Abstract
Mycotoxins are toxic fungal metabolites that exert various toxicities, including leading to death in lethal doses. This study developed a novel high‐pressure acidified steaming (HPAS) for detoxification of mycotoxins in foods and feed. The raw materials, maize and peanut/groundnut, were used for the study. The samples were separated into raw and processed categories. Processed samples were treated using HPAS at different citric acid concentrations (CCC) adjusted to pH 4.0, 4.5, and 5.0. The enzyme‐linked immunosorbent assay (ELISA) kit method for mycotoxins analysis was used to determine the levels of mycotoxins in the grains, with specific focus on total aflatoxins (AT), aflatoxins B1 (AFB1), aflatoxin G1 (AFG1), ochratoxin A (OTA), and citrinin. The mean values of the AT, AFB1, AFG1, OTA, and citrinin in the raw samples were 10.06 ± 0.02, 8.21 ± 0.01, 6.79 ± 0.00, 8.11 ± 0.02, and 7.39 ± 0.01 μg/kg for maize, respectively (p ≤ .05); and for groundnut (peanut), they were 8.11 ± 0.01, 4.88 ± 0.01, 7.04 ± 0.02, 6.75 ± 0.01, and 4.71 ± 0.00 μg/kg, respectively. At CCC adjusted to pH 5.0, the AT, AFB1, AFG1, OTA, and citrinin in the samples significantly reduced by 30%–51% and 17%–38% for maize and groundnut, respectively, and were reduced to 28%–100% when CCC was adjusted to pH 4.5 and 4.0 (p ≤ .05). The HPAS process either completely detoxified the mycotoxins or at least reduced them to levels below the maximum limits of 4.00–6.00, 2.00, 2.00, 5.00, and 100 μg/kg for AT, AFB1, AFG1, OTA, and citrinin, respectively, set by the European Union, WHO/FAO, and USDA. The study clearly demonstrates that mycotoxins can be completely detoxified using HPAS at CCC adjusted to pH 4.0 or below. This can be widely applied or integrated into many agricultural and production processes in the food, pharmaceutical, medical, chemical, and nutraceutical industries where pressurized steaming can be applied for the successful detoxification of mycotoxins.
Keywords: aflatoxins, citrinin, high‐pressure acidified steaming using citric acid, mycotoxin detoxification, mycotoxins, ochratoxins
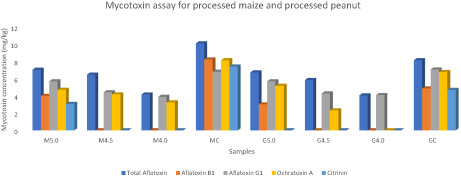
The HPAS process either completely detoxified the mycotoxins or at least reduced them to levels below the maximum limits of 4.00–6.00, 2.00, 2.00, 5.00, and 100 μg/kg for AT, AFB1, AFG1, OTA, and citrinin, respectively, set by the European Union, WHO/FAO, and USDA. The study clearly demonstrates that mycotoxins can be completely detoxified using HPAS at CCC adjusted to pH 4.0 or below. MC = mycotoxin content of the raw (unprocessed) maize samples; M5.0 = mycotoxin content of the maize samples after high‐pressure acidified steaming adjusted to pH 5.0; M4.5 = mycotoxin content of the maize samples after high‐pressure acidified steaming adjusted to pH 4.5; M4.0 = mycotoxin content of the maize samples after high‐pressure acidified steaming adjusted to pH 4.0; GC = mycotoxin content of the raw (unprocessed) groundnut (peanut) samples; G5.0 = mycotoxin content of the groundnut (peanut) samples after high‐pressure acidified steaming adjusted to pH 5.0; G4.5 = mycotoxin content of the groundnut (peanut) samples after high‐pressure acidified steaming adjusted to pH 4.5; and G4.0 = mycotoxin content of the groundnut (peanut) samples after high‐pressure acidified steaming adjusted to pH 4.0.
1. INTRODUCTION
Mycotoxins are toxic fungal metabolites produced by some molds in grains, nuts, spices, dried fruits, etc. Mycotoxins are chemically stable, with most of them posing concerns to humans and livestock, including the potent toxic mycotoxins such as aflatoxins, ochratoxins, zearalenone, nivalenol, deoxynivalenol, citrinin, fumonisins, and patulin (Gab‐Allah et al., 2023; Qing et al., 2022; Vylkova, 2017). Many industrial and domestic processes, including heat treatment (roasting, boiling, and frying), fermentation, and irradiation, among others, have been studied for the removal of mycotoxins in foods (Awuchi, Ondari, Ofoedu, et al., 2021; Ozkan et al., 2023). However, most of these studies reported incomplete or insufficient elimination of these mycotoxins. Mycotoxins are very stable and can withstand the rigors of food processing, thus requiring a technical approach designed for their removal (Bulgaru et al., 2021). Ensuring food and agro‐safety/quality is very important to not just food and agricultural industries but also to pharmaceutical and biomedical industries (Awuchi, 2023; Saeed et al., 2023).
The study aimed at addressing the increasing problems of mycotoxins worldwide, especially in developing and underdeveloped regions of the world. A widely applicable, cost‐effective, and safe method was considered for the thorough decontamination of mycotoxins from foods, to protect public health and animal safety, and successfully demonstrated that high‐pressure acidified steaming (HPAS) can be a reliable method to solve the problem of mycotoxin exposure from dietary and pharmaceutical sources, which are the major sources of human and animal exposure to mycotoxins and their toxic potency. High‐pressure acidified steaming is a combination of three techniques (high‐pressure [physical], steaming [physical], and acidification [chemical]) with the aim of complete detoxification of the mycotoxins. Citric acid is an edible organic acid used in many products directly consumed by humans, and as such, poses no toxicity when present in normal doses in human foods and animal feed. In this study, we developed a citric acid concentration‐dependent high‐pressure acidified steaming to successfully detoxify mycotoxins, with specific focus on total aflatoxins (AT), aflatoxins B1 (AFB1), aflatoxin G1 (AFG1), ochratoxin A (OTA), and citrinin in foods and feeds. The outcome of this study can be widely applied or integrated into many agricultural and production processes in the food, pharmaceutical, medical, chemical, and nutraceutical industries for the successful detoxification of mycotoxins, such as aflatoxins, citrinin, ochratoxins, deoxynivalenol, and fumonisins.
2. MATERIALS AND METHODS
2.1. Study site and sampling area
Samples were randomly drawn from different farm/market outlets in Kampala. The samples were picked using a stainless‐steel container and taken to the laboratory for further analyses and processing. The samples were selected based on the fresh grain produce from farms that were aimed at supplying and/or selling to the public or industries.
2.2. Sample collection and preparation
Three representative samples (three each from three different locations) were randomly collected for each of maize and peanuts from three different agro‐markets in Kampala. The samples were separated into two categories (raw and processed). Processed samples were treated using high‐pressure acidified steaming (HPAS) at different concentrations of citric acid adjusted to pH 4.0, 4.5, and 5.0. Both processed and raw samples were ground into flour using Art's‐Way portable roller mill (PRM30: USA) and labeled accordingly as directed by AOAC (2000a, 2000b). Maximum particle size reduction and thoroughness of mixing of the samples' flour were ensured to achieve effective distribution of contaminated portions.
2.3. High‐pressure acidified steaming
The grain flours were subjected to high‐pressure acidified steaming (HPAS) using autoclave, made by Thermo Fisher Scientific, Waltham, MA, United States, at the pressure of 15 PSI at steaming temperature, at various concentrations of citric acid adjusted to pH of 4.0, 4.5, and 5.0. Before steam generation, aqueous citric acid was added to pure water to acidify the water before steam generation and the pH was clearly noted at 4.0, 4.5, and 5.0. The method described by Jin et al. (2017), Jessica (2019), and Maya and Rao (1998) was used with slight modification.
2.4. Mycotoxin assay
The ELISA kit method for mycotoxins analysis (AOAC, 2000a, 2000b) was employed to determine the concentration of the respective mycotoxins in the samples. Twenty gram of each sample were grinded and added to 100 mL of 70% methanol blended for 3 min. The solutions were filtered through Whatman No. 1 filter and supernatant was collected. Fifty microliter of the filtrate per well was used for the test. Fifty microliter of each of the respective standards (for each mycotoxin assayed) and test samples were added, respectively, to antibody (mycotoxin)‐coated microtiter plate wells. The plates were sealed, gently homogenized, and incubated for 30 min at 37°C. Three hundred (300 μL) of wash buffer was added to each well and washed three times, and the plates were inverted on a layer of absorbent towels to remove residual water. One hundred microliter of HRP conjugate was added to each antibody‐coated well and incubated at ambient temperature for 30 min. After the incubation period, the plates were washed again with the wash buffer, and the plates were inverted on a layer of absorbent towels to remove residual water. One hundred microliter of substrate reagent was added to each well and then gently mixed thoroughly. This was then incubated at 37°C for 15 min in dark. Subsequently, 100 μL of stop solution was added to each well and gently mixed and the result read within 5 min after addition of stop solution. The optical density (OD) value of each well was determined at 450 nm (reference wavelength 630 nm) using a microplate reader. The values (corresponding to the concentration of the samples) were extrapolated from a standard curve obtained by plotting the absorbance percentage of each standard on the y‐axis against the log concentration on the x‐axis.
A: Average absorbance of standard or samples; A 0: Average absorbance of Standard.
2.5. Statistical analysis
The statistical analysis was done using SPSS. Analysis of variance (ANOVA) was conducted to analyze the data and check for any significant differences. Where p < .05, the differences were considered to be significant. Where there was significant difference in means, the least square difference (LSD) was done to separate the means. The values are presented as means and standard deviation.
3. RESULTS AND DISCUSSION
The results, their interpretation, and discussion are presented in this chapter in detail. The statistical analysis is also explained.
3.1. Mycotoxins
The results of the mycotoxins are shown in Table 1. The mycotoxins selected for this study include AT, AFB1, AFG1, OTA, and citrinin. The removal of these mycotoxins in foods will most likely signify the removal of all the mycotoxins in the foods. The effects of the high‐pressure acidified steaming on the mycotoxins were analyzed and reported in this section.
TABLE 1.
High‐pressure acidified steaming with citric acid detoxified mycotoxins.
| Sample | Total aflatoxin (μg/kg) | AFB1 (μg/kg) | AFG1 (μg/kg) | Ochratoxin A (μg/kg) | Citrinin (μg/kg) |
|---|---|---|---|---|---|
| M5.0 | 7.02c ± 0.02 | 4.02c ± 0.00 | 5.68c ± 0.02 | 4.72d ± 0.01 | 3.09c ± 0.01 |
| M4.5 | 6.44e ± 0.05 | ND | 4.43d ± 0.02 | 4.19e ± 0.02 | ND |
| M4.0 | 4.18g ± 0.02 | ND | 3.91g ± 0.01 | 3.26f ± 0.02 | ND |
| MC | 10.06a ± 0.02 | 8.21a ± 0.01 | 6.79b ± 0.00 | 8.11a ± 0.02 | 7.39a ± 0.01 |
| G5.0 | 6.71d ± 0.01 | 3.03d ± 0.02 | 5.68c ± 0.02 | 5.17c ± 0.01 | ND |
| G4.5 | 5.82f ± 0.00 | ND | 4.30e ± 0.14 | 2.34g ± 0.02 | ND |
| G4.0 | 4.07h ± 0.02 | ND | 4.11f ± 0.00 | ND | ND |
| GC | 8.11b ± 0.01 | 4.88b ± 0.01 | 7.04a ± 0.02 | 6.75b ± 0.01 | 4.71b ± 0.00 |
| LSD | 0.09 | 0.41 | 0.10 | 0.36 | 0.84 |
| Limits* | 4.00–6.00 | 2.00 | 2.00 | 5.00 | 100 |
Note: The values are means and standard deviations of the test samples. Mean with different superscripts are significantly different at p ≤ .05. MC and GC, mycotoxin content of the raw maize and groundnut (peanut) samples, respectively; M5.0 and G5.0, mycotoxin content of the maize and peanut samples, respectively, after HPAS at CCC adjusted to pH 5.0; M4.5 and G4.5, mycotoxin content of the maize and peanut samples, respectively, after HPAS at CCC adjusted to pH 4.5; M4.0 and G4.0, mycotoxin content of the maize and peanut samples, respectively, after HPAS at CCC adjusted to pH 4.0. LSD, the least significant difference. ND, not detected, that is, no mycotoxin was found.
3.2. Aflatoxins
The AT, AFB1, and AFG1 before and after processing of the samples were determined and the results are shown Table 1. The mean values of the AT, AFB1, and AFG1 in the raw samples were 10.06 ± 0.02, 8.21 ± 0.01, and 6.79 ± 0.00 μg/kg for maize, respectively (p ≤ .05); for groundnut (peanut), they were 8.11 ± 0.01, 4.88 ± 0.01, and 7.04 ± 0.02 μg/kg, respectively. These values in this study are far above the maximum limits of 4.00, 2.00, and 2.00 μg/kg for AT, AFB1, and AFG1, respectively, in grains and grain products based on the maximum limits set by the European Union, WHO/FAO, and USDA (Agriopoulou et al., 2020; Awuchi, Ondari, Ogbonna, et al., 2021; EFSA, 2020; European Commission, 2019; Giovati et al., 2015). After processing at different citric acid concentrations, adjusted to pH gradients of 4.0, 4.5, and 5.0, the values of the mycotoxins significantly reduced and were not detected in some samples (p ≤ .05). At citric acid concentration adjusted to pH 5.0, the AT, AFB1, and AFG1 in the samples significantly reduced to 7.02 ± 0.02 (30% reduction), 4.02 ± 0.00 (51%), and 5.68 ± 0.02 (16%) μg/kg, and 6.71 ± 0.01 (17%), 3.03 ± 0.02 (38%), and 5.68 ± 0.02 (19%) μg/kg for maize and groundnut, respectively. At citric acid concentration adjusted to pH 4.5, AFB1 was not detected in both samples (i.e., 100% detoxification of AFB1 was achieved), while the AT and AFG1 in the samples significantly reduced to 6.44 ± 0.05 (36%) and 4.43 ± 0.02 (35%) μg/kg, and 5.82 ± 0.00 (28%) and 4.30 ± 0.14 (39%) μg/kg for maize and groundnut, respectively. Similarly, when the citric acid concentration was increased and adjusted to pH of 4.0, AFB1 was also not detected in both samples (i.e., same 100% detoxification of AFB1 was achieved), while AT and AFG1 significantly reduced to 4.18 ± 0.02 (59%) and 3.91 ± 0.01 (42%) μg/kg, and 4.07 ± 0.02 (50%) and 4.11 ± 0.00 (42%) μg/kg for maize and groundnut, respectively. After HPAS processing at citric acid concentration adjusted to pH 4.0, the mycotoxins either completely detoxified or reduced to safe levels, except for AFG1 which was relatively higher than the maximum allowable limit. These levels can be further reduced or completely detoxified by increasing the citric acid concentrations in the high‐pressure steam and adjusting to pH below 4.0 (p ≤ .05). This study has shown reliable model for completely detoxifying aflatoxins or at least reducing them to safe levels. This shows the effectiveness of high‐pressure acidified steaming (HPAS) is completely detoxifying mycotoxins or at least significantly reducing them to safe levels; the efficiency of the detoxification by HPAS is citric acid concentration dependent. The values before processing are comparable to the values reported in other studies. Kholif et al. (2021) reported average contamination levels of 26.9 μg/kg and 2.65 μg/kg in total for AFB1, AFB2, AFB1, and AFG1 in sunflower oilseed and corn samples, respectively. Qing et al. (2022) reported a value of 20.10 mg/kg AFB1 in feed intake in their study. The differences in these values compared to the values in my raw samples may be due to the methods used by Kholif et al. (2021), who made use of size exclusion chromatography followed by HPLC. AFB1 is the most known toxic of all mycotoxins; its removal in foods is very important to food safety and animal/human health. The mean values of the total aflatoxins reported for maize in this study are slightly higher than the mean value of 3.14 ± 3.01 μg/kg reported by Awuchi et al. (2020), but the mean values for peanut in this study are lower than the mean value of 26.30 ± 11.47 μg/kg reported in the same study. These differences may be due to differences in the locations of the sample collection.
In previous studies, aflatoxins, including AFB1 and AFG1, have been shown to be nephrotoxic, immunotoxic, teratogenic, carcinogenic, mutagenic, hepatotoxic, neurotoxic, and genotoxic, among various toxic potencies. This study provides interesting knowledge into how these toxic effects can be zeroed or significantly reduced by applying HPAS against these mycotoxins in foods, especially grains. El‐Mahalaway (2015) and Joubrane et al. (2020) described how AFB1 exposure in adult male albino rats' renal cortex resulted in necrotic changes and degeneration with disrupted basal lamina, glomerular atrophy with light elimination from their capillaries, and enlargement with glomeruli luminal dilation. Li et al. (2018) and Wu et al. (2021) reported the testing of aflatoxins in many renal cell lines for a better understanding of their toxic mechanisms, with “primary fetal bovine kidney cell” and the “MadineDarby bovine kidney cell.” Aflatoxins exerted toxic potencies via several mechanisms. Several recent studies have described the various mechanisms involved in aflatoxins toxicities (Awuchi, Nwozo, et al., 2022; Lai et al., 2022) and their deleterious toxic potency (An et al., 2017; Awuchi, Ondari, et al., 2022). These toxic potencies can be avoided by treating grains with HPAS at the appropriate CCC before consumption. AFB1 potentiates autophagy mediated by ROS in RAW264.7 and THP‐1 cell lines (An et al., 2017; Lai et al., 2022). Navale et al. (2021) described the studies done on AFB1, which mostly focused on evaluating its teratogenic effects on chicks, rats, chickens, and eggs, and found teratogenic activities. Mohamed et al. (2022) reported that ozone can be used as a solution to eliminate aflatoxins' risk in meat and meat products (kofta and luncheon). In their study, they reported that raw Kofta and luncheon samples contained 15.2 ppb and 4.8 ppb of total aflatoxins, respectively (Mohamed et al., 2022), which is significantly different compared to the mean values of 10.06 ± 0.02 and 8.11 ± 0.01 reported in this study for raw maize and peanut samples, respectively. Mohamed et al. (2022) reported that the degree of detoxification depends on the concentration of ozone exposed to the samples. This concentration‐dependent detoxification is similar to the citric acid concentration‐dependent detoxification reported in this study. In Mohamed et al. (2022) study, at 20 ppm ozone concentration, the most detoxified aflatoxins were AFG1 (68.3%) and AFB2 (67.1%), while other aflatoxins reduced in ranges of 61.4% (44.7 ppb) and 55.2% (11.6 ppb) for kofta and luncheon, respectively; at 40 ppm ozone concentration, the most detoxified aflatoxins were AFB2 (91.7%) and AFG1 (100%), while other aflatoxins reduced by ranges from 85.7% (54.6 ppb) and 78.4% (61.4 ppb), respectively. Simões et al. (2023) reported Brazilian table olives as a source of lactic acid bacteria with antifungal and antimycotoxigenic activity. The methods used in this study can complement this treatment in an effort to completely detoxify these mycotoxins.
Due to the persistence of mycotoxins such as AFB1 and AFG1, in foods and feeds, many other studies have been done recently with the aim of harnessing novel ways to control these biological toxins (Awuchi, Ondari, Ofoedu, et al., 2021; Chinaza et al., 2021). The effectiveness of the method developed in this study demonstrates comparative advantage, including cost‐effectiveness, in removing mycotoxins compared with many methods that have been used in previous studies. This method also augments other methods that have been studied, including the promising ones that may not be applied to all foods. Adebo et al. (2019) studied the effect of fermentation on mycotoxins in sorghum and ting and reported promising results. Another recent study was done by Lorán et al. (2022), aimed at evaluating the in vitro effect of essential oils from Origanum virens, Rosmarinus officinalis, and lavandins Grosso and Abrial, as well as natural phenolic acids, such as chlorogenic, caffeic, p‐coumaric, and ferulic acids, on the production of AFB1, AFB2, AFG1, and AFG2. Lorán et al. (2022) reported a significant reduction in levels of aflatoxins after treatment with essential oils and phenolic acids, although some did not have any effect. The initial values of AFB1, AFB2, AFG1, and AFG2 in their raw samples were 0.52, 0.09, 0.94, and 0.26 μg/mL, respectively. These values are less than the values found in the raw samples in this study. Park (2002) reported a 40%–80% reduction in aflatoxins achieved using physical cleaning by removing damaged, mold‐infested nuts, seeds, or kernels from the whole grains. Although the levels of aflatoxins still remained unsafe after this removal. In addition to the physical cleaning, the method developed in this study can be used to either completely remove these mycotoxins or at least reduce them to safe levels. Kaushik (2015) evaluated the effects of food processing operations on my mycotoxin detoxification, including extrusion, roasting, flaking, frying, baking, cleaning, cooking, sorting, and trimming, and reported that processing operations such as thermal, physical, and chemical conditions play important role in detoxifying mycotoxins, with high‐temperature processes having more effects. However, they also concluded that all the processes evaluated significantly reduced the concentrations of mycotoxins, but did not completely eliminate them (Kaushik, 2015). This gives the HPAS method used in this study a comparative advantage, as it can completely detoxify most mycotoxins at CCC adjusted to pH 4 or below.
3.3. Ochratoxin A
The values of OTA in the raw samples were 8.11 ± 0.02 and 6.75 ± 0.01 μg/kg for maize and groundnut (peanut), respectively (see Table 1). These values are far higher than the maximum limits of 5 μg/kg for OTA in grains and grain products set by the European Union, WHO/FAO, and USDA (Agriopoulou et al., 2020; EFSA, 2020; Giovati et al., 2015). After HPAS processing at citric acid concentration adjusted to pH 5.0, the OTA levels significantly decreased to 4.72 ± 0.01 μg/kg (42% detoxification) and 5.17 ± 0.01 μg/kg (23% detoxification) for maize and groundnut, respectively (p ≤ .05). At citric acid concentration adjusted to pH 4.5, the OTA further significantly decreased to 4.19 ± 0.02 μg/kg (48% detoxification) and 2.34 ± 0.02 μg/kg (65% detoxification) for maize and groundnut, respectively. Interestingly, after the citric acid concentration was adjusted to pH 4.0, OTA was not detected in groundnut (100% elimination/detoxification), and in maize, it significantly decreased to 3.26 ± 0.02 μg/kg (60% detoxification) (p ≤ .05). The high‐pressure acidified steaming process either completely detoxified the OTA or at least reduced it to levels far below the maximum limit of 5 μg/kg for OTA set by the European Union, WHO/FAO, and USDA. It was observed that increasing the citric acid concentration significantly reduced the levels of OTA in the grain samples in a manner that shows that increasing the citric acid concentration in pressured steaming process can completely detoxify mycotoxins such as OTA in foods and feeds; that is, the efficiency of the detoxification by HPAS is citric acid concentration dependent (p ≤ .05). The values in the raw samples are relatively lower than the average value of 101.41 mg/kg of OTA in samples from feed intake reported by Qing et al. (2022). OTA and aflatoxins often cooccur in foods and feeds, often along with other mycotoxins (Agriopoulou et al., 2020; Awuchi et al., 2020). Their removal is very important for food safety and public health. This study has developed a reliable method and processing regimen for completely detoxifying mycotoxins in foods and feeds or at least reducing them to safe levels (i.e., levels at which the body can easily detoxify them without them exerting any form of toxicity). Awuchi et al. (2020) and Awuchi, Owuamanam, and Ogueke (2021) reported the effects of ochratoxins on the nutritional and functional properties of foods. Gan et al. (2017) reported that in vitro OTA nephrotoxic effects in primary porcine splenocytes and PK15 cells showed that 0.5–4.0 and 2.0–8.0 mg/mL per day, respectively, induced apoptosis and cytotoxicity by phosphorylation and signaling of ERK and p38. These toxic effects can be avoided by employing the HPAS method to completely detoxify OTA or reduce its levels to safe levels.
OTA exerts many toxic effects in humans and animals, including nephrotoxic, hepatotoxic, carcinogenic, immunotoxic, neurotoxic, genotoxic, and teratogenic effects, among other toxic effects. Loboda et al. (2017) reported the nephrotoxic effects of OTA on porcine tubular epithelial cells after Nrf2 inhibition with reduced vascular endothelial growth factor and claudin‐2 levels and increased expression of proapoptotic, profibrotic, and proinflammatory factors. In other studies, DNA microarray analysis following the proximal tubular cells double fluorescence labeling of primary rat and Wistar rats treated with several ochratoxin A doses showed transcriptional changes in genes involved in apoptosis, inflammatory reactions, and DNA damage responses (Gan et al., 2022; Ráduly et al., 2021). Real‐time polymerase chain reactions application in studying the expression of gene responsible for cell division and cell control in OTA‐treated male F344 rats at doses of 210, 70, 21, and 0 mg/kg of body weight showed that OTA induces the expression of excess mitosis key regulators, such as aurora B kinase, cyclin's kinase‐dependent inhibitors, serine/threonine protein kinase PLK1, surviving, topoisomerase II, cyclin‐dependent kinase 1 (Cdk1Cdc2), and cyclins with some key regulators' upregulation in the proximal tubular S3 cell, where OTA‐induced tumors appear (EFSA Panel on Contaminants in the Food Chain (CONTAM) et al., 2020; Khoi et al., 2021). High and medial doses of ochratoxin A exert liver toxicity. In a study involving OTA toxicity on HepG2 cell lines, Gayathri et al. (2015) reported increase in intracellular ROS accompanied by breaks in DNA strands and mitochondria‐mediated intrinsic apoptosis. Neural stem/progenitor cells (NSCs) formulated from adult mouse brains' hippocampus were tested for their OTA vulnerability in vitro, and 0.01–100 mg OTA/mL concentrations showed that OTA causes time‐ and dose‐dependent (6–72 h) reduction in differential and proliferative viability; differentiated neurons have less vulnerability to toxins compared to proliferating NSCs (Bhat et al., 2016; Gill & Kumara, 2019). These toxicities can simply be avoided by either eliminating or at least reducing the levels of OTA using high‐pressure acidified steaming methods as described in this study.
Many methods have also been explored in other studies in an attempt to reduce the toxic effects of ochratoxin A and other mycotoxins. Leitão and Enguita (2021) conducted research on filamentous fungal proteomes' enzymes that can degrade ochratoxins in a systematic structure‐based manner. They concluded that filamentous fungi are rich in hydrolases that can potentially degrade ochratoxins, and may detoxify many food commodities. However, the successful application of these enzymes in real‐time is yet to be ascertained. The method developed in this study can easily be applied in real time and large‐scale food and agricultural production, as many industries already make use of steam generation and application in many processes.
3.4. Citrinin
Table 1 shows that the values of citrinin in the raw samples were 7.39 ± 0.01 and 4.71 ± 0.00 μg/kg for maize and groundnut (peanut), respectively. There were significant differences in the levels of citrinin in the raw samples, as shown in Table 1. It was observed that these values are within the maximum limit of 100 μg/kg set by the European Union and WHO/FAO (EFSA, 2020; EFSA Panel on Contaminants in the Food Chain (CONTAM) et al., 2020). After HPAS processing at citric acid concentration adjusted to pH 5.0, the level of citrinin in maize significantly reduced to 3.09 ± 0.01 μg/kg (58% detoxification), while it was not detected in groundnut (100% elimination/detoxification). Very interestingly, at HPAS processing of citric acid concentration adjusted to pH 4.5 and 4.0, no citrinin was detected thereafter (100% elimination/detoxification). This shows that the HPAS processing method completely eliminates/detoxifies mycotoxins such as citrinin in foods and feeds. The mean values reported in the raw samples are relatively less than the mean values of 14.6–23.8 μg/kg in corn and wheat samples reported by Čulig et al. (2017), who analyzed the citrinin levels in grains along with its health effects. Many foods, feeds, and supplements have been proven to contain high levels of citrinin. Ali et al. (2015) found that the average urine level for citrinin and its metabolite (dihydrocitrinone) were 0.03 ng/mL and 0.06 ng/mL respectively, which when adjusted to the creatinine level, 20.2 and 60.9 ng/g creatinine for citrinin and dihydrocitrinone, respectively; it became clear that the metabolite appearance in urine was 3× higher. Silva et al. (2020) described various foods that contain citrinin, from low to extremely high amounts, including grains. The presence of citrinin and other mycotoxins can be eliminated or at least significantly reduced to safe levels using the HPAS developed in this study. Magro et al. (2016) simulated citrinin contamination of 625 μg/L and studied its removal using naked magnetic nanoparticles. The results showed that the efficiency of the decontamination using naked magnetic nanoparticles is 70% (Magro et al., 2016), which still leaves citrinin at unsafe levels; this unsafe level can be eliminated or reduced to safe levels using the methods developed in this study. Piemontese et al. (2018) have also described the use of nanoparticles and their magnetic nanocomposites to reduce the levels of mycotoxins in grains. Citrinin is among the rarely studied mycotoxins (Narváez et al., 2021). Tangni et al. (2021) studied citrinin in food supplements produced from red yeast rice and Ginkgo biloba leaves and evaluated the citrinin stability under storage. They reported that citrinin values changed after storage at different temperature ranges between 4 and 24°C. Bartkiene et al. (2021) proceed wheat bran by the combination of extrusion (at screw speeds of 25, 20, and 16 rpm, and temperature of 115–130°C) and fermentation using L. uvarum and Lactobacillus plantarum strains; they reported 29.8 μg/kg total mycotoxin concentration after the combined processing. The HPAS in this study proved more effective in mycotoxin detoxification than many other methods, such as the combined extrusion and fermentation methods.
Citrinin exerts many toxicities on both humans and animals, including genotoxicity, nephrotoxicity, carcinogenicity, immune suppression, acute toxicity, etc. The toxicity of citrinin is further worsened by the fact that it usually occurs along with other mycotoxins, especially OTA and AFB1, as they are produced by the same species of fungi. It mostly occurs with OTA, with both synergistically exerting nephrotoxic effects, and may have influence on necrosis and apoptosis in hepatocytes (Gayathri et al., 2015; Jaus et al., 2022). Many in vitro studies showed that citrinin toxicity involves decreased cytokine production nitride oxide gene expression's inhibition, increase in ROS, inhibition of DNA and RNA synthesis, oxidative stress induction, and apoptotic cell death activation through the caspase cascade system and signal transduction pathways (European Food Safety Authority, 2012). Citrinin and its metabolite (dihydrocitrinon) have been detected in urine by Ali et al. (2015) in 82% and 84% of urine samples, respectively. Citrinin mostly targets the kidney (Jaus et al., 2022). These toxic effects of citrinin and other mycotoxins can be prevented by treating foods and feeds using HPAS in citric acid concentration adjusted to pH 4.5 or 4.0 or below prior to further processing and/or consumption.
4. CONCLUSION
Mycotoxins are very toxic and persistent in foods and feeds. They escape the rigors of many processes used in food, pharmaceutical, and nutraceutical industries. We developed widely applicable, cost‐effective, and safe method for mycotoxins decontamination, and successfully demonstrated that high‐pressure acidified steaming (HPAS) can be a reliable method to solve the problem of mycotoxin exposure from dietary and pharmaceutical sources. The results of this study showed that the HPAS can completely detoxify mycotoxins, such as AT, AFB1, AFG1, OTA, and citrinin, or at least reduce them to safe levels. The study strongly recommends and encourages the application of the HPAS method at industrial scale in the food, pharmaceutical, nutraceutical, medical, and chemical industries where citric acid‐containing pressurized steaming can be applied for the detoxification of mycotoxins.
CONFLICT OF INTEREST STATEMENT
All authors declare no conflict of interest.
ETHICAL APPROVAL
The study does not involve any human or animal testing.
ACKNOWLEDGMENTS
The authors are thankful to Kampala International University, Bushenyi, Uganda, and all the labs in both Uganda and Nigeria that provided/assisted with the research facilities used for this study. The authors are also thankful to all the academic staff at Kampala International University who contributed to the success of this study, in one way or the other.
Awuchi, C. G. , Nwozo, O. S. , Aja, P. M. , & Odongo, G. A. (2023). High‐pressure acidified steaming with varied citric acid dosing can successfully detoxify mycotoxins. Food Science & Nutrition, 11, 2677–2685. 10.1002/fsn3.3324
DATA AVAILABILITY STATEMENT
Additional data for this study can be made available from the corresponding author upon request.
REFERENCES
- Adebo, O. A. , Kayitesi, E. , & Njobeh, P. B. (2019). Reduction of mycotoxins during fermentation of whole grain sorghum to whole grain ting (a southern African food). Toxins, 11(3), 180. 10.3390/toxins11030180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agriopoulou, S. , Stamatelopoulou, E. , & Varzakas, T. (2020). Advances in occurrence, importance, and mycotoxin control strategies: Prevention and detoxification in foods. Food, 9, 137. 10.3390/foods9020137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali, N. , Blaszkewicz, M. , & Degen, G. H. (2015). Occurrence of mycotoxin citrinin and its metabolite dihydrocitrinone in urines of German adults. Archives of Toxicology, 89(4), 573–578. 10.1007/s00204-014-1363-y [DOI] [PubMed] [Google Scholar]
- An, Y. , Shi, X. , Tang, X. , Wang, Y. , Shen, F. , Zhang, Q. , Wang, C. , Jiang, M. , Liu, M. , & Yu, L. (2017). Aflatoxin B1 induces reactive oxygen species‐mediated autophagy and extracellular trap formation in macrophages. Frontiers in Cellular and Infection Microbiology, 7, 53. 10.3389/fcimb.2017.00053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- AOAC . (2000a). Ochratoxin in roasted coffee immunoaffinity column HPLC method first action 2000. A.O.A.C. [Google Scholar]
- AOAC . (2000b). Official method 971.22. Standards of aflatoxin, subpara E, preparation and storage of TLC standards (17th ed.). A.O.A.C. [Google Scholar]
- Awuchi, C. G. (2023). HACCP, quality, and food safety management in food and agricultural systems. Cogent Food & Agriculture, 9(1), 2176280. 10.1080/23311932.2023.2176280 [DOI] [Google Scholar]
- Awuchi, C. G. , Nwozo, S. , Salihu, M. , Odongo, G. A. , Sarvarian, M. , & Okpala, C. O. R. (2022). Mycotoxins' toxicities – from consumer health safety concerns, to mitigation/treatment strategies: A perspective review. Journal of Chemical Health Risks, 12(3), 427–464. 10.22034/jchr.2022.1939170.1399 [DOI] [Google Scholar]
- Awuchi, C. G. , Ondari, E. N. , Nwozo, S. , Odongo, G. A. , Eseoghene, I. J. , Twinomuhwezi, H. , Ogbonna, C. U. , Upadhyay, A. K. , Adeleye, A. O. , & Okpala, C. O. R. (2022). Mycotoxins' toxicological mechanisms involving humans, livestock and their associated health concerns: A review. Toxins, 14, 167. 10.3390/toxins14030167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awuchi, C. G. , Ondari, E. N. , Ofoedu, C. E. , Chacha, J. S. , Rasaq, W. A. , Morya, S. , & Okpala, C. O. R. (2021). Grain processing methods' effectiveness to eliminate mycotoxins: An overview. Asian Journal of Chemistry, 33(10), 2267–2275. 10.14233/ajchem.2021.23374 [DOI] [Google Scholar]
- Awuchi, C. G. , Ondari, E. N. , Ogbonna, C. U. , Upadhyay, A. K. , Baran, K. , Okpala, C. O. R. , Korzeniowska, M. , & Guiné, R. P. F. (2021). Mycotoxins affecting animals, foods, humans and plants: Types, occurrence, toxicities, action mechanisms, prevention and detoxification strategies—A revisit. Food, 10, 1279. 10.3390/foods10061279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awuchi, C. G. , Owuamanam, I. C. , & Ogueke, C. C. (2021). Ochratoxins' effects on the functional properties and nutritional compositions of grains. Journal La Lifesciences, 2(4), 32–53. 10.37899/journallalifesci.v2i4.421 [DOI] [Google Scholar]
- Awuchi, C. G. , Owuamanam, I. C. , Ogueke, C. C. , & Hannington, T. (2020). The impacts of mycotoxins on the proximate composition and functional properties of grains. European Academic Research, 8(2), 1024–1071. [Google Scholar]
- Bartkiene, E. , Zokaityte, E. , Lele, V. , Starkute, V. , Zavistanaviciute, P. , Klupsaite, D. , Cernauskas, D. , Ruzauskas, M. , Bartkevics, V. , Pugajeva, I. , Bērziņa, Z. , Gruzauskas, R. , Sidlauskiene, S. , Santini, A. , & Juodeikiene, G. (2021). Combination of extrusion and fermentation with Lactobacillus plantarum and L. uvarum strains for improving the safety characteristics of wheat bran. Toxins, 13(2), 163. 10.3390/toxins13020163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat, P. V. , Pandareesh, M. D. , Khanum, F. , & Tamatam, A. (2016). Cytotoxic effects of ochratoxin A in neuro‐2a cells: Role of oxidative stress evidenced by N‐acetylcysteine. Frontiers in Microbiology, 7, 1142. 10.3389/fmicb.2016.01142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulgaru, C. V. , Marin, D. E. , Pistol, G. C. , & Taranu, I. (2021). Zearalenone and the immune response. Toxins, 13(4), 248. 10.3390/toxins13040248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinaza, G. A. , Erick, N. O. , Hannington, T. , Victory, S. I. , & Ikechukwu, O. A. (2021). Aflatoxin B1 production, toxicity, mechanism of carcinogenicity, risk management, and regulations. Archives of Animal and Poultry Sciences, 1(4), 555568. [Google Scholar]
- Čulig, B. , Bevardi, M. , Bošnir, J. , Serdar, S. , Lasić, D. , Racz, A. , Galić, A. , & Kuharić, Ž. (2017). Presence of Citrinin in grains and its possible health effects. African Journal of Traditional, Complementary, and Alternative Medicines: AJTCAM, 14(3), 22–30. 10.21010/ajtcam.v14i3.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA . (2020). Risk assessment of aflatoxins in food. EFSA Journal, 18(3), 6040. 10.2903/j.efsa.2020.6040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Panel on Contaminants in the Food Chain (CONTAM) , Dieter, S. , Bodin, L. , Chipman, J. J. , Jesús del, M. , Grasl‐Kraupp, B. , Hogstrand, C. , Hoogenboom, L. , Jean‐Charles, L. , Nebbia, C. S. , Nielsen, E. , Ntzani, E. , Petersen, A. , Sand, S. , Schwerdtle, T. , Vleminckx, C. , Wallace, H. , Alexander, J. , Dall'Asta, C. , … Bignami, M. (2020). Risk assessment of ochratoxin A in food. EFSA Journal, 18(5), e06113. 10.2903/j.efsa.2020.6113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El‐Mahalaway, A. M. (2015). Protective effect of curcumin against experimentally induced aflatoxicosis on the renal cortex of adult male albino rats: A histological and immunohisochemical study. International Journal of Clinical and Experimental Pathology, 8(2015), 6019–6030. [PMC free article] [PubMed] [Google Scholar]
- European Commission . (2019). Commission regulation (EU) 2019/1901 of 7 November 2019 amending regulation (EC) No 1881/2006 as regards maximum levels of citrinin in food supplements based on rice fermented with red yeast Monascus purpureus. Official Journal of the European Union, 62, 2–4. [Google Scholar]
- European Food Safety Authority . (2012). Scientific opinion on the risks for public and animal health related to the presence of citrinin in food and feed. EFSA Journal, 10, 3. 10.2903/j.efsa.2012.2605 [DOI] [Google Scholar]
- Gab‐Allah, M. A. , Choi, K. , & Kim, B. (2023). Type B Trichothecenes in cereal grains and their products: Recent advances on occurrence, toxicology, analysis and post‐harvest decontamination strategies. Toxins, 15(2), 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan, F. , Hou, L. , Xu, H. , Liu, Y. , Chen, X. , & Huang, K. (2022). PCV2 infection aggravates OTA‐induced immunotoxicity in vivo and in vitro. Ecotoxicology and Environmental Safety, 235, 113447. 10.1016/j.ecoenv.2022.113447 [DOI] [PubMed] [Google Scholar]
- Gan, F. , Hou, L. , Zhou, Y. , Liu, Y. , Huang, D. , Chen, X. , & Huang, K. (2017). Effects of ochratoxin A on ER stress, MAPK signaling pathway and autophagy of kidney and spleen in pigs. Environmental Toxicology, 32, 2277–2286. 10.1002/tox.22443 [DOI] [PubMed] [Google Scholar]
- Gayathri, L. , Dhivya, R. , Dhanasekaran, D. , Periasamy, V. S. , Alshatwi, A. A. , & Akbarsha, M. A. (2015). Hepatotoxic effect of ochratoxin A and citrinin, alone and in combination and protective effect of vitamin: In vitro study in HepG2 cell. Food and Chemical Toxicology, 83, 151–163. 10.1016/j.fct.2015.06.009 [DOI] [PubMed] [Google Scholar]
- Gill, S. , & Kumara, V. (2019). Detecting neurodevelopmental toxicity of domoic acid and ochratoxin A using rat fetal neural stem cells. Marine Drugs, 17(10), 566. 10.3390/md17100566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovati, L. , Magliani, W. , Ciociola, T. , Santinoli, C. , Conti, S. , & Polonelli, L. (2015). AFM₁ in milk: Physical, biological, and prophylactic methods to mitigate contamination. Toxins, 7(10), 4330–4349. 10.3390/toxins7104330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaus, A. , Rhyn, P. , Haldimann, M. , Brüschweiler, B. J. , Fragnière Rime, C. , Jenny‐Burri, J. , & Zoller, O. (2022). Biomonitoring of ochratoxin A, 2'R‐ochratoxin A and citrinin in human blood serum from Switzerland. Mycotoxin Research, 38(2), 147–161. 10.1007/s12550-022-00456-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessica, G. (2019). Steaming (Moist‐Heat Cooking Method) . Available from https://www.thespruceeats.com/steaming‐moist‐heat‐cooking‐method‐995849. Accessed 4th September 2021
- Jin, W. Y. , Dae, H. K. , & Min, S. K. (2017). Experimental study of lab‐scale steam generation heat pump with waste heat recovery. 12th IEA Heat Pump Conference (2017) P.3.7.8 .
- Joubrane, K. , Mnayer, D. , El Khoury, A. , El Khoury, A. , & Awad, E. (2020). Co‐occurrence of aflatoxin B1 and ochratoxin A in Lebanese stored wheat. Journal of Food Protection, 83(9), 1547–1552. 10.4315/JFP-20-110 [DOI] [PubMed] [Google Scholar]
- Kaushik, G. (2015). Effect of processing on mycotoxin content in grains. Critical Reviews in Food Science and Nutrition, 55(12), 1672–1683. 10.1080/10408398.2012.701254 [DOI] [PubMed] [Google Scholar]
- Khoi, C. S. , Chen, J. H. , Lin, T. Y. , Chiang, C. K. , & Hung, K. Y. (2021). Ochratoxin A‐induced nephrotoxicity: Up‐to‐date evidence. International Journal of Molecular Sciences, 22(20), 11237. 10.3390/ijms222011237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kholif, O. T. , Sebaei, A. S. , Eissa, F. I. , & Elhamalawy, O. H. (2021). Size‐exclusion chromatography selective cleanup of aflatoxins in oilseeds followed by HPLC determination to assess the potential health risk. Toxicon: Official Journal of the International Society on Toxinology, 200, 110–117. 10.1016/j.toxicon.2021.07.009 [DOI] [PubMed] [Google Scholar]
- Lai, Y. , Sun, M. , He, Y. , Lei, J. , Han, Y. , Wu, Y. , Bai, D. , Guo, Y. , & Zhang, B. (2022). Mycotoxins binder supplementation alleviates aflatoxin B1 toxic effects on the immune response and intestinal barrier function in broilers. Poultry Science, 101(3), 101683. 10.1016/j.psj.2021.101683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitão, A. L. , & Enguita, F. J. (2021). Systematic structure‐based search for ochratoxin‐degrading enzymes in proteomes from filamentous fungi. Biomolecules, 11(7), 1040. 10.3390/biom11071040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, H. , Xing, L. , Zhang, M. , Wang, J. , & Zheng, N. (2018). The toxic effects of aflatoxin B1 and aflatoxin M1 on kidney through regulating L‐proline and downstream apoptosis. BioMed Research International, 2018, 9074861. 10.1155/2018/9074861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loboda, A. , Stachurska, A. , Sobczak, M. , Podkalicka, P. , Mucha, O. , Jozkowicz, A. , & Dulak, J. (2017). Nrf2 deficiency exacerbates ochratoxin A‐induced toxicity in vitro and in vivo . Toxicology, 15, 42–52. 10.1016/j.tox.2017.07.004 [DOI] [PubMed] [Google Scholar]
- Lorán, S. , Carramiñana, J. J. , Juan, T. , Ariño, A. , & Herrera, M. (2022). Inhibition of Aspergillus Parasiticus growth and aflatoxins production by natural essential oils and phenolic acids. Toxins, 14(6), 384. 10.3390/toxins14060384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magro, M. , Moritz, D. E. , Bonaiuto, E. , Baratella, D. , Terzo, M. , Jakubec, P. , Malina, O. , Čépe, K. , Aragao, G. , Zboril, R. , & Vianello, F. (2016). Citrinin mycotoxin recognition and removal by naked magnetic nanoparticles. Food Chemistry, 203, 505–512. 10.1016/j.foodchem.2016.01.147 [DOI] [PubMed] [Google Scholar]
- Maya, P. , & Rao, H. P. (1998). Effect of steaming on the rheological characteristics of wheat flour dough. European Food Research and Technology, 209, 122–125. [Google Scholar]
- Mohamed, M. H. , Ammar, M. A. M. , Zaki, Z. M. , & Youssef, A. E. K. (2022). Ozone as a solution for eliminating the risk of aflatoxins detected in some meat products. Current Research in Nutrition and Food Science, 10(1), 334–348. 10.12944/CRNFSJ.10.1.28 [DOI] [Google Scholar]
- Narváez, A. , Izzo, L. , Rodríguez‐Carrasco, Y. , & Ritieni, A. (2021). Citrinin dietary exposure assessment approach through human biomonitoring high‐resolution mass spectrometry‐based data. Journal of Agricultural and Food Chemistry, 69(22), 6330–6338. 10.1021/acs.jafc.1c01776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navale, V. , Vamkudoth, K. R. , Ajmera, S. , & Dhuri, V. (2021). Aspergillus derived mycotoxins in food and the environment: Prevalence, detection, and toxicity. Toxicology Reports, 8, 1008–1030. 10.1016/j.toxrep.2021.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozkan, G. , Subasi, B. G. , Capanoglu, E. , & Esatbeyoglu, T. (2023). Application of high pressure processing in ensuring food safety. In Non‐thermal food processing operations (pp. 319–357). Woodhead Publishing. [Google Scholar]
- Park, D. L. (2002). Effect of processing on aflatoxin. Advances in Experimental Medicine and Biology, 504, 173–179. 10.1007/978-1-4615-0629-4_17 [DOI] [PubMed] [Google Scholar]
- Piemontese, L. , Messia, M. C. , Marconi, E. , Falasca, L. , Zivoli, R. , Gambacorta, L. , Perrone, G. , & Solfrizzo, M. (2018). Effect of gaseous ozone treatments on DON, microbial contaminants and technological parameters of wheat and semolina. Food Additives & Contaminants. Part A, Chemistry, Analysis, Control, Exposure & Risk Assessment, 2018(35), 760–771. [DOI] [PubMed] [Google Scholar]
- Qing, H. , Huang, S. , Zhan, K. , Zhao, L. , Zhang, J. , Ji, C. , & Ma, Q. (2022). Combined toxicity evaluation of ochratoxin A and aflatoxin B1 on kidney and liver injury, immune inflammation, and gut microbiota alteration through pair‐feeding pullet model. Frontiers in Immunology, 13, 920147. 10.3389/fimmu.2022.920147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ráduly, Z. , Price, R. G. , Dockrell, M. E. C. , Csernoch, L. , & Pócsi, I. (2021). Urinary biomarkers of mycotoxin induced nephrotoxicity—Current status and expected future trends. Toxins, 13(12), 848. 10.3390/toxins13120848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeed, F. , Afzaal, M. , Niaz, B. , Rasheed, A. , Umar, M. , Hussain, M. , Nayik, G. A. , & Ansari, M. J. (2023). Quality and safety aspects of cereal grains. In Cereal grains (pp. 297–308). CRC Press. [Google Scholar]
- Silva, L. , Pereira, A. , Pena, A. , & Lino, C. M. (2020). Citrinin in foods and supplements: A review of occurrence and analytical methodologies. Foods (Basel, Switzerland), 10(1), 14. 10.3390/foods10010014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simões, L. , Fernandes, N. , Teixeira, J. , Abrunhosa, L. , & Dias, D. R. (2023). Brazilian table olives: A source of lactic acid bacteria with antimycotoxigenic and antifungal activity. Toxins, 15(1), 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tangni, E. K. , Van Hove, F. , Huybrechts, B. , Masquelier, J. , Vandermeiren, K. , & Van Hoeck, E. (2021). Citrinin determination in food and food supplements by LC‐MS/MS: Development and use of reference materials in an international collaborative study. Toxins, 13(4), 245. 10.3390/toxins13040245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vylkova, S. (2017). Environmental pH modulation by pathogenic fungi as a strategy to conquer the host. PLoS Pathogens, 13(2), e1006149. 10.1371/journal.ppat.1006149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, K. , Jia, S. , Zhang, J. , Zhang, C. , Wang, S. , Rajput, S. A. , Sun, L. , & Qi, D. (2021). Transcriptomics and flow cytometry reveals the cytotoxicity of aflatoxin B1 and aflatoxin M1 in bovine mammary epithelial cells. Ecotoxicology and Environmental Safety, 209, 111823. 10.1016/j.ecoenv.2020.111823 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Additional data for this study can be made available from the corresponding author upon request.


