Abstract
The immune response to COVID-19 booster vaccinations during pregnancy for mothers and their newborns and the functional response of vaccine-induced antibodies against Omicron variants are not well characterized. We conducted a prospective, multicenter cohort study of participants vaccinated during pregnancy with primary or booster mRNA COVID-19 vaccines from July 2021 to January 2022 at 9 academic sites. We determined SARS-CoV-2 binding and live virus and pseudovirus neutralizing antibody (nAb) titers pre- and post-vaccination, and at delivery for both maternal and infant participants. Immune responses to ancestral and Omicron BA.1 SARS-CoV-2 strains were compared between primary and booster vaccine recipients in maternal sera at delivery and in cord blood, after adjusting for days since last vaccination.
A total of 240 participants received either Pfizer or Moderna mRNA vaccine during pregnancy (primary 2-dose series:167; booster dose:73). Booster vaccination resulted in significantly higher binding and nAb titers, including to the Omicron BA.1 variant, in maternal serum at delivery and in cord blood compared to a primary 2-dose series (range 0.44 to 0.88 log10 higher, p<0.0001 for all comparisons). Live virus nAb to Omicron BA.1 were present at delivery in 9% (GMT ID50 12.7) of Pfizer and 22% (GMT ID50 14.7) of Moderna primary series recipients, and in 73% (GMT ID50 60.2) of mRNA boosted participants (p<0.0001), although titers were significantly lower than to the D614G strain. Transplacental antibody transfer was efficient for all regimens with median transfer ratio range: 1.55-1.77 for IgG, 1.00-1.78 for live virus nAb and 1.79-2.36 for pseudovirus nAb. COVID-19 mRNA vaccination during pregnancy elicited robust immune responses in mothers and efficient transplacental antibody transfer to the newborn. A booster dose during pregnancy significantly increased maternal and cord blood binding and neutralizing antibody levels, including against Omicron BA.1. Findings support the use of a booster dose of COVID-19 vaccine during pregnancy.
Keywords: SARS-CoV-2, COVID-19, booster vaccination, pregnancy, neutralizing antibodies, transplacental antibody, newborn
1. Introduction
Pregnant individuals are at increased risk of severe disease and obstetric complications after SARS-CoV-2 infection.[1], [2], [3], [4] With the emergence of Omicron variants in late 2021, it has become apparent that infants younger than 6 months of age who become infected with SARS-CoV-2 are also at increased risk of hospitalization.[5], [6] During the Omicron (BA.1, BA.4 and BA.5) variant waves, COVID-19 hospitalization rates for infants 0 through 5 months of age increased above rates in older children, adolescents and adults <65years old.[6] This is likely due to immunity in older age groups increasing through vaccination and prior infection, while young infants remain immunologically naïve and not eligible for vaccination until 6 months of age.
Importantly, COVID-19 vaccination during pregnancy is critical to mitigate the burden of disease for mothers and simultaneously represents the best approach to address this gap in protection for their infants.[7], [8], [9], [10], [11] Vaccine-induced antibodies transferred transplacentally to the infant reduces the risk of severe COVID-19 disease and hospitalization in infants in the first months of life.[12], [13] In October 2021, pregnant individuals became eligible for booster vaccinations in the United States, yet the response to a booster dose and how it translates into neonatal antibody transfer and potential maternal and infant protection has not been well characterized.[14]
In this prospective cohort study, we measured the binding and neutralizing antibody responses to COVID-19 mRNA vaccines in pregnant participants and antibody levels in cord blood. We report the effect of primary series versus booster vaccination in pregnant mothers and on transplacental antibody levels in the newborn, and describe the functional immune response to Omicron in these groups.
2. Materials and Methods
This United States (U.S.)-based multicenter cohort study enrolled pregnant participants with and without medical comorbidities from July 6, 2021 to January 31, 2022. Eligible participants received a primary 2-dose series of Pfizer-BioNTech (Pfizer) or Moderna mRNA-1273 (Moderna) vaccine, or a monovalent booster dose of either vaccine, at any time during pregnancy as per current recommendations. Sera for antibody assays were derived from maternal blood collected pre- and post-vaccination (from 2 weeks post-vaccination to delivery), and maternal and cord blood collected at delivery. Maternal history of SARS-CoV-2 infection was collected at enrollment and at each study visit. Follow-up to 12 months post-delivery is ongoing and results will be reported separately. Detailed protocol and study procedures are described elsewhere (DMID 21-0004).[15]
2.1. Immunogenicity
Binding immunoglobulin G (IgG) levels to full-length Spike (Spike) and to the receptor binding domain (RBD) of Spike evaluated using the validated Meso Scale Discovery (MSD) V-PLEX® SARS-CoV-2 Panel 2 IgG assay (MSD #K15383U)[16] were bridged to international standards and reported as Binding Antibody Units (BAU/mL). SARS-CoV-2 neutralizing antibody (nAb) titers were evaluated by a pseudovirus neutralizing assay using a replication-incompetent lentivirus coding for luciferase and containing the SARS-CoV-2 Spike protein (Wuhan-Hu-1) in the viral envelope (expressed as an IC50 value indicating the sample antibody titer capable of inhibiting viral entry and replication by 50%)[17], and a live virus focus reduction neutralization titer (FRNT) assay with viruses representing SARS-CoV-2 Spike mutation D614G and Delta and Omicron BA.1 variants [expressed as the serum inhibitory dilution required to achieve 50% neutralization (ID50)].[18] Detailed assay methods are in Supplementary Materials. Transplacental antibody transfer was evaluated by calculating the ratio of specific antibody levels in maternal and cord blood sera at the time of delivery.
2.2. Statistical Analysis
Medians and interquartile ranges (IQRs) for binding IgG, IC50 for pseudovirus nAb levels, and ID50 for live virus nAb levels were summarized by study visit and vaccine type. Differences in antibody levels between groups at delivery were tested using regression analyses controlling for days since last vaccine dose and prior self-reported SARS-CoV-2 infection or N-protein positive at delivery, as well as sensitivity analyses that were restricted to participants with vaccination in the same time interval between last vaccination and delivery.
2.3. Patient and Public Involvement
Patients or the public were not involved in the design, conduct, reporting, or dissemination plans of this research.
3. Results
This analysis describes 240 pregnant participants who gave birth and their newborns: 100 Pfizer (102 infants) and 67 Moderna (68 infants) 2-dose vaccine recipients, and 73 booster dose participants (75 infants) (Table 1 ). Booster doses were mostly homologous with the primary series (80.8%). The median age of participants was 34 years (range, 22-51). Participants completed their primary 2-dose series at a median of 17.1 weeks of gestation, while booster vaccination was received at a median of 28.6 weeks of gestation. Post-vaccination sera were collected at a median of 18.7 (range: 1.6-33.3) weeks following completion of the 2-dose series and 6.0 (range: 1.1-19.9) weeks following the booster dose. The interval (median weeks) between last vaccine dose and delivery was shorter for booster dose recipients (10.4) than primary 2-dose recipients (21.7). Overall, 14.4% of primary 2-dose recipients and 17.8% of booster dose recipients had self-reported SARS-CoV-2 infection or were N-protein positive up to delivery.
Table 1.
Study Participant Characteristics by Group Assigned at Enrollment
| Maternal Characteristic | Pfizer-BioNTechn=100Median (IQR)% (n) | Modernan=67Median (IQR)% (n) | Boostern=73Median (IQR)% (n) |
|---|---|---|---|
| Age, years | 35 (31, 37) | 34 (31, 37) | 34 (31, 37) |
| RaceAsianBlack/African AmericanWhiteOther | 12.0 (12)10.0 (10)75.0 (75)3.0 (3) | 4.5 (3)16.4 (11)73.1 (49)6.0 (4) | 9.6 (7)1.4 (1)83.6 (61)5.5 (4) |
| Hispanic or Latino | 10.0 (10) | 14.9 (10) | 9.6 (7) |
| Vaccine Exposure up to Delivery2 doses Pfizer only2 doses Pfizer+Pfizer boost2 doses Pfizer+Moderna boost2 doses Moderna only2 doses Moderna+Moderna boost2 doses Moderna+Pfizer boost | 98.0 (98)2.0 (2)0.0 (0) | 92.5 (62)6.0 (4)1.5 (1) | 76.7 (56)16.4 (12)4.1 (3)2.7 (2) |
| Weeks Between Last Dose and Post-Vaccination Visit | 18.8 (14.6, 23.4) | 18.3 (12.9, 23.6) | 6.0 (3.3, 8.1) |
| Weeks Between Primary Series Completion and Delivery | 21.9 (18.0, 26.4) | 22.9 (18.3, 27.3) | 46.7 (40.4, 50.9) |
| Weeks Between Last Dose and Delivery | 21.6 (17.1, 25.7) | 21.9 (15.4, 24.6) | 10.4 (6.9, 14.1) |
| Gestational Age at First Dose During Pregnancy, weeks | 13.1 (9.3, 17.9) | 12.7 (7.6, 15.7) | -10.9 (-14.9, -4.6) |
| Gestational Age at Last Dose During Pregnancy, weeks | 16.9 (13.4, 21.3) | 17.1 (14.0, 23.7) | 28.6 (24.9, 31.7) |
| Gestational Age at Delivery, weeks | 39.3 (37.9, 40.1) | 39.1 (38.7, 39.9) | 39.1 (38.3, 39.6) |
| SARS-CoV-2 infection prior to or during study, up to delivery, self-reported OR N protein positive at delivery | 15.0 (15) | 13.4 (9) | 17.8 (13) |
| GravidityPrimigravidaAt least one prior pregnancyAt least two prior pregnancies | 30.0 (30)70.0 (70)32.0 (32) | 44.8 (30)55.2 (37)29.9 (20) | 42.5 (31)57.5 (42)28.8 (21) |
| Maternal obstetric comorbiditiesGestational diabetesHypertensive disordersObesityPre-eclampsia | 8.0 (8)10.0 (10)9.0 (9)0.0 (0) | 13.4 (9)9.0 (6)10.4 (7)0.0 (0) | 6.8 (5)13.7 (10)13.7 (10)2.7 (2) |
| Mode of deliveryC-sectionVaginal | 33.0 (33)67.0 (67) | 43.3 (29)56.7 (38) | 30.1 (22)69.9 (51) |
| Infant Characteristic | Pfizer-BioNTechn=102% (n) | Modernan=68% (n) | Boostern=75% (n) |
| Preterm birth (<37 weeks) | 6.9 (7) | 7.4 (5) | 14.7 (11) |
| Infant Birthweight (grams) | 3370 (3040, 3640) | 3340 (3030, 3560) | 3280 (2860, 3540) |
3.1. SARS-CoV-2 binding antibodies
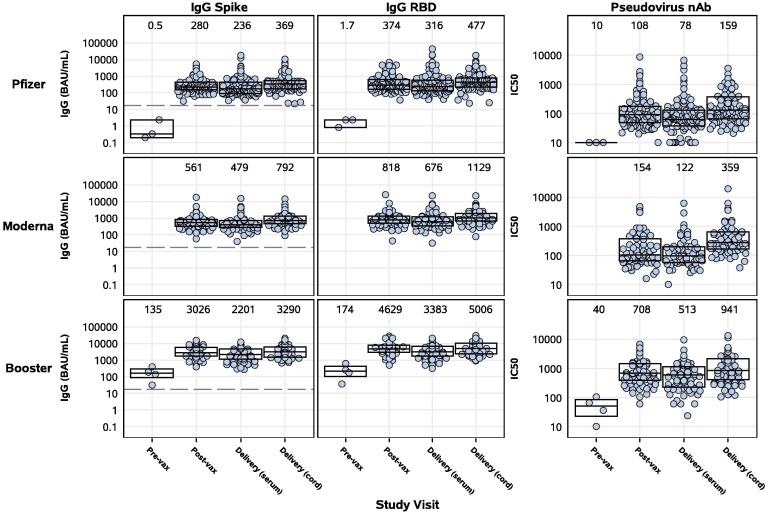
Serum binding IgG to Spike and RBD were detected in all primary 2-dose and booster dose recipients at the post-vaccination and delivery visits, and in all cord blood samples (Figure 1 ). Significantly higher antibody levels were measured post-vaccination and at delivery in participants who received a booster vaccination during pregnancy compared to those who received only a primary 2-dose series (Table 2 ). At delivery, the geometric mean titer (GMT) of IgG to Spike in booster vaccine recipients was 2,201 BAU/mL (n=73), 9.3-fold higher than in those receiving two doses of Pfizer (GMT 236 BAU/mL, n=100), and 4.6-fold higher than in those receiving two doses of Moderna (479 BAU/mL, n=67) vaccines (Figure 1). Booster vaccination also elicited significantly higher levels of Spike IgG in cord blood, where the GMT was 3,290 BAU/mL, 8.9-fold and 4.2-fold higher than in cord blood from those vaccinated with two doses of Pfizer (GMT 369 BAU/mL) or Moderna (GMT 792 BAU/mL), respectively (Figure 1). Similar trends were observed for RBD IgG in cord blood and at the post-vaccination visit to both Spike and RBD IgG (Figure 1, Table 2).
Figure 1.
SARS-CoV-2 binding IgG and pseudovirus nAb activity in maternal and cord blood sera by study group and study visit. Pregnant participants received a 2-dose series of an mRNA vaccine (top – Pfizer, middle – Moderna) or a booster mRNA vaccine (bottom panel). Sera derived from maternal blood collected pre- and post-vaccination and at delivery, and cord blood, were evaluated for binding IgG to full-length Spike (left panels) and RBD (middle panels), or pseudovirus nAb titers (IC50) (right panels). Binding IgG titers were bridged to international standards and reported as Binding Antibody Units (BAU/mL). Box plots represent median (horizontal line within the box) and interquartile range; GMT is displayed at the top of each panel and the dashed line is the cutoff for positivity (17 BAU/mL).
Table 2.
Differences in Antibody Levels Between Primary 2-Dose and Booster Groups at Delivery
| Lab Assessment | Sample | Primary GMT(95% CI) | BoosterGMT(95% CI) | Mean Log10Difference*(95% CI) | p-value |
|---|---|---|---|---|---|
| Spike IgG BAU/mL | Maternal Delivery | 313.8(260.7, 377.8) | 2200.6(1764.9, 2743.8) | 0.55(0.42, 0.68) | <0.0001 |
| Cord Blood | 500.5(424.1, 590.7) | 3290.4(2713.6, 3989.9) | 0.57(0.45, 0.69) | <0.0001 | |
| RBD IgG BAU/mL | Maternal Delivery | 428.6(351.9, 522.1) | 3382.9(2703.7, 4232.86) | 0.59(0.45, 0.73) | <0.0001 |
| Cord Blood | 673.1(564.5, 802.6) | 5005.7(4126.5, 6072.1) | 0.62(0.49, 0.75) | <0.0001 | |
| D614G live virus nAb ID50 | Maternal Delivery | 83.4(65.4, 106.4) | 446.4(342.1, 582.5) | 0.43(0.26, 0.60) | <0.0001 |
| Cord Blood | 96.4(80.2, 115.9) | 742.5(588.5, 936.8) | 0.65(0.52, 0.79) | <0.0001 | |
| Delta live virus nAb ID50 | Maternal Delivery | 39.0(30.9, 49.2) | 390.2(299.2, 508.9) | 0.73(0.57, 0.89) | <0.0001 |
| Cord Blood | 52.1(42.3, 64.2) | 644.5(507.5, 818.4) | 0.84(0.69, 0.99) | <0.0001 | |
| Omicron live virus nAb ID50 | Maternal Delivery | 13.5(11.9, 15.3) | 60.2(43.6, 83.1) | 0.52(0.39, 0.64) | <0.0001 |
| Cord Blood | 13.8(12.2, 15.6) | 109.2(81.6, 146.1) | 0.79(0.67, 0.91) | <0.0001 | |
| Pseudotyped WT nAb IC50 | Maternal Delivery | 93.4(77.3, 112.9) | 512.5(399.1, 658.3) | 0.52(0.36, 0.67) | <0.0001 |
| CordBlood | 220.8(183.5, 265.7) | 941.4(735.7, 1204.7) | 0.42(0.27, 0.57) | <0.0001 |
From regression analysis adjusted for days since last vaccination prior to delivery and prior self-reported SARS-CoV-2 infection or N protein positive at delivery.
Overall, the booster group and their infants had ∼0.6 log10 higher Spike and RBD IgG levels at delivery compared to the combined primary mRNA vaccine group (Pfizer and Moderna) after adjusting for days since last vaccination (p<0.0001) (Table 2). Sensitivity analyses showed similar results (data not shown).
3.2. SARS-CoV-2 neutralizing antibodies
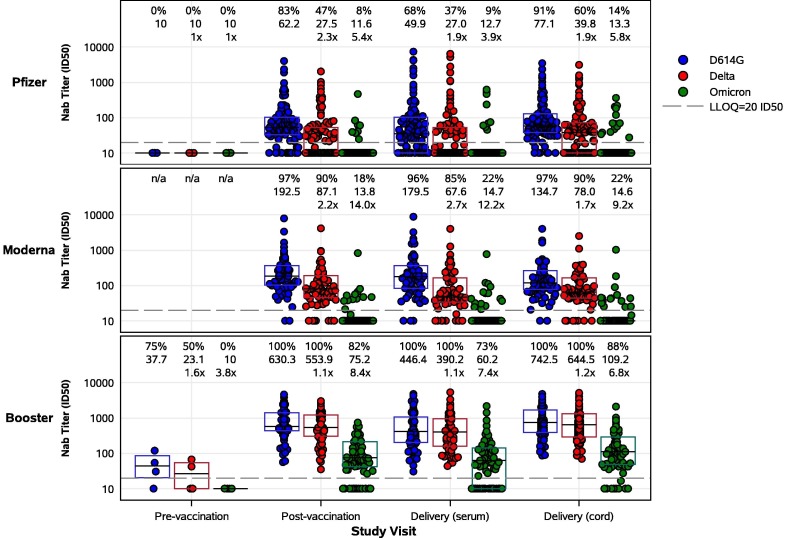
Significantly higher live virus nAb titers to D614G were measured post-vaccination in pregnant participants who received a booster (GMT ID50 630.3) compared to those receiving a primary 2-dose series (GMT ID50 62.2 for Pfizer, 192.5 for Moderna) (Figure 2 ). High live virus nAb titers persisted at delivery and were detectable in 100% of boosted participants (GMT ID50 446.4), compared to 68% (GMT ID50 49.9) and 96% (GMT ID50 179.5) of participants receiving 2 doses of Pfizer or Moderna, respectively (Figure 2, Table 2, Table 3 ). While live virus nAb titers to Omicron BA.1 were present in only 9% (GMT ID50 12.7) of Pfizer and 22% (GMT ID50 14.7) of Moderna dosed participants at delivery, 73% (GMT ID50 60.2) of boosted participants had detectable live virus nAb titers to Omicron BA.1 (p<0.0001). Live virus nAb activity against Delta was intermediate between D614G and Omicron BA.1. Similarly, pseudovirus nAb titers were significantly higher post-vaccination and at delivery in those who received a booster (GMT IC50 708 and 513, respectively) compared to those who received a 2-dose series (GMT IC50 108 and 78, respectively, for Pfizer; 154 and 122, respectively, for Moderna) (Figure 1, Table 2, Table 3).
Figure 2.
SARS-CoV-2 live virus nAb activity of maternal and cord blood sera by study group and study visit. Pregnant participants received a 2-dose series of an mRNA vaccine (top – Pfizer, middle – Moderna) or a booster mRNA vaccine (bottom panel). Sera derived from maternal blood collected pre- and post-vaccination and at delivery, and cord blood, were evaluated for neutralization of D614G, Delta, and Omicron (BA.1) variants. Each point represents the GMT ID50 from two duplicates per specimen (within the same assay run). A value equivalent to half the lower limit of detection (LLOD = 20) was assigned to observations with no detectable response. A specimen was considered as having a positive response if at least one of the duplicates was above the LLOD. Box plots represent median (horizontal line within the box) and interquartile range. Response rate (% with responses > 20 ID50), GMT, and GMT fold reduction compared to D614G are displayed at the top of each panel.
Table 3.
Differences in Response Rate for Neutralizing Antibodies Between Primary 2-Dose and Booster Groups at Delivery
| Lab Assessment | Sample | PrimaryResponse Rate* | BoosterResponse Rate* | Prevalence Ratio**(95% CI) | p-value |
|---|---|---|---|---|---|
| D614G live virus nAb | Maternal Delivery | 79.0% (132/167) | 100% (73/73) | 1.10 (1.03, 1.18) | 0.0039 |
| Cord Blood | 93.5% (159/170) | 100% (75/75) | 1.04 (1.00, 1.09) | 0.0553 | |
| Delta live virus nAb | Maternal Delivery | 56.3% (94/167) | 100% (73/73) | 1.46 (1.28, 1.68) | <0.0001 |
| Cord Blood | 71.8% (122/170) | 100% (75/75) | 1.22 (1.11, 1.33) | <0.0001 | |
| Omicron live virus nAb | Maternal Delivery | 14.4% (24/167) | 72.6% (53/73) | 3.16 (2.13, 4.71) | <0.0001 |
| Cord Blood | 17.1% (29/170) | 88.0% (66/75) | 3.71 (2.53, 5.44) | <0.0001 | |
| Pseudotyped WT nAb | Maternal Delivery | 92.8% (155/167) | 100% (73/73) | 1.05 (1.01, 1.09) | 0.0138 |
| Cord Blood | 100% (169/169) | 100% (75/75) | 1.00 (1.00, 1.00) | n/a |
Response rate defined as number of samples above the lower limit of detection divided by number of samples tested.
From robust Poisson regression analysis adjusted for days since last vaccination prior to delivery and prior self-reported SARS-CoV-2 infection or N protein positive at delivery.
Live virus nAb titers to D614G were also significantly higher in cord blood in the booster group (GMT ID50 742.5) compared to participants receiving 2 doses of Pfizer (GMT ID50 77.1) or Moderna (GMT ID50 134.7) (p<0.0001) (Figure 2, Table 2, Table 3). Notably, live virus nAb titers to Omicron BA.1 were significantly higher in cord blood from the booster group (88% response rate, GMT ID50 109.2) compared to those receiving 2 doses of Pfizer (14% response rate, GMT ID50 13.3) or Moderna (22% response rate, GMT ID50 14.6) (p<0.0001). Sensitivity analyses showed similar results (data not shown). Significantly higher pseudovirus nAb titers were also observed in cord blood in the booster group (GMT IC50 941) compared to those receiving 2 doses of Pfizer (GMT IC50 159) or Moderna (GMT IC50 359) (Figure 1, Table 2, Table 3).
3.3. Transplacental antibody transfer
Efficient transplacental transfer (ratio ≥ 1.0) was observed with both primary and booster vaccination during pregnancy, with median antibody transfer ratios between 1.55 and 1.77 for binding IgG, between 1.00 and 1.78 for live virus nAb, and between 1.79 and 2.36 for pseudovirus nAb (Table 4 ).
Table 4.
Antibody Transfer Ratios and Cord Blood and Maternal Sera Levels at Delivery for Primary 2-Dose and Booster Groups
| Lab Assessment | Group | Transfer RatioMedian (IQR) | Cord Blood SerumGMT(95% CI) | Maternal SerumGMT(95% CI) |
|---|---|---|---|---|
| Spike IgG BAU/mL | Primary | 1.77 (1.38, 2.25) | 500.5(424.1, 590.7) | 313.8(260.7, 377.8) |
| Booster | 1.55 (1.17, 1.98) | 3290.4(2713.6, 3989.9) | 2200.6(1764.9, 2743.8) | |
| RBD IgG BAU/mL | Primary | 1.76 (1.33, 2.15) | 673.1(564.5, 802.6) | 428.6(351.9, 522.1) |
| Booster | 1.58 (1.05, 1.93) | 5005.7(4126.5, 6072.1) | 3382.9(2703.7, 4232.86) | |
| D614G live virus nAb ID50* | Primary | 1.10 (0.76, 1.89) | 96.4(80.2, 115.9) | 83.4(65.4, 106.4) |
| Booster | 1.78 (1.12, 2.25) | 742.5(588.5, 936.8) | 446.4(342.1, 582.5) | |
| Delta live virus nAb ID50* | Primary | 1.10 (1.00, 1.69) | 52.1(42.3, 64.2) | 39.0(30.9, 49.2) |
| Booster | 1.74 (1.06, 2.38) | 644.5(507.5, 818.4) | 390.2(299.2, 508.9) | |
| Omicron live virus nAb ID50* | Primary | 1.00 (1.00, 1.00) | 13.8(12.2, 15.6) | 13.5(11.9, 15.3) |
| Booster | 1.69 (1.00, 2.75) | 109.2(81.6, 146.1) | 60.2(43.6, 83.1) | |
| Pseudotyped WT nAb IC50* | Primary | 2.36 (1.96, 2.84) | 220.8(183.5, 265.7) | 93.4(77.3, 112.9) |
| Booster | 1.79 (1.46, 2.20) | 941.4(735.7, 1204.7) | 512.5(399.1, 658.3) |
All data are included in the calculation of the transfer ratio including those below the LLOQ of the assay.
4. Discussion
In this large, multicenter prospective cohort study, robust antibody responses to mRNA COVID-19 vaccines were detected in pregnant participants immunized across all gestational ages. The substantial increase in binding and neutralizing antibody titers measured in mothers and newborns at the time of delivery after a booster vaccination is a key finding which strongly supports the administration of booster doses during pregnancy. In addition to the D614G vaccine strain, this finding was also observed in the nAb response to Delta and Omicron BA.1 where levels were significantly higher in booster recipients at delivery and in cord blood compared to those receiving a primary 2-dose series only. This booster effect is particularly relevant given the persistence of Omicron subvariants in current phases of the pandemic. However, the nAb levels to Omicron BA.1 were significantly lower than to the vaccine-matched D614G variant, as expected and observed in non-pregnant populations.[19]
Additionally, as reported by other investigators, maternal binding IgG antibodies against both Spike and RBD SARS-CoV-2 proteins were efficiently transferred across the placenta and concentrated in the infant.[8], [20] . [21] This latter finding is particularly important given high hospitalization rates among infants <6 months old during the Omicron BA.1 and BA.5 surges.[5], [6] Transplacental antibody transfer is the key component of newborn protection from SARS-CoV-2 infection, and parallels demonstrated neonatal protection from other respiratory pathogens such as influenza and pertussis.[22], [23] A recent study in Israel showed that IgG antibody titers in infants in the first few weeks of life correlated with SARS-CoV-2 IgG levels at birth.[23] Additionally, vaccine effectiveness studies have shown a reduction in hospitalization risk for the infant in the first few months of life following maternal vaccination during pregnancy.[12] Achieving higher antibody titers at birth could therefore provide protection against disease in the infant for a period of time until active vaccination. While an absolute correlate of protection is unknown, our study’s findings taken in the context of these other studies supports the likelihood of infant protection during a period of high vulnerability and current gap in vaccine eligibility for infants less than 6 months old.
Our study findings demonstrate that both maternal and infant protection can be enhanced with booster vaccination during pregnancy. Similar to our results, Kugelman et al. reported significantly higher, IgG responses in pregnant women in Israel who received a booster dose of the Pfizer-BioNTech mRNA vaccine compared to a historical control group of pregnant women who received only two doses of vaccine in the same gestational age window recipients.[24] Our study extends these findings to include pseudo- and live nAb data, which confirm the functional activity and potential protective effect of these antibodies in the newborn.
While some differences were observed by vaccine type, the clinical significance of these findings is unknown, and additional research is necessary to further characterize the immune responses of both mRNA and non-mRNA SARS-CoV-2 vaccines administered during pregnancy. Furthermore, efforts leading towards understanding the effect on maternal and infant immunity beyond the neonatal period and the effect of factors such as the timing of maternal vaccination before and during pregnancy should continue. The optimal timing of vaccination and waning of immunity in pregnancy deserve further evaluation, particularly given the higher risk of severe maternal disease occurring in the third trimester of gestation.[12], [25], [26] In a large study or maternal infant dyads (N=402) also conducted in Israel, Rottenstreich A, et al., reported substantial waning of anti-S and anti RBD-specific IgG responses in pregnant women at delivery if they were vaccinated in the first trimester compared to the second or third trimester.[25] These observations support booster vaccinations during pregnancy, particularly among mothers who might have completed their primary series prior to or early in pregnancy.[27]
Additional research is also needed to better elucidate the immune response following co-administration with other maternal vaccines, and the influence of maternal health status. Further, it remains important to characterize maternal immune responses to mixed vaccine platform regimens, next generation vaccines formulated against different SARS-CoV-2 variants, and the effect of administering subsequent booster vaccinations, possibly during every pregnancy.
4.1. Strengths and Limitations
Our multicenter prospective cohort study included diverse populations in various geographic regions of the U.S. and utilized an adaptive design which provided a unique opportunity for real world evaluation of the safety and immunogenicity of primary and booster vaccinations during pregnancy. We conducted a systematic, protocol-driven data and sample collection process encompassing periods of high transmission of SARS-CoV-2 variants with impact and relevance to pregnant women and infants. The study was strengthened by a central laboratory assessment of immune responses to vaccine strain as well as Delta and Omicron BA.1 variants, including binding and live and pseudovirus nAb.
Given the observational design of our study, the timing of vaccination during pregnancy was not pre-specified and the timing of sera collection post-vaccination was opportunistic. However, this investigation purposefully took a real-world scenario and inclusive enrollment approach and our analyses controlled for interval between vaccination and delivery, as well as prior maternal infection, to assess immune responses at the time of delivery. Thus, these data are generalizable and help delineate the potential impact of vaccination throughout pregnancy. Our results are limited to the evaluation of mRNA vaccines given current vaccine availability and recommendations in the U.S.
5. Conclusions
Pregnant women are appropriately included among the risk groups targeted for a bivalent booster dose of mRNA vaccines by the Centers for Disease Control and Prevention (CDC) and American College of Obstetrics and Gynecology (ACOG).[8], [14] While an absolute correlate of protection against SARS-CoV-2 infection is still unknown, increases in antibody responses in non-pregnant adults are associated with protection from symptomatic severe COVID-19.[28] Higher binding and neutralizing antibody responses to vaccine and emerging strains of SARS-CoV-2 have the potential to provide protection to both mothers and infants during a period of risk and high vulnerability. Our study supports that COVID-19 vaccination, and particularly booster doses, should be strongly recommended during pregnancy for maternal and neonatal protection.
Ethics Approval and Consent to Participate
Ethical approval of this protocol was received on May 28, 2021 by Vanderbilt University Medicine Center IRB, a single IRB as part of an NIH-funded consortium, IDCRC (IRB #210718). Written informed consent was obtained from each participant.
Data Sharing
Data collected for the study will be made available to others as a de-identified patient data set after finalization of clinical study report at the discretion of the IDCRC. Analyses of data, including data from staged analyses, will be available for presentation at scientific meetings and publication to inform the scientific community. If preliminary analyses are considered of public health importance or relevant to inform research, development, and implementation of SARS-CoV-2 vaccine in pregnancy, results may be shared with public health officials and partners to inform the global scientific community. The study will be conducted in accordance with the NIH Public Access Policy publication and data sharing policies and regulations. To request study data once complete, contact Flor M. Munoz, florm@bcm.edu.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
All the authors contributed to study design and have read and approved the final manuscript. We acknowledge Jeannie A. Murray, MS for contributions to the preparation and submission of this manuscript. We would like to thank study participants for their contributions.
Data availability
The authors do not have permission to share data.
References
- 1.Allotey J., Stallings E., Bonet M., et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ. 2020 Sep;1(370) doi: 10.1136/bmj.m3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zambrano L.D., Ellington S., Strid P., et al. Update: Characteristics of Symptomatic Women of Reproductive Age with Laboratory-Confirmed SARS-CoV-2 Infection by Pregnancy Status — United States, January 22–October 3, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1641–1647. doi: 10.15585/mmwr.mm6944e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Metz T.D., Clifton R.G., Hughes B.L., et al. Association of SARS-CoV-2 Infection With Serious Maternal Morbidity and Mortality From Obstetric Complications. JAMA. 2022 Feb 22;327(8):748–759. doi: 10.1001/jama.2022.1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vousden N, Ramakrishnan R, Bunch K, et al. Management and implications of severe COVID-19 in pregnancy in the UK: data from the UK Obstetric Surveillance System national cohort. Acta Obstet Gynecol Scand. 2022 Apr;101(4):461-470. doi: 10.1111/aogs.14329. Epub 2022 Feb 25. PMID: 35213734; PMCID: PMC9111211. [DOI] [PMC free article] [PubMed]
- 5.Marks K.J., Whitaker M., Agathis N.T., et al. Hospitalization of Infants and Children Aged 0–4 Years with Laboratory-Confirmed COVID-19- COVID-NET, 14 States, March 2020-February 2022. MMWR Morb Mortal Wkly Rep. 2022;71:429–436. doi: 10.15585/mmwr.mm7111e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamid S., Woodworth K., Pham H., et al. COVID-19 Associated Hospitalizations Among U.S. Infants Aged <6 Months – COVID-Net, 13 States, June 2021-August 2022. MMWR Morb Mortal Wkly Rep. 2022;71:1442–1448. doi: 10.15585/mmwr.mm7145a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carlsen E.Ø., Magnus M.C., Oakley L., et al. Association of COVID-19 Vaccination During Pregnancy With Incidence of SARS-CoV-2 Infection in Infants. JAMA. Intern Med. 2022 Jun 1 doi: 10.1001/jamainternmed.2022.2442. Epub ahead of print. PMID: 35648413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Centers for Disease Control (CDC). COVID-19 Vaccines While Pregnant or Breastfeeding [Internet]. Last Updated October 20, 2022. Available from: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/pregnancy.html.
- 9.Stock SJ, Carruthers J, Calvert C, et al. SARS-CoV-2 infection and COVID-19 vaccination rates in pregnant women in Scotland. Nat Med. 2022 Mar;28(3):504-512. doi: 10.1038/s41591-021-01666-2. Epub 2022 Jan 13. Erratum in: Nat Med. 2022 Feb 4;: PMID: 35027756; PMCID: PMC8938271 [DOI] [PMC free article] [PubMed]
- 10.Goldshtein I., Steinberg D.M., Kuint J., et al. Association of BNT162b2 COVID-19 Vaccination During Pregnancy With Neonatal and Early Infant Outcomes. JAMA Pediatr. 2022;176(5):470–477. doi: 10.1001/jamapediatrics.2022.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dagan N., Barda N., Biron-Shental T., et al. Effectiveness of the BNT162b2 mRNA COVID-19 vaccine in pregnancy. Nat Med. 2021;27(10):1693–1695. doi: 10.1038/s41591-021-01490-8. Epub 2021 Sep 7 PMID: 34493859. [DOI] [PubMed] [Google Scholar]
- 12.Halasa NB, Olson SM, Staat MA, et al. Maternal Vaccination and Risk of Hospitalization for Covid-19 among Infants. N Engl J Med. 2022 Jul 14;387(2):109-119. doi: 10.1056/NEJMoa2204399. Epub 2022 Jun 22. PMID: 35731908; PMCID: PMC9342588. [DOI] [PMC free article] [PubMed]
- 13.Zerbo O, Ray GT, Fireman B, et al. Maternal SARS-CoV-2 Vaccination and Infant Protection Against SARS-CoV-2 During the First 6 Months of Life. Res Sq [Preprint]. 2022 Oct 18:rs.3.rs-2143552. doi: 10.21203/rs.3.rs-2143552/v1. PMID: 36299419; PMCID: PMC9603829 [DOI] [PMC free article] [PubMed]
- 14.The American College of Obstetricians and Gynecologists (ACOG). COVID-19 Vaccination Considerations for Obstetric-Gynecologic Care [Internet]. December 2020. Last Updated September 20, 2022. Available from: https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/12/covid-19-vaccination-considerations-for-obstetric-gynecologic-care.
- 15.Munoz F.M., Beigi R.H., Posavad C.M., et al. Multi-site observational maternal and infant COVID-19 vaccine study (MOMI-vax): a study protocol. BMC Pregnancy Childbirth. 2022 May 12;22(1):402. doi: 10.1186/s12884-022-04500-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gilbert PB, Montefiori DC, McDermott AB, et al. Immune correlates analysis of the mRNA-1273 COVID-19 vaccine efficacy clinical trial. Science. 2022 Jan 7;375(6576):43-50. doi: 10.1126/science.abm3425. Epub 2021 Nov 23. PMID: 34812653; PMCID: PMC9017870. [DOI] [PMC free article] [PubMed]
- 17.Larsen SE, Berube BJ, Pecor T, et al. Qualification of ELISA and neutralization methodologies to measure SARS-CoV-2 humoral immunity using human clinical samples. J Immunol Methods. 2021 Dec;499:113160. doi: 10.1016/j.jim.2021.113160. Epub 2021 Sep 30. PMID: 34599915; PMCID: PMC8481082 [DOI] [PMC free article] [PubMed]
- 18.Edara V.V., Pinsky B.A., Suthar M.S., et al. Infection and Vaccine-Induced Neutralizing-Antibody Responses to the SARS-CoV-2 B.1.617 Variants. N Engl J Med. 2021;385(7):664–666. doi: 10.1056/NEJMc2107799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Belik M., Jalkanen P., Lundberg R., et al. Comparative analysis of COVID-19 vaccine responses and third booster dose-induced neutralizing antibodies against Delta and Omicron variants. Nat Commun. 2022;13(1):2476. doi: 10.1038/s41467-022-30162-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rottenstreich A., Zarbiv G., Oiknine-Djian E., Zigron R., Wolf D.G., Porat S. Efficient Maternofetal Transplacental Transfer of Anti- Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Spike Antibodies After Antenatal SARS-CoV-2 BNT162b2 Messenger RNA Vaccination. Clin Infect Dis. 2021;73(10):1909–1912. doi: 10.1093/cid/ciab266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kugelman N., Nahshon C., Shaked-Mishan P., et al. Maternal and Neonatal SARS-CoV-2 Immunoglobulin G Antibody Levels at Delivery After Receipt of the BNT162b2 Messenger RNA COVID-19 Vaccine During the Second Trimester of Pregnancy. JAMA Pediatr. 2022;176(3):290–295. doi: 10.1001/jamapediatrics.2021.5683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Omer S.B. Maternal Immunization. N Engl J Med. 2017;376(13):1256–1267. doi: 10.1056/NEJMra1509044. PMID: 28355514. [DOI] [PubMed] [Google Scholar]
- 23.Kugelman N, Nahshon C, Shaked-Mishan P, et al. SARS-CoV-2 immunoglobulin G antibody levels in infants following messenger RNA COVID-19 vaccination during pregnancy. Am J Obstet Gynecol. 2022 Dec;227(6):911-913. doi: 10.1016/j.ajog.2022.07.016. Epub 2022 Jul 19. PMID: 35863460; PMCID: PMC9293368. [DOI] [PMC free article] [PubMed]
- 24.Kugelman N., Nahshon C., Shaked-Mishan P., et al. Maternal and Neonatal Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Immunoglobulin G Levels After the Pfizer-BioNTech Booster Dose for Coronavirus Disease 2019 (COVID-19) Vaccination During the Second Trimester of Pregnancy. Obstet Gynecol. 2022 Aug 1;140(2):187–193. doi: 10.1097/AOG.0000000000004867. Epub 2022 May 27 PMID: 35852268. [DOI] [PubMed] [Google Scholar]
- 25.Rottenstreich A, Zarbiv G, Oiknine-Djian E, et al. Timing of SARS-CoV-2 vaccination during the third trimester of pregnancy and transplacental antibody transfer: a prospective cohort study. Clin Microbiol Infect. 2022 Mar;28(3):419-425. doi: 10.1016/j.cmi.2021.10.003. Epub 2021 Nov 3. PMID: 34740773; PMCID: PMC8563509. [DOI] [PMC free article] [PubMed]
- 26.Rottenstreich A., Zarbiv G., Oiknine-Djian E., et al. The Effect of Gestational Age at BNT162b2 mRNA Vaccination on Maternal and Neonatal Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Antibody Levels. Clin Infect Dis. 2022 Aug 24;75(1):e603–e610. doi: 10.1093/cid/ciac135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Faust JS, Rasmussen SA, Jamieson DJ. Pregnancy should be a condition eligible for additional doses of COVID-19 messenger RNA vaccines. Am J Obstet Gynecol MFM. 2022 Nov 9;5(2):100801. doi: 10.1016/j.ajogmf.2022.100801. Epub ahead of print. PMID: 36371035; PMCID: PMC9645060. [DOI] [PMC free article] [PubMed]
- 28.DeSilva MB, Mitchell PK, Klein NP, et al. Protection of 2 and 3 mRNA Vaccine Doses Against Severe Outcomes Among Adults Hospitalized with COVID-19 - VISION Network, August 2021 - March 2022. J Infect Dis. 2022 Nov 23:jiac458. doi: 10.1093/infdis/jiac458. Epub ahead of print. PMID: 36415904. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors do not have permission to share data.