Abstract
Objective
In the Middle East and North Africa region, the nutrition transition has resulted in drastic increases in excess adiposity, particularly among women, while some types of undernutrition remain prevalent, especially among pre-school children. We assessed the magnitude, nature and associated factors of the within-household co-occurrence of anaemia in children and excess adiposity in mothers.
Design
Cross-sectional survey using stratified two-stage random cluster sampling to survey households with women aged 20–49 years. BMI≥25·0 kg/m2 defined overweight and BMI≥30·0 kg/m2 obesity, while anaemia for children was defined as Hb<110 g/l. The associations between child anaemia and mother excess adiposity, and sociodemographic and lifestyle factors were estimated by multinomial regression.
Setting
Greater Tunis area, Tunisia, in 2009–2010.
Subjects
Children aged 6–59 months living with their 20–49-year-old mothers (437 child–mother pairs).
Results
The most prevalent double burden of malnutrition in child–mother pairs by far was the anaemic child and overweight mother (24·4 %; 95 % CI 20·1, 29·3 %). A significant proportion of pairs were anaemic child and obese mother (14·4 %; 95 % CI 11·0, 18·5 %). The co-occurrence of anaemia in child and excess adiposity in mother was neither synergetic nor antagonistic (P=0·59 and 0·40 for anaemia–overweight and anaemia–obesity, respectively). This double burden was more frequent among child–mother pairs with younger children, with mothers of higher parity and higher energy intakes.
Conclusions
The high prevalence of anaemic child and overweight or obese mother requires special attention e.g. through interventions which simultaneously target both types of malnutrition within the same household.
Keywords: Overweight, Anaemia, Double burden of malnutrition, Mother–child pairs, Middle East and North Africa
As a consequence of global socio-economic changes and the epidemiological transition, in recent decades middle-income countries have experienced drastic increases in overweight and obesity as well as non-communicable diseases such as diabetes, hypertension and CVD. The Middle East and North Africa (MENA) region has been affected by this evolution and available data indicate that overweight and obesity are now dramatically on the rise, particularly in urban settings; moreover, this increase is especially detrimental to women, as the prevalence of female obesity ranges from 30 to 45 % in most countries of the region( 1 ). Patterns of diet and physical activity that have shifted towards ‘Westernized’ diets characterized by increased consumption of high-energy, nutrient-poor foods and increased sedentary activity are major factors of that increase in overweight/obesity, in the framework of the nutrition transition( 2 ). But these lifestyle factors are themselves determined at a higher level of causation by sociocultural and socio-economic factors either at household or more aggregated levels( 3 ).
Notwithstanding the drastic increase of obesity in the MENA region, child undernutrition, especially in relation to micronutrient deficiencies, remains a public health challenge. Indeed, one of the most prevalent nutrition-related deficiencies in this region is anaemia, which concerns one in two children under 5 years of age( 4 ). Childhood anaemia is of special concern due to its adverse consequences on cognitive development, with long-term impacts on social and economic development in adulthood( 5 ). Beyond the coexistence of these contrasting forms of malnutrition at region or country level, this double burden of malnutrition can also occur at individual level, such as the coexistence of stunting or micronutrient deficiencies with overweight or obesity within the same individual, either children( 6 ) or adults( 7 , 8 ). An intermediate type of double burden can also manifest at household level, e.g. the coexistence of undernourished and overweight members within the same household( 9 ). Most studies of that a priori paradoxical dual burden have focused on mother–child pairs because a mother and her child are in close contact and share the same available resources, which is not necessarily true for other household members( 10 ). The most studied combination is the coexistence of an overweight/obese mother paired with a stunted child( 10 – 14 ). However, stunting among children under 5 years of age is in decline in the MENA region while, at the same time, anaemia is still prevalent( 4 ). Beyond estimates of the prevalence and the associated factors, an important issue for a better understanding of the public health issues related to such double burdens at individual or household level is whether the co-occurrence of the two types of malnutrition is synergetic or antagonistic or independent( 15 , 16 ); all this requires collecting the different types of malnutrition at the level during the same survey.
Tunisia is a typical country of the MENA region which has undergone a rapid epidemiological and nutrition transition, and today features high prevalences of non-communicable diseases including overweight and obesity, especially in urban areas( 7 , 17 ). Although the prevalence of stunting among children aged <5 years is as low as 6·2 % in Tunisia( 18 ), as in other countries of the region, anaemia is still also a public health problem in this age category( 19 ). Therefore the objectives of the present work, carried out among <5 year child and mother pairs in urban areas in Tunisia, were to: (i) assess the magnitude of the double burden, with a special focus on anaemic children and overweight mothers; (ii) understand the interrelationships between the two types of malnutrition; and (iii) investigate anthropometric, socio-economic and behavioural (physical activity and diet quality) associated factors.
Participants and methods
Participants
Study area
Tunisia is a North African country of about 11 million inhabitants( 20 ) and two-thirds of the population is urban. In the last decades, Tunisia has undergone a steady and rapid economic development and currently features an upper middle level of development (ranked 81th out of 169 countries on the Human Development Index composite scale in 2010)( 21 ). The study focused on a mainly urban area around the capital city (Greater Tunis) which comprises a quarter of the country’s population and is also the most developed region.
Sampling
The cross-sectional survey was carried out from March 2009 to January 2010 during the ObeMaghreb research project( 7 ), for which the target population was 20–49-year-old, non-pregnant women. A random stratified two-stage cluster sampling design surveyed 1520 households from seventy-six census districts: all persons aged 6 months to 49 years were included. Our analyses used the sub-sample of child–mother pairs (children aged 6–59 months, living with their mothers).
Measurements and derived variables
Anthropometry
Anthropometric measurements of the children and mothers followed standard procedures( 22 ). Length (for children <2 years) or height was assessed to the nearest 1 mm using a length board or a stadiometer (Person-check®, Kirchner & Wilhelm, Germany). Weight was measured to the nearest 10 g using baby scales (Detecto®, USA) for children under 1 year; otherwise to 100 g using calibrated scales (Detecto®, USA). For children, wasting was defined as weight-for-height Z-score <−2, overweight as BMI-for-age Z-score > 2 and obesity as BMI-for-age Z-score>3( 23 ). Stunting was defined as a height-for-age Z-score<−2( 23 ). For mothers, overweight was defined as BMI≥25·0 kg/m2 and obesity as BMI≥30·0 kg/m2 ( 24 ).
Anaemia
Fasting venous blood samples (2 ml) were collected in tubes with EDTA. All samples were kept at 4–5°C and sent to the Clinical Biology Laboratory of the National Institute of Nutrition and Food Technology in Tunis for Hb analysis using a T540 counter (Beckman Coulter, UK). Anaemia was defined as Hb <110 g/l for children and as Hb <120 g/l for mothers( 25 ).
Double burden of malnutrition in child–mother pairs
We considered several forms of child–mother double burden of malnutrition: anaemic child and overweight or obese mother; overweight or obese child and anaemic mother; stunted child and overweight or obese mother; and wasted child and overweight or obese mother. These were coded as variables with four categories, e.g. for the ‘anaemic child and overweight mother’ double burden: (i) anaemic child and overweight mother (
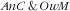 ); (ii) anaemic child and not overweight mother (
); (ii) anaemic child and not overweight mother (
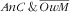 ); (iii) not anaemic child and overweight mother (
); (iii) not anaemic child and overweight mother (
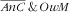 ); and (iv) not anaemic child and not overweight mother (
); and (iv) not anaemic child and not overweight mother (
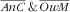 ).
).
Demographic and socio-economic characteristics
Data on age, parity, education level and professional occupation were collected by questionnaire. The birth weight of the child was assessed by the health booklet. The wealth of the household was assessed using an asset-based proxy and classified as low, medium or high according to tertiles of this index( 7 , 26 ).
Dietary intake
Dietary intake was assessed by using the 3d record method for mothers and 24 h recall for children( 27 ). Quantities were estimated using calibrated household utensils (such as cups, spoons, bowls, cups, glasses) and a portion-size guide. For mothers, energy intake was derived from a specific Tunisian food consumption database( 28 ) using the Food Processor Software© version 8.3( 29 ) and divided into tertiles. For children, the dietary diversity score (DDS) was calculated by counting the different food groups consumed the day before the survey, applying a 10 g minimum intake for all food groups( 30 ). For this purpose, we choose the eleven following food groups: (i) cereals and tubers; (ii) dry and green legumes; (iii) fresh and processed vegetables; (iv) fruits and fruit juices; (v) milk and dairy products; (vi) meat and poultry; (vii) fish; (viii) eggs; (ix) sugar and sweet products; (x) oils and fats; and (xi) condiments. The DDS was then divided into tertiles which resulted in the categories: low (1–5), medium (6–7) and high (8–11).
Physical activity
Physical activity of the mother during the 7d preceding the survey was assessed using a validated frequency questionnaire( 31 ). Total energy expenditure was calculated based on time and energy cost of various activities( 32 ) and BMR was computed from predictive equations( 33 ). Physical activity level (PAL) was calculated as the ratio of total energy expenditure to BMR, and PAL <1·70 characterized a sedentary lifestyle( 34 ).
Data management and statistical analysis
Data were entered with Epidata software version 3.1 and were validated by double entry and standard quality check procedures. Data management and statistical analysis were performed using the statistical software package Stata version 14.0. The type I error risk was set at 0·05 for all analyses. All results are expressed as estimates and sampling design-based se or 95 % CI. Descriptive results are expressed as means for interval variables, and as proportions for categorical variables. Based on a conceptual framework of the causes of double burden, the aim of the statistical analyses was to assess the socio-economic and demographic patterning of the double burden, adjusting for individual characteristics.
Associations between the double burdens in four categories and sociodemographic and lifestyle factors were assessed by relative prevalence ratios (RPR) estimated in multinomial logistic regression models(
35
) (mlogit Stata command). The last category was chosen as the response reference category; e.g. for the ‘anaemic child and overweight mother’ double burden, this was the ‘not anaemic child and not overweight mother’ category (
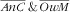 ). This analysis, using double burden four-category response variables, allowed us to estimate associations with covariates of both single and double burdens within the same model (v. the same response reference category, i.e. participants with none of the two). This also enabled the assessment of the specific factors of single and double burdens and how the factors of the single burdens may combine or not as factors of the double burden.
). This analysis, using double burden four-category response variables, allowed us to estimate associations with covariates of both single and double burdens within the same model (v. the same response reference category, i.e. participants with none of the two). This also enabled the assessment of the specific factors of single and double burdens and how the factors of the single burdens may combine or not as factors of the double burden.
Also, this modelling framework enabled us to assess the synergetic, antagonistic or independent coexistence of the two types of malnutrition, by deriving estimates of the ratios of the probability of the double burden to the product of the probabilities of each type of malnutrition (termed in the following IPR, for ‘independence probability ratio’). In the target population, this ratio is equal to 1 if the two types of malnutrition are co-occurring independently, less than 1 if the two types of malnutrition are antagonistic and greater than 1 if they are synergetic.
Descriptive analyses were initially run separately for girls v. boys in child–mother pairs. But as we did not observe significant differences by gender, multivariate analyses were finally performed and presented for the whole sample.
Results
Sample characteristics
Our analyses used the sub-sample of 437 child–mother pairs from the 1520 surveyed households, of whom 90·8 % were from urban areas.
For children, mean age was 2·9 (se 0·1) years, mean weight 14·4 (se 0·2) kg, mean birth weight 3281·7 (se 36·0) g, mean height 92·6 (se 0·7) cm and mean BMI-for-age Z-score 0·6 (se 0·7). Wasting only affected 1·6 % (95 % CI 0·7, 4·0 %) of children (Table 1). One child out of ten (9·7 %; 95 % CI 6·8, 13·7 %) was overweight and 2·4 % (95 % CI 1·2, 4·6 %) were obese. Overall, 5·4 % (95 % CI 3·6, 8·1 %) of children were stunted. Mean Hb of children was 113·7 g/l and one-third of them were anaemic (32·8 %; 95 % CI 28·3, 37·7 %; Table 1). No significant difference between girls and boys was found either for anthropometry or Hb status (see online supplementary material, Supplemental Table 1). Mean DDS was 6·1 (se 0·1).
Table 1.
Sociodemographic, lifestyle and nutritional characteristics of the 6–59-month-old children living with their 20–49-year-old mothers (437 child–mother pairs), Greater Tunis area, Tunisia, 2009/2010
| Characteristic | %* | se † |
|---|---|---|
| Child | ||
| Age (months) | ||
| 6–23 | 29·4 | 2·4 |
| 24–47 | 45·4 | 2·3 |
| 48–59 | 25·3 | 2·4 |
| Sex | ||
| Girls | 48·9 | 2·5 |
| Wasting (weight-for-height Z-score<−2) | 1·6 | 0·6 |
| Overweight (BMI-for-age Z-score>2) | 9·7 | 1·7 |
| Obesity (BMI-for-age Z-score>3) | 2·4 | 0·8 |
| Stunting (height-for-age Z-score<−2) | 5·4 | 1·1 |
| Anaemia (Hb<110 g/l) | 32·8 | 2·4 |
| Dietary diversity score | ||
| Lower tertile (1–5) | 35·7 | 2·6 |
| Intermediate tertile (6–7) | 41·4 | 2·5 |
| Upper tertile (8–11) | 22·9 | 2·3 |
| Mother | ||
| Age (years) | ||
| 20–29 | 20·2 | 2·3 |
| 30–39 | 58·2 | 2·5 |
| 40–49 | 21·6 | 2·1 |
| Overweight (BMI≥25·0 kg/m2) | 77·1 | 2·3 |
| Obesity (BMI≥30·0 kg/m2) | 41·4 | 3·0 |
| Anaemia (Hb<120 g/l) | 43·0 | 3·5 |
| Parity | ||
| 1–2 | 52·6 | 3·0 |
| ≥3 | 47·4 | 3·0 |
| Education | ||
| No schooling | 10·3 | 2·0 |
| Primary school | 40·1 | 2·6 |
| Secondary | 39·7 | 2·8 |
| University | 9·9 | 2·0 |
| Professional activity | ||
| Upper/intermediate | 11·3 | 2·1 |
| Employee/worker | 18·1 | 2·2 |
| Without activity | 70·6 | 2·7 |
| Household economic proxy‡ | ||
| Lower tertile | 35·1 | 3·3 |
| Intermediate tertile | 35·6 | 2·4 |
| Upper tertile | 29·3 | 3·2 |
| Energy intake | ||
| Lower tertile | 31·0 | 2·6 |
| Intermediate tertile | 33·1 | 2·8 |
| Upper tertile | 35·8 | 3·1 |
| Physical activity | ||
| Sedentary (PAL<1·7) | 24·2 | 2·7 |
| Active (PAL≥1·7) | 75·8 | 2·7 |
PAL, physical activity level.
Weighted proportions.
se taking the sampling design into account.
Asset-based household wealth score: increasing wealth from lower to upper tertile.
For mothers, mean age was 35·3 (se 0·3) years, mean weight 72·7 (se 0·7) kg, mean height 158·9 (se 0·3) cm and mean BMI 28·8 (se 0·3) kg/m2. Among mothers, 77·1 % (95 % CI 72·1, 81·4 %) were overweight and 41·4 % (95 % CI 35·6, 47·3 %) were obese (Table 1). Mean Hb was 121·1 g/l and almost half of mothers were anaemic. Half of mothers had more than three children, a tenth had never attended school but half had secondary education or more. A large proportion of mothers had no professional activity (70·6 %; 95 % CI 65·0, 75·6 %) and only 11·3 % (95 % CI 7·7, 16·3 %) had upper level or intermediate level professional activity. Mean daily energy intake was 10 083 (se 99·2) kJ (2410 (se 23·7) kcal), ranging from 6406 to 16 297 kJ (1531 to 3895 kcal). A quarter of the mother had a sedentary lifestyle (Table 1).
Double burden of malnutrition among child–mother pairs
One child–mother pair out of four (24·4 %; 95 % CI 20·1, 29·3 %) presented a double burden of
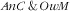 , prevalence of
, prevalence of
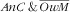 was 8·4 % (95 % CI 5·7, 12·2 %), and that of
was 8·4 % (95 % CI 5·7, 12·2 %), and that of
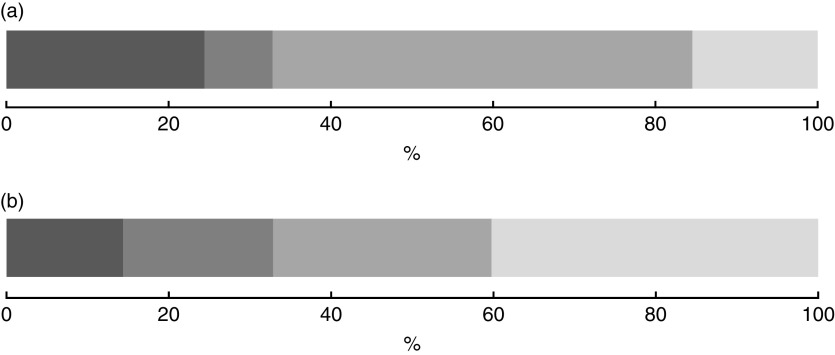
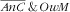 was 51·7 % (95 % CI 46·5, 56·9 %). One child–mother pair out of seven suffered from double burden of anaemic child and obese mother (14·4 %; 95 % CI 11·0, 18·5 %), while the proportion of anaemic child and not obese mother was 18·5 % (95 % CI 14·9, 22·7 %) and that of not anaemic child and obese mother was 26·9 % (95 % CI 22·0, 32·4 %; Fig. 1). There was no synergetic nor antagonistic co-occurrence for either anaemia in child and overweight in mother (IPR=1·0; 95 % CI 0·9, 1·1, P=0·59) or anaemic child and obesity in mother (IPR=1·1; 95 % CI 0·9, 1·2, P=0·40).
was 51·7 % (95 % CI 46·5, 56·9 %). One child–mother pair out of seven suffered from double burden of anaemic child and obese mother (14·4 %; 95 % CI 11·0, 18·5 %), while the proportion of anaemic child and not obese mother was 18·5 % (95 % CI 14·9, 22·7 %) and that of not anaemic child and obese mother was 26·9 % (95 % CI 22·0, 32·4 %; Fig. 1). There was no synergetic nor antagonistic co-occurrence for either anaemia in child and overweight in mother (IPR=1·0; 95 % CI 0·9, 1·1, P=0·59) or anaemic child and obesity in mother (IPR=1·1; 95 % CI 0·9, 1·2, P=0·40).
Fig. 1.
Distribution of the coexistence of anaemia in the child and overweight in the mother (a) and anaemia in the child and obesity in the mother (b) among 6–59-month-old children living with their 20–49-year-old mothers (437 child–mother pairs), Greater Tunis area, Tunisia, 2009/2010 ( , anaemic child and overweight/obese mother;
, anaemic child and overweight/obese mother;  , anaemic child and not overweight/not obese mother;
, anaemic child and not overweight/not obese mother;  , not anaemic child and overweight/obese mother;
, not anaemic child and overweight/obese mother;  , not anaemic child and not overweight/not obese mother)
, not anaemic child and not overweight/not obese mother)
The other forms of double burden were infrequent: the prevalence of the double burden of overweight child and anaemic mother was 3·7 % (95 % CI 2·2, 6·3 %); obese child and anaemic mother 1·2 % (95 % CI 0·5, 2·9 %); stunted child and overweight mother 3·7 % (95 % CI 2·1, 6·4 %); stunted child and obese mother 1·2 % (95 % CI 0·5, 3·3 %); wasted child and overweight mother 1·1 % (95 % CI 0·4, 2·7 %); wasted child and obese mother (0·9 %; 95 % CI 0·3, 2·5 %; Table 2).
Table 2.
Prevalence of double burden of malnutrition in 6–59-month-old children living with their 20–49-year-old mothers (437 child–mother pairs), Greater Tunis area, Tunisia, 2009/2010
| All (n 437) | Girls (n 215) | Boys (n 222) | Girls v. boys | ||
|---|---|---|---|---|---|
| %* | 95 % CI† | %* | %* | P value‡ | |
| Anaemic§ child and overweight║ mother | 24·4 | 20·1, 29·3 | 24·9 | 24·0 | 0·80 |
| Anaemic child and obese¶ mother | 14·4 | 11·0, 18·5 | 14·5 | 14·2 | 0·95 |
| Overweight child and anaemic mother | 3·7 | 2·2, 6·3 | 4·1 | 3·4 | 0·72 |
| Obese child and anaemic mother | 1·2 | 0·5, 2·9 | 2·0 | 0·4 | 0·15 |
| Stunted** child and overweight mother | 3·7 | 2·1, 6·4 | 3·2 | 4·2 | 0·60 |
| Stunted child and obese mother | 1·2 | 0·5, 3·3 | 0·7 | 1·8 | 0·40 |
| Wasted†† child and overweight mother | 1·1 | 0·4, 2·7 | 0·6 | 1·6 | 0·33 |
| Wasted child and obese mother | 0·9 | 0·3, 2·5 | 0·6 | 1·3 | 0·47 |
Weighted proportions.
95 % CI taking the sampling design into account.
P value for girls v. boys contrast.
Anaemia: for children, Hb<110 g/l; for mothers, Hb<120 g/l.
Overweight: for children, BMI-for-age Z-score>2; for mothers, BMI≥25·0·kg/m2.
Obesity: for children, BMI-for-age Z-score>3; for mothers, BMI≥30·0·kg/m2.
Stunting: height-for-age Z-score<−2.
Wasting: weight-for-height Z-score<−2.
For all these latter types of child–mother double burden, no synergetic nor antagonistic co-occurrences of the two types of malnutrition were observed either: confidence intervals for IPR all included 1, thus presenting no evidence at the α=5 % level against the null hypothesis of independence (detailed data not shown).
Associations with sociodemographic and lifestyle factors
Due to the low prevalence of the other types of double burden, we analysed in detail only the association of covariates with the coexistence of anaemic child and overweight or obese mother (with a special focus on anaemia–overweight; results for anaemia–obese are presented in the online supplementary material). Table 3 displays, for the 437 child–mother pairs, the unadjusted and adjusted relationships between socio-economic and lifestyle factors and the categories of the ‘anaemic child and overweight mother’ four-category variable (using the
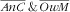 as reference category).
as reference category).
Table 3.
Multinomial regression: crude and adjusted associations between categories of the ‘anaemic child and overweight mother’ double burden and sociodemographic characteristics among 6–59-month-old children living with their 20–49-year-old mothers (437 child–mother pairs), Greater Tunis area, Tunisia, 2009/2010
| Anaemic child and not overweight mother* | Not anaemic child and overweight mother* | Anaemic child and overweight mother* | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crude | Adjusted | Crude | Adjusted | Crude | Adjusted | |||||||||||
| n | %† | RPR§ | 95 % CI║ | RPR¶ | 95 % CI║ | %† | RPR§ | 95 % CI║ | RPR¶ | 95 % CI║ | %† | RPR§ | 95 % CI║ | RPR¶ | 95 % CI║ | |
| Child’s age (months) | P **=0·0003 | P **=0·0081 | P **=0·99 | P **=0·72 | P ** <0·0001 | P ** <0·0001 | ||||||||||
| 6–23 | 128 | 14·4 | 14·3 | 4·0, 50·8 | 13·1 | 2·6, 64·9 | 32·3 | 1·0 | 0·5, 2·0 | 1·4 | 0·6, 3·4 | 43·6 | 11·8 | 4·9, 28·4 | 16·4 | 5·8, 46·3 |
| 24–47 | 197 | 8·0 | 4·7 | 1·2, 17·9 | 4·9 | 1·2, 20·8 | 54·4 | 1·0 | 0·5, 2·0 | 1·2 | 0·6, 2·5 | 21·2 | 3·4 | 1·5, 7·9 | 4·1 | 1·8, 9·1 |
| 48–59 | 112 | 2·1 | 1 | 1 | 69·4 | 1 | 1 | 7·9 | 1 | 1 | ||||||
| Child’s sex | P **=0·44 | P **=0·77 | P **=0·94 | P **=0·51 | P **=0·92 | P **=0·45 | ||||||||||
| Female | 215 | 7·1 | 1 | 1 | 52·5 | 1 | 1 | 24·9 | 1 | 1 | ||||||
| Male | 222 | 9·7 | 1·4 | 0·6, 3·1 | 1·1 | 0·5, 2·8 | 51·0 | 1·0 | 0·5, 1·8 | 0·8 | 0·4, 1·6 | 24·0 | 1·0 | 0·5, 1·8 | 0·8 | 0·4, 1·5 |
| Child’s birth weight | P **=0·70 | P **=0·98 | P **=0·65 | P **=0·87 | P **=0·89 | P **=0·65 | ||||||||||
| <2500 g | 41 | 9·6 | 1·4 | 0·2, 8·0 | 1·0 | 0·2, 5·5 | 55·4 | 1·3 | 0·4, 4·3 | 1·1 | 0·3, 4·0 | 22·1 | 1·1 | 0·3, 3·7 | 0·7 | 0·2, 2·7 |
| ≥2500 g | 396 | 8·3 | 1 | 1 | 51·3 | 1 | 1 | 24·7 | 1 | 1 | ||||||
| Mother’s age (years) | P **=0·43 | P **=0·63 | P **=0·0079 | P **=0·0043 | P **=0·25 | P **=0·24 | ||||||||||
| 20–29 | 106 | 17·4 | 1 | 1 | 34·1 | 1 | 1 | 25·6 | 1 | 1 | ||||||
| 30–39 | 248 | 6·0 | 0·6 | 0·3, 1·5 | 0·6 | 0·2, 1·8 | 55·0 | 2·9 | 1·4, 5·8 | 3·4 | 1·6, 7·0 | 26·3 | 1·9 | 0·8, 4·3 | 2·1 | 0·9, 5·2 |
| 40–49 | 83 | 5·5 | 0·5 | 0·1, 2·2 | 0·7 | 0·1, 2·9 | 61·9 | 2·8 | 1·2, 6·4 | 3·7 | 1·3, 10·3 | 17·6 | 1·1 | 0·4, 2·9 | 1·4 | 0·5, 4·2 |
| Mother’s parity | P **=0·62 | P **=0·65 | P **=0·06 | P **=0·34 | P **=0·0224 | P **=0·0101 | ||||||||||
| 1–2 | 264 | 11·0 | 1 | 1 | 48·9 | 1 | 1 | 21·3 | 1 | 1 | ||||||
| ≥3 | 173 | 5·4 | 0·8 | 0·3, 1·9 | 1·2 | 0·6, 2·6 | 55·0 | 1·8 | 1·0, 3·5 | 1·4 | 0·7, 2·7 | 28·0 | 2·2 | 1·1, 4·1 | 2·5 | 1·2, 4·8 |
| Mother’s education | P **=0·56 | P **=0·67 | P **=0·28 | P **=0·20 | P **=0·86 | P **=0·50 | ||||||||||
| No schooling | 38 | 4·8 | 0·5 | 0·1, 3·0 | 0·4 | 0·0, 4·8 | 66·2 | 2·5 | 0·6, 10·6 | 1·9 | 0·3, 13·7 | 19·0 | 1·5 | 0·2, 9·0 | 1·1 | 0·1, 10·6 |
| Primary school | 175 | 7·9 | 0·4 | 0·1, 1·4 | 0·5 | 0·1, 4·1 | 45·9 | 0·9 | 0·3, 2·5 | 0·9 | 0·2, 4·9 | 27·1 | 1·1 | 0·3, 3·5 | 1·3 | 0·2, 8·0 |
| Secondary | 179 | 7·8 | 0·7 | 0·2, 1·9 | 1·0 | 0·1, 6·6 | 55·7 | 1·7 | 0·6, 5·0 | 2·3 | 0·5, 10·5 | 23·8 | 1·5 | 0·4, 5·3 | 2·3 | 0·4, 13·5 |
| University | 45 | 16·1 | 1 | 1 | 45·0 | 1 | 1 | 21·8 | 1 | 1 | ||||||
| Mother’s professional activity | P **=0·31 | P **=0·44 | P=0·83 | P **=0·96 | P **=0·57 | P **=0·67 | ||||||||||
| Upper/intermediate | 45 | 12·5 | 1 | 1 | 49·8 | 1 | 1 | 24·6 | 1 | 1 | ||||||
| Employee/worker | 76 | 9·8 | 0·8 | 0·2, 3·4 | 0·6 | 0·1, 4·4 | 49·8 | 1·1 | 0·3, 4·0 | 0·8 | 0·1, 5·3 | 28·1 | 1·2 | 0·3, 5·5 | 0·7 | 0·1, 5·6 |
| Without activity | 316 | 7·5 | 0·5 | 0·2, 1·4 | 0·4 | 0·1, 2·1 | 52·5 | 0·8 | 0·3, 2·5 | 0·8 | 0·1, 4·3 | 23·5 | 0·8 | 0·2, 2·8 | 0·5 | 0·1, 3·4 |
| Household economic proxy | P **=0·50 | P **=0·14 | P **=0·70 | P **=0·55 | P **=0·40 | P **=0·22 | ||||||||||
| Lower tertile | 159 | 10·6 | 1·4 | 0·6, 3·6 | 2·9 | 0·9, 9·3 | 49·0 | 1·2 | 0·6, 2·7 | 1·6 | 0·6, 4·1 | 26·2 | 1·7 | 0·7, 4·0 | 2·3 | 0·8, 6·3 |
| Intermediate tertile | 152 | 5·3 | 0·7 | 0·2, 2·3 | 1·1 | 0·3, 3·9 | 54·7 | 1·4 | 0·6, 3·4 | 1·5 | 0·6, 3·5 | 26·1 | 1·7 | 0·7, 4·3 | 2·0 | 0·8, 5·3 |
| Upper tertile | 126 | 9·6 | 1 | 1 | 51·4 | 1 | 1 | 20·2 | 1 | 1 | ||||||
| Child’s dietary diversity score | P **=0·13 | P **=0·29 | P **=0·73 | P **=0·76 | P **=0·05 | P **=0·61 | ||||||||||
| Lower tertile (1–5) | 155 | 10·4 | 1 | 1 | 45·0 | 1 | 1 | 32·5 | 1 | 1 | ||||||
| Intermediate tertile (6–7) | 186 | 5·9 | 0·4 | 0·1, 1·0 | 0·6 | 0·2, 1·8 | 56·4 | 0·8 | 0·5, 1·5 | 0·9 | 0·5, 1·8 | 19·3 | 0·4 | 0·2, 0·8 | 0·7 | 0·3, 1·7 |
| Upper tertile (8–11) | 96 | 9·9 | 0·8 | 0·3, 2·1 | 1·2 | 0·4, 4·1 | 53·8 | 0·9 | 0·4, 2·4 | 1·2 | 0·4, 3·5 | 21·1 | 0·5 | 0·2, 1·6 | 1·1 | 0·3, 3·7 |
| Mother’s energy intake | P **=0·21 | P **=0·21 | P **=0·0002 | P **= 0·0003 | P **=0·0001 | P **=0·0011 | ||||||||||
| Lower tertile | 148 | 10·7 | 1 | 1 | 41·4 | 1 | 1 | 19·7 | 1 | 1 | ||||||
| Intermediate tertile | 143 | 11·3 | 2·3 | 0·9, 5·7 | 2·4 | 0·9, 6·4 | 54·4 | 2·8 | 1·4, 5·8 | 3·8 | 1·6, 9·0 | 21·1 | 2·3 | 1·1, 4·9 | 2·7 | 1·1, 6·5 |
| Upper tertile | 146 | 3·9 | 1·5 | 0·5, 4·8 | 1·3 | 0·4, 4·7 | 58·0 | 5·8 | 2·4, 14·0 | 7·9 | 2·7, 23·0 | 31·3 | 6·6 | 2·9, 15·2 | 7·6 | 2·5, 22·8 |
| Mother’ physical activity | P **=0·64 | P **=0·79 | P **=0·59 | P **=0·33 | P **=0·74 | P **=0·21 | ||||||||||
| Sedentary (PAL <1·7) | 103 | 7·8 | 0·8 | 0·3, 2·2 | 0·9 | 0·3, 2·5 | 50·0 | 0·8 | 0·4, 1·7 | 1·5 | 0·7, 3·5 | 25·0 | 0·9 | 0·4, 1·8 | 1·8 | 0·7, 4·6 |
| Active (PAL≥1·7) | 334 | 8·6 | 1 | 1 | 52·2 | 1 | 1 | 24·3 | 1 | 1·0 | ||||||
PAL, physical activity level.
Compared with the response variable reference category: not anaemic child and not overweight mother (anaemia, Hb <110 g/l for children; overweight, BMI≥25·0 kg/m2 for mothers).
Prevalence proportion (weighted estimates).
Relative prevalence ratio (v. reference category for which RPR=1), taking the sampling design into account.
95 % CI taking the sampling design into account.
Adjusted for all variables in column 1.
Crude or adjusted P value for multinomial regression models.
As for the factors pertaining to
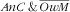 , once adjusted, there was a gradient of increasing prevalence with age of the child (P=0·0081): the child–mother pairs with younger children (6–23 months) were especially prone (RPR=13·1; 95 % CI 2·6, 64·9) v. those aged 48–59 months. The observed trend of a decrease in proportion of
, once adjusted, there was a gradient of increasing prevalence with age of the child (P=0·0081): the child–mother pairs with younger children (6–23 months) were especially prone (RPR=13·1; 95 % CI 2·6, 64·9) v. those aged 48–59 months. The observed trend of a decrease in proportion of
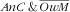 with household economic proxy was not statistically significant (e.g. lower v. upper tertile: RPR=2·9; 95 % CI 0·9, 9·3).
with household economic proxy was not statistically significant (e.g. lower v. upper tertile: RPR=2·9; 95 % CI 0·9, 9·3).
Age of the mother was strongly associated with the
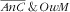 category (P=0·0043): pairs with mothers above 30 years of age were more prone (v. those <30 years), RPR=3·4 (95 % CI 1·6, 7·0) for 30–39 years and RPR=3·7 (95 % CI 1·3, 10·3) for 40–49 years. Also, there was a strong increasing gradient with total energy intake of the mother: v. the lower tertile of intake, RPR=2·8 (95 % CI 1·4, 5·8) for the second tertile and RPR=7·9 (95 % CI 2·7, 23·0) for the third tertile.
category (P=0·0043): pairs with mothers above 30 years of age were more prone (v. those <30 years), RPR=3·4 (95 % CI 1·6, 7·0) for 30–39 years and RPR=3·7 (95 % CI 1·3, 10·3) for 40–49 years. Also, there was a strong increasing gradient with total energy intake of the mother: v. the lower tertile of intake, RPR=2·8 (95 % CI 1·4, 5·8) for the second tertile and RPR=7·9 (95 % CI 2·7, 23·0) for the third tertile.
Regarding the association of covariates with
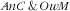 , as for the
, as for the
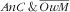 category, there was a strong decreasing gradient with child age: child–mother pairs featuring younger children were especially affected (RPR=16·4; 95 % CI 5·8, 46·3 v. the younger ones). Analogous to the
category, there was a strong decreasing gradient with child age: child–mother pairs featuring younger children were especially affected (RPR=16·4; 95 % CI 5·8, 46·3 v. the younger ones). Analogous to the
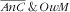 category, there was a strong increasing trend with mother’s total energy intake (P=0·0011): v. the lower tertile of intake, RPR=2·7 (95 % CI 1·1, 6·5) for the second tertile and RPR=7·6 (95 % CI 2·5, 22·8) for the third tertile. On the other hand, the strong association with mother’s age observed for
category, there was a strong increasing trend with mother’s total energy intake (P=0·0011): v. the lower tertile of intake, RPR=2·7 (95 % CI 1·1, 6·5) for the second tertile and RPR=7·6 (95 % CI 2·5, 22·8) for the third tertile. On the other hand, the strong association with mother’s age observed for
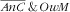 was not observed for the double burden. Nevertheless, parity was specifically associated with the double burden as mothers with three children or more (v. one child or two) were much more prone (RPR=2·5; 95 % CI 1·2, 4·8).
was not observed for the double burden. Nevertheless, parity was specifically associated with the double burden as mothers with three children or more (v. one child or two) were much more prone (RPR=2·5; 95 % CI 1·2, 4·8).
Associations with the three categories of anaemic child and obese mother were quite similar with the exception that, contrary to what observed for overweight, low birth weight (v. normal) decreased the prevalence of the double burden (RPR=0·2; 95 % CI 0·0, 0·9; see online supplementary material, Supplemental Table 2).
Disaggregation of IPR by categories of covariates (to assess whether the absence or presence of synergetic or antagonistic co-occurrence of the two types of malnutrition depended on sociodemographic and lifestyle factors) did not underline significant departures from independence either for anaemic–overweight or anaemic–obese.
Discussion
In the present study, we assessed the magnitude, the nature and the socio-economic and lifestyle covariates of several double burdens of malnutrition among child–mother pairs. The main finding of our study was a high prevalence of the co-occurrence of
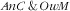 in the same household as this double burden concerned one child–mother pair out of four. We also underlined that these two types of malnutrition were co-occurring independently without specific synergetic or antagonistic association. Finally, we showed that the patterning with sociodemographic and lifestyle variables was not marked except that this double burden was more frequent among child–mother pairs with younger children and with mothers of higher parity and with higher energy intakes.
in the same household as this double burden concerned one child–mother pair out of four. We also underlined that these two types of malnutrition were co-occurring independently without specific synergetic or antagonistic association. Finally, we showed that the patterning with sociodemographic and lifestyle variables was not marked except that this double burden was more frequent among child–mother pairs with younger children and with mothers of higher parity and with higher energy intakes.
Different types of malnutrition among children and mothers
Beyond the estimates of double burdens, regarding the distinct types of malnutrition involved, child undernutrition such as wasting (1·6 %) and stunting (5·4 %) were residual in our population. Indeed, they were largely below the WHO lower cut-off values for public health interpretation because for wasting <5 % is considered ‘acceptable’ and for stunting <20 % is considered ‘low’. These low prevalences are in line with a decreasing trend in our study area of Greater Tunis, e.g. the prevalence of stunting has declined from 7 % in 2000 to 3·7 %( 36 ) (this latter estimate is in line with our observed prevalence of 5·4 (95 % CI 3·6, 8·1) % when taking into account sampling variability). Nevertheless, as stunting has been shown to be strongly associated with socio-economic conditions( 37 ), given the current international economic difficulties (and specifically in Tunisia also linked to the still ongoing political and economic transition post 2011 revolution), it is important that stunting continues be monitored even in such nutrition transition contexts. For childhood overnutrition such as overweight and obesity, our study showed that prevalence rates are lower than in neighbouring countries like Egypt with 20·5 % in 2008 and Libya with 22·4 % in 2007( 38 ). The rise in childhood overweight in almost middle-income countries nevertheless highlights the need for monitoring levels and trends of overweight and obesity in children, especially during pre-school age considering it is a critical period( 38 ). On the other hand, our data confirm that anaemia is still a major public health problem among Tunisian children because we showed that one-third of children under 5 years of age in the Greater Tunis area were anaemic. Our observed rate of anaemia among children is even higher than reported in a previous study conducted in 2000( 19 ).
As for mothers, we found that three women out of four were overweight and more than a third were obese; this is consistent with the prevalence derived from the larger sample of urban women from which the mothers included in our study are a sub-sample( 7 , 16 ). The burden of overweight and obesity is well documented in the MENA region, all the more in urban areas, and women are considered a group especially at risk of obesity in that context( 1 , 17 , 39 ). At the same time, our data confirm that anaemia among Tunisian women is a public health problem even in this mostly urban nutrition transition context: our finding is consistent with the prevalence in the whole sample of women( 7 ) but higher than in a previous study in 2000( 40 ).
Double burden of malnutrition among child–mother pairs
The most documented form of double burden at household level is stunted child and overweight mother, especially in countries with historically high rates of childhood stunting( 12 – 15 , 41 , 42 ). In the MENA region, this type of double burden was as high as 14 % in Egypt( 43 ). But the prevalence of the double burden of stunted child and overweight mother observed in the present study was low at only 3·7 %, despite a high prevalence of overweight among mothers, in relationship with the observed low rate of stunting. The forms of the double burden, such as overweight/obese child and anaemic mother, stunted child and overweight/obese mother, and wasted child and overweight/obese mother, were infrequent in our study area; their prevalence ranged from 0·9 to 3·7 %.
The most remarkably prevalent combination in our study was
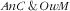 as a quarter of the child–mother pairs were affected; there was also a sizeable proportion (about one-sixth) of anaemic child and obese mother. This is in coherence with the observed high prevalence of both anaemia among children and overweight or obesity among mothers. Nevertheless, how the prevalence of double burden relates to the prevalence of each type of malnutrition depends on whether the co-occurrence of the two types of malnutrition is synergetic (e.g. the probability of an anaemic child increases if the mother is overweight or vice versa), antagonistic (e.g. the probability of an anaemic child decreases if the mother is overweight and vice versa) or independent.
as a quarter of the child–mother pairs were affected; there was also a sizeable proportion (about one-sixth) of anaemic child and obese mother. This is in coherence with the observed high prevalence of both anaemia among children and overweight or obesity among mothers. Nevertheless, how the prevalence of double burden relates to the prevalence of each type of malnutrition depends on whether the co-occurrence of the two types of malnutrition is synergetic (e.g. the probability of an anaemic child increases if the mother is overweight or vice versa), antagonistic (e.g. the probability of an anaemic child decreases if the mother is overweight and vice versa) or independent.
In the current study we tackled that issue using the original IPR derived from the parameters of the multinomial model. For both the
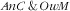 (IPR=1·0; 95 % % CI 0·9, 1·1) and anaemic child obese mother double burdens (IPR=1·1; 95 % CI 0·9, 1·2), our data were strongly in accordance with the independent coexistence of the two types of malnutrition in child–mother pairs. This is not unexpected, but there could also be synergetic or antagonistic co-occurrences. For example, in the study in Mexico, the observed prevalence of double burden at individual or household level was either higher or lower than expected under the independence assumption, depending on the malnutrition studied(
15
).
(IPR=1·0; 95 % % CI 0·9, 1·1) and anaemic child obese mother double burdens (IPR=1·1; 95 % CI 0·9, 1·2), our data were strongly in accordance with the independent coexistence of the two types of malnutrition in child–mother pairs. This is not unexpected, but there could also be synergetic or antagonistic co-occurrences. For example, in the study in Mexico, the observed prevalence of double burden at individual or household level was either higher or lower than expected under the independence assumption, depending on the malnutrition studied(
15
).
As for
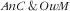 , in the context of the nutrition transition, two opposite hypotheses could be put forward. First, the ‘Westernization’ of diets linked to the nutrition transition, with higher energy intakes, also includes increases in consumption of animal products, such as red meat, that can increase Fe intakes: that could favour the hypothesis of an antagonistic co-occurrence of
, in the context of the nutrition transition, two opposite hypotheses could be put forward. First, the ‘Westernization’ of diets linked to the nutrition transition, with higher energy intakes, also includes increases in consumption of animal products, such as red meat, that can increase Fe intakes: that could favour the hypothesis of an antagonistic co-occurrence of
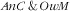 (assuming the diet of the mother is at least partly predictive of that of their children). On the other hand, hypotheses have also been put forward that these high-energy diets are ‘empty-calories’ type diets, which do not protect from micronutrient deficiencies(
44
): that would be in favour of a synergetic co-occurrence of the two types malnutrition. Our results do not support any of these hypotheses (also taking into account that insufficient Fe intake is not likely the only cause of anaemia among children in the context(
19
)).
(assuming the diet of the mother is at least partly predictive of that of their children). On the other hand, hypotheses have also been put forward that these high-energy diets are ‘empty-calories’ type diets, which do not protect from micronutrient deficiencies(
44
): that would be in favour of a synergetic co-occurrence of the two types malnutrition. Our results do not support any of these hypotheses (also taking into account that insufficient Fe intake is not likely the only cause of anaemia among children in the context(
19
)).
Factors associated with the double burden of anaemic child and overweight mother
In Table 3 we present the association of covariates with
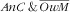 and
and
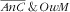 , as well as double burden of
, as well as double burden of
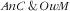 .
.
For the first category, after adjustment for all sociodemographic and lifestyle variables, there was a marked increase in prevalence of
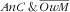 below the age of 24 months, because the RPR v. the 48–59 months age class was greater than ten: this is consistent with findings related to variation of child anaemia with age in other countries(
45
–
47
). This vulnerability is possibly due to the increased need for Fe at this stage of the child’s growth and the possibly inadequate amount of Fe in infant diets. As is known, when term infants are born, their Fe reserves can last for the first 4–6 months of life, after which the infant’s dietary intake is the major determinant of Fe status. Several studies have shown that maternal educational level is strongly associated with the child’s nutritional status and it was suggested that better education contributes to better knowledge about dietary practices for their children and themselves as well as general caring practices(
45
,
48
,
49
). We did not find such an association. In our study we did not observe an association of child DDS with
below the age of 24 months, because the RPR v. the 48–59 months age class was greater than ten: this is consistent with findings related to variation of child anaemia with age in other countries(
45
–
47
). This vulnerability is possibly due to the increased need for Fe at this stage of the child’s growth and the possibly inadequate amount of Fe in infant diets. As is known, when term infants are born, their Fe reserves can last for the first 4–6 months of life, after which the infant’s dietary intake is the major determinant of Fe status. Several studies have shown that maternal educational level is strongly associated with the child’s nutritional status and it was suggested that better education contributes to better knowledge about dietary practices for their children and themselves as well as general caring practices(
45
,
48
,
49
). We did not find such an association. In our study we did not observe an association of child DDS with
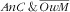 . This is concordant with other studies(
50
–
52
). The point estimate for the RPR of the lower v. upper tertile of household wealth was about three, consistent with some studies which showed the family’s wealth as one of the important determinants of child anaemia(
48
). Nevertheless in our study this relationship was borderline statistically significant (95 % CI 0·9, 9·3), possibly due to sample size issues (as the study is a secondary analysis based on a sub-sample of the participants of a study which did not specifically target our studied age class).
. This is concordant with other studies(
50
–
52
). The point estimate for the RPR of the lower v. upper tertile of household wealth was about three, consistent with some studies which showed the family’s wealth as one of the important determinants of child anaemia(
48
). Nevertheless in our study this relationship was borderline statistically significant (95 % CI 0·9, 9·3), possibly due to sample size issues (as the study is a secondary analysis based on a sub-sample of the participants of a study which did not specifically target our studied age class).
For the second category (
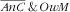 ), there was a strong increasing association with age of the mother because it was much more frequent for child–mother pairs featuring mothers over 30 years old. This is consistent with the increase in excess adiposity with age among women, often reported in the MENA region, including in Tunisia(
7
,
17
). Although in nutrition transition contexts and more specifically in the MENA region especially among women, excess adiposity has been documented to increase with household welfare (e.g. estimated through a welfare proxy)(
17
,
53
,
54
), we did not observe such an association with the
), there was a strong increasing association with age of the mother because it was much more frequent for child–mother pairs featuring mothers over 30 years old. This is consistent with the increase in excess adiposity with age among women, often reported in the MENA region, including in Tunisia(
7
,
17
). Although in nutrition transition contexts and more specifically in the MENA region especially among women, excess adiposity has been documented to increase with household welfare (e.g. estimated through a welfare proxy)(
17
,
53
,
54
), we did not observe such an association with the
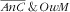 category. This result is nevertheless coherent with what was observed over the whole sample of women in a previous study(
7
) and also with the assumption that within advanced nutrition transitions (such as in our Tunisian mostly urban context) there could be a shift in the relationship with socio-economic status towards an inversion of the relationship (with no or a little association in intermediate stages)(
55
). There was also a strong association of this category with energy intake of the mother (adjusted for all other covariates, including PAL); this is not unexpected because dietary and especially energy intake is among the established proximal causes of excess adiposity(
56
).
category. This result is nevertheless coherent with what was observed over the whole sample of women in a previous study(
7
) and also with the assumption that within advanced nutrition transitions (such as in our Tunisian mostly urban context) there could be a shift in the relationship with socio-economic status towards an inversion of the relationship (with no or a little association in intermediate stages)(
55
). There was also a strong association of this category with energy intake of the mother (adjusted for all other covariates, including PAL); this is not unexpected because dietary and especially energy intake is among the established proximal causes of excess adiposity(
56
).
Overall, the factors associated with the two categories
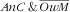 and
and
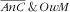 were coherent with those reported in the literature for each type of malnutrition studied separately, as discussed above. This is not unexpected; as we demonstrated above that the co-occurrence of these two types of malnutrition among child–mother pairs was consistent with the independence hypothesis, in this case it can be shown for these two categories that the association estimated by RPR in multinomial models should be identical to those estimated using OR in binary logistic models, for each malnutrition separately(
16
).
were coherent with those reported in the literature for each type of malnutrition studied separately, as discussed above. This is not unexpected; as we demonstrated above that the co-occurrence of these two types of malnutrition among child–mother pairs was consistent with the independence hypothesis, in this case it can be shown for these two categories that the association estimated by RPR in multinomial models should be identical to those estimated using OR in binary logistic models, for each malnutrition separately(
16
).
From the same reasoning it also stems that, in the case of probabilistic independence, the associations (assessed as RPR) of covariates with the third category (i.e. double burden
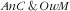 ) should be close to the product of the RPR for the
) should be close to the product of the RPR for the
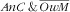 and
and
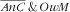 categories. Our observations were mostly consistent with that reasoning, in relation that we demonstrated neither the synergetic nor the antagonistic anaemic child × overweight mother co-occurrence. Child–mother pairs with younger children (<24 months) were then the more prone to the
categories. Our observations were mostly consistent with that reasoning, in relation that we demonstrated neither the synergetic nor the antagonistic anaemic child × overweight mother co-occurrence. Child–mother pairs with younger children (<24 months) were then the more prone to the
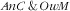 double burden (stemming for the strong association of age of the child with the
double burden (stemming for the strong association of age of the child with the
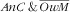 category and the absence of association with the
category and the absence of association with the
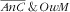 ). Age of the mother was not associated with the double burden because its strong increasing association with
). Age of the mother was not associated with the double burden because its strong increasing association with
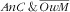 was compounded by the decreasing (even if not significant) association with the
was compounded by the decreasing (even if not significant) association with the
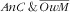 mother category. Similar to what was observed for age of the children, the strong increasing association of mother’s energy intake with the
mother category. Similar to what was observed for age of the children, the strong increasing association of mother’s energy intake with the
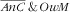 category translated to the
category translated to the
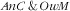 category. There were no specific factors of the double burden of
category. There were no specific factors of the double burden of
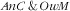 , except for parity: child–mother pairs featuring mothers with three children or more were more prone (RPR=2·5; 95 % CI 1·2, 4·8) to that double burden while marginally so for the first two categories of the response variable. For child–mother pairs with three children or more, this specific association may result from the combination of different pathways, e.g. pertaining to: (i) augmented risk of anaemia for the children via an augmented risk of anaemia for the mother due to multiple pregnancies(
57
); (ii) augmented risk of child anaemia related to diminished caring as we discussed above in the link with education(
45
,
48
,
49
); and (iii) traditional intra-household and social roles which may promote excess adiposity of the mother(
3
,
16
). Although this does not concern the same level of double burden, some of these pathways were also discussed at the individual level in the association of parity with the double burden of anaemia and/or Fe deficiency with excess adiposity among women aged 20–49 in Morocco and Tunisia(
7
).
, except for parity: child–mother pairs featuring mothers with three children or more were more prone (RPR=2·5; 95 % CI 1·2, 4·8) to that double burden while marginally so for the first two categories of the response variable. For child–mother pairs with three children or more, this specific association may result from the combination of different pathways, e.g. pertaining to: (i) augmented risk of anaemia for the children via an augmented risk of anaemia for the mother due to multiple pregnancies(
57
); (ii) augmented risk of child anaemia related to diminished caring as we discussed above in the link with education(
45
,
48
,
49
); and (iii) traditional intra-household and social roles which may promote excess adiposity of the mother(
3
,
16
). Although this does not concern the same level of double burden, some of these pathways were also discussed at the individual level in the association of parity with the double burden of anaemia and/or Fe deficiency with excess adiposity among women aged 20–49 in Morocco and Tunisia(
7
).
As for the anaemic child and obese mother in four categories (shown in the online supplementary material, Supplemental Table 2), the associated factors were mostly the same, except for the increasing relationship of birth weight with the not anaemic child and obese mother, as well as the anaemic child and obese mother categories. This is likely due to a reverse causal association because it has been shown that women featuring excess adiposity tend to have babies with higher birth weight( 58 ).
Beyond internal coherence discussed above, external comparisons are difficult for this specific issue of anaemia–overweight or anaemia–obesity double burden at the child–mother pairs level since published data are almost non-existent, especially in the MENA region.
Strengths and limitations of the study
A strength of our study is being the first, in a typical nutrition transition context of the MENA region, to assess the double burden of anaemia and overweight or obesity in child–mother pairs, performing detailed analyses of the nature of the co-occurrence (v. independence) and of the associations with sociodemographic factors. However, the cross-sectional design of the study limits the causal interpretation of observed associations. The study pertains to the most urbanized and developed region of Tunisia, as a case study of an ‘advanced nutrition transition’ situation for which the issue of such double burdens would seem especially relevant. So the generalizability of the results would rather pertain to such urban areas in similar contexts (in North Africa and likely more generally the MENA region) than to Tunisia as a whole (and especially not the 30 % rural population where the nutrition transition is less advanced). Also, our secondary analysis of data from a study which initially did not target specifically the 6–59 months age class was based on a sample of only about 400 pairs which may explain some borderline significant associations. As for all data collected by questionnaire, some of the covariates are prone to reporting bias.
Conclusion
In a typical context of nutrition transition in the MENA region, we have reported original findings regarding the double burden of undernutrition among children <5 years of age and overnutrition in mothers, especially for child anaemia and excess adiposity among mothers. Beyond the co-existence of these two types of malnutrition at population level, which is well documented in the MENA region, we specifically underlined at household level a significant proportion of pairs featuring both an anaemic child and an overweight (or obese) mother. These findings highlight a paradoxical situation among mother–child pairs sharing the same available resources and living environment, but which may have different negative impacts on children and women. Our data did not demonstrate a higher risk of child anaemia when the mother is overweight, nor vice versa, nor a very marked sociodemographic patterning of this double burden. Nevertheless, we showed that the prevalence of their co-occurrence is very significant and thus requires special attention, such as through prevention programmes which simultaneously target anaemia in children together with excess adiposity among mothers.
Acknowledgements
Financial support: This work was supported by the CORUS programme (Coopération pour la Recherche Universitaire et Scientifique, contract number 6028-2) of the French Ministry of Foreign Affairs; the National Institute of Nutrition and Food Technology of the Ministry of Health, Tunis, Tunisia; and IRD (French National Research Institute for Sustainable Development), Marseille, France. The funders had no role in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript; or in the decision to publish the results. Conflict of interest: The authors declare no conflicts of interest. Authorship: All authors designed the research; H.B.G., F.D. and J.E.A. supervised data collection in the field; S.S., M.M.A., P.T. and J.E.A. analysed the data and performed the statistical analyses; S.S., M.M.A., P.T. and J.E.A. wrote the paper; and S.S. had primary responsibility for final content. All authors read and approved the final manuscript. Ethics of human subject participation: This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the Ethics Committee on Human Research of the National Institute of Nutrition and Food Technology, the Tunisian National Council of Statistics and the Ethical and Deontological Consultative Committee of the French National Research Institute for Sustainable Development. Informed consent was obtained from all subjects in writing or verbally when not possible otherwise (e.g. illiteracy, verbal consent was then witnessed and formally recorded). Data were analysed anonymously. The study was registered at clinicaltrials.gov as NCT01844349.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S1368980018002495.
click here to view supplementary material
References
- 1. NCD Risk Factor Collaboration (2016) Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet 387, 1377–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Popkin BM (2001) The nutrition transition and obesity in the developing world. J Nutr 131, issue 3, 871S–873S. [DOI] [PubMed] [Google Scholar]
- 3. El Ati J, Traissac P, Delpeuch F et al. (2012) Gender obesity inequities are huge but differ greatly according to environment and socio-economics in a North African setting: a national cross-sectional study in Tunisia. PLoS One 7, e48153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. World Health Organization (2015) The Global Prevalence of Anaemia in 2011. Geneva: WHO. [Google Scholar]
- 5. Balarajan Y, Ramakrishnan U, Ozaltin E et al. (2011) Anaemia in low-income and middle-income countries. Lancet 378, 2123–2135. [DOI] [PubMed] [Google Scholar]
- 6. Bates K, Gjonca A & Leone T (2017) Double burden or double counting of child malnutrition? The methodological and theoretical implications of stuntingoverweight in low and middle income countries. J Epidemiol Community Health 71, 779–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gartner A, El Ati J, Traissac P et al. (2014) A double burden of overall or central adiposity and anemia or iron deficiency is prevalent but with little socioeconomic patterning among Moroccan and Tunisian urban women. J Nutr 144, 87–97. [DOI] [PubMed] [Google Scholar]
- 8. Zeba AN, Delisle HF, Renier G et al. (2012) The double burden of malnutrition and cardiometabolic risk widens the gender and socio-economic health gap: a study among adults in Burkina Faso (West Africa). Public Health Nutr 15, 2210–2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Doak CM, Adair LS, Monteiro C et al. (2000) Overweight and underweight coexist within households in Brazil, China and Russia. J Nutr 130, 2965–2971. [DOI] [PubMed] [Google Scholar]
- 10. Jehn M & Brewis A (2009) Paradoxical malnutrition in mother–child pairs: untangling the phenomenon of over- and under-nutrition in underdeveloped economies. Econ Hum Biol 7, 28–35. [DOI] [PubMed] [Google Scholar]
- 11. Garrett JL & Ruel MT (2005) Stunted child–overweight mother pairs: prevalence and association with economic development and urbanization. Food Nutr Bull 26, 209–221. [DOI] [PubMed] [Google Scholar]
- 12. Oddo VM, Rah JH, Semba RD et al. (2012) Predictors of maternal and child double burden of malnutrition in rural Indonesia and Bangladesh. Am J Clin Nutr 95, 951–958. [DOI] [PubMed] [Google Scholar]
- 13. Lee J, Houser RF, Must A et al. (2010) Disentangling nutritional factors and household characteristics related to child stunting and maternal overweight in Guatemala. Econ Hum Biol 8, 188–196. [DOI] [PubMed] [Google Scholar]
- 14. Deleuze Ntandou Bouzitou G, Fayomi B & Delisle H (2005) Child malnutrition and maternal overweight in same households in poor urban areas of Benin. Sante 15, 263–270. [PubMed] [Google Scholar]
- 15. Kroker-Lobos MF, Pedroza-Tobias A, Pedraza LS et al. (2014) The double burden of undernutrition and excess body weight in Mexico. Am J Clin Nutr 100, issue 6, 1652S–168S. [DOI] [PubMed] [Google Scholar]
- 16. Traissac P, El Ati J, Gartner A et al. (2016) Gender inequalities in excess adiposity and anaemia combine in a large double burden of malnutrition gap detrimental to women in an urban area in North Africa. Public Health Nutr 19, 1428–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Atek M, Traissac P, El Ati J et al. (2013) Obesity and association with area of residence, gender and socio-economic factors in Algerian and Tunisian adults. PLoS One 8, e75640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. UNICEF, Ministère de la Santé Publique & Office National de la Famille et de la Population (2008) Enquête sur la santé et le bien être de la mère et l’enfant. Tunisia Multiple Indicator Cluster Survey 3 (MICS 3). https://mics-surveys-prod.s3.amazonaws.com/MICS3/Middle%20East%20and%20North%20Africa/Tunisia/2006/Final/Tunisia%202006%20MICS_French.pdf (accessed September 2017).
- 19. Institut National de Nutrition et de Technologie Alimentaire (2002) Anémie en Tunisie: Causes et mesures d’interventions. Tunis: Ministry of Public Health.
- 20. Institut National de la Statistique (2005) Recensement général des habitants et de l’habitat en 2004. Tunis: INS. [Google Scholar]
- 21. United Nations Development Programme (2010) Human Development Report 2010. New York: UNDP. [Google Scholar]
- 22. Lohman TG, Roche AF & Martorell R (1988) Anthropometric Standardization Reference Manual. Champaign IL: Human Kinetics. [Google Scholar]
- 23. de Onis M, Onyango AW, Borghi E et al. (2006) Comparison of the World Health Organization (WHO) Child Growth Standards and the National Center for Health Statistics/WHO international growth reference: implications for child health programmes. Public Health Nutr 9, 942–947. [DOI] [PubMed] [Google Scholar]
- 24. World Health Organization (2000) Obesity: Preventing and Managing the Global Epidemic. Report of WHO Consultation on Obesity. WHO Technical Report Series no. 894. Geneva: WHO. [PubMed] [Google Scholar]
- 25. World Health Organization, UNICEF & United Nations University (2001) Iron Deficiency Anaemia: Assessment, Prevention, and Control. A Guide for Programme Managers (WHO/NHD/01.3). Geneva: WHO. [Google Scholar]
- 26. Traissac P & Martin-Prevel Y (2012) Alternatives to principal components analysis to derive asset-based indices to measure socio-economic position in low- and middle-income countries: the case for multiple correspondence analysis. Int J Epidemiol 41, 1207–1208. [DOI] [PubMed] [Google Scholar]
- 27. Willett W (2013. ) Nutritional Epidemiology , 3rd ed. New York: Oxford University Press. [Google Scholar]
- 28. El Ati J, Béji C, Farhat A et al. (2007) Table de composition des aliments tunisiens. Tunisia: INNTA/IRD. [Google Scholar]
- 29. ESHA Research, Inc. (2003) Food Processor Software Version 8.3. Salem, OR: ESHA Research, Inc. [Google Scholar]
- 30. Hatloy A, Hallund J, Diarra MM et al. (2000) Food variety, socioeconomic status and nutritional status in urban and rural areas in Koutiala (Mali). Public Health Nutr 3, 57–65. [DOI] [PubMed] [Google Scholar]
- 31. El Ati J, Houti L, Farhat A et al. (2004) Development, reproducibility and validity of a physical activity frequency questionnaire in North Africa. Arab J Food Nutr 5, 148–167. [Google Scholar]
- 32. Ainsworth BE, Haskell WL, Whitt MC et al. (2000) Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc 32, 9 Suppl., S498–S504. [DOI] [PubMed] [Google Scholar]
- 33. Henry CJ (2005) Basal metabolic rate studies in humans: measurement and development of new equations. Public Health Nutr 8, 1133–1152. [DOI] [PubMed] [Google Scholar]
- 34. Food Agriculture Organization of the United Nations, World Health Organization & United Nations University (2004) Human Energy Requirements: Energy Requirement of Adults. Joint FAO/WHO/UNU Expert Consultation. Food and Nutrition Technical Report Series no. 1. Rome: FAO. [Google Scholar]
- 35. Hosmer DW & Lemeshow S (2000) Applied Logistic Regression, 2nd ed. New York: John Wiley & Sons. [Google Scholar]
- 36. UNICEF, Ministère de la Santé Publique & Direction des Soins de Santé de Base (2000) Enquête sur la santé et le bien être de la mère et de l’enfant. Tunisia Multiple Indicator Cluster Survey 2 (MICS 2). Tunis: Ministère de la Santé Publique.
- 37. Keino S, Plasqui G, Ettyang G et al. (2014) Determinants of stunting and overweight among young children and adolescents in sub-Saharan Africa. Food Nutr Bull 35, 167–178. [DOI] [PubMed] [Google Scholar]
- 38. de Onis M, Blossner M & Borghi E (2010) Global prevalence and trends of overweight and obesity among preschool children. Am J Clin Nutr 92, 1257–1264. [DOI] [PubMed] [Google Scholar]
- 39. Kanter R & Caballero B (2012) Global gender disparities in obesity: a review. Adv Nutr 3, 491–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. El Ati J, Lefevre P, Beji C et al. (2008) Aetiological factors and perception of anaemia in Tunisian women of reproductive age. Public Health Nutr 11, 729–736. [DOI] [PubMed] [Google Scholar]
- 41. Leroy JL, Habicht JP, Gonzalez de Cossio T et al. (2014) Maternal education mitigates the negative effects of higher income on the double burden of child stunting and maternal overweight in rural Mexico. J Nutr 144, 765–770. [DOI] [PubMed] [Google Scholar]
- 42. Aitsi-Selmi A (2015) Households with a stunted child and obese mother: trends and child feeding practices in a middle-income country, 1992–2008. Matern Child Health J 19, 1284–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Garrett J & Ruel MT (2005) The coexistence of child undernutrition and maternal overweight: prevalence, hypotheses, and programme and policy implications. Matern Child Nutr 1, 185–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Samaniego-Vaesken ML, Partearroyo T, Olza J et al. (2017) Iron intake and dietary sources in the Spanish population: findings from the ANIBES study. Nutrients 9, E203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Leal LP, Batista Filho M, Lira PI et al. (2011) Prevalence of anemia and associated factors in children aged 6–59 months in Pernambuco, Northeastern Brazil. Rev Saude Publica 45, 457–466. [DOI] [PubMed] [Google Scholar]
- 46. Semedo RM, Santos MM, Baiao MR et al. (2014) Prevalence of anaemia and associated factors among children below five years of age in Cape Verde, West Africa. J Health Popul Nutr 32, 646–657. [PMC free article] [PubMed] [Google Scholar]
- 47. Kuziga F, Adoke Y & Wanyenze RK (2017) Prevalence and factors associated with anaemia among children aged 6 to 59 months in Namutumba district, Uganda: a cross- sectional study. BMC Pediatr 17, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ngnie-Teta I, Receveur O & Kuate-Defo B (2007) Risk factors for moderate to severe anemia among children in Benin and Mali: insights from a multilevel analysis. Food Nutr Bull 28, 76–89. [DOI] [PubMed] [Google Scholar]
- 49. Tympa-Psirropoulou E, Vagenas C, Dafni O et al. (2008) Environmental risk factors for iron deficiency anemia in children 12–24 months old in the area of Thessalia in Greece. Hippokratia 12, 240–250. [PMC free article] [PubMed] [Google Scholar]
- 50. Zhao A, Zhang Y, Peng Y et al. (2012) Prevalence of anemia and its risk factors among children 6–36 months old in Burma. Am J Trop Med Hyg 87, 306–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Halileh S & Gordon NH (2006) Determinants of anemia in pre-school children in the occupied Palestinian territory. J Trop Pediatr 52, 12–18. [DOI] [PubMed] [Google Scholar]
- 52. McDonald CM, McLean J, Kroeun H et al. (2015) Household food insecurity and dietary diversity as correlates of maternal and child undernutrition in rural Cambodia. Eur J Clin Nutr 69, 242–246. [DOI] [PubMed] [Google Scholar]
- 53. Jones-Smith JC, Gordon-Larsen P, Siddiqi A et al. (2011) Cross-national comparisons of time trends in overweight inequality by socioeconomic status among women using repeated cross-sectional surveys from 37 developing countries, 1989–2007. Am J Epidemiol 173, 667–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Subramanian SV, Perkins JM, Ozaltin E et al. (2011) Weight of nations: a socioeconomic analysis of women in low- to middle-income countries. Am J Clin Nutr 93, 413–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Monteiro CA, Moura EC, Conde WL et al. (2004) Socioeconomic status and obesity in adult populations of developing countries: a review. Bull World Health Organ 82, 940–946. [PMC free article] [PubMed] [Google Scholar]
- 56. Hill JO, Wyatt HR & Peters JC (2012) Energy balance and obesity. Circulation 126, 126–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ru Y, Pressman EK, Cooper EM et al. (2016) Iron deficiency and anemia are prevalent in women with multiple gestations. Am J Clin Nutr 104, 1052–1060. [DOI] [PubMed] [Google Scholar]
- 58. McCloskey K, Ponsonby AL, Collier F et al. (2016) The association between higher maternal pre-pregnancy body mass index and increased birth weight, adiposity and inflammation in the newborn. Pediatr Obes 13, 46–53. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit https://doi.org/10.1017/S1368980018002495.
click here to view supplementary material