Abstract
Lignocellulosic biomass resource has been widely used as a natural resource for the synthesis of biofuels and bio-based products through pre-treatment, saccharification and fermentation processes. In this review, we delve into the environmental implications of bioethanol production from the widely utilized lignocellulosic biomass resource. The focus of our study is the critical stage of pre-treatment in the synthesis process, which also includes saccharification and fermentation. By collecting scientific data from the available literature, we conducted a comprehensive life cycle analysis. Our findings revealed substantial differences in the environmental burdens associated with diverse pre-treatment methods used for lignocellulosic biomass. These results highlight the importance of selecting environmentally benign pre-treatment techniques to promote the sustainability of bioethanol production. Future research directions are suggested, emphasizing the optimization of pre-treatment processes to further mitigate their environmental impact.
Keywords: Lignocellulose biomass: bioethanol, Pretreatment methods, Life cycle assessment, Environmental sustainability
Introduction
The increased consumption of non-renewable resources prompted the present globe to exploit alternative renewable energy sources for fuels and chemical production, known as biorefinery (Meng et al. 2020). The development of biorefineries facilitates the conversion of inedible renewable resources to platform chemicals to replace petroleum refinery products and biofuels with non-renewable petroleum resources providing sustainable benign (Mthembu et al. 2021). The global interest in biofuel production has intensified recently due to its environmental friendliness (Sewsynker-Sukai and Gueguim Kana 2018). The first generation of biofuels (ethanol and biodiesel) is produced using edible feedstocks, namely sugar and maize biomass. Other biomass sources such as starchy, sugary, and fatty crops were also used for the production of first-generation biofuels especially bioethanol and biodieselClick or tap here to enter text.. The widely used feedstocks for first-generation biofuel production are mainly sugar/starch-based crops such as fruit, sugar cane molasess, grains, sweet potatoes, cassava and maize. In addition, due to the capacity to mix with current petroleum-based fuels, first-generation biofuel has already been marketed with an annual output of 50 billion litres. However, the primary concern of first-generation biofuels is the food-versus-fuel dilemma. The utilization of food crops as feedstock for bioenergy production leads to a land conflict between food and fuel production (Chong and Ng 2021). In India, a vast amount of crop cultivation takes place all year round, resulting in the production of a significant amount of biomass that is utilised for various purposes, but the residual biomass is mainly discarded as waste upon processing. In recent five years, various waste biomass generated by several human activities such as agro-industry processing residues, food processing by-products, municipal solid waste, sewage sludge, and livestock manure are utilized for conversion to high-value-added products (Zhou and Wang 2020). The biomasses can be classified based on the nature of biomass as: agriculture residues, food residues, industrial waste, municipal waste, and algae biomass, etc. (Khaire et al. 2021) (Fig. 1). They often comprise starch, lignocellulosic, and lipids, among other things that set the platform to produce other alternative fuels such as biogas, bioethanol and biodiesel. The sugar biomass requires only a single-step fermentation process, whereas the starch feedstock needs to undergo fermentation after hydrolysis to facilitate the conversion of starch into simple sugar (Zabed et al. 2017). Despite being the second-largest compound produced by plants after cellulose, starch biomass has less consideration for biofuel production at an industrial scale.
Fig. 1.
Classification of biomasses based on their nature
The inedible feedstocks rich in lignocellulose namely forest residues, bagasse and other marginal land-grown energy crop meets the principle of green chemistry to produce renewable carbon and hydrogen for the conversion to second-generation biofuels for the establishment of sustainable green chemistry (Li et al. 2019). Lignocellulosic biomass is a plant-derived substance composed of three components: lignin (10–25%), cellulose (40–60%), and hemicellulose (20–40%). The refractory nature of the lignin prevents the hydrolysing enzyme from accessing the core cellulose coated by lignin, which represents a major challenge in biorefineries (Banerjee et al. 2017). Table 1 compares the advantages and disadvantages of producing biofuels from various types of biomass sources. The generation of biofuels from agricultural residues, industrial/domestic and forest wastes have dual benefits of waste treatment and energy production.
Table 1.
Comparison of different biomass valorisation approaches for biofuels production
| Types of biomasses | Origin of biomass | Processing method | Biofuels produced | Advantages | Disadvantages | References |
|---|---|---|---|---|---|---|
| First generation biomass | Starchy, sugary, and fatty crops | Single-step extraction process | Biodiesel (Bio-esters), bioethanol and Biogas | Has the ability to blend with existing petroleum-based fuels; Meet the domestic energy needs | Using food crops for bioenergy production leads to an increase in food prices | Balat (2011) |
| Second generation biomass | Energy crops, agriculture waste, Forest waste, and organic residues of domestic waste | Pre-treatment, hydrolysis and fermentation | Bioethanol, Fischer–Tropsch bio-diesel, bio-DME and bio-SNG | Abundantly available, Cheap and non-food material from plants | Recalcitrance nature of the lignocellulosic biomass makes it difficult in digestion and conversion, leading to low product yields | Millati et al. (2020) |
| Third generation biomass | Algae | Anaerobic digestion, gasification, pyrolysis, hydrothermal liquefaction | Biodiesel, biogas and bioethanol |
Shorter doubling time of the algal biomass (2–5 days); No competition with food and water resources; No need of pre-treatment as it contains no lignin |
High costs for the fermentation process, and for harvesting and drying of algae | Orr et al. (2016) |
Agricultural and forestry residues are the most common types of biomass feedstock for biofuel production. The agro-forestry residues are rich in lignocellulosic components consisting of homopolymeric (cellulose) and heteropolymeric (hemicellulose and lignin) content (lignin, cellulose and hemicellulose) that can be converted into various bioproducts to substitute petroleum refinery products, referred to as biorefineries, and its utilization for biorefineries differs based on their composition and accessibility (Alawad and Ibrahim 2022). These biorefineries must be environmentally sustainable, preserving exhausted resources while lowering greenhouse gas and other harmful pollutant emissions.
However, there are conditions whereby these biomass-derived products might create environmental harm, such as eutrophication of water, increased land use, and the use of extra minerals and chemicals during the manufacturing process, all of which pose a danger to the environment. Furthermore, the complicated heterogeneous lignocellulosic structure (cellulose, lignin, hemicelluloses) of lignocellulosic feedstock necessitates numerous treatment methods (Zabed et al. 2017). To overcome these issues, the pre-treatment process must be improved by minimising the use of auxiliary materials and chemicals, boosting yield, and reducing manufacturing cost and time (Soam et al. 2018; Gundupalli et al. 2022). The cost of a biomass pre-treatment process is almost 40% sharing in the total production cost in biofuel production from lignocellulosic biomass. Moreover, lab-scale conventional pre-treatments use harsh chemical and high energy consumption, which affects the development of environmentally friendly large-scale plants (Smullen et al. 2019). Recently, the life cycle assessment (LCA) technique has been applied to examine the impacts of raw materials and processes on environmental performance, in particularly greenhouse gas (GHG) emissions, acidification, and eutrophication, and sustainability of a particular stage process (Rewlay-ngoen et al. 2021). LCA is carried out using the functional unit data output/input of a process or a technology, and it enables to assess resource utilization rates and environmental emissions to water, soil and air (Smullen et al. 2019). LCA widely assesses the environmental impacts of different feedstocks and technologies employed for bioethanol production (Abinandan et al. 2020). A comprehensive LCA has been extensively used to identify the environmental impacts of various pre-treatment biomass methods (Ubando et al. 2019). The environmental effects of the treatment approaches were found to vary significantly according to the chemical agents, process conditions and types of biomasses used (Ferreira et al. 2018). LCA can thus be used to determines whether the chosen biorefineries are sustainable compared to the conventional petroleum-based refineries.
Although current bioethanol manufacturing has a variety of problems, industrial research has focused on increasing output, lowering reaction time and cost, and drastically reducing the number of unit operations, most notably for the starch and lignocellulosic conversion processes (Zabed et al. 2017). To overcome the above challenges, research is mainly focused to implement several integrated technological processes, such as pre-hydrolysis followed by saccharification and fermentation (PSSF), simultaneous saccharification and co-fermentation (SSCF), and simultaneous saccharification and fermentation (SSF). The perusal of the literature indicates significant knowledge gaps exist in sustainable bioethanol production from lignocellulosic feedstocks. Thus, this review intends to discuss in detail about the characteristics of various lignocellulose biomass from wastes, their pre-treatment methods and saccharification techniques for sustainable bioethanol production, focused on the environmental impacts of the various pre-treatment processes.
Lignocellulosic biomass resources
The lignocellulosic feedstock is the abundantly available and sustainable plant biomass for second-generation biofuel production. It is a cheaper renewable resource and a non-food material from plants, which makes it more promising for fuel production. The lignocellulosic biomass is commonly sourced from energy crops, agriculture residues, forest residues, and organic residues of domestic wastes (e.g., yard wastes). The implementation of agricultural residues to bioethanol is the viable option for decreasing dependence on fossil fuel resources. Agriculture residues are more abundantly produced compared with other lignocellulosic biomasses, generated from wheat, corn, rice, and sugarcane (Hassan et al. 2018; Hernández-Beltrán et al. 2019). Forest residues are another type of lignocellulosic biomass widely used for bioenergy generation, which consists of woody materials such as softwood, hardwood, dead trees, waste branches in trees, and residual leaves and other wastes from forestry operations (Siwal et al. 2021). Domestic wastes contain organic and non-organic materials generated from homes and buildings, which contain cellulosic residues (paper, cardboard, scrap, wood, etc.), food waste, rubber, glass, alloys, plastic, and other substances. Hence, the domestic wastes have heterogeneity properties, not ideal feedstock for biofuel production.
Depending on the type of lignocellulosic biomass used, it contains varying proportions of cellulose, hemicelluloses, and lignin. The cellulose and hemicelluloses contribute approximately of 34.1–42.1% and 28.5–38.8% of the total dry weight of the biomass respectively, and lignin of 5.6–13.8% and other ash content of 0.8–3.8% (Pereira et al. 2015). These two polymers: cellulose and hemicelluloses are bonded together by covalent and hydrogen bonds and linked to lignin polymer which accounts for its recalcitrance property. The main disadvantage of the production of biofuel from lignocellulosic biomass is its recalcitrance nature, making it indigestible and leading to a low yield of biofuel product (Millati et al. 2020). The robust and recalcitrant properties are due to the presence of lignin, the crystalline nature of cellulose, and its heterogeneous composition. Therefore, some pre-treatment methods have been developed to enhance the accessibility of cellulose/hemi-cellulose to the enzyme, by loosening the structure of lignocellulosic material or fractionating it into individual lignin, cellulose, and hemicelluloses fractions. Following the pre-treatment of lignocellulosic biomass (to be detailed in the following chapter) to remove lignin and hemicellulose, the treated biomass containing mainly cellulose can be hydrolysed into glucose monomers as substrates for fermentation to produce bioethanol. Cellulose hydrolysis is primarily done by chemicals (concentrated and dilute acid) or enzymes.
Lignocellulosic biomass pre-treatment methods
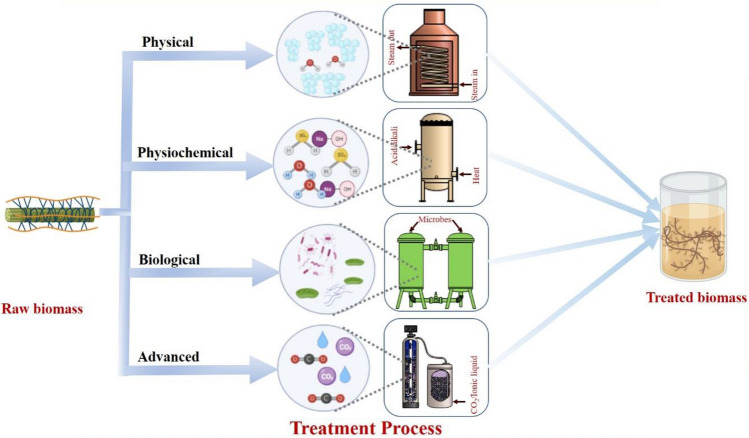
Pre-treatment is a critical step to alter the microscopic, sub-microscopic, and macroscopic structures of a lignocellulosic biomass, making its structure more accessible to hydrolyzing enzymes and increasing the reducing sugar yield (Alvira et al. 2010). The pre-treated lignocellulosic biomass provides up to 90% fermentable sugar, but the untreated biomass yields sugars of less than 20%. The pre-treatment methods can generally be classified into four categories (Fig. 2): (1) physical method, (2) chemical/physiochemical method, (3) biological method, and (4) advanced method. Advanced pre-treatment method has been developed to overcome the disadvantages of the above general pre-treatment methods. The advanced pre-treatment method has several advantages, including the generation of less inhibitory products, high specificity, and being cost-effective. Several critical aspects to consider during lignocellulosic biomass pre-treatment include the plant cell wall's recalcitrance, the development of inhibitors and by-products, and other difficulties such as establishing the physicochemical structure of the feedstock cell walls (Bhatia et al. 2021). However, an ideal pre-treatment process for lignocellulosic biomass should be cost-effective with minimized generation of inhibitors. Table 2 provides a summary of the advantages and disadvantages of different methods for lignocellulosic biomass pre-treatment.
Fig. 2.
Types of lignocellulosic biomass pre-treatment methods
Table 2.
Advantages and disadvantages of different methods for lignocellulosic biomass pre-treatment
| Pre-treatment methods | Advantages | Disadvantages | Operating condition | References |
|---|---|---|---|---|
| Mechanical extrusion |
Has a crucial effect on the disruption of cellulose and hemicellulose fraction The overall yield of sugar can be increased by combining it with other methods |
High energy requirement Cost intensive method, is difficult to scale up for industrial purpose |
Temperature > 300 °C 25–350 rpm |
Kumar and Sharma (2017) |
| Milling/Grinding or Chipping | No production of inhibitors like hydroxymethyl furfuraldehyde (HMF) and levulinic acid |
High energy requirement Need subsequent processing procedure |
Power requirements vary depending upon the biomass material: 130 kWh/ton (Hardwood), 14 kWh/ton (Corn stover) | Lin et al. (2010), |
| Microwave assisted |
Less energy requirement Minimum production of toxic/inhibitor compound Short processing time, ease of operation |
Need a chemical method to increase its efficiency | Irradiation time: 5–30 min, power requirements: 550–900 W | Boonmanumsin et al. (2012), Zhu et al. (2015) |
| Chemical treatment |
Most widely used method at the industrial scale High solubilization of the substrate for easy uptake by microorganism Economical and highly efficient |
Generation of a high number of inhibitory products (furfurals, phenolic acids, and aldehydes) Corrosion of the reactor |
Temperature > 180 °C and time 1 to 5 min Or Temperature < 120° C and time 30–90 min |
Galbe and Zacchi (2002), Sun and Cheng (2002), Tomás-Pejó et al. (2008) |
| Physio-chemical pretreatment: Steam explosion |
Decreased production of inhibitory compounds and improved enzymatic hydrolysis Less usage of chemicals Low energy requirement |
Possibility of formation of inhibitor at high temperature Incomplete digestion of lignin-carbohydrate matrix |
Pressure 0.7–4.8 MPa Temperature 160–260 °C Time: few seconds to minutes |
Wang et al. 2018) |
| Physio-chemical pretreatment: Liquid hot water |
Avoids the formation of inhibitor at high temperature Low-temperature requirement Low cost of solvent |
Requires a high amount of energy in downstream processing | Temperature 170–230 °C, pressure up to 5 MPa | Hongdan et al. (2013) |
| Biological pretreatment |
Environmentally safe and friendly Less energy requirement |
Rate of hydrolysis is low High production cost |
Incubation time: 4 to 8 weeks | Baramee et al. (2020), Heap et al. (2014) |
| Advanced pretreatment—Sc-CO2 |
Low cost of carbon dioxide Low temperature No toxin formation |
High cost of the reactor | Temperature 25–200 °C, Pressure 1000–4000 psi, Residence time 5 to 72 h | Cha et al. 2014), Alayoubi et al. (2020) |
Physical pre-treatment
Physical pre-treatment comprises milling, grinding, or chipping the biomass particle to reduce its size and decrease the crystallinity of the cellulose, hence increasing its surface availability to the hydrolyzing enzyme. Mechanical extrusion, microwave, ultrasound, pyrolysis, and pulsed electric field are some of the additional pre-treatment processes that are widely adopted in practice. Mechanical extrusion is a conventional pre-treatment procedure that utilises temperatures of more than 300 °C combined with shears mixing to generate gaseous product and char. Microwave-assisted approach has been widely used, as it has various benefits, including a quick process time, low energy consumption, and low inhibitor formation (Jasmine et al. 2023). Ultrasound is another promising choice for physical pre-treatment since it has both physical and chemical influences on the biomass, modifying the lignocellulosic structure by disrupting the lignin and hemicellulose fractions, hence enhancing the enzymatic scarification process, leading to the production of more fermentable sugars. Thermal pyrolysis pre-treatment method employs temperatures of 500–800 °C in the absence of oxygen to convert lignocellulosic biomass to bio-oil, which was reported in only a few studies (Gundupalli et al. 2022). In pulsed electric field (PEF) procedures, a high voltage of 5.0–20.0 kV/cm is employed to produce holes in the cell membrane surface, allowing the cellulose to be readily accessed by the hydrolyzing enzyme.
Chemical pre-treatment
Numerous chemicals, including acid, alkali, organic solvents, and oxidising agents, have been employed to pre-treat biomass to facilitate the hydrolysis process. Among them, acid treatment is the most extensively used traditional pre-treatment procedure on an industrial scale. Different chemicals employed in the pre-treatment process have varying impacts on the structure of the biomass. Acid treatment effectively removes lignin more than other chemical methods, e.g., alkali treatment, ozonolysis, wet oxidation, and hemicellulose solubilization but it has less attention due to the production of inhibitor such as phenolic acids, aldehyde and furan derivatives (Kumar and Sharma 2017).
Acid treatment
Acid treatment could effectively break the complex structure of biomass and remove inhibitory substances (furfural, acetic acid, and formic acid), as well as precipitate lignin(Lopez-Hidalgo et al. 2017). Sulfuric acid, hydrochloric acid, and nitric acid are often used to treat lignocellulosic biomass under two conditions: dilute acid at a high temperature, and concentrated acid at a low temperature. The high-temperature condition (> 150 ºC) with dilute acid treatment is effective in the removal of hemicellulose from lignocellulose biomass. (Kundu et al. 2021) carried out mild temperature (90 ºC) dilute acid treatment of hardwood and softwood biomass results in 90% of delignification with increased biomass digestibility of 32% and 23% for hardwood and softwood respectively. Sulfuric and hydrochloric acid are commonly used acid whereas dicarboxylic acids oxalic and maleic acid having high pKa and pH value effectively hydrolyze the biomass compared to other conventional acids. (Lee and Jeffries 2011) reported that maleic and oxalic acid highly solubilized the biomass compared to sulfuric acid.
Alkali treatment
The use of various alkali solutions (sodium, calcium, and ammonium hydroxide) to treat lignocellulosic biomass could effectively eliminate the undesirable components (lignin). Amongst, sodium hydroxide is the most often used alkali solution for lignocellulosic biomass treatment (Kim et al. 2016). These alkaline solutions readily dissolve and removes the lignin, where cellulose decrystallization can be realized by degrading esters and glycolic side chains in the biomass (Loow et al. 2016). It usually operates in two ways: with a short pre-treatment period at a high temperature (85–135 °C) and for a lengthy treatment time at a low temperature (50–135 °C). This process has a major benefit over others in that it uses lower temperatures and pressure. However, the application is limited due to the high cost of the alkali. Utilising low-cost and regeneratable alkali solutions such as calcium hydroxide (lime), sodium carbonate and ammonia, could be an effective solution to the aforementioned limitation (Xia et al. 2020).
Physio-chemical pre-treatment
The physio-chemical treatment breaks the refractory biomass structure utilising liquid hot water, ammonia fibre explosion (AFEX), subcritical water (SCW), or steam explosion (SE). Such treatment is performed prior to enzymatic hydrolysis to increase sugar production yield. Liquid ammonia is used in both the ammonia fibre and steam explosions, whereas the former uses high-temperature and pressure ammonia while the latter uses high-pressure steam (where ammonia serves a catalyst) at high temperature. The steam explosion approach, on the other hand, releases many inhibitors (Koppram et al. 2014). Similarly, the influence of pre-treatments such as liquid hot water and steam explosion on enzymatic hydrolysis and found that the liquid hot water system resulted in 76.2% sugar yield, compared with 62.9% for the steam explosion approach (Muharja et al. 2018). The microwave treatment of sugarcane bagasse assisted with 0.2 M FeCl3 for 5 min treatment released glucose of 48% which is higher compared to conventional acid H2SO4 and FeCl3 specifically targets the lignin removal effectively than H2SO4 assisted microwave treatment (Zhu et al. 2021).
Biological pre-treatment
In contrast to the other approaches, the biological method is a low-energy, efficient, and ecologically friendly procedure. Biological pre-treatment uses whole-cell bacteria or enzymes to remove lignin from lignocellulosic biomass. The whole-cell microorganisms employed are generally lignocellulosic degrading bacteria or fungi that secrete enzymes such as ligninase, manganese peroxidase, laccase, and other enzymes that breakdown lignin, hemicellulose, and polyphenols in biomass (Behera et al. 2014). The environment rich in cellulolytic and hemicellulolytic bacteria can be used to treat biomass efficiently. The lignin-modifying enzymes (laccase and heme-containing peroxidases) and lignin-degrading auxiliary enzymes achieved lignin degradation (Janusz et al. 2017). To breakdown lignin and hemicellulose, soft-rot, white, and brown fungi can also be used. Because of their lignification mechanism, white fungi are the most efficient microorganism for delignification (Ren et al. 2016). The ideal microbe must have a strong affinity to lignin and must delignify quicker than carbohydrate molecules are eliminated. Other research has shown that acid pre-treatment followed by enzymatic pre-treatment with commercial enzymes (Celluclast®, Novozymes, etc.) resulted in a higher sugar yield (Sigurbjornsdottir and Orlygsson 2012; Arreola-Vargas et al. 2014; Gonzales and Kim 2017; Muharja et al. 2018).
Advanced pre-treatment method
Some advanced technologies employing Ionic Liquids (ILs), supercritical fluid (SCFs) such as supercritical CO2 (Sc-CO2), oxygen/H2O2 or sodium chlorite, and organic solvents for delignification of lignocellulosic biomass are emerging owing to their advantages such as eco-friendly, low-cost, high-specificity, and low-inhibitory production. Ionic liquids (ILs) potentially breaks and dissolve lignin and partly hemicellulose in lignocellulosic biomass and liberate the cellulose component (Sun et al. 2016; Bhatia et al. 2020). ILs may be basic ([Ch][Lys]), acidic (TEA][HSO4]), or near to neutral in nature (C2C1Im] [OAc]). The traditional pre-treatment approaches of treating agricultural wastes generate minimal yields of reducing sugars, but these ILs pre-treatment led to yields of over 90% of the reducing sugar and roughly 25% of the hemicellulose content from various grass biomass pine, willow, miscanthus and switchgrass (Sun et al. 2017; Bhatia et al. 2021). The usage of ILs for pre-treatment of biomass provides less utilization of harmful chemicals, requires mild operation conditions (Sharma et al. 2020b). Sc-CO2 could quickly penetrate the lignocellulosic surface, lowering resistance and increasing cellulose accessibility, allowing the production of more reducing sugar (Relvas et al. 2015). Oxygen and sodium chlorite treatments of hardwood yielded substantial improvements in lignin yields, but the former was able to achieve more than two-fold lignin removal (Park et al. 2015). Similarly, a Cosolvent Enhanced Lignocellulosic Fractionation (CELF) pre-treatment method to separate lignin from the biomass using an organic solvent and mild sulfuric acid and obtained a high yield of reducing sugar during the enzymatic hydrolysis phase, whereas this pre-treatment approach resulted in the production of inhibitors due to its high-temperature (60–180 °C) (Meng et al. 2018). Even though the advanced pre-treatment techniques have various benefits as described above, they still have a significant drawback in high operating cost, which would restrict their implementation.
Saccharification of biomass
Chemical hydrolysis
The cellulose and hemicellulose can be chemically hydrolyzed into sugar monomers, often with acids as catalysts. A two-stage approach is often used to treat the hemicellulose and cellulose separately during dilute acid hydrolysis: a low temperature is used in the first step to hydrolyze hemicellulose into five-carbon sugars, and high temperature used in the second stage to hydrolyze cellulose into six-carbon sugars. The key benefits of the dilute acid hydrolysis technique are that it led to increased glucose production by more than 70%, while also balancing the high cellulose hydrolysis rate with limited glucose degradation and concentrated acid hydrolysis using 10–30 wt% concentrated acid could convert cellulose to sugar with 100% yields and minimum sugar degradation, (Thangavelu et al. 2019) performed microwave-assisted acid hydrolysis of sago pith waste using dilute sulphuric acid yields glucose of 0.67 g/g of biomass and ethanol yield of 0.31 g/g with fermentation efficiency of 91%. However, some major drawbacks associated with acid hydrolysis, e.g., generation of inhibitor chemicals, the high expense and environmental problems, as well as reactor corrosion and acid recovery difficulty restrict its use of in the industrial sector.
Enzyme hydrolysis
Enzyme hydrolysis using carbohydrate degrading enzymes like cellulases and hemicelluloses to yield-reducing sugars from cellulose, is widely investigated in the industry for saccharification of cellulose as it avoids the generation of inhibitor compounds, which is the primary disadvantage of acid hydrolysis. The benefits of saccharification using enzymes are less or no inhibitor in the hydrolysate, low chance of toxicity, mild operating condition and less dependence on a corrosive resistant reactor (Manzanares et al. 2012). Enzyme hydrolysis process can attain a high yield of reducing sugars as high as 75–85% yield or even up to 95% yield, (Sharma et al. 2017) obtained a maximum fermentable sugar yield of 464.2 mg/g with substrate loading of 10% using three cellulase enzyme isolated from three different fungal sources Aspergillus niger, Fusarium oxysporum and Trichoderma harzianum. The factors that affect the final yield of reducing sugar are reaction time, solid-to-liquid loading ratio, temperature, pre-treatment condition and the residues bounded to the surface of pretreated biomass, particle size of biomass, degree of polymerization of cellulose, position and linkage of cellulose to other heteropolymer present in biomass (Das et al. 2021). When compared to acid/alkali hydrolysis, the overall cost of utilising enzyme is cheaper. The cellulase enzyme found in bacteria namely Erwinia, Clostridium, Cellulomonas, Thermomonospora, and Bacillus.Nevertheless, for commercial cellulase production, most researchers focused on fungi rather than bacteria.Formerly (Islam and Roy 2018) isolated cellulase-producing strain Paenibacillus sp., Bacillus sp. and Aeromonas sp. And the cellulase enzyme obtained from strain Paenibacillus sp. Showed maximum production 0.9 µmol ml−1 min−1 at pH 7 and 40 ºC with an incubation period of 24 h. Cellulase enzyme contains endoglucanases, exoglucanases or cellobiohydrolases and β-glucosidases enzymes that depolymerize the crystalline structure of cellulose in a synergistic manner. Process parameters, such as temperature and pH, have an influence on the efficiency of enzymatic hydrolysis of cellulose. The use of surfactants, poymers and Bovine serum albumin (BSA) and the addition of secondary enzymes (auxiliary activity (AA9), xylanases, feruloyl esterase, and laccase) may further improve the performance of cellulase (Zhang et al. 2017; Sun et al. 2015). The disadvantage of cellulase enzyme is that it reduces activity in the presence of inhibitors, that overcomes by Simultaneous Saccharification and Fermentation (SSF), hydrolyzes the cellulose to mono sugars and simultaneous fermentation of the sugars to the final bioethanol (Zhang et al. 2021). Hemicellulases (xylanases) need a multi-enzyme system comprising ferulic acid esterase, endo–xylanase, acetyl xylan esterase, and exo–xylanase to enzymatically hydrolyze xylan. As the chemical structure of xylan is more complicated than that of cellulose, numerous enzymes are required to hydrolyse the substrate. For the bioethanol production process the utilization of pentose sugar is the crucial factor to be considered but its application in the fermentation process is not effective due to the requirement of microaerophilic growth condition required by several-xylose utilizing microorganism and its low resistance towards high concentrations of ethanol and inhibitors (Rehman et al. 2020).
Environmental sustainability analysis of pre-treatment methods
Life cycle analysis (LCA) has been used to assess the environmental impacts of various pre-treatment techniques involved in the conversion of lignocellulosic biomass to bioethanol based on TRACI method, in which different methods for lignocellulose biomass pre-treatment such as physical (mechanical extrusion, ultrasound and microwave-assisted), chemical (acid treatment and alkali treatment), physio-chemical (acid with a steam explosion), biological (microbial and enzymatic) and advanced (supercritical CO2 and ionic liquid) were assessed (Kuila and Sharma 2017; Rebello et al. 2020). The process conditions and the amount of energy required for various methods for lignocellulosic biomass pre-treatments are summarized in Table 3.
Table 3.
Process conditions and amount of energy utilized in different methods for lignocellulosic biomass pre-treatment
| Treatment method | Amount of chemical used | Operating condition | Energy used (kWh/reducing sugar yield—100%) | References |
|---|---|---|---|---|
| Mechanical extrusion | – | 200 rpm, 1 min, 75 °C | 0.35 | Karunanithy et al. (2013) |
| Ultrasound | Sodium hydroxide-2.9% | 25 kHz, 47 min, 70 °C, 400W power | 0.25 | Velmurugan and Muthukumar (2012) |
| Microwave-assisted | Sulfuric acid-1.0 mol /L | 1 min, 900 W power | 0.02 | Thangavelu et al. (2019) |
| Microwave-assisted | Hydrochloric acid- 1.5 mol/L | 2 min, 700 W power | 0.04 | Thangavelu et al. (2019) |
| Acid treatment | Sulfuric acid-2%(w/v) | 60 min, Biomass loading 10 wt.%, 121 °C | 0.96 | Li et al. (2016) |
| Acid treatment | Oxalic acid-0.5 wt % | 40 min, Solid-to-liquid ratio 7.5% (w/v), 140 °C | 0.95 | Qing et al. (2015) |
| Alkali treatment | Sodium hydroxide-2% |
120 min, Substrate concentration 20 g/L, 80 °C |
1.34 | Wang et al. (2020) |
| Alkali treatment | Sodium hydroxide-pH—11.5 | 1 h, Solid loading 3:50 (w/v), H2O2 loading 7.5% (w/v), 90 °C | 3.68 | Díaz et al. (2014) |
| Steam explosion | Sulfuric acid – -2.2% | 5 min, 170 °C | 1.58 | Wang et al. (2018) |
| Liquid Hot Water | Water |
Up to 5 MPa, 30 min, 170–230 °C |
0.83 | Hongdan et al. (2013) |
| Biological pretreatment | Bacillus firmus K-1- 2% (v/v) | pH 10.5, 250 rpm, 15 min, 37 °C | Baramee et al. (2020) | |
| Biological pretreatment | Laccase-150 Ug−1 | 1-HBT 5% (w/w), 40 h, 37 °C | 0.24 | Heap et al. (2014) |
| Advanced pretreatment method | Supercritical CO2 | 15 bar, 50 min, 160 °C | 153.85 | Cha et al. (2014) |
| Advanced pretreatment method | Ionic liquid- [Emim][OAc] | 40 min, 45 °C | 3.66 | Alayoubi et al. (2020) |
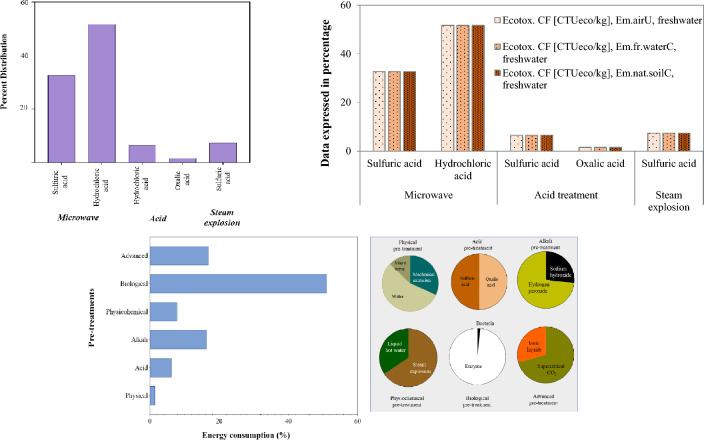
The environmental impacts of various pre-treatment methods following TRACI (USEPA) method describes the impact through several parameters such as acidification Air (kg SO2 eq/kg substance), Human Health (HH) Particulate Air (PM2.5 eq/kg substance), eutrophication potential and energy demand (Vahidi and Zhao 2017; Abinandan et al. 2020). The acidification potential was the greatest (0.51 kg SO2 eq/kg substance) when diluted hydrochloric acid was used in microwave-assisted pre-treatment, and diluted sulfuric acid combined with the microwave-assisted treatment process yielded the second highest SO2 emissions (Fig. 3a). Besides, in comparison with microwave-assisted acid treatment, diluted sulfuric and oxalic acid alone showed relatively low acidification potential (0.06 kg SO2 eq/kg substance). Similarly, diluted sulfuric acid combined with the steam explosion treatment presented a slightly higher acidification potential (0.07 kg SO2 eq/kg substance). According to a study, (Prasad et al. 2016), diluted acid treatment has a greater acidification effect than liquid hot water, organosolv, or steam explosion treatments. When compared with acid treatment alone or steam explosion treatment alone, microwave-assisted hydrochloric acid or sulfuric acid treatment showed greater potential for eutrophication (> 35%) (Fig. 3B). This might be because the microwave-assisted approach requires more acid in the pre-treatment. The energy usage for each pre-treatment method, estimated based on data from the same study is shown in Fig. 3c. Surprisingly, although being regarded as an ecologically favourable method, biological treatment has the highest energy requirement when compared with other pre-treatment approaches. This could be due to the biological process longer treatment time consuming more energy than other treatments. Compared with acid, physical, and physiochemical treatments, the supercritical CO2 and alkali treatment have the second greatest energy consumption. In contrast, pre-treatment with diluted sulfuric acid or oxalic acid alone required the least amount of energy.
Fig. 3.
TRACI (US-EPA) Environmental impacts of various pre-treatment methods, i.e., acidification Air/HH Particulate Air (A), eutrophication potential (B) Energy consumption and distribution (C)
Bio-based products from lignocellulose biomass
The valorization of renewable feedstock lignocellulose components like cellulose (C6H10O5)n, hemicellulose (C5H8O4)m and lignin (C81H92O28) are converted to valuable commodities products. The transformation of lignocellulosic sugars (cellulose and hemicellulose) to platform chemicals such as lactic acid, levulinic acid and, 5-hydroxymethyl furfural widely used in agriculture, food, health and sanitation (Zhou et al. 2021). The U.S Department of Energy stated 12 platform chemicals derived from cellulose namely glycerol, sorbitol, xylitol/arabinitol (bio-based alcohols),1,4-diacids, 2,5-furan dicarboxylic acid, 3-hydroxypropionic acid, aspartic acid, glucaric acid, glutamic acid, itaconic acid, levulinic acid and hydroxy butyrolactone (Galbe and Wallberg 2019). Recently most of high-value product and biofuels synthesis from cellulose-derived glucose by chemo-selective catalytic conversion methods (Li et al. 2019). Moreover, L-lactic acid synthesis from cellulose-derived monomers by thermophilic anaerobic bacterium Caldicellulosiruptor sp. DIB 104ºC (Svetlitchnyi et al. 2022). Similarly, chemo-catalytic conversion of glucose to lactic acid using heterogenous β-zeolite catalyst under optimized catalyst loading of 1.2 mmol/g at 190º C for 3 h yields 45.2% of lactic acid (Shen et al. 2022). (Dedes et al. 2021) The 79.9% of fructose conversion to 44.6% yield of Hydroxymethyl furfural (HMF) from organosolv pre-treated beech wood biomass. Similarly, another top high-value chemical ethylene glycol (polyols) obtained via direct conversion of wood biomass to 41.5% of ethylene glycol using Ru and W catalysts supported carbon nanotubes (CNT) (Ribeiro et al. 2021). Secondly, Renewable lignin resources are complete valorization to high-value aromatic chemicals and fuels by cost-effective biorefinery process (Xu et al. 2014). (Sharma et al. 2020a) reported 99% degradation of kraft lignin selectively converted to 20% 3-methoxybenzoic acid and 16.25% p-hydroxybenzoic acid (PHBA) using heterogeneous nanocomposite TiO2/CQDs under 6 h of solar light irradiation.
Recently, (Dhar et al. 2020) investigated combined ultrasonic photocatalytic degradation of kraft lignin into cinnamyl alcohol,3,4-dihydroxy benzaldehyde, succinic acid and 4-methyl-3-hexanone using TiO2 catalyst.
Future prospects
Bioethanol has emerged as a green fuel to replace routinely used fossil fuels, as it can meet the current energy demand while reducing carbon emissions in the environment. Pre-treatment is essentially needed to produce bioethanol from sustainable raw substrates—lignocellulosic feedstocks (agricultural/crop residues or forestry residues or organic domestic wastes) (Jury et al. 2022). As discussed previously different methods for lignocellulosic biomass pre-treatment have advantages and disadvantages (Table 2) as well as environmental impacts with respect to acidification Air/HH Particulate Air, eutrophication potential and energy demand (Borrion et al. 2012) (Fig. 3). By far, the low substrate conversion and ethanol production yield, and high energy cost (due to the pre-treatment) are the major barriers limiting the industrial production of bioethanol from lignocellulosic feedstock’s (Rijn et al. 2018). Thus, further optimization studies are needed on the pretreatment process to improve the final bioethanol yield and reduce energy (power) consumption (Fig. 4). Innovative conversion processes and environmentally benign cost-effective pretreatment approaches are certainly required. In addition, for large-scale deployment of bioethanol production from lignocellulosic feedstocks, further techno-economic assessment and LCA studies and more extensive analyses of cost-effective feedstocks are necessary (Costa et al. 2018; Vijayalakshmi et al. 2021).
Fig. 4.
Total power consumption and final bioethanol yield in the conversion of lignocellulosic feedstocks involving different pre-treatment methods
Conclusions
Increasing GHG emissions and severe environmental consequences resulting from the use of fossil fuels have intensified the demand for the production of 2nd generation biofuel (bioethanol) from the widely available non-food sustainable biomass resources—lignocellulosic biomass (agricultural residues, industrial/domestic and forest wastes). To utilize lignocellulosic biomass (containing lignin, hemicellulose and cellulose) as a feedstock for bioethanol production, there are still obstacles to overcome, mainly associated with the costly and inefficient pretreatment. The LCA studies show that the present environmentally favourable pretreatment techniques (biological, acid treatment) have higher energy demands and acidification potential. By far, the low substrate conversion and ethanol production yield, and high energy cost (due to the pretreatment) are the major barriers limiting the industrial production of bioethanol from lignocellulosic feedstocks. Thus, further optimization studies are needed on the pretreatment process to improve the final bioethanol yield and reduce energy (power) consumption.
Acknowledgements
The authors are grateful for providing research facilities from the SRM Institute of Science and Technology and one of the authors (Chunbao –Xu) acknowledge the Discovery grant from the Natural Science and Engineering Research Council of Canada (NSERC).
Abbreviations
- AFEX
Ammonia fibre explosion
- SCW
Subcritical water
- SE
Steam explosion
- PEF
Pulsed electric field
- SSCF
Simultaneous saccharification and co-fermentation
- SSF
Simultaneous saccharification and fermentation
- PSSF
Prehydrolysis followed by saccharification and fermentation
- LCA
Life cycle assessment
- GHG
Greenhouse gas
- ILs
Ionic liquids
- SCFs
Supercritical fluid
- LTSD
Low-temperature steep delignification
- CELF
Cosolvent enhanced lignocellulosic fractionation
Author contributions
AJD: writing—original draft, SA: software, data curation, VKV: analysis and interpretation of data, CCX: review & editing, KT: supervision, validation.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Availability of data and materials
This research work not generate any datasets, data and material availability not applicable.
Declarations
Conflict of Interest
The authors declare that they have no competing financial interests that could have appeared to influence the report in this paper.
Ethical Approval
The research work related to agro biomass pre-treatment work, not applicable for any human and animal studies.
References
- Abinandan S, Praveen K, Subashchandrabose SR, et al. Life cycle assessment for the environmental sustainability of the immobilized acid-adapted microalgal technology in iron removal from acid mine drainage. ACS Sustain Chem Eng. 2020;8:15670–15677. doi: 10.1021/acssuschemeng.0c05341. [DOI] [Google Scholar]
- Alawad I, Ibrahim H. Pretreatment of agricultural lignocellulosic biomass for fermentable sugar: opportunities, challenges, and future trends. Biomass Convers Biorefin. 2022;1:1–29. [Google Scholar]
- Alayoubi R, Mehmood N, Husson E, et al. Low temperature ionic liquid pretreatment of lignocellulosic biomass to enhance bioethanol yield. Renew Energy. 2020;145:1808–1816. doi: 10.1016/j.renene.2019.07.091. [DOI] [Google Scholar]
- Alvira P, Tomás-Pejó E, Ballesteros M, Negro MJ. Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: a review. Bioresour Technol. 2010;101:4851–4861. doi: 10.1016/j.biortech.2009.11.093. [DOI] [PubMed] [Google Scholar]
- Arreola-Vargas J, Razo-Flores E, Celis LB, Alatriste-Mondragón F. Sequential hydrolysis of oat straw and hydrogen production from hydrolysates: Role of hydrolysates constituents. Int J Hydrogen Energy. 2014;40:10756–10765. doi: 10.1016/j.ijhydene.2015.05.200. [DOI] [Google Scholar]
- Balat M. Production of bioethanol from lignocellulosic materials via the biochemical pathway: a review. Energy Convers Manag. 2011;52:858–875. doi: 10.1016/j.enconman.2010.08.013. [DOI] [Google Scholar]
- Banerjee R, Chintagunta AD, Ray S. A cleaner and eco-friendly bioprocess for enhancing reducing sugar production from pineapple leaf waste. J Clean Prod. 2017;149:387–395. doi: 10.1016/j.jclepro.2017.02.088. [DOI] [Google Scholar]
- Baramee S, Siriatcharanon AK, Ketbot P, et al. Biological pretreatment of rice straw with cellulase-free xylanolytic enzyme-producing Bacillus firmus K-1: structural modification and biomass digestibility. Renew Energy. 2020;160:555–563. doi: 10.1016/j.renene.2020.06.061. [DOI] [Google Scholar]
- Behera S, Arora R, Nandhagopal N, Kumar S. Importance of chemical pretreatment for bioconversion of lignocellulosic biomass. Renew Sustain Energy Rev. 2014;36:91–106. doi: 10.1016/j.rser.2014.04.047. [DOI] [Google Scholar]
- Bhatia SK, Jagtap SS, Bedekar AA, et al. Recent developments in pretreatment technologies on lignocellulosic biomass: effect of key parameters, technological improvements, and challenges. Bioresour Technol. 2020;300:122724. doi: 10.1016/J.BIORTECH.2019.122724. [DOI] [PubMed] [Google Scholar]
- Bhatia SK, Jagtap SS, Bedekar AA, et al. Renewable biohydrogen production from lignocellulosic biomass using fermentation and integration of systems with other energy generation technologies. Sci Total Environ. 2021;765:144429. doi: 10.1016/j.scitotenv.2020.144429. [DOI] [PubMed] [Google Scholar]
- Boonmanumsin P, Treeboobpha S, Jeamjumnunja K, et al. Release of monomeric sugars from Miscanthus sinensis by microwave-assisted ammonia and phosphoric acid treatments. Bioresour Technol. 2012;103:425–431. doi: 10.1016/j.biortech.2011.09.136. [DOI] [PubMed] [Google Scholar]
- Borrion AL, McManus MC, Hammond GP. Environmental life cycle assessment of bioethanol production from wheat straw. Biomass Bioenergy. 2012;47:9–19. doi: 10.1016/j.biombioe.2012.10.017. [DOI] [Google Scholar]
- Costa D, Jesus J, Virgínio Silva J, Silveira M. Life cycle assessment of bioethanol production from sweet potato (Ipomoea batatas L.) in an experimental plant. BioEnergy Res. 2018;11:715–725. doi: 10.1007/s12155-018-9932-1. [DOI] [Google Scholar]
- Cha YL, Yang J, Ahn JW, et al. The optimized CO2-added ammonia explosion pretreatment for bioethanol production from rice straw. Bioprocess Biosyst Eng. 2014;37:1907–1915. doi: 10.1007/s00449-014-1165-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong CT, Ng J-H (2021) Sustainability of aviation biofuels. In: Biojet fuel in aviation applications, pp 287–335. 10.1016/B978-0-12-822854-8.00005-6
- Das N, Jena PK, Padhi D, et al. A comprehensive review of characterization, pretreatment and its applications on different lignocellulosic biomass for bioethanol production. Biomass Convers Biorefin. 2023;13:1503–1527. doi: 10.1007/s13399-021-01294-3. [DOI] [Google Scholar]
- Dedes G, Karnaouri A, Marianou AA, et al. Conversion of organosolv pretreated hardwood biomass into 5-hydroxymethylfurfural (HMF) by combining enzymatic hydrolysis and isomerization with homogeneous catalysis. Biotechnol Biofuels. 2021;14:1–11. doi: 10.1186/S13068-021-02022-9/FIGURES/4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhar P, Teja V, Vinu R. Sonophotocatalytic degradation of lignin: production of valuable chemicals and kinetic analysis. J Environ Chemi Eng. 2020;8:104286. doi: 10.1016/J.JECE.2020.104286. [DOI] [Google Scholar]
- Díaz AB, Blandino A, Belleli C, Caro I. An effective process for pretreating rice husk to enhance enzyme hydrolysis. Ind Eng Chem Res. 2014;53:10870–10875. doi: 10.1021/ie501354r. [DOI] [Google Scholar]
- Ferreira JA, Brancoli P, Agnihotri S, et al. A review of integration strategies of lignocelluloses and other wastes in 1st generation bioethanol processes. Process Biochem. 2018;75:173–186. doi: 10.1016/j.procbio.2018.09.006. [DOI] [Google Scholar]
- Galbe M, Wallberg O. Pretreatment for biorefineries: a review of common methods for efficient utilisation of lignocellulosic materials. Biotechnol Biofuels. 2019 doi: 10.1186/S13068-019-1634-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galbe M, Zacchi G. A review of the production of ethanol from softwood. Appl Microbiol Biotechnol. 2002;59:618–628. doi: 10.1007/s00253-002-1058-9. [DOI] [PubMed] [Google Scholar]
- Gonzales RR, Kim SH. Dark fermentative hydrogen production following the sequential dilute acid pretreatment and enzymatic saccharification of rice husk. Int J Hydrogen Energy. 2017;42:27577–27583. doi: 10.1016/j.ijhydene.2017.08.185. [DOI] [Google Scholar]
- Gundupalli MP, Tantayotai P, Panakkal EJ, et al. Hydrothermal pretreatment optimization and deep eutectic solvent pretreatment of lignocellulosic biomass: an integrated approach. Bioresour Technol Rep. 2022;17:100957. doi: 10.1016/j.biteb.2022.100957. [DOI] [Google Scholar]
- Hassan SS, Williams GA, Jaiswal AK. Emerging technologies for the pretreatment of lignocellulosic biomass. Bioresour Technol. 2018;262:310–318. doi: 10.1016/J.BIORTECH.2018.04.099. [DOI] [PubMed] [Google Scholar]
- Heap L, Green A, Brown D, et al. Role of laccase as an enzymatic pretreatment method to improve lignocellulosic saccharification. Catal Sci Technol. 2014;4:2251–2259. doi: 10.1039/c4cy00046c. [DOI] [Google Scholar]
- Hernández-Beltrán JU, Hernández-De Lira IO, Cruz-Santos MM, et al. Insight into pretreatment methods of lignocellulosic biomass to increase biogas yield: current state, challenges, and opportunities. Appl Sci. 2019;9:3721. doi: 10.3390/APP9183721. [DOI] [Google Scholar]
- Hongdan Z, Shaohua X, Shubin W. Enhancement of enzymatic saccharification of sugarcane bagasse by liquid hot water pretreatment. Bioresour Technol. 2013;143:391–396. doi: 10.1016/j.biortech.2013.05.103. [DOI] [PubMed] [Google Scholar]
- Islam F, Roy N. Screening, purification and characterization of cellulase from cellulase producing bacteria in molasses. BMC Res Notes. 2018;11:1–6. doi: 10.1186/S13104-018-3558-4/FIGURES/2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janusz G, Pawlik A, Sulej J, et al. Lignin degradation: microorganisms, enzymes involved, genomes analysis and evolution. FEMS Microbiol Rev. 2017;41:941–962. doi: 10.1093/femsre/fux049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasmine A, Rajendran M, Thirunavukkarasu K, et al. Microwave-assisted alkali pre-treatment medium for fractionation of rice straw and catalytic conversion to value-added 5-hydroxymethyl furfural and lignin production. Int J Biol Macromol. 2023;236:123999. doi: 10.1016/J.IJBIOMAC.2023.123999. [DOI] [PubMed] [Google Scholar]
- Jury C, Thomas HL, Carrere H. Life cycle assessment of two alkaline pretreatments of sorghum and miscanthus and of their batch co-digestion with cow manure. BioEnergy Res. 2022;15:1–24. doi: 10.1007/s12155-021-10369-y. [DOI] [Google Scholar]
- Karunanithy C, Muthukumarappan K, Gibbons WR. Effect of extruder screw speed, temperature, and enzyme levels on sugar recovery from different biomasses. ISRN Biotechnol. 2013;2013:1–13. doi: 10.5402/2013/942810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaire KC, Moholkar VS, Goyal A. Bioconversion of sugarcane tops to bioethanol and other value added products: An overview. Mater Sci Energy Technol. 2021;4:54–68. [Google Scholar]
- Kim JS, Lee YY, Kim TH. A review on alkaline pretreatment technology for bioconversion of lignocellulosic biomass. Bioresour Technol. 2016;199:42–48. doi: 10.1016/J.BIORTECH.2015.08.085. [DOI] [PubMed] [Google Scholar]
- Koppram R, Tomás-Pejó E, Xiros C, Olsson L. Lignocellulosic ethanol production at high-gravity: challenges and perspectives. Trends Biotechnol. 2014;32:46–53. doi: 10.1016/j.tibtech.2013.10.003. [DOI] [PubMed] [Google Scholar]
- Kuila A, Sharma V. Lignocellulosic production and industrial applications. Wiley; 2017. [Google Scholar]
- Kumar AK, Sharma S. Recent updates on different methods of pretreatment of lignocellulosic feedstocks: a review. Bioresour Bioprocess. 2017;4:7. doi: 10.1186/s40643-017-0137-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundu C, Samudrala SP, Kibria MA, Bhattacharya S. One-step peracetic acid pretreatment of hardwood and softwood biomass for platform chemicals production. Sci Rep. 2021;11:1–11. doi: 10.1038/s41598-021-90667-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JW, Jeffries TW. Efficiencies of acid catalysts in the hydrolysis of lignocellulosic biomass over a range of combined severity factors. Bioresour Technol. 2011;102:5884–5890. doi: 10.1016/J.BIORTECH.2011.02.048. [DOI] [PubMed] [Google Scholar]
- Li P, Cai D, Luo Z, et al. Effect of acid pretreatment on different parts of corn stalk for second generation ethanol production. Bioresour Technol. 2016;206:86–92. doi: 10.1016/j.biortech.2016.01.077. [DOI] [PubMed] [Google Scholar]
- Li S, Deng W, Li Y, et al. Catalytic conversion of cellulose-based biomass and glycerol to lactic acid. J Energy Chem. 2019;32:138–151. doi: 10.1016/j.jechem.2018.07.012. [DOI] [Google Scholar]
- Lin Z, Huang H, Zhang H, et al. Ball milling pretreatment of corn stover for enhancing the efficiency of enzymatic hydrolysis. Appl Biochem Biotechnol. 2010;162:1872–1880. doi: 10.1007/s12010-010-8965-5. [DOI] [PubMed] [Google Scholar]
- Loow YL, Wu TY, Jahim JM, et al. Typical conversion of lignocellulosic biomass into reducing sugars using dilute acid hydrolysis and alkaline pretreatment. Cellulose. 2016;23:1491–1520. doi: 10.1007/s10570-016-0936-8. [DOI] [Google Scholar]
- Lopez-Hidalgo AM, Sánchez A, De León-Rodríguez A. Simultaneous production of bioethanol and biohydrogen by Escherichia coli WDHL using wheat straw hydrolysate as substrate. Fuel. 2017;188:19–27. doi: 10.1016/j.fuel.2016.10.022. [DOI] [Google Scholar]
- Manzanares P, Ballesteros I, Negro MJ, et al. Biological conversion of forage sorghum biomass to ethanol by steam explosion pretreatment and simultaneous hydrolysis and fermentation at high solid content. Biomass Convers Biorefin. 2012;2:123–132. doi: 10.1007/s13399-012-0040-8. [DOI] [Google Scholar]
- Meng X, Parikh A, Seemala B, et al. Chemical transformations of poplar lignin during cosolvent enhanced lignocellulosic fractionation process. ACS Sustain Chem Eng. 2018;6:8711–8718. doi: 10.1021/acssuschemeng.8b01028. [DOI] [Google Scholar]
- Meng X, Pu Y, Li M, Ragauskas AJ. A biomass pretreatment using cellulose-derived solvent Cyrene. Green Chem. 2020;22:2862–2872. doi: 10.1039/D0GC00661K. [DOI] [Google Scholar]
- Millati R, Wikandari R, Ariyanto T, et al. Pretreatment technologies for anaerobic digestion of lignocelluloses and toxic feedstocks. Bioresour Technol. 2020;304:122998. doi: 10.1016/j.biortech.2020.122998. [DOI] [PubMed] [Google Scholar]
- Mthembu LD, Gupta R, Deenadayalu N, et al. Conversion of cellulose into value-added products. Cellul Sci Deriv. 2021 doi: 10.5772/INTECHOPEN.100022. [DOI] [Google Scholar]
- Muharja M, Junianti F, Ranggina D, et al. An integrated green process: subcritical water, enzymatic hydrolysis, and fermentation, for biohydrogen production from coconut husk. Bioresour Technol. 2018;249:268–275. doi: 10.1016/j.biortech.2017.10.024. [DOI] [PubMed] [Google Scholar]
- Orr VCA, Plechkova NV, Seddon KR, Rehmann L. Disruption and wet extraction of the microalgae chlorella vulgaris using room-temperature ionic liquids. ACS Sustain Chem Eng. 2016;4:591–600. doi: 10.1021/acssuschemeng.5b00967. [DOI] [Google Scholar]
- Park J, Shin H, Yoo S, et al. Delignification of lignocellulosic biomass and its effect on subsequent enzymatic hydrolysis. BioResources. 2015;10:2732–2743. doi: 10.15376/biores.10.2.2732-2743. [DOI] [Google Scholar]
- Pereira SC, Maehara L, Machado CMM, Farinas CS. 2G ethanol from the whole sugarcane lignocellulosic biomass. Biotechnol Biofuels. 2015;8:1–16. doi: 10.1186/S13068-015-0224-0/FIGURES/7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad A, Sotenko M, Blenkinsopp T, Coles SR. Life cycle assessment of lignocellulosic biomass pretreatment methods in biofuel production. Int J Life Cycle Assess. 2016;21:44–50. doi: 10.1007/s11367-015-0985-5. [DOI] [Google Scholar]
- Qing Q, Huang M, He Y, et al. Dilute oxalic acid pretreatment for high total sugar recovery in pretreatment and subsequent enzymatic hydrolysis. Appl Biochem Biotechnol. 2015;177:1493–1507. doi: 10.1007/s12010-015-1829-2. [DOI] [PubMed] [Google Scholar]
- Rebello S, Anoopkumar AN, Aneesh EM, et al. Sustainability and life cycle assessments of lignocellulosic and algal pretreatments. Bioresour Technol. 2020;301:122678. doi: 10.1016/j.biortech.2019.122678. [DOI] [PubMed] [Google Scholar]
- Rehman O, Shahid A, Liu CG, et al. Optimization of low-temperature energy-efficient pretreatment for enhanced saccharification and fermentation of Conocarpus erectus leaves to produce ethanol using Saccharomyces cerevisiae. Biomass Convers Biorefin. 2020;10:1269–1278. doi: 10.1007/s13399-019-00529-8. [DOI] [Google Scholar]
- Relvas FM, Morais ARC, Bogel-Lukasik R. Selective hydrolysis of wheat straw hemicellulose using high-pressure CO2 as catalyst. RSC Adv. 2015;5:73935–73944. doi: 10.1039/c5ra14632a. [DOI] [Google Scholar]
- Ren NQ, Zhao L, Chen C, et al. A review on bioconversion of lignocellulosic biomass to H2: Key challenges and new insights. Bioresour Technol. 2016;215:92–99. doi: 10.1016/j.biortech.2016.03.124. [DOI] [PubMed] [Google Scholar]
- Rewlay-ngoen C, Papong S, Onbhuddha R, Thanomnim B. Evaluation of the environmental performance of bioethanol from cassava pulp using life cycle assessment. J Clean Prod. 2021;284:124741. doi: 10.1016/j.jclepro.2020.124741. [DOI] [Google Scholar]
- Ribeiro LS, de Órfão JJM, Pereira MFR. Direct catalytic conversion of agro-forestry biomass wastes into ethylene glycol over CNT supported Ru and W catalysts. Ind Crops Prod. 2021;166:113461. doi: 10.1016/J.INDCROP.2021.113461. [DOI] [Google Scholar]
- Rijn RV, Nieves IU, Shanmugam KT, et al. Techno-economic evaluation of cellulosic ethanol production based on pilot biorefinery data: a case study of sweet sorghum bagasse processed via L+SScF. Bioenergy Res. 2018;11:414–425. doi: 10.1007/s12155-018-9906-3. [DOI] [Google Scholar]
- Sewsynker-Sukai Y, Gueguim Kana EB. Simultaneous saccharification and bioethanol production from corn cobs: Process optimization and kinetic studies. Bioresour Technol. 2018;262:32–41. doi: 10.1016/j.biortech.2018.04.056. [DOI] [PubMed] [Google Scholar]
- Sharma S, Kuila A, Sharma V. Enzymatic hydrolysis of thermochemically pretreated biomass using a mixture of cellulolytic enzymes produced from different fungal sources. Clean Technol Environ Policy. 2017;19:1577–1584. doi: 10.1007/S10098-017-1346-9/FIGURES/4. [DOI] [Google Scholar]
- Sharma S, Kumar S, Arumugam SM, Elumalai S. Promising photocatalytic degradation of lignin over carbon quantum dots decorated TiO2 nanocomposite in aqueous condition. Appl Catal A Gen. 2020;602:117730. doi: 10.1016/J.APCATA.2020.117730. [DOI] [Google Scholar]
- Sharma V, Nargotra P, Sharma S, Bajaj BK. Efficient bioconversion of sugarcane tops biomass into biofuel-ethanol using an optimized alkali-ionic liquid pretreatment approach. Biomass Convers Biorefin. 2020 doi: 10.1007/s13399-020-01123-z. [DOI] [Google Scholar]
- Shen Z, Chen W, Zhang W, et al. Efficient catalytic conversion of glucose into lactic acid over Y-β and Yb-β zeolites. ACS Omega. 2022;7:25200–25209. doi: 10.1021/ACSOMEGA.2C02051/ASSET/IMAGES/LARGE/AO2C02051_0012.JPEG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigurbjornsdottir MA, Orlygsson J. Combined hydrogen and ethanol production from sugars and lignocellulosic biomass by Thermoanaerobacterium AK54, isolated from hot spring. Appl Energy. 2012;97:785–791. doi: 10.1016/j.apenergy.2011.11.035. [DOI] [Google Scholar]
- Siwal SS, Zhang Q, Devi N, et al. Recovery processes of sustainable energy using different biomass and wastes. Renew Sustain Energy Reviews. 2021;150:111483. doi: 10.1016/j.rser.2021.111483. [DOI] [Google Scholar]
- Smullen E, Finnan J, Dowling D, Mulcahy P. The environmental performance of pretreatment technologies for the bioconversion of lignocellulosic biomass to ethanol. Renew Energy. 2019;142:527–534. doi: 10.1016/j.renene.2019.04.082. [DOI] [Google Scholar]
- Soam S, Kapoor M, Kumar R, et al. Life cycle assessment and life cycle costing of conventional and modified dilute acid pretreatment for fuel ethanol production from rice straw in India. J Clean Prod. 2018;197:732–741. doi: 10.1016/j.jclepro.2018.06.204. [DOI] [Google Scholar]
- Sun Y, Cheng J. Hydrolysis of lignocellulosic materials for ethanol production: a review. Bioresour Technol. 2002;83:1–11. doi: 10.1016/S0960-8524(01)00212-7. [DOI] [PubMed] [Google Scholar]
- Sun FF, Hong J, Hu J, et al. Accessory enzymes influence cellulase hydrolysis of the model substrate and the realistic lignocellulosic biomass. Enzyme Microb Technol. 2015;79–80:42–48. doi: 10.1016/J.ENZMICTEC.2015.06.020. [DOI] [PubMed] [Google Scholar]
- Sun X, Sun X, Zhang F. Combined pretreatment of lignocellulosic biomass by solid base (calcined Na2SiO3) and ionic liquid for enhanced enzymatic saccharification. RSC Adv. 2016;6:99455–99466. doi: 10.1039/c6ra22055j. [DOI] [Google Scholar]
- Sun J, Shi J, Murthy Konda NVSN, et al. Efficient dehydration and recovery of ionic liquid after lignocellulosic processing using pervaporation. Biotechnol Biofuels. 2017;1:1–14. doi: 10.1186/s13068-017-0842-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svetlitchnyi VA, Svetlichnaya TP, Falkenhan DA, et al. Direct conversion of cellulose to l-lactic acid by a novel thermophilic Caldicellulosiruptor strain. Biotechnol Biofuels Bioprod. 2022;15:1–12. doi: 10.1186/s13068-022-02137-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thangavelu SK, Rajkumar T, Pandi DK, et al. Microwave assisted acid hydrolysis for bioethanol fuel production from sago pith waste. Waste Manag. 2019;86:80–86. doi: 10.1016/j.wasman.2019.01.035. [DOI] [PubMed] [Google Scholar]
- Tomás-Pejó E, Oliva JM, Ballesteros M. Realistic approach for full-scale bioethanol production from lignocellulose: a review. J Sci Ind Res (india) 2008;67:874–884. [Google Scholar]
- Ubando AT, Rivera DRT, Chen WH, Culaba AB. A comprehensive review of life cycle assessment (LCA) of microalgal and lignocellulosic bioenergy products from thermochemical processes. Bioresour Technol. 2019;291:121837. doi: 10.1016/j.biortech.2019.121837. [DOI] [PubMed] [Google Scholar]
- Vahidi E, Zhao F. Environmental life cycle assessment on the separation of rare earth oxides through solvent extraction. J Environ Manag. 2017;203:255–263. doi: 10.1016/j.jenvman.2017.07.076. [DOI] [PubMed] [Google Scholar]
- Velmurugan R, Muthukumar K. Ultrasound-assisted alkaline pretreatment of sugarcane bagasse for fermentable sugar production: optimization through response surface methodology. Bioresour Technol. 2012;112:293–299. doi: 10.1016/j.biortech.2012.01.168. [DOI] [PubMed] [Google Scholar]
- Vijayalakshmi S, Govindarajan M, Al-Mulahim N, et al. Cellulase immobilized magnetic nanoparticles for green energy production from Allamanda schottii L: Sustainability research in waste recycling. Saudi J Biol Sci. 2021;28:901–910. doi: 10.1016/j.sjbs.2020.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Wu G, Jönsson LJ. Effects of impregnation of softwood with sulfuric acid and sulfur dioxide on chemical and physical characteristics, enzymatic digestibility, and fermentability. Bioresour Technol. 2018;247:200–208. doi: 10.1016/j.biortech.2017.09.081. [DOI] [PubMed] [Google Scholar]
- Wang W, Wang X, Zhang Y, et al. Effect of sodium hydroxide pretreatment on physicochemical changes and enzymatic hydrolysis of herbaceous and woody lignocelluloses. Ind Crops Prod. 2020 doi: 10.1016/j.indcrop.2020.112145. [DOI] [Google Scholar]
- Xia F, Gong J, Lu J, et al. Combined liquid hot water with sodium carbonate-oxygen pretreatment to improve enzymatic saccharification of reed. Bioresour Technol. 2020;297:122498. doi: 10.1016/J.BIORTECH.2019.122498. [DOI] [PubMed] [Google Scholar]
- Xu C, Arancon RAD, Labidi J, Luque R. Lignin depolymerisation strategies: towards valuable chemicals and fuels. Chem Soc Rev. 2014;43:7485–7500. doi: 10.1039/C4CS00235K. [DOI] [PubMed] [Google Scholar]
- Zabed H, Sahu JN, Suely A, et al. Bioethanol production from renewable sources: Current perspectives and technological progress. Renew Sustain Energy Rev. 2017;71:475–501. doi: 10.1016/j.rser.2016.12.076. [DOI] [Google Scholar]
- Zhang H, Ye G, Wei Y, et al. Enhanced enzymatic hydrolysis of sugarcane bagasse with ferric chloride pretreatment and surfactant. Bioresour Technol. 2017;229:96–103. doi: 10.1016/J.BIORTECH.2017.01.013. [DOI] [PubMed] [Google Scholar]
- Zhang H, Han L, Dong H. An insight to pretreatment, enzyme adsorption and enzymatic hydrolysis of lignocellulosic biomass: experimental and modeling studies. Renew Sustain Energy Rev. 2021;140:110758. doi: 10.1016/J.RSER.2021.110758. [DOI] [Google Scholar]
- Zhou C, Wang Y (2020) Recent progress in the conversion of biomass wastes into functional materials for value-added applications. http://www.tandfonline.com/action/journalInformation?show=aimsScope&journalCode=tsta20#VmBmuzZFCUk 21:787–804. 10.1080/14686996.2020.1848213 [DOI] [PMC free article] [PubMed]
- Zhou Z, Liu D, Zhao X. Conversion of lignocellulose to biofuels and chemicals via sugar platform: an updated review on chemistry and mechanisms of acid hydrolysis of lignocellulose. Renew Sustain Energy Rev. 2021;146:111169. doi: 10.1016/J.RSER.2021.111169. [DOI] [Google Scholar]
- Zhu Z, Simister R, Bird S, et al. Microwave assisted acid and alkali pretreatment of Miscanthus biomass for biorefineries. AIMS Bioeng. 2015;2:449–468. doi: 10.3934/bioeng.2015.4.449. [DOI] [Google Scholar]
- Zhu Z, Liu Y, Yang X, et al. Comparative evaluation of microwave-assisted acid, alkaline, and inorganic salt pretreatments of sugarcane bagasse for sugar recovery. Biomass Convers Biorefin. 2021;11:2681–2693. doi: 10.1007/s13399-020-00680-7. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This research work not generate any datasets, data and material availability not applicable.