Ho RC (2020). Immediate Psychological Responses and Associated Factors during the Initial Stage of the 2019 Coronavirus Disease (COVID-19) Epidemic among the General Population in China. International Journal of Environmental Research and Public Health, 17(5). 10.3390/ijerph17051729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Pan R, Wan X, Tan Y, Xu L, McIntyre RS, Choo FN, Tran B, Ho R, Sharma VK, & Ho C (2020). A longitudinal study on the mental health of general population during the COVID-19 epidemic in China. Brain, Behavior, and Immunity, 87, 40–48. 10.1016/j.bbi.2020.04.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright L, Steptoe A, & Fancourt D (2020). Are we all in this together? Longitudinal assessment of cumulative adversities by socioeconomic position in the first 3 weeks of lockdown in the UK. J Epidemiol Community Health, 74(9), 683–688. 10.1136/jech-2020-214475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong J, Lipsitz O, Nasri F, Lui LMW, Gill H, Phan L, Chen-Li D, Iacobucci M, Ho R, Majeed A, & McIntyre RS (2020). Impact of COVID-19 pandemic on mental health in the general population: A systematic review. Journal of Affective Disorders, 277, 55–64. 10.1016/j.jad.2020.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap Y, Slavish DC, Taylor DJ, Bei B, & Wiley JF (2020). Bi-directional relations between stress and self-reported and actigraphy-assessed sleep: A daily intensive longitudinal study. Sleep, 43(zsz250). 10.1093/sleep/zsz250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu R (2016). Stress potentiates decision biases: A stress induced deliberation-to-intuition (SIDI) model. Neurobiology of Stress, 3, 83–95. 10.1016/j.ynstr.2015.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu K, Devine A, Dick IM, & Prince RL (2007). Association of Back Pain Frequency With Mortality, Coronary Heart Events, Mobility, and Quality of Life in Elderly Women. Spine, 32(18), 2012–2018. 10.1097/BRS.0b013e318133fb82 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


