Abstract
Objective
The present review aimed to quantify the association of dietary intake and circulating concentration of major dietary antioxidants with risk of total CVD mortality.
Design
Systematic review and meta-analysis.
Setting
Systematic search in PubMed and Scopus, up to October 2017.
Participants
Prospective observational studies reporting risk estimates of CVD mortality across three or more categories of dietary intakes and/or circulating concentrations of vitamin C, vitamin E and β-carotene were included. A random-effects meta-analysis was conducted.
Results
A total of fifteen prospective cohort studies and three prospective evaluations within interventional studies (320 548 participants and 16 974 cases) were analysed. The relative risks of CVD mortality for the highest v. the lowest category of antioxidant intakes were as follows: vitamin C, 0·79 (95 % CI 0·68, 0·89; I 2=46 %, n 10); vitamin E, 0·91 (95 % CI 0·79, 1·03; I 2=51 %, n 8); β-carotene, 0·89 (95 % CI 0·73, 1·05; I 2=34 %, n 4). The relative risks for circulating concentrations were: vitamin C, 0·60 (95 % CI 0·42, 0·78; I 2=65 %, n 6); α-tocopherol, 0·82 (95 % CI 0·76, 0·88; I 2=0 %, n 5); β-carotene, 0·68 (95 % CI 0·52, 0·83; I 2=50 %, n 6). Dose–response meta-analyses demonstrated that the circulating biomarkers of antioxidants were more strongly associated with risk of CVD mortality than dietary intakes.
Conclusions
The present meta-analysis demonstrates that higher vitamin C intake and higher circulating concentrations of vitamin C, vitamin E and β-carotene are associated with a lower risk of CVD mortality.
Keywords: Antioxidants, CVD, Meta-analysis, Observational studies
Antioxidants are of interest in the CVD. Several observational studies have shown that higher consumption of different fruits and vegetables, the two main dietary sources of antioxidants( 1 ), was significantly and inversely associated with risk of CVD including CHD( 2 – 4 ), stroke( 5 , 6 ), myocardial infarction( 7 ) and heart failure( 8 ). Higher consumption of fruits and vegetables is accompanied by lower glycaemic load, higher fibre intake, higher dietary anti-inflammatory properties and higher electrolyte intake; which in turn are associated with a lower risk of CVD( 9 ). However, besides these beneficial features, a potentially cardioprotective role for dietary antioxidants has been proposed( 10 , 11 ).
It was shown in two recent prospective cohort studies in US and European populations that adherence to a diet with high antioxidant capacity was associated with a lower CVD mortality risk( 12 , 13 ). Oxidative stress, vascular inflammation and low-grade systemic inflammation are some of the mediatory pathways through which a possible link has been proposed between dietary antioxidants and CVD risk( 14 – 17 ). In accordance with this evidence, several observational studies have suggested an inverse relationship of higher intakes of vitamin E and vitamin C with the risk of CHD and stroke( 18 – 21 ). A pooled analysis of nine prospective cohort studies showed a weak inverse association between higher intake of vitamin E and the risk of CHD, but failed to show such a protective effect for total and individual carotenoids( 22 ). Another meta-analysis of prospective cohort studies suggested an inverse association of higher intakes of vitamin E and vitamin C with the risk of CHD, and like the previous review, found a non-significant association for β-carotene( 23 ).
Although observational studies have suggested an association between a higher intake of antioxidants and a lower risk of CVD, interventional studies have failed to show that supplementation with vitamin C, vitamin E and β-carotene can decrease the risk of CVD( 24 – 26 ). By contrast, a recent meta-regression analysis of fifty-three randomized controlled trials indicated that high-dose supplementations with vitamin E and β-carotene might increase the risk of all-cause mortality( 27 ). High-dose supplementations, short-term follow-up durations and prior history of CVD or other chronic diseases are some of the proposed explanations for the inconsistent findings in interventional studies( 28 ). Thus, determining the shape of the dose–response relationships between the abovementioned antioxidants and the risk of CVD mortality may clarify whether higher dietary intakes of these antioxidants are associated with a higher CVD mortality risk. To our knowledge, no systematic review and meta-analysis has assessed the association of dietary vitamin E, vitamin C and β-carotene with the risk of CVD mortality. The extent to which these antioxidants are associated with the risk of CVD mortality is still unclear. Furthermore, a recent meta-analysis of prospective cohort studies has suggested that the circulating biomarkers of carotenoids were more strongly associated with risk of breast cancer than dietary intakes( 29 ), but this hypothesis has not been examined in the context of CVD. Therefore, the objective of the present study was to summarize data about the associations of dietary intake and circulating concentration of vitamin C, vitamin E and β-carotene with the risk of total CVD mortality in the general population, with the use of prospective observational studies.
Methods
The present systematic review has been reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement( 30 ).
Search strategy
A systematic literature search was done in PubMed and Scopus, with studies published from 1966 up to October 2017. The search was performed as part of a larger search on the association between antioxidants and mortality. The systematic search included combinations of keywords relevant to dietary antioxidants intake, circulating antioxidants concentration, mortality and study design (see online supplementary material, Supplemental Table 1). The reference lists of all related articles and reviews were also manually searched. The search was restricted to articles published in English.
Eligibility and study selection
Two authors (A.J., M.S.Z.) independently reviewed the title and abstract of all studies identified. Prospective cohort, nested case–control, case–cohort studies or prospective reports within randomized controlled trials were obtained and included in the current review if they: (i) were conducted among adults aged 18 years or older; (ii) measured and reported baseline dietary intake or serum/plasma concentration of vitamin C, vitamin E or β-carotene in at least three categories; (iii) reported the outcome of interest as total CVD mortality at follow-up; (iv) reported risk estimates (relative risk (RR) or hazard ratio or odds ratio) and the corresponding 95 % CI of CVD mortality for each category of the abovementioned dietary/circulating antioxidants; and (v) reported the number of cases and participants/non-cases or person-years in each category of the abovementioned exposures, or reported sufficient information to estimate those numbers. Studies that reported results per unit increment in any of the dietary/circulating antioxidants or per sd increment were also included. We excluded studies that were: (i) conducted in children and adolescents; and (ii) conducted among patients with specific diseases such as hypertension and type 2 diabetes, or in institutionalized elders.
Data extraction
Two independent authors (A.J., M.P.) reviewed the full text of selected eligible studies and extracted the following information: first author’s name, publication year, location, follow-up duration, number of participants/cases, mean age and/or age range, sex, exposures, exposure assessment method, covariates adjusted for in the multivariate analyses, exposure levels, number of cases/participants, and reported risk estimates and 95 % CI of CVD mortality across different categories of each dietary/circulating antioxidant. The models with the most comprehensive covariate adjustments were selected and included in the meta-analysis.
Quality of meta-evidence
A nine-point Newcastle–Ottawa Scale was used to assess the quality of included studies and studies with more than seven stars were considered high quality( 31 ). In the main analyses, both low- and high-quality studies were included. However, we conducted sensitivity analyses by restricting only to the high-quality studies (≥7 stars). Furthermore, to provide more reliable measures for judgement about the quality of meta-evidence, we applied the new NutriGrade scoring system (a maximum of 10 points) developed by Schwingshackl et al.( 32 ). Based on this scoring system, we recommended four categories to judge the meta-evidence: high (≥8 points), moderate (6 to 8 points), low (4 to 6 points) and very low (0 to 4 points).
Statistical analysis
The RR and 95 % CI were considered as the effect size of all studies. The reported odds ratios and hazard ratios were considered equal to the RR. For the highest v. lowest category meta-analysis, the reported risk estimates for the highest compared with the lowest category of dietary/circulating antioxidants were combined using the DerSimonian and Laird random-effects model( 33 ). If studies reported results by sex or other subgroups separately, we combined subgroup-specific risk estimates using a fixed-effects model and used the combined effect size for meta-analysis. If studies reported risk estimates per sd increment of dietary/circulating antioxidants, we used the following method to translate the per sd increment risk estimate to the high v. low RR: first, we calculated the differences between the median points of the highest and lowest categories of that dietary/circulating antioxidant in other studies included in the relevant analysis. Then, mean difference between the medians of the highest and lowest categories was calculated. Finally, per sd increment risk estimate was translated to per ‘calculated mean difference’ and was included in the relevant analysis. If the exact amount of sd was not reported in the primary study, we assumed the difference between the highest and lowest categories as 2·18 times the sd ( 34 ). Potential small-study effects such as publication bias were explored by funnel plots and tested by Egger’s test( 35 ) and Begg’s test( 36 ) (P<0·10) when there were sufficient studies (n≥10)( 37 ). To test the potential effect of each study on pooled effect size, influence analysis was done by stepwise exclusion of each study at a time. Subgroup analyses were performed based on sex, geographical location, baseline age, follow-up duration, number of cases, dietary assessment method (in the analyses of dietary antioxidants) and adjustment for main confounders. We included all eligible studies in the main analyses. However, to provide more reliable results, we performed additional sensitivity analyses by restricting only to studies that controlled for main confounders including BMI, physical activity, smoking status and energy intake in their multivariate analyses.
We tested the linear dose–response relationship using generalized least-squares trend estimation, according to the methods developed by Greenland and Longnecker( 38 , 39 ). The method needs the numbers of cases and participants/non-cases or person-years and adjusted risk estimates and their 95 % CI in each category of dietary/circulating antioxidants. Study-specific results were combined using a random-effects model. The median point in each category of dietary/circulating antioxidants was assigned. If medians were not reported, we estimated approximate medians by using the midpoint of the lower and upper bounds. If the highest category was open-ended, we considered it to have the same widths as the closest category. If the lowest category was open-ended, we considered the lower bound as equal to zero. If only the mean of each category was reported, we considered it the same as the median. If the median point of each category was reported per specific amount of energy intake (e.g. per 4184 kJ/1000 kcal) or per specific amount of another variable (e.g. per serum cholesterol in the analysis of α-tocopherol), we recalculated the median point taking the reported mean or median energy intake or serum cholesterol of that category into account. If the numbers of participants/cases or person-years had not been reported in the primary studies, we estimated them by dividing the total number of participants/cases or person-years by the number of categories, if the exposures were defined as quantiles( 40 ). For studies in which the reference category was not the lowest one, we recalculated risk estimates assuming the lowest category as reference, if the numbers of participants and cases across different categories were reported( 41 ). A potential non-linear association was examined by modelling dietary/circulatory antioxidant levels using restricted cubic splines with three knots at fixed percentiles (10, 50 and 90 %) of the distribution( 42 ). A P value for non-linearity of the meta-analysis was calculated by testing the null hypothesis that the coefficient of the second spline was equal to zero. All analyses were conducted with the statistical software package Stata version 13. P<0·05 was considered statistically significant.
Results
As presented in the online supplementary material, Supplemental Fig. 1, the systematic search identified 17 296 articles, plus six articles through hand-searching. Of these, 2161 articles were duplicates and another 14 995 were not relevant, which were eliminated based on screening the title and abstract. Of the remaining 146 articles, another 129 articles were excluded by assessing their full text and respective reasons for study exclusion are detailed in Supplemental Fig. 1. Eventually, seventeen articles were considered eligible for inclusion in the present review( 43 – 59 ). One article reported the results of the two separate cohort studies and was regarded as two separate studies( 59 ). Thus, eighteen studies, comprising a total of 320 548 participants and 16 974 cases of CVD mortality, were included in the final analyses. Five studies were from the USA( 47 – 49 , 54 , 56 ), three studies (two articles) were from Asia( 53 , 59 ) and ten studies were from Europe( 43 – 46 , 50 – 52 , 55 , 57 , 58 ). Five studies included only men( 44 , 50 , 52 , 58 , 59 ), one study included only women( 59 ), and the remainder included both sexes. Seven studies (six articles) assessed dietary intakes of antioxidants( 7 , 44 , 47 , 53 , 57 , 59 ), three studies measured plasma concentrations( 45 , 49 , 51 ), five studies measured serum concentrations( 48 , 50 , 52 , 54 , 58 ), and three studies reported both dietary and circulating antioxidants as exposure( 43 , 46 , 56 ). Three studies were prospective evaluations within interventional studies( 46 , 49 , 58 ), with the remainder being prospective cohort studies. Follow-up duration ranged from 4 to 22 years. The general characteristics of the studies are presented in Table 1.
Table 1.
General characteristics of studies included in the present meta-analysis of dietary/circulating antioxidants and risk of cardiovascular mortality
| Author, year, country | Study | Follow-up (years) | Participants/cases | Sex | Mean age (years) | Age range (years) | Exposure(s) | Exposure assessment | Study quality (max. 9 points) | Adjustments |
|---|---|---|---|---|---|---|---|---|---|---|
| Bates et al.( 43 ), 2011 UK | British National Diet and Nutrition Survey | 13–14 | 526/189 | W/M | 77 | ≥66 | Plasma vitamin C, α-tocopherol and β-carotene Dietary vitamin C and vitamin E | Liquid chromatographic assay 4 d weighed dietary record | 8 | Age and sex |
| Buijsse et al. ( 44 ), 2008 Netherlands | The Zutphen Elderly Study | 15 | 559/197 | M | 72 | Dietary vitamin C, vitamin E and β-carotene | Dietary history method | 7 | Age, energy intake, smoking, BMI, physical activity, alcohol consumption, socio-economic status, use of multivitamin supplements, use of vitamin C supplements, use of aspirin, use of antihypertensive drugs, use of anticoagulants, diet prescription, intakes of fibre, β-carotene, vitamin C, vitamin E, α-tocopherol, folate, SFA, trans-fatty acids and PUFA | |
| Buijsse et al. ( 45 ), 2005 Europe | SENECA | 10 | 1168/148 | W/M | 73 | Plasma α-tocopherol | HPLC | 8 | Age, sex, BMI, serum TC, serum HDL-C, current smoking, alcohol consumption, physical activity, SENECA centre, and either plasma α-tocopherol or plasma carotene (both continuous) | |
| Fletcher et al. ( 46 ), 2003 UK | Medical Research Council Trial of Assessment and Management of Older People in the Community | 4·4 | 1214/128 | W/M | 78 | 75–84 | Plasma ascorbate, α-tocopherol and β-carotene Dietary vitamin C, vitamin E and β-carotene | HPLC FFQ | 7 | Age, sex, total energy intake, BMI, cholesterol, SBP, smoking, alcohol, DM, history of CVD or cancer, supplement use, physical activity and housing tenure |
| Genkinger( 47 ), 2004 USA | Washington County study | 13 | 6151/378 | W/M | 56 | 30–93 | Dietary vitamin C, vitamin E and β-carotene | FFQ | 7 | Age, smoking status, BMI, cholesterol concentration and energy. Nutrients were energy-adjusted using the residual method |
| Goyal et al. ( 48 ), 2013 USA | NHANES III | 14·2 | 16 008/1891 | W/M | ≥20 | Serum vitamin C and β-carotene | Isocratic HPLC | 8 | Age, sex, race/ethnicity, level of education, annual family income, BMI, smoking status, serum cotinine level, alcohol consumption, fruit and vegetable intake, physical activity, serum TC level, HTN status, DM status, history of heart attack, congestive heart failure, stroke or cancer, hormone use in women and supplement use | |
| Greenberg et al. ( 49 ), 1996 USA | Skin cancer prevention study | 8·2 | 1720/127 | W/M | 63 | Plasma β-carotene | HPLC | 7 | Age, sex, centre, BMI and smoking | |
| Karppi et al. ( 50 ), 2012 Finland | The Kuopio Ischemic Heart Disease Risk Factor Study (KIHD) | 15·9 | 1031/122 | M | 56 | 46–65 | Serum β-carotene | Reversed-phase HPLC | 6 | Age, examination year, BMI, SBP, smoking, alcohol consumption, physical activity, years of education, serum LDL-C, symptomatic CHD or CHD history, use of antihypertensive drugs, use of any beta-blockers, serum hs-CRP and DM |
| Khaw et al. ( 51 ), 2001 UK | EPIC-Norfolk | 4 | 19 496/170 | W/M | 59 | 45–79 | Plasma ascorbic acid | Fluorometric assay | 6 | Age |
| Kilander et al. ( 52 ), 2001 Sweden | Uppsala study | 22·7–25·7 | 2285/301 | M | 49–51 | Serum β-carotene and α-tocopherol | HPLC | 8 | Age | |
| Kubota et al. ( 53 ), 2011 Japan | Japan Collaborative Cohort (JACC) Study | 16·5 | 58 730/2690 | W/M | 56 | 40–79 | Dietary vitamin C and vitamin E | FFQ | 8 | Age, history of HTN and DM, smoking status, alcohol consumption, BMI, mental stress, walking, sports, education level, dietary intakes of total energy, cholesterol, SFA, n-3 fatty acids and Na |
| Loria et al. ( 54 ), 2000 USA | NHANES II | 12–16 | 7071/506 | W/M | 48 | 30–75 | Serum ascorbate | 2,4-Dinitrophenyl hydrazine method | 8 | Age at baseline examination, race, educational level, cigarette smoking, alcohol consumption, DM, serum TC, SBP and BMI |
| Martin-Calvo et al. ( 55 ), 2017 Spain | The SUN project | 11 | 13 421/48 | W/M | 43 | Dietary vitamin C | FFQ | 9 | Age, sex, BMI, total energy intake, physical activity, television watching, smoking, family history of stroke, treatment with aspirin, number of cardiovascular-related diseases at baseline, prevalent cancer, prevalent HTN, prevalent DM, prevalent hypercholesterolaemia, prevalent hypertriacylglycerolamia, dietary fibre, and MDS without fruit and vegetable intake-related items | |
| Sahyoun et al. ( 56 ), 1996 USA | Massachusetts Nutrition Status Survey | 9–12 | 725/108 | W/M | 73 | ≥60 | Plasma vitamin C Dietary vitamin C | HPLC 3 d food record | 6 | Age, sex, serum cholesterol, disease status and disabilities affecting shopping |
| Stepaniak et al. ( 57 ), 2016 Three Central and Eastern European countries | HAPIEE study | 8 | 26 993/997 | W/M | 58 | 45–69 | Dietary vitamin C, vitamin E and β-carotene | FFQ | 8 | Age, country, education, smoking status, alcohol intake, BMI, HTN, DM, hypercholesterolaemia, history of CVD or cancer, and total energy intake |
| Wright et al. ( 58 ), 2006 Finland | Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study | 19 | 29 092/5776 | M | 57 | 50–69 | Serum α-tocopherol | HPLC | 8 | Age, cigarettes smoked/d, years smoked, intervention assignment, serum TC, serum HDL-C and history of CVD |
| Zhao et al. ( 59 ), 2017 China | Shanghai Women’s Health Study | 14·2 | 74 619/1819 | W | 53 | 40–70 | Dietary vitamin C and vitamin E | FFQ | 8 | Age, energy, birth cohort, education, income, occupation, smoking status, alcohol intake, BMI, WHR, physical activity, history of HTN, DM, CHD and stroke, vitamin supplements use, menopause status, hormone replacement therapy, dietary total carotenes and vitamin C |
| Zhao et al. ( 59 ), 2017 China | Shanghai Men’s Health Study | 8·3 | 59 739/1379 | M | 55 | 40–74 | Dietary vitamin C and vitamin E | FFQ | 8 | Age, energy, birth cohort, education, income, occupation, smoking status, alcohol intake, BMI, WHR, physical activity, history of HTN, DM, CHD and stroke, vitamin supplements use, dietary total carotenes and vitamin C |
SENECA, Survey in Europe on Nutrition and the Elderly; NHANES, National Health and Nutrition Examination Survey; EPIC, European Prospective Investigation into Cancer and Nutrition; SUN, Seguimiento University of Navarra; HAPIEE, Health, Alcohol and Psychosocial factors In Eastern Europe; W, women; M, men; HDL-C, TC, total cholesterol; HDL-cholesterol; SBP, systolic blood pressure; DM, diabetes mellitus; HTN, hypertension; LDL-C, LDL-cholesterol; hs-CRP, high-sensitivity C-reactive protein; MDS, Mediterranean diet score; WHR, waist-to-hip ratio.
Vitamin C and CVD mortality
Ten studies (nine articles) with a total of 242 677 participants and 7933 cases were included in the analysis of dietary vitamin C and CVD mortality risk( 43 , 44 , 46 , 47 , 53 , 55 – 57 , 59 ). Participants belonging to the highest category of dietary vitamin C had a 21 % lower risk of CVD mortality compared with those in the lowest category (RR=0·79; 95 % CI 0·68, 0·89; Table 2), with moderate evidence of heterogeneity in the data (I 2=45·7 %, P heterogeneity=0·06; see online supplementary material, Supplemental Fig. 2). In the sensitivity analysis removing each study at a time, none of the excluded studies altered the summary result materially (RR ranged between 0·77 and 0·82). In a sensitivity analysis by restricting only to studies with high quality score (≥7 points), the RR altered to 0·82 (0·95 % CI 0·76, 0·89; I 2=0 %, n studies 9). Additionally, we performed a sensitivity analysis by restricting only to studies that controlled for BMI, physical activity, energy intake and smoking status in their multivariate analyses; the summary result remained significant (RR=0·80; 95 % CI 0·72, 0·87; I 2=0 %, n studies 6).
Table 2.
Meta-analysis of dietary/circulatory antioxidants (highest v. lowest category analysis) and risk of total cardiovascular mortality
| Antioxidant | No. of studies | RR | 95 % CI | I 2 (%) | 95 % CI | P heterogeneity | Meta-evidence | NutriGrade score | Explanation of the results according to recommendations of the NutriGrade scoring system |
|---|---|---|---|---|---|---|---|---|---|
| Dietary vitamin C | 10 | 0·79 | 0·68, 0·89 | 46 | 0, 74 | 0·06 | High | 8·4 | There is high confidence in the effect estimate, and further research probably will not change the confidence in the effect estimate |
| Circulating vitamin C | 6 | 0·60 | 0·42, 0·78 | 65 | 15, 85 | 0·01 | Moderate | 6·4 | There is moderate confidence in the effect estimate; further research could add evidence on the confidence and may change the effect estimate |
| Dietary vitamin E | 8 | 0·91 | 0·79, 1·03 | 51 | 0, 81 | 0·04 | Low | 5·4 | There is low confidence in the effect estimate; further research will provide important evidence on the confidence and likely change the effect estimate |
| Circulating α-tocopherol | 5 | 0·82 | 0·76, 0·88 | 0 | 0, 79 | 0·55 | Low | 4·0 | There is low confidence in the effect estimate; further research will provide important evidence on the confidence and likely change the effect estimate |
| Dietary β-carotene | 4 | 0·89 | 0·73, 1·05 | 35 | 0, 77 | 0·21 | Low | 5·5 | There is low confidence in the effect estimate; further research will provide important evidence on the confidence and likely change the effect estimate |
| Circulating β-carotene | 6 | 0·68 | 0·52, 0·83 | 50 | 0, 80 | 0·07 | Low | 5·9 | There is low confidence in the effect estimate; further research will provide important evidence on the confidence and likely change the effect estimate |
RR, relative risk.
In the subgroup analyses, a significant inverse association persisted across most of the subgroups apart from studies conducted in the USA, studies with older participants (mean age >60 years v. <60 years), studies without adjustment for BMI and physical activity, and studies that used methods other than FFQ to assess dietary intake (see online supplementary material, Supplemental Table 2). Stratifying by sources of vitamin C, the relative risk of CVD mortality was 0·83 (95 % CI 0·76, 0·90; I 2=0 %, n studies 7) when only dietary intake was taken into consideration and was 0·55 (95 % CI 0·26, 0·85; I 2=54·5 %, n studies 3) when intake from both food and supplements was taken into account. The subgroup analyses suggested that number of cases, geographical region, follow-up duration, mean age, prior exclusion of participants with a history of CVD and adjustment for main confounders were potential sources of the heterogeneity. No evidence of publication bias was found with Egger’s test (P=0·33) and with Begg’s test (P=0·86), but the funnel plot showed some evidence of asymmetry (Supplemental Fig. 3).
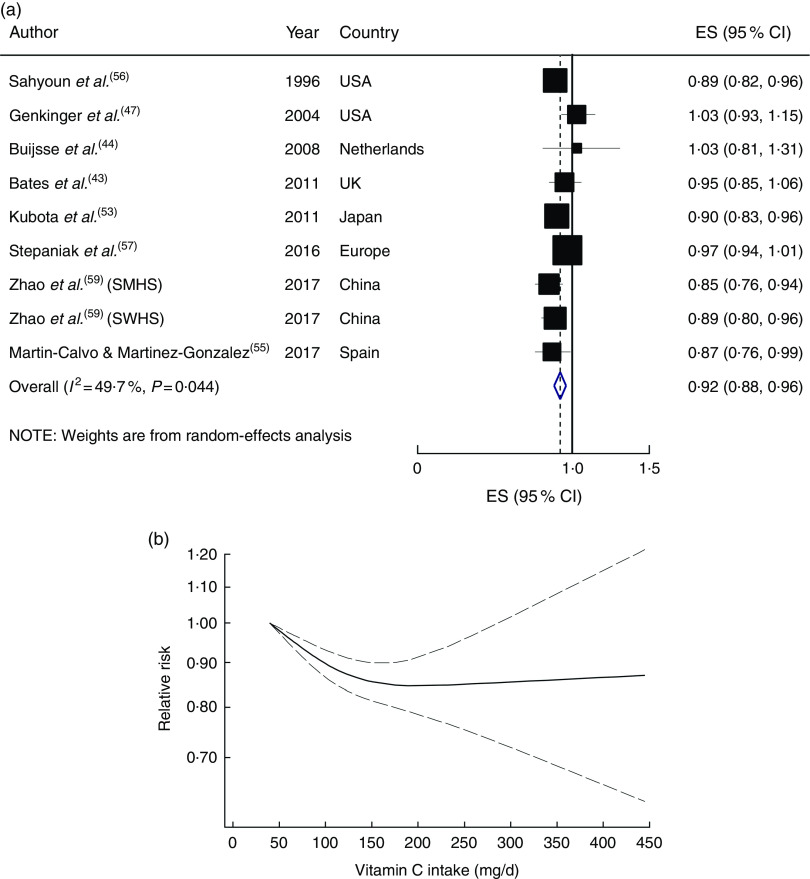
In the linear dose–response meta-analysis, a 50mg/d increment in dietary vitamin C intake was associated with an 8 % lower risk (RR=0·92; 95 % CI 0·88, 0·96), with moderate evidence of heterogeneity (I 2=49·7 %, P heterogeneity=0·04; Fig. 1(a)). Sequential exclusion of each study from the pooled analysis minimally altered the association (RR changed between 0·91 and 0·93). A non-linear dose–response meta-analysis demonstrated a significant dose–dependent association, in which the risk decreased linearly from a baseline of 40mg/d up to an intake of ~200mg/d, and then reached a plateau (P non-linearity<0·001; Fig. 1(b)).
Fig. 1.
(a) Forest plot showing relative risk of cardiovascular mortality for a 50 mg/d increment in vitamin C intake. The study-specific effect size (ES) and 95 % CI are represented by the black square and horizontal line, respectively; the area of the black square is proportional to the specific-study weight to the overall meta-analysis. The centre of the blue open diamond and the vertical dashed line represent the pooled ES, and the width of the blue open diamond represents the pooled 95 % CI (SMHS, Shanghai Men’s Health Study; SWHS, Shanghai Women’s Health Study). (b) Dose–response association of vitamin C intake and risk of total cardiovascular mortality (———), with 95 % CI (— — —)
Six studies with 45 040 participants and 2992 cases were analysed for the association between circulating vitamin C concentration and risk of CVD mortality( 43 , 46 , 48 , 51 , 54 , 56 ). The relative risk of CVD mortality for the highest compared with the lowest category of circulating vitamin C was 0·60 (0·95 % CI 0·42, 0·78), with high heterogeneity (I 2=64·7 %, P heterogeneity=0·01; see online supplementary material, Supplemental Fig. 4). In the sensitivity analysis excluding each study sequentially from the pooled analysis, the association ranged from 0·55 (95 % CI 0·36, 0·74) with the exclusion of the NHANES III study( 48 ) to 0·71 (95 % CI 0·59, 0·82) with the exclusion of the EPIC-Norfolk study( 51 ); and the latter study explained all of the observed heterogeneity (I 2=0 %). In the sensitivity analyses, when the analysis was restricted only to the high-quality studies and studies that controlled for main confounders, the relative risk changed to 0·72 (95 % CI 0·60, 0·85; I 2=0 %, n studies 4) and 0·74 (0·95 % CI 0·58, 0·90; I 2=0 %, n studies 2), respectively. In the subgroup analyses, a significant inverse association persisted across all subgroups, and appeared stronger among European studies compared with US studies (RR=0·46 v. 0·75), studies with <500 cases v. >500 cases (RR=0·46 v. 0·78), follow-up durations <10 years v. >10 years (RR=0·37 v. 0·72), studies that controlled for vitamin supplementation v. studies without adjustment (RR=0·55 v. 0·66), as well as among older participants with a mean age of >60 years v. <60 years (RR=0·58 v. 0·62; Supplemental Table 3). The subgroup analyses suggested that baseline mean age and adjustment for BMI and vitamin supplementation were potential sources of the heterogeneity. Publication bias tests were not performed (n<10).
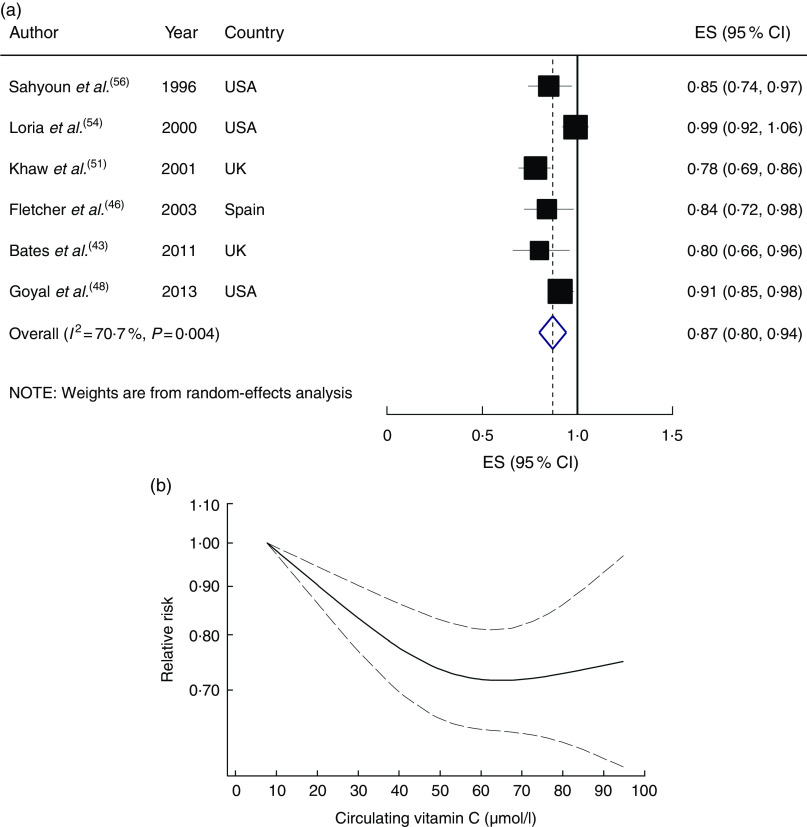
All studies were eligible for inclusion in the dose–response meta-analysis, with the results showing that a 20µmol/l increment in circulating vitamin C concentration was associated with a 13 % lower risk (RR=0·87; 95 % CI 0·80, 0·94), with high heterogeneity (I 2=70·7 %, P heterogeneity=0·004; Fig. 2(a)). A significant inverse association persisted when each study was sequentially excluded from the pooled analysis (RR ranged between 0·84 and 0·90). In the stepwise exclusion of each study, most of the heterogeneity was explained by the NHANES II study( 54 ); when this study was excluded, the heterogeneity was reduced and the association changed to 0·84 (95 % CI 0·79, 0·90; I 2=36·6 %, P heterogeneity=0·18). A non-linear dose–response meta-analysis demonstrated that the risk of CVD mortality decreased linearly with increasing circulatory vitamin C concentration from baseline up to about 60µmol/l, without further changes in effect estimate (P non-linearity<0·001; Fig. 2(b)).
Fig. 2.
(a) Forest plot showing relative risk of cardiovascular mortality for a 20 µmol/l increment in circulating vitamin C concentration. The study-specific effect size (ES) and 95 % CI are represented by the black square and horizontal line, respectively; the area of the black square is proportional to the specific-study weight to the overall meta-analysis. The centre of the blue open diamond and the vertical dashed line represent the pooled ES, and the width of the blue open diamond represents the pooled 95 % CI. (b) Dose–response association of circulating vitamin C concentration and risk of total cardiovascular mortality (———), with 95 % CI (— — —)
Vitamin E and CVD mortality
Eight studies (seven articles) involving a total of 228 531 participants with 7777 cases were included in the analysis of dietary vitamin E and CVD mortality risk( 43 , 44 , 46 , 47 , 53 , 57 , 59 ). All studies were at high quality. We did not include the Massachusetts Nutrition Status Survey in the analysis of dietary vitamin E because of the very high vitamin E consumption in comparison with other studies (almost twofold)( 56 ). The relative risk of CVD mortality for the highest compared with the lowest category of dietary vitamin E intake was 0·91 (95 % CI 0·79, 1·03), with moderate heterogeneity (I 2=51·3 %, P heterogeneity=0·04; see online supplementary material, Supplemental Fig. 5). The association did not reach statistical significance when each study was sequentially excluded from the pooled analysis. We performed an additional sensitivity analysis by restricting only to studies that controlled for main confounders, but the risk did not reach statistical significance (RR=0·86; 95 % CI 0·69, 1·03). A non-significant association persisted across all subgroups and between sexes. The subgroup analyses suggested that number of cases, geographical region, follow-up duration, baseline age, dietary assessment method and adjustment for main confounders were potential sources of the heterogeneity in the data (Supplemental Table 4).
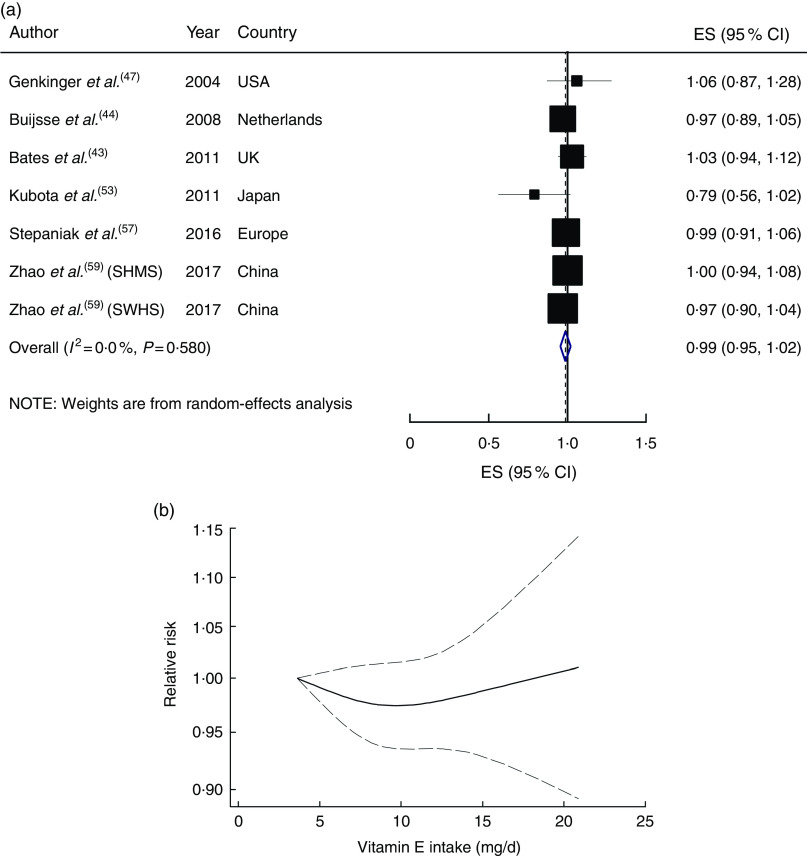
The linear trend estimation indicated that a 5mg/d increment in dietary vitamin E intake was not associated with the risk of CVD mortality (RR=0·99; 95 % CI 0·95, 1·02), with no evidence of heterogeneity (I 2=0 %, P heterogeneity=0·58; Fig. 3(a)). The risk of CVD mortality did not change materially with the increase in dietary vitamin E intake (P non-linearity=0·44; Fig. 3(b)).
Fig. 3.
(a) Forest plot showing relative risk of cardiovascular mortality for a 5 mg/d increment in vitamin E intake. The study-specific effect size (ES) and 95 % CI are represented by the black square and horizontal line, respectively; the area of the black square is proportional to the specific-study weight to the overall meta-analysis. The centre of the blue open diamond and the vertical dashed line represent the pooled ES, and the width of the blue open diamond represents the pooled 95 % CI (SMHS, Shanghai Men’s Health Study; SWHS, Shanghai Women’s Health Study). (b) Dose–response association of vitamin E intake and risk of total cardiovascular mortality (———), with 95 % CI (— — —)
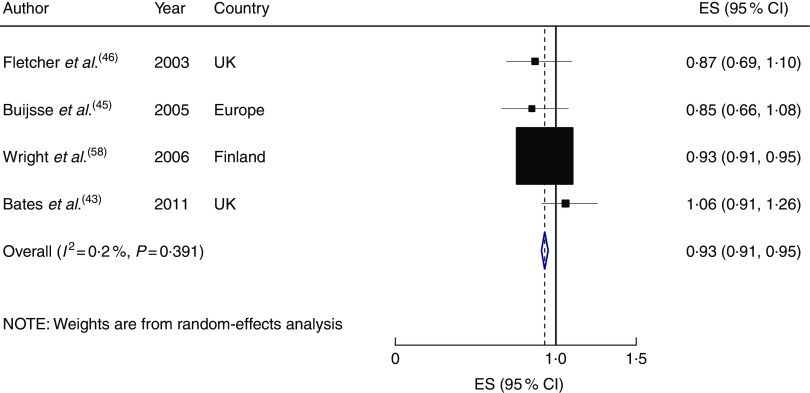
Five studies with 34 285 participants and 6542 cases were included in the analysis of circulating α-tocopherol( 43 , 45 , 46 , 52 , 58 ). Four studies reported an inverse association, which was statistically significant in one study, and another study showed a non-significant positive relationship. A significant inverse association was found for the highest compared with the lowest category of circulating α-tocopherol concentration (RR=0·82; 95 % CI 0·76, 0·88; I 2=0 %, P heterogeneity=0·55, n studies 5; see online supplementary material, Supplemental Fig. 6) and for a 10µmol/l increment in circulating concentration (RR=0·93; 95 % CI 0·91, 0·95; I 2=0·20 %, P heterogeneity=0·39, n 4 studies; Fig. 4). However, the weight of the Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study( 58 ) was much bigger than other studies and when this study was excluded from the pooled analyses, the association became non-significant in both high v. low (RR=0·86; 95 % CI 0·69, 1·03) and in the linear dose–response analyses (RR=0·94; 95 % CI 0·80, 1·07). Only two studies reported sufficient information( 46 , 58 ), so we were unable to test the potential non-linear dose–response relationship.
Fig. 4.
Forest plot showing relative risk of cardiovascular mortality for a 10 µmol/l increment in circulating α-tocopherol concentration. The study-specific effect size (ES) and 95 % CI are represented by the black square and horizontal line, respectively; the area of the black square is proportional to the specific-study weight to the overall meta-analysis. The centre of the blue open diamond and the vertical dashed line represent the pooled ES, and the width of the blue open diamond represents the pooled 95 % CI
β-Carotene and CVD mortality
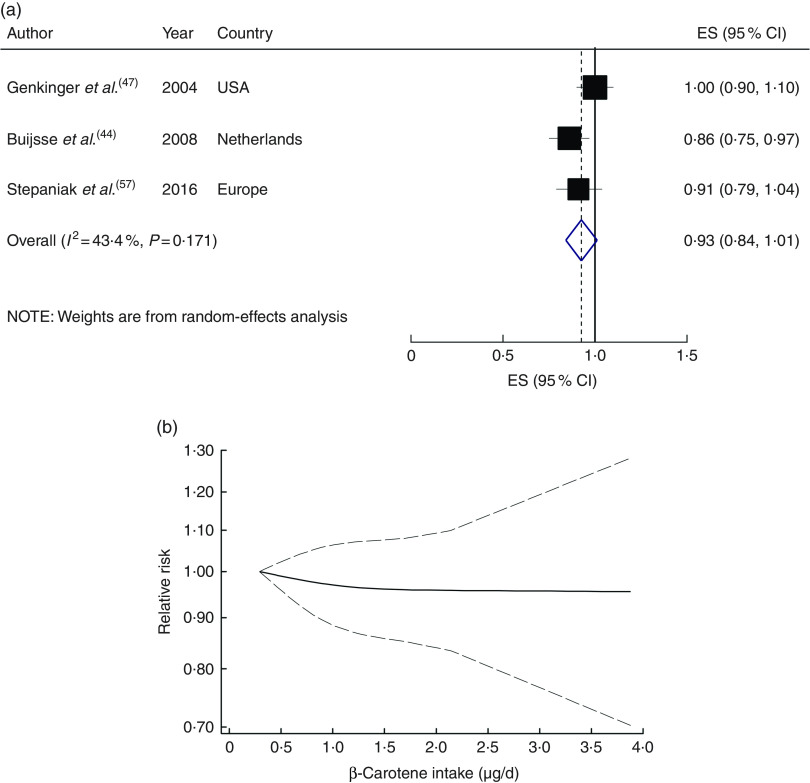
Four studies with 34 917 participants and 1700 cases were included in the analysis of dietary β-carotene with risk of CVD mortality( 43 , 46 , 47 , 57 ). A non-significant inverse association was found for the highest compared with the lowest category of dietary β-carotene intake (RR=0·89; 95 % CI 0·73, 1·05; I 2=34·3 %, P heterogeneity=0·21; see online supplementary material, Supplemental Fig. 7) and for a 1µg/d increment in dietary β-carotene intake (RR=0·93; 95 % CI 0·84, 1·01; I 2=43·4 %, P heterogeneity=0·17; n studies 3; Fig. 5(a)). A non-linear dose–response meta-analysis demonstrated that the risk of CVD mortality did not change with increasing dietary β-carotene intake (P non-linearity=0·78; Fig. 5(b)).
Fig. 5.
(a) Forest plot showing relative risk of cardiovascular mortality for a 1 µg/d increment in β-carotene intake. The study-specific effect size (ES) and 95 % CI are represented by the black square and horizontal line, respectively; the area of the black square is proportional to the specific-study weight to the overall meta-analysis. The centre of the blue open diamond and the vertical dashed line represent the pooled ES, and the width of the blue open diamond represents the pooled 95 % CI. (b) Dose–response association of β-carotene intake and risk of total cardiovascular mortality (———), with 95 % CI (— — —)
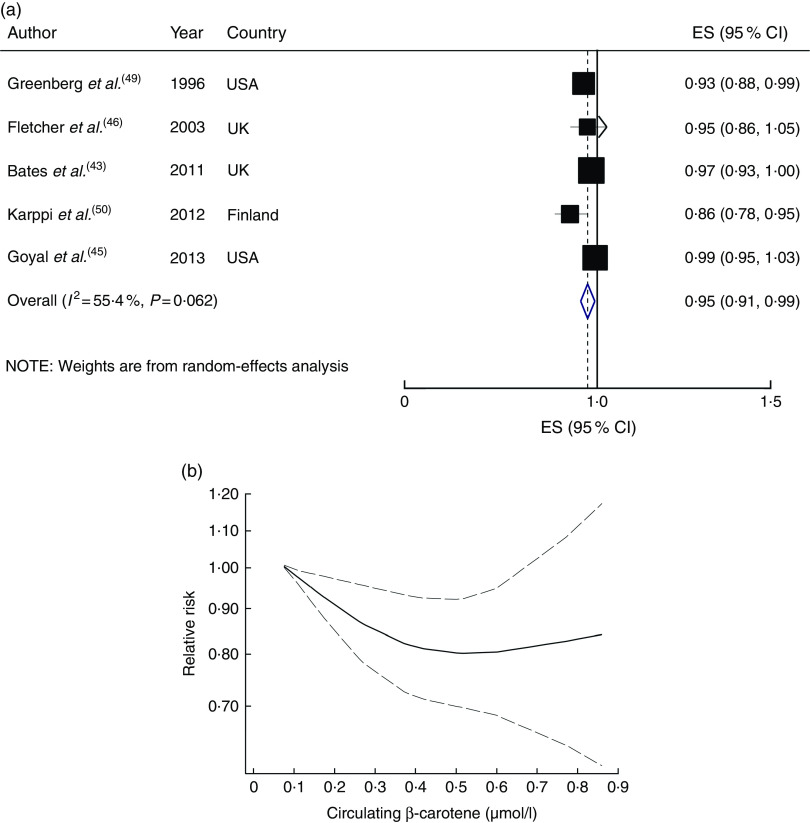
Six studies with 22 784 participants and 2758 cases were included in the analysis of circulating β-carotene( 43 , 46 , 48 , 49 , 50 , 52 ). A higher circulating β-carotene concentration was associated with a 32 % lower risk of CVD mortality (RR=0·68; 95 % CI 0·52, 0·83), with moderate heterogeneity (I 2=50·1 %, P heterogeneity=0·08; see online supplementary material, Supplemental Fig. 8). In the sensitivity analysis by removing each study in turn, none of the excluded studies changed the summary result materially (RR ranged between 0·63 and 0·72). A significant inverse association persisted among high-quality studies (RR=0·73; 95 % CI 0·58, 0·87; I 2=36·7 %, n studies 5), but not among studies that controlled for main confounders (RR=0·67; 95 % CI 0·33, 1·01; I 2=65·0 %, n studies 3). The subgroup analyses suggested follow-up duration, baseline age and adjustment for main confounders as the potential sources of the heterogeneity (Supplemental Table 5).
A 0·10µmol/l increment in circulating β-carotene concentration was associated with a 5 % lower risk (RR=0·95; 95 % CI 0·91, 0·99; I 2=55·4 %, P heterogeneity=0·06; Fig. 6(a)). In the sensitivity analysis by sequential exclusion of each study, none of the excluded studies negated the significance. There was evidence of a non-linear association between circulatory β-carotene concentration and risk of CVD mortality (P non=linearity=0·007; Fig. 6(b)).
Fig. 6.
(a) Forest plot showing relative risk of cardiovascular mortality for a 0·10 µmol/l increment in circulating β-carotene concentration. The study-specific effect size (ES) and 95 % CI are represented by the black square and horizontal line, respectively; the area of the black square is proportional to the specific-study weight to the overall meta-analysis. The centre of the blue open diamond and the vertical dashed line represent the pooled ES, and the width of the blue open diamond represents the pooled 95 % CI. (b) Dose–response association of circulating β-carotene concentration and risk of total cardiovascular mortality (———), with 95 % CI (— — —)
Quality of meta-evidence
The NutriGrade meta-evidence rating was ‘high’ for dietary vitamin C; ‘moderate’ for circulating vitamin C; and ‘low’ for dietary vitamin E, circulating α-tocopherol, and dietary and circulating β-carotene (Table 2).
Discussion
The present study summarized data about the association of dietary intake and circulating concentration of major antioxidants with the risk of total CVD mortality. The results of the present review indicate that higher dietary/circulating vitamin C and higher circulating β-carotene and α-tocopherol concentrations were significantly and inversely associated with the risk of CVD mortality, whereas higher dietary intake of β-carotene and vitamin E did not show such protective effects. The study suggests that the circulating biomarkers of antioxidants may have better predictive value in relation to the risk of total CVD mortality.
Vitamin C has powerful antioxidant, anti-inflammatory and immune-modulatory properties( 60 – 62 ). Also, by increasing nitric oxide synthesis and release, it is associated with arterial dilation, lower blood pressure and better endothelial function( 60 , 62 ). The non-linear dose–response meta-analysis suggested a possible threshold at an intake of about 200mg/d. This finding is in agreement with some evidence suggesting an intake of about 200 mg/d as the optimal vitamin C intake; the dose at which the plasma is relatively saturated and the blood cells are completely saturated with vitamin C( 63 ). However, only two studies reported risk estimates of CVD mortality for an intake of ≥300mg/d( 55 , 56 ). Hence, there was insufficient data for judgement about the cardiovascular outcomes of high vitamin C consumption. A previous meta-analysis of prospective cohort studies suggested a U-shaped association between vitamin C intake and risk of stroke( 18 ). Thus, our results regarding the cardiovascular outcomes of vitamin C intake ≥300mg/d should be interpreted cautiously.
The analyses of vitamin C, vitamin E and β-carotene suggested that the circulating biomarkers of antioxidants may be more strongly associated with the risk of CVD mortality compared with dietary intakes. These findings are similar to those of a recent meta-analysis of prospective cohort studies on dietary and circulating carotenoids and the risk of breast cancer( 29 ). However, it should be noted that the circulating biomarkers of antioxidants do not necessarily reflect dietary intakes because several dietary and non-dietary factors such as age, method of storage and food preparation, alcohol consumption, cigarette smoking, physical activity, socio-economic status, co-morbidities and even genetic factors may effectively affect their circulatory concentrations( 28 , 64 – 66 ). In fact, higher circulating concentration of antioxidants may be a consequence of several dietary and non-dietary factors such as higher diet quality, healthier lifestyle-related behaviours, better health status and lower prevalence of co-morbidities; which in turn are associated with better survival.
The analyses of vitamin E demonstrated that higher circulating α-tocopherol concentration, but not higher dietary vitamin E intake, was associated with a lower risk of CVD mortality. The finding of the current review regarding the null association in the analysis of dietary vitamin E is consistent with those of previous investigations which have indicated a non-significant inverse association between higher dietary vitamin E intake and the risk of all-cause mortality and CVD events( 67 – 69 ). A pooled analysis of nine relatively large-scale prospective cohort studies involving 4647 incident cases of CHD among ~300 000 participants in the USA and Europe showed similar results, in which only a weak marginally significant inverse association was found between higher dietary vitamin E intake and the risk of incident CHD (RR=0·84; 95 % CI 0·71, 1·00; P=0·17)( 22 ). Vitamin E is a fat-soluble antioxidant and anti-inflammatory vitamin, and by inhibition of LDL oxidation has direct anti-atherogenic properties( 70 – 72 ). Thus, it is reasonable to expect the observational studies to show a significant inverse association between higher vitamin E intake and the risk of heart disease.
Also, such inconsistent findings were observed in the analyses of dietary and circulating β-carotene. β-Carotene has strong antioxidant activity( 73 ) and therefore, by decreasing the oxidation of LDL and subsequent risk of developing and progression of atherosclerosis( 74 – 76 ), may be associated with a lower risk of CVD. In addition, β-carotene and other dietary carotenoids, through their functions against oxidative stress, are associated with a lower risk of developing CVD( 77 ). A possible explanation may be the measurement errors in the self-reported assessment of dietary intake, which might result in a weaker effect size. These errors do not exist in the measurement of circulating biomarkers; therefore, studies that evaluated the circulating biomarkers generally showed stronger inverse associations.
In the present meta-analysis of prospective observational studies, we found significant inverse associations of dietary vitamin C and circulating vitamin C, α-tocopherol and β-carotene concentrations with risk of total CVD mortality. However, our results are inconsistent with those of interventional studies which have indicated null findings in this regard( 24 – 26 ). Several explanations have been proposed to explain such inconsistent findings in observational and interventional studies. In general, interventional studies use high-dose supplementations, which may be associated with adverse health effects( 78 ). In addition, the follow-up duration of most interventional studies was inadequate to show benefits of antioxidants supplementation( 28 ). Also, trials generally include participants with a prior history of CVD or other chronic diseases and these inconsistent findings may show that antioxidants may be useful for primary prevention of CVD, not for secondary prevention.
In the context of public health implications, the results of the present review support current dietary recommendations that promote an intake of at least five servings of fruits and vegetables per day( 79 ). Fruits and vegetables are the two main dietary sources of antioxidants( 80 , 81 ). However, despite all efforts to increase the consumption of fruits and vegetables, current reports indicate that the prevalence of low fruit and vegetable consumption is high in both low- and middle-income and high-income countries. The World Health Survey (2002–2003) in fifty-two mainly low- and middle-income countries reported that the prevalence of low fruit and vegetable consumption, defined as less than a minimum of five servings of fruits and/or vegetables daily, was about 78 %( 82 ). This prevalence is similar to that of high-income countries, in which the prevalence of low fruit and vegetable consumption was about 75 and 76 % in the USA( 83 ) and England( 84 ), respectively. Therefore, increasing the consumption of fruits and vegetables may be good and simple advice in order to reduce the risk of CVD deaths.
The present study has several strengths. We assessed the association of both dietary intakes and circulating concentrations of major antioxidants with the risk of total CVD mortality. Previous reviews have shown a null or weak inverse association between higher vitamin E and β-carotene consumption and the risk of CHD. However, we showed a significant inverse association between circulating α-tocopherol and β-carotene concentrations and risk of CVD mortality, which suggests that the null or weak inverse associations found in the analyses of dietary vitamin E and β-carotene may in part be due to measurement errors.
Some important limitations were experienced in the current study. First, the results were accompanied by some evidence of heterogeneity in the analyses of dietary and circulating vitamin C, dietary vitamin E and dietary β-carotene. However, the subgroup analyses suggested that some of the study and participant characteristics were potential sources of the heterogeneity in the data. Second, publication bias tests were performed only in the analysis of dietary vitamin C (n≥10). Therefore, our results may have been affected by publication bias. Third, higher antioxidant intakes are related to higher diet quality and better compliance with dietary guidelines, which leads to higher intakes of cardioprotective nutrients such as fibres, K, Mg and flavonoids; as well as lower intakes of unhealthy foods. Thus, owing to the inadequate adjustments for these dietary variables in the primary studies, we may have reached a biased conclusion. Finally, we were unable to test the associations across sexes.
Conclusion
In conclusion, the current meta-analysis suggests that a higher dietary intake and a higher circulating concentration of vitamin C are each associated with a lower CVD mortality risk. The analyses of vitamin E and β-carotene showed that higher circulating concentrations of these antioxidants are significantly and inversely associated with a lower CVD mortality risk, whereas higher dietary intakes did not show such protective effects. Considering that the rating of the meta-evidence was low in the analyses of vitamin E and β-carotene, there is a low confidence in effect estimates and future well-designed prospective cohort studies may be required to provide more reliable data for a more confident judgement about the degree and the direction of the associations.
Acknowledgements
Financial support: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors. Conflict of interest: None. Authorship: A.J. and S.S.-B. contributed to the conception and design of the work. A.J., M.S.Z. and M.P. contributed to the acquisition of data for the work. A.J., A.R.P. and S.S.-B. contributed to analysis and interpretation of the data. A.J., A.R.P., M.P., M.S.Z. and S.S.-B. drafted the manuscript. A.R.P. and S.S.-B. critically revised the manuscript. All authors gave final approval and agreed to be accountable for all aspects of work ensuring integrity and accuracy. Ethics of human subject participation: Not applicable.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S1368980018003725.
click here to view supplementary material
References
- 1. Carlsen MH, Halvorsen BL, Holte K et al. (2010) The total antioxidant content of more than 3100 foods, beverages, spices, herbs and supplements used worldwide. Nutr J 9, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Aune D, Giovannucci E, Boffetta P et al. (2017) Fruit and vegetable intake and the risk of cardiovascular disease, total cancer and all-cause mortality – a systematic review and dose-response meta-analysis of prospective studies. Int J Epidemiol 46, 1029–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Crowe FL, Roddam AW, Key TJ et al. (2011) Fruit and vegetable intake and mortality from ischaemic heart disease: results from the European Prospective Investigation into Cancer and Nutrition (EPIC)-Heart study. Eur Heart J 32, 1235–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Liu S, Lee IM, Ajani U et al. (2001) Intake of vegetables rich in carotenoids and risk of coronary heart disease in men: the Physicians’ Health Study. Int J Epidemiol 30, 130–135. [DOI] [PubMed] [Google Scholar]
- 5. Gillman MW, Cupples LA, Gagnon D et al. (1995) Protective effect of fruits and vegetables on development of stroke in men. JAMA 273, 1113–1117. [DOI] [PubMed] [Google Scholar]
- 6. Oude Griep LM, Verschuren WM, Kromhout D et al. (2011) Raw and processed fruit and vegetable consumption and 10-year stroke incidence in a population-based cohort study in the Netherlands. Eur J Clin Nutr 65, 791–799. [DOI] [PubMed] [Google Scholar]
- 7. Martinez-Gonzalez MA, Fernandez-Jarne E, Martinez-Losa E et al. (2002) Role of fibre and fruit in the Mediterranean diet to protect against myocardial infarction: a case–control study in Spain. Eur J Clin Nutr 56, 715–722. [DOI] [PubMed] [Google Scholar]
- 8. Rautiainen S, Levitan EB, Mittleman MA et al. (2015) Fruit and vegetable intake and rate of heart failure: a population-based prospective cohort of women. Eur J Heart Fail 17, 20–26. [DOI] [PubMed] [Google Scholar]
- 9. Alissa EM & Ferns GA (2017) Dietary fruits and vegetables and cardiovascular diseases risk. Crit Rev Food Sci Nutr 57, 1950–1962. [DOI] [PubMed] [Google Scholar]
- 10. Nunez-Cordoba JM & Martinez-Gonzalez MA (2011) Antioxidant vitamins and cardiovascular disease. Curr Top Med Chem 11, 1861–1869. [DOI] [PubMed] [Google Scholar]
- 11. Voutilainen S, Nurmi T, Mursu J et al. (2006) Carotenoids and cardiovascular health. Am J Clin Nutr 83, 1265–1271. [DOI] [PubMed] [Google Scholar]
- 12. Bastide N, Dartois L, Dyevre V et al. (2017) Dietary antioxidant capacity and all-cause and cause-specific mortality in the E3N/EPIC cohort study. Eur J Nutr 56, 1233–1243. [DOI] [PubMed] [Google Scholar]
- 13. Kim K, Vance TM, Chen MH et al. (2017) Dietary total antioxidant capacity is inversely associated with all-cause and cardiovascular disease death of US adults. Eur J Nutr 57, 2469–2476. [DOI] [PubMed] [Google Scholar]
- 14. Aviram M, Kaplan M, Rosenblat M et al. (2005) Dietary antioxidants and paraoxonases against LDL oxidation and atherosclerosis development. Handb Exp Pharmacol issue 170, 263–300. [DOI] [PubMed] [Google Scholar]
- 15. Mangge H, Becker K, Fuchs D et al. (2014) Antioxidants, inflammation and cardiovascular disease. World J Cardiol 6, 462–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Siti HN, Kamisah Y & Kamsiah J (2015) The role of oxidative stress, antioxidants and vascular inflammation in cardiovascular disease (a review). Vascul Pharmacol 71, 40–56. [DOI] [PubMed] [Google Scholar]
- 17. Zhang PY, Xu X & Li XC (2014) Cardiovascular diseases: oxidative damage and antioxidant protection. Eur Rev Med Pharmacol Sci 18, 3091–3096. [PubMed] [Google Scholar]
- 18. Chen GC, Lu DB, Pang Z et al. (2013) Vitamin C intake, circulating vitamin C and risk of stroke: a meta-analysis of prospective studies. J Am Heart Assoc 2, e000329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Osganian SK, Stampfer MJ, Rimm E et al. (2003) Vitamin C and risk of coronary heart disease in women. J Am Coll Cardiol 42, 246–252. [DOI] [PubMed] [Google Scholar]
- 20. Rimm EB, Stampfer MJ, Ascherio A et al. (1993) Vitamin E consumption and the risk of coronary heart disease in men. N Engl J Med 328, 1450–1456. [DOI] [PubMed] [Google Scholar]
- 21. Stampfer MJ, Hennekens CH, Manson JE et al. (1993) Vitamin E consumption and the risk of coronary disease in women. N Engl J Med 328, 1444–1449. [DOI] [PubMed] [Google Scholar]
- 22. Knekt P, Ritz J, Pereira MA et al. (2004) Antioxidant vitamins and coronary heart disease risk: a pooled analysis of 9 cohorts. Am J Clin Nutr 80, 1508–1520. [DOI] [PubMed] [Google Scholar]
- 23. Ye Z & Song H (2008) Antioxidant vitamins intake and the risk of coronary heart disease: meta-analysis of cohort studies. Eur J Cardiovasc Prev Rehabil 15, 26–34. [DOI] [PubMed] [Google Scholar]
- 24. Al-Khudairy L, Flowers N, Wheelhouse R et al. (2017) Vitamin C supplementation for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev 3, CD011114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pham DQ & Plakogiannis R (2005) Vitamin E supplementation in cardiovascular disease and cancer prevention: Part 1. Ann Pharmacother 39, 1870–1878. [DOI] [PubMed] [Google Scholar]
- 26. Ye Y, Li J & Yuan Z (2013) Effect of antioxidant vitamin supplementation on cardiovascular outcomes: a meta-analysis of randomized controlled trials. PLoS One 8, e56803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bjelakovic G, Nikolova D & Gluud C (2013) Meta-regression analyses, meta-analyses, and trial sequential analyses of the effects of supplementation with β-carotene, vitamin A, and vitamin E singly or in different combinations on all-cause mortality: do we have evidence for lack of harm? PLoS One 8, e74558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lawlor DA, Smith GD, Bruckdorfer KR et al. (2004) Those confounded vitamins: what can we learn from the differences between observational versus randomised trial evidence? Lancet 363, 1724–1727. [DOI] [PubMed] [Google Scholar]
- 29. Aune D, Chan DS, Vieira AR et al. (2012) Dietary compared with blood concentrations of carotenoids and breast cancer risk: a systematic review and meta-analysis of prospective studies. Am J Clin Nutr 96, 356–373. [DOI] [PubMed] [Google Scholar]
- 30. Moher D, Liberati A, Tetzlaff J et al. (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6, e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Stang A (2010) Critical evaluation of the Newcastle–Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 25, 603–605. [DOI] [PubMed] [Google Scholar]
- 32. Schwingshackl L, Knüppel S, Schwedhelm C et al. (2016) Perspective: NutriGrade: a scoring system to assess and judge the meta-evidence of randomized controlled trials and cohort studies in nutrition research. Adv Nutr 7, 994–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. DerSimonian R & Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7, 177–188. [DOI] [PubMed] [Google Scholar]
- 34. Danesh J, Collins R, Appleby P et al. (1998) Association of fibrinogen, C-reactive protein, albumin, or leukocyte count with coronary heart disease: meta-analyses of prospective studies. JAMA 279, 1477–1482. [DOI] [PubMed] [Google Scholar]
- 35. Egger M, Smith GD, Schneider M et al. (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315, 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Begg CB & Mazumdar M (1994) Operating characteristics of a rank correlation test for publication bias. Biometrics 50, 1088–1101. [PubMed] [Google Scholar]
- 37. Higgins JP & Green S (editors) (2011) Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. https://handbook-5-1.cochrane.org/ (accessed December 2018).
- 38. Berlin JA, Longnecker MP & Greenland S (1993) Meta-analysis of epidemiologic dose–response data. Epidemiology 4, 218–228. [DOI] [PubMed] [Google Scholar]
- 39. Orsini N, Bellocco R & Greenland S (2006) Generalized least squares for trend estimation of summarized dose–response data. Stata J 6, 40–57. [Google Scholar]
- 40. Schwingshackl L, Schwedhelm C, Hoffmann G et al. (2017) Food groups and risk of all-cause mortality: a systematic review and meta-analysis of prospective studies. Am J Clin Nutr 105, 1462–1473. [DOI] [PubMed] [Google Scholar]
- 41. Hamling J, Lee P, Weitkunat R et al. (2008) Facilitating meta‐analyses by deriving relative effect and precision estimates for alternative comparisons from a set of estimates presented by exposure level or disease category. Stat Med 27, 954–970. [DOI] [PubMed] [Google Scholar]
- 42. Orsini N, Li R, Wolk A et al. (2012) Meta-analysis for linear and nonlinear dose-response relations: examples, an evaluation of approximations, and software. Am J Epidemiol 175, 66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bates CJ, Hamer M & Mishra GD (2011) Redox-modulatory vitamins and minerals that prospectively predict mortality in older British people: the National Diet and Nutrition Survey of people aged 65 years and over. Br J Nutr 105, 123–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Buijsse B, Feskens EJ, Kwape L et al. (2008) Both α- and β-carotene, but not tocopherols and vitamin C, are inversely related to 15-year cardiovascular mortality in Dutch elderly men. J Nutr 138, 344–350. [DOI] [PubMed] [Google Scholar]
- 45. Buijsse B, Feskens EJ, Schlettwein-Gsell D et al. (2005) Plasma carotene and α-tocopherol in relation to 10-y all-cause and cause-specific mortality in European elderly: the Survey in Europe on Nutrition and the Elderly, a Concerted Action (SENECA). Am J Clin Nutr 82, 879–886. [DOI] [PubMed] [Google Scholar]
- 46. Fletcher AE, Breeze E & Shetty PS (2003) Antioxidant vitamins and mortality in older persons: findings from the nutrition add-on study to the Medical Research Council Trial of Assessment and Management of Older People in the Community. Am J Clin Nutr 78, 999–1010. [DOI] [PubMed] [Google Scholar]
- 47. Genkinger JM, Platz EA, Hoffman SC et al. (2004) Fruit, vegetable, and antioxidant intake and all-cause, cancer, and cardiovascular disease mortality in a community-dwelling population in Washington County, Maryland. Am J Epidemiol 160, 1223–1233. [DOI] [PubMed] [Google Scholar]
- 48. Goyal A, Terry MB & Siegel AB (2013) Serum antioxidant nutrients, vitamin A, and mortality in US adults. Cancer Epidemiol Biomarkers Prev 22, 2202–2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Greenberg ER, Baron JA, Karagas MR et al. (1996) Mortality associated with low plasma concentration of beta carotene and the effect of oral supplementation. JAMA 275, 699–703. [DOI] [PubMed] [Google Scholar]
- 50. Karppi J, Laukkanen J, Mäkikallio T et al. (2012) Low β-carotene concentrations increase the risk of cardiovascular disease mortality among Finnish men with risk factors. Nutr Metab Cardiovasc Dis 22, 921–928. [DOI] [PubMed] [Google Scholar]
- 51. Khaw KT, Bingham S, Welch A et al. (2001) Relation between plasma ascorbic acid and mortality in men and women in EPIC-Norfolk prospective study: a prospective population study. European Prospective Investigation into Cancer and Nutrition. Lancet 357, 657–663. [DOI] [PubMed] [Google Scholar]
- 52. Kilander L, Berglund L, Boberg M et al. (2001) Education, lifestyle factors and mortality from cardiovascular disease and cancer. A 25-year follow-up of Swedish 50-year-old men. Int J Epidemiol 30, 1119–1126. [DOI] [PubMed] [Google Scholar]
- 53. Kubota Y, Iso H, Date C et al. (2011) Dietary intakes of antioxidant vitamins and mortality from cardiovascular disease. Stroke 42, 1665–1672. [DOI] [PubMed] [Google Scholar]
- 54. Loria CM, Klag MJ, Caulfield LE et al. (2000) Vitamin C status and mortality in US adults. Am J Clin Nutr 72, 139–145. [DOI] [PubMed] [Google Scholar]
- 55. Martin-Calvo N & Martinez-Gonzalez MA (2017) Vitamin C intake is inversely associated with cardiovascular mortality in a cohort of Spanish graduates: the SUN Project. Nutrients 9, E954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sahyoun NR, Jacques PF & Russell RM (1996) Carotenoids, vitamins C and E, and mortality in an elderly population. Am J Epidemiol 144, 501–511. [DOI] [PubMed] [Google Scholar]
- 57. Stepaniak U, Micek A, Grosso G et al. (2016) Antioxidant vitamin intake and mortality in three Central and Eastern European urban populations: the HAPIEE study. Eur J Nutr 55, 547–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wright ME, Lawson KA, Weinstein SJ et al. (2006) Higher baseline serum concentrations of vitamin E are associated with lower total and cause-specific mortality in the Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study. Am J Clin Nutr 84, 1200–1207. [DOI] [PubMed] [Google Scholar]
- 59. Zhao LG, Shu XO, Li HL et al. (2017) Dietary antioxidant vitamins intake and mortality: a report from two cohort studies of Chinese adults in Shanghai. J Epidemiol 27, 89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ellulu MS (2017) Obesity, cardiovascular disease, and role of vitamin C on inflammation: a review of facts and underlying mechanisms. Inflammopharmacology 25, 313–328. [DOI] [PubMed] [Google Scholar]
- 61. Naidu KA (2003) Vitamin C in human health and disease is still a mystery? An overview. Nutr J 2, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Padayatty SJ, Katz A, Wang Y et al. (2003) Vitamin C as an antioxidant: evaluation of its role in disease prevention. J Am Coll Nutr 22, 18–35. [DOI] [PubMed] [Google Scholar]
- 63. Frei B, Birlouez-Aragon I & Lykkesfeldt J (2012) Authors’ perspective: What is the optimum intake of vitamin C in humans? Crit Rev Food Sci Nutr 52, 815–829. [DOI] [PubMed] [Google Scholar]
- 64. Dehghan M, Akhtar-Danesh N, McMillan CR et al. (2007) Is plasma vitamin C an appropriate biomarker of vitamin C intake? A systematic review and meta-analysis. Nutr J 6, 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Galan P, Viteri F, Bertrais S et al. (2005) Serum concentrations of β-carotene, vitamins C and E, zinc and selenium are influenced by sex, age, diet, smoking status, alcohol consumption and corpulence in a general French adult population. Eur J Clin Nutr 59, 1181–1190. [DOI] [PubMed] [Google Scholar]
- 66. Giovannucci E (2013) Nutrient biomarkers are not always simple markers of nutrient intake. Am J Clin Nutr 97, 657–659. [DOI] [PubMed] [Google Scholar]
- 67. Agudo A, Cabrera L, Amiano P et al. (2007) Fruit and vegetable intakes, dietary antioxidant nutrients, and total mortality in Spanish adults: findings from the Spanish cohort of the European Prospective Investigation into Cancer and Nutrition (EPIC-Spain). Am J Clin Nutr 85, 1634–1642. [DOI] [PubMed] [Google Scholar]
- 68. Henriquez-Sanchez P, Sanchez-Villegas A, Ruano-Rodriguez C et al. (2016) Dietary total antioxidant capacity and mortality in the PREDIMED study. Eur J Nutr 55, 227–236. [DOI] [PubMed] [Google Scholar]
- 69. Todd S, Woodward M, Tunstall-Pedoe H et al. (1999) Dietary antioxidant vitamins and fiber in the etiology of cardiovascular disease and all-causes mortality: results from the Scottish Heart Health Study. Am J Epidemiol 150, 1073–1080. [DOI] [PubMed] [Google Scholar]
- 70. Badiou S, Cristol JP, Morena M et al. (2003) Vitamin E supplementation increases LDL resistance to ex vivo oxidation in hemodialysis patients. Int J Vitam Nutr Res 73, 290–296. [DOI] [PubMed] [Google Scholar]
- 71. Mafra D, Santos FR, Lobo JC et al. (2009) α-Tocopherol supplementation decreases electronegative low-density lipoprotein concentration [LDL(–)] in haemodialysis patients. Nephrol Dial Transplant 24, 1587–1592. [DOI] [PubMed] [Google Scholar]
- 72. Rahmani E, Samimi M, Ebrahimi FA et al. (2017) The effects of omega-3 fatty acids and vitamin E co-supplementation on gene expression of lipoprotein(a) and oxidized low-density lipoprotein, lipid profiles and biomarkers of oxidative stress in patients with polycystic ovary syndrome. Mol Cell Endocrinol 439, 247–255. [DOI] [PubMed] [Google Scholar]
- 73. Paiva SA & Russell RM (1999) β-Carotene and other carotenoids as antioxidants. J Am Coll Nutr 18, 426–433. [DOI] [PubMed] [Google Scholar]
- 74. Dugas TR, Morel DW & Harrison EH (1999) Dietary supplementation with βeta-carotene, but not with lycopene, inhibits endothelial cell-mediated oxidation of low-density lipoprotein. Free Radic Biol Med 26, 1238–1244. [DOI] [PubMed] [Google Scholar]
- 75. Levy Y, Kaplan M, Ben-Amotz A et al. (1996) Effect of dietary supplementation of β-carotene on human monocyte-macrophage-mediated oxidation of low density lipoprotein. Isr J Med Sci 32, 473–478. [PubMed] [Google Scholar]
- 76. Levy Y, Zaltsberg H, Ben-Amotz A et al. (2000) Dietary supplementation of a natural isomer mixture of β-carotene inhibits oxidation of LDL derived from patients with diabetes mellitus. Ann Nutr Metab 44, 54–60. [DOI] [PubMed] [Google Scholar]
- 77. Willcox JK, Ash SL & Catignani GL (2004) Antioxidants and prevention of chronic disease. Crit Rev Food Sci Nutr 44, 275–295. [DOI] [PubMed] [Google Scholar]
- 78. Verkaik-Kloosterman J, McCann MT, Hoekstra J et al. (2012) Vitamins and minerals: issues associated with too low and too high population intakes. Food Nutr Res 2012, 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. World Health Organization (2003) Diet, Nutrition, and the Prevention of Chronic Diseases. Report of a Joint WHO/FAO Expert Consultation. WHO Technical Report Series no. 916. Geneva: WHO. [PubMed] [Google Scholar]
- 80. Landete JM (2013) Dietary intake of natural antioxidants: vitamins and polyphenols. Crit Rev Food Sci Nutr 53, 706–721. [DOI] [PubMed] [Google Scholar]
- 81. Reboul E, Richelle M, Perrot E et al. (2006) Bioaccessibility of carotenoids and vitamin E from their main dietary sources. J Agric Food Chem 54, 8749–8755. [DOI] [PubMed] [Google Scholar]
- 82. Hall JN, Moore S, Harper SB et al. (2009) Global variability in fruit and vegetable consumption. Am J Prev Med 36, 402–409.e5. [DOI] [PubMed] [Google Scholar]
- 83. Blanck HM, Gillespie C, Kimmons JE et al. (2008) Trends in fruit and vegetable consumption among US men and women, 1994–2005. Prev Chronic Dis 5, A35. [PMC free article] [PubMed] [Google Scholar]
- 84. Blake M, Chaudhury M, Deverill C et al. (2004) Health Survey England 2003. vol. 2: Risk Factors for Cardiovascular Disease. Norwich: TSO. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit https://doi.org/10.1017/S1368980018003725.
click here to view supplementary material