Abstract
Objective
To evaluate Ca intake and its association with cardiometabolic risk factors during childhood.
Design
A cross-sectional study with a representative sample. Food consumption was assessed through three 24 h dietary recalls. Anthropometry, body composition and biochemical measurements were also conducted.
Setting
Viçosa, Minas Gerais, Brazil.
Subjects
Children between 8 and 9 years old (n 350) enrolled in public and private schools in the urban area of the municipality of Viçosa.
Results
Almost all children had inadequate intake of Ca (97·4 %), especially those with low income, non-white and who studied in public schools. Foods that contributed most to Ca intake were ‘milk’ and ‘cheeses and yoghurts’ (R 2=0·66 and 0·13, respectively), and intake of ‘milk’ was correlated with ‘chocolate milk powder’ intake (r=0·538, P<0·01). Children with lower Ca intake had a higher prevalence of increased C-reactive protein (prevalence ratio=2·93; 95 % CI 1·21, 7·07), increased waist circumference (prevalence ratio=2·86; 95 % CI 1·01, 8·13) and a lower prevalence of high LDL cholesterol (prevalence ratio=0·64; 95 % CI 0·41, 0·99).
Conclusions
Lower Ca intake was associated with excess abdominal adiposity and subclinical inflammation in Brazilian children. Monitoring of adequate Ca intake is important, especially in poorer communities.
Keywords: Child, Food consumption, Inflammation, Obesity, Nutritional epidemiology
The number of children with cardiometabolic risk factors, such as excess abdominal adiposity, insulin resistance, dyslipidaemia and subclinical inflammation, has been increasing in many countries worldwide( 1 , 2 ). These metabolic alterations favour the development and progression of chronic non-communicable diseases during childhood( 3 ) and, consequently, the reduction of life quality and expectancy( 4 ).
Studies with adults have identified low Ca intake to be inversely associated with systemic inflammation( 5 ), adiposity( 6 ) and risk of developing diabetes( 7 , 8 ) and hypertension( 9 ), as well as an increase in mortality rate( 10 ).
In children, adequate Ca intake has been associated with better blood pressure( 11 , 12 ) and lower values of body fat and BMI( 13 – 15 ); however, these results are still controversial( 16 , 17 ). To date, no studies have been found evaluating the relationship between Ca intake and inflammatory markers in children.
Since there is a high prevalence of inadequate Ca intake among children( 18 – 20 ) and an adequate intake of this mineral may be related to better lipid profile, reduced inflammation and lower adiposity, the aim of the present study was to evaluate Ca intake and its association with cardiometabolic risk factors during childhood.
Methodology
Participants
The present study was a cross-sectional, population-based study carried out with children between the ages of 8 and 9 years who were included in the Survey of Health Assessment of Schoolchildren (Pesquisa de Avaliação da Saúde do Escolar, PASE) and enrolled in one of the public or private schools in the city’s urban area of Viçosa, Minas Gerais, Brazil.
In 2015, this city had seventeen public schools and seven private schools in the urban area, with 1464 children aged 8–9 years enrolled. During sample size calculation, we considered the prevalence as 50 % with multiple outcomes for cardiometabolic risk factors in this population, with a 95 % confidence level, a tolerable error of 5 %, 10 % for losses and 10 % for confounding factors, adding up to a sample of 366 children. However, 378 schoolchildren enrolled in the third and fourth year of primary school were selected by stratified random sampling.
The sample from each school was proportional to the total number of enrolled students by age and sex. Students were selected randomly until the required number of each was completed. All urban schools of the city attended by children of this age range were evaluated (n 24). After the draw, the parents or guardians were invited to participate in the study through a telephone call. We explained all the objectives and methodology to them and the first meeting was scheduled. At this point, each step of the study had been clarified to the parents or guardians, and those who agreed to participate signed a written informed consent.
Children using Ca supplementation were not included, nor were those taking medications or with health conditions that modified the nutritional status, body composition, lipid profile, blood pressure or glucose metabolism of the child. Children who had physical, cognitive or multiple disabilities, and those whose legal guardian could not be contacted after three attempts, were also not included in the research. All children with C-reactive protein (CRP) ≥10mg/l (n 28) were excluded since concentrations of this protein above this value are associated with infections and more serious inflammatory conditions, not subclinical inflammation. Thus, the final sample for the present study consisted of 350 children.
The study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the Ethics Committee on Human Research of the Federal University of Viçosa (case number 663.171/2014).
Demographic data
Sociodemographic data, such as sex, age, ethnicity, type of school (public or private), area of residence (urban or rural) and family per capita income, were collected by the researchers through a semi-structured questionnaire. Prior to beginning the research, a pilot study had been performed with 10 % of the sample (n 37) in order to adjust the questionnaire as well as other measures evaluated in the research. Pilot children were not included in the final sample of the present study.
Food consumption
To estimate food consumption, three 24 h dietary recalls were applied on non-consecutive days, including one day of the weekend, and filled in according to information provided by the mother/guardian and the child. To increase data reliability, interviewers went through a specific training and during the interviews participants were shown standard utensils and photographic albums with food preparation and pictures distinguishing different portions( 21 ). Later, the measures done at each individual’s house were transformed into grams or millilitres to analyse intakes of energy (in kilocalories) and Ca (in milligrams).
We estimated nutrient intakes from the 24 h dietary recall using Diet Pro® 5i software version 5.8. For this, the Brazilian Food Composition Table( 22 ) and the US Department of Agriculture’s Food Composition Database( 23 ) were used.
To evaluate the adequacy of Ca intake, we considered the Estimated Average Requirement according to sex and life stage proposed by the Institute of Medicine( 24 ).
Anthropometry and body composition
Child weight was measured using an electronic digital scale with a capacity of 150 kg and sensitivity of 50 g (Tanita® model Ironman BC 553; Tanita Corporation of America, Inc., Arlington Heights, IL, USA), while child height was measured using a vertical stadiometer divided into centimetres and subdivided into millimetres (Alturexata®, Belo Horizonte, MG, Brazil) according to the norms of Jelliffe( 25 ). BMI was calculated based on weight and height. To classify children according to their nutritional status, BMI-for-age cut-off points by Z-score were calculated using the WHO Anthro Plus software( 26 ) and classified according to the WHO( 27 ). Children were considered to have excess weight when they were classified as overweight or obese by BMI.
Waist circumference was measured using an inelastic measuring tape divided into centimetres and subdivided into millimetres, with measurements taken at the midpoint between the iliac crest and the last rib( 28 ). The 90th percentile of the sample was adopted to classify increased waist circumference due to the absence of cut-off points established for this age group. Waist-to-height ratio (WHtR) was calculated as the ratio of waist circumference to height. Cut-off point for WHtR of ≥0·5 was considered as risk of development of cardiometabolic diseases( 29 ).
Body composition of the child was estimated by the dual-energy X-ray absorptiometry method (Lunar Prodigy Advance; GE Medical Systems Lunar, Milwaukee, WI, USA) and was classified according to cut-off points proposed by Lohman( 30 ).
Biochemical variables
Blood collection was performed by a qualified professional after 12 h of fasting through a venepuncture procedure with disposable material. Samples were collected in a vacuum tube and centrifuged for 15 min at 3500 rpm; then the serum samples were separated into 1·5 ml Eppendorf tubes and stored in an ultra-freezer at −80°C.
Serum concentrations of total cholesterol (mg/dl), HDL cholesterol (HDL-C; mg/dl), LDL cholesterol (LDL-C; mg/dl), TAG (mg/dl) and insulin (μU/ml) were determined using BioSystems model 200 Mindray® equipment (Nanchan, China) as recommended by the manufacturer of the Bioclin® kits (Belo Horizonte, MG, Brazil).
Serum insulin was analysed through chemiluminescence immunoassay using the Elecsys Insulin® test (Roche Diagnostics, Indianapolis, IN, USA) with a detection limit of 0·200–1000 μU/ml. Insulin resistance was estimated by the homeostasis model assessment of insulin resistance (HOMA-IR)( 31 ).
Levels of HDL-C<45 mg/dl, LDL-C≥100 mg/dl, TAG≥100 mg/dl, glucose≥100 mg/dl and fasting insulin >15 μU/ml were considered inadequate( 32 , 33 ). The 90th percentile of the sample was used to classify increases in high-sensitivity CRP and HOMA-IR assays due to the absence of cut-off points established for this age group.
Statistical analysis
The analysis was carried out using the statistical software packages IBM SPSS Statistics® version 24 and Stata version 13. The Kolmogorov–Smirnov test was used to evaluate the normality of the variables. Adjustment was made for intra-individual variability( 34 ) and for energy by the residual method( 35 ).
We divided the sample according to the 75th percentile of Ca intake and used the Pearson χ 2 test to verify associations between the variables. ANOVA was used for multiple comparisons in tertiles of Ca intake, with application of the post hoc Tukey test to identify the differences between the groups.
The multiple stepwise regression test was performed to verify which major food items contributed to the variability in Ca intake of the children.
Bivariate analysis was performed based on Poisson regression models with robust variance. Cardiometabolic risk factors were considered dependent variables with daily Ca intake as the explanatory variable. Predictive variables that obtained a P value of <0·20 were inserted by the backward method in the multivariate Poisson regression model with robust variance. Variables with a lower level of significance (P≥0·05) were taken one by one from the model. The models were still adjusted for potential confounding factors. The Hosmer–Lemeshow test was used to verify the adjustment of the final model. Prevalence ratio (PR) with 95 % CI was used as an effect measure. During all analyses, a significance level of 5 % was adopted.
Results
Among the participating children (n 350), 52·6 % were female and 51·4 % were 9 years old. The children with the lowest Ca intake were 9 years old, non-white, public-school students, with low HDL-C and high LDL-C (P<0·05; Table 1).
Table 1.
Sociodemographic, anthropometric and biochemical variables according to calcium intake among urban schoolchildren aged 8–9 years (n 350), Viçosa, Minas Gerais, Brazil, 2015
| Ca intake (mg) | |||||||
|---|---|---|---|---|---|---|---|
| Total | <P75 | ≥P75 | |||||
| Variable | n | % | n | % | n | % | P value |
| Sex | 0·667 | ||||||
| Male | 166 | 47·4 | 123 | 74·1 | 43 | 25·9 | |
| Female | 184 | 52·6 | 140 | 76·1 | 44 | 23·9 | |
| Age (years) | 0·001* | ||||||
| 8 | 170 | 48·6 | 114 | 67·1 | 56 | 32·9 | |
| 9 | 180 | 51·4 | 149 | 82·8 | 31 | 17·2 | |
| Ethnicity | 0·001* | ||||||
| White | 114 | 32·6 | 73 | 64·0 | 41 | 36·0 | |
| Non-white | 236 | 67·4 | 190 | 80·5 | 46 | 19·5 | |
| Type of school | 0·016* | ||||||
| Public | 245 | 70·0 | 193 | 78·8 | 52 | 21·2 | |
| Private | 105 | 30·0 | 70 | 66·7 | 35 | 33·3 | |
| Residence area | 0·307 | ||||||
| Urban | 333 | 95·1 | 252 | 75·7 | 81 | 24·3 | |
| Rural | 17 | 4·9 | 11 | 64·7 | 6 | 35·3 | |
| Maternal education (years) | 0·094 | ||||||
| <8 | 85 | 24·3 | 70 | 82·4 | 15 | 17·6 | |
| ≥8 | 263 | 75·1 | 193 | 73·4 | 70 | 26·6 | |
| BMI (kg/m²) | 0·626 | ||||||
| Adequate | 238 | 68·0 | 177 | 74·4 | 61 | 25·6 | |
| Excess of weight | 112 | 32·0 | 86 | 76·8 | 26 | 23·2 | |
| Waist circumference (cm) | 0·611 | ||||||
| Adequate (<P90) | 317 | 90·6 | 237 | 74·8 | 80 | 25·2 | |
| Increased (≥P90) | 33 | 9·4 | 26 | 78·8 | 7 | 21·2 | |
| WHtR | 0·507 | ||||||
| Adequate | 292 | 83·4 | 217 | 82·5 | 75 | 86·2 | |
| Increased | 58 | 16·6 | 46 | 17·5 | 12 | 13·8 | |
| BF (%) | 0·215 | ||||||
| Adequate | 181 | 51·7 | 131 | 72·4 | 50 | 27·6 | |
| Excess of adiposity | 169 | 48·3 | 132 | 78·1 | 37 | 21·9 | |
| TC (mg/dl) | 0·145 | ||||||
| Adequate | 292 | 83·4 | 132 | 78·6 | 36 | 21·4 | |
| Increased | 58 | 16·6 | 130 | 71·8 | 51 | 28·2 | |
| HDL-C (mg/dl) | 0·041* | ||||||
| Adequate | 251 | 71·7 | 181 | 72·1 | 70 | 27·9 | |
| Low | 98 | 28·0 | 81 | 82·7 | 17 | 17·3 | |
| LDL-C (mg/dl) | 0·011* | ||||||
| Adequate | 256 | 73·1 | 202 | 78·9 | 54 | 21·1 | |
| Increased | 93 | 26·6 | 61 | 65·6 | 32 | 34·4 | |
| TAG (mg/dl) | 0·330 | ||||||
| Adequate | 285 | 81·4 | 217 | 76·1 | 68 | 23·9 | |
| Increased | 64 | 18·3 | 45 | 70·3 | 19 | 29·7 | |
| CRP (mg/l) | 0·053 | ||||||
| Adequate (<P90) | 315 | 90·0 | 232 | 73·7 | 83 | 26·3 | |
| Increased (≥P90) | 35 | 10·0 | 31 | 88·6 | 4 | 11·4 | |
| Glucose (mg/dl) | 0·637 | ||||||
| Adequate | 343 | 98·0 | 257 | 79·4 | 86 | 25·1 | |
| Increased | 6 | 1·7 | 5 | 83·3 | 1 | 16·7 | |
| HOMA-IR | 0·638 | ||||||
| Adequate (<P90) | 341 | 97·4 | 256 | 75·1 | 85 | 29·4 | |
| Increased (≥P90) | 6 | 1·7 | 4 | 66·7 | 2 | 33·3 | |
P75, 75th percentile; P90, 90th percentile; WHtR, waist-to-height ratio; BF (%), body fat percentage; TC, total cholesterol; HDL-C, HDL cholesterol; LDL-C, LDL cholesterol; CRP, C-reactive protein; HOMA-IR, homeostasis model assessment of insulin resistance.
P<0·05 (Pearson’s χ2 test).
After adjusting the results for intra-individual variability, there was a high prevalence of inadequate Ca intake (97·4 %). In addition, the most consumed sources of Ca were ‘milk’ and ‘cheeses and yoghurts’ (R 2=0·66 and 0·13, respectively). There was a moderate correlation between milk consumption and chocolate milk powder (r=0·538, P<0·01). Food items listed in Table 2 explained 66–91 % of the total variability of Ca intake.
Table 2.
Main food items consumed that contributed to calcium intake by urban schoolchildren aged 8–9 years (n 350), Viçosa, Minas Gerais, Brazil, 2015
| Food group | R 2 | Accumulated R 2 |
|---|---|---|
| Milk | 0·66 | 0·66 |
| Cheeses and yoghurts | 0·13 | 0·79 |
| Sweets and desserts† | 0·06 | 0·85 |
| Fortified cereals‡ | 0·04 | 0·89 |
| Fishes | 0·01 | 0·90 |
| Leguminous | 0·01 | 0·91 |
Ice cream, milkshake, chocolate powder, industrialized chocolate milk, chocolate, sweets in general.
Mucilon, breakfast cereal, milk flour, breads, cookies and cakes.
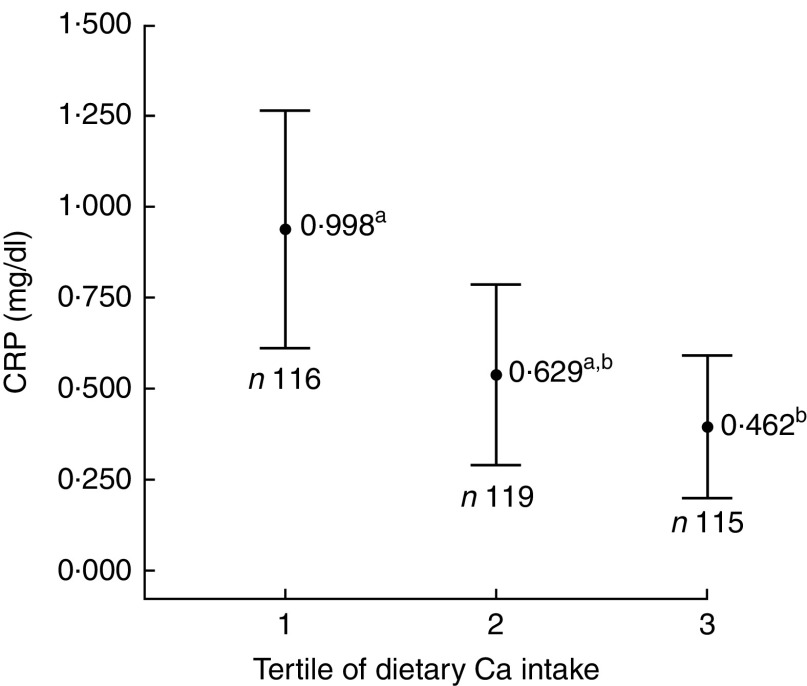
Individuals with Ca intake in the first tertile had lower mean per capita income (P<0·001; Table 3) and higher serum level of CRP (P=0·012; Fig. 1) when compared with the second and third tertiles.
Table 3.
Sociodemographic, anthropometric and biochemical variables according to tertile of calcium intake among urban schoolchildren aged 8–9 years (n 350), Viçosa, Minas Gerais, Brazil, 2015
| Ca intake (mg) | |||||||
|---|---|---|---|---|---|---|---|
| 1st tertile (n 116) | 2nd tertile (n 119) | 3rd tertile (n 115) | |||||
| Variables | Mean | sd | Mean | sd | Mean | sd | P value |
| Income per capita (reais) | 506·08a | 440·20 | 734·51b | 721·19 | 951·93b | 825·73 | <0·001* |
| BMI (kg/m²) | 17·80 | 3·87 | 17·58 | 3·33 | 16·95 | 2·97 | 0·456 |
| Waist circumference (cm) | 61·38 | 10·45 | 60·97 | 9·21 | 58·72 | 8·14 | 0·269 |
| WHtR | 0·45 | 0·06 | 0·44 | 0·06 | 0·43 | 0·05 | 0·213 |
| BF (%) | 24·99 | 11·16 | 23·71 | 9·69 | 23·35 | 9·57 | 0·807 |
| TC (mg/dl) | 150·64 | 22·38 | 152·14 | 27·07 | 156·80 | 29·05 | 0·151 |
| HDL-C (mg/dl) | 49·19 | 9·60 | 50·20 | 10·93 | 52·43 | 9·93 | 0·182 |
| LDL-C (mg/dl) | 84·79 | 20·60 | 85·28 | 20·76 | 90·50 | 24·26 | 0·097 |
| TAG (mg/dl) | 79·56 | 40·66 | 76·99 | 38·90 | 82·83 | 35·78 | 0·536 |
| Glucose (mg/dl) | 84·72 | 8·87 | 84·62 | 7·37 | 85·49 | 7·00 | 0·602 |
| HOMA-IR | 1·19 | 0·72 | 1·19 | 1·02 | 1·11 | 0·66 | 0·490 |
WHtR, waist-to-height ratio; BF (%), body fat percentage; TC, total cholesterol; HDL-C, HDL cholesterol; LDL-C, LDL cholesterol; HOMA-IR. homeostasis model assessment of insulin resistance.
a,bMean values within a row with unlike superscript letters were significantly different.
P<0·05 (ANOVA with post hoc Tukey test).
Fig. 1.
Mean serum concentration of C-reactive protein (CRP), with 95% CI represented by vertical bars, according to tertile of calcium intake among urban schoolchildren aged 8–9 years (n 350), Viçosa, Minas Gerais, Brazil, 2015. a,bMean values with unlike superscript letters were significantly different: P=0·012*. *P<0·05 (ANOVA with post hoc Tukey test)
In the adjusted regression models, children with Ca consumption in the first tertile (0–341·98mg) presented higher prevalence of increased waist circumference (PR=2·86; 95 % CI 1·01, 8·13; P=0·048) and increased CRP (PR=2·93; 95 % CI 1·21, 7·07; P=0·017), as well as a lower prevalence of increased LDL-C (PR=0·64; 95 % CI 0·41, 0·99; P=0·047), compared with children in the third tertile of Ca consumption (≥485·82 mg; Table 4).
Table 4.
Crude and adjusted prevalence ratios (PR) of the association between calcium consumption tertile and cardiometabolic markers among urban schoolchildren aged 8–9 years (n 350), Viçosa, Minas Gerais, Brazil, 2015
| Ca intake (mg) | ||||||
|---|---|---|---|---|---|---|
| 1st tertile | 2nd tertile | 3rd tertile | ||||
| Cardiometabolic marker | PR | 95 % CI | PR | 95 % CI | PR | 95 % CI |
| Waist circumference (≥P90) | ||||||
| Crude | 1·73 | 0·76, 3·98 | 1·33 | 0·55, 3·19 | Reference | |
| P value | 0·194 | 0·524 | ||||
| Adjusted† | 2·86 | 1·01, 8·13 | 1·79 | 0·63, 5·08 | Reference | |
| P value | 0·048* | 0·270 | ||||
| LDL-C (≥100 mg/dl) | ||||||
| Crude | 0·64 | 0·41, 0·99 | 0·75 | 0·50, 1·13 | Reference | |
| P value | 0·049* | 0·176 | ||||
| Adjusted‡ | 0·64 | 0·41, 0·99 | 0·75 | 0·50, 1·13 | Reference | |
| P value | 0·047* | 0·174 | ||||
| CRP (≥P90) | ||||||
| Crude | 2·64 | 1·07, 6·52 | 1·61 | 0·60, 4·29 | Reference | |
| P value | 0·035* | 0·341 | ||||
| Adjusted‡ | 2·93 | 1·21, 7·07 | 1·69 | 0·65, 4·42 | Reference | |
| P value | 0·017* | 0·277 | ||||
P90, 90th percentile; LDL-C, LDL cholesterol; CRP, C-reactive protein.
P < 0·05 (Poisson regression).
Adjusted for sex, age, ethnicity, maternal age and schooling, type of school, sedentary behaviour, and percentage of energy from fat, protein and carbohydrate.
Adjusted for sex, age, ethnicity, maternal schooling, daily number of meals, body fat percentage, sedentary behaviour, and percentage of energy from fat and carbohydrate.
Discussion
Children with lower Ca intake had higher prevalence of increased CRP, increased waist circumference, as well as a lower prevalence of elevated LDL-C.
Higher serum concentration of CRP in children with lower Ca intake may be related to bioactive compounds present in dairy foods, such as Ca, phospholipids, proteins and peptides, which may decrease the inflammatory process in adipose tissue by decreasing the serum concentration of CRP( 36 – 38 ). In research done with adults, Labonté et al. ( 39 ) observed an improvement in concentration of inflammatory markers, including reduction of serum CRP, due to an increased consumption of Ca-containing food sources. On the other hand, other studies with adolescents and adults identified a null effect of dairy intake on the CRP concentration( 5 , 40 – 43 ). Therefore, since these findings are controversial in adults and no studies have been found with children, the present study highlights the need for longitudinal investigations with child populations to elucidate possible mechanisms involved in the relationship between Ca intake and the presence of subclinical inflammation.
The inverse association between Ca intake and abdominal adiposity identified in the current study corroborates other investigations carried out with 10–12-year-old boys( 44 ), 12–19-year-old girls( 6 ) and 7–18-year-old obese children and adolescents( 45 ). Major et al. ( 46 ) identified a possible mechanism to explain the relationship between low Ca intake and abdominal obesity. Adequate Ca intake can reduce the concentration of calcitriol. Therefore, autocrine activation of cortisol synthesis would decrease, leading to less fat accumulation( 47 , 48 ). This occurs because calcitriol stimulates the expression of 11-β-hydroxysteroid dehydrogenase-1, which helps in the conversion of cortisone into cortisol, a substance that acts in the accumulation of fat, mainly in the abdominal region( 49 ). Another accepted mechanism is the increase in the oxidative capacity of adipose tissue when there is an adequate intake of Ca. Higher intake of this mineral would induce intracellular Ca content to decrease in adipose tissue, promoting fat oxidation rather than its deposition( 44 ).
Children with Ca consumption in the first tertile had a lower prevalence of increased LDL-C. We believe that sugar-rich chocolate powder addition to milk may have contributed to this finding since, in the present study, milk accounted for 66 % of Ca intake. Furthermore, all children who reported to consume milk (n 213) were recorded to do so with chocolate milk powder, which corresponds to 60·8 % of the total sample (n 350). Frequent intake of powdered chocolate by children has been described (19·6–49 %)( 50 , 51 ) and usually associated with milk( 52 , 53 ). Ingestion of sugar contributes to an increase in adiposity( 54 , 55 ) and LDL-C( 56 , 57 ), since excessive sugar intake is involved in the synthesis of free radicals, such as reactive oxygen species, cytokines and molecular adhesion molecules. These components lead to the formation of oxidized LDL-C, which stimulates the migration of macrophages to form foam cells, resulting in the progression of atherosclerosis( 58 ).
Dairy foods may be high in saturated fats and an elevated consumption of this nutrient is associated with worsening markers of cardiovascular risk( 59 , 60 ). However, some studies did not find a relationship of fat intake from dairy products with worsening of the lipid profile, inflammation and obesity( 61 – 64 ). This may be because Ca forms insoluble soaps with fatty acids, preventing them from being absorbed since Ca causes fatty acids to be bounded to bile salts, preventing their resorption in the intestine( 65 ). Thus, greater fat excretion occurs when there is higher Ca content in the diet( 66 , 67 ).
Another result to be highlighted is the inadequate intake of Ca in 97·4 % of the sample. In fact, Ca is one of the micronutrients with the highest rate of inadequate consumption worldwide( 68 ). In the literature, most studies with children and adolescents have identified low Ca intake (88·6–97·6 %), corroborating the results of the current study( 12 , 69 , 70 ). Low Ca intake is worrying because this mineral is important for child growth and may be involved with early development of cardiometabolic diseases( 38 , 46 , 71 ). However, we do not know if the high prevalence of inadequate Ca intake among children in our study could affect its relationship to the cardiometabolic risk factors, since few children presented adequate Ca intake (n 9·1, 2·6 %). Therefore, studies conducted with a larger sample size are of scientific interest.
In the present study, milk was the main contributor to Ca intake (66 %), followed by cheeses and yoghurts (13 %; Table 3). Milk has a lower cost compared with other dairy products, which may explain its higher consumption( 72 ). On the other hand, dark green leaves did not contribute to explain the variability in Ca intake, since many children (83 %) consumed less than one portion of these foods daily, well below the daily recommendation of three portions( 73 ).
Non-white children with lower socio-economic status (lower income per capita and students from public schools) had lower Ca intakes. In addition, 84·3 % of non-white study children had an income of up to one minimum wage per capita. Epidemiological studies with child and adolescent populations have also shown that individuals with lower income and those studying in public schools present lower intakes of Ca( 70 , 72 ). Another study evaluating dairy intake according to income, using data from the Household Budget Survey (2002–2003), identified a positive association between Ca intake and income in Brazilian families( 74 ). In this sense, acquisition of dairy products, which are the main sources of Ca, may not be affordable to the budget of low-income families. Thus, they opt for other lower-cost foods.
The current study has some limitations. Because of the cross-sectional design, it is not possible to establish a temporal relationship between Ca intake and cardiometabolic risk factors since the direction of causality is unknown; that is, if the lower consumption of Ca causes cardiometabolic changes or vice versa. In addition, the instrument used to estimate dietary intake (24 h recall) may show poor estimates because it depends on the memory of the children and their legal guardian. However, some good points should be highlighted. We used strategies to reduce memory bias and improve portion estimation, such as interviewer training and the use of photo albums and standard tools. Adjustment of Ca intake was also made to account for intra-individual variability and total energy intake. In addition, the present study is one of the few that have evaluated the relationship of Ca intake with cardiometabolic risk factors and inflammatory markers in the child population; and it is the first Brazilian study within this age group to date.
Conclusion
In conclusion, lower intake of Ca in Brazilian children was found to be associated with excess abdominal adiposity and subclinical inflammation. Prevalence of inadequate Ca intake was high, with the lowest intake being observed in low-income, non-white children and those attending public schools. Furthermore, effective performance of health professionals in monitoring adequate Ca intake is important, especially in poorer communities. Lastly, longitudinal studies are needed to better elucidate the causal direction between low Ca intake and cardiometabolic risk factors in the child population, especially in developing countries such as Brazil.
Acknowledgements
Acknowledgements: The authors thank all children who participated in this work and their parents/guardians; BioClin® (Belo Horizonte, MG, Brazil) for their support in the biochemical analyses and Diet Pro® software for the license granted; and the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and the Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG) for the scholarships granted to L.G.S., B.K.S.S. and M.S.F. Financial support: This work was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (grant number 407547/2012-6). H.H.M.H. and M.C.G.P. have a fellowship in Research Productivity from CNPq. The CNPq had no role in the design, analysis or writing of this article. Conflict of interest: The authors declare no conflict of interest. Authorship: L.G.S. assisted the conception and design of this work, analysis and interpretation of the data, conducted the literature search, as well as wrote the manuscript. B.K.S.S. contributed to analysis and interpretation of the data, conducted the literature search, as well as wrote the manuscript. M.S.F. assisted in data collection, analysis and interpretation of the data, and revised and approved the final version to be published. M.C.G.P. revised and approved the final version to be published. H.H.M.H. assisted in the interpretation of results and approved the final version to be published. J.F.N. designed the study including the data collection and coordinated, supervised and approved the final version to be published. Ethics of human subject participation: This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all the procedures involving human subjects were approved by the Ethics Committee on Human Research of the Federal University of Viçosa (case number 663.171/2014). Moreover, this project was presented to the Municipal Department of Education, the Regional Superintendent of Education and principals of schools. All participants, as well as their responsible parents/guardians, were informed about the objectives of the research and written informed consent was obtained from all children’s parents.
References
- 1. Damsgaard CT, Dalskov S, Laursen RP et al. (2014) Provision of healthy school meals does not affect the metabolic syndrome score in 8–11-year-old children, but reduces cardiometabolic risk markers despite increasing waist circumference. Br J Nutr 112, 1826–1836. [DOI] [PubMed] [Google Scholar]
- 2. Wang Y & Lobstein T (2006) Worldwide trends in childhood overweight and obesity. Int J Pediatr Obes 1, 11–25. [DOI] [PubMed] [Google Scholar]
- 3. Freedman DS, Khan LK, Serdula MK et al. (2005) The relation of childhood BMI to adult adiposity: the Bogalusa Heart Study. Pediatrics 115, 22–27. [DOI] [PubMed] [Google Scholar]
- 4. Hulsegge G, Looman M, Smit HA et al. (2016) Lifestyle changes in young adulthood and middle age and risk of cardiovascular disease and all-cause mortality: the Doetinchem Cohort Study. J Am Heart Assoc 5, e002432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zemel MB & Sun X (2008) Dietary calcium and dairy products modulate oxidative and inflammatory stress in mice and humans. J Nutr 138, 1047–1052. [DOI] [PubMed] [Google Scholar]
- 6. Castro Burbano J, Fajardo Vanegas P, Robles Rodríguez J et al. (2016) Relationship between dietary calcium intake and adiposity in female adolescents. Endocrinol Nutr 63, 58–63. [DOI] [PubMed] [Google Scholar]
- 7. Pittas AG, Lau J, Hu FB et al. (2007) The role of vitamin D and calcium in type 2 diabetes. A systematic review and meta-analysis. J Clin Endocrinol Metab 92, 2017–2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Martini LA, Catania AS & Ferreira SRG (2010) Role of vitamins and minerals in prevention and management of type 2 diabetes mellitus. Nutr Rev 68, 341–354. [DOI] [PubMed] [Google Scholar]
- 9. Wang L, Manson JE, Buring JE et al. (2008) Dietary intake of dairy products, calcium, and vitamin D and the risk of hypertension in middle-aged and older women. Hypertension 51, 1073–1079. [DOI] [PubMed] [Google Scholar]
- 10. Asemi Z, Saneei P, Sabihi SS et al. (2015) Total, dietary, and supplemental calcium intake and mortality from all-causes, cardiovascular disease, and cancer: a meta-analysis of observational studies. Nutr Metab Cardiovasc Dis 25, 623–634. [DOI] [PubMed] [Google Scholar]
- 11. Rangan AM, Flood VL, Denyer G et al. (2012) The effect of dairy consumption on blood pressure in mid-childhood: CAPS cohort study. Eur J Clin Nutr 66, 652–657. [DOI] [PubMed] [Google Scholar]
- 12. Magalhães EI, Pessoa MC, Franceschini SD et al. (2017) Dietary calcium intake is inversely associated with blood pressure in Brazilian children. Int J Food Sci Nutr 68, 331–338. [DOI] [PubMed] [Google Scholar]
- 13. Skinner JD, Bounds W, Carruth BR et al. (2003) Longitudinal calcium intake is negatively related to children’s body fat indexes. J Am Diet Assoc 103, 1626–1631. [DOI] [PubMed] [Google Scholar]
- 14. Keast DR, Gallant KMH, Albertson AM et al. (2015) Associations between yogurt, dairy, calcium, and vitamin D intake and obesity among US children aged 8–18 years: NHANES, 2005–2008. Nutrients 7, 1577–1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dixon LB, Pellizzon MA, Jawad AF et al. (2005) Calcium and dairy intake and measures of obesity in hyper- and normocholesterolemic children. Obes Res 13, 1727–1738. [DOI] [PubMed] [Google Scholar]
- 16. Weaver CM, Campbell WW, Teegarden D et al. (2011) Calcium, dairy products, and energy balance in overweight adolescents: a controlled trial. Am J Clin Nutr 94, 1163–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Moreira P, Padez C, Mourão I et al. (2005) Dietary calcium and body mass index in Portuguese children. Eur J Clin Nutr 59, 861–867. [DOI] [PubMed] [Google Scholar]
- 18. Oliveira CF, Silveira CR, Beghetto M et al. (2014) Assessment of calcium intake by adolescents. Rev Paul Pediatr 32, 216–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tylavsky FA, Cowan PA, Terrell S et al. (2010) Calcium Intake and body composition in African-American children and adolescents at risk for overweight and obesity. Nutrients 2, 950–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Santos LC, Martini LA, Freitas SN et al. (2007) Calcium intake and anthropometric indicators in adolescents. Rev Nutr 20, 275–283. [Google Scholar]
- 21. Zabotto CB, Vianna RPT & Gil MF (1996) Registro Fotográfico Para Inquéritos Dietéticos: Utensílios e Porções. Goiânia: Nepa-Unicamp; available at http://www.fcm.unicamp.br/fcm/sites/default/files/2016/page/manual_fotografico.pdf [Google Scholar]
- 22. Núcleo de Estudos e Pesquisas em Alimentação, Universidade Estadual de Campinas (2011) Tabela Brasileira de Composição de Alimentos – TACO, 4ª ed. rev. e ampl. Campinas: Nepa–Unicamp.
- 23. US Department of Agriculture, Agricultural Research Service (2016) USDA National Nutrient Database for Standard Reference (Release 28). http://www.fnic.nal.usda.gov/food-composition/usda-nutrient-data-laboratory (accessed July 2017).
- 24. Institute of Medicine (2011) Dietary Reference Intakes for Calcium and Vitamin D. Washington, DC: The National Academies Press. [PubMed] [Google Scholar]
- 25. Jellife DB & World Health Organization (1968) Evaluación del Estado de Nutrición de la Comunidad. Geneva: WHO; available at http://www.apps.who.int/iris/handle/10665/41408 [Google Scholar]
- 26. World Health Organization (2009) WHO AnthroPlus for Personal Computers Manual: Software for Assessing Growth of the World’s Children and Adolescents. Geneva: WHO. [Google Scholar]
- 27. de Onis M, Onyango AW, Borghi E et al. (2007) Development of a WHO growth reference for school-aged children and adolescents. Bull World Health Organ 85, 660–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. World Health Organization (2000) Obesity: Preventing and Managing the Global Epidemic. Report of a WHO Consultation. WHO Technical Report Series no. 894. Geneva: WHO. [PubMed]
- 29. Ashwell M & Hsieh SD (2005) Six reasons why the waist-to-height ratio is a rapid and effective global indicator for health risks of obesity and how its use could simplify the international public health message on obesity. Int J Food Sci Nutr 56, 303–307. [DOI] [PubMed] [Google Scholar]
- 30. Lohman TG (1992) Advances in Body Composition Assessment: Current Issues in Exercise Science. Champaign, IL: Human Kinetics Publishers. [Google Scholar]
- 31. Matthews DR, Hosker JP, Rudenski AS et al. (1985) Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28, 412–419. [DOI] [PubMed] [Google Scholar]
- 32. Sociedade Brasileira de Cardiologia (2005) I diretriz de prevenção da aterosclerose na infância e na adolescência. Arq Bras Cardiol 85, 4–35. [PubMed] [Google Scholar]
- 33. American Dietetic Association (2006) Diagnosis and classification of diabetes mellitus. Position statement. Diabetes Care 29, Suppl. 1, 43S–48S. [PubMed] [Google Scholar]
- 34. Fisberg RM, Marchioni DML & Colucci ACA (2009) Avaliação do consumo alimentar e da ingestão de nutrientes na prática clínica. Arq Bras Endocrinol Metab 53, 617–624. [DOI] [PubMed] [Google Scholar]
- 35. Willett W (2013) Nutritional Epidemiology, 3th ed. New York: Oxford University Press. [Google Scholar]
- 36. Zemel MB & Sun X (2008) Dietary calcium and dairy products modulate oxidative and inflammatory stress in mice and humans. J Nutr 138, 1047–1052. [DOI] [PubMed] [Google Scholar]
- 37. Beermann C & Hartung J (2013) Physiological properties of milk ingredients released by fermentation. Food Funct 4, 185–199. [DOI] [PubMed] [Google Scholar]
- 38. Astrup A (2014) Yogurt and dairy product consumption to prevent cardiometabolic diseases: epidemiologic and experimental studies. Am J Clin Nutr 99, 5 Suppl., 1235S–1242S. [DOI] [PubMed] [Google Scholar]
- 39. Labonté MÈ, Couture P, Richard C et al. (2013) Impact of dairy products on biomarkers of inflammation: a systematic review of randomized controlled nutritional intervention studies in overweight and obese adults. Am J Clin Nutr 97, 706–717. [DOI] [PubMed] [Google Scholar]
- 40. Ghayour-Mobarhan M, Sahebkar A, Vakili R, Safarian M et al. (2009) Investigation of the effect of high dairy diet on body mass index and body fat in overweight and obese children. Indian J Pediatr 76, 1145–1150. [DOI] [PubMed] [Google Scholar]
- 41. Benatar JR, Sidhu K & Stewart RAH (2013) Effects of high and low fat dairy food on cardio-metabolic risk factors: a meta-analysis of randomized studies. PLoS One 8, e76480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Labonté MÈ, Cyr A, Abdullah MM et al. (2014) Dairy product consumption has no impact on biomarkers of inflammation among men and women with low-grade systemic inflammation. J Nutr 144, 1760–1767. [DOI] [PubMed] [Google Scholar]
- 43. Schmid A, Petry N, Walther B et al. (2015) Inflammatory and metabolic responses to high-fat meals with and without dairy products in men. Br J Nutr 113, 1853–1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chen Y, Liu Y, Xue H et al. (2016) Association between dietary calcium/dairy intakes and overweight/obesity. Wei Sheng Yan Jiu 5, 402–408. [PubMed] [Google Scholar]
- 45. Czerwonogrodzka A, Pyrzak B, Majcher A et al. (2008) Assessment of dietary calcium intake on metabolic syndrome frequency in obese children and adolescents. Pediatr Endocrinol Diabetes Metab 14, 231–235. [PubMed] [Google Scholar]
- 46. Major GC, Chaput JP, Ledoux M et al. (2008) Recent developments in calcium-related obesity research. Obes Rev 9, 428–445. [DOI] [PubMed] [Google Scholar]
- 47. Da Cunha KA, Magalhães ELS, Loureiro lMR et al. (2015) Ingestão de cálcio, níveis séricos de vitamina D e obesidade infantil: existe associação? Rev Paul Pediatr 33, 222–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zemel MB & Miller SL (2004) Dietary calcium and dairy modulation of adiposity and obesity risk. Nutr Rev 62, 125–131. [DOI] [PubMed] [Google Scholar]
- 49. Dougkas A, Reynolds CK, Givens ID et al. (2011) Associations between dairy consumption and body weight: a review of the evidence and underlying mechanisms. Nutr Res Rev 24, 75–95. [DOI] [PubMed] [Google Scholar]
- 50. Aquino RC & Philippi ST (2002) Association of children’s consumption of processed foods and family income in the city of São Paulo, Brazil. Rev Saude Publica 36, 655–660. [DOI] [PubMed] [Google Scholar]
- 51. Hinning PF & Bergamaschi DP (2012) Itens alimentares no consumo alimentar de crianças de 7 a 10 anos. Rev Bras Epidemiol 15, 324–334. [DOI] [PubMed] [Google Scholar]
- 52. Henry C, Whiting SJ, Finch SL et al. (2016) Impact of replacing regular chocolate milk with the reduced-sugar option on milk consumption in elementary schools in Saskatoon, Canada. Appl Physiol Nutr Metab 1, 511–515. [DOI] [PubMed] [Google Scholar]
- 53. Araujo AM, Brandão AS, Araújo AM et al. (2017) Overweight and obesity in preschoolers: prevalence and relation to food consumption. Rev Assoc Med Bras (1992) 63, 124–133. [DOI] [PubMed] [Google Scholar]
- 54. Frantsve-Hawley J, Bader JD, Welsh JA et al. (2017) A systematic review of the association between consumption of sugar-containing beverages and excess weight gain among children under age 12. J Public Health Dent 77, Suppl. 1, S43–S66. [DOI] [PubMed] [Google Scholar]
- 55. Lee AK, Chowdhury R & Welsh JA (2015) Sugars and adiposity: the long-term effects of consuming added and naturally occurring sugars in foods and in beverages. Obes Sci Pract 1, 41–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Murakami K & Livingstone MB (2015) Variability in eating frequency in relation to adiposity measures and blood lipid profiles in British children and adolescents: findings from the National Diet and Nutrition Survey. Int J Obes (Lond) 39, 608–613. [DOI] [PubMed] [Google Scholar]
- 57. Davis MM, Spurlock M, Ramsey K et al. (2017) Milk Options Observation (MOO): a mixed-methods study of chocolate milk removal on beverage consumption and student/staff behaviors in a rural elementary school. J Sch Nurs 33, 285–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Prasad K & Dhar I (2014) Oxidative stress as a mechanism of added sugar-induced cardiovascular disease. Int J Angiol 23, 217–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Astrup A, Dyerberg J, Elwood P et al. (2011) The role of reducing intakes of saturated fat in the prevention of cardiovascular disease: where does the evidence stand in 2010? Am J Clin Nutr 93, 684–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Vafeiadou K, Weech M, Altowaijri H et al. (2015) Replacement of saturated with unsaturated fats had no impact on vascular function but beneficial effects on lipid biomarkers, E-selectin, and blood pressure: results from the randomized, controlled Dietary Intervention and VAScular function (DIVAS) study. Am J Clin Nutr 102, 40–48. [DOI] [PubMed] [Google Scholar]
- 61. Nestel PJ, Chronopulos A & Cehun M (2005) Dairy fat in cheese raises LDL cholesterol less than that in butter in mildly hypercholesterolaemic subjects. Eur J Clin Nutr 59, 1059–1063. [DOI] [PubMed] [Google Scholar]
- 62. Tricon S, Burdge GC, Jones EL et al. (2006) Effects of dairy products naturally enriched with cis-9,trans-11 conjugated linoleic acid on the blood lipid profile in healthy middle-aged men. Am J Clin Nutr 83, 744–753. [DOI] [PubMed] [Google Scholar]
- 63. Drehmer M, Pereira MA, Schmidt MI et al. (2016) Total and full-fat, but not low-fat, dairy product intakes are inversely associated with metabolic syndrome in adults. J Nutr 146, 81–89. [DOI] [PubMed] [Google Scholar]
- 64. O’Sullivan TA, Bremner AP, Mori TA et al. (2016) Regular fat and reduced fat dairy products show similar associations with markers of adolescent cardiometabolic health. Nutrients 8, E22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Vaskonen T (2003) Dietary minerals and modification of cardiovascular risk factors. J Nutr Biochem 14, 492–506. [DOI] [PubMed] [Google Scholar]
- 66. Buchowski MS, Aslam M, Dosset C et al. (2009) Effect of dairy and nondairy calcium on fecal fat excretion in lactose digester and maldigester obese adults. Int J Obes (Lond) 34, 127–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Christensen R, Lorenzen JK, Svith CR et al. (2009) Effect of calcium from dairy and dietary supplements on faecal fat excretion: a meta-analysis of randomized controlled trials. Obes Rev 10, 475–486. [DOI] [PubMed] [Google Scholar]
- 68. Beal T, Massiot E, Arsenault JE et al. (2017) Global trends in dietary micronutrient supplies and estimated prevalence of inadequate intakes. PLoS One 12, e0175554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Zanchett D, Bosco SMD, Arend AJ et al. (2015) Relação entre excesso de peso e consumo de cálcio em crianças e adolescentes. Rev Baiana Saude Publica 39, 64–73. [Google Scholar]
- 70. Assumpção D, Dias MRMG, Barros MBA et al. (2016) Calcium intake by adolescents: a population-based health survey. J Pediatr (Rio J) 92, 251–259. [DOI] [PubMed] [Google Scholar]
- 71. Wang Lang L, Manson JE & Sesso HD (2012) Calcium intake and risk of cardiovascular disease. A review of prospective studies and randomized clinical trials. Am J Cardiovasc Drugs 12, 105–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Levy RB, Claro RM, Mondini L et al. (2012) Regional and socioeconomic distribution of household food availability in Brazil, in 2008–2009. Rev Saude Publica 46, 15–28. [DOI] [PubMed] [Google Scholar]
- 73. Sociedade Brasileira de Pediatria (2012) Manual de Orientação para a Alimentação do Lactente, do Pré-escolar, do Escolar, do Adolescente e na Escola/Sociedade Brasileira de Pediatria. Departamento de Nutrologia, 3a ed. Rio de Janeiro: SBP.
- 74. Levy-Costa RB, Sichieri R, Pontes NS et al. (2005) Disponibilidade domiciliar de alimentos no Brasil: distribuição e evolução (1974–2003). Rev Saude Publica 39, 530–540. [DOI] [PubMed] [Google Scholar]