Abstract
Anastomotic stricture and leakage are common complications after repair of esophageal atresia (EA). A compromised perfusion of the anastomosis is a contributing factor. Hyperspectral imaging (HSI) is an ultrashort noninvasive method to measure tissue perfusion. We present two cases of with tracheoesophageal fistula (TEF)/EA repair, in whom we applied HSI: the first patient was a newborn with EA type C who underwent open TEF repair. The second one had an EA type A and cervical esophagostomy, in whom we performed gastric transposition. In both patients, HSI confirmed a good tissue perfusion of the later anastomosis. The postoperative course was uneventful and both patients are on full enteral feeds. We conclude that HSI is a safe and noninvasive tool that allows near real-time assessment of tissue perfusion and can contribute to the identification of the optimal anastomotic region during pediatric esophageal surgery.
Keywords: perfusion, hyperspectral imaging, esophageal atresia, anastomotic stricture, anastomotic leakage
Introduction
Esophageal atresia (EA) with or without tracheoesophageal fistula (TEF) has an incidence of 1 in 4,099 births. 1 Anastomotic stricture and leakage are frequent problems following repair of EA/TEF, often due to tension on or compromised perfusion of the anastomosis. 2 3 Anastomotic strictures require 5.1 ± 5.6 dilatations on average. Almost all patients require at least one readmission within the first year of life. 4 The intraoperative assessment of tissue perfusion has recently gained increasing interest in gastrointestinal surgery. 5 Different methods have been established, the most prevalent being indocyanine green fluorescence scan. 6 7 A drawback of this and other techniques to assess tissue perfusion is the need to inject contrast agents with potential adverse reactions and toxicity. 8
Hyperspectral imaging (HSI) is a noninvasive tool for the assessment of tissue perfusion and oxygenation based on the tissue-specific reflection of light in hyperspectral ranges of visible and near-infrared light (500–1,000 nm wavelength). 9 It does not require contrast agents and provides near real-time information on tissue perfusion (near-infrared perfusion index [NIR-PI]), tissue hemoglobin index (THI), and tissue oxygen saturation (StO 2 ) with 10 to 15 seconds delay.
HSI has been applied to various fields of surgery including cutaneous flaps in reconstructive surgery, partial nephrectomies, or intestinal anastomoses. 10 11 12 13 We report the first application of HSI in pediatric esophageal surgery.
Case Description
Case 1 : A newborn girl (body weight 1,850 g, gestational age 36 weeks) presented with VACTERL association including EA type C, anorectal malformation with vestibular fistula, and septum defect. She underwent open TEF repair on day 2 of life. After TEF closure, the tip of the lower esophagus showed an impaired perfusion on real-time HSI assessment (NIR-PI 58%; THI 100%; StO 2 40%; Fig. 1A, B ). Thus, an additional 2 mm of the lower esophagus ( Fig. 2 ) were resected to accomplish an anastomosis in a well-perfused area (NIR-PI 92%; THI 100%; StO 2 55%; Fig. 1C, D ). The postoperative course was uneventful. No anastomotic leak or stenosis occurred. The patient was discharged home on postoperative day 41 due to difficulties related to her low birth weight and cardiac situation. During the further course of 22 months, no esophageal dilatations were required.
Fig. 1.
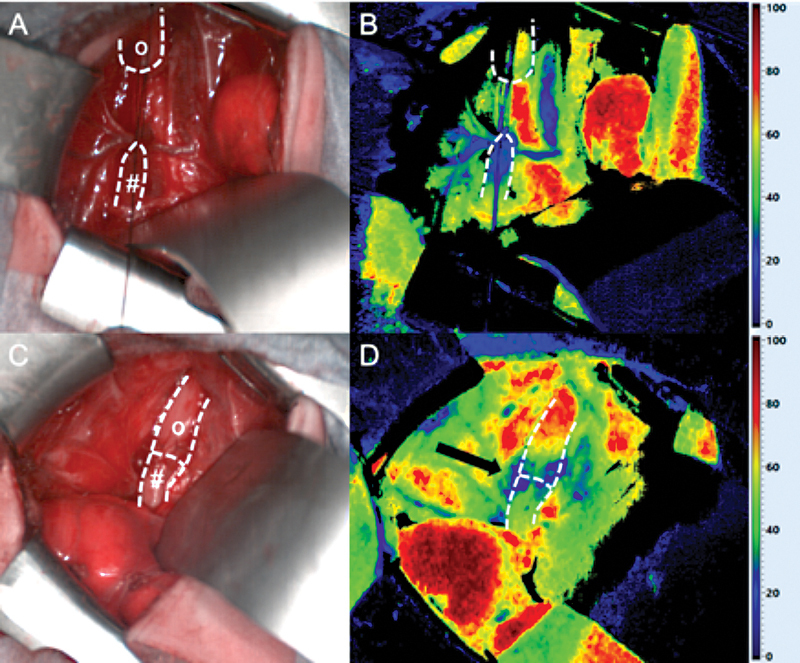
Intraoperative situs ( A, C ) and HSI StO 2 ( B, D ) after TEF ligation: Well-perfused upper esophageal pouch ( o ) and impaired perfusion of the lower esophagus ( # ) before resection of its distal tip ( A, B ). After resection of the tip of the distal pouch (Fig. 2), improved perfusion with only minor impairment at the anastomotic suture line itself ( arrow ) was detected ( C, D ). HSI, hyperspectral imaging; StO 2 , tissue oxygen saturation; TEF, tracheoesophageal fistula.
Fig. 2.
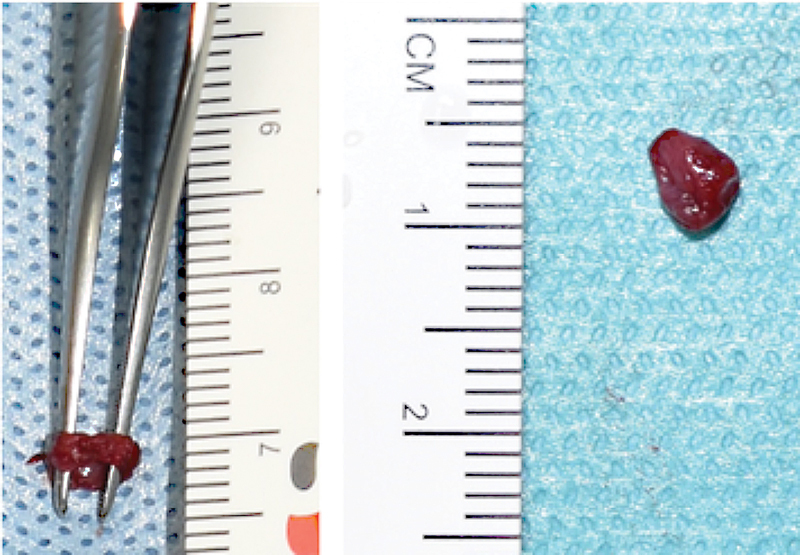
Additional resection of 2 mm distal esophagus after hyperspectral imaging measurement.
Case 2 : A 9-month-old boy with EA type A underwent gastric transposition after receiving a cervical esophagostomy in the newborn period at another institution. After laparotomy, a good perfusion of the gastric fundus was confirmed and a cervical esophagogastric anastomosis was established (StO 2 80%, THI 100%, NIR-PI 80%; Fig. 3 ). The subsequent course remained uneventful for 26 months. No dilation of the cervical anastomosis was required. The patient is on full oral feeds.
Fig. 3.
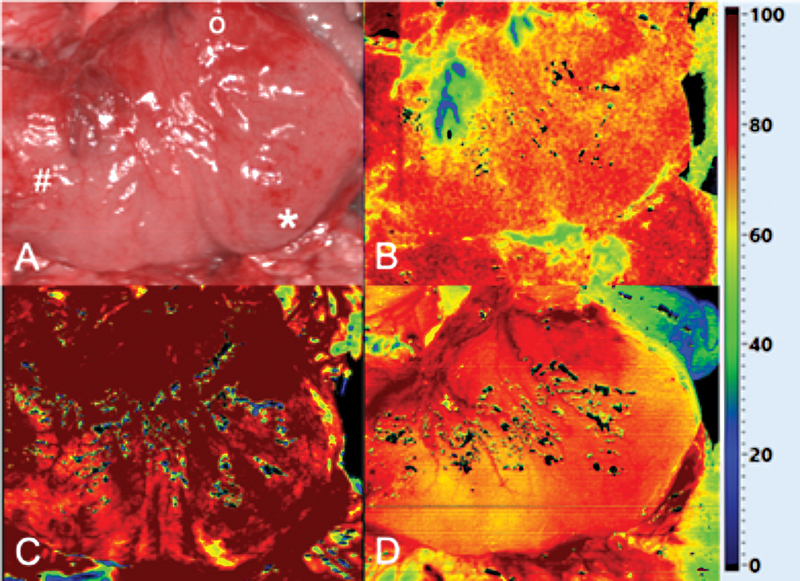
Intraoperative situs ( A ) and HSI assessment (StO 2 [ B ], THI [ C ], and NIR-PI [ D ]) before esophagogastric anastomosis for gastric transposition. o, fundus ; *, greater curvature; #, antrum ; HSI, hyperspectral imaging; NIR-PI, near-infrared perfusion index; StO 2 , tissue oxygen saturation; THI, tissue hemoglobin index.
Discussion
Anastomotic leakage and stricture after repair of EA/TEF occur in ∼20 and 28% of cases, respectively. 14 This is, among other factors, caused by an impaired perfusion of the anastomosis. 15 Although intraoperatively the perfusion of the lower esophagus appeared unaltered macroscopically in patient 1, HSI measurement revealed a diminished perfusion of the distal pouch. Thus, HSI had an additional diagnostic value. We therefore resected additional 2 mm of the distal esophagus to create an anastomosis of the two esophageal ends, which were well perfused. Of note, we recognized an impaired perfusion at the anastomosis itself, most likely from the suture line ( Fig. 1 ), which has been described in adults before. 11
Currently, several clinical studies are ongoing to evaluate the benefits of HSI in gastrointestinal anastomoses. A more peripheral adaptation of the anastomosis based on intraoperative HSI was reported for oncologic esophagectomy in 38% of patients without any postoperative leak. 16 In another study reporting on colorectal resection, a deviation between the transection line planned by the surgeon and the border line visualized by HSI of 1 to 13 mm was found for all patients. Consequently, the resection area was corrected proximally in 21% of patients due to the intraoperative HSI records. Thus, the authors concluded that the determination of the resection margin by HSI provide the surgeon with an objective decision aid for assessment of the best possible perfusion and ideal anastomotic area in colorectal surgery. 17 Its transferability to other gastrointestinal anastomoses is obvious. However, normal or cutoff values for different gastrointestinal anastomotic types especially in pediatric surgery are still required.
Besides HSI, other methods such as indocyanine green fluorescence can also assess intraoperative perfusion and guide surgeons to improve surgical outcomes. 18 However, this technique requires contrast agents, which is not the case in HSI. HSI is a safe, fast, noninvasive technique that can easily be implemented during surgery. The tissue perfusion assessed by HSI is also comparable to that of indocyanine green fluorescence, which has recently been shown for colorectal resections as well as oncologic esophagectomy. 16 19 HSI has also been shown to discriminate tissue perfusion in acute mesenteric ischemia and depict tissue viability via reflectance spectra. 20 Besides, it has been applied to evaluate liver and gastric perfusion during pancreatoduodenectomy and identifying exact resection planes for anatomic liver resection. 21 22
Only recently, also, a HSI system for minimally invasive surgery has been introduced. The HSI laparoscope available has a diameter of 10 mm and is consistent for object distances up to 10 cm. 23 24 It is currently examined for clinical practicability and impact in adult surgery.
These emerging experiences in adult surgery indicate promising applications in pediatric surgery. Future tasks include the implementation of HSI measurements in surgical interventions where adequate tissue perfusion plays a key role. However, to date, there are no reliable normal and cutoff measurements for decision-making. Moreover, prospective studies comparing intraoperative perfusion with postoperative outcomes are also lacking. However, we think that the HSI technique is easy to perform and a promising tool to assess perfusion in pediatric surgery, which may contribute to better in children.
Conclusion
This is the first report on HSI assessment in pediatric surgery as a safe and noninvasive tool to assess tissue perfusion in real time. It can help determine the optimal anastomotic region during pediatric esophageal surgery.
Footnotes
Conflict of Interest None declared.
New Insights and the Importance for a Pediatric Surgeon.
We describe the first use of HSI (a safe and noninvasive tool that allows near real-time assessment of tissue perfusion and can contribute to the identification of the optimal anastomotic region) in pediatric esophageal surgery.
References
- 1.Nassar N, Leoncini E, Amar E. Prevalence of esophageal atresia among 18 international birth defects surveillance programs. Birth Defects Res A Clin Mol Teratol. 2012;94(11):893–899. doi: 10.1002/bdra.23067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Askarpour S, Peyvasteh M, Javaherizadeh H, Askari N. Evaluation of risk factors affecting anastomotic leakage after repair of esophageal atresia. Arq Bras Cir Dig. 2015;28(03):161–162. doi: 10.1590/S0102-67202015000300003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Touloukian R J, Seashore J H.Thirty-five-year institutional experience with end-to-side repair for esophageal atresia Arch Surg 200413904371–374., discussion 374 [DOI] [PubMed] [Google Scholar]
- 4.Dingemann C, Dietrich J, Zeidler J. Early complications after esophageal atresia repair: analysis of a German health insurance database covering a population of 8 million. Dis Esophagus. 2016;29(07):780–786. doi: 10.1111/dote.12369. [DOI] [PubMed] [Google Scholar]
- 5.Urbanavičius L, Pattyn P, de Putte D V, Venskutonis D. How to assess intestinal viability during surgery: a review of techniques. World J Gastrointest Surg. 2011;3(05):59–69. doi: 10.4240/wjgs.v3.i5.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alander J T, Kaartinen I, Laakso A. A review of indocyanine green fluorescent imaging in surgery. Int J Biomed Imaging. 2012;2012:940585. doi: 10.1155/2012/940585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Turner S R, Molena D R. The role of intraoperative fluorescence imaging during esophagectomy. Thorac Surg Clin. 2018;28(04):567–571. doi: 10.1016/j.thorsurg.2018.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alford R, Simpson H M, Duberman J. Toxicity of organic fluorophores used in molecular imaging: literature review. Mol Imaging. 2009;8(06):341–354. [PubMed] [Google Scholar]
- 9.Lu G, Fei B. Medical hyperspectral imaging: a review. J Biomed Opt. 2014;19(01):10901. doi: 10.1117/1.JBO.19.1.010901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Akbari H, Kosugi Y, Kojima K, Tanaka N. Hyperspectral imaging and diagnosis of intestinal ischemia. Annu Int Conf IEEE Eng Med Biol Soc. 2008;2008:1238–1241. doi: 10.1109/IEMBS.2008.4649387. [DOI] [PubMed] [Google Scholar]
- 11.Jansen-Winkeln B, Maktabi M, Takoh J P. Hyperspektral-Imaging bei gastrointestinalen Anastomosen. Chirurg. 2018;89(09):717–725. doi: 10.1007/s00104-018-0633-2. [DOI] [PubMed] [Google Scholar]
- 12.Best S L, Thapa A, Jackson N. Renal oxygenation measurement during partial nephrectomy using hyperspectral imaging may predict acute postoperative renal function. J Endourol. 2013;27(08):1037–1040. doi: 10.1089/end.2012.0683. [DOI] [PubMed] [Google Scholar]
- 13.Gockel I, Jansen-Winkeln B, Holfert N. Möglichkeiten und Perspektiven der Hyperspektralbildgebung in der Viszeralchirurgie. Chirurg. 2020;91(02):150–159. doi: 10.1007/s00104-019-01016-6. [DOI] [PubMed] [Google Scholar]
- 14.Du J, Huang J, Li Y, Chen Y, Guo W, Hou D. The repair of esophageal atresia and major complications—a systematic review and our experience in dealing with the tracheoesophageal fistula. Ann Laparosc Endosc Surg. 2019;4:90–90. [Google Scholar]
- 15.Mayer S, Gitter H, Göbel P. Behandlung der Ösophagusatresie mit unterer tracheoösophagealer Fistel – Zusammenfassung der aktuellen S2K-Leitlinie der DGKCH. Klin Padiatr. 2020;232(04):178–186. doi: 10.1055/a-1149-9483. [DOI] [PubMed] [Google Scholar]
- 16.Hennig S, Jansen-Winkeln B, Köhler H. Novel intraoperative imaging of gastric tube perfusion during oncologic esophagectomy-a pilot study comparing hyperspectral imaging (HSI) and fluorescence imaging (FI) with indocyanine green (ICG) Cancers (Basel) 2021;14(01):97. doi: 10.3390/cancers14010097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jansen-Winkeln B, Holfert N, Köhler H. Determination of the transection margin during colorectal resection with hyperspectral imaging (HSI) Int J Colorectal Dis. 2019;34(04):731–739. doi: 10.1007/s00384-019-03250-0. [DOI] [PubMed] [Google Scholar]
- 18.Lau C T, Au D M, Wong K KY. Application of indocyanine green in pediatric surgery. Pediatr Surg Int. 2019;35(10):1035–1041. doi: 10.1007/s00383-019-04502-4. [DOI] [PubMed] [Google Scholar]
- 19.Jansen-Winkeln B, Germann I, Köhler H. Comparison of hyperspectral imaging and fluorescence angiography for the determination of the transection margin in colorectal resections-a comparative study. Int J Colorectal Dis. 2021;36(02):283–291. doi: 10.1007/s00384-020-03755-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mehdorn M, Köhler H, Rabe S M. Hyperspectral imaging (HSI) in acute mesenteric ischemia to detect intestinal perfusion deficits. J Surg Res. 2020;254:7–15. doi: 10.1016/j.jss.2020.04.001. [DOI] [PubMed] [Google Scholar]
- 21.Moulla Y, Buchloh D C, Köhler H. Hyperspectral imaging (HSI)-a new tool to estimate the perfusion of upper abdominal organs during pancreatoduodenectomy. Cancers (Basel) 2021;13(11):2846. doi: 10.3390/cancers13112846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sucher R, Athanasios A, Köhler H. Hyperspectral imaging (HSI) in anatomic left liver resection. Int J Surg Case Rep. 2019;62:108–111. doi: 10.1016/j.ijscr.2019.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pfahl A, Köhler H, Thomaßen M T. Video: clinical evaluation of a laparoscopic hyperspectral imaging system. Surg Endosc. 2022;36(10):7794–7799. doi: 10.1007/s00464-022-09282-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Köhler H, Kulcke A, Maktabi M. Laparoscopic system for simultaneous high-resolution video and rapid hyperspectral imaging in the visible and near-infrared spectral range. J Biomed Opt. 2020;25(08):86004. doi: 10.1117/1.JBO.25.8.086004. [DOI] [PMC free article] [PubMed] [Google Scholar]


