Abstract
Background
Neoadjuvant immunotherapy combined with chemotherapy has been used to treat locally advanced non–small cell lung cancer (NSCLC). Several systems have been developed for response evaluation. The aim of this study was to evaluate the predictive value of response evaluation criteria in solid tumors (RECIST) and propose modified RECIST (mRECIST).
Methods
Eligible patients received chemotherapy combined with personalized neoadjuvant immunotherapy. Radical resection was subsequently performed for potentially resectable tumors evaluated by RECIST. The resected specimens were evaluated to determine the response to neoadjuvant therapy.
Results
A total of 59 patients received radical resection following neoadjuvant immunotherapy combined with chemotherapy. According to RECIST, four patients had complete remission, 41 had partial remission, and 14 had progressive disease. Postoperative pathological examination showed 31 patients achieved complete pathological remission, and 13 achieved major pathological remission. The final pathological results were uncorrelated with RECIST assessment (p = 0.086). The ycN stage and pN stage were irrelevant (p < 0.001). When the cutoff of sum of diameters (SoD) is 17%, the Youden's index reached its highest value. A correlation was found between mRECIST and final pathological results. Patients with squamous cell lung cancer showed higher proportions in objective response (OR) (p < 0.001) and complete pathological remission (CPR) (p = 0.001). A shorter time to surgery (TTS) was correlated with a better OR (p = 0.014) and CPR (p = 0.010). The decrease in SoD was correlated with better OR (p = 0.008) and CPR (p = 0.002).
Conclusions
mRECIST was effective for patient selection for radical resection after neoadjuvant immunotherapy with advanced NSCLC. Two modifications were suggested for RECIST: (1) the cutoff value was adjusted to 17% for partial remission. (2) Changes in lymph nodes on computed tomography were eliminated. A shorter TTS, a larger decrease in SoD and squamous cell lung cancer (vs. adenocarcinoma) were correlated with better pathological responses.
Keywords: neoadjuvant immunotherapy, non–small cell lung cancer, response evaluation criteria in solid tumors
A modified response evaluation criteria in solid tumors (mRECIST) was effective for patient selection for radical resection after neoadjuvant immunotherapy with advanced non–small cell lung cancer. Two modifications were suggested for RECIST: (1) the cutoff value was adjusted to 17% for partial remission; and (2) changes in lymph nodes on computed tomography were eliminated.
INTRODUCTION
Programmed death‐ligand 1 (PD‐L1) immune checkpoint inhibitors have profoundly changed the treatment paradigm for patients with advanced non–small cell lung cancer (NSCLC). It has been the first‐line treatment for most patients with metastatic NSCLC and negative driver genes. 1 In recent years, it has been used as neoadjuvant therapy for locally advanced NSCLC patients.
The accurate assessment of the response to neoadjuvant therapy is important for making treatment decisions. Several systems, including the response evaluation criteria in solid tumors (RECIST), immune‐related RECIST (irRECIST), immunotherapy‐modified RECIST (iRECIST), positron emission computed tomography response criteria in solid tumors (PERCIST), immunotherapy‐modified PERCIST (imPERCIST), etc., have been developed for response evaluation. However, the predictive value of these systems remains unclear. Here, we report 59 patients with advanced NSCLC who underwent radical resection after neoadjuvant immunotherapy. RECIST was used for response assessment. The aim of this study was to evaluate the predictive value of RECIST and propose modified RECIST (mRECIST) based on our findings.
METHODS
Patients
We retrospectively analyzed the clinical data of patients with advanced NSCLC who were treated at Peking Union Medical College Hospital between December 26, 2019 and October 1, 2022. The inclusion criteria were as follows: age of at least 18 years; NSCLC confirmed by fiber optic bronchoscopy or biopsy; clinical stage II‐IV (8th edition of the tumor‐node‐metastasis [TNM] staging system of the American Joint Committee on Cancer) 2 ; tumors that were unresectable or potentially resectable, but not suitable for immediate surgery; no variation in specific driver genes; no prior cancer treatment; Eastern Cooperative Oncology Group score 0–2; and no organ dysfunction. The exclusion criteria were as follows: immunodeficiency; ongoing systemic immunosuppressive therapy; active autoimmune or infectious disease; or other malignancies. Eligible patients signed the informed consent form before treatment.
Clinical pathway
The detailed clinical pathway for those patients was described in our previous work. 3 Chest computed tomography (CT) was performed every other cycle for tumor assessment, and the results were classified according to RECIST version 1.1 4 as complete remission (CR), partial remission (PR), stable disease (SD), or progressive disease (PD). Objective response (OR) included PR and CR. After neoadjuvant therapy was administered, we identified patients for radical resection based on the following criteria: (1) PR or CR with the possibility of radical resection (i.e., no signs of tumor invasion of any major vessel or the diaphragm, heart, trachea, or carina); or (2) SD or PD regarded as pseudoprogression or potentially resectable. This study was approved by the Ethics Committee of Peking Union Medical College Hospital (S‐K2062) and conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Statistical analysis
The primary end points were major pathological remission (MPR, defined as ≤10% of remaining viable tumor cells on postoperative pathological examination) and complete pathological remission (CPR, defined as tumor regression without residual tumor on pathological examination). 5 , 6 The Mann–Whitney U test was used to compare differences between groups. A receiver operating characteristic (ROC) curve was used to derive an optimal cutoff value. A two‐sided p < 0.05 was considered statistically significant. Statistical analyses were performed using SPSS 23.0 software (IBM).
RESULTS
Patients
A total of 62 patients underwent radical resection after neoadjuvant immunotherapy combined with chemotherapy. Three patients were excluded because they were enrolled in randomized clinical trials that were double‐blinded. Baseline demographics are shown in Table 1. Among the cases, 46 patients (78.0%) were men, and 48 patients (81.4%) were former smokers. Pretreatment biopsy showed squamous cell carcinoma in 48 patients (81.4%). Most of the patients (39 [66.1%]) received paclitaxel + cisplatin + pembrolizumab (PC + K). Thirty‐three patients (48.6%) received 2–4 cycles of immunotherapy. The median time to surgery (TTS, refers to the time interval from the last dose of neoadjuvant immunotherapy to surgery) was 36.5 days.
TABLE 1.
Clinical characteristics of the total cohort
| Characteristic | All, No. (%) | OR, No. (%) | CPR, No. (%) | |
|---|---|---|---|---|
| Gender | Male | 46 (78.0) | 35 (79.5) | 26 (83.9) |
| Female | 13 (22.0) | 9 (20.5) | 5 (16.1) | |
| Median age, years [range] | 63 [39–76] | 63 [39–76] | 63 [51–76] | |
| Preoperative stage | IIB | 2 (3.4) | 2 (45.5) | 2 (6.5) |
| IIIA | 34 (57.6) | 25 (56.8) | 18 (58.1) | |
| IIIB | 21 (35.6) | 15 (34.1) | 10 (32.3) | |
| IIIC | 2 (3.4) | 2 (45.5) | 1 (3.2) | |
| Histology | Squamous cell carcinoma | 48 (81.4) | 41 (93.2) | 30 (96.8) |
| Adenocarcinoma | 10 (16.9) | 2 (45.5) | 1 (3.2) | |
| Adenosquamous carcinoma | 1 (1.7) | 1 (22.7) | 0 | |
| Smoking history (smoking index) | Nonsmokers | 11 (18.6) | 6 (13.6) | 3 (9.7) |
| ≤400 | 11 (18.6) | 9 (20.5) | 7 (22.6) | |
| 401–799 | 11 (18.6) | 8 (18.2) | 6 (1 9.4) | |
| ≥800 | 26 (44.1) | 21 (47.7) | 15 (48.4) | |
| Resected lobe | RUL | 18 (30.5) | 12 (27.3) | 9 (29.0) |
| RML | 2 (3.4) | 0 | 0 | |
| RLL | 7 (11.9) | 5 (11.4) | 3 (9.7) | |
| LUL | 16 (27.1) | 15 (34.1) | 12 (38.7) | |
| LLL | 5 (8.5) | 5 (11.4) | 4 (12.9) | |
| RML + RLL | 11 (18.6) | 7 (15.9) | 3 (9.7) | |
Abbreviations: CPR, complete pathological remission; LLL, left lower lobe; LUL, left upper lobe; OR, objective response; RLL, right lower lobe; RML, right middle lobe; RUL, right upper lobe.
Neoadjuvant therapy response
Ten patients (16.9%) had immune‐related adverse events (irAEs), including rash (n = 6), immune‐related thyroiditis (n = 2), immune‐related enteritis (n = 1) and immune‐related pneumonia (n = 2). Only one grade 3–4 adverse reaction was observed (see our previous case report). 7 Two patients’ surgeries were delayed because of irAEs.
After neoadjuvant therapy, RECIST showed CR in four cases (6.8%), PR in 41 cases (83.7%), SD in 14 cases (23%), and no PD. The sum of diameters (SoD, refers to the sum of the longest diameter and the perpendicular diameter of the tumor's largest cross‐sectional area seen on CT scans) of the tumor was reduced by a median of 40.2% (range, −13.6% to 100%). Reductions in T and TNM stage were observed in 32 (54.2%) and 24 (40.7%) patients, respectively. The N stage was reduced in 12 of 44 patients (27.3%) who underwent an initial assessment of lymph node metastasis.
All patients underwent radical resection after neoadjuvant therapy, including 53 (89.8%) video‐assisted thoracoscopic surgeries, four (6.8%) open surgeries, and two (3.4%) conversion to thoracotomy surgeries. Eight patients (13.6%) received sleeve resection, and three (5.1%) received bronchoplasty. Eleven patients (18.6%) received multilobar resection. Thirty‐nine patients (66.1%) had pleural adhesions. Postoperative complications included atrial fibrillation (3 [5.1%]), poor lung recruitment (1 [1.7%]), continuous air leak (2 [3.4%]) and unilateral vocal cord paralysis (1 [1.7%]).
Pathological examination
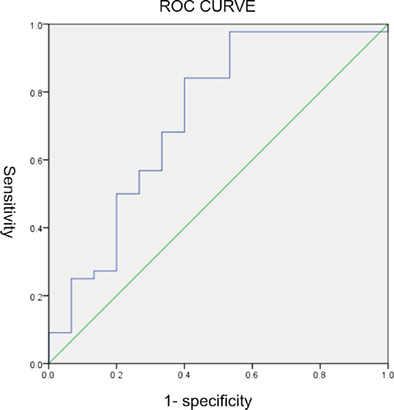
Postoperative pathological examination showed that 31 patients (52.5%) achieved CPR, and 13 patients (22.0%) achieved MPR. The objective response rate (ORR) was 74.6%. A ROC curve (for ORR) was calculated to derive an optimal cutoff value for the SoD (Figure S1). When the cutoff was 17%, the Youden's index reached its highest value. The sensitivity and specificity for OR were 97.7% and 46.7%, respectively (area under the curve [AUC] was 0.726 [95% CI, 0.560–0.891], p = 0.009). When the cutoff was 30% (RECIST), the sensitivity and specificity for OR was 84.1% and 60.0%, respectively. When the cutoff was 47%, the sensitivity and specificity for CPR were 54.8% and 82.1%, respectively (the Youden's index reached the highest, area under the curve [AUC] was 0.730 [95% CI, 0.602–0.859], p = 0.002). ORR were uncorrelated with RECIST assessment (Table S1) (p = 0.086). The ycN (therapy‐clinical‐N) stage and pN (pathologic‐N) stage were irrelevant (p < 0.001).
An mRECIST was proposed according to the results above. Two modifications were applied: (1) the cutoff value was adjusted to 17% for PR; and (2) changes in lymph nodes on CT were eliminated from the criteria. Therefore, it showed CR in four cases (6.8%), PR in 46 cases (78.0%), SD in nine cases (15.3%), and no PD. A correlation was found between mRECIST and final pathological results (Table S2) (p = 0.001).
Table 2 shows the relationships between risk factors and pathological response. No correlations were found between sex, age, smoking history, immunotherapy cycles, reduction in TNM stage (yc vs. clinical (c)), irAEs, and pathological response. Patients with squamous cell lung cancer showed higher proportions in OR (p < 0.001) and CPR (p = 0.001). A shorter TTS was correlated with a better OR (p = 0.014) and CPR (p = 0.010). The decrease in SoD was correlated with better OR (p = 0.008) and CPR (p = 0.002).
TABLE 2.
Relationships between risk factors and pathological response
| OR | CPR | |
|---|---|---|
| Gender | 0.619 | 0.254 |
| Age, years | 0.513 | 0.341 |
| Smoking | 0.249 | 0.120 |
| Pathology | *<0.001 | *0.001 |
| TTS | *0.014 | *0.010 |
| SoD | *0.011 | *0.001 |
| Pleural adhesion | 0.066 | 0.167 |
| Immunotherapy cycles | 0.118 | 0.200 |
| T reduction (yc vs. c) | 0.499 | 0.538 |
| N reduction (yc vs. c) | 0.828 | 0.626 |
| TNM reduction (yc vs. c) | 0.205 | 0.074 |
| irAE | 0.223 | 0.229 |
Abbreviations: CPR, complete pathological remission; irAE, immune‐related adverse events; OR, objective response; SoD, sum of diameters; TNM, tumor‐node‐metastasis; TTS, time to surgery.
p < 0.05.
DISCUSSION
In this study, we retrospectively analyzed 59 patients with advanced NSCLC who underwent radical resection after neoadjuvant immunotherapy. Our cohort used RECIST to select patients. Although the final pathological results were uncorrelated with RECIST assessment, our cohort achieved favorable surgical results and satisfactory pathological remission (ORR = 74.6%). Furthermore, we found: (1) the SoD on CT is an effective predictor of pathological response. Our cohort revealed that when screening for OR, the cutoff should be 17% (with 97.7% sensitivity and 46.7% specificity); when screening for CPR, the cutoff should be 47% (with 54.8% sensitivity and 82.1% specificity). (2) Lymph node grade is an unreliable indicator for patient selection, as ycN stage and pN stage are irrelevant (p < 0.001). We therefore suggest making two modifications to RECIST: (1) the cutoff value was decreased to 17% for PR. (2) Changes in lymph nodes on CT were eliminated. A stronger relationship between preoperative evaluation and pathological findings was observed when using mRECIST (Table S2).
Several criteria have been proposed for response evaluation. RECIST, which is based on CT scans, is an important predictor of OS in NSCLC. 8 As changes in inflammation and interstitial or fibrotic components of tumors may affect the CT results, the predictive value of RECIST may be low. 9 , 10 Therefore, some immunotherapy‐modified classifications were proposed, aiming to avoid reported pseudoprogression as disease progression (0%–5.4%). However, the challenge for these classifications is that they may miss true disease progression. 11 , 12 In our cohort, there were 14 got SD. The lesions of these patients did not shrink after 3 to 10 weeks of observation, suggesting that irRECIST would not provide additional surgical opportunity for these patients. Considering that the ORR is as high as 57.1% (8/14), surgery should be an option for these patients. PERCIST is another potential tool for patient selection that is based on positron emission computed tomography (PET) scans. Current evidence regarding PERCIST is limited and controversial, as a favorable response may also present with an increase in metabolic activity of the lesions. 13 , 14 Therefore, it is suggested that mRECIST is an effective criterion for current practice.
We also studied the correlations between risk factors and pathological response. It was demonstrated that a shorter TTS, larger decrease in SOD and squamous cell lung cancer (vs. adenocarcinoma) were correlated with better responses. Other factors, including sex, age, smoking history, immunotherapy cycles, reduction in TNM stage (yc vs. c), and irAEs, were not correlated. It is difficult to determine the optimal TTS, as clinicians should balance the long tail effect of immunotherapy and the progression of tumors. The TTS in CheckMate 159, LCMC3, and TOP1501 was 1–5 weeks, 9 , 15 whereas in NADIM, NCT02716038 and NEOSTAR, it was 3–7 weeks. 16 In our cohort, patients who achieved OR had a median TTS of 33.5 days, significantly shorter than those who did not (45 days). We thus advise not exceedingly lengthen the TTS.
The limitation of this retrospective study is that patients who showed poor response to neoadjuvant immunotherapy and failed to receive surgery were not included because of a lack of follow‐up. Further, because the follow‐up period was relatively short, we did not have sufficient data on survival outcomes. A prospective design and better follow‐up are needed in future work. Although our study suggests that lymph node grade may not be a reliable indicator for patient selection, further research with larger sample sizes is needed to confirm these findings and determine whether changes in lymph nodes on CT should be eliminated as a criterion for patient selection.
CONCLUSION
Although the final pathological results were uncorrelated with RECIST assessment, mRECIST was effective for patient selection for radical resection after neoadjuvant immunotherapy with advanced NSCLC. Two modifications were suggested for RECIST: (1) the cutoff value was adjusted to 17% for PR; and (2) changes in lymph nodes on CT were eliminated. A shorter TTS, a larger decrease in SoD and squamous cell lung cancer (vs. adenocarcinoma) were correlated with better pathological responses.
AUTHOR CONTRIBUTIONS
Conception and design: Hongsheng Liu. Administrative support: Yingzhi Qin, Ji Li, Ying Jiang, Wuying Cheng, and Ke Hu. Provision of study materials or patients: Shanqing Li, Mengzhao Wang, Yan Xu, Jing Zhao, and Minjiang Chen. Collection and assembly of data: Yuan Xu and Dongjie Ma. Data analysis and interpretation: Yuan Xu. Manuscript writing: All authors. Final approval of manuscript: All authors.
CONFLICT OF INTEREST STATEMENT
The authors have no conflicts of interest to declare.
Supporting information
Figure S1. The receiver operating characteristic (ROC) curve for the objective response rate (ORR).
Table S1. The fourfold table of pathological results and RECIST assessment.
Table S2. The fourfold table of pathological results and modified RECIST assessment
ACKNOWLEDGMENTS
This research is supported by National High Level Hospital Clinical Research Funding (2022‐PUMCH‐B‐012).
Xu Y, Ma D, Qin Y, Li S, Li J, Jiang Y, et al. Is response evaluation criteria in solid tumors (RECIST) effective in patient selection for radical resection after neoadjuvant immunotherapy with advanced NSCLC? Thorac Cancer. 2023;14(17):1635–1639. 10.1111/1759-7714.14909
Yuan Xu and Dongjie Ma contributed equally to the article.
REFERENCES
- 1. Friedlaender A, Naidoo J, Banna GL, Metro G, Forde P, Addeo A. Role and impact of immune checkpoint inhibitors in neoadjuvant treatment for NSCLC. Cancer Treat Rev. 2022;104:102350. [DOI] [PubMed] [Google Scholar]
- 2. Goldstraw P, Chansky K, Crowley J, Rami‐Porta R, Asamura H, Eberhardt WEE, et al. The IASLC lung cancer staging project: proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. J Thorac Oncol. 2016;11(1):39–51. [DOI] [PubMed] [Google Scholar]
- 3. Ma D, Xu Y, Qin Y, Li S, Li J, Jiang Y, et al. Neoadjuvant immunotherapy followed by surgery with curative intent in 35 patients with advanced NSCLC: the retrospective experiences of a multidisciplinary team. Ann Transl Med. 2022;10(10):609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–47. [DOI] [PubMed] [Google Scholar]
- 5. Yuan RY, Yip PK, Liu HM, Hwang BS, Chen RC. The value of duplex and continuous wave Doppler sonography for evaluation of the extracranial vertebral arteries: a prospective comparison with angiography. Chin Med J. 1994;53(1):42–8. [PubMed] [Google Scholar]
- 6. Hellmann MD, Chaft JE, William WN Jr, Rusch V, Pisters KM, Kalhor N, et al. Pathological response after neoadjuvant chemotherapy in resectable non‐small‐cell lung cancers: proposal for the use of major pathological response as a surrogate endpoint. Lancet Oncol. 2014;15(1):e42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Xu Y, Lyu X, Qin Y, Ma D, Wang M, Shi J, et al. Multi‐organs perioperative immune‐related adverse events and postoperative bronchial anastomotic fistula in a patient receiving neoadjuvant immunotherapy with NSCLC. Thorac Cancer. 2022;13(16):2340–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Travis WD, Dacic S, Wistuba I, Sholl L, Adusumilli P, Bubendorf L, et al. IASLC multidisciplinary recommendations for pathologic assessment of lung cancer resection specimens after neoadjuvant therapy. J Thorac Oncol. 2020;15(5):709–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Forde PM, Chaft JE, Smith KN, Anagnostou V, Cottrell TR, Hellmann MD, et al. Neoadjuvant PD‐1 blockade in Resectable lung cancer. N Engl J Med. 2018;378(21):1976–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. William WJ et al. Computed tomography RECIST assessment of histopathologic response and prediction of survival in patients with resectable non‐small‐cell lung cancer after neoadjuvant chemotherapy. J Thorac Oncol. 2013;8(2):222–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tazdait M, Mezquita L, Lahmar J, Ferrara R, Bidault F, Ammari S, et al. Patterns of responses in metastatic NSCLC during PD‐1 or PDL‐1 inhibitor therapy: comparison of RECIST 1.1, irRECIST and iRECIST criteria. Eur J Cancer. 2018;88:38–47. [DOI] [PubMed] [Google Scholar]
- 12. Hicks RJ, Iravani A, Sandhu S. (18)F‐fluorodeoxyglucose positron emission tomography/computed tomography for assessing tumor response to immunotherapy in solid tumors: melanoma and beyond. PET Clin. 2020;15(1):11–22. [DOI] [PubMed] [Google Scholar]
- 13. Humbert O, Cadour N, Paquet M, Schiappa R, Poudenx M, Chardin D, et al. (18)FDG PET/CT in the early assessment of non‐small cell lung cancer response to immunotherapy: frequency and clinical significance of atypical evolutive patterns. Eur J Nucl Med Mol Imaging. 2020;47(5):1158–67. [DOI] [PubMed] [Google Scholar]
- 14. Galldiks N, Kocher M, Ceccon G, Werner JM, Brunn A, Deckert M, et al. Imaging challenges of immunotherapy and targeted therapy in patients with brain metastases: response, progression, and pseudoprogression. Neuro Oncol. 2020;22(1):17–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shen D, Wang J, Wu J, Chen S, Li J, Liu J, et al. Neoadjuvant pembrolizumab with chemotherapy for the treatment of stage IIB‐IIIB resectable lung squamous cell carcinoma. J Thorac Dis. 2021;13(3):1760–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liang W, Cai K, Chen C, Chen H, Chen Q, Fu J, et al. Expert consensus on neoadjuvant immunotherapy for non‐small cell lung cancer. Transl Lung Cancer Res. 2020;9(6):2696–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. The receiver operating characteristic (ROC) curve for the objective response rate (ORR).
Table S1. The fourfold table of pathological results and RECIST assessment.
Table S2. The fourfold table of pathological results and modified RECIST assessment


