Abstract
Objective
To determine the associations of sociodemographic characteristics, diet and outdoor activity as an indicator of sun exposure with serum 25-hydroxyvitamin D (25(OH)D) concentrations in children and their parents from Mesoamerica. We also quantified family aggregation of serum 25(OH)D.
Design
Cross-sectional study. Serum 25(OH)D concentrations were quantified using immunoassay. We compared the distribution of 25(OH)D concentrations in adults and children by levels of each correlate with the use of linear regression. Family aggregation was estimated using Pearson and intraclass correlation coefficients.
Setting
Capital cities of Guatemala, El Salvador, the Dominican Republic, Honduras, Nicaragua, Costa Rica, Panama and Belize, and Tuxtla Gutiérrez in Mexico.
Subjects
Children (n 223) aged 7–12 years and 492 parents.
Results
Mean (sd) 25(OH)D concentrations in adults and children were 81·3 (21·1) and 79·5 (18·1) nmol/l, respectively. Prevalence of vitamin D deficiency (VDD; 25(OH)D <50 nmol/l) was 3·9 % among adults and 3·6 % among children. In adults, adjusted mean 25(OH)D concentrations were highest in Nicaragua (P<0·0001). Serum 25(OH)D was positively related to time spent gardening (P=0·03). Among children, 25(OH)D concentrations were positively associated with male sex (P=0·005), dairy intake (P=0·03) and mother’s serum 25(OH)D concentrations (P<0·0001); and inversely associated with mother’s BMI (P=0·02) and number of home assets (P=0·04). Family membership explained 31 % of the variability in 25(OH)D concentrations; aggregation was highest between mothers and children.
Conclusions
VDD prevalence was low in this study. Sociodemographic characteristics, diet and outdoor activity predict serum 25(OH)D. Family aggregation of serum 25(OH)D is high between mothers and children.
Keywords: Vitamin D, Vitamin D deficiency, Family aggregation, Mesoamerica, Children
Vitamin D deficiency (VDD) is a widespread public health problem, with substantial regional and seasonal variability. The effects of VDD may extend beyond the musculoskeletal system; thus, it is important to characterize the extent of the problem and its determinants at the population level. Few studies have investigated the prevalence of VDD in Mesoamerica. In a study of Guatemalan adolescents, 12·8 % had VDD, defined as serum 25-hydroxyvitamin D (25(OH)D) concentration <50 nmol/l( 1 ). In clinic-based studies of adults in Puerto Rico( 2 , 3 ) and among older adults from Mexico( 4 ), the prevalence of VDD was 36·0 and 36·9 %, respectively.
The main source of vitamin D is skin synthesis from exposure to sunlight, although it can also be obtained from some foods including fatty fish, mushrooms, egg yolks and fortified milk. Although skin exposure and time spent outdoors have been investigated as correlates of vitamin D status( 1 ), the relative contributions of both sunlight and diet to the vitamin D status of Mesoamericans have not been examined. The demographic and socio-economic patterning of vitamin D status in the region is also unknown. Identifying correlates of serum 25(OH)D concentrations in adults and children across the Mesoamerican region is an important initial step to determine which specific subgroups may benefit from public health interventions to prevent and decrease the burden of VDD.
The vitamin D status of family members may be correlated due to shared habits or genetics( 5 , 6 ). Nevertheless, the level of aggregation of this measure between parents and children has not been estimated. It is relevant to understand whether shared environmental or genetic factors may influence vitamin D status because interventions to improve it might be more effective if they are targeted to families rather than individuals.
Table 6.
Multivariable-adjusted correlates of serum 25-hydroxyvitamin D (25(OH)D) concentrations in children from eight Mesoamerican countries, 2011–2013
| Adjusted difference* | 95 % CI | ||
|---|---|---|---|
| nmol/l | P † | ||
| Male v. female | 5·7 | 1·8, 9·7 | 0·005 |
| Mother’s height, quartile (median, cm) | 0·26 | ||
| Q1 (148·8) | Reference | ||
| Q2 (153·1) | 2·1 | −4·0, 8·2 | |
| Q3 (157·0) | 7·6 | 1·8, 13·5 | |
| Q4 (162·4) | 1·6 | −4·2, 7·4 | |
| Mother’s BMI, per 1 kg/m2 | −0·4 | −0·7, −0·1 | 0·02 |
| Mother’s serum 25(OH)D, per 1 nmol/l | 0·3 | 0·2, 0·5 | <0·0001 |
| Number of home assets‡, >4 v. ≤4 | −6·4 | −12·4, −0·3 | 0·04 |
| Country, latitude | 0·27 | ||
| Guatemala, 14·6° | Reference | ||
| El Salvador, 13·7° | 2·0 | −5·5, 9·6 | |
| Dominican Republic, 18·5° | 8·7 | 2·0, 15·5 | |
| Honduras, 14·1° | 4·8 | −2·3, 12·0 | |
| Nicaragua, 12·1° | 8·9 | −1·0, 18·8 | |
| Costa Rica, 9·6° | 2·8 | −5·2, 10·8 | |
| Belize, 17·3° | 6·4 | −0·9, 13·6 | |
| Mexico, 16·8° | 2·6 | −3·2, 8·4 | |
| Frequency of dairy intake§ | 0·03 | ||
| <5/week | Reference | ||
| 5/week to <1·5/d | 1·0 | −4·0, 6·0 | |
| 1·5–<2·5/d | 3·1 | −2·9, 9·1 | |
| ≥2·5/d | 6·8 | 0·7, 12·9 | |
From a multivariable linear regression model with serum 25(OH)D as the continuous outcome. Predictors included all variables presented. Empirical variances were specified in the model.
For height and frequency of dairy intake, test for linear trend when a variable representing ordinal categories of the characteristic was introduced as a continuous predictor. Wald test for sex, mother’s BMI, mother’s serum 25(OH)D concentrations and home assets. χ 2 score test for country.
From a list that included car, bicycle, refrigerator, gas stove, electric stove, blender, microwave, washing machine, colour television, stereo, computer, and having in-home access to the Internet.
Sum of weighted intake frequencies of milk (200 g), cheese (20 g), cream (6 g) and ice cream (60 g).
The aim of the present study was to examine the associations of sociodemographic characteristics, dietary habits and time spent on outdoor activities with serum 25(OH)D concentrations and the aggregation of 25(OH)D concentrations among mothers, fathers and their children in nine Mesoamerican countries.
Methods
Study population and field methods
The present study was conducted in the context of the Nine Mesoamerican Countries Metabolic Syndrome Study (NiMeCoMeS), a cross-sectional survey investigating dietary and other environmental correlates of metabolic syndrome among school-aged children and their two biological parents. Details on the study design have been described previously( 7 ). Briefly, between July 2011 and November 2013, we identified all public primary schools in peri-urban areas of the capital cities of Guatemala, El Salvador, the Dominican Republic, Honduras, Nicaragua, Costa Rica, Panama and Belize, and from the city of Tuxtla Gutiérrez in Chiapas, Mexico, by contacting each country’s Ministry of Education. We considered schools with at least 400 students of both sexes and grouped them by geographic areas roughly representing the four cardinal directions. Next, one to three schools were randomly selected from each geographic area in each city to reach the target enrolment of thirty families per country, a sample size determined by the availability of funding. The study team identified students aged 7–12 years as potentially eligible for recruitment using the list of enrolled students in each school and invited their parents to attend a meeting. The study aims and procedures were explained to the families in attendance and additional eligibility criteria were confirmed. These criteria included living with both biological parents, not being pregnant or having a pregnant mother, and not having a sibling already identified for potential participation in the study. Researchers provided consent forms to families at the end of the meeting and afforded them one week to consider participation. Study personnel collected the signed consent forms at schools and verbally confirmed assent to participate among children of consenting parents. The final sample included 267 families (Guatemala, n 31; El Salvador, n 30; Dominican Republic, n 30; Honduras, n 30; Nicaragua, n 31; Costa Rica, n 27; Panama, n 26; Belize, 31; and Mexico, n 31). The sampling strategy employed did not intend to produce a representative sample of the population in these countries.
Sociodemographic characteristics, food intake and health habits of children and their parents were determined through questionnaires administered during a research visit to the participant’s home or at a health centre. The questionnaires inquired on parental age, education level, maternal parity, and socio-economic status indicators including home and household assets ownership. Household food insecurity was assessed with the use of the Latin American and Caribbean Food Security Scale (ELCSA)( 8 ), a survey validated for the region. We evaluated participants’ usual diet over the past 12 months using a semi-quantitative ninety-seven-item FFQ that was administered separately to mothers, fathers and children. The FFQ had been previously validated in Costa Rican adults( 9 , 10 ) and included major sources of energy and micronutrients in food groups such as fruits, vegetables, animal protein (dairy, eggs and meat), carbohydrates (breads, flours, and cereals), beverages (dairy, sugar-sweetened beverages, coffee and alcohol), fast foods, desserts, sauces and dressings, cooking fats and oils, and dietary supplements. For each item, research assistants described reference portion sizes in natural units or standard measures for commonly consumed servings among this population. There were nine frequency of intake options: ≥6 times/d, 4–5 times/d, 2–3 times/d, 1 time/d, 5–6 times/week, 2–4 times/week, 1 time/week, 1–3 times/month or <1 time/month.
The questionnaires also included questions regarding the time spent on activities typically conducted outdoors as an indicator of sun exposure. For adults, these questions involved the usual frequency of gardening, performing agricultural work or walking outdoors for 1 h during the previous 12 months. Responses were coded into one of ten frequency options: ≥6 times/d, 4–5 times/d, 2–3 times/d, 1 time/d, 5–6 times/week, 2–4 times/week, 1 time/week, 1–3 times/month, <1 time/month or never. For children, mothers reported on their child’s average daily time spent playing outdoors on weekdays and weekends.
Trained research assistants obtained anthropometric measures from each participant. Weight was measured in light clothing to the nearest 0·1 kg on digital scales (Tanita, Arlington Heights, IL, USA) and height was measured without shoes to the nearest 1 mm with the use of portable stadiometers (Seca, Hanover, MD, USA). At the end of the visit, a fasting blood sample was collected by venepuncture from each family member.
Laboratory methods
Blood samples were transported on ice to each country’s collaborating institution where serum was separated and cryopreserved at −20°C until laboratory analysis. Analysis of 25(OH)D was performed at Heartland Assays, Inc. (Ames, IA, USA) with the DiaSorin LIAISON 25-OH Vitamin D Total assay kit, a direct, competitive chemiluminescence immunoassay. This validated method has a sensitivity of 6·25 nmol/l, and an inter- and intra-assay CV of 11·2 and 8·1 %, respectively( 11 ).
Definition of outcomes
The main outcome of interest was mean serum 25(OH)D concentration. A secondary outcome was the prevalence of serum 25(OH)D <50 nmol/l, a cut-off point recommended by the US Endocrine Society to define deficiency( 12 ). A lower cut-off point of 30 nmol/l, proposed to define deficiency by the US Institute of Medicine( 13 ), was not considered because only one person had 25(OH)D concentration under this threshold.
Definition of exposures
Adults
Education level was categorized according to the number of completed years of schooling (incomplete elementary, 1–5 years; complete elementary, 6 years; incomplete secondary, 7–11 years; complete secondary, 12 years; or post-secondary, ≥13 years). We categorized height into quartiles separately for men and women. BMI was calculated as kg/m2 and categorized as <25·0, 25·0–29·9, 30·0–34·9 or ≥35·0( 14 ). A category of <18·5 kg/m2 was not considered because only six people were under this cut-off point. The number of home assets was determined as the total number of affirmative responses to owning a car, bicycle, refrigerator, gas stove, electric stove, blender, microwave, washing machine, colour television, stereo or computer, or having in-house access to the Internet. Household food insecurity was categorized according to the number of affirmative responses to the questions in the ELCSA survey (no insecurity, 0; mild insecurity, 1–5; moderate insecurity, 6–10; severe insecurity, ≥11). From the FFQ, we selected foods with a high vitamin D content as dietary exposures according to food composition tables. These included dairy, eggs, and canned tuna or sardines. Dairy intake frequency was calculated from the sum of weighted intake frequencies of milk, cheese, cream and ice cream. Egg intake represented the sum of weighted intake frequencies of one hard boiled or fried egg. Supplement use was not considered as a dietary correlate of vitamin D because fewer than five participants reported use of supplements that may contain vitamin D. Covariates were categorized as presented in Tables 1 and 2.
Table 1.
Serum 25-hydroxyvitamin D (25(OH)D) concentrations according to sociodemographic characteristics in adults from nine Mesoamerican countries, 2011–2013
| Mean | sd | |||||
|---|---|---|---|---|---|---|
| Sociodemographic characteristic | n * | (nmol/l) | P † | % <50 nmol/l | P ‡ | |
| Sex | 0·15 | 0·18 | ||||
| Female (mothers) | 248 | 82·5 | 21·8 | 2·8 | ||
| Male (fathers) | 244 | 80·1 | 20·4 | 4·9 | ||
| Age (years) | 0·33 | 0·74 | ||||
| <30 | 53 | 83·7 | 20·6 | 3·8 | ||
| 30–34 | 103 | 81·0 | 19·7 | 3·9 | ||
| 35–39 | 152 | 83·1 | 23·4 | 4·0 | ||
| 40–44 | 98 | 78·6 | 20·1 | 2·0 | ||
| 45–54 | 66 | 79·0 | 19·8 | 7·6 | ||
| ≥55 | 19 | 83·6 | 21·4 | 0·0 | ||
| Education level | 0·04 | 0·90 | ||||
| Incomplete elementary | 73 | 83·9 | 24·8 | 4·1 | ||
| Complete elementary | 69 | 84·0 | 21·2 | 2·9 | ||
| Incomplete secondary | 146 | 82·3 | 21·7 | 4·8 | ||
| Complete secondary | 73 | 79·2 | 18·0 | 2·7 | ||
| Post-secondary | 119 | 78·7 | 19·5 | 4·2 | ||
| Height, sex-specific quartile (female/male medians, cm) | 0·67 | 0·33 | ||||
| Q1 (148·8/159·0) | 126 | 82·4 | 19·8 | 2·4 | ||
| Q2 (153·1/165·0) | 126 | 78·9 | 20·0 | 3·2 | ||
| Q3 (157·0/169·7) | 121 | 83·6 | 22·8 | 5·8 | ||
| Q4 (162·7/176·0) | 119 | 80·4 | 21·9 | 4·2 | ||
| BMI (kg/m2) | 0·91 | 0·07 | ||||
| <25·0 | 124 | 79·9 | 17·3 | 2·4 | ||
| 25·0–29·9 | 198 | 82·6 | 22·6 | 3·0 | ||
| 30·0–34·9 | 126 | 81·5 | 21·3 | 4·8 | ||
| ≥35·0 | 44 | 79·1 | 23·6 | 9·1 | ||
| Number of home assets§ | 0·04 | 0·03 | ||||
| 0–4 | 94 | 85·7 | 22·4 | 1·1 | ||
| 5–7 | 190 | 80·7 | 19·7 | 3·2 | ||
| 8–9 | 89 | 81·9 | 24·5 | 4·5 | ||
| 10–12 | 119 | 78·4 | 19·1 | 6·7 | ||
| Food insecurity in the household | 0·80 | 0·24 | ||||
| None | 159 | 81·5 | 22·4 | 5·7 | ||
| Mild | 133 | 80·9 | 16·4 | 2·3 | ||
| Moderate | 115 | 80·5 | 22·5 | 5·2 | ||
| Severe | 83 | 82·9 | 23·8 | 1·2 | ||
| Country, latitude | <0·0001 | 0·15 | ||||
| Guatemala, 14·6° | 58 | 72·4 | 14·1 | 1·7 | ||
| El Salvador, 13·7° | 58 | 81·0 | 16·8 | 3·5 | ||
| Dominican Republic, 18·5° | 60 | 74·2 | 16·6 | 3·3 | ||
| Honduras, 14·1° | 57 | 79·9 | 18·9 | 3·5 | ||
| Nicaragua, 12·1° | 62 | 97·5 | 28·1 | 1·6 | ||
| Panama, 9·0° | 25 | 71·2 | 15·0 | 8·0 | ||
| Costa Rica, 9·6° | 53 | 79·0 | 22·4 | 9·4 | ||
| Belize, 17·3° | 57 | 83·9 | 21·2 | 7·0 | ||
| Mexico, 16·8° | 62 | 85·5 | 19·3 | 0·0 | ||
| Month of measurement | 0·05 | 0·48 | ||||
| Jan–Feb | 26 | 71·6 | 17·1 | 7·7 | ||
| Mar–Apr | 103 | 81·6 | 18·4 | 1·9 | ||
| May–Jun | 44 | 87·0 | 22·5 | 4·6 | ||
| Jul–Sep | 126 | 83·9 | 23·4 | 2·4 | ||
| Oct–Dec | 193 | 79·5 | 20·6 | 5·2 | ||
Totals may be less than 492 because of missing values.
Test for linear trend from linear regression models with serum 25(OH)D as the continuous outcome and a variable representing ordinal categories of each characteristic as the continuous predictor. χ 2 score test for sex, country and month of measurement. An exchangeable correlation structure was specified in all models to account for intrafamily clustering.
Test for linear trend from Poisson regression models with serum 25(OH)D <50 nmol/l as the dichotomous outcome and a variable representing ordinal categories of each characteristic as the continuous predictor. χ 2 score test for sex and month of measurement. An exchangeable correlation structure was specified in all models to account for intrafamily clustering. Fisher’s exact test for country.
From a list that included car, bicycle, refrigerator, gas stove, electric stove, blender, microwave, washing machine, colour television, stereo, computer, and having in-home access to the Internet.
Table 2.
Serum 25-hydroxyvitamin D (25(OH)D) concentrations according to food intake frequency and time spent on outdoor activities in adults from nine Mesoamerican countries, 2011–2013
| Mean | sd | |||||
|---|---|---|---|---|---|---|
| n * | nmol/l | P † | % <50 nmol/l | P ‡ | ||
| Food intake frequency | ||||||
| Dairy§ | 0·50 | 0·62 | ||||
| <3/week | 129 | 81·8 | 21·3 | 2·3 | ||
| 3–6/week | 117 | 78·7 | 18·4 | 5·1 | ||
| 1–<1·5/d | 134 | 81·2 | 20·5 | 3·0 | ||
| ≥1·5/d | 102 | 83·7 | 24·4 | 4·9 | ||
| Eggs║ | 0·32 | 0·54 | ||||
| <3/week | 91 | 83·4 | 23·9 | 3·3 | ||
| 3/week | 140 | 81·0 | 20·5 | 5·0 | ||
| 4–6/week | 132 | 81·1 | 20·7 | 3·8 | ||
| ≥1/d | 119 | 80·3 | 20·2 | 2·5 | ||
| Canned tuna/sardines¶ | 0·65 | 0·25 | ||||
| <1/month | 210 | 82·3 | 20·5 | 2·9 | ||
| 1–3/month | 114 | 80·3 | 22·1 | 3·5 | ||
| 1/week | 94 | 78·3 | 18·9 | 4·3 | ||
| ≥2/week | 64 | 84·1 | 24·1 | 6·3 | ||
| Outdoor activities | ||||||
| Gardening | 0·02 | 0·11 | ||||
| Never | 298 | 79·7 | 20·0 | 3·7 | ||
| 1–3 h/month | 53 | 78·8 | 16·6 | 0·0 | ||
| 1–6 h/week | 76 | 83·6 | 19·4 | 4·0 | ||
| ≥1 h/d | 61 | 88·5 | 29·6 | 8·2 | ||
| Agricultural work | 0·29 | 0·30 | ||||
| Never | 414 | 80·9 | 21·8 | 4·4 | ||
| Any | 66 | 82·4 | 17·1 | 1·5 | ||
| Walking outdoors | 0·22 | 0·82 | ||||
| <1 h/week | 122 | 79·3 | 20·7 | 4·9 | ||
| 1–6 h/week | 130 | 80·4 | 19·5 | 3·1 | ||
| 1 h/d | 116 | 84·1 | 23·4 | 1·7 | ||
| ≥1 h/d | 116 | 81·1 | 21·1 | 6·0 | ||
Totals may be less than 492 because of missing values·
Test for linear trend from linear regression models with serum 25(OH)D as the continuous outcome and a variable representing ordinal categories of each characteristic as the continuous predictor. χ 2 score test for agricultural work. An exchangeable correlation structure was specified in all models to account for intrafamily clustering.
Test for linear trend from Poisson regression models with serum 25(OH)D <50 nmol/l as the dichotomous outcome and a variable representing ordinal categories of each characteristic as the continuous predictor. χ 2 score test for agricultural work. An exchangeable correlation structure was specified in all models to account for intrafamily clustering.
Sum of weighted intake frequencies of milk (200 g), cheese (30 g), cream (12 g) and ice cream (60 g).
Sum of weighted intake frequencies of one hard boiled or fried egg (60 g).
Intake of 60 g.
Children
Children’s height-for-age Z-score and BMI-for-age Z-score were calculated using sex-specific growth references from the WHO( 15 ). The parent who had a higher level of educational attainment determined the highest level of parental education. Maternal height in centimetres was categorized into quartiles and maternal BMI was categorized as described for adults. Mother’s and father’s serum 25(OH)D concentrations were categorized as <50, ≥50 to <75 or ≥75 nmol/l. Covariates were categorized as presented in Tables 4 and 5.
Table 4.
Serum 25-hydroxyvitamin D (25(OH)D) concentrations according to sociodemographic characteristics in children from eight Mesoamerican countries, 2011–2013
| Mean | sd | |||||
|---|---|---|---|---|---|---|
| Sociodemographic characteristic | n * | nmol/l | P † | % <50 nmol/l | P ‡ | |
| Sex | 0·06 | 0·99 | ||||
| Female | 119 | 77·3 | 18·0 | 3·4 | ||
| Male | 104 | 81·9 | 17·9 | 3·9 | ||
| Age (years) | 0·06 | 0·67 | ||||
| <9 | 72 | 83·6 | 20·2 | 4·2 | ||
| 9–11 | 75 | 77·2 | 16·3 | 4·0 | ||
| >11 | 76 | 77·7 | 17·1 | 2·6 | ||
| Height-for-age Z-score§ | 0·87 | 0·99 | ||||
| <−1·0 | 52 | 78·4 | 16·5 | 1·9 | ||
| −1·0–<0·0 | 86 | 81·2 | 19·3 | 4·7 | ||
| 0·0–<1·0 | 57 | 77·7 | 17·1 | 5·3 | ||
| ≥1·0 | 28 | 79·5 | 19·4 | 0·0 | ||
| BMI-for-age Z-score§ | 0·22 | 0·18 | ||||
| <−1·0 | 33 | 82·0 | 14·7 | 0·0 | ||
| −1·0–<0·0 | 45 | 79·5 | 16·3 | 2·2 | ||
| 0·0–<1·0 | 69 | 80·4 | 18·9 | 4·4 | ||
| ≥1·0 | 76 | 77·4 | 19·7 | 5·3 | ||
| Parental highest education level | 0·60 | 0·10 | ||||
| Incomplete elementary | 20 | 79·6 | 19·6 | 5·0 | ||
| Complete elementary | 28 | 75·4 | 19·4 | 10·7 | ||
| Incomplete secondary | 62 | 82·0 | 20·8 | 3·2 | ||
| Complete secondary | 38 | 75·0 | 14·9 | 2·6 | ||
| Post-secondary | 75 | 81·1 | 15·8 | 1·3 | ||
| Mother’s height, quartile (median, cm) | 0·01 | 0·34 | ||||
| Q1 (148·8) | 58 | 74·9 | 15·7 | 1·7 | ||
| Q2 (153·1) | 59 | 77·4 | 19·1 | 3·4 | ||
| Q3 (157·0) | 54 | 85·5 | 17·7 | 3·7 | ||
| Q4 (162·4) | 52 | 80·6 | 18·3 | 5·8 | ||
| Mother’s BMI (kg/m2) | 0·98 | 0·003 | ||||
| <25·0 | 51 | 77·3 | 15·7 | 0·0 | ||
| 25·0–29·9 | 84 | 81·7 | 17·2 | 1·2 | ||
| 30·0–34·9 | 63 | 78·8 | 19·8 | 6·4 | ||
| ≥35·0 | 25 | 77·9 | 20·9 | 12·0 | ||
| Mother’s serum 25(OH)D status | <0·0001 | 0·0004 | ||||
| <50 nmol/l | 5 | 57·5 | 25·6 | 40·0 | ||
| 50–<75 nmol/l | 74 | 70·7 | 15·4 | 6·8 | ||
| ≥75 nmol/l | 138 | 84·9 | 16·5 | 0·7 | ||
| Father’s serum 25(OH)D status | 0·01 | 0·06 | ||||
| <50 nmol/l | 10 | 69·5 | 20·5 | 20·0 | ||
| 50–<75 nmol/l | 76 | 76·5 | 17·8 | 4·0 | ||
| ≥75 nmol/l | 129 | 81·3 | 17·3 | 2·3 | ||
| Number of home assets║ | 0·45 | 0·73 | ||||
| 0–4 | 41 | 86·3 | 20·3 | 0·0 | ||
| 5–7 | 89 | 76·5 | 17·5 | 4·5 | ||
| 8–9 | 41 | 76·8 | 17·9 | 7·3 | ||
| 10–12 | 52 | 81·2 | 15·9 | 1·9 | ||
| Food insecurity in the household | 0·07 | 0·25 | ||||
| None | 73 | 76·9 | 16·2 | 6·9 | ||
| Mild | 60 | 79·7 | 17·2 | 1·7 | ||
| Moderate | 50 | 79·8 | 18·6 | 2·0 | ||
| Severe | 39 | 84·1 | 21·4 | 2·6 | ||
| Country, latitude | 0·005 | 0·10 | ||||
| Guatemala, 14·6° | 28 | 70·7 | 10·0 | 0·0 | ||
| El Salvador, 13·7° | 29 | 79·0 | 18·2 | 6·9 | ||
| Dominican Republic, 18·5° | 29 | 78·5 | 13·3 | 0·0 | ||
| Honduras, 14·1° | 27 | 77·8 | 14·8 | 0·0 | ||
| Nicaragua, 12·1° | 31 | 94·0 | 23·5 | 3·2 | ||
| Costa Rica, 9·6° | 26 | 76·9 | 19·6 | 11·5 | ||
| Belize, 17·3° | 26 | 81·0 | 19·7 | 7·7 | ||
| Mexico, 16·8° | 27 | 76·0 | 13·3 | 0·0 | ||
| Month of measurement | 0·67 | 0·63 | ||||
| Jan–Feb | 13 | 74·2 | 14·4 | 0·0 | ||
| Mar–Apr | 47 | 80·3 | 15·7 | 4·3 | ||
| May–Jun | 20 | 78·1 | 16·0 | 0·0 | ||
| Jul–Sep | 62 | 80·9 | 18·7 | 1·6 | ||
| Oct–Dec | 81 | 79·0 | 19·9 | 6·2 | ||
Totals may be less than 223 because of missing values.
Test for trend for ordinal variables. χ 2 score test for sex, country and month of measurement.
Cochrane–Armitage exact test for trend for ordinal variables. Fisher’s exact test for sex, country and month of measurement.
According to the WHO reference( 15 ).
From a list that included car, bicycle, refrigerator, gas stove, electric stove, blender, microwave, washing machine, colour television, stereo, computer, and having in-home access to the Internet.
Table 5.
Serum 25-hydroxyvitamin D (25(OH)D) concentrations according to food intake frequency and time spent on outdoor activities in children from eight Mesoamerican countries, 2011–2013
| Mean | sd | |||||
|---|---|---|---|---|---|---|
| n * | nmol/l | P † | % <50 nmol/l | P ‡ | ||
| Food intake frequency | ||||||
| Dairy§ | 0·002 | 0·63 | ||||
| <5/week | 58 | 75·0 | 15·1 | 3·5 | ||
| 5/week to <1·5/d | 61 | 76·0 | 16·5 | 6·6 | ||
| 1·5–<2·5/d | 50 | 81·8 | 18·8 | 2·0 | ||
| ≥2·5/d | 43 | 84·2 | 17·9 | 2·3 | ||
| Eggs║ | 0·51 | 0·51 | ||||
| <3/week | 41 | 78·5 | 17·4 | 2·4 | ||
| 3/week | 50 | 76·9 | 19·3 | 8·0 | ||
| 4–6/week | 67 | 79·3 | 16·5 | 3·0 | ||
| ≥1/d | 54 | 79·9 | 16·6 | 1·9 | ||
| Canned tuna/sardines¶ | 0·31 | 0·74 | ||||
| <1/month | 104 | 77·6 | 17·3 | 4·8 | ||
| 1–3/month | 43 | 81·1 | 15·3 | 0·0 | ||
| 1/week | 40 | 75·2 | 16·9 | 7·5 | ||
| ≥2/week | 25 | 85·1 | 19·9 | 0·0 | ||
| Outdoor activities | ||||||
| Time spent playing outdoors (h/week) | 0·10 | 0·64 | ||||
| <1 | 60 | 74·4 | 17·3 | 3·3 | ||
| 1–10 | 55 | 82·3 | 18·6 | 3·6 | ||
| 11–20 | 58 | 82·0 | 18·3 | 1·7 | ||
| ≥21 | 49 | 79·7 | 17·5 | 6·1 | ||
Totals may be less than 223 because of missing values.
Test for trend for ordinal variables.
Cochrane–Armitage exact test for trend for ordinal variables.
Sum of weighted intake frequencies of milk (200 g), cheese (20 g), cream (6 g) and ice cream (60 g).
Sum of weighted intake frequencies of one hard boiled or fried egg (60 g).
Intake of 25 g.
Data analysis
Adults and children were analysed separately. Samples of children from Panama were not available, thus the analytic sample comprised 492 adults and 223 children.
We first compared mean and sd serum 25(OH)D concentrations by categories of sociodemographic characteristics, food intake frequency and time spent on outdoor activities. For ordinal predictors we conducted tests for linear trend using linear regression models, with 25(OH)D concentration as the continuous outcome and a variable representing ordinal categories of the predictor as a continuous covariate. For nominal predictors, we used χ 2 score statistics. Next, we estimated the prevalence of serum 25(OH)D <50 nmol/l by levels of sociodemographic, diet and outdoor activity characteristics. For ordinal predictors in adults, we performed tests for linear trend with the use of Poisson regression models, with serum 25(OH)D <50 nmol/l as the dichotomous outcome and a variable representing ordinal categories of the predictor as a continuous covariate. An exchangeable correlation structure was specified in all models of adults to account for intrafamily clustering. For nominal predictors, we used the χ 2 score test. In children, we used the Cochrane–Armitage exact test for trend for ordinal predictors. For nominal predictors, we used the Fisher’s exact test.
We estimated adjusted differences with 95 % CI in mean serum 25(OH)D concentrations by categories of exposure variables with the use of multivariable linear regression. We included predictors that were significantly associated with the outcome in bivariate analysis at P<0·10; predictors that remained significant (P<0·05) in multivariable analysis were retained in the final models. Robust estimates of the variance were calculated in all models to overcome potential deviations from the multivariate normality assumption( 16 ).
Family aggregation
We estimated Pearson correlations with 95 % CI of serum 25(OH)D concentrations between mothers and fathers, mothers and children, and fathers and children. Next, we used linear random-effects models with serum 25(OH)D as the continuous outcome and a random effect for family to partition the variance into within-family and between-family components. We also considered fixed effects for age and sex to account for extraneous between-person variation. The intraclass correlation coefficient (ICC) was calculated as the ratio of the between-family variance to the sum of the between- and within-family variances. 95 % CI were obtained using the delta method( 17 ). Higher values for an ICC imply that people from the same family would have more similar serum 25(OH)D concentrations than people from different families. We then estimated the ICC among mothers and fathers, mothers and children, and fathers and children, and compared them with the ICC for the whole family to examine which combinations of family membership were associated with greater aggregation. Higher mother–father than mother–child or father–child aggregation could indicate a greater role for shared environment rather than shared genetics in family clustering of 25(OH)D since mothers and fathers are typically not genetically related. In supplemental analysis, we considered whether potential clustering by country could affect the results on family aggregation.
Results
The mean (sd) age of mothers, fathers and children at enrolment was 37·0 (6·4), 40·6 (8·3) and 10·0 (1·7) years, respectively; 53·4 % of children were girls. Sociodemographic characteristics, food intake frequencies and time spent on outdoor activities varied by country (see online supplementary material, Supplemental Table 1).
Vitamin D correlates in adults
Among adults, mean (sd) serum 25(OH)D concentration was 81·3 (21·1) nmol/l; 82·5 (21·8) nmol/l in mothers and 80·1 (20·4) nmol/l in fathers. The prevalence of serum 25(OH)D <30, <50 and <75 nmol/l was, respectively, 0·2, 3·9 and 39·8 %. Mean serum 25(OH)D concentrations were inversely related to education level and number of home assets. Participants from Nicaragua and those with measurements obtained in May or June had the highest concentrations (Table 1). The prevalence of VDD was higher in adults with more household assets compared with that in adults with fewer home assets. Prevalence of VDD in adults varied across countries, ranging from 0·0 % in Mexico to 9·4 % in Costa Rica. Mean serum 25(OH)D concentrations were positively associated with time spent gardening (Table 2). In multivariable analysis, mean serum 25(OH)D concentrations varied significantly by country (P<0·0001); the highest concentrations were in Nicaragua, Mexico and Belize whereas the lowest were in Panama, Guatemala and the Dominican Republic (Table 3). Compared with people who never gardened, adjusted mean serum 25(OH)D concentration was 4·8 nmol/l higher (95 % CI 0·0, 9·6 nmol/l) in people who gardened for 1–6 h/week and 6·4 nmol/l higher (95 % CI −0·6, 13·3 nmol/l) in people who gardened for ≥1 h/d (P-trend=0·03). Month of measurement was not associated with mean serum 25(OH)D concentrations in multivariable analysis.
Table 3.
Multivariable-adjusted correlates of serum 25-hydroxyvitamin D (25(OH)D) concentrations in adults from nine Mesoamerican countries, 2011–2013
| Adjusted difference* | 95 % CI | ||
|---|---|---|---|
| nmol/l | P † | ||
| Country, latitude | <0·0001 | ||
| Guatemala, 14·6° | Reference | ||
| El Salvador, 13·7° | 8·6 | 3·2, 13·9 | |
| Dominican Republic, 18·5° | 1·8 | −3·6, 7·1 | |
| Honduras, 14·1° | 6·7 | 0·4, 13·0 | |
| Nicaragua, 12·1° | 24·7 | 16·8, 32·5 | |
| Panama, 9·0° | −2·3 | −9·0, 4·4 | |
| Costa Rica, 9·6° | 5·8 | −1·5, 13·2 | |
| Belize, 17·3° | 9·7 | 3·4, 15·9 | |
| Mexico, 16·8° | 11·7 | 5·5, 17·8 | |
| Gardening | 0·03 | ||
| Never | Reference | ||
| 1–3 h/month | 0·4 | −4·7, 5·5 | |
| 1–6 h/week | 4·8 | 0·0, 9·6 | |
| ≥1 h/d | 6·4 | −0·6, 13·3 | |
From multivariable linear regression with serum 25(OH)D as the continuous outcome. Predictors included country and gardening. An exchangeable correlation structure was specified to account for intrafamily clustering.
For country, χ 2 score test. For gardening, test for linear trend when a variable representing ordinal categories of gardening time was included as a continuous predictor.
Vitamin D correlates in children
Among children, mean (sd) serum 25(OH)D concentration was 79·5 (18·1) nmol/l. The prevalence of serum 25(OH)D <30, <50 and <75 nmol/l was, respectively, 0·0, 3·6 and 44·4 %. Mean serum 25(OH)D concentrations were positively related to mother’s height, parental 25(OH)D concentrations and Nicaraguan origin (Table 4). The prevalence of VDD was higher in children whose mothers had higher BMI compared with children whose mothers had lower BMI. In addition, the prevalence of VDD was higher in children whose mothers had serum 25(OH)D concentration of <50 or 50–<75 nmol/l compared with children whose mothers’ serum 25(OH)D concentration was ≥75 nmol/l. Prevalence of VDD ranged from 0·0 % in Guatemala, the Dominican Republic, Honduras and Mexico to 11·5 % in Costa Rica. Mean serum 25(OH)D concentrations were positively associated with frequency of dairy intake (Table 5). In multivariable analysis, mean serum 25(OH)D concentrations were positively associated with male sex, mother’s 25(OH)D concentrations and dairy intake frequency (Table 6). Every 1 nmol/l of maternal serum 25(OH)D was related to an adjusted 0·3 nmol/l higher 25(OH)D concentration in the children (95 % CI 0·2, 0·5 nmol/l; P<0·0001). Mean 25(OH)D concentration among children with dairy intake ≥2·5 times/d was 6·8 nmol/l higher than that of children with intake <5 times/week (95 % CI 0·7, 12·9 nmol/l; P=0·03). Finally, mean serum 25(OH)D concentrations were inversely related to mother’s BMI and to having >4 home assets. Maternal height and country of origin were not associated with the children’s serum 25(OH)D concentrations in multivariable analysis.
Family aggregation
Serum 25(OH)D concentrations were positively correlated between family members (Table 7). The mother–child correlation (r=0·49, P<0·0001) was higher than the father–child (r=0·24, P=0·0004) or mother–father (r=0·23, P=0·0004) correlation. Thirty-one per cent of the total variance in serum 25(OH)D was explained by family membership. Adjustment for age and sex did not change the results (adjusted ICC=0·32; 95 % CI 0·24, 0·41). Family membership explained more variability in 25(OH)D concentration than country membership, which only explained 12 %. Mother–child aggregation of serum 25(OH)D concentrations was higher than overall family, father–child or mother–father aggregation.
Table 7.
Family aggregation of serum 25-hydroxyvitamin D (25(OH)D) in eight Mesoamerican countries, 2011–2013
| Mothers, fathers, and children | Mothers and fathers | Mothers and children | Fathers and children | |||||
|---|---|---|---|---|---|---|---|---|
| Correlation, r * (95 % CI) | – | 0·23 | 0·10, 0·35 | 0·49 | 0·38, 0·58 | 0·24 | 0·11, 0·36 | |
| Intraclass correlation† | ||||||||
| Within-family variance | 282·8 | 345·0 | 215·7 | 285·3 | ||||
| Between-family variance | 127·1 | 101·7 | 191·6 | 87·6 | ||||
| ICC‡ (95 % CI) | 0·31 | 0·23, 0·40 | 0·23 | 0·13, 0·37 | 0·47 | 0·37, 0·57 | 0·23 | 0·13, 0·38 |
Pearson correlation coefficient.
Variance partitioned from a linear random-effects model with serum 25(OH)D as the continuous outcome and a random effect for family. Separate models were fitted to partition the variance for each combination of two family members.
Intraclass correlation coefficient (ICC)=
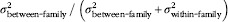 . 95 % CI were calculated using the delta method(
17
).
. 95 % CI were calculated using the delta method(
17
).
Discussion
In the current study of Mesoamerican families, the overall prevalence of serum 25(OH)D concentration <50 nmol/l was relatively low for both adults (3·9 %) and children (3·6 %). Only one person had a serum 25(OH)D concentration <30 nmol/l, the threshold for deficiency proposed by the US Institute of Medicine( 13 ). Although the burden of VDD in Latin America is not well documented, the prevalence of VDD in our study is among the lowest reported in the region. For example, the prevalence of VDD in the National Survey of Nutrition and Health in Mexican adults was 9·8 % in 2006( 18 ). No other country in the Mesoamerican region has conducted a nationwide survey to estimate vitamin D serostatus. Studies of Puerto Rican adults with different underlying health conditions found higher prevalence of VDD, ranging from 14 to 43 %( 2 , 3 , 19 – 21 ). Nevertheless, neither these investigations nor our study were representative and the results may not be directly comparable.
Prevalence estimates of VDD among children and adolescents in the Mesoamerican region are variable. In asthmatic school-aged children from Costa Rica( 22 ) and among Afro-Caribe Guatemalan adolescents( 1 ), the prevalence of VDD was 3·4 and 5·0 %, respectively. These estimates are comparable to ours; however, among children from Southern Mexico (5–11 years old) in 2012 and adolescents (13–19 years old) in 2006 the prevalence of VDD was 29·0 %( 23 ) and 11·5 %( 18 ), respectively. These values are substantially higher than the estimates in our study. Similarly, VDD was much more prevalent in indigenous Mayan adolescents living near the coast (21 %)( 1 ) than in our study from Mesoamerica. These differences suggest that there is high within- and between-country variation in vitamin D status in children from the Mesoamerican region.
In our investigation vitamin D status varied substantially by country, although the country-specific sample sizes were too small to allow for robust statistical inference. Adults and children from Nicaragua had the highest mean serum 25(OH)D concentrations. Reasons to explain this difference are speculative but could be related to socio-economic or dietary differences since Nicaraguans in the present study had fewer home assets and higher dairy intake than participants from other countries and these variables were associated with higher 25(OH)D concentrations.
We found that mean serum 25(OH)D concentrations in adults were positively related to time spent gardening but not to other outdoor activities. Time spent on different outdoor activities may exert distinct effects on serum 25(OH)D levels because they could vary in the factors that ultimately influence the amount of vitamin D produced in the skin, including the amount of skin exposed, the time of day of sun exposure, the use of sunscreen and the absolute amount of time exposed to the sun. For example, among elderly adults in Europe 25(OH)D concentrations were positively associated with gardening and cycling but not with walking outdoors( 24 ). The body postures during gardening may expose a larger skin surface to the sun compared with walking. In addition, the majority of ambient UV radiation occurs between 10.00 and 16.00 hours( 25 ). Perhaps people tend to garden within these hours, whereas other outdoor activities may occur outside this interval. In a study of Puerto Rican medical residents, time spent outdoors between 10.00 and 16.00 hours was positively associated with serum 25(OH)D( 19 ).
We did not find associations between age, sex or dietary sources of vitamin D and serum 25(OH)D concentrations among adults. A relationship of vitamin D status with age or sex has not been consistently demonstrated in previous studies of Mesoamerican adults( 2 , 3 , 18 – 21 , 26 ). Contrary to our findings, in a study of Puerto Rican adults with BMI ≥25·0 kg/m2, a composite measure representing intake of vitamin D supplements, fish, margarine and milk( 20 ) was positively related to serum 25(OH)D concentrations. The main source of dietary vitamin D in that study was supplement intake, whereas in our study population supplement use was negligible. Discrepancies in the results might be explained by differences in supplement or food intake between populations.
In children, mean serum 25(OH)D concentrations were positively associated with male sex, frequency of dairy intake, and both maternal height and serum 25(OH)D concentration. On the other hand, maternal BMI and the number of home assets were inversely related to 25(OH)D concentrations. Consistent with previous studies, boys had higher concentrations of serum 25(OH)D than girls( 1 , 22 , 23 , 27 ). Explanations for this difference are speculative; they could be due to sex-related variation in intake of foods with high vitamin D content or in time spent playing outdoors. Boys in the current study may have been less likely to wear sunscreen or may have had greater body surface exposure to sunlight compared with girls. We did not find a relationship between age and mean serum 25(OH)D in children, possibly because the age range in our study was limited. Mother’s height was positively related to children’s mean serum 25(OH)D concentrations, perhaps reflecting an association with socio-economic status since adult height can be an indicator of socio-economic status( 28 ). There was an inverse association between mothers’ BMI and children’s mean serum 25(OH)D concentrations. An association between mothers’ pre-pregnancy BMI and infants’ vitamin D status has been found previously( 29 ). The mechanisms by which maternal BMI may influence children’s vitamin D status are largely unknown. Obese women transfer vitamin D to their offspring less effectively at birth( 29 ), but this would not necessarily explain an association during middle childhood. On the other hand, the mother’s BMI may be positively correlated with the child’s and adiposity is inversely associated with circulating 25(OH)D concentrations( 25 ). Nevertheless, we did not find an association between the children’s BMI and vitamin D status. Mothers’ and children’s serum 25(OH)D concentrations were strongly correlated, consistent with findings from a cross-sectional study of Korean adolescents( 30 ). This possibly reflects the effect of genetic and environmental exposures that are shared between mothers and their children. Serum 25(OH)D concentrations were lower in children from households with >4 home assets compared with those from households with ≤4 assets. This inverse association between a marker of socio-economic status and 25(OH)D is consistent with results from other studies in the region( 18 , 23 , 27 ). Finally, in children, frequency of dairy intake was positively related to 25(OH)D concentrations. Fortification of milk with vitamin D is required in Guatemala, Honduras and Mexico( 31 ) and in other countries some fortified milk is available. Randomized controlled trials of vitamin D-fortified foods demonstrate that intake of fortified foodstuffs results in higher 25(OH)D concentrations( 32 ). Of note, dairy intake in our study was related to 25(OH)D concentrations in children but not in adults. This could be because the absolute consumption of dairy is usually higher and more variable in children than in adults( 33 ).
In our study, about a third of the variability in serum 25(OH)D concentration was explained by family membership. Family aggregation of serum 25(OH)D concentrations suggests that it may be effective to target families, rather than individuals, for screening of VDD and to implement interventions designed to improve vitamin D status. Family aggregation can be due to both shared genes and shared environment. Estimates of heritability of circulating 25(OH)D from twin studies range from 37 to 86 %( 5 , 6 , 34 – 37 ). This could be due to sharing of gene polymorphisms that are associated with 25(OH)D concentration( 38 ). We found that correlation and aggregation between mothers’ and fathers’ 25(OH)D concentrations were similar to those between fathers and children, but lower than those between mothers and children. Because mothers and fathers are expected to be genetically unrelated, these comparisons could indicate that shared environmental factors play an important role in the vitamin D serostatus of this population, over that played by shared genetics. For example, sun exposure behaviours may aggregate by family. In Denmark, children’s sun exposure-related behaviours were correlated with their mothers’ but not with their fathers’( 39 ).
The present study has several strengths. Serum 25(OH)D concentrations are widely used in epidemiological studies as an indicator of vitamin D status since they integrate dietary and sunlight sources of vitamin D. The design involving families allowed for the examination of serum 25(OH)D correlates in adults and children, as well as aggregation within families. The use of standardized methods allowed for comparisons across countries where data were not previously available. There are limitations as well. First, the cross-sectional nature of the study limits the ability to make causal inference. Second, the samples were not representative of the underlying populations. Selection bias might have occurred if the probability of inclusion in the study was not independent of the exposures and outcomes examined. Also, the relatively small within-country samples prevented country-specific analysis. The FFQ that we used has not been validated in children. In addition, information on skin pigmentation, total time spent outdoors or sunscreen use was not available. Assuming that some adult participants use sunscreen, the magnitude of the underlying effect of gardening on mean serum 25(OH)D concentration could have been underestimated. Finally, the DiaSorin LIAISON 25-OH Vitamin D Total assay we used to measure 25(OH)D may underestimate its concentration compared with the gold standard of liquid chromatography–tandem mass spectrometry. This may lead to an overestimation of VDD at the population level( 40 ).
In conclusion, the prevalence of VDD was low in a group of adults and children from the Mesoamerican region. Serum 25(OH)D concentrations vary by country in both adults and children and exhibit a moderate degree of aggregation by family. Nationally representative surveys of vitamin D status are needed to determine individual- and country-level predictors of VDD in the region, including the effects of skin pigmentation, the use of sunscreen, clothing habits, environmental toxicants and air pollution.
Acknowledgements
Financial support: This study was funded by the US National Heart, Lung, and Blood Institute (contract number BAA-NHLBI-HV-09-12). The funder did not play any role in the design, conduct or interpretation of the study. Conflicts of interest: None of the authors have any conflicts of interest or financial disclosures. Authorship: M.R.-Z., A.V.R. and E.V. designed the research. S.L.R. and E.V. performed the statistical analyses, wrote the paper and had primary responsibility for the final content. All authors have read and approved the final version of the manuscript. Ethics of human subject participation: The research was conducted in accordance with guidelines laid down by the Declaration of Helsinki. The study was approved by the Health Sciences and Behavioral Sciences Institutional Review Board at the University of Michigan and by the ethics committees of all nine collaborating institutions.
Participants in the Nine Mesoamerican Countries Metabolic Syndrome Study (NiMeCoMeS) Group. Universidad de Ciencias y Artes, Tuxtla Gutiérrez, Chiapas, México: Erika López, Liz Peña, Alejandra Maldonado, Aldeni Vásquez, Aldrin López. University of Belize: Lilly Mahung, Diomar Salazar. Instituto de Nutrición de Centroamérica y Panamá (INCAP), Guatemala: Fernanda Kroker, María Alejandra Córdova, Regina García, Lilian Navas. Universidad Nacional de El Salvador: Josefina Sibrián, Mauricio Flores, Noel Avalos. Universidad de Honduras: Astarté Alegría, Jorge A. Sierra, Héctor Murillo. Universidad Nacional Autónoma de Nicaragua: Ana María Gutiérrez, Carmen María Flores, Mario Romero. Universidad de Costa Rica: Emilce Ulate, Natalia Valverde, Andrea Fiatt, Juan Manuel Valverde. Universidad de Panamá: Flavia Fontes, Raisa Rodríguez, Emérita Pons, Lino Chue, Elka González. Universidad de República Dominicana: Rafael Montero, Francisco Torres, Amarilis Then, Melvi Pérez.
Contributor Information
Collaborators: for the Nine Mesoamerican Countries Metabolic Syndrome Study (NiMeCoMeS) Group
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S1368980017001616.
click here to view supplementary material
References
- 1. Naqvi A, Solomons NW, Campos R et al. (2016) Vitamin D status among indigenous Mayan (Kekchi) and Afro-Caribe (Garifuna) adolescents from Guatemala: a comparative description between two ethnic groups residing on the Rio Dulce at the Caribbean coast in Izabal Province, Guatemala. Public Health Nutr (Epublication ahead of print version). [DOI] [PMC free article] [PubMed]
- 2. Diaz GM, Gonzalez L, Ramos-Trautmann G et al. (2016) Vitamin D status is associated with metabolic syndrome in a clinic-based sample of Hispanic adults. Metab Syndr Relat Disord 14, 259–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gonzalez L, Ramos-Trautmann G, Diaz-Luquis GM et al. (2015) Vitamin D status is inversely associated with obesity in a clinic-based sample in Puerto Rico. Nutr Res 35, 287–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Carrillo-Vega MF, Garcia-Pena C, Gutierrez-Robledo LM et al. (2017) Vitamin D deficiency in older adults and its associated factors: a cross-sectional analysis of the Mexican Health and Aging Study. Arch Osteoporos 12, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Orton S, Morris AP, Herrera BM et al. (2008) Evidence for genetic regulation of vitamin D status in twins with multiple sclerosis. Am J Clin Nutr 88, 441–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Karohl C, Su S, Kumari M et al. (2010) Heritability and seasonal variability of vitamin D concentrations in male twins. Am J Clin Nutr 92, 1393–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Villamor E, Finan CC, Ramirez-Zea M et al. (2017) Prevalence and sociodemographic correlates of metabolic syndrome in school-aged children and their parents in nine Mesoamerican countries. Public Health Nutr 20, 255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Perez-Escamilla R, Melgar-Quiñonez H, Nord M et al. (2007) Escala Latinoamericana y Caribeña de Seguridad Alimentaria (ELCSA) (Latinamerican and Caribbean Food Security Scale). Perspect Nutr Hum (Colombia) Suppl., 117–134. [Google Scholar]
- 9. Kabagambe EK, Baylin A, Allan DA et al. (2001) Application of the method of triads to evaluate the performance of food frequency questionnaires and biomarkers as indicators of long-term dietary intake. Am J Epidemiol 154, 1126–1135. [DOI] [PubMed] [Google Scholar]
- 10. Campos H, Willett WC, Peterson RM et al. (1991) Nutrient intake comparisons between Framingham and rural and urban Puriscal, Costa Rica associations with lipoproteins, apolipoproteins, and low density lipoprotein particle size. Arterioscler Thromb 11, 1089–1099. [DOI] [PubMed] [Google Scholar]
- 11. Horst RL (2010) Exogenous versus endogenous recovery of 25-hydroxyvitamins D2 and D3 in human samples using high-performance liquid chromatography and the DiaSorin LIAISON Total-D Assay. J Steroid Biochem Mol Biol 121, 180–182. [DOI] [PubMed] [Google Scholar]
- 12. Holick MF, Binkley NC, Bischoff-Ferrari HA et al. (2011) Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 96, 1911–1930. [DOI] [PubMed] [Google Scholar]
- 13. Institute of Medicine (2011) Dietary Reference Intakes for Calcium and Vitamin D. Washington, DC: The National Academies Press. [PubMed] [Google Scholar]
- 14. World Health Organization (1995) Physical Status: The Use and Interpretation of Anthropometry. Report of a WHO Expert Committee. WHO Technical Report Series no. 854. Geneva: WHO. [PubMed]
- 15. de Onis M (2007) Development of a WHO growth reference for school-aged children and adolescents. Bull World Health Organ 85, 660–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. White H (1980) A heteroskedasticity-consistent covariance matrix estimator and a direct test for heteroskedasticity. Econometrica 48, 817–830. [Google Scholar]
- 17. Hankinson SE, Manson JE, Spiegelman D et al. (1995) Reproducibility of plasma hormone levels in postmenopausal women over a 2–3 year period. Cancer Epidemiol Biomarkers Prev 4, 649–654. [PubMed] [Google Scholar]
- 18. Flores M, Sanchez Romero LM, Macias N et al. (2011) Concentraciones Séricas de Vitamina D en Niños, Adolescentes y Adultos Mexicanos. Resultados de la ENSANUT 2006 (Serum Concentrations of Vitamin D in Mexican Children, Adolescents, and Adults. Results of ENSANUT 2006). Cuernavaca, Mexico; Instituto Nacional de Salud Publica.
- 19. Hernandez Davila L, Rodriguez Rivera N, Lopez Valentin M et al. (2015) Prevalence of vitamin D insufficiency and deficiency among medical residents of the University Hospital in San Juan, Puerto Rico. P R Health Sci J 34, 83–88. [PMC free article] [PubMed] [Google Scholar]
- 20. Palacios C, Gil K, Perez CM et al. (2012) Determinants of vitamin D status among overweight and obese Puerto Rican adults. Ann Nutr Metab 60, 35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Suarez-Martinez EB, Perez CM, Cruz SK et al. (2013) Importance of vitamin D and vitamin D levels status in Puerto Ricans. J Health Care Poor Underserved 24, 38–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Brehm JM, Celedon JC, Soto-Quiros ME et al. (2009) Serum vitamin D levels and markers of severity of childhood asthma in Costa Rica. Am J Respir Crit Care Med 179, 765–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Flores A, Flores M, Macias N et al. (2017) Vitamin D deficiency is common and is associated with overweight in Mexican children aged 1–11 years. Public Health Nutr 20, 1807–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. van den Heuvel EG, van Schoor N, de Jongh RT et al. (2013) Cross-sectional study on different characteristics of physical activity as determinants of vitamin D status; inadequate in half of the population. Eur J Clin Nutr 67, 360–365. [DOI] [PubMed] [Google Scholar]
- 25. Holick MF (2005) The vitamin D epidemic and its health consequences. J Nutr 135, issue 11, 2739S–2748S. [DOI] [PubMed] [Google Scholar]
- 26. Caro Y, Negron V & Palacios C (2013) Association between vitamin D levels and blood pressure in a group of Puerto Ricans. P R Health Sci J 31, 123–129. [PMC free article] [PubMed] [Google Scholar]
- 27. Brehm JM, Acosta-Perez E, Klei L et al. (2012) Vitamin D insufficiency and severe asthma exacerbations in Puerto Rican children. Am J Respir Crit Care Med 186, 140–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Subramanian SV, Ozaltin E & Finlay JE (2011) Height of nations: a socioeconomic analysis of cohort differences and patterns among women in 54 low- to middle-income countries. PLoS One 6, e18962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Josefson JL, Feinglass J, Rademaker AW et al. (2013) Maternal obesity and vitamin D sufficiency are associated with cord blood vitamin D insufficiency. J Clin Endocrinol Metab 98, 114–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kim SH, Oh MK, Namgung R et al. (2014) Prevalence of 25-hydroxyvitamin D deficiency in Korean adolescents: association with age, season and parental vitamin D status. Public Health Nutr 17, 122–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bowley A (editor) (2003) Mandatory food enrichment. NUTRIVIEW, Special Issue 2003. Basel: Roche Vitamins Europe Ltd.
- 32. Black LJ, Seamans KM, Cashman KD et al. (2012) An updated systematic review and meta-analysis of the efficacy of vitamin D food fortification. J Nutr 142, 1102–1108. [DOI] [PubMed] [Google Scholar]
- 33. Quann EE, Fulgoni VL 3rd & Auestad N (2015) Consuming the daily recommended amounts of dairy products would reduce the prevalence of inadequate micronutrient intakes in the United States: diet modeling study based on NHANES 2007–2010. Nutr J 14, 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Arguelles LM, Langman CB, Ariza AJ et al. (2009) Heritability and environmental factors affecting vitamin D status in rural Chinese adolescent twins. J Clin Endocrinol Metab 94, 3273–3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hunter D, De Lange M, MacGregor A et al. (2001) Genetic contribution to bone metabolism, calcium excretion, and vitamin D and parathyroid hormone regulation. J Bone Miner Res 16, 371–378. [DOI] [PubMed] [Google Scholar]
- 36. Mills NT, Wright MJ, Henders AK et al. (2015) Heritability of transforming growth factor-β1 and tumor necrosis factor-receptor type 1 expression and vitamin D levels in healthy adolescent twins. Twin Res Hum Genet 18, 28–35. [DOI] [PubMed] [Google Scholar]
- 37. Snellman G, Melhus H, Gedeborg R et al. (2009) Seasonal genetic influence on serum 25-hydroxyvitamin D levels: a twin study. PLoS One 4, e7747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dastani Z, Li R & Richards B (2013) Genetic regulation of vitamin D levels. Calcif Tissue Int 92, 106–117. [DOI] [PubMed] [Google Scholar]
- 39. Bodekaer Larsen M, Petersen B, Philipsen PA et al. (2014) Sun exposure and protection behavior of Danish farm children: parental influence on their children. Photochem Photobiol 90, 1193–1198. [DOI] [PubMed] [Google Scholar]
- 40. Roth HJ, Schmidt-Gayk H, Weber H et al. (2008) Accuracy and clinical implications of seven 25-hydroxyvitamin D methods compared with liquid chromatography–tandem mass spectrometry as a reference. Ann Clin Biochem 45, 153–159. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit https://doi.org/10.1017/S1368980017001616.
click here to view supplementary material


