Abstract
Objective
Observational studies reported potential associations between different dietary patterns and the risk of metabolic syndrome (MetS); however, a consistent perspective has not been established to date. The current systematic review and meta-analysis aimed to evaluate the relationship between a posteriori dietary patterns and MetS by pooling available data.
Design
MEDLINE and EMBASE databases were searched for relevant articles published up to July 2015 with no time restriction and with English language restriction. Two independent reviewers completed study selection and data extraction. Random-effects models (DerSimonian–Laird method) were used to pool effect sizes of eligible studies. The potential sources of heterogeneity were assessed using the I 2 statistic.
Results
Nineteen papers that identified dietary patterns using an a posteriori method were selected and included in the meta-analysis. The ‘Healthy/Prudent’ dietary pattern was inversely associated with risk of MetS (OR=0·89; 95 % CI 0·84, 0·94, P=0·002). In contrast, the ‘Unhealthy/Western’ dietary pattern had a significant positive association with risk of MetS (OR=1·16; 95 % CI 1·11, 1·22, P<0·001).
Conclusions
Our findings provide evidence that greater adherence to a healthy/prudent dietary pattern is associated with a lower risk of MetS, while an unhealthy/Western dietary pattern is associated with increased risk of MetS. These data suggest that a diet based on healthy food choices is also beneficial for prevention of MetS.
Keywords: Metabolic syndrome, Dietary pattern, Systematic review, Meta-analysis
The metabolic syndrome (MetS) is a cluster of metabolic disorders including abdominal obesity, insulin resistance, hyperglycaemia, dislipidaemia and hypertension( 1 ). It is reported that MetS increases risk of atherosclerotic CVD by threefold and is associated with all-cause mortality( 2 ). It is estimated that 20–25 % of adults suffer from MetS worldwide( 1 ).
Previous investigations have assessed the relationship between consumption of specific foods or nutrients and risk of MetS( 3 – 7 ); however, this assessment may have some limitations. First, people generally consume a combination of various foods and nutrients in each meal, not individual foods and nutrients. Second, single foods and nutrients do not show the interactive and synergistic effects of different foods and nutrients in a diet. Third, there is high correlation between many different nutrients and hence detection of an independent influence is hard. Therefore, another approach called ‘dietary pattern analysis’ has been developed to evaluate the effect of whole diets (instead of specific foods or nutrients) on incidence of chronic diseases. Dietary patterns can be determined using either the a priori or a posteriori approach. A priori approaches such as dietary indices are based on scientific knowledge, while a posteriori approaches such as factor analysis, principal component analysis (PCA) and cluster analysis are based on statistical methods.
Accumulating evidence suggests that there is a relationship between a posteriori dietary patterns and the risk of MetS( 8 – 11 ). However, findings vary substantially across studies. Some studies have found that dietary patterns characterized by high intakes of vegetables, fruits and fish are inversely associated with MetS( 8 ), whereas dietary patterns characterized by high intakes of red meat, processed meat, refined grains, alcohol and fried foods are associated with increased MetS risk( 9 ). In contrast, some studies did not detect a significant association between a dietary pattern characterized by meat and alcohol and MetS( 10 , 11 ).
Therefore, we aimed to conduct a systematic review and meta-analysis of observational studies to assess the association between a posteriori dietary patterns and the risk of MetS in people aged 18–60 years.
Methods
Search strategies
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement was used to conduct the present systematic review and meta-analysis( 12 ). An electronic search for observational studies that investigated the association between a posteriori dietary patterns and risk of MetS was conducted in MEDLINE and EMBASE databases up to July 2015 with no time restriction. Search terms for PubMed included: dietary pattern* (tiab) OR eating pattern* (tiab) OR food* pattern* (tiab) OR dietary habit (tiab) OR dietary (tiab) AND factor analysis (tiab) OR principal component analysis (tiab) AND ‘metabolic syndrome X’ (MeSH) OR metabolic syndrome (tiab) OR MetS (tiab) OR MS (tiab) OR syndrome X (tiab) OR cardio metabolic risk factor (tiab) OR insulin resistance syndrome. (tiab) searches the title and abstract fields only, (MeSH) searches the Medical Subject Headings field only, and the truncation symbol * searches all words with this combination of letters at the beginning. The search strategy was adapted for the other database to fit its specific features. Reference lists of review articles were also checked to identify relevant studies.
Eligibility and study selection
The PICOS (population, intervention, comparator, outcome, study design) framework shown in Table 1 was used. All retrieved articles in the initial search were read independently by two reviewers (M.H. and S.S-B.). Any disagreements were discussed and resolved by consensus or a third independent reviewer (S.M.) if necessary.
Table 1.
Description of the PICOS (population, intervention, comparator, outcome, study design) criteria used to define the research question
| Parameter | Description |
|---|---|
| Participants | Include: presumably healthy adults |
| Intervention/correlate | Dietary patterns |
| Comparison | Not applicable; observational studies were reviewed |
| Outcome | Metabolic syndrome |
| Study design | Include: cross-sectional and case–control studies |
| Exclude: letters, editorials, commentaries |
Relevant articles were included in the meta-analysis if they: (i) identified dietary patterns with factor analysis or PCA; (ii) reported an odds ratio (OR) or risk ratio (RR) for MetS; (iii) were conducted on adults (18–60 years old); (iv) presented their results in percentiles; and (v) used varimax rotation in their methodology. We included only those articles using a posteriori dietary patterns derived by PCA or factor analysis. Studies were excluded if they: (i) examined only individual nutrients or foods; (ii) did not report an OR or RR for MetS; (iii) comprised study samples that were not population based or focused only on a subgroup of individuals with nutritional needs that are different from the general population, including pregnant or lactating women, infants, children or adolescents; (iv) were animal studies; (v) were randomized clinical trials, reviews, case reports, conference and letters; (vi) applied cluster or reduced rank reduction analysis; and (vii) were dissertations.
Data extraction
The following information was extracted from included studies: first author, publication year and country, study design, sampling frame, sample size, number of cases and controls (if available), dietary assessment tool (FFQ or 24h recall), method of identifying dietary patterns, dietary patterns identified, confounders adjusted for in the analysis and main findings, including estimates of the association. When a study provided several estimates with adjustment for different confounders, results were reported for the one adjustment that covered the largest number of factors. Two reviewers independently performed the data extraction and settled differences by consensus. Where further detail was required, we contacted study authors for additional information.
Data synthesis
As all patterns used in the meta-analysis were extracted using varimax rotation, the method which leads to independent components, they can be used in the same model at the same time without having any issues regarding collinearity. Therefore, we extracted patterns and combined them based on the independence hypothesis of patterns and reported two types of patterns separately. We identified the two types of dietary patterns as healthy and unhealthy, which were considered for meta-analysis based on whether they had generally healthy characteristics or not. Because the labelling of dietary patterns varied across studies, so long as the selected patterns were similar with regard to the most frequently consumed foods, these studies were grouped and analysed together regardless of their original label. For example, most studies examined dietary patterns with high factor loadings for fruit and vegetables, fish and whole grains; these studies were pooled and analysed together and the corresponding overall dietary pattern was labelled ‘Healthy/Prudent’. The classification of each food was based on the recommendations of different consensus dietary guidelines such as the seventh edition of the Dietary Guidelines for Americans( 13 ).
Statistical analysis
We used OR of tertiles, quartiles and quintiles among included studies which reported adherence to a posteriori dietary patterns. Then, the pooled OR was used for the highest adherence to each dietary pattern (‘Healthy/Prudent’ and ‘Unhealthy/Western’) in comparison to the lowest adherence to assess the association between dietary patterns and risk of MetS. To pool the OR or RR for dietary patterns, the random-effects model (DerSimonian–Laird method) was used employing the user-written ‘metan’ command in the statistical software package Stata version 11( 14 ). Heterogeneity of studies was determined using Cochrane’s Q test (significant with a P value of <0·10) and the I 2 statistic. I 2 value equal to 25, 50 and 75 % was considered low, moderate and high level of heterogeneity, respectively( 15 ). The 95 % CI were calculated for each I 2 statistic using the ‘heterogi’ command in Stata( 16 ). We also estimated the between-study variance using the τ 2 statistic( 17 ). Outliers were identified through visual inspection of the forest plots. Then, we performed sensitivity analysis by excluding two studies( 10 , 18 ) as outliers. Sensitivity analyses were carried out by disaggregating results with the user-written ‘metan’ command in Stata version 11( 14 ). Publication bias was assessed using funnel plots and Egger’s asymmetry tests with the user-written ‘metabias’ command in Stata version 11( 14 , 19 ). All statistical analyses were conducted using Stata version 11.
Results
Study selection
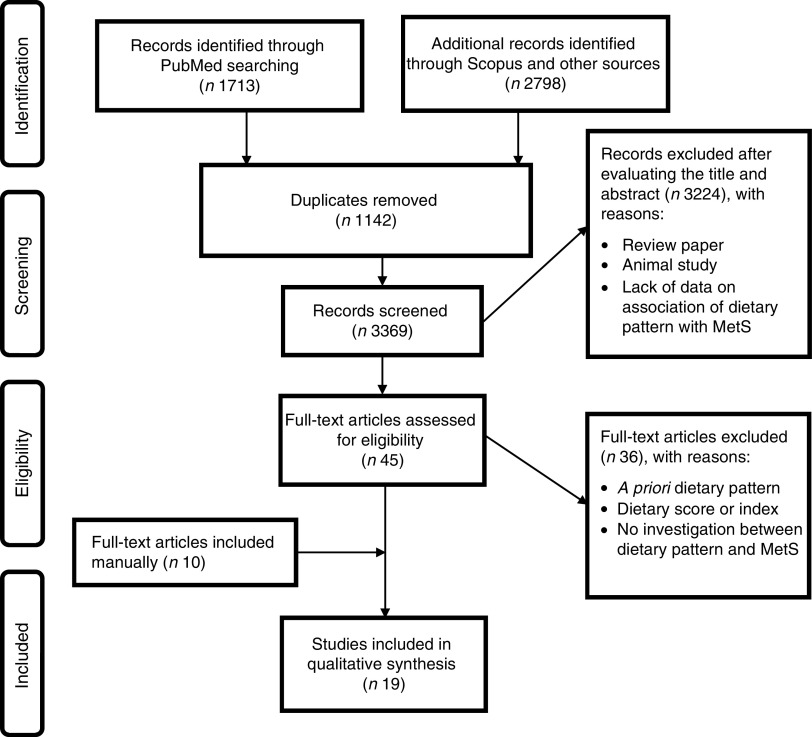
During the initial search 4511 papers were identified, of those 1142 duplicate articles were found. After screening the title and abstract, 3324 articles were excluded and forty-five papers were retrieved for full-text review. Thirty-seven articles were excluded due to lack of information and eleven studies were added manually; thus nineteen cross-sectional studies were included in our meta-analysis. The included articles were published between 2007 to 2015( 9 , 10 , 18 , 20 – 35 ). If studies had several dietary patterns, each extracted dietary pattern considered healthy or unhealthy was included separately; hence twenty-two healthy and twenty-seven unhealthy dietary patterns were included in the meta-analysis. The flowchart of the literature search is shown in Fig. 1.
Fig. 1.
Summary of the study methodology, processes of review, and outcomes of inclusion and exclusion criteria for the present systematic review and meta-analysis on a posteriori dietary patterns and metabolic syndrome (MetS)
Study characteristics
Characteristics of the included studies are shown in Table 2. Included studies were conducted in the USA( 21 , 23 , 24 , 26 , 31 ), Mexico( 28 ), Japan( 18 ), South Korea( 10 , 20 , 29 , 33 , 34 ), Iran( 8 , 27 ) Lebanon( 32 ) and Europe( 9 , 25 , 30 , 35 ). In fifteen studies( 8 , 18 , 21 , 23 – 29 , 31 – 35 ) an FFQ was used to assess dietary intake, while the rest of them( 9 , 10 , 20 , 30 ) used a 24 h recall or food record diary to collect dietary intake. In all studies, dietary patterns were derived using PCA and factor analysis. Regardless of differences in confounding variables and their categorizations among the included studies, the effect size was adjusted for major potential confounding variables including age, sex, BMI, education, energy intake and physical activity in all studies.
Table 2.
Descriptions of the studies included in the present systematic review and meta-analysis on a posteriori dietary patterns (DP) and metabolic syndrome
| Author | Year | Country | Sample size | Study design | Sex | Age (years) | Pattern name | DP assessment | DP method | DP component | Factors adjusted for |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Esmaillzadeh et al.( 8 ) | 2007 | Iran | 388 | Cross-sectional | F | 40–60 | Healthy | FFQ | PCA | Fruits, tomatoes, poultry, legumes, cruciferous and green leafy vegetables, other vegetables, tea, fruit juices and whole grains | Age, PA, smoking, menopausal status, total EI, current oestrogen use |
| Esmaillzadeh et al.( 8 ) | 2007 | Iran | 388 | Cross-sectional | F | 40–60 | Western | FFQ | PCA | Refined grains, red meat, butter, processed meat, high-fat dairy products, sweets and desserts, pizza, potatoes, eggs, hydrogenated fats and soft drinks, and low in other vegetables and low-fat dairy products | As above |
| Esmaillzadeh et al.( 8 ) | 2007 | Iran | 388 | Cross-sectional | F | 40–60 | Traditional | FFQ | PCA | Refined grains, potatoes, tea, whole grains, hydrogenated fats, legumes and broth | As above |
| Lutsey et al.( 26 ) | 2008 | USA | 9514 | Cohort | M/F | 45–64 | Prudent | FFQ | PCA | Cruciferous vegetables, fruit (no juice), other vegetables, fish and seafood, poultry, dark leafy vegetables, whole grains, tomatoes, legumes, low-fat dairy, yoghurt, nuts and peanut butter, fruit juice, potatoes, carotenoid vegetables | Age, sex, current smoker, (packs/year), PA, race, centre, education, EI, behavioural characteristics, AHA guidelines |
| Lutsey et al.( 26 ) | 2008 | USA | 9514 | Cohort | M/F | 45–64 | Western | FFQ | PCA | Refined-grain bread/cereal/rice/pasta, processed meat, fried foods, red meat, eggs, refined-grain desserts, soda and sweetened beverages, cheese and whole milk, legumes, sweets/candy, other vegetables, potatoes, ice cream, yoghurt | As above |
| DiBello et al.( 23 ) (a) | 2009 | Samoan Islands | 366 | Cross-sectional | M/F | 18 | Neo-traditional | FFQ | PCA | Crab and lobster, fish, coconut cream dishes, papaya soup, coconut milk, papaya and taro, and low intakes of sausage, potato chips, Coca Cola, rice and instant noodle soup | Age, sex, modern lifestyle score, current smoking status, PA and total EI |
| DiBello et al.( 23 ) (a) | 2009 | Samoan Islands | 366 | Cross-sectional | M/F | 18 | Factor 2 | FFQ | PCA | A mix of meat and coconut products such as coconut cream dishes and lamb | As above |
| DiBello et al.( 23 ) (a) | 2009 | Samoan Islands | 366 | Cross-sectional | M/F | 18 | Modern | FFQ | PCA | Sausage, eggs, milk, cheese, coconut cream, rice, instant noodle soup, bread, pancakes, cereal, butter/margarine, cake and potato chips, and low intakes of fish, crab, lobster and breadfruit | As above |
| DiBello et al.( 23 ) (b) | 2009 | Samoan Islands | 545 | Cross-sectional | M/F | 18 | Neo-traditional | FFQ | PCA | Crab and lobster, fish, coconut cream dishes, papaya soup, coconut milk, papaya and taro, and low intakes of sausage, potato chips, Coca Cola, rice and instant noodle soup | As above |
| DiBello et al.( 23 ) (b) | 2009 | Samoan Islands | 545 | Cross-sectional | M/F | 18 | Factor 2 | FFQ | PCA | A mix of meat and coconut products such as coconut cream dishes and lamb | As above |
| DiBello et al.( 23 ) (b) | 2009 | Samoan Islands | 545 | Cross-sectional | M/F | 18 | Modern pattern | FFQ | PCA | Sausage, eggs, milk, cheese, coconut cream, rice, instant noodle soup, bread, pancakes, cereal, butter/margarine, cake and potato chips, and low intakes of fish, crab, lobster and breadfruit | As above |
| Noel et al.( 24 ) | 2009 | USA | 1167 | Longitudinal | M/F | 45–75 | Meat, processed meat | FFQ | PCA | Meat, processed meat, French fries, pizza and Mexican foods, eggs, alcohol, and other grains and pasta | Age, sex, smoking, alcohol use, education, PA, total EI, multivitamin use, medication use (lipid-lowering medications) |
| Noel et al.( 24 ) | 2009 | USA | 1167 | Longitudinal | M/F | 45–75 | Rice, beans, and oils | FFQ | PCA | Beans and legumes, rice and oil, and low in high-fat dairy, condiments, and nuts and seeds | As above |
| Noel et al.( 24 ) | 2009 | USA | 1167 | Longitudinal | M/F | 45–75 | Sweets, sugared | FFQ | PCA | Candy, sugar and chocolate candy, soft drinks, sugary beverages, sweet baked goods, dairy desserts and salty snacks | As above |
| Amini et al.( 27 ) | 2010 | Iran | 425 | Cross-sectional | M/F | 35–55 | Prudent | FFQ | PCA | Hydrogenated fat, vegetable oil, liver and organic meat, coconut, juice, peas, barley, non-leafy vegetables, dry fruits, nuts, honey | Age, sex, PA, education |
| Amini et al.( 27 ) | 2010 | Iran | 425 | Cross-sectional | M/F | 35–55 | Western | FFQ | PCA | Sweets, butter, soda, mayonnaise, mutton, juice macaroni, vegetable oil, liver and organic meat, coconut sugar, cookies, tail, hydrogenated fat, egg | As above |
| Denova-Gutiérrez et al.( 28 ) | 2010 | Mexico | 5240 | Cross-sectional | M/F | 20–70 | Prudent | FFQ | PCA | Processed vegetable juices, potatoes, fresh fruits, fresh vegetables, legumes, pastry, fruit juice | Age, sex, current smoker, PA, weight change, place of residence, oestrogen use, menopausal status, EI |
| Denova-Gutiérrez et al.( 28 ) | 2010 | Mexico | 5240 | Cross-sectional | M/F | 20–70 | Western | FFQ | PCA | Legumes, refined cereals, whole cereals, seafood, high-fat dairy products, low-fat dairy products, corn tortilla, sodas | As above |
| Cho et al.( 29 ) | 2011 | South Korea | 4984 | Cross-sectional | F | 30–79 | Healthy | FFQ | PCA | Fried foods, cholesterol-rich foods, green/yellow vegetables, healthy protein foods, seaweeds, bony fish, fruits, dairy products, light-coloured vegetables | Age, menopausal status |
| Cho et al.( 29 ) | 2011 | South Korea | 4984 | Cross-sectional | M/F | 30–79 | Western | FFQ | PCA | Fast foods, animal fat-rich foods, fried foods, grilled meat and seafoods, sweet foods, cholesterol-rich foods, caffeinated drinks | As above |
| Heidemann et al.( 9 ) | 2011 | Germany | 4025 | Cross-sectional | M/F | 50 | Processed foods | Recall | PCA | Refined grains, processed meat, red meat, high-sugar beverages, eggs, potatoes, beer, sweets and cakes, snacks and butter | Age (years), sex (where applicable) and total EI (continuous) |
| Heidemann et al.( 9 ) | 2011 | Germany | 4025 | Cross-sectional | M/F | 50 | Health-conscious | Recall | PCA | Cruciferous vegetables, fruity vegetables, leafy vegetables, all other vegetables, vegetable oils, legumes, fruits, fish and whole grains | As above |
| Kim et al.( 20 ) | 2011 | South Korea | 9850 | Cohort | M/F | 40–69 | White rice and kimchi | Recall | PCA | Vegetables, seaweeds, mushrooms, soya products, salt | Age, sex, BMI, EI, % energy as carbohydrate, alcohol intake, smoking status and PA |
| Kim and Jo( 20 ) | 2011 | South Korea | 9850 | Cohort | M/F | 40–69 | Meat and alcohol | Recall | PCA | Meat and processed meat, seafood | As above |
| Kim and Jo( 20 ) | 2011 | South Korea | 9850 | Cohort | M/F | 40–69 | High fat, sweets and coffee | Recall | PCA | Rice and miso soup, natto (fermented soyabean) | As above |
| Kim and Jo( 20 ) | 2011 | South Korea | 9850 | Cohort | M/F | 40–69 | Grains, vegetables and fish | Recall | PCA | Fruit, vegetables and whole grains | As above |
| Hong et al.( 10 ) | 2012 | South Korea | 460 | Cross-sectional | M/F | 22–78 | Korean traditional | 1×24 h recall and 3 d of food | PCA | Refined and whole grains, Korean seasonings, onions and garlic, vegetable oil, soya products, starch syrup and sugar | Age, sex, taking medications, BMI and EI |
| Hong et al.( 10 ) | 2012 | South Korea | 460 | Cross-sectional | M/F | 22–78 | Alcohol and meat | 1×24 h recall and 3 d of food | PCA | Processed meats, eggs, fish paste, animal fat, and alcohol | As above |
| Hong et al.( 10 ) | 2012 | South Korea | 460 | Cross-sectional | M/F | 22–78 | Sweets and fast foods | 1×24 h recall and 3 d of food | PCA | Fruit juices, chocolate, ice cream, pizza and hamburgers | As above |
| Hong et al.( 10 ) | 2012 | South Korea | 460 | Cross-sectional | M/F | 22–78 | Fruit and dairy | 1×24 h recall and 3 d of food | PCA | Leafy vegetables, fruits and dairy products | As above |
| Wagner et al.( 30 ) | 2012 | France | 3091 | Cross-sectional | Women | 35–74 | Energy-dense | 3 d food diary | PCA | Delicatessen foods, red meat, fruits potatoes, yoghourt, animal fat (butter), sauce and condiments, water, sodas, alcohol | Age, sex, current smoker, BMI, total EI, heart rate (PA), educational level, menopause |
| Wagner et al.( 30 ) | 2012 | France | 3091 | Cross-sectional | Women | 35–74 | Convenience food | 3 d food diary | PCA | Grains, pasta, rice, fruits, vegetables, cheese, cream, cake, junk food, water, fruit and vegetable juices, sodas, diet sodas, commercially cooked or prepared dishes, fresh products prepared at home, pizza, quiches, sausage rolls, pies, etc. | As above |
| Akter et al.( 18 ) | 2013 | Japan | 460 | Cross-sectional | M/F | 21–67 | Healthy Japanese | FFQ | PCA | Vegetables and fruits, soya products mushrooms, and green tea | Age (years, continuous), sex, workplace (A or B), marital status (married or unmarried), job position (low or middle and high), occupational PA (sedentary or active work), current smoking (yes or no) and non-occupational PA (0, >0 to <2 or ≥2 h/week) |
| Akter et al.( 18 ) | 2013 | Japan | 460 | Cross-sectional | M/F | 21–67 | Animal food | FFQ | PCA | Fish and shellfish, meat, processed meat, mayonnaise and egg | As above |
| Akter et al.( 18 ) | 2013 | Japan | 460 | Cross-sectional | M/F | 21–67 | Westernized breakfast | FFQ | PCA | Bread, confectioneries, milk and yoghurt, mayonnaise and egg, and low intakes of rice, alcohol and fish | As above |
| Liu et al.( 31 ) | 2013 | USA | 1775 | Cross-sectional | M/F | 21–94 | Prudent | FFQ | PCA | Cold cereal, dairy desserts, fruit juice, fruit, hot cereal, milk and dairy, nuts and seeds | Age, sex, current smoker, alcohol consumption, PA, education |
| Liu et al.( 31 ) | 2013 | USA | 1775 | Cross-sectional | M/F | 21–94 | Southern | FFQ | PCA | Beans and legumes, bread, chicken and turkey, corn and corn products, eggs, fast food, margarine and butter, meat, miscellaneous fats, organ meats, vegetables, processed meats and poultry, rice and pasta, seafood, soups, potato | As above |
| Naja et al.( 32 ) | 2013 | Lebanon | 323 | Cross-sectional | M/F | >18 | Traditional | FFQ | FA | Desserts, dairy products full-fat, olives, fruits, legumes, grains, eggs, vegetable oil, nuts and dried fruits, traditional sweets, vegetables, dairy products low-fat | Age, sex, current smoker, PA, marital status, education, crowding index |
| Naja et al.( 32 ) | 2013 | Lebanon | 323 | Cross-sectional | M/F | >18 | Fast food/desserts | FFQ | FA | Hamburger, shawarma, pizza and pies, falafel, sandwiches, desserts, carbonated beverages and juices, mayonnaise, butter, alcoholic beverages, fruits, grains, eggs, nuts and dried fruits, chicken, meat | As above |
| Barbaresko et al.( 25 ) | 2014 | Germany | 905 | Cohort | M/F | 25–82 | Unhealthy | FFQ | PCA | Leafy vegetables, fruiting vegetables, root vegetables, cabbage, other vegetables, beef, pork, processed meat, vegetable oil, other fats, sauce and bouillon | Sex, age (years), education (9, 10 or ≥11 years), smoking status (never, former or current smoker), PA (MET-h/week), total EI (kJ/d) and study cohort (random sample of the general population or blood donors) |
| Woo et al.( 33 ) | 2014 | Korea | 1257 | Cross-sectional | M/F | 31–70 | Traditional | FFQ | PCA | Condiments, green and yellow vegetables, light-coloured vegetables, tubers, clams, tofu, soya milk, seaweeds, bony fish, kimchi, lean fish, mushrooms, fruits, nuts, legumes, yoghurt, eggs, pickled vegetables, milk, red meat, other seafood | Age, sex, current smoker, alcohol consumption, PA, total EI |
| Woo et al.( 33 ) | 2014 | Korea | 1257 | Cross-sectional | M/F | 31–70 | Meat | FFQ | PCA | Light-coloured vegetables, clams, lean fish, mushrooms, red meat, red meat by-products, other seafood, high-fat red meat, oil, salted fermented seafood, noodles, poultry, fatty fish, carbonated beverages, dairy products, processed meats, sweets, coffee, tea | As above |
| Yoo et al.( 34 ) | 2014 | Korea | 16 734 | Cross-sectional | M/F | >18 | Dairy-cereal | FFQ | FA | Refined grains, kimchi, dairy, fruit cereal snack, bread, jam | Age, sex, current smoker, alcohol consumption, PA, education, household income, obesity variables, EI, nutrient intake (carbohydrate, protein, fat, crude fibre, Na) |
| Gadgil et al.( 21 ) | 2015 | USA | 892 | Cohort | M/F | 40–84 | Animal protein | FFQ | PCA | Alcohol, coffee, eggs, fish, legumes, low-fat dairy, pasta, pizza, poultry, red meat, refined grains, vegetable oil, whole grains | Age, sex, study site and total EI |
| Gadgil et al.( 21 ) | 2015 | USA | 892 | Cohort | M/F | 40–84 | Fried snacks, sweets and high-fat dairy | FFQ | PCA | Added fat, butter/ghee, fried snacks, fruit juice, high-fat dairy, sugar-sweetened beverages, legumes, nuts, potatoes, refined grains, rice, snacks, sweets, vegetable oil, whole grains | As above |
| Gadgil et al.( 21 ) | 2015 | USA | 892 | Cohort | M/F | 40–84 | Fruits, vegetables, nuts and legumes | FFQ | PCA | Fruit, fruit juice, legumes, low-fat dairy, nuts, vegetable oil, vegetables, whole grains | As above |
| Suliga et al.( 35 ) | 2015 | Poland | 2479 | Cross-sectional | M/F | 45–64 | Healthy | FFQ | PCA | Low-fat milk, cottage cheese, yoghurt, fruit, vegetables, whole grains | Age, current smoker, PA, education level, place of residence |
| Suliga et al.( 35 ) | 2015 | Poland | 2479 | Cross-sectional | M/F | 45–64 | Prudent | FFQ | PCA | Fish, boiled potato, whole grain, refined grain, sugar and sweets, cold cured meat | As above |
| Suliga et al.( 35 ) | 2015 | Poland | 2479 | Cross-sectional | M/F | 45–64 | Fat, meat and alcohol | FFQ | PCA | Eggs, red meat, cold cured meat, lard, fried foods, vegetable oils, mayonnaise | As above |
DP, dietary pattern; F, female; M, male; PCA, principal component analysis; FA, factor analysis; PA, physical activity; EI, energy intake; AHA, American Heart Association; MET, metabolic equivalent of task.
‘Healthy/Prudent’ pattern
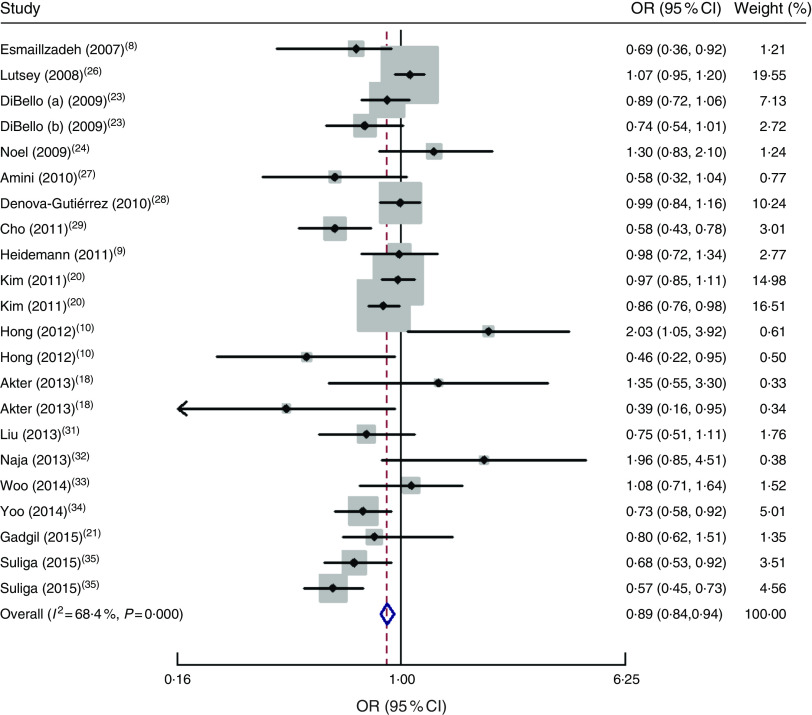
The forest plot of the association between the ‘Healthy/Prudent’ dietary pattern and MetS is indicated in Fig. 2. There was a significant inverse association between the ‘Healthy/Prudent’ pattern and risk of MetS (OR=0·89; 95 % CI 0·84, 0·94, P=0·002). There was moderate heterogeneity among included studies (I 2=68 %; 95 % CI 51, 80 %; P<0·001; τ 2=0·035). Sensitivity analysis showed that two studies significantly affected the pooled effect size( 10 , 18 ).
Fig. 2.
Forest plot of ‘Healthy/Prudent’ dietary pattern and risk of metabolic syndrome for the highest category compared with the lowest. The study-specific OR and 95 % CI are represented by the black diamond and the horizontal line, respectively; the area of the grey square is proportional to the specific-study weight to the overall meta-analysis. The centre of the open diamond/vertical dashed line represents the pooled OR and the width of the open diamond represents the pooled 95 % CI
‘Unhealthy/Western’ pattern
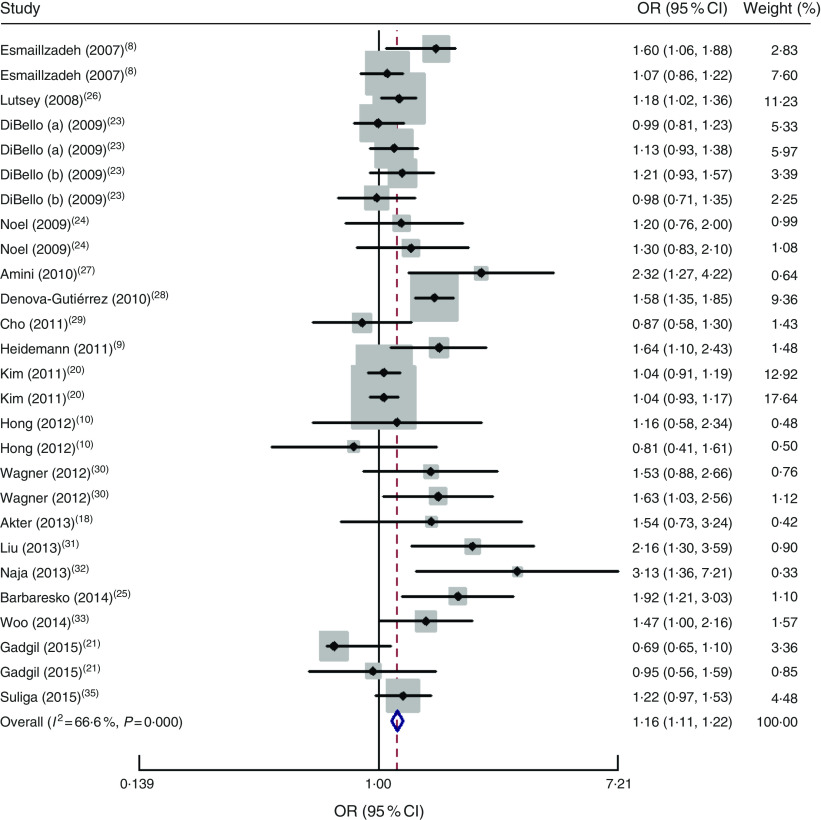
The ‘Unhealthy/Western’ dietary pattern had a significant positive association with risk of MetS (OR=1·16; 95 % CI 1·11, 1·22; P<0·001). There was moderate heterogeneity in studies (I 2=68 %; 95 % CI 50, 78 %; P<0·001; τ 2=0·034), as shown in Fig. 3.
Fig. 3.
Forest plot of ‘Unhealthy/Western’ dietary pattern and risk of metabolic syndrome for the highest category compared with the lowest. The study-specific OR and 95 % CI are represented by the black diamond and the horizontal line, respectively; the area of the grey square is proportional to the specific-study weight to the overall meta-analysis. The centre of the open diamond/vertical dashed line represents the pooled OR and the width of the open diamond represents the pooled 95 % CI
Publication bias
Funnel plots did not reveal asymmetry (Fig. 4). There was no publication bias for the ‘Healthy/Prudent’ dietary pattern (Egger’s test, P=0·65) or the ‘Unhealthy/Western’ dietary pattern (Egger’s test, P=0·34).
Fig. 4.
Publication bias assessment of included studies on a posteriori dietary patterns and metabolic syndrome (MetS): funnel plot (●, individual study) with pseudo 95 % confidence limits (– – – – –) for (a) the ‘Healthy/Prudent’ dietary pattern and (b) the ‘Unhealthy/Western’ dietary pattern
Discussion
To our knowledge, the current systematic review and meta-analysis is the first that has assessed the association between a posteriori dietary patterns and risk of MetS. Our results indicated that a ‘Healthy/Prudent’ dietary pattern is inversely associated with risk of MetS, whereas a ‘Unhealthy/Western’ dietary pattern is positively associated with risk of MetS.
In the current study we used two common dietary patterns, i.e. ‘Healthy/Prudent’ and ‘Unhealthy/Western’, due to large variation in the number and description of dietary patterns. These two dietary patterns share most foods with similar factor loadings. Moreover, in the meta-analysis, we included studies that used PCA and factor analysis to derive dietary patterns. PCA has long-term reproducibility, stability and validity compared with other methods( 36 ) which could minimize the risk of bias and increase the accuracy of our results. We excluded studies which used cluster analysis for extracting dietary patterns. Cluster analysis is about grouping subjects (e.g. people) while factor analysis is about grouping variables. Obviously, cluster analysis and factor analysis yield different information about the data. In contrast to cluster analysis which implies an empirical classification or an a priori theoretically defined cluster structure, factor analysis uses the aspiration of establishing a theoretically based causal relationship between indicators (items)( 37 ). Another a posteriori method to study dietary patterns is reduced rank regression, which finds dietary patterns that are potentially relevant for a disease by using a priori knowledge, for example on biological risk factors or nutrients relevant for the disease of interest. In contrast to PCA and factor analysis, reduced rank regression does not describe naturally occurring patterns of the population under study but explains variation in biologically important risk factors( 38 , 39 ).
In accordance with our study, a recent meta-analysis by Rodríguez-Monforte et al. showed that a prudent/healthy pattern is associated with a lower prevalence of MetS, whereas a Western/unhealthy pattern is associated with an increased risk for MetS( 40 ). The risk for MetS through unhealthy dietary patterns was 1·22 in our study v. 1·28 in their study; however, the risk for MetS through healthy dietary patterns was 0·89 in our study v. 0·83 in Rodríguez-Monforte et al.’s study( 40 ). It should be noted that their meta-analysis contained thirty-one studies including those which used cluster analysis. The dietary patterns identified using cluster methods could not be pooled with those identified by PCA or factor analysis because, as reported in other studies, large differences in factor scores between clusters have been observed, particularly for the factor with the largest variance. In a study by Smith et al. clusters were associated with high or low scores for a particular factor( 41 ).
The ‘Healthy/Prudent’ dietary pattern was associated with lower risk of MetS. The ‘Healthy/Prudent’ dietary pattern included high factor loadings for fruits, vegetables, fish and whole grains, and low factor loadings for red and processed meat. However, we may have misclassification because the factor loadings of individual foods in the ‘Healthy/Prudent’ dietary pattern were not identical between studies and is a limitation for this type of analysis. Even modest amounts of measurement error may have a large impact on measures of MetS risk, and it is likely that the small inverse association shown is due to a combination of dietary measurement error and misclassification of populations into categories of dietary pattern. Additionally, PCA is a subjective technique with opportunities for variation at almost every step (e.g. a variation in the number and type of dietary patterns derived within each study and categories of dietary pattern score)( 36 , 42 ).
The ‘Healthy/Prudent’ dietary pattern is rich in fresh fruits, vegetables, whole grains and fish. It seems that high content of vitamins, minerals, antioxidants, fibre, MUFA and n-3 fatty acids in this dietary pattern is responsible for the protective effect of the ‘Healthy/Prudent’ dietary pattern against MetS and its components. In addition, higher adherence to the ‘Healthy/Prudent’ dietary pattern is associated with a lower risk of glucose intolerance, weight gain, inflammation and insulin resistance and a higher level of HDL cholesterol( 43 ). Furthermore, improvement in lipid profile, antioxidant capacity, systolic and diastolic blood pressure, arrhythmias and insulin sensitivity may be another possible explanation for the observed association( 44 ).
Our results showed that risk of MetS is higher in subjects who had higher level of adherence to an ‘Unhealthy/Western’ dietary pattern compared with subjects who had a lower level of adherence. The ‘Unhealthy/Western’ dietary pattern is composed of red meat, processed meat, refined grains, sweets, French fries, desserts, eggs and high-fat dairy products. The positive association between the ‘Unhealthy/Western’ dietary pattern and MetS may be related to high intakes of Fe( 45 ), red meat( 46 ) and high-glycaemic-index foods that increase the risk of MetS( 47 ).
We found a moderate level of heterogeneity among included studies in both dietary patterns and risk of MetS, which may correlate to different versions of FFQ used to collect dietary intakes among included studies. Using different versions of FFQ could influence the selection of foods loaded on the dietary patterns. Moreover, various models used to control for confounding variables in included studies may explain the heterogeneity observed in the present study. However, in most of studies, the effect size was adjusted for major potential confounding variables including age, sex, BMI, education, energy intake and physical activity. It is possible that unmeasured variables such as cooking methods or food grouping may differ among studies and populations. Then it is inventible that we have very high levels of heterogeneity and some researchers may argue not to combine studies. However, heterogeneity seems to be always present( 48 , 49 ) and it may provide benefits for meta-analysis as we can explore the source of heterogeneity between studies( 48 ). The other reason for heterogeneity in the current meta-analysis could be the broad range of age used in the original studies, because young adults have different dietary habits from older adults. Additionally, in PCA/factor analysis, the extracted components/factors are as many as the initial variables and in the published studies usually the main two or three major components are reported, based on eigenvalue. Then, if in a study an unhealthy pattern was extracted as a fifth component it will be missed out from our meta-analysis. The other limitation is the cross-sectional nature of studies included in the meta-analysis, which precludes causal inference, and another shortcoming of cross-sectional studies is the possibility that the dietary pattern may represent a post hoc event. To minimize and control all types of heterogeneity, individual patient data meta-analyses are recommended( 50 ). Moreover, in our analysis, only the OR of being in the highest and the lowest quantile of healthy or unhealthy patterns have been used. This may be misleading as the presence of any trend cannot be evaluated. Finally, some studies might have used quintiles while others might have used tertiles, and this may have an effect on the OR.
Conclusion
In conclusion, our findings have indicated an inverse association between a ‘Healthy/Prudent’ dietary pattern and risk of MetS and a positive significant association between an ‘Unhealthy/Western’ dietary pattern and risk of MetS. To our knowledge, the present study is the first meta-analysis that has investigated the association between dietary patterns and risk of MetS; however, its limitations should be considered.
Acknowledgements
Financial support: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors. Conflict of interest: The authors declare that there is no conflict of interest. Authorship: S.S.-B. and M.H. designed the study. S.S.-B. and M.H. contributed to the literature searches, data extraction and independent reviewing. M.G. and S.M. performed the statistical analyses. All authors contributed to the writing of the manuscript. M.G. and S.M. wrote a first draft of the manuscript and S.S.-B. prepared the final draft. All authors read the manuscript and approved it. Ethics of human subject participation: Not applicable.
References
- 1. Eckel RH, Grundy SM & Zimmet PZ (2005) The metabolic syndrome. Lancet 365, 1415–1428. [DOI] [PubMed] [Google Scholar]
- 2. Ford ES (2005) Risks for all-cause mortality, cardiovascular disease, and diabetes associated with the metabolic syndrome: a summary of the evidence. Diabetes Care 28, 1769–1778. [DOI] [PubMed] [Google Scholar]
- 3. Radhika G, Van Dam RM, Sudha V et al. (2009) Refined grain consumption and the metabolic syndrome in urban Asian Indians (Chennai Urban Rural Epidemiology Study 57). Metabolism 58, 675–681. [DOI] [PubMed] [Google Scholar]
- 4. Song S, Lee JE, Song WO et al. (2014) Carbohydrate intake and refined-grain consumption are associated with metabolic syndrome in the Korean adult population. J Acad Nutr Diet 114, 54–62. [DOI] [PubMed] [Google Scholar]
- 5. Park S, Ham JO & Lee BK (2015) Effects of total vitamin A, vitamin C, and fruit intake on risk for metabolic syndrome in Korean women and men. Nutrition 31, 111–118. [DOI] [PubMed] [Google Scholar]
- 6. Mirmiran P, Shab-Bidar S, Hosseini-Esfahani F et al. (2012) Magnesium intake and prevalence of metabolic syndrome in adults: Tehran Lipid and Glucose Study. Public Health Nutr 15, 693–701. [DOI] [PubMed] [Google Scholar]
- 7. Pan A, Franco OH, Ye J et al. (2008) Soy protein intake has sex-specific effects on the risk of metabolic syndrome in middle-aged and elderly Chinese. J Nutr 138, 2413–2421. [DOI] [PubMed] [Google Scholar]
- 8. Esmaillzadeh A, Kimiagar M, Mehrabi Y et al. (2007) Dietary patterns, insulin resistance, and prevalence of the metabolic syndrome in women. Am J Clin Nutr 85, 910–918. [DOI] [PubMed] [Google Scholar]
- 9. Heidemann C, Scheidt-Nave C, Richter A et al. (2011) Dietary patterns are associated with cardiometabolic risk factors in a representative study population of German adults. Br J Nutr 106, 1253–1262. [DOI] [PubMed] [Google Scholar]
- 10. Hong S, Song Y, Lee KH et al. (2012) A fruit and dairy dietary pattern is associated with a reduced risk of metabolic syndrome. Metabolism 61, 883–890. [DOI] [PubMed] [Google Scholar]
- 11. Cho YA, Kim J, Cho ER et al. (2011) Dietary patterns and the prevalence of metabolic syndrome in Korean women. Nutr Metabol Cardiovasc Dis 21, 893–900. [DOI] [PubMed] [Google Scholar]
- 12. Ottawa Hospital Research Institute & University of Oxford (2018) PRISMA 2009 Checklist. http://www.prisma-statement.org/ (accessed March 2018).
- 13. Dietary Guidelines Advisory Committee (2010) Report of the Dietary Guidelines Advisory Committee on the Dietary Guidelines for Americans, 2010, to the Secretary of Agriculture and the Secretary of Health and Human Services. Washington, DC: US Department of Agriculture, Agricultural Research Service. [Google Scholar]
- 14. Egger M, Smith GD & Altman D (2008) Systematic Reviews in Health Care: Meta-Analysis in Context, 2nd ed. London: BMJ Books. [Google Scholar]
- 15. Higgins JP, Thompson SG, Deeks JJ et al. (2003) Measuring inconsistency in meta-analyses. BMJ 327, 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Orsini N, Bottai M, Higgins J et al. (2006) Heterogi: Stata module to quantify heterogeneity in a meta-analysis (computer program). Statistical Software Components S449201. https://econpapers.repec.org/software/bocbocode/s449201.htm (accessed February 2018).
- 17. Higgins JP & Green S (2011) Cochrane Handbook for Systematic Reviews of Interventions, Version 5.1.0. http://handbook-5-1.cochrane.org/ (accessed February 2018).
- 18. Akter S, Nanri A, Pham NM et al. (2013) Dietary patterns and metabolic syndrome in a Japanese working population. Nutr Metab (Lond) 10, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Peters JL, Sutton AJ, Jones DR et al. (2008) Contour-enhanced meta-analysis funnel plots help distinguish publication bias from other causes of asymmetry. J Clin Epidemiol 61, 991–996. [DOI] [PubMed] [Google Scholar]
- 20. Kim J & Jo I (2011) Grains, vegetables, and fish dietary pattern is inversely associated with the risk of metabolic syndrome in South Korean adults. J Am Diet Assoc 111, 1141–1149. [DOI] [PubMed] [Google Scholar]
- 21. Gadgil MD, Anderson CAM, Kandula NR et al. (2015) Dietary patterns are associated with metabolic risk factors in South Asians living in the United States. J Nutr 145, 1211–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Esmaillzadeh A, Kimiagar M, Mehrabi Y et al. (2007) Dietary patterns, insulin resistance, and prevalence of the metabolic syndrome in women. Am J Clin Nutr 85, 910–918. [DOI] [PubMed] [Google Scholar]
- 23. DiBello JR, McGarvey ST, Kraft P et al. (2009) Dietary patterns are associated with metabolic syndrome in adult Samoans. J Nutr 139, 1933–1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Noel SE, Newby P, Ordovas JM et al. (2009) A traditional rice and beans pattern is associated with metabolic syndrome in Puerto Rican older adults. J Nutr 139, 1360–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Barbaresko J, Siegert S, Koch M et al. (2014) Comparison of two exploratory dietary patterns in association with the metabolic syndrome in a Northern German population. Br J Nutr 112, 1364–1372. [DOI] [PubMed] [Google Scholar]
- 26. Lutsey PL, Steffen LM & Stevens J (2008) Dietary intake and the development of the metabolic syndrome. Circulation 117, 754–761. [DOI] [PubMed] [Google Scholar]
- 27. Amini M, Esmaillzadeh A, Shafaeizadeh S et al. (2010) Relationship between major dietary patterns and metabolic syndrome among individuals with impaired glucose tolerance. Nutrition 26, 986–992. [DOI] [PubMed] [Google Scholar]
- 28. Denova-Gutiérrez E, Castañón S, Talavera JO et al. (2010) Dietary patterns are associated with metabolic syndrome in an urban Mexican population. J Nutr 140, 1855–1863. [DOI] [PubMed] [Google Scholar]
- 29. Cho YA, Kim J, Cho ER et al. (2011) Dietary patterns and the prevalence of metabolic syndrome in Korean women. Nutr Metab Cardiovasc Dis 21, 893–900. [DOI] [PubMed] [Google Scholar]
- 30. Wagner A, Dallongeville J, Haas B et al. (2012) Sedentary behaviour, physical activity and dietary patterns are independently associated with the metabolic syndrome. Diabetes Metab 38, 428–435. [DOI] [PubMed] [Google Scholar]
- 31. Liu J, Hickson DA, Musani SK et al. (2013) Dietary patterns, abdominal visceral adipose tissue, and cardiometabolic risk factors in African Americans: the Jackson heart study. Obesity (Silver Spring) 21, 644–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Naja F, Nasreddine L, Itani L et al. (2013) Association between dietary patterns and the risk of metabolic syndrome among Lebanese adults. Eur J Nutr 52, 97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Woo HD, Shin A & Kim J (2014) Dietary patterns of Korean adults and the prevalence of metabolic syndrome: a cross-sectional study. PLoS One 9, e111593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yoo K-B, Suh H-J, Lee M-J et al. (2014) Breakfast eating patterns and the metabolic syndrome: the Korea National Health and Nutrition Examination Survey (KNHANES) 2007–2009. Asia Pac J Clin Nutr 23, 128–137. [DOI] [PubMed] [Google Scholar]
- 35. Suliga E, Kozieł D, Cieśla E et al. (2015) Association between dietary patterns and metabolic syndrome in individuals with normal weight: a cross-sectional study. Nutr J 14, 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hu FB (2002) Dietary pattern analysis: a new direction in nutritional epidemiology. Curr Opin Lipidol 13, 3–9. [DOI] [PubMed] [Google Scholar]
- 37. Krebs D, Berger M & Ferligoj A (2000) Approaching achievement motivation – comparing factor analysis and cluster analysis. N Appr Appl Stat Metod Zvezki 16, 147–169. [Google Scholar]
- 38. Jankovic N, Steppel MT, Kampman E et al. (2014) Stability of dietary patterns assessed with reduced rank regression; the Zutphen Elderly Study. Nutr J 13, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tucker KL (2010) Dietary patterns, approaches, and multicultural perspective. This is one of a selection of papers published in the CSCN–CSNS 2009 Conference, entitled Can we identify culture-specific healthful dietary patterns among diverse populations undergoing nutrition transition? Appl Physiol Nutr Metabol 35, 211–218. [DOI] [PubMed] [Google Scholar]
- 40. Rodríguez-Monforte M, Sánchez E, Barrio F et al. (2017) Metabolic syndrome and dietary patterns: a systematic review and meta-analysis of observational studies. Eur J Nutr 56, 925–947. [DOI] [PubMed] [Google Scholar]
- 41. Smith AD, Emmett P, Newby P et al. (2011) A comparison of dietary patterns derived by cluster and principal components analysis in a UK cohort of children. Eur J Clin Nutr 65, 1102–1109. [DOI] [PubMed] [Google Scholar]
- 42. Jacques PF & Tucker KL (2001) Are dietary patterns useful for understanding the role of diet in chronic disease? Am J Clin Nutr 73, 1–2. [DOI] [PubMed] [Google Scholar]
- 43. Fung TT, Willett WC, Stampfer MJ et al. (2001) Dietary patterns and the risk of coronary heart disease in women. Arch Intern Med 161, 1857–1862. [DOI] [PubMed] [Google Scholar]
- 44. Tourlouki E, Matalas A-L & Panagiotakos DB (2009) Dietary habits and cardiovascular disease risk in middle-aged and elderly populations: a review of evidence. Clin Interv Aging 4, 319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Leiva E, Mujica V, Sepulveda P et al. (2013) High levels of iron status and oxidative stress in patients with metabolic syndrome. Biol Trace Elem Res 151, 1–8. [DOI] [PubMed] [Google Scholar]
- 46. Azadbakht L & Esmaillzadeh A (2009) Red meat intake is associated with metabolic syndrome and the plasma C-reactive protein concentration in women. J Nutr 139, 335–339. [DOI] [PubMed] [Google Scholar]
- 47. Finley CE, Barlow CE, Halton TL et al. (2010) Glycemic index, glycemic load, and prevalence of the metabolic syndrome in the cooper center longitudinal study. J Am Diet Assoc 110, 1820–1829. [DOI] [PubMed] [Google Scholar]
- 48. Berlin JA (1995) Invited commentary: Benefits of heterogeneity in meta-analysis of data from epidemiologic studies. Am J Epidemiol 142, 383–387. [DOI] [PubMed] [Google Scholar]
- 49. Kontopantelis E, Springate DA & Reeves D (2013) A re-analysis of the Cochrane Library data: the dangers of unobserved heterogeneity in meta-analyses. PLoS One 8, e69930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Oxman AD, Clarke MJ & Stewart LA (1995) From science to practice: meta-analyses using individual patient data are needed. JAMA 274, 845–846. [DOI] [PubMed] [Google Scholar]