Abstract
Objective
Epidemiological studies have indicated that dietary patterns during pregnancy are associated with adverse pregnancy and birth outcomes such as hypertensive disorders of pregnancy (HDP), gestational diabetes mellitus (GDM), preterm birth (PTB) and low birth weight (LBW). However, the results of these studies are varied and inconsistent. The present study aimed to assess the association between dietary patterns and the risk of adverse pregnancy and birth outcomes.
Design
Systematic review and meta-analysis. Seven databases were searched for articles. Two reviewers performed the study selection and data extraction. A random-effects model was used to estimate pooled effect sizes of eligible studies.
Setting
Studies conducted all over the world were incorporated.
Subjects
The review focused on pregnant women.
Results
A total of twenty-one studies were identified. Adherence to a healthy dietary pattern (intake of vegetables, fruits, legumes, whole grains) was significantly associated with lower odds (OR; 95 % CI) of pre-eclampsia (0·78; 0·70, 0·86; I 2=39·0 %, P=0·178), GDM (0·78; 0·56, 0·99; I 2=68·6 %, P=0·013) and PTB (0·75; 0·57, 0·93; I 2=89·6 %, P=0·0001).
Conclusions
Our review suggests that dietary patterns with a higher intake of fruits, vegetables, legumes, whole grains and fish are associated with a decreased likelihood of adverse pregnancy and birth outcomes. Further research should be conducted in low-income countries to understand the impact of limited resources on dietary intake and adverse pregnancy and birth outcomes.
Keywords: Dietary patterns, Dietary intake, Pregnancy, Pregnant women
Hypertensive disorders of pregnancy (HDP) are a group of conditions related to high blood pressure during pregnancy, proteinuria and in some cases convulsions( 1 ). HDP are responsible for increased morbidity and mortality in mothers and newborns, accounting for approximately 14 % of maternal deaths globally between 2003 and 2009( 2 ). According to an analysis of international cohorts from six countries (Australia/New Zealand, Canada, Israel, Japan, Spain and Sweden), the incidence rate of HDP was 13 % (ranging from 10·3 to 16·4 %)( 3 ).
Preterm birth (PTB) is the premature delivery of a neonate before 37 weeks of gestation( 4 ). PTB is most common in low- and middle-income countries and is one of the leading causes of direct neonatal deaths and complications( 4 ), responsible for more than 50 % of neonatal mortality in 2010( 5 ). According to a systematic analysis and estimation of PTB, the rate of PTB was 11 % in 2010 globally, ranging from 5 % in European countries to 18 % in some African countries( 6 ). Likewise, low birth weight (LWB), which refers to a newborn birth weight of less than 2·5 kg, is common (15 %). High rates are reported in many developing countries, especially South Asia (25 %), sub-Saharan Africa (12 %)( 7 ), Pakistan (35 %), Nepal (30 %) and Jordan (22 %)( 8 ).
Evidence has shown that dietary patterns have an influence on adverse pregnancy and birth outcomes( 9 , 10 ). When individuals consume foods, they consume a combination of nutrients, not single nutrients( 11 ). The whole diet with its expected synergistic effects may have a greater influence on the occurrence of health outcomes than single nutrients( 11 ). Hence, it appears more complete to examine the effect of the whole diet by applying a more all-inclusive method of dietary pattern analysis, because dietary patterns evaluate the usual diet as one complete dietary exposure( 12 , 13 ).
Dietary pattern analysis aims to assess the usual foods consumed as one overall dietary exposure( 12 , 14 ). Dietary patterns are defined as the quantities, proportions, variety or combinations of different foods and beverages in diets and the frequency with which they are regularly consumed( 15 ). Dietary patterns can be determined by three approaches. The first is the a priori approach, which constructs dietary scores or indices based on predefined dietary recommendations( 12 , 14 , 16 ). The second is the a posteriori approach, which identifies data-driven dietary patterns using statistical methods (cluster analysis and principal component analysis (PCA))( 12 , 14 , 16 ). The third approach consists of hybrid methods such as reduced rank regression, which combine aspects of the a priori and a posteriori approaches( 16 ).
Previous studies have indicated that dietary patterns during pregnancy have a varied effect on maternal health and pregnancy outcomes such as HDP( 10 , 17 ), GDM( 18 , 19 ), PTB( 9 , 20 , 21 ) and LBW( 22 ). For HDP, intake of vegetables, legumes, nuts, tofu, rice, pasta, rye bread, fish, milk, green leafy vegetables and pulses/beans was associated with a lower odds of pre-eclampsia/eclampsia( 10 , 23 ), while the consumption of meat and potatoes, processed meat, sweet drinks and salty snacks increased the likelihood of pre-eclampsia( 10 , 24 , 25 ). Other studies have reported contradictory findings; a cohort study in the USA( 17 ) reported that a higher Alternate Healthy Eating Index (AHEI) score comprising vegetables, fruit, fibre, trans fat, high PUFA:SFA, folate, Ca and Fe from foods was not associated with pre-eclampsia. For GDM, a Western dietary pattern (high intake of red meat, processed meat, refined grain products, sweets, French fries and pizza) among pregnant women in the USA( 26 ), a pasta–cheese–processed-meat pattern( 18 ) in a Singaporean population and a sweet and seafood pattern in China( 19 ) have been associated with increased odds of GDM.
Regarding the birth outcomes, a ‘prudent’ dietary pattern with a high intake of vegetables, fruits, oils, water (beverage), wholegrain cereals and fibre-rich breads was associated with a reduced occurrence of PTB( 9 ). In contrast, a Western pattern (salty and sweet snacks, white bread, desserts and processed meat products)( 9 ) and a Mediterranean diet with a high intake of fish, fruit, vegetables, olive/canola oil, and a low intake of red meat and coffee had no effect on PTB( 20 ). Contrary to this, in a Danish birth cohort study, the odds of PTB increased in women who adhered to a Western pattern (high in meat and fats and low in fruits and vegetables)( 21 ). A study from the USA( 27 ) revealed that birth weight and fetal growth were not associated with the maternal AHEI score (high intakes of vegetables, fruit, whole grains, nuts and legumes, long-chain (n-3) fats, polyunsaturated fats, folate, Ca and Fe).
Current epidemiological studies show some evidence for an association between dietary patterns and adverse pregnancy and birth outcomes. However, the findings are inconsistent and there is a need to identify which dietary patterns could have health benefits for pregnant women in preventing adverse pregnancy and birth outcomes. Therefore, our aim was to determine the association between dietary patterns during pregnancy and the risk of pregnancy (HDP, GDM) and birth (PTB and LBW) outcomes through a systematic review and meta-analysis.
Methods
Search strategy
Seven databases were searched, including MEDLINE, EMBASE, CINAHL, Scopus, Cochrane Library, Web of Science, and Maternity and Infant Care. The reference lists of all previous articles were hand-searched.
The following terms, words and combinations of words were searched: (‘diet’ OR ‘nutrition’ OR ‘food pattern’ OR ‘meal pattern’ OR ‘eating practice’ OR ‘food intake’ OR ‘food habits’ OR ‘eating behaviour’ OR ‘dietary pattern’ OR ‘dietary diversity score’ AND ‘pregnancy’ OR ‘pregnant women’ OR ‘gravid’ OR ‘gestation’ OR ‘prenatal care’ OR ‘antenatal care’ AND ‘gestational hypertension’ OR ‘pregnancy-induced hypertension’ OR ‘preeclampsia’ OR ‘pre-eclampsia’ OR ‘low birth weight’ OR ‘premature infant’ OR ‘premature birth’ OR ‘preterm birth’ OR ‘pregnancy in diabetics’ OR ‘gestational diabetes mellitus’).
The search was comprised of free text words, title and Medical Subject Heading for outcomes, exposure and participants, as well as applying limits including English language and human subjects.
Study selection
The studies were screened by title and then by abstract by two reviewers (K.T.K., T.K.T.). The full texts of all selected studies were critically reviewed based on the inclusion/exclusion criteria summarized in Table 1.
Table 1.
Inclusion and exclusion criteria for the current systematic review and meta-analysis on maternal dietary patterns and risk of adverse pregnancy and birth outcomes
| Inclusion criteria |
| ∙ Pregnant women |
| ∙ No date restrictions |
| ∙ Original articles (randomized trials and observational studies) |
| ∙ Dietary pattern as the exposure variable |
| ∙ Included one or more of the following outcome variables: HDP, GDM, LBW, PTB |
| Exclusion criteria |
| ∙ High-risk populations: women with heart diseases, diabetes, pre-eclampsia or gestational hypertension at baseline |
| ∙ Unpublished papers |
| ∙ Animal studies |
| ∙ Brief communications, case series, editorials, review studies |
| ∙ Studies that focused on single nutrients |
HDP, hypertensive disorders of pregnancy (gestational hypertension, pre-eclampsia and eclampsia); GDM, gestational diabetes mellitus; LBW, low birth weight; PTB, preterm birth.
Data extraction
The following variables were extracted by two reviewers (K.T.K., T.K.T.): authors, publication year, study period, study design, settings/country, sample, dietary patterns with food details, dietary assessment methods and periods, main outcomes (HDP, GDM, LBW and PTB) and adjustment for confounding factors.
Quality assessments
The quality of selected full-text articles was rated by two reviewers independently (K.T.K., T.K.T.) using the Academy of Nutrition and Dietetics quality appraisal tool( 28 ). This tool has four relevance questions and ten validity questions. The validity questions appraise the selection, comparability of groups, assessment of exposures or outcomes and statistical analysis for each study separately( 28 ). The validity of a study is assessed as the responses to all relevant questions being ‘yes’. The response for all validity questions is ‘yes’ if the criterion was fulfilled, ‘no’ if not fulfilled, ‘unclear’ if not precisely stated and ‘N/A’ (not applicable) if the criterion does not apply to the articles( 28 ). The rating scores of studies were positive (+) if the responses to the validity questions were ‘yes’ for six or more responses (including all four relevance questions). If the articles did not fulfil the relevance criterion of selection, comparability of groups and measurement of exposures or outcomes, the rating score was neutral (Ø) and if the responses for the validity questions are ‘no’ or ‘unclear’ for six or more responses, a negative (−) rating score was given( 28 ).
Statistical analysis
The data were entered into a Microsoft® Excel spreadsheet version 16 and exported to the statistical software package Stata version 13 for analysis. The OR was used as a measure of effect estimate. If an incidence of outcome variable was less than or equal to 20 %, the risk ratio (RR) and OR were pooled together in the meta-analysis; otherwise RR was converted to OR using the proposed methods of Zhang and Yu( 29 ) and Cochrane( 30 ). If the studies did not report OR/RR but reported the coefficient (β) of the regression, it was converted into OR/RR by exponentiation of the coefficient (i.e. OR=exp(β))( 31 ).
Some articles reported OR/RR based on different references. Some used lower adherence to dietary patterns, while some used good adherence. To make this consistent and unify all results using either the higher or lower group as reference, the new OR/RR was calculated by taking the reciprocal of the reported OR/RR. The lower limit of the new OR/RR is the reciprocal of the upper limit of the old OR/RR and the upper limit of the new OR/RR is reciprocal of the lower limit of the old OR/RR( 32 ).
The random-effects model was used for calculating pooled estimates. Statistical heterogeneity was evaluated by Cochran’s Q test (I 2), which shows the amount of heterogeneity between studies. An I 2 value reflects between-study variation (values of 25, 50 and 75 % refer to low, medium and high variation, respectively)( 33 ).
Subgroup analyses were conducted to detect potential sources of heterogeneity. The possible effects of between-study variance of dietary assessment methods (dietary diversity score (DDS), Mediterranean diet score (MDS), PCA) and dietary assessment periods/trimesters (first trimester (1st–12th weeks), second trimester (13th–27th weeks), third trimester (28th–40th weeks)) were assessed.
Dietary patterns detected in each study were different regarding to the country of origin and the approaches used for identifying dietary patterns; however, they had similarities among commonly consumed food items. For instance, most articles identified a prudent, traditional, Mediterranean or healthy dietary pattern which commonly consisted of whole grains, nuts legumes/pulses, vegetables/fruits and fish. These studies were grouped together and analysed by labelling them as ‘healthy dietary pattern’.
Similarly, those patterns comprised mostly of refined grains, processed meats or snacks, high-sugar and high-fat dairy products, eggs and white potatoes were grouped together, labelled as the ‘Western dietary pattern’ and then analysed.
Using the available articles, pooled estimates were determined for the effect of the healthy pattern on HDP, GDM, PTB and LBW. Likewise, meta-analysis was performed for a Western dietary pattern and GDM, HDP and PTB.
Results
Identified studies
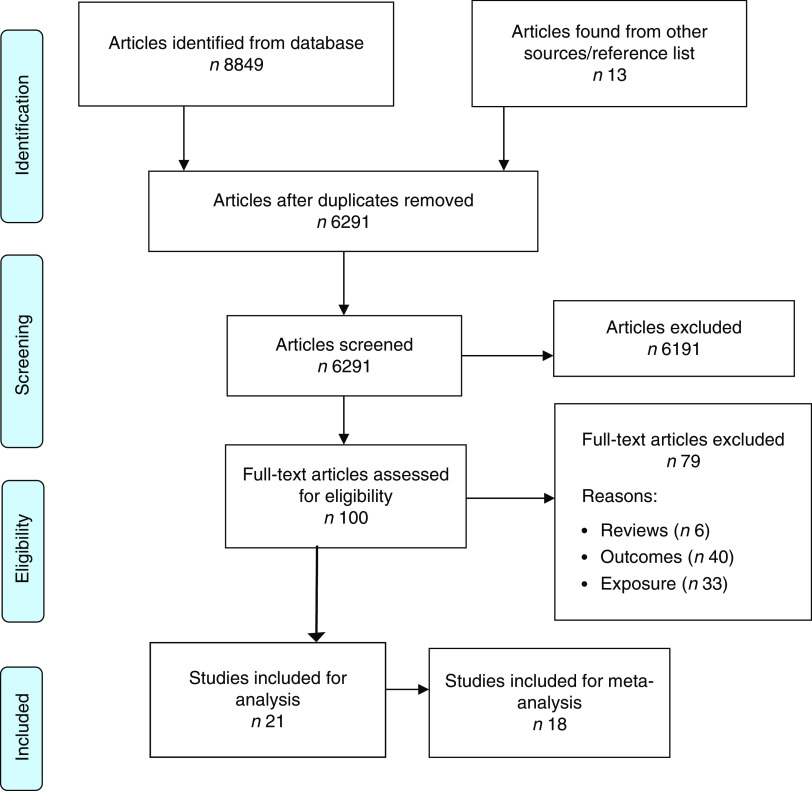
Our search identified 6291 records after removal of duplicates. One hundred articles were identified for full-text review, with twenty-one articles incorporated in the systematic review and meta-analysis (Fig. 1).
Fig. 1.
(colour online) Flowchart of the study selection process for the current systematic review and meta-analysis on maternal dietary patterns and risk of adverse pregnancy and birth outcomes
Study characteristics
Of the twenty-one articles included, the majority (n 15) were conducted in developed countries, with the remainder in developing countries. Out of all included articles, eighteen were cohort studies and three were cross-sectional studies. The articles were published between 2008 and 2016. The sample in each study ranged from 168( 34 ) to 66 000( 9 ) with 302 450 pregnant women in total. In the included articles, six reported the effect of dietary patterns on HDP( 10 , 25 , 35 – 38 ), six reported on GDM( 18 , 19 , 34 , 39 – 41 ), nine reported on PTB( 9 , 20 , 21 , 36 , 42 – 46 ) and two reported on LBW( 46 , 47 ) (Table 2).
Table 2.
Characteristics of the articles included in the current systematic review and meta-analysis on maternal dietary patterns and risk of adverse pregnancy and birth outcomes
| Dietary assessment | Methods of defining dietary pattern | Dietary patterns identified | Main findings | Outcomes | Confounding factors | ||||
|---|---|---|---|---|---|---|---|---|---|
| Study | Study design; period; country | Sample (n) | Methods | Trimester (period) | |||||
| Brantsaeter et al. (2009)( 10 ) | Cohort; 2002–2006; Norway | 23 423 | 255-item FFQ* | 2nd (17–22 weeks) | PCA | Vegetable; potato and fish; cakes and sweets; processed food | Vegetable (tertile 3 v. tertile 1): OR=0·72 (95 % CI 0·62, 0·85) Processed food (tertile 3 v. tertile 1): OR=1·21 (95 % CI 1·03, 1·42) Potato and fish (tertile 3 v. tertile 1): OR=1·00 (95 % CI 0·84, 1·18) | PE | BMI, education, age, smoking, height, education status, hypertension prior to pregnancy, TEI, dietary supplement use |
| Eshriqui et al. (2016)( 35 ) | Cohort; 2009–2012; Brazil | 299 | Eighty-two item FFQ* | 3rd (28–38 weeks) | PCA | Healthy; processed; common Brazilian | Mixed-effect regression with SBP: Healthy: β=−0·199 (95 % CI −1·28, 0·88); OR=0·82 (95 % CI 0·28, 2·10). Processed: β=−0·268 (95 % CI −1·67, 1·14); OR=0·76 (95 % CI 0·19, 3·13) Mixed-effect regression with DBP:Healthy: β=−0·670 (95 % CI −1·573, 0·232); OR=0·51 (95 % CI 0·21, 1·26). Processed: β=−0·032 (95 % CI −1·202, 1·138); OR=0·97 (95 % CI 0·30, 3·12) | Blood pressure (SBP & DBP) | Age, BMI, education, parity, TEI |
| Mwanri et al. (2015)( 37 ) | Cross-sectional; 2011–2012; Tanzania | 910 | Sixteen-food-group 24 h recall* | 2nd & 3rd (20–36 weeks) | DDS | Sixteen food groups | Medium DDS: OR=2·54 (95 % CI 1·04, 6·16) High DDS: OR=5·84 (95 % CI 2·11, 16·15) | Hypertension during pregnancy | Residence, age, gestational age, MUAC, parity, GDM, education, PA |
| Timmermans et al. (2011)( 25 ) | Prospective cohort; the Netherlands | 3187 | 293-item FFQ* | All (median 13·5 weeks) | PCA | Mediterranean diet pattern (MDP); traditional dietary pattern | For PE: Low adherence to MDP: OR=1·2 (95 % CI 0·6, 2·3); adherence to MDP: OR=0·83 (95 % CI 0·43, 1·60); adherence to traditional: OR=1·1 (0·6, 2·1) For GHTN: Low adherence to MDP: OR=1·3 (95 % CI 0·9, 1·9); adherence to MDP: OR=0·77 (95 % CI 0·53, 1·11); adherence to traditional: OR=1·3 (95 % CI 0·9, 1·9) | PE & GHTN | Maternal BMI, maternal age, parity, educational level, smoking, vomiting, preconception folic acid use |
| Torjusen et al. (2014)( 38 ) | Cohort; 2002–2008; Norway | 28 192 | Six-food-group FFQ | 2nd (17–22 weeks) | PCA | Healthy pattern; organic vegetables pattern | Healthy pattern, tertile 3 v. tertile 1: OR=0·74 (95 % CI 0·64, 0·85) Organic vegetables, tertile 3 v. tertile 1: OR=0·79 (95 % CI 0·62, 0·99) | PE | Hypertension prior to pregnancy, pre-pregnant BMI, height, age, education, household income, smoking in pregnancy, TEI, gestational weight gain |
| Hillesund et al. (2014)( 36 ) | Cohort; Norway | 72 072 | 255-item FFQ* | 25 weeks | NND score | New Nordic dietary index (NND) | With high NND score: Risk of PE: OR=0·86 (95 % CI 0·78, 0·95); risk of early PE: OR=0·71 (95 % CI 0·52, 0·96); risk of PTB: OR=0·91 (95 % CI 0·80, 1·30) | PE & PTB | Maternal age, height, pre-pregnancy BMI, parity, education, smoking status, exercise during pregnancy, chronic hypertension, diabetes, marital status, energy intake |
| Dayeon et al. (2015)( 39 ) | Cross-sectional; USA | 253 | Eight-food-group 24 h recall | All (avg. 20 weeks) | RRR | ‘High refined grains’, ‘high nuts, seeds, fat and soyabeans, low milk’, ‘high added sugar and organ meats’, ‘low fruits, vegetables and seafood’ | ‘High refined grains’ pattern: OR=4·9 (95 % CI 1·4, 17·0) ‘High nuts, seeds, fat and soyabeans, low milk’ pattern: OR=7·5 (95 % CI 1·8, 32·3) ‘High added sugar and organ meats’ pattern: OR=22·3 (95 % CI 3·9, 127·4) | GDM | Age, race/ethnicity, family poverty income ratio, education, marital status, energy intake, pre-pregnancy BMI, gestational weight gain, log-transformed CRP |
| De Seymour et al. (2016)( 18 ) | Multi-ethnic Asian cohort; Singapore | 909 | Sixty-eight-food-group 24 h recall* | 2nd & 3rd (26–28 weeks) | PCA | Three patterns: vegetable–fruit–rice-based-diet; seafood–noodle-based-diet; pasta–cheese–processed-meat diet | Vegetable–fruit–rice-based-diet: OR=1·10 (95 % CI 0·90, 1·35) Seafood–noodle-based-diet: OR=0·74 (95 % CI 0·59, 0·93) Pasta–cheese–processed-meat diet: OR=0·96 (95 % CI 0·79, 1·17) | GDM | Energy intake, pregnancy BMI, birth order, smoking, alcohol intake, age, ethnicity, education, previous GDM, family history of diabetes, household monthly income, other dietary patterns |
| He et al. (2015)( 19 ) | Prospective cohort, China | 3063 | Sixty-four-item FFQ* | 2nd (24–27 weeks) | PCA | Four dietary patterns: vegetable pattern; protein-rich food pattern; prudent pattern; sweets and seafood pattern | Vegetable pattern: RR=0·79 (95 % CI 0·64, 0·97) Sweets and seafood pattern: RR=1·23 (95 % CI 1·02, 1·49) Protein-rich food pattern: RR=0·95 (95 % CI 0·78, 1·16) Prudent pattern: RR=1·00 (95 % CI 0·82, 1·22) | GDM | Maternal age, education level, monthly income, parity, pre-pregnancy BMI, family history of diabetes |
| Karamanos et al. (2014)( 40 ) | Prospective cohort; Jan 2010–Jul 2011; ten Mediterranean countries | 1076 | Seventy-eight-item FFQ* | 2nd & 3rd (24–32 weeks) | MDS | Mediterranean diet index | Mediterranean diet: OR=0·618 (95 % CI 0·401, 0·950) | GDM | Age, BMI, diabetes in the family, weight gain, energy intake |
| Nascimento et al. (2016)( 41 ) | Prospective cohort; Nov 2011–Feb 2014; Spain | 841 | Eighty-one-item FFQ* | 2nd (15–20 weeks) | PCA | Three patterns: traditional pattern; vegetable and Western pattern; mixed pattern | High tertile v. low tertile (3 v. 1): Traditional pattern: RR=0·88 (95 % CI 0·49, 1·58) Mixed pattern: RR=0·93 (95 % CI 0·51, 1·71) Vegetable and Western pattern: RR=0·78 (95 % CI 0·43, 1·43) | GDM | BMI, age, education, monthly income, family history of diabetes, parity |
| Tryggvadottir et al. (2016)( 34 ) | Prospective cohort; Apr 2012–Oct 2013; Iceland | 168 | Eighteen-food-group & 4 d weighed food record | 2nd (19–24 weeks) | PCA | Prudent pattern | Adhering to the prudent pattern: OR=0·44 (95 % 0·21, 0·90) | GDM | Age, parity, pre-pregnancy weight, energy intake, weekly weight gain, total metabolic equivalents of task |
| Chia et al. (2016)( 42 ) | Cohort study; 2009–2010; Singapore | 923 | Sixty-eight-food-group 24 h recalls and 3 d food diaries | 2nd & 3rd (26–28 weeks) | PCA | Vegetable, fruit and white rice; seafood and noodle; pasta, cheese and processed meat | Adherence to vegetable, fruit and white rice pattern: OR=0·67 (95 % CI 0·50, 0·91) Adherence to seafood and noodle pattern: OR=1·27 (95 % CI 0·93, 1·74) Adherence to pasta, cheese and processed meat pattern: OR=0·79 (95 % CI 0·55, 1·12) | PTB | Infant sex, birth order, maternal TEI, maternal age, ethnicity, pre-pregnancy BMI, weight gain until 26–28 week of gestation, height, GDM status, educational status, alcohol use, smoking during pregnancy, other dietary patterns |
| Englund-Ogge et al. (2014)( 9 ) | Prospective cohort; 2002–2008; Norway | 66 000 | 255-item FFQ* | 2nd (17–22 weeks) | PCA | ‘Prudent’; ‘Western’; ‘traditional’ | Prudent: RR=0·88 (95 % CI 0·80, 0·97) Western: RR=1·02 (95 % CI 0·92, 1·13) Traditional: RR=0·91 (95 % CI 0·83, 0·99) | PTB | Maternal age, pre-pregnancy BMI, height, parity, TEI, maternal education, marital status, smoking, previous preterm delivery, household income, other dietary patterns |
| Haugen et al. (2008)( 20 ) | Cohort; Norway | 569 | 255-item FFQ* | 2nd (18–22 weeks) | MDS | Mediterranean diet criteria | Mediterranean diet criteria 5 v. 0: OR=0·73 (95 % CI 0·32, 1·68) | PTB | Parity, BMI, maternal height, SES; cohabitant status |
| Martin et al. (2015)( 43 ) | Prospective cohort; USA | 3143 | Ninety-five-item FFQ | 2nd & 3rd (26–29 weeks) | PCA and DASH | Factor 1; Factor 2; Factor 3; Factor 4 | Factor 1: OR=0·87 (95 % CI 0·60, 1·27) Factor 2: OR=1·53 (95 % CI 1·02, 2·30) Factor 3: OR=1·55 (95 % CI 1·07, 2·24) Adherence to DASH diet: OR=0·59 (95 % CI 0·40, 0·85) | PTB | Maternal age, race, maternal pre-pregnancy BMI status, educational level, household income, parity, marital status, smoking status, energy intake |
| Rasmussen et al. (2014)( 21 ) | Longitudinal cohort; Denmark | 59 949 | 360-item FFQ* | 2nd & 3rd (avg. 25 weeks) | PCA | Vegetable/prudent; Western; Seafood | Western pattern: OR=1·30 (95 % CI 1·13, 1·49) Vegetable/prudent pattern: OR=1·40 (95 % CI 0·80, 1·62) Seafood pattern: OR=0·90 (95 % CI 0·72, 1·11) | PTB | Maternal age, maternal height, pre-pregnancy BMI, parity, civil status, SES, smoking during pregnancy |
| Zerfu et al. (2016)( 46 ) | Prospective cohort; Ethiopia | 432 | Nine-food-group 24 h WDDS | 2nd & 3rd (24–28 weeks) | DDS | Nine food groups | Low DDS: RR=4·61 (95 % CI 2·31, 9·19) High DDS: RR=0·21 (95 % CI 0·11, 0·43) | PTB | Age, height, MUAC, education, Hb level |
| Mikkelsen et al. (2008)( 44 ) | Cohort; Denmark | 35 530 | 360-item FFQ | 2nd & 3rd (avg. 25 weeks) | MDS | Mediterranean diet criteria: consumption of fish twice/week; intake of olive or rapeseed oil; high consumption of fruits & vegetables (5/d or more); meat (other than poultry and fish) at most twice/week | Mediterranean diet criteria 5 v. 0: OR=0·61 (95 % CI 0·35, 1·05) Mediterranean diet criteria 5 v. 1–4: OR=0·92 (95 % CI 0·69, 1·24) Note: 5 v. 0 means fulfilled ≥5 v. no fulfilled criteria | PTB | Parity, BMI, maternal height, SES, cohabitant status |
| Saunders et al. (2014)( 45 ) | Cohort; 2004–2007; French Caribbean island | 728 (710 with complete data) | 214-item FFQ | Days following delivery | MDS | Nine categories of the Mediterranean diet scale (vegetables, legumes, fruits and nuts, cereals, fish, meat and poultry, dairy products, alcohol, fat) | Adherence to Mediterranean diet: OR=0·9 (95 % CI 0·8, 1·0) | PTB | Maternal place of birth, marital status, pre-pregnancy BMI, maternal education, enrolment site, weight gain during pregnancy, energy intake, maternal smoking during pregnancy |
| Abubakari and Jahn (2016)( 47 ) | Cross-sectional; Ghana | 578 | Fifty-five-item FFQ* | 2nd trimester and 0–1 month post-birth | PCA | Non-health conscious; health conscious | Health conscious diet: OR=0·23 (95 % CI 0·12, 0·45) Non-health conscious diet: OR=1·04 (95 % CI 0·65, 1·67) High DDS: OR=0·10 (95 % CI 0·04, 0·13) | LBW | Gestational age |
| Zerfu et al. ( 46 ) | Cohort; Ethiopia | 432 | Nine-food-group 24 h WDDS | 2nd & 3rd (24–28 weeks) | DDS | Nine food groups | High DDS: RR=2·06 (95 % CI 1·03, 4·11) | LBW | Education, age, height, MUAC, and Hb level |
WDDS, Women Dietary Diversity Score; avg., average; PCA, principal component analysis; DDS, dietary diversity score; RRR, reduced rank regression; MDS, Mediterranean diet score; DASH, Dietary Approaches to Stop Hypertension; SBP, systolic blood pressure; β, regression coefficient; DBP, diastolic blood pressure; PE, pre-eclampsia; GHTN, gestational hypertension; RR, risk ratio; PTB, preterm birth; GDM, gestational diabetes mellitus; LBW, low birth weight; TEI, total energy intake; MUAC, mid-upper arm circumference; PA, physical activity; CPR, C-reactive protein; SES, socio-economic status.
Validated FFQ or recall was used.
Most of the articles (n 15) used an FFQ( 9 , 10 , 19 – 21 , 25 , 35 , 36 , 38 , 40 , 41 , 43 – 45 , 47 ) as the method of dietary assessment, five studies used a 24h recall( 18 , 37 , 39 , 42 , 46 ), and one used a four-day food record( 34 ) to assess the dietary intake. Various types of approaches were used to identify dietary patterns. Most studies applied the a posteriori approach (PCA; n 13)( 9 , 10 , 18 , 19 , 21 , 25 , 34 , 35 , 38 , 41 – 43 , 47 ); seven studies used the a priori method, DDS( 37 , 46 ) or MDS( 20 , 40 , 44 , 45 ) or New Nordic Diet (NND)( 36 ); and one study used the rank reduced regression method( 39 ) to identify the dietary pattern of the women (Table 2). All studies had a positive score for the quality assessment.
The association of dietary patterns with adverse pregnancy outcomes (hypertensive disorders of pregnancy and gestational diabetes mellitus)
Dietary pattern and hypertensive disorders of pregnancy
Six articles( 10 , 25 , 35 – 38 ) assessed the association between dietary pattern and HDP. These articles identified a range of different dietary patterns like healthy, traditional, Mediterranean and Western patterns, and therefore the results could not be pooled in meta-analysis except the healthy dietary pattern.
Healthy dietary pattern. Four studies( 10 , 25 , 36 , 38 ) were available for meta-analysis that reported the association between a healthy dietary pattern with a high intake of fruits, vegetables, whole-grain foods, fish and poultry and HDP. Based on this pooled analysis, study participants who adhered to a healthy dietary pattern were shown to have significantly lower odds of pre-eclampsia (OR=0·78, 95 % CI 0·70, 0·86; I 2=39·0 %, P=0·178; Fig. 2).
Fig. 2.
(colour online) Forest plot for the pooled OR of the association between a healthy dietary pattern and pre-eclampsia. The study-specific OR and 95 % CI are represented by the black diamond and the horizontal line, respectively; the area of the grey square is proportional to the specific-study weight to the overall meta-analysis. The centre of the blue open diamond and the red dashed vertical line represent the pooled OR; and the width of the blue open diamond represents the pooled 95 % CI
However, one( 37 ) cross-sectional study in Tanzania indicated that having a high DDS (OR=5·84; 95 % CI 2·11, 16·15) or a medium DDS (OR=2·54; 95 % CI 1·04, 6·16) was associated with increased odds of gestational hypertension. On the contrary, in a cohort study, no association was observed between gestational hypertension and adherence to a Mediterranean pattern (OR=0·77; 95 % CI 0·53, 1·11) or a traditional pattern (OR=1·3; 95 % CI 0·9, 1·9)( 25 ). Likewise, a cohort study from Brazil( 35 ) revealed that adherence to a healthy dietary pattern did not have an effect on systolic blood pressure (OR=0·82; 95 % CI 0·28, 2·21) or diastolic blood pressure (OR=0·94; 95 % CI 0·18, 1·28).
Western dietary pattern. In a cohort study in Norway( 10 ), a potato and fish dietary pattern (lean fish, cooked potatoes, processed fish; fish burgers, margarine, fish soufflé, meat spread, lean fish and poultry) was not associated with pre-eclampsia (OR=1·00: 95 % CI 0·84, 1·18). Likewise, a cohort study in Brazil( 35 ) reported that adherence to a processed food pattern was not significantly associated with the change in systolic blood pressure (OR=0·76; 95 % CI 0·19, 3·13) and diastolic blood pressure (OR=0·97; 95 % CI 0·30, 3·10) during pregnancy.
Dietary pattern and gestational diabetes mellitus
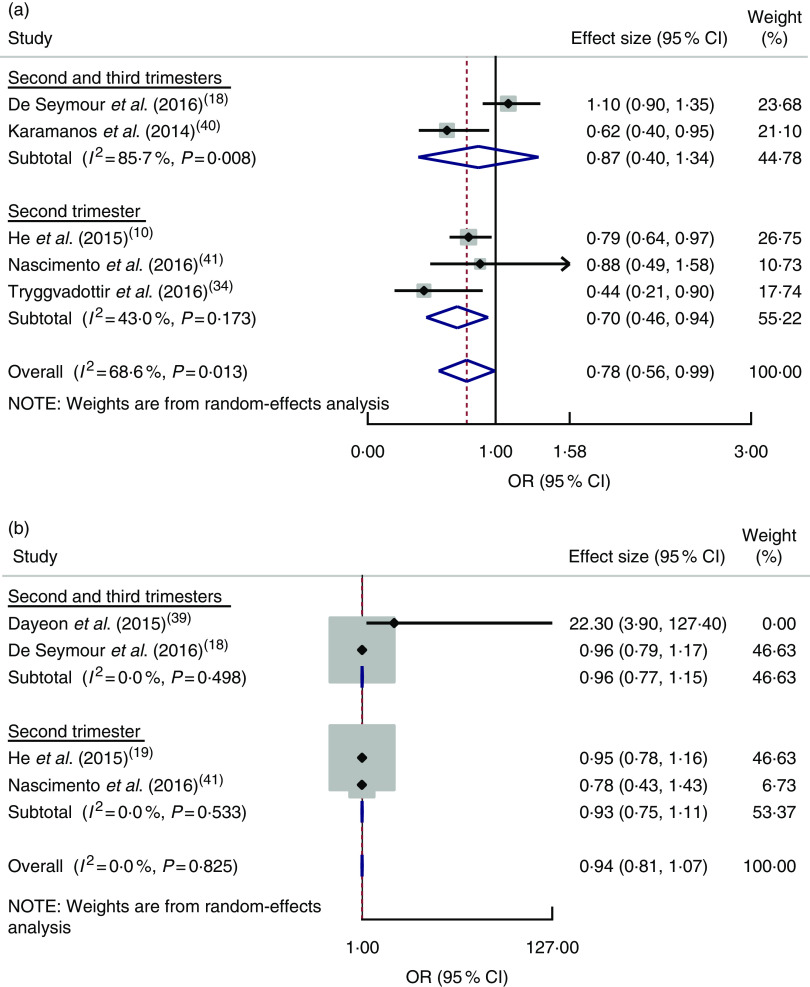
Healthy dietary pattern. Six studies( 18 , 19 , 34 , 39 – 41 ) assessed the effect of dietary patterns on GDM. A cohort study in Singapore( 18 ) indicated that a seafood–noodle-based diet was related with lower odds of GDM (OR=0·74; 95 % CI 0·59, 0·93). However, higher v. lower adherence to a vegetable–fruit–rice-based diet (OR=1·10; 95 % CI 0·90, 1·35) and a pasta–cheese–processed-meat diet (OR=0·96; 95 % CI 0·79, 1·17) was not associated with GDM. Similarly, adherence to a traditional pattern (RR=0·88; 95 % CI 0·49, 1·58) as well as adherence to a mixed pattern (RR=0·93; 95 % CI 0·51, 1·71) was not associated with the incidence of GDM among Brazilian women( 41 ).
The pooled estimate of a healthy dietary pattern on GDM was determined by using five studies( 18 , 19 , 34 , 40 , 41 ). Based on this estimate, women who had higher adherence to a healthy dietary pattern had lower odds of GDM (OR=0·78; 95 % CI 0·56, 0·99), with significant heterogeneity detected between studies (I 2=68·6 %, P=0·013; Fig. 3(a)).
Fig. 3.
(colour online) Forest plot for the pooled OR of the association between gestational diabetes mellitus (GDM) and different dietary patterns, with subgroup analysis regarding period of dietary assessment (second trimester v. both second and third trimesters): (a) association between GDM and healthy dietary pattern; (b) association between GDM and Western dietary pattern. The study-specific OR and 95 % CI are represented by the black diamond and the horizontal line, respectively; the area of the grey square is proportional to the specific-study weight to the overall meta-analysis. The centre of the blue open diamond and the red dashed vertical line represent the pooled OR; and the width of the blue open diamond represents the pooled 95 % CI
Western dietary pattern. Four studies( 18 , 19 , 39 , 41 ) were combined, showing no relationship between adherence to a Western dietary pattern and odds of GDM (OR=0·94; 95 % CI 0·81, 1·07) and no heterogeneity between studies (I 2=0·0 %, P=0·825; Fig. 3(b)).
A cross-sectional survey in the USA( 39 ) and a prospective cohort study in China( 19 ) reported that adherence to dietary patterns of refined grains (OR=4·9; 95 % CI 1·4, 17·0), high nuts, seeds, fat and soyabeans, low milk (OR=7·5; 95 % CI 1·8, 32·3), and sweets and seafood (RR=1·23; 95 % CI 1·02, 1·49) during pregnancy was associated with an increased likelihood of GDM.
The association between dietary patterns and adverse birth outcomes (preterm birth and low birth weight)
Dietary pattern and preterm birth
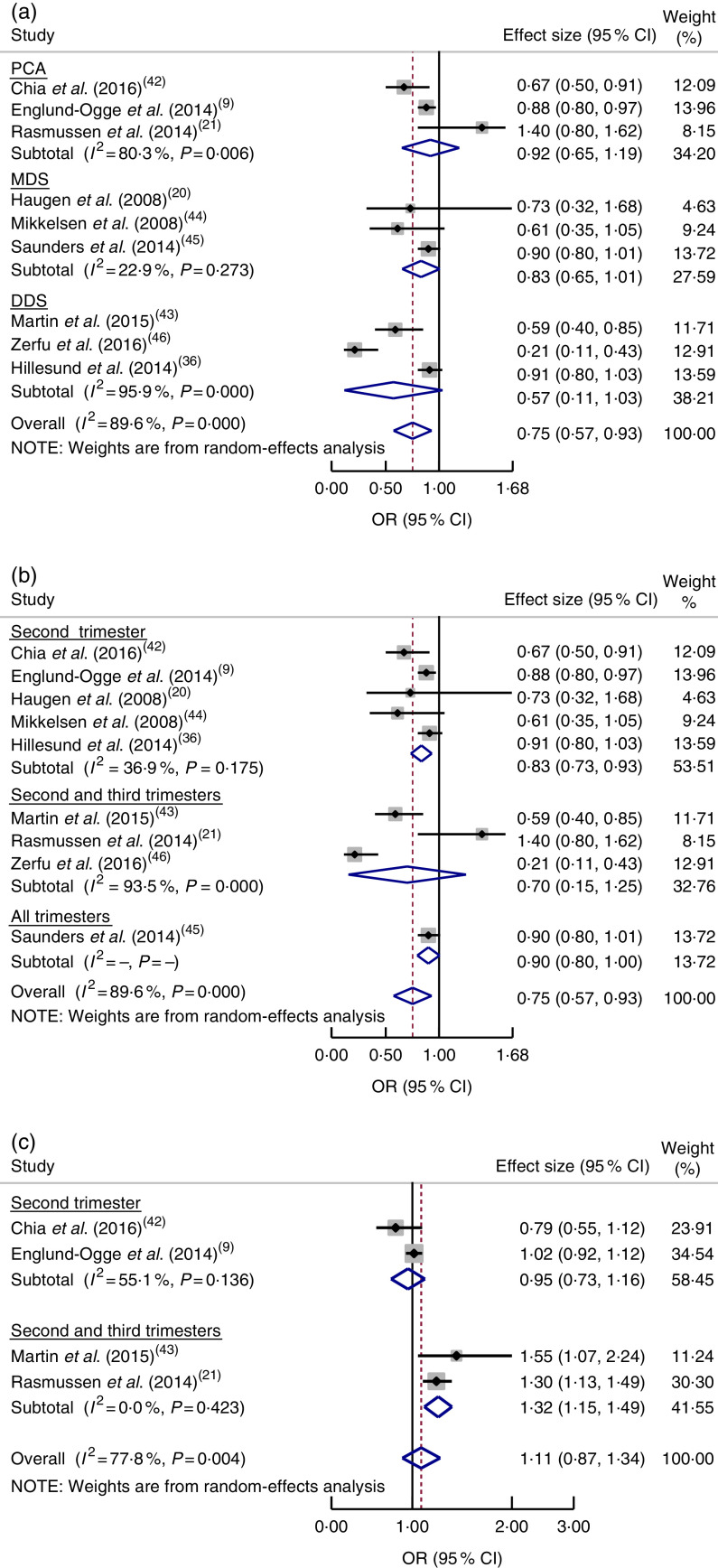
Based on a meta-analysis of nine studies( 9 , 20 , 21 , 36 , 42 – 46 ), women who had good adherence to a healthy dietary pattern were shown to have reduced odds of PTB (OR=0·75; 95 % CI 0·57, 0·93), although significant heterogeneity was observed (I 2=89·6 %, P=0·0001; Fig. 4(a)). Further subgroup analysis indicated a difference in relation to dietary pattern assessment method (MDS, DDS or PCA; P=0·001). There was also a significant subgroup difference regarding dietary assessment period (second trimester or both second and third trimesters; P=0·001; Fig. 4(b)).
Fig. 4.
(colour online) Forest plot for the pooled OR of the association between preterm birth (PTB) and different dietary patterns: (a) association between healthy dietary pattern and PTB, with subgroup analysis in relation to dietary pattern assessment methods (Mediterranean diet score (MDS) v. dietary diversity score (DDS) v. principal component analysis (PCA)); (b) association between healthy dietary pattern and PTB, with subgroup analysis regarding period of dietary assessment (second trimester v. both second and third trimesters v. all trimesters); and (c) association between Western pattern and PTB, with subgroup analysis regarding period of dietary assessment (second trimester v. both second and third trimesters). The study-specific OR and 95 % CI are represented by the black diamond and the horizontal line, respectively; the area of the grey square is proportional to the specific-study weight to the overall meta-analysis. The centre of the blue open diamond and the red dashed vertical line represent the pooled OR; and the width of the blue open diamond represents the pooled 95 % CI
On the other hand, the pooled estimate of four studies( 9 , 21 , 42 , 43 ) showed that a Western dietary pattern did not increase the odds of PTB (OR=1·11; 95 % CI 0·87, 1·34; I 2=77·8 %, P=0·004; Fig. 4(c)). There were subgroup differences between assessing diet in the second trimester and in both the second and third trimesters with respect to risk of PTB (P=0·001). We did not undertake a subgroup analysis regarding study design, as all studies had the same design (cohort).
Dietary patterns and low birth weight
Two studies assessed the effect of dietary pattern during gestation on LBW. A study in Ghana( 47 ) reported that a ‘health conscious’ dietary pattern with a high intake of corn, rice, cassava, yam, fruits, vegetables (carrots, tomatoes, dark green leafy vegetables, cabbage, salad, cucumber), meat and eggs reduced the odds of LBW (OR=0·23; 95 % CI 0·12, 0·45). Similarly, that study reported that women who had a higher DDS were less likely to deliver an LBW baby v. those who had a lower DDS (OR=0·10; 95 % CI 0·04, 0·13). However, a high consumption of sweetened beverages, ice cream, chocolate, energy drinks, milk and local soft drinks, which was labelled the ‘non-health conscious’ dietary pattern, was not significantly associated with LBW (OR=1·04; 95 % CI 0·65, 1·67). Another study in Ethiopia( 46 ) showed that women who had an adequate DDS were less likely to deliver an LBW baby (OR=0·49; 95 % CI 0·24, 0·97).
Discussion
The current systematic review and meta-analysis summarizes evidence focusing on the effects of different dietary patterns during pregnancy on adverse pregnancy (HDP and GDM) and birth (PTB and LBW) outcomes. Globally, adverse pregnancy outcomes and nutritional insufficiencies still remain public health problems( 48 ). Sufficient consumption of energy, protein and micronutrients continues to be essential throughout pregnancy( 49 ).
Hypertensive disorders of pregnancy
The meta-analysis of four studies assessing the healthy diet pattern resulted in pooled estimates suggesting decreased odds of pre-eclampsia. However, other studies reported inconsistent findings on the association between adherence to a healthy dietary pattern and the likelihood of HDP occurrence. A cohort study in the Netherlands( 25 ) revealed that adherence to a Mediterranean diet pattern (vegetables, vegetable oils, pasta, fish, legumes and rice) or a traditional pattern (meat and potatoes) was not associated with gestational hypertension. A cohort study in Brazil( 35 ) revealed that adherence to healthy dietary patterns with high intakes of dairy products, fruit, green vegetables, legumes, fish, cakes, cookies–crackers and tea was not associated with a change in systolic or diastolic blood pressure. On the contrary, a cross-sectional study in Tanzania( 37 ) reported that, compared with a lower score, having a high and medium DDS were associated with increased odds of gestational hypertension.
These inconsistencies might be due to the differences in method and population characteristics. The Tanzanian study was cross-sectional( 37 ) and conducted in a resource-limited setting; however, the other studies were cohort studies conducted in well-resourced settings, except the Brazilian study( 35 ). These studies also assessed dietary intake using a different number of food items and methods. The Tanzanian study applied a 24 h recall method using sixteen food groups, while the studies from Brazil( 35 ) and the Netherlands( 25 ) assessed dietary intake using an eighty-two-item and a 293-item FFQ, respectively.
The healthy diet pattern is in line with dietary guidelines, which suggest consumption of whole grains, vegetables, fruits, potatoes, pasta, cereals, beans, lentils and fish( 50 ). Similarly, the beneficiary influence of diets high in fibre, K, fruits, vegetables, cereals, dark bread and low-fat dairy products was reported as decreasing the odds of pre-eclampsia( 51 ). It was also reported that a lower likelihood of pregnancy-induced hypertension or pre-eclampsia is observed with a diet comprising intake of plant-derived foodstuffs and vegetables( 52 ). The risk of pregnancy complications like pre-eclampsia and LBW has been linked with maternal oxidative stress in the middle of pregnancy( 53 ). Evidence indicates that oxidative stress during pregnancy could be reduced by antioxidant compounds from fruit and vegetables( 54 ). The findings of a multicentre study indicate that oxidative stress could be reduced by sufficient intakes of fruit, vegetables and vitamin C( 54 ). A combination of vitamin C and E might lower the risk of pre-eclampsia( 55 ) through removal of free radicals which may cause oxidative stress in pregnancy( 56 ). Therefore, it could be the cumulative effect of nutrients and their biochemical properties that influence pre-eclampsia risk.
Gestational diabetes mellitus
The meta-analyses of five studies assessing the healthy diet pattern resulted in pooled estimates that indicated reduced odds of GDM, but this was not statistically significant, most likely due to insufficient power, since few articles were included. Additionally, there were inconsistent findings among included studies for meta-analysis regarding the healthy dietary patterns and GDM; three studies showed decreased odds of GDM while the remainder reported no association. This might result from unmeasured factors due to the majority of studies not controlling for all possible confounding factors. For instance, He et al.( 19 ) could not control for parity, energy intake, blood pressure and family history of type 2 diabetes mellitus. Likewise, parity, energy intake and blood pressure were not adjusted for in the other two studies( 40 , 41 ). There was also a difference in assessing dietary intake across these studies, with four studies( 18 , 19 , 40 , 41 ) using a validated FFQ and the other an unvalidated FFQ( 34 ). The dietary intake was assessed at different trimesters of pregnancy, even though there was no significant difference in subgroup analysis based on dietary intake assessment period. This could be a possible explanation for the variations across different studies.
Evidence indicates that pre-pregnancy adherence to a Mediterranean pattern style, with intake of fruit, vegetables, legumes, nuts, fish and cereals, and to the Dietary Approaches to Stop Hypertension (DASH) diet decreases the odds of GDM( 57 , 58 ). Similarly, a clinical trial reported that adhering to the DASH diet, which is high in fruits, vegetables, whole grains and low-fat dairy products, with low amounts of saturated fats, cholesterol and refined grains, reduced the need for insulin treatment( 59 ). Intake of fibre, fruits and cereals reduced the odds of GDM( 60 ).
A cohort study reported that higher odds of GDM was observed with adherence to a Western dietary pattern, which contained higher intake of refined-grain products, processed meat, red meat, French fries and pizza, sweets and desserts( 26 ). However, our pooled estimate of four studies did not show a significant relationship between the Western pattern and GDM occurrence. The possible explanation may be the difference in the dietary pattern investigation methods (two studies used FFQ( 19 , 41 ) and two studies used 24 h recall methods( 18 , 39 )) and population (one study was conducted in a Western population( 39 ) and three studies were done in an Asian population( 18 , 19 , 41 )).
Preterm birth
In the current systematic review, a pooled estimate of nine studies indicated that compared with low adherence, higher adherence to a healthy dietary pattern significantly decreased the odds of PTB. Likewise, the pooled estimates of four studies on vegetable pattern and three studies on the Mediterranean diet indicated decreased odds of PTB, but this was not statistically significant. However, the meta-analysis result of four studies assessing the Western pattern and PTB showed that adherence to the Western pattern was not significantly associated with PTB. There were significant differences in subgroup analysis based on dietary intake assessment period. In two articles, the dietary intake was assessed in the second (13–27 weeks) and third (28–40 weeks) trimesters and reported that the Western dietary pattern significantly increased the odds of PTB. Nevertheless, the other two studies assessed the dietary intake in the second trimester (13–27 weeks) and the Western dietary pattern did not significantly increase the odds of PTB. A previous systematic review of randomized controlled trials revealed that macronutrient dietary interventions have reduced PTB( 61 ).
Low birth weight
Two articles assessed the effect of dietary patterns on LBW. A dietary pattern labelled as ‘health conscious’, characterized by intake of local dishes made from corn flour, vegetables (carrot, tomatoes, dark green leafy vegetables, cabbage, salad, cucumber), rice, meat, a mixture of corn and cassava dough, yam, fruits, water and eggs, was associated with reduced odds of LBW( 47 ). Similarly, women who had a higher DDS were less likely to deliver an LBW baby( 46 , 47 ). However, high consumption of sweetened beverages, ice cream, chocolate, energy drinks, milk and local soft drinks, which was labelled as a ‘non-health conscious’ dietary pattern, showed a significant effect on risk of LBW( 47 ). This is in line with the evidence that suggests the occurrence of LBW has decreased through the consumption of foods and fortified foodstuffs( 62 ).
It is suggested that pregnant women should be advised to eat a diet rich in fruits and vegetables, whole grains, beans, lean meats and fish/seafood, and low in added sugar, red meat and processed foods( 63 ). Intake of vegetables, fruits and legumes improves micronutrient and antioxidant intakes, which could improve pregnancy and birth outcomes( 63 ), particularly at the second trimester since oxidative stress has been shown to reach high levels mid-pregnancy( 64 ). Pregnancy complications and adverse outcomes like pre-eclampsia and PTB have been related to oxidative stress and associated inflammation( 53 ). Antioxidant vitamins (C and E) and essential trace elements (Cu and Zn) through dietary intake of legumes and fruits, which are rich in these nutrients, could decrease this risk( 65 – 67 ). Oxidative stress-linked adverse pregnancy outcomes could be reduced by antioxidants through an intake of vegetables and fruits( 68 ).
Study limitations
The limitations of the present systematic review must be acknowledged. To acquire complete dietary data, most of the articles reviewed applied FFQ followed by diet scores. Nevertheless, there are unavoidable dietary intake misclassifications, which probably bias the degree of detecting real effects. Furthermore, problems of recall bias are also unavoidable because dietary information is dependent on memory. Including articles written only in the English language is another shortcoming of the systematic review. Due to the nature of nutritional research, it is difficult to make all dietary exposures similar for all study subjects. Heterogeneity among studies is a further issue; however, meta-analysis permits the inconsistent findings among studies to be evaluated, even with heterogeneity( 69 ). As all included studies were observational epidemiological studies, the effect of confounders may be another limitation of the current review, despite controlling for some possible confounding factors. Publication bias is always a concern in any review. Reviewed studies that had negative results might not have been submitted for publication, and thus are less likely to have been published.
Conclusion
The evidence presented in the current systematic review indicates the inconsistent associations between different dietary patterns and pregnancy and birth outcomes. Some results in the systemic review show the importance of healthy dietary intake during gestation to improve pregnancy and birth outcomes for the mother and infant, even though inconsistencies have been observed among studies. Essentially, the review suggests that dietary patterns with higher intake of whole grains, vegetables/fruits, legumes and fish are associated with lower likelihood of adverse pregnancy and birth outcomes. However, as the evidence presented herein is inconsistent regarding the association between dietary intake and pregnancy and birth outcomes, caution should be given during advising pregnant women about diet. Since most of the articles included in the review were conducted in resource-rich settings, additional studies need to be done in resource-limited settings to elucidate the impact of limited resources on dietary intake and adverse pregnancy and birth outcomes.
Acknowledgements
Acknowledgements: The authors would like to thank Debbie Booth for her help with developing the literature search strategy and use of Endnote. The authors would also like to thank Dr Ryan O’Neill for his unreserved help in editing the English language. Financial support: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors. Conflict of interest: The authors declare that they have no conflicting interests. Authorship: K.T.K., C.C., D.L. and E.G. formulated the research questions. K.T.K., C.C., D.L. and E.G. designed the study. K.T.K., C.C., D.L., E.G. and T.K.T. carried out the analysis. K.T.K., C.C., D.L., E.G. and T.K.T. analysed the data and wrote the manuscript. Ethics of human subject participation: Not applicable.
References
- 1. American College of Obstetricians and Gynecologists (2013) Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obst Gynecol 122, 1122–1131. [DOI] [PubMed] [Google Scholar]
- 2. Say L, Chou D, Gemmill A et al. (2014) Global causes of maternal death: a WHO systematic analysis. Lancet Glob Health 2, e323–e333. [DOI] [PubMed] [Google Scholar]
- 3. Gemmell L, Martin L, Murphy KE et al. (2016) Hypertensive disorders of pregnancy and outcomes of preterm infants of 24 to 28 weeks’ gestation. J Perinatol 36, 1067–1072. [DOI] [PubMed] [Google Scholar]
- 4. Lawn JE, Gravett MG, Nunes TM et al. (2010) Global report on preterm birth and stillbirth (1 of 7): definitions, description of the burden and opportunities to improve data. BMC Pregnancy Childbirth 10, Suppl. 1, S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Blencowe H, Cousens S, Chou D et al. (2013) Born too soon: the global epidemiology of 15 million preterm births. Reprod Health 10 , Suppl. 1, S2. [DOI] [PMC free article] [PubMed]
- 6. Blencowe H, Cousens S, Oestergaard MZ et al. (2012) National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet 379, 2162–2172. [DOI] [PubMed] [Google Scholar]
- 7. Ramakrishnan U (2004) Nutrition and low birth weight: from research to practice. Am J Clin Nutr, 79, 17–21. [DOI] [PubMed] [Google Scholar]
- 8. Mahumud RA, Sultana M & Sarker AR (2017) Distribution and determinants of low birth weight in developing countries. J Prev Med Public Health 50, 18–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Englund-Ogge L, Brantsaeter AL, Sengpiel V et al. (2014) Maternal dietary patterns and preterm delivery: results from large prospective cohort study. BMJ 348, g1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brantsaeter AL, Haugen M, Samuelsen SO et al. (2009) A dietary pattern characterized by high intake of vegetables, fruits, and vegetable oils is associated with reduced risk of preeclampsia in nulliparous pregnant Norwegian women. J Nutr 139, 1162–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jacques PF & Tucker KL (2001) Are dietary patterns useful for understanding the role of diet in chronic disease? Am J Clin Nutr 73, 1–2. [DOI] [PubMed] [Google Scholar]
- 12. Hu FB (2002) Dietary pattern analysis: a new direction in nutritional epidemiology. Curr Opin Lipidol 13, 3–9. [DOI] [PubMed] [Google Scholar]
- 13. Wirfält E, Drake I & Wallström P (2013) What do review papers conclude about food and dietary patterns? Food Nutr Res 57, 20523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kant AK (2004) Dietary patterns and health outcomes. J Am Diet Assoc 104, 615–635. [DOI] [PubMed] [Google Scholar]
- 15. Food Agriculture and Organization of the United Nations & Family Health International 360 (2016) Minimum Dietary Diversity for Women: A Guide for Measurement. Rome: FAO. [Google Scholar]
- 16. Ocke MC (2013) Evaluation of methodologies for assessing the overall diet: dietary quality scores and dietary pattern analysis. Proc Nutr Soc 72, 191–199. [DOI] [PubMed] [Google Scholar]
- 17. Rifas-Shiman SL, Rich-Edwards JW, Kleinman KP et al. (2009) Dietary quality during pregnancy varies by maternal characteristics in Project Viva: a US cohort. J Am Diet Assoc 109, 1004–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. De Seymour J, Chia A, Colega M et al. (2016) Maternal dietary patterns and gestational diabetes mellitus in a multi-ethnic Asian cohort: the GUSTO study. Nutrients 8, E574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. He JR, Yuan MY, Chen NN et al. (2015) Maternal dietary patterns and gestational diabetes mellitus: a large prospective cohort study in China. Br J Nutr 113, 1292–1300. [DOI] [PubMed] [Google Scholar]
- 20. Haugen M, Meltzer HM, Brantsaeter AL et al. (2008) Mediterranean-type diet and risk of preterm birth among women in the Norwegian Mother and Child Cohort Study (MoBa): a prospective cohort study. Acta Obstet Gynecol Scand 87, 319–324. [DOI] [PubMed] [Google Scholar]
- 21. Rasmussen MA, Maslova E, Halldorsson TI et al. (2014) Characterization of dietary patterns in the Danish national birth cohort in relation to preterm birth. PLoS One 9, e93644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kjøllesdal MKR & Holmboe-Ottesen G (2014) Dietary patterns and birth weight – a review. AIMS Public Health 1, 211–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Agrawal S, Fledderjohann J, Vellakkal S et al. (2015) Adequately diversified dietary intake and iron and folic acid supplementation during pregnancy is associated with reduced occurrence of symptoms suggestive of pre-eclampsia or eclampsia in Indian women. PLoS One 10, e0119120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Borgen I, Aamodt G, Harsem N et al. (2012) Maternal sugar consumption and risk of preeclampsia in nulliparous Norwegian women. Eur J Clin Nutr 66, 920–925. [DOI] [PubMed] [Google Scholar]
- 25. Timmermans S, Steegers-Theunissen RPM, Vujkovic M et al. (2011) Major dietary patterns and blood pressure patterns during pregnancy: the Generation R Study. Am J Obstet Gynecol 205, 337.e1–e12. [DOI] [PubMed] [Google Scholar]
- 26. Zhang C, Schulze MB, Solomon CG et al. (2006) A prospective study of dietary patterns, meat intake and the risk of gestational diabetes mellitus. Diabetologia 49, 2604–2613. [DOI] [PubMed] [Google Scholar]
- 27. Poon AK, Yeung E, Boghossian N et al. (2013) Maternal dietary patterns during third trimester in association with birthweight characteristics and early infant growth. Scientifica (Cairo) 2013, 786409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Academy of Nutrition and Dietetics (2016) Evidence Analysis Manual: Steps in the Academy Evidence Analysis Process. Chicago, IL: Research, International and Strategic Business Development Team, Academy of Nutrition and Dietetics; available at https://www.andeal.org/vault/2440/web/files/2016_April_EA_Manual.pdf [Google Scholar]
- 29. Zhang J & Yu KF (1998) What’s the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA 280, 1690–1691. [DOI] [PubMed] [Google Scholar]
- 30. Higgins JP & Green S (editors) (2011) Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration; available at http://www.handbook.cochrane.org
- 31. Wiest MM, Lee KJ & Carlin JB (2015) Statistics for clinicians: An introduction to logistic regression. J Paediatr Child Health 51, 670–673. [DOI] [PubMed] [Google Scholar]
- 32. Pryor ER (2010) Logistic Regression: A Self‐Learning Text, 3rd ed. New York: Springer. [Google Scholar]
- 33. Borenstein M, Hedges LV, Higgins JPT et al. (2009) Introduction to Meta-Analysis, 1st ed. Chichester: John Wiley & Sons Ltd. [Google Scholar]
- 34. Tryggvadottir EA, Medek H, Birgisdottir BE et al. (2016) Association between healthy maternal dietary pattern and risk for gestational diabetes mellitus. Eur J Clin Nutr 70, 237–242. [DOI] [PubMed] [Google Scholar]
- 35. Eshriqui I, Vilela AAF, Rebelo F et al. (2016) Gestational dietary patterns are not associated with blood pressure changes during pregnancy and early postpartum in a Brazilian prospective cohort. Eur J Nutr 55, 21–32. [DOI] [PubMed] [Google Scholar]
- 36. Hillesund ER, Overby NC, Engel SM et al. (2014) Associations of adherence to the New Nordic Diet with risk of preeclampsia and preterm delivery in the Norwegian Mother and Child Cohort Study (MoBa). Eur J Epidemiol 29, 753–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mwanri AW, Kinabo JL, Ramaiya K et al. (2015) High blood pressure and associated risk factors among women attending antenatal clinics in Tanzania. J Hypertens 33, 940–947. [DOI] [PubMed] [Google Scholar]
- 38. Torjusen H, Brantsaeter AL, Haugen M et al. (2014) Reduced risk of pre-eclampsia with organic vegetable consumption: results from the prospective Norwegian Mother and Child Cohort Study. BMJ Open 4, e006143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dayeon S, Kyung Won L & Won OS (2015) Dietary patterns during pregnancy are associated with risk of gestational diabetes mellitus. Nutrients 7, 9369–9382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Karamanos B, Thanopoulou A, Anastasiou E et al. (2014) Relation of the Mediterranean diet with the incidence of gestational diabetes. Eur J Clin Nutr 68, 8–13. [DOI] [PubMed] [Google Scholar]
- 41. Nascimento GR, Alves LV, Fonseca CL et al. (2016) Dietary patterns and gestational diabetes mellitus in a low income pregnant women population in Brazil – a cohort study. Arch Latinoam Nutr 66, 301–308. [Google Scholar]
- 42. Chia AR, de Seymour JV, Colega M et al. (2016) A vegetable, fruit, and white rice dietary pattern during pregnancy is associated with a lower risk of preterm birth and larger birth size in a multiethnic Asian cohort: the Growing Up in Singapore Towards healthy Outcomes (GUSTO) cohort study. Am J Clin Nutr 104, 1416–1423. [DOI] [PubMed] [Google Scholar]
- 43. Martin CL, Sotres-Alvarez D & Siega-Riz AM (2015) Maternal dietary patterns during the second trimester are associated with preterm birth. J Nutr 145, 1857–1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mikkelsen TB, Osterdal ML, Knudsen VK et al. (2008) Association between a Mediterranean-type diet and risk of preterm birth among Danish women: a prospective cohort study. Acta Obstet Gynecol Scand 87, 325–330. [DOI] [PubMed] [Google Scholar]
- 45. Saunders L, Guldner L, Costet N et al. (2014) Effect of a Mediterranean diet during pregnancy on fetal growth and preterm delivery: results from a French Caribbean Mother–Child Cohort Study (TIMOUN). Paediatr Perinat Epidemiol 28, 235–244. [DOI] [PubMed] [Google Scholar]
- 46. Zerfu TA, Umeta M & Baye K (2016) Dietary diversity during pregnancy is associated with reduced risk of maternal anemia, preterm delivery, and low birth weight in a prospective cohort study in rural Ethiopia. Am J Clin Nutr 103, 1482–1488. [DOI] [PubMed] [Google Scholar]
- 47. Abubakari A & Jahn A (2016) Maternal dietary patterns and practices and birth weight in northern Ghana. PLoS One 11, e0162285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gernand AD, Schulze KJ, Stewart CP et al. (2016) Micronutrient deficiencies in pregnancy worldwide: health effects and prevention. Nat Rev Endocrinol 12, 274–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wakimoto P, Akabike A & King JC (2015) Maternal nutrition and pregnancy outcome – a look back. Nutr Today 50, 221–229. [Google Scholar]
- 50. World Health Organization (2001) Healthy Eating during Pregnancy and Breastfeeding: Booklet for Mothers. Copenhagen: WHO Regional Office for Europe, Nutrition and Food Security.
- 51. Frederick IO, Williams MA, Dashow E et al. (2005) Dietary fiber, potassium, magnesium, and calcium in relation to preeclampsia risk. J Reprod Med 50, 322–344. [PubMed] [Google Scholar]
- 52. Pistollato F, Sumalla Cano S, Elio I et al. (2015) Plant-based and plant-rich diet patterns during gestation: beneficial effects and possible shortcomings. Adv Nutr 6, 581–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hsieh TT, Chen SF, Lo LM et al. (2012) The association between maternal oxidative stress at mid-gestation and subsequent pregnancy complications. Reprod Sci 19, 505–512. [DOI] [PubMed] [Google Scholar]
- 54. Kim H, Hwang JY, Ha EH et al. (2011) Fruit and vegetable intake influences the association between exposure to polycyclic aromatic hydrocarbons and a marker of oxidative stress in pregnant women. Eur J Clin Nutr 65, 1118–1125. [DOI] [PubMed] [Google Scholar]
- 55. Raijmakers MT, Dechend R & Poston L (2004) Oxidative stress and preeclampsia: rationale for antioxidant clinical trials. Hypertension 44, 374–380. [DOI] [PubMed] [Google Scholar]
- 56. Siddiqui IA, Jaleel A, Tamimi W et al. (2010) Role of oxidative stress in the pathogenesis of preeclampsia. Arch Gynecol Obstet 282, 469–474. [DOI] [PubMed] [Google Scholar]
- 57. Tobias DK, Cuilin Z, Chavarro J et al. (2012) Prepregnancy adherence to dietary patterns and lower risk of gestational diabetes mellitus. Am J Clin Nutr 96, 289–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Schoenaker D, Soedamah-Muthu SS & Mishra GD (2016) Quantifying the mediating effect of body mass index on the relation between a Mediterranean diet and development of maternal pregnancy complications: the Australian Longitudinal Study on Women’s Health. Am J Clin Nutr 104, 638–645. [DOI] [PubMed] [Google Scholar]
- 59. Asemi Z, Samimi M, Tabassi Z et al. (2014) The effect of DASH diet on pregnancy outcomes in gestational diabetes: a randomized controlled clinical trial. Eur J Clin Nutr 68, 490–495. [DOI] [PubMed] [Google Scholar]
- 60. Zhang C, Liu S, Solomon CG et al. (2006) Dietary fiber intake, dietary glycemic load, and the risk for gestational diabetes mellitus. Diabetes Care 29, 2223–2230. [DOI] [PubMed] [Google Scholar]
- 61. Gresham E, Bisquera A, Byles JE et al. (2016) Effects of dietary interventions on pregnancy outcomes: a systematic review and meta-analysis. Matern Child Nutr 12, 5–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Gresham E, Byles JE, Bisquera A et al. (2014) Effects of dietary interventions on neonatal and infant outcomes: a systematic review and meta-analysis. Am J Clin Nutr 100, 1298–1321. [DOI] [PubMed] [Google Scholar]
- 63. King JC (2006) Maternal obesity, metabolism, and pregnancy outcomes. Annu Rev Nutr 26, 271–291. [DOI] [PubMed] [Google Scholar]
- 64. Casanueva E & Viteri FE (2003) Iron and oxidative stress in pregnancy. J Nutr 133, 5 Suppl. 2, 1700S–1708S. [DOI] [PubMed] [Google Scholar]
- 65. Mistry HD & Williams PJ (2011) The importance of antioxidant micronutrients in pregnancy. Oxid Med Cell Longev 2011, 841749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Al-Gubory KH, Fowler PA & Garrel C (2010) The roles of cellular reactive oxygen species, oxidative stress and antioxidants in pregnancy outcomes. Int J Biochem Cell Biol 42, 1634–1650. [DOI] [PubMed] [Google Scholar]
- 67. Zhang Y, Zhou H, Perkins A et al. (2017) Maternal dietary nutrient intake and its association with preterm birth: a case–control study in Beijing, China. Nutrients 9, E221. [Google Scholar]
- 68. Asemi Z, Samimi M, Tabassi Z et al. (2013) A randomized controlled clinical trial investigating the effect of DASH diet on insulin resistance, inflammation, and oxidative stress in gestational diabetes. Nutrition 29, 619–624. [DOI] [PubMed] [Google Scholar]
- 69. Higgins JP, Thompson SG, Deeks JJ et al. (2003) Measuring inconsistency in meta-analyses BMJ 327, 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]