Abstract
Prevalence of survivors of breast cancer has been steadily increasing in the last 20 years. Currently, more than 90% of women diagnosed with early-stage breast cancer are expected to be alive at 5 years from diagnosis thanks to early detection and breakthrough innovations in multimodal treatment strategies. Alongside this advancement in clinical outcomes, survivors of breast cancer might experience several specific challenges and present with unique needs. Survivorship trajectories after diagnosis and treatment of breast cancer can be significantly impacted by long-lasting and severe treatment-related side effects, including physical problems, psychological distress, fertility issues in young women, and impaired social and work reintegration, which add up to patients’ individual risk of cancer recurrence and second primary malignancies. Alongside cancer-specific sequelae, survivors still present with general health needs, including management of chronic preexisting or ensuing conditions. Survivorship care should implement high-quality, evidence-based strategies to promptly screen, identify, and address survivors’ needs in a comprehensive way and minimize the impact of severe treatment sequelae, preexisting comorbidities, unhealthy lifestyles, and risk of recurrence on quality of life. This narrative review focuses on core areas of survivorship care and discuss the state of the art and future research perspectives in key domains including selected long-term side effects, surveillance for recurrences and second cancers, well-being promotion, and specific survivors’ needs.
Keywords: Breast cancer, Survivorship, Supportive care
Key Summary Points
| 1. Prevalence of breast cancer survivors has been steadily increasing thanks to early detection and improvement of oncological outcomes. |
| 2. Over the course of the survivorship trajectory, survivors of breast cancer face several challenges that can lead to quality-of-life deterioration. |
| 3. Survivorship care is articulated in five main areas: management of physical and psychological sequelae of treatment, health promotion, management of chronic conditions, and surveillance for recurrences and second cancers. |
| 4. To accelerate progress in the field and improve quality of survivorship care, future research in the field should aim at integrating high-quality data to better characterize determinants of long-term toxicities, deliver novel and effective interventions, and test novel models of care. |
Introduction
Survivors of breast cancer (BC) are currently estimated at 4 millions in the USA [1] and 2 millions in Europe [2], and are expected to further increase in the next decades [3]. The progressive increase in prevalence of survivors is multifactorial, being linked, on the one hand, to increased prevalence of unhealthy lifestyles [4] and earlier detection due to implementation of mammographic screening [5], and on the other hand, to striking advances in the multimodal treatment of BC [6, 7].
Over the last 20 years, the treatment landscape of BC has been repeatedly revolutionized by novel agents that have radically modified patterns of care and significantly improved clinical outcomes [8]. However, novel agents can pose significant challenges for survivors of BC.
First, the use of novel treatments in the early setting has significantly extended the active treatment phase, requiring more frequent medical evaluations in the hospital setting [9–12]. Second, although pivotal studies and patient-reported outcomes (PROs) data suggest that most novel agents are tolerable and not associated with substantial deterioration in quality of life (QoL), adverse events and long-term physical and psychological effects might still place a significant burden on survivors in terms of interference with daily activities, potential need for medical care, and risk of long-lasting toxicities [13–15]. Third, the potential impact of novel agents on fundamental aspects of survivorship care, such as fertility issues in young women, long-term physical effects, psychological distress, and social and work reintegration, are still largely unknown.
Overall, following completion of primary treatment and along the survivorship trajectory, survivors of BC face several challenges and present different needs that have to be addressed in a comprehensive model of care rooted in an evidence-based framework [16]. Consequently, current guidelines for survivorship care recommend appropriate assessment and management of survivors’ needs in five main areas: physical and psychological sequelae of treatments, identification of recurrences and second cancers, general health promotion, and management of chronic conditions [16–18].
Recently, a consensus statement at the European level reinforced the need to promote high-quality survivorship care and research in the context of an evidence-based framework, underlying the need for better identification and management of survivors’ needs [19]. A similar attention toward survivorship care has been raised by policymakers in the context of the Europe Beating Cancer Plan [20], with a specific flagship aimed at improving QoL in cancer survivors.
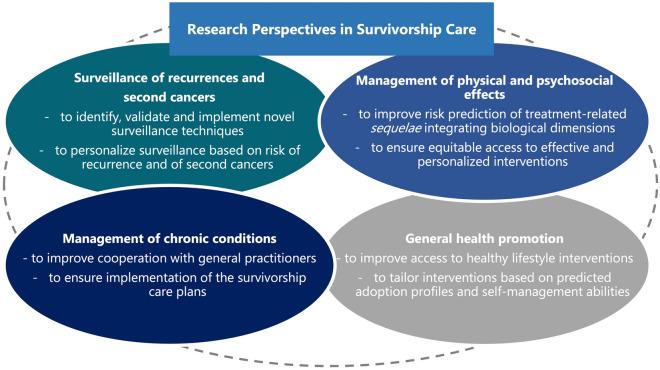
In this narrative review, we discuss state of the art and research perspectives (Fig. 1) in the field of survivorship care, focusing on selected issues including long-term side effects, surveillance protocols, well-being and general health promotion, and specific survivors’ needs.
Fig.1.
Future research perspectives in the four main areas of survivorship care
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Long-Term Side Effects
Survivors of BC are at risk of developing a variety of physical effects whose appearance, severity, and persistence may be related to different treatment exposures and underlying risk factors.
Multimodal treatment of BC requires administration of different agents, each associated with a specific spectrum of physical effects that can appear during treatment and frequently persist afterward. The most common long-term physical symptoms reported by survivors of BC include, among others, fatigue, insomnia, musculoskeletal pain, vasomotor symptoms and sexual dysfunction, cognitive dysfunction, peripheral neuropathy, and weight gain. Treatment of BC also carries a risk of long-term physician-assessed conditions such as cardiotoxicity and loss of reproductive potential in young women.
Long-term physical effects can be associated with a significant time-dependent deterioration of QoL [21], loss of physical and role function, and with reduced adherence to adjuvant treatment as well, especially endocrine therapy [22].
Considering the broad spectrum of potential long-term physical effects, we selected some of the most prevalent and burdensome.
Fatigue
Cancer-related fatigue (CRF) is one of the most common and distressing long-term sequela among survivors of BC [23]. CRF is distinct from regular fatigue since it is less responsive to rest, is more intense and distressing [24–26], and has multidimensional manifestations involving the physical, emotional, and cognitive domains [23]. CRF can place a substantial burden on survivors and lead to deterioration in QoL due to physical, psychological, and socioeconomic repercussions [27].
The highest prevalence of CRF, around 60%, has been described during primary treatment and in the following year; however, up to 30% of survivors of BC report persistent fatigue up to 10 years after diagnosis and treatment for BC [27–30].
A complex interplay between several biobehavioral factors has been associated with the development of CRF. Previous data reported on significant associations between CRF and social (e.g., age, marital status, education, and income), psychological and cognitive (e.g., preexisting depression and fatigue), and medical (e.g. comorbidities and type of treatment) factors [27]. Furthermore, dysregulation of relevant biological pathways may play a significant role in the development of CRF. Higher levels of proinflammatory cytokines, alterations in the neuroendocrine system, and higher expression of aging biomarkers have been described in survivors reporting persistent and severe CRF [23, 31–36]. Additionally, preliminary data show a potential role of single nucleotide polymorphism on the risk of developing CRF after BC [37].
Current guidelines recommend a systematic screening of cancer survivors using PROs, aiming at prompt identification of CRF and concomitant symptoms (e.g., pain, emotional distress, and insomnia) that frequently cluster with it [17, 38, 39]. Despite these recommendations, the high prevalence, and the significant impact on survivors’ lives, data show that CRF remains a largely under-addressed issue [23, 27].
Despite the fact that the mechanisms underlying development and persistence of CRF are not completely understood, different therapeutic approaches have been tested and are endorsed by guidelines [39]. Optimized management of underlying medical conditions (e.g., anemia, nutritional deficits) and of concomitant symptoms clustering with fatigue (e.g., insomnia, emotional distress) should always be considered in a global approach to CRF [39]. In addition, specific interventions are effective in mitigating CRF and should be proactively proposed. Initiation and/or maintenance of adequate physical activity (including both aerobic and resistance training) reduce CRF, probably through modulation of systemic inflammation [38, 40–43]. Psychosocial interventions including cognitive behavioral therapy, educational therapies, and mindfulness reduce CRF by regulating maladaptive thoughts [38, 44, 45]. Finally, data from randomized clinical trials support a potential role of acupuncture in relieving CRF [46–49]. Despite availability of interventions targeting CRF, several barriers, including limited awareness of healthcare professionals and lack of resources and reimbursement for supportive care interventions [50, 51], significantly hamper their implementation in routine clinical practice [52].
Recent data published using the CANTO (NCT01993498) cohort [53] shed new light on the evolution of CRF over time and on risk factors associated with reporting severe CRF. Using longitudinal self-reported PROs among 4173 patients, the authors identified clusters of patients at different risk for severe global CRF: one cluster of patients (high-risk group, 21%) with a consistently high risk at diagnosis and 4 years after, and a second cluster (deteriorating group, 19%) of patients with low risk at diagnosis but with a significant 64% risk 4 years after. Patients belonging to these two clusters were more frequently young, single, had more comorbidities, and higher body mass index (BMI), reporting symptoms at diagnosis and receiving endocrine therapy. Interestingly, dimensions of CRF (i.e., physical, emotional, and cognitive) showed that different clustering patterns and specific risk factors could be identified, although emotional distress and endocrine therapy were common to all dimensions [54].
The same authors developed an online tool exploiting risk factors collected at diagnosis to predict the probability of reporting severe global fatigue at year 2 and year 4 after diagnosis. Specific risk factors for reporting severe fatigue at year 2 included presence of severe fatigue at baseline, younger age, higher BMI, current smoking behavior, and presence of anxiety, insomnia, and pain at diagnosis, while endocrine therapy and premenopausal status emerged as significant risk factors at year 4 after diagnosis [55].
Overall, these studies reinforce the notion that CRF should be addressed in the context of a patient-specific framework to systematically screen for CRF, with the overachieving goal of identifying both patients with severe fatigue at diagnosis to propose therapeutic interventions and patients at risk of developing severe fatigue over time to implement symptom monitoring and address modifiable risk factors.
Future research in the field should aim for better understanding the neurobiological mechanism underlying development and persistence of CRF to inform the design of novel therapeutic approaches for this prevalent symptom. Development of CRF is multifactorial and several risk factors identified in large cohorts are related to unhealthy behaviors that are, therefore, modifiable. Future interventions should evaluate whether early implementation of therapeutic approaches for CRF and targeting of modifiable risk factors is able to prevent development of severe fatigue in survivors at risk. Finally, considering that PROs represent the most reliable tool to adequately detect the subjective experience of CRF, integration in clinical practice of electronic solutions to periodically and remotely monitor survivors might offer the chance for early identification of patients with symptom deterioration to promptly offer supportive and therapeutic measures.
Infertility
A relevant number of young patients diagnosed with early BC receive gonadotoxic chemotherapy treatments [56], leading to a potential risk of premature ovarian insufficiency (POI) and consequent infertility [57].
International guidelines recommend adequate counseling on gonadotoxicity risk for patients diagnosed with cancer during their reproductive years, and treatment planning should guarantee access to fertility preservation techniques before treatment start for all of those are interested in these strategies [58, 59].
Medical Gonadoprotection
Administration of a gonadotropin-releasing hormone agonist (GnRHa) during chemotherapy is not a fertility preservation technique per se, but should be proposed to premenopausal patients that wish to preserve ovarian function and following cryopreservation options in those concerned about risk of infertility [58]. Medical gonadoprotection using GnRHa should start at least 1 week before cytotoxic chemotherapy with the aim of reducing the risk of POI and its fertility and endocrine-related consequences [58].
A systematic review and meta-analysis of 14 randomized trials in premenopausal patients with BC demonstrated the efficacy of this strategy [60]. Efficacy was also confirmed in a patient-level meta-analysis showing that risk of POI was significantly reduced by concurrent GnRHa administration during chemotherapy (POI rates 30.9% versus 14.1%, adjusted OR 0.38; 95% CI 0.26–0.57; P < 0.001) [61].
While a proper counseling of patients on the risk of developing POI with the use of chemotherapy is possible, to date there are no data in humans on the gonadotoxicity of new targeted treatments and on the safety of pregnancy following their administration [62]. Evidence in this regard is urgently needed, especially considering that new treatments approved in early BC (e.g., immune checkpoint inhibitors and PARP inhibitors) have shown to reduce ovarian reserve and reproductive potential in animal models [63, 64].
Fertility Preservation Techniques
Cryopreservation of oocytes/embryos following controlled ovarian stimulation (COS) is the first fertility preservation technique. Ovarian tissue cryopreservation is an alternative that can be proposed to patients who cannot receive COS, who cannot delay gonadotoxic therapy because of disease aggressiveness, or in prepuberal girls [59, 65].
Increasingly reassuring data on the safety of these techniques are emerging. A recent meta-analysis by Arecco et al. showed the safety of COS before the start of cancer treatment among 3980 patients [66]. COS was not associated with inferior outcomes in terms of risk of recurrence (RR 0.58, 95% CI 0.46–0.73), event-free survival (EFS, HR 0.76, 95% CI 0.55–1.06), or death (RR 0.54, 95% CI 0.38–0.76), neither in the overall population nor in the hormone-receptor positive subtype (HR for EFS 0.36, 95% CI 0.20–0.65). Most of the efficacy and safety data on cryopreservation options in young women with BC derive from single-center retrospective studies with a limited sample size. Results from multicenter prospective studies, such as the ongoing Italian PREFER study [67], are awaited to provide more solid answers on this topic.
In recent years, several studies have shown that pregnancy following proper treatment and follow-up is safe for both the mother and the baby [68], including in the case of prior history of hormone-receptor positive BC [69, 70]. Recently, the POSITIVE trial (NCT02308085) has also shown, at short-term follow-up, no alarming signals for a temporary interruption of endocrine therapy for up to 2 years in order to become pregnant for women (≤ 42 years) who completed at least 18 months of endocrine therapy [71–74]. Longer-term follow-up from this trial will be crucial before revolutionizing our approach to endocrine therapy in all patients seeking to become pregnant.
The oncofertility counseling of patients with hereditary breast and ovarian cancer syndrome (HBOC) syndromes is complex considering the additional issues that they have to face, including the indication for risk-reducing gynecological surgeries at a young age [75]. Moreover, as shown in preclinical studies, carriers of BRCA1/2 pathogenic variants (PVs) might have an accelerated involution of the ovarian follicular reserve leading to POI, an increased risk of aneuploidies, and ultimately, earlier menopause [76, 77].
The biological mechanism underlying this negative effect on reproductive potential is potentially related to DNA damage in the primordial oocytes that can occur in the form of both single- and double-strand breaks (DSBs) [78]. DSBs are the most deleterious damage that can result in failed rearrangements and chromosomal instability [79]. The relevance of this damage is particularly high in the presence of BRCA1/2 PVs since ATM-pathway repair system is defective and inevitably leads to cell cycle arrest and apoptosis [80], resulting in an early reduction of ovarian reserve. Preliminary evidence in young women with BC suggests that BRCA carriers have worse ovarian reserve (measured with the levels of anti-müllerian hormone, AMH) at breast cancer diagnosis than women without genetic defects [81].
This observation has raised concerns of a possible higher risk of treatment-induced gonadotoxicity in BRCA1/2 carriers compared with non-carriers, but very limited data are available [82–84]. Similarly, in the specific cohort of patients with BC carrying BRCA PVs, data on the efficacy and safety of the available options for fertility and/or ovarian function preservation [85–92] and on the safety of pregnancy after BC [82, 93, 94] are very limited. Further research focused on fertility- and pregnancy-related issues in BRCA carriers with BC is needed. Moreover, evidence on how to properly counsel young women with BC harboring other high-to-moderate penetrance genes is warranted.
Sexual Dysfunction
Available options for adjuvant endocrine therapy in premenopausal women include ovarian function suppression (OFS) in combination with an aromatase inhibitor or tamoxifen, or tamoxifen alone. Treatment should be tailored on individual risk of recurrence and patients’ characteristics [95–97]. Addition of OFS to endocrine therapy has proven effective in reducing risk of recurrence and death in premenopausal women diagnosed with high-risk disease [98].
However, OFS leads to a significant increase in side effects including hot flashes, sexual dysfunction, weight gain, musculoskeletal symptoms, bone density loss, depression, cognitive dysfunction, and fatigue [99, 100].
Consequently, an accurate evaluation of the risk–benefit ratio, discussion with patients, and availability of a framework [101] to address endocrine therapy-related side effects are fundamental aspects to consider when proposing addition of OFS in women who are premenopausal.
Sexual concerns are highly prevalent among survivors of BC and include different symptoms such as poor body image, sexual inactivity, vulvovaginal symptoms, low sexual desire, and reduced sexual satisfaction. These symptoms can be temporary or permanent and have a negative impact on QoL [21, 102]. Risk factors for reporting sexual concerns include OFS-related side effects, coexistence of anxiety and depression, and partner satisfaction [103, 104]. Radical breast surgery is certainly associated with poorer body image, which might negatively affect sexual health, but evidence of its direct impact on it is less compelling [103–106]. In a recent meta-analysis, breast reconstruction was associated with improved body image or satisfaction compared with mastectomy, but no difference was observed for sexual health, although relevant heterogeneity was observed among included studies [107].
Despite their prevalence and relevance for survivors, these concerns remain largely unaddressed, and only a small percentage of survivors are referred to specific counseling [108], although several strategies, both pharmacological and non-pharmacological, have proven effective in treating these disorders [101].
Local treatments using vaginal lubricants (water-based formulations, polycarbophil, and hyaluronic acid moisturizers) can provide support to treat genitourinary symptoms [109, 110]. Lubricants reduce friction and discomfort during penetrative sexual activity, and their availability, low cost, and irrelevant side effects make them a valid first approach to all survivors reporting sexual dysfunction and vaginal symptoms.
Whenever other pharmacological or non-pharmacological hormonal-free approaches fail, use of local estrogen can be considered. Absorption of local treatments varies by the amount of active ingredient applied and by the product’s formulation, and these agents can cause an elevation of serum estradiol concentrations [111], which might be reason of concern in women receiving adjuvant endocrine therapy for BC [112]. Recently, results of an observational cohort of women receiving local or systemic estrogens for endocrine-related genitourinary symptoms have been reported. With a median follow-up of 9.8 years for recurrences and 15.2 years for mortality, the authors did not observe an increased risk of events in the general population, neither with administration of systemic nor local estrogens; however, an increased risk of recurrence was found in patients receiving aromatase inhibitors [113]. More robust data from studies investigating these strategies are needed to definitely establish safety of local estrogen treatments [114].
Ospemifene is a systemic oral selective estrogen receptor modulator approved for the treatment of moderate/severe menopause-related dyspareunia and vaginal dryness; despite its efficacy in the general population, there are no safety data to support its use in survivors of BC [114].
Aqueous lidocaine compresses applied to the vulvar vestibule before vaginal penetration can effectively reduce dyspareunia and sexual distress in patients receiving endocrine therapy. Lidocaine might, therefore, be a useful temporary strategy for patients reporting insertion pain [115].
The effect of vaginal laser for genitourinary symptoms related to sexual dysfunction (such as vaginal dryness, dyspareunia, vaginal itching, and burning) remains a topic to be further investigated. Results of single-arm cohorts have reported improvement in vaginal atrophy and related symptoms [116–118]. However, in a recent randomized clinical trial, no significant improvement of overall vaginal symptoms after use of laser was observed [119]. Several additional trials are ongoing and will help to better place the role of vaginal lasers in this setting.
Injections of autologous platelet-rich plasma have also been reported to improve vaginal atrophy and sexual distress in survivors; however, additional evidence is needed before this approach can be considered in clinical practice [120].
Several trials have evaluated the role of cognitive behavioral therapy in survivors facing sexual dysfunction, reporting improvements in overall sexual function, sexual desire, arousal, and vaginal lubrication, as well as improvement in sexual pleasure, discomfort during sex, and sexual distress [121]. Telephone counseling intervention or in-person 6-week sexual life reframing programs (including interventions not only on psychological but also on physical aspects of sexual health) have also shown to have a positive effect on sexual dysfunction [122, 123]. On the basis of available evidence, cognitive behavioral therapy should be highly encouraged for survivors facing sexual dysfunction. Dedicated and experienced counseling in this regard should be made available to all patients facing these side effects.
Considering that there are no clear data on the safety of locoregional hormonal treatments in patients reporting sexual dysfunction during endocrine therapy, non-hormonal approaches should always be the first option for the management of genitourinary symptoms. However, pharmacological strategies using temporary application of low-dose local estrogens may be considered in the case of severe and refractory symptoms after careful discussion with the patient about the risk–benefit ratio.
Future research and education in the field of sexual dysfunction is needed. First, determinants and risk factors should be further elucidated using data from prospective studies, particularly to assess the impact of different surgical and reconstructive techniques on long-term outcomes of sexual health. Second, the approval of novel agents in the adjuvant setting could potentially modify incidence and severity of this toxicity; therefore, clinical trials should collect high-quality data to allow for adequate counseling. Third, personal and cultural barriers of patients and physicians might hamper appropriate management of sexual dysfunction; additional education and awareness are needed to quickly capture sexual dysfunction onset to address causes and promptly treat patients or refer them to gynecologists or sexologists. Finally, additional data regarding the safety of local hormonal treatments for patients diagnosed with hormone receptor-positive (HR+)/HER2-negative (HER2−) are needed, considering their efficacy when non-hormonal therapies are ineffective.
Well-Being Promotion
Weight Management
Approximately 50% of patients are overweight at BC diagnosis, and over 25% experience substantial weight gain (≥ 5% of baseline) by year 4 post-diagnosis [124, 125].
Clinicobehavioral factors for weight gain after diagnosis include younger age at diagnosis, lower pretreatment body mass index (BMI), receipt of chemotherapy, chemotherapy-induced menopause, reduced energy expenditure, and excess caloric intake [126–128].
BC treatments may also alter body composition [129] and lead to metabolic (e.g., reduction of adiponectin and elevation of leptin and resistin) and inflammatory (e.g., elevation in CRP, IL6, IL2, TNFα) alterations that could trigger or accelerate weight gain [130]. A genetic predisposition may also play a role in the risk of gaining weight, as previous data showed a link between SNPs in fat mass and obesity-associated protein gene (FTO), adiponectin gene (ADIPOQ), and its receptor (ADIPOR1) with obesity and BC [131, 132].
Several studies demonstrated a link between excess weight at diagnosis, weight gain after BC treatment, and poorer outcomes among survivors of early-stage BC [133]. Moreover, obesity and weight gain increase risk of cardiovascular disease, severe treatment-related sequelae, deterioration of QOL [134], and impaired social reintegration [135].
Additionally, the prognostic impact of BMI has been identified in several studies and most of them showed that being obese at any time (e.g., before, at, and after BC diagnosis) is associated with poorer prognosis in terms of recurrence rate, disease-specific, and overall survival [136–139].
The most extensive meta-analysis evaluated data from 82 studies and reported relative risks (RRs) of 1.41 (95% CI, 1.29–1.53) and 1.35 (95% CI, 1.24–1.47) for breast cancer-specific mortality and overall mortality, respectively, for patients who were obese at diagnosis compared with normal weight. Lower RRs were observed for patients who were overweight, who were still significantly at higher risk compared with those with normal weight. Furthermore, a linear relationship between BMI at diagnosis and outcomes was observed with RRs of 1.17 and 1.18 for total mortality and breast-cancer-specific mortality, respectively, for each 5-unit increment of baseline BMI. Similarly, weight gain after BC diagnosis was associated with higher risk of BC recurrence (HR 1.24, 95% CI, 1.00–1.53) and weight loss with lower risk (RR 0.67, 95% CI, 0.42–1.05), although these last data were not significant and not confirmed in other studies [140].
Overall, the role of intentional weight loss in overweight and obese survivors in improving oncological outcomes after BC diagnosis remains to be determined. The Breast cancer WEight Loss Study (BWEL, NCT02750826) is a clinical trial that randomized overweight and obese survivors of BC (baseline BMI ≥ 27 kg/m2) to a telephone-based intervention versus a health educational control to test the effect of weight loss on invasive disease-free survival (iDFS); accrual has been completed and results of the study will provide more information on this specific aspect [141].
Similarly to BWEL, several studies investigated the role of weight loss interventions based on behavioral and lifestyle changes to improve energy balance by reducing caloric intake and increasing physical activity and energy expenditure [142, 143]. Multimodal behavioral interventions are associated with the most significant and sustained weight loss, while also improving health-related outcomes and QOL [144]. Data from the CANTO cohort also confirmed that obese survivors of BC losing weight after primary treatment had lower odds of reporting dyspnea, breast symptoms, and deterioration in physical functioning compared with survivors with stable or increasing weight [125].
Recently, several studies suggested that digitally-delivered weight loss interventions, including both self-managed and provider-based solutions, are effective and safe for survivors of BC [145]. Nevertheless, despite the proven efficacy of weight management interventions, several limitations to their implementation exist, including high heterogeneity in adoption of behavioral weight loss strategies, overtime adherence, and long-term post-intervention maintenance of lifestyle changes [143].
Current guidelines state that survivors of BC should aim at achieving and maintaining a healthy weight through increased physical activity and healthy dietary habits, adopting recommendations from cancer prevention programs [17, 18, 146].
Future research in the field should focus on improving prediction of weight gain risk after BC, as well as integrating biological data, on patients’ stratification on the basis of adoption of weight loss interventions and on improving integration of digital solutions to deliver them and promote sustained behavioral change. Finally, the use of digital solutions may facilitate and accelerate research toward the use of body composition analysis, which might provide additional information on survival outcomes when compared with BMI evaluation [147].
Physical Activity
Survivors of BC should be encouraged and counseled toward adoption of a healthier lifestyle that includes at least 150 min per week of physical exercise including both aerobic and resistance training, with an ideal target of 300 min per week [18, 146, 148].
The beneficial effect of engaging in physical activity have been proven both during active treatment and over the course of the survivorship trajectory.
Active Treatment Phase
Exercise during active treatment is safe, although a medical evaluation could be considered to tailor training type and intensity based on individuals’ characteristics [149].
Patients with BC engaging in higher level of physical activity during cancer treatment experience improvement in cardiorespiratory fitness [150], physical function [151], and quality of life [152], and less severe treatment-related side effects including fatigue [153], anxiety and depression [152]; conversely, conflicting evidence exists on the impact of exercise in facilitating chemotherapy completion and maintaining chemotherapy dose intensity [154, 155].
Survivorship Trajectory
Consistent evidence suggests that higher levels of physical activity before and after diagnosis of BC are associated with improved survival outcomes.
A recent meta-analysis showed that pre-diagnosis level of physical activity has an inverse dose–response relationship with both breast-cancer-specific and overall mortality. Specifically, a significant reduction in breast cancer (HR 0.86, 95% CI, 0.78–0.94) and overall mortality (HR 0.82, 95% CI, 0.76–0.87) was observed in survivors with the highest levels of physical activity compared with the lowest. Additionally, this relationship was observed both for recreational (i.e., physical activity undertaken for the sake of exercise) and total physical activity (i.e., including occupational, household, and transportation physical activity) [156].
A positive association between improved clinical outcomes and appropriate levels of physical activity has been observed post-diagnosis and along the survivorship trajectory, with a higher magnitude of risk reduction compared with pre-diagnosis physical activity.
Friedenreich et al. conducted a meta-analysis investigating associations between risk of death and physical activity in cancer survivors. Among survivors of BC, the meta-analysis showed a significant reduction in breast cancer-specific mortality (HR 0.63, 95% CI, 0.50–0.78) and overall mortality (HR 0.58, 95% CI, 0.52–0.65) for the most active survivors, irrespective of BMI at diagnosis. Levels of physical activity showed a positive dose–response relationship with survival outcomes and risk of death showed a significant decline starting from 10 metabolic equivalent (MET) hours per week, which corresponds to approximately 3 hours of light walking per week, consistent with current recommendations [156].
A meta-analysis by Lee et al. specifically explored the association between recommended levels of physical activity and prognosis. Results of the study showed that survivors adhering to recommendations experienced a risk reduction of 21% and 28% in breast cancer-specific and all-cause mortality, respectively [157].
Although adherence to recommendations confers the greatest benefit, another meta-analysis showed that even low amounts of physical activity are associated with reduced mortality (HR 0.60, 95% CI, 0.50–0.69), although only three studies looked specifically at survivors of BC [158].
Several studies investigated the effect of reducing physical activity and of sedentary behavior after BC diagnosis. One meta-analysis of two prospective studies found a significant increase in the risk of all-cause mortality for survivors of BC who decreased their levels of physical activity after diagnosis (RR 2.36, 95% CI, 1.09–5.12) [157]. In one prospective cohort study including more than 8000 cancer survivors, total sedentary time, evaluated through the use of accelerometers, was associated with increased risk of cancer mortality when comparing survivors in the third (HR 1.52, 95% CI, 1.01–2.27) and second tertile (1.45, 95% CI, 1.00–2.11) with those in the first [159]. Similarly, in a prospective cohort of more than 1500 cancer survivors, self-reported levels of physical inactivity were associated with worse survival outcomes. Survivors sitting for more than 8 h/day, compared with those sitting less than 4 h, had higher risk of all-cause (HR 1.81, 95% CI, 1.05–3.14) and cancer-specific mortality (HR 2.27, 95% CI, 1.08–4.79) [160]. Finally, recent data from the CANTO cohort suggested an inverse association between levels of physical activity and QOL deterioration among survivors of BC who received adjuvant chemotherapy. Using data from more than 4000 survivors, patients were clustered in four distinct trajectories on the basis of self-reported QOL evaluated through the EORTC QLQ-C30 questionnaire: excellent (51.7%), very good (31.7%), deteriorating (10.0%), and poor (6.6%). Patients with deteriorating and poor QOL trajectories were more frequently non-adherent to physical activity recommendations and had reduced levels of physical activity over the survivorship trajectory [161].
Overall, considering the positive benefits associated with physical activity, survivors should be counseled on meeting current exercise recommendations and eventually referred to specific programs of adapted physical activity. Several limitations remain to be addressed in the field. The use of passively collected physical activity data through electronic devices (smartphones, smartwatches, and accelerometers) could improve reliability of findings compared with self-reported questionnaires on physical activity. Despite recommendations, the overall levels of physical activity in survivors of BC remain suboptimal. Data from the CANTO cohort showed that more than 30% of survivors were insufficiently active at years 1 and 2 after diagnosis [125], and several barriers explaining this low adherence to recommendations have been described [162, 163]. Future research should aim at designing tailored interventions that take into account patient preferences and individual characteristics as well as self-management solutions to improve adherence to recommendations.
Smoking and Alcohol Cessation
Smoking Cessation
Patients diagnosed with BC as well as survivors of BC who have an active smoking habit should be advised to avoid smoking and eventually be referred to smoking-cessation programs [17].
Active smokers have a significantly lower overall and breast cancer-specific survival (HR 1.33, 95% CI: 1.12–1.58) compared with former and never smokers [164]. Appropriate screening of survivors with a former or active smoking habit is fundamental considering that smoke is one of the main risk factors for a number of malignancies, including lung, esophagus, and head and neck, and that survivors with heavy smoking history could be referred to specific screening programs for lung cancer [165].
In addition to inferior oncological outcomes, smoking confers higher risk of several treatment-related complications including higher symptom burden during adjuvant chemotherapy, increased rate of surgical complications, worse physical, social, and emotional functioning, cognitive deterioration, sleep and mood alterations, and accelerated aging [36, 166–170]. Active smoking habit at diagnosis was associated with increased odds of reporting poor QOL at diagnosis, at 2 years, and 4 years after in a cohort of survivors of BC receiving adjuvant chemotherapy compared with never smokers (OR 1.82, 95% CI 1.49–2.22) [161]. Similarly, being a current or former smoker at diagnosis conferred increased odds of reporting severe pre-treatment fatigue at diagnosis and persistent severe fatigue at 2 and 4 years thereafter compared with never smokers (OR 1.65, 95% CI 1.26–2.15); interestingly, smoking status emerged as a relevant risk factor not only for global fatigue, but also for the physical, emotional, and cognitive dimensions [54].
Despite the existence of public policy in place to reduce smoking in the general population and of specific recommendations regarding smoking cessation in survivors of BC, approximately 15–20% of patients are active smokers at diagnosis and around 10% of them maintain this unhealthy habit along the survivorship trajectory [125, 171, 172]. Furthermore, when looking specifically at smoking discontinuation rates after BC diagnosis, the available evidence consistently identifies a proportion of survivors who continue smoking, and some studies reported higher prevalence of smoking habits at longer follow-up [172]. Increased awareness among oncologists and primary care physicians in referring patients to smoking-cessation programs are needed.
Alcohol Cessation
The latest World Cancer Research Fund and American Institute for Cancer Research (WCRF/AICR) summary report identified alcohol consumption as a significant risk factor for BC development, in both pre- and post-menopausal women, and a significant dose–response association was identified [173]. Current dietary recommendations for survivors of BC suggest that, similarly to what is recommended for cancer prevention, alcohol consumption should be avoided or at least limited [146].
Evidence regarding the association between alcohol intake and BC outcomes is inconsistent. Higher pre-diagnosis alcohol consumption was associated with increased risk of BC recurrences in two studies [174]. Only one study reported an association between higher post-diagnosis intake (≥ 6 g/day versus no consumption) and recurrence rate (HR 1.35, 95% CI, 1.00–1.83) [175]. After pooling results from two other studies, no association between consumption ≥ 6 g/day and recurrence was observed in the overall population, but a significant risk increase was observed among women who were postmenopausal (HR 1.19, 95% CI, 1.01–1.40) [174]. When looking specifically at the risk of late recurrences (i.e., ≥ 5 years from diagnosis) an increased risk of recurrence was observed among women consuming at least one drink per day compared with non-drinkers (HR 1.28, 95% CI, 1.01–1.62) [136]. No significant association between higher alcohol intake and overall mortality was observed [136, 176].
Unhealthy alcohol consumption appears to be prevalent in survivors of BC, reported by approximately 10% of patients at diagnosis and persisting in the vast majority of them along the survivorship trajectory [125, 177]. Factors associated with persistent unhealthy behavior include older age, depression, higher education, and partnered status [125].
Frequent alcohol consumption has been linked with increased risk of developing second non-breast cancers [173] as well as higher incidence of cardiometabolic diseases and obesity [178], osteoporosis [179], vasomotor symptoms, and poor sleep quality [180]. Furthermore, higher alcohol consumption frequently clusters with other unhealthy behaviors, including smoking habits [181], suggesting the need for additional health education in a subset of patients persisting in multiple unhealthy behaviors that might negatively impact outcomes over the survivorship trajectory.
Surveillance for Recurrences
Survivors of BC are at risk of developing local, regional, and distant recurrences as well as second primary cancers. Consequently, surveillance for early diagnosis and treatment of these recurrence events is a fundamental domain of survivorship care.
Current guidelines recommend a follow-up protocol based on physical examination, yearly mammograms, and additional tests based on previous and ongoing medical treatments (e.g., echocardiogram for previous anthracycline-based chemotherapy and/or anti-HER2 therapy exposure or laboratory tests to monitor endocrine therapy side effects). Imaging tests are indicated only to investigate symptoms potentially related to recurrence [56, 182]. This approach is supported by trials and meta-analyses that demonstrated no survival benefit from systematic imaging testing to identify recurrences in survivors of BC [183]. A recent study showed that asymptomatic imaging positively impact on BC death only in the HER2+ and triple-negative subtypes, while no benefit was seen in the HR+ subgroup [184]. Patterns of relapse over time vary significantly according to BC subtype and for the HR+ subtype it is constant over time with late relapses occurring up to 20 years from diagnosis [185].
New surveillance strategies to identify recurrences while reducing radiation exposure are needed to improve outcomes of BC survivors.
ctDNA
Recently, detection of circulating tumor DNA (ctDNA) has emerged as a potential tool to personalize adjuvant treatments and surveillance strategies. ctDNA refers to the part of circulating cell-free DNA (cfDNA) originating from tumor tissue that can be identified in several biological fluids, although the most used is blood [186].
Currently, administration of adjuvant treatment after surgery is based on tumor–node–metastasis (TNM) staging, additional histopathological risk factors and results of genomic signatures. Identification of ctDNA after surgery (frequently referred to as molecular residual disease, MRD) might help stratify patients according to the expected risk of recurrence and thus potentially allow for the personalization of adjuvant therapies using (de)-escalation approaches. This approach was recently tested in a randomized clinical trial in stage II colon cancer and showed safety of a ctDNA-based approach to guide decision in adjuvant treatment without increasing risk of recurrence [187].
In early BC, detection of ctDNA after surgery has shown ability to predict risk of relapse and survival outcomes after surgery and (neo)adjuvant chemotherapy, both using a single timepoint and with serial monitoring [188–192]. Interestingly, ctDNA identification appears to retain predictive value for late recurrences as well. In a recent study, patients with HR+ stage II–III BC free from recurrence at least 5 years from diagnosis underwent serial evaluation of ctDNA with a personalized assay based on the genomic landscape of the primary tumor. The study demonstrated that detection of MRD predicted development of distant metastasis with a median diagnostic anticipation of 1 year [193].
Recently, results of the phase II multicenter c-TRAK-TN (NCT03145961) study have been reported. The study enrolled 208 patients who completed treatment for triple-negative breast cancer and started thrice monthly ctDNA-based surveillance. Rate of MRD at 12 months, the primary endpoint of the study, was 27.3%, and 72% of the patients already had metastatic disease at time of ctDNA positivity [194]. These results will be fundamental to design future studies, considering that several clinical trials (Table 1) are evaluating the use of ctDNA to inform surveillance protocols and de-escalating or escalating adjuvant treatments on the basis of MRD identification.
Table 1.
Ongoing clinical studies testing ctDNA-based surveillance and treatment strategies in early-stage breast cancer
| Trial number | No. of patients | Setting | Timing of ctDNA test | Primary endpoint | Study intervention/objective |
|---|---|---|---|---|---|
| NCT05058183 | 400 | Adjuvant—Stage I HER2+ and TNBC | Before and after surgery | Incidence of pts with positive ctDNA after surgery | Chemotherapy de-escalation in case of negative ctDNA |
| NCT04768426 | 25 | Adjuvant—TNBC receiving adjuvant capecitabine | Baseline and 6 months | Detection of ctDNA at baseline and 6 months | Identification of patients not benefiting from capecitabine |
| NCT04530890 | 1000a | Adjuvant and neoadjuvant | Start of treatment, during treatment, at progression | Prognostic impact of ctDNA on mortality | Evaluation of the prognostic role of ctDNA on risk of death |
| NCT04567420 | 100 | Adjuvant—Stage II–III HR+/HER2− BC at high risk of relapse | Every 4/6 months concomitant to follow-up visits | ctDNA positivity and RFS | Patients receiving adjuvant endocrine treatment with ctDNA positivity will be randomized to fulvestrant and palbociclib or to continue standard of care treatment |
| NCT03357120 | 180 | Surveillance after neoadjuvant chemotherapy | After surgery and every 6 months thereafter for 5 years | Prognostic impact on RFI at 3 years | NA |
| NCT04985266 (TRAK-ER) | 1100 | Adjuvant—HR+/HER2− at high risk of relapse | Every 3 months for up to 3 years | Incidence of ctDNA detection during surveillance and RFS | Patients with ctDNA detection under endocrine therapy and without evidence of metastasis will be randomized to fulvestrant and palbociclib or standard endocrine therapy |
| NCT04501523 (Apollo) | 460 | Adjuvant—TNBC with and without RD | Baseline, after NACT, and after surgery | 5-year DFS | Patients with ctDNA detection will be randomized to adjuvant therapy with capecitabine ± tislelizumab |
| NCT04849364 (PERSEVERE) | 197 | Adjuvant—TNBC with RD | After surgery | 2-year DFS | Patients with residual disease after neoadjuvant chemotherapy and with ctDNA detection will be randomized to different treatments on the basis of identification of genomic targets |
| NCT05512364 (EORTC – Treat ctDNA) | 220 | Adjuvant—Stage IIB and III HR+/HER2− | Baseline and every 6 months for up to 3 years | DMFS | Patients with ctDNA detection under endocrine therapy and without evidence of metastasis will be randomized to elacestrant or standard endocrine therapy |
| NCT03709134 | 100 | Neoadjuvant | NA | pCR | Determination of the prognostic and predictive role of genomic markers |
| NCT04803539 (Artemis) | 260 | Adjuvant—TNBC | After surgery and/or adjuvant chemotherapy | iDFS | Patients with positive ctDNA will be randomized to capecitabine ± camrelizumab + apatinib |
| NCT05433753 (Harmony) | 60 | Surveillance—Stage IIA–IIIC HER2+ | NA | iDFS | Identify a relation between ctDNA detection and recurrence |
| NCT03285412 (Leader) | 120 | Adjuvant—HR+/HER2− | Baseline | ctDNA clearance | Patients with ctDNA detection will receive ribociclib in addition to standard adjuvant endocrine therapy |
| NCT04915755 (ZEST) | 800 | Adjuvant—TNBC and HER2− BC with BRCA 1/2 mutations | Longitudinal monitoring of ctDNA after surgery or adjuvant treatment | DFS | Patients with ctDNA detection will be randomized to treatment with niraparib for 3 years or placebo |
| NCT05388149 | 15 | Adjuvant—Stage I–III HER2+ | After 2–6 cycles of T-DM1 | ctDNA clearance | Patients with ctDNA detection will receive adjuvant neratinib and T-DM1 |
| NCT04434040 (ASPRIA) | 40 | Adjuvant—TNBC with residual disease | After completion of all local and neoadjuvant therapies | ctDNA clearance after 18 weeks | Patients with ctDNA detection will receive adjuvant atezolizumab and sacituzumab govitecan |
ctDNA circulating tumor DNA, TNBC triple-negative breast cancer, pCR pathological complete response, RFS relapse-free survival, RFI relapse-free interval, RD residual disease, NACT neoadjuvant chemotherapy, DFS disease-free survival, DMFS distant metastasis-free survival, iDFS invasive disease-free survival
aIncluding patients with digestive and gynecological malignancies as well
Brain Metastases
Brain metastases negatively impact the prognosis of BC: expected survival ranges from 6–36 months, depending on several prognostic factors including age, performance status, number of brain metastases, molecular subtype, and presence of concomitant extra-cranial disease [195, 196].
Despite the prognostic implications, the high risk of their development in specific subgroups, and the current availability of effective local and systemic treatments, imaging to detect brain metastasis is recommended only in the presence of symptoms suggestive of central nervous system (CNS) involvement, both at staging and during surveillance [56, 182].
Main factors associated with the risk of developing brain metastasis include disease stage and type of metastatic involvement, molecular subtype, and age. In metastatic BC, 15% of patients present with brain metastases at diagnosis; in patients without CNS involvement at diagnosis, 25% will subsequently develop brain metastases after a median of 2–3 years; conversely, in early BC, the estimated incidence of brain metastases is around 3% [197, 198]. Subtype is a major risk factor for brain metastasis development. In a cohort of 1434 patients treated with curative intent, brain metastases were observed in 2.5% of patients overall; however, the risk of brain metastasis at 10 years was highly dependent on BC subtype, ranging from 0.7% in low-intermediate grade HR+/HER2− to 7% in HR−/HER2− and 12% in the HR−/HER2+ subtypes [199]. Similar results are observed in advanced disease where 15%, 30%, and 50% of patients with HR+ , HR−/HER2−, and HR−/HER2+ subtypes are expected to develop brain metastases, respectively [200].
In the HER2+ subtype, brain metastases frequently represent the first site of relapse, even in the absence of extracranial metastases. Among 3400 women randomized in the HERA trial to receive adjuvant trastuzumab or no anti-HER2 therapy [201], CNS involvement as first site of recurrence was around 2% in both treatment arms at 4 years of follow-up [202]. The KATHERINE trial in patients with residual disease after neoadjuvant chemotherapy demonstrated no differences in brain metastasis rate between patients receiving trastuzumab or T-DM1 (~ 5% in both arms); CNS was the only site of recurrence in 4.8% of patients in the T-DM1 arm versus 2.8% with trastuzumab [203].
Whether the increased risk of brain metastases in patients with HER2+ is due to improved disease control leading to increased survival, to a biological predisposition of HER2+ clones for the brain, or to poor penetration of monoclonal antibodies across the blood–brain barriers is still a matter of debate [204].
Molecular structure of tyrosine-kinase inhibitors (TKI) allows for better penetration in the CNS. However, in the ALTTO trial, investigating the addition of lapatinib to trastuzumab as adjuvant treatment for HER2+ disease, CNS involvement as first site of relapse (~ 2%) was identical between patients treated with lapatinib plus trastuzumab or trastuzumab alone [205].
The ExteNET trial evaluated the role of adjuvant neratinib after adjuvant chemotherapy and trastuzumab. At the final report with a median 8 years of follow-up, incidence of brain metastases was slightly lower in the neratinib arm (1.3%) compared with the placebo (1.8%) [206].
Risk of CNS involvement for patients diagnosed with HR−/HER2− BC is highly dependent on stage at diagnosis. Approximately 3%, 5%, and 10% of patients diagnosed with stage I, II, and III, respectively, will develop brain metastases as first site of recurrence at 5 years from diagnosis, and the cumulative incidence of brain metastases over the natural history ranges between 25% and 45% [199]. Interestingly, in the TNBC subtype, occurrence of brain metastases is usually associated with systemic disease progression [207].
TNBC is frequently associated with germline pathogenic variants (PVs) in the BRCA genes; however, whether harboring a PV increases risk of brain metastasis is debated as conflicting results have been reported in the literature, mainly from retrospective cohorts [208–210]. Recently, the PARP inhibitor olaparib has been approved in TNBC and HR+/HER2− breast cancer as adjuvant treatment for BRCA carriers with high-risk disease after (neo-)adjuvant chemotherapy. Olaparib significantly improved DFS and OS in the intention-to-treat population, and a lower rate of brain metastases as first site of recurrence was observed in patients treated with olaparib (2.6%) compared with placebo (4.2%) [10, 211].
Overall, future research in this field should be focused on refined identifications of patients at risk of developing brain metastases, development of personalized surveillance protocols, identification and integration of novel biomarkers for CNS disease, and assessment of the potential protective role of novel agents that reported high efficacy data on CNS involvement in the metastatic setting (e.g., tucatinib and trastuzumab deruxtecan in the HER2+ setting).
Hereditary Cancer Syndromes
Approximately 10% of all diagnosed BC cases are related to HBOC, defined by the presence of a suggestive family history and identification of germline PVs in genes that increase the risk of developing breast and/or ovarian cancer [75, 212, 213]. According to the increased risk of tumor development, high- (e.g., BRCA1, BRCA2, PALB2, TP53) and moderate- (e.g., ATM, CHECK2, RAD51C, RAD51D) risk genes have been identified [214, 215].
Identification of PVs associated with HBOC holds several medical and psychological implications for patients, survivors, and unaffected relatives. The main aspects related to identification of HBOC include: tailored medical treatments in BRCA1/2 carriers and risk management of breast and ovarian cancer as well as of other malignancies based on the identified PV, management of estrogen-deprivation associated sequelae after risk-reducing bilateral salpingo-oophorectomy (RRBSO), fertility, and psychological issues [75].
Risk management strategies for BC in PV carriers include bilateral risk-reducing mastectomy (BRRM), risk-reducing medications, and intensified screening [75]. Adequate counseling for PV carriers is fundamental when HBOC is identified, and should include appropriate discussion regarding individualized risk and physical, psychological, and social consequences of each risk-reducing strategy [75].
The most effective strategy for BC risk management is represented by BRRM [216]. Radical risk-reducing surgery can be proposed to all carriers of PVs in high-risk genes, although individual risk assessment should be carefully weighed against the risk and complications to assure informed decision-making [217, 218]. The only effective strategy available for risk management of ovarian cancer is RRBSO [219, 220]. There is no effective screening strategy for ovarian cancer in PV carriers, although current guidelines recommend transvaginal ultrasound and Ca125 testing every 6 months in patients not receiving RRBSO [75].
Intensified screening has been evaluated in the context of retrospective studies that proved its efficacy in early identification of BC, improved outcomes [221], and cost-effectiveness [222].
Adequate planning of intensified screening should include: age of screening onset (based on age at diagnosis in the youngest affected family member), screening intervals (based on the HBOC gene identified), and imaging modality [75].
All international guidelines indicate that surveillance of carriers harboring PVs in high-risk genes should include magnetic resonance imaging (MRI) [223, 224], but screening intervals and duration vary significantly across guidelines [75, 225, 226]. Annual MRI among carriers of high-risk PVs between 30 and 49 years of age is recommended by all guidelines [75, 225, 226]. Extension of MRI screening after 50 years is more debated as some guidelines indicate that annual mammography could be sufficient as surveillance technique with the exclusion of carriers with dense breast tissue [225], while others indicate the need for continued annual MRI surveillance as long as carriers are in good health status [227], since breast density is not the only determinant of lower sensitivity of imaging techniques [75, 226].
Recent data suggest the potential interest of further intensifying surveillance in BRCA1 carriers using MRI every 6 months [228] considering the aggressive biology and rapid growth rates of BC associated with PVs in this gene.
Future research in this field should be directed toward improved and individualized risk definition, potentially using single nucleotide polymorphisms (SNPs) in the context of polygenic risk scores (PRS) to further refine surveillance and risk-reducing strategies [229, 230]. Application of liquid biopsy in patients with PVs might be highly relevant for early detection of malignancies that are diagnosed in advanced stage in the interval between screening exams (e.g., pancreatic cancer) or without reliable screening strategies (e.g., ovarian cancer). However, the diagnostic accuracy of these techniques is not optimal, false positives are common, and these strategies should be further validated before introduction in clinical practice [231].
Discussion and Future Perspectives
Long-term prognosis of BC has significantly improved in the last 40 years. However, alongside the striking improvement in clinical outcomes, treatments for BC have a substantial risk of long-term physical and psychosocial sequelae that negatively impact QoL.
Several barriers to delivering high-quality survivorship care exist, including lack of awareness among physicians on the long-term consequences of BC treatments and their management, shortage of human and financial resources to refer survivors toward supportive care and behavioral interventions, and lack of a comprehensive and multidisciplinary approach.
Future research should aim at upfront identification of the multifactorial and multidimensional determinants of long-term sequelae to move toward more personalized surveillance and management strategies. Lack of high-quality, multimodal, and prospective data still represent a significant limitation in the field and reinforce the need for a collaborative research effort to improve the quality of survivorship research, particularly in the strengthening of methodological quality and analytic standards for QoL studies, but also in collecting high-quality, multimodal data that integrate multiple sources and intersect biology data with clinical and patient-reported ones. Contemporary longitudinal cohorts of survivors of BC such as CANTO (NCT01993498) [53] and the Young Women’s Breast Cancer Study (YWS, NCT01468246) are examples of real-world efforts that integrate biological, clinical, and patient-reported data to address these limitations.
Understanding determinants of long-term sequelae could also help accelerate interventional research in the field by developing novel approaches for long-lasting toxicities with more limited management options such as cognitive dysfunction and neuropathy.
Additionally, interventional research in the field should be focused on evaluating the efficacy of lifestyle interventions on the basis of behavioral change in mitigating long-term sequelae of BC treatment in underrepresented populations, including carriers of PVs, and on implementing coordinated research efforts to test in a reliable and high-quality framework the potential benefit of non-pharmacological strategies, including complementary and alternative medicine solutions.
Research in the field should also focus on determining the feasibility, acceptability, and efficacy of different models of delivering survivorship care on the basis of patients’ individual needs and ability to self-manage. Self-management involves behavioral change and ability to apply different tasks that pertain to medical, emotional, and role management, and is an essential pillar in the management of several chronic diseases [232, 233]; it could be appropriate for cancer survivors as well, although less evidence on the topic is available in this setting [145]. Testing the implementation of novel models of care in empowering cancer survivors to self-manage the consequences of treatments over the course of the survivorship trajectory could improve health outcomes and optimize the use of resources [234]. However, ability to self-manage is strictly dependent on self-efficacy, defined as the patient belief in their capacity to self-manage, and on health literacy. Future study should stratify patients on the basis of these characteristics to assess whether personalized models of care can be implemented in clinical practice.
Development of digital health solutions has increased steadily in recent years and some of these solutions have also been evaluated in survivors of BC [235], proving effective in the management of several symptoms including fatigue, menopausal symptoms, sexual dysfunction, weight gain, cognitive dysfunction, and emotional distress [121, 236–241]. Digital health solutions could allow continuous assessment over time, intercepting patients with deteriorating symptoms or quality of life that could then be referred to digitally delivered self-management interventions, in-person interventions, or to a medical evaluation, on the basis of severity of deterioration.
Conclusions
Future research in the survivorship field should aim at better defining the needs and psychosocial profile of cancer survivors in terms of risk of long-term toxicities, adoption of behavioral and digital interventions, self-efficacy in managing consequences of cancer care, and health literacy. These research efforts would ultimately allow for the development and implementation of personalized care models able to simultaneously tackle consequences of cancer care and optimize resources by providing the best framework and tools based on survivors’ characteristics.
Acknowledgements
Matteo Lambertini acknowledges the Italian Association for Cancer Research (“Associazione Italiana per la Ricerca sul Cancro,” AIRC; MFAG 2020 ID 24698) for supporting his research in the field of breast cancer in young women and oncofertility.
Funding
No funding or sponsorship was received for this study or publication of this article.
Author Contributions
Conceptualization: Davide Soldato, Luca Arecco, Matteo Lambertini. Writing—Original draft preparation: Davide Soldato, Luca Arecco, Matteo Lambertini. Writing—Review and editing: all authors. Supervision: Matteo Lambertini.
Disclosures
Davide Soldato, Luca Arecco, Maria Alice Franzoi, Elene Mariamidze, Salome Begijanashvili, Nicole Brunetti, Stefano Spinaci, Cinzia Solinas, Ines Vaz-Luis, and Antonio Di Meglio declare that they have no competing interests. Elisa Agostinetto received consultancy fees/honoraria from Eli Lilly, Sandoz, AstraZeneca, and support for attending medical conferences from Novartis, Roche, Eli Lilly, Genetic, Istituto Gentili, Daiichi Sankyo (all outside the submitted work). Matteo Lambertini reports advisory role for Roche, Lilly, Novartis, Astrazeneca, Pfizer, Seagen, Gilead, MSD, and Exact Sciences and speaker honoraria from Roche, Lilly, Novartis, Pfizer, Sandoz, Libbs, Daiichi Sankyo, and Takeda, travel grants from Gilead and research support (to the institution) from Gilead outside the submitted work.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
- 1.Giaquinto AN, Sung H, Miller KD, Kramer JL, Newman LA, Minihan A, et al. Breast cancer statistics, 2022. CA Cancer J Clin [Internet]. 2022 Oct 3 [cited 2022 Oct 22];caac.21754. 10.3322/caac.21754.
- 2.World Health Organization. Global Cancer Observatory. International agency for research on cancer. 2020. International Agency for Research on cancer, World Health Organization. Available at https://gco.iarc.fr/. [Internet]. [cited 2022 Oct 22]. Available from http://gco.iarc.fr/today/home.
- 3.Bluethmann SM, Mariotto AB, Rowland JH. Anticipating the “Silver Tsunami”: prevalence trajectories and co-morbidity burden among older cancer survivors in the United States. Cancer Epidemiol Biomarkers Prev. 2016;25(7):1029–1036. doi: 10.1158/1055-9965.EPI-16-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Islami F, Goding Sauer A, Miller KD, Siegel RL, Fedewa SA, Jacobs EJ, et al. Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States: potentially preventable cancers in US. CA Cancer J Clin. 2018;68(1):31–54. doi: 10.3322/caac.21440. [DOI] [PubMed] [Google Scholar]
- 5.Berry DA, Cronin KA, Plevritis SK, Fryback DG, Clarke L, Zelen M, et al. Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med. 2005;353(17):1784–1792. doi: 10.1056/NEJMoa050518. [DOI] [PubMed] [Google Scholar]
- 6.Abe O, Abe R, Enomoto K, Kikuchi K, Koyama H, Masuda H, et al. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365(9472):1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 7.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100 000 women in 123 randomised trials. Lancet. 2012;379(9814):432–444. doi: 10.1016/S0140-6736(11)61625-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Agostinetto E, Gligorov J, Piccart M. Systemic therapy for early-stage breast cancer: learning from the past to build the future. Nat Rev Clin Oncol. 2022;19(12):763–774. doi: 10.1038/s41571-022-00687-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.von Minckwitz G, Huang CS, Mano MS, Loibl S, Mamounas EP, Untch M, et al. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N Engl J Med. 2019;380(7):617–628. doi: 10.1056/NEJMoa1814017. [DOI] [PubMed] [Google Scholar]
- 10.Geyer CE, Garber JE, Gelber RD, Yothers G, Taboada M, Ross L, et al. Overall survival in the OlympiA phase III trial of adjuvant olaparib in patients with germline pathogenic variants in BRCA1/2 and high risk, early breast cancer. Ann Oncol. 2022;33(12):1250–1268. doi: 10.1016/j.annonc.2022.09.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harbeck N, Rastogi P, Martin M, Tolaney SM, Shao ZM, Fasching PA, et al. Adjuvant abemaciclib combined with endocrine therapy for high-risk early breast cancer: updated efficacy and Ki-67 analysis from the monarchE study. Ann Oncol. 2021;32(12):1571–1581. doi: 10.1016/j.annonc.2021.09.015. [DOI] [PubMed] [Google Scholar]
- 12.Schmid P, Cortes J, Dent R, Pusztai L, McArthur H, Kümmel S, et al. Event-free survival with pembrolizumab in early triple-negative breast cancer. N Engl J Med. 2022;386(6):556–567. doi: 10.1056/NEJMoa2112651. [DOI] [PubMed] [Google Scholar]
- 13.Delaloge S, Cella D, Ye Y, Buyse M, Chan A, Barrios CH, et al. Effects of neratinib on health-related quality of life in women with HER2-positive early-stage breast cancer: longitudinal analyses from the randomized phase III ExteNET trial. Ann Oncol. 2019;30(4):567–574. doi: 10.1093/annonc/mdz016. [DOI] [PubMed] [Google Scholar]
- 14.Rugo HS, O’Shaughnessy J, Boyle F, Toi M, Broom R, Blancas I, et al. Adjuvant abemaciclib combined with endocrine therapy for high-risk early breast cancer: safety and patient-reported outcomes from the monarchE study. Ann Oncol. 2022;33(6):616–627. doi: 10.1016/j.annonc.2022.03.006. [DOI] [PubMed] [Google Scholar]
- 15.Dent RA, Cortés J, Pusztai L, McArthur HL, Kuemmel S, Bergh J, et al. 135MO HRQoL with neoadjuvant pembrolizumab + chemotherapy vs placebo + chemotherapy, followed by adjuvant pembrolizumab vs placebo for early-stage TNBC: results from KEYNOTE-522. Ann Oncol. 2022;33:S600–S601. doi: 10.1016/j.annonc.2022.07.170. [DOI] [Google Scholar]
- 16.Nekhlyudov L, Mollica MA, Jacobsen PB, Mayer DK, Shulman LN, Geiger AM. Developing a quality of cancer survivorship care framework: implications for clinical care, research, and policy. JNCI J Natl Cancer Inst [Internet]. 2019;111(11):1120–1130. doi: 10.1093/jnci/djz089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Runowicz CD, Leach CR, Henry NL, Henry KS, Mackey HT, Cowens-Alvarado RL, et al. American Cancer Society/American Society of Clinical Oncology Breast Cancer Survivorship Care Guideline. J Clin Oncol. 2016;34(6):611–635. doi: 10.1200/JCO.2015.64.3809. [DOI] [PubMed] [Google Scholar]
- 18.National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines. Survivorship (Version 1.2022). Available at https://www.nccn.org/professionals/physician_gls/pdf/survivorship.pdf.
- 19.Vaz-Luis I, Masiero M, Cavaletti G, Cervantes A, Chlebowski RT, Curigliano G, et al. ESMO expert consensus statements on cancer survivorship: promoting high-quality survivorship care and research in Europe. Ann Oncol. 2022;33(11):1119–1133. doi: 10.1016/j.annonc.2022.07.1941. [DOI] [PubMed] [Google Scholar]
- 20.Europe’s Beating Cancer Plan Communication from the commission to the European Parliament and the Council. European Commission. Available at https://health.ec.europa.eu/publications/europes-beating-cancer-plan_en.
- 21.Ferreira AR, Di Meglio A, Pistilli B, Gbenou AS, El-Mouhebb M, Dauchy S, et al. Differential impact of endocrine therapy and chemotherapy on quality of life of breast cancer survivors: a prospective patient-reported outcomes analysis. Ann Oncol. 2019;30(11):1784–1795. doi: 10.1093/annonc/mdz298. [DOI] [PubMed] [Google Scholar]
- 22.Pistilli B, Paci A, Ferreira AR, et al. Serum detection of nonadherence to adjuvant tamoxifen and breast cancer recurrence risk. J Clin Oncol. 2020;38:2762–2772. doi: 10.1200/JCO.19.01758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bower JE. Cancer-related fatigue—mechanisms, risk factors, and treatments. Nat Rev Clin Oncol. 2014;11(10):597–609. doi: 10.1038/nrclinonc.2014.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poulson MJ. Not just tired. J Clin Oncol. 2001;19(21):4180–4181. doi: 10.1200/JCO.2001.19.21.4180. [DOI] [PubMed] [Google Scholar]
- 25.Andrykowski MA, Donovan KA, Laronga C, Jacobsen PB. Prevalence, predictors, and characteristics of off-treatment fatigue in breast cancer survivors. Cancer. 2010;116(24):5740–5748. doi: 10.1002/cncr.25294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cella D, Lai JS, Chang CH, Peterman A, Slavin M. Fatigue in cancer patients compared with fatigue in the general United States population. Cancer. 2002;94(2):528–538. doi: 10.1002/cncr.10245. [DOI] [PubMed] [Google Scholar]
- 27.Abrahams HJG, Gielissen MFM, Schmits IC, Verhagen CAHHVM, Rovers MM, Knoop H. Risk factors, prevalence, and course of severe fatigue after breast cancer treatment: a meta-analysis involving 12,327 breast cancer survivors. Ann Oncol. 2016;27(6):965–974. doi: 10.1093/annonc/mdw099. [DOI] [PubMed] [Google Scholar]
- 28.Bower JE, Ganz PA, Desmond KA, Bernaards C, Rowland JH, Meyerowitz BE, et al. Fatigue in long-term breast carcinoma survivors. Cancer. 2006;106(4):751–758. doi: 10.1002/cncr.21671. [DOI] [PubMed] [Google Scholar]
- 29.Bower JE, Ganz PA, Desmond KA, Rowland JH, Meyerowitz BE, Belin TR. Fatigue in breast cancer survivors: occurrence, correlates, and impact on quality of life. J Clin Oncol. 2000;18(4):743–753. doi: 10.1200/JCO.2000.18.4.743. [DOI] [PubMed] [Google Scholar]
- 30.Ferreira AR, Di Meglio A, Pistilli B, Gbenou AS, El-Mouhebb M, Everhard S, et al. Differential impact of endocrine therapy (ET) and chemotherapy (CT) on quality of life (QoL) of 4,262 breast cancer (BC) survivors: a prospective patient-reported outcomes (PRO) analysis. J Clin Oncol. 2019;37(15 suppl):512. doi: 10.1200/JCO.2019.37.15_suppl.512. [DOI] [PubMed] [Google Scholar]
- 31.Collado-Hidalgo A. Inflammatory biomarkers for persistent fatigue in breast cancer survivors. Clin Cancer Res. 2006;12(9):2759–2766. doi: 10.1158/1078-0432.CCR-05-2398. [DOI] [PubMed] [Google Scholar]
- 32.Bower JE, Ganz PA, Irwin MR, Arevalo JMG, Cole SW. Fatigue and gene expression in human leukocytes: Increased NF-κB and decreased glucocorticoid signaling in breast cancer survivors with persistent fatigue. Brain Behav Immun. 2011;25(1):147–150. doi: 10.1016/j.bbi.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Orre IJ, Reinertsen KV, Aukrust P, Dahl AA, Fosså SD, Ueland T, et al. Higher levels of fatigue are associated with higher CRP levels in disease-free breast cancer survivors. J Psychosom Res. 2011;71(3):136–141. doi: 10.1016/j.jpsychores.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 34.Bower JE, Ganz PA, Tao ML, Hu W, Belin TR, Sepah S, et al. Inflammatory biomarkers and fatigue during radiation therapy for breast and prostate cancer. Clin Cancer Res. 2009;15(17):5534–5540. doi: 10.1158/1078-0432.CCR-08-2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Geinitz H, Zimmermann FB, Stoll P, Thamm R, Kaffenberger W, Ansorg K, et al. Fatigue, serum cytokine levels, and blood cell counts during radiotherapy of patients with breast cancer. Int J Radiat Oncol. 2001;51(3):691–698. doi: 10.1016/S0360-3016(01)01657-1. [DOI] [PubMed] [Google Scholar]
- 36.Carroll JE, Bower JE, Ganz PA. Cancer-related accelerated ageing and biobehavioural modifiers: a framework for research and clinical care. Nat Rev Clin Oncol. 2022;19(3):173–187. doi: 10.1038/s41571-021-00580-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee S, Deasy JO, Oh JH, Di Meglio A, Dumas A, Menvielle G, et al. Prediction of breast cancer treatment–induced fatigue by machine learning using genome-wide association data. JNCI Cancer Spectr. 2020;4(5):pkaa039. doi: 10.1093/jncics/pkaa039/5835872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bower JE, Bak K, Berger A, Breitbart W, Escalante CP, Ganz PA, et al. Screening, assessment, and management of fatigue in adult survivors of cancer: an American Society of Clinical Oncology clinical practice guideline adaptation. J Clin Oncol. 2014;32(17):1840–1850. doi: 10.1200/JCO.2013.53.4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.National Comprehensive Cancer Network (NCCN) Clinical practice guidelines. Cancer-related fatigue version 2.2022. Available at https://www.nccn.org/professionals/physician_gls/pdf/fatigue.pdf.
- 40.Cramp F, Byron-Daniel J. Exercise for the management of cancer-related fatigue in adults. New Jersey: John Wiley and Sons Ltd; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Patel AV, Friedenreich CM, Moore SC, Hayes SC, Silver JK, Campbell KL, et al. American College of Sports Medicine roundtable report on physical activity, sedentary behavior, and cancer prevention and control. Med Sci Sports Exerc. 2019;51(11):2391–2402. doi: 10.1249/MSS.0000000000002117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brown JC, Huedo-Medina TB, Pescatello LS, Pescatello SM, Ferrer RA, Johnson BT. Efficacy of exercise interventions in modulating cancer-related fatigue among adult cancer survivors: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2011;20(1):123–133. doi: 10.1158/1055-9965.EPI-10-0988. [DOI] [PubMed] [Google Scholar]
- 43.Speck RM, Courneya KS, Mâsse LC, Duval S, Schmitz KH. An update of controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. J Cancer Surviv. 2010;4(2):87–100. doi: 10.1007/s11764-009-0110-5. [DOI] [PubMed] [Google Scholar]
- 44.Duijts SFA, Faber MM, Oldenburg HSA, Van Beurden M, Aaronson NK. Effectiveness of behavioral techniques and physical exercise on psychosocial functioning and health-related quality of life in breast cancer patients and survivors-a meta-analysis. Psychooncology. 2011;20:115–126. doi: 10.1002/pon.1728. [DOI] [PubMed] [Google Scholar]
- 45.Bower JE, Partridge AH, Wolff AC, Thorner ED, Irwin MR, Joffe H, et al. Targeting depressive symptoms in younger breast cancer survivors: the pathways to wellness randomized controlled trial of mindfulness meditation and survivorship education. J Clin Oncol. 2021;39(31):3473–3484. doi: 10.1200/JCO.21.00279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Greenlee H, DuPont-Reyes MJ, Balneaves LG, Carlson LE, Cohen MR, Deng G, et al. Clinical practice guidelines on the evidence-based use of integrative therapies during and after breast cancer treatment. CA Cancer J Clin. 2017;67(3):194–232. doi: 10.3322/caac.21397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Towler P, Molassiotis A, Brearley SG. What is the evidence for the use of acupuncture as an intervention for symptom management in cancer supportive and palliative care: an integrative overview of reviews. Supportive Care Cancer. 2013;21:2913–2923. doi: 10.1007/s00520-013-1882-8. [DOI] [PubMed] [Google Scholar]
- 48.Posadzki P, Moon TW, Choi TY, Park TY, Lee MS, Ernst E. Acupuncture for cancer-related fatigue: a systematic review of randomized clinical trials. Supportive Care Cancer. 2013;21:2067–2073. doi: 10.1007/s00520-013-1765-z. [DOI] [PubMed] [Google Scholar]
- 49.Lau CHY, Wu X, Chung VCH, Liu X, Hui EP, Cramer H, et al. Acupuncture and related therapies for symptom management in palliative cancer care: systematic review and meta-analysis. Med U S. 2016;95(9):e2901. doi: 10.1097/MD.0000000000002901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cardoso F, MacNeill F, Penault-Llorca F, Eniu A, Sardanelli F, Nordström EB, et al. Why is appropriate healthcare inaccessible for many European breast cancer patients?—The EBCC 12 manifesto. Breast Edinb Scotl. 2021;55:128–135. doi: 10.1016/j.breast.2020.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang W, Williams JH, Hogan PF, Bruinooge SS, Rodriguez GI, Kosty MP, et al. Projected supply of and demand for oncologists and radiation oncologists through 2025: an aging, better-insured population will result in shortage. J Oncol Pract. 2014;10(1):39–45. doi: 10.1200/JOP.2013.001319. [DOI] [PubMed] [Google Scholar]
- 52.Di Meglio A, Charles C, Martin E, Havas J, Gbenou A, Flaysakier JD, et al. Uptake of recommendations for posttreatment cancer-related fatigue among breast cancer survivors. J Natl Compr Canc Netw. 2021;19(13):98–110. doi: 10.6004/jnccn.2021.7051. [DOI] [PubMed] [Google Scholar]
- 53.Vaz-Luis I, Cottu P, Mesleard C, Martin AL, Dumas A, Dauchy S, et al. UNICANCER: French prospective cohort study of treatment-related chronic toxicity in women with localised breast cancer (CANTO) ESMO Open. 2019;4(5):e000562. doi: 10.1136/esmoopen-2019-000562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vaz-Luis I, Di Meglio A, Havas J, El-Mouhebb M, Lapidari P, Presti D, et al. Long-term longitudinal patterns of patient-reported fatigue after breast cancer: a group-based trajectory analysis. J Clin Oncol Off J Am Soc Clin Oncol. 2022;40(19):2148–2162. doi: 10.1200/JCO.21.01958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Di Meglio A, Havas J, Soldato D, Presti D, Martin E, Pistilli B, et al. Development and validation of a predictive model of severe fatigue after breast cancer diagnosis: toward a personalized framework in survivorship care. J Clin Oncol. 2022;40(10):1111–1123. doi: 10.1200/JCO.21.01252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cardoso F, Kyriakides S, Ohno S, Penault-Llorca F, Poortmans P, Rubio IT, et al. Early breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019;30(8):1194–1220. doi: 10.1093/annonc/mdz173. [DOI] [PubMed] [Google Scholar]
- 57.Martelli V, Latocca MM, Ruelle T, Perachino M, Arecco L, Beshiri K, et al. Comparing the gonadotoxicity of multiple breast cancer regimens: important understanding for managing breast cancer in pre-menopausal women. Breast Cancer Targets Ther. 2021;13:341–351. doi: 10.2147/BCTT.S274283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lambertini M, Peccatori FA, Demeestere I, Amant F, Wyns C, Stukenborg JB, et al. Fertility preservation and post-treatment pregnancies in post-pubertal cancer patients: ESMO clinical practice guidelines. Ann Oncol. 2020;31(12):1664–1678. doi: 10.1016/j.annonc.2020.09.006. [DOI] [PubMed] [Google Scholar]
- 59.The ESHRE Guideline Group on Female Fertility Preservation. Anderson RA, Amant F, Braat D, D’Angelo A, Chuva de Sousa Lopes SM, et al. ESHRE guideline: female fertility preservation. Hum Reprod Open. 2020;2020(4):52. doi: 10.1093/hropen/hoaa052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lambertini M, Horicks F, Mastro LD, Partridge AH, Demeestere I. Ovarian protection with gonadotropin-releasing hormone agonists during chemotherapy in cancer patients: from biological evidence to clinical application. Cancer Treat Rev. 2019;72:65–77. doi: 10.1016/j.ctrv.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 61.Lambertini M, Moore HCF, Leonard RCF, Loibl S, Munster P, Bruzzone M, et al. Gonadotropin-releasing hormone agonists during chemotherapy for preservation of ovarian function and fertility in premenopausal patients with early breast cancer: a systematic review and meta-analysis of individual patient-level data. J Clin Oncol Off J Am Soc Clin Oncol. 2018;36(19):1981–1990. doi: 10.1200/JCO.2018.78.0858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lambertini M, Marrocco C, Spinaci S, Demeestere I, Anderson RA. Risk of gonadotoxicity with immunotherapy and targeted agents remains an unsolved but crucial issue. Eur J Clin Invest. 2022 doi: 10.1111/eci.13779. [DOI] [PubMed] [Google Scholar]
- 63.Winship AL, Alesi LR, Sant S, Stringer JM, Cantavenera A, Hegarty T, et al. Checkpoint inhibitor immunotherapy diminishes oocyte number and quality in mice. Nat Cancer. 2022;3(8):1–13. doi: 10.1038/s43018-022-00413-x. [DOI] [PubMed] [Google Scholar]
- 64.Nakamura K, Takae S, Shiraishi E, Shinya K, Igualada AJ, Suzuki N. Poly (ADP-ribose) polymerase inhibitor exposure reduces ovarian reserve followed by dysfunction in granulosa cells. Sci Rep. 2020;10(1):17058. doi: 10.1038/s41598-020-74087-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.On behalf of the ESMO Guidelines Committee. Lambertini M, Peccatori FA, Demeestere I, Amant F, Wyns C, Stukenborg JB, Paluch-Shimon S, Halaska MJ, Uzan C, Meissner J, vonWolff M, Anderson RA. Fertility preservation and post-treatment pregnancies in post-pubertal cancer patients: ESMO clinical practice guidelines. Ann Oncol. 2020;31(12):1664–1678. doi: 10.1016/j.annonc.2020.09.006. [DOI] [PubMed] [Google Scholar]
- 66.Arecco L, Blondeaux E, Bruzzone M, Ceppi M, Latocca MM, Marrocco C, et al. Safety of fertility preservation techniques before and after anticancer treatments in young women with breast cancer: a systematic review and meta-analysis. Hum Reprod. 2022;37(5):954–968. doi: 10.1093/humrep/deac035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Blondeaux E, Massarotti C, Fontana V, Poggio F, Arecco L, Fregatti P, et al. The PREgnancy and FERtility (PREFER) study investigating the need for ovarian function and/or fertility preservation strategies in premenopausal women with early breast cancer. Front Oncol. 2021;11:690320. doi: 10.3389/fonc.2021.690320/full. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lambertini M, Blondeaux E, Bruzzone M, Perachino M, Anderson RA, de Azambuja E, et al. Pregnancy after breast cancer: a systematic review and meta-analysis. J Clin Oncol. 2021;39(29):3293–3305. doi: 10.1200/JCO.21.00535. [DOI] [PubMed] [Google Scholar]
- 69.Lambertini M, Kroman N, Ameye L, Cordoba O, Pinto A, Benedetti G, et al. Long-term safety of pregnancy following breast cancer according to estrogen receptor status. J Natl Cancer Inst. 2018;110(4):426–429. doi: 10.1093/jnci/djx206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Anderson RA, Lambertini M, Hall PS, Wallace WH, Morrison DS, Kelsey TW. Survival after breast cancer in women with a subsequent live birth: influence of age at diagnosis and interval to subsequent pregnancy. Eur J Cancer. 2022;173:113–122. doi: 10.1016/j.ejca.2022.06.048. [DOI] [PubMed] [Google Scholar]
- 71.Pagani O, Ruggeri M, Manunta S, Saunders C, Peccatori F, Cardoso F, et al. Pregnancy after breast cancer: are young patients willing to participate in clinical studies? Breast. 2015;24(3):201–207. doi: 10.1016/j.breast.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 72.Sun Z, Niman SM, Pagani O, Partridge AH, Azim HA, Peccatori FA, et al. Estimation of historical control rate for a single arm de-escalation study—application to the POSITIVE trial. Breast Edinb Scotl. 2020;53:1–7. doi: 10.1016/j.breast.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Partridge AH, Niman SM, Ruggeri M, Peccatori FA, Azim HA, Colleoni M, et al. Who are the women who enrolled in the POSITIVE trial: a global study to support young hormone receptor positive breast cancer survivors desiring pregnancy. Breast. 2021;59:327–328. doi: 10.1016/j.breast.2021.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Partridge AH et al. GS4–09 pregnancy outcome and safety of interrupting therapy for women with endocrine responsive breast cancer: primary results from the POSITIVE Trial (IBCSG 48-14/BIG 8-13) presented at SABCS 2022. 2022 Sep 12.
- 75.Sessa C, Balmaña J, Bober SL, Cardoso MJ, Colombo N, Curigliano G, et al. Risk reduction and screening of cancer in hereditary breast-ovarian cancer syndromes: ESMO Clinical Practice Guideline. Ann Oncol. 2022;34:33–47. doi: 10.1016/j.annonc.2022.10.004. [DOI] [PubMed] [Google Scholar]
- 76.Lin F, Ma XS, Wang ZB, Wang ZW, Luo YB, Huang L, et al. Different fates of oocytes with DNA double-strand breaks in vitro and in vivo. Cell Cycle. 2014;13(17):2674–2680. doi: 10.4161/15384101.2015.945375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Turan V, Oktay K. BRCA-related ATM-mediated DNA double-strand break repair and ovarian aging. Hum Reprod Update. 2020;26(1):43–57. doi: 10.1093/humupd/dmz043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lips J, Kaina B. DNA double-strand breaks trigger apoptosis in p53-deficient fibroblasts. Carcinogenesis. 2001;22(4):579–585. doi: 10.1093/carcin/22.4.579. [DOI] [PubMed] [Google Scholar]
- 79.Vilenchik MM, Knudson AG. Endogenous DNA double-strand breaks: production, fidelity of repair, and induction of cancer. Proc Natl Acad Sci. 2003;100(22):12871–12876. doi: 10.1073/pnas.2135498100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Titus S, Li F, Stobezki R, Akula K, Unsal E, Jeong K, et al. Impairment of BRCA1-related DNA double-strand break repair leads to ovarian aging in mice and humans. Sci Transl Med. 2013 doi: 10.1126/scitranslmed.3004925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Turan V, Lambertini M, Lee DY, Wang E, Clatot F, Karlan BY, et al. Association of germline BRCA pathogenic variants with diminished ovarian reserve: a meta-analysis of individual patient-level data. J Clin Oncol. 2021;39(18):2016–2024. doi: 10.1200/JCO.20.02880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Valentini A, Finch A, Lubiński J, Byrski T, Ghadirian P, Kim-Sing C, et al. Chemotherapy-induced amenorrhea in patients with breast cancer with a BRCA1 or BRCA2 mutation. J Clin Oncol. 2013;31(31):3914–3919. doi: 10.1200/JCO.2012.47.7893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lambertini M, Olympios N, Lequesne J, Calbrix C, Fontanilles M, Loeb A, et al. Impact of taxanes, endocrine therapy, and deleterious germline BRCA mutations on anti-Müllerian hormone levels in early breast cancer patients treated with anthracycline- and cyclophosphamide-based chemotherapy. Front Oncol. 2019;9:575. doi: 10.3389/fonc.2019.00575/full. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Oktay KH, Bedoschi G, Goldfarb SB, Taylan E, Titus S, Palomaki GE, et al. Increased chemotherapy-induced ovarian reserve loss in women with germline BRCA mutations due to oocyte deoxyribonucleic acid double strand break repair deficiency. Fertil Steril. 2020;113(6):1251–1260.e1. doi: 10.1016/j.fertnstert.2020.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lambertini M, Goldrat O, Ferreira AR, Dechene J, Azim HA, Jr, Desir J, et al. Reproductive potential and performance of fertility preservation strategies in BRCA-mutated breast cancer patients. Ann Oncol. 2018;29(1):237–243. doi: 10.1093/annonc/mdx639. [DOI] [PubMed] [Google Scholar]
- 86.Turan V, Bedoschi G, Emirdar V, Moy F, Oktay K. Ovarian stimulation in patients with cancer: impact of letrozole and BRCA mutations on fertility preservation cycle outcomes. Reprod Sci. 2018;25(1):26–32. doi: 10.1177/1933719117728800. [DOI] [PubMed] [Google Scholar]
- 87.Gunnala V, Fields J, Irani M, D’Angelo D, Xu K, Schattman G, et al. BRCA carriers have similar reproductive potential at baseline to noncarriers: comparisons in cancer and cancer-free cohorts undergoing fertility preservation. Fertil Steril. 2019;111(2):363–371. doi: 10.1016/j.fertnstert.2018.10.014. [DOI] [PubMed] [Google Scholar]
- 88.Porcu E, Cillo GM, Cipriani L, Sacilotto F, Notarangelo L, Damiano G, et al. Impact of BRCA1 and BRCA2 mutations on ovarian reserve and fertility preservation outcomes in young women with breast cancer. J Assist Reprod Genet. 2020;37(3):709–715. doi: 10.1007/s10815-019-01658-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kim J, Turan V, Oktay K. Long-term safety of letrozole and gonadotropin stimulation for fertility preservation in women with breast cancer. J Clin Endocrinol Metab. 2016;101(4):1364–1371. doi: 10.1210/jc.2015-3878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Buonomo B, Massarotti C, Dellino M, Anserini P, Ferrari A, Campanella M, et al. Reproductive issues in carriers of germline pathogenic variants in the BRCA1/2 genes: an expert meeting. BMC Med. 2021;19(1):205. doi: 10.1186/s12916-021-02081-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lambertini M, Boni L, Michelotti A, Magnolfi E, Cogoni AA, Mosconi AM, et al. Long-term outcomes with pharmacological ovarian suppression during chemotherapy in premenopausal early breast cancer patients. JNCI J Natl Cancer Inst. 2022;114(3):400–408. doi: 10.1093/jnci/djab213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shapira M, Raanani H, Meirow D. IVF for fertility preservation in breast cancer patients—efficacy and safety issues. J Assist Reprod Genet. 2015;32(8):1171–1178. doi: 10.1007/s10815-015-0519-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lambertini M, Ameye L, Hamy AS, Zingarello A, Poorvu PD, Carrasco E, et al. Pregnancy after breast cancer in patients with germline BRCA mutations. J Clin Oncol. 2020;38(26):3012–3023. doi: 10.1200/JCO.19.02399. [DOI] [PubMed] [Google Scholar]
- 94.Condorelli M, Bruzzone M, Ceppi M, Ferrari A, Grinshpun A, Hamy AS, et al. Safety of assisted reproductive techniques in young women harboring germline pathogenic variants in BRCA1/2 with a pregnancy after prior history of breast cancer. ESMO Open. 2021;6(6):100300. doi: 10.1016/j.esmoop.2021.100300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Burstein HJ, Lacchetti C, Anderson H, Buchholz TA, Davidson NE, Gelmon KA, et al. Adjuvant endocrine therapy for women with hormone receptor–positive breast cancer: ASCO clinical practice guideline focused update. J Clin Oncol. 2019;37(5):423–438. doi: 10.1200/JCO.18.01160. [DOI] [PubMed] [Google Scholar]
- 96.Paluch-Shimon S, Cardoso F, Partridge AH, Abulkhair O, Azim HA, Bianchi-Micheli G, et al. ESO–ESMO fifth international consensus guidelines for breast cancer in young women (BCY5) Ann Oncol. 2022;33(11):1097–1118. doi: 10.1016/j.annonc.2022.07.007. [DOI] [PubMed] [Google Scholar]
- 97.Lambertini M, Blondeaux E, Perrone F, Del Mastro L. Improving adjuvant endocrine treatment tailoring in premenopausal women with hormone receptor–positive breast cancer. J Clin Oncol. 2020;38(12):1258–1267. doi: 10.1200/jco.19.02242. [DOI] [PubMed] [Google Scholar]
- 98.Bradley R, Braybrooke J, Gray R, Hills RK, Liu Z, Pan H, et al. Aromatase inhibitors versus tamoxifen in premenopausal women with oestrogen receptor-positive early-stage breast cancer treated with ovarian suppression: a patient-level meta-analysis of 7030 women from four randomised trials. Lancet Oncol. 2022;23(3):382–392. doi: 10.1016/S1470-2045(21)00758-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bernhard J, Luo W, Ribi K, Colleoni M, Burstein HJ, Tondini C, et al. Patient-reported outcomes with adjuvant exemestane versus tamoxifen in premenopausal women with early breast cancer undergoing ovarian suppression (TEXT and SOFT): a combined analysis of two phase 3 randomised trials. Lancet Oncol. 2015;16(7):848–858. doi: 10.1016/S1470-2045(15)00049-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Saha P, Regan MM, Pagani O, Francis PA, Walley BA, Ribi K, et al. Treatment efficacy, adherence, and quality of life among women younger than 35 years in the international breast cancer study group TEXT and SOFT adjuvant endocrine therapy trials. J Clin Oncol. 2017;35(27):3113–3122. doi: 10.1200/JCO.2016.72.0946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Franzoi MA, Agostinetto E, Perachino M, Del Mastro L, de Azambuja E, Vaz-Luis I, et al. Evidence-based approaches for the management of side-effects of adjuvant endocrine therapy in patients with breast cancer. Lancet Oncol. 2021;22(7):e303–e313. doi: 10.1016/S1470-2045(20)30666-5. [DOI] [PubMed] [Google Scholar]
- 102.Nimbi FM, Magno S, Agostini L, Di Micco A, Maggiore C, De Cesaris BM, et al. Sexuality in breast cancer survivors: sexual experiences, emotions, and cognitions in a group of women under hormonal therapy. Breast Cancer Tokyo Jpn. 2022;29(3):419–428. doi: 10.1007/s12282-021-01320-2. [DOI] [PubMed] [Google Scholar]
- 103.von Hippel C, Rosenberg SM, Austin SB, Sprunck-Harrild K, Ruddy KJ, Schapira L, et al. Identifying distinct trajectories of change in young breast cancer survivors’ sexual functioning. Psychooncology. 2019;28(5):1033–1040. doi: 10.1002/pon.5047. [DOI] [PubMed] [Google Scholar]
- 104.Oberguggenberger A, Martini C, Huber N, Fallowfield L, Hubalek M, Daniaux M, et al. Self-reported sexual health: breast cancer survivors compared to women from the general population—an observational study. BMC Cancer. 2017;17(1):599. doi: 10.1186/s12885-017-3580-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Raggio GA, Butryn ML, Arigo D, Mikorski R, Palmer SC. Prevalence and correlates of sexual morbidity in long-term breast cancer survivors. Psychol Health. 2014;29(6):632–650. doi: 10.1080/08870446.2013.879136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Brédart A, Dolbeault S, Savignoni A, Besancenet C, This P, Giami A, et al. Prevalence and associated factors of sexual problems after early-stage breast cancer treatment: results of a French exploratory survey. Psychooncology. 2011;20(8):841–850. doi: 10.1002/pon.1789. [DOI] [PubMed] [Google Scholar]
- 107.Zehra S, Doyle F, Barry M, Walsh S, Kell MR. Health-related quality of life following breast reconstruction compared to total mastectomy and breast-conserving surgery among breast cancer survivors: a systematic review and meta-analysis. Breast Cancer. 2020;27(4):534–566. doi: 10.1007/s12282-020-01076-1. [DOI] [PubMed] [Google Scholar]
- 108.Hungr C, Sanchez-Varela V, Bober SL. Self-image and sexuality issues among young women with breast cancer: practical recommendations. Rev Investig Clin. 2017;69(2):99. doi: 10.24875/ric.17002200. [DOI] [PubMed] [Google Scholar]
- 109.Loprinzi CL, Abu-Ghazaleh S, Sloan JA, vanHaelst-Pisani C, Hammer AM, Rowland KM, et al. Phase III randomized double-blind study to evaluate the efficacy of a polycarbophil-based vaginal moisturizer in women with breast cancer. J Clin Oncol. 1997;15(3):969–973. doi: 10.1200/JCO.1997.15.3.969. [DOI] [PubMed] [Google Scholar]
- 110.Advani P, Brewster AM, Baum GP, Schover LR. A pilot randomized trial to prevent sexual dysfunction in postmenopausal breast cancer survivors starting adjuvant aromatase inhibitor therapy. J Cancer Surviv. 2017;11(4):477–485. doi: 10.1007/s11764-017-0606-3. [DOI] [PubMed] [Google Scholar]
- 111.Mitchell CM, Larson JC, Crandall CJ, Bhasin S, LaCroix AZ, Ensrud KE, et al. Association of vaginal estradiol tablet with serum estrogen levels in women who are postmenopausal: secondary analysis of a randomized clinical trial. JAMA Netw Open. 2022;5(11):e2241743. doi: 10.1001/jamanetworkopen.2022.41743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Holmberg L, Anderson H. HABITS (hormonal replacement therapy after breast cancer—is it safe?), a randomised comparison: trial stopped. Lancet. 2004;363(9407):453–455. doi: 10.1016/S0140-6736(04)15493-7. [DOI] [PubMed] [Google Scholar]
- 113.Cold S, Cold F, Jensen MB, Cronin-Fenton D, Christiansen P, Ejlertsen B. Systemic or vaginal hormone therapy after early breast cancer: a Danish Observational Cohort Study. J Natl Cancer Inst. 2022;114(10):1347–1354. doi: 10.1093/jnci/djac112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Faubion SS, Larkin LC, Stuenkel CA, Bachmann GA, Chism LA, Kagan R, et al. Management of genitourinary syndrome of menopause in women with or at high risk for breast cancer: consensus recommendations from The North American Menopause Society and The International Society for the Study of Women’s Sexual Health. Menopause. 2018;25(6):596–608. doi: 10.1097/GME.0000000000001121. [DOI] [PubMed] [Google Scholar]
- 115.Goetsch MF, Lim JY, Caughey AB. A practical solution for dyspareunia in breast cancer survivors: a randomized controlled trial. J Clin Oncol. 2015;33(30):3394–3400. doi: 10.1200/JCO.2014.60.7366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bober SL, Fine E, Recklitis CJ. Sexual health and rehabilitation after ovarian suppression treatment (SHARE-OS): a clinical intervention for young breast cancer survivors. J Cancer Surviv. 2020;14(1):26–30. doi: 10.1007/s11764-019-00800-x. [DOI] [PubMed] [Google Scholar]
- 117.Arêas F, Valadares ALR, Conde DM, Costa-Paiva L. The effect of vaginal erbium laser treatment on sexual function and vaginal health in women with a history of breast cancer and symptoms of the genitourinary syndrome of menopause: a prospective study. Menopause. 2019;26(9):1052–1058. doi: 10.1097/GME.0000000000001353. [DOI] [PubMed] [Google Scholar]
- 118.Becorpi A, Campisciano G, Zanotta N, Tredici Z, Guaschino S, Petraglia F, et al. Fractional CO2 laser for genitourinary syndrome of menopause in breast cancer survivors: clinical, immunological, and microbiological aspects. Lasers Med Sci. 2018;33(5):1047–1054. doi: 10.1007/s10103-018-2471-3. [DOI] [PubMed] [Google Scholar]
- 119.Li FG, Maheux-Lacroix S, Deans R, Nesbitt-Hawes E, Budden A, Nguyen K, et al. Effect of fractional carbon dioxide laser vs sham treatment on symptom severity in women with postmenopausal vaginal symptoms: a randomized clinical trial. JAMA. 2021;326(14):1381–1389. doi: 10.1001/jama.2021.14892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hersant B, SidAhmed-Mezi M, Belkacemi Y, Darmon F, Bastuji-Garin S, Werkoff G, et al. Efficacy of injecting platelet concentrate combined with hyaluronic acid for the treatment of vulvovaginal atrophy in postmenopausal women with history of breast cancer: a phase 2 pilot study. Menopause. 2018;25(10):1124–1130. doi: 10.1097/GME.0000000000001122. [DOI] [PubMed] [Google Scholar]
- 121.Hummel SB, van Lankveld JJDM, Oldenburg HSA, Hahn DEE, Kieffer JM, Gerritsma MA, et al. Efficacy of internet-based cognitive behavioral therapy in improving sexual functioning of breast cancer survivors: results of a randomized controlled trial. J Clin Oncol. 2017;35(12):1328–1340. doi: 10.1200/JCO.2016.69.6021. [DOI] [PubMed] [Google Scholar]
- 122.Marcus AC, Garrett KM, Cella D, Wenzel L, Brady MJ, Fairclough D, et al. Can telephone counseling post-treatment improve psychosocial outcomes among early stage breast cancer survivors?: psychosocial telephone counseling for breast cancer survivors. Psychooncology. 2010;19(9):923–932. doi: 10.1002/pon.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Jun EY, Kim S, Chang SB, Oh K, Kang HS, Kang SS. The effect of a sexual life reframing program on marital intimacy, body image, and sexual function among breast cancer survivors. Cancer Nurs. 2011;34(2):142–149. doi: 10.1097/NCC.0b013e3181f1ab7a. [DOI] [PubMed] [Google Scholar]
- 124.Soldato D, Meglio AD, Pradon C, Noce AD, Presti D, Havas J, et al. Abstract P4-11-34: an integrated clinical, behavioral and biological model to predict the risk of weight gain among breast cancer survivors (BCS). Cancer Res 2022;82 (4):P4-11-34
- 125.Meglio AD, Gbenou AS, Martin E, Pistilli B, Ligibel JA, Crane TE, et al. Unhealthy behaviors after breast cancer: capitalizing on a teachable moment to promote lifestyle improvements. Cancer. 2021;127(15):2774–2787. doi: 10.1002/cncr.33565. [DOI] [PubMed] [Google Scholar]
- 126.Gandhi A, Copson E, Eccles D, Durcan L, Howell A, Morris J, et al. Predictors of weight gain in a cohort of premenopausal early breast cancer patients receiving chemotherapy. Breast Edinb Scotl. 2019;45:1–6. doi: 10.1016/j.breast.2019.02.006. [DOI] [PubMed] [Google Scholar]
- 127.Irwin ML, McTiernan A, Baumgartner RN, Baumgartner KB, Bernstein L, Gilliland FD, et al. Changes in body fat and weight after a breast cancer diagnosis: influence of demographic, prognostic, and lifestyle factors. J Clin Oncol Off J Am Soc Clin Oncol. 2005;23(4):774–782. doi: 10.1200/JCO.2005.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.van den Berg MMGA, Winkels RM, de Kruif JTCM, van Laarhoven HWM, Visser M, de Vries JHM, et al. Weight change during chemotherapy in breast cancer patients: a meta-analysis. BMC Cancer. 2017;17(1):259. doi: 10.1186/s12885-017-3242-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Demark-Wahnefried W, Peterson BL, Winer EP, Marks L, Aziz N, Marcom PK, et al. Changes in weight, body composition, and factors influencing energy balance among premenopausal breast cancer patients receiving adjuvant chemotherapy. J Clin Oncol Off J Am Soc Clin Oncol. 2001;19(9):2381–2389. doi: 10.1200/JCO.2001.19.9.2381. [DOI] [PubMed] [Google Scholar]
- 130.Taylor EB. The complex role of adipokines in obesity, inflammation, and autoimmunity. Clin Sci Lond Engl 1979. 2021;135(6):731–752. doi: 10.1042/CS20200895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kaklamani V, Yi N, Sadim M, Siziopikou K, Zhang K, Xu Y, et al. The role of the fat mass and obesity associated gene (FTO) in breast cancer risk. BMC Med Genet. 2011;12(1):52. doi: 10.1186/1471-2350-12-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kaklamani VG, Sadim M, Hsi A, Offit K, Oddoux C, Ostrer H, et al. Variants of the adiponectin and adiponectin receptor 1 genes and breast cancer risk. Cancer Res. 2008;68(9):3178–3184. doi: 10.1158/0008-5472.CAN-08-0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Jiralerspong S, Goodwin PJ. Obesity and breast cancer prognosis: evidence, challenges, and opportunities. J Clin Oncol. 2016;34(35):4203–4216. doi: 10.1200/JCO.2016.68.4480. [DOI] [PubMed] [Google Scholar]
- 134.Meglio AD, Michiels S, Jones LW, El-Mouhebb M, Ferreira AR, Martin E, et al. Changes in weight, physical and psychosocial patient-reported outcomes among obese women receiving treatment for early-stage breast cancer: a nationwide clinical study. Breast. 2020;52:23–32. doi: 10.1016/j.breast.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Meglio AD, Menvielle G, Dumas A, Gbenou A, Pinto S, Bovagnet T, et al. Body weight and return to work among survivors of early-stage breast cancer. ESMO Open. 2020;5(6). [DOI] [PMC free article] [PubMed]
- 136.Nechuta S, Chen WY, Cai H, Poole EM, Kwan ML, Flatt SW, et al. A pooled analysis of post-diagnosis lifestyle factors in association with late estrogen-receptor-positive breast cancer prognosis. Int J Cancer. 2016;138(9):2088–2097. doi: 10.1002/ijc.29940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Parekh N, Chandran U, Bandera EV. Obesity in cancer survival. Annu Rev Nutr. 2012;21(32):311–342. doi: 10.1146/annurev-nutr-071811-150713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Jackson SE, Heinrich M, Beeken RJ, Wardle J. Weight loss and mortality in overweight and obese cancer survivors: a systematic review. PLoS ONE. 2017;12(1):e0169173. doi: 10.1371/journal.pone.0169173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Martel S, Lambertini M, Agbor-Tarh D, Ponde NF, Gombos A, Paterson V, et al. Body mass index and weight change in patients with HER2-positive early breast cancer: exploratory analysis of the ALTTO BIG 2–06 trial. J Natl Compr Cancer Netw JNCCN. 2021;19(2):181–189. doi: 10.6004/jnccn.2020.7606. [DOI] [PubMed] [Google Scholar]
- 140.Chan DSM, Vieira AR, Aune D, Bandera EV, Greenwood DC, McTiernan A, et al. Body mass index and survival in women with breast cancer—systematic literature review and meta-analysis of 82 follow-up studies. Ann Oncol. 2014;25(10):1901–1914. doi: 10.1093/annonc/mdu042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ligibel JA, Barry WT, Alfano C, Hershman DL, Irwin M, Neuhouser M, et al. Randomized phase III trial evaluating the role of weight loss in adjuvant treatment of overweight and obese women with early breast cancer (Alliance A011401): study design. NPJ Breast Cancer. 2017;3:37. doi: 10.1038/s41523-017-0040-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Goodwin PJ, Segal RJ, Vallis M, Ligibel JA, Pond GR, Robidoux A, et al. The LISA randomized trial of a weight loss intervention in postmenopausal breast cancer. NPJ Breast Cancer. 2020 doi: 10.1038/s41523-020-0149-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ligibel JA, Basen-Engquist K, Bea JW. Weight management and physical activity for breast cancer prevention and control. Am Soc Clin Oncol Educ Book. 2019;39:e22–e33. doi: 10.1200/EDBK_237423. [DOI] [PubMed] [Google Scholar]
- 144.Shaikh H, Bradhurst P, Ma LX, Tan SY, Egger SJ, Vardy JL. Body weight management in overweight and obese breast cancer survivors. Cochrane Database Syst Rev. 2020 doi: 10.1002/14651858.CD012110.pub2/full. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Chan RJ, Crawford-Williams F, Crichton M, Joseph R, Hart NH, Milley K, et al. Effectiveness and implementation of models of cancer survivorship care: an overview of systematic reviews. J Cancer Surviv. 2021;17(1):197–221. doi: 10.1007/s11764-021-01128-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Rock CL, Thomson CA, Sullivan KR, Howe CL, Kushi LH, Caan BJ, et al. American Cancer Society nutrition and physical activity guideline for cancer survivors. CA Cancer J Clin. 2022;72(3):230–262. doi: 10.3322/caac.21719. [DOI] [PubMed] [Google Scholar]
- 147.Caan BJ, Cespedes Feliciano EM, Prado CM, Alexeeff S, Kroenke CH, Bradshaw P, et al. Association of muscle and adiposity measured by computed tomography with survival in patients with nonmetastatic breast cancer. JAMA Oncol. 2018;4(6):798–804. doi: 10.1001/jamaoncol.2018.0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ligibel JA, Bohlke K, May AM, Clinton SK, Demark-Wahnefried W, Gilchrist SC, et al. Exercise, diet, and weight management during cancer treatment: ASCO guideline. J Clin Oncol. 2022;40(22):2491–2507. doi: 10.1200/JCO.22.00687. [DOI] [PubMed] [Google Scholar]
- 149.Campbell KL, Winters-Stone KM, Wiskemann J, May AM, Schwartz AL, Courneya KS, et al. Exercise guidelines for cancer survivors: consensus statement from international multidisciplinary roundtable. Med Sci Sports Exerc. 2019;51(11):2375–2390. doi: 10.1249/MSS.0000000000002116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Maginador G, Lixandrão ME, Bortolozo HI, Vechin FC, Sarian LO, Derchain S, et al. Aerobic exercise-induced changes in cardiorespiratory fitness in breast cancer patients receiving chemotherapy: a systematic review and meta-analysis. Cancers. 2020;12(8):2240. doi: 10.3390/cancers12082240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Juvet LK, Thune I, Elvsaas IKØ, Fors EA, Lundgren S, Bertheussen G, et al. The effect of exercise on fatigue and physical functioning in breast cancer patients during and after treatment and at 6 months follow-up: a meta-analysis. Breast. 2017;33:166–177. doi: 10.1016/j.breast.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 152.Singh B, Spence RR, Steele ML, Sandler CX, Peake JM, Hayes SC. A systematic review and meta-analysis of the safety, feasibility, and effect of exercise in women with stage II + breast cancer. Arch Phys Med Rehabil. 2018;99(12):2621–2636. doi: 10.1016/j.apmr.2018.03.026. [DOI] [PubMed] [Google Scholar]
- 153.Ehlers DK, DuBois K, Salerno EA. The effects of exercise on cancer-related fatigue in breast cancer patients during primary treatment: a meta-analysis and systematic review. Expert Rev Anticancer Ther. 2020;20(10):865–877. doi: 10.1080/14737140.2020.1813028. [DOI] [PubMed] [Google Scholar]
- 154.van Waart H, Stuiver MM, van Harten WH, Geleijn E, Kieffer JM, Buffart LM, et al. Effect of low-intensity physical activity and moderate- to high-intensity physical exercise during adjuvant chemotherapy on physical fitness, fatigue, and chemotherapy completion rates: results of the PACES randomized clinical trial. J Clin Oncol. 2015;33(17):1918–1927. doi: 10.1200/JCO.2014.59.1081. [DOI] [PubMed] [Google Scholar]
- 155.Baker JL, Di Meglio A, Gbenou AS, El Mouhebb M, Iyengar NM, Michiels S, et al. Association between physical activity and neoadjuvant chemotherapy completion and pathologic complete response in primary breast cancer: the CANTO study. Br J Cancer. 2022;127(5):886–891. doi: 10.1038/s41416-022-01870-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Friedenreich CM, Stone CR, Cheung WY, Hayes SC. Physical activity and mortality in cancer survivors: a systematic review and meta-analysis. JNCI Cancer Spectr. 2020;4(1):pkz080. doi: 10.1093/jncics/pkz080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lee J. A meta-analysis of the association between physical activity and breast cancer mortality. Cancer Nurs. 2019;42(4):271–285. doi: 10.1097/NCC.0000000000000580. [DOI] [PubMed] [Google Scholar]
- 158.Wang Y, Song H, Yin Y, Feng L. Cancer survivors could get survival benefits from postdiagnosis physical activity: a meta-analysis. Evid Based Complement Alternat Med. 2019;2019:e1940903. doi: 10.1155/2019/1940903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Gilchrist SC, Howard VJ, Akinyemiju T, Judd SE, Cushman M, Hooker SP, et al. Association of sedentary behavior with cancer mortality in middle-aged and older US adults. JAMA Oncol. 2020;6(8):1210–1217. doi: 10.1001/jamaoncol.2020.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Cao C, Friedenreich CM, Yang L. Association of daily sitting time and leisure-time physical activity with survival among US cancer survivors. JAMA Oncol. 2022;8(3):395–403. doi: 10.1001/jamaoncol.2021.6590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Di Meglio A, Havas J, Gbenou AS, Martin E, El-Mouhebb M, Pistilli B, et al. Dynamics of long-term patient-reported quality of life and health behaviors after adjuvant breast cancer chemotherapy. J Clin Oncol. 2022;40(27):3190–3204. doi: 10.1200/JCO.21.00277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Demark-Wahnefried W, Rogers LQ, Alfano CM, Thomson CA, Courneya KS, Meyerhardt JA, et al. Practical clinical interventions for diet, physical activity, and weight control in cancer survivors. CA Cancer J Clin. 2015;65(3):167–189. doi: 10.3322/caac.21265. [DOI] [PubMed] [Google Scholar]
- 163.Ng AH, Ngo-Huang A, Vidal M, Reyes-Garcia A, Liu DD, Williams JL, et al. Exercise barriers and adherence to recommendations in patients with cancer. JCO Oncol Pract. 2021;17(7):e972–e981. doi: 10.1200/OP.20.00625. [DOI] [PubMed] [Google Scholar]
- 164.Bérubé S, Lemieux J, Moore L, Maunsell E, Brisson J. Smoking at time of diagnosis and breast cancer-specific survival: new findings and systematic review with meta-analysis. Breast Cancer Res. 2014;16(2):R42. doi: 10.1186/bcr3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.US Preventive Services Task Force Screening for lung cancer: US preventive services task force recommendation statement. JAMA. 2021;325(10):962–970. doi: 10.1001/jama.2021.1117. [DOI] [PubMed] [Google Scholar]
- 166.Peppone LJ, Mustian KM, Morrow GR, Dozier AM, Ossip DJ, Janelsins MC, et al. The effect of cigarette smoking on cancer treatment-related side effects. Oncologist. 2011;16(12):1784–1792. doi: 10.1634/theoncologist.2011-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Warren GW, Sobus S, Gritz ER. The biological and clinical effects of smoking by patients with cancer and strategies to implement evidence-based tobacco cessation support. Lancet Oncol. 2014;15(12):e568–e580. doi: 10.1016/S1470-2045(14)70266-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Zhan M, Flaws JA, Gallicchio L, Tkaczuk K, Lewis LM, Royak-Schaler R. Profiles of tamoxifen-related side effects by race and smoking status in women with breast cancer. Cancer Detect Prev. 2007;31(5):384–390. doi: 10.1016/j.cdp.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 169.Goodwin SJ, McCarthy CM, Pusic AL, Bui D, Howard M, Disa JJ, et al. Complications in smokers after postmastectomy tissue expander/implant breast reconstruction. Ann Plast Surg. 2005;55(1):16–20. doi: 10.1097/01.sap.0000168282.81348.b3. [DOI] [PubMed] [Google Scholar]
- 170.Jang S, Prizment A, Haddad T, Robien K, Lazovich D. Smoking and quality of life among female survivors of breast, colorectal and endometrial cancers in a prospective cohort study. J Cancer Surviv. 2011;5(2):115–122. doi: 10.1007/s11764-010-0147-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Blanchard CM, Courneya KS, Stein K. Cancer survivors’ adherence to lifestyle behavior recommendations and associations with health-related quality of life: results from the American Cancer Society’s SCS-II. J Clin Oncol. 2008;26(13):2198–2204. doi: 10.1200/JCO.2007.14.6217. [DOI] [PubMed] [Google Scholar]
- 172.Zhao G, Li C, Okoro CA, Li J, Wen XJ, White A, et al. Trends in modifiable lifestyle-related risk factors following diagnosis in breast cancer survivors. J Cancer Surviv. 2013;7(4):563–569. doi: 10.1007/s11764-013-0295-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.World Cancer Research Fund/American Institute for Cancer Research. Continuous Update Project Expert Report 2018. Diet, nutrition, physical activity and breast cancer. Available at dietandcancerreport.org. 2017;124.
- 174.Simapivapan P, Boltong A, Hodge A. To what extent is alcohol consumption associated with breast cancer recurrence and second primary breast cancer?: a systematic review. Cancer Treat Rev. 2016;50:155–167. doi: 10.1016/j.ctrv.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 175.Kwan ML, Kushi LH, Weltzien E, Tam EK, Castillo A, Sweeney C, et al. Alcohol consumption and breast cancer recurrence and survival among women with early-stage breast cancer: the life after cancer epidemiology study. J Clin Oncol Off J Am Soc Clin Oncol. 2010;28(29):4410–4416. doi: 10.1200/JCO.2010.29.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Schwedhelm C, Boeing H, Hoffmann G, Aleksandrova K, Schwingshackl L. Effect of diet on mortality and cancer recurrence among cancer survivors: a systematic review and meta-analysis of cohort studies. Nutr Rev. 2016;74(12):737–748. doi: 10.1093/nutrit/nuw045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Bidstrup PE, Dalton SO, Christensen J, Tjonneland A, Larsen SB, Karlsen R, et al. Changes in body mass index and alcohol and tobacco consumption among breast cancer survivors and cancer-free women: a prospective study in the Danish Diet, Cancer and Health Cohort. Acta Oncol Stockh Swed. 2013;52(2):327–335. doi: 10.3109/0284186X.2012.746466. [DOI] [PubMed] [Google Scholar]
- 178.Lahti-Koski M, Pietinen P, Heliövaara M, Vartiainen E. Associations of body mass index and obesity with physical activity, food choices, alcohol intake, and smoking in the 1982–1997 FINRISK Studies. Am J Clin Nutr. 2002;75(5):809–817. doi: 10.1093/ajcn/75.5.809. [DOI] [PubMed] [Google Scholar]
- 179.Coleman R, Hadji P, Body JJ, Santini D, Chow E, Terpos E, et al. Bone health in cancer: ESMO clinical practice guidelines. Ann Oncol. 2020;31(12):1650–1663. doi: 10.1016/j.annonc.2020.07.019. [DOI] [PubMed] [Google Scholar]
- 180.Lowery-Allison AE, Passik SD, Cribbet MR, Reinsel RA, O’Sullivan B, Norton L, et al. Sleep problems in breast cancer survivors 1–10 years posttreatment. Palliat Support Care. 2018;16(3):325–334. doi: 10.1017/S1478951517000311. [DOI] [PubMed] [Google Scholar]
- 181.Ligibel J. Lifestyle factors in cancer survivorship. J Clin Oncol. 2012;30(30):3697–3704. doi: 10.1200/JCO.2012.42.0638. [DOI] [PubMed] [Google Scholar]
- 182.Gradishar WJ, Moran MS, Abraham J, Aft R, Agnese D, Allison KH, et al. Breast cancer, version 3.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2022;20(6):691–722. doi: 10.6004/jnccn.2022.0030. [DOI] [PubMed] [Google Scholar]
- 183.Moschetti I, Cinquini M, Lambertini M, Levaggi A, Liberati A. Follow-up strategies for women treated for early breast cancer. Cochrane Database Syst Rev. 2016 doi: 10.1002/14651858.CD001768.pub3/full. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Schumacher JR, Neuman HB, Yu M, Vanness DJ, Si Y, Burnside ES, et al. Surveillance imaging vs symptomatic recurrence detection and survival in stage II–III breast cancer (AFT-01) JNCI J Natl Cancer Inst. 2022;114(10):1371–1379. doi: 10.1093/jnci/djac131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Pan H, Gray R, Braybrooke J, Davies C, Taylor C, McGale P, et al. 20-Year risks of breast-cancer recurrence after stopping endocrine therapy at 5 years. N Engl J Med. 2017;377(19):1836–1846. doi: 10.1056/NEJMoa1701830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Corcoran RB, Chabner BA. Application of cell-free DNA analysis to cancer treatment. N Engl J Med. 2018;379(18):1754–1765. doi: 10.1056/NEJMra1706174. [DOI] [PubMed] [Google Scholar]
- 187.Tie J, Cohen JD, Lahouel K, Lo SN, Wang Y, Kosmider S, et al. Circulating tumor DNA analysis guiding adjuvant therapy in stage II colon cancer. N Engl J Med. 2022;386(24):2261–2272. doi: 10.1056/NEJMoa2200075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Garcia-Murillas I, Schiavon G, Weigelt B, Ng C, Hrebien S, Cutts RJ, et al. Mutation tracking in circulating tumor DNA predicts relapse in early breast cancer. Sci Transl Med. 2015 doi: 10.1126/scitranslmed.aab0021. [DOI] [PubMed] [Google Scholar]
- 189.Garcia-Murillas I, Chopra N, Comino-Méndez I, Beaney M, Tovey H, Cutts RJ, et al. Assessment of molecular relapse detection in early-stage breast cancer. JAMA Oncol. 2019;5(10):1473–1478. doi: 10.1001/jamaoncol.2019.1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Coombes RC, Page K, Salari R, Hastings RK, Armstrong A, Ahmed S, et al. Personalized detection of circulating tumor DNA antedates breast cancer metastatic recurrence. Clin Cancer Res Off J Am Assoc Cancer Res. 2019;25(14):4255–4263. doi: 10.1158/1078-0432.CCR-18-3663. [DOI] [PubMed] [Google Scholar]
- 191.Magbanua MJM, Swigart LB, Wu HT, Hirst GL, Yau C, Wolf DM, et al. Circulating tumor DNA in neoadjuvant-treated breast cancer reflects response and survival. Ann Oncol Off J Eur Soc Med Oncol. 2021;32(2):229–239. doi: 10.1016/j.annonc.2020.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Cailleux F, Agostinetto E, Lambertini M, Rothé F, Wu HT, Balcioglu M, et al. Circulating tumor DNA after neoadjuvant chemotherapy in breast cancer is associated with disease relapse. JCO Precis Oncol. 2022;6:e2200148. doi: 10.1200/PO.22.00148. [DOI] [PubMed] [Google Scholar]
- 193.Lipsyc-Sharf M, de Bruin EC, Santos K, McEwen R, Stetson D, Patel A, et al. Circulating tumor DNA and late recurrence in high-risk hormone receptor-positive, human epidermal growth factor receptor 2–negative breast cancer. J Clin Oncol. 2022;40(22):2408–2419. doi: 10.1200/JCO.22.00908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Turner NC, Swift C, Jenkins B, Kilburn L, Coakley M, Beaney M, et al. Results of the c-TRAK TN trial: a clinical trial utilising ctDNA mutation tracking to detect molecular residual disease and trigger intervention in patients with moderate and high-risk early stage triple negative breast cancer. Ann Oncol. 2022;34(2):200–211. doi: 10.1016/j.annonc.2022.11.005. [DOI] [PubMed] [Google Scholar]
- 195.Sperduto PW, Kased N, Roberge D, Xu Z, Shanley R, Luo X, et al. Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol. 2012;30(4):419–425. doi: 10.1200/JCO.2011.38.0527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Sperduto PW, Mesko S, Li J, Cagney D, Aizer A, Lin NU, et al. Survival in patients with brain metastases: summary report on the updated diagnosis-specific graded prognostic assessment and definition of the eligibility quotient. J Clin Oncol. 2020;38(32):3773–3784. doi: 10.1200/JCO.20.01255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Barnholtz-Sloan JS, Sloan AE, Davis FG, Vigneau FD, Lai P, Sawaya RE. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the metropolitan Detroit cancer surveillance system. J Clin Oncol. 2004;22(14):2865–2872. doi: 10.1200/JCO.2004.12.149. [DOI] [PubMed] [Google Scholar]
- 198.Darlix A, Louvel G, Fraisse J, Jacot W, Brain E, Debled M, et al. Impact of breast cancer molecular subtypes on the incidence, kinetics and prognosis of central nervous system metastases in a large multicentre real-life cohort. Br J Cancer. 2019;121(12):991–1000. doi: 10.1038/s41416-019-0619-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Arvold ND, Oh KS, Niemierko A, Taghian AG, Lin NU, Abi-Raad RF, et al. Brain metastases after breast-conserving therapy and systemic therapy: incidence and characteristics by biologic subtype. Breast Cancer Res Treat. 2012;136(1):153–160. doi: 10.1007/s10549-012-2243-x. [DOI] [PubMed] [Google Scholar]
- 200.Kuksis M, Gao Y, Tran W, Hoey C, Kiss A, Komorowski AS, et al. The incidence of brain metastases among patients with metastatic breast cancer: a systematic review and meta-analysis. Neuro Oncol. 2021;23(6):894–904. doi: 10.1093/neuonc/noaa285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353(16):1659–1672. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 202.Pestalozzi BC, Holmes E, de Azambuja E, Metzger-Filho O, Hogge L, Scullion M, et al. CNS relapses in patients with HER2-positive early breast cancer who have and have not received adjuvant trastuzumab: a retrospective substudy of the HERA trial (BIG 1-01) Lancet Oncol. 2013;14(3):244–248. doi: 10.1016/S1470-2045(13)70017-2. [DOI] [PubMed] [Google Scholar]
- 203.Untch M, Geyer CE, Huang C, Loibl S, Wolmark N, Mano MS, et al. Peripheral neuropathy (PN), thrombocytopenia (TCP) and central nervous system (CNS) recurrence: an update of the phase III KATHERINE trial of post-neoadjuvant trastuzumab emtansine (T-DM1) or trastuzumab (H) in patients (pts) with residual invasive HER2-positive breast cancer (BC) Ann Oncol. 2019;30:ix183. doi: 10.1093/annonc/mdz446.003. [DOI] [Google Scholar]
- 204.Lin NU, Winer EP. Brain metastases: the HER2 paradigm. Clin Cancer Res. 2007;13(6):1648–1655. doi: 10.1158/1078-0432.CCR-06-2478. [DOI] [PubMed] [Google Scholar]
- 205.Piccart-Gebhart M, Holmes E, Baselga J, de Azambuja E, Dueck AC, Viale G, et al. Adjuvant lapatinib and trastuzumab for early human epidermal growth factor receptor 2-positive breast cancer: results from the randomized phase III adjuvant lapatinib and/or trastuzumab treatment optimization trial. J Clin Oncol. 2016;34(10):1034–1042. doi: 10.1200/JCO.2015.62.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Holmes FA, Moy B, Delaloge S, Chia S, Ejlertsen B, Mansi J, et al. Abstract PD3-03: continued efficacy of neratinib in patients with HER2-positive early-stage breast cancer: final overall survival analysis from the randomized phase 3 ExteNET trial. Cancer Res. 2021;81(4):PD3-03. doi: 10.1158/1538-7445.SABCS20-PD3-03. [DOI] [Google Scholar]
- 207.Lin NU, Claus E, Sohl J, Razzak AR, Arnaout A, Winer EP. Sites of distant recurrence and clinical outcomes in patients with metastatic triple-negative breast cancer: high incidence of central nervous system metastases. Cancer. 2008;113(10):2638–2645. doi: 10.1002/cncr.23930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Albiges L, André F, Balleyguier C, Gomez-Abuin G, Chompret A, Delaloge S. Spectrum of breast cancer metastasis in BRCA1 mutation carriers: highly increased incidence of brain metastases. Ann Oncol. 2005;16(11):1846–1847. doi: 10.1093/annonc/mdi351. [DOI] [PubMed] [Google Scholar]
- 209.Song Y, Barry WT, Seah DS, Tung NM, Garber JE, Lin NU. Patterns of recurrence and metastasis in BRCA1/BRCA2-associated breast cancers. Cancer. 2020;126(2):271–280. doi: 10.1002/cncr.32540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Garber HR, Raghavendra AS, Lehner M, Qiao W, Gutierrez-Barrera AM, Tripathy D, et al. Incidence and impact of brain metastasis in patients with hereditary BRCA1 or BRCA2 mutated invasive breast cancer. NPJ Breast Cancer. 2022;8(1):1–8. doi: 10.1038/s41523-022-00407-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Tutt ANJ, Garber JE, Kaufman B, Viale G, Fumagalli D, Rastogi P, et al. Adjuvant olaparib for patients with BRCA1 - or BRCA2 -mutated breast cancer. N Engl J Med. 2021;384(25):2394–2405. doi: 10.1056/NEJMoa2105215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Chen S, Parmigiani G. Meta-analysis of BRCA1 and BRCA2 penetrance. J Clin Oncol. 2007;25(11):1329–1333. doi: 10.1200/JCO.2006.09.1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Copson ER, Maishman TC, Tapper WJ, Cutress RI, Greville-Heygate S, Altman DG, et al. Germline BRCA mutation and outcome in young-onset breast cancer (POSH): a prospective cohort study. Lancet Oncol. 2018;19(2):169–180. doi: 10.1016/S1470-2045(17)30891-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Breast Cancer Association Consortium. Dorling L, Carvalho S, Allen J, González-Neira A, Luccarini C, et al. Breast cancer risk genes—association analysis in more than 113,000 women. N Engl J Med. 2021;384(5):428–439. doi: 10.1056/NEJMoa1913948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Hu C, Hart SN, Gnanaolivu R, Huang H, Lee KY, Na J, et al. A population-based study of genes previously implicated in breast cancer. N Engl J Med. 2021;384(5):440–451. doi: 10.1056/NEJMoa2005936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Carbine NE, Lostumbo L, Wallace J, Ko H. Risk-reducing mastectomy for the prevention of primary breast cancer. Cochrane Breast Cancer Group, editor. Cochrane Database Syst Rev. 2018;2019(1). 10.1002/14651858.CD002748.pub4. [DOI] [PMC free article] [PubMed]
- 217.Metcalfe KA, Cil TD, Semple JL, Li LDX, Bagher S, Zhong T, et al. Long-term psychosocial functioning in women with bilateral prophylactic mastectomy: does preservation of the nipple-areolar complex make a difference? Ann Surg Oncol. 2015;22(10):3324–3330. doi: 10.1245/s10434-015-4761-3. [DOI] [PubMed] [Google Scholar]
- 218.Wood ME, McKinnon W, Garber J. Risk for breast cancer and management of unaffected individuals with non-BRCA hereditary breast cancer. Breast J. 2020;26(8):1528–1534. doi: 10.1111/tbj.13969. [DOI] [PubMed] [Google Scholar]
- 219.Berek JS, Chalas E, Edelson M, Moore DH, Burke WM, Cliby WA, et al. Prophylactic and risk-reducing bilateral salpingo-oophorectomy: recommendations based on risk of ovarian cancer. Obstet Gynecol. 2010;116(3):733–743. doi: 10.1097/AOG.0b013e3181ec5fc1. [DOI] [PubMed] [Google Scholar]
- 220.Domchek SM. Association of risk-reducing surgery in BRCA1 or BRCA2 mutation carriers with cancer risk and mortality. JAMA. 2010;304(9):967. doi: 10.1001/jama.2010.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Hadar T, Mor P, Amit G, Lieberman S, Gekhtman D, Rabinovitch R, et al. Presymptomatic awareness of germline pathogenic BRCA variants and associated outcomes in women with breast cancer. JAMA Oncol. 2020;6(9):1460. doi: 10.1001/jamaoncol.2020.2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Geuzinge HA, Obdeijn IM, Rutgers EJT, Saadatmand S, Mann RM, Oosterwijk JC, et al. Cost-effectiveness of breast cancer screening with magnetic resonance imaging for women at familial risk. JAMA Oncol. 2020;6(9):1381. doi: 10.1001/jamaoncol.2020.2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Mann RM, Kuhl CK, Moy L. Contrast-enhanced MRI for breast cancer screening. J Magn Reson Imaging. 2019;50(2):377–390. doi: 10.1002/jmri.26654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Gao Y, Reig B, Heacock L, Bennett DL, Heller SL, Moy L. Magnetic resonance imaging in screening of breast cancer. Radiol Clin North Am. 2021;59(1):85–98. doi: 10.1016/j.rcl.2020.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Familial breast cancer: classification, care and managing breast cancer and related risks in people with a family history of breast cancer (CG164)—NICE Clinical Guidelines. Available at https://www.nice.org.uk/guidance/cg164/resources/familial-breast-cancer-classification-care-and-managing-breast-cancer-and-related-risks-in-people-with-a-family-history-of-breast-cancer-pdf-35109691767493. 2019;51. [PubMed]
- 226.National Comprehensive Cancer Network (NCCN). Clinical practice guidelines. Genetic/familial high-risk assessment: breast, ovarian, and pancreatic. Version 1.2023. Available at https://www.nccn.org/professionals/physician_gls/pdf/genetics_bop.pdf. [DOI] [PubMed]
- 227.Lee CS, Monticciolo DL, Moy L. Screening guidelines update for average-risk and high-risk women. Am J Roentgenol. 2020;214(2):316–323. doi: 10.2214/AJR.19.22205. [DOI] [PubMed] [Google Scholar]
- 228.Guindalini RSC, Zheng Y, Abe H, Whitaker K, Yoshimatsu TF, Walsh T, et al. Intensive surveillance with biannual dynamic contrast-enhanced magnetic resonance imaging downstages breast cancer in BRCA1 mutation carriers. Clin Cancer Res. 2019;25(6):1786–1794. doi: 10.1158/1078-0432.CCR-18-0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Lee A, Mavaddat N, Wilcox AN, Cunningham AP, Carver T, Hartley S, et al. BOADICEA: a comprehensive breast cancer risk prediction model incorporating genetic and nongenetic risk factors. Genet Med. 2019;21(8):1708–1718. doi: 10.1038/s41436-018-0406-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Barnes DR, Rookus MA, McGuffog L, Leslie G, Mooij TM, Dennis J, et al. Polygenic risk scores and breast and epithelial ovarian cancer risks for carriers of BRCA1 and BRCA2 pathogenic variants. Genet Med. 2020;22(10):1653–1666. doi: 10.1038/s41436-020-0862-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Wentzensen N, Clarke MA. Liquid biopsy for cancer detection: clinical and epidemiologic considerations. Clin Cancer Res. 2021;27(21):5733–5735. doi: 10.1158/1078-0432.CCR-21-2426. [DOI] [PubMed] [Google Scholar]
- 232.McCabe C, McCann M, Brady AM. Computer and mobile technology interventions for self-management in chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2017;5:CD011425. doi: 10.1002/14651858.CD011425.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Ballester M, Orrego C, Heijmans M, Alonso-Coello P, Versteegh MM, Mavridis D, et al. Comparing the effectiveness and cost-effectiveness of self-management interventions in four high-priority chronic conditions in Europe (COMPAR-EU): a research protocol. BMJ Open. 2020;10(1):e034680. doi: 10.1136/bmjopen-2019-034680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Cuthbert CA, Farragher JF, Hemmelgarn BR, Ding Q, McKinnon GP, Cheung WY. Self-management interventions for cancer survivors: a systematic review and evaluation of intervention content and theories. Psychooncology. 2019;28(11):2119–2140. doi: 10.1002/pon.5215. [DOI] [PubMed] [Google Scholar]
- 235.Chan RJ, Crichton M, Crawford-Williams F, Agbejule OA, Yu K, Hart NH, et al. The efficacy, challenges, and facilitators of telemedicine in post-treatment cancer survivorship care: an overview of systematic reviews. Ann Oncol Off J Eur Soc Med Oncol. 2021;32(12):1552–1570. doi: 10.1016/j.annonc.2021.09.001. [DOI] [PubMed] [Google Scholar]
- 236.Abrahams HJG, Gielissen MFM, Donders RRT, Goedendorp MM, van der Wouw AJ, Verhagen CAHHVM, et al. The efficacy of Internet-based cognitive behavioral therapy for severely fatigued survivors of breast cancer compared with care as usual: a randomized controlled trial. Cancer. 2017;123(19):3825–3834. doi: 10.1002/cncr.30815. [DOI] [PubMed] [Google Scholar]
- 237.Singleton AC, Raeside R, Hyun KK, Partridge SR, Tanna GLD, Hafiz N, et al. Electronic health interventions for patients with breast cancer: systematic review and meta-analyses. J Clin Oncol. 2022 doi: 10.1200/JCO.21.01171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Zachariae R, Amidi A, Damholdt MF, Clausen CDR, Dahlgaard J, Lord H, et al. Internet-delivered cognitive-behavioral therapy for insomnia in breast cancer survivors: a randomized controlled trial. J Natl Cancer Inst. 2018;110(8):880–887. doi: 10.1093/jnci/djx293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Atema V, van Leeuwen M, Kieffer JM, Oldenburg HSA, van Beurden M, Gerritsma MA, et al. Efficacy of internet-based cognitive behavioral therapy for treatment-induced menopausal symptoms in breast cancer survivors: results of a randomized controlled trial. J Clin Oncol Off J Am Soc Clin Oncol. 2019;37(10):809–822. doi: 10.1200/JCO.18.00655. [DOI] [PubMed] [Google Scholar]
- 240.Bray VJ, Dhillon HM, Bell ML, Kabourakis M, Fiero MH, Yip D, et al. Evaluation of a web-based cognitive rehabilitation program in cancer survivors reporting cognitive symptoms after chemotherapy. J Clin Oncol Off J Am Soc Clin Oncol. 2017;35(2):217–225. doi: 10.1200/JCO.2016.67.8201. [DOI] [PubMed] [Google Scholar]
- 241.Holtdirk F, Mehnert A, Weiss M, Mayer J, Meyer B, Bröde P, et al. Results of the Optimune trial: a randomized controlled trial evaluating a novel Internet intervention for breast cancer survivors. PLoS ONE. 2021;16(5):e0251276. doi: 10.1371/journal.pone.0251276. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.