Abstract
Objective
The present review aimed to examine the association of eating frequency with body weight or body composition in adults of both sexes.
Design
PubMed, EMBASE and Scopus databases were searched. PRISMA and MOOSE protocols were followed. Observational studies published up to August 2016 were included. The methodological quality of the studies was assessed with the Downs and Black checklist.
Setting
A systematic review of the literature.
Subjects
Adults (n 136 052); the majority of studies were developed in the USA and Europe.
Results
Thirty-one articles were included in the review: two prospective and twenty-nine cross-sectional studies. Thirteen per cent of the studies received quality scores above 80 %. The assessment of eating frequency and body composition or body weight varied widely across the studies. Potential confounders were included in 73 % of the studies. Fourteen studies reported an inverse association between eating frequency and body weight or body composition, and seven studies found a positive association. The majority of studies applied multiple analyses adjusted for potential confounders, such as sex, age, education, income, smoking, physical activity and alcohol intake. Six studies took into account under-reporting of eating frequency and/or energy intake in the analysis, and one investigated the mediation effect of energy intake.
Conclusions
There is not sufficient evidence confirming the association between eating frequency and body weight or body composition when misreporting bias is taken into account. However, in men, a potential protective effect of high eating frequency was observed on BMI and visceral obesity.
Keywords: Systematic literature review Obesity, Eating frequency, Body weight, Meal pattern, Body composition
Obesity is increasing at alarming rates worldwide( 1 ). The media, health professionals and guidelines for health and weight management have postulated that higher eating frequency may be good for weight management( 2 ), but such a recommendation lacks solid evidence to justify it.
Since the 1960s, some scientists have suggested an inverse association between the consumption of a greater number of small meals per day and body weight maintenance( 3 ). According to them, the consumption of more meals per day might lead to greater thermogenesis, higher insulin sensitivity and lower total energy intake( 4 , 5 ).
Since then, studies that have attempted to determine the effects of eating frequency on weight have reached different conclusions. Some experimental and observational studies of eating patterns and body weight status conducted in the 1960s and 1970s found an inverse relationship between eating frequency and adiposity, supporting the claim for an association between lower body weight and higher eating frequency( 3 , 6 ). More recently, mainly in the 2000s, studies have shown mixed conclusions. A meta-analysis on meal frequency with respect to changes in fat and lean mass based on experimental research, published in 2015, found only a small potential benefit of increased feeding frequency for fat mass and body fat percentage( 7 ). Two observational studies showed results in the same direction( 8 , 9 ); while others have also reported a sex difference( 10 , 11 ). On the other hand, some studies found a positive association between eating frequency and body weight status( 12 , 13 ) or did not find any relationship( 14 , 15 ).
Overall energy intake also has a relevant role in the causal pathway that links meal frequency and weight maintenance, although the results of studies evaluating the effect of eating frequency on energy intake were inconclusive. Edelstein et al.( 16 ) and Howarth et al.( 17 ) showed that total energy intake increased with increasing frequency of meals or snacks, in both men and women. Meanwhile, Westerterp-Plantenga et al.( 18 ) described that healthy young men with a high habitual meal frequency had lower total energy intake. Additionally, a study reported that meal frequency and a period of fasting have no major impact on energy intake( 19 ).
Considering the need to organize these divergent evidences, the aim of the present systematic literature review (SLR) was to examine the association between eating frequency and body weight and body composition in adults of both sexes.
Methods
An SLR was conducted aiming to find original articles on the association between eating frequency and body composition or body weight. The PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses)( 20 ) and MOOSE (Guidelines for Meta-Analyses and Systematic Reviews of Observational Studies)( 21 ) protocols were followed. Thus, the research protocol was identified using the PICO (patient, intervention, comparison, outcome) strategy. The articles retrieved from the literature met the following inclusion criteria: (i) the study design was observational; (ii) the article was derived from original research; (iii) the measured outcome included at least one of weight, change in body weight, overweight, obesity, BMI, adiposity, waist circumference, waist-to-hip ratio or abdominal obesity; (iv) the exposure measurement included eating frequency as number of meals per day; and (v) the samples comprised adults (aged >18 years).
PubMed, EMBASE and Scopus databases were searched. Articles published from 1960 to August 2016 were included. The search strategies are shown in Table 1. Terms relative to eating frequency and body weight were used. Additional papers were identified in the reference lists of selected articles that met the inclusion criteria. All records identified were uploaded or manually entered into EndNote X4. The searches were conducted by two independent investigators (R.C. and A.S.G.) and their results were compared.
Table 1.
Search strategy for Pubmed, EMBASE and Scopus
| Exposure | |
|---|---|
| EMBASE | ‘meal frequency’ OR ‘meal frequencies’ OR ‘meals’/exp OR ‘meals’ OR ‘meal time’/exp OR ‘meal time’ OR ‘mealtime’ OR ‘mealtimes’ OR ‘meal times’ OR ‘eating frequency’ OR ‘eating frequencies’ OR ‘eating episodes’ OR ‘meal pattern’ OR ‘meal patterns’ OR ‘eating pattern’ OR ‘eating patterns’ OR ‘eating behaviors’ OR ‘dietary pattern’ OR ‘dietary patterns’ OR ‘dietary habits’ OR ‘diet habit’ OR’ diet habits’ |
| Scopus | ‘meal frequency’ OR ‘meal frequencies’ OR ‘meals’ OR ‘meal time’ OR ‘mealtime’ OR ‘mealtimes’ OR ‘meal times’ OR ‘eating frequency’ OR ‘eating frequencies’ OR ‘eating episodes’ OR ‘meal pattern’ OR ‘meal patterns’ OR ‘eating pattern’ OR ‘eating patterns’ OR ‘eating behaviors’ OR ‘dietary pattern’ OR ‘dietary patterns’ OR ‘dietary habits’ OR ‘diet habit’ OR ‘diet habits’ |
| Outcome | |
| PubMed | ‘weight’ [all fields] OR ‘overweight’ [all fields] OR ‘obesity ’[all fields] OR ‘adiposity’ [all fields] OR ‘waist circumference’ [all fields] OR ‘BMI’ [all fields] OR ‘waist-to-hip ratio’ [all fields] OR ‘abdominal obesity ’[all fields] OR ‘change body weight’ [all fields] |
| EMBASE | ‘weight’/exp OR ‘weight’ OR ‘overweight’/exp OR ‘overweight’ OR ‘obesity’/exp OR ‘obesity’ OR ‘adiposity’/exp OR ‘adiposity’ OR ‘waist circumference’/exp OR ‘waist circumference’ OR ‘BMI’/exp OR ‘BMI’ OR ‘waist-to-hip ratio’/exp OR ‘waist-to-hip ratio’ OR ‘abdominal obesity’/exp OR ‘abdominal obesity’ OR ‘change body weight’ |
| Scopus | ‘weight’ OR ‘overweight’ OR ‘obesity’ OR ‘adiposity’ OR ‘waist circumference’ OR ‘ BMI’ OR ‘waist-to-hip ratio’ OR ‘abdominal obesity’ OR ‘change body weight’ |
| Design | |
| PubMed | Case-Control Study [all fields] OR Case Control Study [all fields] OR Epidemiological Studies [all fields] OR Retrospective Studies [all fields] OR Cohort Study [all fields] OR Incidence Study [all fields] OR Cross-Sectional Study [all fields] OR Cross Sectional Study [all fields] OR Prevalence Study [all fields] OR Longitudinal Study [all fields] OR Follow-Up Study [all fields] OR Prospective Study [all fields] |
| EMBASE | ‘case control study’/de OR ‘cohort analysis’/de OR ‘cross-sectional study’/de OR ‘longitudinal study’/de OR ‘observational study’/de OR ‘prospective study’/de OR ‘retrospective study’/de |
| Scopus | case-control study OR case control study OR epidemiological studies OR retrospective studies OR cohort study OR incidence study OR cross-sectional study OR cross sectional study OR prevalence study OR longitudinal study OR follow-up study OR prospective study |
| Limits | |
| PubMed | (‘adult’ [all fields] OR ‘adults’ [all fields]) AND (‘humans’ [MeSH terms] AND ‘adult’ [MeSH terms]) |
| EMBASE | ([article]/lim OR [article in press]/lim) AND [adult]/lim AND [humans]/lim AND [embase]/lim |
| Scopus | (LIMIT-TO (DOCTYPE, ‘ar’)) AND (LIMIT-TO (SRCTYPE, ‘j’)) AND (EXCLUDE (EXACTKEYWORD, ‘Adolescent’) OR EXCLUDE (EXACTKEYWORD, ‘Child’)) |
MeSH, medical subject heading.
The articles that met all the established criteria were included. Two reviewers (R.C. and A.S.G.) independently read all titles and abstracts. At a second stage, the reviewers read in full all manuscripts that had consensus about their inclusion. If consensus between the two reviewers could not be reached, a third reviewer (M.T.A.O.) was called upon to make a final decision. In four instances the full text of the article was not available. In theses cases we contacted the authors by email up to three times.
The data were extracted and summarized according to the following variables: first author, date of publication, study design, sample size, subjects’ age, follow-up duration (prospective studies), outcomes and exposure assessment, statistical analysis including confounders and mediators used in the adjusted analysis, and numerical results.
Guidelines for SLR and meta-analysis have drawn attention to the importance of evaluating the possible bias in the key methodology domains of the primary studies( 22 ). In the present SLR, a validated checklist originally proposed by Downs and Black was used in order to assess the quality of the selected articles, especially regarding possible bias. This checklist, originally proposed to rate the quality of clinical trials, consists of twenty-seven items that evaluate the risk of bias in five domains: reporting, external validity, internal validity, confounding and power. Subsequently, this checklist was adapted for observational studies( 23 ), and items 8, 13, 23 and 24 were eliminated for longitudinal studies, while items 8, 9, 13, 17, 23 and 24 were excluded for the assessment of cross-sectional studies. In the present SLR, items 14 and 15 were also eliminated for both designs because they evaluate the blinding process and most observational studies do not take blinding into consideration. The final scale ranged from 0 (poorest quality) to 21 points (best quality) for longitudinal studies and 19 points (best quality) for cross-sectional studies. All items received scores of 0 or 1 (1 if the item was contemplated in the study and 0 if the item was not contemplated or was not able to be determined), with the exception of item 5. Item 5 evaluates if a list of main confounders was provided, ranging from 0 to 2 (0=no; 1=partially; 2=yes). In item 27, the score (0 or 1) depended on whether the statistical power of the survey was explicitly stated in the article.
A score of quality was created as follows: the number given by the total sum of the questions was then divided by the number of total applicable items in the study and finally multiplied by 100.
In the second stage of the quality assessment, in the same way, a general assessment of the quality of the articles was performed for each item of the evaluation instrument. The studies with questions that had scores of 1 or 2 were classified as having a ‘low risk of bias’, whereas scores of 0 reflected a ‘risk of bias’.
The two reviewers (R.C. and A.S.G.) independently made use of the checklist to assess the quality of the retrieved articles. When a consensus could not be reached between them, the third reviewer (M.T.A.O.) was called upon to make a final decision.
Results
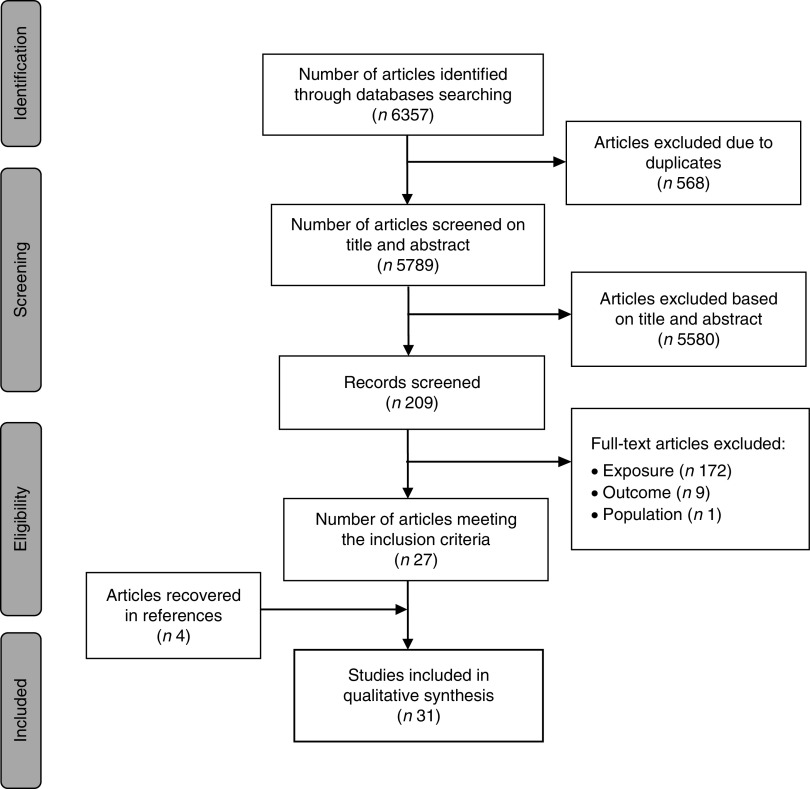
The search strategies resulted in 6357 articles (2503 from PubMed; 2380 from EMBASE; 1474 from Scopus). After excluding duplications, 5789 titles and abstracts were examined; 209 full texts were selected for reading. One hundred and eighty-two articles were excluded for the following reasons: outcome and exposure measurements did not meet the inclusion criteria (n172); the study population was not adult (n1); and the article did not show the statistical results for the analysis of the relationship between exposure and outcome (n1). Twenty-seven articles met all of the inclusion criteria. The references of these articles were checked, resulting in four additional articles. As a result, a total of thirty-one articles were included in the present SLR (Fig. 1).
Fig. 1.
The search and selection process in the present systematic literature review according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement
The studies had different sample characteristics. The majority of the studies were conducted in the USA( 6 , 9 , 12 , 13 , 15 , 17 , 24 – 28 ) and European countries( 8 , 10 , 24 , 29 – 38 ). Two studies included only men( 13 , 36 ), five included only women( 32 , 35 , 37 , 39 , 40 ) and twenty-four included both sexes( 6 , 8 – 12 , 14 , 15 , 17 , 24 – 31 , 33 , 34 , 37 , 38 , 41 – 43 ). The sample sizes of the studies ranged between eighty-two( 37 ) and 34974 individuals( 33 ), and the age of the participants was between 18 and 90 years old. Two prospective( 13 , 15 ) and twenty-nine cross-sectional studies were retrieved. The follow-up of prospective studies was 8 and 10 years( 13 , 15 ) (Table 2).
Table 2.
Summary of population and design characteristics of the studies sorted according to quality scores
| Study | Year(s) | Population | Sample size | Age (years) | Study design |
|---|---|---|---|---|---|
| Aljuraiban et al. (2015)( 24 ) | 1996–1999 | Women and men from INTERMAP | 2696 | 40–59 | Cross-sectional |
| Murakami & Livingstone (2015)( 12 ) | 2003–2012 | Women and men from NHANES 2003–2012 | 18 696 | ≥60 | Cross-sectional |
| Gigante et al. (1997)( 42 ) | 1994 | Brazilian women and men | 1035 | 20–69 | Cross-sectional |
| Karatzi et al. (2015)( 29 ) | NA | Greek women and men | 164 | Mean 46·8 (sd 9·3) | Cross-sectional |
| Oliveira et al. (2009)( 44 ) | NA | Brazilian women and men | 570 | 19–59 | Cross-sectional |
| Kant et al. (1995)( 15 ) | 1971–1975 to 1982–1984 | Women and men from NHANES I and NHEFS | 7147 | 25–74 | Prospective: 8–10-year follow-up |
| Holmback et al. (2010)( 10 ) | 1991–1995 | Women and men from Sweden Diet and Cancer cohort | 3009 | 47–68 | Cross-sectional |
| Kim et al. (2014)( 14 ) | 2005 | Women and men from Third Korean National Health and Nutrition Examination Survey | 4625 | ≥19 | Cross-sectional |
| Mohindra et al. (2009)( 25 ) | 1998–1991 | US adults from Louisiana Bogalusa Heart Study | 504 | 19–28 | Cross-sectional |
| Howarth et al. (2005)( 17 ) | 1994–1996 | Women and men from US Continuing Survey of Food Intake | 2685 | Younger: 20–59 Older: 60–90 | Cross-sectional |
| Ma et al. (2003)( 9 ) | NA | American women and men | 299 | 20–70 | Cross-sectional |
| Mills et al. (2011)( 40 ) | 2008 | American women | 1099 | 40–60 | Cross-sectional |
| Smith et al. (2012)( 11 ) | 2004–2006 | Australian women and men | 2775 | 26–36 | Cross-sectional |
| Titan et al. (2011)( 30 ) | 1993 and 1997 | Women and men from Norfolk cohort of EPIC | 14 666 | 45–75 | Cross-sectional |
| Yannakoulia et al. (2007)( 32 ) | NA | Greek women | 64 pre- and 50 postmenopausal | 24–74 | Cross-sectional |
| Murakami and Livingstone (2014)( 38 ) | 2000–2001 | British women and men | 1487 | 19–64 | Cross-sectional |
| Berg et al. (2009)( 31 ) | 2001–2004 | Swiss women and men | 3610 | 25–74 | Cross-sectional |
| Drummond et al. (1998)( 8 ) | NA | Women and men workers of Scotland | 95 | Mean 20 (sd 55) | Cross-sectional |
| Marín-Guerrero et al. (2008)( 33 ) | 1999 | Spanish women and men | 34 974 | 25–64 | Cross-sectional |
| Peixoto et al. (2007)( 43 ) | 2001 | Brazilian women and men | 1252 | 20–64 | Cross-sectional |
| Ruidavets et al. (2002)( 36 ) | 1996–1997 | French men | 330 | 45–64 | Cross-sectional |
| Teichmann et al. (2006)( 39 ) | NA | Brazilian women | 981 | 20–60 | Cross-sectional |
| van der Heijden et al. (2007)( 13 ) | 1992–2002 | Men from HPSF | 20 064 | 46–81 | Prospective: 10-year follow-up |
| Bachman et al. (2011)( 26 ) | 2006–2007 | American women and men | 257 | 18–65 | Cross-sectional |
| Bertéus Forslund et al. (2005)( 34 ) | Reference: 1994–1999 Obese: 1997–2001 | Swiss women and men | Obese: 4470 Reference: 1092 | Obese: 30–60 Reference: 37–60 | Cross-sectional |
| Bertéus-Forslund et al. (2002)( 35 ) | Obese: 1994–1999 Reference: NA | Swiss obese women | Obese: 83 Reference: 94 | 37–60 | Cross-sectional |
| Metzner et al. (1997)( 6 ) | 1967–1969 | American women and men | 2028 | 35–69 | Cross-sectional |
| Pearcey and de Castro (2002)( 27 ) | NA | Women and men from research pool at Georgia State University | 19 weight-gaining men and women and 19 weight-stable | NA | Cross-sectional |
| Reicks et al. (2014)( 28 ) | 2013 | American women and men | 2702 | 18–80 | Cross-sectional |
| Amosa et al. (2001)( 37 ) | 1994 | Polynesian and European women | 82 | 18–27 | Cross-sectional |
| Al-Isa (1999)( 41 ) | 1997–1998 | Kuwait women and men university students | 842 | 18–23 | Cross-sectional |
NA, not available; INTERMAP, International Study on Macro/Micronutrients and Blood Pressure; NHANES, National Health and Nutrition Examination Survey; NHEFS, NHANES Epidemiologic Follow-up Study; EPIC, European Prospective Investigation into Cancer and Nutrition; HPSF, Health Professionals Follow-up Study.
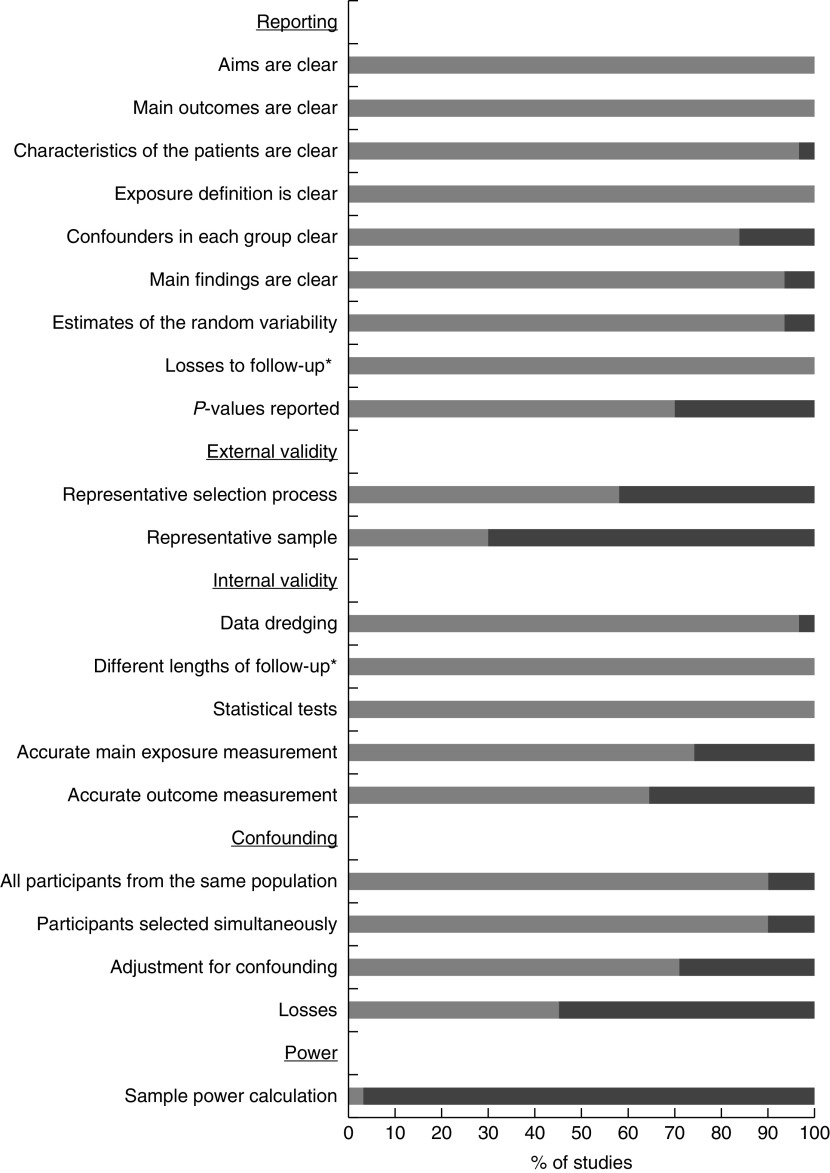
In the reporting items, most articles were classified as having a ‘low risk of bias’. On the other hand, in the external validity domain, several of the articles were not clear about how their participants were selected (42 %) or/and did not rely on representative samples (70 %). As regards internal validity, 34·8 % of the studies did not use an accurate method (valid and reliable) to measure the outcomes, using self-reported measures. In the confounding domain, 54·8 % of the articles did not describe characteristics of participants lost between the initial selection process and the final sample, and 29·0 % of the studies did not perform any adjustment for confounding in the analysis. Finally, almost all studies (96·7 %) did not report a power calculation for their sample size and were classified as having a ‘risk of bias’ in the power domain (Fig. 2).
Fig. 2.
Summary of quality assessment ( , low risk of bias;
, low risk of bias;  , risk of bias) of the studies (n 31) included in the present systematic literature review. *Items ‘different lengths of follow-up’ and ‘losses to follow-up’ were evaluated only in prospective studies
, risk of bias) of the studies (n 31) included in the present systematic literature review. *Items ‘different lengths of follow-up’ and ‘losses to follow-up’ were evaluated only in prospective studies
Considering the fourteen studies that found inverse associations between eating frequency and the outcomes, the following was observed: all studies were cross-sectional; eleven had scores of quality above 70 %( 8 – 11 , 24 , 25 , 30 , 33 , 36 , 43 , 44 ); and eight studies found an association between eating frequency and outcomes measured as body weight, BMI, overweight or obesity( 8 – 10 , 24 , 25 , 30 ), five as waist circumference or waist-to-hip ratio( 10 , 11 , 25 , 36 , 44 ) and one as adiposity index( 6 ). Only one study used self-reported measurements( 28 ). In most studies (n 11) multiple recalls or meal pattern questionnaires were used in order to assess the eating frequency( 6 , 8 – 11 , 24 – 26 , 28 , 33 , 36 ). Five studies classified the exposure as a continuous variable( 8 , 11 , 25 , 26 , 30 ); seven according to the three major meals (breakfast, lunch and dinner) and compared the intake of three meals with a greater or lower number of meals( 9 , 10 , 24 , 33 , 36 , 43 , 44 ). Most studies (n 10) performed multiple analyses or other statistical methods to adjust for possible confounding, including sociodemographic variables such as sex( 8 – 10 , 24 , 25 , 30 , 33 , 36 , 43 , 44 ), age( 9 – 11 , 24 , 25 , 30 , 33 , 36 , 43 , 44 ), education( 10 , 11 , 24 , 33 , 36 , 44 , 45 ), income( 10 , 43 , 44 ) and race/ethnicity( 25 , 44 ); and behavioural variables such as smoking( 24 , 25 , 30 , 33 , 36 , 43 , 44 ), physical activity( 9 – 11 , 16 , 24 , 30 , 33 , 36 , 43 , 44 ), alcohol intake( 10 , 11 , 30 , 33 , 43 , 44 ) and dietary characteristics( 9 – 11 , 24 , 25 , 30 , 36 , 43 , 44 ). Seven studies took into account energy intake as a confounder( 6 , 9 , 10 , 24 , 25 , 30 , 36 ) and in three of them misreporters of energy were excluded( 10 , 24 , 36 ) (Table 3).
Table 3.
Summary of the main results of studies that found an inverse association between eating frequency and body weight or body composition (n 14)
| Study | Quality score (%) | Outcomes | Exposure assessment | Exposure classification | Statistical analysis | Results | |
|---|---|---|---|---|---|---|---|
| Aljuraiban et al. (2015)( 24 ) | 94·7 | BMI: continuous variable | No. of meals: main meals and snacks Assessment: 4×24 h recall | <4 v. 4 v. 5 v. ≥6 meals and continuous variable | BMI: generalized linear model adjusted for sex, age, population sample, educational level, PA, smoking, diet, dietary supplement and EI BMI difference: multiple linear regression model adjusted for sex, age, population sample and EI Energy misreporting was defined based on EI:EER and <2092 kJ/24 h (<500 kcal/24 h) or >20 920 kJ/24 h (>5000 kcal/24 h) for women and >33 472 kJ/24 h (>8000 kcal/24 h) for men | Men and women BMI (kg/m2), mean (95 % CI): >4 meals: 29·0 (28·8, 29·5) 4 meals: 28·4 (28·0, 28·5) 5 meals: 28·1 (27·7, 28·4) ≥6 meals: 27·3 (26·8, 27·8) P<0·01 BMI difference (kg/m2): β=−1·1 (95 % CI −1·6, −0·7) P<0·0001 | |
| Oliveira et al. (2009)( 44 ) | 89·5 | Overweight: BMI ≥ 25·0 kg/m2 WC risk category: men>94 cm, women>80 cm | Total no. of meals: main meals and snacks Assessment: simple question | ≤3 v. ≥4 meals | Logistic regression model adjusted for sex, age, race, marital status, education, income, occupational status, self-rated health, PA, smoking, alcohol intake, parity, morbidities, dietary practices, frequency of fruits, vegetables, meats, sausages and derivatives intake | Men WC, OR (95 % CI): ≥4 v. ≤3 meals: 3·5 (1·3, 9·3) BMI: NS | Women WC and BMI: NS |
| Holmback et al. (2010)( 10 ) | 84·2 | Underweight: BMI<18·5 kg/m2 Normal weight: BMI=18·5–25·0 kg/m2 Overweight: BMI=25·0–29·9 kg/m2 Obesity: BMI≥30 kg/m2 WC increased risk: 80 cm (women), 94 cm (men) WC greatly increased risk: 88 cm (women), 102 cm (men) | No. of meals: main meals and snacks Assessment: meal pattern questionnaire | ≤3 v. ≥6 meals 4–5 v. ≥6 meals | Logistic regression model adjusted for age, education, SES, smoking, alcohol intake, PA activity and EI Energy misreporting was defined based on EI:BMR | Men Obese, OR (95 % CI): ≥6 v. ≤3 meals: 2·4 (1·02, 5·7) ≥6 v. 4–5 meals: 1·1 (0·6, 2·2) WC greatly increased risk, OR (95 % CI): ≥6 v. ≤3 meals: 2·1 (1·0, 4·3) ≥6 v. 4–5 meals: 1·6 (0·9, 2·8) | Women Obese: P=0·12 WC greatly increased risk: P=0·07 |
| Mohindra et al. (2009)( 25 ) | 84·2 | Overweight: BMI=≥25·0–29·0 kg/m2 Obesity: BMI≥30·0 kg/m2 | Total no. of meals: main meals and snacks Assessment: 1×24 h dietary recall | Continuous variable | Logistic regression model adjusted for sex, age, ethnicity and EI | Men and women Normal v. overweight, OR (95 % CI): 0·9 (0·8,1·1) Normal v. obese, OR (95 % CI): 0·8 (0·7,1·0) | |
| Titan et al. (2001)( 30 ) | 78·9 | BMI (kg/m2) and WHR: continuous variables | Total no. of meals: main meals, snacks, biscuits with coffee breaks Assessment: simple question | Continuous variable | Multiple linear regression adjusted for age, obesity, smoking, PA, intakes of alcohol, fat, protein and carbohydrate, and EI | Men BMI (kg/m2): β=−0·08 (se 0·03) P=0·02 WHR: β=−0·01 (se 0·01) P=0·42 | Women BMI (kg/m2): β=0·05 (se 0·04) P=0·27 WHR: β=−0·01 (se 0·01) P=0·02 |
| Ma et al. (2003)( 9 ) | 78·9 | Obesity: BMI≥30·0 kg/m2 | Total no. of meals: main meals and snacks Assessment: 10–15×24 h dietary recall | ≤3 v. ≥4 meals | Logistic regression model adjusted for sex, age, PA and EI | Men and women ≤3v. ≥4 meals, OR (95 % CI): 0·5 (0·3, 0·9) | |
| Smith et al. (2012)( 11 ) | 78·9 | BMI (kg/m2) and WC (cm): continuous variables | Total no. of meals: main meals and snacks Assessment: meal pattern questionnaire | Continuous variable | Multiple linear regression adjusted for age, education, PA, alcohol intake, diet quality and overall dietary quality | Men WC (cm): β=−0·7 (95 % CI −1·1, −0·3) BMI (kg/m2): β=−0·3 (95 % CI −0·4, −0·1) | Women WC (cm): β=−0·1 (95 % CI −0·5, 0·3) BMI (kg/m2): β=−0·1 (95 % CI −0·2, 0·9) |
| Drummond et al. (1998)( 8 ) | 73·7 | Body weight (kg), BMI (kg/m2) and body fat %: continuous variables | Total no. of meals: any occasion when food was taken Assessment: 7 d food record | Continuous variable | Pearson’s correlation without adjustments | Men Body weight: r=−0·34 P=0·03 BMI: P=0·09 Body fat %: P=0·17 | Women Body weight: P=0·41 BMI: P=0·35 Body fat %: P=0·43 |
| Marín-Guerrero et al. (2008)( 33 ) | 73·7 | Obesity: BMI ≥30·0 kg/m2 | 3–4 meals: 3 main meals and afternoon tea 2 meals: 2 main meals 1 meal: 1 main meal Snack: small amounts of food many times over the course of the day Assessment: meal pattern questionnaire | 1 v. ≥3–4 meals ≤2 v. ≥3–4 meals 3–4 v. ≥5 meals | Logistic regression model adjusted for age, education, size of town of residence, marital status, PA, smoking, alcohol intake and health status | Men 3–4 v. 2 meals, OR (95 % CI): 1·6 (CI 1·4, 1·9) 3–4 v. 1 meals, OR (95 % CI): 1·4 (0·9, 2·1) 3–4 v. ≥5 meals, OR (95 % CI): 1·4 (1·0, 2·0) | Women 3–4 v. 2 meals, OR (95 % CI): 1·3 (1·0, 1·6) 3–4 v. 1 meals, OR (95 % CI): 1·1 (0·7, 1·8) 3–4 v. ≥5 meals, OR (95 % CI): 1·5 (1·2, 1·9) |
| Peixoto et al. (2007)( 43 ) | 73·7 | BMI (kg/m2): continuous variables | No. of meals Assessment: simple question | ≤3 v. ≥4 meals | Multiple linear regression adjusted for age, income, smoking, alcohol intake, PA, frequency of meat and vegetable intake | Men <3 v. ≥4 meals: β=−0·8 (95 % CI −1·5, −2·1) | Women NS |
| Ruidavets et al. (2002)( 36 ) | 73·7 | BMI (kg/m2) and WHR: continuous variables | Total no. of meals: any food or drink intake providing energy Assessment: 3 d food record | 1–2 v. ≥5 meals 3 v. ≥5 meals 3 v. ≥5 meals | Multiple linear regression adjusted for age, education, PA, smoking, habits of dieting and EI Energy misreporting was defined based on EI:BMR | Men BMI (kg/m2): ≥5 meals: reference 4 meals: β=1·7 (se 0·7) 3 meals: β=1·7 (se 0·7) 1–2 meals: β=3·7 (se 1·1) P=0·05 WHR: ≥5 meals: reference 4 meals: β=0·01 (se 0·01) 3 meals: β=0·01 (se 0·01) 1–2 meals: β=0·04 (se 0·02) P<0·01 | |
| Bachman et al. (2011)( 26 ) | 68·4 | Overweight/obese (OW): BMI=27·0–45·0 kg/m2 Weight-loss maintainers (WLM): BMI>25·0 kg/m2 at some point in life, lost>10 % of maximum body weight and maintained that for at least 5 years, and were weight-stable within the previous 2 years Normal weight (NW): BMI=19·0–24·9 kg/m2 at entry into the trial, never overweight or obese and were weight-stable within the previous 2 years | Main meals: breakfast, lunch and dinner Snack: any food eaten outside habitual meal times Assessment: 3 ×24 h dietary recalls | Continuous variable | Covariance analyses adjusted for sex, age, PA and Pearson correlation without adjustment. | Men and women Snacks, mean (sd): WLM: 1·9 (1·1) NW: 2·3 (1·1) OW: 1·5 (1·3) P<0·05 Meals, mean (sd): WLM: 2·7 (0·4) NW: 2·7 (0·4) OW: 2·7 (0·5) P>0·05 All participants BMI v. snacks: r=−0·20 P<0·01 | |
| Metzner et al. (1977)( 6 ) | 68·4 | Adiposity index: continuous variable | Total no. of meals: main meals and snacks Assessment: 1 × 24 h dietary recall | 2 v. 6 meals | ANCOVA with energy (calories) per kilogram of ideal weight as the covariate | Men and women Mean adiposity index gets smaller as the number of meals increases from 2 to 6 (numerical results not shown) | |
| Reicks et al. (2014)( 28 ) | 63·2 | BMI (kg/m2): continuous variable Self-reported | No. of meals: main meals and snacks Assessment: self-administered meal pattern questionnaire and meal pattern history | 1–5 v. 6–10 v. ≥11 meals | Quantile regression of the median without adjustment | Men and women BMI (kg/m2), median: 1–5 meals: 27·0 6–10 meals: 26·5 ≥11 meals: 26·2 P=0·008 | |
WC, waist circumference; WHR, waist-to-hip ratio; PA, physical activity; EI, energy intake; EER, estimated energy requirement; SES, socio-economic status; β, linear regression coefficient; NS, not statistically significant and P value not available; r, correlation coefficient.
Eating frequency was positively associated with the outcomes in seven studies and one of them presented a prospective design( 13 ). Five studies received quality scores above 70 %( 12 , 13 , 17 , 32 , 38 ). The outcomes were measured as weight, BMI, overweight/obesity in six studies( 12 , 13 , 17 , 34 , 35 , 38 ); two studies used self-reported measurements( 13 , 17 ), including the prospective study. In the majority of studies (n 5), eating frequency was assessed through multiple recalls or meal pattern questionnaires. In four studies, the exposure was classified as a continuous variable( 32 , 34 , 35 , 38 ), and in two according to the three major meals and compared with a greater number of meals( 12 , 17 ). Four studies that provided a complete list of sociodemographic (age, race/ethnicity, education and income) and behavioural (smoking, physical activity and dietary characteristics) confounders were included in the analysis( 12 , 13 , 17 , 38 ). In five studies, energy intake was investigated as a confounder( 12 , 17 , 32 , 34 , 38 ), and one investigated the mediation effect of energy intake( 17 ). In three of them its measurement took into account misreporting( 12 , 32 , 38 ) (Table 4).
Table 4.
Summary of the main results of studies that found a positive association between eating frequency and body weight or body composition (n 6)
| Study | Quality score (%) | Outcome | Exposure assessment | Exposure classification | Statistical methods and confounders | Results | |
|---|---|---|---|---|---|---|---|
| Murakami and Livingstone (2015)( 12 ) | 94·7 | Overweight: BMI ≥ 25·0 kg/m2 Central obesity: Men, WC >102 cm; women, WC>88 cm | EFall: all eating occasions EFenergy: all eating occasions except for those providing no energy EF≥50 kcal: all eating occasions providing ≥50 kcal (≥209 kJ) Assessment: 2×24 h dietary recalls | EFall and EFenergy: ≤3·5 v. 4 v. 4·5 v. 5 v. ≥5·5 meals EF≥50 kcal: ≤3 v. 3·5 v. 5 v. 4·5 v. ≥5 meals | Logistic regression model adjusted for age, race/ethnicity, education, income, smoking, PA, intakes of alcohol, protein, fat, sugar and dietary fibre, EI:EER Energy misreporting was defined based on EI:EER | Overweight, Men EFall, OR (95 % CI): ≤3·5 meals: 1·0 (ref.) 4 meals: 1·2 (0·9, 1·6) 4·5 meals: 1·1 (0·9, 1·3) 5 meals: 1·2 (0·9, 1·5) ≥5·5: 1·4 (1·2, 1·7) P=0·0006 EFenergy, OR (95 % CI): ≤3·5meals: 1·0 (ref.) 4 meals: 1·3 (1·0, 1·6) 4·5 meals: 1·1 (0·8, 1·3) 5 meals: 1·1 (0·9, 1·5) ≥5·5 meals: 1·4 (1·2, 1·7) P=0·002 EF≥50 kcal, OR (95 % CI): ≤3 meals: 1·0 (ref.) 3·5 meals: 1·4 (1·1, 1·7) 4 meals: 1·3 (1·0, 1·7) 4·5 meals: 1·4 (1·15, 1·8) ≥5meals: 1·5 (1·2, 1·9) P=0·003 | Overweight, Women EFall: P=0·40 EFenergy: P=0·56 EF≥50 kcal, OR (95 % CI): ≤3 meals: 1·0 (ref.) 3·5 meals: 1·2 (0·9, 1·5) 4 meals: 1·3 (1·0, 1·7) 4·5 meals: 1·3 (1·0, 1·6) ≥5 meals: 1·4(1·2, 1·8) P=0·001 |
| Central obesity, Men EFall, OR (95 % CI): ≤3·5 meals: 1·0 (ref.) 4 meals: 14 (0·9, 1·4) 4·5 meals: 1·1 (0·9, 1·4) 5 meals: 1·4 (1·1, 1·7) ≥5·5 meals: 1·4 (1·2, 1·7) P=0·0001 EFenergy, OR (95 % CI): ≤3·5 meals: 1·0 (ref.) 4 meals: 1·1 (0·9, 1·4) 4·5 meals: 1·1 (0·9, 1·4) 5 meals: 1·4 (1·1, 1·7) ≥5·5 meals: 1·4 (1·1, 1·7) P=0·0001 EF≥50 kcal, OR (95 % CI): ≤3 meals: 1·0 (ref.) 3·5 meals: 1·0 (0·9, 1·2) 4 meals: 1·3 (1·0, 1·6) 4·5 meals: 1·4 (1·1, 1·8) ≥5 meals: 1·4 (1·1, 1·7) P=0·002 | Central obesity, Women EFall: P=0·31 EFenergy: P=0·52 EF≥50 kcal, OR (95 % CI): ≤3 meals: 1·0 (ref.) 3·5 meals: 1·1 (0·9, 1·4) 4 meals: 1·4 (1·1, 1·7) 4·5 meals: 1·3 (1·0, 1·6) ≥5 meals: 1·3 (1·0, 1·6) P=0·03 | ||||||
| Howarth et al. (2007)( 17 ) | 78·9 | BMI (kg/m2): continuous variable Self-reported | No. of meals: breakfast, brunch, lunch, dinner, supper or snack Assessment: self-administered meal pattern questionnaire | ≤3 v. 3·5–6 meals ≤3 v. >6 meals | Multiple linear regression adjusted for sex, age, ethnicity, education, income urbanity, geographic region, smoking, self-reported chronic disease, TV viewing, fibre and fat | Younger men and women ≤3 v. 3·5–6 meals: β=0·37 P=0·19 ≤3 v. >6 meals: β=1·28 P=0·006 | Older men and women ≤3 v. 3·5–6 meals: β=0·87 P=0·022 ≤3 v. >6 meals: β=2·32 P=0·004 |
| Yannakoulia et al. (2007)( 32 ) | 78·9 | BMI (kg/m2), WC (cm), WHR and body fat %: continuous variables | Total no. of meals: any eating occasion when food or drink was taken Assessment: 3 d food record | Continuous variable | Multiple linear regression adjusted for age, PA and EI Energy misreporting was defined based on EI:BMR | Postmenopausal Body fat %: β=0·41 P=0·01 BMI, WC, WHR: P<0·05 | Premenopausal Body fat %, BMI, WC, WHR: P<0·05 |
| Murakami & Livingstone (2014)( 38 ) | 73·7 | BMI (kg/m2) and WC (cm): continuous variables | EFall: all eating occasions EFenergy: all eating occasions except for those providing no energy EF≥210 kJ: all eating occasions providing ≥210 kJ Assessment: 7 d weighed food record | Continuous variable | Multiple linear regression adjusted for age, social class, smoking, PA, intakes of protein, fat, total sugar, alcohol, dietary fibre and EI:EER Energy misreporting was defined based on EI:EER | Men EFall BMI (kg/m2): β=0·19 (se 0·08) P=0·03 WC (cm): β=0·35 (se 0·23) P=0·12 EFenergy BMI (kg/m2): β=0·23 (se 0·09) P≤0·01 WC (cm): β=0·47 (se 0·25) P=0·07 EF≥210 kJ BMI (kg/m2): β=0·37 (se 0·13) P=0·004 WC (cm): β=0·80 (se 0·35) P=0·02 | Women EFall BMI (kg/m2): β=0·05 (se 0·11) P=0·67 WC (cm): β=0·12 (se 0·25) P=0·63 EFenergy BMI (kg/m2): β=0·06 (se 0·13) P=0·63 WC (cm): β=0·14 (se 0·29) P=0·63 EF≥210 kJ BMI (kg/m2): β=0·61 (se 0·21) P=0·004 WC (cm): β=1·22 (se 0·47) P=0·01 |
| Bertéus Forslund et al. (2005)( 34 ) | 68·4 | Reference: BMI<30·0 kg/m2 Obesity: BMI≥30·0 kg/m2 | Total no. of meals: main meal, light meal/breakfast, snacks or drink-only Assessment: self-administered meal pattern questionnaire | Continuous variable | Logistic regression model adjusted for age and EI | Men and women Reference v. obesity, OR (95 % CI): 1·2 (1·1, 1·3) | |
| Bertéus Forslund et al. (2002)( 35 ) | 68·4 | Obesity: BMI≥30·0 kg/m2 | Total no. of meals: main meals, light meal/breakfast, snacks or drink-only Assessment: self-administered meal pattern questionnaire | Continuous variable | Two-sample t test without adjustment | Women Obese v. Reference, meals (mean): 6·1 v. 5·2 P<0·0001 | |
WC, waist circumference; WHR, waist-to-hip ratio; PA, physical activity; EI, energy intake; EER, estimated energy requirement; TV, television; ref., reference category; β, linear regression coefficient.
Ten studies did not show associations between eating frequency and outcomes, one being prospective( 15 ). Seven received quality scores above 70 %( 14 , 15 , 29 , 31 , 39 , 40 , 42 ). Six studies used a simple question or meal pattern questionnaire to access eating frequency( 14 , 15 , 31 , 39 , 41 , 42 ) and three used multiple recalls( 27 , 29 , 37 ). Five studies provided a complete list of possible sociodemographic confounders (age, race/ethnicity, education and income)( 15 , 39 – 42 ) and four of behavioural confounders (smoking, physical activity and alcohol intake)( 15 , 39 , 41 , 42 ). Investigation of energy intake as confounder was included in the analyses of only two studies( 15 , 40 ) (Table 5).
Table 5.
Summary of the main results of studies that did not find an association between eating frequency and body weight or body composition (n 10)
| Study | Quality score (%) | Outcome | Exposure assessment | Exposure classification | Confounders | Results | |
|---|---|---|---|---|---|---|---|
| Gigante et al. (1997)( 42 ) | 89·5 | Obesity: BMI≥30·0 kg/m2 | No. of meals: breakfast, lunch, dinner and snacks. Assessment: meal pattern questionnaire | ≤3 v. 4–6 meals | Logistic regression model adjusted for sex, age, race, marital status, education, income, occupational status, self-rated health, PA, smoking, alcohol intake, parental overweight status, parity and morbidities | Men and women <3 v. 4–6 meals, OR (95 % CI): 0·8 (0·4, 1·7) | |
| Karatzi et al. (2015)( 29 ) | 89·5 | BMI (kg/m2) and WC (cm): continuous variables | No. of meals: food or drink Assessment: 3 d food record | Continuous variable | Multiple linear regression model adjusted for age, intakes of carbohydrates and fat, and EI | BMI (kg/m2), one-meal increase: β=−0·01 (95 % CI −0·01, +0·01) WC (cm), one-meal increase: β=−0·01 (95 % CI −0·01, +0·01) | |
| Kant et al. (1995)( 15 ) | 85·7 | Weight (kg): continuous variable | Total no. of meals: main meals and snacks Assessment: 1971: 1×24 h dietary recall 1982: simple question | Continuous variable | Multiple linear regression model adjusted for age, education, race, baseline BMI, length of follow-up, smoking, alcohol intake, PA, parity, morbidity and EI | Men β=0·08 (se 0·13) P=0·52 | Women β=0·2299 (se 0·15) P=0·13 |
| Kim et al. (2014)( 14 ) | 84·2 | BMI (kg/m2) and WC (cm): continuous variables | No. of meals Assessment: simple question | ≤2 v. 3 v. 4 v. ≥5 meals | General linear model without adjustments | WC (cm), mean (sd): ≤2 meals: 79·9 (0·6) 3 meals: 81·2 (0·3) 4 meals: 80·7 (0·3) ≥5 meals: 79·5 (0·4) P=0·007 BMI (kg/m2), mean (sd): ≤2 meals: 23·4 (0·2) 3 meals: 23·6 (0·1) 4 meals: 23·6 (0·1) ≥5 meals 23·3 (0·1) P=0·212 | |
| Mills et al. (2011)( 40 ) | 78·9 | Overweight: BMI≥25·0–29·9 kg/m2 Obesity: BMI≥30·0 kg/m2 Self-reported | Total no. of meals: breakfast, lunch, dinner and snack Assessment: 1 d food record | Continuous variable | Logistic regression model adjusted for age, income, marital status, race, education, menopausal status and EI Energy misreporting was defined based on EI:BMR | Men and women Meals Normal v. overweight/obesity, OR (95 % CI): 0·9 (0·7, 1·2) Snacking Normal v. overweight/obesity, OR (95 % CI): 1·0 (0·7, 1·2) | |
| Berg et al. (2009)( 31 ) | 73·7 | Reference: BMI<30·0 kg/m2 Obesity: BMI ≥ 30·0 kg/m2 | Total no. of meals (1–8): morning coffee, breakfast, between-meal snack, lunch, between-meal snack, dinner, supper and night meal Assessment: self-administered meal pattern questionnaire | Continuous variable | Logistic regression model adjusted for sex, age, smoking and PA | Men and women Reference v. obesity, OR (95 % CI): 0·9 (0·9, 1·0) | |
| Teichmann et al. (2006)( 39 ) | 73·7 | Obesity: BMI≥30·0 kg/m2 | Total no. of meals: main meals and snacks Assessment: simple question | 1–2 v. 3 meals 1–2 v. 4 meals 1–2 v. ≤5 meals | Poisson regression model adjusted for sex, age, race, marital status, education, income, occupational status, self-rated health, PA, smoking, alcohol intake, parental overweight status, parity and morbidities | Women Obesity, PR (95 % CI): 1–2 meals: 1·0 (ref.) 3 meals: 1·7 (0·9, 3·2) 4 meals: 1·3 (CI 0·7, 2·5) ≤5 meals: 0·9 (0·5, 1·8) | |
| Pearcey and de Castro (2002)( 27 ) | 63·2 | Weight-gaining: a weight gain of >5 % during the previous 6 months Weight-stable: a weight gain of <5 % during the previous 6 months | Total no. of meals: main meals and snacks Assessment: 7 d food record | Continuous variable | Student’s t test without adjustments | Men and women Weight-stable group, mean (se): 3·30 (0·14) P<0·05 Weight-gaining group, mean (se): 3·52 (0·13) P<0·05 | |
| Amosa et al. (2001)( 37 ) | 57·9 | Obesity: BMI ≥30·0 kg/m2 | Total no. of meals in the seven records: breakfast, lunch, dinner and snacks Assessment: 7 d food record | Continuous variable | Mann–Whitney test without adjustments | European women Meals, median (range): Non-obese: 31 (20–40) Obese: 29 (13–39) P>0·05 | Polynesian women Meals, median (range): Non-obese: 31 (17–58) Obese: 28 (17–53) P>0·05 |
| Al-Isa et al. (1999)( 41 ) | 42·1 | Non-obesity: BMI≤25·0 kg/m2 Obesity I: BMI=25·0–30·0 kg/m2 Obesity II: BMI≥30·0 kg/m2 | No. of the main meals: 1, 2 or 3 Assessment: simple question | 1 v. 2 v. 3 meals | Logistic regression model adjusted for sex, age, marital status, obesity among parents, parents’ education, parents’ occupation, dieting, last dental or physical check-up, education, number of male, female and total siblings, college major, number of obese relatives, number of people residing at home, number of servants, birth order, countries prefer visiting, family income, chronic disease, PA and eating between meals | Men and women P>0·05 | |
WC, waist circumference; PA, physical activity; EI, energy intake; β, linear regression coefficient; PR: prevalence ratio; ref., reference category.
Among the ten studies that showed analyses in men separately, five found inverse associations between high eating frequency and waist circumference( 10 , 11 , 44 ) or waist-to-hip ratio( 30 , 36 ) and seven found inverse associations for body weight( 8 , 10 , 11 , 30 , 33 , 36 , 43 , 46 ). All of these studies adjusted for physical activity and dietary characteristics in multiple analyses, but only two of them took into account dietary intake misreporting( 10 , 36 ). On the other hand, when the results were analysed only in women, no pattern was observed in the results.
Finally, when the results were analysed according to exposure and outcomes, no association pattern was observed.
Discussion
Our SLR focused on the association of eating frequency with body composition or body weight. We concluded that, to date, there is not sufficient evidence for establishing a clear association between eating frequency and body composition or body weight. However, among men, a potential protective effect of high eating frequency on these outcomes was observed.
The findings should be interpreted in light of the methodological characteristics of the articles included. First, the outcome and exposure measurement might not be accurate in some studies. Moreover, the outcome measurement varied among the studies, thereby limiting the comparability among them. For example, the role of eating frequency on body weight might be different from the one it has on central adiposity. With respect to exposure, different methods were used for data collection. Some studies used methods such as multiple recalls or food diaries and meal pattern questionnaires, and may be more accurate than others, such as simple questions, for eating frequency assessment. Although dietary records and 24 h dietary recalls are subject to misreporting, particularly under-reporting( 47 ), there is no information about the validity of most of the meal pattern questionnaires and simple questions used in these studies. Furthermore, different cut-offs were used to determine high or low eating frequency. Only six authors classified eating frequency according to the three major meals (breakfast, lunch and dinner) and compared intake of three meals with intake of a greater number of meals. Other studies assessed the exposure as a continuous variable or compared the extremes of eating frequency (e.g. two v. six meals per day).
Second, the results are based mostly on cross-sectional studies, with only two studies selected having a longitudinal design, which should be considered a limitation in the current available literature. Longitudinal studies are well known for being a better study design to investigate the temporal relationship between the exposure and change in outcome status. The issue of reverse causality is especially important in this case, because people skip meals, thus reducing eating frequency, when they become overweight in an attempt to lose weight or to prevent further gain( 47 ).
Finally, obesity is a multifactorial disorder arising from genetic, environmental, socio-economic and behavioural factors. These differ in their respective contributions to the obesity epidemic( 48 ). In this sense, another methodological issue that is very important in this type of epidemiological investigation is the inclusion of main confounders and mediators in the analysis. A confounding variable is an extraneous variable in a statistical model that correlates (positively or negatively) with both the exposure and the outcome variable; meanwhile, a mediator factor is a variable that occurs in the causal pathway between the exposure and the outcome( 49 ). The majority of studies compiled in the present SLR applied multiple analyses adjusted for potential confounders, such as sex, age, education, income, smoking, physical activity and alcohol intake.
The effect of dietary characteristics (energy intake and quality of diet) in the causal pathway linking eating frequency to body weight and body composition was adjusted for in the multiple analyses of most studies. However, in order to understand the role of a variable in a causal chain, sometimes it is more informative to stratify the analyses according to this variable, rather than adjusting for it in multiple analyses. In this sense, only one study investigated the mediation effect of energy intake by stratifying the analysis according to it( 17 ). However, several studies included in the present SLR showed that higher eating frequency is positively associated with energy intake( 10 , 17 , 30 , 34 , 40 ). In Holmback et al.( 10 ), eating frequency and carbohydrate energy percentage, as well as relative fibre intake, increased with higher eating frequency; while the energy percentage from fat, protein and alcohol decreased. Bertéus Forslund et al.( 34 ) found that sweet and fatty food groups were associated with snacking and contributed considerably to energy intake. Regarding quality of diet, Mills et al. ( 40 ) showed that intakes of fruit and vegetables, whole grains, dietary fibre, dairy and added sugars also increased as eating frequency increased. Other aspects of diet, such as meal timing, are also important in the control and reduction of body weight, total body fat and visceral fat( 50 ).
In our SLR, fourteen studies reported an inverse association between eating frequency and body weight or body composition. Murakami and Livingstone and Bellisle et al.( 12 , 51 ) have called attention to this apparent inverse relationship between eating frequency and adiposity measures, suggesting it is an artifact that in part can be attributed to the under-reporting of eating frequency concomitant with the under-reporting of energy intake by overweight or obese subjects. In this regard, Murakami and Livingstone’s( 12 ) study showed the importance of evaluating energy intake misreporting when examining the association between eating frequency and overweight/obesity and central obesity. In their study, energy intake misreporting was evaluated based on ratio of energy intake to estimated energy requirement (EI:EER). In the multiple analyses, without taking into account energy intake or EI:EER, eating frequency showed an inverse or null association with the outcomes. However, after full adjustment including EI:EER, a completely different picture emerged: eating frequency was positively associated with overweight/obesity and central obesity. Only three studies that reported an inverse association between eating frequency and body weight or body composition in the present SLR took into account under-reporting of energy intake and/or eating frequency; and three of the six studies that found a positive association between eating frequency and outcomes took into account misreporting of energy intake.
Physical activity also plays a role in the association that links eating frequency with body weight and body composition( 52 ). Physical activity might be a potential confounder in this association, since physical activity practice may improve diet quality( 53 , 54 ); although physical activity has been well described in the literature as an independent factor in the control and reduction of body weight, total body fat and visceral fat( 55 , 56 ).
Even though it was not the objective of our SLR to investigate differences between sexes, when analysing the results of the articles included, a potential protective effect of high eating frequency on the outcomes was observed among men. In general, increased eating frequency has been postulated to increase metabolism( 4 ), appetite control and food intake( 46 , 57 ), and to improve glucose and insulin control( 58 , 59 ). However, this difference could be due to the fact that men who have high eating frequency also have a healthier lifestyle, including practice of physical activity and healthier eating habits, which results in reduced body fat and waist circumference. In Holmback et al.( 10 ), a high fibre intake was the clearest diet-quality indicator associated with a high eating frequency among men. In two other studies, men were more physically active than women, which may help to explain the protective effect found only in men( 11 , 43 ). However, only in Smith et al.’s study( 11 ) was physical activity measured by a direct method (pedometer). In the other two studies( 10 , 43 ), self-reported measurements were used, which can be inaccurate. In addition, there is evidence that energy intake compensation is poor in women, a factor normally associated with obesity( 60 ). Drummond et al. ( 8 ) showed that men compensated for extra eating occasions by reducing the mean energy per eating episode. Although all studies adjusted for physical activity and dietary characteristics in the multiple analyses, it is possible that residual confounding of both lifestyle factors could contribute to the results.
The present review is the first SLR of observational studies that examines specifically the association of eating frequency with body weight and body composition in adults with a systematic approach. Several narrative reviews have been conducted( 2 , 52 , 61 – 63 ) and these concluded that the evidence available to suggest the presence of an association between eating frequency and weight, BMI and body fatness is limited. In addition, a meta-analysis evaluating experimental research suggested that eating frequency is positively associated with reductions in fat mass and body fat percentage, as well as an increase in fat-free mass; however, the positive findings were the product of a single study and need to be interpreted with circumspection( 7 ).
Considering there is contradictory evidence about the association between eating frequency and body weight, it is important to assess the whole body of evidence about this topic and in particular to do so systematically. In addition, researchers have suggested that nutrition policy decisions will have to be made using the totality of the available evidence( 64 ). It is almost inevitable that causal chains in nutrition outcomes involve long periods of latency, complex individual variability in the biological response, and cultural, economic and geographic influences; aspects that observational studies may help to understand, since observational studies for food habits indicate what happens over a lifetime of consumption( 45 , 64 , 65 ).
In this respect, at the same time as we encourage the conduct of more clinical trials to help to examine and potentially determine this causal relationship, future high-quality observational studies are needed to understand the role of eating frequency on loss and maintenance of weight and body composition and to guide clinical recommendations. However, evaluating eating behaviour is also a complex task and we demonstrated substantial heterogeneity in the methodological quality of studies. Thus, in Table 6, we call attention to some important methodological issues that should be considered in future observational studies. It is necessary to conduct studies with long-term longitudinal design and representative samples. Outcome and exposure should be measured with accurate methods and classified based on clinically relevant aspects. Moreover, it is important that statistical analyses should be stratified by sex, if the sample size allows, and to include the potentially relevant confounders and mediators, with special attention to nutrients and energy intake, and these should be appropriately measured.
Table 6.
Methodological recommendations for future observational studies
| Design | Long-term longitudinal studies |
|---|---|
| Sample characteristics | Population-based studies with representative samples and sample size to perform analysis by sex |
| Exposure assessment | Accurate methods should be used for data collection, such as recalls and food diaries, or standardized pattern of meal questionnaires, which can measure food habits; and misreporting should be taken into account in the analyses It is necessary to standardize the method of classifying the eating occasion (main meal and snacks) in order to use a reproducible method between studies The analysis should compare the intake of the three major meals (breakfast, lunch and dinner) with a greater number of meals, in order to propose health recommendations based on results |
| Outcome assessment | Anthropometric measurements of weight, height to determine BMI, as well as abdominal adiposity measurements (waist, abdominal and hip circumferences), should be obtained using standard techniques, avoiding using self-reported measures, especially in cross-sectional studies In addition to BMI and abdominal adiposity, measures of body composition should be included, such as fat mass and fat-free mass, in order to understand the role of eating frequency on body composition. Body composition should be evaluated by objective measurements, such as dual-energy X-ray absorptiometry, impedance or densitometry, when it is possible |
| Confounders and mediators | The analyses should be stratified by sex, if the sample size allows for this, in order to explore whether there are differences in the association between eating frequency and adiposity according to sex Should apply multivariate analyses or other statistical methods to control for possible confounding, such as by age, education, race/ethnicity, marital status, income, physical activity, smoking and sleep duration When associations between eating frequency and body weight or body composition are found, it is very important to investigate the role of food habits, with special attention to energy intake, quality of diet, meal composition (macro- and micronutrients) and meal timing as mediator factors. Furthermore, they should be appropriately measured and under-reporting bias should be taken in account |
Acknowledgements
Financial support: This study was supported by the Foundation for Research Support of the State of Rio Grande do Sul (grant number 1220-2551/13-3). M.T.A.O. and G.K. received research productivity grants from the Brazilian National Council for Scientific and Technological Development (grant numbers 307257/2013-4 and 304182/2013-3). Conflict of interest: none. Authorship: R.C. and M.T.A.O. conceptualized the study. R.C. and A.S.G. completed the searches, abstract/title screening, data extraction and quality assessment. R.C. and M.T.A.O. drafted the manuscript. G.K. and P.I.C.L. assisted in drafting and revision of the manuscript. All authors read and approved the final manuscript. Ethics of human subject participation: Not applicable.
References
- 1. Finucane MM, Stevens GA, Cowan MJ et al. (2011) National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet 377, 557–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Seagle HM, Strain GW, Makris A et al. (2009) Position of the American Dietetic Association: weight management. J Am Diet Assoc 109, 330–346. [DOI] [PubMed] [Google Scholar]
- 3. Fabry P, Hejl Z, Fodor J et al. (1964) The frequency of meals. Its relation to overweight, hypercholesterolaemia, and decreased glucose-tolerance. Lancet 2, 614–615. [DOI] [PubMed] [Google Scholar]
- 4. Jenkins DJ, Wolever TM, Vuksan V et al. (1989) Nibbling versus gorging: metabolic advantages of increased meal frequency. N Engl J Med 321, 929–934. [DOI] [PubMed] [Google Scholar]
- 5. Verboeket-van de Venne WP & Westerterp KR (1991) Influence of the feeding frequency on nutrient utilization in man: consequences for energy metabolism. Eur J Clin Nutr 45, 161–169. [PubMed] [Google Scholar]
- 6. Metzner HL, Lamphiear DE, Wheeler NC et al. (1977) The relationship between frequency of eating and adiposity in adult men and women in the Tecumseh Community Health Study. Am J Clin Nutr 30, 712–715. [DOI] [PubMed] [Google Scholar]
- 7. Schoenfeld JB, Aragon AA & Krieger JW (2015) Effects of meal frequency on weight loss and body composition: a meta-analysis. Nutr Rev 73, 69–82. [DOI] [PubMed] [Google Scholar]
- 8. Drummond SE, Crombie NE, Cursiter MC et al. (1998) Evidence that eating frequency is inversely related to body weight status in male, but not female, non-obese adults reporting valid dietary intakes. Int J Obes Relat Metab Disord 22, 105–112. [DOI] [PubMed] [Google Scholar]
- 9. Ma Y, Bertone ER, Stanek EJ 3rd et al. (2003) Association between eating patterns and obesity in a free-living US adult population. Am J Epidemiol 158, 85–92. [DOI] [PubMed] [Google Scholar]
- 10. Holmback I, Ericson U, Gullberg B et al. (2010) A high eating frequency is associated with an overall healthy lifestyle in middle-aged men and women and reduced likelihood of general and central obesity in men. Br J Nutr 104, 1065–1073. [DOI] [PubMed] [Google Scholar]
- 11. Smith KJ, Blizzard L, McNaughton SA et al. (2012) Daily eating frequency and cardiometabolic risk factors in young Australian adults: cross-sectional analyses. Br J Nutr 108, 1086–1094. [DOI] [PubMed] [Google Scholar]
- 12. Murakami K & Livingstone MB (2015) Eating frequency is positively associated with overweight and central obesity in US adults. J Nutr 145, 2715–2724. [DOI] [PubMed] [Google Scholar]
- 13. van der Heijden AA, Hu FB, Rimm EB et al. (2007) A prospective study of breakfast consumption and weight gain among US men. Obesity (Silver Spring) 15, 2463–2469. [DOI] [PubMed] [Google Scholar]
- 14. Kim S, Park GH, Yang JH et al. (2014) Eating frequency is inversely associated with blood pressure and hypertension in Korean adults: analysis of the Third Korean National Health and Nutrition Examination Survey. Eur J Clin Nutr 68, 481–489. [DOI] [PubMed] [Google Scholar]
- 15. Kant AK, Schatzkin A, Graubard BI et al. (1995) Frequency of eating occasions and weight change in the NHANES I Epidemiologic Follow-up Study. Int J Obes Relat Metab Disord 19, 468–474. [PubMed] [Google Scholar]
- 16. Edelstein SL, Barrett-Connor EL, Wingard DL et al. (1992) Increased meal frequency associated with decreased cholesterol concentrations; Rancho Bernardo, CA, 1984–1987. Am J Clin Nutr 55, 664–669. [DOI] [PubMed] [Google Scholar]
- 17. Howarth NC, Huang TT, Roberts SB et al. (2007) Eating patterns and dietary composition in relation to BMI in younger and older adults. Int J Obes (Lond) 31, 675–684. [DOI] [PubMed] [Google Scholar]
- 18. Westerterp-Plantenga MS, Kovacs EM & Melanson KJ (2002) Habitual meal frequency and energy intake regulation in partially temporally isolated men. Int J Obes Relat Metab Disord 26, 102–110. [DOI] [PubMed] [Google Scholar]
- 19. Taylor MA & Garrow JS (2001) Compared with nibbling, neither gorging nor a morning fast affect short-term energy balance in obese patients in a chamber calorimeter. Int J Obes Relat Metab Disord 25, 519–528. [DOI] [PubMed] [Google Scholar]
- 20. Moher D, Shamseer L, Clarke M et al. (2015) Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 4, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Stroup DF, Berlin JA, Morton SC et al. (2000) Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 283, 2008–2012. [DOI] [PubMed] [Google Scholar]
- 22. Higgins JPT & Green S (editors) (2011) Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. http://handbook.cochrane.org/ (accessed February 2015).
- 23. Monteiro PO & Victora CG (2005) Rapid growth in infancy and childhood and obesity in later life – a systematic review. Obes Rev 6, 143–154. [DOI] [PubMed] [Google Scholar]
- 24. Aljuraiban GS, Chan Q, Oude Griep LM et al. (2015) The impact of eating frequency and time of intake on nutrient quality and body mass index: the INTERMAP Study, a population-based study. J Acad Nutr Diet 115, 528–536.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mohindra NA, Nicklas TA, O’Neil C E et al. (2009) Eating patterns and overweight status in young adults: the Bogalusa Heart Study. Int J Food Sci Nutr 60, Suppl. 3, 14–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bachman JL, Phelan S, Wing RR et al. (2011) Eating frequency is higher in weight loss maintainers and normal-weight individuals than in overweight individuals. J Am Diet Assoc 111, 1730–1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pearcey SM & de Castro JM (2002) Food intake and meal patterns of weight-stable and weight-gaining persons. Am J Clin Nutr 76, 107–112. [DOI] [PubMed] [Google Scholar]
- 28. Reicks M, Degeneffe D, Rendahl A et al. (2014) Associations between eating occasion characteristics and age, gender, presence of children and BMI among US adults. J Am Coll Nutr 33, 315–327. [DOI] [PubMed] [Google Scholar]
- 29. Karatzi K, Yannakoulia M, Psaltopoulou T et al. (2015) Meal patterns in healthy adults: inverse association of eating frequency with subclinical atherosclerosis indexes. Clin Nutr 34, 302–308. [DOI] [PubMed] [Google Scholar]
- 30. Titan SM, Bingham S, Welch A et al. (2001) Frequency of eating and concentrations of serum cholesterol in the Norfolk population of the European prospective investigation into cancer (EPIC-Norfolk): cross sectional study. BMJ 323, 1286–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Berg C, Lappas G, Wolk A et al. (2009) Eating patterns and portion size associated with obesity in a Swedish population. Appetite 52, 21–26. [DOI] [PubMed] [Google Scholar]
- 32. Yannakoulia M, Melistas L, Solomou E et al. (2007) Association of eating frequency with body fatness in pre- and postmenopausal women. Obesity (Silver Spring) 15, 100–106. [DOI] [PubMed] [Google Scholar]
- 33. Marín-Guerrero AC, Gutierrez-Fisac JL, Guallar-Castillon P et al. (2008) Eating behaviours and obesity in the adult population of Spain. Br J Nutr 100, 1142–1148. [DOI] [PubMed] [Google Scholar]
- 34. Bertéus Forslund BH, Torgerson JS, Sjostrom L et al. (2005) Snacking frequency in relation to energy intake and food choices in obese men and women compared to a reference population. Int J Obes (Lond) 29, 711–719. [DOI] [PubMed] [Google Scholar]
- 35. Bertéus-Forslund H, Lindroos AK, Sjostrom L et al. (2002) Meal patterns and obesity in Swedish women – a simple instrument describing usual meal types, frequency and temporal distribution. Eur J Clin Nutr 56, 740–747. [DOI] [PubMed] [Google Scholar]
- 36. Ruidavets JB, Bongard V, Bataille V et al. (2002) Eating frequency and body fatness in middle-aged men. Int J Obes Relat Metab Disord 26, 1476–1483. [DOI] [PubMed] [Google Scholar]
- 37. Amosa T, Rush E & Plank L (2001) Frequency of eating occasions reported by young New Zealand Polynesian and European women. Pac Health Dialog 8, 59–65. [PubMed] [Google Scholar]
- 38. Murakami K & Livingstone MB (2014) Eating frequency in relation to body mass index and waist circumference in British adults. Int J Obes (Lond) 38, 1200–1206. [DOI] [PubMed] [Google Scholar]
- 39. Teichmann L, Olinto MTA, Costa JSD et al. (2006) Risk factors associated with overweight and obesity in women living in São Leopoldo, RG. Rev Bras Epidemiol 9, 360–373. [Google Scholar]
- 40. Mills JP, Perry CD & Reicks M (2011) Eating frequency is associated with energy intake but not obesity in midlife women. Obesity (Silver Spring) 19, 552–559. [DOI] [PubMed] [Google Scholar]
- 41. Al-Isa AN (1999) Obesity among Kuwait University students: an explorative study. J R Soc Promot Health 119, 223–227. [DOI] [PubMed] [Google Scholar]
- 42. Gigante DP, Barros FC, Post CLA et al. (1997) Prevalence and risk factors of obesity in adults. Rev Saude Publica 31, 236–246. [DOI] [PubMed] [Google Scholar]
- 43. Peixoto Mdo R, Benicio MH & Jardim PC (2007) The relationship between body mass index and lifestyle in a Brazilian adult population: a cross-sectional survey. Cad Saude Publica 23, 2694–2740. [DOI] [PubMed] [Google Scholar]
- 44. Oliveira LP, Assis AM, Silva Mda C et al. (2009) Factors associated with overweight and abdominal fat in adults in Salvador, Bahia State, Brazil. Cad Saude Publica 25, 570–582. [DOI] [PubMed] [Google Scholar]
- 45. Blumberg J, Heaney RP, Huncharek M et al. (2010) Evidence-based criteria in the nutritional context. Nutr Rev 68, 478–484. [DOI] [PubMed] [Google Scholar]
- 46. Leidy HJ & Campbell WW (2011) The effect of eating frequency on appetite control and food intake: brief synopsis of controlled feeding studies. J Nutr 141, 154–157. [DOI] [PubMed] [Google Scholar]
- 47. Summerbell CD, Moody RC, Shanks J et al. (1996) Relationship between feeding pattern and body mass index in 220 free-living people in four age groups. Eur J Clin Nutr 50, 513–519. [PubMed] [Google Scholar]
- 48. Cohen DA (2008) Obesity and the built environment: changes in environmental cues cause energy imbalances. Int J Obes (Lond) 32, Suppl. 7, S137–S142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rothman K & Lash T (2008) Modern Epidemiology, 3rd ed. Philadelphia, PA: Lippincott Williams & Wilkins. [Google Scholar]
- 50. Jakubowicz D, Froy O, Wainstein J et al. (2012) Meal timing and composition influence ghrelin levels, appetite scores and weight loss maintenance in overweight and obese adults. Steroids 77, 323–331. [DOI] [PubMed] [Google Scholar]
- 51. Bellisle F, McDevitt R & Prentice AM (1997) Meal frequency and energy balance. Br J Nutr 77, Suppl. 1, S57–S70. [DOI] [PubMed] [Google Scholar]
- 52. Cohen DA (2008) Obesity and the built environment: changes in environmental cues cause energy imbalances. Int J Obes (Lond) 32, Suppl. 7, S137–S142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Holmes MD, Chen WY, Hankinson SE et al. (2009) Physical activity’s impact on the association of fat and fiber intake with survival after breast cancer. Am J Epidemiol 170, 1250–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Pearson N & Biddle SJ (2011) Sedentary behavior and dietary intake in children, adolescents, and adults. A systematic review. Am J Prev Med 41, 178–188. [DOI] [PubMed] [Google Scholar]
- 55. Ross R & Janssen I (2001) Physical activity, total and regional obesity: dose–response considerations. Med Sci Sports Exerc 33, 6 Suppl., S521–S527. [DOI] [PubMed] [Google Scholar]
- 56. Slentz CA, Houmard JA & Kraus WE (2009) Exercise, abdominal obesity, skeletal muscle, and metabolic risk: evidence for a dose response. Obesity (Silver Spring) 17, Suppl. 3, S27–S33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Leidy HJ, Armstrong CL, Tang M et al. (2010) The influence of higher protein intake and greater eating frequency on appetite control in overweight and obese men. Obesity (Silver Spring) 18, 1725–1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Schwarz NA, Rigby BR, La Bounty P et al. (2011) A review of weight control strategies and their effects on the regulation of hormonal balance. J Nutr Metab 2011, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Farshchi HR, Taylor MA & Macdonald IA (2005) Beneficial metabolic effects of regular meal frequency on dietary thermogenesis, insulin sensitivity, and fasting lipid profiles in healthy obese women. Am J Clin Nutr 81, 16–24. [DOI] [PubMed] [Google Scholar]
- 60. Lissner L, Levitsky DA, Strupp BJ et al. (1987) Dietary fat and the regulation of energy intake in human subjects. Am J Clin Nutr 46, 886–892. [DOI] [PubMed] [Google Scholar]
- 61. Kulovitz MG, Kravitz LR, Mermier C et al. (2014) Potential role of meal frequency as a strategy for weight loss and health in overweight or obese adults. Nutrition 30, 386–392. [DOI] [PubMed] [Google Scholar]
- 62. La Bounty PM, Campbell BI, Wilson J et al. (2011) International Society of Sports Nutrition position stand: meal frequency. J Int Soc Sports Nutr 8, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hutfless S, Gudzune KA, Maruthur N et al. (2013) Strategies to prevent weight gain in adults: a systematic review. Am J Prev Med 45, e41–e51. [DOI] [PubMed] [Google Scholar]
- 64. Mitchell HL, Aggett PJ, Richardson DP et al. (2011) Food & health forum meeting: evidence-based nutrition. Br J Nutr 105, 322–328. [DOI] [PubMed] [Google Scholar]
- 65. Mann JI (2010) Evidence-based nutrition: does it differ from evidence-based medicine? Ann Med 42, 475–486. [DOI] [PubMed] [Google Scholar]