Abstract
Objective
To examine the associations of vegetable and/or fruit consumption with metabolic syndrome (MetS).
Design
Meta-analysis of observational studies.
Setting
The electronic databases of PubMed, Web of Science and EMBASE were searched up to September 2017 for observational studies concerning the associations of vegetable and/or fruit consumption with MetS. The pooled relative risk (RR) of MetS for the highest v. the lowest category of vegetable and/or fruit consumption, as well as their corresponding 95 % CI, were calculated.
Results
A total of twenty-six observational studies (twenty cross-sectional, one case–control and five cohort studies) were included in the meta-analysis. Specifically, sixteen studies were related to vegetable consumption and the overall multivariable-adjusted RR evidenced a negative association between vegetable consumption and MetS (RR=0·89, 95 % CI 0·85, 0·93; P<0·001). For fruit consumption, sixteen studies were included and the overall multivariable-adjusted RR demonstrated that fruit consumption was inversely associated with MetS (RR=0·81, 95 % CI 0·75, 0·88; P<0·001). For vegetable and fruit consumption, eight studies were included; the overall multivariable-adjusted RR showed that vegetable and fruit consumption was also negatively associated with MetS (RR=0·75, 95 % CI 0·63, 0·90; P=0·002).
Conclusions
The existing evidence suggests that vegetable and/or fruit consumption is negatively associated with MetS. More well-designed prospective cohort studies are needed to elaborate the concerned issues further.
Keywords: Vegetables, Fruits, Metabolic syndrome, Meta-analysis, Observational studies
Metabolic syndrome (MetS) is associated with the development of CVD( 1 ). MetS involves at least three of the five following metabolic alterations: elevated waist circumference, high serum TAG, low HDL cholesterol, elevated fasting plasma glucose and elevated blood pressure( 2 ). With its prevalence increasing exponentially in recent decades, MetS has been regarded as an important public health issue in the 21st century, affecting about 25 % of the population in developed countries in parallel with obesity and diabetes( 3 ). Recently, increasing evidence has shown that alcohol consumption( 4 ), soft drink intake( 5 ) and coffee and tea consumption( 6 ) are closely associated with MetS. Therefore, dietary factors are considered to play an important role in MetS( 7 ).
As important sources for a wide range of beneficial nutrients and non-nutrient substances, fruits and vegetables are rich in fibre, vitamins (particularly A, B and C), minerals (Se and K), antioxidants (carotenoids and tocopherols) and phytochemicals (flavonoids, glucosinolates and isothiocyanates)( 8 ). A survey report, which was based on a sample extracted from fifty-two low- and middle-income countries, showed that 77·6 % of men and 78·4 % of women had daily fruit and vegetable intake lower than 400 g (the minimum intake recommended by the WHO)( 9 ). Low intake of fruits and vegetables is deemed a risk factor for many health problems, such as cancer, CVD, stroke and all-cause mortality( 10 ). The consumption of vegetables and fruits should also be considered with regard to MetS. Vitamin C and fibre, which are two of the primary constituents in vegetables and fruits, are believed conducive to the control of MetS( 11 – 14 ). In addition, antioxidants and anti-inflammatory components from fruits and vegetables are also considered to be beneficial for MetS( 15 ). Therefore, it is natural to speculate that vegetable and/or fruit consumption is inversely associated with MetS. To our best knowledge, the effect of vegetable and/or fruit intake on MetS has been investigated by numerous epidemiological studies( 7 , 16 – 40 ), but conclusions are still controversial. In view of this, the present meta-analysis of observational studies aimed to further examine the associations of vegetable and/or fruit consumption with MetS. It was hypothesized that vegetable and/or fruit consumption would be inversely associated with MetS.
Materials and methods
Search strategy
The current meta-analysis was conducted according to the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) guidelines( 41 ). The electronic databases of PubMed, Web of Science and EMBASE were searched up to September 2017, using a series of logic combinations of keywords and in-text words that are related to MetS (‘metabolic syndrome’, ‘metabolism syndrome’), vegetables and fruits (‘vegetable’, ‘vegetables’, ‘fruit’, ‘fruits’). No language restrictions were set in the search strategy. We first screened the title and abstract of all the articles to identify eligible studies and then read the full article to include eligible studies. Moreover, the reference lists from retrieved articles were reviewed to identify additional studies. The corresponding author of the potential relevant study was contacted if the full text was not available.
Study selection
The title, abstract and full text of all retrieved studies were reviewed by two researchers (Y.Z. and D.Z.Z.) independently. Disagreements were resolved by discussion and mutual consultation. The included studies were required to meet the following criteria: (i) observational studies in the general population; (ii) the exposure of interest was vegetable and/or fruit consumption; (iii) the study outcome included MetS; and (iv) hazard ratio (HR), relative risk (RR) or odds ratio (OR) and 95 % CI were reported. The exclusion criteria were as follows: (i) duplicated or irrelevant articles; (ii) reviews, letters or case reports; (iii) randomized controlled trials; and (iv) non-human studies.
Data extraction
Data extraction was conducted by two independent reviewers (Y.Z. and D.Z.Z.) and disagreements were resolved by consensus. The following information was collected: first author, year of publication, location, age and gender of the study population, sample size, study design, adjustments, exposure, exposure assessment and diagnostic criteria of MetS. The corresponding effect estimates adjusted for the maximum number of confounding variables with corresponding 95 % CI for the highest v. lowest level were extracted. For the studies that did not report direct effect estimates, we calculated pooled effect estimates using the natural logarithm of the RR and 95 % CI.
Quality assessment
Quality assessment was conducted according to the Newcastle–Ottawa criteria for non-randomized studies( 42 ), which are based on three broad perspectives: (i) the selection process of study cohorts, (ii) the comparability among different cohorts and (iii) the identification of either the exposure or outcome of study cohorts. Disagreements with respect to the methodological quality were resolved by discussion and mutual consultation.
Statistical analyses
The RR was considered as the common measure of the associations of vegetable and fruit consumption with MetS, and OR and HR were directly converted into RR. The homogeneity of effect size across trials was tested by Q statistics (P<0·05 was considered heterogeneous). The I 2 statistic, which measures the percentage of the total variation across studies due to heterogeneity, was also examined (I 2>50 % was considered heterogeneity). If significant heterogeneity was observed among studies, the random-effects model was used; otherwise, the fixed-effects model was acceptable. Begg’s tests were performed to assess the publication bias( 43 ). Meta-regression was performed to explore the potentially important covariates that might exert substantial impacts on between-study heterogeneity( 44 ). Subgroup analyses were performed by gender, study design, geographical region, age of the population, diagnostic criteria of MetS, sample size and type of vegetable (only for vegetables). In addition, a sensitivity analysis was also conducted to determine whether an individual study affected the pooled result. All statistical analyses were performed using the statistical software package STATA version 11.0. A P value ≤0·05 was accepted as statistically significant, unless otherwise specified.
Results
Study identification and selection
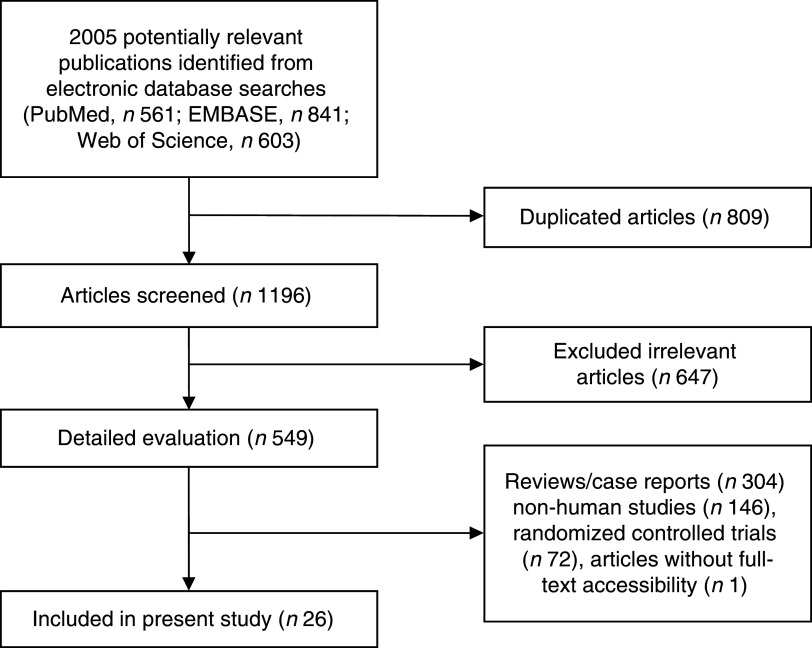
Figure 1 presents the detailed flow diagram of articles included in the present meta-analysis. A total of 2005 potentially relevant articles (PubMed, n 561; EMBASE, n 841; Web of Science, n 603) were retrieved during the initial literature search. After eliminating 809 duplicated articles, 1169 articles were screened by title and abstract, leading to initial exclusion of 647 irrelevant studies. Then, 304 reviews, case reports or letters, 146 non-human studies, seventy-two randomized control trials studies and one articles without full-text accessibility were removed. Eventually, a total of twenty-six observational studies were selected for the current meta-analysis( 7 , 16 – 40 ).
Fig. 1.
Flowchart for the identification of studies included in the present meta-analysis on associations of vegetable and/or fruit consumption with metabolic syndrome
Study characteristics
Table 1 shows the main characteristics of the included studies. These studies were published between 2007 and 2017, and include twenty cross-sectional, one case–control and five cohort studies. Four of the included studies were performed in European countries (Finland( 25 , 37 ), Poland( 26 ) and Portugal( 21 )), fourteen studies were conducted in Asian countries (Korea( 16 , 17 , 20 , 24 , 27 , 30 , 38 ), India( 22 ), Taiwan( 32 , 40 ), Iran( 34 , 39 ), Japan( 33 ) and China( 29 )), four studies were conducted in the USA( 7 , 19 , 28 , 31 ) and the other four studies were from Chile( 35 ), Suriname( 18 ) and Brazil( 23 , 36 ). Twenty-three articles included both male and female participants( 7 , 17 – 26 , 28 – 33 , 35 – 40 ), with three articles including only female or male participants( 16 , 27 , 34 ). The sample size ranged from 305 to 27656 for a total number of 115727. The criteria for MetS were those of the National Cholesterol Education Program Adult Treatment Panel III in eighteen articles( 16 – 18 , 21 – 25 , 27 , 28 , 30 , 32 , 34 – 36 , 38 – 40 ), the International Diabetes Federation in three( 19 , 20 , 33 ) and the American Heart Association in two studies( 7 , 26 ). Moreover, the criteria proposed by Alberti et al. were used in two studies( 29 , 37 ). Finally, sixteen( 16 , 17 , 20 , 21 , 23 – 25 , 27 , 30 , 32 , 34 , 35 , 37 – 40 ), sixteen( 16 , 17 , 20 – 24 , 27 – 30 , 33 , 34 , 35 , 37 , 39 ) and eight articles( 7 , 16 , 18 , 19 , 26 , 31 , 36 , 39 ) were related to the associations of vegetable, fruit, and vegetable and fruit consumption with MetS, respectively.
Table 1.
Characteristics of the individual studies included in the present meta-analysis on associations of vegetable and/or fruit consumption with metabolic syndrome (MetS)
| Study | Location | Age (years) | Male (%) | Sample size | Study design | Adjustments | Exposure | Exposure assessment | Diagnostic criteria of MetS | NOS score |
|---|---|---|---|---|---|---|---|---|---|---|
| Esmaillzadeh et al. (2006)( 34 ) | Iran | 40–60 | NA | 486 | Cross-sectional | Age, BMI, energy intake, cholesterol intake, percentage of energy from fat, cigarette smoking, physical activity level, current oestrogen use, menopausal status, family history of diabetes or stroke, intakes of whole grains, refined grains, dairy products, meat and fish, mutual effects of fruit and vegetable intakes, C-reactive protein | Vegetable Fruit | FFQ | NCEP ATP III | 6 |
| Pan and Pratt (2008)( 28 ) | USA | 12–19 | 50·8 | 4450 | Cross-sectional | Age, BMI, sex, ethnicity, poverty status, physical activity level | Fruit | 24 h dietary recall | NCEP ATP III | 6 |
| Lutsey et al. (2008)( 7 ) | USA | 45–64 | 44·1 | 9514 | Cohort | Age, sex, race, education, centre, total energy intake, smoking status, pack-years, physical activity level, intakes of meat, dairy, whole grains and refined grains | Vegetable and fruit | FFQ | AHA | 8 |
| Kelishadi et al. (2008)( 39 ) | Iran | 6–18 | 46·7 | 4811 | Cross-sectional | Age | Vegetable Fruit Vegetable and fruit | FFQ | NCEP ATP III | 6 |
| Kwaśniewska et al. (2009)( 26 ) | Poland | 20–74 | 47·4 | 1187 | Cross-sectional | BMI, smoking, physical activity | Vegetable and fruit | FFQ | AHA | 6 |
| Shin et al. (2009)( 27 ) | Korea | >30 | 100 | 7081 | Cross-sectional | Age, family history of type 2 diabetes, smoking status, physical activity | Vegetable Fruit | FFQ | NCEP ATP III | 6 |
| Kouki et al. (2001)( 25 ) | Finland | 57–78 | 49·7 | 1334 | Cross-sectional | Age, smoking, alcohol consumption, education | Vegetable | 4 d food record | NCEP ATP III | 6 |
| Jung et al. (2011)( 24 ) | Korea | 30–59 | 43 | 596 | Case–control | Age, sex, energy intake | Vegetable Fruit | 3 d food record | NCEP ATP III | 8 |
| Cho et al. (2012)( 38 ) | Korea | >30 | 42·9 | 1388 | Cross-sectional | Age | Vegetable | FFQ | NCEP ATP III | 6 |
| Jaaskelainen et al. (2012)( 37 ) | Finland | 3–18 | 45·6 | 2128 | Cohort | Age, sex | Vegetable Fruit | FFQ | Criteria proposed by Alberti et al. | 8 |
| de Oliveira et al. (2012)( 23 ) | Brazil | >35 | 73·4 | 305 | Cross-sectional | Age, sex, total energy intake, BMI | Vegetable Fruit | 24 h dietary recall | NCEP ATP III | 6 |
| Prasad et al. (2012)( 22 ) | India | 20–80 | 50·1 | 1178 | Cross-sectional | Not mentioned | Fruit | FFQ | NCEP ATP III | 6 |
| Castanho et al. (2013)( 21 ) | Portugal | 15–88 | 25 | 636 | Cross-sectional | Sex, total energy intake | Vegetable Fruit | 24 h dietary recall | NCEP ATP III | 5 |
| Baik et al. (2013)( 20 ) | Korea | 40–69 | NA | 5251 | Cohort | Age, sex, income, occupation, education, smoking status, alcohol intake, quartiles of MET-h/d, study site, FTO genotypes, quartiles of energy intake, quintiles of food groups or food items that are presented in their table | Vegetable Fruit | FFQ | IDF | 7 |
| Masaki (2013)( 33 ) | Japan | 20–69 | NA | 534 | Cross-sectional | Age, physical activity level, smoking and drinking status, other confounding variables | Fruit | NA | IDF | 6 |
| Boucher et al. (2013)( 31 ) | USA | NA | NA | 1059 | Cohort | Age, education, gender, diabetes, heart disease status | Vegetable and fruit | NA | NA | 7 |
| Lin et al. (2013)( 32 ) | Taiwan | >65 | 67·8 | 888 | Cohort | Age, gender, blood pressure, serum creatinine, ALT, uric acid, urine protein, initial MetS score, smoking, alcohol drinking, exercise, teeth brushing, milk intake | Vegetable | FFQ | NCEP ATP III | 8 |
| Chen et al. (2014)( 41 ) | Taiwan | >15 | 48 | 6591 | Cross-sectional | Age, sex | Vegetable | FFQ | NCEP ATP III | 6 |
| Neia Martini et al. (2014)( 36 ) | Brazil | >20 | NA | 1112 | Cross-sectional | NA | Vegetable and fruit | NA | NCEP ATP III | 6 |
| Park et al. (2015)( 30 ) | Korea | >20 | 40·8 | 27 656 | Cross-sectional | Age, BMI, residence area, education level, smoking, drinking status, menopausal status, exercise, walking, serum AST and ALT | Vegetable Fruit | FFQ | NCEP ATP III | 6 |
| Dussaillant et al. (2015)( 35 ) | Chile | >18 | 48 | 2561 | Cross-sectional | Sex, BMI, age, education level, activity level | Vegetable Fruit | FFQ | NCEP ATP III | 6 |
| Krishnadath et al. (2016)( 18 ) | Suriname | 39·2 | 48·5 | 2646 | Cross-sectional | Age, sex | Vegetable and fruit | FFQ | NCEP ATP III | 6 |
| Fletcher et al. (2016)( 19 ) | USA | 12–19 | 53·1 | 1379 | Cross-sectional | Age, sex, ethnicity, socio-economic position, self-reported physical activity level, dietary intake under-reporting, television viewing time | Vegetable and fruit | 24 h dietary recall | IDF | 6 |
| Wu et al. (2016)( 29 ) | China | 18–79 | 46·2 | 16 831 | Cross-sectional | Sex, age, location distribution | Fruit | FFQ | Criteria proposed by Alberti et al. | 6 |
| Hong and Kim (2017)( 16 ) | Korea | 40–64 | 0 | 2999 | Cross-sectional | Age, education level, household income, living with spouse, current smoker, current alcohol drinker, multivitamin use, menopausal status, energy intake, energy-adjusted carbohydrate intake, energy-adjusted Na intake | Vegetable Fruit Vegetable and fruit | 24 h dietary recall | NCEP ATP III | 6 |
| Kim and Choi (2016)( 11 ) | Korea | 30–64 | 39·8 | 11 029 | Cross-sectional | Age, sex, total energy intake, diet modification, education level | Vegetable Fruit | FFQ | NCEP ATP III | 6 |
NOS, Newcastle–Ottawa scale; NA, not applicable; MET, metabolic equivalent of task; ALT, alanine aminotransferase; AST, aspartate aminotransferase; NCEP ATP III, National Cholesterol Education Program–Adult Treatment Panel III; AHA, American Heart Association; IDF, International Diabetes Federation.
Association between vegetable consumption and metabolic syndrome
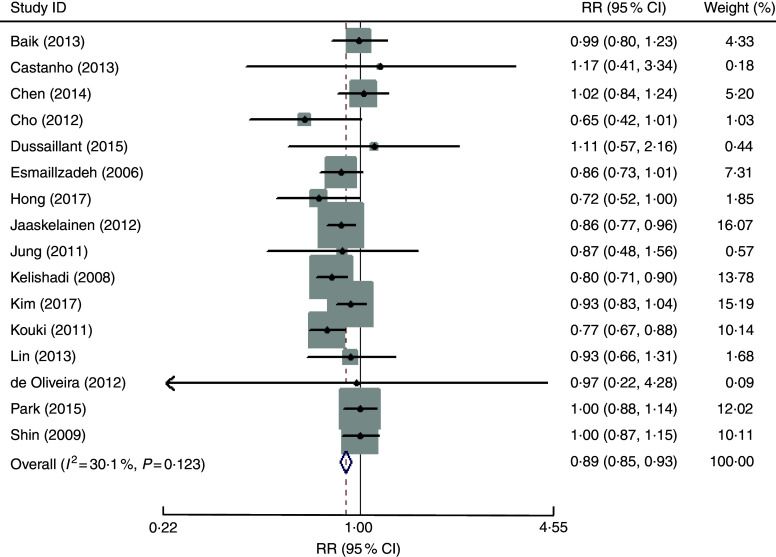
The overall multivariable-adjusted RR evidenced a negative association between vegetable consumption and MetS (RR=0·89, 95 % CI 0·85, 0·93; P<0·001; Fig. 2). No substantial level of heterogeneity was observed among studies (P=0·123, I 2=30·1 %). No evidence of publication bias was observed among the included studies according to the Begg rank-correlation test (P=0·964). The results of subgroup analysis for vegetable consumption are shown in Table 2. No significant relationship between green vegetable consumption and MetS was found according to the overall multivariable-adjusted RR (RR=1·10, 95 % CI 0·98, 1·24; P=0·12). No substantial level of heterogeneity was observed among studies (P=0·47, I 2=0 %). No evidence of publication bias was observed among the included studies according to the Begg rank-correlation test (P=1·000).
Fig. 2.
Forest plot of the overall multivariable-adjusted relative risk (RR) of metabolic syndrome for the highest v. the lowest category of vegetable consumption. The study-specific RR and 95 % CI are represented by the black diamond and the horizontal line, respectively; the area of the grey square is proportional to the specific-study weight to the overall meta-analysis. The centre of the open diamond and the vertical dashed line represent the pooled RR and the width of the open diamond represents the pooled 95 % CI
Table 2.
Subgroup analyses of vegetable consumption and metabolic syndrome (MetS)
| Stratification | No. of studies | Pooled RR | 95 % CI | Heterogeneity |
|---|---|---|---|---|
| All studies | 16 | 0·89 | 0·85, 0·93 | P=0·12; I 2=30·1 % |
| Type of vegetable | ||||
| Green vegetables | 3 | 1·10 | 0·98, 1·24 | P=0·47; I 2=0 % |
| Gender | ||||
| Male | 6 | 0·91 | 0·81, 1·03 | P=0·005; I 2=70 % |
| Female | 8 | 0·89 | 0·83, 0·95 | P=0·12; I 2=39 % |
| Design | ||||
| Cross-sectional or case–control | 13 | 0·89 | 0·85, 0·94 | P=0·07; I 2=40 % |
| Cohort | 3 | 0·89 | 0·81, 0·98 | P=0·50; I 2=0 % |
| Diagnostic criteria of MetS | ||||
| NCEP ATP III | 14 | 0·89 | 0·85, 0·94 | P=0·09; I 2=35 % |
| IDF | 1 | 0·99 | 0·80, 1·23 | – |
| Other | 1 | 0·86 | 0·77, 0·96 | – |
| Geographical region | ||||
| Asia | 11 | 0·92 | 0·87, 0·96 | P=0·13; I 2=34 % |
| Europe | 3 | 0·83 | 0·76, 0·90 | P=0·38; I 2=0 % |
| South America | 2 | 1·09 | 0·59, 1·99 | P=0·87; I 2=0 % |
| Sample size | ||||
| <1000 | 5 | 0·88 | 0·76, 1·01 | P=0·98; I 2=0 % |
| >1000 | 11 | 0·89 | 0·83, 0·96 | P=0·02; I 2=52 % |
| Age of population | ||||
| Adult | 14 | 0·92 | 0·87, 0·97 | P=0·21; I 2=22 % |
| Adolescents | 2 | 0·83 | 0·77, 0·90 | P=0·38; I 2=0 % |
RR, relative risk; NCEP ATP III, National Cholesterol Education Program–Adult Treatment Panel III; IDF, International Diabetes Federation.
Association between fruit consumption and metabolic syndrome
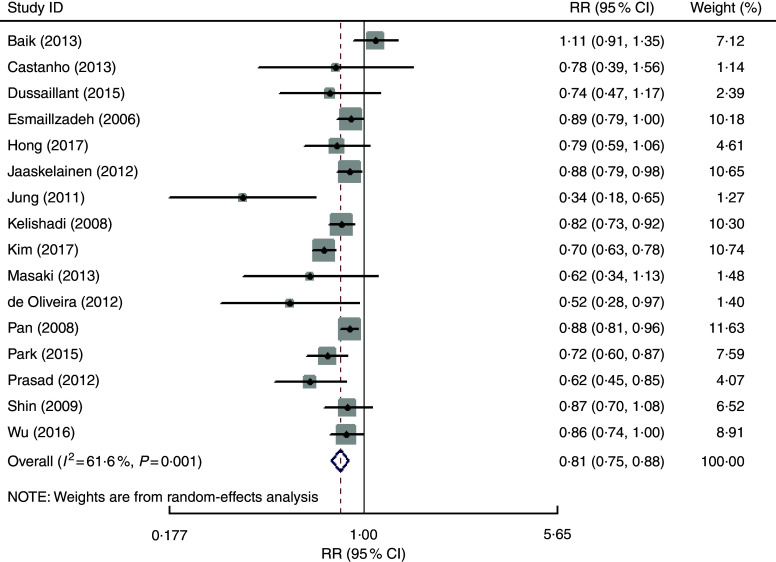
The overall multivariable-adjusted RR showed that fruit consumption was negatively associated with MetS (RR=0·81, 95 % CI 0·75, 0·88; P<0·001; Fig. 3). A substantial level of heterogeneity was observed among studies (P=0·001, I 2=61·6 %). No evidence of publication bias was observed among the included studies according to the Begg rank-correlation test (P=0·079). The results of subgroup analysis for fruit consumption are shown in Table 3.
Fig. 3.
Forest plot of the overall multivariable-adjusted relative risk (RR) of metabolic syndrome for the highest v. the lowest category of fruit consumption. The study-specific RR and 95 % CI are represented by the black diamond and the horizontal line, respectively; the area of the grey square is proportional to the specific-study weight to the overall meta-analysis. The centre of the open diamond and the vertical dashed line represent the pooled RR and the width of the open diamond represents the pooled 95 % CI
Table 3.
Subgroup analyses of fruit consumption and metabolic syndrome (MetS)
| Stratification | No. of studies | Pooled RR | 95 % CI | Heterogeneity |
|---|---|---|---|---|
| All studies | 16 | 0·81 | 0·75, 0·88 | P=0·12; I 2=61·6 % |
| Gender | ||||
| Male | 4 | 0·83 | 0·76, 0·90 | P=0·91; I 2=0 % |
| Female | 6 | 0·80 | 0·70, 0·91 | P=0·02; I 2=63 % |
| Design | ||||
| Cross-sectional or case–control | 14 | 0·78 | 0·72, 0·85 | P=0·008; I 2=54 % |
| Cohort | 2 | 0·97 | 0·78, 1·22 | P=0·04; I 2=75 % |
| Diagnostic criteria of MetS | ||||
| NCEP ATP III | 12 | 0·78 | 0·71, 0·85 | P=0·004; I 2=59 % |
| IDF | 2 | 0·89 | 0·51, 1·55 | P=0·07; I 2=69 % |
| Other | 2 | 0·87 | 0·80, 0·95 | P=0·81; I 2=0 % |
| Geographical region | ||||
| Asia | 11 | 0·92 | 0·87, 0·96 | P=0·13; I 2=34 % |
| Europe | 2 | 0·88 | 0·79, 0·98 | P=0·74; I 2=0 % |
| North and South America | 3 | 0·87 | 0·80, 0·94 | P=0·20; I 2=38 % |
| Sample size | ||||
| <1000 | 5 | 0·63 | 0·44, 0·92 | P=0·02; I 2=66 % |
| >1000 | 11 | 0·82 | 0·76, 0·89 | P=0·002; I 2=64 % |
| Age of population | ||||
| Adult | 13 | 0·78 | 0·69, 0·87 | P=0·001; I 2=65 % |
| Adolescents | 3 | 0·87 | 0·82, 0·92 | P=0·38; I 2=0 % |
RR, relative risk; NCEP ATP III, National Cholesterol Education Program–Adult Treatment Panel III; IDF, International Diabetes Federation.
Association of vegetable and fruit consumption with metabolic syndrome
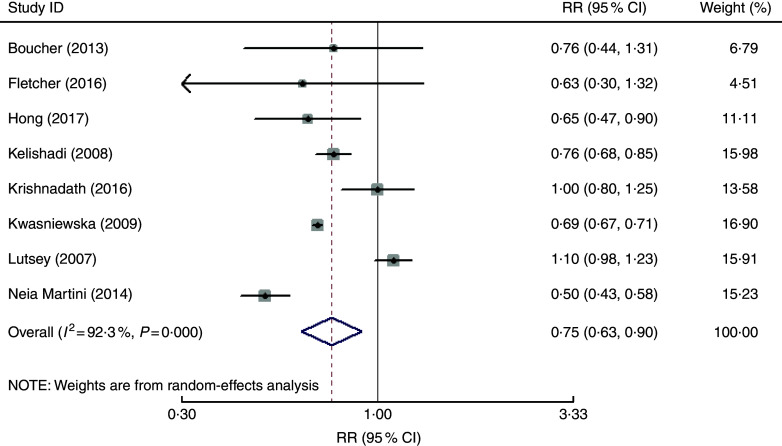
The overall multivariable-adjusted RR showed that vegetable and fruit consumption was negatively associated with MetS (RR=0·75, 95 % CI 0·63, 0·90; P=0·002; Fig. 4). A substantial level of heterogeneity was observed among studies (P<0·001, I 2=92·3 %). No evidence of publication bias was observed among the included studies according to the Begg rank-correlation test (P=0·127). The results of subgroup analysis for vegetable and fruit consumption are shown in Table 4.
Fig. 4.
Forest plot of the overall multivariable-adjusted relative risk (RR) of metabolic syndrome for the highest v. the lowest category of vegetable and fruit consumption. The study-specific RR and 95 % CI are represented by the black diamond and the horizontal line, respectively; the area of the grey square is proportional to the specific-study weight to the overall meta-analysis. The centre of the open diamond and the vertical dashed line represent the pooled RR and the width of the open diamond represents the pooled 95 % CI
Table 4.
Subgroup analyses of vegetable and fruit consumption and metabolic syndrome (MetS)
| Stratification | No. of studies | Pooled RR | 95 % CI | Heterogeneity |
|---|---|---|---|---|
| All studies | 8 | 0·75 | 0·63, 0·90 | P<0·001; I 2=92·3 % |
| Gender | ||||
| Male | 3 | 0·67 | 0·56, 0·80 | P=0·007; I 2=69 % |
| Female | 4 | 0·66 | 0·58, 0·76 | P=0·10; I 2=52 % |
| Design | ||||
| Cross-sectional or case–control | 6 | 0·69 | 0·60, 0·81 | P<0·001; I 2=84 % |
| Cohort | 2 | 1·08 | 0·97, 1·21 | P=0·19; I 2=41 % |
| Diagnostic criteria of MetS | ||||
| NCEP ATP III | 4 | 0·70 | 0·53, 0·94 | P<0·001; I 2=90 % |
| IDF | 1 | 0·63 | 0·30, 1·32 | – |
| AHA | 2 | 0·87 | 0·55, 1·37 | P<0·001; I 2=98 % |
| Geographical region | ||||
| Asia | 2 | 0·75 | 0·67, 0·83 | P=0·37; I 2=0 % |
| Europe | 1 | 0·69 | 0·67, 0·71 | – |
| USA | 3 | 1·07 | 0·96, 1·20 | P =0·16; I 2=46 % |
| South America | 2 | 0·70 | 0·36, 1·39 | P<0·001; I 2=96 % |
| Sample size | ||||
| <1000 | 0 | – | – | – |
| >1000 | 8 | 0·75 | 0·63, 0·90 | P<0·001; I 2=92 % |
| Age of population | ||||
| Adult | 5 | 0·76 | 0·58, 0·99 | P<0·001; I 2=95 % |
| Adolescents | 2 | 0·76 | 0·68, 0·84 | P=0·62; I 2=0 % |
RR, relative risk; NCEP ATP III, National Cholesterol Education Program–Adult Treatment Panel III; IDF, International Diabetes Federation; AHA, American Heart Association.
Sensitivity analysis
The results of the sensitivity analysis showed only minimal changes in magnitude of the pooled RR and heterogeneity when any one study was excluded from the meta-analysis, indicating that no individual study had excessive influence on these robust aggregated results (data not shown).
Meta-regression
Low (P=0·123; I 2=30·1 %), moderate (P<0·001; I 2=61·6 %) and high (P<0·001; I 2=92 %) heterogeneity was demonstrated for the associations of vegetable, fruit, and vegetable and fruit consumption with MetS, respectively. To explore the sources of heterogeneity, univariate meta-regression with covariates was performed. For vegetable consumption, the results showed the following: publication year (P=0·289), sample size (P=0·045), gender (P=0·515), age of the population (P=0·195), geographical region (P=0·316), study design (P=0·809), diagnostic criteria of MetS (P=0·872). Only sample size (P=0·045) seemed to contribute to the heterogeneity in this analysis. With regard to fruit consumption, the results showed the following: publication year (P=0·269), sample size (P=0·581), gender (P=0·695), age of the population (P=0·377), geographical region (P=0·871), study design (P=0·068), diagnostic criteria of MetS (P=0·192). None of these covariates was found to contribute to the moderate heterogeneity. In addition, concerning fruit and vegetable consumption, the results showed the following: publication year (P=0·419), sample size (P=0·05), gender (P=0·046), age of the population (P=0·870), geographical region (P=0·857), study design (P=0·152), diagnostic criteria of MetS (P=0·698). Gender (P=0·046) and sample size (P=0·05) seemed to contribute to the high heterogeneity in this analysis.
Discussion
In the present meta-analysis, a total of twenty-six observational studies were included for examination. The pooled analysis showed that vegetable and/or fruit consumption were negatively associated with MetS. However, the consumption of green vegetables might not be associated with MetS.
The underlying mechanism behind the negative associations of vegetable and/or fruit consumption with MetS may be explained as follows. First, as an established biomarker for vegetable and fruit consumption, vitamin C was found to be associated with a lower risk of MetS( 11 ). Second, fibre, another important substance in vegetables and fruits, was proved to be inversely associated with MetS( 12 – 14 ). Third, the fat content in vegetables was also reported to be associated with a lower risk of MetS( 45 ). Finally, as fruits and vegetables are good sources of antioxidants and anti-inflammatory agents, their intake may be beneficial for MetS patients( 15 , 46 ). On the other hand, the Mediterranean diet and the Dietary Approaches to Stop Hypertension (DASH) diet, which are rich in vegetable and fruit consumption, were reported to be negatively associated with either risk or prevalence of MetS( 47 – 52 ). These findings are strongly consistent with the results of the present study. Therefore, it is speculated that vegetable and fruit consumption may exert a positive effect on MetS.
Recently, an earlier meta-analysis including eight randomized controlled trials explored the influence of fruits and vegetables on MetS( 46 ). Interestingly, it reported that fruit and vegetable intake was associated only with a reduction in diastolic blood pressure, but not in waist circumference, systolic blood pressure, fasting glucose, HDL cholesterol and TAG levels in MetS patients. However, that meta-analysis only investigated the effect of fruit and vegetable interventions on the components of MetS, with no trial reporting changes in the prevalence of MetS. Therefore, further randomized controlled trials that aim at MetS directly are still needed.
Generally, radish leaf, spinach, cucumber and pepper are regarded as ‘green vegetables’, and cabbage, radish, sprout, carrot, pumpkin and tomato are regarded as ‘white vegetables’( 30 ). Luo et al. found that the consumption of white vegetables was inversely associated with the risk of colorectal cancer, while green vegetable intake was not( 53 ). Therefore, it is speculated that the biological effect of vegetables may vary with variety. This was also the subject that the present study intended to address. However, due to the limited number of included studies (only three), subgroup analysis was only conducted for green vegetables in the present meta-analysis. Surprisingly, the results showed that green vegetable consumption was not associated with MetS. With respect to this obvious difference between the results for vegetables as a whole and for green vegetables, several speculations were raised as follows. First of all, the reliability of the results might be weakened since only three studies related to green vegetables were included for subgroup analysis. Second, white vegetables might have a significant contribution to the anti-MetS effect of vegetables as a whole. Furthermore, the components in green vegetables are complicated, and some neglected substances might work against the effect of vitamin C or fibre. Although some inconsistency in results with regard to gender, study design, diagnostic criteria of MetS and geographical region was found in subgroup analysis (Tables 2–4), it might be due to the high heterogeneity or limited number of included studies. As a consequence, more well-designed studies with detailed specification of vegetable varieties are needed.
The strengths of the present meta-analysis are mainly reflected in the following aspects. First, it is the first meta-analysis of observational studies aiming at the associations of vegetable and/or fruit consumption with MetS based on the most comprehensive literature search to date. Second, the included studies were analysed based on adjusted results and large samples. Third, the present study can serve as a reference and indication for further research (specify the variety of vegetable). Limitations of the present study should also be acknowledged. First, the substantial level of heterogeneity might have distorted the results. Second, due to the limitation of relevant literature, only a limited number of observational studies qualified for the current meta-analysis. Third, the classification of exposure may also vary greatly among individuals. Fourth, the diagnostic criteria of MetS and the selection of adjusted factors were not uniform. Fifth, since only a few studies specified the varieties of vegetable, some issues could not be addressed. Finally, due to the limitation of insufficient data at present, a dose–response analysis could not be performed in the current meta-analysis. These limitations might weaken the significance of the present study.
Conclusions
The existing evidence suggests that vegetable and/or fruit consumption are negatively associated with MetS. However, due to the limited number of included studies, the consumption of green vegetables might not be associated with MetS. More well-designed prospective cohort studies that specify vegetable varieties are needed to elaborate the concerned issues further.
Acknowledgements
Financial support: This work was supported by the China Scholarship Council (student ID: 201706370196) and the Fundamental Research Funds for the Central Universities of Central South University (2017zzts233). The funders had no role in the design, analysis or writing of this article. Conflict of interest: The authors declare that there are no conflicts of interest. Authorship: Y.Z. conceived the idea, performed the statistical analysis and drafted this meta-analysis. Y.Z. and D.Z.Z. selected and retrieved relevant papers. D.Z.Z. assessed each study. D.Z.Z. was the guarantor of the overall content. All authors revised and approved the final manuscript. Ethics of human subject participation: Not applicable.
References
- 1. Kaur J (2014) A comprehensive review on metabolic syndrome. Cardiol Res Pract 2014, 943162. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 2. O’Neill S & O’Driscoll L (2015) Metabolic syndrome: a closer look at the growing epidemic and its associated pathologies. Obes Rev 16, 1–12. [DOI] [PubMed] [Google Scholar]
- 3. Athyros VG, Ganotakis ES, Elisaf M et al. (2005) The prevalence of the metabolic syndrome using the National Cholesterol Educational Program and International Diabetes Federation definitions. Curr Med Res Opin 21, 1157–1159. [DOI] [PubMed] [Google Scholar]
- 4. Sun K, Ren M, Liu D et al. (2014) Alcohol consumption and risk of metabolic syndrome: a meta-analysis of prospective studies. Clin Nutr 33, 596–602. [DOI] [PubMed] [Google Scholar]
- 5. Narain A, Kwok CS & Mamas MA (2017) Soft drink intake and the risk of metabolic syndrome: a systematic review and meta-analysis. Int J Clin Pract 71, e12927. [DOI] [PubMed] [Google Scholar]
- 6. Marventano S, Salomone F, Godos J et al. (2016) Coffee and tea consumption in relation with non-alcoholic fatty liver and metabolic syndrome: a systematic review and meta-analysis of observational studies. Clin Nutr 35, 1269–1281. [DOI] [PubMed] [Google Scholar]
- 7. Lutsey PL, Steffen LM & Stevens J (2008) Dietary intake and the development of the metabolic syndrome: the atherosclerosis risk in communities study. Circulation 117, 754–761. [DOI] [PubMed] [Google Scholar]
- 8. Duthie SJ, Duthie GG, Russell WR et al. (2017) Effect of increasing fruit and vegetable intake by dietary intervention on nutritional biomarkers and attitudes to dietary change: a randomised trial. Eur J Nutr. Published online: 30 May 2017. doi: 10.1007/s00394-017-1469-0. [DOI] [PMC free article] [PubMed]
- 9. Hall JN, Moore S, Harper SB et al. (2009) Global variability in fruit and vegetable consumption. Am J Prev Med 36, 402–409.e5. [DOI] [PubMed] [Google Scholar]
- 10. Aune D, Giovannucci E, Boffetta P et al. (2017) Fruit and vegetable intake and the risk of cardiovascular disease, total cancer and all-cause mortality – a systematic review and dose–response meta-analysis of prospective studies. Int J Epidemiol 46, 1029–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kim J & Choi YH (2016) Physical activity, dietary vitamin C, and metabolic syndrome in the Korean adults: the Korea National Health and Nutrition Examination Survey 2008 to 2012. Public Health 135, 30–37. [DOI] [PubMed] [Google Scholar]
- 12. Hosseinpour-Niazi S, Mirmiran P, Sohrab G et al. (2011) Inverse association between fruit, legume, and cereal fiber and the risk of metabolic syndrome: Tehran Lipid and Glucose Study. Diabetes Res Clin Pract 94, 276–283. [DOI] [PubMed] [Google Scholar]
- 13. Hosseinpour-Niazi S, Mirmiran P, Mirzaei S et al. (2015) Cereal, fruit and vegetable fibre intake and the risk of the metabolic syndrome: a prospective study in the Tehran Lipid and Glucose Study. J Hum Nutr Diet 28, 236–245. [DOI] [PubMed] [Google Scholar]
- 14. Hosseinpour Niazi S, Mirmiran P, Sohrab G et al. (2012) Association between dietary fiber intake and metabolic syndrome: Tehran Lipid and Glucose Study. Iran J Epidemiol 7, 19–28. [Google Scholar]
- 15. Qiao Q (2006) Comparison of different definitions of the metabolic syndrome in relation to cardiovascular mortality in European men and women. Diabetologia 49, 2837–2846. [DOI] [PubMed] [Google Scholar]
- 16. Hong SA & Kim MK (2017) Relationship between fruit and vegetable intake and the risk of metabolic syndrome and its disorders in Korean women according to menopausal status. Asia Pac J Clin Nutr 26, 514–523. [DOI] [PubMed] [Google Scholar]
- 17. Kim OY, Kwak SY, Kim B et al. (2017) Selected food consumption mediates the association between education level and metabolic syndrome in Korean Adults. Ann Nutr Metab 70, 122–131. [DOI] [PubMed] [Google Scholar]
- 18. Krishnadath IS, Toelsie JR, Hofman A et al. (2016) Ethnic disparities in the prevalence of metabolic syndrome and its risk factors in the Suriname health study: a cross-sectional population study. BMJ Open 6, e013183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fletcher EA, McNaughton SA, Lacy KE et al. (2016) Mediating effects of dietary intake on associations of TV viewing, body mass index and metabolic syndrome in adolescents. Obes Sci Pract 2, 232–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Baik I, Lee M, Jun NR et al. (2013) A healthy dietary pattern consisting of a variety of food choices is inversely associated with the development of metabolic syndrome. Nutr Res Pract 7, 233–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Castanho GK, Marsola FC, McLellan KC et al. (2013) Consumption of fruit and vegetables associated with the metabolic syndrome and its components in an adult population sample. Cien Saude Colet 18, 385–392. [DOI] [PubMed] [Google Scholar]
- 22. Prasad DS, Kabir Z, Dash AK et al. (2012) Prevalence and risk factors for metabolic syndrome in Asian Indians: a community study from urban Eastern India. J Cardiovasc Dis Res 3, 204–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. de Oliveira EP, McLellan KC, Vaz de Arruda Silveira L et al. (2012) Dietary factors associated with metabolic syndrome in Brazilian adults. Nutr J 11, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jung HJ, Han SN, Song S et al. (2011) Association between adherence to the Korean Food Guidance System and the risk of metabolic abnormalities in Koreans. Nutr Res Pract 5, 560–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kouki R, Schwab U, Hassinen M et al. (2011) Food consumption, nutrient intake and the risk of having metabolic syndrome: the DR’s EXTRA Study. Eur J Clin Nutr 65, 368–377. [DOI] [PubMed] [Google Scholar]
- 26. Kwasniewska M, Kaleta D, Dziankowska-Zaborszczyk E et al. (2009) Healthy behaviours, lifestyle patterns and sociodemographic determinants of the metabolic syndrome. Cent Eur J Public Health 17, 14–19. [DOI] [PubMed] [Google Scholar]
- 27. Shin A, Lim SY, Sung J et al. (2009) Dietary intake, eating habits, and metabolic syndrome in Korean men. J Am Diet Assoc 109, 633–640. [DOI] [PubMed] [Google Scholar]
- 28. Pan Y & Pratt CA (2008) Metabolic syndrome and its association with diet and physical activity in US adolescents. J Am Diet Assoc 108, 276–286. [DOI] [PubMed] [Google Scholar]
- 29. Wu Y, Yu Y, Zhao T et al. (2016) Interactions of environmental factors and APOA1–APOC3–APOA4–APOA5 gene cluster gene polymorphisms with metabolic syndrome. PLoS One 11, e0147946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Park S, Ham JO & Lee BK (2015) Effects of total vitamin A, vitamin C, and fruit intake on risk for metabolic syndrome in Korean women and men. Nutrition 31, 111–118. [DOI] [PubMed] [Google Scholar]
- 31. Boucher JL, Sidebottom AC, Sillah A et al. (2013) Short-term changes in lifestyle risk factors and incident metabolic syndrome in the heart of new Ulm project. Circulation 128, A13983. [Google Scholar]
- 32. Lin YH, Chang HT, Tseng YH et al. (2013) Characteristics and health behavior of newly developed metabolic syndrome among community-dwelling elderly in Taiwan. Int J Gerontol 7, 90–96. [Google Scholar]
- 33. Masaki M (2013) Dietary patterns and risk for metabolic syndrome. J Diabetes 5, 195. [Google Scholar]
- 34. Esmaillzadeh A, Kimiagar M, Mehrabi Y et al. (2006) Fruit and vegetable intakes, C-reactive protein, and the metabolic syndrome. Am J Clin Nutr 84, 1489–1497. [DOI] [PubMed] [Google Scholar]
- 35. Dussaillant C, Echeverría G, Villarroel L et al. (2015) Unhealthy food intake is linked to higher prevalence of metabolic syndrome in Chilean adult population: cross sectional study in 2009–2010 national health survey. Nutr Hosp 32, 2098–2104. [DOI] [PubMed] [Google Scholar]
- 36. Neia Martini FA, Borges MB & Guedes DP (2014) Eating habit and metabolic syndrome in a sample of Brazilian adults. Arch Latinoam Nutr 64, 161–173. [PubMed] [Google Scholar]
- 37. Jaaskelainen P, Magnussen CG, Pahkala K et al. (2012) Childhood nutrition in predicting metabolic syndrome in adults. Diabetes Care 35, 1937–1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cho YC, Kwon IS, Park JY et al. (2012) Prevalence of metabolic syndrome and Its associated factors among health checkup examinees in a university hospital. J Korea Academia–Industrial Cooperation Soc 13, 5317–5325. [Google Scholar]
- 39. Kelishadi R, Gouya MM, Adeli K et al. (2008) Factors associated with the metabolic syndrome in a national sample of youths: CASPIAN study. Nutr Metab Cardiovasc Dis 18, 461–470. [DOI] [PubMed] [Google Scholar]
- 40. Chen TH, Hsiao HP, Chiu YW et al. (2014) Maternal diabetes or hypertension and lifestyle factors may be associated with metabolic syndrome: a population-based study in Taiwan. Kaohsiung J Med Sci 30, 86–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Liberati A, Altman DG, Tetzlaff J et al. (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 339, b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wells GA, Shea B, O’Connell D et al.2010) The Newcastle–Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed February 2018).
- 43. Begg CB & Mazumdar M (1994) Operating characteristics of a rank correlation test for publication bias. Biometrics 50, 1088–1101. [PubMed] [Google Scholar]
- 44. Higgins JP & Thompson SG (2004) Controlling the risk of spurious findings from meta-regression. Stat Med 23, 1663–1682. [DOI] [PubMed] [Google Scholar]
- 45. Um YJ, Oh SW, Lee CM et al. (2015) Dietary fat intake and the risk of metabolic syndrome in Korean adults. Korean J Fam Med 36, 245–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Shin JY, Kim JY, Kang HT et al. (2015) Effect of fruits and vegetables on metabolic syndrome: a systematic review and meta-analysis of randomized controlled trials. Int J Food Sci Nutr 66, 416–425. [DOI] [PubMed] [Google Scholar]
- 47. Steffen LM, Van Horn L, Daviglus ML et al. (2014) A modified Mediterranean diet score is associated with a lower risk of incident metabolic syndrome over 25 years among young adults: the CARDIA (Coronary Artery Risk Development in Young Adults) study. Br J Nutr 112, 1654–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Babio N, Bullo M & Salas-Salvado J (2009) Mediterranean diet and metabolic syndrome: the evidence. Public Health Nutr 12, 1607–1617. [DOI] [PubMed] [Google Scholar]
- 49. Esposito K, Ciotola M & Giugliano D (2007) Mediterranean diet and the metabolic syndrome. Mol Nutr Food Res 51, 1268–1274. [DOI] [PubMed] [Google Scholar]
- 50. Godos J, Zappalà G, Bernardini S et al. (2017) Adherence to the Mediterranean diet is inversely associated with metabolic syndrome occurrence: a meta-analysis of observational studies. Int J Food Sci Nutr 68, 138–148. [DOI] [PubMed] [Google Scholar]
- 51. Drehmer M, Odegaard AO, Schmidt MI et al. (2017) Brazilian dietary patterns and the dietary approaches to stop hypertension (DASH) diet-relationship with metabolic syndrome and newly diagnosed diabetes in the ELSA-Brasil study. Diabetol Metab Syndr 9, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Saneei P, Fallahi E, Barak F et al. (2015) Adherence to the DASH diet and prevalence of the metabolic syndrome among Iranian women. Eur J Nutr 54, 421–428. [DOI] [PubMed] [Google Scholar]
- 53. Luo WP, Fang YJ, Lu MS et al. (2015) High consumption of vegetable and fruit colour groups is inversely associated with the risk of colorectal cancer: a case–control study. Br J Nutr 113, 1129–1138. [DOI] [PubMed] [Google Scholar]