Abstract
Drug delivery devices which can control the release of drugs on demand allow for improved treatment to a patient. These smart drug delivery devices allow for the release of drugs to be turned on and off as needed, thereby increasing the control over the drug concentration within the patient. The addition of electronics to the smart drug delivery devices increases the functionality and applications of these devices. Through the use of 3D printing and 3D-printed electronics, the customizability and functions of such devices can also be greatly increased. With the development in such technologies, the applications of the devices will be improved. In this review paper, the application of 3D-printed electronics and 3D printing in smart drug delivery devices with electronics as well as the future trends of such applications are covered.
Keywords: 3D printing, Smart drug delivery device, Printed electronics
1. Introduction
144Drug delivery devices are used to transport a drug either into a patient or to specific sites within a patient’s body. This can be achieved through different routes and methods. The role of a drug delivery device is also to ensure that the drug concentration within the patient or at the specific sites is within a certain functional range known as the therapeutic window[1]. The therapeutic window refers to a range of drug concentration within a patient which allows for the optimal treatment. Above this range, the drug becomes toxic or causes intolerable adverse side effects and below which the drug is ineffective and unable to produce the desired effects[2]. Thus ensuring that the drug concentration stays within the therapeutic window is essential for providing effective treatment to the patient. Traditional drug delivery devices, however, once administered would not be able to control the amount and duration of drug release. This would affect the control of drug concentration within a patient[3]. Drug delivery devices which can directly control the release of drug allow for the better control over the drug concentration that is within the patient or at the specific sites[4].
1.1. Smart drug delivery devices
145Smart drug delivery devices are a type of drug delivery devices which can trigger the release of drugs as needed from the device. These devices can control when the start and end of the drug release from the device is, allowing for the drug release to be turned “on” and “off” as needed. Thus, the duration, the rate, as well as the location of the drug release can be controlled. This improves the control over the concentration of drugs within the patient, increasing the effectiveness of the drug therapy and decreasing the possibility of adverse side effects[5]. There are mainly two categories to classify smart drug delivery devices. These two categories are as follows.
The first category is smart drug delivery devices with a “passive” triggering of drug release from the device. For these “passive” devices, the trigger of the drug release from the device requires an external stimulus. External stimuli such as infrared light (IR), ultraviolet light (UV), ultrasound, magnetic field, or heat can be used[6-9]. Besides these external stimuli, a microchip can also be used to trigger the drug release. The microchip allows for either the manual or wireless control of the drug release by the doctor and nurse or by the patient[10]. For this type of smart drug delivery device, while it allows for the control of drug release, it requires an external source to trigger the drug release. Without any external interference, drug release would not be triggered by the device.
The second category of smart drug delivery devices is “active” smart drug delivery devices. Drug release from “active” smart drug delivery devices does not require an external stimulus or external interference. Instead, changes in the physiological conditions of the patient or in the surrounding area of the device can cause the triggering of the drug release. One way that the physiological condition changes can be used to trigger the drug release is through the use of electronic sensors. The sensors can detect these physiological changes and control the drug release through a microchip[11]. “Smart” materials can also be used to detect changes in physiological conditions and trigger drug release[12]. For the “active” smart drug delivery devices, the drug release can be triggered and controlled in a selfregulated, closed loop system. These devices can detect changes in the patient or in the surrounding area of the device and trigger the drug release as needed without any external interference[13].
“Smart” materials are materials which undergo a phase or property change when subjected to certain stimulus. For the purpose of drug delivery, these could be physiological stimuli such as changes in body temperature, pH, enzymes, or biomarkers[14,15]. Other non-physiological stimuli such as ultrasound, magnetic fields, IR, and UV lights could also be used[16,17]. When exposed to such stimuli, these “smart” materials would undergo changes to their properties such as phase, solubility, or shape. These property changes can then be used to control or trigger the release of drugs when the “smart” material is exposed to the necessary stimuli. The drug intended for delivery could be directly loaded into the “smart” materials for release or the “smart” material can function as a controlling mechanism to control the release of drugs stored in a drug reservoir[18]. For non-physiological stimuli-triggered “smart” materials, an external interference or source would be needed to trigger the drug release. However, for physiological stimuli-triggered “smart” materials, the drug release can be self-regulated by changes in the physiological conditions of the patient or in the surrounding area of the material[19].
1.2. Addition of electronics to smart drug delivery devices
For the “active” category of the smart drug delivery devices, electronics can be added for use as both the monitoring and triggering components of the device. Doing so would increase the areas of application of the devices and would also confer several advantages to the devices. The addition of electronics and sensors to the smart drug delivery device would allow for the patient’s wound or medical condition to be monitored. This could be done by tracking the certain physiological conditions such as pH, temperature, or the amount of various biomarkers of the patient or in the surrounding area. The information on the patient’s condition can then be directly sent to the healthcare professional taking care of the patient or to the patients themselves. This allows for the healthcare professional to have more information on the patient’s condition and to provide better care for the patient[20]. It would also allow for the patients to keep track of their own physiological information which could help the patients better monitor their own conditions[21]. The electronics which are added to the devices can also be used to control the drug release. This can be done either through information from the sensors or directly by the healthcare professional or patient[22]. Using sensors to monitor the condition of the patient and supply the necessary dose of drug as needed would also increase the compliance of the patient. This is especially applicable for illness which requires multiple drug doses daily such as diabetes[23]. The electronics can also control the start and end of drug release, controlling the timing, duration, and amount of drug released. This would allow for the precise control over the amount of drugs which enter a patient’s circulatory system so as to reduce possible side effects[24].
The focus of this review paper is on the smart drug delivery devices which have electronics. The review paper will cover the use of 3D printing for smart drug delivery 146devices which contains or are designed to be used with electronics as well as 3D printing fabrication of electronics that are used in smart drug delivery devices.
2. Applying 3D printing to smart drug delivery devices with electronics
2.1. 3D printing
Three-dimensional (3D) printing is a fabrication technique which uses layer-by-layer deposition to fabricate the final product. The 3D printing techniques used in smart drug delivery devices can be classified into three main categories based on the method of deposition. These categories are extrusion-based, droplet-based, and vat-based 3D printing.
In extrusion-based 3D printing, mechanical force is used to extrude material out through a nozzle. Different methods can be used to provide the necessary mechanical force to extrude the material such as pneumatics, screwbased, or mechanical pistons[25,26]. For extrusion-based 3D printing, materials which exhibit shear thinning properties are preferred. Shear thinning materials undergo a decrease in viscosity under high shear forces. At the extrusion nozzle, where the material experiences high shear forces, the material will have a decreased viscosity allowing for easy extrusion. After being extruded, the high shear force is removed and the increase in viscosity of the material allows for it to retain the printed shape[27]. Alternatively, heat can be used to soften the printing material. The heat can be applied at the nozzle or in the cartridge housing the printing material. When the material leaves the nozzle, the heat is removed and the material hardens, holding the printed shape[28].
For droplet-based printing, micro-droplets of the printing or binding material are jetted onto a substrate or material powder bed forming each layer of the product. Examples of droplet-based 3D printing include microvalve, acoustic wave jetting, thermal, and piezoelectric inkjet[29]. For binder-based droplet 3D printing, binding material is dropped onto a powder bed of the desired printing material. After the desired pattern is formed on the layer, another layer of powder is deposited on top and the process is repeated[30]. In material-based droplet-based printing, droplets of the printing material are deposited and subsequently crosslinked. This can be achieved through deposition of the printing material into a pool of crosslinking material or the crosslinking can be achieved after deposition through methods such as UV crosslinking. The use of micro-droplets to form the final product allows for a higher resolution of the printing design[31]. However, due to the need for a lower viscosity in order to form the droplets, the types of material which can be printed using this method are limited. The lower viscosity of the printing material also causes the initial printed design to have a lower mechanical strength and be less able to hold its shape. Thus, crosslinking of the printed design immediately after printing could help to retain the shape.
Vat-based 3D printing involves the use of selective curing of a vat of liquid crosslinkable material for the fabrication of the product. In most vat-based printing, a printing platform is lowered into the vat to the desired printing thickness. Light in the desired printing pattern or lasers are then shown across the surface crosslinking the material forming the design. The platform is then raised to allow for the liquid material to fill the void for the printing of the next layer. This process is repeated until the final product is formed[32]. Examples of vat-based printing include stereolithography, digital light processing, and two-photon polymerization (TPP)[33,34]. In TPP, the material is cured only at the focal point of a high-power pulse laser. This allows for the fabrication of products with nanometer-scale resolution[35]. However, the drawback of TPP is the scalability of the process for printing larger objects. For vat-based 3D printing, the optical properties of the printing material will affect the interaction between the material and lasers and change the degree of crosslinking of the material[36].
The 3D printing methods described above are mainly applied to the fabrication of the non-electronic parts of the smart drug delivery devices. The applications of the 3D printing methods used for the direct fabrication of printed electronics are different. A more detailed description and examples of the 3D printing methods used for 3D-printed electronics will be given in subsequent chapter.
2.1.1. Benefits of using 3D printing
The use of 3D printing for the fabrication of products confers several advantages. One such advantage is that the product can be highly customizable. As the printing materials used in 3D printing can be deposited or crosslinked on demand, it allows for free-form designs. Thus, the design of the product can be customized or tailored to individuals. 3D printing also allows for the fabrication of designs which are hard or impossible to fabricate using traditional fabrication methods. Due to the layer-by-layer fabrication process of 3D printing, 3D printing can be used to fabricate certain unique designs which cannot be done by traditional fabrication due to design restrictions[37]. The fabrication of unique designs also applies the internal designs of products, known as the infill. By customizing the infill of a printed item, different infill designs can be fabricated. Different infill designs can affect different properties of the printed item such as weight, mechanical strength, diffusion rate, and density[38]. Using 3D printing to fabricate the product also greatly reduces the amount of material waste. Traditional fabrication methods 147typically use material removal methods from a larger block to fabricate the products, leading to material wastage and increased cost. The layer-by-layer fabrication method of 3D printing only deposits printing material as needed. This leads to minimal waste generated from the fabrication process. 3D printing has also been used for prototyping or testing of product design[39]. As 3D printing can be used for the fabrication of small quantity of products on demand, it allows for prototypes or products with different design changes to be easily fabricated. These products can be used for testing or for physical visualization of how the final product would be.
2.2. 3D-printed parts for integration with electronics
3D printing can be applied to smart drug delivery devices which contain electronics in various ways. These includes the use of 3D printing to fabricate different parts, casings or shells which are used with the electronic components in smart drug delivery devices. This section covers 3D printing fabricated parts that are used for integration with electronic components for smart drug delivery. The next section will cover casings and shells that are fabricated using 3D printing for containing electronic components in smart drug delivery devices.
There are a few different types of 3D-printed parts that are fabricated to be integrated with electronic components which are used for smart drug delivery. One of them is 3D-printed microneedles. These microneedles are often used with microelectronic control systems or micropumps in order to control the on-demand release of drugs. In one such example, a hollow microneedle array, which could be attached to micro-electromechanical systems (MEMS) with integrated electronics and pumps, was fabricated using vat-based 3D printing[40]. This allows accurate control of the amount of drugs delivered through the microneedles. The hollow microneedle patch was specially designed and fabricated using stereolithography. The microneedles were designed to have an internal reservoir which is used to store the liquid drug before distribution through the hollow microneedles. The patch was also fabricated with a tubular opening for connection to the pump. The microneedle combined with the MEMS allows for the controlled delivery of microliter volumes of drugs though a noninvasive transdermal route. In another example, hollow microneedle patches, which were integrated with wireless electronic control system and pumps, were fabricated using extrusion-based 3D printing[41]. The system is also equipped with two separate reservoirs which can be used to store and deliver different drugs. The wireless electronic system allows for the control and delivery of separate release profile from the two different drugs. Through the use of 3D printing, the design of the microneedles, such as the needle length and spacing, can be easily customized.
This is to facilitate the delivery of drugs into chronic wounds. The thickness of the crust and necrotic tissue of a chronic wound differs with time, type and stage of the wound. By having customizable length of microneedle, the needles could pierce and deliver drugs under the wound crust and necrotic tissue. This would help increase the bioavailability of the drugs within the deeper layers of the wound, improving the treatment of chronic wounds.
The customizability of 3D printing has also been used to fabricate specially designed features to be used with microneedles for smart drug delivery. In one such example, vat-based 3D printing was used to fabricate a microneedle patch which contained microfluidic channels[42]. The microfluidic channels were connected to a specialized mixing channel which would allow for the content flowing from the different microfluidic channels to mix before being delivered through the microneedles (Figure 1). For the purposes of drug delivery, this would allow the mixing and combination of different drug compounds to be delivered to the patient. The patient would be able to receive multiple different drug treatment simultaneously without having to go through complicated process of taking several drugs separately. Additionally, by controlling the input flow rate of the individual microfluidic channels, the final composition of the mixed fluid can be changed. The mixed fluid can then be transdermally delivered to the patient through the microneedle patch attached to the end of the mixing channel. The 3D-printed microfluidic and mixing channels can also be used for drug testing of different drug compositions for development of drug therapies. In another work regarding 3D-printed microfluidic channels, 3D printing was used to fabricate different microfluidic chips with varying channel geometries and dimensions[43]. Due to the customizability of 3D printing, the different geometries and designs of the microfluidic chips can be easily fabricated. These differently designed microfluidic chips were used to investigate and optimize the use of electrotactic drug delivery for drug-loaded ionic liquid microdroplets. These ionic liquid microdroplets would then be used for drug delivery.
Figure 1.
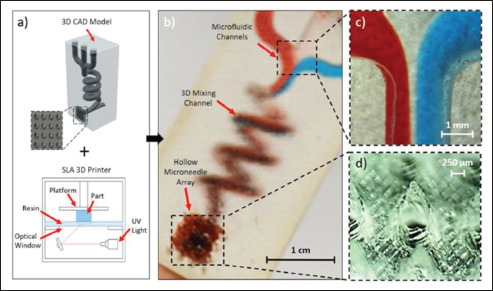
(a) The use of a stereolithography 3D printer for fabrication of the microfluidic microneedle and the 3D model. (b) Printed microneedle device with three separate microfluidic inlets which leads to a mixing chamber and subsequently to the hollow microneedles. (c) Zoomed-in image of the three inlets converge junction. (d) Zoomed-in image of the hollow microneedles. (Reprinted with permission from[42]. Copyright (2019) AIP Publishing.)
2.3. 3D-printed casing for containing electronics
3D printing has also been used to fabricate different casing and shells for containing electronics for smart drug delivery purposes. The customizability of 3D printing allows for unique design of the casing which would be used to house the components of the drug delivery device, such as the drug reservoir, electronic sensors, and triggers. One area of applications of these 3D-printed casings is in smart pills. Smart pills use various different types of electronic components to sense and trigger the release of drugs from the pill. In one such example, 3D printing was used to fabricate a pill which contained a miniature electrolytic pump connected to a drug reservoir[44]. When the drug is to be released from the pill, the 148electrolysis from the pump will cause a membrane to swell, pushing the drug from the reservoir out through a nozzle in the pill to be released. By controlling the current supplied to the pump, the deflection of the membrane changes, controlling the amount of drugs released. 3D printing allowed for the pill capsule to be designed and fabricated with compartments to contain all the necessary parts, such as the pump and the electronic controlling components. In another example, 3D printing was used to fabricate a capsule for a smart pill which contains multiple near-infrared (NIR) LEDs[45]. The NIR LEDs allow for the tracking of the location of the pill as it passes through the digestive system. The capsule was designed with slots into it for placing the LEDs. The slots also contained opening to allow for electrical connections. By tracking the location of the pill, it would allow for the release of the drugs to be triggered at specific areas within the digestive system. This would allow for targeted drug delivery to specific areas or for drugs to be released in certain area for increased drug efficacy.
The use of 3D printing for drug delivery shells allows for the fabrication of specially designed shells. For example, multi-material 3D printing was used to fabricate a gastric-resident electronic device[46]. The device was uniquely designed with different materials forming different parts to allow for the device to be delivered orally and to reside in the stomach for long periods of time. The joints of the arms of the device were printed with a flexible material, allowing for the stiff arms to be folded in a more compact shape for oral delivery. When the device reaches the stomach, the arms unfold. This increases the overall size of the device, preventing the device from leaving the stomach. The 3D-printed shell of the device is also designed with compartments which allows for the addition of electronics, sensors, and drug reservoirs, allowing for long-term monitoring, wireless feedback, and drug release. After a period of time, the arms detach from the device, allowing for the device and the arms to pass through the digestive system. Casings for smart drug delivery devices using other methods of drug delivery have also been fabricated using 3D printing. In one such example, vat-based 3D printing was used to fabricate microreservoirs with customizable shape and volume to operate with a 3D-printed micropump for on-demand drug release (Figure 2a)[47]. The entire device was housed in a 3D-printed biocompatible casing (Figure 2b). The scalability, customizable design, and biocompatibility of the printed device allows for the device to be implanted into different parts of the body for long-term drug release[48]. In another example, 3D printing was used to fabricate a flexible bandage used for drug delivery for wound management[49]. The patch was designed to contain the wireless electronic control component, the flexible heater and the drug reservoir. The flexibility of the patch allows for the bandage to better conform to the wound area, providing better interface and conformance to the wound.
Figure 2.
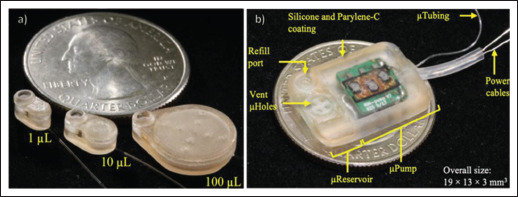
(a) Scalable 3D-printed microreservoir with customizable shape and storage volume[47]. (b) Completed device with a 3D-printed microreservoir, micropump, and biocompatible 3D-printed casing for implantation. (Reprinted with permission from[48].)
2.4. Advantages of 3D printing in the fabrication of smart drug delivery devices
One of the advantages of using 3D printing for the fabrication of smart drug delivery devices with electronics is its ability to fabricate design, which is difficult or impossible to achieve with traditional fabrication methods. 149This includes certain internal structure of certain parts of the smart drug delivery device. Examples of such include the microneedle patch which has the internal structure of the microfluidic mixing channel or internal reservoir[40,42]. This allows for the addition of beneficial functions which are difficult to or cannot be achieved through traditional fabrication methods. 3D printing also allows for the fabrication of customized design better tailored to suit the function of the smart drug delivery device. The customizations include certain design aspects, such as the length of the microneedle for optimal piercing of chronic wounds, or certain slots and compartments for the addition of electronics. These customizations allow for the devices to function optimally or allow for the addition of components which can aid the device’s function.
The usage of 3D printing also allows for the embedding of electronics and sensors to the device due to the layer-by-layer fabrication. The layer-by-layer and on-demand fabrication of 3D printing allows for the electronic component to be put into place by pausing the printing process, after which the printing process can be continued[50]. This allows for the electronic components, such as temperature, strain, or stress sensors, to be not only placed on the surface of the device but also within the device[51,52]. These components can be used for monitoring and feedback of the patient condition in order to allow for healthcare professionals to provide better care for the patient. Other components of the smart drug delivery device, such as drug reservoirs and actuators for controlling drug release, can also be embedded into the device through the use of 3D printing[53].
3. Applying 3D-printed electronics to smart drug delivery devices
3.1. 3D-printed electronics
Another way in which 3D printing can be used for smart drug delivery devices which contain electronics is the direct fabrication of the electronic components. This is known as 3D-printed electronics. 3D-printed electronics is a niche field within the landscape of 3D printing or additive manufacturing that mainly deals with the fabrication of electrically and electronically functional components that use electrical signals to realize an intended purpose. Unlike conventional 3D printing, 3D electronic printing typically involves the use of a singular or a combination of electrically conducting, semiconducting, and dielectric feedstock materials to produce different electronic architectures ranging from single-material components to multi-layer and multiple materials components. To date, 3D printing has been successfully applied in different fields, such as aerospace, automobile, and biomedical sectors, to fabricate various types of active and passive electronic components, circuits, sensors, antennas, and energy harvesting and storage devices, just to name a few. Examples and descriptions of 3D-printed electronics used in smart drug delivery devices will be given in subsequent section.
3.1.1. Benefits of using 3D-printed electronics
Conventionally, electronic system and devices are fabricated using silicon technology, printed circuit board technology, and microfabrication technology. These electronic manufacturing technologies are known to be capable of manufacturing advanced and high-performance electronic devices. Although these processes are suitable and effective for mass production of electronics, but they have several drawbacks, such as (i) high energy consumption, (ii) tedious fabrication process, (iii) being non-environmentally friendly because the processes produce harmful waste products, (iv) limited material choices due to the high processing temperature, and (v) lesser design freedom. With the rising demands for wider applications of electronics across various fields such as biomedical, wearable, and implantable applications, conventional 150electronics fail to meet the increasing requirements of these devices in terms of the material softness and stretchability, biocompatibility, and freeform fabrication. So far, the more common electronic fabrication techniques that are being used and compatible with the fabrication of conventional electronics for soft and flexible drug delivery devices (DDD) are the microfabrication[10,49,54], selective electrodeposition[55], and screen printing techniques[56,57]. These fabrication methods have several drawbacks such as the tedious fabrication process, the need for stencil or mask, and being limited to 2D patterning. For the case of microfabrication and electrodeposition, these methods are also incompatible to process advanced materials such as composite material incorporated with novel nanomaterials like graphene and MXene[58].
In contrast, 3D electronic printing techniques which often utilize a layer-by-layer fabrication approach offer added benefits, such as low processing temperature, compatibility with various kinds of soft and flexible substrate, ability to process various kinds of functional inks, and greater design flexibility to the electronic designers. In addition, 3D electronic printing techniques produces lesser waste compared to conventional electronic fabrication methods as the material and energy are used to deposit materials where necessary and do not require stencil or tooling. The simple fabrication process of 3D printing techniques makes it more convenient for rapid prototyping of smart DDD and it also opens new opportunities for the development of highly customized DDDs are individual-specific. The following section discusses the more common 3D electronic printing techniques that have been used for the fabrication of smart DDDs.
In general, 3D electronic printing techniques can be categorized as material extrusion printing techniques, droplet-jetting printing techniques, vat polymerization techniques, and powder-bed fusion techniques[59]. Interestingly, it was found that majority of the works that are related to smart DDDs have used the extrusion-based printing techniques and droplet-jetting printing techniques. As such, the discussion in this paper will mainly focus on these two techniques and briefly cover some works on vat polymerization techniques for 3D-printed electronics for use in smart DDDs.
Material extrusion 3D printing techniques deposit materials layer-by-layer through a coordinate-controlled nozzle. The feedstock materials mainly come in two forms, namely filament and viscous liquid. The printing technique for the former is usually called fused filament fabrication (FFF) technique, whereas the printing technique for the latter is commonly known as direct ink writing (DIW) technique.
For FFF technique, the process involves the use of a heated nozzle to melt the filament into molten state. The high temperature of the extruded material aids in forming bonds between the newly deposited materials and the previous layer (Figure 3a). For 3D electronic printing, conductive filaments in the form of polymeric nanocomposite are often used. Thus far, the nanocomposite filaments that have been developed for 3D printing of physiological sensors include graphene-polylactic acid (PLA)[60,61], graphene nanorods-PLA[62], carbon nanotube (CNT)-PLA[63], and carbon black (CB)-PLA[64], just to name a few. The sensors that were fabricated through FFF printing technique include electrochemical sensor[64], pressure sensor[63], and temperature sensor[62,63], and biosensors[60,61]. The FFF techniques are suitable for the fabrication of 3D and freestanding electronic structures, but FFF-printed devices usually suffer from poor electrical properties due to the nature of nanocomposite materials.
Figure 3.
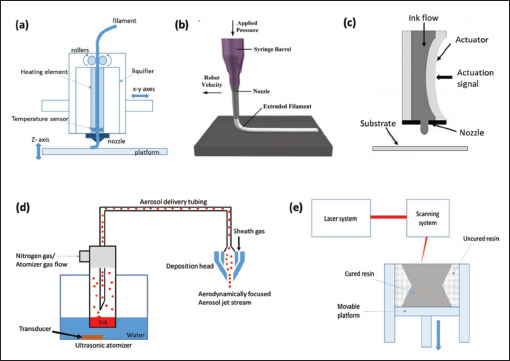
Schematics showing the various 3D printing processes, namely (a) fused filament fabrication (FFF)[96], (b) direct ink writing (DIW)[97], (c) inkjet printing (IJP)[98], (d) aerosol jet printing (AJP)[98], and (e) stereolithography[96].
DIW techniques, on the other hand, typically involve the extrusion of the functional inks through a nozzle by means of screw, pneumatic, and microvalve dispensing methods (Figure 3b)[58]. The functional inks can exist in the form of nanomaterial dispersions or nanocomposite resins. The former usually forms high-purity structure with good electrical properties after the solvent completely evaporates, but these materials are used to manufacture thin film structures that are attached to a substrate. The latter, on the other hand, can be used to manufacture freeform and freestanding electronic structures but suffers in terms of the electrical properties due to the presence of the matrix materials similar to the materials used for FFF technique. Some examples of functional inks for DIW include silver-based ink[58], CNT-polydimethylsiloxane (PDMS) nanocomposite ink[65], graphene-PDMS[66], and PEDOT:PSS/HPU hydrogel[67], just to name a few. For photosensitive resin-based functional inks, a UV curing laser or light source will be used to cure the resin in situ to ensure that the extruded filament can retain its geometry immediately after being extruded from the nozzle to prevent the structure from collapsing. Typically, the DIW-printed electronics are fabricated on a substrate and then subsequently removed from the substrate for use. In certain case, a solution support bath may be used to achieve more complex or freestanding electronic structures[68]. To date, DIW technology has been reported to be used for printing heaters, temperature sensors, pH sensors, sweat sensors, UV sensors, etc.[65-70]
Droplet-jetting techniques are a class of 3D printing techniques that deposit materials layer-by-layer in the form of liquid droplets. In general, there are two main types of droplet-jetting techniques for electronic printing, namely inkjet printing (IJP) and aerosol jet printing (AJP) 151techniques. These techniques are widely used for 3D electronic printing to manufacture high-performance and advanced electronics components and devices. Typically, these printing techniques produce thin-film electronic architectures that conform to the surface of the substrate.
IJP technique is a process that deposit the ink by ejecting a series of droplets through a nozzle using an appropriate droplet ejecting mechanism (Figure 3c)[71]. Generally, there are two main ejecting mechanisms, namely piezoelectric actuation and thermal actuation. IJP techniques tend to have strict requirements on the ink properties, such as surface tension, viscosity, and particle size due to the small nozzle size and the dominating surface tension effect that can affect the printability of the ink significantly[72]. Typically, the particle size has to be at least one-tenths or smaller than the nozzle size to prevent nozzle clogging issues and to ensure successful and continuous printing. IJP techniques are more commonly used for printing 2D circuits and sensors that are structurally flat. To date, many works have used IJP techniques to fabricate physiological sensors that can be applied to drug delivery devices, such as CNT-based pH sensor for wound monitoring[73], silver nanowire-based transparent strain sensor and heater for human wearables[74], just to name a few[75].
AJP technique, on the other hand, uses inert gas flows like nitrogen gas to direct and deposit the droplets of ink onto the substrate (Figure 3d)[76]. AJP process consists of several stages, which are the atomization, aerosol delivery, aerosol focusing, and deposition[77,78]. The atomization of the inks can be performed by means of pneumatic atomization or ultrasonic atomization, depending on the viscosity of the ink. Typically, the working ranges of ink’s viscosity for the pneumatic atomizer and ultrasonic atomizer are 1–1000 cP and <10 cP, respectively. The formed aerosols will then be transported to the printhead by the carrier gas through a tubing. Once the aerosols reach the printhead, a sheath flow is used to aerodynamically focus the ink flow into a fine jet before exiting the nozzle. Due to the high exit velocity of the aerosols, the standoff distance between the nozzle and the substrate can be as far as 5 mm which makes it very suitable for conformal printing as the alignment of part becomes less critical compared to other contact printing processes like the DIW techniques[79]. Besides, another advantage of AJP technique is that the process can achieve a line resolution as high as 20 μm which could be useful for high-performance electronic applications[80]. Unlike IJP technique, AJP technique is less prone to nozzle clogging issue due to the presence of sheath flow to prevent the ink from coming in contact directly with the inner wall of the nozzle. Similar to IJP, AJP technique also works with many kinds of nanomaterials, such as nanoparticle[81], nanowires[82], and 2D nanomaterials[83]. So far, AJP technique has been used to develop and fabricate sensors, 152such as pH sensor[84] and electrochemical biosensor[85-88], that can be potentially used in smart DDDs.
Vat polymerization 3D printing technique is a process where a light or a laser source is used to cure photosensitive resin layer-by-layer (Figure 3e). Generally, there are different types of vat polymerization techniques, namely stereolithography, digital light processing, and TPP techniques. The 3D printers usually contain a vat filled with uncured resin. Due to the nature of the printing technique, it is normally limited to printing structures with single material only. As a result, it possesses restrictions in terms of electronic fabrication where patterning of conductive material is required. Interestingly, this technique has also been shown useful for the fabrication of sensing electrodes for smart DDDs. For instance, Stassi et al. have used digital light processing 3D printing technique to 3D print a polymeric functional microcantilever that can be used as mass-sensitive biosensors[89]. In other works, researchers have demonstrated using stereolithography printing technique to fabricate microneedle and achieve patterning of electrodes via selective deposition method[90,91].
Table 1 summarizes and compares the different 3D printing techniques that have been used for the fabrication of sensors and electronics. Their advantages and disadvantages of each printing technique are also highlighted in the table.
Table 1. Comparison among the 3D printing techniques in terms of the typical material and printing specifications, and their pros and cons.
| Printing technique | Form of feedstock materials | Ink viscosity (cP) | Minimum feature size (μm) | Layer thickness (μm) | Advantages | Disadvantages |
|---|---|---|---|---|---|---|
| Fused filament fabrication[92] | Polymeric-based nanocomposite filaments | - | 400-600 | 100-300 |
|
|
| Direct ink writing[93] | Nanocomposite resin, conductive inks | 102-106 | Dependent on the nozzle size | 100 |
|
|
| Inkjet printing[94] | Conductive nanomaterial inks | 5-20 | 30-50 | <0.5 |
|
|
| Aerosol jet printing[94] | Conductive nanomaterial inks | 1-1000 | ~10 | 0.1-2 |
|
|
| Stereolithography[95] | Photosensitive resin | Typically <600 | ~80 | ~25 |
|
|
3.2. 3D-printed electronics used in smart drug delivery devices
Apart from utilizing 3D printing in the fabrication of parts and casing for smart drug delivery devices which contains electronics, 3D printing can also be used for the direct fabrication of 3D-printed electronic components or systems to incorporate “smartness” to the DDD. Besides triggering the drug delivery using “smart” materials, the triggering process can also be achieved via the use of electronic circuits equipped with sensors and microheaters. So far, there have been many types of 3D-printed heaters and 3D-printed sensors that have been demonstrated for potential smart drug delivery purposes. In this review paper, the area of focus of the electronic components will be on these 3D-printed sensors for detecting physiological changes and 3D-printed microheaters. There are other technologies for the fabrication of such electronics such as hybrid electronic manufacturing and transfer technique; however, these will not be the focus of this review paper. Readers are encouraged to read about these techniques through other papers[99-104]. This section discusses the 3D-printed electronics that are used in smart drug delivery devices, which include printed microheaters and various physiological parameter sensors, such as temperature, glucose, sweat, and electrochemical sensors.
Microheater is typically used for triggering drug delivery in thermo-responsive DDDs[10]. The heating 153mechanism is achieved by Joule heating in which the electrical energy is converted into heat energy when it passes through an electrical resistive element. Therefore, it is imperative that the materials used for the microheaters must be electrical conducting. Although any electrical conducting materials can be used as the heating element for microheater, the performance and durability differ depending on the material properties. Usually, the resistive materials should have properties such as high resistivity for low energy consumption and good oxidation property for longer working lifespan[105]. Conventionally, these resistive elements can be fabricated on substrates using sputtering, plasma vapor deposition, etching, direct laser writing, and selective deposition[55,105]. More recently, 3D printing has also been explored for the fabrication of microheaters, but there are not many of them. For instance, Cai et al. developed a technique that combines DIW technique with laser sintering technique to process, print, and sinter the platinum (Pt) ink on a ceramic substrate for the fabrication of microheaters[106]. They have demonstrated using different laser parameters to produce different microstructure of the Pt material to optimize the heating performance. Similarly, Vasiliev et al. demonstrated the fabrication of microheaters via AJP, and they showed that the printed Pt microheaters can operate at a temperature as high as 450°C[107]. Other than printing pure metallic materials, Yin et al. demonstrated the use of nanocomposite ink (PDMS-CNT) as the resistive materials of the microheater (Figure 4)[65]. The DIW-printed microheater exhibits high stretchability and conformity that can be worn on human skin for drug-encapsulated patch system for pain management.
Figure 4.
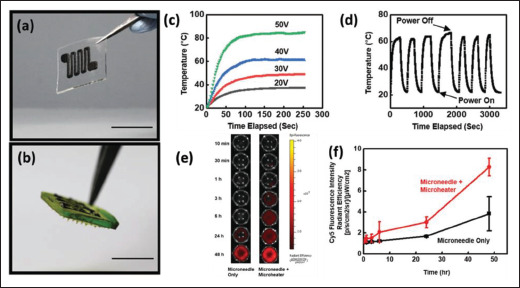
(a and b) Optical images showing the PDMS-CNT-based microheater fabricated using DIW technique. (c and d) Graphs showing the heating performance of the microheater. (e and f) Comparison of the drug release with and without the microheater. (Reprinted with permission from[65]. Copyright (2019) Wiley, John & Sons, Inc.)
Temperature sensor is essential for temperature monitoring applications for regulating the microheater to stimulate drug release, and for monitoring purposes[108]. Generally, there four main types of temperature sensors that have been manufactured through 3D printing technologies, namely resistance temperature detector (RTD), thermistor, thermocouple, and fiber grating sensor[109,110]. RTDs and thermistors work similarly, and they detect the temperature change based on the change in its resistance at different temperature. The main difference is the type of materials that they are made of; RTDs are usually made of pure conducting metal, whereas thermistors are typically made of a mixture of metal oxides. Thermocouple, on the other hand, is formed by joining two dissimilar electrically conducting materials to form electrical junctions that are used for temperature detection[111]. The temperature is detected via the electrical voltages that are generated across the hot and cold ends of the thermocouples. In general, it is observed that the more common 3D-printed temperature sensors are based on RTDs and thermistors[109]. So far, researchers have attempted various materials as the temperature-sensitive materials for 3D-printed temperature sensors, including various types of nanocomposite materials such as PEDOT:PSS-CNT[112], PDMS-graphene[66], PLA-CNT[63], etc. It was found that the thermal sensitivity of the sensing element can be improved through the use of nanocomposite inks[112]. Interestingly, it was also found that raw feedstock printing material and the as-printed materials can have different thermal responsiveness due to the residual stress induced by the printing technique[63]. Other than that, 154Wang et al. demonstrated that stretchable temperature sensor can be fabricated using PDMS nanocomposite ink with DIW technique (Figure 5)[66]. They also showed that the use of DIW technique to fabricate grid-like structure of the temperature sensor can help to decouple the effect of motions to enhance the accuracy of temperature measurement.
Figure 5.
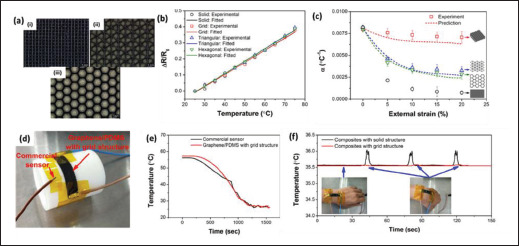
(a) Various 3D-printed porous structures: (i) grid, (ii) triangular, and (iii) hexagonal. (b and c) Temperature sensing performance of various 3D-printed temperature sensors with different porous structures. (d) Optical image of the 3D-printed temperature sensor. (e) Comparison between a commercial temperature sensor and a 3D-printed temperature sensor. (f) Demonstration of strain-insensitive temperature sensor. (Reprinted with permission from[66]. Copyright (2019) ACS Publications.)
pH sensors are very useful for wound monitoring and management as it allows for in situ monitoring of the wound condition and triggering of drug release as early as possible. pH sensing can be achieved via different sensing mechanisms such as conductometry, voltammetry, and potentiometry, just to name a few[57]. In terms of 3D-printed pH sensors, many different types of sensors have been reported[57,67,69,73,78,84]. Typically, the pH-sensitive materials that have been used for 3D printing of pH sensors include PEDOT:PSS, PANI, graphene, and CNT. Naficy et al. fabricated a hydrogel-based pH sensor using a composite functional inks made of 5 wt.% of PEDOT:PSS and hydrophilic polyurethane and 3D printed the pH sensors using material-extrusion printing method[67]. They showed that the performance of printed pH sensors was comparable to those prepared by conventional method. In another work, Jose et al. compared three types of printed pH sensors, which are conductometric PEDOT:PSS, voltametric carbon-alizarin and potentiometric graphene/PANI sensors, and they found that the potentiometric PANI-based sensors outperformed the other two sensors in terms of linearity, repeatability, stability, and leaching resistance (Figure 6)[57]. Also, Goh and Agarwala et al. 155attempted the fabrication of CNT-based resistive pH sensor via AJP and IJP, respectively[73,78,84]. The pH-responsive property of CNT allows the detection of pH through the monitoring of electrical resistance of the CNT-based pH sensing electrodes. On the other hand, Fan et al. demonstrated the fabrication of organic electrochemical transistor (OECT) that is pH-sensitive on a 3D-printed PLA substrate[69]. The OECT has a multi-layered architecture and contains multiple materials, such as silver conductor and PEDOT:PSS, and it is fabricated through DIW technique. They found that the performance of the sensor is comparable to other devices that are fabricated using microfabrication techniques.
Figure 6.
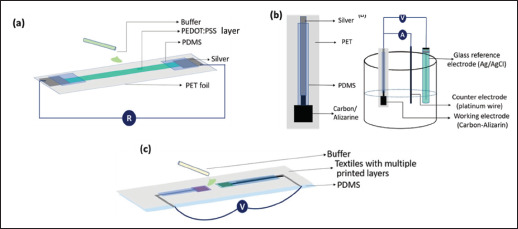
Schematic showing various printed pH sensors, namely (a) conductometric PEDOT:PSS, (b) voltametric carbon-alizarin, and (c) potentiometric graphene/PANI sensors. (Reprinted with permission from[57]. Copyright (2021) Wiley, John, and Sons Inc.)
Glucose sensor is an essential component of glucose monitoring for diabetic patients. It enables on-demand triggering of insulin release whenever the glucose level of the patients is at an unhealthy level. So far, there are many works that have demonstrated using 3D-printed sensors for glucose level detection[60,61,113,114]. Interestingly, most of these works involve the use of FFF 3D printing techniques to fabricate the 3D electrodes. The electrode materials that have been attempted include copper filament[114], graphene–PLA nanocomposite filament[60,61], and copper–zinc oxide-PVDF nanocomposite filament[113]. Redondo et al. found that the 3D-printed copper electrode is very suitable for non-enzymatic glucose sensing due to their high conductivity and high catalytic activity resulting from the porous structure[114]. However, it should be noted that the drawback of the 3D-printed copper electrode is the high energy consumption as it requires a high sintering temperature of greater than 1000°C. In contrast, Kumar et al. demonstrated that the use of copper-zinc oxide-reinforced PVDF as nonenzymatic glucose electrode can function adequately with heat treatment at only 60°C[113]. Graphene–PLA-based electrodes, on the other hand, are usually used to produce enzymatic glucose sensors. The fabrication of these sensors involves certain post-processing steps such as polishing, cleaning, and surface activation, and enzyme immobilization. The oxygenated groups from the nanocomposite material provides suitable conditions for the enzyme (glucose oxidase) to immobilize on its surface via crosslinkers such as glutaraldehyde[60,61].
Other than 3D-printed microheater, temperature sensors, pH sensors and glucose sensors, there are other types of 3D-printed sensors, such as sweat sensor[58], biomolecule sensor[60], and DNA sensor[115] for other physiological parameters. For instance, Kim et al. fabricated a 3D-printed wearable bioelectronic patch that allows in situ sweat electrolyte monitoring purposes[58]. The sweat sensing relies on the DIW-printed ion-selective electrodes to detect the concentrations of Na+, K+, and Ca2+ ions in the sweat electrolyte (Figure 7). The main ingredients of the ion-selective electrodes are sodium ionophore, potassium ionophore, and calcium ionophore, which are the lipophilic complexing agents that reversibly bind ions. These ingredients are usually incorporated into a membrane before binding to the 3D-printed electrodes. Other than ions, there is another work that describes the detection of biologically-produced molecules such as uric acid and nitrite using 3D-printed graphene–PLA electrode[116]. They have tested the 3D-printed sensor with saliva and urine and shown that the sensor exhibits satisfactory linear range, sensitivity, limit of detection. Another interesting example of 3D-printed sensor is on DNA sensing. Loo et al. used selective laser melting printing technology to fabricate a helical-shape stainless 156steel electrode, which is then electroplated with gold for the detection of DNA hybridization. They have shown that the 3D-printed electrode is superior against a non-complementary DNA target and capable of achieving a detection range of 1–1000 nM[115].
Figure 7.
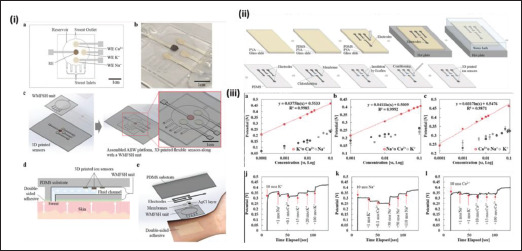
(i) Schematic showing the design of the sweat sensors. (ii) Schematic showing the fabrication steps of the sweat sensors. (iii) Graphs showing the detection Na+, K+ and Ca2+ ions and the selectivity of the sensors. (Reprinted with permission from[58]. Copyright (2021) from John, Wiley and Sons Inc.)
3.3. Advantages of using 3D-printed electronics in smart drug delivery devices
Technological advances in smart drug delivery systems are being propelled by impressive developments in the synergistic integration of 3D printing technologies with soft bioelectronics. This paves the way for a personalized, direct therapeutic and diagnostic intervention. Integrating electronic devices into the body enables remote health monitoring, management as well as data collection for evaluation of results. Flexible electronics plays an increasingly important role in drug delivery. Miniature systems offer unique advantages with high response time, stylized manipulation of complex structures, low power consumption, reduced material waste, higher packing efficiency, and minimal cost[117,118]. 3D-printed microheaters are beneficial due to their small size, mass-fabrication capacity, good mechanical stability and performance[119]. Integrated microheaters have demonstrated precisely controlled drug diffusion and subsequent drug release on a periodic basis via heating function with a potential to manufacture and design sensor-controlled medical devices. This study provides a new arena to support the critical demands of managing pain with on-demand dose requirement for clinical use[120].
3D printing technology has become the key player in the development of biosensors, thanks to its versatility[121]. Different types of sensors offer advantages, depending on the unique properties of the material, functionality and sensor response. Biosensors with precise designs can be made using special inks, and improved surface properties with fewer flaws can be tailored to increase the sensor’s sensitivity to pick up signals better than those manufactured through conventional methods[122]. These properties remain an asset in designing biosensors for improved drug delivery. Moreover, 3D manufacturing causes minimal material waste and can be readily customized to the patient’s needs. Flexible biosensors that simultaneously detect multiple metabolites in biological samples through continuous and non-invasive measurements not only saves time, but also have the potential to monitor disease progression and determine an individual’s health parameters[123]. Lin et al. developed a biocompatible gastric resident electronic device made from poly-l-lactic acid and thermoplastic polyurethane capable of prolonged residence in the gastric environment and maintains wireless electronic communications. When coupled with drug delivery modules, the implanted biosensors will possibly eliminate opportunistic infections prior to their growth and enable an automated, on-demand closed loop systems that can increase the efficacy of an intervention[46]. 3D printing also allows creation of other kinds of drug delivery devices entirely fitting our needs. A microneedle system integrated with MEMS has shown potential for transdermal insulin delivery and has a promising scope for evolution of medical devices for personalized treatment[40]. A programmable wirelessly controlled microsystem with a refillable microreservoir and a phase-changing peristaltic micropump as a single embodiment has shown promising results for delivering drug into murine inner ear[48]. The structure was fabricated with a dental resin to minimize inflammatory or immune response and implanted transdermally. This opens up the possibility of building DDDs by choosing a raw material from the wide spectrum of biocompatible materials that can be placed into the human body either permanently or temporarily to support functions[124,125]. It also enables scalability for use ranging from small animal models to direct clinical translation[126].
4. Future trends of 3D printing and 3D-printed electronics in smart drug delivery devices
The current application of 3D printing and 3D-printed electronics in smart drug delivery devices has been more focused on the “passive” type of smart drug delivery devices. This allows for the fabrication of many different types of drug delivery devices, which allow for controllable, on-demand drug release. However, the use of 3D printing and 3D-printed electronics in “active” smart drug delivery devices with electronics, which has a self-regulated, closed loop system, are currently more limited. “Active” smart drug delivery device can use sensors to detect and monitor physiological changes in a patient, and then start and stop the release of drug as needed. The future direction and trends in 3D printing and 3D-printed electronics in “active” smart drug delivery devices will be discussed in the subsequent sections in two main parts. The two parts are concerning the use of 3D printing for the fabrication of different parts or even the entire smart drug delivery device, and the fabrication and application of 3D-printed electronics in smart drug delivery devices.
4.1. Future trends in smart drug delivery devices for 3D printing
The application of 3D printing in the fabrication of smart drug delivery devices has allowed for an increase in the customizability and types of design of the smart drug delivery devices. 3D printing has also allowed for different 157methods of triggering drug release through the use of special 3D-printed designs. In one such example, 3D printing was used to fabricate oral drug capsule, which was designed to safely break and release the stored drug under the physiological pressures of the antropyloric region in the gastrointestinal tract[127]. This allows for the drug release to be targeted at that region. The customizability of the dimensions and design of the printed capsule through the use of 3D printing allows for pressure at which the capsule will break to be easily changed for other purposes[128]. However, currently the use of 3D printing has mostly been limited to single material or single 3D printing process fabrication. This has limited the type of components that can be fabricated. A way to overcome this is the use of multi-material or multiple different 3D printing processes, also known as hybrid or multi-process 3D printing, in a singular fabrication process. Hybrid 3D printing allows for the printing and fabrication of multiple different materials and designs, some of which are difficult for a singular 3D process to achieve. This allows for the combination of the different advantages brought about by using different 3D printing methods. The use of different 3D printing methods also allows for materials with drastically different properties, such as a soft, flexible material and a hard, rigid material, to be fabricated into a single product (Figure 8)[129]. This allows for a higher degree of customization in terms of the design and application of the printed part as a greater number of different materials can be used. The use of hybrid and multi-material 3D printing also allows for more parts of a product to be fabricated using 3D printing due to the improved customization and freedom in material selection and design.
Figure 8.
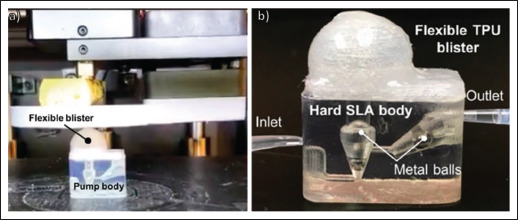
(a) 3D-printed rigid and hard stereolithography-printed body with a soft and flexible thermoplastic polyurethane (TPU) blister being 3D printed on top. (b) Photo of the hybrid soft and hard multi-material microfluidic pump with fingered power actuation. (Reprinted with permission from[129].)
Currently, hybrid 3D printing has some applications in traditional drug delivery devices, such as the fabrication of liquid-filled solid capsules using alternating extrusion and droplet-based 3D printing[130]. In another example, a drug delivery system with locally incorporated drug depots, which can be selectively placed within a printed drug delivery matrix, was fabricated using hybrid 3D printing. Vat-based 3D printing was used to fabricate the drug delivery matrix, and droplet-based 3D printing was used to deposit the drug depots. This drug delivery system allowed for the customization of the drug release profile as well as multiple drug releases by controlling the position, size and type of drug depots[131]. The use of hybrid 3D printing in smart drug delivery devices, however, has been limited. Hybrid 3D printing has also been applied to the fabrication of some electronic healthcare devices. However, most of the electronic components are not printed but are placed on to the device[132]. These pick-and-place methods of fabricating 3D-printed electronics devices use the traditional hard and rigid electronic components. By using this method, not only would the customizability of the design of the product be limited, but it would also negatively impact the flexibility and conformity of the devices as the electronic components might delaminate from the substrate[133]. Thus, 3D-printed electronics could be used in order to overcome such issues. The future trends or direction of the use of 3D-printed electronics in smart drug delivery devices will be explored in the next section.
4.2. Future trends in smart drug delivery devices for 3D-printed electronics
As discussed in previous sections, flexible electronics has clearly demonstrated rapid progress and its unique advantages for a plethora of drug delivery applications. These features have offered several benefits, such as minimally invasive acute delivery methods for implantation, small size, easy manipulation, enhanced performance, reduced immune response as well as greater compatibility with the in vivo systems. However, a few aspects need to be carefully considered. In case of 158choosing biodegradable materials for device fabrication, residues of the degradation should be harmless to human body following implantation. Synthetic polymers such as polylactic acid and polycaprolactone are extensively explored for this purpose due to their biocompatibility and safe degradation by-products[90,134]. Moreover, implantable devices can be coated with an immunomodulatory or immunoprotective coating in order to evade immune response. In addition, the developed system should also facilitate implantation in small as well as large animals prior to clinical trials. A broad range of materials can be used for the development of ink-based electronics devices to achieve complicated geometric features and site-specific drug delivery applications[135]. 3D-printed electronics will bring unprecedented opportunities for smart drug delivery by facilitating long-term implantation of the device with periodic delivery of active compounds. Further developments in the field of 3D-printed bioelectronics should exploit some unconventional architectures and materials for use in drug delivery.
5. Conclusion
“Active” smart drug delivery devices offer many benefits over traditional drug delivery devices and have the potential for use as a closed loop, self-regulating drug delivery system. By including electronics into this type of system, functions such as monitoring of a patient’s condition can be achieved. This not only allows for on-demand drug release but also provides more information to the healthcare professional to further improve the care to be provided. The use of 3D printing and 3D-printed electronics in “active” smart drug delivery devices has increased the customizability and versatility of the functions which these devices can provide. These additions allow for devices to be tailored to suit individuals and also for different sensors and circuits with varying functions to be printed onto the device. By increasing the types of sensors that can be printed onto a device, the range of applications of the device would also increase. With the further development in hybrid 3D printing, flexible and 3D-printed bioelectronics, the applications of such devices in not only long-term “active” drug delivery but also in healthcare monitoring can be greatly improved.
Acknowledgments
The authors are thankful for the support by Singapore Centre for 3D Printing, Nanyang Technological University, Singapore. The content is solely the responsibility of the authors.
Conflict of interest
The authors declare no conflicts of interest.
Author contributions
Conceptualization: Wai Cheung Ma, Guo Liang Goh, Wai Yee Yeong
Writing – original draft: Wai Cheung Ma, Guo Liang Goh, Balasankar Meera Priyadarshini
Writing – review & editing: Wai Cheung Ma, Guo Liang Goh
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data
Not applicable.
References
- 1.Wen H, Jung H, Li X. Drug delivery approaches in addressing clinical pharmacology-related issues: Opportunities and challenges. AAPS J. 2015;17(6):1327–1340. doi: 10.1208/s12248-015-9814-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mehrotra N, Gupta M, Kovar A, et al. The role of pharmacokinetics and pharmacodynamics in phosphodiesterase-5 inhibitor therapy. Int J Impot Res. 2007;19(3):253–264. doi: 10.1038/sj.ijir.3901522. [DOI] [PubMed] [Google Scholar]
- 3.McCoy CP, Brady C, Cowley JF, et al. 2010. Triggered drug delivery from biomaterials. Exp Opin Drug Deliv 7 5 605-616 [DOI] [PubMed] [Google Scholar]
- 4.Wang Y, Kohane DS. External triggering and triggered targeting strategies for drug delivery. Nat Rev Mater. 2017;2(6):17020. [Google Scholar]
- 5.Alvarez-Lorenzo C, Concheiro A. Smart drug delivery systems: From fundamentals to the clinic. Chem Commun, 2014;50(58):7743–7765. doi: 10.1039/c4cc01429d. [DOI] [PubMed] [Google Scholar]
- 6.Ghani M, Heiskanen A, Kajtez J, et al. On-demand reversible UV-triggered interpenetrating polymer network-based drug delivery system using the Spiropyran-Merocyanine hydrophobicity switch. ACS Appl Mater Interfaces. 2021;13(3):3591–3604. doi: 10.1021/acsami.0c19081. [DOI] [PubMed] [Google Scholar]
- 7.Zhou C, Xie X, Yang H, et al. Novel class of ultrasound-triggerable drug delivery systems for the improved treatment of tumors. Mol Pharm, 2019;16(7):2956–2965. doi: 10.1021/acs.molpharmaceut.9b00194. [DOI] [PubMed] [Google Scholar]
- 8.Hoare T, Timko BP, Santamaria J, et al. Magnetically triggered nanocomposite membranes: A versatile platform for triggered drug release. Nano Letters. 2011;11(3):1395–1400. doi: 10.1021/nl200494t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Teodorescu F, Queniat G, Foulon C, et al. Transdermal skin patch based on reduced graphene oxide: A new approach for photothermal triggered permeation of ondansetron across porcine skin. J Control Release. 2017;245:137–146. doi: 10.1016/j.jconrel.2016.11.029. [DOI] [PubMed] [Google Scholar]
- 10.159Bagherifard S, Tamayol A, Mostafalu P, et al. Dermal patch with integrated flexible heater for on demand drug delivery. Adv Healthc Mater. 2016;5(1):175–184. doi: 10.1002/adhm.201500357. [DOI] [PubMed] [Google Scholar]
- 11.Lee H, Choi TK, Lee YB, et al. A graphene-based electrochemical device with thermoresponsive microneedles for diabetes monitoring and therapy. Nat Nanotechnol. 2016;11(6):566–572. doi: 10.1038/nnano.2016.38. [DOI] [PubMed] [Google Scholar]
- 12.Lawrence M, Séguin C, Price A. 3D printed polypyrrole scaffolds for pH-dependent drug delivery for bone regeneration. SPIE Smart Structures + Nondestructive Evaluation. 2021;11590 Vol. SPIE. [Google Scholar]
- 13.Feng G, Tseng W. PZT and PNIPAM film-based flexible and stretchable electronics for knee health monitoring and enhanced drug delivery. IEEE Sens J. 2018;18(23):9736–9743. [Google Scholar]
- 14.Derakhshankhah H, Jahanban-Esfahlan R, Vandghanooni S, et al. A bio-inspired gelatin-based pH- and thermal-sensitive magnetic hydrogel for in vitro chemo/hyperthermia treatment of breast cancer cells. J Appl Polym Sci. 2021;138(24):50578. [Google Scholar]
- 15.Tiryaki E, Baçaran Elalmiç Y, Karakuzu ikizler B, et al. Novel organic/inorganic hybrid nanoparticles as enzyme-triggered drug delivery systems: Dextran and dextran aldehyde coated silica aerogels. J Drug Deliv Sci Technol. 2020;56:101517. [Google Scholar]
- 16.Liu C, Wang Z, Wei X, et al. 2021. 3D printed hydrogel/PCL core/shell fiber scaffolds with NIR-triggered drug release for cancer therapy and wound healing. Acta Biomater 131, 314 325 [DOI] [PubMed] [Google Scholar]
- 17.Yang Y, Zeng W, Huang P, et al. Smart materials for drug delivery and cancer therapy. VIEW. 2021;2(2):20200042. [Google Scholar]
- 18.SSoppimath KS, Aminabhavi TM, Dave AM, et al. Stimulus-responsive “smart” hydrogels as novel drug delivery systems. Drug Dev Ind Pharm, 2002;28(8):957–974. doi: 10.1081/ddc-120006428. [DOI] [PubMed] [Google Scholar]
- 19.Lodhi BA, Hussain MA, Ashraf MU, et al. Basil (Ocimum basilicum L.) seeds engender a smart material for intelligent drug delivery: On-off switching and real-time swelling, in vivo transit detection, and mechanistic studies. Ind Crops Prod. 2020;155:112780. [Google Scholar]
- 20.Yadav KS, Kapse-Mistry S, Peters GJ, et al. E-drug delivery: A futuristic approach. Drug Discov Today. 2019;24(4):1023–1030. doi: 10.1016/j.drudis.2019.02.005. [DOI] [PubMed] [Google Scholar]
- 21.Appelboom G, Camacho E, Abraham ME, et al. Smart wearable body sensors for patient self-assessment and monitoring. Arch Public Health. 2014;72(1):28. doi: 10.1186/2049-3258-72-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu G, Lu Y, Cheng C, et al. Battery-free and wireless smart wound dressing for wound infection monitoring and electrically controlled on-demand drug delivery. Adv Funct Mater. 2021;31(26):2100852. [Google Scholar]
- 23.Lee H, Song C, Hong YS, et al. Wearable/disposable sweat-based glucose monitoring device with multistage transdermal drug delivery module. Sci Adv. 2017;3(3):e1601314. doi: 10.1126/sciadv.1601314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Timko BP, Dvir T, Kohane DS. Remotely triggerable drug delivery systems. Adv Mater, 2010;22(44):4925–4943. doi: 10.1002/adma.201002072. [DOI] [PubMed] [Google Scholar]
- 25.Placone JK, Engler AJ. Recent advances in extrusion-based 3D printing for biomedical applications. Adv Healthc Mater. 2018;7(8):1701161. doi: 10.1002/adhm.201701161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schouten M, Wolterink G, Dijkshoorn A, et al. A review of extrusion-based 3D printing for the fabrication of electro- and biomechanical sensors. IEEE Sens J, 2021;21(11):12900–12912. [Google Scholar]
- 27.Jiang Z, Diggle B, Tan ML, et al. Extrusion 3D printing of polymeric materials with advanced properties. Adv Sci. 2020;7(17):2001379. doi: 10.1002/advs.202001379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dumpa N, Butreddy A, Wang H, et al. 3D printing in personalized drug delivery: An overview of hot-melt extrusion-based fused deposition modeling. Int J Pharm. 2021;600:120501. doi: 10.1016/j.ijpharm.2021.120501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gudapati H, Dey M, Ozbolat I. A comprehensive review on droplet-based bioprinting: Past, present and future. Biomaterials. 2016;102:20–42. doi: 10.1016/j.biomaterials.2016.06.012. [DOI] [PubMed] [Google Scholar]
- 30.Wang Y, Xu Z, Wu D, et al. Current status and prospects of polymer powder 3D printing technologies. Materials (Basel) 2020;13(10):2406. doi: 10.3390/ma13102406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abdolmaleki H, Kidmose P, Agarwala S. Dropletbased techniques for printing of functional inks for flexible physical sensors. Adv Mater. 2021;33(20):2006792. doi: 10.1002/adma.202006792. [DOI] [PubMed] [Google Scholar]
- 32.Li W, Mille LS, Robledo JA, et al. Recent advances in formulating and processing biomaterial inks for vat polymerization-based 3D printing. Adv Healthc Mater. 2020;9(15):2000156. doi: 10.1002/adhm.202000156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pagac M, Hajnys J, Ma Q-P, et al. A review of vat photopolymerization technology: Materials, applications, challenges, and future trends of 3D printing. Polymers. 2021;13(4):598. doi: 10.3390/polym13040598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu X, Awad A, Robles-Martinez P, et al. Vat photopolymerization 3D printing for advanced drug delivery and medical device applications. J Control Release. 2021;329:743–757. doi: 10.1016/j.jconrel.2020.10.008. [DOI] [PubMed] [Google Scholar]
- 35.Xing J-F, Zheng M-L, Duan X-M. Two-photon polymerization microfabrication of hydrogels: An advanced 3D printing technology for tissue engineering and drug delivery. Chem Soc Rev. 2015;44(15):5031–5039. doi: 10.1039/c5cs00278h. [DOI] [PubMed] [Google Scholar]
- 36.Piedra-Cascon W, Krishnamurthy VR, Att W, et al. 3D printing parameters, supporting structures, slicing, and post-processing procedures of vat-polymerization additive manufacturing technologies: A narrative review. J Dentist. 2021;109:103630. doi: 10.1016/j.jdent.2021.103630. [DOI] [PubMed] [Google Scholar]
- 37.Sadia M, Arafat B, Ahmed W, et al. Channelled tablets: An innovative approach to accelerating drug release from 3D printed tablets. J Control Release. 2018;269:355–363. doi: 10.1016/j.jconrel.2017.11.022. [DOI] [PubMed] [Google Scholar]
- 38.Kyobula M, Adedeji A, Alexander MR, et al. 3D inkjet printing of tablets exploiting bespoke complex geometries 160for controlled and tuneable drug release. J Control Release. 2017;261:207–215. doi: 10.1016/j.jconrel.2017.06.025. [DOI] [PubMed] [Google Scholar]
- 39.Goyanes A, Robles MP, Buanz A, et al. Effect of geometry on drug release from 3D printed tablets. Int J Pharm. 2015;494(2):657–663. doi: 10.1016/j.ijpharm.2015.04.069. [DOI] [PubMed] [Google Scholar]
- 40.Economidou SN, Uddin MJ, Marques MJ, et al. A novel 3D printed hollow microneedle microelectromechanical system for controlled, personalized transdermal drug delivery. Addit Manuf, 2021;38:101815. [Google Scholar]
- 41.Derakhshandeh H, Aghabaglou F, McCarthy A, et al. A wirelessly controlled smart bandage with 3D-printed miniaturized needle arrays. Adv Funct Mater. 2020;30(13):1905544. doi: 10.1002/adfm.201905544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yeung C, Chen S, King B, et al. A 3D-printed microfluidic-enabled hollow microneedle architecture for transdermal drug delivery. Biomicrofluidics. 2019;13(6):064125. doi: 10.1063/1.5127778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dalvand K, Ghiasvand A, Gupta V, et al. Chemotaxis-based smart drug delivery of epirubicin using a 3D printed microfluidic chip. J Chromatogr B. 2021;1162:122456. doi: 10.1016/j.jchromb.2020.122456. [DOI] [PubMed] [Google Scholar]
- 44.Goffredo R, Pecora A, Maiolo L, et al. A swallowable smart pill for local drug delivery. J Microelectromech Syst. 2016;25(2):362–370. [Google Scholar]
- 45.Jiang H, Kim A, Zhou J, et al. Real-time tracking of a 3D-printed smart capsule using on-board near-infrared led array. 2019 20th International Conference on Solid-State Sensors, Actuators and Microsystems & Eurosensors XXXIII (TRANSDUCERS & EUROSENSORS XXXIII) 2019 [Google Scholar]
- 46.Kong YL, Zou X, McCandler CA, et al. 3D-printed gastric resident electronics. Adv Mater Technol. 2019;4(3):1800490. doi: 10.1002/admt.201800490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Forouzandeh F, Ahamed NN, Hsu M-C, et al. A 3D-printed modular microreservoir for drug delivery. Micromachines. 2020;11(7):648. doi: 10.3390/mi11070648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Forouzandeh F, Ahamed NN, Zhu X, et al. A wirelessly controlled scalable 3D-printed microsystem for drug delivery. Pharmaceuticals. 2021;14(6):538. doi: 10.3390/ph14060538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mostafalu P, Amugothu S, Tamayol A, et al. Smart flexible wound dressing with wireless drug delivery. 2015 IEEE Biomedical Circuits and Systems Conference (BioCAS) 2015 [Google Scholar]
- 50.Ota H, Emaminejad S, Gao Y, et al. Application of 3D printing for smart objects with embedded electronic sensors and systems. Adv Mater Technol. 2016;1(1):1600013. [Google Scholar]
- 51.Ota H, Chao M, Gao Y, et al. 3D printed “earable” smart devices for real-time detection of core body temperature. ACS Sens. 2017;2(7):990–997. doi: 10.1021/acssensors.7b00247. [DOI] [PubMed] [Google Scholar]
- 52.Muth JT, Vogt DM, Truby RL, et al. Embedded 3D printing of strain sensors within highly stretchable elastomers. Adv Mater. 2014;26(36):6307–6312. doi: 10.1002/adma.201400334. [DOI] [PubMed] [Google Scholar]
- 53.Akmal JS, Salmi M, Makitie A, et al. Implementation of industrial additive manufacturing: Intelligent implants and drug delivery systems. J Funct Biomater. 2018;9(3):41. doi: 10.3390/jfb9030041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Punjiya M, Mostafalu P, Sonkusale S. Smart bandages for chronic wound monitoring and on-demand drug delivery. 2017 IEEE 60th International Midwest Symposium on Circuits and Systems (MWSCAS) 2017 IEEE. [Google Scholar]
- 55.Yang Y, Li S, Xu H, et al. Fabrication of flexible microheater with tunable heating capabilities by direct laser writing and selective electrodeposition. J Manuf Process. 2022;74:88–99. [Google Scholar]
- 56.Karimi MA, Arsalan M, Shamim A. Live demonstration: Screen printed, microwave based level sensor for automated drug delivery. 2017 IEEE SENSORS 2017 [Google Scholar]
- 57.Jose M, Mylavarapu SK, Bikkarolla SK, et al. Printed pH sensors for textile-based wearables: A conceptual and experimental study on materials, deposition technology, and sensing principles. Adv Eng Mater. 2022;24(5):2101087. [Google Scholar]
- 58.Kim T, Yi Q, Hoang E, et al. A 3D printed wearable bioelectronic patch for multi-sensing and in situ sweat electrolyte monitoring. Adv Mater Technol. 2021;6(4):2001021. [Google Scholar]
- 59.Goh GL, Zhang H, Chong TH, et al. 3D printing of multilayered and multimaterial electronics: A review. Adv Electron Mater. 2021;7(10):2100445. [Google Scholar]
- 60.Cardoso RM, Silva PR, Lima AP, et al. 3D-printed graphene/polylactic acid electrode for bioanalysis: Biosensing of glucose and simultaneous determination of uric acid and nitrite in biological fluids. Sens Actuators B Chem. 2020;307:127621. [Google Scholar]
- 61.Wang L, Pumera M. Covalently modified enzymatic 3D-printed bioelectrode. Microchim Acta. 2021;188(11):1–8. doi: 10.1007/s00604-021-05006-6. [DOI] [PubMed] [Google Scholar]
- 62.Sajid M, Gul JZ, Kim SW, et al. Development of 3D-printed embedded temperature sensor for both terrestrial and aquatic environmental monitoring robots. 3D Print Addit Manuf. 2018;5(2):160–169. [Google Scholar]
- 63.Kim H-G, Hajra S, Oh D, et al. Additive manufacturing of high-performance carbon-composites: An integrated multi-axis pressure and temperature monitoring sensor. Compos Part B Eng. 2021;222:109079. [Google Scholar]
- 64.Silva-Neto HA, Santhiago M, Duarte LC, et al. Fully 3D printing of carbon black-thermoplastic hybrid materials and fast activation for development of highly stable electrochemical sensors. Sens Actuators B Chem. 2021;349:130721. [Google Scholar]
- 65.Yin M, Xiao L, Liu Q, et al. 3D printed microheater sensor-integrated, drug-encapsulated microneedle patch system for pain management. Adv Healthc Mater. 2019;8(23):1901170. doi: 10.1002/adhm.201901170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang Z, Gao W, Zhang Q, et al. 3D-printed graphene/polydimethylsiloxane composites for stretchable and strain-insensitive temperature sensors. ACS Appl Mater Interfaces. 2018;11(1):1344–1352. doi: 10.1021/acsami.8b16139. [DOI] [PubMed] [Google Scholar]
- 67.Naficy S, Oveissi F, Patrick B, et al. Printed, flexible pH sensor hydrogels for wet environments. Adv Mater Technol. 2018;3(11):1800137. [Google Scholar]
- 68.161Liu Y, Li H, Feng Q, et al. A three-dimensional-printed recyclable, flexible, and wearable device for visualized UV, temperature, and sweat pH sensing. ACS Omega. 2022;7(11):9834–9845. doi: 10.1021/acsomega.2c00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fan J, Montemagno C, Gupta M. 3D printed high transconductance organic electrochemical transistors on flexible substrates. Org Electron. 2019;73:122–129. [Google Scholar]
- 70.Agarwala S, Lee JM, Yeong WY, et al. 3D printed bioelectronic platform with embedded electronics. MRS Adv. 2018;3(50):3011–3017. [Google Scholar]
- 71.Goh GL, Ma J, Chua KLF, et al. Inkjet-printed patch antenna emitter for wireless communication application. Virtual Phys Prototyping. 2016;11(4):289–294. [Google Scholar]
- 72.Du Z, Yu X, Han Y. Inkjet printing of viscoelastic polymer inks. Chin Chem Lett. 2018;29(3):399–404. [Google Scholar]
- 73.Iversen M, Monisha M, Agarwala S. Flexible, wearable and fully-printed smart patch for pH and hydration sensing in wounds. Int J Bioprint. 2022;8(1):447. doi: 10.18063/ijb.v8i1.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nair NM, Pakkathillam JK, Kumar K, et al. Printable silver nanowire and PEDOT: PSS nanocomposite ink for flexible transparent conducting applications. ACS Appl Electron Mater. 2020;2(4):1000–1010. [Google Scholar]
- 75.Gao M, Li L, Song Y. Inkjet printing wearable electronic devices. J Mater Chem C. 2017;5(12):2971–2993. [Google Scholar]
- 76.Sinha AK, Goh GL, Yeong WY, et al. Ultra-low-cost, crosstalk-free, fast-responding, wide-sensing-range tactile fingertip sensor for smart gloves. Adv Mater. 2022;9(21):2200621. Interfaces. [Google Scholar]
- 77.Goh GL, Agarwala S, Yeong WY. Proceedings of the 3rd International Conference on Progress in Additive Manufacturing (PRO-AM) Nanyang Technological University; Singapore: 2018. High resolution aerosol jet printing of conductive ink for stretchable electronics. [Google Scholar]
- 78.Agarwala S, Goh GL, Yeong WY. 2018. Aerosol jet printed pH sensor based on carbon nanotubes for flexible electronics. Proceedings of the 3rd International Conference on Progress in Additive Manufacturing (PRO-AM). Nanyang Technological University; Singapore [Google Scholar]
- 79.Goh GL, Dikshit V, Koneru R, et al. Fabrication of design-optimized multifunctional safety cage with conformal circuits for drone using hybrid 3D printing technology. Int J Adv Manuf Technol, 2022;120(3):2573–2586. [Google Scholar]
- 80.Goh GL, Agarwala S, Yeong WY. Aerosol-jet-printed preferentially aligned carbon nanotube twin-lines for printed electronics. ACS Appl Mater Interfaces. 2019;11(46):43719–43730. doi: 10.1021/acsami.9b15060. [DOI] [PubMed] [Google Scholar]
- 81.Goh GL, Zhang H, Goh GD, et al. Multi-objective optimization of intense pulsed light sintering process for aerosol jet printed thin film. MSAM. 2022;1(2):10. [Google Scholar]
- 82.Wang F-X, Lin J, Gu W-B, et al. Aerosol-jet printing of nanowire networks of zinc octaethylporphyrin and its application in flexible photodetectors. Chem Commun. 2013;49(24):2433–2435. doi: 10.1039/c3cc38996k. [DOI] [PubMed] [Google Scholar]
- 83.Zeng M, Kuang W, Khan I, et al. Colloidal nanosurfactants for 3D conformal printing of 2D van der Waals materials. Adv Mater. 2020;32(39):2003081. doi: 10.1002/adma.202003081. [DOI] [PubMed] [Google Scholar]
- 84.Goh GL, Agarwala S, Tan YJ, et al. A low cost and flexible carbon nanotube pH sensor fabricated using aerosol jet technology for live cell applications. Sens Actuators B Chem. 2018;260:227–235. [Google Scholar]
- 85.Parate K, Pola CC, Rangnekar SV, et al. Aerosol-jet-printed graphene electrochemical histamine sensors for food safety monitoring. 2D Mater. 2020;7(3):034002. [Google Scholar]
- 86.Pola CC, Rangnekar SV, Sheets R, et al. Aerosol-jet-printed graphene electrochemical immunosensors for rapid and label-free detection of SARS-CoV-2 in saliva. 2D Mater. 2022;9(3):035016. doi: 10.1088/2053-1583/ac7339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Di Novo NG, Cantu E, Tonello S, et al. Supportmaterial-free microfluidics on an electrochemical sensors platform by aerosol jet printing. Sensors. 2019;19(8):1842. doi: 10.3390/s19081842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tonello S, Bianchetti A, Braga S, et al. Impedancebased monitoring of mesenchymal stromal cell three-dimensional proliferation using aerosol jet printed sensors: A tissue engineering application. Materials. 2020;13(10):2231. doi: 10.3390/ma13102231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Stassi S, Fantino E, Calmo R, et al. Polymeric 3D printed functional microcantilevers for biosensing applications. ACS Appl Mater Interfaces. 2017;9(22):19193–19201. doi: 10.1021/acsami.7b04030. [DOI] [PubMed] [Google Scholar]
- 90.Rezapour Sarabi M, Nakhjavani SA, Tasoglu S. 3D-printed microneedles for point-of-care biosensing applications. Micromachines. 2022;13(7):1099. doi: 10.3390/mi13071099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Azim N, Kundu A, Royse M, et al. Fabrication and characterization of a 3D printed, microelectrodes platform with functionalized electrospun nano-scaffolds and spin coated 3D insulation towards multi-functional biosystems. J Microelectromech Syst. 2019;28(4):606–618. [Google Scholar]
- 92.Grimm T. Fused deposition modeling: A technology evaluation. Time-compression technologies. 2003;11(2):1–6. [Google Scholar]
- 93.Saadi MASR, Maguire A, Pottackal NT, et al. Direct ink writing: A 3D printing technology for diverse materials. Adv Mater. 2022;34(28):2108855. doi: 10.1002/adma.202108855. [DOI] [PubMed] [Google Scholar]
- 94.Tan H, Tran T, Chua C. A review of printed passive electronic components through fully additive manufacturing methods. Virtual Phys Prototyping. 2016;11(4):271–288. [Google Scholar]
- 95.Huang J, Qin Q, Wang J. A review of stereolithography: Processes and systems. Processes. 2020;8(9):1138. [Google Scholar]
- 96.Goh GD, Agarwala S, Goh G, et al. 2017. Additive manufacturing in unmanned aerial vehicles (UAVs): Challenges and potential. Aerosp Sci Technol 63, 140 151 [Google Scholar]
- 97.Wan X, Luo L, Liu Y, et al. Direct ink writing based 4D printing of materials and their applications. Adv Sci, 2020;7(16):2001000. doi: 10.1002/advs.202001000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Goh GL, Tay MF, Lee JM, et al. Potential of printed electrodes for electrochemical impedance spectroscopy 162(EIS): Toward membrane fouling detection. Adv Electron Mater. 2021;7(10):2100043. [Google Scholar]
- 99.Khan Y, Thielens A, Muin S, et al. A new frontier of printed electronics: Flexible hybrid electronics. Adv Mater. 2020;32(15):1905279. doi: 10.1002/adma.201905279. [DOI] [PubMed] [Google Scholar]
- 100.Sarobol P, Cook A, Clem PG, et al. Additive manufacturing of hybrid circuits. Annu Rev Mater Res. 2016;46(1):41–62. [Google Scholar]
- 101.Ma Y, Zhang Y, Cai S, et al. Flexible hybrid electronics for digital healthcare. Adv Mater. 2020;32(15):1902062. doi: 10.1002/adma.201902062. [DOI] [PubMed] [Google Scholar]
- 102.Carlson A, Bowen AM, Huang Y, et al. Transfer printing techniques for materials assembly and micro/nanodevice fabrication. Adv Mater. 2012;24(39):5284–5318. doi: 10.1002/adma.201201386. [DOI] [PubMed] [Google Scholar]
- 103.Zheng C, Huang L, Zhang H, et al. Fabrication of ultrasensitive field-effect transistor DNA biosensors by a directional transfer technique based on CVD-grown graphene. ACS Appl Mater Interfaces. 2015;7(31):16953–16959. doi: 10.1021/acsami.5b03941. [DOI] [PubMed] [Google Scholar]
- 104.Linghu C, Zhang S, Wang C, et al. Transfer printing techniques for flexible and stretchable inorganic electronics. npj Flex Electron. 2018;2(1):26. [Google Scholar]
- 105.Jeroish ZE, Bhuvaneshwari KS, Samsuri F, et al. Microheater: Material, design, fabrication, temperature control, and applications—A role in COVID-19. Biomed Microdevices. 2021;24(1):3. doi: 10.1007/s10544-021-00595-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cai Z, Zeng X, Duan J. Fabrication of platinum microheater on alumina substrate by micro-pen and laser sintering. Thin Solid Films. 2011;519(11):3893–3896. [Google Scholar]
- 107.Vasiliev AA, Nisan AV, Samotaev NN. Aerosol/ink jet printing technology for high-temperature MEMS sensors. Multidisciplinary Digital Publishing Institute Proceedings. 2017;1(4):617. [Google Scholar]
- 108.Honda W, Harada S, Arie T, et al. Wearable, human-interactive, health-monitoring, wireless devices fabricated by macroscale printing techniques. Adv Funct Mater. 2014;24(22):3299–3304. [Google Scholar]
- 109.Khan S, Ali S, Khan A, et al. Wearable printed temperature sensors: Short review on latest advances for biomedical applications. IEEE Reviews in Biomedical Engineering. 2021 doi: 10.1109/RBME.2021.3121480. [DOI] [PubMed] [Google Scholar]
- 110.Lee J, Kim Y, Lee JH. A 3-D-printed, temperature sensor based on mechanically-induced long period fibre gratings. J Mod Optics. 2020;67(5):469–474. [Google Scholar]
- 111.Rahman MT, Cheng C-Y, Karagoz B, et al. High performance flexible temperature sensors via nanoparticle printing. ACS Appl Nanomater. 2019;2(5):3280–3291. [Google Scholar]
- 112.Honda W, Harada S, Arie T, et al. Printed wearable temperature sensor for health monitoring. SENSORS. 2014 IEEE. [Google Scholar]
- 113.Kumar V, Kumar R, Singh R, et al. On 3D printed biomedical sensors for non-enzymatic glucose sensing applications. Proc Inst Mech Eng Part H J Eng Med, 2022;236(8):1057–1069. doi: 10.1177/09544119221100116. [DOI] [PubMed] [Google Scholar]
- 114.Redondo E, Pumera M. Fully metallic copper 3D-printed electrodes via sintering for electrocatalytic biosensing. Appl Mater Today. 2021;25:101253. [Google Scholar]
- 115.Loo AH, Chua CK, Pumera M. DNA biosensing with 3D printing technology. Analyst. 2017;142(2):279–283. doi: 10.1039/c6an02038k. [DOI] [PubMed] [Google Scholar]
- 116.Abdalla A, Patel BA. 3D-printed electrochemical sensors: A new horizon for measurement of biomolecules. Curr Opin Electrochem, 2020;20:78–81. [Google Scholar]
- 117.Hsu T-R. Miniaturization—A paradigm shift in advanced manufacturing and education. 2002 Proceedings of the IEEE/ASME international conference on advanced manufacturing technologies and education in the 21st Century 1-19. [Google Scholar]
- 118.Ikuta K, Hirowatari K, Ogata T. Three dimensional micro integrated fluid systems (MIFS) fabricated by stereo lithography. Proceedings IEEE Micro Electro Mechanical Systems an Investigation of Micro Structures, Sensors, Actuators, Machines and Robotic Systems 1994 [Google Scholar]
- 119.Udofia EN. Microextrusion 3D Printing of Optical Waveguides and Microheaters 2019 [Google Scholar]
- 120.Yin M, Xiao L, Liu Q, et al. 3D printed microheater sensor-integrated, drug-encapsulated microneedle patch system for pain management. Adv Healthc Mater. 2019;8(23):1901170. doi: 10.1002/adhm.201901170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Remaggi G, Zaccarelli A, Elviri L. 3D printing technologies in biosensors production: Recent developments. Chemosensors. 2022;10(2):65. [Google Scholar]
- 122.Kim T, Yi Q, Hoang E, et al. A 3D printed wearable bioelectronic patch for multi-sensing and in situ sweat electrolyte monitoring. Adv Mater Technol. 2021;6(4):2001021. [Google Scholar]
- 123.Liu M, Yang M, Wang M, et al. A flexible dual-analyte electrochemical biosensor for salivary glucose and lactate detection. Biosensors. 2022;12(4):210. doi: 10.3390/bios12040210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Samson OA, Margaret OI, Oluwashina PG, et al. Biomaterials for drug delivery: Sources, classification, synthesis, processing, and applications. Adv Funct Mater. 2020 IntechOpen, Ch. 10. [Google Scholar]
- 125.Fenton OS, Olafson KN, Pillai PS, et al. Advances in biomaterials for drug delivery. Adv Mater. 2018;30(29):1705328. doi: 10.1002/adma.201705328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Picco CJ, Dominguez-Robles J, Utomo E, et al. 3D-printed implantable devices with biodegradable ratecontrolling membrane for sustained delivery of hydrophobic drugs. Drug Delivery. 2022;29(1):1038–1048. doi: 10.1080/10717544.2022.2057620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Berg S, Krause J, Bjorkbom A, et al. In vitro and in vivo evaluation of 3D printed capsules with pressure triggered release mechanism for oral peptide delivery. J Pharm Sci. 2021;110(1):228–238. doi: 10.1016/j.xphs.2020.10.066. [DOI] [PubMed] [Google Scholar]
- 128.Krause J, Bogdahn M, Schneider F, et al. Design and characterization of a novel 3D printed pressure-controlled drug delivery system. Eur J Pharm Sci. 2019;140:105060. doi: 10.1016/j.ejps.2019.105060. [DOI] [PubMed] [Google Scholar]
- 129.163Ruiz C, Kadimisetty K, Yin K, et al. Fabrication of hard-soft microfluidic devices using hybrid 3D printing. Micromachines. 2020;11(6):567. doi: 10.3390/mi11060567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Rupp H, Binder WH. 3D printing of core-shell capsule composites for post-reactive and damage sensing applications. Adv Mater Technol. 2020;5(11):2000509. [Google Scholar]
- 131.Konasch J, Riess A, Mau R, et al. A novel hybrid additive manufacturing process for drug delivery systems with locally incorporated drug depots. Pharmaceutics. 2019;11(12):661. doi: 10.3390/pharmaceutics11120661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lin R, Li Y, Mao X, et al. Hybrid 3D printing all-in-one heterogenous rigidity assemblies for soft electronics. Adv Mater Technol. 2019;4(12):1900614. [Google Scholar]
- 133.Valentine AD, Busbee TA, Boley JW, et al. Hybrid 3D printing of soft electronics. Adv Mater. 2017;29(40):1703817. doi: 10.1002/adma.201703817. [DOI] [PubMed] [Google Scholar]
- 134.Sarabi MR, Bediz B, Falo LD, et al. 3D printing of microneedle arrays: Challenges towards clinical translation. J 3D Print Med. 2021;5(2):65–70. [Google Scholar]
- 135.Joyee EB, Pan Y. Additive manufacturing of multi-material soft robot for on-demand drug delivery applications. J Manuf Process, 2020;56:1178–1184. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


