Abstract
Throughout 2020, COVID-19 interventions prioritised symptomatic individuals despite growing evidence of pre-symptomatic and asymptomatic transmission. From the pandemic we have learned that global health is slow to quantify asymptomatic disease transmission and slow to implement relevant interventions. While asymptomatic infectious periods exist for nearly all pathogens, it is frequently ignored during case finding, and there are limited research efforts to understand its potential to drive small scale outbreaks, epidemics and pandemics.
We conducted a pragmatic review on 15 key pathogens including SARS-CoV-2 and Ebola to demonstrate substantial variation in terminology around asymptomatic infectious individuals, and varying proportions of asymptomatic amongst prevalent infectious cases (0-99%) and their contribution to transmission (0-96%). While no pattern was discernible by pathogen type (virus, bacteria, parasite) or mode of transmission (direct, indirect or mixed), there are multiple lessons to learn from previous and current control programmes.
As found during the COVID-19 pandemic, overlooking asymptomatic infectious individuals can impede disease control. Improving our understanding of how asymptomatic individuals can drive epidemics can strengthen our efforts to control current pathogens, and improve our preparedness for when the next new pathogen emerges.
Keywords: Asymptomatic, HIV, TB, malaria, SARS-CoV-2
1. Introduction
Early in 2020 it was thought that more than half of prevalent SARS-CoV-2 infections were either asymptomatic or pre-symptomatic, and that more than half of transmission occurred during these periods (Liu et al., 2020). However, case definitions and strategies remained focussed on symptomatic individuals (Sayampanathan et al., 2021, Pollock and Lancaster, 2020). For example, in the UK testing facilities were only made available to those who exhibited two or more symptoms despite other risk factors, creating a missed opportunity to better understand transmission of the pathogen (Pollock and Lancaster, 2020). New variants of the SARS-CoV-2 virus with higher transmissibility are now becoming dominant, complicating existing control strategies and shifting existing knowledge of the disease (Gabbat et al., 2020).
Asymptomatic infectious periods are known to exist for nearly all viral, bacterial and parasitic infections, and new research continues to expand our understanding of transmission from individuals without clinical disease. For example in Ebola, a recent genome sequencing study has found that the June 2021 outbreak in Guinea was likely due to a persistent infection with a period of latency rather than a new spill-over event, previously not considered to be possible (Keita et al., 2021). Additional studies by other research groups also add to the evidence base for shifting our understanding of Ebola transmission (Glynn et al., 2017).
Asymptomatic individuals are unlikely to seek care or take preventative measures (Bosch et al., 2018, Drakeley et al., 2018, Espinoza et al., 2021), while disease control programs often omit asymptomatic infectious individuals from case definitions and control strategies (Frascella et al., 2020). From the COVID-19 pandemic and the latest research on Ebola we have learned that this risks ongoing transmission and sustains the epidemic, with limited tools/prospects to address it.
Better understanding the role of asymptomatic infectious periods in natural history may lead to improved control of existing pathogens, and eventually their elimination or eradication. To illustrate the importance of the asymptomatic infectious period and the between pathogen variation that exists, we collated data on COVID-19 and compared information with 14 other key diseases in terms of global burden, vaccine programmes or elimination, to answer two questions - how common is the asymptomatic infectious periods occur, and does it matter for transmission?
2. Methods
In addition to COVID-19, we included key diseases in terms of burden (HIV, TB and malaria), relevance for current or past eradication programmes (smallpox, schistosomiasis), routine immunisation programmes (measles, rubella and typhoid) and recurrent/emerging epidemics (Ebola and Dengue). For each we collated case definitions, terminology used, prevalence and relative infectiousness of asymptomatic infectious individuals as compared to those with symptoms. Where the disease is caused by different species of the same pathogen (e.g. malaria), or transmission driven by asymptomatic infectious states, we used weighted averages to calculate overall burden and infectiousness. We combined the information to estimate the percentage contribution of asymptomatic infectious periods to transmission.
3. Results
3.1. Language and definitions
An ‘asymptomatic’ infection is a term commonly used that insinuates reference to individuals infected with a pathogen but either without symptoms or unaware of them. The intent of the term asymptomatic is to group those who do not currently meet the symptom component of a case definition (Espinoza et al., 2021, Lindblade et al., 2013).
COVID-19 asymptomatic infections were important to capture especially at the beginning of the pandemic when symptoms aside from a cough and general malaise were not very well known or documented (England, P. H). It is now understood that individuals with very mild or no symptoms make up a large proportion of infected individuals (Chappell et al., 2021). We also now understand that an individual may become infectious before developing symptoms, referred to as pre-symptomatic infection (Liu et al., 2020, Emery et al., 2020). Progression can also be non-linear, with individuals moving between symptomatic and asymptomatic phases over time. A classic example is HIV, where an individual at their primary infection may develop fever, fatigue and night sweats at which point they are more infectious, live without symptoms for 10 years with limited infectiousness, before progressing to symptoms of AIDS (Hollingsworth et al., 2008).
We found a wide range of terminology used around infectious asymptomatic individuals, both within and between disease fields, including in-apparent infections (Heymann, 2015), subclinical cases (Frascella et al., 2020), carriers (Watson, 2018), or clinical case contacts (Mach et al., 2014) (see Table 1 for complete list).
Table 1.
Burden and infectiousness of symptomatic and non-symptomatic states in 15 pathogens.
| Disease | Agent | Transmission mode | Transmission route | Terminology |
|---|---|---|---|---|
| Covid-19 | Virus | Direct | Respiratory | Asymptomatic, pre-symptomatic |
| HIV | Virus | Direct, indirect | Bloodborne and sexual transmission | Asymptomatic, chronic infection, chronic latency, presymptomatic |
| TB | Bacteria | Direct | Respiratory | Subclinical, asymptomatic |
| Malaria | Parasite | Indirect | Vector (mosquito) | Subpatent, asymptomatic |
| Polio | Virus | Indirect | Faecal - oral | Asymptomatic, clinical case contacts |
| Measles | Virus | Direct, indirect | Respiratory | Subclinical |
| Smallpox | Virus | Direct | Droplet and skin to skin contact | N/A |
| Typhoid | Bacteria | Indirect | Contaminated food and water | Asymptomatic, short-term carrier |
| Ebola | Virus | Indirect | Contact with body fluids | Asymptomatic |
| Dengue | Virus | Indirect | Vector (mosquito) | Asymptomatic, subclinical, pre-symptomatic, inapparent |
| Monkeypox | Virus | Direct | Droplet and skin to skin contact | Asymptomatic |
| Schistosomiasis | Parasite | Indirect | Contact with infected water | Asymptomatic, light infection, egg-negative |
| Rubella | Virus | Direct | Respiratory | N/A |
| Influenza | Virus | Direct | Respiratory | Asymptomatic, inapparent |
| Norovirus | Virus | Direct | Contaminated food and contact with body fluids | Asymptomatic |
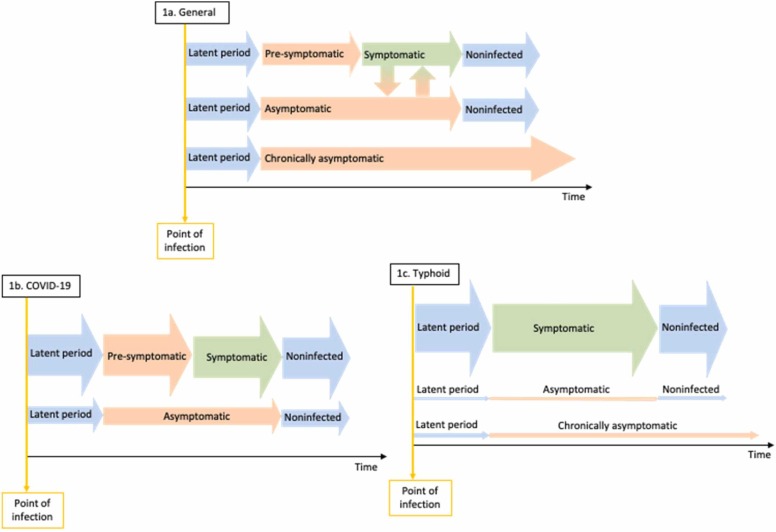
For clarity, we propose to define pre-symptomatic infections as infectious individuals who are not experiencing or aware of symptoms following the latent period but will eventually progress to developing some physical sign of their disease. An asymptomatic individual is an individual who will not develop symptoms for the duration of their infection but will otherwise progress through to recovery in the same duration as a symptomatic individual. A chronic asymptomatic individual (sometimes referred to as a carrier), however, is an individual who will have a longer duration of infection relative to a symptomatic individual. In some cases, an individual may also be infectious following clinical recovery from symptomatic disease such as for Hepatitis B (Heymann, 2015). In line with other publications, we define any individual not currently displaying or aware of symptoms as non-symptomatic infections (Espinoza et al., 2021). In Fig. 1 (top row) we provide a simplified visualisation of the different pathways for non-symptomatic but infectious progression.
Fig. 1.
Visualisation of non-symptomatic but infectious stages of infection (orange arrows) A) in general, with examples of B) COVID-19, and C) Typhoid. Width of arrow demonstrates the relative contribution to transmission from each pathway.
Direct transmission occurs through transfer of an agent from a host by direct contact (e.g. bites or sexual intercourse), through direct projections such as droplet spread onto the mucous membranes of the eyes, nose or mouth (e.g. coughing, sneezing or singing). Direct transmission may also occur transplacentally. Conversely, indirect transmission occurs through transfer of an agent from a host by contaminated inanimate objects (i.e. fomites), transfer occurring through vectors by a mechanical mode (e.g. transfer of agent by insect through soiling of its feet) or biological mode (e.g. propagation or cyclic development in an arthropod followed by injection of salivary gland fluid during biting or regurgitation or deposition). Indirect transmission may also occur through airborne modes through microbial aerosols which remain suspended in the air for prolonged periods of time and then drawn into the alveoli of the lungs (e.g. dust rising from fungus spores or pathogens spread through atomised devices), these are not to be considered droplets which settle promptly (Heymann, 2015).
3.2. Prevalence and relative infectiousness of asymptomatic infections
Using the categories described above, we quantified the different non-symptomatic infectious periods for different pathogens. As Figs. 1b and 1c show, there is a wide range across pathogens. For example, after infection with SARS-CoV-2, around 50% of infectious individuals will not develop symptoms, and 30% of those who do develop symptoms will be infectious in the pre-symptomatic stage for an average of 5 days (Liu et al., 2020, He et al., 2020). While for Salmonella Typhi approximately 16% will develop symptoms for an average of 14 days, whereas 80% will be asymptomatic for the same period, and approximately 4% will be chronically asymptomatic for up to 10 years (Watson, 2018).
A summary of burden and the characteristics of non-symptomatic and infectious states are outlined in Table 2. Using these values, we estimated the contribution of non-symptomatic cases to overall transmission.
Table 2.
Burden and characteristics of non-symptomatic and infectious states for 15 pathogens.
| Disease | Incidence, 2019 (Confidence intervals) [with exceptions] |
Mortality, 2019 (Confidence intervals) [with exceptions] |
Proportion infected who are symptomatic | Proportion infected who are pre-symptomatic and infectious |
Proportion infected who are asymptomatic | Proportion infected who are chronically asymptomatic | Relative infectiousness of asymptomatic cases compared to symptomatic cases | Time spent pre-symptomatic and infectious (days) | Time spent asymptomatic and infectious (days) | Time spent chronically asymptomatic and infectious (days) | References |
|---|---|---|---|---|---|---|---|---|---|---|---|
| COVID-19 | 102,083,344 | 2,209,195 | 0.5 | 0.3 | 0.5* | 0 | 0.75* | 5* | 7* | 0 | (He et al., 2020, Johansson et al., 2021, WHO, 2021) |
| HIV | 1,989,282(1,760,906 - 2,259,348) | 863,837 (786,074 - 996,044) |
0.99 | 0 | 0.99 | 0.01 | 0.24 | 90 | 2920 | 18250 | (Hollingsworth et al., 2008, Evaluation, 2019) |
| TB | 8,497,316(7,445,681 - 9,727,992) | 1,179,766 (1,078,546 - 1,292,664) |
0.47 | 0 | 0.53 | 0 | 0.5 | 548 | 548 | 0 | (Frascella et al., 2020, Evaluation, 2019, Emery et al., 2022) |
| Malaria | 231,357,372(186,034,444 - 290,217,184) | 643,381 (301,600 - 1,153,663) |
0.5 | 0.5 | 0.25 | 0 | 0.44 | 18 | 194 | 0 | (Lindblade et al., 2013, Evaluation, 2019, Filipe et al., 2007, Tadesse et al., 2018) |
| Polio | 1047** | 0 | 0.01 | 0 | 0.97 | 0.02 | 1 | 14 | 43 | 475 | (Evaluation, 2019, Nathanson and Kew, 2010, Fine and Carneiro, 1999, Initiative, 2021) |
| Measles | 12,806,077 (4,548,907 - 27,689,595) |
83,392 (30,998 - 180,402) |
0.33 | 0 | 0.67 | 0 | 0.1 | 11 | 4 | 0 | (Heymann, 2015, Evaluation, 2019, Glass and Grenfell, 2004) |
| Smallpox | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 12 | 7 | 0 | (Heymann, 2015) |
| Typhoid | 9,237,224 (5,942,766 - 14,132,773) |
133,366 (65,708 - 228,866) |
0.16 | 0 | 0.8 | 0.04 | 0.03 | 18 | 14 | 3650 | (Watson, 2018, Evaluation, 2019) |
| Ebola | 2,229*** | 3,481*** | 0.97 | 0 | 0.03 | 0 | 0 | 0 | 9 | 0 | (Glynn et al., 2017, WHO & newsroom, 2021) |
| Dengue | 56,878,729 (37,083,098 - 101,350,556) |
36,055 (9,176 - 44,467) |
0.23 | 0.18 | 0.77 | 0 | 0.8 | 8 | 11 | 0 | (Bosch et al., 2018, Evaluation, 2019) |
| Monkeypox | 4,594 | 171 | 0.9 | 0 | 0.1 | 0 | 0.2 | 0 | 9 | 0 | (Guagliardo et al., 2020, response, 2020) |
| Schisto | 139,967,777 (166,093,920 - 117,172,099) |
11,514 (10,135 - 13,275) |
0.6 | 0 | 0.4 | 0 | 0.25 | 0 | 3650 | 0 | (Evaluation, 2019, Farooq et al., 1966) |
| Rubella | 47,556 | 0 | 0.5 | 0 | 0.5 | 0 | 0 | 0 | 0 | 0 | (Heymann, 2015, Edmunds et al., 2000, Distribution, 2021) |
| Influenza | 54,481,000**** (38,465,000 - 73,864,000) |
145,000**** (99,000 - 200,000) |
0.56 | 0 | 0.44 | 0 | 0.35 | 0 | 6 | 0 | (Cohen et al., 2021, Collaborators, 2017) |
| Norovirus | 685,000,000 | 50,000 | 0.75 | 0 | 0.25 | 0 | 0.05 | 0 | 19 | 0 | (Lopman et al., 2014, Prevention, 2021) |
** 14 April 2020 to 13 April 2021
*** 2018-2020 North Kivu, Democratic Republic of Congo outbreak
**** Estimate from 2017
^Prevalence estimate rather than incidence
Estimates for the proportion of SARS-CoV-2 infections leading to asymptomatic disease vary with each new variant and changes in the background immunity of the general population. We use initial estimates from the D614 strain for the purposes of this exercise.
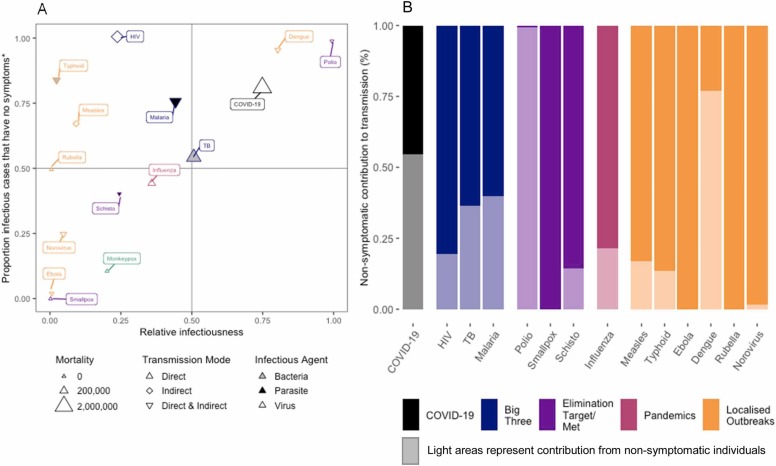
Fig. 2a shows for each pathogen the proportion of prevalent (i.e. current) infectious cases that are non-symptomatic (y-axis) and the relative infectiousness (x-axis). Among the diseases included in this exercise, the proportion of prevalent non-symptomatic infectious cases ranged from 0 - 100% and the relative infectiousness also ranged from 0 - 100%. Fig. 2b shows what proportion of current transmission is driven by non-symptomatic individuals, which varied by nearly all (Polio) to none (Smallpox, Rubella, Ebola). With both SARS-CoV-2 and Dengue, individuals in the pre-symptomatic period are considered as infectious compared to the symptomatic period that follows, indicating a high transmission potential (Liu et al., 2020, Duong et al., 2015).
Fig. 2.
: Estimation of the contribution of non-symptomatic cases to overall transmission of 15 key pathogens. A) the relative infectiousness of infectious cases without symptoms compared to the proportion of infectious cases that have no symptoms; B) the contribution of non-symptomatic cases and symptomatic cases to transmission. *Non-symptomatic/symptomatic case parameters are in absence of treatment where this data is available (exception includes HIV). **Ebola - recent studies find that non-symptomatic Ebola may pose a risk of sustaining transmission. ***Monkeypox - not enough data to calculate contribution to transmission. **** Dengue - some lab studies show relative infectiousness of pre-symptomatics may be higher than symptomatic cases. *****Raw data in appendix. Point values shown for clarity.
While we provide population-level averages to enable between pathogen comparison, it is important to note that for some pathogens the proportion of infections that progress asymptomatically will vary by age. E.g. SARS-CoV-2 infected children (<15yo) are more likely to proceed asymptomatically compared to older populations (Lavezzo et al., 2020). In Malaria-endemic populations this is reversed, although this is likely driven by immunity due repeat infections, and no age specific pattern for non-symptomatic infectious states has been found for other pathogens, e.g. TB and HIV.
Fig. 2a also shows the type of pathogen (bacteria, virus or parasite) and its mode of transmission (direct, indirect or both) for the diseases included in this review i.e. SARS-CoV-2 leads to a viral infection primarily spread through direct transmission. There was no discernible pattern for the association with contribution of non-symptomatic groups to transmission by pathogen type, or mode of transmission.
Fig. 2b provides an estimate for the proportion of all transmission that comes from non-symptomatic infectious individuals, we estimate that 55% of all transmission of SARS-CoV-2 occurs from non-symptomatic individuals. There is a stark contrast in contribution of transmission between Polio (99%) and Smallpox (0%), and for 10 out of 15 diseases at least 10% of transmission occurs from a reservoir of individuals without noticeable symptoms.
The graphs in Fig. 2 highlight the role non-symptomatic infectious cases play, highlighted by the quadrants in Fig. 2a. COVID-19 and other diseases in the upper right quadrant (Polio, Dengue), are where half or more of the total transmission stems from non-symptomatic infectious individuals (Fig. 1b). The upper left quadrant highlights diseases where the majority of prevalent infectious individuals are non-symptomatic, but due to a low relative infectiousness, they are less large proportion and so are less of a problem (HIV, Typhoid, Measles and Malaria). Infectiousness of HIV is dependent on viral load, individuals infected with HIV are highly infectious in the first 3 months after infection (3.6 times relative to those with AIDS) after which their viral load decreases until late stage infection (AIDS) which precedes death by 2 years (Hollingsworth et al., 2008).
Smallpox and Monkeypox are all in the lower left quadrant, and unlike SARS-CoV-2 infection there is little to no non-symptomatic infectious cases and low relative infectiousness. Ebola is also currently in this quadrant while we await new data and research. No diseases in our study had a low proportion of non-symptomatic individuals with a high relative infectiousness, i.e. the bottom right quadrant.
4. Discussion
This high-level non-exhaustive review demonstrates the wide range in role non-symptomatic infectious periods play, and there is no single applicable approach to take with regard to control or research, which will depend on context.
4.1. Consequences for elimination and outbreak management
The behaviour of infected individuals can play a large or negligible impact on transmission of infection from symptomatic and non-symptomatic individuals. For example, following positive diagnoses individuals with COVID-19 related symptoms may be more inclined to wear a mask or self-isolate. In the case of HIV, another pathogen with a direct transmission route, individuals may undertake protective efforts, and continuation of ART suppresses viral load thus reducing the infectiousness of many individuals. In contrast, behaviour may not be as apparent for indirectly transmitted pathogens such as malaria or dengue.
Contrasting positions of pathogens in Fig. 2 illustrate the further challenges asymptomatic infectious individuals can bring to elimination and outbreak control.
Smallpox elimination (far bottom left quadrant) was officially achieved in 1980, through a concerted effort of ring vaccination around symptomatic cases (Henderson and Klepac, 2013). While elimination of wild poliovirus (top right quadrant) has been achieved in all but two countries, a major hindrance to the program remains the shedding of vaccine derived polio virus from asymptomatic individuals most of whom are vaccinated (Nathanson and Kew, 2010). Transmission studies in the UK have found that vaccinated and unvaccinated individuals with SARS-CoV-2 infections carry similar viral loads (Singanayagam et al., 2021). Therefore, asymptomatic transmission remains an important end-point or indicator for existing and new COVID-19 vaccines.
4.2. Implications for evolutionary advantages
While Fig. 2 shows there is a wide range across pathogens for the prevalence and relative infectiousness of non-symptomatic individuals, the lack of diseases in the bottom right quadrant is striking. While one hypothesis may be a simple correlation between pathogens transmission and the causation of symptoms, alternatively it may indicate an evolutionary disadvantage of a relatively rare but highly infectious asymptomatic period.
Gene loss and gain of functional virulence genes, such as those associated with host-specific pathogenicity, has been identified as an evolutionary process in multiple pathogens. The arms-race between human immunity and a given pathogens‘ ability to invade a human host is perpetually on-going, thus applying selection pressure upon a pathogens’ genome i.e. the emergence of multiple variants and sub-variants of SARS-CoV-2 with key differences in immune escape and virulence (UKHSA, 2023). Additionally, for clonal pathogen species with low recombination, it is shown that the loss and acquisition of virulence genes over time is a key factor in increasing the genetic variation within these species, allowing it to adapt to changes in the hosts immunity and behaviour (Bolotin and Hershberg, 2015).
Ancient DNA studies have allowed researchers to track the evolution of pathogens over thousands of years. An interesting case study is Yersinia pestis, which has shown genome loss occurring in multiple historical epidemics and outbreaks. Specifically, a previously unknown lineage, known as the LNBA lineage, the earliest evidence of which was ~5000 years ago, is shown to have been widespread across Eurasia and exhibited a genome loss of ∼83 kb, ~2% of the total chromosomal genome across a ~2000 year time period (Rasmussen et al., 2015, Spyrou et al., 2018). This gene loss could have led to a restricted ecological niche, making it difficult for the pathogen to adapt, which could explain why the LNBA lineage is not found in current outbreaks, despite indications of high-transmissibility from its wide geographical spread (McNally et al., 2016). Transmission dynamics of this lineage is not fully understood, due to the lack of a key virulence gene associated with flea-mediated transmission, and the pathogens ability to be spread via respiratory route and via contact with contaminated fluid or tissue from an infected animal.
During this time period, we see acquisition of the ymt gene, allowing for the transmission of plague from the rodent reservoirs into humans, in a lineage that gave rise to three well known historical epidemics and is consequently, present in all modern-day strains of the pathogen (Spyrou et al., 2018).
The example of Yersinia pestis illustrates the links between genomics and transmission dynamics, and the need to explore the question of non-symptomatic transmission in a cross-disciplinary approach.
4.3. Uncertainty and unknowns
For new pathogens such as SARS-CoV-2, the impact of newly emerging and dominant variants on key asymptomatic indicators (what proportion, how infectious, how distributed by age) is generally unknown, and estimates vary due to bias of study populations (Johansson et al., 2021). While for some diseases the asymptomatic period is reasonably well understood (e.g. HIV), substantial uncertainty remains for others.
Since the eradication of smallpox in the 1980s and waning of Smallpox (Vaccinia) Vaccine induced immunity in the general population, monkeypox has become the dominant Orthopoxvirus in humans with approximately 5,000 new cases in 2019 (response, 2020). Although clinically very similar to smallpox, new research is highlighting a potential non-symptomatic infectious stage of disease (Guagliardo et al., 2020). As such, the methods of control which worked for smallpox eradication, including ring vaccinations around symptomatic cases, may not be sufficient for the control of monkeypox. As new data emerges, the position of monkeypox on Fig. 2a should be reviewed to support design of new control strategies. Similarly, the understanding of asymptomatic infectious cases of schistosomiasis is limited, and may contribute towards the difficulty in reaching elimination despite its position in the lower left quadrant in Fig. 2a.
4.4. Research priorities for new (and existing) pathogens
In the drive to control the morbidity and mortality of a new or existing pathogen, asymptomatic individuals are not usually considered a priority. However, we have shown that this may be a mistake and lead to wasted resources or opportunity to interrupt ongoing transmission, especially in the context of outbreaks or a pandemic.
While there are many aspects of asymptomatic infectious period that warrant investigation, the two key metrics for control and prioritisation are what proportion of infections will include an asymptomatic infectious period, and how infectious are these individuals relative to the symptomatic individuals.
Several research approaches exist to generate estimates for the proportion of infections with a pathogen that experience an asymptomatic infectious period. These include surveys that detect the evidence of prevalent (e.g. PCR) or past (e.g. antibodies) infections. If these are repeated in the same population, incident infections may be found, which provide an opportunity to study the natural history over time. The challenge of such surveys is the sampling frame, which is often biased due to non-random participation from subpopulations, and the open nature of most populations such as a village or region. One opportunity that overcomes such limitations in the case of rapidly progressing infections are outbreaks in relatively closed populations, such as cruise ships or naval vessels, where the population is well-delineated, and can be tested and interviewed appropriately.
Infectiousness of asymptomatic individuals is often measured through the relative number of secondary infections (the numerator) that emanate from well-defined symptomatic and non-symptomatic source cases, within a well-described at-risk populations such as household contacts (the denominator). Such studies can be complemented by molecular epidemiology which ideally confirms that the strain in the secondary case matches that of the source case.
Based on those findings, an informed prioritisation of research efforts can be made, as well as consideration of including the appropriate population measures. For example, symptom-based self-isolation as widely implemented in countries during the current COVID-19 pandemic would have limited impact if the majority of transmission comes from pre-symptomatic or asymptomatic cases. This is further complicated by changing transmission patterns from new variants.
A multitude of research employing these epidemiological techniques exist for multiple pathogens, yet research into non-symptomatic infectious periods is not standard practice. This scoping review was designed to explore key similarities and differences between the chosen pathogens and highlight key gaps in research. While our estimates of non-symptomatic infectious contribution to transmission align with other research (Bosch et al., 2018, Lindblade et al., 2013, Tadesse et al., 2018), exhaustive reviews are needed for many pathogens to better quantify how asymptomatic and pre-symptomatic disease states can proliferate infections, quantify the uncertainty and variation in these metrics, and to improve communication around the risk of non-symptomatic infectious disease states to colleagues working in disease control. As shown with the example of Yersinia Pestis, key evolutionary changes led to the extinction of a directly transmitted lineage to the proliferation of a lineage with gene-mutations allowing for a vector borne disease transmission route. Such examples illustrate that further research into disease transmission should not take place in silos, as there may be value in understanding the pathways for the mechanism of non-symptomatic infections by species of microbe, or mode of transmission.
5. Conclusion
We have shown that there are non-symptomatic infectious individuals for nearly all diseases, and from the COVID-19 pandemic it has become evident non-symptomatic infections can play a major role in driving transmission and sustaining the disease burden while evading routine disease control measures. There are still substantial gains to be made in our understanding of disease transmission, and understanding varies strongly between diseases, which is reflected in unclear language and risks ineffective surveillance and control programmes.
We argue here that disease research should routinely and proactively incorporate non-symptomatic infectious states of disease when investigating the epidemiology and transmission for all pathogens. Efforts should be made to develop relevant protocols and terminology should be clearly aligned to support health professionals implementing adequate response measures. Further work is also required to understand symptom classification errors based on screening tools and reporting habits of suspected cases.
Eradication of a disease where there are no clear clinical manifestations and poor symptoms screening tools is increasingly difficult. Epidemiologists and policy makers alike should utilise shared learning on this topic across the silos of disease research.
Funding
N.S. and R.M.G.J.H received funding from the European Research Council (ERC) under the Horizon 2020 research and innovation programme (ERC Starting Grant No. 757699) while conducting this study. The funder did not contribute to the design, analysis or interpretation of this study.
CRediT authorship contribution statement
N.S. and R.M.G.J.H conceptualised the study and interpreted the results, N.S. conducted the analysis and developed the figures, all authors contributed to writing and review of the manuscript.
Declaration of Competing Interest
N.S. reports current employment at Sanofi Pasteur, her Sanofi employment includes work on COVID-19, but does not benefit from the research conducted in this study. The authors declare that they have no competing interests.
Acknowledgements
We thank David Heymann for his input with interpretation of the results and his insights into the epidemiology of multiple pathogens. We are also grateful to colleagues at LSHTM who kindly discussed their views and insight into important research on non-symptomatic infectious phase for specific pathogens.
Declarations
None
Competing interests
The authors declare that they have no competing interests.
Data availability
No data was used for the research described in the article.
References
- Liu Y., Group C. for M.M. of I.D., nCoV W., Funk S., Flasche S. The contribution of pre-symptomatic infection to the transmission dynamics of COVID-2019. [version 1; peer review: 3 approved]. Wellcome Open Res. 2020 doi: 10.12688/wellcomeopenres.15788.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayampanathan A.A., et al. Infectivity of asymptomatic versus symptomatic COVID-19. Lancet. 2021;397:93–94. doi: 10.1016/S0140-6736(20)32651-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock A.M., Lancaster J. Asymptomatic transmission of covid-19. Bmj. 2020;371:m4851. [Google Scholar]
- Gabbat, A., Grover, N. & Glenza, J. ‘The war has changed’: CDC paper warns Delta variant is far more transmissible. The Guardian https://www.theguardian.com/world/2021/jul/30/covid-delta-variant-guidelines-masks-cdc-paper-transmission?CMP=Share_AndroidApp_Other (2020).
- Keita A.K., et al. Resurgence of Ebola virus in 2021 in Guinea suggests a new paradigm for outbreaks. Nature. 2021;597:539–543. doi: 10.1038/s41586-021-03901-9. [DOI] [PubMed] [Google Scholar]
- Glynn J.R., et al. Asymptomatic infection and unrecognised Ebola virus disease in Ebola-affected households in Sierra Leone: a cross-sectional study using a new non-invasive assay for antibodies to Ebola virus. Lancet Infect Dis. 2017;17:645–653. doi: 10.1016/S1473-3099(17)30111-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch Q.A. ten, et al. Contributions from the silent majority dominate dengue virus transmission. Plos Pathog. 2018;14 doi: 10.1371/journal.ppat.1006965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drakeley, C., Gonçalves, B., Okell, L. & Slater, H. Towards Malaria Elimination - A Leap Forward. (2018) doi:10.5772/intechopen.77293.
- Espinoza B., Marathe M., Swarup S., Thakur M. Asymptomatic individuals can increase the final epidemic size under adaptive human behavior. Sci Rep-uk. 2021;11:19744. doi: 10.1038/s41598-021-98999-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frascella B., et al. Subclinical tuberculosis disease - a review and analysis of prevalence surveys to inform definitions, burden, associations and screening methodology. Clin Infect Dis. 2020;73:ciaa1402. doi: 10.1093/cid/ciaa1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindblade K.A., Steinhardt L., Samuels A., Kachur S.P., Slutsker L. The silent threat: asymptomatic parasitemia and malaria transmission. Expert Rev Anti-infe. 2013;11:623–639. doi: 10.1586/eri.13.45. [DOI] [PubMed] [Google Scholar]
- England, P.H. COVID-19: investigation and initial clinical management of possible cases. https://www.gov.uk/government/publications/wuhan-novel-coronavirus-initial-investigation-of-possible-cases/investigation-and-initial-clinical-management-of-possible-cases-of-wuhan-novel-coronavirus-wn-cov-infection.
- Chappell J.G., et al. Retrospective screening of routine respiratory samples revealed undetected community transmission and missed intervention opportunities for SARS-CoV-2 in the United Kingdom. J Gen Virology. 2021;102 doi: 10.1099/jgv.0.001595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery J.C., et al. The contribution of asymptomatic SARS-CoV-2 infections to transmission - a model-based analysis of the Diamond Princess outbreak. Medrxiv 2020.05.07.20093849. 2020 doi: 10.1101/2020.05.07.20093849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingsworth T.D., Anderson R.M., Fraser C. HIV-1 Transmission, by Stage of Infection. J Infect Dis. 2008;198:687–693. doi: 10.1086/590501. [DOI] [PubMed] [Google Scholar]
- Heymann D.L. Apha Press; 2015. Control of Communicable Diseases Manual an Official Report of the American Public Health Association. [Google Scholar]
- Watson, C. Seroepidemiological investigations of Salmonella enterica serovar Typhi infection and the potential role of vaccination in the control of typhoid fever in Fiji. (London School of Hygiene & Tropical Medicine., 2018).
- Mach O., et al. Prevalence of Asymptomatic Poliovirus Infection in Older Children and Adults in Northern India: Analysis of Contact and Enhanced Community Surveillance, 2009. J Infect Dis. 2014;210:S252–S258. doi: 10.1093/infdis/jit234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X., et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med. 2020;26:672–675. doi: 10.1038/s41591-020-0869-5. [DOI] [PubMed] [Google Scholar]
- Johansson M.A., et al. SARS-CoV-2 Transmission From People Without COVID-19 Symptoms. Jama Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2020.35057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. WHO Coronavirus (COVID-19) Dashboard. https://covid19.who.int/ (2021).
- Evaluation, I. for H. M. and. GBD Results Tool. (2019).
- Emery J.C., et al. Estimating the contribution of subclinical tuberculosis disease to transmission – an individual patient data analysis from prevalence surveys. Medrxiv 2022.06.09.22276188. 2022 doi: 10.1101/2022.06.09.22276188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipe J.A.N., Riley E.M., Drakeley C.J., Sutherland C.J., Ghani A.C. Determination of the Processes Driving the Acquisition of Immunity to Malaria Using a Mathematical Transmission Model. Plos Comput Biol. 2007;3 doi: 10.1371/journal.pcbi.0030255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadesse F.G., et al. The Relative Contribution of Symptomatic and Asymptomatic Plasmodium vivax and Plasmodium falciparum Infections to the Infectious Reservoir in a Low-Endemic Setting in Ethiopia. Clin Infect Dis. 2018;66:1883–1891. doi: 10.1093/cid/cix1123. [DOI] [PubMed] [Google Scholar]
- Nathanson N., Kew O.M. From Emergence to Eradication: The Epidemiology of Poliomyelitis Deconstructed. Am J Epidemiol. 2010;172:1213–1229. doi: 10.1093/aje/kwq320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine P.E.M., Carneiro I.A.M. Transmissibility and Persistence of Oral Polio Vaccine Viruses: Implications for the Global Poliomyelitis Eradication Initiative. Am J Epidemiol. 1999;150:1001–1021. doi: 10.1093/oxfordjournals.aje.a009924. [DOI] [PubMed] [Google Scholar]
- Initiative, P.E. Polio Now. https://polioeradication.org/polio-today/polio-now/ (2021).
- Glass K., Grenfell B.T. Waning immunity and subclinical measles infections in England. Vaccine. 2004;22:4110–4116. doi: 10.1016/j.vaccine.2004.02.047. [DOI] [PubMed] [Google Scholar]
- WHO & newsroom. Ebola Virus Disease. Chronology of previous Ebola virus disease outbreaks. https://www.who.int/news-room/fact-sheets/detail/ebola-virus-disease (2021).
- Guagliardo S.A.J., et al. Asymptomatic Orthopoxvirus Circulation in Humans in the Wake of a Monkeypox Outbreak among Chimpanzees in Cameroon. Am J Tropical Medicine Hyg. 2020;102:206–212. doi: 10.4269/ajtmh.19-0467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- response, W.H.O.E. preparedness. Monkeypox - Democratic Republic of the Congo. https://www.who.int/csr/don/01-october-2020-monkeypox-drc/en/ (2020).
- Farooq M., Samaan S.A., Nielsen J. Assessment of severity of disease caused by Schistosoma haematobium and S. mansoni in the Egypt-49 project area. B World Health Organ. 1966;35:389–404. [PMC free article] [PubMed] [Google Scholar]
- Edmunds W.J., Heijden O.G.V., de, Eerola M., Gay N.J. Modelling rubella in Europe. Epidemiology Amp Infect. 2000;125:617–634. doi: 10.1017/s0950268800004660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- W.H.O. Distribution of rubella cases by country and by month, 2015-2021. https://www.who.int/teams/immunization-vaccines-and-biologicals/immunization-analysis-and-insights/surveillance/monitoring/provisional-monthly-measles-and-rubella-data (2021).
- Cohen C., et al. Asymptomatic transmission and high community burden of seasonal influenza in an urban and a rural community in South Africa. 2017–18 (PHIRST): a population cohort study. Lancet Global Heal. 2021;9:e863–e874. doi: 10.1016/S2214-109X(21)00141-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collaborators G. I. et al. Mortality, morbidity, and hospitalisations due to influenza lower respiratory tract infections, 2017: an analysis for the Global Burden of Disease Study 2017. Lancet Respir Medicine. 2017;7(69–89):2019. doi: 10.1016/S2213-2600(18)30496-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopman B., Simmons K., Gambhir M., Vinjé J., Parashar U. Epidemiologic Implications of Asymptomatic Reinfection: A Mathematical Modeling Study of Norovirus. Am J Epidemiol. 2014;179:507–512. doi: 10.1093/aje/kwt287. [DOI] [PubMed] [Google Scholar]
- Prevention, C. for D. C. and. Norovirus Worldwide. Norovirus, Trends and Outbreaks https://www.cdc.gov/norovirus/trends-outbreaks/worldwide.html (2021).
- Duong V., et al. Asymptomatic humans transmit dengue virus to mosquitoes. Proc National Acad Sci. 2015;112:14688–14693. doi: 10.1073/pnas.1508114112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavezzo E., et al. Suppression of a SARS-CoV-2 outbreak in the Italian municipality of Vo’. Nature. 2020;584:425–429. doi: 10.1038/s41586-020-2488-1. [DOI] [PubMed] [Google Scholar]
- Henderson D.A., Klepac P. Lessons from the eradication of smallpox: an interview with D. A. Henderson. Philosophical Transactions Royal Soc B Biological Sci. 2013;368 doi: 10.1098/rstb.2013.0113. 20130113. 20130113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singanayagam A., et al. Community transmission and viral load kinetics of the SARS-CoV-2 delta (B.1.617.2) variant in vaccinated and unvaccinated individuals in the UK: a prospective, longitudinal, cohort study. Lancet Infect Dis. 2021 doi: 10.1016/s1473-3099(21)00648-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UKHSA. Investigation of SARS-CoV-2 variants of concern: variant risk assessments. https://www.gov.uk/government/publications/investigation-of-sars-cov-2-variants-of-concern-variant-risk-assessments (2023).
- Bolotin E., Hershberg R. Gene Loss Dominates As a Source of Genetic Variation within Clonal Pathogenic Bacterial Species. Genome Biol Evol. 2015;7:2173–2187. doi: 10.1093/gbe/evv135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen S., et al. Early Divergent Strains of Yersinia pestis in Eurasia 5,000 Years Ago. Cell. 2015;163:571–582. doi: 10.1016/j.cell.2015.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spyrou M.A., et al. Analysis of 3800-year-old Yersinia pestis genomes suggests Bronze Age origin for bubonic plague. Nat Commun. 2018;9:2234. doi: 10.1038/s41467-018-04550-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally A., Thomson N.R., Reuter S., Wren B.W. “Add, stir and reduce”: Yersinia spp. as model bacteria for pathogen evolution. Nat Rev Microbiol. 2016;14:177–190. doi: 10.1038/nrmicro.2015.29. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.