Abstract
Objective
Several epidemiological studies have been performed to evaluate the association of fruit and vegetable consumption with risk of the metabolic syndrome (MetS), but the results remain controversial. Thus, we conducted a systematic meta-analysis to assess the associations of fruit or/and vegetable consumption with risk of MetS, separately.
Design
We searched PubMed, EMBASE and Web of Science databases up to July 2017 for relevant available articles. Pooled OR with 95 % CI were calculated with the fixed- or random-effects model.
Results
A total of nine studies for fruit consumption, nine studies for vegetable consumption and seven studies for fruit and vegetable consumption were identified as eligible for the present meta-analysis. The pooled OR (95 % CI) of MetS for the highest v. lowest category were 0·87 (0·82, 0·92; I 2=46·7 %) for fruit consumption, 0·85 (0·80, 0·91; I 2=0·0 %) for vegetable consumption and 0·76 (0·62, 0·93; I 2=83·5 %) for fruit and vegetable consumption. In subgroup analyses stratified by continent where the study was conducted, the inverse association of fruit consumption (0·86 (0·77, 0·96)) and vegetable consumption (0·86 (0·80, 0·92)) with risk of MetS remained significant in Asia. There was no evidence of small-study effect.
Conclusions
Our meta-analysis indicates that fruit or/and vegetable consumption may be inversely associated with risk of MetS. It suggests that people should consume more fruits and vegetables to decrease the risk of MetS.
Keywords: Fruits, Vegetables, Metabolic syndrome, Meta-analysis
Metabolic syndrome (MetS) is identified by the presence of abdominal obesity, hypertension, fasting hyperglycaemia and dyslipidaemia (reduced HDL cholesterol and increased TAG)( 1 ). In the USA, MetS is a common disease with a prevalence of 32·9 % in 2003–2004 and 34·7 % in 2011–2012( 2 ); this increasing tendency is also developing throughout the world( 3 ). Persons with MetS have an increased risk of death from all causes as well as CVD( 4 – 8 ). Increasing evidence indicates that MetS is affected by genetic( 9 , 10 ) and lifestyle factors, such as alcohol consumption, soft drink intake, coffee consumption and sedentary behaviours( 11 – 14 ). As the two important determinants of body weight, diet and physical activity can influence obesity and most of the MetS components directly( 15 ).
Fruits and vegetables are important components of the diet and have been reported to have many potential health benefits, since they are rich in fibre, minerals, vitamins and phytochemicals. Several studies have indicated fruits and vegetables significantly decrease the risk of inflammatory bowel disease( 16 ), lung cancer( 17 ), depression( 18 ), hypertension( 19 , 20 ), type 2 diabetes mellitus( 21 ) and all-cause mortality( 22 ). Antioxidants and anti-inflammatory components from fruits and vegetables are hypothesized to play an important role in MetS( 23 ). A previous study investigated circulatory levels of vitamins C and E, as well as Zn, Mg and Se, finding them inversely correlated with obesity and body fat mass( 24 ). Meanwhile, obesity is a crucial component of MetS. Thus, it is speculated that fruits and vegetables may have relationship with MetS. Many epidemiological studies have been performed to investigate the relationship between fruit or/and vegetable consumption and risk of MetS. However, the results remain controversial. Some studies( 25 – 32 ) have revealed that fruit or/and vegetable consumption is significantly associated with a decreased risk of MetS, while other studies( 33 – 37 ) showed no significant relationship.
Therefore, we performed a systematic meta-analysis combining all of the available data from observational studies to assess the associations of fruit or/and vegetable consumption with risk of MetS, separately.
Methods
The PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines were consulted in the current analysis( 38 ).
Literature search strategy
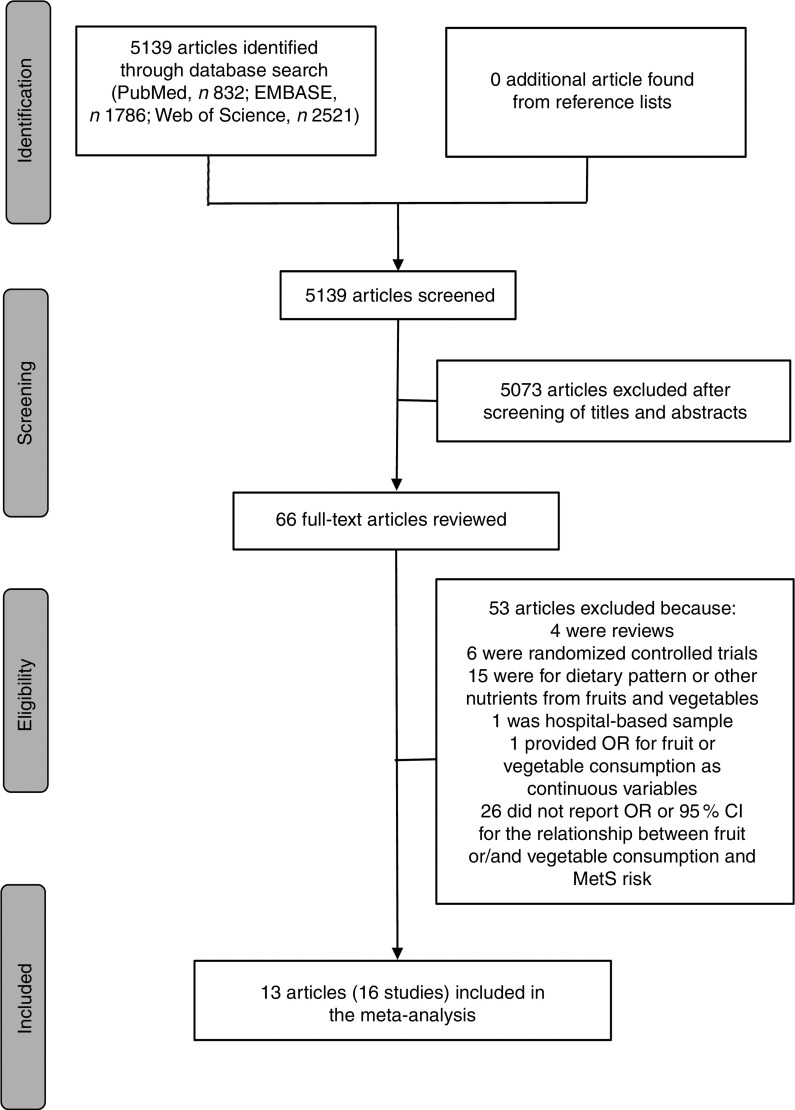
A literature search was conducted for relevant available articles published in English from three databases: PubMed, EMBASE and Web of Science, up to July 2017. We used the search terms ‘fruit’, ‘fruits’, ‘vegetable’ or ‘vegetables’ in combination with ‘metabolic syndrome’ or ‘MetS’. The reference lists from the reviews and articles included were also reviewed for undetected relevant articles. The detailed steps of the literature search are shown in Fig. 1.
Fig. 1.
Flow diagram of the literature search for studies included in the present meta-analysis on fruit or/and vegetable consumption and risk of metabolic syndrome (MetS)
Inclusion criteria and exclusion criteria
The inclusion criteria were as follows: (i) observational study published as an original study; (ii) population-based sample; (iii) exposure of interest was defined as fruit or/and vegetable consumption; (iv) outcome of interest was defined as MetS; (v) study presented estimates of OR, relative risk (RR) or hazard ratio (HR) with corresponding 95 % CI for the associations of fruit or/and vegetable consumption with risk of MetS; and (vi) the most recent and complete study was selected if data from the same population had been published more than once.
The exclusion criteria were as follows: (i) study did not report OR, RR or HR with 95 % CI for the relationship between fruit or/and vegetable consumption and MetS risk; and (ii) study provided OR, RR or HR for fruit or/and vegetable consumption as continuous variables, not categorical variables.
All identified studies were carefully reviewed independently by two investigators, and discrepancies were discussed and resolved by a third investigator.
Data extraction and quality assessment
Two investigators were required to extract and tabulate key data from retrieved studies independently, and any discrepancies were resolved by a third investigator. The following information was extracted from studies: the first author’s name, publication year, country where the study was conducted, study design, baseline age, gender, number of cases and sample size, fruit or/and vegetable assessment method, MetS assessment method, adjusted OR with 95 % CI (we present all results with OR for simplicity) of MetS for fruit or/and vegetable consumption, and adjusted covariates. If the overall results were not provided, results for men and women were included as separate study results. The Newcastle–Ottawa quality assessment scale was used to assess the quality of original studies.
Statistical analysis
The pooled effect was calculated as the inverse variance-weighted mean of the logarithm of adjusted OR with 95 % CI to assess the strength of the associations between fruit or/and vegetable consumption and risk of MetS. The I 2 statistic of Higgins and Thompson was adopted to assess the heterogeneity between studies; I 2 values of 0, 25, 50 and 75 % represented no, low, moderate and high heterogeneity, respectively( 39 ). The random-effects model (REM) was adopted if moderate or higher heterogeneity (I 2≥50 %) was found; otherwise (I 2<50 %), the fixed-effects model (FEM) was used( 40 ). Meta-regression with restricted maximum likelihood estimation was performed to explore the potentially important covariates that might exert substantial impacts on between-study heterogeneity(41). Influence analysis was also conducted to determine whether an individual study affected the aggregate result( 42 ). Potential small-study effect was evaluated using Egger’s regression asymmetry test( 43 ) and visual inspection of the funnel plot. Subgroup analyses were performed by continent where the study was conducted, age of the population, MetS assessment method, and whether adjusted for BMI and physical activity. All statistical analyses were performed using the statistical software package STATA version 12.0. All reported P values were two-sided, with P<0·05 considered statistically significant.
Results
Literature search and study characteristics
As shown in Fig. 1, the search strategy identified 832 articles from PubMed, 1786 articles from EMBASE and 2521 articles from Web of Science, but no additional article was found from reference lists. After reviewing the titles and abstracts, 5073 articles were excluded. In all, sixty-six potentially relevant full-text articles were reviewed, with fifty-three articles were excluded because: four were reviews; six were randomized controlled trials; fifteen were for dietary pattern or other nutrients from fruits and vegetables; one was not a population-based sample, but a hospital-based sample( 44 ); one provided OR for fruit or vegetable consumption as continuous variables( 45 ); and twenty-six did not report OR or 95 % CI for the relationship between fruit or/and vegetable consumption and MetS risk. As a result, a total of thirteen published articles( 25 – 37 ) including sixteen studies were identified as eligible for the present meta-analysis.
The basic characteristics of the included studies for fruit or/and vegetable consumption with risk of MetS are shown in Table 1. Among these studies, seven studies were conducted in Asia, four in Europe, four in North America and one in South America. Considering the age of the population, four were adolescents and twelve were adults. As for study design, there were three cohort studies and thirteen cross-sectional studies included in the meta-analysis. The major adjustment confounding factors included age, gender, BMI, physical activity level, race, education, smoking, alcohol use and energy intake. After assessing the study quality by the Newcastle–Ottawa scale, for fruit consumption, eight studies( 25 , 26 , 29 – 31 , 33 , 37 ) received a quality score of ≥7 and the remaining one study( 27 ) received a quality score of 6; for vegetable consumption, all nine studies( 25 , 29 – 31 , 33 , 35 , 37 ) had a quality of ≥7; for fruit and vegetable consumption, six studies( 28 , 29 , 34 , 36 ) received a quality score of ≥7 and the remaining one study( 32 ) received a quality score of 5 (see online supplementary material, Supplemental Table 1).
Table 1.
Characteristics of the studies included in the present meta-analysis on fruit or/and vegetable consumption and risk of metabolic syndrome (MetS)
| Author(s), year, | Country | Age range (years) | No. of | Exposure | MetS | Outcome | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| reference | (continent) | Study design | Gender | Exposure | participants | No. of cases | assessment | assessment | OR | 95 % CI | Adjusted covariates | |
| Shin et al. | Korea (Asia) | ≥30 | Cross-sectional | Male | Fruits | 5337 | 904 | FFQ | NCEP ATPIII | 0·87 | 0·70, 1·07 | Age, family history of T2DM, smoking |
| (2009)( 33 ) | Yellow vegetables | 0·95 | 0·79, 1·14 | status, regular PA | ||||||||
| Park et al. (2015)( 25 ) | Korea (Asia) | ≥20 | Cross-sectional | Female | Fruits White vegetables | 16 378 | 3018 | FFQ | NCEP ATPIII | 0·72 0·85 | 0·60, 0·87 0·71, 1·01 | Age, BMI, smoking and drinking status, menopausal status, exercise, residence area, education level, walking, serum aspartate aminotransferase, serum alanine aminotransferase |
| Kelishadi et al. | Iran (Asia) | 6–18 | Cross-sectional | Male | Fruits | 2248 | 317 | FFQ | NCEP ATPIII | 0·80 | 0·70, 1·04 | Age |
| (2008)( 29 ) | Vegetables | 0·80 | 0·70, 0·90 | |||||||||
| Fruits and vegetables | 0·80 | 0·70, 0·90 | ||||||||||
| Female | Fruits | 2563 | 364 | 0·90 | 0·70, 1·04 | |||||||
| Vegetables | 0·80 | 0·60, 0·90 | ||||||||||
| Fruits and vegetables | 0·70 | 0·60, 0·90 | ||||||||||
| Esmaillzadeh et al. (2006)( 30 ) | Iran (Asia) | 40–60 | Cross-sectional | Female | Fruits | 486 | NA | FFQ | NCEP ATPIII | 0·89 | 0·79, 1·02 | Age, BMI, energy intake, cigarette smoking, PA level, cholesterol intake, %E from fat, current oestrogen use, menopausal status, family history of diabetes or stroke, intakes of whole grains, refined grains, dairy products, meat and fish, mutual effects of fruit and vegetable intakes, CRP concentration |
| Vegetables | 0·86 | 0·73, 0·99 | ||||||||||
| de Oliveira et al. (2012)( 31 ) | Brazil (South America) | >35 | Cross-sectional | Both | Fruits | 305 | 121 | 24 h dietary recall | NCEP ATPIII | 0·52 | 0·28, 0·98 | Age, BMI, gender, total energy intake |
| Vegetables | 0·97 | 0·22, 4·27 | ||||||||||
| Baik et al. (2013)( 37 ) | Korea (Asia) | 40–69 | Cohort | Both | Fruits | 5251 | 1325 | FFQ | IDF | 1·11 | 0·91, 1·35 | Age, sex, income, occupation, education, smoking status, alcohol intake, quartiles of MET-h/d, study site, FTO genotypes, quartiles of energy intake, quintiles of food groups or food items that are presented in their table |
| Vegetables and seaweed | 0·99 | 0·80, 1·22 | ||||||||||
| Pan and Pratt (2008)( 26 ) | USA (North America) | 12–19 | Cross-sectional | Both | Fruits | 4450 | 156 | 24 h dietary recall | NCEP ATPIII | 0·88 | 0·81, 0·97 | Age, BMI, sex, ethnicity, poverty status, PA level |
| Masaki (2013)( 27 ) | Japan (Asia) | 20–69 | Cross-sectional | Male | Fruits | NA | NA | NA | IDF | 0·62 | 0·34, 0·96 | Age, PA level, smoking and drinking status, other confounding variables |
| Kouki et al. | Finland (Europe) | 57–78 | Cross-sectional | Male | Vegetables | 663 | 182 | 4 d food record | NCEP ATPIII | 0·71 | 0·44, 1·15 | Age, smoking, alcohol consumption, |
| (2011)( 35 ) | Female | Vegetables | 671 | 169 | 0·82 | 0·51, 1·32 | education, VO2max | |||||
| Lutsey et al. (2008)( 34 ) | USA (North America) | 45–64 | Cohort | Both | Fruits and vegetables | 9514 | 3782 | FFQ | AHA | 1·10 | 0·98, 1·24 | Age, sex, race, education, centre, total energy intake, smoking status, pack-years, PA, intakes of meat, dairy, whole grains and refined grains |
| Kwasniewska | Poland (Europe) | 20–74 | Cross-sectional | Male | Fruits and vegetables | 563 | 102 | FFQ/24 h food | AHA | 0·69 | 0·67, 0·98 | BMI, smoking, PA |
| et al. (2009)( 28 ) | Female | Fruits and vegetables | 624 | 123 | questionnaire | 0·72 | 0·65, 0·99 | |||||
| Fletcher et al. (2016)( 36 ) | USA (North America) | 12–19 | Cross-sectional | Both | Fruits and vegetables | 1379 | 126 | Two 24 h recalls | IDF | 0·63 | 0·30, 1·37 | Age, sex, ethnicity, SEP, self-reported PA intensity, dietary intake under-reporting, TV viewing time |
| Boucher et al. (2013)( 32 ) | USA (North America) | NA | Cohort | Both | Fruits and vegetables | 1059 | 123 | NA | NA | 0·19 | 0·06, 0·66 | Age, education, gender, diabetes, heart disease status |
NA, not available; NCEP ATPIII, National Cholesterol Education Program Adult Treatment Panel III; IDF, International Diabetes Federation; AHA, American Heart Association; T2DM, type 2 diabetes mellitus; PA, physical activity; %E, percentage of energy; CRP, C-reactive protein; MET, metabolic equivalent of task; FTO, fat mass and obesity-associated (gene); SEP, socio-economic position; TV, television.
Quantitative synthesis
Fruit consumption and risk of metabolic syndrome
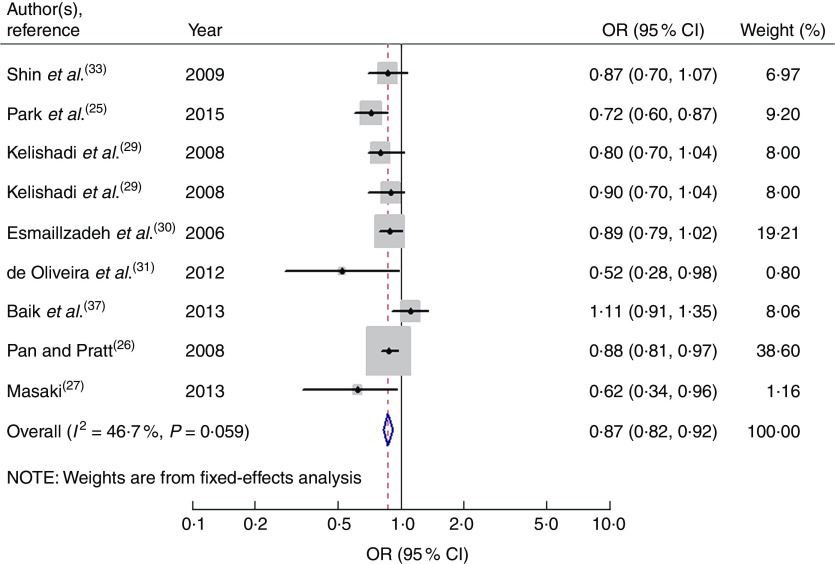
Nine studies from eight articles involving 37 018 participants (6205 cases) evaluated the association of fruit consumption with risk of MetS. Four of the nine studies( 25 – 27 , 31 ) revealed an inverse association, while the other five studies( 29 , 30 , 33 , 37 ) indicated no relationship. For the highest v. lowest category of fruit consumption, the pooled OR of MetS was 0·87 (95 % CI 0·82, 0·92; I 2=46·7 %, P heterogeneity=0·059, FEM; Fig. 2). For subgroup analysis stratified by continent where the study was conducted, the result indicated that higher consumption of fruits was inversely associated with risk of MetS for studies conducted in Asia (OR=0·86; 95 % CI 0·77, 0·96). With regard population age, higher consumption of fruits was inversely associated with risk of MetS in both adults (OR=0·84; 95 % CI 0·71, 0·99) and adolescents (OR=0·87; 95 % CI 0·81, 0·94). With regard to MetS assessment method, higher consumption of fruits was inversely associated with risk of MetS in the National Cholesterol Education Program Adult Treatment Panel III (NCEP APTIII) subgroup (OR=0·85; 95 % CI 0·81, 0·91). The remaining results of subgroup analysis are shown in Table 2.
Fig. 2.
Forest plot for the pooled OR of the association of fruit consumption with risk of metabolic syndrome. The study-specific OR and 95 % CI are represented by the black diamond and the horizontal line, respectively; the area of the grey square is positively proportional to the weight assigned to each study, which is inversely proportional to the se of the OR. The centre of the open diamond and the vertical dashed line represent the pooled OR, and the width of the open diamond represents the pooled 95 % CI
Table 2.
Summary of pooled OR for the association of fruit consumption and vegetable consumption with risk of metabolic syndrome (MetS)
| Stratification | No. of studies | OR | 95 % CI | I 2 (%) | P heterogeneity | Effect model |
|---|---|---|---|---|---|---|
| Fruits | ||||||
| All studies | 9 | 0·87 | 0·82, 0·92 | 46·7 | 0·059 | FEM |
| Geographical region | ||||||
| Asia | 7 | 0·86 | 0·77, 0·96 | 51·4 | 0·055 | REM |
| Other continents (North America and South America) | 2 | 0·74 | 0·46, 1·20 | 62·3 | 0·103 | REM |
| Age of the population | ||||||
| Adults | 6 | 0·84 | 0·71, 0·99 | 64·6 | 0·015 | REM |
| Adolescents | 3 | 0·87 | 0·81, 0·94 | 0 | 0·649 | FEM |
| MetS assessment method | ||||||
| NCEP ATPIII | 7 | 0·85 | 0·81, 0·91 | 15·9 | 0·309 | FEM |
| IDF | 2 | 0·87 | 0·50, 1·53 | 76·3 | 0·040 | REM |
| Adjustment for BMI | ||||||
| Yes | 4 | 0·83 | 0·73, 0·93 | 53·2 | 0·093 | REM |
| No | 5 | 0·90 | 0·82, 1·00 | 48·6 | 0·100 | FEM |
| Adjustment for physical activity | ||||||
| Yes | 6 | 0·87 | 0·78, 0·97 | 56·6 | 0·042 | REM |
| No | 3 | 0·83 | 0·72, 0·95 | 31·4 | 0·233 | FEM |
| Vegetables | ||||||
| All studies | 9 | 0·85 | 0·80, 0·91 | 0 | 0·729 | FEM |
| Geographical region | ||||||
| Asia | 6 | 0·86 | 0·80, 0·92 | 0 | 0·463 | FEM |
| Other continents (Europe and South America) | 3 | 0·77 | 0·56, 1·07 | 0 | 0·874 | FEM |
| Age of the population | ||||||
| Adults | 7 | 0·89 | 0·82, 0·97 | 0 | 0·816 | FEM |
| Adolescents | 2 | 0·80 | 0·72, 0·89 | 0 | 1·000 | FEM |
| MetS assessment method | ||||||
| NCEP ATPIII | 8 | 0·84 | 0·78, 0·90 | 0 | 0·871 | FEM |
| IDF | 1 | 0·99 | 0·80, 1·22 | – | – | FEM |
| Adjustment for BMI | ||||||
| Yes | 3 | 0·85 | 0·76, 0·96 | 0 | 0·977 | FEM |
| No | 6 | 0·85 | 0·79, 0·93 | 4·1 | 0·391 | FEM |
| Adjustment for physical activity | ||||||
| Yes | 4 | 0·90 | 0·82, 0·98 | 0 | 0·590 | FEM |
| No | 5 | 0·80 | 0·72, 0·88 | 0 | 0·989 | FEM |
NCEP ATPIII, National Cholesterol Education Program Adult Treatment Panel III; IDF, International Diabetes Federation; FEM, fixed-effects model; REM, random-effects model;
When men and women were analysed separately, the result indicated that higher consumption of fruits was inversely associated with risk of MetS in both men (OR=0·81; 95 % CI 0·71, 0·94; I 2=0·0 %, P heterogeneity=0·481, FEM) and women (OR=0·85; 95 % CI 0·77, 0·93; I 2=47·5 %, P heterogeneity=0·149, FEM).
Vegetable consumption and risk of metabolic syndrome
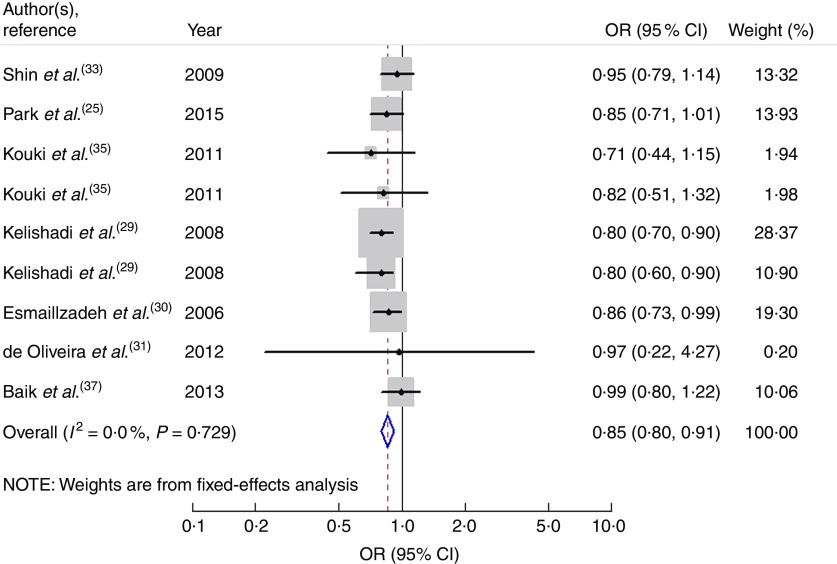
Nine studies from seven articles involving 33 902 participants (6400 cases) investigated the association of vegetable consumption with risk of MetS. Three of the nine studies( 29 , 30 ) revealed an inverse association, while the other six studies( 25 , 31 , 33 , 35 , 37 ) indicated no relationship. For the highest v. lowest category of vegetable consumption, the pooled OR of MetS was 0·85 (95 % CI 0·80, 0·91; I 2=0·0 %, P heterogeneity=0·729, FEM; Fig. 3). For subgroup analysis stratified by continent where the study was conducted, the result indicated that higher consumption of vegetables was inversely associated with risk of MetS for studies conducted in Asia (OR=0·86; 95 % CI 0·80, 0·92). With regard to population age, higher consumption of vegetables was inversely associated with risk of MetS in both adults (OR=0·89; 95 % CI 0·82, 0·97) and adolescents (OR=0·80; 95 % CI 0·72, 0·89). With regard to MetS assessment method, higher consumption of vegetables was inversely associated with risk of MetS in the NCEP APTIII subgroup (OR=0·84; 95 % CI 0·78, 0·90). The remaining results of subgroup analysis are shown in Table 2.
Fig. 3.
Forest plot for the pooled OR of the association of vegetable consumption with risk of metabolic syndrome. The study-specific OR and 95 % CI are represented by the black diamond and the horizontal line, respectively; the area of the grey square is positively proportional to the weight assigned to each study, which is inversely proportional to the se of the OR. The centre of the open diamond and the vertical dashed line represent the pooled OR, and the width of the open diamond represents the pooled 95 % CI
When men and women were analysed separately, the result indicated that higher consumption of vegetables was inversely associated with risk of MetS in both men (OR=0·84; 95 % CI 0·76, 0·93; I 2=28·0 %, P heterogeneity=0·249, FEM) and women (OR=0·84; 95 % CI 0·76, 0·93; I 2=0·0 %, P heterogeneity=0·954, FEM).
Fruit and vegetable consumption and risk of metabolic syndrome
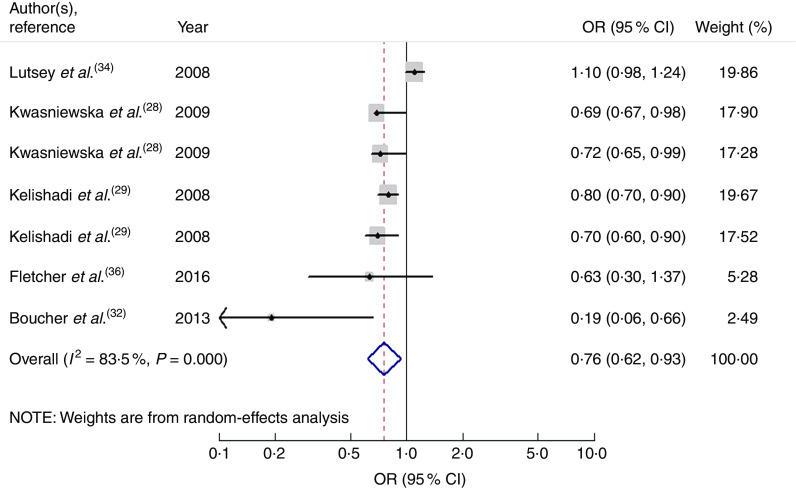
Seven studies from five articles involving 17 950 participants (4937 cases) explored the association of fruit and vegetable consumption with risk of MetS. Five of the seven studies( 28 , 29 , 32 ) revealed an inverse association, while the other two studies( 34 , 36 ) indicated no relationship. For the highest v. lowest category of fruit and vegetable consumption, the pooled OR of MetS was 0·76 (95 % CI 0·62, 0·93; I 2=83·5 %, P heterogeneity=0·000, REM; Fig. 4).
Fig. 4.
Forest plot for the pooled OR of the association of fruit and vegetable consumption with risk of metabolic syndrome. The study-specific OR and 95 % CI are represented by the black diamond and the horizontal line, respectively; the area of the grey square is positively proportional to the weight assigned to each study, which is inversely proportional to the se of the OR. The centre of the open diamond and the vertical dashed line represent the pooled OR, and the width of the open diamond represents the pooled 95 % CI
Meta-regression
For the association between fruit consumption and risk of MetS, in order to explore the sources of heterogeneity, we performed univariate meta-regression with covariates of publication year (P=0·554), gender (P=0·308), age of the population (P=0·907), continent where the study was conducted (P=0·791), study design (P=0·056), MetS assessment method (P=0·178), whether adjusted for BMI (P=0·467) and whether adjusted for physical activity (P=0·605). However, none of these covariates were found to contribute to the low heterogeneity in the analysis.
High heterogeneity was demonstrated for the association between fruit and vegetable consumption (I 2=83·5 %, P heterogeneity=0·000) and risk of MetS. In order to explore the sources of heterogeneity, we performed univariate meta-regression with covariates of publication year (P=0·242), gender (P=0·627), age of the population (P=0·844), continent where the study was conducted (P=0·185), study design (P=0·026), MetS assessment method (P=0·634), whether adjusted for BMI (P=0·642) and whether adjusted for physical activity (P=0·552). Only study design (P=0·026) was found to contribute to the high heterogeneity in the analysis.
Influence analysis and small-study effect
In the influence analyses, all of the point estimates lay within the 95 % CI of the combined analysis, indicating that no individual study had excessive influence on the above-mentioned aggregate results.
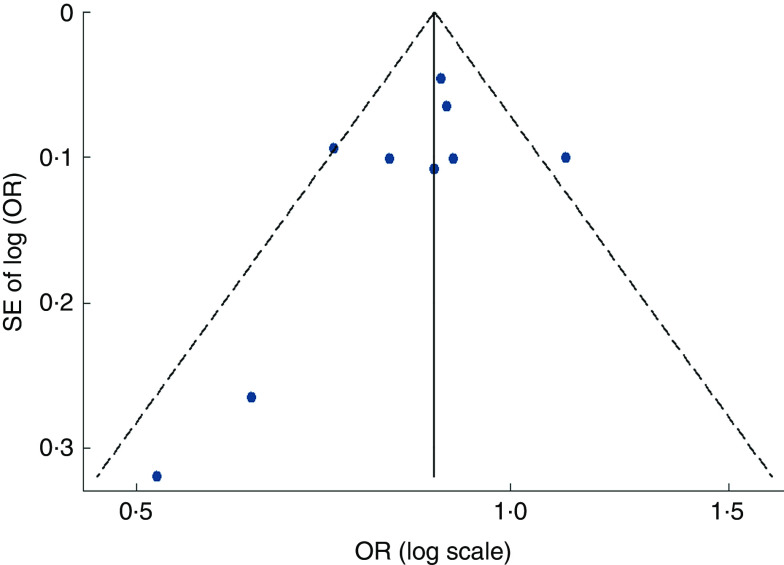
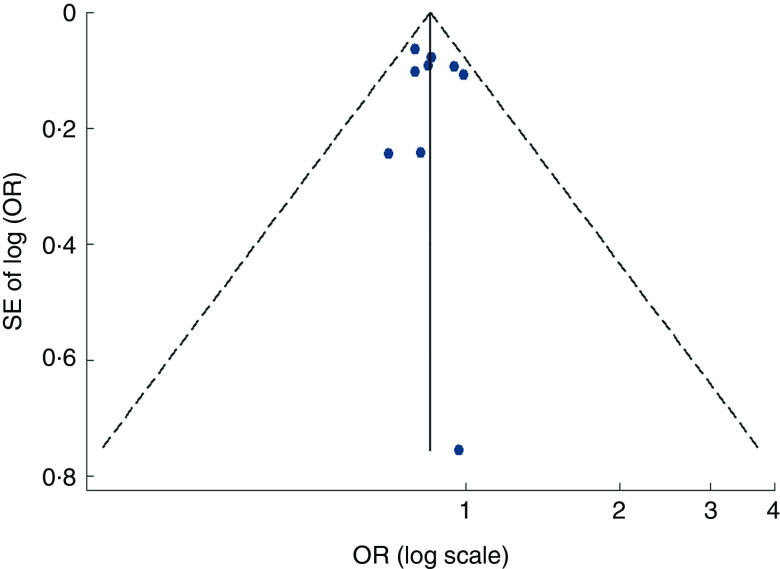
Visual inspection of the funnel plot and Egger’s test suggested there was no evidence of significant small-study effects for the associations of fruit consumption (P=0·247; Fig. 5), vegetable consumption (P=0·902; Fig. 6) and fruit and vegetable consumption (P=0·113) with risk of MetS.
Fig. 5.
Funnel plot, with pseudo 95 % CI represented by dashed lines, for the analysis of fruit consumption with risk of metabolic syndrome. Each dot represents a different study
Fig. 6.
Funnel plot, with pseudo 95 % CI represented by dashed lines, for the analysis of vegetable consumption with risk of metabolic syndrome. Each dot represents a different study
Discussion
Our meta-analysis included sixteen studies from thirteen available articles, based on observational evidence. The results indicated that fruit consumption, vegetable consumption as well as fruit and vegetable consumption was associated with a significantly decreased risk of MetS. Subgroup analyses indicated that the results for studies in the adult subgroup and the adolescent subgroup, studies conducted in Asia and studies with NCEP APTIII assessment remained significant. The results for studies conducted in other continents were statistically insignificant, possibly because few studies were included. There was no sufficient evidence to suggest small-study effect.
There are several potential mechanisms underlying the impact of fruit and vegetable consumption on risk of MetS. First, MetS is associated with oxidative stress and insulin resistance( 46 ). Fruits and vegetables are abundant in antioxidants, such as vitamins C and E, Mg, K, folic acid and phytochemicals. A higher intake of antioxidants can reduce reactive oxygen species and improve antioxidant status in both man and animal models( 23 , 47 ), which can slow the development of systemic oxidative damage causing the overproduction of reactive oxygen species( 48 , 49 ); phytochemicals can increase the body’s production of insulin( 50 ), suggesting the possibility that these components may have a role in the prevention of insulin resistance, and then have an important function in the prevention of MetS; the MetS-protective effects of fruits and vegetables also may be mediated through the effect of their components (such as antioxidants) on inflammatory markers, such as C-reactive protein( 51 , 52 ). A higher consumption of fruits and vegetables is associated with lower plasma concentrations of C-reactive protein( 30 , 53 ), which may have protective effect for MetS. Second, increased fruit and vegetable consumption may have an impact on raising dietary fibre consumption and reducing fat intake, and this diet structure has been shown to be significantly associated with decreased risk of MetS( 54 ).
Between-study heterogeneity is common in meta-analysis( 55 ) and it is essential to explore the sources of between-study heterogeneity. High heterogeneity was found from the association between fruit and vegetable consumption and risk of MetS. Meta-regression revealed that the heterogeneity was associated with study design. The factors accounting for the heterogeneity between studies are complicated. First, there were differences in fruit and vegetable variety, methods of preservation and cooking methods, which might contribute to the heterogeneity. One study by Kelishadi et al.( 29 ) classified juice into the fruit group, but other studies did not. One study by Baik et al.( 37 ) classified seaweed into the vegetable group, which was different from other studies. Second, variables adjusted for varied among studies, including age, smoking status, BMI, energy intake, fat intake, protein intake, dietary fibre intake, education level and physical activity level.
Several strengths must be noticed in our meta-analysis. First, a relatively larger number of participants included, with a reduction in sampling error to a huge extent, enabled a much greater possibility of reaching reasonable conclusions. Second, the interference of potential confounding factors, such as age, smoking status, BMI and physical activity level, was controlled to the greatest extent due to the most fully adjusted OR being extracted. Third, almost consistent associations were identified after undertaking extensive subgroup analyses, identifying the results as robust and credible.
Nevertheless, our study has potential limitations as well. First, most of the studies we included were cross-sectional in design; larger prospective cohort studies are needed to confirm these results. Second, although most major confounders had been adjusted for in most of the included studies, unmeasured and residual confounding was still possible. Confounders adjusted for in each study were also different, which might affect the observed association. Third, the MetS assessment methods differed among studies, which might have a certain influence on the results. However, due to the limited number of studies, we could not further explore the impact of inconsistent assessment methods on the strength of the associations. Fourth, due to insufficient data at present, we could not identify a dose–response relationship between fruit and vegetable consumption and the risk of MetS in the current meta-analysis.
Conclusions
The present meta-analysis indicates that fruit or/and vegetable consumption may be inversely associated with risk of MetS, separately. It suggests that people should consume more fruits and vegetables to decrease the risk of MetS.
Acknowledgements
Financial support: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors. Conflict of interest: None. Authorship: Y.T. and X.J. conceived the study and participated in its design and coordination. Y.T. and L.S. carried out the literature search and data extraction. Y.T. and X.D. were involved in the interpretation of the data. Y.T. and J.W. drafted the manuscript and conducted its critical revision for important intellectual content. Ethics of human subject participation: Ethical approval was not required.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S136898001700310X.
click here to view supplementary material
References
- 1. Alberti KG, Eckel RH, Grundy SM et al. (2009) Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 120, 1640–1645. [DOI] [PubMed] [Google Scholar]
- 2. Aguilar M, Bhuket T, Torres S et al. (2015) Prevalence of the metabolic syndrome in the United States, 2003–2012. JAMA 313, 1973–1974. [DOI] [PubMed] [Google Scholar]
- 3. Eckel RH, Grundy SM & Zimmet PZ (2005) The metabolic syndrome. Lancet 365, 1415–1428. [DOI] [PubMed] [Google Scholar]
- 4. Resnick HE, Jones K, Ruotolo G et al. (2003) Insulin resistance, the metabolic syndrome, and risk of incident cardiovascular disease in nondiabetic American Indians: the Strong Heart Study. Diabetes Care 26, 861–867. [DOI] [PubMed] [Google Scholar]
- 5. Isomaa B, Almgren P, Tuomi T et al. (2001) Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care 24, 683–689. [DOI] [PubMed] [Google Scholar]
- 6. Lakka HM, Laaksonen DE, Lakka TA et al. (2002) The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA 288, 2709–2716. [DOI] [PubMed] [Google Scholar]
- 7. McNeill AM, Rosamond WD, Girman CJ et al. (2005) The metabolic syndrome and 11-year risk of incident cardiovascular disease in the Atherosclerosis Risk in Communities study. Diabetes Care 28, 385–390. [DOI] [PubMed] [Google Scholar]
- 8. Trevisan M, Liu J, Bahsas FB et al. (1998) Syndrome X and mortality: a population-based study. Risk Factor and Life Expectancy Research Group. Am J Epidemiol 148, 958–966. [DOI] [PubMed] [Google Scholar]
- 9. Povel CM, Boer JM, Reiling E et al. (2011) Genetic variants and the metabolic syndrome: a systematic review. Obes Rev 12, 952–967. [DOI] [PubMed] [Google Scholar]
- 10. Gao M, Ding D, Huang J et al. (2013) Association of genetic variants in the adiponectin gene with metabolic syndrome: a case–control study and a systematic meta-analysis in the Chinese population. PLoS One 8, e58412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sun K, Ren M, Liu D et al. (2014) Alcohol consumption and risk of metabolic syndrome: a meta-analysis of prospective studies. Clin Nutr 33, 596–602. [DOI] [PubMed] [Google Scholar]
- 12. Narain A, Kwok CS & Mamas MA (2017) Soft drink intake and the risk of metabolic syndrome: a systematic review and meta-analysis. Int J Clin Pract 71, e12927. [DOI] [PubMed] [Google Scholar]
- 13. Shang F, Li X & Jiang X (2016) Coffee consumption and risk of the metabolic syndrome: a meta-analysis. Diabetes Metab 42, 80–87. [DOI] [PubMed] [Google Scholar]
- 14. Yamaoka K & Tango T (2012) Effects of lifestyle modification on metabolic syndrome: a systematic review and meta-analysis. BMC Med 10, 138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cornier MA, Dabelea D, Hernandez TL et al. (2008) The metabolic syndrome. Endocr Rev 29, 777–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Li F, Liu X, Wang W et al. (2015) Consumption of vegetables and fruit and the risk of inflammatory bowel disease: a meta-analysis. Eur J Gastroenterol Hepatol 27, 623–630. [DOI] [PubMed] [Google Scholar]
- 17. Vieira AR, Abar L, Vingeliene S et al. (2016) Fruits, vegetables and lung cancer risk: a systematic review and meta-analysis. Ann Oncol 27, 81–96. [DOI] [PubMed] [Google Scholar]
- 18. Liu X, Yan Y, Li F et al. (2016) Fruit and vegetable consumption and the risk of depression: a meta-analysis. Nutrition 32, 296–302. [DOI] [PubMed] [Google Scholar]
- 19. Li B, Li F, Wang L et al. (2016) Fruit and vegetables consumption and risk of hypertension: a meta-analysis. J Clin Hypertens (Greenwich) 18, 468–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wu L, Sun D & He Y (2016) Fruit and vegetables consumption and incident hypertension: dose–response meta-analysis of prospective cohort studies. J Hum Hypertens 30, 573–580. [DOI] [PubMed] [Google Scholar]
- 21. Wu Y, Zhang D, Jiang X et al. (2015) Fruit and vegetable consumption and risk of type 2 diabetes mellitus: a dose–response meta-analysis of prospective cohort studies. Nutr Metab Cardiovasc Dis 25, 140–147. [DOI] [PubMed] [Google Scholar]
- 22. Wang X, Ouyang Y, Liu J et al. (2014) Fruit and vegetable consumption and mortality from all causes, cardiovascular disease, and cancer: systematic review and dose–response meta-analysis of prospective cohort studies. BMJ 349, g4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Qiao Q & Group DS (2006) Comparison of different definitions of the metabolic syndrome in relation to cardiovascular mortality in European men and women. Diabetologia 49, 2837–2846. [DOI] [PubMed] [Google Scholar]
- 24. Hosseini B, Saedisomeolia A & Allman-Farinelli M (2017) Association between antioxidant intake/status and obesity: a systematic review of observational studies. Biol Trace Elem Res 175, 287–297. [DOI] [PubMed] [Google Scholar]
- 25. Park S, Ham JO & Lee BK (2015) Effects of total vitamin A, vitamin C, and fruit intake on risk for metabolic syndrome in Korean women and men. Nutrition 31, 111–118. [DOI] [PubMed] [Google Scholar]
- 26. Pan Y & Pratt CA (2008) Metabolic syndrome and its association with diet and physical activity in US adolescents. J Am Diet Assoc 108, 276–286. [DOI] [PubMed] [Google Scholar]
- 27. Masaki M (2013) Dietary patterns and risk for metabolic syndrome. J Diabetes 5, 195. [Google Scholar]
- 28. Kwasniewska M, Kaleta D, Dziankowska-Zaborszczyk E et al. (2009) Healthy behaviours, lifestyle patterns and sociodemographic determinants of the metabolic syndrome. Cent Eur J Public Health 17, 14–19. [DOI] [PubMed] [Google Scholar]
- 29. Kelishadi R, Gouya MM, Adeli K et al. (2008) Factors associated with the metabolic syndrome in a national sample of youths: CASPIAN Study. Nutr Metab Cardiovasc Dis 18, 461–470. [DOI] [PubMed] [Google Scholar]
- 30. Esmaillzadeh A, Kimiagar M, Mehrabi Y et al. (2006) Fruit and vegetable intakes, C-reactive protein, and the metabolic syndrome. Am J Clin Nutr 84, 1489–1497. [DOI] [PubMed] [Google Scholar]
- 31. de Oliveira EP, McLellan KC, Vaz de Arruda Silveira L et al. (2012) Dietary factors associated with metabolic syndrome in Brazilian adults. Nutr J 11, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Boucher JL, Sidebottom AC, Sillah A et al. (2013) Short-term changes in lifestyle risk factors and incident metabolic syndrome in the Heart of New Ulm Project. Circulation 128, A13983. [Google Scholar]
- 33. Shin A, Lim SY, Sung J et al. (2009) Dietary intake, eating habits, and metabolic syndrome in Korean men. J Am Diet Assoc 109, 633–640. [DOI] [PubMed] [Google Scholar]
- 34. Lutsey PL, Steffen LM & Stevens J (2008) Dietary intake and the development of the metabolic syndrome: the Atherosclerosis Risk in Communities study. Circulation 117, 754–761. [DOI] [PubMed] [Google Scholar]
- 35. Kouki R, Schwab U, Hassinen M et al. (2011) Food consumption, nutrient intake and the risk of having metabolic syndrome: the DR’s EXTRA Study. Eur J Clin Nutr 65, 368–377. [DOI] [PubMed] [Google Scholar]
- 36. Fletcher EA, McNaughton SA, Lacy KE et al. (2016) Mediating effects of dietary intake on associations of TV viewing, body mass index and metabolic syndrome in adolescents. Obes Sci Pract 2, 232–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Baik I, Lee M, Jun NR et al. (2013) A healthy dietary pattern consisting of a variety of food choices is inversely associated with the development of metabolic syndrome. Nutr Res Pract 7, 233–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Moher D, Liberati A, Tetzlaff J et al. (2010) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg 8, 336–341. [DOI] [PubMed] [Google Scholar]
- 39. Higgins JP & Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21, 1539–1558. [DOI] [PubMed] [Google Scholar]
- 40. Higgins JP, Thompson SG, Deeks JJ et al. (2003) Measuring inconsistency in meta-analyses. BMJ 327, 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Higgins JP & Thompson SG (2004) Controlling the risk of spurious findings from meta-regression. Stat Med 23, 1663–1682. [DOI] [PubMed] [Google Scholar]
- 42. Tobias A (1999) Assessing the influence of a single study in the meta-analysis estimate. Stata Tech Bull 8, 7526–7529. [Google Scholar]
- 43. Egger M, Davey Smith G, Schneider M et al. (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315, 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Jung HJ, Han SN, Song S et al. (2011) Association between adherence to the Korean Food Guidance System and the risk of metabolic abnormalities in Koreans. Nutr Res Pract 5, 560–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jaaskelainen P, Magnussen CG, Pahkala K et al. (2012) Childhood nutrition in predicting metabolic syndrome in adults: the Cardiovascular Risk in Young Finns Study. Diabetes Care 35, 1937–1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ando K & Fujita T (2009) Metabolic syndrome and oxidative stress. Free Radic Biol Med 47, 213–218. [DOI] [PubMed] [Google Scholar]
- 47. Bokov A, Chaudhuri A & Richardson A (2004) The role of oxidative damage and stress in aging. Mech Ageing Dev 125, 811–826. [DOI] [PubMed] [Google Scholar]
- 48. Grundy SM (2007) Metabolic syndrome: a multiplex cardiovascular risk factor. J Clin Endocrinol Metab 92, 399–404. [DOI] [PubMed] [Google Scholar]
- 49. Palmieri VO, Grattagliano I, Portincasa P et al. (2006) Systemic oxidative alterations are associated with visceral adiposity and liver steatosis in patients with metabolic syndrome. J Nutr 136, 3022–3026. [DOI] [PubMed] [Google Scholar]
- 50. Jayaprakasam B, Vareed SK, Olson LK et al. (2005) Insulin secretion by bioactive anthocyanins and anthocyanidins present in fruits. J Agric Food Chem 53, 28–31. [DOI] [PubMed] [Google Scholar]
- 51. Brighenti F, Valtueña S, Pellegrini N et al. (2007) Total antioxidant capacity of the diet is inversely and independently related to plasma concentration of high-sensitivity C-reactive protein in adult Italian subjects. Br J Nutr 93, 619–625. [DOI] [PubMed] [Google Scholar]
- 52. Wannamethee SG, Lowe GD, Rumley A et al. (2006) Associations of vitamin C status, fruit and vegetable intakes, and markers of inflammation and hemostasis. Am J Clin Nutr 83, 567–574. [DOI] [PubMed] [Google Scholar]
- 53. Watzl B, Kulling SE, Moseneder J et al. (2005) A 4-wk intervention with high intake of carotenoid-rich vegetables and fruit reduces plasma C-reactive protein in healthy, nonsmoking men. Am J Clin Nutr 82, 1052–1058. [DOI] [PubMed] [Google Scholar]
- 54. Singh RB, Niaz MA & Ghosh S (1994) Effect on central obesity and associated disturbances of low-energy, fruit- and vegetable-enriched prudent diet in north Indians. Postgrad Med J 70, 895–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Munafo MR & Flint J (2004) Meta-analysis of genetic association studies. Trends Genet 20, 439–444. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit https://doi.org/10.1017/S136898001700310X.
click here to view supplementary material