Abstract
Bioprinting is a very useful tool that has a huge application potential in different fields of science and biotechnology. In medicine, advances in bioprinting are focused on the printing of cells and tissues for skin regeneration and the manufacture of viable human organs, such as hearts, kidneys, and bones. This review provides a chronological overview of some of the most relevant developments of bioprinting technique and its current status. A search was carried out in SCOPUS, Web of Science, and PubMed databases, and a total of 31,603 papers were found, of which 122 were finally chosen for analysis. These articles cover the most important advances in this technique at the medical level, its applications, and current possibilities. Finally, the paper ends with conclusions about the use of bioprinting and our expectations of this technique. This paper presents a review on the tremendous progress of bioprinting from 1998 to the present day, with many promising results indicating that our society is getting closer to achieving the total reconstruction of damaged tissues and organs and thus solving many healthcare-related problems, including the shortage of organ and tissue donors.
Keywords: Bioprinting, 3D printing, Tissue engineering, Organ culture, Tissue regeneration
1. Introduction
367Three-dimensional (3D) bioprinting, which can be defined as a technique used to print living cells in a pre-designed pattern[1], is an innovative technology that is still in its infancy. Bioprinting has a huge application potential in many disciplines of science and biotechnology. Particularly, in the field medicine, bioprinting is utilized to print cells and tissues for the purposes of skin regeneration and manufacture of viable human organs, including hearts, kidneys, and bones.
Bioprinting has undergone an enormous evolution within the recent decade, and it is expected to bring about revolution to the modern medicine. Specifically, bioprinting can be applied to develop new therapies in the form of newly generated organs for autologous transplants in patients.
In this review, we aim to present a chronological overview of the most relevant developments of bioprinting technique and its current status after a thorough search of368 papers in SCOPUS, Web of Science (WOS), and PubMed databases and the subsequent analysis. The achievements of bioprinting spanning more than two decades, from 1998 to the present day, have been tremendous, with promising results that help steer the world toward achieving the total reconstruction of damaged tissues and organs, thereby addressing the long-standing issues of organ and tissue donor shortage.
2. Methodology
Before carrying out the article search and analysis, access to the main bibliographic databases and scientific journals such as SCOPUS, WOS, and PubMed was obtained. The articles required for this review were retrieved from these databases.
A global search was carried out in the three databases using the following criteria: (i) publications in English (only publications in English were selected); (ii) papers related to 3D bioprinting; (iii) papers related to materials for 3D bioprinting; and (iv) papers related to bioprinting techniques. A total of 31,603 results were obtained, leaving 17,603 after duplicates were removed. A total of 8244 articles were excluded and 4863 articles were discarded because they dealt with other reviews or belonged to fields of study unrelated to bioprinting. Finally, a total of 853 articles were obtained, from which 122 were selected for the analysis required for this review (Figure 1).
Figure 1.

PRISMA flow diagram depicting literature search, exclusion process, eligibility criteria, and final included papers. One hundred and nineteen papers were included in this review without publication date restriction.
3. Results
Of the 122 publications selected, 120 are articles. A simple analysis of these publications categorized by year shows that a high number of papers pertaining to the application of bioprinting in medicine were published in 2004, 2009, 2016, and 2018. From 2019 onward, there has been a decrease in the number of articles that are suitable for this review due to the fact that no relevant discoveries were made. Furthermore, due to the COVID-19 pandemic caused by SARS-CoV-2, there has been a significant decrease in the publication of research articles and a considerable increase in review articles since 2020.
Despite this, Figure 2 shows a general upward trend in the number of important publications.
Figure 2.

Number of publications (of the 122 selected articles) by year of publication.
3.1. Geography of bioprinting research
The analysis of the articles according to the place of publication shows that most of the studies were carried out in the United States, followed by China, Germany, and the United Kingdom.
This section shows that the researchers from the most developed countries are leading research in the important field of bioprinting (Figure 3).
Figure 3.

Representative map of the number of publications (of 122 selected articles) per country/region. The actual counts are as follows: United States (53), China (11), Germany (9), United Kingdom (9), Netherlands (8), South Korea (6), Japan (5), India (3), Israel (2), Beirut (1), South Africa (1), Canada (1), Qatar (1), Russia (1), Australia (1), Sweden (1), Belgium (1), France (1), Ireland (1), Italy (1), Thailand (1), Taiwan (1), Latvia (1), Turkey (1), and Iran (1).
3.2. Timeline of bioprinting research
3.2.1. Beginning phase of bioprinting research
The first attempts to achieve cell growth on a pre-fabricated, biodegradable, and survivable 3D surface began in 1998, when the surface of biodegradable polylactic acid (PLA) polymers was modified using polyethylene oxide (PEO) and polypropylene oxide (PPO) to achieve adhesion of liver cells and fibroblasts to their surface[2].
In the same year, survival and function of hepatocytes in a new 3D synthetic biodegradable polymer scaffold with an intrinsic network of interconnected channels under continuous flow conditions that allowed for adequate oxygen diffusion were studied[3]. It was in 1999 that the idea of replacing damaged or diseased organs with artificial tissues from a combination of living cells and biocompatible scaffolds began to be considered, thanks to the multidisciplinary efforts and the increasing availability of tools to study the control of cell behavior[4]. In 2000, printing methodologies based on high-precision 3D micropositioning with syringes that can deposit volumes of up to nanoliters were already being developed[5].
In 2001, the importance of determining the spatial organization of the surface of polymers when they were to be modified for use as scaffolds[6], as well as the influence of porosity and pore size on these scaffolds for proper fabric formation[7] was demonstrated. From 2002 onward, studies related to cell-to-cell fabrication of living tissue using low- energy laser pulses to achieve the construction of complex 3D tissues, such as living mammalian cells, active proteins, extracellular matrix materials, or materials for semi-rigid scaffolds[8], began to emerge.
3.2.2. Take-off phase of bioprinting research
In 2003, the first article unifying the concepts of printing cells layer by layer on a thermo-reversible gel to form 3D organs was published, proposing this method as a possible solution to the organ shortage crisis[9]. Afterward, the possibility of using thermo-sensitive 3D gels to generate sequential layers for cell printing was proposed by the same researchers[10].
The concept of bioprinting first appeared in 2004, when a system of 12 piezoelectric ejectors capable of printing biological materials by droplet ejection on an XY platform was developed, allowing the printing of any desired pattern[11]. Numerous articles on different 3D printing techniques applied to biological materials were also released that year[12-17]. Thereafter, it was realized that viable cells could be printed using something as simple as commercial inkjet printers, where multiple nozzles would be able to create arbitrary structures made up of mixed cell types[18]. It was at this time that bioprinting became an increasingly important research target, to the point that the369370 first International Workshop on Bioprinting, Biodesign, and Bioassembly was held at the Medical University of South Carolina, with its second workshop held in 2005[19]. Several studies were also carried out for the realization of biocompatible scaffolds for bone tissue regeneration[20,21].
The first print associated with the encapsulation of cells in hydrogels was produced in 2006 by bioprinting liver cells for an in vitro model for pharmacokinetic studies[22]. In the same year, a growing demand for assembling different relevant biological materials into prescribed371 3D hierarchical organizations with the aim of recreating multicellular tissues and organs was observed, and new developments in material transfer processes at micrometer and nanometer scales were seen[23]. In addition, the key message from the First Annual Charleston Bioprinting Symposium, organized by the Medical University of South Carolina’s Bioprinting Research Center, demonstrated that despite many technological challenges, bioprinting which was a rapidly evolving technology at that time is a feasible solution to organ shortage[24].
In 2007, articles concerning the huge potential of bioprinting and its possibilities in tissue engineering began to surface[25,26], and new symposia were held on the subject[27]. In 2008, owing to the emergence of new studies connecting bioprinting and bone regeneration and studies on the use of hydroxyapatite in scaffolds, the importance of exploring the use of bioprinting in bone regeneration was repeatedly highlighted[28-33].
In 2009, a multi-drug implant containing isoniazid and timed-release rifampicin was developed using 3D printing to treat bone tuberculosis, implying another important potential application of this technique[34]. Direct printing of living cells in alginate gel was also performed with an inkjet printing system[35], and the recreation of skin grafts was achieved by printing collagen hydrogel precursors, fibroblasts, and keratinocytes[36]. Besides, the introduction of light-curing inks[37,38] and the successful bioprinting of microvasculature[39] and also vascular tissue without the use of scaffolds[40] had catapulted bioprinting technique to a new height.
Throughout 2010, there was a continuous development of high-performance laser printing of cells and biomaterials[41], and the hydrogels were established as the materials of choice for the future development of direct biofabrication techniques[42,43]. In addition, a 3D microscale liver tissue analog was biofabricated to evaluate pharmacokinetic profiles[44]. In 2011, a bioprinter that was quite similar to the current inkjet printers was developed; its mechanism was based on the layer-by-layer deposition of customized ink in adherence to complex image data, increasing the printer’s ability to mimic the conformation of tissue structures[45].
In 2012, the possibility of using amniotic fluid- derived cells in bioprinting was studied, with very positive results in the treatment of wounds, in which the rate of angiogenesis was higher than that of the single application of mesenchymal cells or fibrocollagen gel[46]. The great importance of endowing 3D engineered tissues with perfused vascular channels is also demonstrated, which solved the problem of nuclear necrosis to which densely populated tissues lacking such channels were subjected[47]. The importance of dopants in hydrogels, such as silica and zinc oxide in tricalcium phosphate scaffolds, was also uncovered, and the dopants allowed faster cell proliferation compared to pure tricalcium phosphate scaffolds[48]. In addition, printing of cardiac tissue with a combination of biomaterials and cardiomyocyte progenitor cells[49], as well as the 3D printing of an aortic valve[49], was tested, and good results were obtained.
3.2.3. Early achievements in bioprinting
In 2013, a bioabsorbable airway splint was created to treat tracheobronchomalacia, a condition that makes some newborns difficult to breathe[50]. Apart from that, the feasibility of manufacturing complex heterogeneous tissue constructs containing multiple cell types was also demonstrated using inkjet printing technologies[51], and with regard to laser-assisted bioprinting, successful 3D printing of a cellular construct with subsequent in vivo tissue formation was achieved for the creation of a skin-like tissue consisting of different cell types forming a complex pattern[52].
A very important milestone was represented by the bioprinting of a heterogeneous aortic valve using alginate-gelatin hydrogels, demonstrating that anatomically complex and heterogeneously encapsulated aortic valve hydrogel conduits could be fabricated with 3D bioprinting[53]. The time most people saw the budding, real potential of hydrogels in the field of bioprinting is when the use of methacrylated gelatine (GelMA) started to gain traction; upon exposure to ultraviolet light, GelMA is able to increase its stiffness and swelling properties, which are the mechanical properties conducive for bioprinting[54]. Another breakthrough in the development of bionic tissues and organs is represented by the reproduction of a human ear that was achieved by 3D-printing a cell-seeded hydrogel matrix with an inductive coil antenna inside, which provides radiofrequency reception capability[55].
In 2014, a printing method was developed to build vascular channels inside the printed structures, which allows cell viability to be maintained in thicker tissues, allowing the cells to grow and mature after printing[56-58]. Tumor recreations were also carried out for study. For instance, HeLa cells were 3D-printed to generate an in vitro cervical tumor model[59], and it was shown that the pressure and shape of the needle used in bioprinting could affect cell viability[60]. Furthermore, due to the complexity of the composition of the extracellular matrix and its important role in cell development and survival, bioinks made of decellularized extracellular matrix were created and became more tissue-specific[61].
3.2.4. Progression toward four-dimensional (4D) bioprinting
372During 2015, it was demonstrated that the tissue construct made of soft hydrogels reinforced with high- porosity microfiber networks had stiffness and elasticity comparable to that of articular cartilage tissue, providing a basis for reproducing tissue constructs with biological and mechanical compatibility[62]. Besides, the composition of a bioink containing graphene was manipulated to alter a range of parameters such as adhesion, viability, proliferation, and differentiation of mesenchymal cells to neurological tissue due to its high conductivity[63].
The use of alginate bioink with nanocellulose, which has excellent shear-thinning properties, as a matrix for the printing of cartilage tissue, was seen as a possibility[64], and another bioink composed of polyethylene glycol, sodium alginate, and nanoclay with high cell strength and viability was also developed for the printing of cartilage tissue[65]. A major breakthrough was the bioprinting of encapsulated primary neural cells into brain-like structures using gellan gum as bioink[66], and hydrogels with adjustable mechanical properties through light irradiation were becoming increasingly important[67].
During 2016, an integrated organ and tissue printer was developed, capable of manufacturing stable humanscale tissue constructs of any shape by printing cell-loaded hydrogels together with biodegradable polymers[68], and in general, the printing of complex tissues with good vascularization was increasingly perfected[69,70]. The combination of different hydrogels to optimize the properties of the printed structure as much as possible was also gaining importance[71].
A 3D biomimetic liver model that mimics the characteristic morphological organization of liver cells for use in disease replication and early drug detection was successfully developed[72]. A bioprinting method to create 3D human renal proximal tubules in vitro that are completely embedded within extracellular matrix and housed in perfusable tissue chips was also developed; the viability of these bioprinted tubules was successfully maintained for more than 2 months[73,74].
The use of sound waves as “acoustic tweezers” has been explored for precise and non-invasive manipulation of single and whole cells to create two-dimensional (2D) and 3D structures[75]. In vivo monitoring of bioprinted tissues using sensors, such as non-invasive electronic readout of contractile stresses and drug responses of cardiac tissues, was first started in 2017[76].
The impression quality of cardiac tissue was improved by pre-vascularizing it prior to implantation, achieving rapid vascularization after patch application to the affected tissue, and enabling repair of infarcted areas[77].
A technique that allows the creation of scaffolds morphologically and structurally similar to the extracellular matrix by thermally induced autoagglomeration of nanofibers and electro-spinning PLA and polycaprolactone (PCL) nanofibers was developed[78]. Furthermore, the importance of pore geometry and the effect it has on cell behavior and function have been demonstrated, with 30° and 60° scaffolds restoring ovarian tissue in sterilized mice, which were able to produce offspring by natural mating[79]. The possibility of printing functional living components with bacteria signifies the possibility of obtaining materials that can perform different functions, as in the case of the 3D printing of bioinks with Acetobacter xylinum and Pseudomonas putida, capable of producing cellulose for medical use and degrading pollutants, respectively, at the same time[80]. Separately, the development of a functional mouse thyroid gland capable of normalizing thyroxine levels in the blood and regulating body temperature after engraftment is another noteworthy achievement[81].
In 2018, the refinement of bioprinting materials for bone regeneration[82], as well as the study of possible combinations of hydrogels and their printing parameters to obtain the best possible results continued[83,84]. The study of the tumor microenvironment and its role in cell communication in cancer development continued, in order to recreate this type of tissue as faithfully as possible using bioprinting technologies, and thus achieve more specific and realistic assays to combat the disease[85].
Bioprinting of a full-thickness human skin model was achieved using skin-derived extracellular matrix composite bioinks[86]. New studies on the use of graphene in 3D printing of neural tissue showed that it promoted axonal growth and remyelination after peripheral nerve injury, with great potential for preclinical and clinical applications[87]. In addition, research on producing transplant-ready corneal prostheses has begun; although a suitable final structure has not yet been achieved, obtaining bioprinted keratocytes with high cell viability was the successful first step toward this goal[88]. The bioprinting of oligodendrocytes together with precisely and concretely printed spinal neuronal progenitor cells also opened the door to the reconstruction of functional axonal connections in areas of tissue damage in the central nervous system[89].
In 2019, pH-driven gelation control was found to provide 20-μm filament resolution, a porous microstructure that enables rapid cell infiltration and microvascularization, and mechanical strength for vasculature fabrication and perfusion in cardiac tissue regeneration[90]. The printing of cellularized human hearts with a natural architecture was 373also achieved by reprogramming cells for differentiation into cardiomyocytes and endothelial cells[91]. 3D printing of central nervous system structures, which is difficult to achieve due to the high complexity of the central nervous system, was made possible on a small scale using the microscale continuous projection printing. With these biomimetic scaffolds, regeneration of damaged axons from the spinal cord of mice was achieved, which synapse with the neural progenitor cells of the scaffold[92].
“Tumor-on-a-chip,” in which the reconstruction of a glioblastoma tumor from patient-derived cells reproduces the structure, biochemistry, and biophysical properties of the native tumor, was then developed and could be used for identifying effective treatments[93]. An in situ skin bioprinter was also developed, where skin layers were printed directly onto the patient, resulting in rapid wound closure, reduced shrinkage, and accelerated re-epithelialization[94].
It has been shown that the imprinting of cartilage extracellular matrix scaffolds, GelMA, and mesenchymal stem cell-derived exosomes can be an effective treatment for osteoarthritis by regulating disease-causing mitochondrial function and facilitating cartilage regeneration[95]. In addition, the design of biomimetic muscles created by 3D printing with tissue-derived cells and bioinks has been found to improve the treatment of irrecoverable volumetric muscle wasting[96].
The year of 2020 has seen a shortfall of original research papers because of the COVID-19 pandemic, and most of the articles on this topic were review articles.
The topic of most recent review articles revolves around 4D bioprinting, which integrates the concept of time as the fourth dimension within traditional 3D bioprinting technology and facilitates the fabrication of functional and complex biological architectures[97]. This is made possible by the fact that such 3D-printed structures are able to produce changes in their structure after receiving a certain stimulus. Apart from that, reviews on bioprinting in the study of the complex tumor microenvironment, with a better understanding of its functionality and the goal to tailor personalized treatment for each patient, also started to accumulate[98,99].
The use of poloxamer composites in bioprinting was first proposed due to their bioactivity, temperaturedependent self-assembly, thermo-reversible behavior, and physicochemical properties, which make them promising drug carriers and good mimics of various tissue types[100]. Also in 2020, the bioprinting of a meniscus that fulfilled the necessary characteristics to be implanted was achieved[101].
Throughout 2021 and 2022, reviews and studies on the use of decellularized cellular matrix in bioprinting were again published in abundance. A large number of articles focus on the characterization of hydrogels, impressions of small parts of organs that, thanks to all the advances made each year, are becoming more functional, and, above all, small additions, modifications, or changes in the materials to make bioprinted constructs as similar as possible to real biological structures. In addition, articles related to robotic- assisted in situ bioprinting started to emerge, thanks to the generation of new robots capable of correctly positioning the nozzles for dexterous and precise bioprinting[102]. Also, as an example of progress in this field, pancreatic islets were bioprinted using extracellular decellularized composite bioinks and subsequently implanted in mouse models; it was observed that there were no inflammatory reactions, and the neovascular processes started in week 8 of the experiment, and the cell viability was 120%[103].
Many of the aforementioned studies continue to be carried out and evolve, leading to the development of new protocols with important changes that bring us ever closer to the reality of using bioprinting in medicine on a routine basis (Figure 4).
Figure 4.
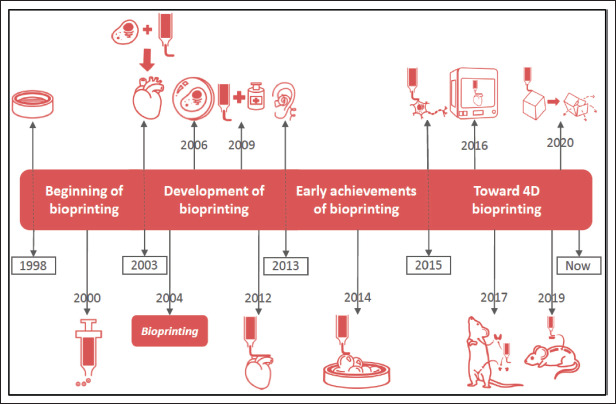
Relevant milestones in the evolution of bioprinting. 1998: cell growth on prefabricated 3D structure. 2000: methodologies based on nanodepositions with syringes. 2003: approach of joining cells with hydrogels to form organs. 2004: emergence of the term “bioprinting.” 2006: encapsulation of cells in hydrogels. 2009: drug binding and bioprinting. 2012: bioprinting of heart tissue. 2013: bioprinting of human ear with coil. 2014: bioprinting with HeLa cells for tumor studies. 2015: bioprinting of brain-like structures with encapsulated primary neurons. 2016: development of integrated organ and tissue bioprinter. 2017: bioprinting of functional thyroid gland and ovarian tissue in mice. 2019: bioprinting of biomimetic scaffolds that en able regeneration of damaged axons in the spinal cord of mice. 2020: the start of 4D bioprinting, whereby bioprinted elements can change after reacting with the environment.
4. Main applications of bioprinting
4.1. Tissue regeneration
The worldwide shortage of organ and tissue donors, owing the high demand for organs and tissues and the need for using immunosuppressants for a long time after implantation, is a problem that needs to be addressed[104]. Therefore, the use of bioprinting is increasingly being considered a possible solution to this problem, whereby transplants are created from the patient’s own cells, obviating the need for a donor or the use of various immunosuppressants that could induce negative side effects. To create tissues using bioprinting, three central approaches, namely biomimicry, autonomous self-assembly, and microtissue building blocks, are considered[105].
-
(i)
Biomimicry. Biomimicry involves the fabrication of identical reproductions of the cellular and extracellular components of a tissue or organ[106].
-
(ii)
Autonomous self-assembly. Autonomous selfassembly relies on the cell as the main driver of histogenesis, directing the composition, localization, functional, and structural properties of the tissue[107].
-
(iii)
Microtissue building blocks. Microtissue building block is defined as the smallest structural and functional component of a tissue. There are two main strategies: first, self-assembled cell spheres (similar to microtissues) are assembled into a macrotissue using biologically inspired design and organization[108]; second, accurate, high-resolution374 tissue units are designed and then allowed to selfassemble into a functional macrotissue. Examples of these approaches include the self-assembly of vascular building blocks to form branched vascular networks[109].
In order to be able to reproduce different tissues or organs to be developed, it is essential to have a perfect understanding of the organization and interaction of their components. Medical imaging technology provides information on the 3D structure and its functioning at the cellular, tissue, organ, and organism levels. This knowledge helps to determine the optimal parameters and conditions for bioprinting each type of tissue so that it survives to perform its functions, making it increasingly easier to recreate and mimic the tissues to perfection so that they can be transplanted in organisms.
4.2. Pharmacokinetic studies
The manufacturing process for medicines can be lengthy, as there are multiple steps from laboratory-based investigations to commercial exploitation that can delay marketing a product and make the process more expensive.
3D printing and high-throughput techniques can not only improve the product model, but also reduce the manufacturing time, production cost, and time to market[110]. This is achieved by including 3D-bioprinted tissue models for high-throughput drug testing[111]. Such 3D-bioprinted tissues allow for the most accurate possible recreation of the target organ on which the drugs will act, simulating in vitro response to drug administration and allowing for faster assessment of the results obtained. Through the integration of 3D in vitro cell culture models with cell lines, advances such as microfluidic devices and tissues and organs on a chip have been launched to recapitulate the biological properties and functions of native human tissues, organs, and circulation[112].
4.3. Study of infectious diseases
Infectious diseases have traditionally been treated with antibiotics, anti-fungals, and anti-virals when the host’s immune system is incapable of fighting off the infection on its own. Therefore, understanding the host response to pathogen entry as well as the different interactions that occur between the host and the pathogen is key375 to the successful design and development of effective drugs.
Traditionally, 2D cultures have been used to understand the host–pathogen interaction, but in these cultures, the cells are not exposed to the same conditions as in the organism, and the studies performed in them, although useful and absolutely necessary, do not faithfully represent the processes that take place in tissues in vivo[113]. Therefore, 3D models may serve as a better platform for the development of drugs and vaccines[114], as they mimic the microenvironment that occurs in the organism better than 2D culture systems.
A succinct example of the usefulness of bioprinting against infectious diseases is the recreation of human respiratory tissue to study SARS-CoV-2 infection and test potential anti-viral drugs[115]. By using lung and colonic organoids, it was discovered that not only respiratory tract cells, but several types of colon cells also express the ACE2 receptor, indicating that both kinds of cells are permissive for virus entry[116]. Moreover, these models also helped to test and verify the usefulness of several existing drugs in reducing SARS-CoV-2 infection[115] and were used to develop the first drug approved for the treatment of COVID-19 at the same time[117].
4.4. Tumor studies
As with pharmacokinetic studies, 3D engineered tissue printing can be of great help in the fight against cancer. The efficiency of current strategies for studying different tumors may be limited, as cells may become inactive or even acquire new mutations while growing in the lab during in vitro studies[118].
Currently, the preclinical process for drug development is carried out by evaluating the toxicity or efficacy of the drug to be tested in vitro in a 2D culture, followed by animal studies. Such cultures, despite their great value in medical research, are not compatible with the 3D architecture of tumor tissue in the human body and do not maintain the different functions of the multiple cell types that play an important role in disease progression[119].
Therefore, the best option for the development of antitumor therapies is the use of 3D bioprinting technology, which makes it possible to develop tumor models that recapitulate the conditions to which cells in tumors are exposed, such as hydrostatic pressure, shear stress, and compressive stress and forces, which play important roles in the regulation of tissue and cellular behavior[120].
4.5. 4D bioprinting and future directions
One of the huge challenges for bioprinting is to establish itself as a routine tool in the field of medicine. Currently, the most relevant studies are those related to organ and tissue regeneration, and much research is needed to study the bioprinting of complete and functional organs ready for transplantation in real patients. Bioprinting and subsequent implantation of simpler tissues in animal models have already been achieved, but more investigations are warranted to optimize bioprinting of more complex tissues.
The exploration of 4D bioprinting, which creates 3D structures that are able to respond to a stimulus by changing shape, color, function, and so on, has already begun. 4D bioprinting is a technique that, thanks to the improvement of hydrogels and their printing conditions, could increase the functionality of bioprinted tissues by allowing changes in them in response to external stimuli. This is of particular interest in medicine because these structures could form part of smart orthopedic implants, targeted drug delivery methods, or smart scaffolds in tissue engineering. This is the new line of research direction in bioprinting, which will bring breakthroughs in biomedical engineering and provide new solutions to the currently unsolvable problems.
Stimuli that can help transform 3D-printed structures to 4D can be physical (temperature, light, magnetism, and electric fields), chemical (pH response and ionic concentration), and biological[121]. Other factors that induce shape change in 3D-printed parts are the type of material, the stimulus exerted on the 3D structure, the mechanism by which the transformation of the structure occurs, and the theoretical and numerical modeling or software[122]. Thus, by understanding the dynamics of human tissues and fluids, and the kinetics of smart polymers used in 4D printing, it would be possible to monitor tissue remodeling for the benefit of organ regeneration[121], or even to benefit the production of pharmaceutical products with new properties when in contact with various fluids, as in the case of Spritam®, the first drug generated using 3D technology and developed by Aprecia Pharmaceuticals[123], which facilitates the intake of medicines. In light of the new advances in this regard and the possibilities of 4D printing in organ and tissue regeneration and new drug formulations, the concept of 4D printing represents a new approach for biomedical applications.
Despite the great advances made, the problems facing bioprinting include the long processing time to print and generate structures, the need to maintain printed structures under very specific conditions to ensure cell survival and structural integrity, and the inability to directly create dynamic structures that more closely resemble living body tissues, which are of great complexities. Such a conundrum necessitates continued research into the different hydrogels available, new formulations, and the conditions under376 which each should be used, which is to ensure good mechanical properties and high cell viability at the same time. In the future, these discoveries will make it possible to reduce printing time while increasing cell viability, as well as increasing the resemblance of bioprinted tissues to real tissues of the organism.
5. Conclusion
Bioprinting is developing at an exponential rate, with new and important discoveries being made every day. With the research on bioprinting first published in 1998 when the term “bioprinting” had not yet been coined, and with the publication of only very few articles in the following years, the humble start of bioprinting research has gradually piqued the interest of the scientific community, and the tremendous exploration of this technique has eventually generated more than 1000 publications in each of the last 2 years. It is anticipated that, in a matter of a few years, it will undergo another round of evolution, accompanied by more impactful and beneficial applications. Moreover, it is also expected that bioprinting will be applied in medical regeneration, as a routine practice in hospitals and laboratories.
Great successes of bioprinting, such as in situ reconstruction of tissues on the patient that led to excellent outcomes in skin regeneration[94], reconstruction of ovarian tissue that allows the gestation of offspring in a natural way[79], reconstruction of a thyroid gland capable of normalizing thyroxine levels in the blood[81], and regeneration of damaged axons of a spinal cord[92], are all indicators that the continued evolution of bioprinting will eventually aid in the development of new therapies, such as the generation of complete organs for autologous transplants and the new solutions to currently incurable diseases. This also corroborates the fact that bioprinting is an impetus to the revolution in modern medicine, thanks to current and future advances as well as any new applications that are yet to be discovered or conceived.
Acknowledgments
We want to thank the European Regional Development Fund (ERDF) in the framework of the project (BIOSIMPRO. IB20158) with the code 2021/00110/001 for funding this publication.
Funding
This work was funded by the European Regional Development Fund (ERDF) in the framework of the project (BIOSIMPRO. IB20158) with the code 2021/00110/001.
Conflict of interest
The authors declare no conflicts of interest.
Author contributions
Conceptualization: Jesús M. Rodríguez-Rego, Laura
Mendoza-Cerezo
Funding acquisition: Alfonso C. Marcos-Romero
Investigation: Jesús M. Rodríguez-Rego, Laura Mendoza- Cerezo
Project administration: Alfonso C. Marcos-Romero, Antonio Macías-García
Supervision: Antonio Macías-García, Antonio Díaz- Parralejo
Writing – original draft: Laura Mendoza-Cerezo
Writing – review & editing: Jesús M. Rodríguez-Rego, Alfonso C. Marcos-Romero
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data
Not applicable.
References
- 1.Ozbolat IT, Yu Y. Bioprinting toward organ fabrication: Challenges and future trends. IEEE Trans Biomed Eng. 2013;60(3):691–699. doi: 10.1109/TBME.2013.2243912. http://doi.org/10.1109/TBME.2013.2243912. [DOI] [PubMed] [Google Scholar]
- 2.Park A, Wu B, Griffith LG. Integration of surface modification and 3D fabrication techniques to prepare patterned poly(L-lactide) substrates allowing regionally selective cell adhesion. J Biomater Sci Polym Ed. 1998;9(2):89–110. doi: 10.1163/156856298x00451. http://doi.org/10.1163/156856298×00451. [DOI] [PubMed] [Google Scholar]
- 3.Kim SS, Utsunomiya H, Koksi JA, et al. Survival and function of hepatocytes on a novel three-dimensional synthetic biodegradable polymer scaffold with an intrinsic network of channels. Ann Surg. 1998;228(1):8. doi: 10.1097/00000658-199807000-00002. http://doi.org/10.1097/00000658-199807000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhatia SN, Chen CS. Tissue engineering at the microscale. Biomed Microdevices. 1999;2(2):131–144. http://doi.org/10.1023/A:1009949704750. [Google Scholar]
- 5.Vozzi G, Ahluwalia A, de Rossi D, et al. Microsyringe based fabrication of high resolution organic structures for bioengineering applications. Proceedings of the First Annual International IEEE-EMBS Special Topic Conference on Microtechnologies in Medicine and Biology. 2000:141–145. [Google Scholar]
- 6.Quirk RA, Briggs D, Davies MC, et al. Characterization of the spatial distributions of entrapped polymers following the surface engineering of poly(lactic acid). Surf Interface Anal, 2001;31(1):46–50. http://doi.org/10.1002/SIA.951. [Google Scholar]
- 7.377Zeltinger J, Sherwood JK, Graham DA, et al. Effect of pore size and void fraction on cellular adhesion, proliferation, and matrix deposition. Tissue Eng. 2001;7(5):557–572. doi: 10.1089/107632701753213183. http://doi.org/10.1089/107632701753213183. [DOI] [PubMed] [Google Scholar]
- 8.Ringeisen BR, Chrisey DB, Krizman DB, et al. Cell- by-cell construction of living tissue by ambient laser transfer. Proceedings of the Second Annual International IEEE-EMBS Special Topic Conference on Microtechnologies in Medicine and Biology. 2002:120–125. http://doi.org/10.1109/MMB.2002.1002277. [Google Scholar]
- 9.Mironov V, Boland T, Trusk T, et al. Organ printing: Computer-aided jet-based 3D tissue engineering. Trends Biotechnol. 2003;21(4):157–161. doi: 10.1016/S0167-7799(03)00033-7. http://doi.org/10.1016/S0167-7799(03)00033-7. [DOI] [PubMed] [Google Scholar]
- 10.Boland T, Mironov V, Gutowska A, et al. Cell and organ printing 2: Fusion of cell aggregates in threedimensional gels. Anat Rec. 2003;272(2):497–502. doi: 10.1002/ar.a.10059. http://doi.org/10.1002/AR.A.10059. [DOI] [PubMed] [Google Scholar]
- 11.ben Hsieh H, Fitch J, White D, et al. Ultra-high- throughput microarray generation and liquid dispensing using multiple disposable piezoelectric ejectors. J Biomol Screen. 2004;9(2):85–94. doi: 10.1177/1087057103260943. http://doi.org/10.1177/1087057103260943. [DOI] [PubMed] [Google Scholar]
- 12.Pfister A, Landers R, Laib A, et al. Biofunctional rapid prototyping for tissue-engineering applications: 3D bioplotting versus 3D printing. J Polym Sci A Polym Chem. 2004;42(3):624–638. http://doi.org/10.1002/POLA.10807. [Google Scholar]
- 13.Jakab K, Neagu A, Mironov V, et al. Engineering biological structures of prescribed shape using self-assembling multicellular systems. Proc Natl Acad Sci U S A. 2004;101(9):2864. doi: 10.1073/pnas.0400164101. http://doi.org/10.1073/PNAS.0400164101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ackermann J, Videlot C, Nguyen TN, et al. Gellayer-assisted directional electropolymerization: A versatile method for high-resolution volume and surface patterning of flexible substrates with conjugated polymers. Adv Mater. 2004;16(19):1709–1712. http://doi.org/10.1002/ADMA.200306705. [Google Scholar]
- 15.Fridman G, Li M, Friedman G, et al. Non-thermal plasma bioPrinter with nano-scale precision. Proceedings of the IEEE International Conference on Plasma Science. 2004:170. http://doi.org/10.1109/PLASMA.2004.1339724. [Google Scholar]
- 16.Hackney PM, Pancholi KP. Application of the Z-Corps three-dimensional printing processes using novel material to manufacture bio-scaffold for bone replacement. Proceedings of the Fifth National Conference on Rapid Design, Prototyping, and Manufacturing. 2004:53–60. [Google Scholar]
- 17.Komatsu H, Iwasawa N, Citterio D, et al. Design and synthesis of highly sensitive and selective fluorescein-derived magnesium fluorescent probes and application to intracellular 3D Mg2+ imaging. J Am Chem Soc. 2004;126(50):16353–16360. doi: 10.1021/ja049624l. http://doi.org/10.1021/JA049624L. [DOI] [PubMed] [Google Scholar]
- 18.Ball P. Body painting. Nat Mater, 2005;4(8):582. doi: 10.1038/nmat1439. http://doi.org/10.1038/nmat1439. [DOI] [PubMed] [Google Scholar]
- 19.Mironov V. The Second International Workshop on bioprinting, biopatterning and bioassembly. Expert Opin Biol Ther. 2005;5(8):1111–1115. doi: 10.1517/14712598.5.8.1111. http://doi.org/10.1517/14712598.5.8.1111. [DOI] [PubMed] [Google Scholar]
- 20.Leukers B, Gülkan H, Irsen SH, et al. Biocompatibility of ceramic scaffolds for bone replacement made by 3D printing. Materwiss Werksttech. 2005;36(12):781–787. http://doi.org/10.1002/MAWE.200500968. [Google Scholar]
- 21.Leukers B, Gülkan H, Irsen SH, et al. Hydroxyapatite scaffolds for bone tissue engineering made by 3D printing. J Mater Sci Mater Med. 2005;16(12):1121–1124. doi: 10.1007/s10856-005-4716-5. http://doi.org/10.1007/S10856-005-4716-5. [DOI] [PubMed] [Google Scholar]
- 22.Chang R, Starly B, Sun W, et al. 2006. Freeform bioprinting of liver encapsulated in alginate hydrogels tissue constructs for pharmacokinetic study. Proceedings of the 2006 International Solid Freeform Fabrication Symposium. [Google Scholar]
- 23.Mironov V, Reis N, Derby B. Review: Bioprinting: A beginning. Tissue Eng. 2006;12(4):631–634. doi: 10.1089/ten.2006.12.631. http://doi.org/10.1089/TEN.2006.12.631. [DOI] [PubMed] [Google Scholar]
- 24.Mironov V. Toward human organ printing: Charleston Bioprinting Symposium. ASAIO J. 2006;52(6):27–30. doi: 10.1097/01.mat.0000248999.25334.6a. http://doi.org/10.1097/01.MAT.0000248999.25334.6A. [DOI] [PubMed] [Google Scholar]
- 25.Fakhrzadeh H, Larikani B. Bioprinting: Prospects of a rapidly growing technology. J Diabetes Lipid Disord. 2007;7(1):1–7. [Google Scholar]
- 26.Mironov V, Kasyanov V, Markwald R. Bioprinting: Directed tissue self-assembly. Chem Eng Prog. 2007;103:S12–S17. [Google Scholar]
- 27.IEEE. 2007 International Symposium on VLSI Technology, Systems and Applications (VLSI-TSA) 2007. http://lib.ugent.be/catalog/ebk01:1000000000525455 Hsinchu, Taiwan IEEE.
- 28.Suwanprateeb J, Sanngam R, Suwanpreuk W. Fabrication of bioactive hydroxyapatite/bis-GMA based composite via three dimensional printing. J Mater Sci Mater Med. 2008;19(7):2637–2645. doi: 10.1007/s10856-007-3362-5. http://doi.org/10.1007/S10856-007-3362-5. [DOI] [PubMed] [Google Scholar]
- 29.Igawa K, Chung U-i, Tei Y. Custom-made artificial bones fabricated by an inkjet printing technology. Clin Calcium. 2008;18(12):1737–1743. http://doi.org/clica081217371743. [PubMed] [Google Scholar]
- 30.378Sachlos E, Wahl DA, Triffitt JT, et al. The impact of critical point drying with liquid carbon dioxide on collagenhydroxyapatite composite scaffolds. Acta Biomater. 2008;4(5):1322–1331. doi: 10.1016/j.actbio.2008.03.016. http://doi.org/10.1016/J.ACTBIO.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 31.Fedorovich NE, de Wijn JR, Verbout AJ, et al. Threedimensional fiber deposition of cell-laden, viable, patterned constructs for bone tissue printing. Tissue Eng Part A. 2008;14(1):127–133. doi: 10.1089/ten.a.2007.0158. http://doi.org/10.1089/TEN.A.2007.0158. [DOI] [PubMed] [Google Scholar]
- 32.Ryan GE, Pandit AS, Apatsidis DP. Porous titanium scaffolds fabricated using a rapid prototyping and powder metallurgy technique. Biomaterials. 2008;29(27):3625–3635. doi: 10.1016/j.biomaterials.2008.05.032. http://doi.org/10.1016/J.BIOMATERIALS.2008.05.032. [DOI] [PubMed] [Google Scholar]
- 33.Fierz FC, Beckmann F, Huser M, et al. The morphology of anisotropic 3D-printed hydroxyapatite scaffolds. Biomaterials. 29(28):3799–3806. doi: 10.1016/j.biomaterials.2008.06.012. http://doi.org/10.1016/J.BIOMATERIALS.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 34.Wu W, Zheng Q, Guo X, et al. A programmed release multi-drug implant fabricated by three-dimensional printing technology for bone tuberculosis therapy. Biomed Mater. 2009;4(6):065005. doi: 10.1088/1748-6041/4/6/065005. http://doi.org/10.1088/1748-6041/4/6/065005. [DOI] [PubMed] [Google Scholar]
- 35.Nishiyama Y, Nakamura M, Henmi C, et al. Development of a three-dimensional bioprinter: Construction of cell supporting structures using hydrogel and state-of-the- art inkjet technology. J Biomech Eng. 2009;131(3):035001. doi: 10.1115/1.3002759. http://doi.org/10.1115/1.3002759. [DOI] [PubMed] [Google Scholar]
- 36.Lee W, Debasitis JC, Lee VK, et al. Multi-layered culture of human skin fibroblasts and keratinocytes through three-dimensional freeform fabrication. Biomaterials. 2009;30(8):1587–1595. doi: 10.1016/j.biomaterials.2008.12.009. http://doi.org/10.1016/J.BIOMATERIALS.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 37.Barry RA, Shepherd R, Hanson JN, et al. Direct-write assembly of 3D hydrogel scaffolds for guided cell growth. Adv Mater. 2009;21(23):2407–2410. http://doi.org/10.1002/ADMA.200803702. [Google Scholar]
- 38.Fedorovich NE, Swennen I, Girones J, et al. Evaluation of photocrosslinked Lutrol hydrogel for tissue printing applications. Biomacromolecules. 2009;10(7):1689–1696. doi: 10.1021/bm801463q. http://doi.org/10.1021/BM801463Q. [DOI] [PubMed] [Google Scholar]
- 39.Cui X, Boland T. Human microvasculature fabrication using thermal inkjet printing technology. Biomaterials. 2009;30(31):6221–6227. doi: 10.1016/j.biomaterials.2009.07.056. http://doi.org/10.1016/J.BIOMATERIALS.2009.07.056. [DOI] [PubMed] [Google Scholar]
- 40.Norotte C, Marga FS, Niklason LE, et al. Scaffold-free vascular tissue engineering using bioprinting. Biomaterials. 2009;30(30):5910–5917. doi: 10.1016/j.biomaterials.2009.06.034. http://doi.org/10.1016/J.BIOMATERIALS.2009.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guillemot F, Souquet A, Catros S, et al. High- throughput laser printing of cells and biomaterials for tissue engineering. Acta Biomater. 2010;6(7):2494–2500. doi: 10.1016/j.actbio.2009.09.029. http://doi.org/10.1016/J.ACTBIO.2009.09.029. [DOI] [PubMed] [Google Scholar]
- 42.Nakamura M, Iwanaga S, Henmi C, et al. Biomatrices and biomaterials for future developments of bioprinting and biofabrication. Biofabrication. 2010;2(1):014110. doi: 10.1088/1758-5082/2/1/014110. http://doi.org/10.1088/1758-5082/2/1/014110. [DOI] [PubMed] [Google Scholar]
- 43.Lee W, Lee V, Polio S, et al. On-demand threedimensional freeform fabrication of multi-layered hydrogel scaffold with fluidic channels. Biotechnol Bioeng. 2010;105(6):1178–1186. doi: 10.1002/bit.22613. http://doi.org/10.1002/BIT.22613. [DOI] [PubMed] [Google Scholar]
- 44.Chang R, Emami K, Wu H, et al. Biofabrication of a three-dimensional liver micro-organ as an in vitro drug metabolism model. Biofabrication. 2010;2(4):045004. doi: 10.1088/1758-5082/2/4/045004. http://doi.org/10.1088/1758-5082/2/4/045004. [DOI] [PubMed] [Google Scholar]
- 45.Arai K, Iwanaga S, Toda H, et al. Three-dimensional inkjet biofabrication based on designed images. Biofabrication. 2011;3(3):034113. doi: 10.1088/1758-5082/3/3/034113. http://doi.org/10.1088/1758-5082/3/3/034113. [DOI] [PubMed] [Google Scholar]
- 46.Skardal A, Mack D, Kapetanovic E, et al. Bioprinted amniotic fluid-derived stem cells accelerate healing of large skin wounds. Stem Cells Transl Med. 2012;1(11):792–802. doi: 10.5966/sctm.2012-0088. http://doi.org/10.5966/SCTM.2012-0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miller JS, Stevens KR, Yang MT, et al. Rapid casting of patterned vascular networks for perfusable engineered three-dimensional tissues. Nat Mater. 2012;11(9):768–774. doi: 10.1038/nmat3357. http://doi.org/10.1038/NMAT3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miller JS, Stevens KR, Yang MT, et al. Effects of silica and zinc oxide doping on mechanical and biological properties of 3D printed tricalcium phosphate tissue engineering scaffolds. Dent Mater. 2012;28(2):113–122. doi: 10.1016/j.dental.2011.09.010. http://doi.org/10.1016/J.DENTAL.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hockaday LA, Kang KH, Colangelo NW, et al. Rapid 3D printing of anatomically accurate and mechanically heterogeneous aortic valve hydrogel scaffolds. Biofabrication. 2012;4(3):035005. doi: 10.1088/1758-5082/4/3/035005. http://doi.org/10.1088/1758-5082/4/3/035005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.379Zopf DA, Hollister SJ, Nelson ME, et al. Bioresorbable airway splint created with a three-dimensional printer. N Engl J Med. 2013;368(21):2043–2045. doi: 10.1056/NEJMc1206319. http://doi.org/10.1056/NEJMC1206319. [DOI] [PubMed] [Google Scholar]
- 51.Xu T, Zhao W, Zhu JM, et al. Complex heterogeneous tissue constructs containing multiple cell types prepared by inkjet printing technology. Biomaterials. 2013;34(1):130–139. doi: 10.1016/j.biomaterials.2012.09.035. http://doi.org/10.1016/J.BIOMATERIALS.2012.09.035. [DOI] [PubMed] [Google Scholar]
- 52.Michael S, Sorg H, Peck CT, et al. Tissue engineered skin substitutes created by laser-assisted bioprinting form skin-like structures in the dorsal skin fold chamber in mice. PLoS One. 2013;8(3):0057741. doi: 10.1371/journal.pone.0057741. http://doi.org/10.1371/JOURNAL.PONE.0057741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Duan B, Hockaday LA, Kang KH, et al. 3D bioprinting of heterogeneous aortic valve conduits with alginate/gelatin hydrogels. J Biomed Mater Res A. 2013;101(5):1255–1264. doi: 10.1002/jbm.a.34420. http://doi.org/10.1002/JBM.A.34420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schuurman W, Levett PA, Pot MW, et al. Gelatin- methacrylamide hydrogels as potential biomaterials for fabrication of tissue-engineered cartilage constructs. Macromol Biosci. 2013;13(5):551–561. doi: 10.1002/mabi.201200471. http://doi.org/10.1002/MABI.201200471. [DOI] [PubMed] [Google Scholar]
- 55.Mannoor MS, Jiang Z, James T, et al. 3D printed bionic ears. Nano Lett. 2013;13(6):2634–2639. doi: 10.1021/nl4007744. http://doi.org/10.1021/NL4007744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee VK, Lanzi AM, Ngo H, et al. Generation of multiscale vascular network system within 3D hydrogel using 3D bio-printing technology. Cell Mol Bioeng. 2014;7(3):460–472. doi: 10.1007/s12195-014-0340-0. http://doi.org/10.1007/S12195-014-0340-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee VK, Kim DY, Ngo H, et al. Creating perfused functional vascular channels using 3D bio-printing technology. Biomaterials. 2014;35(28):8092–8102. doi: 10.1016/j.biomaterials.2014.05.083. http://doi.org/10.1016/J.BIOMATERIALS.2014.05.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kolesky DB, Truby RL, Gladman AS, et al. 3D bioprinting of vascularized, heterogeneous cell-laden tissue constructs. Adv Mater. 2014;26(19):3124–3130. doi: 10.1002/adma.201305506. http://doi.org/10.1002/ADMA.201305506. [DOI] [PubMed] [Google Scholar]
- 59.Zhao Y, Yao R, Ouyang L, et al. Three-dimensional printing of Hela cells for cervical tumor model in vitro. Biofabrication. 2014;6(3):035001. doi: 10.1088/1758-5082/6/3/035001. http://doi.org/10.1088/1758-5082/6/3/035001. [DOI] [PubMed] [Google Scholar]
- 60.Billiet T, Gevaert E, de Schryver T, et al. The 3D printing of gelatin methacrylamide cell-laden tissue-engineered constructs with high cell viability. Biomaterials. 2014;35(1):49–62. doi: 10.1016/j.biomaterials.2013.09.078. http://doi.org/10.1016/J.BIOMATERIALS.2013.09.078. [DOI] [PubMed] [Google Scholar]
- 61.Pati F, Jang J, Ha DH, et al. Printing three-dimensional tissue analogues with decellularized extracellular matrix bioink. Nat Commun, 2014;5(1):1–11. doi: 10.1038/ncomms4935. http://doi.org/10.1038/ncomms4935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Visser J, Melchels FPW, Jeon JE, et al. Reinforcement of hydrogels using three-dimensionally printed microfibres. Nat Commun, 2015;6(1):1–10. doi: 10.1038/ncomms7933. http://doi.org/10.1038/ncomms7933. [DOI] [PubMed] [Google Scholar]
- 63.Jakus AE, Secor EB, Rutz AL, et al. Three-dimensional printing of high-content graphene scaffolds for electronic and biomedical applications. ACS Nano. 2015;9(4):4636–4648. doi: 10.1021/acsnano.5b01179. http://doi.org/10.1021/ACSNANO.5B01179. [DOI] [PubMed] [Google Scholar]
- 64.Markstedt K, Mantas A, Tournier I, et al. 3D bioprinting human chondrocytes with nanocellulosealginate bioink for cartilage tissue engineering applications. Biomacromolecules. 2015;16(5):1489–1496. doi: 10.1021/acs.biomac.5b00188. http://doi.org/10.1021/ACS.BIOMAC.5B00188/ASSET/ IMAGES/LARGE/BM-2015-00188Z_0007.JPEG. [DOI] [PubMed] [Google Scholar]
- 65.Hong S, Sycks D, Fai Chan H, et al. 3D printing of highly stretchable and tough hydrogels into complex, cellularized structures. Adv Mater. 2015;27(27):4035–4040. doi: 10.1002/adma.201501099. http://doi.org/10.1002/ADMA.201501099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lozano R, Stevens L, Thompson BC, et al. 2015. 3D printing of layered brain-like structures using peptide modified gellan gum substrates. Biomaterials 67, 264 273 http://doi.org/10.1016/J.BIOMATERIALS.2015.07.022 [DOI] [PubMed] [Google Scholar]
- 67.Yue K, Trujillo-de Santiago G, Alvarez MM, et al. 2015. Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. Biomaterials 73, 254 271 http://doi.org/10.1016/J.BIOMATERIALS.2015.08.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kang HW, Lee SJ, Ko IK, et al. A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat Biotechnol, 2016;34(3):312–319. doi: 10.1038/nbt.3413. http://doi.org/10.1038/nbt.3413. [DOI] [PubMed] [Google Scholar]
- 69.Kolesky DB, Homan KA, Skylar-Scott MA, et al. Three-dimensional bioprinting of thick vascularized tissues. Proc Natl Acad Sci U S A. 2016;113(12):3179–3184. doi: 10.1073/pnas.1521342113. http://doi.org/10.1073/PNAS.1521342113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mandrycky C, Wang Z, Kim K, et al. 3D bioprinting for engineering complex tissues. Biotechnol Adv. 2016;34(4):422–434. doi: 10.1016/j.biotechadv.2015.12.011. http://doi.org/10.1016/J.BIOTECHADV.2015.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jia W, Gungor-Ozkerim PS, Zhang YS, et al. 2016. Direct 3D bioprinting of perfusable vascular constructs using a blend bioink. Biomaterials 106, 58 68 http://doi.org/10.1016/J.BIOMATERIALS.2016.07.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.380Ma X, Qu X, Zhu W, et al. Deterministically patterned biomimetic human iPSC-derived hepatic model via rapid 3D bioprinting. Proc Natl Acad Sci U S A. 2016;113(8):2206–2211. doi: 10.1073/pnas.1524510113. http://doi.org/10.1073/PNAS.1524510113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Homan KA, Kolesky DB, Skylar-Scott MA, et al. Bioprinting of 3D convoluted renal proximal tubules on perfusable chips. Sci Rep. 2016;6(1):1–13. doi: 10.1038/srep34845. http://doi.org/10.1038/srep34845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bhise NS, Manoharan V, Massa S, et al. A liver- on-a-chip platform with bioprinted hepatic spheroids. Biofabrication. 2016;8(1):014101. doi: 10.1088/1758-5090/8/1/014101. http://doi.org/10.1088/1758-5090/8/1/014101. [DOI] [PubMed] [Google Scholar]
- 75.Guo F, Mao Z, Chen Y, et al. Three-dimensional manipulation of single cells using surface acoustic waves. Proc Natl Acad Sci U S A. 2016;113(6):1522–1527. doi: 10.1073/pnas.1524813113. http://doi.org/10.1073/PNAS.1524813113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lind JU, Busbee TA, Valentine AD, et al. Instrumented cardiac microphysiological devices via multi-material 3D printing. Nat Mater. 2017;16(3):303. doi: 10.1038/nmat4782. http://doi.org/10.1038/NMAT4782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jang J, Park HJ, Kim SW, et al. 2017. 3D printed complex tissue construct using stem cell-laden decellularized extracellular matrix bioinks for cardiac repair. Biomaterials 112, 264 274 http://doi.org/10.1016/J.BIOMATERIALS.2016.10.026 [DOI] [PubMed] [Google Scholar]
- 78.Yao Q, Cosme JGL, Xu T, et al. 2017. Three dimensional electrospun PCL/PLA blend nanofibrous scaffolds with significantly improved stem cells osteogenic differentiation and cranial bone formation. Biomaterials 115, 115 127 http://doi.org/10.1016/J.BIOMATERIALS.2016.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Laronda MM, Rutz AL, Xiao S, et al. A bioprosthetic ovary created using 3D printed microporous scaffolds restores ovarian function in sterilized mice. Nat Commun. 2017;8(1):15261. doi: 10.1038/ncomms15261. http://doi.org/10.1038/NCOMMS15261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schaffner M, Rühs PA, Coulter F, et al. 3D printing of bacteria into functional complex materials. Sci Adv. 2017;3(12):eaao6804. doi: 10.1126/sciadv.aao6804. http://doi.org/10.1126/SCIADV.AAO6804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bulanova EA, Koudan EV, Degosserie J, et al. Bioprinting of a functional vascularized mouse thyroid gland construct. Biofabrication. 2017;9(3):034105. doi: 10.1088/1758-5090/aa7fdd. http://doi.org/10.1088/1758-5090/AA7FDD. [DOI] [PubMed] [Google Scholar]
- 82.Turnbull G, Clarke J, Picard F, et al. 3D bioactive composite scaffolds for bone tissue engineering. Bioact Mater. 2017;3(3):278–314. doi: 10.1016/j.bioactmat.2017.10.001. http://doi.org/10.1016/J.BIOACTMAT.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gopinathan J, Noh I. Recent trends in bioinks for 3D printing. Biomater Res. 2018;22(1)) doi: 10.1186/s40824-018-0122-1. http://doi.org/10.1186/S40824-018-0122-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gao T, Gillipsie GJ, Copus JS, et al. Optimization of gelatin-alginate composite bioink printability using rheological parameters: A systematic approach. Biofabrication. 2018;10(3):034106. doi: 10.1088/1758-5090/aacdc7. http://doi.org/10.1088/1758-5090/AACDC7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hoarau-Véchot J, Rafii A, Touboul C, et al. Halfway between 2D and animal models: Are 3D cultures the ideal tool to study cancer-microenvironment interactions? Int J Mol Sci. 2018;19(1):181. doi: 10.3390/ijms19010181. http://doi.org/10.3390/IJMS19010181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kim BS, Kwon YW, Kong JS, et al. 2018. 3D cell printing of in vitro stabilized skin model and in vivo pre-vascularized skin patch using tissue-specific extracellular matrix bioink: A step towards advanced skin tissue engineering. Biomaterials 168, 38 53 http://doi.org/10.1016/J.BIOMATERIALS.2018.03.040 [DOI] [PubMed] [Google Scholar]
- 87.Qian Y, Zhao X, Han Q, et al. An integrated multilayer 3D-fabrication of PDA/RGD coated graphene loaded PCL nanoscaffold for peripheral nerve restoration. Nat Commun. 2018;9(1):1–16. doi: 10.1038/s41467-017-02598-7. http://doi.org/10.1038/s41467-017-02598-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Isaacson A, Swioklo S, Connon CJ. 2018. 3D bioprinting of a corneal stroma equivalent. Exp Eye Res 173, 188 193 http://doi.org/10.1016/J.EXER.2018.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Joung D, Truong V, Neitzke CC, et al. 3D printed stem-cell derived neural progenitors generate spinal cord scaffolds. Adv Funct Mater. 2018;28(39):1801850. doi: 10.1002/adfm.201801850. http://doi.org/10.1002/ADFM.201801850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lee A, Hudson AR, Shiwarski DJ, et al. 3D bioprinting of collagen to rebuild components of the human heart. Science. 2019;365(6452):482–487. doi: 10.1126/science.aav9051. http://doi.org/10.1126/SCIENCE.AAV9051. [DOI] [PubMed] [Google Scholar]
- 91.Noor N, Shapira A, Edri R, et al. 3D printing of personalized thick and perfusable cardiac patches and hearts. Adv Sci. 2019;6(11):1900344. doi: 10.1002/advs.201900344. http://doi.org/10.1002/ADVS.201900344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Koffler J, Zhu W, Qu X, et al. Biomimetic 3D-printed scaffolds for spinal cord injury repair. Nat Med. 2019;25(2):263–269. doi: 10.1038/s41591-018-0296-z. http://doi.org/10.1038/S41591-018-0296-Z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yi HG, Jeong YH, Kim Y, et al. A bioprinted human- glioblastoma-on-a-chip for the identification of patientspecific responses to chemoradiotherapy. Nat Biomed Eng. 2019;3(7):509–519. doi: 10.1038/s41551-019-0363-x. http://doi.org/10.1038/S41551-019-0363-X. [DOI] [PubMed] [Google Scholar]
- 94.381Albanna M, Binder KW, Murphy SV, et al. In situ bioprinting of autologous skin cells accelerates wound healing of extensive excisional full-thickness wounds. Sci Rep. 2019;9(1):1856. doi: 10.1038/s41598-018-38366-w. http://doi.org/10.1038/S41598-018-38366-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chen P, Zheng L, Wang Y, et al. Desktop-stereolithography 3D printing of a radially oriented extracellular matrix/ mesenchymal stem cell exosome bioink for osteochondral defect regeneration. Theranostics. 2019;9(9):2439–2459. doi: 10.7150/thno.31017. http://doi.org/10.7150/THNO.31017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Choi YJ, Jun YJ, Kim DY, et al. 2019. A 3D cell printed muscle construct with tissue-derived bioink for the treatment of volumetric muscle loss. Biomaterials 206, 160 169 http://doi.org/10.1016/J.BIOMATERIALS.2019.03.036 [DOI] [PubMed] [Google Scholar]
- 97.Wan Z, Zhang P, Liu Y, et al. 2020. Four-dimensional bioprinting: Current developments and applications in bone tissue engineering. Acta Biomater 101, 26 42 http://doi.org/10.1016/J.ACTBIO.2019.10.038 [DOI] [PubMed] [Google Scholar]
- 98.Pan S, Yin J, Yu L, et al. 2D MXene‐integrated 3D‐printing scaffolds for augmented osteosarcoma phototherapy and accelerated tissue reconstruction. Adv Sci. 2020;7(2):1901511. doi: 10.1002/advs.201901511. http://doi.org/10.1002/ADVS.201901511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Datta P, Dey M, Ataie Z, et al. 3D bioprinting for reconstituting the cancer microenvironment. NPJ Precis Oncol. 2020;4(1):18. doi: 10.1038/s41698-020-0121-2. http://doi.org/10.1038/S41698-020-0121-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zarrintaj P, Ramsey JD, Samadi A, et al. 2020. Poloxamer: A versatile tri-block copolymer for biomedical applications. Acta Biomater 110, 37 67 http://doi.org/10.1016/J.ACTBIO.2020.04.028 [DOI] [PubMed] [Google Scholar]
- 101.Sun Y, You Y, Jiang W, et al. 2020. Generating ready-to- implant anisotropic menisci by 3D-bioprinting proteinreleasing cell-laden hydrogel-polymer composite scaffold. Appl Mater Today 18: 100469 http://doi.org/10.1016/J.APMT.2019.100469 [Google Scholar]
- 102.Dong H, Hu B, Zhang W, et al. Robotic-assisted automated in situ bioprinting. Int J Bioprint. 2023;9(1):98–108. doi: 10.18063/ijb.v9i1.629. http://doi.org/10.18063/IJB.V9I1.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Klak M, Kosowska K, Bryniarski T, et al. Bioink based on the dECM for 3D bioprinting of bionic tissue, the first results obtained on murine model. Bioprinting. 2022;28(1):e00233. http://doi.org/10.1016/J.BPRINT.2022.E00233. [Google Scholar]
- 104.Pavan Kalyan B, Kumar L. 3D printing: Applications in tissue engineering, medical devices, and drug delivery. AAPS PharmSciTech. 2022;23(4):92. doi: 10.1208/s12249-022-02242-8. http://doi.org/10.1208/S12249-022-02242-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Murphy Sv, Atala A. 3D bioprinting of tissues and organs. Nat Biotechnol. 2014;32(8):773–785. doi: 10.1038/nbt.2958. http://doi.org/10.1038/NBT.2958. [DOI] [PubMed] [Google Scholar]
- 106.Ingber DE, Mow VC, Butler D, et al. Tissue engineering and developmental biology: Going biomimetic. Tissue Eng. 2006;12(12):3265–3283. doi: 10.1089/ten.2006.12.3265. http://doi.org/10.1089/TEN.2006.12.3265. [DOI] [PubMed] [Google Scholar]
- 107.Derby B. Printing and prototyping of tissues and scaffolds. Science. 2012;338(6109):921–926. doi: 10.1126/science.1226340. http://doi.org/10.1126/SCIENCE.1226340. [DOI] [PubMed] [Google Scholar]
- 108.Kelm JM, Lorber V, Snedeker JG, et al. A novel concept for scaffold-free vessel tissue engineering: Self-assembly of microtissue building blocks. J Biotechnol. 2010;148(1):46–55. doi: 10.1016/j.jbiotec.2010.03.002. http://doi.org/10.1016/J.JBIOTEC.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 109.Alajati A, Laib AM, Weber H, et al. Spheroid-based engineering of a human vasculature in mice. Nat Methods. 2008;5(5):439–445. doi: 10.1038/nmeth.1198. http://doi.org/10.1038/NMETH.1198. [DOI] [PubMed] [Google Scholar]
- 110.Gao G, Ahn M, Cho WW, et al. 3D printing of pharmaceutical application: Drug screening and drug delivery. Pharmaceutics. 2021;13(9):1373. doi: 10.3390/pharmaceutics13091373. http://doi.org/10.3390/PHARMACEUTICS13091373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Vaidya M. Startups tout commercially 3D-printed tissue for drug screening. Nat Med. 2015;21(1):2. doi: 10.1038/nm0115-2. http://doi.org/10.1038/NM0115-2. [DOI] [PubMed] [Google Scholar]
- 112.Zhang B, Korolj A, Lai BFL, et al. Advances in organ-on-a- chip engineering. Nat Rev Mater. 3(8):257–278. http://doi.org/10.1038/S41578-018-0034-7. [Google Scholar]
- 113.Saleh FA, Genever PG. Turning round: Multipotent stromal cells, a three-dimensional revolution? Cytotherapy. 2011;13(8):903–912. doi: 10.3109/14653249.2011.586998. http://doi.org/10.3109/14653249.2011.586998. [DOI] [PubMed] [Google Scholar]
- 114.Joseph JS, Malindisa ST, Ntwasa M. Two-dimensional (2D) and three-dimensional (3D) cell culturing in drug discovery, Cell Culture. 2018 in. IntechOpen. http://doi.org/10.5772/INTECHOPEN.81552. [Google Scholar]
- 115.Harb A, Fakhreddine M, Zaraket H, et al. Threedimensional cell culture models to study respiratory virus infections including COVID-19. Biomimetics (Basel) 2021;7(1):3. doi: 10.3390/biomimetics7010003. http://doi.org/10.3390/BIOMIMETICS7010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rijsbergen LC, van Dijk LLA, Engel MFM, et al. In vitro modelling of respiratory virus infections in human airway epithelial cells – A systematic review. Front Immunol. 2021;12(1):683002. doi: 10.3389/fimmu.2021.683002. http://doi.org/10.3389/FIMMU.2021.683002/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.382Krüger J, Groß R, Conzelmann C, et al. Drug inhibition of SARS-CoV-2 replication in human pluripotent stem cell-derived intestinal organoids. Cell Mol Gastroenterol Hepatol. 2021;11(4):935–948. doi: 10.1016/j.jcmgh.2020.11.003. http://doi.org/10.1016/J.JCMGH.2020.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ben-David U, Siranosian B, Ha G, et al. Genetic and transcriptional evolution alters cancer cell line drug response. Nature. 2018;560(7718):325–330. doi: 10.1038/s41586-018-0409-3. http://doi.org/10.1038/S41586-018-0409-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Neufeld L, Yeini E, Reisman N, et al. Microengineered perfusable 3D-bioprinted glioblastoma model for in vivo mimicry of tumor microenvironment. Sci Adv. 2021;7(34):eabi9119. doi: 10.1126/sciadv.abi9119. http://doi.org/10.1126/SCIADV.ABI9119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Butcher DT, Alliston T, Weaver VM. A tense situation: Forcing tumour progression. Nat Rev Cancer. 2009;9(2):108–122. doi: 10.1038/nrc2544. http://doi.org/10.1038/NRC2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Agarwal K, Srinivasan V, Lather V, et al. Insights of 3D bioprinting and focusing the paradigm shift towards 4D printing for biomedical applications. J Mater Res, 2022;38(1):112–141. http://doi.org/10.1557/S43578-022-00524-2. [Google Scholar]
- 122.Tamay DG, Usal TD, Alagoz AS, et al. 3D and 4D printing of polymers for tissue engineering applications. Front Bioeng Biotechnol. 2019;7(JUL):164. doi: 10.3389/fbioe.2019.00164. http://doi.org/10.3389/FBIOE.2019.00164/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Aprecia. 2015. https://www.aprecia.com/technology/zipdose Spritam, viewed March 02, 2023,
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


