Abstract
Objective
Wheat and rye, the most consumed whole grains (WG) in the Nordic countries, contain alkylresorcinols (AR) in their bran. AR concentrations in human adipose tissue might reflect long-term WG rye and wheat intake. We aimed to evaluate AR concentrations in adipose tissue biopsies as a long-term biomarker of WG wheat and rye intake in free-living Swedish men and women.
Design
Cross-sectional study. AR concentrations in adipose tissue biopsies were analysed and compared with long-term WG intake assessed by three FFQ (repeated over a period of 14 years in men, 17 years in women) and with plasma AR concentrations.
Setting
The Cohort of Swedish Men between 1997 and 2010 and the Swedish Mammography Cohort between 1987 and 2003, Sweden.
Subjects
Men (n 149) and women (n 109).
Results
Long-term WG rye intake estimated with repeated FFQ correlated (r=0·31–0·41, P<0·01) with adipose-tissue AR concentrations, while WG wheat intake correlated only weakly (r=0·17–0·33, P<0·05). Total AR concentration in adipose tissue was 61 % lower in women than in men at similar energy-adjusted WG wheat and rye intakes, but plasma concentrations were similar. AR concentrations in adipose tissue correlated well with plasma concentrations (r=0·49–0·81, P<0·001).
Conclusions
AR in adipose tissue reflected long-term WG rye but not WG wheat intake, probably due to poor precision in estimating WG wheat intake by FFQ. AR in adipose tissue appears promising as a biomarker of long-term WG rye intake but should be adjusted for sex.
Keywords: Dietary biomarker, Alkylresorcinol, Whole grain, Adipose tissue, Plasma
Epidemiological studies have consistently shown inverse associations between whole-grain (WG) intake and risk of developing CVD( 1 ), type 2 diabetes( 2 ) and colorectal cancer( 3 ). WG wheat and rye are the two main sources of WG intake in the Nordic countries( 4 ). Among cereals, WG rye has the highest content and broadest composition of different dietary fibres( 5 – 7 ) and phytochemicals( 5 , 8 ). Differences in chemical composition and physiochemical properties between cereals may cause different health outcomes, and it is therefore relevant to study them separately. However, due to difficulties in assessing separate grains accurately in observational studies( 9 , 10 ), and because only a few dietary interventions have been conducted with whole grains from different cereals separately, such investigations are currently lacking to a large extent. Specific dietary biomarkers could facilitate such studies and could be used as a complement to conventional dietary assessment in epidemiological studies and to address compliance in dietary interventions( 11 , 12 ).
Alkylresorcinols (AR) are phenolic lipids mainly present in the bran of wheat and rye in common food items in the Nordic diet( 13 , 14 ). They are removed during the refining process but remain in WG products. AR are stable during food processing, absorbed mainly in the small intestine and present in a dose–response manner in the plasma of WG wheat and rye consumers( 15 ). Thus, AR concentrations in plasma have been evaluated and applied as a biomarker of total WG wheat and rye intake in various epidemiological studies( 15 – 17 ), and they are used as a tool to assess and judge compliance in WG intervention studies( 18 – 20 ). AR in wheat and rye mainly consist of a saturated and straight alkyl chain with an odd number of carbon atoms ranging from 17 to 25 (C17:0 to C25:0) attached to the resorcinol-type phenolic ring. AR C17:0 content is similar to C21:0 content in WG rye, but ten times less abundant in relation to C21:0 content in WG wheat. Thus, the C17:0/C21:0 ratio is associated with relative WG rye intake and has been used to evaluate specific grains in relation to intermediate end points( 15 , 21 , 22 ). Due to its short half-life (5 h), use of plasma AR as a WG biomarker is problematic in populations with low and infrequent WG intake( 23 ). AR are accumulated and present in adipose tissue, and their concentrations are reported to correlate with WG intake estimated from food records over an intervention of 12 weeks( 24 ) and reported WG bread intake over 1 year, estimated by FFQ( 25 ). However, no study so far has investigated the contribution of long-term WG wheat and rye intake and non-dietary factors to AR concentrations in adipose tissue among free-living men and women.
Thus, the aim of the present study was to evaluate AR in adipose tissue biopsies as a biomarker of long-term WG wheat and rye intake among free-living men and women. Correlations of AR concentrations in subcutaneous adipose tissue with estimates of long-term WG wheat and rye intakes based on FFQ repeated over a period of 14–17 years and with plasma AR concentrations as well as with non-dietary factors, including BMI, age and sex, were examined.
Methods
Participants
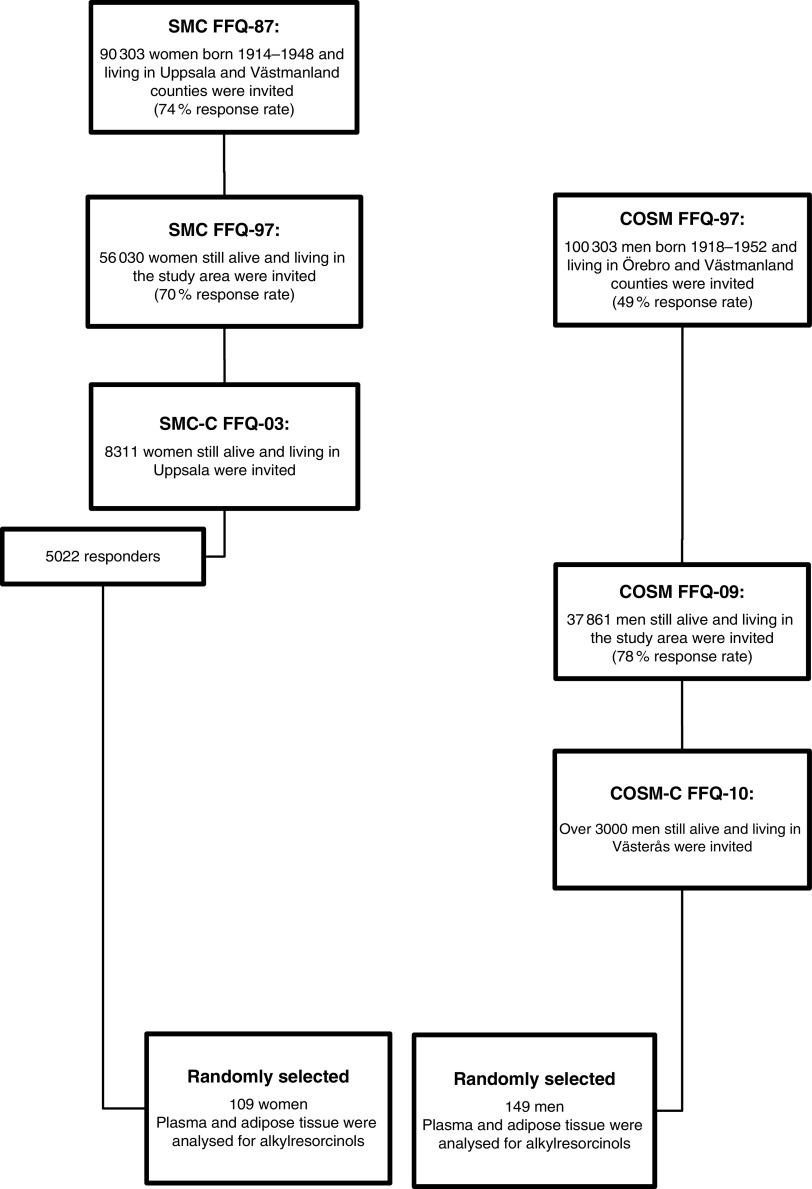
Data and measurements were obtained for women (n 109) and men (n 149) who participated in two clinical sub-studies: the Swedish Mammography Cohort-Clinical (SMC-C) and the Cohort of Swedish Men-Clinical (COSM-C; Fig. 1)( 26 ). Both are clinical sub-studies of two population-based prospective cohorts: the Swedish Mammography Cohort (SMC; clinicaltrials.gov: NCT01127698) and the Cohort of Swedish Men (COSM; clinicaltrials.gov: NCT01127711), respectively. Participants included in the present study were randomly selected among men and women whose subcutaneous adipose tissue samples had previously been thawed and analysed for fatty acid composition, and where more than 10 mg of sample and dietary data were available.
Fig. 1.
The Swedish Mammography Cohort (SMC) and the Cohort of Swedish Men (COSM), as well as the 109 women and 149 men used in the present study (SMC-C, Swedish Mammography Cohort-Clinical; COSM-C, Cohort of Swedish Men-Clinical)
SMC comprises women living in the counties of Västmanland and Uppsala, central Sweden. All women born between 1914 and 1948 were mailed a questionnaire concerning diet (sixty-seven food items), height, weight, education and marital status between 1987 and 1990 (FFQ-87), and the response rate was 74 %. More comprehensive questionnaires on issues including dietary supplements, physical activity and smoking status were sent to all SMC participants who were still alive and residing in the study area during 1997 (FFQ-97)( 27 ). Between 2003 and 2009, the SMC women living in Uppsala County were invited to the SMC-C and asked to fill out a 123-item diet and lifestyle questionnaire (FFQ-03) and to undergo a health examination that included: dual energy X-ray absorptiometry scan for estimation of bone density and body fat; recording of body weight, height, blood pressure, and waist and hip circumferences; and collection of adipose tissue biopsies, fasting blood and urine samples (65 % participated). Over a period of 17 years the women filled in three FFQ.
COSM was initiated in 1997 by inviting all men who were born between 1918 and 1952 and lived in the counties of Västmanland and Örebro, central Sweden( 28 ). Men were asked to fill out a questionnaire on lifestyle, including diet (as in the SMC, FFQ-97). In total, 48 850 of men returned a completed questionnaire. A second FFQ was sent to all COSM members who were still residing in the study area in 2009 (FFQ-09). The COSM-C started in 2010 (ongoing), by inviting men from the COSM who lived in Västmanland County to complete a 132-item FFQ (FFQ-10) and a clinical examination. Over a period of 14 years the men filled in three FFQ.
Dietary intake from FFQ
Data on dietary intake were collected with self-administered semi-quantitative FFQ distributed in 1987, 1997 and 2003 for women and in 1997, 2009 and 2010 for men (FFQ-87, FFQ-97, FFQ-03 and FFQ-97, FFQ-09, FFQ-10, respectively). Questions were asked on predefined frequency of consumption (3/d, 2/d, 1/d, 5–6/week, 3–4/week, 1–2/week, 1–3/month or never) of WG products, including ‘crispbread’, ‘white bread’, ‘whole-meal bread’, ‘porridge’, ‘muesli’, ‘pasta’ and ‘pancake’, during the previous year. The reported frequency of WG food item intake per day was calculated and multiplied by age-specific portion sizes (based on records of scale-weighed portions in 213 women over 4 weeks and in 159 men over 2 weeks distributed over 1 year) in order to estimate the WG product intake (g/d), as described before( 29 , 30 ). WG product intake was then converted to intakes of total whole grains (TWG), WG wheat (WGW), WG rye (WGR) and the sum of WG rye and wheat (WGR&W), using the mean content of total whole grains, WG wheat and WG rye from corresponding food products listed in the Swedish National Food Composition Database( 31 ).
Long-term WG intakes (intakes of WG products, TWG, WGR&W, WGR and WGW) during the years prior to adipose/plasma biopsy samplings were calculated as follows. For men, the mean of FFQ-09 and FFQ-10, i.e. 2 years prior to biopsy; and the mean of FFQ-97, FFQ-09 and FFQ-10, i.e. 14 years prior to biopsy. For women, the mean of FFQ-97 and FFQ-03, i.e. 7 years prior to biopsy; and the mean of FFQ-87, FFQ-97 and FFQ-03, i.e. 17 years prior to biopsy.
Alkylresorcinols in adipose tissue and plasma
Subcutaneous adipose tissue and venous blood samples were collected after a 12 h overnight fast. The subcutaneous adipose tissue samples were taken from the upper-outer quadrant of a buttock of participants with a needle attached to a vacuum tube and stored at −80 °C prior to analysis. Venous blood samples were collected into EDTA-coated evacuated tubes and centrifuged at 3000 g for 10 min at 4 °C. Plasma samples were aliquoted, protected from light and stored at −80 °C until analysis. AR in plasma (150 μl) and adipose tissue samples (11–14 mg) were analysed with a recently developed GC–MS method( 32 ). The intra- and inter-assay CV estimated by inclusion of a quality control sample (n 4) in every batch were <10 and 15 %, respectively. Plasma samples were missing for nineteen women. Total AR concentrations were calculated as the sum of the individual AR concentrations from C17:0 to C25:0 and evaluated as a biomarker of total WG rye and wheat intake in the diet. AR C17:0/C21:0 ratio was calculated as an indicator of the proportion of WG rye intake to total WG rye and wheat intake of the diet.
Statistical analyses
Selected features of participants from the two cohorts at biopsy were compared using Student’s t test. Concentration biomarkers were skewed and therefore log-transformed when used as dependent variables in statistical models or when comparing means with Student’s t test.
Spearman’s rank correlation coefficients between total AR in adipose tissue or plasma and FFQ-based WG intakes over different time periods were calculated. Variations in WG wheat and rye intake over 14 years and 17 years for men and women, respectively (FFQ-87, FFQ-97 and FFQ-03 for women; FFQ-97, FFQ-09, and FFQ-10 for men), were examined at group level using repeated-measures ANOVA. Intraclass correlations (ICC) using the %icc9 SAS macro( 33 ) were calculated to assess reproducibility, i.e. intra-individual fluctuation in WG intakes over time. Individual AR homologues in adipose tissue were correlated with WG intakes from single FFQ and compared with corresponding correlation coefficients for AR in plasma (adjusted for sex). Dietary (WGW and WGR from the single FFQ closest to biopsy) and non-dietary determinants (age, sex and BMI) of AR concentrations in adipose tissue or plasma were assessed by two multiple linear regression models. In Model 1, log AR concentration in adipose tissue or plasma was used as dependent variable; WGW and WGR intakes were energy-adjusted (residual method), divided into quartiles, centred by the median values of each quartile for each sex and used as continuous prediction variables. In Model 2, sex, BMI (entered as a continuous variable) and age (entered as a continuous variable) were added to Model 1 as covariates. The statistical software package SAS release 9.4 was used for all statistical analyses and two-sided P<0·05 was considered significant. All statistical analyses were done with missing data excluded.
Results
Characteristics of the 258 participants at the time when adipose tissue and plasma samples were taken (2003 for the women and 2010 for the men) are presented in Table 1. Compared with women, men were older (P<0·001). Total and individual AR homologue concentrations in adipose tissue were significantly higher (P<0·05) among men compared with women, whereas BMI, total and individual AR in plasma, and AR C17:0/C21:0 ratio in adipose tissue or plasma did not differ significantly between men and women.
Table 1.
Characteristics of the sub-sample of men (n 149) from the Cohort of Swedish Men-Clinical (COSM-C) in 2010 and women (n 109) from the Swedish Mammography Cohort-Clinical (SMC-C) in 2003 included in the present study
| Men (n 149) | Women (n 109) | ||||
|---|---|---|---|---|---|
| Mean | 95 % CI | Mean | 95 % CI | P † | |
| Age (years)‡ | 87 | 85, 88 | 66 | 60, 71 | *** |
| BMI (kg/m2)‡ | 24·6 | 22·6, 27·2 | 24·3 | 23·0, 25·7 | NS |
| Plasma§ | |||||
| Total AR (nmol/l) | 51·4 | 30·8, 82·4 | 46·7 | 30·8, 70·8 | NS |
| C17:0 (nmol/l) | 6·3 | 2·6, 10·8 | 5·0 | 2·5, 10·4 | NS |
| C19:0 (nmol/l) | 16·9 | 10·5, 27·5 | 15·4 | 8·7, 24·8 | NS |
| C21:0 (nmol/l) | 18·0 | 11·1, 27·4 | 17·1 | 12·1, 24·6 | NS |
| C23:0 (nmol/l) | 4·8 | 2·6, 8·2 | 5·2 | 3·2, 7·9 | NS |
| C25:0 (nmol/l) | 4·1 | 2·3, 7·8 | 5·5 | 2·7, 9·0 | NS |
| C17:0/C21:0 | 0·34 | 0·23, 0·46 | 0·27 | 0·20, 0·47 | NS |
| Adipose tissue | |||||
| Total AR (pmol/g) | 1142·4 | 710·7, 1754·5 | 883·1 | 565·3, 1303·1 | ** |
| C17:0 (pmol/g) | 107·9 | 52·1, 161·5 | 75·7 | 45·5, 110·6 | ** |
| C19:0 (pmol/g) | 328·9 | 216·4, 519·2 | 247·5 | 167·7, 373·9 | ** |
| C21:0 (pmol/g) | 398·3 | 249·2, 611·8 | 308·0 | 204·1, 473·7 | ** |
| C23:0 (pmol/g) | 124·3 | 76·7, 203·9 | 103·2 | 63·2, 149·8 | * |
| C25:0 (pmol/g) | 149·3 | 85·0, 256·6 | 116·0 | 80·8, 198·7 | * |
| C17:0/C21:0 | 0·26 | 0·19, 0·34 | 0·26 | 0·17, 0·35 | NS |
AR, alkylresorcinol.
*P<0·05, **P<0·01, ***P<0·001.
Student’s t test was used to compare means between men and women. AR concentrations were log-transformed prior to Student’s t test.
Variables were obtained or calculated from a 132-item FFQ for men (COSM-C) in 2010 and a 123-item FFQ for women (SMC-C) in 2003.
Plasma samples were missing for nineteen women.
The mean intakes of WG rye and wheat and their ICC are shown in Table 2. FFQ-based WG intakes and energy intake did not differ significantly between FFQ-97, FFQ-09 and FFQ-10 among men, while women reported higher WG intakes and lower energy intake in FFQ-87 than in FFQ-97 or FFQ-03. Modest ICC were found for intake of WG products (ICC=0·24–0·52), TWG (ICC=0·36–0·54), WGR (ICC=0·30–0·53), WGR&W (ICC=0·25–0·47) and energy (ICC=0·31–0·53) for both sexes. ICC of WGW were modest between FFQ-97 and FFQ-03 among women (ICC=0·47) but poor between FFQ-87 and FFQ-97 among women (ICC=0·18) as well as between all FFQ among men (ICC=0·10–0·28).
Table 2.
FFQ-based whole-grain (WG) and energy intakes, as well as intraclass correlations (ICC) between different FFQ†, among free-living Swedish men (n 149) and women (n 109)
| Men‡ (n 149) | Women§ (n 109) | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FFQ-97 | FFQ-09 | FFQ-10 | FFQ-97,09║ | FFQ-09,10¶ | FFQ-97,09,10†† | FFQ-87 | FFQ-97 | FFQ-03 | FFQ-87,97║ | FFQ-97,03¶ | FFQ-87,97,03†† | |||||||||||||
| FFQ-based | Mean | sd | Mean | sd | Mean | sd | ICC | 95 % CI | ICC | 95 % CI | ICC | 95 % CI | Mean | sd | Mean | sd | Mean | sd | ICC | 95 % CI | ICC | 95 % CI | ICC | 95 % CI |
| WGP | 304 | 114 | 311 | 152 | 292 | 148 | 0·31 | 0·18, 0·47 | 0·37 | 0·24, 0·52 | 0·31 | 0·22, 0·42 | 248 | 103 | 186 | 85 | 196 | 93 | 0·24 | 0·10, 0·45 | 0·52 | 0·38, 0·65 | 0·44 | 0·34, 0·54 |
| TWG | 116 | 53 | 124 | 66 | 115 | 62 | 0·36 | 0·24, 0·51 | 0·42 | 0·30, 0·56 | 0·39 | 0·29, 0·49 | 99 | 47 | 74 | 37 | 81 | 40 | 0·43 | 0·29, 0·58 | 0·54 | 0·41, 0·67 | 0·52 | 0·43, 0·61 |
| WGW | 28 | 14 | 29 | 17 | 27 | 16 | 0·28 | 0·16, 0·45 | 0·10 | 0·02, 0·39 | 0·14 | 0·06, 0·28 | 28 | 13 | 21 | 11 | 21 | 11 | 0·18 | 0·06, 0·43 | 0·44 | 0·30, 0·59 | 0·30 | 0·21, 0·41 |
| WGR | 51 | 31 | 50 | 30 | 47 | 34 | 0·46 | 0·33, 0·58 | 0·53 | 0·42, 0·64 | 0·48 | 0·38, 0·57 | 56 | 30 | 34 | 22 | 32 | 19 | 0·30 | 0·16, 0·49 | 0·46 | 0·32, 0·60 | 0·36 | 0·27, 0·47 |
| WGR&W | 79 | 36 | 79 | 42 | 74 | 44 | 0·42 | 0·30, 0·56 | 0·40 | 0·28, 0·54 | 0·39 | 0·30, 0·50 | 84 | 38 | 55 | 27 | 53 | 26 | 0·25 | 0·11, 0·46 | 0·47 | 0·33, 0·62 | 0·34 | 0·25, 0·45 |
| Energy | 2395 | 651 | 2436 | 709 | 2443 | 753 | 0·31 | 0·18, 0·47 | 0·47 | 0·35, 0·60 | 0·34 | 0·25, 0·45 | 1625 | 411 | 1764 | 477 | 1742 | 502 | 0·46 | 0·32, 0·61 | 0·53 | 0·40, 0·66 | 0·42 | 0·33, 0·53 |
Daily WG intake (g/d), including WG products (WGP), total whole grains (TWG), WG wheat (WGW), WG rye (WGR), and WG rye and wheat (WGR&W), and energy (kcal/d). All WG intakes among men in FFQ-10 were significantly higher (Student’s t test, P<0·001) than among women in FFQ-03.
WGP, TWG, WGW, WGR, WGR&W and energy intakes in FFQ-97, FFQ-09 and FFQ-10 were not significantly different.
WGP, TWG, WGW, WGR and WGR&W intakes in FFQ-87 were higher than corresponding intakes in FFQ-97 or FFQ-03, while energy intake in FFQ-87 was lower than in FFQ-97 or FFQ-03.
Intraclass correlation between intakes from FFQ in 1997 and 2009 for men; 1987 and 1997 for women.
Intraclass correlation between intakes from FFQ in 2009 and 2010 for men; 1997 and 2003 for women.
Intraclass correlation between intakes from FFQ in 1997, 2009 and 2010 for men; 1987, 1997 and 2003 for women.
In general, total AR concentration in adipose tissue was modestly to well correlated with WG intakes over a period of 14 years in men and 17 years in women (Table 3). Associations were overall stronger when mean intakes over longer time periods, rather than intakes from the single FFQ closest to sampling, were correlated with total AR concentration. Moreover, correlations were weaker for WGR and plasma AR when using WG intakes more distant from the blood sampling occasion. The correlations were also stronger for women than for men. Notably, TWG and WGR&W were more strongly correlated with AR in adipose tissue than was WG products, for both men and women. Similar findings were observed for WG intakes and plasma AR concentration, except for WGW, which was not correlated with plasma AR among men.
Table 3.
Correlations between FFQ-based whole-grain (WG) intakes and total alkylresorcinol (AR) concentrations in adipose tissue or plasma over 1, 2 or 14 years among free-living Swedish men (n 149) and over 1, 7 or 17 years among free-living Swedish women (n 109)†
| Men | Years | AR in adipose | AR in plasma | Women | Years | AR in adipose | AR in plasma |
|---|---|---|---|---|---|---|---|
| WGP | WGP | ||||||
| FFQ-10 | 1 | 0·15 | 0·16 | FFQ-03 | 1 | 0·28** | 0·27* |
| FFQ-09,10‡ | 2 | 0·25** | 0·23** | FFQ-97,03║ | 7 | 0·32** | 0·31** |
| FFQ-97,09,10§ | 14 | 0·25** | 0·26** | FFQ-87,97,03¶ | 17 | 0·36*** | 0·33** |
| TWG | TWG | ||||||
| FFQ-10 | 1 | 0·22** | 0·21* | FFQ-03 | 1 | 0·35*** | 0·32** |
| FFQ-09,10‡ | 2 | 0·29*** | 0·27** | FFQ-97,03║ | 7 | 0·42*** | 0·36** |
| FFQ-97,09,10§ | 14 | 0·30*** | 0·28** | FFQ-87,97,03¶ | 17 | 0·42*** | 0·34** |
| WGR&W | WGR&W | ||||||
| FFQ-10 | 1 | 0·27** | 0·21* | FFQ-03 | 1 | 0·39*** | 0·42** |
| FFQ-09,10‡ | 2 | 0·36*** | 0·30*** | FFQ-97,03║ | 7 | 0·43*** | 0·36*** |
| FFQ-97,09,10§ | 14 | 0·37*** | 0·30*** | FFQ-87,97,03¶ | 17 | 0·44*** | 0·36*** |
| WGW | WGW | ||||||
| FFQ-10 | 1 | 0·09 | 0·04 | FFQ-03 | 1 | 0·26** | 0·23* |
| FFQ-09,10‡ | 2 | 0·21* | 0·12 | FFQ-97,03║ | 7 | 0·27** | 0·23* |
| FFQ-97,09,10§ | 14 | 0·17* | 0·15 | FFQ-87,97,03¶ | 17 | 0·33*** | 0·28** |
| WGR | WGR | ||||||
| FFQ-10 | 1 | 0·31*** | 0·26** | FFQ-03 | 1 | 0·41*** | 0·44*** |
| FFQ-09,10‡ | 2 | 0·37*** | 0·32*** | FFQ-97,03║ | 7 | 0·42*** | 0·36** |
| FFQ-97,09,10§ | 14 | 0·37*** | 0·31*** | FFQ-87,97,03¶ | 17 | 0·40*** | 0·33** |
WGP, WG products; TWG, total whole grains; WGR&W, WG rye and wheat; WGW, WG wheat; WGR, WG rye.
*P<0·05, **P<0·01, ***P<0·001.
Plasma samples were missing for nineteen women.
Mean WG wheat or rye intake from FFQ in 2009 and 2010.
Mean WG wheat or rye intake from FFQ in 1997, 2009 and 2010.
Mean WG wheat or rye intake from FFQ in 1997 and 2003.
Mean WG wheat or rye intake from FFQ in 1987, 1997 and 2003.
Individual AR homologue concentrations, especially shorter homologues, were significantly correlated with WG wheat and/or rye intakes from single FFQ, with correlation coefficient: WGR>WGR&W>WGW (Table 4). However, WGW was not associated with AR C21:0 in adipose tissue or with AR C19:0, C21:0, C23:0 or C25:0 in plasma. Overall, correlations for AR homologues in adipose tissue and plasma were stronger for shorter homologues than for longer homologues.
Table 4.
Spearman’s rank correlation coefficients between intakes of whole-grain (WG) wheat (WGW), WG rye (WGR) and WG rye and wheat (WGR&W) from the FFQ closest to the sample draw/aspiration (FFQ-10 for men and FFQ-03 for women) and alkylresorcinol (AR) concentrations in plasma or adipose tissue among free-living Swedish men (n 149) and women (n 109)†
| AR | WGR&W | WGW | WGR | Plasma v. adipose‡ |
|---|---|---|---|---|
| C17:0 | ||||
| Adipose | 0·35*** | 0·14* | 0·40*** | 0·81*** |
| Plasma | 0·36*** | 0·15* | 0·40*** | |
| C19:0 | ||||
| Adipose | 0·29*** | 0·14* | 0·32*** | 0·70*** |
| Plasma | 0·28*** | 0·09 | 0·32*** | |
| C21:0 | ||||
| Adipose | 0·26*** | 0·12 | 0·30*** | 0·61*** |
| Plasma | 0·19** | 0·06 | 0·23*** | |
| C23:0 | ||||
| Adipose | 0·32*** | 0·18** | 0·33*** | 0·58*** |
| Plasma | 0·26*** | 0·12 | 0·28*** | |
| C25:0 | ||||
| Adipose | 0·28*** | 0·17** | 0·29*** | 0·49*** |
| Plasma | 0·28** | 0·12 | 0·31*** |
WGW and WGR were calculated from FFQ by multiplying the frequency of intake of every WG-containing item by its specific portion size and WG content (WG wheat content for WGW; and WG rye content for WGR); WGR&W was the sum of WGW and WGR.
*P<0·05, **P<0·01, ***P<0·001.
n 239 for AR concentration in plasma; plasma samples were missing for nineteen women.
The correlation coefficients between AR concentrations.
On examining potential determinants, it was found that energy-adjusted WGR was significantly associated with total AR concentration in adipose tissue in Model 1 and Model 2 (P<0·001) (Table 5). Total AR in adipose tissue increased by approximately 12 % per 10 g increment in daily energy-adjusted WGR intake (P<0·001). For women, total AR concentration in adipose tissue was approximately 61 % lower than for men (P<0·05) under similar energy-adjusted WGR and WGW intake and further adjusting for BMI and age in Model 2 (Table 5). In the present study, age, BMI or WGW did not appear to be a significant determinant of AR concentration in adipose tissue in models with both sexes (Table 5) and stratified by sex (data not shown). Total AR concentration in plasma was associated only with energy-adjusted WGR (about 11 % per 10 g increment, P<0·001) and not with WGW, sex, age or BMI.
Table 5.
Intakes of whole-grain (WG) wheat (WGW), WG rye (WGR) and non-dietary determinants of total alkylresorcinol (AR) concentration in adipose tissue and plasma among free-living Swedish men (n 149) and women (n 109)†
| Total AR concentration in adipose tissue | Total AR concentration in plasma | |||||||
|---|---|---|---|---|---|---|---|---|
| Model 1‡ | Model 2‡ | Model 1‡ | Model 2‡ | |||||
| Determinant | % | 95 % CI | % | 95 % CI | % | 95 % CI | % | 95 % CI |
| WGW (per 10-g increase)§ | −7·7 | −17·2, 2·9 | −8·0 | −17·5, 2·6 | −7·8 | −17·8, 3·5 | −7·7 | −17·8, 3·6 |
| WGR (per 10-g increase)§ | 11·9*** | 6·0, 18·2 | 12·5*** | 6·5, 18·7 | 11·2*** | 5·0, 17·9 | 11·5*** | 5·2, 18·1 |
| Energy (per 1-Mcal/d increase) | 23·5** | 8·8, 40·1 | 16·5* | 1·1, 34·4 | 19·7** | 4·7, 36·9 | 24·6** | 7·3, 44·6 |
| Sex (female v. male) | – | – | −61·3* | −83·3, −10·5 | – | – | −52·7 | −82·9, 31·1 |
| BMI (per 1-kg/m2 increase) | – | – | −1·1 | −3·8, 1·6 | – | – | 1·3 | −1·6, 4·2 |
| Age (per 1-year increase) | – | – | −2·1 | −4·2, 0·0 | – | – | −2·3 | −4·8, 0·2 |
| R 2 | 0·098 | 0·120 | 0·080 | 0·104 | ||||
WGW and WGR were calculated from FFQ by multiplying the frequency of intake of every WG-containing item by its specific portion size and WG content (WG wheat content for WGW; WG rye content for WGR). Estimated effects (95 % CI) were calculated using general linear models with log-transformed total AR concentration as dependent variable.
*P<0·05, **P<0·01 ***P<0·001.
Plasma samples were missing for nineteen women.
Model 1 included energy-adjusted dietary determinants (with the residual method) and total energy intake as dependent variables; Model 2 included energy-adjusted dietary determinants (with the residual method), sex, age, BMI and total energy intake as dependent variables.
WGW and WGR were calculated from FFQ-10 for men and FFQ-03 for women.
Discussion
In the present study, we evaluated AR concentrations in adipose tissue as a long-term biomarker of WG rye and wheat intake by correlating intake data derived from repeated FFQ over 14 years (for men) or 17 years (for women) with AR concentrations in adipose tissue biopsies in free-living Swedish men and women. We also compared correlations with plasma AR concentrations.
Intakes of WG products, TWG, WGR and WGW were more than twice as high as reported for Swedish men and women in a previous Nordic study( 4 ). This may be due to the participants being older than in the previous study, since higher age has been associated with higher intake of WG products( 34 ). The higher intake may also be due to inclusion of more food categories in the present study and because some products in these food categories contained WG wheat and/or rye, according to the Swedish National Food Composition Database. Except for FFQ-87 among women, participants in the present study reported relatively stable intakes of WG products, TWG, WGR and WGR&W across repeated FFQ, which is in agreement with reported intake of cereal fibre on occasions 1 year apart among SMC participants( 30 ) and of cereal fibre intake over a 4-year period among premenopausal US women (ICC≈0·50)( 35 ). Higher intake of WG in FFQ-87 than FFQ-97 or FFQ-03 among female participants might have led to the poor ICC of WG intakes found between FFQ-87 and FFQ-97.
The AR concentrations in adipose tissue were similar to those reported in a previous study in Swedish women( 25 ) and in a Finnish intervention study including men and women( 24 ). Plasma AR concentrations were within the range of what has been previously reported in other Nordic studies( 19 , 36 – 42 ). Plasma AR C17:0/C21:0 ratio was about 0·1 or more than 0·6 in subjects who mainly consumed WG wheat or WG rye, respectively( 21 , 42 – 44 ). In the present study, AR C17:0/C21:0 ratios in adipose tissue and plasma were about 0·3, which is in agreement with a previous Swedish study( 45 ). Modest AR C17:0/C21:0 ratio suggests that both WG rye and WG wheat are included in the habitual diet of the participants, which agrees with the reported WGR and WGW from the FFQ.
TWG and WGR&W were more strongly correlated with AR in adipose tissue than was WG product intake, showing the advantage of deriving WG intakes instead of just using WG product intake as a proxy. A reason for the weaker correlations for WG product intake may also be the inclusion of ‘white bread’ in this category due to the presence of whole grains in several breads that fall into this category. This may have contributed to misclassification of the WG product intake, since most ‘white bread’ contains only minor amounts of whole grains. Correlation coefficients for WGR&W were not stronger than for TWG, which also includes WG oats with no AR. This could be due to low overall intake of WG oats and a lack of precision in estimating WG wheat. The strongest correlations were observed for WGR, probably due to the main source being crispbread (contributing 67–74 % of WGR), for which intake was stable (ICC=0·37–0·56). In contrast, the correlation between WGW and AR in adipose tissue was weak probably because WGW was derived mainly from whole-meal bread and pasta (contributing 58–73 % of WGW), for which the intakes were unstable (ICC=0·01–0·28). A reduction in random measurement error when using the mean of repeated FFQ instead of a single FFQ resulted in stronger correlations between intakes and AR in adipose tissue, despite including intakes over longer periods. This is in line with findings of a correlation between fatty acid intakes in repeated FFQ and corresponding fatty acids in erythrocytes( 46 ). Although the time interval between repeated FFQ was longer for women than for men, correlations between WG intakes and AR in adipose tissue were overall stronger for women, suggesting more accurate intake reporting of WG intakes and/or more stable intakes among women than men.
Among non-dietary determinants, age has in previous studies been suggested to be associated with higher plasma AR concentrations( 20 , 47 ). Moreover, age has been shown to affect age-related central fat accumulation of some lipophilic compounds such as vitamin D in response to intake in elderly subjects( 48 , 49 ). However, we found that neither adipose tissue/plasma AR concentration nor body fat content measured with dual-energy X-ray absorptiometry (data not shown) was associated with age in the present study. One reason could be that the present study subjects were older and that the age range was rather narrower than in previous studies.
Sex has been shown to affect plasma AR concentration with higher concentrations in men than in women( 20 , 50 ), but this was not observed in the present study. However, adipose tissue biopsies from men contained significantly higher AR concentrations than those from women after adjusting for differences in WG intake. This strongly suggests sex-related differences in AR accumulation in adipose tissue and should be taken into account if using AR in adipose tissue as a biomarker. AR are believed to be metabolized similarly to γ-tocopherol( 51 ), which is cleared faster and leads to lower accumulation in women than in men( 52 ). However, this was not supported by any differences in plasma AR concentrations between men and women in the present study. The adipose tissue AR concentration was lower among women, which suggests that the lower AR concentrations in adipose tissue could be due to mechanisms unrelated to plasma AR concentrations. Women, particularly postmenopausal women, usually have a larger body pool of fat than men( 53 ). A larger volume of distribution could explain the lower AR adipose tissue concentrations in women. Unfortunately, we did not have dual-energy X-ray absorptiometry measurements for men and therefore could not investigate whether this was the reason.
Because of longer anticipated turnover rate of AR in adipose tissue, we expected stronger correlations between long-term WG intake assessed by FFQ and AR in adipose tissue than for plasma( 24 , 54 ). The finding that AR in adipose tissue correlated similarly as AR in plasma with estimated long-term WG wheat and rye intake could be due to regular and frequent WG consumption in the present population (Table 1). Under such conditions, plasma AR concentration also reflects long-term intake, as shown in previous studies on Swedish populations( 39 , 47 ). Average WGR&W intakes from repeated FFQ were better correlated with AR in adipose tissue, but more weakly correlated with AR in plasma. This suggests that AR in plasma might be more prone to being affected by recent WG intake, although the difference was small. The fact that AR concentrations in adipose tissue and plasma were more weakly correlated with FFQ-based WG intake (r=0·22–0·42; Table 3) than with previously reported WG intake estimated with food records (r=0·60–0·72)( 24 ) suggests that FFQ-based WG intake contains larger measurement errors, which might mask the potential difference between AR in adipose tissue and in plasma as a biomarker of long-term WG rye and wheat intake. Another possible reason is that AR in fasting plasma may to a large extent reflect AR liberated from the adipose tissue pool and longer homologues may be liberated to a lower extent than shorter homologues (Table 4), as reported for fatty acids( 55 ).
Our study has several limitations. First, potential differences in performance of AR in adipose tissue and plasma may have been more easily detectable by comparing their correlations with estimated intake in a population with low and infrequent WG intake and using repeated biopsies. However, studies with such sampling are scarce. Second, misclassification of WG rye and WG wheat intake, particularly the latter, due to large variation of WG content in food products, is likely to be the reason for the poor correlations to adipose tissue AR concentrations, particularly for WG wheat( 31 ). Third, we lacked dual-energy X-ray absorptiometry data for men and therefore could not test the hypothesis that lower concentrations of AR in adipose tissue among women are due to large distribution volume. Fourth, since the adipose tissue available was scarce, the sample size of the present study was relatively small, thus results should be interpreted with caution.
The present study also has several strengths. First, we monitored intake of WG food products and calculated WG wheat and WG rye intakes over a long period, 14 years in men and 17 years in women, with repeated FFQ. Second, WG intake range in the current population was broad and diverse, which made it suitable for the present purposes. Moreover, we evaluated AR in adipose tissue as a long-term biomarker of WG wheat and rye intake in comparison with other exposure measurements, i.e. FFQ-derived WG intakes and plasma AR concentrations.
In summary, we found that AR in adipose tissue appears to be promising as a long-term dietary biomarker of WG rye intake, after adjusting for differences in sex. The lack of correlation between WG wheat and AR concentration is most likely due to large-scale misclassification of WG wheat intake by FFQ, errors inherent in the large variation in WG content in WGW products, combined with few questions in the FFQ and the fact that WG wheat contains much lower amounts of AR than WG rye( 56 ). Future studies should evaluate AR in adipose tissue as a long-term biomarker of WG wheat and rye intake in populations with more irregular WG intake.
Acknowledgements
Acknowledgements: The authors are grateful to Janicka Nilsson, Emma Myhré Herelius and Elisabeth Sekli, who assisted with AR analysis in plasma and adipose tissue. Financial support: This work was supported by the Swedish Research Council for Environment, Agricultural Sciences and Spatial Planning (FORMAS, 2011-520) and the Swedish Research Council – Medicine. The funders had no role in the design, analysis or writing of this article. Conflict of interest: None of the other authors declare any conflict of interest. Authorship: H.W. planned and conducted the study, including the analysis of AR in adipose tissue and plasma, derived WG intakes from dietary data, conducted the statistical analysis, and drafted the paper; N.A.M.O. and N.H. contributed to the analysis of dietary data, statistical analysis and drafting of the manuscript; A.W. and K.M. designed the cohorts, provided data and samples, and supervised the data interpretation and manuscript preparation; R.L. conceived the study and supervised analysis of samples and data, as well as manuscript preparation. All authors were involved in data interpretation and manuscript preparation. Ethics of human subject participation: This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the Regional Ethical Review Board in Stockholm. Written informed consent was obtained from all subjects.
References
- 1. Aune D, Keum N, Giovannucci E et al. (2016) Whole grain consumption and risk of cardiovascular disease, cancer, and all cause and cause specific mortality: systematic review and dose–response meta-analysis of prospective studies. BMJ 353, i2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Qi L, van Dam RM, Liu S et al. (2006) Whole-grain, bran, and cereal fiber intakes and markers of systemic inflammation in diabetic women. Diabetes Care 29, 207–211. [DOI] [PubMed] [Google Scholar]
- 3. Kyrø C, Skeie G, Loft S et al. (2013) Intake of whole grains from different cereal and food sources and incidence of colorectal cancer in the Scandinavian HELGA cohort. Cancer Causes Control 24, 1363–1374. [DOI] [PubMed] [Google Scholar]
- 4. Kyrø C, Skeie G, Dragsted LO et al. (2012) Intake of whole grain in Scandinavia: intake, sources and compliance with new national recommendations. Scand J Public Health 40, 76–84. [DOI] [PubMed] [Google Scholar]
- 5. Andersson AAM, Andersson R, Piironen V et al. (2013) Contents of dietary fibre components and their relation to associated bioactive components in whole grain wheat samples from the HEALTHGRAIN diversity screen. Food Chem 136, 1243–1248. [DOI] [PubMed] [Google Scholar]
- 6. Saastamoinen M, Plaami S & Kumpulainen J (1989) Pentosan and β-glucan content of Finnish winter rye varieties as compared with rye of six other countries. J Cereal Sci 10, 199–207. [Google Scholar]
- 7. Hansen HB, Rasmussen CV, Bach Knudsen KE et al. (2003) Effects of genotype and harvest year on content and composition of dietary fibre in rye (Secale cereale L.) grain. J Sci Food Agric 83, 76–85. [Google Scholar]
- 8. Nurmi T, Nyström L, Edelmann M et al. (2008) Phytosterols in wheat genotypes in the HEALTHGRAIN Diversity Screen. J Agric Food Chem 56, 9710–9715. [DOI] [PubMed] [Google Scholar]
- 9. Kristal AR, Peters U & Potter JD (2005) Is it time to abandon the food frequency questionnaire? Cancer Epidemiol, Biomarkers Prev 14, 2826–2828. [DOI] [PubMed] [Google Scholar]
- 10. Thompson FE & Subar AF (2008) Dietary assessment methodology. Nutr Prev Treat Dis 2, 3–39. [Google Scholar]
- 11. Kristensen M, Toubro S, Jensen MG et al. (2012) Whole grain compared with refined wheat decreases the percentage of body fat following a 12-week, energy-restricted dietary intervention in postmenopausal women. J Nutr 142, 710–716. [DOI] [PubMed] [Google Scholar]
- 12. Ross AB, Bruce SJ, Blondel-Lubrano A et al. (2011) A whole-grain cereal-rich diet increases plasma betaine, and tends to decrease total and LDL-cholesterol compared with a refined-grain diet in healthy subjects. Br J Nutr 105, 1492–1502. [DOI] [PubMed] [Google Scholar]
- 13. Tłuścik F (2015) Localization of the alkylresorcinols in rye and wheat caryopses. Acta Soc Bot Pol 47, 211–218. [Google Scholar]
- 14. Kozubek A & Tyman JH (1999) Resorcinolic lipids, the natural non-isoprenoid phenolic amphiphiles and their biological activity. Chem Rev 99, 1–26. [DOI] [PubMed] [Google Scholar]
- 15. Chen Y, Ross AB, Åman P et al. (2004) Alkylresorcinols as markers of whole grain wheat and rye in cereal products. J Agric Food Chem 52, 8242–8246. [DOI] [PubMed] [Google Scholar]
- 16. Linko AM, Juntunen KS, Mykkanen HM et al. (2005) Whole-grain rye bread consumption by women correlates with plasma alkylresorcinols and increases their concentration compared with low-fiber wheat bread. J Nutr 135, 580–583. [DOI] [PubMed] [Google Scholar]
- 17. Kyrø C, Olsen A, Landberg R et al. (2014) Plasma alkylresorcinols, biomarkers of whole-grain wheat and rye intake, and incidence of colorectal cancer. J Natl Cancer Inst 106, djt352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Marklund M, Magnusdottir OK, Rosqvist F et al. (2014) A dietary biomarker approach captures compliance and cardiometabolic effects of a healthy Nordic diet in individuals with metabolic syndrome. J Nutr 144, 1642–1649. [DOI] [PubMed] [Google Scholar]
- 19. Landberg R, Kamal-Eldin A, Andersson SO et al. (2009) Reproducibility of plasma alkylresorcinols during a 6-week rye intervention study in men with prostate cancer. J Nutr 139, 975–980. [DOI] [PubMed] [Google Scholar]
- 20. Ross AB, Bourgeois A, Macharia HN et al. (2012) Plasma alkylresorcinols as a biomarker of whole-grain food consumption in a large population: results from the WHOLEheart Intervention Study. Am J Clin Nutr 95, 204–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Linko-Parvinen A-M, Landberg R, Tikkanen MJ et al. (2007) Alkylresorcinols from whole-grain wheat and rye are transported in human plasma lipoproteins. J Nutr 137, 1137–1142. [DOI] [PubMed] [Google Scholar]
- 22. Magnusdottir OK, Landberg R, Gunnarsdottir I et al. (2014) Plasma alkylresorcinols C17:0/C21:0 ratio, a biomarker of relative whole-grain rye intake, is associated to insulin sensitivity: a randomized study. Eur J Clin Nutr 68, 453–458. [DOI] [PubMed] [Google Scholar]
- 23. Aune D, Norat T, Romundstad P et al. (2013) Whole grain and refined grain consumption and the risk of type 2 diabetes: a systematic review and dose–response meta-analysis of cohort studies. Eur J Epidemiol 28, 845–858. [DOI] [PubMed] [Google Scholar]
- 24. Wu H, Kolehmainen M, Mykkanen H et al. (2015) Alkylresorcinols in adipose tissue biopsies as biomarkers of whole-grain intake: an exploratory study of responsiveness to advised intake over 12 weeks. Eur J Clin Nutr 69, 1244–1248. [DOI] [PubMed] [Google Scholar]
- 25. Jansson E, Landberg R, Kamal-Eldin A et al. (2010) Presence of alkylresorcinols, potential whole grain biomarkers, in human adipose tissue. Br J Nutr 104, 633–636. [DOI] [PubMed] [Google Scholar]
- 26. Harris H, Håkansson N, Olofsson C et al. (2013) The Swedish mammography cohort and the cohort of Swedish men: study design and characteristics of two population-based longitudinal cohorts. OA Epidemiol 1, 16. [Google Scholar]
- 27. Wolk A, Larsson SC, Johansson J et al. (2006) Long-term fatty fish consumption and renal cell carcinoma incidence in women. JAMA 296, 1371–1376. [DOI] [PubMed] [Google Scholar]
- 28. Larsson SC, Giovannucci E & Wolk A (2005) Diabetes and colorectal cancer incidence in the cohort of Swedish men. Diabetes Care 28, 1805–1807. [DOI] [PubMed] [Google Scholar]
- 29. Terry P, Giovannucci E, Michels KB et al. (2001) Fruit, vegetables, dietary fiber, and risk of colorectal cancer. J Natl Cancer Inst 93, 525–533. [DOI] [PubMed] [Google Scholar]
- 30. Khani BR, Ye W, Terry P et al. (2004) Reproducibility and validity of major dietary patterns among Swedish women assessed with a food-frequency questionnaire. J Nutr 134, 1541–1545. [DOI] [PubMed] [Google Scholar]
- 31. Bergström L, Kylberg E, Hagman U et al. (1991) The food composition database KOST: the National Administration’s information system for nutritive values of food. Vår Föda 43, 439–447. [Google Scholar]
- 32. Wierzbicka R, Wu H, Franek M et al. (2015) Determination of alkylresorcinols and their metabolites in biological samples by gas chromatography–mass spectrometry. J Chromatogr B 1000, 120–129. [DOI] [PubMed] [Google Scholar]
- 33. Hertzmark E & Spiegelman D (2010) The SAS ICC9 Macro. Cambridge, MA: Channing Laboratory, Harvard University. [Google Scholar]
- 34. Sandvik P (2017) Rye bread in Sweden: health-related and sensory qualities, consumer perceptions and consumption patterns. PhD Thesis, Uppsala University.
- 35. Landberg R, Townsend MK, Neelakantan N et al. (2012) Alkylresorcinol metabolite concentrations in spot urine samples correlated with whole grain and cereal fiber intake but showed low to modest reproducibility over one to three years in US women. J Nutr 142, 872–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. McKeown NM, Marklund M, Ma J et al. (2016) Comparison of plasma alkylresorcinols (AR) and urinary AR metabolites as biomarkers of compliance in a short-term, whole-grain intervention study. Eur J Nutr 55, 1235–1244. [DOI] [PubMed] [Google Scholar]
- 37. McKeown NM, Hruby A, Landberg R et al. (2016) Plasma alkylresorcinols, biomarkers of whole-grain intake, are not associated with progression of coronary artery atherosclerosis in postmenopausal women with coronary artery disease. Public Health Nutr 19, 326–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nybacka S, Lindroos AK, Wirfält E et al. (2016) Carotenoids and alkylresorcinols as objective biomarkers of diet quality when assessing the validity of a web-based food record tool and a food frequency questionnaire in a middle-aged population. BMC Nutr 2, 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Andersson A, Marklund M, Diana M et al. (2011) Plasma alkylresorcinol concentrations correlate with whole grain wheat and rye intake and show moderate reproducibility over a 2- to 3-month period in free-living Swedish adults. J Nutr 141, 1712–1718. [DOI] [PubMed] [Google Scholar]
- 40. Marklund M, Landberg R, Andersson A et al. (2013) Alkylresorcinol metabolites in urine correlate with the intake of whole grains and cereal fibre in free-living Swedish adults. Br J Nutr 109, 129–136. [DOI] [PubMed] [Google Scholar]
- 41. Cuff J, Sanders T, Reidlinger D et al. (2015) Urinary alkylresorcinol metabolites as a biomarker of dietary wholegrain intake and of compliance in a randomised dietary intervention trial: results from the CRESSIDA Study. Proc Nutr Soc 74, E42. [Google Scholar]
- 42. Kyrø C, Olsen A, Skeie G et al. (2014) Plasma alkylresorcinol concentrations, biomarkers of whole-grain wheat and rye intake, in the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort. Br J Nutr 111, 1881–1890. [DOI] [PubMed] [Google Scholar]
- 43. Ross AB, Pineau N, Kochhar S et al. (2009) Validation of a FFQ for estimating whole-grain cereal food intake. Br J Nutr 102, 1547–1551. [DOI] [PubMed] [Google Scholar]
- 44. Landberg R (2009) Alkylresorcinols as biomarkers of whole grain wheat and rye intake. PhD Thesis, Swedish University of Agricultural Sciences.
- 45. Landberg R, Kamal-Eldin A, Andersson A et al. (2008) Alkylresorcinols as biomarkers of whole-grain wheat and rye intake: plasma concentration and intake estimated from dietary records. Am J Clin Nutr 87, 832–838. [DOI] [PubMed] [Google Scholar]
- 46. Sun Q, Ma J, Campos H et al. (2007) Comparison between plasma and erythrocyte fatty acid content as biomarkers of fatty acid intake in US women. Am J Clin Nutr 86, 74–81. [DOI] [PubMed] [Google Scholar]
- 47. Landberg R, Åman P, Hallmans G et al. (2013) Long-term reproducibility of plasma alkylresorcinols as biomarkers of whole-grain wheat and rye intake within Northern Sweden Health and Disease Study Cohort. Eur J Clin Nutr 67, 259–263. [DOI] [PubMed] [Google Scholar]
- 48. Mazahery H & von Hurst PR (2015) Factors affecting 25-hydroxyvitamin D concentration in response to vitamin D supplementation. Nutrients 7, 5111–5142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Toth MJ & Tchernof A (2000) Lipid metabolism in the elderly. Eur J Clin Nutr 54, Suppl. 3, S121–S125. [DOI] [PubMed] [Google Scholar]
- 50. Montonen J, Landberg R, Kamal-Eldin A et al. (2010) Reliability of fasting plasma alkylresorcinol concentrations measured 4 months apart. Eur J Clin Nutr 64, 698–703. [DOI] [PubMed] [Google Scholar]
- 51. Ross AB, Chen Y, Frank J et al. (2004) Cereal alkylresorcinols elevate γ-tocopherol levels in rats and inhibit γ-tocopherol metabolism in vitro . J Nutr 134, 506–510. [DOI] [PubMed] [Google Scholar]
- 52. Frank J, Lee S, Leonard SW et al. (2008) Sex differences in the inhibition of γ-tocopherol metabolism by a single dose of dietary sesame oil in healthy subjects. Am J Clin Nutr 87, 1723–1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ley CJ, Lees B & Stevenson JC (1992) Sex- and menopause-associated changes in body-fat distribution. Am J Clin Nutr 55, 950–954. [DOI] [PubMed] [Google Scholar]
- 54. Strawford A, Antelo F, Christiansen M et al. (2004) Adipose tissue triglyceride turnover, de novo lipogenesis, and cell proliferation in humans measured with 2H2O. Am J Physiol Endocrinol Metab 286, E577–E588. [DOI] [PubMed] [Google Scholar]
- 55. Halliwell KJ, Fielding BA, Samra JS et al. (1996) Release of individual fatty acids from human adipose tissue in vivo after an overnight fast. J Lipid Res 37, 1842–1848. [PubMed] [Google Scholar]
- 56. Menzel C, Kamal-Eldin A, Marklund M et al. (2012) Alkylresorcinols in Swedish cereal food products. J Food Compost Anal 28, 119–125. [Google Scholar]