Abstract
Objective
Findings from cohort studies investigating the association between rice consumption and risk of chronic diseases or mortality have been inconsistent. We performed a comprehensive systematic review and meta-analysis on all published cohort studies examining white rice consumption in relation to incidence of chronic diseases or risk of mortality.
Design
A systematic literature search of MEDLINE, Embase, Cochrane review, Google Scholar and Scopus databases for relevant cohort studies published until July 2014. For systematic review, we found nineteen studies examining the association between rice intake and risk of chronic diseases (obesity, hypertension, metabolic syndrome, diabetes, CVD and cancers) or mortality. Cohort studies which reported relative risk (RR) or odds ratio for highest v. lowest intake of rice and chronic diseases or mortality were included in the meta-analysis.
Results
In a meta-analysis on seventeen risk estimates for highest v. lowest category of rice intake, provided from twelve studies, we found a trend towards a positive association (RR; 95 % CI) between rice consumption and risk of all chronic diseases (1·11; 0·96, 1·29); however, significant between-study heterogeneity was found (I 2=70·3 %, P<0·001). Stratified analysis by gender showed a significant positive association between rice consumption and risk of chronic diseases in women (1·40; 1·13, 1·73), but not in men (0·95; 0·72, 1·24). Combining ten effect sizes from five studies showed that high consumption of rice was not significantly associated with mortality (0·97; 0·88, 1·06). Subgroup analysis by gender indicated an inverse association between rice consumption and mortality in men (0·87; 0·81, 0·94), but a trend towards a positive association in women (1·08; 0·97, 1·19).
Conclusions
Although white rice consumption was not found to be associated with individual chronic conditions, we observed a positive association between white rice intake and risk of all overall chronic diseases in women. High rice consumption was related to a modest reduction in risk of mortality in men but not in women. Further studies of these relationships, in different populations, are needed.
Keywords: Rice consumption, Chronic disease, Mortality, Meta-analysis
Worldwide, chronic diseases such as CVD, stroke, diabetes and cancer account for 60 % of all deaths( 1 ). The rising prevalence of chronic diseases and the projected public health and economic consequences imply the need for identification of modifiable risk factors, including habitual diet, as a priority( 2 ).
White rice is the major staple food for more than half of the world’s population, in particular for those living in Asian countries( 3 ). As white rice consumption affects insulin secretion and postprandial glycaemia( 4 ), it is implicated in the aetiology of many chronic diseases. As a major refined carbohydrate source, it may increase the risk of cardiometabolic conditions directly by straining the glucose homeostatic system or indirectly by displacing the consumption of wholegrain foods that may exert protective effects( 5 ). However, findings from earlier cohort studies that investigated the association between rice consumption and risk of chronic diseases or mortality have been inconsistent. With respect to diabetes, a positive association was documented in a meta-analysis of cohort studies( 5 ); this association was stronger for Asians than for Western populations. Another recent meta-analysis revealed no significant association between rice intake and diabetes( 6 ). In terms of CVD, one study has reported a positive association( 7 ) while another one found no relationship( 8 ). Most studies that investigated rice intake in relation to different cancers have reported no associations( 9 – 12 ); however, some have reached significant associations with the risk of prostate and upper aerodigestive tract cancer( 13 , 14 ). Finally, while three studies have reported no association between rice consumption and mortality( 8 , 15 , 16 ), two other investigations found an inverse relationship between rice intake and mortality in men, but not in women( 17 , 18 ). Some publications have shown that the replacement of rice with fruit, vegetables or whole-wheat bread might reduce the risk of mortality from CVD( 15 ). On the other hand, a cohort study in a Japanese population showed that rice intake was associated with reduced risks of mortality from CHD, heart failure and total CVD in men( 17 ). Overall, findings on rice intake, incident chronic diseases and risk of mortality are inconclusive. Given the high contribution of rice intake to total energy intake and to help resolve these inconsistencies, we performed a comprehensive systematic review and meta-analysis on all published cohort studies examining white rice consumption in relation to incidence of chronic diseases or risk of mortality.
Methods
Search strategy
We conducted a literature search, up to July 2014, of MEDLINE, Embase, Cochrane review, Google Scholar and Scopus databases for prospective or retrospective cohort studies examining the association between rice intake and risk of chronic diseases or mortality. The search terms were (rice(tiab) OR rice(MeSH) OR grain(tiab) OR ‘oryza stativa’ (MeSH)) AND (‘cohort’ OR ‘longitudinal’ OR ‘prospective’ OR ‘future’) for MEDLINE and (‘rice’/exp OR rice OR ‘grain’/exp OR grain) for Embase. The search was restricted to human studies. Because of resource limitations, we did not include grey literature (such as dissertations and patents) in our search. There were no language restrictions. We did not include the outcome in our search to have complete information about the studies that had been performed on rice. We also performed a manual search of references cited by the published original studies and relevant review articles. After excluding 1362 duplicate studies from different databases, the contents of 1255 abstracts or full-text manuscripts identified through the literature search were reviewed independently by two investigators (P.S. and A.E.) in duplicate to determine whether they met the inclusion criteria. We conformed as much as practicable to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines in the reporting of the present systematic review and meta-analysis.
Inclusion criteria
Studies were eligible for inclusion in the current analysis if: (i) their main exposure was rice consumption; (ii) they had a prospective or retrospective cohort design; (iii) their final outcome was occurrence of a chronic disease or mortality; and (iv) estimates of relative risks (RR), hazard ratios (HR) or OR with corresponding 95 % CI for the highest v. lowest category of rice consumption were provided. Because of the frequent consumption of white rice across the world, we considered only white rice consumption in the current study. Several studies that had reported the association between major dietary patterns (and rice was an ingredient of these dietary patterns) and risk of chronic diseases or mortality were not included in the current study. Chronic diseases that were considered in the study were incidence of obesity, hypertension, metabolic syndrome (MetS), diabetes, CVD, CHD, IHD, stroke and different types of cancer. Although we found sufficient records for risk of type 2 diabetes and mortality to pool specifically for these outcomes, there were insufficient reports to pool for stroke or even CVD, due to very limited studies. So, we pooled incidence of certain chronic diseases together to get a general conclusion of the association between white rice consumption with risk of chronic disease, as estimating the combined risk of several chronic diseases was common in previous meta-analysis( 19 ).
Excluded studies
One report reported RR for all-cause and cancer mortality in 2007 and again for cardiovascular mortality in 2010 based on the same study population( 17 , 18 ). Therefore, we decided to include RR for cancer( 18 ) and cardiovascular mortality( 17 , 18 ) in the present meta-analysis and excluded the RR for all-cause mortality. If a study reported data for CVD as well as CHD and stroke, CVD was included in the analysis. One cohort study( 20 ) provided RR for hypertension, abdominal obesity and MetS; since hypertension and abdominal obesity are components of MetS, we used only the RR for MetS in the meta-analysis. Some studies that reported OR or RR for rice intake in combination with pasta or refined grain intake were excluded from the analysis( 7 , 13 ).
Data extraction
We extracted the following information from each paper: (i) study’s characteristics (first author, year of publication, study location, duration of follow-up, person-years, and number of participants and incident cases); (ii) participants’ characteristics (age and gender); (iii) data on exposure (rice intake as the main exposure) and dietary assessment method; (iv) information about outcome (incidence of CVD, CHD, IHD, stroke, MetS, diabetes and different types of cancer, as well as mortality from any cause) and its ascertainment; (v) covariates adjusted for in the analyses; and (vi) risk estimates and their 95 % CI. Two investigators (P.S. and A.E.) extracted data independently, and any discrepancies were resolved by discussion. For studies that expressed data separately for men and women( 4 , 9 , 11 , 15 – 18 ) or included data from multiple cohorts( 21 ), we considered the analysis for each gender or cohort as an independent report and extracted data separately.
Assessment of methodological quality
The quality of studies included in the meta-analysis was examined by using a score previously developed for quality assessment of cohort studies( 5 ). The score assigns a maximum of 15 points to each cohort study for study design, response rate, follow-up time, exposure and outcome measurements, and statistical analysis (see online supplementary material, Supplemental Table 1). In the current analysis, when a study received more than a median score, it was considered as relatively high quality; otherwise it was deemed to have low quality. Any discrepancies were resolved by discussion.
Table 1.
Characteristics of cohort studies included in the systematic review
| Study | Cohort name | Country | Age range (years) | Gender | Sample size | Cases | Duration follow-up (years) | Person-years | Exposure assessment | Outcome (ascertainment) | Comparison | OR or RR | 95 % CI | Adjustments* |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rebello et al. (2014)( 15 ) | Singapore Chinese Health Study | China | 45–74 | M F | 23 501 29 968 | 1022 838 | 15 | 804 433 | FFQ, 165-item | IHD mortality (ICD-9, codes 410·0–414·9) | Q5 v. Q1 (6·74 v. 2·35 servings/d) | 1·02 1·10 | 0·79, 1·31 0·77, 1·58 | 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15 |
| Eshak et al. (2014)( 8 ) | Japan Public Health Centre-based (JPHC) Study I & II | Japan | 40–69 | M/F M/F M F | 91 223 91 223 NR NR | 4395 1088 NR NR | 15–18 | 1 401 401 1 401 401 NR NR | FFQ, 44-, 52, 138-item | Stroke (National Survey of Stroke 1981) IHD (Monitoring Trends and Determinants of Cardiovascular Disease project 1994 diagnostic criteria) CVD mortality (ICD-10, codes I60–I69, I21–23, I46 and I50) | Q5 v. Q1(542 v. 251 g/d) | 1·01 1·08 0·89 1·26 | 0·90, 1·14 0·84, 1·38 0·65, 1·15 0·87, 1·64 | 1, 4, 5, 6, 7, 10, 11, 12, 14, 16, 17, 18, 19, 20, 21 |
| Eshak et al. (2011)( 17 ) | Japan Collaborative Cohort (JACC) Study | Japan | 40–79 | M F | 35 064 48 688 | 1927 1587 | 14·1 | 434 272 621 551 | FFQ, 40-item | CVD mortality (ICD-10, codes I01–I99) | Q5 v. Q1 (M: 711 v. 280, F: 560 v. 279 g/d) | 0·82 1·07 | 0·70, 0·97 0·88, 1·34 | 1, 4, 5, 6, 7, 8, 9, 11, 14, 18, 21, 22, 23 |
| Oba et al. (2010)( 16 ) | Takayama Study | Japan | ≥35 | M F | 12 561 15 301 | 120 127 | 7 | NR | FFQ, 169-item | Stroke mortality (ICD-9, codes 430–438 and ICD-10, codes I60–I69) | Q4 v. Q1 (M: 4·0 v. 2·3, F: 3·2 v. 1·9 servings/d) | 0·84 1·37 | 0·43, 1·62 0·64, 2·94 | 1, 4, 5, 6, 7, 9, 10, 11, 21, 24, 25 |
| Iso & Kubota (2007)( 18 ) | JACC Study | Japan | 40–79 | M F | 44 703 61 491 | 3760 2220 | 13–15 | NR | FFQ, 39-item | Cancer mortality (NR) | M: ≥5 v. <3, F: ≥4 v. <3 bowls/d | 0·87 1·04 | 0·80, 0·96 0·91, 1·18 | 1, 16, 17 |
| Bahadoran et al. (2014)( 23 ) | Tehran Lipid and Glucose Study (TLGS) | Iran | 19–70 | M/F | 1476 | 249 | 3 | NR | FFQ, 168-item | MetS (NCEP ATP III diagnostic criteria) | Q4 v.Q1 (432 v. 93 g/d) | 1·66 | 1·04, 2·66 | 1, 4, 10, 16, 24, 26 |
| Shi et al.( 20 ) | Jiangsu Nutrition Study (JIN) | China | ≥20 | M/F | 1231 935 683 873 | – 127 206 140 | 5 | NR | FFQ, 149-item | Weight change MetS (IDF diagnostic criteria) Hypertension Abdominal obesity | ≥401 v. <200 g/d | −2·08 0·76 0·58 0·64 | −2·75, −1·41 0·43, 1·36 0·36, 0·93 0·34, 1·19 | 1, 4, 5, 6, 7, 9, 16, 20 |
| Hodge et al. (2004)( 27 ) | Melbourne Collaborative Cohort Study (MCCS) | Australia | 40–69 | M/F | 31 641 | 365 | 4 | 129 190 | FFQ, 121-item | Type 2 diabetes (self-reported disease confirmed by medical practitioners) | Q4 v. Q1 (≥2·5 v. <1·0 times/week) | 0·93 | 0·68, 1·27 | 1, 4, 6, 7, 9, 10, 16, 17, 27, 28, 29 |
| Villegas et al. (2007)( 26 ) | Shanghai Women’s Health Study (SWHS) | China | 40–70 | F | 64 191 | 1608 | 5 | 297 755 | FFQ, 77-item | Type 2 diabetes (self-reported disease based on ADA 1997 diagnostic criteria) | Q5 v. Q1 (≥300 v. <200 g/d) | 1·78 | 1·48, 2·15 | 1, 4, 5, 6, 7, 9, 10, 11, 20, 29 |
| Sun et al. (2010)( 21 ) | Health Professionals Follow-up Study (HPFS) | USA | 32–87 | M | 39 765 | 2648 | 20 | 702 920 | FFQ, 116–131-item | Type 2 diabetes (self-reported disease confirmed by validated supplementary questionnaire; National Diabetes Data Group or ADA 1997 diagnostic criteria) | Q5 v. Q1 (≥5/week v. <1/month) | 1·02 | 0·77, 1·34 | 1, 4, 5, 6, 7, 10, 14, 15, 27, 30, 31 |
| Nurses’ Health Study (NHS) I | USA | 37–65 | F | 69 120 | 5500 | 22 | 1 404 373 | FFQ, 116–131-item | Type 2 diabetes (self-reported disease confirmed by validated supplementary questionnaire; National Diabetes Data Group or ADA 1997 diagnostic criteria) | Q5 v. Q1 (≥5/week v. <1/month) | 1·11 | 0·87, 1·43 | 1, 4, 5, 6, 7, 10, 12, 14, 15, 27, 30, 31, 32 | |
| NHS II | USA | 26–45 | F | 88 343 | 2359 | 14 | 1 210 903 | FFQ, 116–131-item | Type 2 diabetes (self-reported disease confirmed by validated supplementary questionnaire; National Diabetes Data Group or ADA 1997 diagnostic criteria) | Q5 v. Q1 (≥5/week v. <1/month) | 1·40 | 1·09, 1·80 | 1, 4, 5, 6, 7, 10, 12, 14, 15, 27, 30, 31, 32 | |
| Nanri et al. (2010)( 4 ) | JPHC Study | Japan | 45–75 | M F | 25 666 33 622 | 625 478 | 5 | 128 330 168 110 | FFQ, 147-item | Type 2 diabetes (self-reported disease confirmed by medical records; Japan Diabetes Society 1982 diagnostic criteria) | Q4 v. Q1 (M: 700 v. 280, F: 560 v. 165 g/d) | 1·19 1·65 | 0·85, 1·68 1·06, 2·57 | 1, 4, 5, 6, 7, 10, 11, 14, 17, 20, 24 ,27, 31 |
| Rosa et al. (2014)( 22 ) | CAMELIA (retrospective cohort study) | Brazil | ≥20 | M/F | 409 | 30 | 4–5 | NR | FFQ, 73-item | Type 2 diabetes (self-reported disease or use of antidiabetic drug) | g/d | 0·998 | 0·988, 1·009 | 4 |
| Soriguer et al. (2013)( 24 ) | The Pizarra Study | Spain | 18–65 | M/F | 605 | 54 | 6 | NR | FFQ, NR | Type 2 diabetes (WHO 1998 diagnostic criteria) | 2–3/week v. ≤1/week | 0·41 | 0·17, 0·98 | 1, 10, 16, 26, 33 |
| Yu et al. (2011)( 25 ) | Hong Kong Dietary Survey | China | 25–74 | M/F | 1010 | 74 | 11·8 | NR | FFQ, 266-item | Type 2 diabetes (WHO 1998 diagnostic criteria) | g/week | 0·87 | 0·67, 1·13 | 1, 5, 6, 7, 10, 14, 16, 27, 29 |
| Abe et al. (2014)( 9 ) | JPHC Study | Japan | 45–74 | M F | 34 559 38 942 | 777 499 | 11 | 801 937 | FFQ, 147-item | Colorectal cancer (hospital records & population-based cancer registries, ICD-3, codes C18–C20) | Q4 v. Q1 (M: 305 v. 122, F: 244 v. 96 g/d) | 0·77 1·10 | 0·56, 1·07 0·71, 1·68 | 1, 4, 5, 6, 7, 10, 12, 13, 14, 17, 24, 27 |
| Giles et al. (2006)( 10 ) | MCCS | Australia | 40–69 | F | 12 273 | 324 | 9·1 | NR | FFQ, 121-item | Breast cancer (Victorian Cancer Registry) | times/week | 0·96 | 0·88, 1·05 | 1, 4, 12, 17 |
| Chyou et al. (1995)( 14 ) | Honolulu Heart Program | Japan | 45–68 | M | 7995 | 92 | 24 | NR | FFQ, 20-item | Upper aerodigestive tract cancer (histological tissue confirmation of diagnosis) | ≥3 v. ≤1 times/d | 1·43 | 0·56, 3·67 | 1, 5, 6 |
| Kato et al. (1992)( 11 ) | Aichi Cancer Center Hospital | Japan | 40–79 | M/F | 3914 | 45 | 4·4 | 17 285 | Questionnaire, 10-item | Stomach cancer (gastroendoscopic records in Aichi Cancer Center Hospital & Aichi Cancer Registry) | ≥4 v. ≤2 cups/d | 1·22 | 0·53, 2·78 | 1, 16, 17 |
| Severson et al. (1989)( 12 ) | Honolulu Heart Program | Japan | 46–65 | M | 7825 | 174 | 18–21 | 139 727 | FFQ, 20-item | Prostate cancer (histological confirmed disease in Oahu hospitals & Hawaii Tumor Registry) | ≥3 v. ≤1 times/d | 0·38 | 0·14, 1·04 | 1 |
RR, relative risk; M, male; F, female; NR, not reported; ICD, International Classification of Diseases; MetS, metabolic syndrome; NCEP ATP III, National Cholesterol Education Program Adult Treatment Panel III; IDF, International Diabetes Federation; ADA, American Diabetes Association; Q5, quintile 5; Q4, quartile 4; Q1, quintile/quartile1.
Adjustments were: 1= age; 2= year of interview; 3= father’s dialect; 4= total energy intake; 5= smoking; 6= alcohol consumption; 7= physical activity; 8= sleep duration; 9= education; 10= BMI; 11= history of hypertension; 12= menopausal status and hormone replacement therapy use (for women only); 13= ratio of polyunsaturated to saturated fat; 14= consumption of noodles, vegetables, fruit, fish, red meat, poultry, eggs, legumes, soya protein, white bread and whole-wheat bread; 15= ethnicity/sociodemographic; 16= gender; 17= public health centre area or area of study; 18= history of diabetes; 19= use of lipid-lowering drugs; 20= occupation or income level; 21= intake of sodium or salt; 22= perceived mental stress; 23= Key’s dietary score; 24= dietary fibre; 25= intake of total fat; 26= intake of carbohydrate and protein; 27= family history of diabetes; 28= 5-year weight change; 29= waist-to-hip ratio; 30= multivitamin use; 31= intake of coffee; 32= oral contraceptive use; 33= abnormal glucose regulation.
Statistical analysis
RR, HR or OR for comparison of the highest v. the lowest category of rice intake were used as the measure of association between rice consumption and risk of developing a chronic disease or mortality. Meta-analyses were performed using the random-effects model, calculating both Q statistics and I 2 as indicators of heterogeneity. In the case of significant between-study heterogeneity, we used subgroup analysis to find out possible sources of heterogeneity. Between-subgroup heterogeneity was examined through fixed-effects modelling. To assess the potential for publication bias, we constructed funnel plots for each outcome in which log RR values were plotted v. their se. We also conducted a sensitivity analysis in which each prospective cohort study was excluded in turn to evaluate the influence of that prospective cohort study on the overall estimate. Statistical analyses were conducted using the statistical software package Stata version 11.2. P values less than 0·05 were considered statistically significant.
Results
Study characteristics
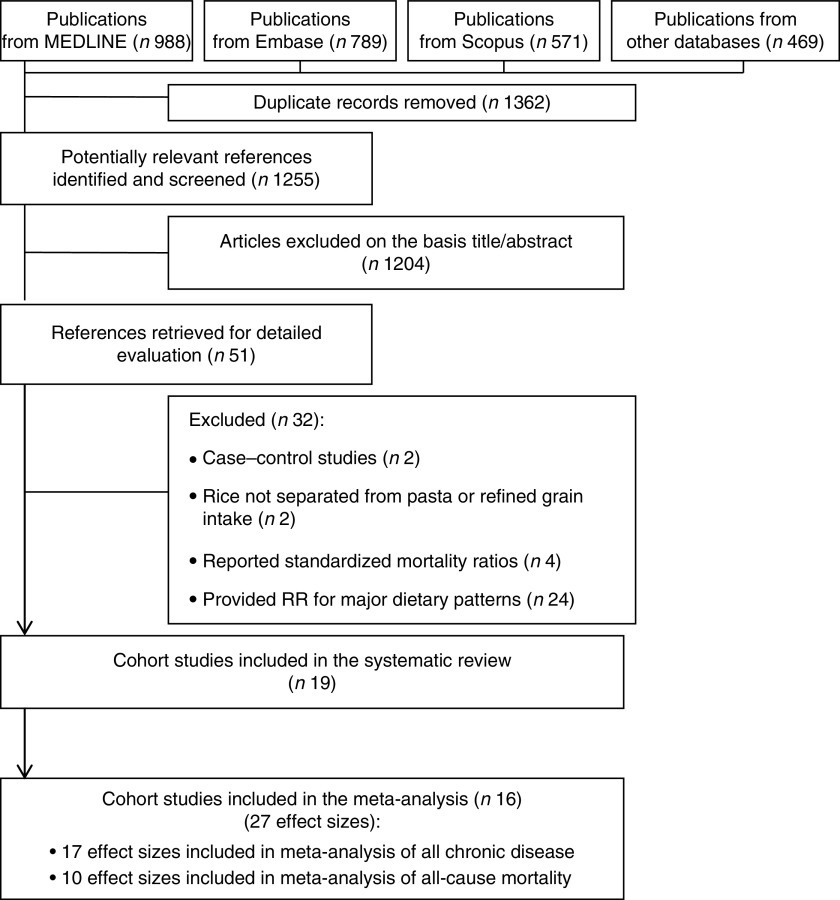
The preliminary literature search yielded 1255 unique publications. Of these, 1204 were excluded on the basis of the title or abstract. Of the remaining fifty-one studies, thirty-two were excluded for the following reasons: case–control study (n 2), provided RR for rice in combination with pasta or refined grain intake (n 2), study reported standardized mortality ratios (n 4) or study assessed major dietary patterns in relation to the risk (n 24). Therefore, we included a total of nineteen cohort studies( 4 , 8 – 12 , 14 – 18 , 20 – 27 ) in the systematic review. Three studies( 10 , 22 , 25 ) that reported RR for rice consumption as a continuous variable were included in the systematic review, but not in the meta-analysis. Finally, sixteen prospective cohort studies( 4 , 8 , 9 , 11 , 12 , 14 – 18 , 20 , 21 , 23 , 24 , 26 , 27 ) (out of nineteen papers included in systematic review) were included in the meta-analysis. Overall, twenty-seven effect sizes were extracted from these sixteen publications; seven studies provided subgroup analysis based on gender( 4 , 8 , 9 , 15 – 18 ), one publication reported data separately from three independent cohorts( 21 ) and another study reported RR for two different CVD (CHD and stroke) and mortality( 8 ). The flow diagram of the study selection process is indicated in Fig. 1. The characteristics of studies included in the systematic review are presented in Table 1.
Fig. 1.
The flow diagram of study selection (RR, relative risk)
Findings from systematic review
Out of the nineteen cohort studies, published between 1989 and 2014, nine were conducted in Japan( 4 , 8 , 9 , 11 , 12 , 14 , 16 – 18 ), four in China( 15 , 20 , 25 , 26 ), two in Australia( 10 , 27 ), one was reported from three independent cohorts in the USA( 21 ), and the remainder came from Brazil( 22 ), Spain( 24 ) and Iran( 23 ). The number of participants ranged from 409 to 91 223, with age range from 18 to 79 years. Eighteen cohort studies had a prospective design, whereas one study( 22 ) had a retrospective design. Of the nineteen studies, fourteen publications reported RR for incident chronic diseases, four papers for mortality and one publication for both chronic diseases and mortality. Among those that reported RR for chronic diseases, seven studies considered incident type 2 diabetes as the outcome, five reported risks for different cancer subtypes (prostate, stomach, upper aerodigestive tract, breast and colorectal cancer), one considered cardiovascular events (along with mortality from CVD) and two reported risks for MetS. Those that reported RR for mortality had considered all CVD (n 2), IHD (n 1), stroke (n 1) and all cancers (n 1) as their outcome. All publications used an FFQ for dietary assessment except for the study by Kato et al.( 11 ), which used a ten-item short questionnaire for assessing dietary intakes including white rice. In total, 553 518 individuals were included in the fifteen studies that reported risks for chronic disease and 362 500 individuals were included in the five reports on mortality. Among the participants, 21 511 incident cases of all chronic diseases and 14 306 deaths occurred during follow-up periods ranging from 3 to 24 years. Reported OR or RR for chronic diseases were in the range of 0·38 to 1·78, and for mortality between 0·82 and 1·37. Nearly all studies that reported OR or RR used multivariable logistic or Cox regression analysis. However, the number and type of potential confounders controlled for were different between studies. Most studies had controlled for age and gender (when relevant). Several studies had done further adjustments for total energy intake, smoking, alcohol consumption, physical activity and BMI. In addition, education, history of hypertension, area of study and dietary intakes were also considered in some studies. Results from the quality assessment of the sixteen studies included in the meta-analysis are presented in the online supplementary material, Supplemental Table 1; half of the studies achieved a score of 12 or above.
Findings from meta-analysis on rice consumption and risk of chronic diseases
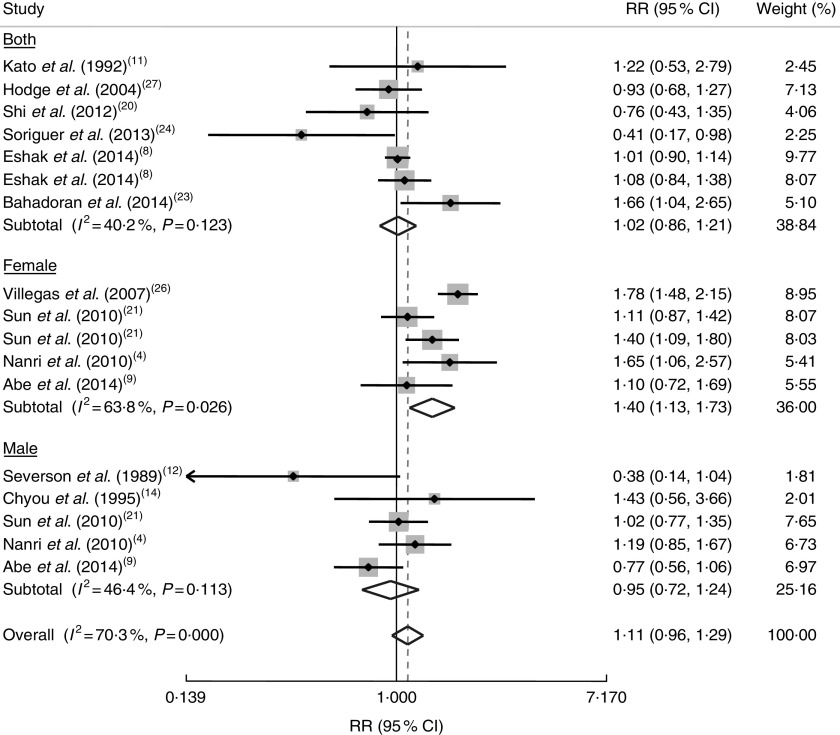
In a meta-analysis on seventeen risk estimates for the highest v. the lowest category of rice intake, provided from twelve studies( 4 , 8 , 9 , 11 , 12 , 14 , 20 , 21 , 23 , 24 , 26 , 27 ), we found a trend towards a positive association between rice consumption and risk of all chronic diseases (RR=1·11; 95 % CI 0·96, 1·29; Fig. 2); however, significant between-study heterogeneity was found (I 2=70·3 %, P<0·001). When we conducted stratified analysis by gender, we found a significant positive association between rice consumption and risk of chronic diseases in women (RR=1·40; 95 % CI 1·13, 1·73), but not in men (OR=0·95; 95 % CI 0·72, 1·24; Fig. 2).
Fig. 2.
Forest plot of the association between rice consumption and chronic disease risk, stratified by gender. The study-specific RR and 95 % CI are represented by the grey square and horizontal line, respectively; the area of the grey square is proportional to the specific-study weight to the overall meta-analysis. The centre of the diamond represents the pooled RR and its width represents the pooled 95 % CI
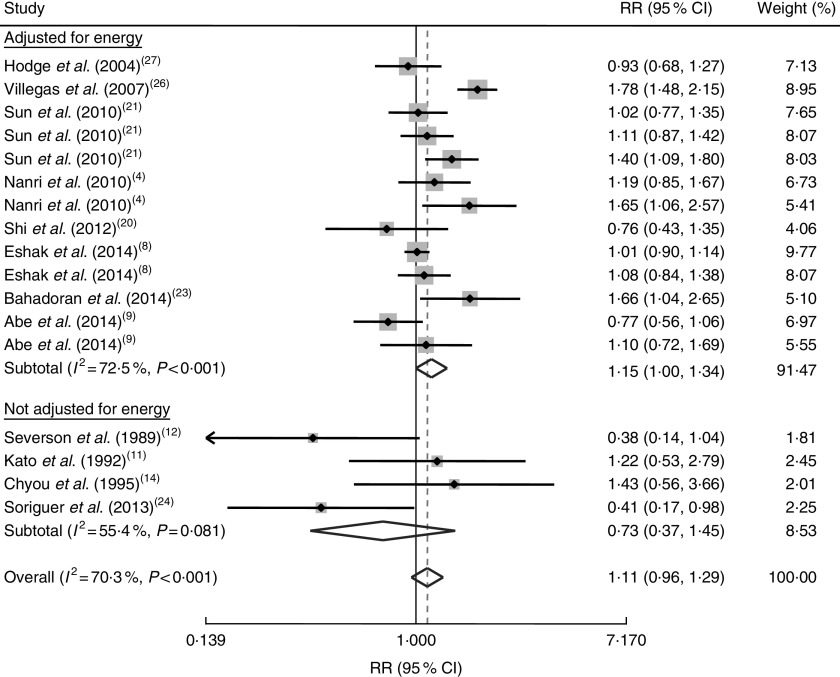
Further analysis based on adjustment for energy intake as a possible source of between-study heterogeneity revealed a significant association between rice consumption and increased risk of chronic diseases for studies that controlled for energy intake (RR=1·15; 95 % CI 1·00, 1·34); however, for publications that did not take energy intake into account in their analyses, we failed to find any significant association (RR=0·73; 95 % CI 0·37, 1·45; Fig. 3).
Fig. 3.
Forest plot of the association between rice consumption and chronic disease risk, stratified by energy adjustment status in the studies. The study-specific RR and 95 % CI are represented by the grey square and horizontal line, respectively; the area of the grey square is proportional to the specific-study weight to the overall meta-analysis. The centre of the diamond represents the pooled RR and its width represents the pooled 95 % CI
Findings from subgroup analyses on main outcome, study quality and geographical area are presented in Table 2. The combined RR for high-quality cohort studies (quality score >12) showed an elevated risk of chronic diseases with high rice intake (RR=1·18; (5 % CI 1·00, 1·38). However, the analysis on geographical area revealed no significant association between rice consumption and risk of individual chronic diseases. Also, subgroup analysis based on the main outcome revealed no significant association between rice consumption and CVD (RR=1·02; 95 % 0·92, 1·14; I 2=0·0 %, P=0·63), MetS (RR=1·14; 95 % CI 0·53, 2·46; I 2=76·5 %, P=0·04), type 2 diabetes (RR=1·20; 95 % CI 0·97, 1·49; I 2=75·2 %, P<0·001) and different cancers (RR=0·91; 95 % CI 0·65, 1·25; I 2=33·3 %, P=0·20; Table 2). In sensitivity analysis, we found that the exclusion of any single prospective cohort study from the analysis did not alter the overall association. There was also no evidence of publication bias (P=0·56 by Egger’s test, P=0·68 by Begg’s test).
Table 2.
Results of subgroup-analysis for rice consumption and risk of chronic disease and mortality
| No. of effect sizes | Reference(s) | RR | 95 % CI | P within* | I 2 (%) | P between† | |
|---|---|---|---|---|---|---|---|
| Subgroup analyses for all chronic disease | |||||||
| Main outcome | |||||||
| CVD | 2 | 8 | 1·02 | 0·92, 1·14 | 0·63 | 0·0 | 0·002 |
| Metabolic syndrome | 2 | 20, 23 | 1·14 | 0·53, 2·46 | 0·04 | 76·5 | |
| Type 2 diabetes | 8 | 4, 21, 24, 26–27 | 1·20 | 0·97, 1·49 | <0·001 | 75·2 | |
| Cancers | 5 | 9, 11–12, 14 | 0·91 | 0·65, 1·25 | 0·20 | 33·3 | |
| Quality score‡ | <0·001 | ||||||
| Score ≥median (12) | 10 | 4, 8–9, 21, 26 | 1·18 | 1·00, 1·38 | <0·001 | 76·0 | |
| Score<median (12) | 7 | 11–12, 14, 20, 23–24, 27 | 0·91 | 0·63, 1·32 | 0·03 | 56·5 | |
| Geographical area | 0·58 | ||||||
| Asian countries | 12 | 4,8–9,11–12,14,20,23,26 | 1·15 | 0·94, 1·40 | <0·001 | 74·8 | |
| Non-Asian countries | 5 | 21,24, 27 | 1·05 | 0·84, 1·31 | 0·04 | 59·3 | |
| Subgroup analyses for mortality | |||||||
| Energy adjustment status | 0·47 | ||||||
| Energy adjusted | 8 | 8, 15–17 | 0·99 | 0·87, 1·11 | 0·22 | 26·0 | |
| Not adjusted | 2 | 18 | 0·95 | 0·79, 1·13 | 0·03 | 79·4 | |
| Quality score‡ | 0·52 | ||||||
| Score ≥median (13) | 6 | 8, 15, 17 | 0·99 | 0·86, 1·13 | 0·13 | 41·0 | |
| Score <median (13) | 4 | 16, 18 | 0·95 | 0·62, 1·10 | 0·11 | 49·7 | |
P for heterogeneity, within subgroup.
P for heterogeneity, between subgroups.
Quality scores were according to Hu et al.’s criteria( 5 ).
Findings from meta-analysis on rice consumption and risk of mortality
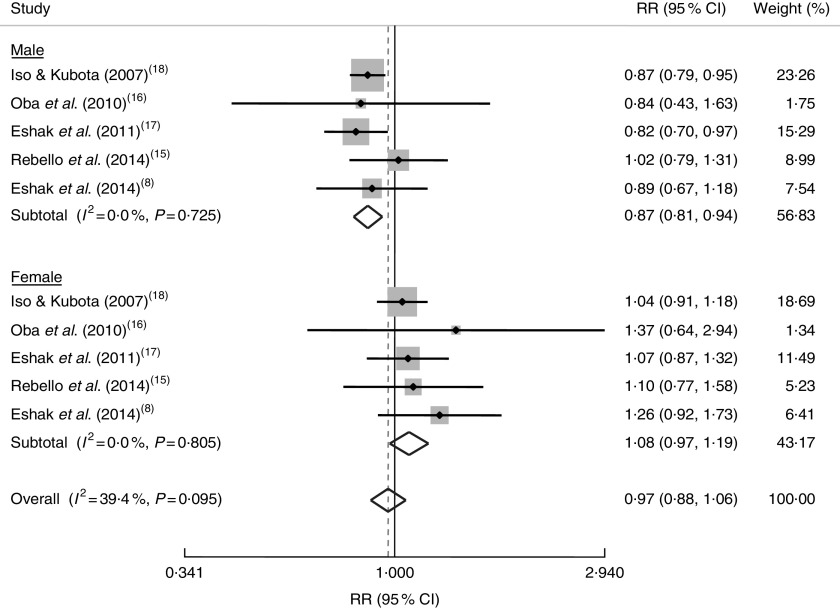
Overall, combining ten effect sizes from five studies( 8 , 15 – 18 ) showed that high consumption of rice was not significantly associated with mortality (RR=0·97; 95 % CI 0·88, 1·06; Fig. 4). Stratified analysis by gender indicated an inverse association between rice consumption and mortality in men (RR=0·87; 5 % CI 0·81, 0·94), but a trend towards a positive association in women (RR=1·08; 95 % CI 0·97, 1·19; Fig. 4). Out of five included studies, all had reported mortality from CVD events, except for one study that had reported RR for cancer mortality. We conducted a sensitivity analysis excluding that study( 18 ) and found no significant association in the whole population (RR=0·99; 95 % CI 0·87, 1·11) or in women (RR=1·13; 95 % CI 0·97, 1·32), but a slight protective association in men (RR=0·88; 95 % CI 0·78, 0·99). Subgroup analysis by energy adjustment as well as study quality (Table 2) revealed no alteration in the findings. A sensitivity analysis was performed to examine the influence of each individual study on the overall results. These findings showed no significant effect of any individual study on the overall findings. There was also no significant asymmetry in the funnel plots, suggesting no evidence of publication bias (P=0·15 by Egger’s test, P=0·42 by Begg’s test).
Fig. 4.
Forest plot of the association between rice consumption and mortality risk, stratified by gender. The study-specific RR and 95 % CI are represented by the grey square and horizontal line, respectively; the area of the grey square is proportional to the specific-study weight to the overall meta-analysis. The centre of the diamond represents the pooled RR and its width represents the pooled 95 % CI
Discussion
The present meta-analysis of prospective cohort studies indicated that high rice consumption was associated with a 40 % greater risk of chronic diseases in women, but not in men. A significant association between high rice consumption and increased risk of chronic diseases was also found for cohort studies that adjusted their analyses for total energy intake as well as for high-quality studies. With regard to mortality, we found a 13 % lower risk of mortality among men with the highest rice intake compared with those with the lowest intake; however, the combined estimate for both genders did not reach statistical significance. To our knowledge, the current is the first systematic review and meta-analysis of cohort studies that has summarized the prior evidence regarding the association between rice consumption and risk of chronic diseases and mortality.
We found a positive association between high rice intake and chronic diseases in women but not in men. This finding reflects the unfavourable metabolic consequences of consuming rice, as a high-glycaemic-index or high-glycaemic-load food, in women( 28 , 29 ). Previous investigations among Dutch women have shown that consuming high-glycaemic-index foods increases the risk of CVD, particularly in overweight women( 28 , 29 ). Another possible explanation for this finding might be that the number of events in females was more frequent than in males (10 444 v. 4316 events of chronic diseases). In addition, women use more health-care services than men and they live longer than men; albeit with greater disabilities. Moreover, high consumption of white rice has resulted in reduced circulating HDL cholesterol concentrations( 30 ) and thus disproportionately increased CVD risk in women, particularly in postmenopausal women( 30 ). It seems that high-glycaemic-index diets strongly affect metabolic profile and might increase insulin resistance in women compared with men. This might be explained by the interaction between these foods and hormonal profiles of women( 31 ). Insulin resistance is negatively correlated with oestrogen levels in women( 32 ); therefore, an increased demand for insulin after rice intake might reduce the beneficial effects of oestrogen( 32 ). One might assume that the high incidence of chronic diseases in women, due to their limited activity and their unhealthy diet, might explain the positive association between rice consumption and these conditions. This is particularly relevant for developing nations where most women are housewives; however, some previous studies have shown that the prevalences of some chronic diseases such as CVD and diabetes are not significantly different in men and women, at least in South-East Asia( 33 – 35 ). Further studies in different societies are needed to shed light on this finding.
The present meta-analysis revealed an inverse relationship between rice intake and mortality in men; but a non-significant elevated risk of mortality was reached in women. A careful review of five prospective cohort studies included in the meta-analysis highlighted the point that all of these investigations were conducted in Asian countries. Rice has been a staple food in Asian populations for thousands of years, but it may be difficult to disentangle the effects of high rice consumption per se from the effects of foods typically consumed along with rice in mixed dishes in Asian countries( 15 , 17 ). So, there is a need for further large-scale prospective studies in different countries to determine whether rice intake can decrease the risk of mortality. Furthermore, investigators of earlier publications on rice and mortality have claimed that the fibre content of rice in some Asian countries, like Japan, is a bit higher than that in other countries( 17 ). However, the type of rice frequently consumed in these countries has not been well introduced in their publications( 36 ). Out of five cohort studies that assessed rice intake and mortality( 8 , 15 – 18 ), two studies( 17 , 18 ) reached a significant inverse association in men. These studies had been done on the same study population in Japan and their findings need further clarifications( 36 ).
In the current meta-analysis, we found no significant association between white rice consumption and risk of CVD, MetS, type 2 diabetes or cancers; this might be due to the very limited studies in each category of individual chronic diseases. However, findings from a meta-analysis of seven distinct prospective cohort studies( 5 ) documented a positive association between rice consumption and risk of type 2 diabetes; this association was stronger for Asians than for Western populations. In line with our findings, that meta-analysis( 5 ) reported that the association was more pronounced among women (pooled RR=1·46; 95 % CI 1·16 to 1·83) than men (RR=1·08; 95 % CI 0·87 to 1·34). In contrast, we found no significant difference in the associations between rice consumption and risk of chronic diseases in Asian v. non-Asian countries. Studies included in the current analysis were reported from Japan, China, Brazil, Australia, Spain and the USA; however, the number of studies included was not sufficient to analyse reports from these countries separately. It is worth noting that the cultures, in particular the dietary intakes, of people of these countries are very different and might further affect the findings. In addition, other risk factors, such as lower BMI in the Japanese v. Brazilian population, additives and pesticides (whose presence might transform a healthy food into a poison), might influence the results.
Since consumption of brown rice is not common in most parts of the world, previous studies did not distinguish between white and brown rice in the questionnaires. Among studies included in the present systematic review, only four studies( 16 , 21 , 23 , 24 ) indicated RR for white rice and others reported the risk for rice. Data on the association between brown rice intake and chronic diseases are limited. A recent meta-analysis has documented that brown rice intake was associated with an 11 % reduction in risk of type 2 diabetes( 6 ), but this summary effect was based on only three cohort studies in Western populations. Asian populations consume white rice frequently and there are no data available on the relationship between brown rice intake and risk of chronic diseases in these populations. Further prospective cohort studies are needed to shed light on whether substituting brown rice for white rice can affect chronic diseases or mortality risk, especially in Asian populations.
There are plausible mechanisms linking the development of chronic diseases with rice consumption. The high glycaemic index and glycaemic load of white rice may contribute to elevated risk of chronic diseases through chronically increasing insulin demand and insulin resistance( 37 ). High glucose and insulin concentrations are associated with increased risk of CVD, including decreased concentrations of HDL cholesterol, increased glycosylated proteins, oxidative status, haemostatic variables, poor endothelial function and cell proliferation( 38 ). Furthermore, high rice consumption is associated with high concentrations of inflammatory markers including plasminogen activator inhibitor type 1, C-reactive protein and fibrinogen( 39 ), which might mediate the risk for chronic diseases. In addition, due to the milling process, white rice has low content of many nutrients including insoluble fibre, magnesium, vitamins, lignans, plant stanols and sterols, and phyto-oestrogens( 40 ). Most of these nutrients have been associated with lower risk of chronic diseases in prospective cohort studies( 40 – 42 ).
The present meta-analysis has notable strengths. A major one is that all original studies included in the meta-analysis had a prospective cohort design that could minimize the potential recall and selection bias. Almost all studies were done on large population samples, which can help detection of significant associations, if any. A significant limitation, however, is the fact that the current data on rice intake and mortality were restricted to Asian populations and the summarized estimate of the five prospective cohorts in the current study needs to be further verified. Although we systematically searched the online databases, as with other meta-analyses, some published or unpublished literature, including grey literature, might be difficult to trace via conventional channels and missing these publications must be taken into account in the interpretation of our findings. In addition, despite comprehensive adjustments for confounders in all publications, residual confounding in observational studies is always a concern. Furthermore, we were unable to separate data on brown rice from those on white rice in the current meta-analysis because this has not been well separated in earlier publications. Lastly, almost all studies used an FFQ to assess white rice consumption; although validation studies in most studies had shown reasonable validity of self-reported rice intake, misclassification is inevitable in epidemiological studies and may attenuate the true associations.
Conclusion
In conclusion, although the present meta-analysis of cohort studies did not find white rice consumption to be associated with individual chronic conditions, we observed a positive association between white rice intake and risk of all overall chronic diseases in women. High intake of rice was also associated with a modest reduction in risk of mortality in men, but not in women.
Acknowledgements
Acknowledgements: The authors thank the Food Security Research Center, Isfahan University of Medical Sciences, Isfahan, Iran for financial support. Financial support: This research was supported by the Food Security Research Center, Isfahan University of Medical Sciences, Isfahan, Iran. The Food Security Research Center had no role in the design/conduct of the study, collection/analysis/interpretation of the data and preparation/review/approval of the manuscript. Conflict of interest: None of the authors had any personal or financial conflicts of interest. Authorship: P.S., B.L. and A.E. contributed in conception, design, statistical analyses, data analysis, data interpretation and manuscript drafting. All authors approved the final manuscript for submission. Ethics of human subject participation: Not applicable.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S1368980016002172.
click here to view supplementary material
References
- 1. World Health Organization (2006) World Health Statistics 2006. Geneva: WHO. [Google Scholar]
- 2. World Health Organization (2003) Diet, Nutrition and the Prevention of Chronic Diseases. WHO Technical Report Series no. 916. Geneva: WHO; [PubMed] [Google Scholar]
- 3. US Department of Agriculture, Economic Research Service (2010) Data sets. http://www.ers.usda.gov/Data/ (accessed August 2016).
- 4. Nanri A, Mizoue T, Noda M et al. (2010) Rice intake and type 2 diabetes in Japanese men and women: the Japan Public Health Center-based Prospective Study. Am J Clin Nutr 92, 1468–1477. [DOI] [PubMed] [Google Scholar]
- 5. Hu EA, Pan A, Malik V et al. (2012) White rice consumption and risk of type 2 diabetes: meta-analysis and systematic review. BMJ 344, e1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Aune D, Norat T, Romundstad P et al. (2013) Whole grain and refined grain consumption and the risk of type 2 diabetes: a systematic review and dose–response meta-analysis of cohort studies. Eur J Epidemiol 28, 845–858. [DOI] [PubMed] [Google Scholar]
- 7. Yu D, Shu XO, Li H et al. (2013) Dietary carbohydrates, refined grains, glycemic load, and risk of coronary heart disease in Chinese adults. Am J Epidemiol 178, 1542–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Eshak ES, Iso H, Yamagishi K et al. (2014) Rice consumption is not associated with risk of cardiovascular disease morbidity or mortality in Japanese men and women: a large population-based, prospective cohort study. Am J Clin Nutr 100, 199–207. [DOI] [PubMed] [Google Scholar]
- 9. Abe SK, Inoue M, Sawada N et al. (2014) Rice, bread, noodle and cereal intake and colorectal cancer in Japanese men and women: the Japan Public Health Center-based prospective Study (JPHC Study). Br J Cancer 110, 1316–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Giles GG, Simpson JA, English DR et al. (2006) Dietary carbohydrate, fibre, glycaemic index, glycaemic load and the risk of postmenopausal breast cancer. Int J Cancer 118, 1843–1847. [DOI] [PubMed] [Google Scholar]
- 11. Kato I, Tominaga S, Ito Y et al. (1992) A prospective study of atrophic gastritis and stomach cancer risk. Jpn J Cancer Res 83, 1137–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Severson RK, Nomura AM, Grove JS et al. (1989) A prospective study of demographics, diet, and prostate cancer among men of Japanese ancestry in Hawaii. Cancer Res 49, 1857–1860. [PubMed] [Google Scholar]
- 13. Drake I, Sonestedt E, Gullberg B et al. (2012) Dietary intakes of carbohydrates in relation to prostate cancer risk: a prospective study in the Malmo Diet and Cancer cohort. Am J Clin Nutr 96, 1409–1418. [DOI] [PubMed] [Google Scholar]
- 14. Chyou PH, Nomura AM & Stemmermann GN (1995) Diet, alcohol, smoking and cancer of the upper aerodigestive tract: a prospective study among Hawaii Japanese men. Int J Cancer 60, 616–621. [DOI] [PubMed] [Google Scholar]
- 15. Rebello SA, Koh H, Chen C et al. (2014) Amount, type, and sources of carbohydrates in relation to ischemic heart disease mortality in a Chinese population: a prospective cohort study. Am J Clin Nutr 100, 53–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Oba S, Nagata C, Nakamura K et al. (2010) Dietary glycemic index, glycemic load, and intake of carbohydrate and rice in relation to risk of mortality from stroke and its subtypes in Japanese men and women. Metabolism 59, 1574–1582. [DOI] [PubMed] [Google Scholar]
- 17. Eshak ES, Iso H, Date C et al. (2011) Rice intake is associated with reduced risk of mortality from cardiovascular disease in Japanese men but not women. J Nutr 141, 595–602. [DOI] [PubMed] [Google Scholar]
- 18. Iso H & Kubota Y (2007) Nutrition and disease in the Japan Collaborative Cohort Study for Evaluation of Cancer (JACC). Asian Pac J Cancer Prev 8, Suppl., 35–80. [PubMed] [Google Scholar]
- 19. Barclay AW, Petocz P, McMillan-Price J et al. (2008) Glycemic index, glycemic load, and chronic disease risk – a meta-analysis of observational studies. Am J Clin Nutr 87, 627–637. [DOI] [PubMed] [Google Scholar]
- 20. Shi Z, Taylor AW, Hu G, Gill T et al. (2012) Rice intake, weight change and risk of the metabolic syndrome development among Chinese adults: the Jiangsu Nutrition Study (JIN). Asia Pac J Clin Nutr 21, 35–43. [PubMed] [Google Scholar]
- 21. Sun Q, Spiegelman D, van Dam RM et al. (2010) White rice, brown rice, and risk of type 2 diabetes in US men and women. Arch Intern Med 170, 961–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rosa ML, Falcao PM, Yokoo EM et al. (2014) Brazil’s staple food and incident diabetes. Nutrition 30, 365–368. [DOI] [PubMed] [Google Scholar]
- 23. Bahadoran Z, Mirmiran P, Delshad H et al. (2014) White rice consumption is a risk factor for metabolic syndrome in Tehrani adults: a prospective approach in Tehran lipid and glucose study. Arch Iran Med 17, 435–440. [PubMed] [Google Scholar]
- 24. Soriguer F, Colomo N, Olveira G et al. (2013) White rice consumption and risk of type 2 diabetes. Clin Nutr 32, 481–484. [DOI] [PubMed] [Google Scholar]
- 25. Yu R, Woo J, Chan R et al. (2011) Relationship between dietary intake and the development of type 2 diabetes in a Chinese population: the Hong Kong Dietary Survey. Public Health Nutr 14, 1133–1141. [DOI] [PubMed] [Google Scholar]
- 26. Villegas R, Liu S, Gao YT et al. (2007) Prospective study of dietary carbohydrates, glycemic index, glycemic load, and incidence of type 2 diabetes mellitus in middle-aged Chinese women. Arch Intern Med 167, 2310–2316. [DOI] [PubMed] [Google Scholar]
- 27. Hodge AM, English DR, O’Dea K et al. (2004) Glycemic index and dietary fiber and the risk of type 2 diabetes. Diabetes Care 27, 2701–2706. [DOI] [PubMed] [Google Scholar]
- 28. Beulens JW, de Bruijne LM, Stolk RP et al. (2007) High dietary glycemic load and glycemic index increase risk of cardiovascular disease among middle-aged women: a population-based follow-up study. J Am Coll Cardiol 50, 14–21. [DOI] [PubMed] [Google Scholar]
- 29. Mirrahimi A, de Souza RJ, Chiavaroli L et al. (2012) Associations of glycemic index and load with coronary heart disease events: a systematic review and meta-analysis of prospective cohorts. J Am Heart Assoc 1, e000752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ahn Y, Park SJ, Kwack HK et al. (2013) Rice-eating pattern and the risk of metabolic syndrome especially waist circumference in Korean Genome and Epidemiology Study (KoGES). BMC Public Health 13, 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Reardon MF, Nestel PJ, Craig IH et al. (1985) Lipoprotein predictors of the severity of coronary artery disease in men and women. Circulation 71, 881–888. [DOI] [PubMed] [Google Scholar]
- 32. Matsui S, Yasui T, Tani A et al. (2013) Associations of estrogen and testosterone with insulin resistance in pre- and postmenopausal women with and without hormone therapy. Int J Endocrinol Metab 11, 65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kashima S, Inoue K, Matsumoto M et al. (2015) Prevalence and characteristics of non-obese diabetes in Japanese men and women: the Yuport Medical Checkup Center Study. J Diabetes 7, 523–530. [DOI] [PubMed] [Google Scholar]
- 34. Yang W, Lu J, Weng J et al.; China National Diabetes and Metabolic Disorders Study Group (2010) Prevalence of diabetes among men and women in China. N Engl J Med 362, 1090–1101. [DOI] [PubMed] [Google Scholar]
- 35. He L, Tang X, Song Y et al. (2012) Prevalence of cardiovascular disease and risk factors in a rural district of Beijing, China: a population-based survey of 58,308 residents. BMC Public Health 12, 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rouhani MH, Azadbakht L & Esmaillzadeh A (2011) Inverse association between rice consumption and cardiovascular mortality: additional data are required. J Nutr 141, 1918. [DOI] [PubMed] [Google Scholar]
- 37. Zuniga YL, Rebello SA, Oi PL et al. (2014) Rice and noodle consumption is associated with insulin resistance and hyperglycaemia in an Asian population. Br J Nutr 111, 1118–1128. [DOI] [PubMed] [Google Scholar]
- 38. Brand-Miller JC (2003) Glycemic load and chronic disease. Nutr Rev 61, 5 Pt 2, S49–S55. [DOI] [PubMed] [Google Scholar]
- 39. Masters RC, Liese AD, Haffner SM et al. (2010) Whole and refined grain intakes are related to inflammatory protein concentrations in human plasma. J Nutr 140, 587–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Slavin J (2003) Why whole grains are protective: biological mechanisms. Proc Nutr Soc 62, 129–134. [DOI] [PubMed] [Google Scholar]
- 41. Schulze MB, Schulz M, Heidemann C et al. (2007) Fiber and magnesium intake and incidence of type 2 diabetes: a prospective study and meta-analysis. Arch Intern Med 167, 956–965. [DOI] [PubMed] [Google Scholar]
- 42. Weickert MO, Mohlig M, Schofl C et al. (2006) Cereal fiber improves whole-body insulin sensitivity in overweight and obese women. Diabetes Care 29, 775–780. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit https://doi.org/10.1017/S1368980016002172.
click here to view supplementary material