Abstract
Objective
To examine the hypothesis that the association between vitamin D deficiency and depressive symptoms is dependent upon total cholesterol level in a representative national sample of the South Korean population.
Design
This was a population-based cross-sectional study.
Setting
The Fifth Korean National Health and Nutrition Examination Survey (KNHANES V, 2010–2012).
Subjects
We included 7198 adults aged 20–88 years.
Results
The incidence of depressive symptoms in individuals with vitamin D deficiency (serum 25-hydroxyvitamin D<20 ng/ml) was 1·54-fold (95 % CI 1·20, 1·98) greater than in individuals without vitamin D deficiency (serum 25-hydroxyvitamin D ≥20 ng/ml). The relationship was stronger in individuals with normal-to-borderline serum total cholesterol (serum total cholesterol<240 mg/dl; OR=1·60; 95 % CI 1·23, 2·08) and non-significant in individuals with high serum total cholesterol (OR=0·97; 95 % CI 0·52, 1·81) after adjustment for confounding variables (age, sex, BMI, alcohol consumption, smoking status, regular exercise, income level, education level, marital status, changes in body weight, perceived body shape, season of examination date and cholesterol profiles).
Conclusions
The association between vitamin D deficiency and depressive symptoms was weakened by high serum total cholesterol status. These findings suggest that both vitamin D and total cholesterol are important targets for the prevention and treatment of depression.
Keywords: Serum 25-hydroxyvitamin D, TAG, Total cholesterol, Depression
Vitamin D is involved in regulating serum concentrations of calcium and phosphate as well as bone mineralization( 1 – 3 ). Vitamin D is also involved in various pathophysiological processes in the human body; research has shown that vitamin D deficiency is a risk factor for CVD, cancer, diabetes mellitus, autoimmune disease and cognitive disorders( 4 – 6 ). In addition, vitamin D supplementation has been reported to reduce the risk of these chronic diseases( 7 – 9 ). Therefore, vitamin D deficiency is an important and relevant health issue in modern society.
Depression is a disorder that has been correlated with vitamin D deficiency in several studies( 10 , 11 ). The pathological mechanism explaining this phenomenon potentially involves the ability of vitamin D to cross the blood–brain barrier and bind to vitamin D receptors in the brain( 12 , 13 ). Similarly, depression has also been related to serum cholesterol status( 14 , 15 ). Increasing evidence suggests that cholesterol affects cognitive function and mood status( 16 , 17 ). Low serum cholesterol has been identified as a risk factor for depression in some studies( 14 , 18 ); yet, other studies indicate an opposite (positive) correlation between serum cholesterol and depression( 15 ).
Vitamin D and cholesterol are not completely independent of one another. Several studies have demonstrated that high serum 25-hydroxyvitamin D (25(OH)D) is associated with a favourable serum cholesterol profile( 19 ). Research has also indicated a positive correlation between vitamin D and total cholesterol levels( 19 – 22 ); however, other studies have also suggested an opposite (negative) correlation between these variables( 23 ).
Clearly, there exists controversy over the relationships between vitamin D, cholesterol and depression; furthermore, no study to date has investigated correlations among these three factors simultaneously. Therefore, in the present study, we examined associations among vitamin D deficiency, serum cholesterol and depressive symptoms in a general population of South Korean adults using data from the Fifth Korean National Health and Nutritional Examination Survey (KNHANES V) conducted from 2010 to 2012. Our data characterize the relationship between serum 25(OH)D and depressive symptoms, as well as the interaction of this relationship with serum total cholesterol level.
Methods
Study population
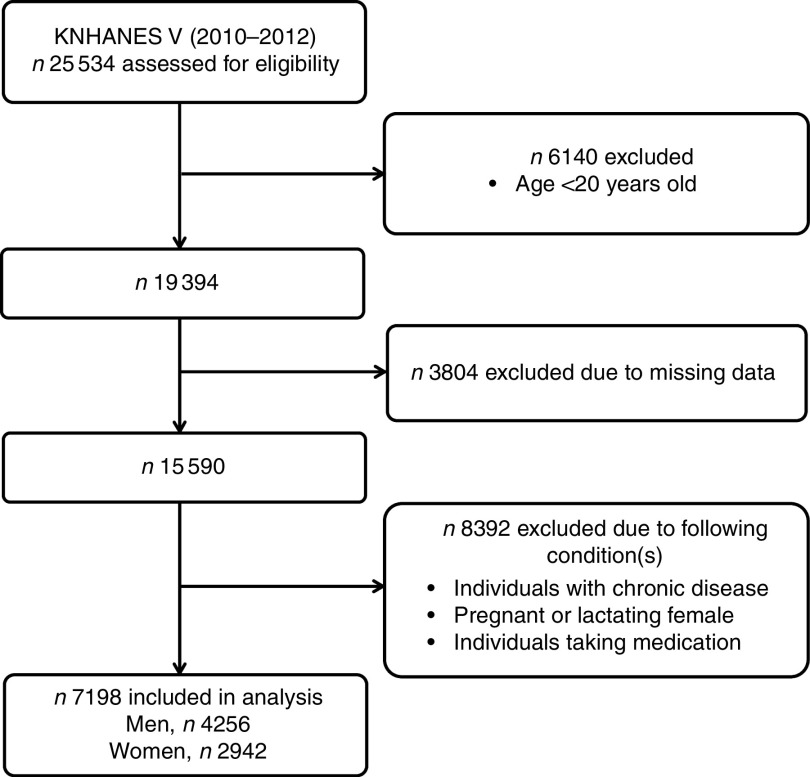
The present study was performed on data from the KNHANES V, which was conducted by the Division of Chronic Disease Surveillance of the Korea Centers for Disease Control and Prevention between January 2010 and December 2012. The KNHANES is a survey for the assessment of health status, health behaviour, nutritional status and sociodemographic data in the civilian non-institutionalized population of South Korea. A stratified, multistage probability sampling design was used for the selection of household units. Each year, 192 survey sections were randomly sampled and twenty households were selected from each section. Finally, 25 534 individuals participated in the examination. For the purposes of our study, we selected individuals who were over the age of 20 years (Fig. 1). We excluded individuals who did not report essential variables, such as serum 25(OH)D concentration, serum cholesterol profiles or mental health questionnaire responses. We also excluded individuals with chronic diseases that might affect serum 25(OH)D concentration or mood status, including hypertension, diabetes mellitus, ischaemic heart disease, stroke, cancer, liver cirrhosis, renal failure, asthma, thyroid disease and arthritis. Additionally, we excluded females who were pregnant or lactating and individuals who were taking medications such as vitamins, hormones and statins. In total, 7198 individuals were included in our statistical analysis. The KNHANES was approved by the Institutional Review Board of the Centers for Disease Control and Prevention in South Korea. All participants who took part in the survey provided written informed consent.
Fig. 1.
Selection of the study population (KNHANES V, Fifth Korean National Health and Nutrition Examination Survey)
General characteristics of participants
Sex, age, BMI, marital status, income level, education level, alcohol consumption, smoking status, regular exercise, changes in body weight, perceived body shape and season were considered as confounding variables potentially affecting depression( 24 – 27 ). BMI (kg/m2) was defined as body weight divided by height squared. A standard mercury sphygmomanometer (Baumanometer; W.A. Baum Co., Inc., Copiague, NY, USA) was used on the right arm to measure blood pressure. Systolic blood pressure and diastolic blood pressure were measured with the participant in a seated position; measurements were taken twice with a 5 min interval and the values were averaged. Individuals who met the 7th Joint National Committee guideline criteria for hypertension (systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg( 28 )) were classified as hypertensive and excluded from the analysis. Participants were divided into two groups according to marital status (single or married). Income level was divided into quartiles (lower, lower middle, upper middle and highest) based on questionnaire responses. Education level was classified according to four categories: graduation from elementary school, middle school, high school and university or higher. Participants were categorized as either non-smokers or current smokers. Alcohol consumption was evaluated using the Alcohol Use Disorders Identification Test (AUDIT) and individuals were divided into three groups according to the results: normal user (0–7 points), hazardous user (8–15 points) and problematic user (≥16 points)( 29 ). Changes in body weight were classified according to three categories: weight gain, weight maintenance and weight loss. Perceived body shape was categorized as lean, normal or obese based on questionnaire responses. Regular exercise was defined as participation in a form of vigorous exercise (running, climbing, rapid cycling, rapid swimming, football, basketball, jumping rope, squash, singles tennis or carrying heavy objects) for ≥20 min at least three times per week; moderate exercise (slow swimming, team tennis, volleyball or carrying light objects) for ≥30 min at least five times per week; or walking for ≥30 min more than five times per week. The season of the examination date was classified as spring (March to May), summer (June to August), autumn (September to November), or winter (December to February).
Vitamin D and cholesterol levels measurement
Fasting blood samples were collected for assessment. To evaluate vitamin D status, serum concentrations of 25(OH)D were measured by RIA (DiaSorin Inc., Stillwater, MN, USA) using a 1470 WIZARD gamma-counter (Perkin Elimer, Turku, Finland). Vitamin D status was categorized into two groups based on the American guideline( 30 ): vitamin D deficiency (25(OH)D<20 ng/ml) and no deficiency (25(OH)D ≥20 ng/ml). Total cholesterol, TAG and HDL cholesterol (HDL-C) were measured by an enzymatic technique using a Hitachi Automatic Analyser 7600 (Hitachi, Tokyo, Japan). Because LDL cholesterol data were not available for all participants in the KNHANES V, we excluded LDL cholesterol levels from our analysis. Total cholesterol level was categorized into three groups: desirable (<200 mg/dl), borderline high (200–239 mg/dl) and high (≥240 mg/dl). HDL-C level was categorized into two groups: low (<40 mg/dl) and normal (≥40 mg/dl). TAG level was also categorized into two groups: high (≥200 mg/dl) and normal to borderline (<200 mg/dl). Cholesterol classifications were based on the National Cholesterol Education Program (NCEP) Adult Treatment Panel III (ATP III) guideline( 31 ).
Depressive symptoms assessment
Depressive symptoms were assessed by self-reporting. Participants were asked whether they had experienced feelings of sadness or despair that interfered with everyday life and persisted for longer than 2 weeks in the past year. Those who answered ‘yes’ were classified as exhibiting depressive symptoms( 32 , 33 ).
Statistical analysis
All statistical analyses were performed using the statistical software package PASW Statistics Version 18.0. P values of <0·05 were considered to be statistically significant. Clinical and demographic characteristics were analysed according to the presence or absence of self-reported depressive symptoms. Continuous variables were analysed using t tests and nominal variables were analysed using χ 2 tests. Associations between serum 25(OH)D and depressive symptoms were analysed using multiple logistic regressions. Odds ratios and 95 % confidence intervals were compared to assess changes in the association between serum 25(OH)D and depressive symptoms according to total cholesterol level. Confounding factors potentially affecting serum 25(OH)D and depressive symptoms were defined previously( 18 , 34 – 39 ). Age, sex, BMI, marital status, income level, education level, alcohol consumption, smoking status, regular exercise, changes in body weight, perceived body shape, season of the examination date, total cholesterol, HDL-C and TAG were adjusted for in the logistic regression analysis. To assess associations between different cholesterol profiles and depressive symptoms, odds ratios and 95 % confidence intervals of each cholesterol profile for depressive symptoms were calculated using multiple logistic regressions. We classified cholesterol profiles into two groups based on the NCEP ATP III guideline( 31 ): total cholesterol level and TAG level were divided into high and normal-to-borderline groups, and HDL-C level was divided into low and normal groups. We adjusted for the same confounding factors as in the analysis of serum 25(OH)D and depressive symptoms except for cholesterol profile. A correlation between serum 25(OH)D and total cholesterol level was analysed using a multiple linear regression analysis adjusted for age, sex, BMI, income level, education level, alcohol consumption, smoking status, regular exercise and changes in body weight, as these factors have been correlated with serum lipid concentrations in previous studies( 40 – 43 ). Changes in the correlation between serum 25(OH)D and total cholesterol level were analysed by examining the strength of this correlation in the normal-to-borderline total cholesterol group and the high total cholesterol group.
Results
Characteristics of study participants
Study participants’ characteristics are summarized according to the presence or absence of depressive symptoms in Table 1. The depressive group consisted of 695 individuals (9·7 %). The mean age of each group (depressive and non-depressive) was approximately 39 years (range 20–88 years). There was a higher proportion of women in the depressive group relative to the non-depressive group. Moreover, income and education levels were lower in the depressive group than in the non-depressive group. Problematic alcohol consumption and changes in weight were more prevalent whereas regular exercise was lower in the depressive group relative to the non-depressive group. No seasonal variation in the reporting of depressive symptoms was noted.
Table 1.
Study participants’ characteristics according to the presence or absence of depressive symptoms*; adults (n 7198) aged 20–88 years, Fifth Korean National Health and Nutrition Examination Survey (KNHANES V, 2010–2012)
| Individuals without depressive symptoms† (n 6503) | Individuals with depressive symptoms (n 695) | ||||
|---|---|---|---|---|---|
| Characteristic | Mean or % | sd | Mean or % | sd | P value‡ |
| Age (years), mean and sd | 39·04 | 13·16 | 39·23 | 13·46 | 0·739 |
| Sex, female (%) | 39·3 | – | 55·5 | – | <0·001 |
| BMI (kg/m2), mean and sd | 23·52 | 3·31 | 23·21 | 3·46 | 0·099 |
| Marital status, married (%) | 30·5 | – | 31·6 | – | 0·648 |
| Income level (%) | 0·004 | ||||
| Lowest | 25·5 | – | 33·2 | – | |
| Lower middle | 25·5 | – | 24·5 | – | |
| Upper middle | 25·3 | – | 22·2 | – | |
| Highest | 23·7 | – | 20·1 | – | |
| Education level (%) | 0·002 | ||||
| Elementary school | 6·7 | – | 10·6 | – | |
| Middle school | 6·9 | – | 8·1 | – | |
| High school | 43·7 | – | 45·6 | – | |
| University | 42·8 | – | 35·7 | – | |
| Smoking status, current smoker (%) | 32·9 | – | 34·7 | – | 0·423 |
| Alcohol consumption (%) | <0·001 | ||||
| Normal user | 58·4 | – | 54·2 | – | |
| Hazardous user | 27·1 | – | 22·8 | – | |
| Problematic user | 14·4 | – | 23·0 | – | |
| Regular exercise§ (%) | 50·7 | – | 43·6 | – | 0·004 |
| Perceived body shape (%) | 0·278 | ||||
| Lean | 17·8 | – | 18·6 | – | |
| Normal | 40·2 | – | 36·4 | – | |
| Obese | 41·9 | – | 45·0 | – | |
| Changes in body weight (%) | <0·001 | ||||
| Weight loss | 12·5 | – | 17·4 | – | |
| Maintenance | 64·1 | – | 54·6 | – | |
| Weight gain | 23·4 | – | 28·1 | – | |
| Season (%) | 0·187 | ||||
| Spring | 23·4 | – | 26·9 | – | |
| Summer | 27·3 | – | 27·0 | – | |
| Autumn | 25·0 | – | 21·4 | – | |
| Winter | 24·3 | – | 24·8 | – | |
| 25(OH)D (ng/ml), mean and sd | 17·14 | 5·76 | 15·80 | 5·84 | <0·001 |
| Vitamin D deficiency (%) | 70·7 | – | 80·3 | – | <0·001 |
| Total cholesterol (mg/dl), mean and sd | 187·10 | 34·92 | 188·18 | 37·97 | 0·562 |
| TAG (mg/dl), mean and sd | 130·42 | 90·51 | 134·53 | 93·72 | 0·586 |
| HDL-C (mg/dl), mean and sd | 52·71 | 12·50 | 54·52 | 13·59 | 0·009 |
25(OH)D, serum 25-hydroxyvitamin D; HDL-C, HDL cholesterol.
Values are presented as means and standard deviations or as percentages.
Depressive symptoms were defined as feelings of sadness or despair that interfered with everyday life and persisted for longer than 2 weeks in the previous year.
The t test was used to analyse continuous variables and the χ 2 test was used to analyse categorical variables.
Regular exercise was defined as vigorous physical activity lasting ≥20 min at least three times per week, moderate physical activity lasting ≥30 min at least five times per week, or walking for ≥30 min more than five times per week.
With regard to biochemical characteristics, serum 25(OH)D was lower and the prevalence of vitamin D deficiency was higher in the depressive group than in the non-depressive group. Moreover, HDL-C levels were higher in the depressive group than in the non-depressive group. There were no significant differences in total cholesterol or TAG levels between the two groups.
Association between serum 25-hydroxyvitamin D and depressive symptoms, and the interaction of this association with total cholesterol level
We first analysed the association between vitamin D deficiency and depressive symptoms using a multiple logistic regression analysis. Odds ratios and 95 % confidence intervals are presented in Table 2. First, we analysed all participants independent of total cholesterol level. In the unadjusted model, there was a positive association between vitamin D deficiency and depressive symptoms (OR=1·69; 95 % CI 1·35, 2·11) and this positive association was preserved after adjusting for age, sex, BMI, alcohol consumption, smoking status, regular exercise, income level, education level, marital status, changes in body weight, perceived body shape, season and cholesterol profiles (OR=1·54; 95 % CI 1·20, 1·98). After dividing participants according to total cholesterol level, the positive association changed: the number of individuals with normal-to-borderline total cholesterol was 6673 while the number with high total cholesterol was 525. In the normal-to-borderline total cholesterol group (total cholesterol <240 mg/dl), the positive association between vitamin D deficiency and depressive symptoms was preserved (Model 1: OR=1·75; 95 % CI 1·38, 2·23, Model 2: OR=1·60; 95 % CI 1·23, 2·08); however, this association became non-significant in the high total cholesterol group (Model 1: OR=1·29; 95 % CI 0·69, 2·44, Model 2: OR=0·97; 95 % CI 0·52, 1·81). No changes in the association between vitamin D deficiency and depressive symptoms were observed when participants were divided according to HDL-C or TAG level.
Table 2.
Association between serum 25-hydroxyvitamin D (25(OH)D) and depressive symptoms according to serum total cholesterol (TC) level among adults (n 7198) aged 20–88 years, Fifth Korean National Health and Nutrition Examination Survey (KNHANES V, 2010–2012)
| Depressive symptoms | |||||||
|---|---|---|---|---|---|---|---|
| Model 1* | Model 2† | ||||||
| Category | 25(OH)D (ng/ml) | OR | 95 % CI | P value | OR | 95 % CI | P value‡ |
| All participants (n 7198) | <20 | 1·69 | 1·35, 2·11 | <0·001 | 1·54 | 1·20, 1·98 | <0·001 |
| ≥20§ | 1·00 | 1·00 | |||||
| TC<240 mg/dl group | <20 | 1·75 | 1·38, 2·23 | <0·001 | 1·60 | 1·23, 2·08 | 0·001 |
| (n 6673) | ≥20§ | 1·00 | 1·00 | ||||
| TC≥240 mg/dl group | <20 | 1·29 | 0·69, 2·44 | 0·426 | 0·97 | 0·52, 1·81 | 0·927 |
| (n 525) | ≥20§ | 1·00 | 1·00 | ||||
Unadjusted.
Adjusted for age, sex, BMI, alcohol consumption, smoking status, regular exercise, income level, education level, marital status, changes in body weight, perceived body shape, season of the examination date and cholesterol profiles.
A multiple logistic regression model was used.
Reference group.
Association between cholesterol profiles and depressive symptoms
We evaluated the association between cholesterol profiles and the presence of depressive symptoms using a multiple logistic regression analysis. The odds ratios and 95 % confidence intervals are presented in Table 3. No significant associations between cholesterol profiles and depressive symptoms were observed even after adjusting for confounding factors.
Table 3.
Associations between serum cholesterol profiles and depressive symptoms among adults (n 7198) aged 20–88 years, Fifth Korean National Health and Nutrition Examination Survey (KNHANES V, 2010–2012)
| Depressive symptoms | ||||||
|---|---|---|---|---|---|---|
| Model 1* | Model 2† | |||||
| Cholesterol profile | OR | 95 % CI | P value | OR | 95 % CI | P value‡ |
| Total cholesterol (mg/dl) | ||||||
| ≥240 | 1·32 | 0·93, 1·85 | 0·117 | 1·30 | 0·91, 1·85 | 0·146 |
| <240§ | 1·00 | 1·00 | ||||
| TAG (mg/dl) | ||||||
| ≥200 | 0·97 | 0·72, 1·29 | 0·557 | 1·04 | 0·77, 1·43 | 0·785 |
| <200§ | 1·00 | 1·00 | ||||
| HDL-C (mg/dl) | ||||||
| <40 | 0·87 | 0·65, 1·16 | 0·350 | 1·14 | 0·83, 1·55 | 0·423 |
| ≥40§ | 1·00 | 1·00 | ||||
HDL-C, HDL cholesterol.
Unadjusted.
Adjusted for age, sex, BMI, alcohol consumption, smoking status, regular exercise, income level, education level, marital status, changes in body weight, perceived body shape and season of the examination date.
A multiple logistic regression model was used.
Reference group.
Correlation between serum 25-hydroxyvitamin D and total cholesterol level
We evaluated the association between serum 25(OH)D and total cholesterol level using a multiple linear regression analysis. We also analysed the change of correlation between serum 25(OH)D and total cholesterol level according to total cholesterol status. The regression coefficients (β) between serum 25(OH)D and total cholesterol are presented in Table 4. In the unadjusted model, significant positive correlations were observed between serum 25(OH)D and total cholesterol for all participants (β=0·481, P<0·001) and in the normal-to-borderline total cholesterol group (β=0·413, P<0·001). However, these correlations became non-significant after adjusting for age, sex, BMI, alcohol consumption, smoking status, regular exercise, income, education and changes in body weight (β=0·034, P=0·714 v. β=0·080, P=0·288). Alternatively, there was a significant negative correlation between serum 25(OH)D and total cholesterol level in the high total cholesterol group that was preserved even after adjustment for the abovementioned factors (β=−0·480, P=0·038).
Table 4.
Correlation between serum 25-hydroxyvitamin D (25(OH)D) and serum total cholesterol (TC) level according to total cholesterol status among adults (n 7198) aged 20–88 years, Fifth Korean National Health and Nutrition Examination Survey (KNHANES V, 2010–2012)
| Model 1* | Model 2† | |||
|---|---|---|---|---|
| Category | β | P value | β | P value‡ |
| All participants (n 7198) | 0·481 | <0·001 | 0·034 | 0·714 |
| TC<240 mg/dl group (n 6673) | 0·413 | <0·001 | 0·080 | 0·288 |
| TC≥240 mg/dl group (n 525) | −0·408 | 0·058 | −0·480 | 0·038 |
β, regression coefficient.
Unadjusted.
Adjusted for age, sex, BMI, alcohol consumption, smoking status, regular exercise, income level, education level and changes in body weight.
A multiple linear regression model was used.
Discussion
In the current population-based cross-sectional study, we confirmed the presence of an association between vitamin D deficiency and depressive symptoms in a population of South Korean adults after controlling for confounding variables. Vitamin D-deficient persons with normal-to-borderline serum total cholesterol had an approximately 1·6-fold increased risk of depressive symptoms. Furthermore, we demonstrated that this association was absent in a sub-population of individuals with high serum total cholesterol. These data suggest that individuals with high serum total cholesterol may be less susceptible to the effects of vitamin D deficiency on mood status and particularly depression. Previous studies that have investigated serum 25(OH)D or total cholesterol in the context of depression have focused on only one factor and yielded inconsistent results( 10 , 11 , 44 ). These previous studies were limited by small sample sizes and confounding socio-economic and demographic factors. The population-based nature of our study and our consideration of a variety of confounding factors related to serum 25(OH)D, cholesterol status and depression therefore bolster the strength of our findings.
The underlying pathophysiology of depression remains unclear; however, several potential mechanisms have been put forth. Vitamin D receptors are abundant in many brain areas including the cingulate cortex and hippocampus. Vitamin D plays a role in neuroimmunomodulation, the regulation of neurotrophic factors, neuroprotection, neuroplasticity and brain development( 12 , 13 ). To this end, vitamin D has been hypothesized to play a direct role in the aetiology of depression.
Many studies have examined the relationship between serum cholesterol and depression. In some studies, individuals with depressive symptoms were noted to have lower serum total cholesterol( 14 , 15 ). One study suggested that lower total cholesterol might produce depression by altering the micro-viscosity of plasma membranes as a mechanism to decrease central serotonin transmission( 45 ). Alternatively, other studies have suggested that depression decreases serum total cholesterol by suppressing appetite and inhibiting cholesterol synthesis( 16 , 17 ). Dysregulation of the hypothalamic–pituitary–adrenal axis observed in depression can also alter cholesterol metabolism( 46 ). In the present study we failed to observe any relationship between serum total cholesterol and the presence of depressive symptoms.
It is known that both vitamin D and total cholesterol are factors relevant to depression; however, the correlation between these factors has been reported inconsistently in previous studies. While some studies reported a negative correlation( 23 , 47 , 48 ), others indicated a positive correlation between vitamin D and total cholesterol( 19 – 22 ). Vitamin D can influence serum cholesterol levels via a direct effect on adipogenesis( 49 , 50 ). Indirectly, vitamin D can increase intestinal calcium absorption and thus fat absorption( 51 , 52 ), which has reciprocal effects on serum parathyroid hormone and lipolysis( 53 ). Baseline total cholesterol level has also been identified as a determinant of 25(OH)D synthesis after UV-B exposure( 54 ). In our study, vitamin D and total cholesterol were negatively correlated only in individuals with high total cholesterol status. The reason for this observation is unclear; however, it may partly explain the inconsistent results of previous studies investigating the relationship between vitamin D and serum total cholesterol.
Although we did not observe a significant association between total cholesterol and depression symptoms in the present study, a recent meta-analysis confirmed the inverse association of total cholesterol with depression in a total of thirty previous studies( 18 ). Thus, we can suppose that both vitamin D and total cholesterol are independent factors related to depression in individuals with normal-to-borderline total cholesterol. However, our data suggest that in individuals with high total cholesterol as classified by the NCEP ATP III guideline, vitamin D and total cholesterol influence one another in a manner that alters their correlations with depression.
There were several limitations in the present study. First, the cross-sectional design of the study prohibited us from making conclusions regarding the causality of relationships among vitamin D deficiency, total cholesterol and depressive symptoms. The directionality of associations between these factors also remains unclear. A second limitation is related to the definition of depressive symptoms in our study. Individuals were included in the depressive group if they answered ‘yes’ to a questionnaire regarding the experience of depressive symptoms for a period of at least 2 weeks in the previous year. While previous studies have shown the validity of single-question screening for depression( 33 , 55 ), there still exists some controversy concerning this method( 56 ). Future studies should validate our findings through the use of well-accepted scoring systems for the diagnosis of depression. Third, other potential confounding factors affecting depressive symptoms, vitamin D and total cholesterol may have been overlooked in our study. Future analyses should consider information about dietary intake (energy and saturated fat intakes) and menopause status.
To the best of our knowledge, the present study is the first to examine the association of vitamin D deficiency and depressive symptoms according to serum total cholesterol status. As aforementioned, it is likely that the presence of vitamin D receptors in the brain contributes to the pathophysiology of depression and that total cholesterol can influence these processes. However, it was beyond the scope of our study to identify the definite mechanisms explaining our result. Nevertheless, our results have important implications for the use of vitamin D supplementation in vitamin D-deficient individuals with depression. In conclusion, our data indicate that high serum total cholesterol weakens the association between vitamin D deficiency and depressive symptoms in adults.
Acknowledgements
Acknowledgements: The authors are thankful to the Division of Chronic Disease Surveillance, Korea Centers for Disease Control and Prevention for providing the KNHANES V data. Financial support: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors. Conflict of interest: The authors declare no conflict of interest relevant to this article. Authorship: S.-H.L. drafted the article. K.-C.P. conceived and designed the study and takes full responsibility for the work as a whole. E.S., J.-H.H., K.K. and H.S.K. contributed to data interpretation and gave valuable suggestions and feedback. B.Y.W., K.-H.P., S.-H.L. and K.-S.P. analysed the data. M.-J.K., Y.-S.K. and B.C. revised the article critically for important intellectual content. Ethics of human subject participation: This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the Institutional Review Board of the Centers for Disease Control and Prevention in South Korea. Written informed consent was obtained from all subjects.
References
- 1. Dawson-Hughes B (2004) Racial/ethnic considerations in making recommendations for vitamin D for adult and elderly men and women. Am J Clin Nutr 80, 6 Suppl., 1763S–1766S. [DOI] [PubMed] [Google Scholar]
- 2. DeLuca HF (2004) Overview of general physiologic features and functions of vitamin D. Am J Clin Nutr 80, 6 Suppl., 1689S–1696S. [DOI] [PubMed] [Google Scholar]
- 3. Khazai N, Judd SE & Tangpricha V (2008) Calcium and vitamin D: skeletal and extraskeletal health. Curr Rheumatol Rep 10, 110–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Holick MF (2004) Vitamin D: importance in the prevention of cancers, type 1 diabetes, heart disease, and osteoporosis. Am J Clin Nutr 79, 362–371. [DOI] [PubMed] [Google Scholar]
- 5. Holick MF (2007) Vitamin D deficiency. N Engl J Med 357, 266–281. [DOI] [PubMed] [Google Scholar]
- 6. Przybelski RJ & Binkley NC (2007) Is vitamin D important for preserving cognition? A positive correlation of serum 25-hydroxyvitamin D concentration with cognitive function. Arch Biochem Biophys 460, 202–205. [DOI] [PubMed] [Google Scholar]
- 7. Teng M, Wolf M, Ofsthun MN et al. (2005) Activated injectable vitamin D and hemodialysis survival: a historical cohort study. J Am Soc Nephrol 16, 1115–1125. [DOI] [PubMed] [Google Scholar]
- 8. Shoji T, Shinohara K, Kimoto E et al. (2004) Lower risk for cardiovascular mortality in oral 1α-hydroxy vitamin D3 users in a haemodialysis population. Nephrol Dial Transplant 19, 179–184. [DOI] [PubMed] [Google Scholar]
- 9. Vacek JL, Vanga SR, Good M et al. (2012) Vitamin D deficiency and supplementation and relation to cardiovascular health. Am J Cardiol 109, 359–363. [DOI] [PubMed] [Google Scholar]
- 10. Wilkins CH, Sheline YI, Roe CM et al. (2006) Vitamin D deficiency is associated with low mood and worse cognitive performance in older adults. Am J Geriatr Psychiatry 14, 1032–1040. [DOI] [PubMed] [Google Scholar]
- 11. May HT, Bair TL, Lappe DL et al. (2010) Association of vitamin D levels with incident depression among a general cardiovascular population. Am Heart J 159, 1037–1043. [DOI] [PubMed] [Google Scholar]
- 12. Anglin RE, Samaan Z, Walter SD et al. (2013) Vitamin D deficiency and depression in adults: systematic review and meta-analysis. Br J Psychiatry 202, 100–107. [DOI] [PubMed] [Google Scholar]
- 13. Fernandes de Abreu DA, Eyles D & Feron F (2009) Vitamin D, a neuro-immunomodulator: implications for neurodegenerative and autoimmune diseases. Psychoneuroendocrinology 34, Suppl. 1, S265–S277. [DOI] [PubMed] [Google Scholar]
- 14. Tedders SH, Fokong KD, McKenzie LE et al. (2011) Low cholesterol is associated with depression among US household population. J Affect Disord 135, 115–121. [DOI] [PubMed] [Google Scholar]
- 15. Manfredini R, Caracciolo S, Salmi R et al. (2000) The association of low serum cholesterol with depression and suicidal behaviours: new hypotheses for the missing link. J Int Med Res 28, 247–257. [DOI] [PubMed] [Google Scholar]
- 16. Terao T, Nakamura J, Yoshimura R et al. (2000) Relationship between serum cholesterol levels and meta-chlorophenylpiperazine-induced cortisol responses in healthy men and women. Psychiatry Res 96, 167–173. [DOI] [PubMed] [Google Scholar]
- 17. Feingold KR & Grunfeld C (1992) Role of cytokines in inducing hyperlipidemia. Diabetes 41, Suppl. 2, 97–101. [DOI] [PubMed] [Google Scholar]
- 18. Shin JY, Suls J & Martin R (2008) Are cholesterol and depression inversely related? A meta-analysis of the association between two cardiac risk factors. Ann Behav Med 36, 33–43. [DOI] [PubMed] [Google Scholar]
- 19. Jorde R, Figenschau Y, Hutchinson M et al. (2010) High serum 25-hydroxyvitamin D concentrations are associated with a favorable serum lipid profile. Eur J Clin Nutr 64, 1457–1464. [DOI] [PubMed] [Google Scholar]
- 20. Kumar J, Muntner P, Kaskel FJ et al. (2009) Prevalence and associations of 25-hydroxyvitamin D deficiency in US children: NHANES 2001–2004. Pediatrics 124, e362–e370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Giovannucci E, Liu Y, Hollis BW et al. (2008) 25-Hydroxyvitamin D and risk of myocardial infarction in men: a prospective study. Arch Intern Med 168, 1174–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bolland MJ, Bacon CJ, Horne AM et al. (2010) Vitamin D insufficiency and health outcomes over 5 y in older women. Am J Clin Nutr 91, 82–89. [DOI] [PubMed] [Google Scholar]
- 23. Melamed ML, Michos ED, Post W et al. (2008) 25-Hydroxyvitamin D levels and the risk of mortality in the general population. Arch Intern Med 168, 1629–1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dixon JB, Dixon ME & O’Brien PE (2003) Depression in association with severe obesity: changes with weight loss. Arch Intern Med 163, 2058–2065. [DOI] [PubMed] [Google Scholar]
- 25. Kim O & Kim K (2001) Body weight, self-esteem, and depression in Korean female. Adolescence 36, 315–322. [PubMed] [Google Scholar]
- 26. Strohle A (2009) Physical activity, exercise, depression and anxiety disorders. J Neural Transm (Vienna) 116, 777–784. [DOI] [PubMed] [Google Scholar]
- 27. Blazer DG, Kessler RC, McGonagle KA et al. (1994) The prevalence and distribution of major depression in a national community sample: the National Comorbidity Survey. Am J Psychiatry 151, 979–986. [DOI] [PubMed] [Google Scholar]
- 28. Chobanian AV, Bakris GL, Black HR et al. (2003) Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 42, 1206–1252. [DOI] [PubMed] [Google Scholar]
- 29. Saunders JB, Aasland OG, Babor TF et al. (1993) Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption – II. Addiction 88, 791–804. [DOI] [PubMed] [Google Scholar]
- 30. Dawson-Hughes B, Heaney RP, Holick MF et al. (2005) Estimates of optimal vitamin D status. Osteoporos Int 16, 713–716. [DOI] [PubMed] [Google Scholar]
- 31. National Cholesterol Education Program Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) (2002) Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 106, 3143–3421. [PubMed] [Google Scholar]
- 32. Watkins C, Daniels L, Jack C et al. (2001) Accuracy of a single question in screening for depression in a cohort of patients after stroke: comparative study. BMJ 323, 1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Le Strat Y & Dubertret C (2013) A single question to screen for major depression in the general population. Compr Psychiatry 54, 831–834. [DOI] [PubMed] [Google Scholar]
- 34. Rhee SY, Hwang YC, Chung HY et al. (2012) Vitamin D and diabetes in Koreans: analyses based on the Fourth Korea National Health and Nutrition Examination Survey (KNHANES), 2008–2009. Diabetic Med 29, 1003–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Saliba W, Barnett-Griness O & Rennert G (2013) The relationship between obesity and the increase in serum 25(OH)D levels in response to vitamin D supplementation. Osteoporos Int 24, 1447–1454. [DOI] [PubMed] [Google Scholar]
- 36. Cutillas-Marco E, Fuertes-Prosper A, Grant WB et al. (2012) Vitamin D deficiency in South Europe: effect of smoking and aging. Photodermatol Photoimmunol Photomed 28, 159–161. [DOI] [PubMed] [Google Scholar]
- 37. Lorant V, Deliege D, Eaton W et al. (2003) Socioeconomic inequalities in depression: a meta-analysis. Am J Epidemiol 157, 98–112. [DOI] [PubMed] [Google Scholar]
- 38. Thuesen B, Husemoen L, Fenger M et al. (2012) Determinants of vitamin D status in a general population of Danish adults. Bone 50, 605–610. [DOI] [PubMed] [Google Scholar]
- 39. Alvisa-Negrin J, Gonzalez-Reimers E, Santolaria-Fernandez F et al. (2009) Osteopenia in alcoholics: effect of alcohol abstinence. Alcohol Alcohol 44, 468–475. [DOI] [PubMed] [Google Scholar]
- 40. Wamala SP, Wolk A, Schenck-Gustafsson K et al. (1997) Lipid profile and socioeconomic status in healthy middle aged women in Sweden. J Epidemiol Community Health 51, 400–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Whitehead TP, Robinson D & Allaway SL (1996) The effects of cigarette smoking and alcohol consumption on blood lipids: a dose-related study on men. Ann Clin Biochem 33, 99–106. [DOI] [PubMed] [Google Scholar]
- 42. Leenen R, van der Kooy K, Meyboom S et al. (1993) Relative effects of weight loss and dietary fat modification on serum lipid levels in the dietary treatment of obesity. J Lipid Res 34, 2183–2191. [PubMed] [Google Scholar]
- 43. Monda KL, Ballantyne CM & North KE (2009) Longitudinal impact of physical activity on lipid profiles in middle-aged adults: the Atherosclerosis Risk in Communities Study. J Lipid Res 50, 1685–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pan A, Lu L, Franco OH et al. (2009) Association between depressive symptoms and 25-hydroxyvitamin D in middle-aged and elderly Chinese. J Affect Disord 118, 240–243. [DOI] [PubMed] [Google Scholar]
- 45. Engelberg H (1992) Low serum cholesterol and suicide. Lancet 339, 727–729. [DOI] [PubMed] [Google Scholar]
- 46. Chrousos GP (2000) The role of stress and the hypothalamic–pituitary–adrenal axis in the pathogenesis of the metabolic syndrome: neuro-endocrine and target tissue-related causes. Int J Obes Relat Metab Disord 24, Suppl. 2, S50–S55. [DOI] [PubMed] [Google Scholar]
- 47. Delvin EE, Lambert M, Levy E et al. (2010) Vitamin D status is modestly associated with glycemia and indicators of lipid metabolism in French-Canadian children and adolescents. J Nutr 140, 987–991. [DOI] [PubMed] [Google Scholar]
- 48. Gaddipati VC, Bailey BA, Kuriacose R et al. (2011) The relationship of vitamin D status to cardiovascular risk factors and amputation risk in veterans with peripheral arterial disease. J Am Med Dir Assoc 12, 58–61. [DOI] [PubMed] [Google Scholar]
- 49. Dace A, Martin-el Yazidi C, Bonne J et al. (1997) Calcitriol is a positive effector of adipose differentiation in the OB 17 cell line: relationship with the adipogenic action of triiodothyronine. Biochem Biophys Res Commun 232, 771–776. [DOI] [PubMed] [Google Scholar]
- 50. Shi H, Norman AW, Okamura WH et al. (2002) 1α,25-Dihydroxyvitamin D3 inhibits uncoupling protein 2 expression in human adipocytes. FASEB J 16, 1808–1810. [DOI] [PubMed] [Google Scholar]
- 51. Welberg JW, Monkelbaan JF, de Vries EG et al. (1994) Effects of supplemental dietary calcium on quantitative and qualitative fecal fat excretion in man. Ann Nutr Metab 38, 185–191. [DOI] [PubMed] [Google Scholar]
- 52. Christensen R, Lorenzen JK, Svith CR et al. (2009) Effect of calcium from dairy and dietary supplements on faecal fat excretion: a meta-analysis of randomized controlled trials. Obes Rev 10, 475–486. [DOI] [PubMed] [Google Scholar]
- 53. Zemel MB, Shi H, Greer B et al. (2000) Regulation of adiposity by dietary calcium. FASEB J 14, 1132–1138. [PubMed] [Google Scholar]
- 54. Bogh MK, Schmedes AV, Philipsen PA et al. (2010) Vitamin D production after UVB exposure depends on baseline vitamin D and total cholesterol but not on skin pigmentation. J Invest Dermatol 130, 546–553. [DOI] [PubMed] [Google Scholar]
- 55. Watkins CL, Lightbody CE, Sutton CJ et al. (2007) Evaluation of a single-item screening tool for depression after stroke: a cohort study. Clin Rehabil 21, 846–852. [DOI] [PubMed] [Google Scholar]
- 56. Blozik E, Scherer M, Lacruz ME et al. (2013) Diagnostic utility of a one-item question to screen for depressive disorders: results from the KORA F3 study. BMC Fam Pract 14, 198. [DOI] [PMC free article] [PubMed] [Google Scholar]