Abstract
Background:
Haploidentical bone marrow transplant (haplo-BMT) offers near universal donor availability as a curative modality for individuals with severe sickle cell disease (SCD). However, the required intense immunodepletion is associated with increased infectious complications. A paucity of data exists on immune reconstitution following haplo-BMT for SCD.
Methods:
A multi-institution learning collaborative was developed in the context of a phase II clinical trial of a non-myeloablative, related haplo-BMT with post-transplant cyclophosphamide for SCD. We report results from a cohort of 23 patients for whom immune reconstitution data up to one year were available.
Results:
Median age was 14.8 years. Out of 23, 18 participants received pre-conditioning with azathioprine, hydroxyurea, and hypertransfusions. 70% (16/23) of participants had multiple indications for haplo-BMT. We observed excellent immune reconstitution of CD4, CD8, CD19, and CD56 cellular subsets by 6 months post transplant. Engraftment rate and event-free survival in this cohort were 100% and 96%, respectively. 70% (16/23) of patients had at least one viral reactivation or infection, including CMV 35% (8/23), HHV-6 22% (5/23), and polyoma virus 17% (4/23), with no cases of post-transplant lymphoproliferative disease.
Conclusion:
Further prospective studies are needed to better characterize immune reconstitution and the immunologic basis for increased viral reactivation following haplo-BMT with post-transplant cyclophosphamide for SCD.
Keywords: haploidentical bone marrow transplant, immune reconstitution, sickle cell disease, viral reactivation
1 |. INTRODUCTION
Sickle cell disease (SCD), the most common hereditary hemoglobinopathy in the world, results in extensive morbidity and early mortality resulting from progressive multi-system end-organ damage.1–4 Haploidentical bone marrow transplant (haplo-BMT) is an evolving platform that provides near universal donor availability for children and adults with severe SCD who lack a matched sibling donor.5–9 However, the intense immunoablation required to mitigate the risk of severe acute and chronic graft-versus-host disease (GvHD) and graft rejection is associated with increased infectious complications, namely viral reactivation and infection, along with variable consequences for immune reconstitution.10–18
Myeloablative conditioning followed by infusion of bone marrow allografts from matched siblings donors remains the gold standard for achieving optimal transplant-related outcomes for children and adults with sickle cell disease. In the longest follow-up study to date, Bernaudin et al detail the outcomes of 234 patients with sickle cell anemia younger than 30 years who underwent transplant.19 The 5-year cumulative incidence of transplant-related mortality was 3% (95% CI 0.8%-5.2%).19 In addition, graft rejection was found to be 3.1% (95% CI 0.7%-5.5%). The incidence of acute GvHD grade ≥ II was 20.1% (95% CI 14.9%-25.3%) and of chronic GvHD 10.5% (95% CI 6.5%-14.5%).19 The main infection complications were CMV reactivation, seen in 23.9% of patients, and asymptomatic EBV viral replication was observed in 6% of patients.19 An international survey of human leukocyte antigen (HLA) identical sibling hematopoietic cell transplant for sickle cell disease conducted by Gluckman et al revealed similar transplant-related outcomes.20
Immune reconstitution following T-cell deplete and T-cell replete haploidentical hematopoietic cell transplant is still poorly understood.10,15–18 Adults notably have impaired thymic function and depend more on expansion of mature T cells in the graft rather than through de novo thymus pathways, with unclear implications for pathogen-specific immunity.10,18 T-cell depletion has been shown to significantly reduce the incidence of GvHD, though is associated with delayed immune recovery with increased risk of graft rejection and infectious complications.21 Secondly, haplo-BMT has been known to be associated with clinically significant viral reactivations and infections, which has been postulated to result from impaired recovery of adaptive immunity post transplant.11–14 Crocchiolo et al showed a cumulative incidence of early viral reactivation or infection between 70% and 77%, with rates of CMV 65%, EBV 11%, and polyoma virus 19%, following T-cell replete haplo-BMT.12 Individuals with SCD undergo this curative therapy in the context of functional asplenia and increased susceptibility to invasive bacterial infections, including bacteremia, pneumonia, and meningitis.23 The impact of this immune modulatory approach on immune reconstitution and infection risk in individuals with SCD remains unknown.
Here, we report on the outcome of haplo-BMT with post-transplant cyclophosphamide (PTCy) plus thiotepa for individuals with severe SCD who lacked appropriate response to disease-modifying therapy with hydroxyurea and/or regular exchange blood transfusions.24 We hypothesized that T-cell replete haplo-BMT with PTCy would result in comparable outcomes compared to matched sibling donor transplants.
2 |. MATERIALS AND METHODS
2.1 |. Study design
We utilized a prospective, phase II study of reduced intensity conditioning haplo-BMT with PTCy plus thiotepa in the context of a multi-institutional learning collaborative. The primary objective was to estimate transplant-related mortality and progression-free survival in patients with severe hemoglobinopathies, already published. Secondary objectives included determining infectious complications, assessing immune reconstitution, characterizing hematologic and non-hematologic toxicities, determining donor hematopoietic chimerism in peripheral blood, assessing impact on pre-existing organ dysfunction, and SCD-related outcomes such as stroke and acute chest syndrome, following haplo-BMT.
2.2 |. Learning collaborative and study setting
We created a multi-disciplinary haplo-BMT consortium for SCD, including three clinical sites (one each from France, United Kingdom, and the United States). The learning collaborative had a common Data Safety and Monitoring Committee (DSMC), whose members included a statistician, adult and pediatric transplant physicians, and pediatric hematologists. Project oversight was reviewed by an advisory board. Three sites enrolled participants, and the following strategy was undertaken, namely a) use of common eligibility criteria, b) monthly phone conferences, c) collection of minimal and accurate data, d) common objectives and stopping rules, and e) spirit of collaboration. The main objective was to improve engraftment using reduced intensity conditioning haplo-BMT with PTCy. The stopping rules included >20% mortality rate, graft rejection, or severe chronic GvHD.
2.3 |. Participants
Eligible patients were between 1 and 70 years of age with severe SCD, including HbSS, HbSbeta thal, HbSC, HbSE, and HbSD, with an Eastern Cooperative Oncology Group performance status of 0 or 1, or Karnofsky performance status of at least 70%. Patients had no HLA-matched related donors, but had suitable and available first-degree haploidentical donor. A total of 33 patients have been transplanted using our haplo-BMT platform with thiotepa in conditioning, with 69% (23/33) having immune reconstitution data available for one year (Figure 1). Fifty-five percent (18/33) received pre-conditioning with azathioprine, hydroxyurea, and hypertransfusions. Forty-five percent (15/33) did not receive pre-conditioning. For study inclusion, patients had to have a life-expectancy of at least one year. Only patients with immune reconstitution data available for one year are included for analysis. For patients younger than 16, assent was obtained before enrollment, in accordance with the Declaration of Helsinki. Informed consent was obtained for participants at least 16 years of age. Patients were referred for transplant by independent hematologists after a full discussion of other available treatment options for severe SCD. The risks and benefits of haplo-BMT were discussed extensively with each patient and caregivers prior to giving assent or signing informed consent.
FIGURE 1.
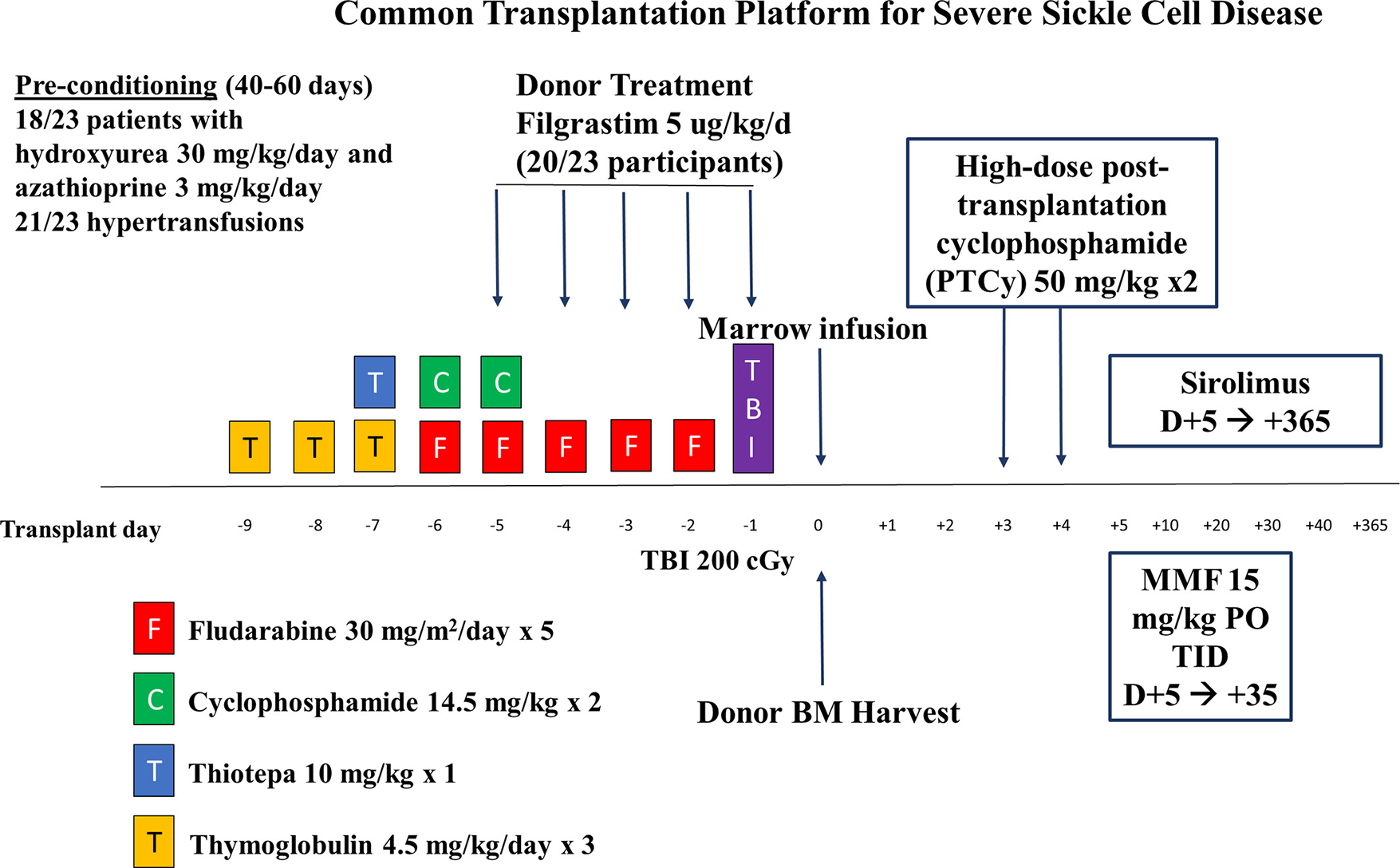
Common Transplantation Platform for Severe Sickle Cell Disease. PTCy (post-transplant cyclophosphamide), MMF (mycophenolate mofetil), BM (bone marrow), TBI (total body irradiation)
Patients also had at least one of the following criteria: stroke or central nervous system event lasting longer than 24 hours, magnetic resonance angiographic evidence of cerebrovascular disease despite regular transfusion therapy for at least 12 months, silent cerebral infarcts (defined as magnetic resonance imaging of the brain with signal abnormality of at least 3 mm in one dimension and visible in two planes on FLARE T2-weighted images) with associated evidence of vasculopathy or progression based on local interpretation, recurrent acute chest syndrome despite hydroxyurea therapy, recurrent vaso-occlusive crises requiring frequent hospitalizations (at least two per year for at least two years) despite hydroxyurea or regular blood transfusion therapy, and stage I or II sickle chronic lung disease. Patients were deemed to have appropriate end-organ functioning prior to transplant, defined as left ventricular shortening fraction of at least 26%, and absence of liver cirrhosis, defined as Ishak stage of at least 5.
2.4 |. Treatments
Pre-conditioning included azathioprine and hydroxyurea in 18/23 participants and hypertransfusions in 21/23 patients. The conditioning regimen included fludarabine, cyclophosphamide, thiotepa, thymoglobulin, and total body irradiation. Graft-versus-host disease prophylaxis included post-transplant cyclophosphamide, mycophenolate mofetil, and sirolimus (Figure 1).
2.5 |. Donors and grafts
Bone marrow stem cells were harvested from consenting first-degree relatives who must a) have shared at least one HLA-haplotype with the patient, b) not have sickle cell disease or other hemoglobinopathies, and c) were deemed to be otherwise healthy. Donors with sickle cell trait were not excluded. Potential donors were initially typed at the HLA-A, HLA-B, HLA-C, and HLA-DRB1 loci at intermediate or high-resolution level. Loci HLA-DRB1 and HLA-DQB1 alleles were typed at a high-resolution level. Haplotypes were determined based on family studies when able. A total of 20/23 donors underwent priming with granulocyte colony stimulating factor (G-CSF) at 10 ug/kg/day for 5 consecutive days (days −5 to −1). The target nucleated cell (TNC) dose was 8–16 × 108 TNC/kg of recipient ideal body weight, with the volume not to exceed 20 mL/kg donor weight once the minimum target dose was reached, and infused on day 0. In patients with multiple potential donors, the priority was as follows: donor age less than 40 years, donors with the fewest HLA-allele mismatches, major or minor ABO mismatches, cytomegalovirus (CMV) mismatched donor-recipient pairs, and those with the fewest donor-specific anti-HLA antibodies. Bone marrow was unmanipulated except in major ABO incompatible grafts. Graft volume of over 20 mL/recipient kg was plasma depleted.
2.6 |. Supportive care
Supportive care measures included use of G-CSF based on institutional standards and anti-microbial prophylaxis at the time of transplantation to prevent bacterial, viral, and fungal infections. Patients were monitored for signs/symptoms of infection, and serial viral studies were performed at periodic intervals, usually weekly. Serum quantitative polymerase chain reaction (qPCR) was used to diagnose viral reactivation given its high sensitivity and increased specificity compared to antigenemia assays. In recipients who were CMV seropositive or whose donors were CMV seropositive, whole blood CMV PCR was performed weekly starting at day + 14 until day + 100. Subsequent monitoring was performed per physician discretion. Other viral screening studies, including serum EBV PCR, were obtained after engraftment and checked biweekly. Urine specimens were used to test for BK virus when patients exhibited symptoms of cystitis, and blood assays were used to detect other viruses based on symptoms. Cultures were drawn from specific sites to diagnose bacterial and fungal infections, in addition to biopsies taken to confirm the diagnosis of fungal disease. The drugs used for anti-microbial prophylaxis are as follows (Table S1). For antibiotic prophylaxis, levofloxacin 500 mg once daily was started on day −1 and changed to penicillin VK 500 mg twice daily at engraftment, which was administered until patients had discontinued all immunosuppression.25–28 Alternatively, ciprofloxacin 7.5 mg/kg was given twice daily from day −8 until engraftment. Valacyclovir or acyclovir was used for anti-viral prophylaxis, and doses were escalated to provide coverage for both herpes simplex virus (HSV) and varicella zoster virus (VZV) in addition to CMV.29,30 Primary fungal prophylaxis was with either micafungin 50 mg once daily, which was started day −1 and continued until discontinuation of immunosuppression therapy.31 Itraconazole 5 mg/kg was also used and was continued until day + 100. Patients with a history of invasive fungal infections (IFI) or at high risk of IFI were discharged on posaconazole 300 mg or fluconazole 400 mg once daily for secondary prophylaxis.32 Pneumocystis prophylaxis was given from day + 30 to + 180 or until discontinuation of immunosuppression therapy and consisted of trimethoprim-sulfamethoxazole, dapsone, atovaquone, or inhaled pentamidine.33–36 All patients received 50 mg/kg PTCy on days + 3 and +4, mycophenolate mofetil, and either tacrolimus or sirolimus starting on day + 5 for GvHD prophylaxis.37 Furthermore, patients underwent strict blood pressure control and received seizure prophylaxis for the duration of immunosuppressive therapy. In terms of blood counts, platelets were maintained at least 50 × 109/L to reduce the risk of intracranial hemorrhage.
2.7 |. Outcomes measurements
Chimerism was assessed by polymerase chain reaction analysis of variable number of nucleotide tandem repeats unique to donors or recipients on total peripheral blood and isolated CD3 T cells on days 30, 60, 100, 180, and 365 (±7 days), and annually for two years thereafter. Primary graft rejection was defined as the presence of less than 5% donor cells as assessed by peripheral blood chimerism of any lineage by day + 60. Secondary graft rejection was defined as the presence of less than 5% donor cells as assessed by peripheral blood chimerism of any lineage, with prior evidence of at least 5% donor cells. Overall survival was time from transplant to death and was censored at the last follow-up. Neutrophil recovery was defined as the first of three days of neutrophil count of at least 0.5 × 109/L. Platelet recovery was defined as platelet count of at least 50 × 109/L in the first seven days post transplant without a platelet transfusion. GvHD severity was graded using the established National Institute of Health consensus criteria. Transplant-related mortality was defined as death because of any transplant-related cause other than disease recurrence.
2.8 |. Flow cytometry analysis of immune reconstitution
Peripheral blood was collected into EDTA tubes and processed within 24 hours to preserve cell surface markers. Cellular immunity was assessed by measuring the number and frequency of total lymphocytes and their subsets by flow cytometry using standardized laboratory protocols (FACSCanto II). Anti-CD3-phycoerythrin (PE), anti-CD19-fluorescein isothiocyanate (FITC), and anti-CD16/CD56-FITC monoclonal antibodies were used to identify T, B, and NK cells, respectively. All monoclonal antibodies were purchased from Becton-Dickinson (Heidelberg, Germany). Data were analyzed with FACS Diva Software (Becton-Dickinson, Heidelberg, Germany). To ensure comparable results between different time points of measurements, a calibration according to manufacturer instructions was performed on a monthly basis. To prevent potential bias and varaibility in the analysis, all measurements were gated by the same two persons in the two centers. The analyses were not centralized. Results were interpreted with respect to age appropriate reference ranges established in a healthy reference population.38 Immune reconstitution following hematopoietic cell transplant is defined as reconstitution of the donor-derived immune system. This refers to normal values of cellular subset by flow cytometry and occurs at different time points summarized in Table S2.
2.9 |. Statistical analysis
Baseline patient characteristics were manually extracted from the electronic patient record and are described with median values. Data were collected using Microsoft Excel spreadsheets that were checked by the different principal investigators from enrolling sites. Numbers of viral reactivations and infections were manually extracted from the EPR. Median time to viral reactivation and infection were calculated manually also. Median and interquartile ranges were calculated with a statistician. The Wilcoxon rank-sum test was used to detect statistically significant differences between patients who received pre-conditioning compared with those who did not. Survival analyses with Kaplan-Meier curves were not performed, since only patients who were alive at the time of analysis were included in the study.
3 |. RESULTS
3.1 |. Participant and donor characteristics
The majority of patients (16/23) had multiple indications for haplo-BMT. All but 2 donors had sickle cell trait. None of the donors with sickle cell trait suffered any toxicity with G-CSF priming. Donor-specific anti-HLA antibodies were assessed in all participants and detected in 2 patients. The two patient who had anti-HLA antibodies had donor-specific anti-HLA antibodies. Both underwent desensitization to reduce mean fluorescence intensity (MFI) to <2,000 units using the John Hopkins approach without modifications.39 Patient characteristics are shown in Table 1.
Table 1.
Baseline patient characteristics in cohort
| Metric | Data (n = 23) |
|---|---|
| Median age years (range) | 14.8 (8 to 25) |
| Disease phenotype of patients | 21 SS, 1 Sbeta-thal 0, 1 SD-Punjab |
| Pre-transplant comorbidities as indication for haplo-BMT | 10—overt stroke 21—moyamoya/cerebral vasculopathy 17—recurrent vaso-occlusive crises 9—recurrent acute chest syndrome 1—pulmonary hypertension 4—red blood cell alloimmunization 16/23 had multiple indications for BMT |
| Pre-transplant disease-modifying therapy | 11—hydroxyurea 19—exchange blood transfusion |
| Family donors | 21 parental donors (with sickle cell trait) 2 sibling donors |
| CMV serostatus (data for 22/23 patients) | 15—donor positive and recipient positive 3—donor positive and recipient negative |
Abbreviations: CMV, Cytomegalovirus; haplo-BMT, haploidentical bone marrow transplant; SS, sickle cell disease; thal, thalassemia.
3.2 |. Favorable overall outcomes following haplo-BMT
All patients had primary engraftment. However, one patient had secondary graft failure at day + 604. All engrafted patients had reversal of the SCD phenotype following haplo-BMT. Incidence of acute graft-versus-host disease grades II-IV was 17% (4/23) and for mild to moderate chronic graft-versus-host disease was 22% (5/23) and severe 4.3% (1/23). Eighty-six percent (18/21) patients were off immunosuppression at twelve months post transplant (Table 2).
TABLE 2.
Patient outcomes post–Haplo-BMT
| Metric | Data (n = 23) |
|---|---|
| Median TNC dose | 9.8 × 108 (5.9 to 11.7 × 108) |
| Median CD34 dose | 4.7 × 106 (3 to 6 × 106) |
| Median time to neutrophil engraftment (days) | 17 |
| Median time to platelet engraftment (days) | 32.5 |
| Incidence of acute graft-versus-host disease | Skin—Grade III-IV—4.3% (1/23) GI —Grade II-IV—8.7% (2/23) |
| Incidence of chronic graft-versus-host disease | Mild/moderate—21.7% (5/23) Severe—4.3% (1/23) |
| Graft failure rate | Secondary—4.3% (1/23) day + 604 post transplant (with return of autologous hematopoiesis) |
| Donor chimerism at 6 months and 1 year | Whole blood B and T cell > 95% |
| Sickle cell disease-related symptoms post transplant | None in engrafted patients |
| Number of patients off immune suppression therapy at 12 months | 86% (18/21) |
Abbreviations: haplo-BMT, Haploidentical bone marrow transplant; PTLD, post-transplant lymphoproliferative disorder; TNC, target nucleated cell.
3.2.1 |. Increased viral reactivations and infections post–haplo-BMT
In our cohort, we observed early viral reactivation and infections following haplo-BMT (Table 3, Figure 2). The majority of patients, 70%, (16/23) had at least one viral reactivation or infection, with 35 total cases of either noted (Table 3, Figure 2). CMV serostatus data were available in 22/23 patients included in the study. 68% (15/22) donor/recipient pair were CMV-positive while 14% (3/22) were donor-positive, recipient-negative pairs (Table 1). 35% (8/23) patients had CMV reactivation, predominantly in patients with CMV donor and/or recipient seropositive status. The remaining cases were mostly nosocomial pathogens, noted in just over 20% of patients. None of these viral reactivations or infections impacted transplant outcomes. Twenty-three total cases of suspected or documented bacterial infection were found, with 57% (13/23) of patients having at least one infection. Thirty-nine percent (9/23) patients in our study had documented or suspected fungal disease in the context of ongoing post-transplant care. Diagnosis was based on high-degree of clinical suspicion along with imaging correlates and positive cultures when available (Table S3). In terms of location of infection, four patients had pulmonary, two had sinus, one had suspected pulmonary and sinus, one had oropharyngeal candidiasis, and one had pulmonary involvement and documented Candida glabrata sepsis (Table S3).
TABLE 3.
Increased viral reactivation and infection post–Haplo-BMT
| Virus | Number of cases | Date range post–Haplo-BMT (median) |
|---|---|---|
| CMV reactivation | 35% (8 cases) | 4–118 (59) |
| HHV-6 reactivation/infection | 22% (5 cases) | 63–214 (153) |
| BK hemorrhagic cystitis | 17% (4 cases) | 31–184 (79.5) |
| HSV-1 stomatitis | 13% (3 cases) | 73–168 (125) |
| Parvovirus infection | 13% (3 cases) | 63–214 (98) |
| EBV reactivation (no PTLD) | 13% (3 cases) | 60–100 (80) |
| Influenza A | 9% (2 cases) | 54–116 (85) |
| Adenovirus | 9% (2 cases) | 35–68 (51.5) |
| CMV cystitis | 9% (2 cases) | 31–39 (35) |
Abbreviations: CMV, Cytomegalovirus; Haplo-BMT, haploidentical bone marrow transplant; PTLD, post-transplant lymphoproliferative disorder; TNC, total nucleated cell.
FIGURE 2.
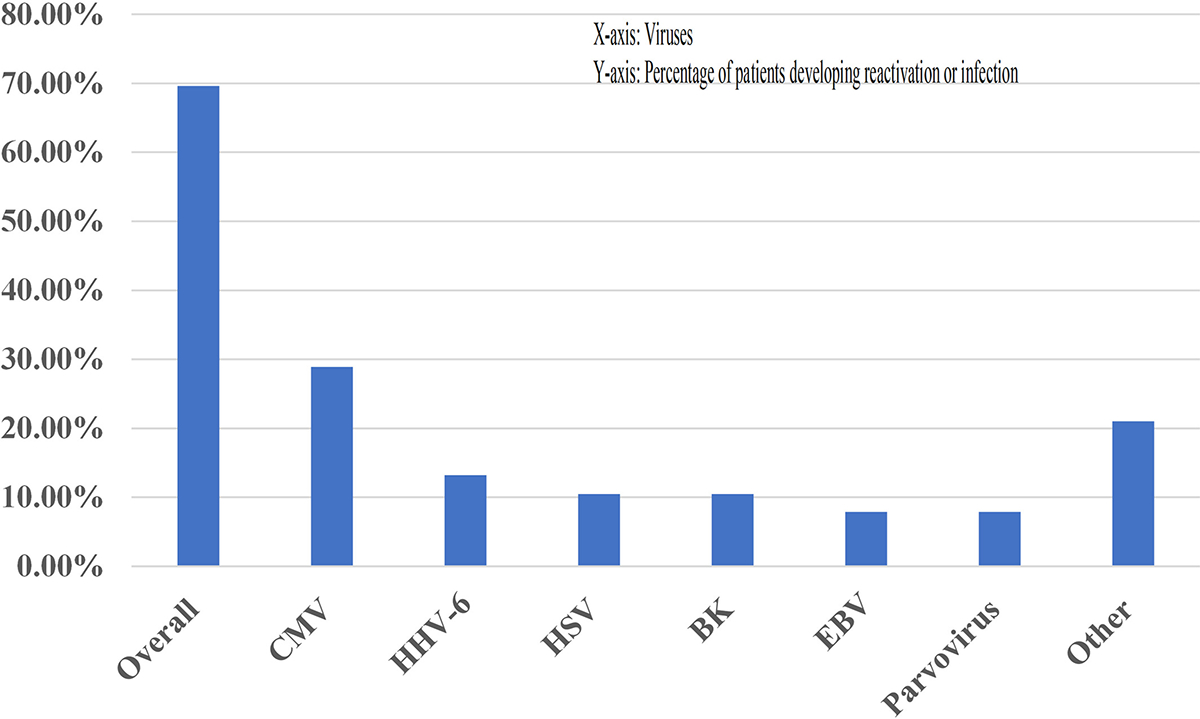
Increased Early Viral Reactivation Post–Haplo-BMT. Haplo-BMT (haploidentical bone marrow transplant), X-axis (viruses), Y-axis (percentage of patients developing reactivation or infection)
3.2.2 |. Prompt immune reconstitution was observed in cohort
As shown in Table S2, neutrophil engraftment was achieved at a mean of 17 days, and immune reconstitution data for CD4, CD8, CD19, and NK cells (Figures 3 and 4) showed prompt and robust reconstitution by 60 days post transplant.40 Immune reconstitution was observed in all 23 patients in the study (Figure 3A–D) with a total of 115 measurements. Most patients, 86% (18/21), were off immune suppression therapy by 12 months. Recovery of CD4, CD8, and CD19 cells increased slowly, though more rapidly after 6 months (Figure 3). Subgroup analysis was performed to determine whether immune reconstitution differed for patients who received pre-conditioning (n = 18) compared with those who did not (n = 5) (Figure 4A–D). No statistically significant differences were found.
FIGURE 3.
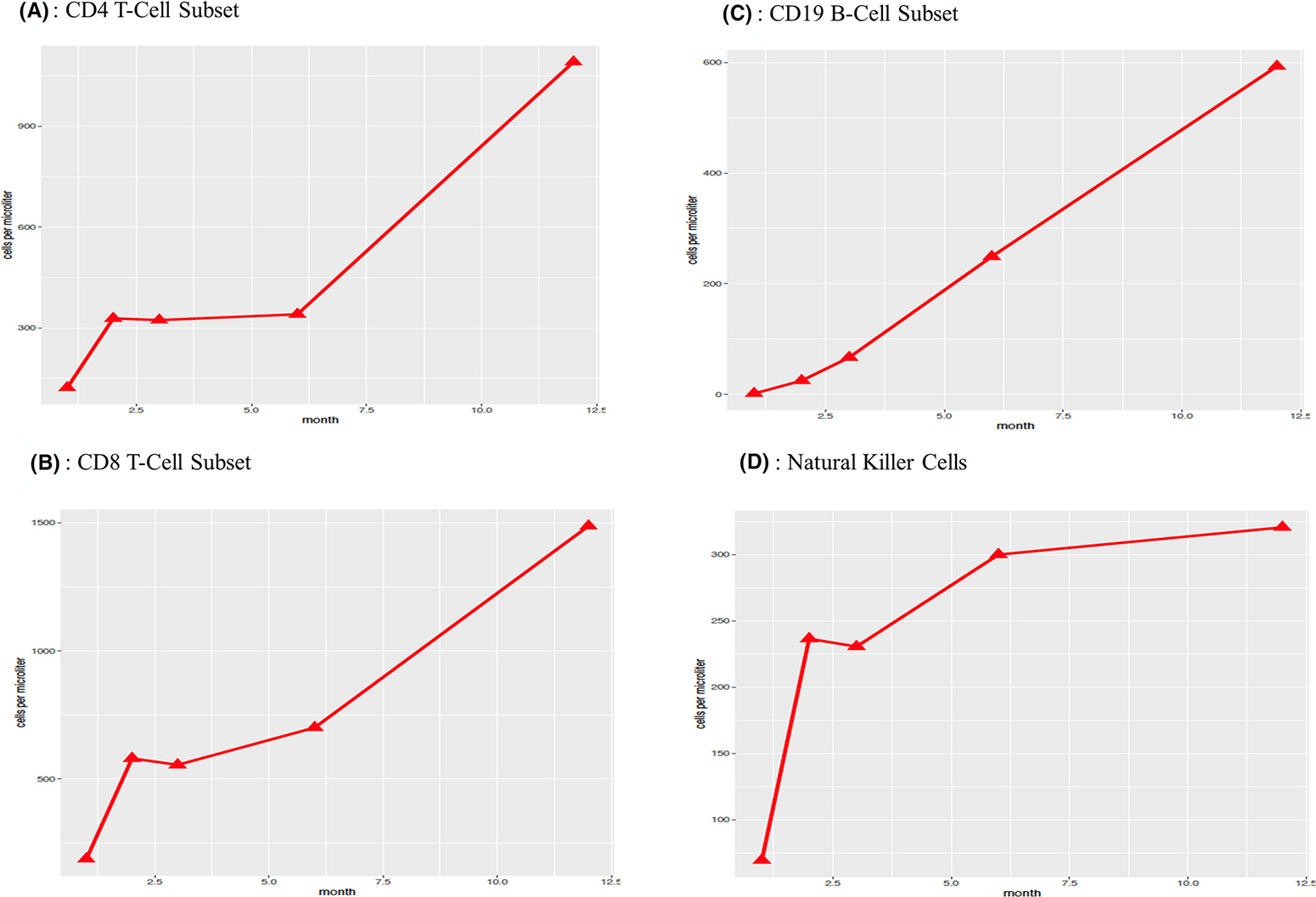
Immune Reconstitution Data for Cellular Subsets. A-D: Haplo-BMT (haploidentical bone marrow transplant), X-axis (months following haplo-BMT), Y-axis (cells per microliter)
FIGURE 4.
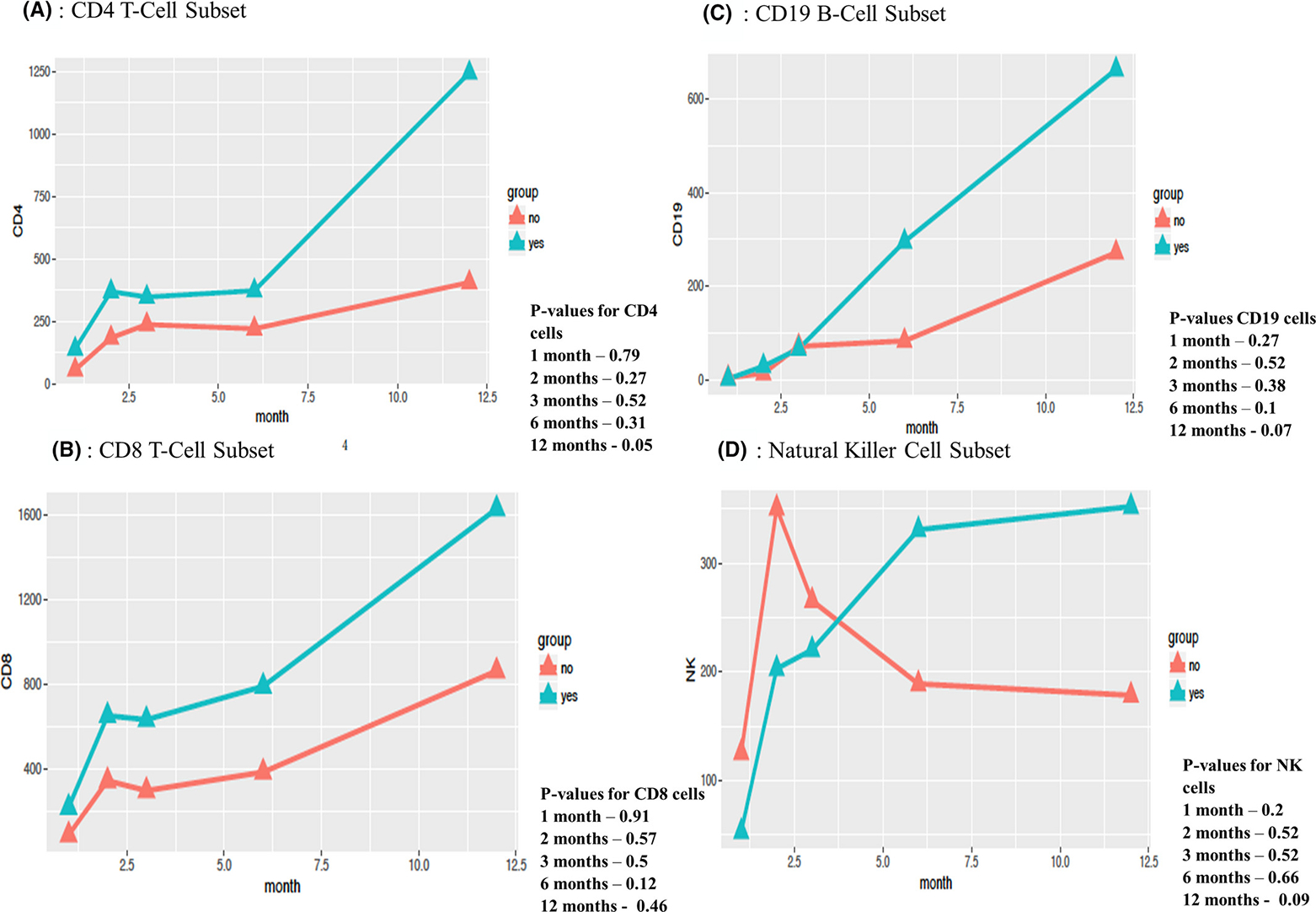
Immune Reconstitution With or Without Pre-Conditioning. A-D: Haplo-BMT (haploidentical bone marrow transplant), X-axis (months following haplo-BMT), y-axis (cells per microliter), no (lack of pre-conditioning, depicted in orange), yes (pre-conditioning received, depicted in teal)
4 |. DISCUSSION
To our knowledge, this is the first analysis of immune reconstitution and viral reactivation following haploidentical transplantation with PTCy. We found increased viral reactivation and infection following haplo-BMT for SCD despite excellent immune reconstitution. We also have shown favorable outcomes following this reduced intensity conditioning approach, a platform that offers curative potential for most eligible patients, even those with chronic organ dysfunction.5–8 As hypothesized, transplant-related outcomes in terms of engraftment and incidence of acute and chronic GvHD were comparable to trials involving matched sibling donor grafts.19,20 The drug-induced immunologic tolerance with PTCy has greatly reduced historic life-limiting severe acute and chronic GvHD.7,10 Graft rejection with haplo-BMT has improved based on recent approaches, including the addition of thiotepa to conditioning regimens, without increasing morbidity or mortality.24 To our knowledge, our consortium data include one of the largest datasets on immune reconstitution following haplo-BMT with PTCy for SCD.
A novel finding in our study was the early immune reconstitution of T-, B-, and NK-cell subsets despite intense immunodepletion.10,16–19,22 Multiple reasons exist for favorable immune reconstitution in the setting of haplo-BMT, which historically has required intense immunosuppression either pre-transplant with T-cell depletion strategies or post transplant with drug-induced immunologic tolerance, most commonly with PTCy.10,15–18 It has been postulated that PTCy permits naïve and non-activated memory cells to reconstitute in the later post-transplant period, allowing for late protection against infectious events.10,12 In addition, the high early viral reactivation incidence has been attributed to low numbers of adoptively transferred memory T cells in the early phase after haplo-BMT, contributing to a deficiency in cell-mediated immunity, though with rapid recovery based on selective deletion of alloreactive rather than pathogen-specific immune cells with PTCy.10,41,42 The rate of improvement characteristically increased for each subset (CD4, CD8, CD19, NK) after a median of 6 months, which correlated with the cessation of immunosuppression. Immune reconstitution in our cohort compared favorably to that found by Gaziev et al, though requires confirmation in larger prospective studies.21,22
A key and unexpected finding in our study was an increased incidence of early viral reactivations and infections before day + 100, which occurred in 70% (16/23) of patients, including 43% (10/23) with multiple viral reactivations or infections, the majority of which were due to CMV. The median time of CMV reactivation was 59 days (range 4–118), suggesting a contribution of impaired cell-mediated immunity in the immediate post-transplant period.18,43–45 However, CMV reactivation at even low levels (250 IU/mL) is still associated with an increased risk of mortality in the post-transplant period.45–47 Therefore, efforts to identify patients who are at higher risk of viral reactivation is imperative to improve transplant-related outcomes. With improved immunoprophylaxis, the non-relapse mortality from viral infections has decreased markedly since the initial trials of allogeneic hematopoietic cell transplant.46–48
The number of reactivations and infections in our study seems high compared to the gold standard of matched sibling donor transplant platforms. In a cohort of 234 patients younger than age 30, Bernaudin et al showed a CMV reactivation rate of 24% without CMV-related end-organ damage with pre-emptive therapy.19 In the second cohort treated with ATG, asymptomatic EBV reactivation occurred in 6% (15/234) patients.19 However, our data compare favorably to other haplo-BMT platforms. Crocchiolo et al conducted a study in patients undergoing T-cell replete haplo-BMT with PTCy as GvHD prophylaxis for hematologic malignancies or non-hematologic conditions such as aplastic anemia and found a 77% cumulative incidence of reactivations or infections.12 Gaziev et al, on the other hand, conducted a retrospective analysis on patients with hemoglobinopathies undergoing T-cell receptor α/β deplete haplo-BMT, with a similar 70% cumulative incidence of viral reactivations or infections, though with a 23% incidence of EBV-related post-transplant lymphoproliferative disorder.22 Our data showed a near 70% cumulative incidence also, with slightly less CMV reactivation of 28.9%, but higher rates of HHV-6 and HSV1/2, the implications of which still needs to be determined in larger studies.47 No cases of post-transplant lymphoproliferative disorder were seen in our cohort.
Our study has several limitations, which include but are not limited to small sample size, non-randomized nature, mainly children, and possible restriction to patients and families who live in high resource settings who were referred for consideration of haplo-BMT and cared for by knowledgeable pediatric hematologists. Certain aspects of supportive care were also institutionally based and may have varied some among the multiple sites included. However, we do not have evidence that institutional preferences varied in terms of the frequency or quality of assessment of pathogens post transplant.
Our results need to be confirmed in larger prospective trials, strategies are needed to mitigate against early infectious complications following this approach, and new prophylactic strategies such as letermovir or viral specific T cells are increasingly being utilized.45 Methodology similar to that used by Russo et al for NK-cell phenotyping after haplo-BMT with PTCy may provide insights into the reconstitution of viral specific CD4 and CD8 cells, with potential for enhanced ex vivo manipulation and infusion for patients with multiple reactivations or infections.49
Supplementary Material
ACKNOWLEDGEMENTS
We acknowledged Katie Gatwood for figure creation.
Footnotes
CONFLICT OF INTEREST
Authors do not disclose any competing financial interests in relation to the work described.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section.
REFERENCES
- 1.Platt OS, Brambilla DJ, Rosse WF, et al. Mortality in sickle cell disease – life expectancy and risk factors for early death. N Engl J Med. 1994;330:1639–1644. [DOI] [PubMed] [Google Scholar]
- 2.Rees D, Williams T, Gladwin M. Sickle-cell disease. Lancet. 2010;376:2018–2031. [DOI] [PubMed] [Google Scholar]
- 3.Weatherall D The inherited diseases of hemoglobin are an emerging global health burden. Blood. 2010;115(22):4331–4336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Piel FB, Steinberg MH, Rees DC. Sickle cell disease. N Engl J Med. 2017;376(16):1561–1573. [DOI] [PubMed] [Google Scholar]
- 5.Bolaños-Meade J, Fuchs EJ, Luznik L, et al. HLA-haploidentical bone marrow transplantation with posttransplant cyclophosphamide expands the donor pool for patients with sickle cell disease. Blood. 2012;120(22):4285–4291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Joseph J, Abraham A, Fitzhugh C. When there is no match, the game is not over: alternative donor options for hematopoietic stem cell transplantation in sickle cell disease. Semin Hematol. 2018;55:94–101. [DOI] [PubMed] [Google Scholar]
- 7.Bolanos-Meade J, Brodsky R. Blood and marrow transplantation for sickle cell disease: overcoming barriers to success. Curr Opin Oncol. 2009;21(2):158–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kassim A, Sharma D. Hematopoietic stem cell transplantation for sickle cell disease: the changing landscape. Hematol Oncol Stem Cell Ther. 2017;10:259–266. [DOI] [PubMed] [Google Scholar]
- 9.Gragert L, Eapen M, Williams E, et al. Match likelihoods for hematopoietic stem-cell grafts in the U.S. Registry. N Engl J Med. 2014;371(4):339–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kanakry C, Fuchs E, Luznik L. Modern approaches to HLA-haploidentical blood or marrow transplantation. Nat Rev Clin Oncol. 2016;13(1):10–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Slade M, Goldsmith S, Romee R, et al. Epidemiology of infections following haploidentical peripheral blood hematopoietic cell transplantation. Transpl Infect Dis. 2017;19(1):e12629. 10.1111/tid.12629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crocchiolo R, Bramanti S, Vai A, et al. Infections after T-replete haploidentical transplantation and high-dose cyclophosphamide as graft-versus-host disease prophylaxis. Transpl Infect Dis. 2015;17:242–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Atilla E, Atilla P, Bozdag S, Demirer T. A review of infectious complications after haploidentical hematopoietic stem cell transplantations. Infection. 2017;45:403–411. [DOI] [PubMed] [Google Scholar]
- 14.Aversa F, Prezioso L, Manfra I, Galaverna F, Spolzino A, Monti A. Immunity to infections after haploidentical hematopoietic stem cell transplantation. Mediterr J Hematol Infect Dis. 2016;8(1):e2016057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Servais S, Lengline E, Porcher R, et al. Long-term immune reconstitution and infection burden after mismatched hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2014;20:507–517. [DOI] [PubMed] [Google Scholar]
- 16.Chang Y-J, Zhao X-Y, Huang X-J. Immune reconstitution after haploidentical hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2014;20:440–449. [DOI] [PubMed] [Google Scholar]
- 17.Chang Y-J, Zhao X-Y, Huo M-R, et al. Immune reconstitution following unmanipulated HLA-mismatched/haploidentical transplantation compared with HLA-identical sibling transplantation. J Clin Immunol. 2012;32:268–280. [DOI] [PubMed] [Google Scholar]
- 18.Luo X-H, Chang Y-J, Huang X-J. Improving cytomegalovi-rus-specific T cell reconstitution after haploidentical stem cell transplantation. J Immunol Res. 2014;2014:1–12. 10.1155/2014/631951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bernaudin F, Dalle J-H, Bories D, et al. Long-term event-free survival, chimerism and fertility outcomes in 234 patients with sickle-cell anemia younger than 30 years after myeloablative conditioning and matched-sibling transplantation in France. Haematologica. 2019;104:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gluckman E, Cappelli B, Bernaudin F, et al. Sickle cell disease: an international survey of results of HLA-identical sibling hematopoietic stem cell transplantation. Blood. 2017;129(11):1548–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bertaina A, Pitisci A, Sinibaldi M, Algeri M. T Cell-depleted and T-cell replete HLA-haploidentical stem cell transplantation for non-malignant disorders. Curr Hematol Rep. 2017;12:68–78. [DOI] [PubMed] [Google Scholar]
- 22.Gaziev J, Isgro A, Sodani P, et al. Haploidentical HSCT for hemoglobinopathies: improved outcomes with TCR alpha/beta+/CD19+- depleted grafts. Blood Adv. 2017;2(3):263–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Payne AB, Link-Gelles R, Azonobi I, et al. Invasive pneumo-coccal disease among children with and without sickle cell disease in the United States, 1998 to 2009. Pediatr Infect Dis J. 2013;32(12):1308–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De la Fuente J, Dhedin N, Koyama T, et al. Haploidentical bone marrow transplant with post-transplant cyclophosphamide plus thiotepa improves donor engraftment in patients with sickle cell anemia: results of an international learning collaborative. Biol Blood Marrow Transplant. 2018;25:1197–1209. [DOI] [PubMed] [Google Scholar]
- 25.Bucaneve G, Micozzi A, Menichetti F, et al. Levofloxacin to prevent bacterial infection in patients with cancer and neutropenia. N Engl J Med. 2005;353:977–987. [DOI] [PubMed] [Google Scholar]
- 26.Bucaneve G, Castagnola E, Viscoli C, Leibovici L, Menichetti F. Quinolone prophylaxis for bacterial infections in afebrile high risk neutropenic patients. Eur J Cancer. 2007;5(2):5–12. [Google Scholar]
- 27.Cruciani M, Rampazzo R, Malena M, et al. Prophylaxis with fluoroquinolones for bacterial infections in neutropenic patients: a meta-analysis. Clin Infect Dis. 1996;23:795–805. [DOI] [PubMed] [Google Scholar]
- 28.Tomblyn M, Chiller T, Einsele H, et al. Guidelines for preventing infectious complications among hematopoietic cell transplantation recipients: a global perspective. Biol Blood Marrow Transplant. 2009;15:1143–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dignani MC, Mykietiuk A, Michelet M, et al. Valacyclovir prophylaxis for the prevention of Herpes simplex virus reactivation in recipients of progenitor cells transplantation. Bone Marrow Transplant. 2002;29(3):263–267. [DOI] [PubMed] [Google Scholar]
- 30.Ljungman P, de la Camara R, Milpied N, et al. Valacyclovir International Bone Marrow Transplant Study Group. Randomized study of valacyclovir as prophylaxis against cytomegalovirus reactivation in recipients of allogeneic bone marrow transplants. Blood. 2002;99(8):3050–3056. [DOI] [PubMed] [Google Scholar]
- 31.van Burik J-a h, Ratanatharathorn V, Stepan DE, et al. National Institute of Allergy and Infectious Diseases Mycoses Study Group. Micafungin versus fluconazole for prophylaxis against invasive fungal infections during neutropenia in patients undergoing hematopoietic stem cell transplantation. Clin Infect Dis. 2004;39:1407–1416. [DOI] [PubMed] [Google Scholar]
- 32.Ullmann AJ, Lipton JH, Vesole DH, et al. Posaconazole or fluconazole for prophylaxis in severe graft-versus-host disease. N Engl J Med. 2007;356:335–347. [DOI] [PubMed] [Google Scholar]
- 33.Green H, Paul M, Vidal L, Leibovici L. Prophylaxis of Pneumocystis pneumonia in immunocompromised non-HIV-infected patients: systematic review and meta-analysis of randomized controlled trials. Mayo Clin Proc. 2007;82:1052–1059. [DOI] [PubMed] [Google Scholar]
- 34.Souza J, Boeckh M, Gooley T, Flowers M, Crawford S. High rates of Pneumocystis carinii pneumonia in allogeneic blood and marrow transplant recipients receiving dapsone prophylaxis. Clin Infect Dis. 1999;29:1467–1471. [DOI] [PubMed] [Google Scholar]
- 35.Link H, Vohringer H, Wingen F, et al. Pentamidine aerosol for prophylaxis of Pneumocystis carinii pneumonia after BMT. Bone Marrow Transplant. 1993;11:403–406. [PubMed] [Google Scholar]
- 36.Chan C, Montaner J, Lefebvre EA, et al. Atovaquone suspension compared with aerosolized pentamidine for prevention of Pneumocystis carinii pneumonia in human immunodeficiency virus-infected subjects intolerant of trimethoprim or sulfonamides. J Infect Dis. 1999;180:369–376. [DOI] [PubMed] [Google Scholar]
- 37.Luznik L, O’Donnell PV, Symons HJ, et al. HLA-Haploidentical Bone Marrow Transplantation for Hematologic Malignancies Using Nonmyeloablative Conditioning and High-Dose, Posttransplantation Cyclophosphamide. Biol Blood Marrow Transplant. 2008;149(6):641–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Comans-Bitter W, de Groot R, van den Beemd R, et al. Immunophenotyping of blood lymphocytes in children. Reference values for lymphocyte subpopulations. J Pediatr. 1997;130(3):388–393. [DOI] [PubMed] [Google Scholar]
- 39.Gladstone DE, Zachary AA, Fuchs EJ, et al. Partially mismatched transplantation and human leukocyte antigen donor-specific antibodies. Biol Blood Marrow Transplant. 2013;19(4):647–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ogonek J, Kralj Juric M, Ghimire S, et al. Immune reconstitution after allogeneic hematopoietic stem cell transplantation. Front Immunol. 2016;7:507. 10.3389/fimmu.2016.00507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kanakry CG, Coffey DG, Towlerton AMH, et al. Origin and evolution of the T cell repertoire after posttransplantation cyclophosphamide. JCI Insight. 2016;1(5):e86252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McCurdy SR, Vulic A, Symons HJ, et al. Comparable and robust immune reconstitution after HLA-haploidentical or HLA-matched allogeneic transplantation (BMT) utilizing posttransplantation cyclophosphamide. Biol Blood Marrow Transplant. 2015;21(2):S71. [Google Scholar]
- 43.Crocchiolo R, Castagna L, Furst S, et al. The patient’s CMV serological status affects clinical outcome after T-cell replete haplo-HSCT and post-transplant cyclophosphamide. Bone Marrow Transplant. 2016;51:1134–1136. [DOI] [PubMed] [Google Scholar]
- 44.Suessmuth Y, Koura D, Finstermeier K, et al. Exhaustive TCR Deep Sequencing Reveals that CMV Reactivation Fundamentally Resets Immune Reconstitution after Transplant and Results in Significant Deficits in the Effector Memory TCR Repertoire. BMT Tandem “Scientific” Meeting. 2015;21(2):S69–S70. [Google Scholar]
- 45.Camargo JF, Wieder ED, Kimble E, et al. Deep functional immunophenotyping predicts risk of cytomegalovirus reactivation after hematopoietic cell transplantation. Blood. 2019;133(8):867–877. [DOI] [PubMed] [Google Scholar]
- 46.Green M, Leisenring W, Xie H, et al. Cytomegalovirus viral load and mortality after hematopoietic stem cell transplantation in the era of pre-emptive therapy: a retrospective cohort study. Lancet Haematol. 2016;3(3):e119–e127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jain N, Lu K, Ito S, et al. The clinical and financial burden of pre-emptive management of cytomegalovirus disease after allogeneic stem cell transplantation-implications for preventative treatment approaches. Cytotherapy. 2014;16(7):927–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Patel DA, Dhedin N, Chen H, et al. Delayed Immune Reconstitution and Increased Viral Infections Following Haploidentical BMT with Post-Transplant Cyclophosphamide for Sickle Cell Disease: Results of a Haploidentical Transplant Consortium for Hemoglobinopathies (ICHH). Biol Blood Marrow Transplant. 2019;25(3):S39–S40. [Google Scholar]
- 49.Russo A, Oliveira G, Berglund S, et al. NK cell recovery after haploidentical HSCT with posttransplant cyclophosphamide: dynamics and clinical implications. Blood. 2018;131(2):247–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


