Abstract
Objective
There is limited knowledge on vitamin D status of children residing in the Andes and its association with undernutrition. We evaluated the vitamin D status of children residing in a low socio-economic status (SES) setting in the Ecuadorian Andes and assessed the association between vitamin D status, stunting and underweight. We hypothesized that children who were underweight would have lower serum 25-hydroxyvitamin D (25(OH)D) levels and lower 25(OH)D levels would be associated with a higher risk of stunting.
Design
We conducted a cross-sectional secondary analysis of a randomized controlled trial, the Vitamin A, Zinc and Pneumonia study. Children had serum 25(OH)D concentrations measured. A sensitivity analysis was undertaken to determine a vitamin D cut-off specific for our endpoints. Associations between serum 25(OH)D and underweight (defined as weight-for-age Z-score≤−1) and stunting (defined as height-for-age Z-score≤−2) were assessed using multivariate logistic regression.
Setting
Children residing in five low-SES peri-urban neighbourhoods near Quito, Ecuador.
Subjects
Children (n 516) aged 6–36 months.
Results
Mean serum 25(OH)D concentration was 58·0 (sd 17·7) nmol/l. Sensitivity analysis revealed an undernutrition-specific 25(OH)D cut-off of <42·5 nmol/l; 18·6 % of children had serum 25(OH)D<42·5 nmol/l. Children who were underweight were more likely to have serum 25(OH)D<42·5 nmol/l (adjusted OR (aOR)=2·0; 95 % CI 1·2, 3·3). Children with low serum 25(OH)D levels were more likely to be stunted (aOR=2·8; 95 % CI 1·6, 4·7).
Conclusions
Low serum 25(OH)D levels were more common in underweight and stunted Ecuadorian children.
Keywords: Vitamin D deficiency, 25-Hydroxyvitamin D, Children, Stunting, Underweight, Undernutrition, Ecuador
Vitamin D deficiency has increasingly been reported in many regions of the world in both adults and children( 1 – 6 ). In comparison to North America and Europe, Latin America is a region where vitamin D research has been under-represented, particularly in the paediatric population( 5 , 7 , 8 ). In young children (<5 years), data from the Patagonia region of Argentina( 9 ) (6–23 months) and Mexico (2–5 years)( 10 ) show a prevalence of vitamin D deficiency (<50 nmol/l) of nearly 24 %. However, there is limited knowledge of the prevalence of vitamin D status among young children living in the high-altitude Andean countries. One study published in the Andean capital of Bogota, Colombia among pre-adolescent girls (5–12 years) showed that 12 % of girls were vitamin D deficient (<50 nmol/l) and 51 % were insufficient (50–72·5 nmol/l)( 11 ).
Since exposure of skin to solar UVB radiation and cutaneous production is a major source of vitamin D, regions with low latitudes should theoretically have a low prevalence of vitamin D deficiency. With its equatorial latitude, the Ecuadorian capital Quito receives nearly 12 h of sunlight per day year-round, and has an altitude of 2800 m above sea level. Both conditions increase the solar UV radiation index( 12 ) and are conducive to epidermal vitamin D synthesis( 13 ). However, previous studies in countries with low latitude( 4 , 14 , 15 ) and/or high altitude( 16 – 18 ) show that this does not necessarily translate into a low population prevalence of vitamin D deficiency.
Vitamin D deficiency is of particular concern in growing children( 19 , 20 ) and limited data are available on the association between vitamin D and nutritional status. The Generation R study, a large multi-ethnic cohort of 6-year-olds in the Netherlands, found that children who were underweight (defined by BMI Z-score) had a higher risk of vitamin D deficiency (<50 nmol/l) compared with normal-weight children( 21 ). Another study in a tertiary care centre in Tanzania found that 30·6 % (41/134) of children <5 years old had vitamin D deficiency (defined as <50 nmol/l) and that children with marasmus were more likely to have vitamin D deficiency compared with children with kwashiorkor or marasmic kwashiorkor( 22 ).
Vitamin D status influences linear growth, even in children with no clinical signs of rickets. A study among South African children aged 2–5 years found that stunted children were less likely to consume vitamin D along with other key components of milk such as Ca, riboflavin and fat than non-stunted children( 23 ). In India, a randomized placebo-controlled trial with 2709 full-term, low-birth-weight infants found that infants randomized to a weekly cholecalciferol (vitamin D3) dose of 35 µg (1400 IU) from 7 d to 6 months of age had significant increases in length, weight and mid-upper arm circumference compared with infants given placebo( 24 ). However, the anthropometric gains from the short-term supplementation trial did not appear to be sustained when a sub-sample of these children had their anthropometrics remeasured at 3–6 years of age( 25 ). Each of these studies underlines the importance of vitamin D for normal growth of young children.
Given the lack of information on the vitamin D status among Ecuadorian children and its potentially important effects on growth, we performed a secondary analysis using a convenience sample of children participating in a clinical trial in Ecuador. The objectives of the current analysis were to: (i) measure the prevalence of vitamin D deficiency in a low socio-economic status (SES) weight-stratified sample of children aged 6–36 months residing in the Ecuadorian Andes; (ii) assess whether vitamin D status differs between underweight children and normal-weight children; and (iii) determine whether children with lower levels of vitamin D were more likely to be stunted than those with higher levels.
Methods
Study setting
The study setting was low-SES peri-urban neighbourhoods, called barrios, in Quito, Ecuador. The barrios are located on the hilly outskirts about 20 km north-west of downtown Quito with elevations greater than 2800 m above sea level. The study was carried out in five adjacent neighbourhoods: Atucucho, Caminos de la Libertad, Colinas del Norte, Pisuli and Roldos. These impoverished barrios had poor infrastructure and limited health-care facilities. In 2000, a baseline survey of the barrios showed that households had a mean monthly income of US $54, which was 50 % below the mean basic income in Ecuador. Only 52 % of households had a municipal source of potable water while 62 % had sewer access.
Study design
The current study was a cross-sectional analysis of subjects who participated in a larger randomized control trial, the Vitamin A, Zinc and Pneumonia (VAZPOP) study (clinicaltrial.gov identifier number NCT00228254). During 2000–2003, children were recruited in groups of 600 to 660 in four serial cohorts with each cohort beginning in July. Individual participation lasted one year. To be enrolled in the trial, children had to be 6–36 months old, have had no recent micronutrient supplementation, and have resided in one of the five study neighbourhoods for at least one year. After the initial baseline survey of the neighbourhoods, a site map with geographic divisions was developed which included all eligible children in each of the five neighbourhoods. Children were randomly chosen from geographic divisions in a proportional fashion. In households with two or more eligible children, the youngest child was considered eligible and older siblings were excluded from enrolment. Parents of eligible children were contacted and children were invited to participate in the study and attend an anthropometric measurement session.
Study enrolment was weight-stratified, with approximately 200–220 children selected (forty to forty-five children in each of the five study neighbourhoods) in each of the following weight-for-age Z-score (WAZ) strata: underweight (WAZ≤−2), mildly underweight (–1≤WAZ>−2) and well nourished (WAZ>−1) based on the National Institute of Child Health and Human Development reference growth curves (Epi-Info 2002 software; Centers for Disease Control, Atlanta, GA, USA). Severely malnourished children (weight <60 % of predicted) were excluded and their entry into nutritional rehabilitation centres was facilitated. The current cross-sectional study was limited to subjects from the last year of VAZPOP who had a minimum of 100 µl of serum from their baseline blood draw. A flowchart showing the selection of study participants is available in the online supplementary material, Supplemental Fig. 1.
Anthropometric measurements
Anthropometric measures were collected at baseline (conducted between 12 June and 2 July 2003). Trained study personnel performed height, length and weight measures. Length for children aged <24 months was measured using horizontal scales. Standing height was measured for children aged ≥24 months using non-distensible plastic tape fixed on to a vertical board. Height and length were measured to the nearest 0·1 cm. Weight was measured using Detecto® Health-o-meters balance scale (Webb City, IA, USA) to the nearest 0·1 kg. Anthropometric devices for weight and height measurements were calibrated annually for accuracy by the National Bureau of Standards of Ecuador. WAZ, height-for-age Z-score (HAZ) and weight-for-height Z-score (WHZ) were calculated using the 2007 WHO growth standards (WHO Anthro version 3.2.2, January 2011; WHO, http://www.who.int/childgrowth/software/en/). Children who had a height-for-age≤−2 sd below the median of the WHO child growth standards (i.e. HAZ≤−2) were categorized as stunted. Children who had a weight-for-age≤−1 sd below the median reference growth standards (i.e. WAZ≤−1) were categorized as underweight; this group included both mildly and significantly underweight children. We compared children having WAZ≤−1 (instead of children having WAZ≤−2) and children having WAZ>–1 in our models, since a stronger association between WAZ≤−1 and vitamin D status was observed in initial analyses.
Biochemical analysis
Venous blood was collected from children at baseline into trace-element-free tubes (Sarstedt AG, Nümbrecht, Germany). The blood samples were transported in a cooler with ice packs to the laboratory of Pontificia Universidad Católica del Ecuador, where they were stored in freezers at −80°C and subsequently shipped to Boston for vitamin D analyses.
Hb was measured at baseline with a finger prick sample using the HemaCue Hb 201+ assay. Serum 25-hydroxyvitamin D (25(OH)D) and intact parathyroid hormone (iPTH; amino acids 1–84) were measured at the Vitamin D Lab at the Boston University School of Medicine (BUSM, Boston, MA, USA) using an automated enzyme immunoassay (IDS-iSYS; Immunodiagnostic Systems Ltd, Boldon, UK) which uses an ester magnetic particle/chemiluminescence immunoassay technique. The assay for 25(OH)D was validated at the Vitamin D Lab against HPLC with tandem MS (LC/MS/MS), the gold standard for 25(OH)D determination, using additional samples not obtained for the study. A single determination of 25(OH)D and iPTH was measured. However, of those samples that had >400 µl of serum, a 20 % random sub-sample was used to measure 25(OH)D in duplicate.
The assay recognizes 25-hydroxycholecalciferol (25(OH)D3) and 25-hydroxyergocalciferol (25(OH)D2) equally well, with a dynamic range of 15–315 nmol/l. The lower limit of detection of the 25(OH)D assay was 15 nmol/l. Subjects below the detection limit of the assay were assigned a value of 15 nmol/l (n 2) for their 25(OH)D concentration. Subjects below the detection limit of the iPTH assay (<0·5 pmol/l) were assigned a value of 0·5 pmol/l (n 9) with a dynamic range of 0·5–10·5 pmol/l.
Control samples were inserted at periodic intervals for quality control purposes. The inter-class CV for serum 25(OH)D at the high, medium and low levels was 11·1, 5·7 and 6·0 %, respectively and for iPTH it was 7·4, 4·1 and 5·0 %, respectively. The intra-assay CV for the current analysis was 4·9 % for 25(OH)D.
Interpretation of vitamin D status
Existing recommendations for interpreting vitamin D status focus on bone metabolism( 26 – 30 ) and no guidance exists for linking serum levels to undernutrition. We conducted a sensitivity analysis to ascertain the 25(OH)D concentration that showed the strongest associations between 25(OH)D, HAZ and WAZ (see online supplementary material, Supplemental Table 1). For the sensitivity analysis, a series of univariate logistic regression models was carried out with 25(OH)D cut-offs changing from 30 to 75 nmol/l in 2·5 nmol/l increments (e.g. 30, 32·5, 35 nmol/l, etc.). In these analyses, a cut-off value for serum 25(OH)D of 42·5 nmol/l was found to be most strongly associated with WAZ and very strongly associated with HAZ, and was therefore used in the current study. For comparison to other studies, we also used the Institute of Medicine definitions (at risk of classic vitamin D deficiency states such as rickets, <30 nmol/l (<12 ng/ml); risk of inadequacy, 30 to 47·5 nmol/l (12 to 19 ng/ml); sufficiency, ≥50 nmol/l (≥20 ng/ml)( 30 )) and the Endocrine Society Practice Guidelines (deficiency, <50 nmol/l (<20 ng/ml); insufficiency, 50 to 72·5 nmol/l (20 to 29 ng/ml); sufficiency, ≥75 nmol/l (≥30 ng/ml)( 26 )).
Statistical analyses
Data analysis was performed using the statistical software package SAS release 9.3®. Results are presented as mean and standard deviation for continuous variables, or as number and percentage for categorical variables. The mean difference (MD) and 95 % confidence interval were calculated. Linear regression was performed to examine the relationship between 25(OH)D (continuous) and iPTH (continuous) concentrations.
Two analyses were performed. The first was to examine WAZ as a predictor of vitamin D status. We hypothesized that underweight children are at greater risk of lower serum 25(OH)D levels due to less dietary intake. The second analysis examined vitamin D status as a predictor of stunting since lower vitamin D levels may contribute to less than optimal bone growth.
Multiple logistic regression analyses were performed to examine the relationship between underweight as measured by WAZ (independent variable) and vitamin D status (dependent variable) as well as between vitamin D status (independent variable) and stunting (dependent variable). To control for confounding, each potential confounder was added individually to the model. Variables that changed the main exposure effect estimate by more than 10 % were included in the multivariate model. The following potential variables were considered for the model: height, weight, sex, age, SES variables, Hb concentration and iPTH. SES variables were available in a subset of children. These included maternal education and paternal education (illiterate, primary school, high school or higher education), access to sanitary facilities (household access to toilet v. non-toilet (field, latrine or well)), source of water supply (access to potable water v. no access to potable water), type of household construction (block construction v. other type of construction (e.g. wooden)), household ownership (own, rent, other), number of persons per household, number of rooms per household and a crowding index (calculated as the number of persons per household/number of rooms per household). To avoid multicollinearity, a Spearman correlation was calculated for binary and ordinal data while a Pearson correlation was calculated for continuous variables. Variables that were highly correlated were not included in the same model. The most parsimonious model which controlled for all known confounders was chosen for our final multivariate models. Since children were recruited from one of five neighbourhoods, additional cluster effects analyses were performed for comparison with our multivariate logistic regression models. The cluster effects analyses allowed us to account for the possibility that children residing in the same neighbourhood may exhibit similarities in characteristics. To do this, generalized estimating equation models were used and we adjusted for covariates obtained from the confounding analysis.
Sample size and power calculation
The study sample was obtained by convenience sampling. In total, 526 of the 645 children who participated in the final phase of VAZPOP had baseline serum 25(OH)D analysed. The remaining 119 subjects were excluded due to inadequate (n 76) or protein precipitation samples (n 43). A further ten subjects were also excluded for having missing measures of weight and/or height, leaving 516 for analysis.
Post hoc power calculations were based on the minimal detectable difference for the sample size of the study. This was performed using the ‘PS: Power and Sample Size’ program version 3.1.2 assuming a 5 % significance level and 80 % power and using an uncorrected χ 2 test. For the analysis examining WAZ as a predictor of vitamin D status, we were able to detect a difference in the OR of 1·7 with a sample size of 516. For the second analysis examining vitamin D status as a predictor of stunting, the minimal detectable difference was 1·3.
Ethical review
Parental informed consent was obtained for each child who participated in the study. The Boston University Institutional Review Board and the Ethical Committee of the Corporación Ecuatoriana de Biotecnología (CEB) approved this study.
Results
Subject characteristics
A similar number of males and females participated in the study (Table 1); their mean age was 17·9 (sd 8·0) months with >70 % of children being >12 months of age. Underweight and normal-weight children had similar age distributions. There were no important differences in household characteristics between the underweight and normal-weight groups. As expected for this weight-stratified sample, 65·3 % (337/516) were underweight (WAZ≤−1) and the rest were normal weight (WAZ>–1). About two-thirds (62·2 %, 321/516) of children were stunted (HAZ≤−2) and 3·1 % (16/516) were wasted (WHZ≤−2). Children who were underweight were shorter (MD=3·4 cm; 95 % CI 1·9, 4·8 cm) and had lower mean HAZ scores (MD=1·3; 95 % CI 1·2, 1·5) than children who were normal weight. The mean WHZ scores also differed between the two WAZ categories (MD=1·2; 95 % CI 1·1, 1·3).
Table 1.
Baseline characteristics, stratified by weight status, among the study population of children aged 6–36 months (n 516) residing in five low-SES peri-urban neighbourhoods near Quito, Ecuador, June–July 2003
| Overall | Underweight* (WAZ≤− 1) (n 337) | Normal weight (WAZ>−1) (n 179) | |||||
|---|---|---|---|---|---|---|---|
| Variable | n | Mean or n | sd or % | Mean or n | sd or % | Mean or n | sd or % |
| Demographic and household characteristics | |||||||
| Age (months), mean and sd | 516 | 17·9 | 7·7 | 17·8 | 8·5 | ||
| Age group, n and % | |||||||
| 6–12 months | 134 | 26·0 | 83 | 24·6 | 51 | 28·5 | |
| >12 months | 382 | 74·0 | 254 | 75·4 | 128 | 71·5 | |
| Sex, n and % | 516 | ||||||
| Male | 261 | 50·6 | 174 | 51·6 | 87 | 48·6 | |
| Female | 255 | 49·4 | 163 | 48·4 | 92 | 51·4 | |
| Maternal education, n and % | 377 | ||||||
| Illiterate | 34 | 9·0 | 19 | 7·7 | 15 | 11·5 | |
| Primary school | 190 | 50·4 | 128 | 52·1 | 62 | 47·3 | |
| High school or higher education | 153 | 40·6 | 99 | 40·2 | 54 | 41·2 | |
| Paternal education, n and % | 361 | ||||||
| Illiterate | 27 | 7·5 | 19 | 8·0 | 8 | 6·5 | |
| Primary school | 179 | 49·6 | 115 | 48·3 | 64 | 52·0 | |
| High school or higher education | 155 | 42·9 | 104 | 43·7 | 51 | 41·5 | |
| Household ownership, n and % | 381 | ||||||
| Own | 193 | 50·7 | 123 | 49·8 | 70 | 52·2 | |
| Rent | 119 | 31·2 | 79 | 32·0 | 40 | 29·9 | |
| Other | 69 | 18·1 | 45 | 18·2 | 24 | 17·9 | |
| Access to sanitary facilities, n and % | 380 | ||||||
| Access to toilet | 190 | 50·0 | 113 | 45·6 | 77 | 58·3 | |
| No access to toilet | 190 | 50·0 | 135 | 54·4 | 55 | 41·7 | |
| Crowding, mean and sd | 369 | 2·7 | 1·6 | 2·7 | 1·5 | 2·6 | 1·7 |
| Water source, n and % | 381 | ||||||
| Potable | 230 | 60·4 | 150 | 60·5 | 80 | 60·2 | |
| Non-potable | 151 | 39·6 | 98 | 39·5 | 53 | 39·8 | |
| Anthropometric measures | |||||||
| Weight (kg), mean and sd | 516 | 9·2 | 1·8 | 8·6 | 1·5 | 10·2 | 1·9 |
| Height (cm), mean and sd | 516 | 74·8 | 7·6 | 73·6 | 7·0 | 77·0 | 8·3 |
| HAZ, mean and sd | 516 | −2·3 | 1·2 | −2·8 | 1·0 | −1·5 | 1·0 |
| HAZ category, n and % | |||||||
| Normal height-for-age | 61 | 11·8 | 8 | 2·4 | 53 | 29·6 | |
| Mildly stunted | 134 | 26·0 | 54 | 16·0 | 80 | 44·7 | |
| Stunted | 321 | 62·2 | 275 | 81·6 | 46 | 25·7 | |
| WHZ or WLZ, mean and sd | 516 | −0·18 | 1·0 | −0·63 | 0·82 | 0·55 | 0·68 |
| WHZ or WLZ category, n and % | |||||||
| Normal weight-for-height/length | 411 | 79·7 | 235 | 69·7 | 176 | 98·3 | |
| Mildly wasted | 89 | 17·2 | 86 | 25·5 | 3 | 1·7 | |
| Wasted | 16 | 3·1 | 16 | 4·8 | 0 | 0 | |
| Laboratory measures | |||||||
| 25(OH)D (nmol/l)†, mean and sd | 516 | 58·0 | 17·7 | 57·7 | 18·5 | 58·1 | 16·0 |
| 25(OH)D cut-off, n and % | |||||||
| <42·5 nmol/l | 96 | 18·6 | 74 | 22·0 | 22 | 12·3 | |
| ≥42·5 nmol/l | 420 | 81·4 | 263 | 78·0 | 157 | 87·7 | |
| iPTH (pmol/l), mean and sd | 396 | 1·9 | 1·5 | 1·9 | 1·5 | 1·9 | 1·4 |
| Hb (g/l), mean and sd | 491 | 12·0 | 1·2 | 12·0 | 1·2 | 12·2 | 1·2 |
SES, socio-economic status; WAZ, weight-for-age Z-score; HAZ, height-for-age Z-score; WHZ, weight-for-height Z-score; WLZ, weight-for-length Z-score; 25(OH)D, 25-hydroxyvitamin D; iPTH, intact parathyroid hormone.
Underweight (WAZ≤–1) includes both mildly underweight and moderately underweight children.
To convert units of 25(OH)D from nmol/l to ng/ml divide by 2·496.
We explored potential confounding by a wide range of SES-related variables (shown in Table 1). Since many subjects had missing SES variables, we compared the SES-adjusted models with the SES-unadjusted models in the subset of subjects with non-missing data and the effect estimates were virtually identical. Therefore, we removed all SES variables from the final models. There was also no effect modification by SES or other variables. Subjects who had data on SES variables did not differ significantly from those subjects who did not have SES variables (see online supplementary material, Supplemental Table 2). This result is consistent with the objectives of the parent VAZPOP study, which sought to minimize SES effects by conducting the study in poor neighbourhoods.
Distribution of serum 25-hydroxyvitamin D concentrations among children
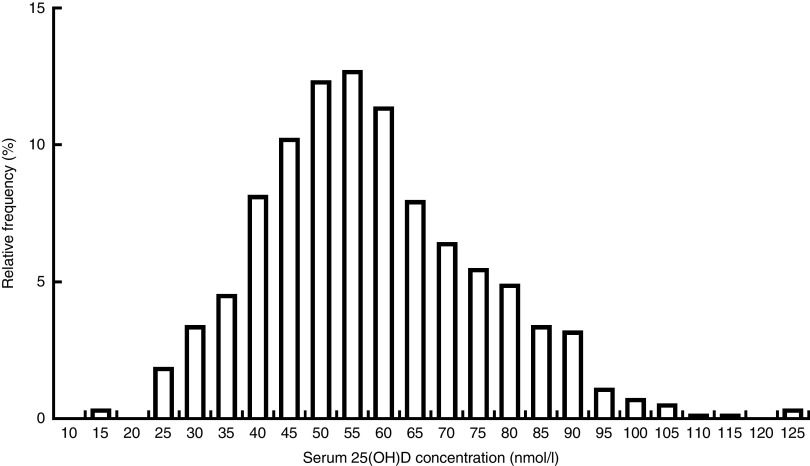
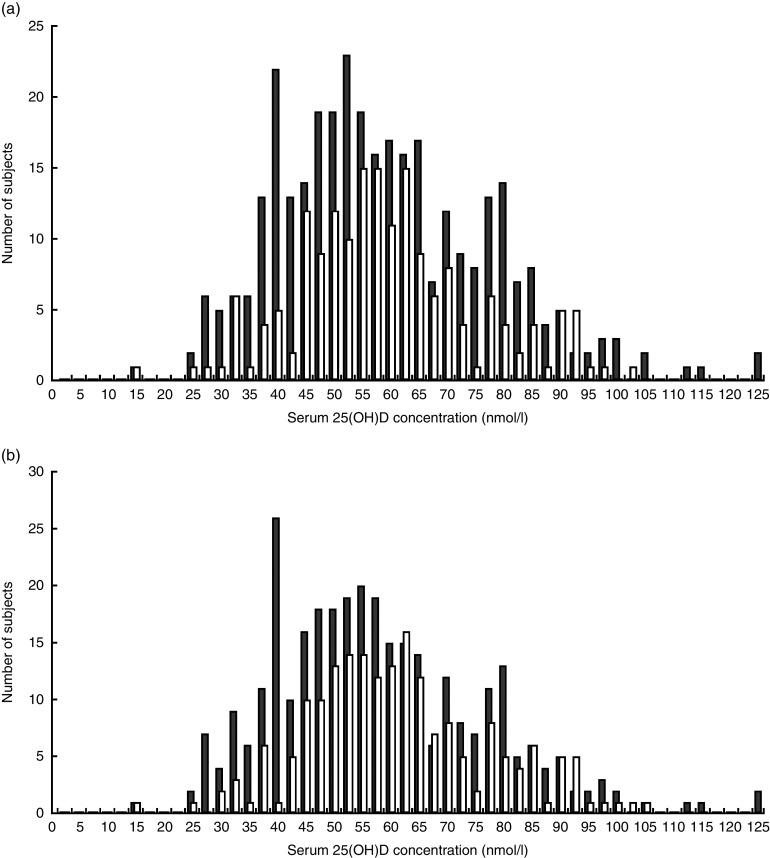
Figure 1 shows the distribution of serum 25(OH)D among study participants. The mean serum 25(OH)D concentration was 58·0 (sd 17·7) nmol/l. Using our study-specific cut-off of 42·5 nmol/l, 18·6 % of children had a serum 25(OH)D level <42·5 nmol/l (Table 2). According to the Institute of Medicine cut-offs( 30 ), only 3·5 % of children were at risk of deficiency (<30 nmol/l) and 31·6 % were at risk of inadequacy (30–47·5 nmol/l). Using the Endocrine Society Practice Guidelines definitions( 26 ), 35·1 % were deficient (<50 nmol/l) and 44·4 % were insufficient (50–72·5 nmol/l). Overall, the mean 25(OH)D concentration was similar in children who were underweight and normal weight (MD=0·40 nmol/l; 95 % CI −2·7, 3·5 nmol/l), although the likelihood of being classified with 25(OH)D level <42·5 nmol/l was higher in underweight than normal-weight children (22·0 v. 12·3 %, respectively; Table 1). This is because the distribution of serum 25(OH)D differed between the two weight groups (Fig. 2). Children who were stunted had mean serum 25(OH)D concentration of 56·7 (sd 18·5) nmol/l and those who were not stunted had a mean of 60·0 (sd 16·0) nmol/l (MD=3·3 nmol/l; 95 % CI 0·32, 6·5 nmol/l). Mean 25(OH)D serum concentrations were highest in infants aged 6–12 months compared with older children (>12 months; MD=10·0 nmol/l; 95 % CI 6·7, 13·5 nmol/l) but did not differ by sex (MD=−0·60 nmol/l; 95 % CI −3·7, 2·4 nmol/l).
Fig. 1.
Distribution of serum 25(OH)D levels in the study population of children aged 6–36 months (n 516) residing in five low-SES peri-urban neighbourhoods near Quito, Ecuador, June–July 2003 (25(OH)D, 25-hydroxyvitamin D; SES, socio-economic status)
Table 2.
Mean 25(OH)D levels, based on different cut-off points defined using a study-specific sensitivity analysis cut-off, the Institute of Medicine definitions and the Endocrine Society Practice Guidelines, among the study population of children aged 6–36 months (n 516) residing in five low-SES peri-urban neighbourhoods near Quito, Ecuador, June–July 2003
| Overall | 25(OH)D (nmol/l) | ||||
|---|---|---|---|---|---|
| Cut-off (nmol/l) | n | % | Mean | sd | |
| Study-specific cut-off for undernutrition | |||||
| Low 25(OH)D | <42·5 | 96 | 18·6 | 34·9 | 5·7 |
| Referent | ≥42·5 | 420 | 81·4 | 63·1 | 15·0 |
| Institute of Medicine( 30 ) | |||||
| At risk of deficiency | <30 | 18 | 3·5 | 25·5 | 4·2 |
| Risk of inadequacy | 30–47·5 | 163 | 31·6 | 41·9 | 5·2 |
| Sufficient | ≥50 | 335 | 64·9 | 67·4 | 14·0 |
| Endocrine Society Practice Guidelines( 26 ) | |||||
| Deficient | <50 | 181 | 35·1 | 40·4 | 7·2 |
| Insufficient | 50–72·5 | 229 | 44·4 | 59·4 | 6·0 |
| Sufficient | >72·5 | 106 | 20·5 | 84·6 | 10·2 |
25(OH)D, 25-hydroxyvitamin D; SES, socio-economic status.
Fig. 2.
Distribution of serum 25(OH)D by (a) WAZ group ( , underweight (WAZ≤−1), n 337;
, underweight (WAZ≤−1), n 337;  , normal weight (WAZ>–1), n 179) and (b) HAZ group (
, normal weight (WAZ>–1), n 179) and (b) HAZ group ( , stunted (HAZ≤−2), n 321;
, stunted (HAZ≤−2), n 321;  , not stunted (HAZ>–2), n 195) among children aged 6–36 months (n 516) residing in five low-SES peri-urban neighbourhoods near Quito, Ecuador, June–July 2003 (25(OH)D, 25-hydroxyvitamin D; WAZ, weight-for-age Z-score; HAZ, height-for-age Z-score; SES, socio-economic status)
, not stunted (HAZ>–2), n 195) among children aged 6–36 months (n 516) residing in five low-SES peri-urban neighbourhoods near Quito, Ecuador, June–July 2003 (25(OH)D, 25-hydroxyvitamin D; WAZ, weight-for-age Z-score; HAZ, height-for-age Z-score; SES, socio-economic status)
Weight and vitamin D status
Significantly, underweight children were twice as likely to have a serum 25(OH)D level <42·5 nmol/l compared with children who were normal weight (unadjusted OR=2·0; 95 % CI 1·2, 3·4; Table 3). After adjusting for age and sex, the association was unchanged (adjusted OR (aOR)=2·0; 95 % CI 1·2, 3·3). After accounting for the clustering of data by neighbourhood, the association remained the same although the 95 % CI were slightly wider (aOR=2·0; 95 % CI 1·2, 3·5). Further, sex (male v. female) was not a predictor of vitamin D status (OR=1·1; 95 % CI 0·69, 1·7). Older (>12 months) children had a non-statistically significant 50 % increased risk (OR=1·5; 95 % CI 0·87, 2·6) of serum 25(OH)D level <42·5 nmol/l compared with younger children (6–12 months).
Table 3.
Multivariate models for predictors of vitamin D status (25(OH)D <42·5 v. ≥42·5 nmol/l) among the study population of children aged 6–36 months (n 516) residing in five low-SES peri-urban neighbourhoods near Quito, Ecuador, June–July 2003
| Univariate analysis | Multivariate analysis | Cluster effects analysis | |||||||
|---|---|---|---|---|---|---|---|---|---|
| n | OR | 95 % CI | n | OR | 95 % CI | n | OR | 95 % CI | |
| WAZ | |||||||||
| Normal weight (WAZ>−1) | 179 | Reference | 179 | Reference | 179 | Reference | |||
| Underweight* (WAZ≤–1) | 337 | 2·0 | 1·2, 3·4 | 337 | 2·0 | 1·2, 3·3 | 337 | 2·0 | 1·2, 3·5 |
| Age | |||||||||
| 6–12 months | 134 | Reference | 134 | Reference | 134 | Reference | |||
| >12 months | 382 | 1·5 | 0·89, 2·6 | 382 | 1·5 | 0·87, 2·6 | 382 | 1·5 | 0·86, 2·6 |
| Sex | |||||||||
| Female | 255 | Reference | 255 | Reference | 255 | Reference | |||
| Male | 261 | 1·1 | 0·69, 1·7 | 261 | 1·1 | 0·69, 1·7 | 261 | 1·1 | 0·69, 1,7 |
25(OH)D, 25-hydroxyvitamin D; SES, socio-economic status; WAZ, weight-for-age Z-score.
Unadjusted model: vitamin D status (<42·5 v ≥42·5 nmol/l)=WAZ (≤−1 v. >–1).
Multivariable model: adjusted for age (dichotomous) and sex.
Underweight (WAZ≤−1) includes both mildly underweight and moderately underweight children.
Stunting and vitamin D status
A significantly greater proportion of children who had serum 25(OH)D concentration <42·5 nmol/l were stunted than were children with 25(OH)D level ≥42·5 nmol/l (79·2 v. 58·3 %, respectively; Table 4). After adjusting for age and sex, children with 25(OH)D concentration <42·5 nmol/l were more likely to be stunted than children with higher serum concentration (≥42·5 nmol/l; aOR=2·8; 95 % CI 1·6, 4·7; Table 5). Nearly identical results were found when the analysis accounted for the clustering of data by neighbourhood (aOR=2·8; 95 % CI 1·6, 4·8). In addition, boys were 1·6 times (95 % CI 1·1, 2·3) more likely to be stunted than girls, but age was not associated with stunting (OR=0·9; 95 % CI 0·57, 1·3).
Table 4.
Baseline characteristics, stratified by vitamin D status, of the study population of children aged 6–36 months (n 516) residing in five low-SES peri-urban neighbourhoods near Quito, Ecuador, June–July 2003
| <42·5 nmol/l (n 96) | ≥42·5 nmol/l (n 420) | ||||
|---|---|---|---|---|---|
| Variable | n | Mean or n | sd or % | Mean or n | sd or % |
| Demographic and household characteristics | |||||
| Age (months), mean and sd | 516 | 18·4 | 7·7 | 17·7 | 8·1 |
| Age group, n and % | |||||
| 6–12 months | 134 | 19 | 19·8 | 115 | 27·4 |
| >12 months | 382 | 77 | 80·2 | 305 | 72·6 |
| Sex, n and % | 516 | ||||
| Male | 261 | 50 | 52·1 | 211 | 50·2 |
| Female | 255 | 46 | 47·9 | 209 | 49·8 |
| Maternal education, n and % | 377 | ||||
| Illiterate | 34 | 5 | 7·3 | 29 | 9·4 |
| Primary school | 190 | 38 | 55·9 | 152 | 49·2 |
| High school or higher education | 153 | 25 | 36·8 | 128 | 41·4 |
| Paternal education, n and % | 361 | ||||
| Illiterate | 27 | 4 | 6·2 | 23 | 7·8 |
| Primary school | 179 | 34 | 52·3 | 145 | 49·0 |
| High school or higher education | 155 | 27 | 41·5 | 128 | 43·2 |
| Crowding, mean and sd | 369 | 2·5 | 1·5 | 2·7 | 1·6 |
| Household ownership, n and % | 381 | ||||
| Own | 193 | 38 | 55·9 | 155 | 49·5 |
| Rent | 119 | 14 | 20·6 | 105 | 33·6 |
| Other | 69 | 16 | 23·5 | 53 | 16·9 |
| Access to sanitary facilities, n and % | 380 | ||||
| Access to toilet | 190 | 33 | 48·5 | 157 | 50·3 |
| No access to toilet | 190 | 35 | 51·5 | 155 | 49·7 |
| Water source, n and % | 381 | ||||
| Potable | 159 | 38 | 55·9 | 121 | 38·7 |
| Non-potable | 222 | 30 | 44·1 | 192 | 61·3 |
| Anthropometric measures | |||||
| Weight (kg), mean and sd | 516 | 9·0 | 1·6 | 9·2 | 1·9 |
| Height (cm), mean and sd | 516 | 74·4 | 7·0 | 74·9 | 7·8 |
| WAZ, mean and sd | 516 | −1·6 | 0·9 | −1·4 | 1·0 |
| WAZ category, n and % | |||||
| Normal weight | 179 | 22 | 22·9 | 157 | 37·4 |
| Underweight | 337 | 74 | 77·1 | 263 | 62·6 |
| HAZ, mean and sd | 516 | −2·6 | 1·1 | −2·3 | 1·2 |
| HAZ category, n and % | |||||
| Not stunted | 195 | 20 | 20·8 | 175 | 41·7 |
| Stunted | 321 | 76 | 79·2 | 245 | 58·3 |
| WHZ or WLZ, mean and sd | 516 | −0·26 | 0·89 | −0·21 | 0·97 |
| WHZ or WLZ category | |||||
| Normal weight-for-height/length | 411 | 78 | 81·3 | 333 | 79·3 |
| Mildly wasted | 89 | 15 | 15·6 | 74 | 17·6 |
| Wasted | 16 | 3 | 3·1 | 13 | 3·1 |
| Laboratory measures | |||||
| 25(OH)D (nmol/l)*, mean and sd | 516 | 35·0 | 5·7 | 63·2 | 15·0 |
| iPTH (pmol/l), mean and sd | 396 | 2·1 | 1·6 | 1·9 | 1·5 |
| Hb (g/l), mean and sd | 491 | 12·1 | 1·0 | 12·0 | 1·2 |
SES, socio-economic status; WAZ, weight-for-age Z-score; HAZ, height-for-age Z-score; WHZ, weight-for-height Z-score; WLZ, weight-for-length Z-score; 25(OH)D, 25-hydroxyvitamin D; iPTH, intact parathyroid hormone.
To convert units of 25(OH)D from nmol/l to ng/ml divide by 2·496.
Table 5.
Multivariate models for predictors of stunting (HAZ≤−2) among the study population of children aged 6–36 months (n 516) residing in five low-SES peri-urban neighbourhoods near Quito, Ecuador, June–July 2003
| Univariate analysis | Multivariate analysis | Cluster effects analysis | |||||||
|---|---|---|---|---|---|---|---|---|---|
| n | OR | 95 % CI | n | OR | 95 % CI | n | OR | 95 % CI | |
| 25(OH)D cut-off | |||||||||
| ≥42·5 nmol/l | 420 | Reference | 420 | Reference | 420 | Reference | |||
| <42·5 nmol/l | 96 | 2·7 | 1·6, 4·6 | 96 | 2·8 | 1·6, 4·7 | 96 | 2·8 | 1·6, 4·8 |
| Age | |||||||||
| 6–12 months | 134 | Reference | 134 | Reference | 134 | Reference | |||
| >12 months | 382 | 0·89 | 0·59, 1·3 | 382 | 0·87 | 0·57, 1·3 | 382 | 0·90 | 0·58, 1·4 |
| Sex | |||||||||
| Female | 255 | Reference | 255 | Reference | 255 | Reference | |||
| Male | 261 | 1·6 | 1·1, 2·2 | 261 | 1·6 | 1·1, 2·3 | 261 | 1·6 | 1·1, 2·3 |
HAZ, height-for-age Z-score; SES, socio-economic status; 25(OH)D, 25-hydroxyvitamin D.
Unadjusted model: HAZ (≤−2 v. >−2)=vitamin D status (<42·5 v. ≥42·5 nmol/l).
Multivariate model: adjusted for age (dichotomous) and sex.
Serum 25-hydroxyvitamin D and parathyroid hormone
Intact PTH was measured in the subjects (n 396) who had sufficient serum to allow both 25(OH)D and iPTH assessment. Mean iPTH was similar in the two vitamin D status categories (Table 4; MD=0·17 pmol/l; 95 % CI −0·21, 0·55 pmol/l). Similarly, there was no difference in mean iPTH among children who were normal weight v. underweight (MD=−0·013 pmol/l; 95 % CI −0·33, 0·29 pmol/l; Table 1). The linear regression analysis showed an inverse relationship between 25(OH)D and iPTH, although this was not statistically significant (β=−0·20, P=0·062).
Discussion
To our knowledge, the present study is the first to measure vitamin D status in Ecuadorian children. Children who were underweight were 2·0 (95 % CI 1·2, 3·3) times more likely to have serum 25(OH)D level <42·5 nmol/l compared with normal-weight children. We report a novel association between lower vitamin D status and stunting (aOR=2·8; 95 % CI 1·6, 4·7). Accounting for clustering by site from which children were recruited did not alter these findings.
Reports on the association of vitamin D deficiency with stunting in young children not diagnosed with rickets are limited. There is evidence from studies in adolescents which supports an association between vitamin D deficiency and reduced linear growth( 17 , 31 , 32 ). Among older adolescent girls (16–22 years), Kremer et al. found a positive correlation between circulating 25(OH)D and height( 32 ). In adolescents in Bogota, Colombia, a longitudinal study by Gilbert-Diamond et al.( 17 ) found that vitamin D deficiency (<50 nmol/l) was associated with impaired growth in girls but not in boys. Infants whose mothers received vitamin D supplements during gestation showed improved birth weight and linear growth in the first year of life compared with infants born to non-supplemented mothers( 33 – 37 ). Vitamin D may thus be an important modifiable nutritional factor to prevent stunting.
Defining low vitamin D status and which cut-off to use has been extensively debated, especially in the paediatric population( 27 , 28 ). Currently, there are insufficient data to support a specific cut-off in children. The definitions set forth by the Institute of Medicine relate to serum levels needed to prevent rickets and demonstrate optimal bone mineral density( 27 , 30 ). The Endocrine Society Practice Guidelines definitions focus on levels associated with improved bone mineral density, muscle mass and plateauing of PTH levels( 13 , 26 ). The validity of applying these traditional cut-offs to our endpoints of undernutrition was unclear. Thus, we chose to conduct a sensitivity analysis to examine the consistency of the effects across a wide range of serum 25(OH)D concentrations. These analyses suggested an optimum cut-off value for serum 25(OH)D of <42·5 nmol/l in this data set.
Overall, 18·6 % of children had a serum 25(OH)D level <42·5 nmol/l. By contrast, using the cut-offs from the Endocrine Society Practice Guidelines( 26 ), we found that 35·0 % of children had serum 25(OH)D level <50 nmol/l and 46·1 % were vitamin D insufficient (50 to <75 nmol/l). This level of deficiency is slightly higher than in children (2–5 years) in Mexico (<50 nmol/l: 24 %)( 10 ), infants (6–23 months) in the Patagonia region of Argentina (<50 nmol/l: 24 %)( 9 ) and children (1–8 years) in the USA (<50 nmol/l: 9–11 %)( 38 ). However, it is similar to the deficiency level in children and adolescents (7–18 years) in Brazil (<50 nmol/l: 36·3 %)( 39 ). In comparison to findings from Colombia (5–12 years)( 17 ), the prevalence of vitamin D deficiency (<50 nmol/l) in Ecuador is higher than in Colombian children (35·0 v. 10·2 %) but the level of insufficiency (50–72·5 nmol/l) is comparable (46·1 v. 46·4 %). These findings concur with studies which examined vitamin D status in low-SES children in the USA (1–5 years)( 40 ) and the Netherlands (6 years)( 21 ). Severe deficiency, defined by the Institute of Medicine as serum 25(OH)D level <30 nmol/l, was present in only 3·5 % of the children we assessed. This is similar to rates found in the Patagonia region of Argentina( 9 ) (<27·5 nmol/l: 2·8 %), Costa Rica( 41 ) (<30 nmol/l: 3·5 %) and US children (<30 nmol/l: 1 %)( 38 ).
There may be several reasons for such a high level of vitamin D deficiency and insufficiency in Ecuadorian children. The oversampling of underweight children due to the weight-stratified sampling design of the original randomized controlled trial might have resulted in a selection bias in favour of a higher prevalence of vitamin D deficiency. In addition, the high altitude in Quito causes a cooler climate which leads to less skin exposure and cutaneous vitamin D synthesis. Further, in Ecuador, dairy products are not fortified with vitamin D, although there is a minor amount of vitamin D in margarine (50 µg (2000 IU)/kg). Alternative dietary sources of vitamin D (primarily oily fish, and to a minor extent beef liver, cheese and egg yolks) for children residing in the barrios were infrequently consumed. An evaluation of food intake of elderly living in the same neighbourhoods found minimal dietary sources of vitamin D in their diets( 42 ).
Underweight children were twice as likely to have lower serum 25(OH)D concentration (<42·5 nmol/l) compared with normal-weight children (aOR=2·0; 95 % CI 1·2, 3·3). Studies of low-SES children in the Netherlands( 21 ), German adults( 43 ) and the elderly in England( 44 ) found a similar risk of lower vitamin D status in underweight subjects compared with normal-weight subjects. The mechanism by which this occurs is unclear. A logical hypothesis is that being underweight is the result of reduced intake of both energy and micronutrients such as vitamin D. Our findings suggest that improved dietary vitamin D intake may have an important impact on vitamin D status in the underweight population.
In line with previous studies, we found that mean 25(OH)D serum concentration was higher in younger children (6–12 months) than older children (>12–36 months; MD=10·0 nmol/l; 95 % CI 6·7, 13·5 nmol/l)( 21 , 38 , 45 – 48 ). However, in the logistic regression models this was not significant (OR=1·5; 95 % CI 0·87, 2·6). Sex was not associated with vitamin D status, which confirms findings in previous studies in young children( 21 , 40 , 49 , 50 ).
Stunting has been repeatedly observed in the Andes( 51 ). A report by the World Bank found that 23·2 % of Ecuadorian children under the age of 5 years are stunted( 52 ). We found much higher levels of stunting in our study population (62·2 %), which is partly due to sampling in a low-SES region of Quito as well as oversampling of underweight children. The study population resides in the Andean highlands of Ecuador, with topographically high altitudes. Prior studies have found that stunting is more common at high altitude( 53 , 54 ). National data show that children residing at 1500 m or higher above sea level are more likely to be stunted than those residing at lower altitudes( 52 ).
We also found that boys were more likely to be stunted than girls, which is consistent with the international literature( 55 , 56 ). Although improved access to sanitary facilities and potable water has been reported to reduce stunting( 57 – 59 ), we did not find any association between these household SES factors and stunting.
A strength of the current study is that children’s serum 25(OH)D concentrations were ascertained within a narrow time period (June–July). Thus, the effect of seasonality did not play a role in differences in vitamin D status. Other strengths include the large sample size and careful attention to anthropometric measurements. Furthermore, oversampling of undernourished children allowed us to assess differences between normal and undernourished groups more robustly than had we used a population-representative sampling strategy. We believe our empirical result of a cut-off of 42·5 nmol/l, which had the strongest statistical relationship to WAZ≤−1 and to HAZ≤−2 (online supplementary material, Supplemental Table 1), may be an important methodological finding.
It is important to note that our study has some inherent limitations. First, the findings may not be generalizable to all Ecuadorian children. The weight-stratified nature in which subjects were recruited into the original trial resulted in oversampling children in the underweight category. In addition, the study participants live in a specific peri-urban area of Quito with lower SES than other parts of Ecuador. A nationally representative study would better assess the vitamin D status in the country and is warranted based on our results.
Another limitation of the study is the lack of data on potential determinants of vitamin D status such as time spent outdoors, breast-feeding status, dietary sources, use of sunscreen and skin pigmentation( 60 ). Although cow’s milk is not fortified with vitamin D, several infant formula options are available in Ecuador and some are fortified with vitamin D. The duration of breast-feeding, whether infants were exclusively breast-fed and whether infant formula and/or cow’s milk were consumed once weaning occurred were not measured in this study population. This is a limitation in our study design as these would have been important variables to include in our analysis. Since breast-feeding and infant formula consumption were not assessed, it is not clear if differences in breast-feeding status or infant formula-feeding differed between malnourished and well-nourished children. Future studies should account for this in the study design.
Moreover, it is possible that genetic differences contribute to differences in vitamin D status and polymorphisms were not tested in the current study. There is a possibility that low vitamin D status could act as a marker for overall micronutrient deficiency. Due to limited serum volume, serum Zn levels could not be measured so we were unable to adjust for serum Zn levels, which may be a confounder in the observed association between low vitamin D levels and stunting, in our models. Finally, it is important to note that the cross-sectional design cannot ascertain causality between undernutrition and lower vitamin D status and cannot rule out reverse causation.
Conclusion
We empirically found that a cut-off of <42·5 nmol/l had the highest statistical association with underweight and stunting. In the current cross-sectional analysis of poor children residing in Quito which oversampled underweight children, we found 18·6 % of children had serum 25(OH)D level <42·5 nmol/l, 35·0 % of children with vitamin D level <50 nmol/l, and 46·1 % with serum level between 50 and 72·5 nmol/l. This is higher than expected given the equatorial latitude and high altitude. Since there are no nationally representative data on vitamin D deficiency in the Andes, studies evaluating other paediatric populations in Ecuador are warranted.
We found that children who were underweight were more likely to have lower level of serum 25(OH)D (<42·5 nmol/l) than normal-weight children. In addition, serum 25(OH)D level <42·5 nmol/l was strongly associated with stunting. These findings have important public health implications especially in low-SES populations. The role of vitamin D in stunting should be further evaluated. Our results suggest that strategies to improve vitamin D status in children through dietary diversification or food fortification could benefit the Ministry of Health’s efforts to reduce stunting in Ecuador.
Acknowledgements
Acknowledgements: The authors thank Danielle M. Enserro for her consultation with statistical analysis and Susan K. Fried for her thorough review of the manuscript. Financial support: This work was supported by the National Institutes of Health (grant number R01 HD038327); and a Sight and Life education grant. The funders had no role in the design, analysis or writing of this article. Conflict of interest: None. Authorship: R.R.M., F.S., J.K.G., B.E. and D.H.H. conceived this sub-study and study design; M.F.H. and D.H.H. had study oversight; F.S., J.K.G., B.E. and D.H.H. consulted for database clarification; R.R.M. and M.F.H. were responsible for laboratory analysis of assays and laboratory results; R.R.M., L.L.M. and M.P.F. designed the statistical analysis; R.R.M. analysed data; R.R.M. and D.H.H. drafted the manuscript; D.H.H. and M.F.H. had primary responsibility for final content of the manuscript; R.R.M., M.F.H., J.K.G., B.E., L.L.M., M.P.F. and D.H.H. revised the manuscript; and all authors read and approved the final version. Ethics of human subject participation: This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects/patients were approved by the Boston University Institutional Review Board and the Ethical Committee of the Corporación Ecuatoriana de Biotecnología (CEB). Written parental informed consent was obtained for each child who participated in the study.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S1368980017002816.
click here to view supplementary material
References
- 1. Holick MF & Chen TC (2008) Vitamin D deficiency: a worldwide problem with health consequences. Am J Clin Nutr 87, issue 4, 1080S–1086S. [DOI] [PubMed] [Google Scholar]
- 2. Mithal A, Wahl DA, Bonjour J-P et al. (2009) Global vitamin D status and determinants of hypovitaminosis D. Osteoporos Int 20, 1807–1820. [DOI] [PubMed] [Google Scholar]
- 3. Hilger J, Friedel A, Herr R et al. (2014) A systematic review of vitamin D status in populations worldwide. Br J Nutr 111, 23–45. [DOI] [PubMed] [Google Scholar]
- 4. Lips P (2010) Worldwide status of vitamin D nutrition. J Steroid Biochem Mol Biol 121, 297–300. [DOI] [PubMed] [Google Scholar]
- 5. Palacios C & Gonzalez L (2014) Is vitamin D deficiency a major global public health problem? J Steroid Biochem Mol Biol 144, 138–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. van Schoor NM & Lips P (2011) Worldwide vitamin D status. Best Pract Res Clin Endocrinol Metab 25, 671–680. [DOI] [PubMed] [Google Scholar]
- 7. Brito A, Cori H, Olivares M et al. (2013) Less than adequate vitamin D status and intake in Latin America and the Caribbean: a problem of unknown magnitude. Food Nutr Bull 34, 52–64. [DOI] [PubMed] [Google Scholar]
- 8. Mokhtar R, Brito A & Holick MF (2013) Vitamin D across the life cycle with special emphasis on Latin America. Sight and Life 27, issue 2, 24–30. [Google Scholar]
- 9. Durán P, Mangialavori G, Biglieri A et al. (2009) Nutrition status in Argentinean children 6 to 72 months old: results from the National Nutrition and Health Survey (ENNyS). Arch Argent Pediatr 107, 397–404. [DOI] [PubMed] [Google Scholar]
- 10. Flores M, Macias N, Lozada A et al. (2013) Serum 25-hydroxyvitamin D levels among Mexican children ages 2 y to 12 y: a national survey. Nutrition 29, 802–804. [DOI] [PubMed] [Google Scholar]
- 11. Villamor E, Marin C, Mora-Plazas M et al. (2011) Vitamin D deficiency and age at menarche: a prospective study. Am J Clin Nutr 94, 1020–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Engelsen O, Brustad M, Aksnes L et al. (2005) Daily duration of vitamin D synthesis in human skin with relation to latitude, total ozone, altitude, ground cover, aerosols and cloud thickness. Photochem Photobiol 81, 1287–1290. [DOI] [PubMed] [Google Scholar]
- 13. Holick MF, Chen TC, Lu Z et al. (2007) Vitamin D and skin physiology: a D-lightful story. J Bone Miner Res 22, Suppl. 2, V28–V33. [DOI] [PubMed] [Google Scholar]
- 14. Marwaha R, Tandon N, Reddy H et al. (2005) Vitamin D and bone mineral density status of healthy schoolchildren in Northern India. Am J Clin Nutr 82, 477–482. [DOI] [PubMed] [Google Scholar]
- 15. Reesukumal K, Manonukul K, Jirapongsananuruk O et al. (2015) Hypovitaminosis D in healthy children in Central Thailand: prevalence and risk factors. BMC Public Health 15, 248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hirschler V, Maccallini G, Molinari C et al. (2013) Low vitamin D concentrations among indigenous Argentinean children living at high altitudes. Pediatr Diabetes 14, 203–210. [DOI] [PubMed] [Google Scholar]
- 17. Gilbert-Diamond D, Baylin A, Mora-Plazas M et al. (2010) Vitamin D deficiency and anthropometric indicators of adiposity in school-age children: a prospective study. Am J Clin Nutr 92, 1446–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sud SR, Montenegro-Bethancourt G, Bermúdez OI et al. (2010) Older Mayan residents of the western highlands of Guatemala lack sufficient levels of vitamin D. Nutr Res 30, 739–746. [DOI] [PubMed] [Google Scholar]
- 19. Rauch F (2007) Bone accrual in children: adding substance to surfaces. Pediatrics 119, Suppl. 2, S137–S140. [DOI] [PubMed] [Google Scholar]
- 20. Bachrach LK (2001) Acquisition of optimal bone mass in childhood and adolescence. Trends Endocrinol Metab 12, 22–28. [DOI] [PubMed] [Google Scholar]
- 21. Voortman T, van den Hooven EH, Heijboer AC et al. (2015) Vitamin D deficiency in school-age children is associated with sociodemographic and lifestyle factors. J Nutr 145, 791–798. [DOI] [PubMed] [Google Scholar]
- 22. Walli NZ, Munubhi EK, Aboud S et al. (2016) Vitamin D levels in Malnourished children under 5 years in a tertiary care center at Muhimbili National Hospital, Dar es Salaam, Tanzania – a cross-sectional study. J Trop Pediatr 63, 203–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. van Stuijvenberg ME, Nel J, Schoeman SE et al. (2015) Low intake of calcium and vitamin D, but not zinc, iron or vitamin A, is associated with stunting in 2- to 5-year-old children. Nutrition 31, 841–846. [DOI] [PubMed] [Google Scholar]
- 24. Kumar GT, Sachdev HS, Chellani H et al. (2011) Effect of weekly vitamin D supplements on mortality, morbidity, and growth of low birthweight term infants in India up to age 6 months: randomised controlled trial. BMJ 342, d2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Trilok-Kumar G, Kaur M, Rehman AM et al. (2015) Effects of vitamin D supplementation in infancy on growth, bone parameters, body composition and gross motor development at age 3–6 years: follow-up of a randomized controlled trial. Int J Epidemiol 44, 894–905. [DOI] [PubMed] [Google Scholar]
- 26. Holick MF, Binkley NC, Bischoff-Ferrari HA et al. (2011) Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 96, 1911–1930. [DOI] [PubMed] [Google Scholar]
- 27. Ross AC, Manson JE, Abrams SA et al. (2011) The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab 96, 53–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rosen CJ, Abrams SA, Aloia JF et al. (2012) IOM committee members respond to Endocrine Society vitamin D guideline. J Clin Endocrinol Metab 97, 1146–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Heaney RP & Holick MF (2011) Why the IOM recommendations for vitamin D are deficient. J Bone Miner Res 26, 455–457. [DOI] [PubMed] [Google Scholar]
- 30. Institute of Medicine (2011) Dietary Reference Intakes for Calcium and Vitamin D. Washington, DC: National Academies Press. [PubMed] [Google Scholar]
- 31. Hatun S, Islam O, Cizmecioglu F et al. (2005) Subclinical vitamin D deficiency is increased in adolescent girls who wear concealing clothing. J Nutr 135, 218–222. [DOI] [PubMed] [Google Scholar]
- 32. Kremer R, Campbell PP, Reinhardt T et al. (2009) Vitamin D status and its relationship to body fat, final height, and peak bone mass in young women. J Clin Endocrinol Metab 94, 67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Brooke OG, Butters F & Wood C (1981) Intrauterine vitamin D nutrition and postnatal growth in Asian infants. Br Med J (Clin Res Ed) 283, 1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Marya RK, Rathee S, Lata V et al. (1981) Effects of vitamin D supplementation in pregnancy. Gynecol Obstet Invest 12, 155–161. [DOI] [PubMed] [Google Scholar]
- 35. Roth DE, Perumal N, Al Mahmud A et al. (2013) Maternal vitamin D3 supplementation during the third trimester of pregnancy: effects on infant growth in a longitudinal follow-up study in Bangladesh. J Pediatr 163, 1605–1611.e3. [DOI] [PubMed] [Google Scholar]
- 36. Morley R, Carlin JB, Pasco JA et al. (2006) Maternal 25-hydroxyvitamin D and parathyroid hormone concentrations and offspring birth size. J Clin Endocrinol Metab 91, 906–912. [DOI] [PubMed] [Google Scholar]
- 37. Harvey NC, Holroyd C, Ntani G et al. (2014) Vitamin D supplementation in pregnancy: a systematic review. Health Technol Assess 18, issue 45, 1–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Looker AC, Johnson CL, Lacher DA et al. (2011) Vitamin D status: United States, 2001–2006. NCHS Data Brief issue 59, 1–8. [PubMed] [Google Scholar]
- 39. Santos BR, Mascarenhas LPG, Satler F et al. (2012) Vitamin D deficiency in girls from South Brazil: a cross-sectional study on prevalence and association with vitamin D receptor gene variants. BMC Pediatr 12, 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cole CR, Grant FK, Tangpricha V et al. (2010) 25-Hydroxyvitamin D status of healthy, low-income, minority children in Atlanta, Georgia. Pediatrics 125, 633–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Brehm JM, Celedón JC, Soto-Quiros ME et al. (2009) Serum vitamin D levels and markers of severity of childhood asthma in Costa Rica. Am J Respir Crit Care Med 179, 765–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hamer DH, Sempértegui F, Estrella B et al. (2009) Micronutrient deficiencies are associated with impaired immune response and higher burden of respiratory infections in elderly Ecuadorians. J Nutr 139, 113–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hintzpeter B, Mensink GBM, Thierfelder W et al. (2008) Vitamin D status and health correlates among German adults. Eur J Clin Nutr 62, 1079–1089. [DOI] [PubMed] [Google Scholar]
- 44. Hirani V & Primatesta P (2005) Vitamin D concentrations among people aged 65 years and over living in private households and institutions in England: population survey. Age Ageing 34, 485–491. [DOI] [PubMed] [Google Scholar]
- 45. Absoud M, Cummins C, Lim MJ et al. (2011) Prevalence and predictors of vitamin D insufficiency in children: a Great Britain population based study. PLoS One 6, e22179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Carpenter TO, Herreros F, Zhang JH et al. (2012) Demographic, dietary, and biochemical determinants of vitamin D status in inner-city children. Am J Clin Nutr 95, 137–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mansbach JM, Ginde AA & Camargo CA (2009) Serum 25-hydroxyvitamin D levels among US children aged 1 to 11 years: do children need more vitamin D? Pediatrics 124, 1404–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Weng FL, Shults J, Leonard MB et al. (2007) Risk factors for low serum 25-hydroxyvitamin D concentrations in otherwise healthy children and adolescents. Am J Clin Nutr 86, 150–158. [DOI] [PubMed] [Google Scholar]
- 49. Gordon CM, Feldman HA, Sinclair L et al. (2008) Prevalence of vitamin D deficiency among healthy infants and toddlers. Arch Pediatr Adolesc Med 162, 505–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sioen I, Mouratidou T, Kaufman J-M et al. (2012) Determinants of vitamin D status in young children: results from the Belgian arm of the IDEFICS (Identification and Prevention of Dietary- and Lifestyle-Induced Health Effects in Children and Infants) Study. Public Health Nutr 15, 1093–1099. [DOI] [PubMed] [Google Scholar]
- 51. Larrea C & Freire W (2002) Social inequality and child malnutrition in four Andean countries. Rev Panam Salud Publica/Pan Am J Public Health 11, 356–364. [DOI] [PubMed] [Google Scholar]
- 52. The World Bank (2007) Nutrition Failure in Ecuador: Causes, Consequences and Solutions. Washington, DC: World Bank. [Google Scholar]
- 53. Dang S, Yan H & Yamamoto S (2007) High altitude and early childhood growth retardation: new evidence from Tibet. Eur J Clin Nutr 62, 342–348. [DOI] [PubMed] [Google Scholar]
- 54. Román EM, Bejarano IF, Alfaro EL et al. (2015) Geographical altitude, size, mass and body surface area in children (1–4 years) in the Province of Jujuy (Argentina). Ann Hum Biol 42, 431–438. [DOI] [PubMed] [Google Scholar]
- 55. Shrimpton R, Victora CG, de Onis M et al. (2001) Worldwide timing of growth faltering: implications for nutritional interventions. Pediatrics 107, E75. [DOI] [PubMed] [Google Scholar]
- 56. Grantham-McGregor S, Cheung YB, Cueto S et al. (2007) Developmental potential in the first 5 years for children in developing countries. Lancet 369, 60–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Dangour AD, Watson L, Cumming O et al. (2013) Interventions to improve water quality and supply, sanitation and hygiene practices, and their effects on the nutritional status of children. Cochrane Database Syst Rev issue 8, CD009382. [DOI] [PubMed] [Google Scholar]
- 58. Fink G, Günther I & Hill K (2011) The effect of water and sanitation on child health: evidence from the Demographic and Health Surveys 1986–2007. Int J Epidemiol 40, 1196–1204. [DOI] [PubMed] [Google Scholar]
- 59. Spears D (2013) How Much International Variation in Child Height Can Sanitation Explain? Policy Research Working Paper no. WPS 6351. Washington, DC: World Bank.
- 60. Hossein-nezhad A & Holick MF (2013) Vitamin D for health: a global perspective. Mayo Clin Proc 88, 720–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit https://doi.org/10.1017/S1368980017002816.
click here to view supplementary material