Abstract
Background:
Prognostic biomarker research neonatal sepsis is lacking. We assessed the utility of a validated pediatric prognostic tool called PERSEVERE II that uses decision tree methodology to predict mortality at discharge in neonates who experienced sepsis.
Methods:
Prospective study in a dual-center cohort of neonates with sepsis admitted between June 2020 and December 2021. Biomarker analysis was done on serum samples obtained at the time of evaluation for the event.
Results:
In a cohort of 59 neonates with a mortality rate of 15.3%, PERSEVERE II was 67% sensitive and 59% specific for mortality, p 0.27. Amongst PERSEVERE II biomarkers, IL-8 showed good prognostic performance for mortality prediction with a cutoff of 300 pg/mL (sensitivity 100%, specificity 65%, negative predictive value 100%, AUC 0.87, p 0.0003). We derived a new decision tree that is neonate specific (nPERSEVERE) with improved performance compared to IL-8 (sensitivity 100%, specificity 86%, negative predictive value 100%, AUC 0.95, p <0.0001).
Conclusions:
IL-8 and nPERSEVERE demonstrated good prognostic performance in a small cohort of neonates with sepsis. Moving towards precision medicine in sepsis, our study proposes an important tool for clinical trial prognostic enrichment which needs to be validated in larger studies.
Category of study: Clinical research
Introduction
Sepsis remains a major cause of mortality and morbidity in neonates, with the very low birth weight population being the most vulnerable1, 2. Biologically plausible interventions failed to show impact on mortality from neonatal sepsis3, 4, which could be explained -in part- by heterogeneity of neonatal sepsis and unequal baseline mortality risk in study arms. Biomarker research could help in prognostication in neonatal sepsis, yet studies are heavily skewed towards early diagnosis of culture-positive sepsis. Biomarkers such as CRP5, IL-66, IL-87, and IL-108 have been tested in various settings for this purpose, but study findings have not changed current clinical practice.
PERSEVERE (Pediatric Sepsis Biomarker Risk Model) was developed as a tool to identify pediatric patients with sepsis at high mortality risk in the PICU based on five serum biomarkers: CCL3 (CC Chemokine Ligand 3), IL-8 (Interleukin-8), HSP A1b (Heatshock Protein A1b), GZMB (Granzyme B), and MMP-8 (Matrix Metallopeptidase 8)9. These biomarkers were chosen after identifying gene probes that were differentially expressed in pediatric patients with septic shock that did not survive and were reported to have a role in the pathophysiology of septic shock10. This prognostic tool has been validated in pediatric and adult cohorts with good performance in identifying high-risk patients early in the disease course9, 11, 12, 13. The most recent iteration of PERSEVERE is called PERSEVERE II9, which includes platelet count in addition to the abovementioned biomarkers, and it has shown good performance in a large pediatric cohort of 461 patients. PERSEVERE II is 86% sensitive and 69% specific for mortality with a negative predictive value of 97% and an area under the curve of 0.83.
Given the paucity of prognostic biomarker research in neonatal sepsis, we conducted a prospective study to assess the utility of PERSEVERE II and its biomarkers as possible prognostic tools in neonatal sepsis.
Material and Methods
Study Population
This prospective cohort study was approved by the institutional review board at Cincinnati Children’s Hospital Medical Center and the University of Cincinnati prior to data and specimen collection. The study was approved with waiver of consent given that serum for analysis was obtained from residual whole blood samples in the clinical laboratory and the study protocol did not alter or inform clinical care. Neonates were enrolled from the Cincinnati Children’s Hospital Medical Center level IV NICU and the level III NICU at the University of Cincinnati Medical Center between June 2020 to December 2021. All neonates who had a complete blood count performed were screened for enrollment using Vigilanz reporting system (Vigilanz Corp, MN). Clinical and laboratory data was obtained and stored in a secured REDCap14 database.
Definitions
Neonates in the sepsis cohort were enrolled if they fit the following criteria:
- Suspected to have an infection as evident by obtaining whole blood counts, initiating antibiotic therapy, and with evidence of systemic inflammatory response by having two of the following (one must be number 1 or 2):
- Leukocytosis, or leukopenia, or immature shift, or elevated CRP in preterm infants only
- Temperature instability
- Elevated respiratory rate for greater than 2 hours
- Elevated or depressed heart rate for greater than 2 hours
Cardiac, respiratory, hepatic, neurologic, and hematologic dysfunctions were defined as suggested by Wynn et al., 201015. Renal dysfunction was defined as acute kidney injury according to KIDGO modified neonatal definitions16. Organ injury data was collected over the course of 7 days from the time of enrollment. The neonate was assumed to not have an organ-specific dysfunction if the clinical team did not obtain the laboratory test that is used to define that dysfunction. Please refer to the supplementary document for full details on enrollment and definitions.
Neonates were excluded if antibiotics were discontinued before 48 hours or if the neonate was found to have an underlying lethal diagnosis or a cardiac defect requiring intervention in the neonatal period. We did not exclude infants with congenital anomalies.
Primary outcome of the study was in hospital mortality. Secondary outcomes were the occurrence of complicated course (defined as in hospital mortality or 2 or more organ dysfunctions on day 7 of illness), vasopressor use, and duration of vasopressor use.
Neonates without inflammation were identified if they had whole blood count obtained without having a blood culture drawn or having antibiotics initiated. Furthermore, they did not meet criteria for systemic inflammatory response as defined above.
Serum Samples and Biomarkers Assay
Whole blood samples obtained at the time of evaluation for sepsis (the time when blood culture was obtained and antibiotics were ordered) were collected from the clinical laboratory within 72 hours of acquisition, then centrifuged for 5 minutes at 500 G. Serum was isolated and stored at −80° C. Biomarkers were measured using a multiplex magnetic bead platform designed by EMD Millipore Corporation (Millipore Sigma, MA) specifically for PERSEVERE. Concentrations of markers were obtained using a Luminex 100/200 plate reader (Luminex Corporation, Austin, TX) according to the manufacturer’s protocol.
Statistical Analysis
Comparisons between survivors and non-survivors were performed using the Fisher’s exact test or the Mann-Whitney U test when appropriate. We used the PERSEVERE II classification tree9 to stratify patients into terminal nodes that were deemed high- or low-risk. High- and low-risk patient comparisons were done using the Fisher’s exact test or the Mann-Whitney test when appropriate. Prism v9.0 (GraphPad Software, San Diego, CA) was used for generating receiving-operator-curves and calculating the area under the curve. RStudio v1.4 (RStudio Team, MA) were used to derive and validate the new decision tree. The new tree was derived using the tree package in R studio by instructing the algorithm to classify neonates according to mortality outcome and to continue branching until there is 5% of the cohort in the terminal node using all available PERSEVERE II serum biomarker levels and gestational age at birth.
Results
Cohort Demographics
71 events were evaluated for enrollment. 1 neonate was excluded for terminal diagnosis and 6 neonates were excluded for discontinuation of antibiotics within 48 hours of evaluation. 58 neonates were included in the analysis who had a total of 64 events. Four neonates who had multiple sepsis events during their NICU stay (n=4), and we included only the last event they had in our analysis (Figure 1). The overall mortality rate was 15.3%.
Figure 1. Study Flow Chart.
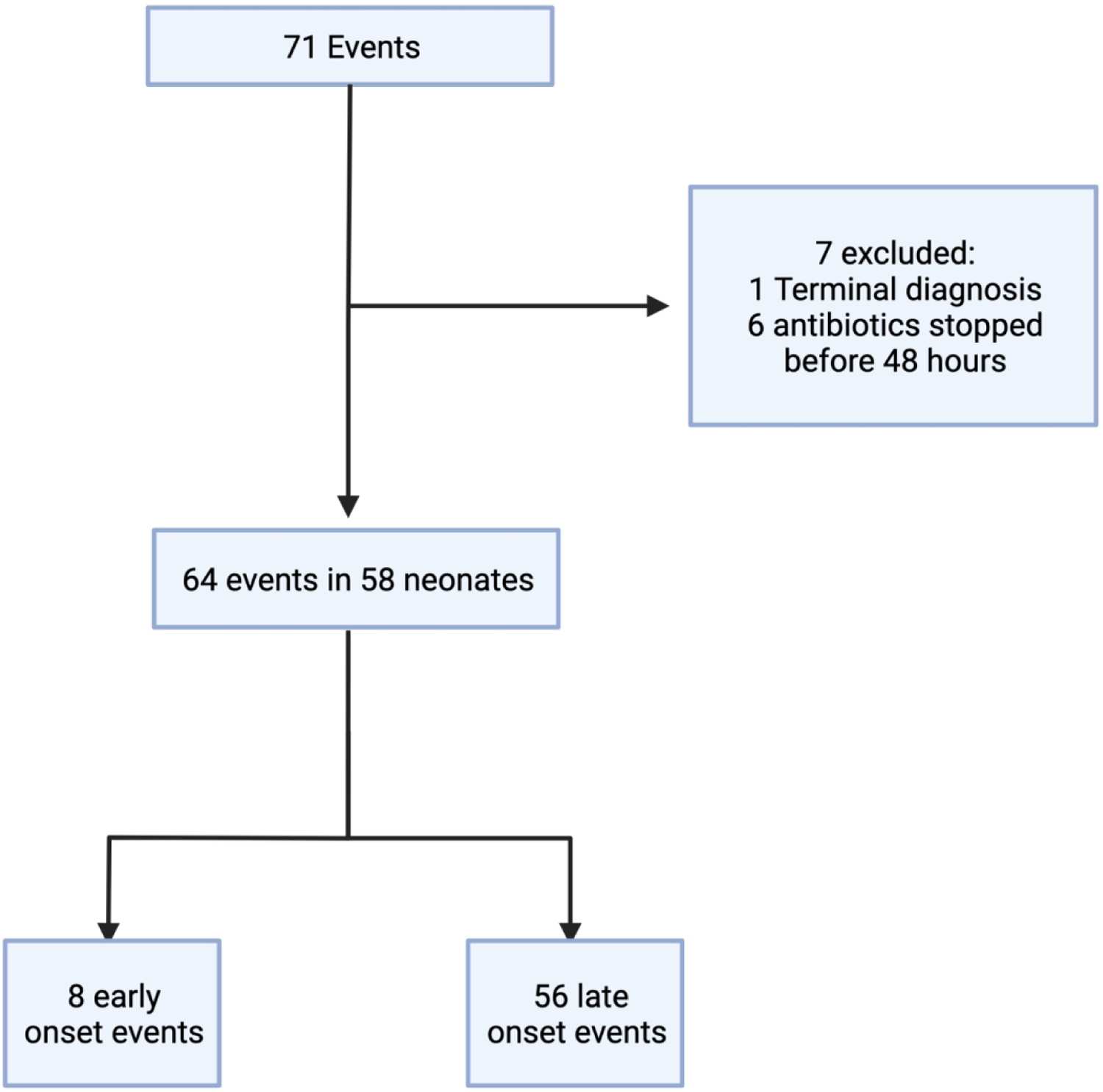
71 events were evaluated, after exclusions, 58 neonates were included in the analysis.
When comparing the median values of PERSEVERE biomarkers between neonates with sepsis and neonates without inflammation (n=13), all of the biomarkers were higher in neonates with sepsis, but only IL-8 and MMP-8 reached statistical significance (please refer to Table S1 in the supplementary materials).
Amongst neonates with sepsis, survivors and non-survivors were comparable in their characteristics. There was no statistical difference between both groups in all variables, including culture positivity rate. Although not statistically significant, non-survivors were born at earlier gestational ages and had lower birth weights (Table 1). Further details regarding culture results can be found in supplementary materials (Table S2).
Table 1. Characteristics of Neonates.
Survivors and non-survivors are comparable.
| Characteristic | Mortality n=9 (16%) | Survival n=49 (84%) | p-value |
|---|---|---|---|
| Sex: Female n (%) | 7 (78%) | 24 (49%) | 0.15a |
| Male n (%) | 2 (22%) | 25 (51%) | |
| Race: Black | 3 (33%) | 16 (33%) | |
| Others | 1 (11%) | 1 (2%) | >0.99b |
| White | 5 (56%) | 32 (65%) | |
| Birth Weight gram [IQR] | 1030 [605–2289] | 1638 [799–2925] | 0.29c |
| GA at Birth Weeks [IQR] | 26 [25–35] | 34 [26–37] | 0.19c |
| Congenital Anomalies | 1 (11%) | 20 (41%) | 0.14a |
| Positive Culture | 7 (78%) | 42 (86%) | 0.61a |
| Gram Negative | 3/7 (43%) | 18/42 (43%) | >0.99d |
| Gram Positive | 3/7 (43%) | 21/42 (50%) | >0.99d |
| Coagulase Negative Staph | 1/7 (14%) | 7/42 (17%) | >0.99d |
| Fungal | 1/7 (14%) | 1/42 (2%) | 0.26d |
| Early Vs. Late | 3 (33%) | 5 (10%) | 0.10a |
| Chronological Age at Event Days [IQR]e | 12 [8–41] | 27 [13–70] | 0.10c |
Calculated using the Fisher’s exact test
Calculated using the Fisher’s exact test comparing neonates who are black vs. white
Calculated using the Mann-Whitney test
Calculated using the Fisher’s exact test comparing each category to the rest of cases with positive cultures
Included only late sepsis events
Performance of PERSEVERE II for Neonates with Sepsis
We classified neonates according to the PERSEVERE II decision tree as described previously (Figure S1). Neonates who were classified to terminal nodes one, two, and eight were considered low-risk (predicted survivors), and those classified to terminal nodes three, four, six, seven, and nine were considered high-risk (predicted non-survivors). The entire cohort had a mortality rate of 15.3%, and those who were classified as low-risk had a mortality rate of 9.4% while high-risk patients had a rate of 23.1%. PERSEVERE II was 67% sensitive and 59% specific for mortality, p 0.27 (Table 2). High-risk patients also had higher complicated course rate nearing statistical significance compared to low-risk ones, 42.3% vs. 18.8% respectively, p 0.08.
Table 2. Performance of Candidate Predictors of Mortality in Neonatal Sepsis.
Summary of candidate predictors of mortality. IL-8 is superior to PERSEVERE II with improved sensitivity, specificity, negative predictive value, and area under the curve. nPERSEVERE is superior to IL-8 with improved specificity, + likelihood ratio, and area under the curve.
| Characteristic | PERSEVERE | IL-8 | nPERSEVERE |
|---|---|---|---|
| Sensitivity (95% CI) | 67% (35–88%) | 100% (70–100%) | 100% (71–100%) |
| Specificity (95% CI) | 59% (45–72%) | 65% (51–77%) | 85% (73–93%) |
| NPV (95% CI) | 91% (76–97%) | 100% (89–100%) | 100% (92–100%) |
| PPV (95% CI) | 23% (11–42%) | 35% (19–54%) | 56% (33–77%) |
| + Likelihood Ratio | 1.6 | 2.9 | 7 |
| AUC (95% CI) | 0.65 (0.46–0.84) | 0.87 (0.77–0.98) | 0.95 (0.89–1.00) |
| The Fisher’s Exact test p value | 0.27 | 0.0003 | <0.0001 |
IL-8 is a Candidate Marker for Mortality Prediction
We wondered if any of PERSEVERE II biomarkers alone would perform better than PERSEVERE II to identify high-risk patients at the time of evaluation for sepsis. Amongst PERSEVERE II biomarkers, IL-8 was the most statistically different between non-survivors and survivors with a median of 10,114 pg/mL [IQR 531–81,355 pg/mL] vs. 207 pg/mL [IQR 89–494, pg/mL] respectively, p 0.0001 (Figure 2a). The area under the ROC curve for mortality was 0.88 (95% CI 0.77 to 0.98), p 0.0004 (Figure 2b). Based on ROC calculations, a cutoff of 300 pg/mL had the highest specificity (65%) for mortality while retaining a 100% sensitivity, p 0.0003 (Table 2).
Figure 2. IL-8 Is a Candidate Biomarker for Mortality Prediction.
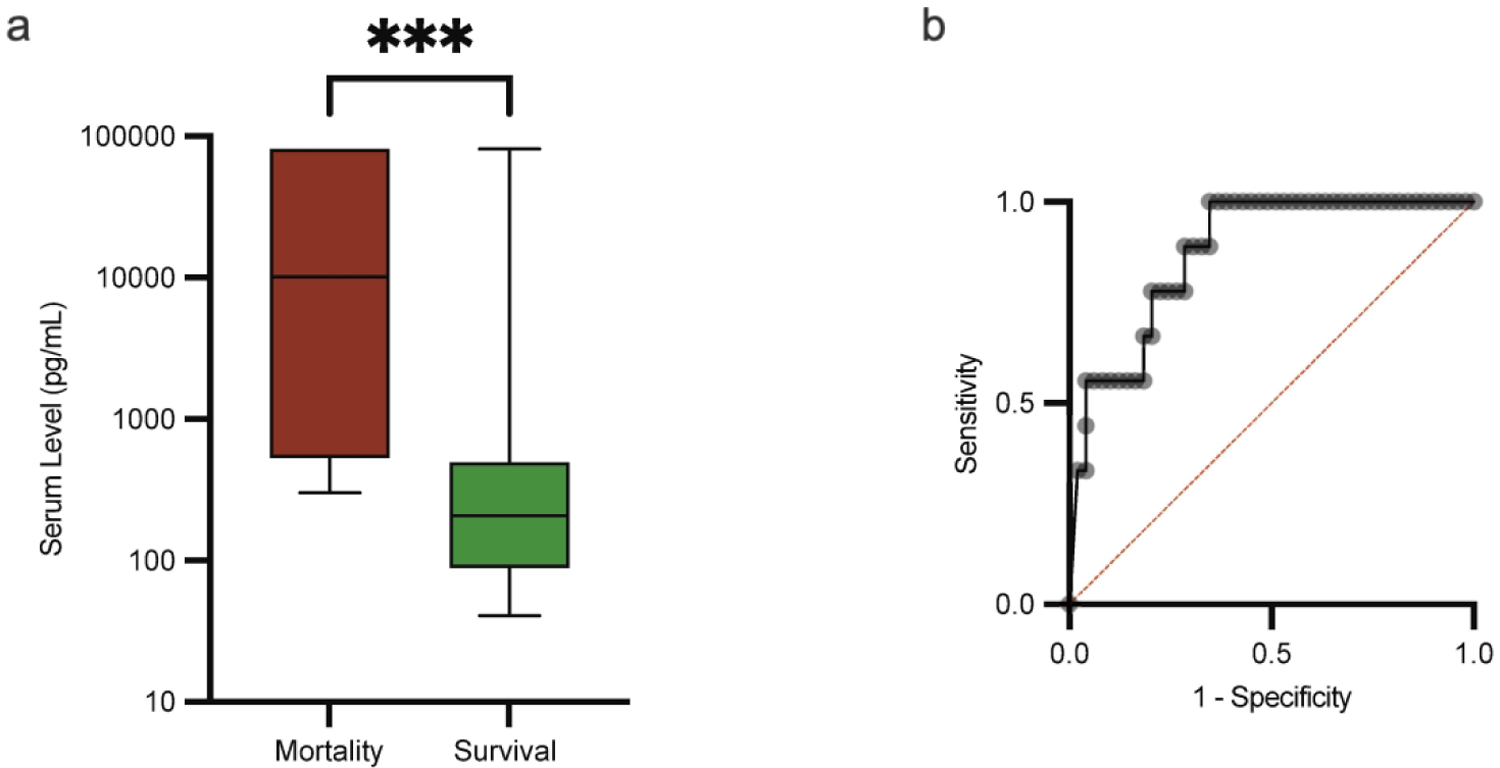
a. The median and the minimum to maximum range of IL-8 levels in non-survivors (red) vs. survivors (green), 10,114 pg/mL vs. 207 pg/mL respectively, p 0.0001 using the Mann-Whitney U test. b. Receiving-operator curve for IL-8, area under the curve 0.87 (95% CI 0.77 to 0.98).
The next most statistically different biomarker between survivors and non-survivors was the platelet count. Non-survivors had a median count of 104,000 per mm3 [71,000–213,000 per mm3] compared to 246,000 per mm3 [180,000–365,000 per mm3] in survivors, p 0.006 (Supplementary Figure S2). Other markers were also statistically different between non-survivors and survivors, CCL3 was higher in non-survivors compared to survivors, 104 pg/mL [IQR 62–181 pg/mL] vs. 39 pg/mL [30–67 pg/mL] respectively, p 0.03 (Supplementary Figure S3). HSP A1b was also different between non-survivors and survivors and had predictive mortality capacity comparable to CCL-3; 1,390,000 pg/mL [IQR 497,047–2,415,000 pg/mL] vs. 433,874 pg/mL [IQR 232,115–963,855 pg/mL] respectively, p 0.02 (Supplementary Figure S4). GZMB and MMP8 were not statistically different between survivors and non-survivors (data not shown).
nPERSEVERE: A Decision Tree That Is Specific for Neonates
Since IL-8 showed good performance for mortality prediction in neonatal sepsis, we wanted to test if a new decision tree that is neonate-specific can perform better than IL-8. Using classification tree methodology, we rederived a new neonatal tree, nPERSEVERE, that utilizes IL-8, platelet count, CCL3, MMP8, and GZMB to classify neonates according to mortality (Figure S5). One of the main downfalls of classification trees is overfitting. To overcome this issue, we pruned the original tree by decreasing the number of branching and increasing the number of subjects in terminal nodes (Figure 3). Neonates classified to terminal nodes one and three (predicted survivors) had a mortality rate of 0% and considered low-risk. Meanwhile, neonates classified to nodes two and four (predicted non-survivors) had a mortality rate of 56% and considered high-risk. The new pruned tree was 100% sensitive and 86% specific for mortality with a misclassification rate of 6%, p <0.0001. The area under the ROC curve for mortality was 0.95 (95% CI 0.89 to 1.00), p <0.0001. To validate our model, we performed 5-fold cross validation, the average misclassification rate was 7% and the calculated area under the curve showed good predictive capacity with an average of 0.86. Furthermore, neonates who were low-risk had a complicated course rate of 14% compared to 69% in high-risk neonates, p <0.0001.
Figure 3. The Pruned Final Version of nPERSEVERE.
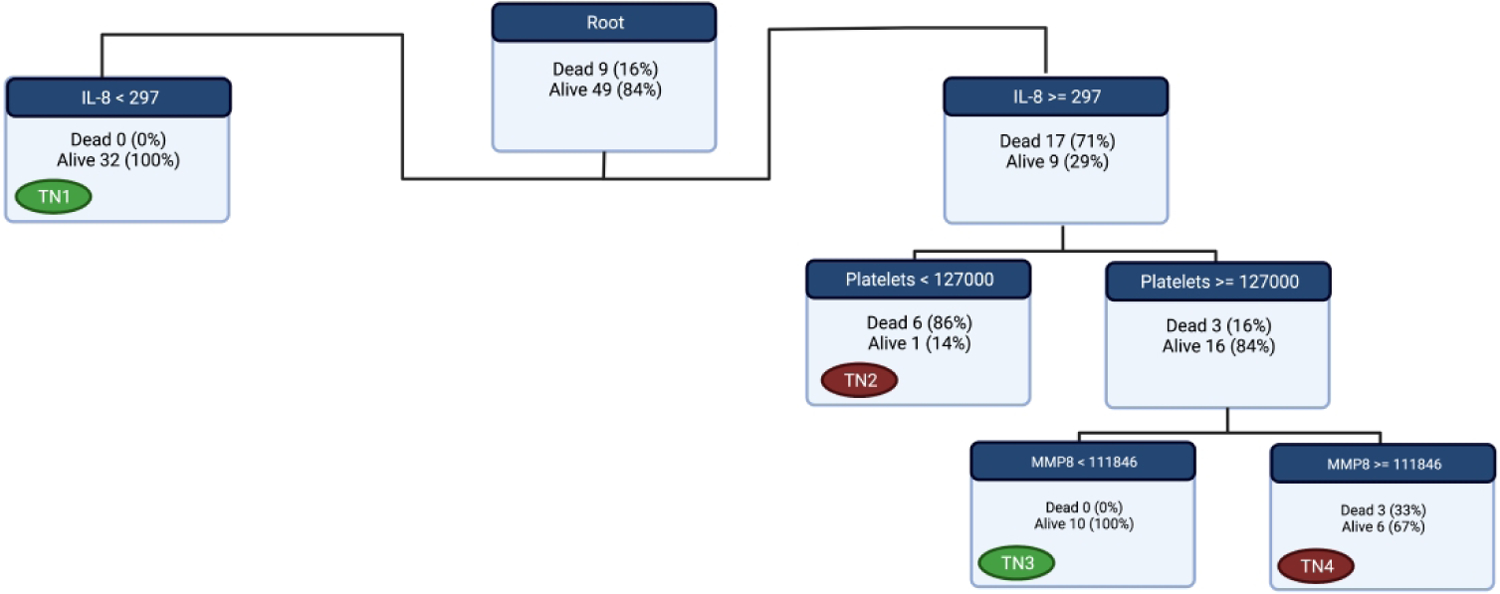
After pruning, we decided on this tree to test in the cohort. The Terminal node that are labeled with a green tag are low-risk (predicted survivors) while the ones labeled with red tags are high-risk (predicted non-survivors). The survival rate of those classified in the green nodes is 100% compared to 44% of those classified in red nodes.
Beyond 5-fold cross validation, when we applied nPERSEVERE to the 6 events that were not included in the derivation cohort (prior multiple events), the model showed good performance with a misclassification rate of 15% and an area under the curve for all the events (n=65) was 0.88 (95% CI 0.77–0.99), p <0.0001. Also, there was a non-statistically significant trend of higher vasopressor use in predicted non-survivors compared to predicted survivors, 35% vs 24% respectively, p 0.25.
Since there were patients that were classified as predicted non-survivors and did survive, we wondered if they indeed had a higher baseline mortality risk, but the clinical care provided to them mitigated that outcome. We compared those who were predicted non-survivors and survived (n=7) to those who were predicted survivors and survived (n= 42). Predicted non-survivors were more likely to experience a complicated course of illness than predicted survivors, 29% vs. 22% respectively, but this difference was not significant, p 0.25. Furthermore, neonates who were predicted non-survivors had more organs injured compared to predicted survivors, 2 [IQR 1–3] vs. 1 [IQR 0–2], p 0.06. Amongst survivors, the use of vasopressor was comparable between the predicted survivors and predicted non-survivors, 28% vs. 24% respectively, p >0.99.
Survival Curves of High- and Low-Risk Patients According to nPERSEVERE
We generated the survival curves of neonates classified as low-risk and high-risk according to nPERSEVERE to give a visual representation of the difference between the two groups. Comparison of survival curves using the Mantel-Cox test showed that the curves of high- and low-risk groups were statistically significant, p <0.0001 (Figure 4a). Although most death events occurred early in the course (median day of death = 12 days), some events ended with late death after 30 days (3 events, 33% of total deaths) (Figure 4b).
Figure 4. Survival Trends of Neonates According to nPERSEVERE Classification.

a. Neonates classified as high-risk (nHR red square) had a survival rate of 44% compared to 100% survival for neonates classified as low-risk (nLR green square), p <0.0001. The entire cohort’s (EC black square) survival curve is depicted for comparison. b. Distribution of mortality timing shows that most deaths occur within the first month of the sepsis event (67%) with the majority occurring in the first two weeks.
Discussion
Our main goal for this study is to demonstrate the feasibility of stratification in neonates with sepsis at the time of evaluation according to their baseline mortality risk. First, we tested PERSEVERE II and showed that PERSEVERE II was 67% sensitive and 59% specific for mortality in our cohort compared to a sensitivity of 86% and specificity of 69% in the pediatric cohort9. It is not surprising that PERSEVERE II did not perform as well in a neonate only population compared to its prior performance in various pediatric cohorts, since neonates have a unique early response in sepsis that does not match older pediatric age groups17. Second, using PERSEVERE biomarkers, we demonstrated that IL-8 is promising as a single biomarker for mortality prediction in neonatal sepsis. IL-8 is a potent neutrophil chemoattractant and has been shown to be elevated in sepsis and septic shock and corelated with other pro-inflammatory cytokines such as IL-618, thus it is plausible to state that higher levels of IL-8 corelate with higher inflammatory burden in sepsis and potentially worse outcomes. Also, a prospective cohort study of adults with sepsis demonstrated that a cutoff IL-8 level of 94 pg/mL was 66% sensitive and 61% specific for mortality with a negative predictive value of 77%, p <0.000119.
Finally, using classification tree methodology, we devised a new decision tree, nPERSEVERE, that performed well in this cohort of neonates from two centers. nPERSEVERE is 100% sensitive and has a negative predictive value of 100% for mortality with AUC of 0.95. We also demonstrated that neonates classified as high-risk had a higher disease burden as evident by having more organ dysfunction and higher rate of complicated course.
Biomarker research in neonatal sepsis has largely been focused on identifying a marker that can diagnose culture positive sepsis early in the course, but this approach has not yet translated to meaningful change in clinical practice. In this study we focus on the utility of prognostication in biomarker research, and we foresee many applications for nPERSEVERE. It could provide risk assessment at the time of evaluation rather than after certain time has lapsed since the diagnosis of sepsis, allowing for mortality risk allocation as soon as the neonate starts to show clinical evidence of the disease. This could be the first step towards precision medicine in neonatal sepsis and could provide meaningful data at the bedside. It alerts the clinical team very early in the course of the infection about those who are at high risk of dying, and this is helpful in counseling families of neonates with sepsis, as it provides information about how sick they are from the time of sepsis evaluation.
Another meaningful and important use for nPERSEVERE would be in randomized clinical trials that ask whether certain interventions reduce mortality in neonates with sepsis. It could allow for enrollment of neonates who have high baseline mortality risk, so that the intervention and placebo arms are equitable while also providing prognostic enrichment that reduces the number of subjects needed to answer the same question with equal power. Furthermore, these high-risk interventions, such as immunomodulation20, 21 and higher dose antibiotic prescription22, would be targeted towards patients who are more likely to see benefit from such interventions and would spare those who are at low risk of dying from potential side effects.
Finally on a healthcare system level, it could serve as a valuable tool in quality improvement efforts that aims to reduce neonatal mortality from infections, as it will not only provide a metric to gage the effect of the effort’s interventions, but also focuses these interventions on those who have a high baseline risk of mortality. Also, this assay could guide clinicians to determine which neonates would benefit from transferring to a center with higher capabilities, as this test allows to identify neonates who are not only at higher risk of dying, but also developing multi-organ dysfunction that requires support not available at the transferring center.
There are limitations to our study. First, and this is true for all ongoing research in neonatal sepsis, there is no consensus definition for neonatal sepsis comparable to the ones that exist for adult and pediatric patients23, 24. To overcome this, we elected to use a definition that was proposed by an expert in the field and allows for prospective enrollment at the time of evaluation without relying on blood culture results15, which usually occurs hours to days after the initial suspicion. Secondly, this is a relatively small cohort of patients (n=58), but it is from two centers that represent a level III mostly inborn unit and a level IV mostly outborn unit. Nonetheless, validation in a multi-center cohort is needed to confirm our findings.
Supplementary Material
Impact Statement:
Prognostic and predictive biomarker research is lacking in the newborn intensive care unit.
Biomarkers can be used at the time of evaluation for neonatal sepsis (blood culture acquisition) to identify neonates with high baseline mortality risk.
Stratification is an important step towards precision medicine in neonatal sepsis.
Acknowledgements:
Near the end of this work Dr. Hector Wong passed away unexpectedly. He was a good mentor, friend, husband, father, and physician scientist. He will be forever remembered by those who had the privilege to work with him. We also appreciate Dr. Basillia Zingarelli for reviewing the final drafts of the manuscript and Andrew M. Smith for his input on the analysis. Some of the figures were generated using www.BioRender.com
Funding
This presented work is supported by Dr. Wong’s R35GM126943 grant from the NIH.
Footnotes
Competing interest:
Cincinnati Children’s Research foundation and Hector Wong hold U.S patents for the PERSEVERE biomarkers. Cincinnati Children’s Research foundation and Faris Al Gharaibeh have filed for a U.S patent for nPERSEVERE.
Consent Statement
This study was reviewed by the institutional review board at Cincinnati Children’s Hospital Medical Center and the University of Cincinnati and consent was waived.
Data Availability
Datasets generated from this work are available upon reasonable request to the corresponding author.
References:
- 1.Stoll BJ et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics 126, 443–456 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Camacho-Gonzalez A, Spearman PW & Stoll BJ Neonatal infectious diseases: evaluation of neonatal sepsis. Pediatr Clin North Am 60, 367–389 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carr R, Modi N & Dore C G-CSF and GM-CSF for treating or preventing neonatal infections. Cochrane Database Syst Rev, CD003066 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ohlsson A & Lacy JB Intravenous immunoglobulin for suspected or proven infection in neonates. Cochrane Database Syst Rev 1, CD001239 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown JVE, Meader N, Wright K, Cleminson J & McGuire W Assessment of C-Reactive Protein Diagnostic Test Accuracy for Late-Onset Infection in Newborn Infants: A Systematic Review and Meta-analysis. JAMA Pediatr 174, 260–268 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ye Q, Du LZ, Shao WX & Shang SQ Utility of cytokines to predict neonatal sepsis. Pediatr Res 81, 616–621 (2017). [DOI] [PubMed] [Google Scholar]
- 7.Zhou M, Cheng S, Yu J & Lu Q Interleukin-8 for diagnosis of neonatal sepsis: a meta-analysis. PLoS One 10, e0127170 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sherwin C et al. Utility of interleukin-12 and interleukin-10 in comparison with other cytokines and acute-phase reactants in the diagnosis of neonatal sepsis. Am J Perinatol 25, 629–636 (2008). [DOI] [PubMed] [Google Scholar]
- 9.Wong HR et al. Prospective clinical testing and experimental validation of the Pediatric Sepsis Biomarker Risk Model. Sci Transl Med 11 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wong HR et al. The pediatric sepsis biomarker risk model. Crit Care 16, R174 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wong HR et al. Testing the prognostic accuracy of the updated pediatric sepsis biomarker risk model. PLoS One 9, e86242 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wong HR et al. A multibiomarker-based outcome risk stratification model for adult septic shock*. Crit Care Med 42, 781–789 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jacobs L et al. The Pediatric Sepsis Biomarker Risk Model (PERSEVERE) Biomarkers Predict Clinical Deterioration and Mortality in Immunocompromised Children Evaluated for Infection. Sci Rep 9, 424 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harris PA et al. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 42, 377–381 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wynn JL & Wong HR Pathophysiology and treatment of septic shock in neonates. Clin Perinatol 37, 439–479 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jetton JG et al. Incidence and outcomes of neonatal acute kidney injury (AWAKEN): a multicentre, multinational, observational cohort study. Lancet Child Adolesc Health 1, 184–194 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wynn JL et al. The influence of developmental age on the early transcriptomic response of children with septic shock. Mol Med 17, 1146–1156 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hack CE et al. Interleukin-8 in sepsis: relation to shock and inflammatory mediators. Infect Immun 60, 2835–2842 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anderson BJ et al. Plasma sTNFR1 and IL8 for prognostic enrichment in sepsis trials: a prospective cohort study. Crit Care 23, 400 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bao Q, Lv R & Lei M IL-33 attenuates mortality by promoting IFN-gamma production in sepsis. Inflamm Res 67, 531–538 (2018). [DOI] [PubMed] [Google Scholar]
- 21.Vanden Berghe T et al. Simultaneous targeting of IL-1 and IL-18 is required for protection against inflammatory and septic shock. Am J Respir Crit Care Med 189, 282–291 (2014). [DOI] [PubMed] [Google Scholar]
- 22.Lertwattanachai T et al. Clinical outcomes of empirical high-dose meropenem in critically ill patients with sepsis and septic shock: a randomized controlled trial. J Intensive Care 8, 26 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singer M et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 315, 801–810 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goldstein B, Giroir B, Randolph A & International Consensus Conference on Pediatric, S. International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med 6, 2–8 (2005). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Datasets generated from this work are available upon reasonable request to the corresponding author.


