Abstract
Objective:
Dietary acid load (DAL) might contribute to change the levels of cardiometabolic risk factors; however, the results are conflicting. The present review was conducted to determine the relationship between DAL and cardiometabolic risk factors.
Design:
Systematic review and meta-analysis.
Setting:
A systematic search was conducted in electronic databases including ISI Web of Science, PubMed/MEDLINE, Scopus and Google Scholar for observational studies which assessed cardiometabolic risk factors across DAL. Outcomes were lipid profile, glycaemic factors and anthropometric indices. Effect sizes were derived using a fixed- or random-effect model (DerSimonian–Laird). Also, subgroup analysis was performed to find the probable source of heterogeneity. Egger’s test was performed for finding any publication bias.
Results:
Thirty-one studies were included in the current review with overall sample size of 92 478. There was a significant relationship between systolic blood pressure (SBP; weighted mean difference (WMD) = 1·74 (95 % CI 0·25, 3·24) mmHg; P = 0·022; I 2 = 95·3 %), diastolic blood pressure (DBP; WMD = 0·75 (95 % CI 0·07, 1·42) mmHg; P = 0·030; I 2 = 80·8 %) and DAL in cross-sectional studies. Serum lipids, glycaemic parameters including fasting blood sugar, glycated Hb, serum insulin, homeostatic model assessment of insulin resistance and waist circumference had no significant relationship with DAL. No publication bias was found. BMI was not associated with DAL in both cross-sectional and cohort studies.
Conclusions:
Higher DAL is associated with increased SBP and DBP. More studies are needed to find any relationship of DAL with lipid profile and glycaemic factors.
Keywords: Dietary acid load, Cardiometabolic risk factors, Observational studies, Systematic review, Meta-analysis
Chronic diseases such as CVD, diabetes mellitus and metabolic syndrome are major causes of disabilities and global deaths(1). Lipid profile, diabetic characteristics, blood pressure, anthropometric indices and inflammatory markers are some of the cardiometabolic risk factors. In fact, over 60 % of deaths from chronic diseases such as diabetes and chronic kidney disease in 2010 were attributable to cardiometabolic risk factors(2). These risk factors are associated with a variety of genetic and environmental factors. One of the important environmental factors is diet, and various studies have shown the links between diet and these risk factors(1). Nutritionists believe in evaluating the whole diet rather than individual nutrients because of the potential interaction among nutrients. One of the indices that assesses the whole diet is dietary acid load (DAL).
DAL can be related to cardiometabolic factors such as insulin resistance(1,3,4). Diets with a high acid load such as the Western dietary pattern can cause alteration or disequilibrium in blood pH and acid–base balance(4). A high amount of animal proteins in the diet and inadequate bicarbonate intake from vegetables and fruits can exacerbate this condition(5,6). There are three methods for calculating DAL: (i) potential renal net acid load (PRAL) that, when positive, indicates an acid-forming potential(7); (ii) net endogenous acid production (NEAP) that indicates the high consumption of animal proteins(8); and (iii) net acid excretion (NAE) that indicates excess of dietary anions(9).
High serum lactate has been associated with insulin resistance and type 2 diabetes incidence(4,10). Moreover, increasing DAL is positively related to glycated Hb (HbA1c)(11). On the other hand, there is some research that has indicated no relationship between DAL and insulin resistance, fasting glucose and HbA1c(12,13). Also, DAL has been shown to have a positive relationship with hypertension, hepatic TAG accumulation, total cholesterol (TC), LDL-cholesterol (LDL-C), BMI and waist circumference (WC)(1,12,14). Acid–base balance can impact on the Mg and Ca mechanism that is related to control of blood pressure(1,12). On the other hand, higher protein intake from animal-based foods can increase blood pressure(15).
To our knowledge, there is no systematic review examining the association of DAL with cardiometabolic risk factors. Therefore, we conducted the present systematic review and meta-analysis aimed at determining the relationship between DAL and cardiometabolic risk factors.
Materials and methods
The Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) checklist was followed in conducting the current review.
Search strategy
Scopus, ISI Web of Science, PubMed/MEDLINE and Google Scholar databases were searched systematically to obtain studies published up to December 2017. Medical subject headings (MeSH) and several text words were used as a search syntax for searching: dietary acid load, potential renal acid load, net endogenous acid production, insulin, blood sugar, abdominal obesity, central obesity, visceral obesity, waist circumference, BMI, Quetelet Index, HbA1c, glycosylated haemoglobin A, triacylglycerol, diabetes, high blood pressure, low density lipoprotein cholesterol, beta-lipoprotein cholesterol, body weight, high density lipoprotein cholesterol, alpha-lipoprotein cholesterol, HDL cholesterol (see online supplementary material, Supplemental Table S1). Reference lists of all studies were checked for any relationship with the topic. Two authors (E.D. and F.H.) did all of these steps independently and the third author (L.A.) checked them to resolve any disagreement.
Inclusion and exclusion criteria
All observational studies including cross-sectional, case–control and cohort studies that investigated the association of DAL with cardiometabolic factors were selected for inclusion in the present systematic review and meta-analysis. We considered articles which assessed both genders. Also, we had no restriction on age, BMI, study setting, socio-economic status and education level, type of disease or sample size. No restriction was set on publication date. Non-English papers, clinical trials, books, conference papers, reviews, non-related studies and studies which had insufficient data were excluded. The PICOS (participants, intervention, comparison, outcomes and study design) criteria used to define the research question were as follows. P: all apparently healthy people or individuals with diabetes and hypertension; however, pregnant women, infants, and individuals with malignant diseases such as cancers were excluded. I: dietary acid load. C: highest n-tile v. lowest n-tile. O: BMI, WC, HDL-cholesterol (HDL-C), LDL-C, TAG, TC, diastolic blood pressure (DBP), systolic blood pressure (SBP), fasting blood sugar (FBS), HbA1c, serum insulin and homeostatic model assessment of insulin resistance (HOMA-IR). S: observational studies.
Data extraction
For each relevant study, E.D. and F.H extracted data about the first author’s name, publication date, country, sample size, participants’ age and gender, type of study, method used to assess DAL and study duration. Also extracted were the means, sd and se of all study variables including weight, BMI, WC, LDL-C, HDL-C, TC, TAG, FBS, HbA1c, serum insulin, DBP and SBP according to the lowest and highest levels of DAL from descriptive tables. Because of limited data on odds ratios or hazard ratios, we considered means and corresponding sd according to n-tiles of DAL score. The Newcastle–Ottawa Scale was used to evaluate the quality of included studies and this score for each study is shown in Table 1. Studies that obtained six or more points were considered to be of high quality(16).
Table 1.
Characteristics of observational studies included in the current systematic review and meta-analysis on dietary acid load and cardiometabolic risk factors
| Study | Design | Country | Number of participants | Age range (years) | Gender | DAL assessment method | Dietary intake assessment tool | Outcome | Comparison | NOS score |
|---|---|---|---|---|---|---|---|---|---|---|
| Wright et al. (2005)(17) | Cohort | Finland | 27 096 Q5 & Q1: 5419 |
50–69 | M | NAE | 24 h urine collection | BMI | Q5 v. Q1 | 9 |
| Welch et al. (2007)(31) * | Cross-sectional | UK | 6375 Q5 & Q1: 1275 |
42–82 | M | PRAL | FFQ | BMI | Q5 v. Q1 | 6 |
| Welch et al. (2007)(31) * | Cross-sectional | UK | 8188 Q5: 1639 Q1: 1640 |
42–82 | F | PRAL | FFQ | BMI | Q5 v. Q1 | 6 |
| Murakami et al. (2008)(12) | Cross-sectional | Japan | 1136 Q5 & Q1: 227 |
18–22 | F | PRAL | DHQ | BMI, WC, SBP, DBP, TC, HDL-C, LDL-C, TAG, FBS, HbA1c | Q5 v. Q1 | 5 |
| Wynn et al. (2008)(32) | Cross-sectional | Switzerland | 256 T3: 86 T1: 92 |
≥70 | F | NEAP | FFQ | BMI | T3 v. T1 | 5 |
| Zhang et al. (2009)(18) | Cohort | USA | 87 293 D10 & D1: 8729 |
32–40 | F | NEAP | FFQ | BMI | D10 v. D1 | 7 |
| Scialla et al. (2011)(33) | Cross-sectional | USA | 462 Q4: 86 Q1: 85 |
50–72 | M | NEAP | 24 h urine collection | BMI | Q4 v. Q1 | 7 |
| Berg et al. (2012)(34) | Cross-sectional | Netherlands | 707 T3 & T1: 236 |
≥18 | Both | NAE | 24 h urine collection | SBP, DBP, TC, HDL-C, TAG, HbA1c | T3 v. T1 | 7 |
| Engberink et al. (2012)(6) | Cohort | Netherlands | 2241 T3 & T1: 747 |
≥55 | Both | PRAL | FFQ | BMI, SBP, DBP, TC, HDL-C | T3 v. T1 | 7 |
| Scialla et al. (2012)(19) | Cohort | USA | 632 Q4 & Q1: 185 |
22–70 | Both | NEAP | 24 h urine collection | BMI | Q4 v. Q1 | 6 |
| Amodu and Abramowitz (2013)(35) | Cross-sectional | USA | 9781 Q4: 2490 Q1: 2477 |
≥20 | Both | NEAP | 24 h recall | BMI | Q4 v. Q1 | 7 |
| Krupp et al. (2013)(36) | Cross-sectional | Germany | 267 T3 & T1: 89 |
4–14 | Both | PRAL | 3 d dietary record | SBP | T3 v. T1 | 7 |
| Krupp et al. (2013)(20) | Cohort | Germany | 257 | 4–10 | Both | PRAL, NAE | 7 d dietary record | SBP, DBP | T3 v. T1 | 7 |
| Bahadoran et al. (2015)(37) | Cross-sectional | Iran | 5620 Q4 & Q1: 351 |
20–70 | Both | PRAL | FFQ | BMI, weight, WC, SBP, DBP, TAG, FBS | Q4 v. Q1 | 7 |
| Fagherazzi et al. (2014)(21) | Cohort | France | 66 485 Q4: 16 621 Q1: 16 622 |
44–59 | F | PRAL | DHQ | BMI | Q4 v. Q1 | 6 |
| Xu et al. (2014)(13) | Cohort | Sweden | 911 T3: 304 T1: 303 |
70–71 | M | PRAL | 7 d dietary record | BMI,FBS | T3 v. T1 | 7 |
| Akter et al. (2015)(38) | Cross-sectional | Japan | 2028 T3 & T1: 676 |
18–70 | Both | PRAL | DHQ | BMI | T3 v. T1 | 9 |
| Chan et al. (2015)(22) | Cohort | China | 3122 Q4: 780 Q1: 779 |
≥65 | Both | NEAP | FFQ | BMI | Q4 v. Q1 | 8 |
| Garcia et al. (2015)(23) * | Cohort | Netherlands | 2850 T3 & T1: 950 |
27–36 | F | PRAL | FFQ | BMI | T3 v. T1 | 8 |
| Garcia et al. (2015)(23) * | Cohort | Netherlands | 2850 T3 & T1: 950 |
1–6 | Both | PRAL | FFQ | BMI | T3 v. T1 | 8 |
| Haghighatdoost et al. (2015)(11) | Cross-sectional | Iran | 547 High: 274 Low: 273 |
50–70 | Both | PRAL | FFQ | BMI, WC, SBP, TC, LDL-C, TAG, FBS, HbA1c, HOMA-IR, insulin | High v. Low group | 7 |
| Huston et al. (2015)(39) | Cross-sectional | USA | 16 906 Q4: 4616 Q1: 3804 |
≥17 | Both | NEAP | 24 h recall | BMI | Q4 v. Q1 | 7 |
| Iwase et al. (2015)(40) | Cross-sectional | Japan | 149 High: 75 Low: 74 |
≥50 | Both | PRAL | DHQ | BMI, SBP, LDL-C, TAG, HbA1c | High v. Low group | 4 |
| Jia et al. (2015)(24) | Cohort | Sweden | 861 Q4 & Q1: 215 |
70 | Both | NEAP | 7 d FFQ | BMI | Q4 v. Q1 | 6 |
| Luis et al. (2015)(43) | Cross-sectional | Sweden | 673 T3 & T1: 224 |
70–71 | M | PRAL | 7 d dietary record | BMI, SBP, DBP | T3 v. T1 | 8 |
| Akter et al. (2016)(1) | Cross-sectional | Japan | 1732 Q4 & Q1: 433 |
19–69 | Both | PRAL | DHQ | BMI, FBS, HbA1c, insulin, HOMA-IR | Q4 v. Q1 | 7 |
| Akter et al. (2016)(25) * | Cohort | Japan | 27 809 Q4: 6952 Q1: 6953 |
45–75 | M | PRAL | FFQ | BMI | Q4 v. Q1 | 8 |
| Akter et al. (2016)(25) * | Cohort | Japan | 36 851 Q4: 9212 Q1: 9213 |
45–75 | Female | PRAL | FFQ | BMI | Q4 v. Q1 | 8 |
| Esche et al. (2016)(26) | Cohort | Germany | 200 High & Low: 100 |
6–10 | Both | NAE | 24 h urine collection | BMI, weight | High v. Low group | 8 |
| Han et al. (2016)(41) | Cross-sectional | Korea | 11 601 T3: 4202 T1: 3859 |
40–79 | Both | PRAL | 24 h recall | BMI, WC, SBP, DBP, TC, HDL-C, LDL-C, TAG, FBS | T3 v. T1 | 7 |
| Ikizler et al. (2016)(42) | Cross-sectional | USA | 63 T3 & T1: 21 |
45–75 | Both | NEAP | 3 d prospective food dairies | BMI, SBP, DBP, FBS, insulin | T3 v. T1 | 3 |
| Moghadam et al. (2016)(27) | Cohort | Iran | 925 Q4 & Q1: 231 |
22–80 | Both | PRAL | FFQ | Weight, SBP, DBP, HDL-C, LDL-C, TAG, FBS, HOMA-IR, insulin | Q4 v. Q1 | 8 |
| Scialla et al. (2016)(28) | Cohort | USA | 980 Q4: 246 Q1: 245 |
45–75 | Both | NAE | 24 h urine collection | BMI | Q4 v. Q1 | 6 |
| Xu et al. (2016)(29) * | Cohort | Sweden | 44 957 Q5: 9038 Q1: 8974 |
45–84 | M | PRAL | FFQ | BMI | Q5 v. Q1 | 8 |
| Xu et al. (2016)(29) * | Cohort | Sweden | 36 740 Q5 & Q1: 7294 |
45–84 | F | PRAL | FFQ | BMI | Q5 v. Q1 | 8 |
| Akter et al. (2017)(30) | Cohort | Japan | 92 478 Q4: 23 119 Q1: 23 120 |
45–75 | Both | PRAL | FFQ | BMI | Q4 v. Q1 | 7 |
| Shea et al. (2017)(44) * | Cross-sectional | USA | 162 T3: 13 T1: 19 |
50–58 | Both | NAE | FFQ | BMI | T3 v. T1 | 6 |
| Shea et al. (2017)(44) * | Cross-sectional | USA | 232 T3: 25 T1: 84 |
60–58 | Both | NAE | 24 h recall | BMI | T3 v. T1 | 6 |
DAL, dietary acid load; NOS, Newcastle–Ottawa Scale; Q, quintile or quartile; T, tertile; D, decile; M, male; F, female; NAE, net acid excretion; PRAL, potential renal net acid load; NEAP, net endogenous acid production; DHQ, diet history questionnaire; WC, waist circumference; SBP, systolic blood pressure; DBP, diastolic blood pressure; TC, total cholesterol; HDL-C, HDL-cholesterol; LDL-C, LDL-cholesterol; FBS, fasting blood sugar; HbA1c, glycated Hb; HOMA-IR, homeostatic model assessment of insulin resistance.
indicates consecutive studies by the same authors that come from just one article but with different situations, such as difference in gender or type of outcome assessed.
Statistical analysis
The means and corresponding sd of all variables of all included studies in the lowest and the highest n-tiles of DAL were used to calculate the weighted mean difference (WMD) as effect size in the meta-analysis. In cases that sd was not reported, we calculated sd using se and sample size. Analysis was performed by the fixed-effect model and the DerSimonian–Laird random-effect model was used for variables with high heterogeneity. Subgroup analyses according to type of study, participant age, participant gender, study quality, type of DAL measurement and type of food assessment were performed with Cochran’s Q test and the I 2 statistic for evaluating the possible sources of heterogeneity. Also, publication bias was assessed using funnel plots and Egger’s regression test. Sensitivity analyses were run to determine the extent to which summary estimates might be related to one particular study or a group of studies, done in accordance with the Cochrane handbook for systematic reviews of observational studies.
All statistical analyses were performed using the statistical software package Stata version 14. P < 0.05 for the association of all variables with DAL, also P < 0.1 for heterogeneity, were considered statistically significant.
Results
Systematic review
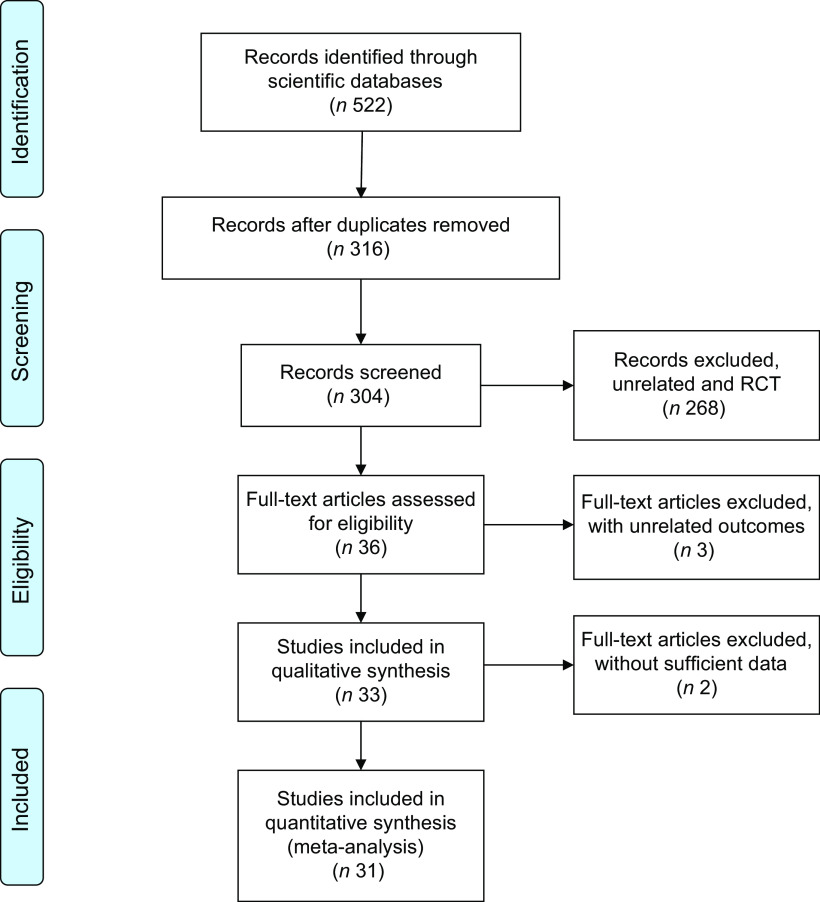
As shown in Fig. 1, 522 records were accessed by searching the scientific databases of which 316 records remained after removing the 206 duplicate references. After exclusion of irrelevant studies, sixteen cohort studies(6,13,17–30) and seventeen cross-sectional studies(1,11,12,31–44) were included in the current systematic review. Characteristics of the included studies, which were published between 2005 and 2017, are shown in Table 1. The sample size of these studies ranged from sixty-three to 92 478 individuals, and totally just 3574 individuals were aged 17 years or younger. Of thirty-three included studies, eleven were conducted in the Asia(1,11,12,22,25,27,30,37,38,41), fourteen in Europe(6,13,17,20,21,23,24,26,29,31,32,34,36,43) and eight in the USA(18,19,28,33,35,39,42,44). Four studies were done on men(13,17,33,43), five on women(12,18,21,23,32) and the remaining studies implied on both genders. Statistical analysis was performed separately for men and women in five studies(23,25,29,31,44). DAL had been assessed by NAE method in six studies(17,20,26,28,34,44), PRAL in nineteen(1,6,11–13,20,21,23,25,27,29–31,36–38,40,41,43) and NEAP in nine studies(18,19,22,24,32,33,35,39,42). These assessment methods were assessed and calculated using FFQ(6,11,18,22–25,27,29–32,37,44), 24 h urine collection(17,19,26,28,33,34), diet history questionnaire(1,12,21,38,40), 24 h food recall(35,39,41,44), 3 d dietary record(36,42) and 7 d dietary record(13,20,43). Included studies had assessed weight(26,27,37), BMI(1,6,11–13,17–19,21–26,28–33,35,37–44), WC(11,12,37,41), TC(6,11,12,34,41), HDL-C(6,12,27,34,41), LDL-C(11,12,27,40,41), TAG(11,12,34,37,40,41), FBS(11–13,27,37,41,42), HbA1c(11,12,34,40), HOMA-IR(11,27), insulin(11,27,42), SBP(6,11,12,20,27,34,36,37,40–43) and DBP(6,12,20,27,34,37,41–43) in relation to n-tiles of DAL. These outcomes were reported as mean and sd across n-tiles of DAL measurement; therefore, we compared the changes of the outcomes’ effect size by conducting a meta-analysis as reported below. Amodu and Abramowitz’s study(35) presented BMI in relation to DAL by percentage in underweight, normal, overweight and obese status. Also, Krupp et al.’s(20) study did not present sufficient data for combining and performing a meta-analysis; therefore, we mentioned these two studies just in the systematic review. Only two studies(1,11) presented considerable outcomes with adjustment, and the rest of the studies had no adjustment for outcomes. All included studies except four of them(12,32,40,42) were of high quality based on the Newcastle–Ottawa Scale.
Fig. 1.
Flow diagram showing the selection of observational studies for the current systematic review and meta-analysis on dietary acid load and cardiometabolic risk factors (RCT, randomized controlled trial)
Totally nine included studies showed a significant(6,21,25,28,30,35,37,39,41) or marginally significant(33) positive association between DAL and BMI. Also, thirteen included studies had no significant association between DAL and BMI(1,11–13,19,22–24,27,31,38,40,43). In addition, one study had no significant(37) relationship between weight and DAL. There were only two studies that reported weight across DAL n-tiles; therefore, we could not perform meta-analysis on this variable. Two studies showed a significant positive relationship between WC and DAL(12,37); however, others(11,41) did not find a significant association.
Six studies(6,11,12,20,36,41) indicated a positive significant relationship between SBP and DAL, whereas five studies had no significant association between SBP and DAL(27,34,37,40,43). In addition, three studies showed a positive significant association between DBP and DAL(12,20,41), while five studies had no significant relationship(6,27,34,37,43).
Five studies found no significant relationship between FBS and DAL(1,12,27,37,41), as well as no association of insulin, HOMA-IR(27,41) and HbA1c(1,12,34,40) with DAL. However, Haghighatdoost et al. found a significant positive relationship between FBS, HbA1c and DAL(11). Akter et al. found a significant positive relationship between insulin, HOMA-IR and DAL(1).
Three studies found no significant relationship between TAG and DAL(27,34,37); however, other studies indicated a significant positive association(11,40,41). Three studies(6,11,41) indicated no significant association between TC and DAL, but Murakami et al. (12) and Berg et al. (34) indicated a significant association. Several studies had no significant relationship between LDL-C and DAL(11,27,40); however, Murakami et al. (12) and Han et al. (41) indicated a significant association. Murakami et al. (12), Berg et al. (34) and Han et al. (41) did not show a significant relationship between HDL-C and DAL, but one study showed a marginally significant(6) positive association between DAL and HDL-C.
Meta-analysis
Of thirty-three studies, two studies were excluded because of insufficient data; thus thirty-one studies (sixteen cohort and seventeen cross-sectional studies) were included in the meta-analysis. Means and sd for mentioned outcomes in all studies were considered in analysis. Meta-analysis on the association of anthropometric indices, lipid profiles, blood sugar and blood pressure with DAL was performed in cross-sectional and cohort studies separately, and results are presented in Figs 2 and 3 and the online supplementary material. Subgroup analyses based on age, gender, method of DAL assessment, dietary intake assessment tool and study quality are presented in Supplemental Tables S2 to S11.
Fig. 2.
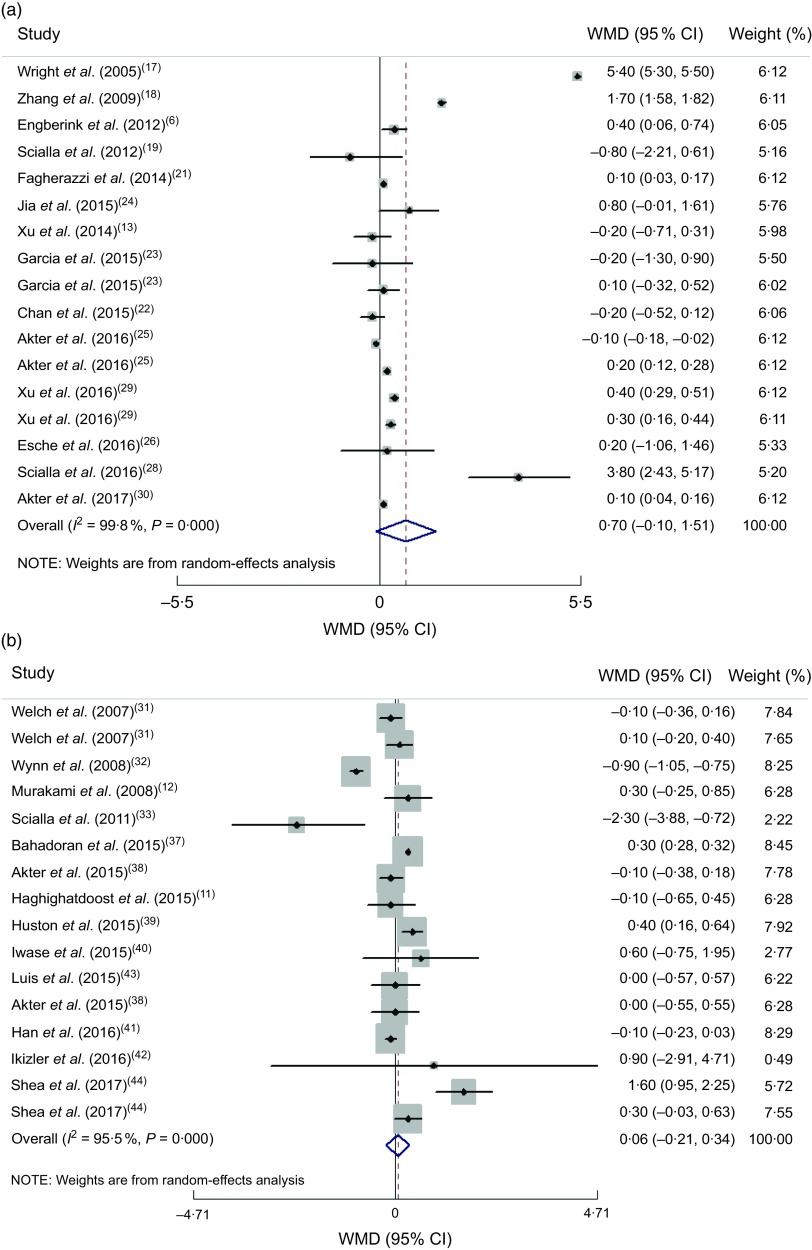
(a) Forest plot for the association between dietary acid load and BMI in cohort studies by the random-effect model; (b) Forest plot for the association between dietary acid load and BMI in cross-sectional studies by the random-effect model. The study-specific effect size (expressed as weighted mean difference (WMD)) and 95 % CI are represented by the solid diamond and horizontal line, respectively; the area of the grey square is proportional to the specific-study weight to the overall meta-analysis. The centre of the open diamond and the vertical dashed line represent the pooled effect size, and the width of the open diamond represents the pooled 95 % CI
Fig. 3.
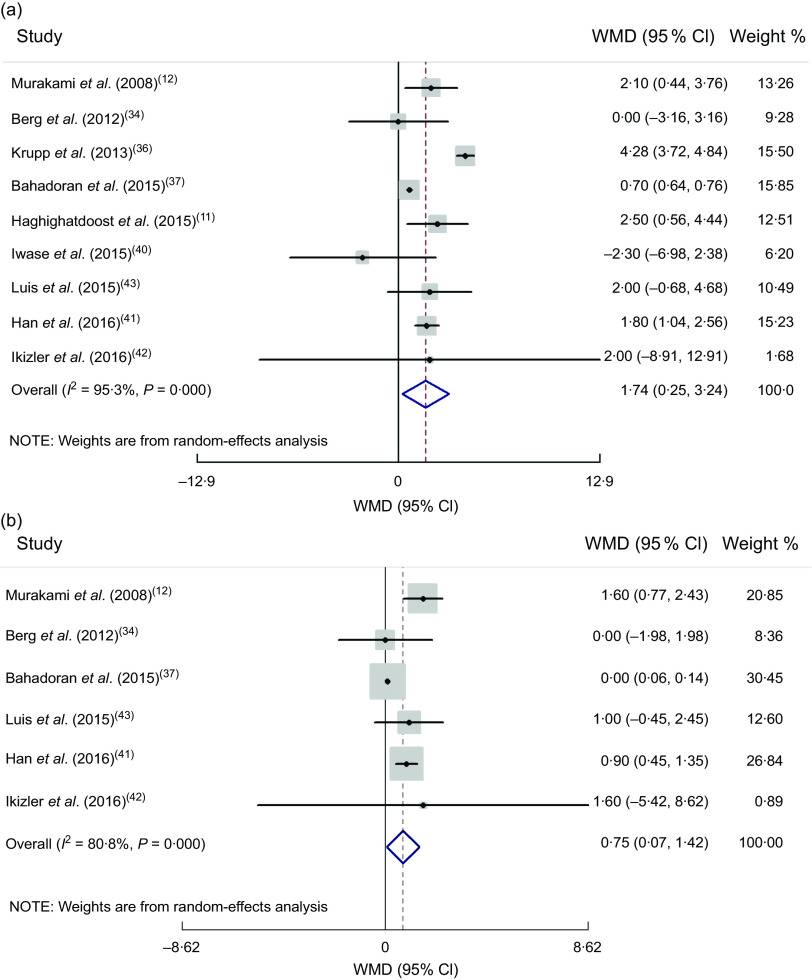
(a) Forest plot for the association between dietary acid load and systolic blood pressure in cross-sectional studies by random-effect model; (b) Forest plot for the association between dietary acid load and diastolic blood pressure in cross-sectional studies by random-effect model. The study-specific effect size (expressed as weighted mean difference (WMD)) and 95 % CI are represented by the solid diamond and horizontal line, respectively; the area of the grey square is proportional to the specific-study weight to the overall meta-analysis. The centre of the open diamond and the vertical dashed line represent the pooled effect size, and the width of the open diamond represents the pooled 95 % CI
Anthropometric measures
Combining effect sizes of both cohort and cross-sectional studies revealed a non-significant association between DAL and BMI by the random-effect model (pooled effect size = 0·39 (95 % CI −0·02, 0·80) kg/m2, P = 0·062; I 2 = 99·7 %, P < 0·0001). Since cross-sectional studies present less power in defining causation, we separated the results based on type of study. Combining the seventeen cohort studies by random-effects analysis indicated that there was no association between DAL and BMI (pooled effect size = 0·70 (95 % CI −0·10, 1·51) kg/m2, P = 0·088; I 2 = 99·8 %, P < 0·0001; Fig. 2(a)).
When we performed subgroup analysis based on age, the significant positive association was seen for individuals older than 17 years (pooled effect size = 0·69 (95 % CI 0·66, 0·72) kg/m2, P < 0·0001; I 2 = 99·9 %, P < 0·0001), while the relationship was not significant in individuals younger than 17 years old (pooled effect size = 0·11 (95 % CI −0·29, 0·51) kg/m2, P = 0·590; I 2 = 0 %, P = 0·883). We found that age was a source of heterogeneity (Supplemental Table S2).
Combining sixteen effect sizes of cross-sectional studies revealed no association between DAL and BMI (pooled effect size by random-effect model = 0·06 (95 % CI −0·21, 0·34) kg/m2, P = 0·651; I 2 = 95·5 %, P < 0·0001; Fig. 2(b)). Based on subgroup analyses, the method of dietary assessment and health status were the sources of the heterogeneity (Supplemental Table S3).
Overall analysis of four cross-sectional studies which reported WC showed that there was no significant relationship between WC and DAL (pooled effect size based on random-effect model = 0·42 (95 % CI −0·80, 1·65) cm, P = 0·495; I 2 = 96·3 %, P < 0·0001). We could not find the source of heterogeneity for this relationship by subgroup analysis (Supplemental Table S4). Moreover, there was not enough effect size to pool for determining the association of WC and DAL in cohort studies.
Blood pressure
By combining two effect sizes of cohort studies, no significant association was observed between SBP and DAL (pooled effect size = 1·12 (95 % CI −0·002, 2·25) mmHg; P = 0·050; I 2 = 0 %, P = 0·443), or between DBP and DAL (pooled effect size = 0·61 (95 % CI −0·17, 1·40) mmHg, P = 0·127; I 2 = 0 %, P = 0·927).
Combining six effect sizes of cross-sectional studies revealed a positive association between SBP, DBP and DAL, in fixed-effect models. Because of high heterogeneity also after performing random-effect models, there was a significant relationship between SBP, DBP and DAL (pooled effect size = 1·74 (95 % CI 0·25, 3·24) mmHg, P = 0·022; I 2 = 95·3 %, P < 0·0001 and pooled effect size = 0·75 (95 % CI 0·07, 1·42) mmHg; P = 0·030; I 2 = 80·8 %, P < 0·0001, respectively; Fig. 3(a) and (b), respectively). Based on subgroup analysis, the method of dietary assessment, study quality and health status were the sources of heterogeneity for SBP and DBP. When we performed subgroup analysis based on dietary assessment, the significant positive association was seen for individuals whose dietary intake was assessed by food records (pooled effect size = 4·18 (95 % CI 3·63, 4·73) mmHg, P < 0·0001; I 2 = 28·9 %, P = 0·245; Supplemental Tables S5 and S6).
Serum lipids
Findings from four cross-sectional studies revealed that there was no association between DAL and TC by the random-effect model (pooled effect size = −1·94 (95 % CI −7·97, 3·90) mg/dl; P = 0·515; I 2= 86·1 %, P < 0·0001). Health status was the source of heterogeneity for the association between TC and DAL (Supplemental Table S7). Moreover, there was not enough effect size to pool for determining the association of TC and DAL in cohort studies.
Findings from three cross-sectional studies (pooled effect size = 0·09 (95 % CI −0·42, 0·61) mg/dl, P = 0·713; I 2 = 0 %, P = 0·998) and two cohort studies (pooled effect size = −0·55 (95 % CI −1·70, 0·60) mg/dl, P = 0·348; I 2 = 3·3 %, P = 0·309) revealed that there was no association between DAL and HDL-C by fixed-effect models.
Findings from four cross-sectional studies revealed that there was no association between DAL and LDL-C by the random-effect model (pooled effect size = 1·61 (95 % CI −2·19, 5·42) mg/dl, P = 0·407; I 2 = 68·5 %, P = 0·023). Gender, dietary assessment and study quality were the sources of heterogeneity for the association of LDL-C with DAL (Supplemental Table S8). Moreover, there was not enough effect size to pool for determining the association of LDL-C with DAL in cohort studies.
Findings from six cross-sectional studies revealed that there was a non-significant association between DAL and TAG by the random-effect model (pooled effect size = 4·46 (95 % CI −0·76, 9·70) mg/dl, P = 0·515; I 2 = 82·9 %, P < 0·0001). We did not find the sources of heterogeneity for the association between TAG and DAL (Supplemental Table S9). Moreover, there was not enough effect size to pool for determining the association of TAG with DAL in cohort studies.
Glycaemic parameters
Overall, combining eight studies that reported FBS by the fixed-effect model showed a significant positive association between FBS and DAL with high heterogeneity; however, after performing a random-effect model, there was no significant association (pooled effect size = −3·73 (95 % CI −9·99, 2·51) mg/dl, P = 0·242; I 2 = 99·5 %, P < 0·0001). Since cross-sectional studies present less power in defining causation, we separated the results based on study type. Combining six effect sizes of cross-sectional studies indicated that there was no association between DAL and FBS (pooled effect size = 0·43 (95 % CI −3·68, 4·54) mg/dl, P = 0·839; I 2 = 98·5 %, P < 0·0001). Dietary assessment and study quality were the sources of heterogeneity for the association of FBS with DAL (Supplemental Table S10). Moreover, combining two effect sizes of cohort studies revealed a non-significant association between FBS and DAL after performing the random-effect model.
By combining five effect sizes of cross-sectional studies, there was no significant relationship between HbA1c and DAL (pooled effect size based on random-effect model = −0·012 (95 % CI −0·046, 0·069) %, P = 0·693; I 2 = 57·7 %, P = 0·051). The method of dietary assessment, health status and study quality were the sources of heterogeneity (Supplemental Table S11). Moreover, there was not enough effect size to pool for determining the association of HbA1c with DAL in cohort studies.
Combining two effect sizes of cross-sectional studies revealed no significant association between HOMA-IR and DAL (pooled effect size = 0·06 (95 % CI −0·02, 0·14), P = 0·165; I 2 = 0 %, P = 0·758). Moreover, there was not enough effect size to pool for determining the association of HOMA-IR with DAL in cohort studies.
Combining three effect sizes of cross-sectional studies revealed no significant association between serum insulin and DAL (pooled effect size based on fixed-effect model = 0·29 (95 % CI −0·04, 0·61) μU/ml, P = 0·084; I 2 = 1·6 %, P = 0·362). Moreover, there was not enough effect size to pool for determining the association of serum insulin with DAL in cohort studies.
Based on the results of Egger’s test, no evidence of publication bias was found for BMI (P for cohort studies = 0·781; P for cross-sectional studies = 0·149), WC (P for cross-sectional studies = 0·381), SBP (P for cross-sectional studies = 0·214), DBP (P for cross-sectional studies = 0·104), TC (P for cross-sectional studies = 0·500), LDL-C (P for cross-sectional studies = 0·464), FBS (P for cross-sectional studies = 0·070), HbA1c (P for cross-sectional studies = 0·502) and serum insulin (P for cross-sectional studies = 0·451). There was a significant publication bias for the association of TAG and DAL (P for cross-sectional studies = 0·030).
Discussion
In the present systematic review and meta-analysis, we found that DAL was positively associated with SBP and DBP. However, there was no significant association of DAL with BMI, WC, glycaemic factors and lipid profile. Subgroup analysis revealed that DAL which was calculated with PRAL from FFQ was positively associated with FBS and HbA1c. To the best of our knowledge, the present review is the first to investigate the association between DAL and cardiometabolic factors.
In the present review, we found that DAL was directly associated with SBP and DBP. In line with our results, several studies reported that higher level of DAL was associated with hypertension risk(20,36,41); nevertheless, some others showed no association(34,37,43). Kidney function is an important factor which regulates the acid–base balance(45), suggesting that glomerular filtration rate and renal function should be assessed for the exact association between DAL and blood pressure. On the other hand, acid–base balance impacts on the absorption of minerals which are effective in hypertension improvement such as Ca and Mg(46). It has been shown that PRAL and NEAP scores have an inverse association with K, Ca and Mg(25). High DAL can increase excretion of Ca and Mg, which leads to increased blood pressure and insulin resistance(47). Another mechanism related to hypertension due to higher DAL and higher renal acid excretion is stimulation of the production of cortisol by DAL, which can increase blood pressure as well as insulin resistance(48). Also decreasing urinary citrate excretion due to higher DAL can lead to hypertension(49). Two other mechanisms which lead to hypertension due to higher DAL are related to increasing BMI and glycaemic factors such as FBS, serum insulin, HOMA-IR and HbA1c. In fact, higher DAL induces metabolic acidosis which leads to impaired secretion of insulin-like growth factor 1(50) and increases insulin resistance(51).
According to previous studies, individuals who had higher DAL tended to have higher BMI and WC(12,21,41,52). Moreover, Maalouf et al. showed that decreased 24 h urine pH is associated with higher HOMA-IR(53). According to a prospective study, higher plasma level of bicarbonate is associated with a lower incidence of type 2 diabetes(54). Akter et al. found no association between DAL and FBS and HbA1c(1). In the present review, we found no association between FBS, serum insulin, HbA1c and HOMA-IR and DAL. However, there was a direct significant association between FBS, HbA1c and DAL in studies that assessed the dietary intakes by FFQ, and diet history questionnaires, but not recalls. Definitely, a dietary assessment with these former methods is more precise than dietary recalls which confirm short daily dietary intake with remembering error(41,55).
The present results indicated no significant association between lipid profiles and DAL. However, there was a significant positive relationship between LDL-C and DAL in studies that assessed dietary intakes by diet history questionnaire. Included studies were different in the methods used to assess dietary intakes and the DAL assessment tool. Inconsistent with our overall results, Murakami et al. (12) found a positive association between PRAL and LDL-C and TC, but did not find a significant association between PRAL and TAG. Metabolic acidosis induced by DAL might influence cardiometabolic factors by increasing cortisol production(56–58). Further investigations are warranted to find the exact relationship. Moreover, according to different mentioned mechanisms, health status should be considered to discuss the real associations regarding higher DAL in patients with diabetes, hypertension or kidney diseases.
The present study has some strengths. A comprehensive systematic literature search was performed to include all relevant studies. Moreover, we considered and analysed different cardiometabolic factors such as HbA1c, serum insulin, lipid profile and BMI. Also, subgroup analyses were conducted to find sources of heterogeneity. Since the present review is the first assessing the association between DAL and cardiometabolic risk factors, its limitations must be kept in mind. Since there was no adjustment of the considered variables for confounders such as energy intake, age and gender, our results should be interpreted carefully. Also, we could not find the source of heterogeneity for TAG and WC. Moreover, although we conducted subgroup analysis based on health status, the study participants were different in health status which may a subject bias. Also, different DAL categories (tertile, quartile or quintile) were used in each included study.
Conclusion
The findings of the current review suggest that high DAL is associated with increased SBP and DBP. More studies with adjustment for risk factors are needed to find any relationship between DAL and lipid profile and glycaemic factors.
Acknowledgements
Financial support: This study was supported by Tehran University of Medical Sciences (grant number 97-01-161-38074). Tehran University of Medical Sciences had no role in the design, analysis or writing of this article. Conflict of interest: The authors declared no conflict of interest. Authorship: E.D. and L.A. contributed in the conception and design of the study. E.D. and F.H. performed the search process, excluded irrelevant studies and conducted data extraction separately, with final checking by L.A. E.D. and F.H. contributed in statistical analyses, data interpretation and manuscript drafting. All authors approved the final manuscript for submission. Ethics of human subject participation: Not applicable.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S1368980019001125.
click here to view supplementary material
References
- 1. Akter S, Eguchi M, Kuwahara K et al. (2016) High dietary acid load is associated with insulin resistance: the Furukawa Nutrition and Health Study. Clin Nutr 35, 453–459. [DOI] [PubMed] [Google Scholar]
- 2. Danaei G, Lu Y, Singh GM et al. (2014) Cardiovascular disease, chronic kidney disease, and diabetes mortality burden of cardio-metabolic risk factors between 1980 and 2010: comparative risk assessment. Lancet Diabetes Endocrinol 2, 634–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Souto G, Donapetry C, Calvino J et al. (2011) Metabolic acidosis-induced insulin resistance and cardiovascular risk. Metab Syndr Relat Disord 9, 247–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Williams R, Heilbronn L, Chen D et al. (2015) Dietary acid load, metabolic acidosis and insulin resistance – lessons from cross-sectional and overfeeding studies in humans. Clin Nutr 35, 1084–1094. [DOI] [PubMed] [Google Scholar]
- 5. Adeva MM & Souto G (2011) Diet-induced metabolic acidosis. Clin Nutr 30, 416–421. [DOI] [PubMed] [Google Scholar]
- 6. Engberink MF, Bakker SJ, Brink EJ et al. (2012) Dietary acid load and risk of hypertension: the Rotterdam Study. Am J Clin Nutr 95, 1438–1444. [DOI] [PubMed] [Google Scholar]
- 7. Remer T & Manz F (1995) Potential renal acid load of foods and its influence on urine pH. J Am Diet Assoc 95, 791–797. [DOI] [PubMed] [Google Scholar]
- 8. Frassetto LA, Todd KM, Morris RC et al. (1998) Estimation of net endogenous noncarbonic acid production in humans from diet potassium and protein contents. Am J Clin Nutr 68, 576–583. [DOI] [PubMed] [Google Scholar]
- 9. Remer T & Manz F (1994) Estimation of the renal net acid excretion by adults consuming diets containing variable amounts of protein. Am J Clin Nutr 59, 1356–1361. [DOI] [PubMed] [Google Scholar]
- 10. Crawford SO, Hoogeveen RC, Brancati FL et al. (2010) Association of blood lactate with type 2 diabetes: the Atherosclerosis Risk in Communities Carotid MRI Study. Int J Epidemiol 39, 1647–1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Haghighatdoost F, Najafabadi M, Bellissimo N et al. (2015) Association of dietary acid load with cardiovascular disease risk factors in patients with diabetic nephropathy. Nutrition 31, 697–702. [DOI] [PubMed] [Google Scholar]
- 12. Murakami K, Sasaki S, Takahashi Y et al. ; Japan Dietetic Students’ Study for Nutrition and Biomarkers Group (2008) Association between dietary acid–base load and cardiometabolic risk factors in young Japanese women. Br J Nutr 100, 642–651. [DOI] [PubMed] [Google Scholar]
- 13. Xu H, Jia T, Huang X et al. (2014) Dietary acid load, insulin sensitivity and risk of type 2 diabetes in community-dwelling older men. Diabetologia 57, 1561–1568. [DOI] [PubMed] [Google Scholar]
- 14. Krupp D, Johner S, Kalhoff H et al. (2012) Long-term dietary potential renal acid load during adolescence is prospectively associated with indices of nonalcoholic fatty liver disease in young women. J Nutr 142, 313–319. [DOI] [PubMed] [Google Scholar]
- 15. Remer T, Zhang L, Curhan GC et al. (2009) Diet-dependent net acid load and risk of incident hypertension in US women. Hypertension 54, 751–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wells GA, Shea B, O’Connell D et al. (2000) The Newcastle–Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed November 2016).
- 17. Wright E, Michaud D, Pietinen P et al. (2005) Estimated urine pH and bladder cancer risk in a cohort of male smokers (Finland). Cancer Causes Control 16, 1117–1123. [DOI] [PubMed] [Google Scholar]
- 18. Zhang L, Curhan G & Forman J (2009) Diet-dependent net acid load and risk of incident hypertension in United States women. Hypertension 54, 751–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Scialla J, Appel L, Astor B et al. (2012) Net endogenous acid production is associated with a faster decline in GFR in African Americans. Kidney Int 82, 106–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Krupp D, Shi L & Remer T (2013) Longitudinal relationships between diet-dependent renal acid load and blood pressure development in healthy children. Kidney Int 85, 204–210. [DOI] [PubMed] [Google Scholar]
- 21. Fagherazzi G, Vilier A, Bonnet F et al. (2014) Dietary acid load and risk of type 2 diabetes: the E3N-EPIC cohort study. Diabetologia 57, 313–320. [DOI] [PubMed] [Google Scholar]
- 22. Chan R, Leung J & Woo J (2015) Association between estimated net endogenous acid production and subsequent decline in muscle mass over four years in ambulatory older Chinese people in Hong Kong: a prospective cohort study. J Gerontol A Biol Sci Med Sci 70, 905–911. [DOI] [PubMed] [Google Scholar]
- 23. Garcia A, Franco O, Voortman T et al. (2015) Dietary acid load in early life and bone health in childhood: the Generation R Study. Am J Clin Nutr 102, 1595–1603. [DOI] [PubMed] [Google Scholar]
- 24. Jia T, Byberg L, Lindholm B et al. (2015) Dietary acid load, kidney function, osteoporosis, and risk of fractures in elderly men and women. Osteoporos Int 26, 563–570. [DOI] [PubMed] [Google Scholar]
- 25. Akter SH, Kurotani K, Kashino I et al. (2016) High dietary acid load score is associated with increased risk of type 2 diabetes in Japanese men: the Japan Public Health Center-based Prospective Study. J Nutr 146, 1076–1083. [DOI] [PubMed] [Google Scholar]
- 26. Esche J, Shi L, Sánchez-Guijo A et al. (2016) Higher diet-dependent renal acid load associates with higher glucocorticoid secretion and potentially bioactive free glucocorticoids in healthy children. Kidney Int 90, 325–333. [DOI] [PubMed] [Google Scholar]
- 27. Moghadam S, Bahadoran Z, Mirmiran P et al. (2016) Association between dietary acid load and insulin resistance: Tehran Lipid and Glucose Study. Prev Nutr Food Sci 21, 104–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Scialla J, Asplin J, Dobre M et al. (2016) Higher net acid excretion is associated with a lower risk of kidney disease progression in patients with diabetes. Kidney Int 91, 204–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Xu H, Akesson A, Orsini N et al. (2016) Modest U-shaped association between dietary acid load and risk of all-cause and cardiovascular mortality in adults. J Nutr 146, 1580–1585. [DOI] [PubMed] [Google Scholar]
- 30. Akter S, Nanri A, Mizoue T et al. (2017) Dietary acid load and mortality among Japanese men and women: the Japan Public Health Center–based Prospective Study. Am J Clin Nutr 106, 146–154. [DOI] [PubMed] [Google Scholar]
- 31. Welch A, Bingham S, Reeve J et al. (2007) More acidic dietary acid–base load is associated with reduced calcaneal broadband ultrasound attenuation in women but not in men: results from the EPIC-Norfolk cohort study. Am J Clin Nutr 85, 1134–1141. [DOI] [PubMed] [Google Scholar]
- 32. Wynn E, Lanham-New S, Krieg M et al. (2008) Low estimates of dietary acid load are positively associated with bone ultrasound in women older than 75 years of age with a lifetime fracture. J Nutr 138, 1349–1354. [DOI] [PubMed] [Google Scholar]
- 33. Scialla J, Appel L, Astor B et al. (2011) Estimated net endogenous acid production and serum bicarbonate in African Americans with chronic kidney disease. Clin J Am Soc Nephrol 6, 1526–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Berg E, Engberink M, Brink E et al. (2012) Dietary acid load and metabolic acidosis in renal transplant recipients. Clin J Am Soc Nephrol 7, 1811–1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Amodu A & Abramowitz M (2013) Dietary acid, age, and serum bicarbonate levels among adults in the United States. Clin J Am Soc Nephrol 8, 2034–2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Krupp D, Shi L, Maser-Gluth C et al. (2013) 11b Hydroxysteroid dehydrogenase type 2 and dietary acid load are independently associated with blood pressure in healthy children and adolescents. Am J Clin Nutr 97, 612–620. [DOI] [PubMed] [Google Scholar]
- 37. Bahadoran Z, Mirmiran P, Khosravi H et al. (2015) Associations between dietary acid–base load and cardiometabolic risk factors in adults: the Tehran Lipid and Glucose Study. Endocrinol Metab 30, 201–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Akter S, Eguchi M, Kurotani K et al. (2015) High dietary acid load is associated with increased prevalence of hypertension: the Furukawa Nutrition and Health Study. Nutrition 31, 298–303. [DOI] [PubMed] [Google Scholar]
- 39. Huston H, Abramowitz M, Zhang Y et al. (2015) Net endogenous acid production and mortality in NHANES III. Nephrology 20, 209–215. [DOI] [PubMed] [Google Scholar]
- 40. Iwase H, Tanaka M, Kobayashi Y et al. (2015) Lower vegetable protein intake and higher dietary acid load associated with lower carbohydrate intake are risk factors for metabolic syndrome in patients with type 2 diabetes: post-hoc analysis of a cross-sectional study. J Diabetes Invest 6, 465–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Han E, Kim G, Hong N et al. (2016) Association between dietary acid load and the risk of cardiovascular disease: nationwide surveys (KNHANES 2008–2011). Cardiovasc Diabetol 15, 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ikizler H, Zelnick L, Ruzinski J et al. (2016) Dietary acid load is associated with serum bicarbonate but not insulin sensitivity in chronic kidney disease. J Ren Nutr 26, 93–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Luis D, Huang X, Riserus U et al. (2015) Estimated dietary acid load is not associated with blood pressure or hypertension incidence in men who are approximately 70 years old. J Nutr 145, 315–321. [DOI] [PubMed] [Google Scholar]
- 44. Shea M, Gilhooly C & Dawson-Hughes B (2017) Food groups associated with measured net acid excretion in community-dwelling older adults. Eur J Clin Nutr 71, 420–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Goraya N & Wesson DE (2012) Acid–base status and progression of chronic kidney disease. Curr Opin Nephrol Hypertens 21, 552–556. [DOI] [PubMed] [Google Scholar]
- 46. Rylander R, Tallheden T & Vormann J (2009) Acid–base conditions regulate calcium and magnesium homeostasis. Magnes Res 22, 262–265. [DOI] [PubMed] [Google Scholar]
- 47. Nielsen TF & Rylander R (2011) Urinary calcium and magnesium excretion relates to increase in blood pressure during pregnancy. Arch Gynecol Obstet 283, 443–447. [DOI] [PubMed] [Google Scholar]
- 48. Kelly JJ, Mangos G, Williamson PM et al. (1998) Cortisol and hypertension. Clin Exp Pharmacol Physiol Suppl 25, 51–56 6. [DOI] [PubMed] [Google Scholar]
- 49. Taylor EN, Mount DB, Forman JP et al. (2006) Association of prevalent hypertension with 24-h urinary excretion of calcium, citrate, and other factors. Am J Kidney Dis 47, 780–789. [DOI] [PubMed] [Google Scholar]
- 50. Ordonez FA, Santos F, Martinez V et al. (2000) Resistance to growth hormone and insulin-like growth factor-I in acidotic rats. Pediatr Nephrol 14, 720–725. [DOI] [PubMed] [Google Scholar]
- 51. Souto G, Donapetry C, Calvino J et al. (2011) Metabolic acidosis-induced insulin resistance and cardiovascular risk. Metab Syndr Relat Disord 9, 247–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Chan R, Wong VW, Chu WC et al. (2015) Higher estimated net endogenous Acid production may be associated with increased prevalence of nonalcoholic fatty liver disease in Chinese adults in Hong Kong. PLoS One 10, e0122406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Maalouf NM, Cameron MA, Moe OW et al. (2007) Low urine pH: a novel feature of the metabolic syndrome. Clin J Am Soc Nephrol 2, 883–888. [DOI] [PubMed] [Google Scholar]
- 54. Mandel EI, Curhan GC, Hu FB et al. (2012) Plasma bicarbonate and risk of type 2 diabetes mellitus. CMAJ 184, 719–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kowalkowska J, Slowinska M, Slowinski D et al. (2013) Comparison of a full food-frequency questionnaire with the three-day unweighted food records in young Polish adult women: implications for dietary assessment. Nutrients 5, 2747–2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Dimitriou T, Maser-Gluth C & Remer T (2003) Adrenocortical activity in healthy children is associated with fat mass. Am J Clin Nutr 77, 731–736. [DOI] [PubMed] [Google Scholar]
- 57. Marin P, Darin N, Amemiya T et al. (1992) Cortisol secretion in relation to body fat distribution in obese premenopausal women. Metabolism 41, 882–886. [DOI] [PubMed] [Google Scholar]
- 58. Fraser R, Ingram MC, Anderson NH et al. (1999) Cortisol effects on body mass, blood pressure, and cholesterol in the general population. Hypertension 33, 1364–1368. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit https://doi.org/10.1017/S1368980019001125.
click here to view supplementary material