Abstract
Objective:
The present study investigated the risks and benefits of routine Fe–folic acid (IFA) supplementation in pregnant women living in low- and high-groundwater-Fe areas in Bangladesh.
Design:
A case-controlled prospective longitudinal study design was used to compare the effect of daily Fe (60 mg) and folic acid (400 μg) supplementation for 3·5 months.
Setting:
A rural community in Bangladesh.
Participants:
Pregnant women living in low-groundwater-Fe areas (n 260) and high-groundwater-Fe areas (n 262).
Results:
Mean Hb and serum ferritin concentrations at baseline were significantly higher in pregnant women in the high-groundwater-Fe areas. After supplementation, the mean change in Hb concentration in the women in the low-groundwater-Fe areas (0·10 mg/dl) was higher than that in the pregnant women in the high-groundwater-Fe areas (–0·08 mg/dl; P = 0·052). No significant changes in the prevalence of anaemia or Fe deficiency (ID) in either group were observed after IFA supplementation; however, the prevalence of Fe-deficiency anaemia (IDA) decreased significantly in the women in the low-groundwater-Fe areas. The risk of anaemia, ID and IDA after supplementation did not differ significantly between the groups. None of the participants had Fe overload. However, a significant proportion of the women in the high- and low-groundwater-Fe areas remained anaemic and Fe-deficient after supplementation.
Conclusion:
IFA supplementation significantly increased the Hb concentration in pregnant women living in the low-groundwater-Fe areas. Routine supplementation with 60 mg Fe and 400 μg folic acid does not pose any significant risk of haemoconcentration or Fe overload. Further research to identify other nutritional and non-nutritional contributors to anaemia is warranted to prevent and treat anaemia.
Keywords: Anaemia, Iron deficiency, Pregnant women, Groundwater-iron, Iron–folic acid supplements
Anaemia is recognized as a major public health problem in Bangladesh(1), affecting nearly half of the pregnant women in the country(2). Fe deficiency (ID) is thought to be the primary cause of anaemia in the country and a routine Fe–folic acid (IFA) supplementation programme has been in effect for the prevention of anaemia and ID in pregnant women for the past few decades(3). The latest National Micronutrient Survey 2011–12 (NMS 2011–12) revealed a very low prevalence of ID (7·1 %) and Fe-deficiency anaemia (IDA; 4·8 %) among non-pregnant and non-lactating Bangladeshi women(4). At present, there are no national-level data on ID in pregnant women in Bangladesh. A small-scale study of women of reproductive age in a rural area of Bangladesh showed a positive association between Fe status, based on plasma ferritin concentration, and daily Fe intake from drinking-water and found no IDA in this population(5). These studies concluded that the low prevalence of ID and IDA could be attributed to drinking Fe-containing groundwater from tube wells(4,5). In a recent study of pregnant women, we also found a significant independent association between Fe status and daily Fe intake from drinking tube-well water(6).
It is important to note that a large proportion of Bangladeshis living in rural areas use groundwater from tube wells for drinking and cooking(7). The Arsenic Contamination of Groundwater in Bangladesh (ACGB) survey reported varying amounts of Fe in tube-well water in Bangladesh, with a very high concentration of Fe in some parts of the country(8). A recent article based on NMS 2011–12 data reported a differential prevalence of anaemia and ID in Bangladeshi non-pregnant and non-lactating women living in areas with high and low levels of Fe in the groundwater, as identified by the ACGB report(9). In a study in preselected high- and low-groundwater-Fe areas, we also observed differential prevalence of anaemia and ID in pregnant women(6).
The evidence of a very low prevalence of IDA in non-pregnant and non-lactating women(4) may have serious implications for the current IFA supplementation programme for controlling anaemia and ID in pregnant women in the country. Giving too much Fe to Fe-replete women may increase their risk of undesirable side-effects. High doses of Fe supplements have been found to be associated with consequences of oxidative stress thought to be due to Fe overload and haemoconcentration(10–12). Both Fe overload and haemoconcentration during pregnancy may be counterproductive to the well-being of the mother and the fetus(12,13). Nevertheless, it is important to recognize that Fe requirements increase significantly during pregnancy to meet the extra demand for Fe due to physiological changes and fetal development(12–14). If the pre-pregnancy Fe store is barely enough to maintain an adequate status during the pre-pregnancy stage, the post-conception additional demand for Fe during pregnancy may not be met by the body store. Routine Fe supplementation is indicated in such a situation. Given the recent findings of low ID in non-pregnant and non-lactating women and children(4) and its association with drinking Fe-containing groundwater from tube wells(4–6), the relevance of the IFA supplementation programme during pregnancy needs further investigation. Thus, the present study investigated the effect of routine IFA supplementation on the prevalence of anaemia, ID and risk of Fe overload in pregnant women living in areas in Bangladesh with high and low levels of Fe in the groundwater.
Methods and participants
Study design
A case-controlled prospective longitudinal study design was used to compare the effect of routine daily supplementation with IFA tablets containing 60 mg elemental Fe and 400 µg folic acid (the current government policy to prevent anaemia and ID in pregnant women) in pregnant women living in areas in Bangladesh with high and low levels of Fe in the groundwater.
Participants and study areas
Pregnant women with a gestational age ≤20 weeks living in rural areas were recruited for the present study. Two upazilas (sub-districts; Sharishabari and Pirgacha) in predominantly high-groundwater-Fe areas and two upazilas (Lalmohon and Badarganj) in predominantly low-groundwater-Fe areas were selected. The ACGB survey report was consulted to identify areas with high and low groundwater-Fe levels(8). First, the tube wells in the selected sub-districts were identified in the ACGB survey data file, and the mean Fe content of the water from the tube wells in each sub-district was calculated. A low-Fe sub-district (area) was defined by a mean value lower than the national median (1·1 mg/l) provided by the ACGB survey, and mean values ≥1·1 mg/l were considered high. A total of twenty-four unions (administrative units, consisting of a cluster of villages), six unions from each upazila, were randomly selected and then a convenience sampling method was applied to recruit the pregnant women. The study was carried out from April to October 2015. The study was conducted according to the guidelines provided by the Declaration of Helsinki and the research proposal was approved by the Faculty of Biological Sciences, University of Dhaka, Dhaka, Bangladesh and the Bangladesh Medical Research Council, Dhaka, Bangladesh.
Sample size
The sample size calculation was based on 90 % power and a 5 % significance level for two variables, namely prevalence of anaemia and prevalence of IDA. Based on the Bangladesh Demographic and Health Survey (BDHS) data(2), we considered a 50 % prevalence of anaemia among pregnant women and assumed that at least half of the pregnant women had IDA. To detect a difference of 25 % in the prevalence of anaemia between the two groups (pregnant women living in low- and high-groundwater-Fe areas) after IFA supplementation, eighty-four pregnant women per group would be required. Detecting a difference of 25 % in the prevalence of IDA between the two groups after IFA supplementation would require 168 pregnant women per group. We considered the larger (n 168) of the above two sample size estimates and, after including a 30 % allowance for dropout during follow-up, each group required 240 pregnant women.
Selection of participants
The field staff identified pregnant women by the date of their last menstrual period and carried out door-to-door visits, listing all eligible pregnant women. Pregnant women who had already visited the antenatal clinic for a check-up during the current pregnancy were excluded from the study. The purpose and exact nature of the study were explained to all prospective participants. The eligible pregnant women were invited to attend the antenatal clinic on a pre-set date for data and blood collection. On the day of data collection, the purpose of the study was explained again, and those who agreed to participate were requested to sign or place their thumb print on the consent form approved by the institutional review board.
Iron–folic acid supplementation
There were two participant groups: Group 1, 260 pregnant women living in areas with low Fe levels in the groundwater; and Group 2, 262 pregnant women living in areas with high Fe levels in the groundwater. Both groups were given IFA supplements daily for 3·5 months; therefore, a maximum of 104 tablets were taken during this period. This procedure was done according to the government protocol at the time and thus no potential harm would be intentionally incurred as a result of the study.
Supplement delivery and compliance recording
The enrolled pregnant women were given 20 IFA tablets (two strips of 10 tablets each) when baseline data were collected. A physician explained to them the benefits of taking the IFA tablets during pregnancy and gave instructions on how and when to take the tablets. The field assistants visited each woman on a weekly basis to record compliance (except for the first visit, which took place two weeks after the baseline blood collection). During each visit, the field assistants provided IFA tablets to the pregnant women to last them for another 10 d. During the house visit, the field assistants also recorded the reasons for any irregular consumption and then provided appropriate counselling to motivate the pregnant women to take the tablets as advised. The field assistants also advised the women to visit the antenatal clinic and receive other antenatal services. The field supervisors regularly attended field visits to oversee the activities of the field assistants, cross-check the compliance, and compare the number of tablets remaining with the number of tablets delivered by the field assistant.
Data collection
All consenting pregnant women were tested for pregnancy using a commercial pregnancy detection kit. A total of 530 pregnant women (268 from low-groundwater-Fe areas and 262 from high-groundwater-Fe areas) were recruited for the study. The response rate was over 90 %. Eight pregnant women were excluded because of either incomplete data or insufficient blood samples for assessment of all biochemical parameters.
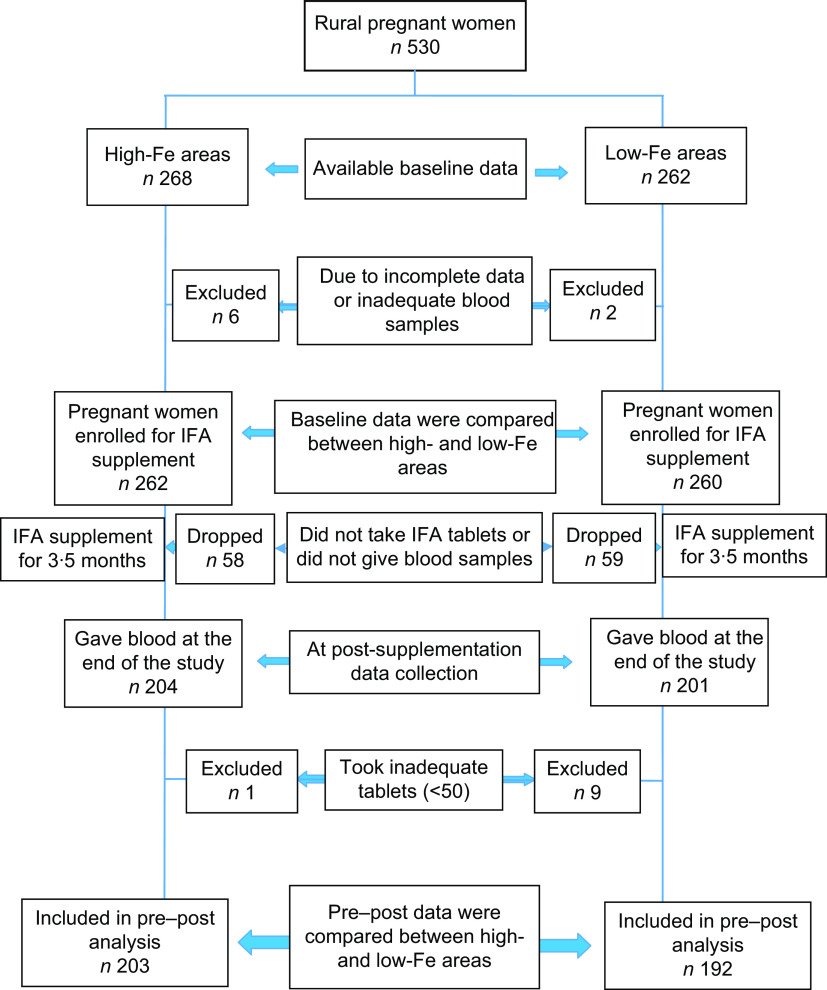
All recruited women were interviewed by trained interviewers at the antenatal clinic before blood collection to obtain socio-economic and pregnancy-related information. Information on the usual consumption pattern of selected food items rich in micronutrients was obtained by an interview, using a 7 d FFQ. A 30 d recall was used to obtain information on the consumption of vitamin and mineral supplements. Furthermore, information on morbidity was also collected. Following the interview, 5 ml of venous blood was collected. A second blood specimen was drawn from the pregnant women at the end of the 3·5 months of IFA supplementation. In addition, information was also collected on dietary pattern, consumption of any vitamin or mineral supplements other than that which was given, and morbidity. Figure 1 shows the participant selection process. Details of the data collection and blood sample processing procedures are described elsewhere(6).
Fig. 1.
Flowchart showing the selection of participants for the present study to determine the effect of iron–folic acid (IFA) supplementation among pregnant women living in low- and high-groundwater-iron areas of Bangladesh
Analytical procedures
Hb concentration was measured by a HemoCue Hb 301 haemoglobinometer (Hemocue, Ängelholm, Sweden). Serum folate concentration was measured using commercial kits from Roche Diagnostics on a clinical chemistry analyser (Cobas C311; Roche Diagnostics, Mannheim, Germany). Serum ferritin and C-reactive protein levels were measured by an ELISA using commercial kits (BioCheck, Inc., San Francisco, CA, USA). Serum α1-acid glycoprotein levels were measured by the immunoturbidimetric method using commercial kits from Roche Diagnostics on Cobas C311. Haemoglobinopathies (HbS, HbE, HbF, HbA and other haemoglobins) were identified by capillary zone electrophoresis of Hb at pH 9·4 and a high voltage of 9600 V (Capillary 2 system; Sebia, Evry, France).
Data analysis
Statistical analysis was carried out using the statistical software packages IBM SPSS Statistics version 20 and SAS for Windows version 9.3. Sociodemographic and pregnancy-related characteristics and general morbidity and diet-related data of the pregnant women by area of residence, i.e. low- or high-groundwater-Fe areas, were compared by the χ2 test.
Anaemia was defined as a Hb concentration <11·0 g/dl(15). ID was defined as a serum ferritin concentration <15·0 μg/l(15). IDA was defined as a Hb concentration <11·0 g/dl and a serum ferritin concentration <15·0 μg/l(15). Haemoconcentration in pregnant women was defined by a Hb concentration >13·0 g/dl(10) and Fe overload was defined by a serum ferritin concentration >150 μg/l(15). Folate deficiency was defined as a serum folate concentration <4·4 ng/ml(16). In the presence of inflammation or infection, serum ferritin can be in the normal range or elevated despite deficient stores(17). Hence, serum ferritin concentrations were adjusted for elevated C-reactive protein (≥10 mg/l) and α1-acid glycoprotein (≥1·0 g/l) concentrations by mathematical correction(18).
Distributions of Hb, serum ferritin and folate concentrations were checked by the Kolmogorov–Smirnov goodness- of-fit test. Because serum ferritin concentrations, both at baseline and post-supplementation, were not normally distributed, data were log-transformed, and the outputs back-transformed to the original scale are presented. All data are presented as means and 95 % CI.
The data from pregnant women who consumed >50 tablets were analysed to compare the effect of IFA supplementation in areas with different groundwater-Fe concentrations. Repeated-measures ANOVA was performed using the generalized estimating equations approach within the SAS GENMOD procedure with time as the repeated measure. The χ2 test score, based on a generalized score function, was used to compare the concentrations of all biochemical variables from baseline to after the supplementation period, both within and between the two groups. A general linear model was used, with the mean change in concentration of each biochemical variable as the dependent variable, to compare the change over time in the two groups. Among the socio-economic variables, the husband’s occupation and possession of cultivable land were found to be significantly different between the two groups of pregnant women. Furthermore, the baseline values of all biochemical parameters and the number of IFA tablets consumed were significantly different between the two groups. Therefore, to account for these potential confounders, the results were adjusted for corresponding baseline values, the number of IFA tablets consumed, the husband’s occupation and possession of cultivable land.
Both at baseline and at the end of the supplementation, the prevalence of anaemia, ID, IDA and folate deficiency in the pregnant women in the two groups were compared by the χ2 test or Fisher’s exact test, as appropriate. Furthermore, differences in prevalence in each group between pre- and post-supplementation were compared by Cochran’s Q test or Fisher’s exact test, as appropriate. The prevalence of anaemia, ID, IDA and folate deficiency at baseline and at the end of the supplementation period in the pregnant women in the low- and high-groundwater-Fe areas were further compared by logistic regression with deficiency status (present/absent) as the dependent variable. The OR and 95 % CI for low-groundwater-Fe areas were estimated by the likelihood ratio method. To account for potential confounders (as mentioned above), the data were adjusted for the baseline status of each variable, the number of tablets consumed, the husband’s occupation and possession of cultivable land. Finally, all analyses were repeated on participants without haemoglobinopathies.
Results
Dropout rate
Of the 522 pregnant women enrolled at baseline, 405 completed the 3·5-month IFA supplementation protocol. The overall dropout rate was 22·4 %. In high-groundwater-Fe areas, the dropout rate was 22·1 %, and in low-groundwater-Fe areas it was 22·7 %; the difference between the groups was not statistically significant.
Comparison between pregnant women who dropped out and those who completed the study
The socio-economic and pregnancy-related characteristics and biochemical parameters of the pregnant women who completed the study and those who did not were not significantly different, except for serum folate concentration which was significantly higher (P = 0·02) in those who did not complete the study.
Compliance
Ten pregnant women (one in a high-Fe area and nine in low-Fe areas) were excluded from the analysis because they took fewer than 50 % of the tablets provided. Three hundred and ninety-five pregnant women (203 in the high-Fe areas and 192 in the low-Fe areas) were included in the final analysis. Thirty-nine per cent of the pregnant women in the high-groundwater-Fe areas consumed 100 or more tablets, 55 % consumed 80–99 tablets and 6 % consumed 50–79 tablets. In the low-groundwater-Fe areas, 14 % of the pregnant women consumed 100 or more tablets, 66 % consumed 80–99 tablets and 20 % consumed 50–79 tablets. The mean (sd) number of tablets consumed by the pregnant women in the high-groundwater-Fe areas (97·8 (11·4)) was significantly higher (P = 0·001) than the number of tablets taken by the pregnant women in the low-groundwater-Fe areas (88·8 (11·6)).
Characteristics of the pregnant women who completed the study
There were no statistically significant differences in age, parity and gestational age between the pregnant women who completed the study in the low- and high-groundwater-Fe areas (Table 1). A significantly (P = 0·012) higher proportion of the participants’ husbands in the low-groundwater-Fe areas was functionally illiterate and either unemployed or daily labourers, compared with the pregnant women’s husbands in the high-groundwater-Fe areas (P = 0·001). Moreover, a significantly higher proportion of the pregnant women in the low-groundwater-Fe areas had no cultivable land.
Table 1.
Sociodemographic and pregnancy-related characteristics of the study participants who completed the supplementation protocol, by areas with low and high iron in the groundwater, Bangladesh, April–October 2015
| High-groundwater-Fe areas (n 203) | Low-groundwater-Fe areas (n 192) | ||||
|---|---|---|---|---|---|
| Characteristic | n | % | n | % | P value* |
| Age (years) | |||||
| 13–19 | 46 | 22·2 | 45 | 23·4 | 0·085 |
| 20–24 | 56 | 27·6 | 69 | 35·9 | |
| ≥25 | 102 | 50·2 | 78 | 40·7 | |
| Parity | |||||
| Nulliparous | 69 | 34·0 | 74 | 38·5 | 0·560 |
| One | 82 | 40·4 | 76 | 39·6 | |
| Two or more | 52 | 25·6 | 42 | 21·9 | |
| Gestational age (weeks) | |||||
| ≤12 | 25 | 12·3 | 22 | 11·5 | 0·793 |
| >12 | 178 | 87·7 | 170 | 88·5 | |
| Participant’s education | |||||
| Functionally illiterate | 76 | 37·5 | 93 | 48·4 | 0·100 |
| Incomplete secondary | 92 | 45·3 | 71 | 37·0 | |
| Complete secondary and higher | 35 | 17·2 | 28 | 14·6 | |
| Husband’s education | |||||
| Functionally illiterate | 105 | 51·8 | 125 | 65·1 | 0·012 |
| Incomplete secondary | 48 | 23·6 | 39 | 20·3 | |
| Complete secondary and higher | 50 | 24·6 | 28 | 14·6 | |
| Husband’s occupation | |||||
| Unemployed/labourer | 54 | 26·6 | 94 | 49·0 | 0·001 |
| Agriculture | 67 | 33·0 | 46 | 24·0 | |
| Business | 49 | 24·1 | 31 | 16·1 | |
| Services | 33 | 16·3 | 21 | 10·9 | |
| Participant’s occupation | |||||
| No | 194 | 95·6 | 184 | 95·8 | 0·896 |
| Yes | 9 | 4·4 | 8 | 4·2 | |
| Household size | |||||
| Small, ≤4 | 125 | 61·6 | 110 | 57·3 | 0·216 |
| Large, ≥5 | 78 | 38·4 | 82 | 42·7 | |
| Homestead ownership | |||||
| Own homestead | 195 | 96·1 | 176 | 91·7 | 0·068 |
| Others | 8 | 3·9 | 16 | 8·3 | |
| Cultivable land ownership (acres†) | |||||
| No land | 104 | 51·2 | 117 | 60·9 | 0·014 |
| Small landholding, <0·50 | 63 | 30·9 | 34 | 17·7 | |
| Sizeable landholding, >0·50 | 36 | 17·9 | 41 | 21·4 | |
From χ2 test.
1 acre = 0·40 ha.
Dietary pattern
During the week preceding the baseline interview, the pregnant women living in the areas with low groundwater Fe had a significantly higher frequency of consumption of chicken, milk and milk products. There were no significant differences in consumption of red meat, green leafy vegetables or fruit between the two groups. No significant differences in the frequency of consumption of red meat, chicken, milk and milk products, green leafy vegetables or fruit were observed between the two groups during the week preceding the post-supplementation interview.
Morbidity pattern
No statistically significant difference was observed in morbidity pattern, except for respiratory distress at baseline, between the pregnant women in the high- and low-groundwater-Fe areas during the week preceding the baseline and post-supplementation data collection visits. In areas with low levels of Fe in the groundwater, a significantly higher proportion of pregnant women had respiratory distress. However, both at the baseline and post-supplementation visits, there were no statistically significant differences in the prevalence of elevated C-reactive protein and elevated α1-acid glycoprotein in the pregnant women in the two areas (data not shown).
Effect of iron–folic acid supplementation on biochemical measures
The mean Hb and serum ferritin concentrations at baseline were significantly lower and the serum folate concentration was significantly higher in the pregnant women living in the low-groundwater-Fe areas than in the pregnant women living in the high-groundwater-Fe areas (Table 2). Both Hb and serum ferritin concentrations decreased significantly after supplementation in the pregnant women living in the high-groundwater-Fe areas (P < 0·05). Conversely, the Hb concentration increased significantly in the pregnant women in the low-groundwater-Fe areas, but no significant change was observed in serum ferritin concentration. The serum folate concentration increased significantly in the pregnant women in both groups after supplementation.
Table 2.
Hb, serum ferritin and folate concentrations in the study participants at baseline and after the 3·5-month supplementation period, and the difference between baseline and post-supplementation, by areas with low and high iron in the groundwater, Bangladesh, April–October 2015
| High-groundwater-Fe areas (n 203) | Low-groundwater-Fe areas(n 192) | ||||
|---|---|---|---|---|---|
| Variable | Mean* | 95 % CI | Mean* | 95 % CI | P value† |
| Hb (mg/dl) | |||||
| Baseline | 11·43a,x | 11·29, 11·57 | 11·21a,y | 11·06, 11·35 | |
| Post | 11·29b | 11·15, 11·42 | 11·37b | 11·24, 11·50 | |
| Change (baseline – post-supplementation) | –0·08 | –0·20, 0·04 | 0·10 | –0·03, 0·22 | 0·052 |
| Serum ferritin (µg/l)‡ | |||||
| Baseline | 25·80a,x | 23·22, 28·67 | 19·25y | 17·06, 21·73 | |
| Post | 22·23b,x | 20·34, 24·31 | 17·86y | 16·22, 19·66 | |
| Change (baseline – post-supplementation) | –4·97 | –6·86, –3·07 | –7·21 | –9·16, –5·26 | 0·120 |
| Serum folate (ng/ml) | |||||
| Baseline | 8·35a,x | 7·90, 8·80 | 9·10a,y | 8·61, 9·60 | |
| Post | 13·44b | 12·88, 14·01 | 13·19b | 12·62, 13·77 | |
| Change (baseline – post-supplementation) | 4·82 | 4·28, 5·37 | 4·40 | 3·84, 4·96 | 0·303 |
a,bMean values within a column for each of the variables (within each groundwater-Fe area) with unlike subscript letters were significantly different (P < 0·05).
x,yMean values within a row for each of the variables (at each time point) with unlike subscript letters were significantly different (P < 0·05).
Repeated-measures ANOVA, with time as the repeated measure, was performed.
Based on the general linear model with change in concentration as the dependent variable and adjusted for corresponding baseline value, total number of tablets consumed, husband’s occupation and possession of cultivable land. Serum ferritin was adjusted for elevated serum C-reactive protein and α1-acid glycoprotein by mathematical correction.
P values based on log-transformed serum ferritin concentrations.
After adjusting for potential confounders, the mean change in Hb concentration after supplementation was greater (P = 0·052) in the pregnant women living in the low-groundwater-Fe areas than in the pregnant women living in the high-groundwater-Fe areas (Table 2). The mean changes in serum ferritin and folate concentrations did not differ significantly between the two groups.
Effect of iron–folic acid supplementation on anaemia and iron and folate deficiency
The prevalence of anaemia and folate deficiency did not differ significantly at baseline between the pregnant women living in high- and low-groundwater-Fe areas, while the prevalence of ID and IDA were significantly higher in the pregnant women living in the low-groundwater-Fe areas (Table 3). After 3·5 months of supplementation, the prevalence of anaemia decreased only in the pregnant women living in the low-groundwater-Fe areas (P = 0·057), while the prevalence of ID remained unchanged in the pregnant women in both areas. Interestingly, the prevalence of IDA in the pregnant women living in the low-groundwater-Fe areas decreased significantly (P = 0·02) after supplementation. After supplementation, the prevalence of folate deficiency decreased significantly (P < 0·007) in both groups.
Table 3.
Prevalence of anaemia, iron and folate deficiencies in the study participants at baseline and after the 3·5-month supplementation period, and the estimates of OR for prevalence of anaemia, iron and folate deficiencies, by areas with low and high iron in the groundwater, Bangladesh, April–October 2015
| High-groundwater-Fe areas(n 203) | Low-groundwater-Fe areas(n 192) | Low- v. high-groundwater-Fe areas | ||||
|---|---|---|---|---|---|---|
| % | % | P value* | OR | 95 % CI | P value† | |
| Anaemia‡ | ||||||
| Baseline | 32·5 | 40·1 | 0·142 | – | – | |
| Post | 37·9 | 32·8 | 0·295 | 0·65 | 0·40, 1·06 | 0·083 |
| P = 0·138** | P = 0·057** | |||||
| Fe deficiency§ | ||||||
| Baseline | 21·7 | 34·4 | 0·006 | – | – | |
| Post | 21·2 | 34·9 | 0·002 | 0·74 | 0·44, 1·22 | 0·234 |
| P = 0·887** | P = 0·896** | |||||
| Fe-deficiency anaemia║ | ||||||
| Baseline | 8·4 | 21·4 | 0·0003 | – | – | |
| Post | 10·3 | 14·1 | 0·283 | 0·73 | 0·35, 1·51 | 0·396 |
| P = 0·371** | P = 0·02** | |||||
| Folate deficiency¶ | ||||||
| Baseline | 7·4 | 8·9 | 0·712 | – | – | |
| Post | 1·0 | 3·1 | 0·166 | 2·83 | 0·51, 15·72 | 0·235 |
| P = 0·001** | P = 0·007** | |||||
Prevalence values within a row (between high- and low-groundwater-Fe areas) were compared with the χ2 test or Fisher’s exact test, as appropriate.
Based on logistic regression and adjusted for the dependent variable, corresponding baseline value, total number of tablets consumed, husband’s occupation and possession of cultivable land.
Hb < 11·0 g/dl.
Serum ferritin < 15·0 µg/l.
Hb < 11·0 g/dl and serum ferritin < 15·0 µg/l.
Serum folate < 4·4 ng/ml.
Prevalence values within a column (within each groundwater-Fe area) were compared by Cochran’s Q test or Fisher’s exact test, as appropriate.
The results of logistic regression analysis, after controlling for potential confounding factors, showed that the risk (OR) for developing anaemia (OR = 0·65; 95 % CI 0·40, 1·06), ID (OR = 0·74; 95 % CI 0·44, 1·22) and IDA (OR = 0·73; 95 % CI 0·35, 1·51) after supplementation was lower in the pregnant women living in areas with low levels of Fe in the groundwater than in the pregnant women living in areas with high levels of Fe in the groundwater. However, the differences between the groups were not statistically significant. Similarly, the risk for developing folate deficiency in the two groups of pregnant women did not differ significantly after supplementation (Table 3).
Haemoconcentration and iron overload
Nearly 6·0 % (n 12) of the pregnant women living in areas with high levels of Fe in the groundwater and 2·6 % (n 5) of the pregnant women living in areas with low levels of Fe in the groundwater had haemoconcentration at baseline. After supplementation, 4·9 % (n 10) of the pregnant women in the high-groundwater-Fe areas and 2·6 % (n 5) of the pregnant women in the low-groundwater-Fe areas were found to have haemoconcentration. Furthermore, only one pregnant woman in an area with a low level of Fe in the groundwater and three pregnant women in areas with high levels of Fe in the groundwater had haemoconcentration at both time points. However, none of the participants had Fe overload after supplementation.
Haemoglobinopathies
The pregnant women who completed the supplementation protocol were screened for haemoglobinopathies post-supplementation. Overall, 17·2 % of the pregnant women were found to have haemoglobinopathies: 9·4 % in the high-groundwater-Fe areas and 7·9 % in the low-groundwater-Fe areas. The difference was not statistically significant (P = 0·47).
Effect of iron–folic acid supplementation on Hb and serum ferritin concentrations in pregnant women without haemoglobinopathies
When data were reanalysed after excluding the pregnant women with haemoglobinopathies, the Hb concentration increased significantly in the pregnant women in the low-groundwater-Fe areas after supplementation, while no significant change was observed in the pregnant women in the high-groundwater-Fe areas (Table 4). Serum ferritin concentration decreased significantly only in the pregnant women living in the high-groundwater-Fe areas after supplementation. After adjusting for potential confounding factors, the mean changes in Hb and serum ferritin concentrations in the pregnant women in the low-groundwater-Fe areas were higher after supplementation than the changes in the pregnant women in the high-groundwater-Fe areas. However, the differences between the two groups were not statistically significant.
Table 4.
Hb and serum ferritin concentrations in the study participants without haemoglobinopathies at baseline and after the 3·5-month supplementation period, and the difference between baseline and post-supplementation, by areas with low and high iron in the groundwater, Bangladesh, April–October 2015
| High-groundwater-Fe areas (n 203) | Low-groundwater-Fe areas(n 192) | ||||
|---|---|---|---|---|---|
| Variable | Mean* | 95 % CI | Mean* | 95 % CI | P value† |
| Hb (mg/dl) | |||||
| Baseline | 11·58x | 11·44, 11·72 | 11·29a,y | 11·14, 11·44 | |
| Post | 11·44 | 11·30, 11·59 | 11·48b | 11·33, 11·62 | |
| Change (baseline – post-supplementation) | –0·06 | –0·19, 0·07 | 0·11 | –0·03, 0·24 | 0·101 |
| Serum ferritin (µg/l)‡ | |||||
| Baseline | 25·46a,x | 22·61, 28·68 | 18·92y | 16·60, 21·57 | |
| Post | 22·39b,x | 20·27, 24·73 | 17·44y | 15·76, 19·30 | |
| Change (baseline – post-supplementation) | –4·71 | –6·82, –2·60 | –7·64 | –9·78, –5·51 | 0·068 |
a,bMean values within a column for each of the variables (within each groundwater-Fe area) with unlike subscript letters were significantly different (P < 0·05).
x,yMean values within a row for each of the variables (at each time point) with unlike subscript letters were significantly different (P < 0·05).
Repeated-measures ANOVA, with time as the repeated measure, was performed.
Based on the general linear model with change in concentration as the dependent variable and adjusted for corresponding baseline value, total number of tablets consumed, husband’s occupation and possession of cultivable land. Serum ferritin was adjusted for elevated serum C-reactive protein and α1-acid glycoprotein, by mathematical correction.
P values based on log-transformed serum ferritin concentrations.
Effect of iron–folic acid supplementation on anaemia, iron deficiency and iron-deficiency anaemia in pregnant women without haemoglobinopathies
After supplementation, the prevalence of anaemia decreased (P = 0·07) only in the pregnant women in the low-groundwater-Fe areas, while the prevalence of ID remained unchanged in the participants from both areas (Table 5). In the low-groundwater-Fe areas, the prevalence of IDA in the pregnant women decreased significantly (P = 0·016) after supplementation, but remained unchanged in those from the high-groundwater-Fe areas.
Table 5.
Prevalence of anaemia, iron deficiency and iron-deficiency anaemia in the study participants without haemoglobinopathies at baseline and after the 3·5-month supplementation period, and the estimates of OR for anaemia, iron deficiency and iron-deficiency anaemia, by areas with low and high iron in the groundwater, Bangladesh, April–October 2015
| High-groundwater-Fe areas(n 165) | Low-groundwater-Fe areas(n 162) | Low- v. high-groundwater-Fe areas | ||||
|---|---|---|---|---|---|---|
| % | % | P value* | OR | 95 % CI | P value† | |
| Anaemia‡ | ||||||
| Baseline | 25·5 | 36·4 | 0·032 | – | – | |
| Post | 30·9 | 29·0 | 0·719 | 0·69 | 0·39, 1·21 | 0·193 |
| P = 0·170¶ | P = 0·070¶ | |||||
| Fe deficiency§ | ||||||
| Baseline | 23·6 | 34·6 | 0·034 | – | – | |
| Post | 21·8 | 35·8 | 0·007 | 0·74 | 0·42, 1·29 | 0·286 |
| P = 0·639¶ | P = 0·782¶ | |||||
| Fe-deficiency anaemia║ | ||||||
| Baseline | 7·9 | 21·0 | 0·001 | – | – | |
| Post | 9·7 | 13·0 | 0·386 | 0·64 | 0·27, 1·52 | 0·312 |
| P = 0·439¶ | P = 0·016¶ | |||||
Prevalence values within a column (within each groundwater-Fe area) were compared by Cochran’s Q test or Fisher’s exact test as appropriate.
Based on logistic regression and adjusted for corresponding baseline value, total number of tablets consumed, husband’s occupation and possession of cultivable land. ‡Hb < 11·0 g/dl.
Serum ferritin < 15·0 µg/l.
Hb < 11·0 g/dl and serum ferritin < 15·0 µg/l.
Prevalence values within a row (between high- and low-groundwater-Fe areas) were compared by the χ2 test or Fisher’s exact test as appropriate.
After 3·5 months of supplementation, the risk (OR) for developing anaemia, ID and IDA was lower in pregnant women in areas with low levels of Fe in the groundwater than in the pregnant women living in areas with high levels of Fe in the groundwater (Table 5). However, the differences between the two groups were not statistically significant.
Discussion
In Bangladesh, recent studies have identified low ID among non-pregnant and non-lactating women(4) and have linked this to the consumption of Fe-containing groundwater from tube wells(5,6). This finding raises concerns for the relevance of the current national IFA supplementation programme for preventing anaemia and ID in pregnant women in the country. The present study examined the associated risks and benefits of routine IFA supplementation in pregnant women living in high- and low-groundwater-Fe areas in Bangladesh. To our knowledge, the present study is the first that has compared the effect of routine IFA supplementation on haematological outcomes between pregnant women living in areas with low and high levels of Fe in the groundwater. In the present study, in areas with low levels of Fe in the groundwater, the mean (sd) Fe concentration in the tube-well water was 0·83 (1·35) mg/l, with a median of 0·31 mg/l. In areas with high levels of Fe in the groundwater, the mean (sd) Fe concentration in the tube-well water was 4·14 (5·76) mg/l, with a median of 1·28 mg/l(6). Notably, the ACGB survey reported that the national median Fe concentration in Bangladesh tube-well water was 1·1 mg/l, with nearly 10 % of tube-well water containing more than 10 mg Fe/l(8). In areas with high levels of Fe in the groundwater, we also found that 15 % of the tube-well water had more than 10 mg Fe/l. Thus, the findings of the present study can be generalized to all Bangladeshi pregnant women living in areas with low and high levels of Fe in the groundwater.
Although the overall compliance was high, the mean number of IFA tablets consumed by the pregnant women living in the areas with low levels of Fe in the groundwater was significantly lower than the number of tablets taken by the pregnant women living in the areas with high levels of Fe in the groundwater. Furthermore, nine pregnant women in areas with low levels of Fe in the groundwater and one woman living in an area with a high level of Fe in the groundwater consumed fewer than 50 IFA tablets, which was considered inadequate; thus, these participants were excluded from the final analysis. By examining the characteristics of these women in the low-groundwater-Fe areas we found that those who came from a relatively lower socio-economic background (lower level of literacy, a husband with a lower level of literacy and a lower socio-economic occupation, and possession of no cultivable land) appeared to be less compliant with the IFA supplementation protocol.
In the present study, there were some differences in the literacy level and work status of the husbands of the participants, the ownership of cultivable land, and baseline biochemical status between the two groups of pregnant women. Furthermore, bivariate analysis revealed significant differences in mean changes in serum concentrations of all biochemical indices by husband’s occupation and possession of cultivable land. Therefore, the study’s results were adjusted for these confounders using multivariate analysis.
The study revealed that the mean Hb concentration increased significantly in the pregnant women living in the low-groundwater-Fe areas after 3·5 months of IFA supplementation, while the mean Hb concentration decreased significantly in the pregnant women in the high-groundwater-Fe areas. After adjustment for corresponding baseline values, the number of IFA tablets consumed, the husband’s occupation and the ownership of cultivable land, the mean change in Hb concentration in the pregnant women living in the low-groundwater-Fe areas was found to be significantly higher than in the pregnant women living in the high-groundwater-Fe areas. Furthermore, the prevalence of anaemia at the post-supplementation visit decreased by approximately 17·0 % from the baseline values in women from low-Fe areas, and there was a 35 % reduced risk of anaemia compared with pregnant women living in the high-groundwater-Fe areas. Although these differences were not statistically significant, the results showed a declining trend in anaemia. Several reviews reported a significant improvement in haematological outcomes in pregnant women living in developing countries as a result of routine Fe supplementation, with or without folic acid, compared with placebo or folic acid alone(10,19,20). The post-supplementation Hb concentration during pregnancy is a result of the combined action of two opposing factors, namely dilution due to an increase in blood volume and stimulation of Hb synthesis by increasing the physiologically available Fe as a result of the increased absorption of Fe following supplementation(21,22). In the present study we did not have any control group due to ethical reasons, which might raise questions as to whether the increase in Hb levels was the effect of increased erythropoiesis after IFA supplementation or if this was due to a lower increase in plasma volume in late pregnancy. If the latter scenario was true, this would have similarly impacted the pregnant women in both groups. In the present study, we found a 0·10 mg/dl increase in Hb concentration only in the pregnant women living in the low-groundwater-Fe areas after IFA supplementation; this finding reinforces the effect of increased erythropoiesis due to increased Fe availability.
Furthermore, the prevalence of IDA at the post-supplementation visit was significantly reduced by 34 % from the baseline prevalence only in pregnant women living in the low-groundwater-Fe areas. This result suggests that the pregnant women in the low-groundwater-Fe areas reaped a greater benefit from IFA supplementation. Several mechanisms regulating intestinal Fe absorption have been identified(22–24). Both human and animal studies have shown that depleted body Fe stores can lead to increased intestinal absorption(23,24). In addition, intestinal Fe absorption is also regulated by bone-marrow erythropoiesis(24). Therefore, possible explanations for the present study findings could be the higher absorption of Fe in the pregnant women living in the low-groundwater-Fe areas due to the fact that their Fe status and/or Hb levels were initially lower than those in the pregnant women in the high-groundwater-Fe areas. It is noteworthy that in addition to IFA supplementation, dietary Fe intake may also contribute to the increased Fe availability(22). In a recent study among rural pregnant women, irrespective of groundwater-Fe area of residence, we found an independent positive association between the frequency of red meat consumption and Fe status(6). Thus, in the present study, we also examined the consumption of Fe-rich foods using a 7 d FFQ during the pre- and post-supplementation visits. There were no differences in red meat, green leafy vegetable or fruit intake between the two groups of pregnant women, at either time point. Therefore, the reduction in the prevalence of IDA in the pregnant women living in the low-groundwater-Fe areas reflects the effects of IFA supplementation.
The prevalence of anaemia and ID remained unchanged in the pregnant women living in areas with high levels of Fe in the groundwater, indicating that IFA supplementation in this group of women resulted in the maintenance of baseline Hb levels and Fe status. Moreover, IFA supplementation in the pregnant women in the high-groundwater-Fe areas did not pose any increased risk of haemoconcentration or Fe overload. Contrary to the present study findings, some studies reported that Fe supplementation could lead to Fe overload, which increases the risk of the formation of reactive oxygen species and induces haemoconcentration(11,12). A possible explanation for the present study finding is that the body’s Fe balance is regulated by Fe absorption through the action of hepcidin(25). In the presence of adequate Fe status, hepatic hepcidin production is increased. Hepcidin binds to ferroprotein on enterocytes, macrophages and hepatocytes, resulting in its internalization and, consequently, to the sequestration of Fe in intracellular stores, which, in turn, inhibits the expression of duodenal cytochrome B and divalent metal transporter 1 on the luminal surface of the enterocytes, thus decreasing dietary Fe absorption(22).
At baseline, the mean serum folate concentration in the pregnant women in the areas with low levels of Fe in the groundwater was significantly higher than that in the pregnant women in the areas with high levels of Fe in the groundwater. Studies have shown increased serum folate concentration as a result of increasing folic acid intake through foods, especially legumes, fruits and vegetables(26). In the present study, we did not find any significant differences in the frequency of consumption of these foods between the two groups. Further studies with more in-depth dietary information (both consumption frequency and amount) would be required to explain the reasons for this difference. However, the prevalence of folate deficiency was not significantly different at baseline between the two groups of pregnant women. As expected, there was a significant increase in serum folate concentration and a decrease in folate deficiency after the 3·5 months of supplementation in both groups.
Genetic disorders such as haemoglobinopathy are known to contribute to the burden of anaemia, distort the estimates of the prevalence of IDA(27,28) and increase the risk of Fe overload(29). Therefore, the pregnant women with haemoglobinopathies were identified from the study participants who completed the IFA supplementation protocol, and the effect of the haemoglobinopathies on Hb and serum ferritin levels was examined by stratifying the pregnant women, irrespective of Fe-level area, into those with and without haemoglobinopathy. The results showed that mean Hb, but not serum ferritin, concentrations were significantly lower at the baseline and post-supplementation visits in the pregnant women with haemoglobinopathy than in the pregnant women without haemoglobinopathy (data not shown). Consequently, all analyses were repeated using sub-samples without haemoglobinopathy. After adjusting for the corresponding baseline values and the number of IFA tablets consumed, the mean increase in Hb in the pregnant women living in the low-groundwater-Fe areas was 0·11 mg/dl, which is very similar to that which was observed in the total group of participants. However, the mean difference in Hb concentration between the two groups did not reach statistical significance. The results of the prevalence of anaemia and IDA in the pregnant women living in the low-groundwater-Fe areas following supplementation were very similar to those that were observed in the total group of participants. Furthermore, there was no case of Fe overload in the pregnant women with haemoglobinopathy. These results indicate that the current recommended dose of IFA supplementation probably does not pose any risk of Fe overload in pregnant women with haemoglobinopathy in this population. A study from Thailand also reported a similar finding that Fe supplementation in pregnant women did not cause Fe overload(30).
Anaemia may also be caused by other non-nutritional factors, such as malaria, hookworm infestation and chronic infection(31–33). We explored whether general morbidity and subclinical inflammation/infection, assessed by elevated levels of C-reactive protein (≥10 mg/l) and α1-acid glycoprotein (≥1·0 g/l), influenced the mean Hb level in the study population. We did not find any significant differences in Hb levels between the pregnant women with and without general morbidities and/or subclinical infection (data not shown). Moreover, the risk of malaria is quite low in Bangladesh and is prevalent only in certain regions of hilly areas. In the present study, we did not assess the hookworm load in pregnant women. A study among Bangladeshi rural non-pregnant women reported only a 1 % prevalence of hookworm infestation(34). It is very unlikely that, with such a low prevalence of hookworm infestation, there would have been any significant impact on anaemia status in this population.
Nevertheless, after 3·5 months of IFA supplementation, a significant proportion of pregnant women in both the high- and low-groundwater-Fe areas remained anaemic and Fe-deficient, even after excluding the women with haemoglobinopathies from the analysis. It is important to note that in addition to deficiency of Fe, deficiency in a number of micronutrients such as vitamin A, vitamin C, riboflavin, folic acid and vitamin B12 can lead to anaemia(35). These micronutrients are known to affect the synthesis of Hb either directly or indirectly by affecting the absorption and/or mobilization of Fe(35). Allen et al.(36) noted that the coexistence of multiple micronutrient (MMN) deficiencies with ID may increase the risk of anaemia and limit the haematological response. Although the 2016 WHO guidelines for antenatal care did not universally recommend supplementation with MMN during pregnancy, they did state that ‘policy-makers in populations with a high prevalence of nutritional deficiencies might consider the benefits of MMN supplements on maternal health to outweigh the disadvantages, and may choose to give MMN supplements that include iron and folic acid’(37). It is noteworthy that a recent meta-analysis of individual patient data from seventeen randomized controlled trials including 112 953 pregnant women living in low- and middle-income countries found that MMN supplementation during pregnancy reduced the risk of low birth weight, preterm birth and being born small for gestational age(38). Thus, in light of the above findings, our study findings suggest that Bangladesh should actively consider the need for implementing MMN supplementation including Fe and folic acid for pregnant women.
Conclusion
In conclusion, routine supplementation with IFA tablets, containing 60 mg elemental Fe and 400 μg folic acid, for 3·5 months improved the Hb concentration in rural Bangladeshi pregnant women living in the low-groundwater-Fe areas. However, there was no significant change observed in the pregnant women living in the high-groundwater-Fe areas. After IFA supplementation, a decreasing trend of anaemia and a significantly reduced prevalence of IDA were observed only in the pregnant women living in the low-groundwater-Fe areas, as expected. Furthermore, IFA supplementation did not pose any significant risk of haemoconcentration or Fe overload in the pregnant women in either area. Nevertheless, a sizeable proportion of pregnant women in high- and low-groundwater-Fe areas remained anaemic and Fe-deficient post-supplementation. Thus, the findings of the present study support the continuation of routine supplementation with IFA tablets during pregnancy to prevent anaemia and ID in this population. Future research to identify other nutritional and non-nutritional contributors to anaemia is warranted to prevent and treat anaemia. In addition, research should focus on developing possible strategies to improve the compliance of pregnant women to the IFA supplementation protocol and scale up the coverage to benefit a larger population.
Acknowledgements
Acknowledgements: The authors thank the staff of the Ministry of Health and Family Welfare for help in recruiting the participants. Financial support: The authors gratefully acknowledge financial support by UNICEF, Dhaka, Bangladesh. UNICEF funders had no role in the analysis or writing of this article. Conflict of interest: I.A.C. works for UNICEF, which funded the study. There are no conflicts of interest. Authorship: F.A. took the lead in study planning and design, provided guidance on the data collection and wrote the manuscript. M.R.K. supervised the fieldwork, data and blood collection. M.R.K., R.R. and A.K.R. were responsible for laboratory analysis. M.R.K. and I.A.C. contributed to the study design. R.C. contributed to the statistical analysis of the data and interpretation. F.A. had primary responsibility for the final content. All authors read and approved the final manuscript. Ethics of human subject participation: This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the Bangladesh Medical Research Council, Dhaka, Bangladesh and the Faculty of Biological Sciences, University of Dhaka, Dhaka, Bangladesh. Written informed consent (signed or thumb impression) was obtained from all subjects.
References
- 1.Ahmed F, Prendiville N & Narayan A (2016) Micronutrient deficiencies among children and women in Bangladesh: progress and challenges. J Nutr Sci 5, e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Institute of Population Research and Training, Mitra and Associates & ICF International (2011) Bangladesh Demographic and Health Survey 2011. Dhaka and Calverton, MD: NIPORT, Mitra and Associates, and ICF International. [Google Scholar]
- 3.Bangladesh National Nutrition Council (1997) Bangladesh National Plan of Action for Nutrition. Dhaka: Ministry of Health and Family Welfare, Government of the People’s Republic of Bangladesh. [Google Scholar]
- 4.Institute of Public Health Nutrition (2014) National Micronutrient Survey 2011–12. Dhaka: Institute of Public Health Nutrition, Ministry of Health and Family Welfare, Government of the People’s Republic of Bangladesh. [Google Scholar]
- 5.Merrill RD, Shamim AA, Ali H et al. (2011) Iron status of women is associated with the iron concentration of potable groundwater in rural Bangladesh. J Nutr 141, 944–949. [DOI] [PubMed] [Google Scholar]
- 6.Ahmed F, Khan MR, Shaheen N et al. (2018) Anemia and iron deficiency in rural Bangladeshi pregnant women living in areas of high and low iron in groundwater. Nutrition 51, 46–52. [DOI] [PubMed] [Google Scholar]
- 7.Milton AH, Rahman H, Smith W et al. (2006) Water consumption patterns in rural Bangladesh: are we underestimating total arsenic load? J Water Health 4, 431–436. [PubMed] [Google Scholar]
- 8.British Geological Survey & Department of Public Health Engineering, Government of the People’s Republic of Bangladesh (2001) Arsenic Contamination of Groundwater in Bangladesh. Keyworth: British Geological Survey. [Google Scholar]
- 9.Rahman S, Ahmed T, Rahman AS et al. (2016) Determinants of iron status and Hb in the Bangladesh population: the role of groundwater iron. Public Health Nutr 19, 1862–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peña-Rosas JP, De-Regil LM, Garcia-Casal MN et al. (2015) Daily oral iron supplementation during pregnancy. Cochrane Database Syst Rev issue 7, CD004736. [DOI] [PMC free article] [PubMed]
- 11.Scholl TO (2005) Iron status during pregnancy: setting the stage for mother and infant. Am J Clin Nutr 81, issue 5, 1218S–1222S. [DOI] [PubMed] [Google Scholar]
- 12.von Tempelhoff G-F, Heilmann L, Rudig L et al. (2008) Mean maternal second-trimester hemoglobin concentration and outcome of pregnancy: a population-based study. Clin Appl Thromb Hemost 14, 19–28. [DOI] [PubMed] [Google Scholar]
- 13.Scanlon KS, Yip R, Schieve LA et al. (2000) High and low hemoglobin levels during pregnancy: differential risks for preterm birth and small for gestational age. Obstet Gynecol 96, 741–748. [DOI] [PubMed] [Google Scholar]
- 14.Scholl TO (2011) Maternal iron status: relation to fetal growth, length of gestation, and iron endowment of the neonate. Nutr Rev 69, Suppl. 1, S23–S29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.UNICEF, United Nations University & World Health Organization (2001) Iron Deficiency Anaemia Assessment, Prevention, and Control: A Guide for Programme Managers. WHO/NHD/01.3. Geneva: WHO. [Google Scholar]
- 16.Rucker RB, Suttie JW & McCormick DB (2001) Handbook of Vitamins, 3rd ed. New York: CRC Press. [Google Scholar]
- 17.Tomkins A (2003) Assessing micronutrient status in the presence of inflammation. J Nutr 133, 5 Suppl. 2, 1649S–1655S. [DOI] [PubMed] [Google Scholar]
- 18.Thurnham DI, McCabe LD, Haldar S et al. (2010) Adjusting plasma ferritin concentrations to remove the effects of subclinical inflammation in the assessment of iron deficiency: a meta-analysis. Am J Clin Nutr 92, 546–555. [DOI] [PubMed] [Google Scholar]
- 19.Imdad A & Bhutta ZA (2012) Routine iron/folate supplementation during pregnancy: effect on maternal anaemia and birth outcomes. Paediatr Perinat Epidemiol 26, 168–177. [DOI] [PubMed] [Google Scholar]
- 20.Haider BA, Olofin I, Wang M et al. (2013) Anaemia, prenatal iron use, and risk of adverse pregnancy outcomes: systematic review and meta-analysis. BMJ 346, f3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chandra S, Tripathi AK, Mishra S et al. (2012) Physiological changes in hematological parameters during pregnancy. Indian J Hematol Blood Transfus 28, 144–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coad J & Pedley K (2014) Iron deficiency and iron deficiency anaemia in women. Scand J Clin Lab Invest 74, Suppl. 244, 82–89. [DOI] [PubMed] [Google Scholar]
- 23.Abbaspour N, Hurrell R & Kelishadi R (2014) Review on iron and its importance for human health. J Res Med Sci 19, 164–174. [PMC free article] [PubMed] [Google Scholar]
- 24.Wallace DF (2016) The regulation of iron absorption and homeostasis. Clin Biochem Rev 37, 51–62. [PMC free article] [PubMed] [Google Scholar]
- 25.Ganz T (2013) Systemic iron homeostasis. Physiol Rev 93, 1721–1741. [DOI] [PubMed] [Google Scholar]
- 26.Hatzis CM, Bertsias GK, Linardakis M et al. (2006) Dietary and other lifestyle correlates of serum folate concentrations in a healthy adult population in Crete, Greece: a cross-sectional study. Nutr J 5, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Merrill RD, Shamim AA, Ali H et al. (2012) High prevalence of anemia with lack of iron deficiency among women in rural Bangladesh: a role of thalassemia and iron in ground water. Asia Pac J Clin Nutr 21, 416–424. [PubMed] [Google Scholar]
- 28.Modell B & Darlison M (2008) Global epidemiology of haemoglobin disorders and derived service indicators. Bull World Health Organ 86, 480–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zimmermann MB, Fucharoen S, Winichagoon P et al. (2008) Iron metabolism in heterozygotes for hemoglobin E (HbE), α-thalassemia 1, or β-thalassemia and in compound heterozygotes for HbE/β-thalassemia. Am J Clin Nutr 88, 1026–1031. [DOI] [PubMed] [Google Scholar]
- 30.Sanchaisuriya K, Fucharoen S, Ratanasiri T et al. (2007) Effect of the maternal βE-globin gene on hematologic responses to iron supplementation during pregnancy. Am J Clin Nutr 85, 474–479. [DOI] [PubMed] [Google Scholar]
- 31.Ganz T & Nemeth E (2012) Hepcidin and iron homeostasis. Biochem Biophys Acta 1823, 1434–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dreyfuss ML, Stoltzfus RJ, Shrestha JB et al. (2000) Hookworms, malaria and vitamin A deficiency contribute to anemia and iron deficiency among pregnant women in the plains of Nepal. J Nutr 130, 2527–2536. [DOI] [PubMed] [Google Scholar]
- 33.Stoltzfus RJ, Chwaya HM, Tielsch JM et al. (1997) Epidemiology of iron deficiency anemia in Zanzibari schoolchildren: the importance of hookworms. Am J Clin Nutr 65, 153–159. [DOI] [PubMed] [Google Scholar]
- 34.Hyder SMZ, Persson L-A, Chowdhury AMR et al. (2001) Anaemia among non-pregnant women in rural Bangladesh. Public Health Nutr 4, 79–83. [DOI] [PubMed] [Google Scholar]
- 35.Fishman SM, Christian P & West KP (2000) The role of vitamins in the prevention and control of anaemia. Public Health Nutr 3, 125–150. [DOI] [PubMed] [Google Scholar]
- 36.Allen LH, Rosado JL, Casterline JE et al. (2000) Lack of hemoglobin response to iron supplementation in anemic Mexican preschoolers with multiple micronutrient deficiencies. Am J Clin Nutr 71, 1485–1494. [DOI] [PubMed] [Google Scholar]
- 37.World Health Organization (2016) WHO Recommendations on Antenatal Care for a Positive Pregnancy Experience. Geneva: WHO. [PubMed] [Google Scholar]
- 38.Smith ER, Shankar AH, Wu LS et al. (2017) Modifiers of the effect of maternal multiple micronutrient supplementation on stillbirth, birth outcomes, and infant mortality: a meta-analysis of individual patient data from 17 randomised trials in low-income and middle-income countries. Lancet Glob Health 5, e1090–e1100. [DOI] [PubMed] [Google Scholar]