Abstract
Phytobioactive compounds are plant secondary metabolites and bioactive compounds abundantly present in medicinal plants and have remarkable therapeutic potential. Oxidative stress and antibiotic resistance are major causes of present‐day ailments such as diabetes, atherosclerosis, cardiovascular disorders, cancer, and inflammation. The data for this review were collected from Google Scholar, PubMed, Directory of Open Access Journals (DOAJ), and Science Direct by using keywords: “Medicinal plants, Phytobioactive compounds, Polyphenols, Alkaloids, Carotenoids etc.” Several studies have reported the pharmacological and therapeutic potential of the phytobioactives. Polyphenols, alkaloids, terpenes, and polysaccharides isolated from medicinal plants showed remarkable antioxidant, anticancer, cytotoxic, anti‐inflammatory, cardioprotective, hepatoprotective, immunomodulatory, neuroprotective, and antidiabetic activities. This literature review was planned to provide comprehensive insight into the biopharmacological and therapeutic potential of phytobioactive compounds. The techniques used for the extraction and isolation of phytobioactive compounds, and bioassays required for their biological activities such as antioxidant, antimicrobial, anti‐inflammatory, and cytotoxic activities, have been discussed. Characterization techniques for the structural elucidation of phytobioactive compounds such as HPLC, TLC, FTIR, GC–MS/MS, and NMR have also been discussed. This review concludes that phytobioactive compounds may be used as potential alternative to synthetic compounds as therapeutic agents for the treatment of various diseases.
Keywords: alkaloids, medicinal plants, phytobioactive compounds, polyphenols, terpenoids
Phytobioactive compounds are plant secondary metabolites and have remarkable therapeutic potential. Polyphenols, alkaloids, terpenes, and polysaccharides isolated from medicinal plants showed remarkable antioxidant, anticancer, cytotoxic, anti‐inflammatory, cardioprotective, hepatoprotective, immunomodulatory, neuroprotective, and antidiabetic activities. Phytobioactive compounds may be used as a potential alternative to synthetic compounds and as therapeutic agents for the treatment of various diseases.

1. INTRODUCTION
According to the Worlds Health Organization (WHO), any plants that have therapeutic potential or which are precursors of pharmaceutical drugs are referred to as medicinal plants (Munir et al., 2014). Since the emergence of life, medicinal agents have been used for the treatment of ailments (Shahid et al., 2016). Most of the traditional medicines are obtained from plants (Ashraf et al., 2015). In spite of huge progress in pharmaceutical industry, plants are major raw materials for synthetic drugs (Shang et al., 2021). Clinical advancements have increased the value of medicinal plants by determining active principles present in them (Noreen, Hussain, & Shahid, 2020). Nowadays, the world is progressively turning toward effective herbal medicines (Aanouz et al., 2021). Synthetic drugs have not only side effects and are inadequate but are also expensive and unaffordable in developing nations (Ashraf et al., 2016).
In the last few decades, natural therapeutic compounds are explored by scientists from all across the world (Jilani et al., 2016). Ethnomedicinal survey of plants sets the basis for discovering new medicinal compounds (Khalid et al., 2016). With the passage of time, a large amount of evidence has been gathered that demonstrates the use of plants in the field of pharmacology (Radha et al., 2021). Since the appearance of life on Earth, plants are a major source of food, clothing, shelter, and medicine (Shahzadi et al., 2018; Yousaf et al., 2017). Due to their nutritive and medicinal potential, plants have been playing a vital role in human life (Yazarlu et al., 2021). From the start of life, human civilization has been using plants, mainly as medicines, and most civilizations still use them (Bano et al., 2017). Currently, 25% of pharmaceutical drugs are derived from plants (Okpuzor et al., 2021). In developing countries, about 70%–95% population uses medicinal plants to treat health issues (Liaqat et al., 2019).
As stated by WHO, 80% of the world population uses phytochemical agents for the treatment of diseases. Bioactive compounds of plants have remarkable potential to treat diseases (Hussain et al., 2018). Secondary metabolites, such as phenolics, flavonoids, alkaloids, saponins, and terpenoids produced by plants, are the constituents of plant's defense system but they have remarkable potential to treat various diseases (Aanouz et al., 2021; Anjum et al., 2020). Plant extracts have been gaining huge attention to control emerging antibacterial resistance (Abbas et al., 2020; Safdar et al., 2021). Crude plant extracts have remarkable antibacterial potential (Romano et al., 2021). Purified plant bioactive compounds are a major research topic among researchers (Lim et al., 2021; Shahid et al., 2021).
Phytobioactive compounds play a significant role in the adaptation of plants to their surrounding environment but are also a major source for the pharmaceutical industry (Merrouni & Elachouri, 2021). Phytobioactives are also known as plant secondary metabolites and bioactive compounds (Fatima et al., 2021). They are present ubiquitously in the plant kingdom and are considered nonnutritional but vital components for the maintenance of human health (Nafees et al., 2017). Phytobioactive compounds are not required for the basic metabolism of plants, are synthesized apart from the primary biosynthetic pathway, and are synthesized from the metabolites of biosynthetic routes (Riaz et al., 2017). Several of them not only play important roles for the plants such as protecting, attracting, signaling, and adapting to their environment but also represent the main source of pharmaceuticals (Jahan & Shahid, 2019; Khairan et al., 2021). The foundation of modern therapeutics is based on the use of plants and their extract in preparing herbal drugs, enabling man in establishing an empirical medicinal system (Gumisiriza et al., 2021). Thus, screening of a number of plant species is being carried out for these herbal compounds (Waseem et al., 2021).
2. HISTORY
The history of medicinal plants is as old as humankind. In the beginning, people exploited herbs for nutrition but following the discovery of medicinal attributes, medicinal plants became a beneficial source for the improvement of health among human communities (Kumar et al., 2021). Herbal products were introduced by the Romanian pharmacopeia during the 19th century by establishing the first institute of medicinal herbs in Cluj city in 1904 (Vinatoru, 2001). The herbal plants' usage in ancient times truly illustrates the history of bioactive molecules (Swargiary et al., 2021). People were unaware of plants' bioactives but the usage of these molecules is sufficiently diverse in various prospects (Zeidali et al., 2021). Typically, secondary metabolites are the bioactive compounds produced by plants. The use of different parts of plants (bark or flowers, leaves, berries, roots, and seeds) for healthcare in treating various diseases is called herbal medicine (Anwar et al., 2013).
During the late 19th century, researchers began to isolate, purify, and identify phytobioactive compounds from plants, and their efforts led them to discover vital drugs from plants that are the base of modern medicine (Tanweer et al., 2018). In this view, for the preparation of semi‐synthetic drugs, bioactive compounds isolated from medicinal plants have been modified to make them effective. In 1953, aspirin was synthesized through the structural modification of an active constituent of a number of medicinal plants, which is salicylic acid, having pain‐relieving effects (Shahid et al., 2012).
3. LITERATURE SEARCH STRATEGY
The data for this review were collected by searching databases including google scholar, PubMed, Science direct, Directory of Open Access Journals (DOAJ), Scopus, and Web of Science by using keywords: “Medicinal plants, Therapeutic potential of plants derived Bioactive compounds, Phytobioactive compounds, phytochemicals, phytoconstituents, Polyphenols, Flavonoids, Alkaloids, Terpenoids, Carotenoids, Gums etc.” Research articles and reviews published in English were only considered for this review study. Publications that extensively investigated the biological activities and therapeutic effects of bioactive compounds present in plants were included. Moreover, the published research conducted on animal models for in vitro and in vivo experimentations were also included.
4. PLANT‐DERIVED BIOACTIVE COMPOUNDS AND THEIR THERAPEUTIC POTENTIAL
Phytobioactive compounds are the compounds present in plants that provide protection against various diseases but are not of dietary importance. These compounds obtained from plants having distinct structural and functional properties are referred to as phytochemicals or secondary metabolites of plants. These are found abundantly in vegetables, grains, fruits, seeds, and nuts. These metabolites are usually classified into six large‐molecule families: phenolics, terpenes, alkaloids, saponins, glycosides, and polysaccharides based on their biosynthetic pathways. The schematic classification of phytobioactive compounds is shown in Figure 1, whereas the overview of the therapeutic potential of phytobioactive compounds is presented in Figure 2. Phytobioactive compounds have been extensively used in traditional medicines for the treatment of various diseases including type 2 diabetes. Higher antidiabetic potential of these phytobioactives has been shown in various animal models, and plant bioactive‐based medicines currently are in great demand in the market due to their multiple efficacies and higher availability. The possible mechanism of molecular action of phytobioactives for the treatment of type 2 diabetes is shown in Figure 3. There are many complications associated with type 2 diabetes, and few of the regularly used phytobioactive compounds through food have shown antidiabetic potential in a variety of ways including the reduction in drug loads during treatment (Ganesan et al., 2017). A summary of the biological/pharmacological properties of phytobioactive compounds and their action mechanism is given in Table 1.
FIGURE 1.
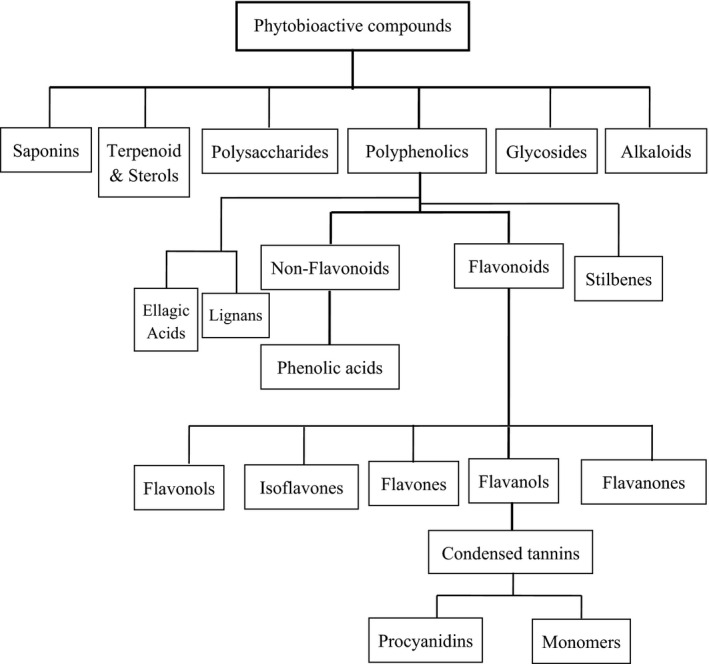
Classification of phytobioactive compounds.
FIGURE 2.
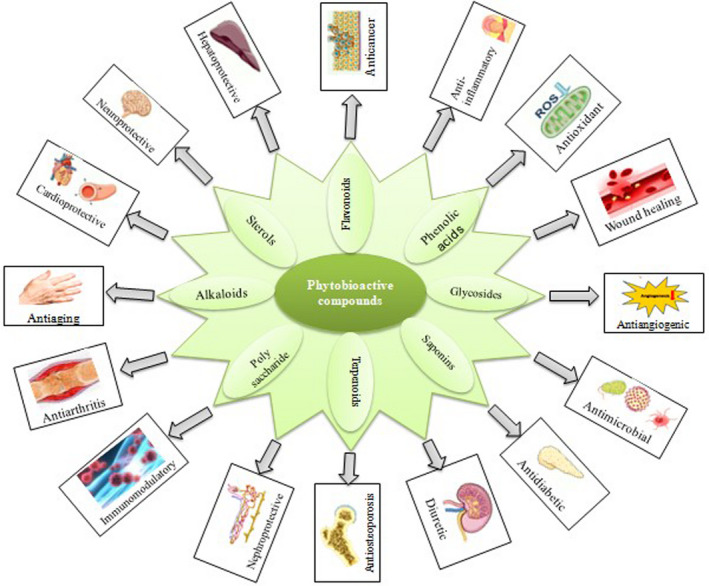
Overview of the therapeutic potential of phytobioactive compounds.
FIGURE 3.
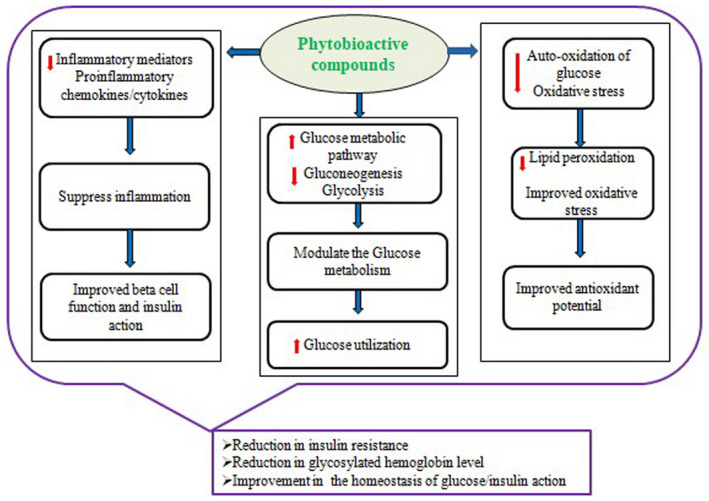
Schematic mechanism of phytobioactive compounds in treating type 2 diabetes.
TABLE 1.
Biological/pharmacological properties of phytobioactive compounds and their mechanism of action.
| Major class of bioactive compounds | Subclasses | Examples | Sources/plants | Biopharmacological activities | Action mechanism | References |
|---|---|---|---|---|---|---|
| Phenolic acids | Hydroxybenzoic acid |
Gallic acids
|
Gallnuts, witch hazel, sumac, tea leaves, bark, oak, and other plants | Induction of antiproliferation and apoptosis | Regulation of mitochondrial‐mediated pathways, induction of the activity of ROS, caspase‐3, and caspase‐9, reduction of the mitochondrial membrane potential, and the elevation of Bcl‐2‐like protein 4 | Jagan et al. (2008) |
| Induce HSC apoptosis | Regulating TNF‐α signaling pathway | Chang et al. (2015) | ||||
|
Ellagic acid
|
Berries, grapes, pomegranates, green tea, wine, walnuts, and chocolate | Anticarcinogenesis | Removal of free radicals and prevention of DNA fragmentation | Hussein and Khalifa (2014) | ||
| Prevention of inflammasome‐associated PAH | Inhibition of NLRP3 inflammasome activation | Tang et al. (2015) | ||||
| Hydroxycinnamic acid |
Chlorogenic acid
|
Hibiscus Sabdariffa leaves, peaches, and eggplants | Inhibition of HepG2 xenograft proliferation and progression | ERK1/2 inactivation and suppression of MMP‐2 and MMP‐9 expression | Yan et al. (2017) | |
| Inhibition of proliferation and profibrogenesis‐related genes in HSCs | Improvement of antioxidant capacity via Nrf2 pathway activation and suppression of profibrotic action through NOX/ROS/MAPK pathway inhibition | Shi et al. (2016) | ||||
| Attenuation of antifibrosis and liver injury | Suppression of the AGEs‐mediated induction of RAGE gene expression by stimulating GSH and increasing PPARγ | Stefanska (2012) | ||||
|
Curcumin
|
Curcuma longa | Antioxidant, anticarcinogenic, and anti‐inflammatory | Decrease oxidative damage and inhibit apoptosis in myocardium (JAK2/STAT3 signal pathway) | Sultana et al. (2021) | ||
|
6‐Gingerol
|
Curcuma longa | Antioxidative, anti‐inflammatory, and antiapoptotic | Alleviate oxidative stress to inhibit cell death (upregulate PI3K/Akt signaling pathway expression) | Zhang et al. (2017) | ||
| Flavonoids | Anthocyanins |
Delphinidin
|
Ribes nigrum L., bilberry and blueberry | Inhibit tumor growth, antiproliferation, apoptosis, and antiangiogenesis | Activation of caspase‐3/9, suppression of Bax, Akt/PI3K/MAPK, VEGFA, cyclin D1, PCNA, Bcl‐2, and EGFR | Pal et al. (2013) |
| Antiangiogenesis | Suppress Akt/mTOR/PI3K/p70S6K, ERK, HIF‐1α, CoCl2, EGF, and VEGF mRNA | Kim et al. (2017) | ||||
|
Cyanidin
|
Ipomoea batatas, Morus alba L. | Antioxidant, antifibrotic and anti‐inflammatory | Attenuate inflammation of the liver cells through the inhibition of NLRP3 inflammasome activation, lowering the cleaved IL‐1β | Wang et al. (2017) | ||
|
Malvidin
|
Ribes nigrum L. | Anti‐inflammatory | Increase the cytosolic IκB which is an NF‐κB inhibitor and inhibit nuclear translocation of NF‐κB | Bishayee et al. (2013) | ||
| Flavanols |
EGCG
|
Tea | Inhibit metastasis | Decrease the half‐life of osteopontin mRNA by reducing osteopontin | Zapf et al. (2015) | |
| Anticarcinogenesis | Regulate the self‐renewal Hh/Gli1, Wnt/beta‐catenin pathways, and related genes EGFR, cMyc, and cyclin D1 along with E‐cadherin downregulation | Sur et al. (2016) | ||||
| Induce apoptosis and antiproliferation | Receptor tyrosine kinase inhibition; PI3K/AKT downregulating; and inactivation of NF‐κB via downregulation of Bcl‐2 alpha and Bcl‐xl | Shimizu et al. (2008) | ||||
| Flavanones |
Hesperidin
|
Citrus fruits | Inhibition of metastasis | Reducing the expression of MMP‐9 via inhibiting the activated AP‐1 and NF‐κB activity by p38, JNK, and I κB signaling pathways | Yeh et al. (2009) | |
| Induce apoptosis | Regulation of mitochondrial pathway and death receptor pathway; trigger the mitochondrial pathway activation by increasing the intracellular ROS, ATP, and Ca2+ levels | Banjerdpongchai et al. (2016), Zhang et al. (2015) | ||||
|
Naringenin
|
Citrus fruits | Inhibition of invasion and metastasis | Suppress MMP‐9 transcription through the inhibition of NF‐κB and AP‐1 activity | Yen et al. (2015) | ||
|
Eriodictyol
|
Eriodictyon californicum | Induction of apoptosis | Upregulating PARP and Bax, and downregulating the Bcl‐2 protein | Wang et al. (2016) | ||
| Flavones |
Apigenin
|
Scutellaria baicalensis Georgi | Inhibition of HSC Activation | Dependent on mitochondrial‐activated Apoptosis | Akram, Riaz, Wadood, et al. (2020), Jing Li et al. (2011) | |
|
Chrysin
|
Passiflora caerulea, the passion flowers, propolis, and honey | Induce apoptosis, decrease the proliferation and cell motility | Downregulate the expression of Skp2 and LRP6; activate p53/Bcl‐2/caspase‐9 pathway | Huang et al. (2016) | ||
| Inhibit proliferation and profibrogenesis‐related genes in HSCs | Suppressing TGF‐β1/Smad pathway | Balta et al. (2015) | ||||
|
Luteolin
|
Green pepper, celery, dandelion, thyme, and parsley | Induction of apoptosis | Regulation of extrinsic and intrinsic caspases along with executioner caspases through ROS‐mediated pathway | Chen et al. (2021) | ||
| Inhibition of proliferation‐ and profibrogenesis‐related genes in HSCs | Suppress the phosphorylation of AKT and Smad pathway simulated by PDGF and TGF1; Induce G1 arrest with reduced expression of cyclin E, bcl‐2, and p‐Cdk‐2; Increase p53 expression and caspase 3 activity | Li et al. (2015) | ||||
| Flavonols |
Quercetin
|
Berries, tea, beans, broccoli, and apples | Inhibit carcinogenesis | Upregulate BAX and p53 via downregulating ROS, COX‐2, PI3K, and PKC | Maurya and Vinayak (2015) | |
| Inhibition of HSC activation | MMPs activation and the regulation of antifibrogenic/ profibrogenic molecules balance | Hernández‐Ortega et al. (2012) | ||||
|
Kaempferol
|
Rosa damascene, delphinium, grapefruit | Autophagy‐mediated cell death | ER stress–CHOP–autophagy signaling pathway | Akram, Riaz, Munir, et al. (2020), Chen et al. (2017) | ||
|
Myricetin
|
Red wine, tea, berries, nuts, fruits, and vegetables | Inhibition of HCC development | Inhibit PAK1 via coordinate abrogation of PI3K/AKT and MAPK/ERK | Iyer et al. (2015) | ||
| Isoflavonoids |
Genistein
|
Soy | Inhibition of metastasis | Reverse the epithelial–mesenchymal transition, partly mediated through nuclear factor of activated T cell 1 | Chen et al. (2021) | |
|
Puerarin
|
Root of Pueraria | Induce apoptosis | Regulating MAPK pathway | Zhang et al. (2017) | ||
| Lignans | – |
Sauchinone
|
Schisandra chinensis | Antioxidant and anti‐inflammatory | Inhibit p38 phosphorylation and signaling pathways of JNK death | Kim et al. (2012) |
|
Isovaleroylbinankadsurin A
|
Schisandra Chinensis | Antioxidative, antiapoptotic and anti‐inflammatory | Inhibit ROS generation and blocked the apoptosis (activating RISK pathway dependent on GR) | Zuo et al. (2020) | ||
| Glycosides | Stilbenes |
Resveratrol
|
Grapes, berries, and red wine | Antiangiogenesis | Inhibit the expression of VEGF via NF‐κB‐mediated mechanism | Yu et al. (2010) |
| Inhibit profibrogenesis‐ and proliferation‐related genes in HSCs | Suppress NF‐κB and Akt activation | Zhang et al. (2016) | ||||
| Inhibit the activation of HSC | Dependent on mitochondrial‐activated apoptosis | Li et al. (2011) | ||||
| Inhibit invasion and metastasis | Regulate HGF‐c‐Met signaling pathway; reduce MMP‐9 via downregulating NF‐κB signaling pathway | Gao et al. (2017) | ||||
|
Polydatin
|
Reynoutria japonica Houtt | Antioxidant and anti‐inflammatory | Promote autophagic flux clearing injured mitochondria reducing ROS and cell death | Ling et al. (2016) | ||
| Terpenes | Triterpenoid |
Asiatic acid
|
Centella asiatica | Anticancer activity | Inhibit cell proliferation, migration, and angiogenesis (inhibition of Src/FAK/ERK and PI3K/Akt signaling pathway). | Mioc et al. (2022) |
| Neuroprotective activity | Downregulate IL‐6, IL‐1β, and TNF‐α; downregulate Bax and upregulate Bcl (NF‐kB/STAT3/ERK signaling pathway inhibition). Antiapoptotic and anti‐inflammatory effects (inhibit TAK1–JNK pathway). | Lu et al. (2019), Mioc et al. (2022) | ||||
| Cardioprotective activity | Antioxidant effect (inhibit MAPK/mitochondria‐dependent apoptotic pathway); reduces myocardial hypertrophy (activation of Akt/GSK‐3β‐mir‐126‐mediated signaling pathway). | Yi et al. (2022) | ||||
| Hepatoprotective activity | Suppression of PERK/ATF6 and IRE1 pathway activation involved in the initiation of apoptosis. Regulatory factor on the Bcl‐2/Bax and PI3K/AKT/mTOR signaling pathways preventing liver fibrosis | Wang, Lao, et al. (2018), Wei et al. (2018) | ||||
| Anti‐inflammatory activity | Inhibits NF‐κB pathway activation and the nucleotide‐binding domain‐like receptor protein 3 (NLRP3) inflammasome, which mediates IL‐1β release and maturation | Yuyun et al. (2018) | ||||
|
Oleanolic acid
|
Ligustrum lucidum, Olea europaea, S. mussotii, and Olives | Anticancer activity | Inhibit cell migration (upregulation of PI3K/Akt pathway; inhibit cell proliferation; inhibition of notch signaling pathway); anti‐inflammatory activity (suppress NF‐kB signaling pathway); and induce apoptosis (activation ERK/JNK/p38 pathway) | Mioc et al. (2022) | ||
| Antidiabetic activity | Reduces inflammatory cytokines TNF‐α and IL‐6 (NF‐kB signaling inhibition); reduces proinflammatory cytokines expression (NLRP3 inflammasome inhibition); and antioxidant effect (inhibition of MAPK signaling) | Mioc et al. (2022), Swanson et al. (2019) | ||||
| Neuroprotective activity | Prevent microglial migration toward neurons and removes intracellular ROS. Decreased production of ROS and MDA Facilitate the Nrf2 signaling activation and upregulate its downstream stress response protein HO‐1 in the injured cortex | Shi et al. (2021) | ||||
|
Ursolic acid
|
Fruits, vegetables, dietary fibers, and herbs of the Lamiaceae family | Anticancer activity | Inhibit cell proliferation, survival, and angiogenesis (inhibit PI3K/Akt signaling pathway); induce apoptosis (activation of JNK signaling pathway) | Guo et al. (2020) | ||
| Antidiabetic activity | Modulate AGEs‐RAGE signaling pathway, upregulation of MMP‐2 protein levels, and inhibition of proinflammatory cytokines TNF‐α, MCP‐1, and TGF‐β1 production | Dai et al. (2021) | ||||
| Anti‐infectious Activity | Stimulate the anti‐inflammatory cytokines GM‐CSF, IL‐10, and IL12 along with the ROS and NO production in parasite‐infected immune cells. Inhibit the expression of proinflammatory cytokines IL‐1β, IL‐6, TNF‐α, and TGF‐β1 | Choi and Lee (2019) | ||||
| Anti‐inflammatory activity | Inhibit PERK activation, resulting in Bax proapoptotic protein downregulation and Bcl‐2 antiapoptotic protein upregulation (inhibit the NF‐kB and MALAT1/miR206/PTGS1 signaling pathway). | Mou et al. (2021) | ||||
| Sesquiterpene lactone |
Artemisinin
|
Artemisia annua L. | Anti‐inflammatory, antioxidant, and antitumor | Suppress NLRP3 inflammasome activation (cleaved IL‐1β, caspase‐1, decrease NLRP3, and ASC) | Wang et al. (2020) | |
| Diterpene lactone |
Ginkgolide B
|
Ginkgo leaves | Anti‐inflammatory, antioxidant, and antiapoptotic | Inhibit apoptosis induced by ER stress via PI3K/AKT/mTOR signaling pathway | Guo et al. (2019) | |
| Alkaloids | – |
Berberine
|
Berberis vulgaris L. and Coptis Chinensis | Antiapoptotic and anti‐inflammatory | Promote proliferation, attenuate apoptosis through mitophagy‐mediated HIF‐1α/BNIP3 pathway | Zhu et al. (2020) |
|
Capsaicin
|
Capsicum annuum L. | Antioxidant and antiapoptotic | Attenuate ROS production, inhibit the opening of mPTP and activation of caspase‐3, downregulation of Bax, upregulation of 14–3‐3η and Bcl‐2, and eventually decreased apoptosis | Huang et al. (2018) | ||
|
Matrine
|
Sophora flavescens Aiton | Antitumor and anti‐inflammatory | Upregulates HSP70 expression and activates JAK2/STAT3 pathway | Guo et al. (2018) | ||
| Saponins | – |
Polyphyllin I
|
Paris polyphylla | Anticancer effect | Inhibit inflammatory responses and oxidative stress (NF‐κB, p65 signaling pathway) | Huang et al. (2020) |
|
Platycodin D
|
Platycodon grandiflorus | Antioxidant, anti‐inflammatory, and antiapoptotic | Inhibit apoptosis and oxidative stress (induce Akt/Nrf2/HO‐1 pathway activation) | Wang, Che, et al. (2018) | ||
|
Gypenoside A
|
Gynostemma pentaphyllum | Anti‐inflammatory, antioxidative, and antitumor | Suppress miR‐143‐3p through AMPK signaling activation | Chang et al. (2020) | ||
|
Ginsenoside Rb3
|
Panax ginseng C. A. Meyer | Antiapoptotic | Inhibit apoptosis (activation of JNK/NF‐κB signaling pathway) | Ma et al. (2014) | ||
| Polysaccharides | – |
Fucoidan
|
Species of brown algae (Phaeophyta) | Anticancer | Block P‐selectin‐mediated neutrophil rolling on the vessel wall | Omata et al. (1997) |
| Carotenoids | – |
Lycopene
|
Tomatoes and tomato products, apricots, watermelon, and guava | Antioxidant, anti‐inflammatory, and cardioprotective | Inhibit inflammation and accumulation of ROS (JNK signaling pathway) | Przybylska and Tokarczyk (2022) |
| Coumarin | – |
Osthole
|
Angelica pubescens Maxim and Cnidium monnieri (L.) Cusson | Antioxidant, antiapoptotic, and anti‐inflammatory | Exerted anti‐inflammatory and antioxidant effect (inhibit the IκB‐α/NF‐κB signaling pathway and HMGB1 expression) | Wang et al. (2013) |
Abbreviations: AGEs: Advanced glycation end products; AMPK: AMP‐activated protein kinase; ATF6: Activating transcription factor 6; BNIP3: BCL2/adenovirus E1B 19 kDa protein‐interacting protein 3; COX‐2: Cyclooxygenase‐2; EGF: Epidermal growth factor; EGFR: Endothelial growth factor receptor; ERK: Extracellular signal‐regulated kinase; ERK: Extracellular signal‐regulated kinase; GM‐CSF: Granulocyte–macrophage colony‐stimulating factor; GSH: Glutathione reductase; HCC: Hepatocellular carcinoma; HGF: Hepatocyte growth factor; HIF‐1α: Hypoxia‐inducible factor 1‐alpha; HMGB1: High‐mobility group box protein 1; HSC: Hepatic stellate cells; HSP70: Heat shock protein 70; IL‐1β: Interleukin 1 β; IL‐6: Interleukin‐6; IRE1: Inositol‐requiring enzyme 1; JAK2: Janus kinase 2; JNK: Jun N‐terminal Kinase; LRP6: LDL receptor‐related protein 6; MAPK: Mitogen‐activated protein kinase; MAPK: Mitogen‐activated protein kinase; MCP‐1: Monocyte chemoattractant protein‐1; MDA: Malondialdehyde; MMP‐2: Matrix metallopeptidase 2; MMP‐9: Matrix metallopeptidase 9; mTOR: mechanistic target of rapamycin; NF‐κB: Nuclear factor kappa B; NLRP3: Nucleotide‐binding oligomerization domain, leucine‐rich repeat‐containing gene family, and pyrin domain‐containing 3; NOX: Nitrogen oxides; Nrf2: Nuclear factor‐erythroid factor 2‐related factor 2; p70S6K: phosphoprotein 70 ribosomal protein S6 kinase; PAH: Pulmonary artery hypertension; PAK1: P21‐activated kinase 1; PARP: poly‐ADP ribose polymerase; PCNA: Proliferating cell nuclear antigen; PERK: Protein kinase‐like endoplasmic reticulum kinase; PI3K: Phosphoinositide 3‐kinase; PKC: Protein kinase C; PPARγ: Peroxisome proliferator‐activated receptor gamma; PTGS1: Prostaglandin‐endoperoxide synthase 1; RAGE: Receptor for advanced glycation end products; RISK pathway: Reperfusion injury salvage kinase pathway; ROS: Reactive oxygen species TNF‐α: Tumor necrosis factor α; STAT3: Signal transducer and activator of transcription 3; TGF‐β1: Transforming growth factor beta 1; VEGF: Vascular endothelial growth factor; VEGFA: Vascular endothelial growth factor A.
4.1. Polyphenols
Polyphenols are plants' secondary metabolites or the plants' nonnutritional natural products found in fruits, seeds, and vegetables. Polyphenols are common in the plant kingdom and are a large family of bioactive metabolites derived from secondary metabolism. Most of the polyphenols are derived through phenylpropanoid pathway from l‐phenylalanine. These are characterized by the presence of one or more phenolic groups that show highly diverse structures, of which, flavonoids, phenolic acids, lignans, stilbenes, tannins, and coumarins are the major structural type of polyphenols (Quideau et al., 2011). Some of the polyphenols are responsible for the color, aroma, and antioxidant properties of nuts, vegetables, fruits, and seeds that we consume in our daily life. The importance of polyphenols is increasing particularly because of their health benefits. Their antioxidant role in preventing and treating various diseases is increasing particularly against oxidative stress, cancer, cardiovascular, inflammatory, neurodegenerative, and age‐related degenerative diseases (Hano & Tungmunnithum, 2020). They have a broad range of uses as pharmaceuticals, food supplements, and cosmetic additives. Identification of more than 8000 polyphenols has been carried out from plants, and several hundred have been characterized from grains, vegetables, and fruits (Shen et al., 2017). Various classes of polyphenols are shown in Figure 1 and are described below.
Polyphenols play an important role to prevent carcinogenesis leading to the decreased incidence of some cancers. They have widespread medicinal applications with reduced toxicity and hence, can be used as chemopreventive agents. Any of the endogenous or exogenous carcinogens, tumor promoters, and inflammatory cytokines can take part in the activation of carcinogenesis through the regulation of transcription factors, AP‐1 and NF‐kB; proapoptotic proteins, PARP and caspases; the protein kinases, MAPK, c‐Jun N‐terminal kinase (JNK), and IκB kinase (IKK); growth factor signaling pathways; and the cell cycle proteins, CDK and cyclins (Shen et al., 2017). During the initiation, carcinogens cause damage by interacting with cellular DNA, therefore, blocking the damage caused by genotoxic chemicals can be an effective approach for the prevention of cancer that might be achieved through the elimination of ROS and induction of phase II conjugating enzymes. In the next stage, inhibition of cell proliferation is a useful strategy, such as through the modulation of apoptosis and cell cycle arrest. In progression step, prevention of malignant cells' progression to metastasis and invasiveness and/or the interruption of angiogenesis are particularly important (Ramos, 2008; Shen et al., 2017). Evidence indicates that polyphenols of dietary importance play a protective role in a multistep process of carcinogenesis through phase I and phase II enzymes, cell proliferation, cell cycle progression, DNA repair, metastasis, angiogenesis, and apoptosis (Shen et al., 2017). The possible action mechanisms through which the carcinogenic processes are inhibited by polyphenols are illustrated in Figure 4.
FIGURE 4.
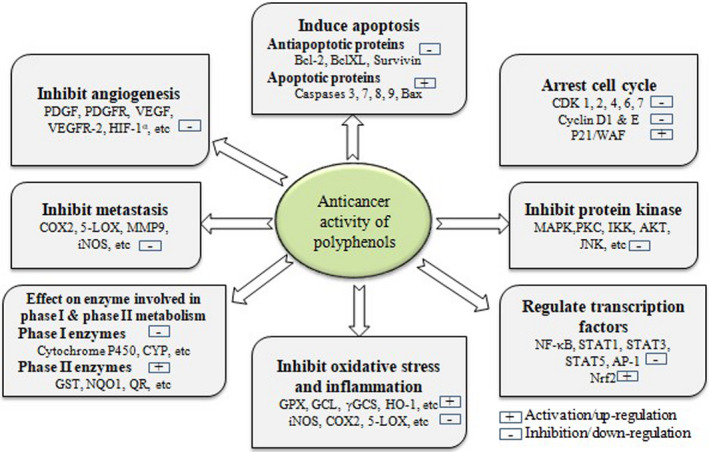
Molecular mechanism of the anticancer activity of polyphenolic compounds. 5‐LOX, lipoxygenase; AP‐1, activator protein‐1; CDK, cyclin‐dependent kinase; COX2, cyclooxygenase 2; CYP, cytochrome P; GCL, glutamate cysteine ligase; GPX, Glutathione peroxidase; GST, glutathione S‐transferase; HIF‐1 α, hypoxia‐inducible factor 1α; HO‐1, hemeoxygenase‐1; IKK, IκB kinase; iNOS, inducible nitric oxide synthase; JNK, c‐Jun N‐terminal Kinase; MAPK, mitogen‐activated protein kinases; MMP9, matrix metalloprotease‐9; NF‐ κB, nuclear factor‐κB; NQO1, nicotinamide adenine dinucleotide phosphate, quinone oxidoreductase; Nrf2, NF‐E2‐related factor 2; PDGF, platelet‐derived growth factor; PDGFR, PDGF receptor; PKC, protein kinase C; QR, quinone reductase; VEGF, vascular endothelial growth factor; VEGFR, VEGF receptor; γGCS, γ‐glutamylcysteine synthetase.
Polyphenols scavenge the excess free radicals (ROS) inhibiting the oxidative stress through interaction with protein kinase‐like endoplasmic reticulum kinase (PERK) dependent on RNA, phosphatidylinositol 3‐kinase (PI3K), protein kinase C (PKC), and mitogen‐activated protein kinases (MAPKs) as these kinases upregulate Nrf2 through phosphorylation. Polyphenols have the capability to suppress the activation of NF‐κB and inhibit the expression of COX2, interleukin (IL)‐1β, and inducible nitric oxide synthase (iNOS), hence decreasing the adoptive cellular responses (Shen et al., 2017). Polyphenols like curcumin, caffeic acid, quercetin, EGCG, and resveratrol have been reported to inhibit oxidative stress. EGCG enhances the antioxidant capacity of cells through MAPK protein activation. Quercetin increases the antioxidant enzymes expression such as γ‐glutamylcysteine synthetase (γGCS), glutathione peroxidase (GPX), and superoxide dismutase (SOD), upregulates glutathione reductase (GSH) level, enhances the stabilization of Nrf2, and promotes the transcription activity mediated by Nrf2. Quercetin also inhibits the activation of NF‐κB. Resveratrol inhibits oxidative stress through the upregulation of γGCS and hemoxygenase‐1 (HO‐1) expression and increases the Nrf2 transcriptional activity (Csiszar et al., 2011; Shen et al., 2017). EGCG reduces the cyclin D1 expression, induces the expression of p27Kip1 and p21waf1/Cip1 genes, and inhibits CDKs 2 and 4 thereby blocking the progression of cell cycle MCF‐7 at G1 phase. Many polyphenols such as curcumin, ellagic acid, apigenin, resveratrol, EGCG, and quercetin induce apoptosis to inhibit carcinogenesis (Shen et al., 2017). Quercetin, theaflavin, catechin, curcumin, and resveratrol through AP‐1 inhibition can inhibit tumor metastasis. Polyphenols including ellagic acid, genistein, quercetin, resveratrol, curcumin, and ECGC can act as inhibitors of NF‐kB (Aggarwal & Shishodia, 2006).
4.1.1. Phenolic acids
Phenolic acids are characterized by the presence of one or more OH groups linked to benzene or any other aromatic ring. Phenolics are responsible for different color development in the plants, help in pollination, and protect plants from pathogenic attack (Ayub et al., 2017). Vegetables, fruits, wine, coffee, and tea are the richest sources of phenolic compounds. Distribution of phenolic compounds is not uniform in plants. Polyphenols are fundamental in ensuring the reproduction, development, and growth of plant (Den et al., 2019). Phenolics have been classified on the basis of the pattern of their basic skeleton into six different classes which were further subdivided into family in order to hierarchize their differences (Naz et al., 2016). The increasing complexity of basic skeleton of the members characterized the first five classes while the sixth class includes a separate group of compounds called hybrid phenolics (Sharif et al., 2018).
Phenolic compounds have important properties, including antimicrobial activity, stabilization of ascorbic acid, inhibition of lipid peroxidation, and carcinogenesis (Sharif et al., 2018). Flavonoids are found in garlic and are known to be helpful in reducing cholesterol level, coronary thrombosis, atherosclerosis, and a variety of other life‐threatening diseases (Ali et al., 2021). The radical‐scavenging activity of flavonoids is ascribed to their capacity of donating hydrogen. Flavonoids contain the phenolic groups which act as a source of hydrogen atoms readily available (Tanweer et al., 2018). Flavonoids have the potential to inhibit fluids that caused diarrhea by targeting the intestinal cystic fibrosis transmembrane regulator (Ahmad et al., 2021). The therapeutic functions of flavonoids comprise protection against allergies, platelet aggregation, free radicals, inflammation, ulcers, microbes, viruses, and tumors (Abbas et al., 2012). Due to physiological, pharmaceutical, and ecological roles, phenolic compounds have been explored and manipulated extensively (Hussain et al., 2018).
Bioavailability of phenolic compounds is limited due to their less water solubility and instability at low pH values (Malode et al., 2021). The bioavailability of polyphenols can be increased by using nanoparticles (Irshad et al., 2020). One of the flavonols called quercetin belongs to flavonoids ubiquitously present in vegetables and fruits. Several in vitro and in vivo studies have demonstrated the antioxidant and anti‐inflammatory potential of quercetin (Afzal, Shahid, & Jamil, 2014). Quercetin has antioxidant activity due to its potential to scavenge free radicals, metal cation chelating ability, and hydrogen atoms or electrons donating ability (Irshad et al., 2017). Quercetin and its glycosidic metabolites modulate the biological processes due to its antioxidant properties and these processes include the reduction of oxidative deoxyribonucleic acid (DNA) damage and cell signaling pathways (Sohaib et al., 2017).
Onion shells (Allium cepa) contain a large amount of quercetin (3, 5, 7, 3′, 4′‐pentahydroxy flavones), a natural dye that imparts yellow‐reddish brown color, exhibiting antioxidant, anticarcinogenic, and good dyeing potential (Rehman et al., 2013). Dietary quercetin can prevent the oxidative deterioration of low‐density lipoproteins (LDL) due to its ability to chelate metal ions produced through lipid oxidation and scavenging of free radicals (Sohaib et al., 2015). Honey showed remarkable antioxidant and antitumor activity (Afzal, Shahid, Iqbal, & Hussain, 2014). The phenolics and flavonoids isolated from honey possessed antioxidant and antitumor properties as immunotherapeutic agents (Noor et al., 2014).
Contrary to this, simple phenols (e.g., catechol, hydroquinone, eugenol, p‐anisaldehyde, and phloroglucinol), the C6‐C3 phenylpropanoids, and their derivatives (myristicin, ferulic acid, cinnamic acid, caffeic acid, and sinapyl alcohol), the C6‐C1 benzoic acids (vanillic acid gallic acid and protocatechuic acid), coumarins (dicoumarol, scopoletin, and warfarin), hydrozable tannis (ellagitannins and gallotannins), lignans, and related compounds all belong to nonflavonoid phenolic compounds (Irshad et al., 2018). Phenolic compounds such as flavonoids are present in all plant parts and have remarkable biological activities due to their redox potential (Khan et al., 2012).
Phenolic compounds have shown significant glucosidase inhibition potential which can be used to treat type 2 diabetes mellitus. Cholinesterase inhibition potential has been used for the treatment of Alzheimer's disease (Rahman et al., 2021). Inflammation is a natural biochemical reaction of the body in response to infections and injuries caused by physical and chemical stimuli (Soulimani et al., 2021). The immune system responds to harmful stimuli by generating proinflammatory mediators, but moderate production of proinflammatory mediators leads to many lethal diseases. Phenolic compounds work in tandem with nonsteroidal anti‐inflammatory drugs (NSAIDs) to inhibit the gene expression or activity of proinflammatory mediators, including cyclooxygenase (COX). Phenolic compounds inhibit the excessive production of proinflammatory cytokine which leads to asthma and cancer (Devi et al., 2015). Phenolic compounds have also been demonstrated to treat skin diseases due to their anti‐inflammatory properties (Działo et al., 2016), rheumatoid arthritis (Nguyen et al., 2019), and inflammatory bowel disease (Singh & Easwari, 2021).
Phenolic compounds have the ability to treat enzyme‐linked human diseases such as neurodegenerative diseases and hypertension. The angiotensin‐converting enzyme (ACE) inhibition by phenolic compounds has been used to treat hypertension. Patten et al. reported the angiotensin‐converting enzyme (ACE) inhibition effect on 74 plant families (Patten et al., 2016). Polyphenols isolated from cocoa showed remarkable antihypertensive properties via ACE inhibition (Sari et al., 2020).
4.1.2. Flavonoids
Flavonoids are widely distributed polyphenols mainly present in plant‐based foods and beverages. These are generally found in grapes, cherries, berries, plums, and crane berries and more than 8000 flavonoids have been identified. Two main categories of flavonoids are anthocyanins and anthoxanthins. Anthocyanins are responsible for plant and fruit color while anthoxanthins are colorless molecules that are further classified as flavones, isoflavones, flavanols, and flavanones. Anthocyanins, kaempferol, hesperidin, naringenin, myricetin, and quercetin are the chief flavonoids that have particular impact on human health due to their biological activities. Flavonoids possess anticancer activity and preventive potential against cell destruction due to oxidation processes. These compounds have been reported as immunomodulatory agents and have beneficial effect on the immune system (Khairan et al., 2021).
Flavanols, particularly monomeric flavanols, including catechin, gallocatechin, epigallocatechin, epicatechin, and proanthocyanidin (polymerization product of flavanols), are found in significant concentration in chocolate and cocoa powder, grapes, and teas. Thearubigin and theaflavin are present in significant concentrations in black tea. The most readily absorbable flavonoids are catechins because of their existence as unbound form and the only flavonoid not bound to sugar. Catechin and epicatechin which are found in high concentration in grape and grape juices are capable of reducing glutamate excitotoxicity and exerting powerful antioxidant activity by reducing the oxidation of low‐density lipoprotein (LDL) and platelet aggregation and ameliorating endothelial function (Mecocci et al., 2014). Among flavonols, quercetin along with myricetin and kaempferol is probably the most abundant flavonol in capers, green teas, apples, and onions with promising antioxidant potential. Quercetin has been considered beneficial for the treatment of neurodegenerative diseases such as Alzheimer's disease (AD) due to its impact on multiple action mechanisms related to these disorders. Isoflavones, such as genistein, puerarin, glycitin, and daidzein, present in increased concentration in soybeans have beneficial activity to prevent cognitive decline and maintain normal brain function which is still debatable. Luteolin, a flavone from the class of flavonoids, found in rosemary, celery, and parsley has demonstrated activity against neurodegenerative diseases (Bonetti et al., 2017).
4.1.3. Lignans and stilbenes
Lignans belong to the wide class of phenolic compounds found in higher plants and are derived from dibenzylbutane as well as produced in vivo from human gut microorganisms. These are phytoestrogens present in legumes, cereals, vegetables, and fruits as glycosides and bio‐oligomers aglycones. The richest sources of lignans are sesame seeds, flaxseed, and linseeds. The most common lignans are syringaresinol, pinoresinol, sesamolin, sesamol, sesamin, matairesinol, secoisolariciresinol, 7‐hydroxymatairesinol, and lariciresinol. Lignans have antifeedant, antiviral, antioxidants, and insecticidal properties (Banwo et al., 2021).
Resveratrol is the most widely studied stilbene known to have cardioprotective and anticarcinogenic activities. Resveratrol is found in berries, grapes, vine, and peanuts. The cardioprotective function of resveratrol stilbene is achieved by the prevention of vascular smooth muscle cell proliferation (Delgado et al., 2019).
4.2. Alkaloids
Plant alkaloids are one of the largest groups of natural entities, representing a diverse group of chemical products. The term alkaloid was introduced for the first time by W. Meisner in the early 19th century to name the natural substances behaving like bases. There is no precise definition of alkaloids, and it is difficult to differentiate alkaloids from other natural metabolites containing nitrogen as an essential component (Fatima et al., 2020). Alkaloids are defined as nitrogen‐containing nonnucleosidic and nonpeptidic compounds. Alkaloids are organic bases that have nitrogen‐containing heterocyclic rings and majority of them possess definite pharmacological activities (Bari et al., 2012).
Alkaloids have a 3000 years golden history in human medicine used as laxative, astringent, and sedatives for snake bites, fever, and insanity. About 5500 alkaloids are known, comprising the largest class of phytobioactive compounds, and are widely distributed (Mehmood et al., 2012). They are known to have therapeutic potential and are used as medicational and recreational drugs. Most of the alkaloids are very toxic and bitter in taste which makes them useful for plants to be used as defensive agents against invertebrate pest attacks, microbial pathogens, and herbivores (Khadka et al., 2021). Several medicinal plants containing alkaloids have been manipulated by human beings as pain relievers (Iqbal et al., 2013).
Alkaloids form salts with organic acids and mineral acids. Alkaloid salts are usually soluble in dilute alcohols and water while rarely insoluble in organic solvents. They are classified according to their chemical nature and natural sources. Alkaloids are commonly classified according to the distribution of their C–N skeleton into the following large groups, such as diterpenoid, steroidal, quinazoline, indole, isoquinoline, quinoline, pyridine, pyrrolidine, and other alkaloids (Hafiz et al., 2018).
Cytotoxicity, carcinogenic or mutagenic activity, antifungal, antiviral, and antibacterial activities, and their possible roles as phytoalexins have been reported. Many alkaloids are highly toxic and may lead to the death of animals if eaten. Several alkaloids such as anabasine and nicotine are used as insecticides (Jahangir et al., 2018). Plants containing berberine alkaloids are known to be used as antiseptics, analgesics, antistomatitis, and sedatives in Chinese folk medicine. In Islamic and Indian folk medicine, such plants are used for eye diseases and bleeding disorders, and as uterine muscle depressants, sedatives, and antiseptics. Both quaternary and their tetra‐alkaloids derivatives possess several biological and therapeutic properties, for example, tetrahydropalmatine, jatrorrhizine, and palmatine have been reported for their in vitro antimalarial activity (Adhikari et al., 2021). In China, tetrahydropalmatine has been tabulated to exhibit sedative, hypotensive, and bradycardial activities and is used as an analgesic. About 40% of modern drugs are derived from natural sources and a wider pharmacological potential is exhibited by alkaloids, especially isoquinoline (Afzal et al., 2018).
Alkaloids have remarkable ability to inhibit animal and human coronaviruses (Khan et al., 2018). Songorine (C20 diterpenoid alkaloid) and its derivatives possess several therapeutic effects including anti‐inflammatory, antiarrhythmic, anticardiac fibrillation, anxiolytic effects, excitation of synaptic transmission, antinociceptive and antiarthritic effects (Fielding et al., 2020). Neurodegenerative diseases are characterized by neuron deterioration which is affecting millions of people worldwide. Currently, pharmaceutical approaches are unable to cure or even halt the neurodegenerative disease progression. For the last two decades, much attention has been paid to the antineurodegenerative and neuroprotective properties of natural compounds with high bioavailability. Several types of research have reported the antineurodegenerative properties of berberine, an isoquinoline alkaloid isolated from medicinal herbs. Berberine has the inhibitory potential against numerous pathogenic enzymes, triggers autophagy, attenuates neuroinflammation, and protects neurons against apoptotic cell death (Fan et al., 2019). Berberine has also shown promising therapeutic potential, including anticancer, anti‐inflammatory, analgesic, antioxidant, cardioprotective, antihyperlipidemic, antidiabetic, antimicrobial, antinociceptive, antidepressant, memory enhancement, and cholesterol‐lowering effects. It also has the ability to lower lipid–glucose levels, hence can be used as antiatherosclerotic and anti‐Alzheimer's disease (Singh et al., 2019).
4.3. Terpenes
Terpenes, also called terpenoids or isoprenoids, constitute the largest class of natural products with over 55,000 known compounds. These chemicals constitute the secondary metabolism of animal and vegetal species resulting from C5 isoprene units connected in a head‐to‐tail fashion via the intermediates mevalonic acid. Typical structures containing carbon skeletons are classified as tetraterpenes (C40), triterpenes (C30), sesterterpenes (C25), diterpenes (C20), sesquiterpenes (C15), monoterpenes (C10), and hemiterpenes (C5; Noreen, Zia, et al., 2020).
Terpenes are widely applied in the industrial sector as spices, fragrances, and flavors and are used in scents, cosmetic products, and as food additives. They are active components of drugs in pharmaceutical industry (Shahid et al., 2020). Increasing interest in the therapeutic application of alkaloids among these pharmaceuticals, the antimalarial (artemisinin) and anticancer (paclitaxel) drugs are two of the well‐known terpene‐based drugs (Ashraf et al., 2020).
Monoterpenes in essential oils have the ability to damage microbial cells and inhibit microbial growth (Aslam et al., 2011). Terpenes have remarkable anti‐inflammatory potential, it perform anti‐inflammatory activity by inhibiting inflammatory pathways associated with numerous diseases (Riaz et al., 2017). Terpenes isolated from turmeric species (Curcuma sp.) showed anti‐inflammatory properties, both in vitro and in vivo analyses (Tanweer et al., 2018). Terpenes have enzyme inhibition activities as they have potential to inhibit lipoxygenase and proteinase, principal enzymes of inflammatory pathways (Irshad et al., 2018). Terpenes inhibit oxidative stress by inhibiting the catalytic activity of enzymes involved in reactive oxygen species generation and by forming metal‐ion chelates. Camphor is a monoterpene widely used in cosmetics, pharmaceutics, and the food industry (Bhatti et al., 2013).
Terpenoids inhibit apoptosis of hepatic cells through the inhibition of cytosolic release of cytochrome c, reducing the Bax/Bcl‐2 ratio, and by the inhibition of I‐κB, ERK, and JNK phosphorylation. They are also involved in the management of disorders caused by obesity, such as insulin resistance, type‐2 diabetes, and hyperlipidemia (Chatterjee et al., 2019). Antioxidant properties of terpenoids are also associated with their hepatoprotective activity (Ielciu et al., 2021). Terpenoids are supposed to be a safe and promising agent for the treatment of diabetes (Panigrahy et al., 2021). The antimalarial activity of terpenoids demonstrated a similar action mechanism to that of chloroquine (a pharmaceutical drug). Curcumin is widely used for the treatment of various diseases such as anti‐inflammatory, antioxidant, anticancer, antiplasmodial, antiseptic, and anticancer (Cox‐Georgian et al., 2019). A schematic molecular mechanism of the anti‐inflammatory activity of terpenes is shown in Figure 5.
FIGURE 5.
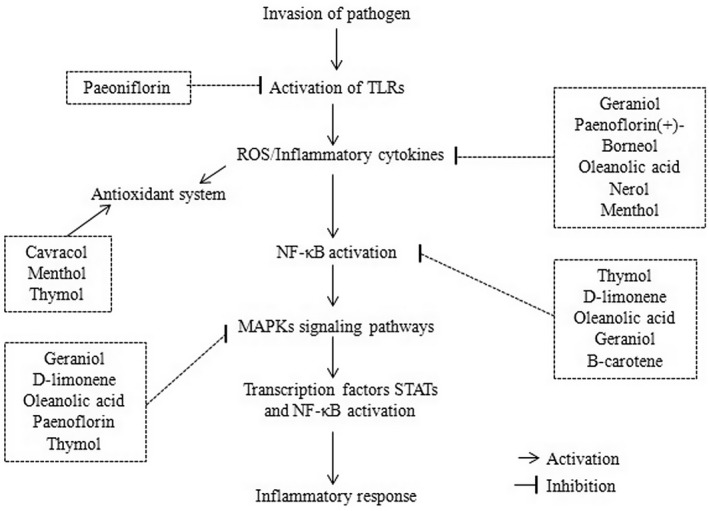
Possible molecular mechanism of anti‐inflammatory activity of plant terpenes.
4.4. Saponins
Saponins are known as surface‐active compounds and are widely distributed in the plant kingdom. Saponins include five‐ring triterpene saponins and four‐ring triterpene saponins which are reported to have cardioprotective potential through diverse mechanisms involving calcium homeostasis, regulation of energy metabolism, and the inhibition of inflammation and oxidative stress (Chen et al., 2017). A steroidal saponin called polyphyllin I (PPI) is extracted from Paris polyphylla roots. PPI is anticancer and cardioprotective. Its anticancer activity is through the inhibition of tumor cell growth and proliferation. It activates NF‐κB and reduces oxidative stress and inflammation resulting in decreased myocardial death (Huang et al., 2020). Platycodin D is another saponin found in Platycodon grandiflorus, which has antioxidant potential and anti‐inflammatory and antiatherosclerotic activities (Wang, Che, et al., 2018). Gypenoside A from Gynostemma pentaphyllum possesses anti‐inflammatory, antioxidative, and antitumor activities (Chang et al., 2020). Ginsenoside Rb3 is a saponin mainly found in Panax ginseng C. A. Meyer plant which is antiapoptotic inhibiting apoptosis through the activation of NK/NF‐κB signaling pathway (Ma et al., 2014).
4.5. Polysaccharides
Polysaccharides are generally found in vegetables and fruits. Several studies reported the cardioprotective potential of polysaccharides through various mechanisms like anticancer, antioxidative stress, anti‐inflammatory, immunomodulatory, and regulating metabolism. The biologically important polysaccharides are gums and fucoidan (Chen et al., 2021). The possible mechanism of polysaccharides action is shown in Figure 6.
FIGURE 6.
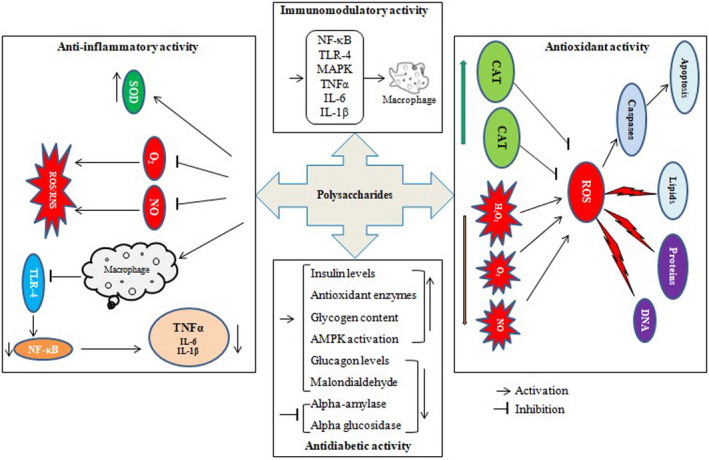
Possible action mechanism of plant polysaccharides as antioxidant, anti‐inflammatory, antidiabetic, and immunomodulatory agents. Plant polysaccharides increase the endogenously produced antioxidant enzymes like catalase and superoxide dismutase levels, whereas significantly reduce ROS‐induced free radicals, which are responsible for the destruction of important biomolecules found in body tissues that lead to apoptosis. These polysaccharides are primarily involved in the downregulation of toll‐like receptor 4 (TLR‐4) and NF‐κB, a nuclear transcription factor involved in inflammatory diseases by triggering the inducible NO synthase, TNFα, IL‐6, and IL‐1β. Antidiabetic activity of plant polysaccharides has been shown through various mechanisms including antioxidative and anti‐inflammatory properties by alleviating β‐cell dysfunctioning, increasing insulin secretion, and inhibiting the α‐amylase and α‐glucosidase activity to improve glucose metabolism. Plant polysaccharides exhibit immune‐modulatory activity by inducing ROS and cell proliferation and increasing cytokines and macrophages phagocytosis.
Gums are polysaccharides obtained through extraction or natural exudation from different plant parts (Naqvi et al., 2011). Different monosaccharide units are linked together through glycosidic linkages forming the gums (Hussain, Ali, et al., 2015; Hussain, Bakalis, et al., 2015). On the basis of structure, composition, behavior, and production differences, gums have been classified into subcategories (Munir et al., 2015), broadly, exudates gums (synthesized by plants for protection against microbial attacks or in response to mechanical injury and process is named as gummosis) and nonexudate gums (artificially procured from plant by suitable extraction method). Gums have remarkable applications at commercial scale (Munir et al., 2016).
Plant gums possess several applications in food industry. Their use in food products to improve quality has been reported in the literature (Munir et al., 2017). However, in addition to numerous functionalities, gums have also been known for their health benefits (Ayub et al., 2018). Gums act as dietary fiber. Consumption of dietary fibers contributed to decreased risk of cardiac issues, promotes immunity and satiety, and contributes to the management of body weight (Irshad et al., 2011). Several studies have documented the role of gums as bioactive such as antioxidant and scavenging activity against free radicals which are necessary for decreasing oxidative stress (Ullah et al., 2019).
Guar gum like other natural gums is hydrophilic nontoxic, economical, and easily available. Being biodegradable has several applications in pharmaceutical, textile, and food industries. Due to biodegradation, it is less stable and cannot be used in its natural form. Surface modification via grafting enhances its uses as grafted gum is an important medium for controlled drug release (Shahid et al., 2013). Crude, purified, and hydrolyzed guar gum characterized through TGA, XRD, FTIR, and SEM demonstrated that hydrolyzed and grafted gums are more crystalline and increase thermal stability (Anjum et al., 2014; Bukhari et al., 2014).
5. EXTRACTION OF BIOACTIVE COMPOUNDS
Considering the variations among phytobioactive compounds and variety of plant species, it is essential to formulate a standard technique for the screening of phytobioactives from plants extract. Different extraction techniques have been used for the selective extraction of bioactive compounds from natural sources (Abbas et al., 2014). Most of the techniques used for bioactive extraction remain almost same through hundreds of years (Hussain, Ali, et al., 2015). All these assays have common objectives such as (a) extraction of target phytobioactives from plants extract, (b) enhancing the analytical bioassay selectivity, (c) enhancement of the sensitivity of bioassay, (d) conversion of phytobioactives into a more suitable form for detection and separation, and (e) the establishment of a strong and reproducible method that is independent of variations in the sample matrix (Iqbal et al., 2015).
Extraction techniques of phytobioactive compounds depend on the nature of the solvents used for extraction, mixing, and application of heat (Jabbar et al., 2012). Extraction techniques are classified into two major groups such as conventional extraction techniques (maceration, Soxhlet extraction, and hydrodistillation) and unconventional extraction techniques (Zia et al., 2012). New and promising techniques for phytobioactive compound extraction are introduced to overcome the limitations of conventional extraction methods. Some of the new and promising extraction techniques are microwave‐assisted extraction, enzyme‐assisted extraction, ultrasound‐assisted extraction, supercritical fluid extraction, pulsed electric field‐assisted extraction, and pressurized liquid extraction (Afzal, Shahid, Iqbal, & Hussain, 2014; Afzal, Shahid, & Jamil, 2014).
Microwave‐assisted extraction (MAE) uses electromagnetic waves that generate heat by penetrating inside the matrix resulting in subsequent cell wall destruction. The heat generated through microwave exposure causes cell wall swelling by inducing an increase in pressure and temperature of water vapor inside the cell, consequently releasing the intracellular compounds. Ultrasound‐assisted extraction (UAE) is capable of improving the transfer of heat and mass by rupturing the plant cell walls due to cavitation effect. Supercritical fluid extraction (SFE) works in two ways i‐e extraction and separation. In the first stage, fluid is brought to the required temperature by compressing to the required pressure and then diffuses into the sample to dissolve the soluble material and transfer the fluid into the next section for separation. In the separation stage, temperature and pressure are readjusted to reduce the solubility of supercritical fluid resulting in precipitation of the analytes (Carpentieri et al., 2021).
6. BIOASSAYS FOR PHYTOBIOACTIVE COMPOUNDS
After a plant has been identified as a medicinal plant, the bioactive compounds are detected by using so‐called “Bioassays” (Zahid et al., 2016). There are several procedures available for in vitro and in vivo screening of plant extract. In this process, successive extraction of medicinal plants is carried out using solvents of increasing polarity and tested by a range of bioassays according to the activity of bioactive molecules (Ali et al., 2016). When the bioactivity has been identified, combination of chromatographic methods has been performed for further separation (Rehman et al., 2013). After obtaining a pure compound, a variety of spectroscopic methods like MS, IR, and NMR are used for the structural elucidation, and X‐ray crystallography is used in certain cases (Riaz et al., 2017). After toxicity study, clinical trial is the last, but the most important part to determine and compare the efficacy and doses of the new medicinal product to the already available products in the market (Rubab et al., 2016).
6.1. Antioxidant assays
Antioxidants are molecules that have the ability to scavenge free radicals produced during oxidative stress (Ashraf et al., 2016). All organisms have natural defense systems that scavenge free radicals but present‐day lifestyle causes overproduction of free radicals which are responsible for oxidative stress. Oxidative stress imposed several deleterious effects such as damaging the cell structures, including proteins, lipids, and DNA (Hina et al., 2017). Many pharmaceutical drugs have been formulated for the treatment of oxidative stress (Iqbal et al., 2017). Synthetic antioxidants have a few limitations as they cause carcinogenicity and are expensive. Natural antioxidants (vitamin C, vitamin E, xanthophylls, tannins, carotenes, and phenolic) have several biological activities (Cheema et al., 2011), which is why scientists have been exploring natural antioxidants for use in medicine and food to replace the synthetic compounds (Riaz, Rasool, Bukhari, Shahid, Zubair, et al., 2012). Bioactive compounds of medicinal plants have antioxidant potential (Irshad et al., 2012) and are being explored by researchers extensively (Asghar et al., 2017). Different bioassays have been performed to explore the antioxidant properties of phytobioactives (Riaz et al., 2017). Some bioassays are as follows:
6.1.1. DPPH assay
The radical scavenging DPPH bioassay (2, 2‐diphenyl‐1‐picrylhydrazine) was described by Blois in 1958 and was slightly modified by numerous researchers (Afzal, Shahid, Iqbal, & Hussain, 2014; Afzal, Shahid, & Jamil, 2014). DPPH assay is one of the most commonly used assays to determine the antioxidant activity of plant extract. The stable free radical DPPH reacts with hydrogen‐donating compounds. In its reduced form, DPPH is violet in color and turned into pale yellow color upon oxidation by an antioxidant. The activity of antioxidants is calculated by a reduction in optical density. In this method, DPPH solution (0.004%) in methanol is prepared and 10 μL of the sample was mixed with 1 mL of 0.004% DPPH solution (0.004%). Following incubation for 30 minutes, absorbance was measured at 517 nm (Irshad et al., 2017).
where A 1 is the unknown sample absorbance and A 0 is the blank absorbance.
6.1.2. ABTS assay
The ABTS radical scavenging assay was developed by Rice Evans and Miller in 1994 and then modified by Re in 1999. The modification is based on the production of a radical cation through metmyoglobin activation with H2O2 in the presence of ABTS•+ (Anwar et al., 2013). This assay is extensively used, generating a blue/green ABTS•+ chromophore via ABTS and potassium persulfate reaction. ABTS radical scavenging method together with DPPH assay is one of the widely used assays for the determination of antioxidant potential of plant samples. The ABTS radical scavenging activity is measured at 734 nm with the help of a spectrophotometer. This bioassay measures the total antioxidant capacity in both hydrophilic and hydrophobic substances. Trolox, a water‐soluble analog of Vitamin E, is used as a positive control and the antioxidant activity is expressed as Trolox‐equivalent antioxidant capacity per mg of extract sample (TEAC/mg; Anjum et al., 2014; Sridhar & Charles, 2019).
6.1.3. TPC assay
Folin–Ciocalteu method is used for the measurement of total phenolic content (TPC) based on phenolic group containing phytobioactives present in plant samples. The reaction for electron transfer occurs between antioxidants and molybdenum, reducing the complex‐forming blue species, which can be calculated by spectrophotometer. Sodium carbonate is added to make the medium alkaline which is required for this reaction, and gallic acid is used as standard (Waseem et al., 2021).
6.1.4. TFC assay
Total flavonoid contents (TFC) in the plant extracts are determined by aluminum chloride colorimetric assay. The sample solution of plant extracts was mixed with 0.1 mL 10% aluminum nitrate, potassium acetate, and 80% ethanol. The reagents were allowed to stand for 40 min after thorough mixing and the absorbance was measured at 510 nm via spectrophotometer. The presence of flavonoids is indicated by the yellow coloration of the reaction mixture and quercetin is used as standard (Ayub et al., 2017).
6.1.5. FRAP assay
Ferric ion‐reducing antioxidant power assay (FRAP) was developed by Iris Benzie and J. J. Strain and has been conducted in laboratories for the analysis of antioxidants due to its simplicity, good reproducibility, and simple instrumentation (Pramila & Julius, 2019). FRAP assay is based on the ability of antioxidants to reduce ferric ions in the presence of 2,4,6‐tris(2‐pyridyl)‐s‐triazine (TPTZ) at pH 3.6, forming an intense‐blue Fe2+ ‐TPTZ complex. The absorbance is measured at 593 nm. The absorbance is inversely proportional to the antioxidant content. For this assay, 0.05 mL of the extract is added to 1 mL of FRAP reagent in acetate buffer and the reaction mixture is incubated at 37°C for 30 min, and absorbance is measured at 593 nm. FRAP reagent is prepared by mixing 0.3 M acetate buffer (pH 3.6) and 10 mM 2,4,6‐tris(2‐pyridyl)‐S‐triazine in 40 mM hydrochloric acid and ferric chloride (20 mM) at a ratio of 10:1:1 (v/v/v; Saini et al., 2020).
6.1.6. CUPRAC assay
Cupric reducing antioxidant capacity (CUPRAC) assay was developed by Apak, Guclu, Ozyurek, and Karademir (Özyürek et al., 2011) from the Department of Analytical, Istanbul University, 7 years following the development of the FRAP assay. This assay was developed mainly for the determination of “total antioxidant” as a nutritional index for food labeling. No doubt, CUPRAC assay has been proven to be an efficient method for a variety of polyphenols (namely phenolic acids including hydroxybenzoic acids and hydroxycinnamic acids, flavonoids, anthocyanins, and carotenoids), in addition to thiols, vitamin E, and vitamin C. Since its development, it has been used over the last few years by many investigators in different laboratories. The light‐blue chromogen Cu (Nc)22+ used in this assay, upon reduction by the antioxidant, is reduced into an orange–yellow bis(neocuproine) copper (I) chelate Cu (Nc)2+. For this assay 100 μL of extract is added to the reaction mixture CuCl2 (200 μL, 10 mM), neocuproine (200 mL, 7.5 mM), and ammonium acetate aqueous buffer (200 μL, 1 M) at pH 7, incubated for 30 min at room temperature and measured the absorbance at 450 nm. CUPRAC capacity is expressed as Trolox, gallic acid, or quercetin equivalent (standard compound; Diniyah et al., 2020).
6.2. Antimicrobial activity
Antibiotics are used for the treatment of microbial infections and also as chemotherapeutic agents against infectious diseases (Hussain et al., 2010). However, few pathogens become resistant to antibiotics with the passage of time (Riaz et al., 2019). These drug‐resistant pathogens contribute to morbidity, mortality, and increased health issues (Mansoor et al., 2019). Resistance to antibiotics has become a global concern (Anwar et al., 2009). The therapeutic potential of several available antibiotics is being threatened by the appearance of multidrug‐resistant pathogens (Shahzadi et al., 2019). The use of crude plant extract and phytobioactive compounds are of huge significance to cope with multidrug‐resistant pathogens (Irshad et al., 2020).
Biofilm formation is a significant virulence strategy for microorganisms to survive in harsh environments (Iram et al., 2013). Bacteria form biofilms both on biotic and abiotic surfaces. Biofilms help bacteria in spreading infections by preventing them from antibiotics and the host immune system (Maqbool et al., 2019). Almost 80% of bacterial infections in humans are caused by biofilm. Biofilms cause numerous diseases, for example, vaginitis, gingivitis, conjunctivitis, colitis, and urethritis. Pseudomonas aeruginosa causes lung infection by forming a biofilm. Staphylococcus aureus and Escherichia coli also cause infections by forming biofilms. Biofilm formation on medical devices poses serious health issues. Plant extracts have been gaining huge attention to control emerging antibacterial resistance. Plants are packed up with bioactive compounds. Scientists have been evaluating various medicinal plants for their antibiofilm potential for the last few decades.
Quorum sensing (QS) is an intercellular communication process adopted by both Gram‐negative and Gram‐positive bacteria which is based on the secretion and detection of external signal molecules (Misbah Bhatti et al., 2021). In the form of biofilm, bacteria communicate with each other via quorum sensing. Quorum sensing is based on bacterial cell density. Inhibition of quorum sensing has the ability to inhibit virulence of bacteria and biofilms also (Afzal et al., 2021). Compounds having anti‐quorum‐sensing properties are able to control microbial infections, which is why researchers in the medical field are interested to explore more antimicrobial compounds (Abbasi et al., 2020). Phytobioactive compounds have remarkable antimicrobial properties (Shah et al., 2019). Lipophilic compounds isolated from Moringa oleifera bind with cytoplasmic membrane and inhibit the growth of filamentous fungi, especially by causing membrane permeabilization (Jabeen et al., 2008; Latif et al., 2011). Proteins purified from Croton tiglium L. showed remarkable antimicrobial activity (Shahid et al., 2008). Phytobioactive compounds isolated from sunflower seeds demonstrated high antibacterial properties (Den et al., 2019).
Broadly speaking, two types of bioassays are available for evaluating the antibacterial activity of natural compounds (Riaz, Rasool, Bukhari, Shahid, Zahoor, et al., 2012). Bioassays used for the detection of antimicrobial activity include diffusion‐based, bioautographic and cell morphology‐based assays. Quantitative bioassays are agar dilution, broth macrodilution, and broth microdilution assays (Abbasi et al., 2019).
6.3. Cytotoxicity analysis
When phytobioactive compounds are isolated as antioxidant and antimicrobial compounds, their toxicity analysis is quite necessary (Rasool et al., 2013; Sadaf et al., 2009). Most lethal poisons in the world, for example, botulinum, maitotoxin, batrachotoxin, and ricin, are naturally occurring compounds (Nighat et al., 2020; Shahid et al., 2020). In vitro bioassays for toxicity testing have increased considerably (Naseem et al., 2020). In vitro assays available for cytotoxicity testing are AMES assay (Jabbar et al., 2012; Rubab et al., 2015), DNA damage protection assay (Riaz et al., 2013), inflammatory assay (Ali et al., 2015), hemolytic assay (Gul et al., 2017; JaEeen et al., 2015; Shahid et al., 2016), thrombolytic assay (Rizwan et al., 2014; Sharif et al., 2018), etc. Several of the commonly used cytotoxicity assays are discussed below:
6.3.1. AMES assay
AMES test has been used to evaluate the mutagenic potential of medicinal plant extract. It was standardized for the identification of mutagenic potential of chemicals in 1970s (Rasgele & Dulger, 2021). For the last few years, this test has been extensively used to assess herbal products. This mutagenic assay uses two mutant strains of Salmonella typhimurium (TA98 and TA100). Potassium dichromate (K2Cr2O7) is used as standard mutagen for TA98 and sodium azide (NaN3) for TA100. Pale‐yellow wells are considered positive wells. A number of positive wells in sample plate (plant extract) should be higher than the number of positive wells in the background plate for a sample to be mutagenic (da Silva Dantas et al., 2020).
6.3.2. DNA damage protection assay
Oxidative stress is the main cause of aging and DNA damage. Nowadays, chemical, biological, and physical exposure and aging can increase DNA damage. DNA damage protection assay is used to assess the protective effects of plant extracts. CT‐DNA (calf thymus) is commonly used to determine DNA damage protection potential of extracts. Fenton's reagent is used to generate oxidative stress (Anjum et al., 2020).
6.3.3. Anti‐inflammatory assay
Inflammation is the response of the immune system to injury and infection. Denaturation of proteins is a well‐documented cause of inflammation. Several phytobioactives possess the ability to inhibit protein denaturation. The in vitro anti‐inflammatory potential of plant extracts is evaluated by the BSA denaturation method. Bovine serum albumin (BSA) solution is prepared in dH2O. In test tubes, 0.45 mL BSA and 0.05 mL of test samples are poured and incubated at 37°C for 20 min after that the temperature is increased to keep samples at 57°C for 3 min. Diclofenac sodium is used as standard. On cooling at room temperature, 2.5 mL of PBS (phosphate buffer saline) is added to test tubes, and absorbance is measured at 255 nm. The anti‐inflammatory potential of plant extracts is compared with the positive control (diclofenac sodium; Yadav & Mohite, 2020).
6.3.4. Antihemolytic assay
The antihemolytic assay is used to evaluate the protective capacity of plant extracts for red blood cells. In this assay, fresh heparinized human blood is used. After removing the serum, the mean packed volume is washed with PBS solution. Blood suspension (180 μL) and plant extract (20 μL) are added in Eppendorf tubes, incubated for 30 min, and centrifuged for 5 min. After centrifugation, 100 μL supernatant from each Eppendorf tube is taken and diluted with 900 μL PBS solution. Subsequently, 100 μL from new Eppendorf tubes is poured into microwells in ELISA plates, and absorbance is measured at 576 nm by an ELISA reader. Triton X‐100 is taken as the positive control while PBS solution is taken as the negative control. The test was executed in triplicate (Kauser et al., 2018).
7. CHARACTERIZATION OF PHYTOBIOACTIVE COMPOUNDS
Phytobioactive compounds are separated and purified from plant extracts employing chromatographic techniques (Qazi et al., 2021). The most common chromatographic methods used are thin‐layer chromatography (TLC) and high‐performance liquid chromatography (HPLC; Alim et al., 2010; Naseem et al., 2020). Gas chromatography (GC) is another technique used for separating, identifying, and quantifying phytobioactive compounds such as flavonoids, phenolic acids, and condensed tannins (Sajid et al., 2016). GC technique is used for the analysis of volatile compounds only (Qasim et al., 2020). FTIR and NMR are used to determine the functional groups of compounds present in the crude plant sample and to detect the number of protons present in the compound.
7.1. Thin‐layer chromatography (TLC)
TLC is the most commonly used adsorption chromatographic technique, employed for the separation and analysis of low‐molecular‐weight phytochemicals where plant extract is separated based on the nature of stationary phase (Ullah, 2020).
7.2. High‐performance liquid chromatography (HPLC)
HPLC is an analytical technique for the separation and identification of organic and inorganic solutes in a variety of samples including pharmaceutical, biological, industrial, and environmental samples. HPLC separation of compounds is based on the interaction of compounds with solid particles of tightly packed columns (stationary phase) and the mobile‐phase solvent (Grybinik & Bosakova, 2021). HPLC is useful for samples that cannot be vaporized or decomposed at high temperatures. Nowadays, this technique is gaining popularity among other analytical techniques for the analysis of medicinal plant extracts (Sahu et al., 2018).
7.3. Gas chromatography (GC) and GC–MS
Gas chromatography is used for the analysis of volatile compounds which provides quantitative and qualitative information on individual components present in the compound such as essential oils, hydrocarbons, and solvents. Gas chromatography is based on the principle of adsorption and partition. In GC, sample is injected onto the top of the chromatographic column and then transported through the column (liquid stationary phase) by the flow of inert, gaseous mobile phase (Al‐Rubaye et al., 2017). GC–MS has become a highly recommended analytical technique in the pharmaceutical industry due to its robustness, selectivity, sensitivity, and reproducibility (Beale et al., 2018).
7.4. UV–Visible spectroscopy
UV–visible spectroscopy is used for the identification and qualitative analysis of biological mixtures and plant extracts. Phytobioactives can be analyzed by using UV–visible spectroscopy because aromatic compounds act as chromophores in the UV range. This technique provides information about the total phenolic contents of plant mixtures (Rocha et al., 2018).
7.5. Fourier transform infrared (FTIR) spectroscopy
FTIR spectroscopy is used for the identification of functional groups of the organic and inorganic compounds from plants extract by using infrared light. It helps in the identification, characterization, and structure determination of unknown molecules. It is a high‐resolution analytical technique for the identification and structural elucidation of phytobioactive compounds (Riaz et al., 2018).
7.6. Nuclear magnetic resonance (NMR) spectroscopy
NMR spectroscopy provides physical, chemical, and biological properties of matter. Due to its accuracy, rapidness, and intactness, NMR spectroscopy has gained a key role in determining the structures and dynamic properties of pharmaceutical drugs. Although NMR has remarkable advantages, sometimes it is combined with other techniques, such as HPLC, FT‐IR, and GC–MS, for more accurate and effective results (Cao et al., 2021).
8. CONCLUSIONS AND FUTURE PERSPECTIVES
Since ancient times, phytobioactive compounds are being used as traditional medicine for the treatment of several diseases around the globe. Several studies on their therapeutic potential are documented, as they are considered to be the natural sources for the production of new drugs with greater efficacy and biocompatibility. Recent publications are gradually adding up new characteristics to their pre‐existing vast spectrum pharmaceutical value. In present‐day lifestyle, oxidative stress has been continuously increasing and causing numerous disorders such as diabetes mellitus, cancer, and cardiovascular problems. Misuse of antibiotics results in antibiotic resistance which poses serious health issues, especially in hospitals. Synthetic drugs are insufficient to cope with these problems. Exploration of natural phytobioactive compounds to cure present‐day diseases is necessary. Polyphenols, alkaloids, and terpenoids have the potential to treat cancer, oxidative stress, inflammation, ulcers, diabetes, platelet aggregation, microbial resistance, and tumors.
This review study concluded that the use of bioactive compounds in foods, beverages, and pharmaceutical products plays a significant role in preventing various disorders including cancer, neurodegenerative diseases, oxidative stress, cardiovascular diseases, diabetes, obesity, etc. The antioxidant and other beneficial effects of phytobioactive compounds can be explained through the modulation of multiple pathways involved in cell signaling. These mechanisms of phytobioactive compound action include the modulation of caspases, NF‐kB, MAPK, and Nrf2 as well as LOX and COX. An important approach to prevent pathological processes is the possible crosstalk between these signaling pathways. One of the critical factors that can affect the potential of these phytobioactives to counteract the oxidative damage in human organs induced by ROS is bioavailability. The application of these phytobioactive compounds as therapeutic agents against human diseases will be facilitated through the understanding of their absorption, distribution, metabolism, and excretion. The complications encountered while using phytobioactives, like their stability, solubility, and bioavailability, need to be addressed to use these metabolites as medicine.
ACKNOWLEDGEMENTS
The authors acknolwledge their resposective institutions for giving them time to work on this review.
CONFLICT OF INTEREST STATEMENT
The authors declared no conflict of interest.
Riaz, M. , Khalid, R. , Afzal, M. , Anjum, F. , Fatima, H. , Zia, S. , Rasool, G. , Egbuna, C. , Mtewa, A. G. , Uche, C. Z. , & Aslam, M. A. (2023). Phytobioactive compounds as therapeutic agents for human diseases: A review. Food Science & Nutrition, 11, 2500–2529. 10.1002/fsn3.3308
Contributor Information
Muhammad Riaz, Email: riazmlt786@gmail.com.
Fozia Anjum, Email: drfoziaanjum@gcuf.edu.pk.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable—no new data are generated and this review article describes entirely the published literature.
REFERENCES
- Aanouz, I. , Belhassan, A. , El‐Khatabi, K. , Lakhlifi, T. , El‐Ldrissi, M. , & Bouachrine, M. (2021). Moroccan medicinal plants as inhibitors against SARS‐CoV‐2 main protease: Computational investigations. Journal of Biomolecular Structure and Dynamics, 39(8), 2971–2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbas, M. , Rana, S. , Shahid, M. , Rana, N. , Mahmood‐ul‐Hassan, M. , & Hussain, M. (2012). Chemical evaluation of weed seeds mixed with wheat grains at harvest. The Journal of Animal & Plant Sciences, 22(2), 283–288. [Google Scholar]
- Abbas, M. , Shahid, M. , Sheikh, M. A. , & Muhammad, G. (2014). Phytochemical screening of plants used in folkloric medicine: Effect of extraction method and solvent. Asian Journal of Chemistry, 26(18), 6194–6198. [Google Scholar]
- Abbas, S. , Sharif, M. K. , Butt, M. S. , & Shahid, M. (2020). Screening of Pakistani sesame cultivars for nutritive value and bioactive components. Pakistan Journal of Agricultural Sciences, 57(3), 743–751. [Google Scholar]
- Abbasi, M. A. , Irshad, M. , Riaz, T. , Siddiqui, S. Z. , & Shahid, M. (2019). In vitro assessment of antibacterial, antifungal, enzyme inhibition and hemolytic activities of various fractions of Rhynchosia pseudo‐cajan. Pakistan Journal of Pharmaceutical Sciences, 32(5), 2003–2010. [PubMed] [Google Scholar]
- Abbasi, M. A. , Zeb, A. , Siddiqui, S. Z. , Shah, S. A. A. , Shahid, M. , & Fatima, H. (2020). Synthesis, bacterial biofilm inhibition and cytotoxicity of new N‐alkyl/aralkyl‐N‐(2, 3‐dihydro‐1, 4‐benzodioxin‐6‐yl)‐4‐nitrobenzenesulfonamides. Pakistan Journal of Pharmaceutical Sciences, 33(1), 41–47. [PubMed] [Google Scholar]
- Adhikari, B. , Marasini, B. P. , Rayamajhee, B. , Bhattarai, B. R. , Lamichhane, G. , Khadayat, K. , Adhikari, A. , Khanal, S. , & Parajuli, N. (2021). Potential roles of medicinal plants for the treatment of viral diseases focusing on COVID‐19: A review. Phytotherapy Research, 35(3), 1298–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afzal, I. , Saleem, S. , Skalicky, M. , Javed, T. , Bakhtavar, M. A. , Kamran, M. , Shahid, M. , Sohail Saddiq, M. , Afzal, A. , Shafqat, N. , Dessoky, E. S. , Gupta, A. , Korczyk‐Szabo, J. , Brestic, M. , & Shafqat, N. (2021). Magnetic field treatments improves sunflower yield by inducing physiological and biochemical modulations in seeds. Molecules, 26(7), 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afzal, M. , Shahid, M. , Iqbal, M. , & Hussain, F. (2014). Influence of Aspergillus niger stress on antimicrobial and biochemical profiling of Cichorium intybus . Oxidation Communications, 37(1), 269. [Google Scholar]
- Afzal, M. , Shahid, M. , & Jamil, A. (2014). Phytochemical spectrum of essential oil of Paganum harmala by GC‐MS and antimicrobial activity using sequential solvents fractions and essential oil. Asian Journal of Chemistry, 26(2), 574–578. [Google Scholar]
- Afzal, M. , Shahid, M. , Riaz, M. , Saleem, M. , Wattoo, J. , Zeshan, B. , & Naveed, M. (2018). Role of fungal‐induced stress in biochemical and phytochemical profiling of Peganum harmala . Journal of Biological Regulators and Homeostatic Agents, 32(2), 379–384. [PubMed] [Google Scholar]
- Aggarwal, B. B. , & Shishodia, S. (2006). Molecular targets of dietary agents for prevention and therapy of cancer. Biochemical Pharmacology, 71(10), 1397–1421. [DOI] [PubMed] [Google Scholar]
- Ahmad, S. , Zahiruddin, S. , Parveen, B. , Basist, P. , Parveen, A. , Parveen, R. , & Ahmad, M. (2021). Indian medicinal plants and formulations and their potential against COVID‐19–preclinical and clinical research. Frontiers in Pharmacology, 11, 2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akram, M. , Riaz, M. , Munir, N. , Akhter, N. , Zafar, S. , Jabeen, F. , Ali Shariati, M. , Akhtar, N. , Riaz, Z. , Altaf, S. H. , Daniyal, M. , Zahid, R. , & Said Khan, F. (2020). Chemical constituents, experimental and clinical pharmacology of Rosa damascena: A literature review. Journal of Pharmacy and Pharmacology, 72(2), 161–174. [DOI] [PubMed] [Google Scholar]
- Akram, M. , Riaz, M. , Wadood, A. W. C. , Hazrat, A. , Mukhtiar, M. , Ahmad Zakki, S. , Daniyal, M. , Ali Shariati, M. , Said Khan, F. , & Zainab, R. (2020). Medicinal plants with anti‐mutagenic potential. Biotechnology & Biotechnological Equipment, 34(1), 309–318. [Google Scholar]
- Ali, A. , Hussain, F. , & Shahid, M. (2015). Investigation of the wound healing potential of Onosma hispidum root extract in rabbit models. Progress in Nutrition, 17(3), 245–249. [Google Scholar]
- Ali, K. , Sagheer, M. , Rashid, A. , & Shahid, M. (2021). Bioactivity of medicinal plant extracts as toxicants and enzyme inhibitors against insect pests of stored commodities. Journal of Crop Protection, 10(1), 95–109. [Google Scholar]
- Ali, S. , Khan, A. S. , Malik, A. U. , & Shahid, M. (2016). Effect of controlled atmosphere storage on pericarp browning, bioactive compounds and antioxidant enzymes of litchi fruits. Food Chemistry, 206, 18–29. [DOI] [PubMed] [Google Scholar]
- Alim, M. , Nawaz, R. , Shahid, M. , Asi, M. R. , Iqbal, T. , & Anwar, F. (2010). HPLC method for the determination of pioglitazone in human plasma and its application to pharmacokinetic study. Asian Journal of Chemistry, 22(1), 213. [Google Scholar]
- Al‐Rubaye, A. F. , Hameed, I. H. , & Kadhim, M. J. (2017). A review: Uses of gas chromatography‐mass spectrometry (GC‐MS) technique for analysis of bioactive natural compounds of some plants. International Journal of Toxicological and Pharmacological Research, 9(1), 81–85. [Google Scholar]
- Anjum, F. , Bukhari, S. A. , Shahid, M. , Bokhari, T. H. , & Talpur, M. M. A. (2014). Exploration of nutraceutical potential of herbal oil formulated from parasitic plant. African Journal of Traditional, Complementary and Alternative Medicines, 11(1), 78–86. [PMC free article] [PubMed] [Google Scholar]
- Anjum, F. , Shahid, M. , Jilani, M. I. , Oranab, S. , Farooq, S. , Nazir, A. , Naz, S. , & Iqbal, M. (2020). Evaluation of antioxidant potential and cytotoxic behavior of different varieties of Allium sativum . Polish Journal of Environmental Studies, 29(6), 4447–4451. [Google Scholar]
- Anwar, F. , Ali, M. , Hussain, A. I. , & Shahid, M. (2009). Antioxidant and antimicrobial activities of essential oil and extracts of fennel (Foeniculum vulgare Mill.) seeds from Pakistan. Flavour and Fragrance Journal, 24(4), 170–176. [Google Scholar]
- Anwar, S. , Zubair, M. , Rashid, U. , Rizwan, K. , Shahid, M. , Bukhari, I. H. , Talha Jamil, M. , Uddin Ahmad, V. , & Rasool, N. (2013). Antimicrobial potential of methanolic extract and various fractions of Jatropha curcas roots. Asian Journal of Chemistry, 25(13), 7477–7480. [Google Scholar]
- Asghar, A. , Butt, M. S. , Shahid, M. , & Huang, Q. (2017). Evaluating the antimicrobial potential of green cardamom essential oil focusing on quorum sensing inhibition of Chromobacterium violaceum . Journal of Food Science and Technology, 54(8), 2306–2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashraf, A. , Sarfraz, R. A. , Rashid, M. A. , Mahmood, A. , Shahid, M. , & Noor, N. (2016). Chemical composition, antioxidant, antitumor, anticancer and cytotoxic effects of Psidium guajava leaf extracts. Pharmaceutical Biology, 54(10), 1971–1981. [DOI] [PubMed] [Google Scholar]
- Ashraf, A. , Sarfraz, R. A. , Rashid, M. A. , & Shahid, M. (2015). Antioxidant, antimicrobial, antitumor, and cytotoxic activities of an important medicinal plant (Euphorbia royleana) from Pakistan. Journal of Food and Drug Analysis, 23(1), 109–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashraf, A. , Zafar, S. , Ashraf, M. Y. , Ijaz, M. U. , Muzammal, S. , Asad, F. , Jabeen, F. , & Shahid, M. (2020). Phytochemical composition and potent biological activities of Ficus benjamina var. comosa leaves extract. Pakistan Journal of Botany, 52(2), 531–535. [Google Scholar]
- Aslam, M. , Shahid, M. , Rehman, F. , Naveed, N. , Batool, A. , Sharif, S. , & Asia, A. (2011). Purification and characterization of bacteriocin isolated from Streptococcus thermophilus . African Journal of Microbiology Research, 5(18), 2642–2648. [Google Scholar]
- Ayub, M. A. , Hanif, M. A. , Sarfraz, R. A. , & Shahid, M. (2018). Biological activity of Boswellia serrata Roxb. oleo gum resin essential oil: Effects of extraction by supercritical carbon dioxide and traditional methods. International Journal of Food Properties, 21(1), 808–820. [Google Scholar]
- Ayub, M. A. , Hussain, A. I. , Hanif, M. A. , Chatha, S. A. S. , Kamal, G. M. , Shahid, M. , & Janneh, O. (2017). Variation in phenolic profile, β‐carotene and flavonoid contents, biological activities of two tagetes species from Pakistani Flora. Chemistry & Biodiversity, 14(6), e1600463. [DOI] [PubMed] [Google Scholar]
- Balta, C. , Herman, H. , Boldura, O. M. , Gasca, I. , Rosu, M. , Ardelean, A. , & Hermenean, A. (2015). Chrysin attenuates liver fibrosis and hepatic stellate cell activation through TGF‐β/Smad signaling pathway. Chemico‐Biological Interactions, 240, 94–101. [DOI] [PubMed] [Google Scholar]
- Banjerdpongchai, R. , Wudtiwai, B. , Khaw‐On, P. , Rachakhom, W. , Duangnil, N. , & Kongtawelert, P. (2016). Hesperidin from citrus seed induces human hepatocellular carcinoma HepG2 cell apoptosis via both mitochondrial and death receptor pathways. Tumor Biology, 37(1), 227–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bano, N. , Afzal, M. , Abdullah, S. , & Shahid, M. (2017). The potential use of citric acid for the improvement in growth of Labeo rohita . International Journal of Agriculture & Biology, 19(2), 363–366. [Google Scholar]
- Banwo, K. , Olojede, A. O. , Adesulu‐Dahunsi, A. T. , Verma, D. K. , Thakur, M. , Tripathy, S. , Singh, S. , Patel, A. R. , Kumar Gupta, A. , Noe Aguilar, C. , & Aguilar, C. N. (2021). Functional importance of bioactive compounds of foods with potential health benefits: A review on recent trends. Food Bioscience, 43, 101320. [Google Scholar]
- Bari, M. N. , Zubair, M. , Rizwan, K. , Rasool, N. , Bukhari, I. H. , Akram, S. , Bokhari, T. H. , Shahid, M. , Hameed, M. , & Ahmad, V. U. (2012). Biological activities of Opuntia monacantha cladodes. Journal of the Chemical Society of Pakistan, 34(4), 990–995. [Google Scholar]
- Beale, D. J. , Pinu, F. R. , Kouremenos, K. A. , Poojary, M. M. , Narayana, V. K. , Boughton, B. A. , Kanojia, K. , Dayalan, S. , Jones, O. A. H. , & Dias, D. A. (2018). Review of recent developments in GC–MS approaches to metabolomics‐based research. Metabolomics, 14(11), 1–31. [DOI] [PubMed] [Google Scholar]
- Bhatti, I. , Iqbal, M. , Anwar, F. , Shahid, S. , & Shahid, M. (2013). Quality characteristics and microbiological safety evaluation of oils extracted from gamma irradiated almond (Prunus dulcis Mill.) seeds. Grasas y Aceites, 64(1), 68–76. [Google Scholar]
- Bishayee, A. , Thoppil, R. J. , Mandal, A. , Darvesh, A. S. , Ohanyan, V. , Meszaros, J. G. , Háznagy‐Radnai, E. , Hohmann, J. , & Bhatia, D. (2013). Black currant phytoconstituents exert chemoprevention of diethylnitrosamine‐initiated hepatocarcinogenesis by suppression of the inflammatory response. Molecular Carcinogenesis, 52(4), 304–317. [DOI] [PubMed] [Google Scholar]
- Bonetti, F. , Brombo, G. , & Zuliani, G. (2017). Nootropics, functional foods, and dietary patterns for prevention of cognitive decline. In Watson R. S. (Ed.), Nutrition and functional foods for healthy aging (pp. 211–232). Elsevier. [Google Scholar]
- Bukhari, S. A. , Shahid, M. , Anjum, F. , Rasheed, A. , & Munir, H. (2014). Purification, fragmentation and characterization of gum from Cyamopsis tetragonolobus to enhance its nutraceutical attributes. Pakistan Journal of Agricultural Sciences, 51(4), 1059–1067. [Google Scholar]
- Cao, R. , Liu, X. , Liu, Y. , Zhai, X. , Cao, T. , Wang, A. , & Qiu, J. (2021). Applications of nuclear magnetic resonance spectroscopy to the evaluation of complex food constituents. Food Chemistry, 342, 128258. [DOI] [PubMed] [Google Scholar]
- Carpentieri, S. , Soltanipour, F. , Ferrari, G. , Pataro, G. , & Donsì, F. (2021). Emerging green techniques for the extraction of antioxidants from agri‐food by‐products as promising ingredients for the food industry. Antioxidants, 10(9), 1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, L. , Shi, R. , Wang, X. , & Bao, Y. (2020). Gypenoside A protects ischemia/reperfusion injuries by suppressing miR‐143‐3p level via the activation of AMPK/Foxo1 pathway. BioFactors, 46(3), 432–440. [DOI] [PubMed] [Google Scholar]
- Chang, Y. J. , Hsu, S. L. , Liu, Y. T. , Lin, Y. H. , Lin, M. H. , Huang, S. J. , Annie Ho, J.‐A. , & Wu, L.‐C. (2015). Gallic acid induces necroptosis via TNF–α signaling pathway in activated hepatic stellate cells. PLoS One, 10(3), e0120713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee, S. , Patra, D. , Ghosh, P. , Prasad, A. , & Chowdhury, K. D. (2019). Terpenoids in treatment of liver disease. In Roy D. N. (Ed.), Terpenoids against human diseases (pp. 61–94). CRC Press. [Google Scholar]
- Cheema, U. B. , Sultan, J. I. , Javaid, A. , Akhtar, P. , & Shahid, M. (2011). Chemical composition, mineral profile and in situ digestion kinetics of fodder leaves of four native trees. Pakistan Journal of Botany, 43(1), 397–404. [Google Scholar]
- Chen, C. , Yu, L.‐T. , Cheng, B.‐R. , Xu, J.‐L. , Cai, Y. , Jin, J.‐L. , Feng, R.‐L. , Xie, L. , Qu, X.‐Y. , Li, D. , Liu, J. , Li, Y. , Cui, X.‐Y. , Lu, J.‐J. , Zhou, K. , Lin, Q. , & Li, D. (2021). Promising therapeutic candidate for myocardial ischemia/reperfusion injury: What are the possible mechanisms and roles of phytochemicals? Frontiers in Cardiovascular Medicine, 8, 792592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Y. , Zeng, L. , Yang, J. , Wang, Y. , Yao, F. , Wu, Y. , Wang, D. , Hu, Y. , & Liu, J. (2017). Anti‐DHAV‐1 reproduction and immuno‐regulatory effects of a flavonoid prescription on duck virus hepatitis. Pharmaceutical Biology, 55(1), 1545–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi, W. H. , & Lee, I. A. (2019). The mechanism of action of ursolic acid as a potential anti‐toxoplasmosis agent, and its immunomodulatory effects. Pathogens, 8(2), 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox‐Georgian, D. , Ramadoss, N. , Dona, C. , & Basu, C. (2019). Therapeutic and medicinal uses of terpenes. In Ekiert H. M., Ramawat K. G. & Arora J. (Eds.), Medicinal plants (pp. 333–359). Springer. [Google Scholar]
- Csiszar, A. , Bagi, Z. , Feher, A. , Recchia, F. A. , Sonntag, W. , Ungvari, Z. , Pearson, K. , de Cabo, R. , & de Cabo, R. (2011). Resveratrol confers endothelial protection via activation of the antioxidant transcription factor Nrf2. The FASEB Journal, 25(S1), 1093.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva Dantas, F. G. , de Castilho, P. F. , de Almeida‐Apolonio, A. A. , de Araújo, R. P. , & de Oliveira, K. M. P. (2020). Mutagenic potential of medicinal plants evaluated by the Ames Salmonella/microsome assay: A systematic review. Mutation Research/Reviews in Mutation Research, 786, 108338. [DOI] [PubMed] [Google Scholar]
- Dai, S. , Meng, X. , Cai, X. , Yuan, C. , Zhao, Z. , Zhong, L. , Shi, Y. , & Yin, F. (2021). Therapeutic effect of ursolic acid on fetal development in pregnant rats with gestational diabetes mellitus via AGEs‐RAGE signaling pathway. Journal of Food Biochemistry, 45(4), e13651. [DOI] [PubMed] [Google Scholar]
- Delgado, A. M. , Issaoui, M. , & Chammem, N. (2019). Analysis of main and healthy phenolic compounds in foods. Journal of AOAC International, 102(5), 1356–1364. [DOI] [PubMed] [Google Scholar]
- Den, N. Z. U. , Mustafa, G. , Bukhari, S. A. , Anjum, F. , Qasim, M. , & Shahid, M. (2019). Enhancement of nutraceutical and antioxidant potential of sunflower hybrid seed varieties through chemical priming. Pakistan Journal of Pharmaceutical Sciences, 32(4(Supplementary)), 1901–1907. [PubMed] [Google Scholar]
- Devi, K. P. , Malar, D. S. , Nabavi, S. F. , Sureda, A. , Xiao, J. , Nabavi, S. M. , & Daglia, M. (2015). Kaempferol and inflammation: From chemistry to medicine. Pharmacological Research, 99, 1–10. [DOI] [PubMed] [Google Scholar]
- Diniyah, N. , Alam, M. B. , & Lee, S.‐H. (2020). Antioxidant potential of non‐oil seed legumes of Indonesian's ethnobotanical extracts. Arabian Journal of Chemistry, 13(5), 5208–5217. [Google Scholar]
- Działo, M. , Mierziak, J. , Korzun, U. , Preisner, M. , Szopa, J. , & Kulma, A. (2016). The potential of plant phenolics in prevention and therapy of skin disorders. International Journal of Molecular Sciences, 17(2), 160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan, D. , Liu, L. , Wu, Z. , & Cao, M. (2019). Combating neurodegenerative diseases with the plant alkaloid berberine: Molecular mechanisms and therapeutic potential. Current Neuropharmacology, 17(6), 563–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatima, H. , Shahid, M. , Jamil, A. , & Naveed, M. (2021). Therapeutic potential of selected medicinal plants against carrageenan induced inflammation in rats. Dose‐Response, 19(4), 15593258211058028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatima, S. , Bhatti, H. N. , Iqbal, M. A. , & Shahid, M. (2020). Synthesis of needle like Mn‐Zn bimetallic nanoparticles and its applications towards photocatalysis and as fuel additives. International Journal of Environmental Analytical Chemistry, 102, 7617–7632. [Google Scholar]
- Fielding, B. C. , Filho, C. D. S. M. B. , Ismail, N. S. , & Sousa, D. P. D. (2020). Alkaloids: Therapeutic potential against human coronaviruses. Molecules, 25(23), 5496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganesan, P. , Arulselvan, P. , & Choi, D. K. (2017). Phytobioactive compound‐based nanodelivery systems for the treatment of type 2 diabetes mellitus–current status. International Journal of Nanomedicine, 12, 1097–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao, F. , Deng, G. , Liu, W. , Zhou, K. , & Li, M. (2017). Resveratrol suppresses human hepatocellular carcinoma via targeting HGF‐c‐met signaling pathway. Oncology Reports, 37(2), 1203–1211. [DOI] [PubMed] [Google Scholar]
- Grybinik, S. , & Bosakova, Z. (2021). An overview of chiral separations of pharmaceutically active substances by HPLC (2018–2020). Monatshefte für Chemie‐Chemical Monthly, 152(9), 1033–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gul, S. , Abbasi, M. A. , Khan, K. M. , Nafeesa, K. , Siddiqa, A. , Akhtar, M. N. , Shahid, M. , & Subhani, Z. (2017). Synthesis, antimicrobial evaluation and hemolytic activity of 2‐[[5‐alkyl/aralkyl substituted‐1, 3, 4‐oxadiazol‐2‐yl] thio]‐N‐[4‐(4‐morpholinyl) phenyl] acetamide derivatives. Journal of Saudi Chemical Society, 21, S425–S433. [Google Scholar]
- Gumisiriza, H. , Sesaazi, C. D. , Olet, E. A. , Kembabazi, O. , & Birungi, G. (2021). Medicinal plants used to treat “African” diseases by the local communities of Bwambara sub‐county in Rukungiri District, Western Uganda. Journal of Ethnopharmacology, 268, 113578. [DOI] [PubMed] [Google Scholar]
- Guo, C. , Zhang, J. , Zhang, P. , Si, A. , Zhang, Z. , Zhao, L. , Lv, F. , & Zhao, G. (2019). Ginkgolide B ameliorates myocardial ischemia reperfusion injury in rats via inhibiting endoplasmic reticulum stress. Drug Design, Development and Therapy, 13, 767–774. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Guo, S. , Gao, C. , Xiao, W. , Zhang, J. , Qu, Y. , Li, J. , & Ye, F. (2018). Matrine protects cardiomyocytes from ischemia/reperfusion injury by regulating HSP70 expression via activation of the JAK2/STAT3 pathway. Shock, 50(6), 664–670. [DOI] [PubMed] [Google Scholar]
- Guo, W. , Xu, B. , Wang, X. , Zheng, B. , Du, J. , & Liu, S. (2020). The analysis of the anti‐tumor mechanism of ursolic acid using connectively map approach in breast cancer cells line MCF‐7. Cancer Management and Research, 12, 3469–3476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafiz, I. , Bhatti, H. , Hanif, M. , & Shahid, M. (2018). In‐vitro antibacterial and antioxidant potential of winged prickly ash, green tea and thyme. Journal of Biological Regulators and Homeostatic Agents, 32(1), 101–106. [PubMed] [Google Scholar]
- Hano, C. , & Tungmunnithum, D. (2020). Plant polyphenols, more than just simple natural antioxidants: Oxidative stress, aging and age‐related diseases (Vol. 7, p. 26). Multidisciplinary Digital Publishing Institute. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández‐Ortega, L. D. , Alcántar‐Díaz, B. E. , Ruiz‐Corro, L. A. , Sandoval‐Rodriguez, A. , Bueno‐Topete, M. , Armendariz‐Borunda, J. , & Salazar‐Montes, A. M. (2012). Quercetin improves hepatic fibrosis reducing hepatic stellate cells and regulating pro‐fibrogenic/anti‐fibrogenic molecules balance. Journal of Gastroenterology and Hepatology, 27(12), 1865–1872. [DOI] [PubMed] [Google Scholar]
- Hina, S. , Shahid, M. , & Jahan, N. (2017). In vitro antioxidant, hepatoprotective potential and chemical profiling of Syzygium aromaticum using HPLC and GC‐MS. Pakistan Journal of Pharmaceutical Sciences, 30, 1031–1039. [PubMed] [Google Scholar]
- Huang, C. , Wei, Y.‐X. , Shen, M.‐C. , Tu, Y.‐H. , Wang, C.‐C. , & Huang, H.‐C. (2016). Chrysin, abundant in Morinda citrifolia fruit water–etoac extracts, combined with apigenin synergistically induced apoptosis and inhibited migration in human breast and liver cancer cells. Journal of Agricultural and Food Chemistry, 64(21), 4235–4245. [DOI] [PubMed] [Google Scholar]
- Huang, J. , Liu, Z. , Xu, P. , Zhang, Z. , Yin, D. , Liu, J. , He, M. , & He, M. (2018). Capsaicin prevents mitochondrial damage, protects cardiomyocytes subjected to anoxia/reoxygenation injury mediated by 14‐3‐3η/Bcl‐2. European Journal of Pharmacology, 819, 43–50. [DOI] [PubMed] [Google Scholar]
- Huang, R. , Shu, J. , Dai, X. , Liu, Y. , Yu, F. , & Shi, G. (2020). The protective effect of polyphyllin I on myocardial ischemia/reperfusion injury in rats. Annals of Translational Medicine, 8(10), 644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain, A. I. , Anwar, F. , Shahid, M. , Ashraf, M. , & Przybylski, R. (2010). Chemical composition, and antioxidant and antimicrobial activities of essential oil of spearmint (Mentha spicata L.) from Pakistan. Journal of Essential Oil Research, 22(1), 78–84. [Google Scholar]
- Hussain, F. , Irshad, A. , & Shahid, M. (2018). Study of selected therapeutic potentials of Momordica charantia fruit. Journal of Biological Regulators and Homeostatic Agents, 32(4), 859–862. [PubMed] [Google Scholar]
- Hussain, M. , Bakalis, S. , Gouseti, O. , Zahoor, T. , Anjum, F. M. , & Shahid, M. (2015). Dynamic and shear stress rheological properties of guar galactomannans and its hydrolyzed derivatives. International Journal of Biological Macromolecules, 72, 687–691. [DOI] [PubMed] [Google Scholar]
- Hussain, S. , Ali, S. , Shahzadi, S. , Rizzoli, C. , & Shahid, M. (2015). Diorganotin (IV) complexes with monohydrate disodium salt of iminodiacetic acid: Synthesis, characterization, crystal structure and biological activities. Journal of the Chinese Chemical Society, 62(9), 793–802. [Google Scholar]
- Hussein, R. H. , & Khalifa, F. K. (2014). The protective role of ellagitannins flavonoids pretreatment against N‐nitrosodiethylamine induced‐hepatocellular carcinoma. Saudi Journal of Biological Sciences, 21(6), 589–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ielciu, I. , Sevastre, B. , Olah, N.‐K. , Turdean, A. , Chișe, E. , Marica, R. , Oniga, I. , Uifălean, A. , Sevastre‐Berghian, A. C. , Niculae, M. , Benedec, D. , & Niculae, M. (2021). Evaluation of hepatoprotective activity and oxidative stress reduction of Rosmarinus officinalis L. shoots tincture in rats with experimentally induced hepatotoxicity. Molecules, 26(6), 1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal, H. , Ali, S. , Shahzadi, S. , Sharma, S. K. , Qanungo, K. , & Shahid, M. (2015). Synthesis and characterization of hetero‐bimetallic complexes with 2‐mercapto‐5‐methyl‐benzimidazole: Theoretical study and biological activities. Journal of Coordination Chemistry, 68(14), 2434–2448. [Google Scholar]
- Iqbal, M. , Nisar, J. , Adil, M. , Abbas, M. , Riaz, M. , Tahir, M. A. , Younus, M. , & Shahid, M. (2017). Mutagenicity and cytotoxicity evaluation of photo‐catalytically treated petroleum refinery wastewater using an array of bioassays. Chemosphere, 168, 590–598. [DOI] [PubMed] [Google Scholar]
- Iqbal, S. Z. , Bhatti, I. A. , Asi, M. R. , Zuber, M. , Shahid, M. , & Parveen, I. (2013). Effect of γ irradiation on fungal load and aflatoxins reduction in red chillies. Radiation Physics and Chemistry, 82, 80–84. [Google Scholar]
- Iram, S. , Ali, S. , Shahzadi, S. , & Shahid, M. (2013). Synthesis, characterization, and antimicrobial activity of heterobimetallic complexes of Sn (IV) and Zn (II) with 4‐aminophenylacetic acid. Russian Journal of General Chemistry, 83(12), 2453–2459. [Google Scholar]
- Irshad, A. , Sarwar, N. , Sadia, H. , Riaz, M. , Sharif, S. , Shahid, M. , & Khan, J. A. (2020). Silver nano‐particles: Synthesis and characterization by using glucans extracted from Pleurotus ostreatus . Applied Nanoscience, 10(8), 3205–3214. [Google Scholar]
- Irshad, A. , Shahid, M. , Asghar, M. , & Khan, J. (2017). Antioxidant potential analysis of P. ostreatus, P. sajor‐caju, P. sapidus and P. columbinus . Journal of Biological Regulators and Homeostatic Agents, 31(3), 705–709. [PubMed] [Google Scholar]
- Irshad, M. , Aziz, S. , Ahmed, M. N. , Asghar, G. , Akram, M. , & Shahid, M. (2018). Comparisons of chemical and biological studies of essential oils of stem, leaves and seeds of Zanthoxylum alatum Roxb growing wild in the state of Azad Jammu and Kashmir, Pakistan. Records of Natural Products, 12(6), 638–642. [Google Scholar]
- Irshad, M. , Aziz, S. , Shahid, M. , Ahmed, M. N. , Minhas, F. A. , & Sherazi, T. (2012). Antioxidant and antimicrobial activities of essential oil of Skimmea laureola growing wild in the state of Jammu and Kashmir. Journal of Medicinal Plants Research, 6(9), 1680–1684. [Google Scholar]
- Irshad, M. , Shahid, M. , Aziz, S. , & Ghous, T. (2011). Antioxidant, antimicrobial and phytotoxic activities of essential oil of Angelica glauca . Asian Journal of Chemistry, 23(5), 1947. [Google Scholar]
- Iyer, S. C. , Gopal, A. , & Halagowder, D. (2015). Myricetin induces apoptosis by inhibiting P21 activated kinase 1 (PAK1) signaling cascade in hepatocellular carcinoma. Molecular and Cellular Biochemistry, 407(1), 223–237. [DOI] [PubMed] [Google Scholar]
- Jabbar, S. , Shahzadi, I. , Rehman, R. , Iqbal, H. , Ul‐Ain, Q. , Jamil, A. , Kousar, R. , Ali, S. , Shahzadi, S. , Choudhary, M. A. , Shahid, M. , Khan, Q. M. , Sharma, S. K. , & Qanungo, K. (2012). Synthesis, characterization, semi‐empirical study, and biological activities of organotin (IV) complexes with cyclohexylcarbamodithioic acid as biological active ligand. Journal of Coordination Chemistry, 65(4), 572–590. [Google Scholar]
- Jabeen, R. , Shahid, M. , Jamil, A. , & Ashraf, M. (2008). Microscopic evaluation of the antimicrobial activity of seed extracts of Moringa oleifera . Pakistan Journal of Botany, 40(4), 1349–1358. [Google Scholar]
- JaEeen, R. , Jamil, A. , Shahid, M. , & Ashraf, M. (2015). Bioactive potential of normal and fungal stressed extracts from Ricinus communis L.(Castor) whole plant relative potential of protein/PeStide. Oxidation Communications, 38(4), 1612–1621. [Google Scholar]
- Jagan, S. , Ramakrishnan, G. , Anandakumar, P. , Kamaraj, S. , & Devaki, T. (2008). Antiproliferative potential of gallic acid against diethylnitrosamine‐induced rat hepatocellular carcinoma. Molecular and Cellular Biochemistry, 319(1), 51–59. [DOI] [PubMed] [Google Scholar]
- Jahan, N. , & Shahid, M. (2019). Starring role of acetylcholinesterase from medicinal plants on lactate dehydrogenase production in cytotoxic hepatic cells. Journal of Biological Regulators and Homeostatic Agents, 33(6), 1797–1801. [DOI] [PubMed] [Google Scholar]
- Jahangir, M. A. , Shehzad, A. , Butt, M. S. , & Bashir, S. (2018). Influence of supercritical fluid extract of Cinnamomum zeylanicum bark on physical, bioactive and sensory properties of innovative cinnamaldehyde‐enriched chocolates. Czech Journal of Food Sciences, 36(1), 28–36. [Google Scholar]
- Jilani, M. , Bhatti, H. , Bhatti, I. , & Shahid, M. (2016). Chemical profiling and biological activities of basil essential oil. Oxidation Communications, 39(4), 2913–2918. [Google Scholar]
- Kauser, A. , Shah, S. M. A. , Iqbal, N. , Murtaza, M. A. , Hussain, I. , Irshad, A. , Nasir, S. , Akram, M. , Munir, N. , & Riaz, M. (2018). In vitro antioxidant and cytotoxic potential of methanolic extracts of selected indigenous medicinal plants. Progress in Nutrition, 20(4), 706–712. [Google Scholar]
- Khadka, D. , Dhamala, M. K. , Li, F. , Aryal, P. C. , Magar, P. R. , Bhatta, S. , Thakur, M. S. , Basnet, A. , Cui, D. , & Shi, S. (2021). The use of medicinal plants to prevent COVID‐19 in Nepal. Journal of Ethnobiology and Ethnomedicine, 17(1), 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khairan, K. , Idroes, R. , Tallei, T. E. , Nasim, M. J. , & Jacob, C. (2021). Bioactive compounds from medicinal plants and their possible effect as therapeutic agents against COVID‐19: A review. Current Nutrition & Food Science, 17(6), 621–633. [Google Scholar]
- Khalid, S. , Malik, A. U. , Khan, A. S. , Shahid, M. , & Shafique, M. (2016). Tree age, fruit size and storage conditions affect levels of ascorbic acid, total phenolic concentrations and total antioxidant activity of ‘Kinnow’ mandarin juice. Journal of the Science of Food and Agriculture, 96(4), 1319–1325. [DOI] [PubMed] [Google Scholar]
- Khan, H. , Nabavi, S. M. , Sureda, A. , Mehterov, N. , Gulei, D. , Berindan‐Neagoe, I. , Taniguchi, H. , & Atanasov, A. G. (2018). Therapeutic potential of songorine, a diterpenoid alkaloid of the genus Aconitum . European Journal of Medicinal Chemistry, 153, 29–33. [DOI] [PubMed] [Google Scholar]
- Khan, M. M. , Iqbal, M. , Hanif, M. A. , Mahmood, M. S. , Naqvi, S. A. , Shahid, M. , & Jaskani, M. J. (2012). Antioxidant and antipathogenic activities of citrus peel oils. Journal of Essential Oil Bearing Plants, 15(6), 972–979. [Google Scholar]
- Kim, M.‐H. , Jeong, Y.‐J. , Cho, H.‐J. , Hoe, H.‐S. , Park, K.‐K. , Park, Y.‐Y. , Choi, Y. H. , Kim, C. H. , Chang, H. W. , Park, Y. J. , Chung, I. K. , & Chang, Y. C. (2017). Delphinidin inhibits angiogenesis through the suppression of HIF‐1α and VEGF expression in A549 lung cancer cells. Oncology Reports, 37(2), 777–784. [DOI] [PubMed] [Google Scholar]
- Kim, S. J. , Jeong, C. W. , Bae, H. B. , Kwak, S. H. , Son, J.‐K. , Seo, C.‐S. , Lee, H. J. , Lee, J. , & Yoo, K. Y. (2012). Protective effect of sauchinone against regional myocardial ischemia/reperfusion injury: Inhibition of p38 MAPK and JNK death signaling pathways. Journal of Korean Medical Science, 27(5), 572–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar, M. , Prakash, S. , Kumari, N. , Pundir, A. , Punia, S. , Saurabh, V. , Choudhary, P. , Changan, S. , Dhumal, S. , Pradhan, P. C. , Alajil, O. , Singh, S. , Sharma, N. , Ilakiya, T. , Singh, S. , & Pradhan, P. C. (2021). Beneficial role of antioxidant secondary metabolites from medicinal plants in maintaining oral health. Antioxidants, 10(7), 1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latif, S. , Anwar, F. , Hussain, A. I. , & Shahid, M. (2011). Aqueous enzymatic process for oil and protein extraction from Moringa oleifera seed. European Journal of Lipid Science and Technology, 113(8), 1012–1018. [Google Scholar]
- Li, J. , Li, X. , Xu, W. , Wang, S. , Hu, Z. , Zhang, Q. , Deng, X. , Wang, J. , Zhang, J. , & Guo, C. (2015). Antifibrotic effects of luteolin on hepatic stellate cells and liver fibrosis by targeting AKT/mTOR/p70S6K and TGFβ/Smad signalling pathways. Liver International, 35(4), 1222–1233. [DOI] [PubMed] [Google Scholar]
- Li, J. , Liu, P. , Zhang, R. , Cao, L. , Qian, H. , Liao, J. , Xu, W. , Wu, M. , & Yin, Z. (2011). Icaritin induces cell death in activated hepatic stellate cells through mitochondrial activated apoptosis and ameliorates the development of liver fibrosis in rats. Journal of Ethnopharmacology, 137(1), 714–723. [DOI] [PubMed] [Google Scholar]
- Liaqat, A. , Zahoor, T. , Atif Randhawa, M. , & Shahid, M. (2019). Characterization and antimicrobial potential of bioactive components of sonicated extract from garlic (Allium sativum) against foodborne pathogens. Journal of Food Processing and Preservation, 43(5), e13936. [Google Scholar]
- Lim, X. Y. , Teh, B. P. , & Tan, T. Y. C. (2021). Medicinal plants in COVID‐19: Potential and limitations. Frontiers in Pharmacology, 12, 355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling, Y. , Chen, G. , Deng, Y. , Tang, H. , Ling, L. , Zhou, X. , Song, X. , Yang, P. , Liu, Y. , Li, Z. , Zhao, C. , Yang, Y. , Wang, X. , Kitakaze, M. , Liao, Y. , & Chen, A. (2016). Polydatin post‐treatment alleviates myocardial ischaemia/reperfusion injury by promoting autophagic flux. Clinical Science, 130(18), 1641–1653. [DOI] [PubMed] [Google Scholar]
- Lu, C. W. , Lin, T. Y. , Wang, S. J. , & Huang, S. K. (2019). Asiatic acid, an active substance of Centella asiatica, presynaptically depresses glutamate release in the rat hippocampus. European Journal of Pharmacology, 865, 172781. [DOI] [PubMed] [Google Scholar]
- Ma, L. , Liu, H. , Xie, Z. , Yang, S. , Xu, W. , Hou, J. , & Yu, B. (2014). Ginsenoside Rb3 protects cardiomyocytes against ischemia‐reperfusion injury via the inhibition of JNK‐mediated NF‐κB pathway: A mouse cardiomyocyte model. PLoS One, 9(8), e103628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malode, L. L. , Manwar, J. V. , Panchale, W. A. , Bartere, S. A. , & Bakal, R. L. (2021). Potential of medicinal plants in management of diabetes: An updates. GSC Advanced Research and Reviews, 8(1), 149–159. [Google Scholar]
- Mansoor, N. , Younus, A. , Jamil, Y. , & Shahid, M. (2019). Impact of nanosized and bulk ZnO on germination and early growth response of Triticum aestivum . Pakistan Journal of Agricultural Sciences, 56(4), 1–17. [Google Scholar]
- Maqbool, M. , Bhatti, H. N. , Sadaf, S. , Zahid, M. , & Shahid, M. (2019). A robust approach towards green synthesis of polyaniline‐Scenedesmus biocomposite for wastewater treatment applications. Materials Research Express, 6(5), 055308. [Google Scholar]
- Maurya, A. K. , & Vinayak, M. (2015). Anticarcinogenic action of quercetin by downregulation of phosphatidylinositol 3‐kinase (PI3K) and protein kinase C (PKC) via induction of p53 in hepatocellular carcinoma (HepG2) cell line. Molecular Biology Reports, 42(9), 1419–1429. [DOI] [PubMed] [Google Scholar]
- Mecocci, P. , Tinarelli, C. , Schulz, R.‐J. , & Polidori, M. C. (2014). Nutraceuticals in cognitive impairment and Alzheimer's disease. Frontiers in Pharmacology, 5, 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehmood, N. , Zubaır, M. , Rızwan, K. , Rasool, N. , Shahid, M. , & Ahmad, V. U. (2012). Antioxidant, antimicrobial and phytochemical analysis of cichoriumintybus seeds extract and various organic fractions. Iranian Journal of Pharmaceutical Research, 11(4), 1145–1151. [PMC free article] [PubMed] [Google Scholar]
- Merrouni, I. A. , & Elachouri, M. (2021). Anticancer medicinal plants used by Moroccan people: Ethnobotanical, preclinical, phytochemical and clinical evidence. Journal of Ethnopharmacology, 266, 113435. [DOI] [PubMed] [Google Scholar]
- Mioc, M. , Milan, A. , Malița, D. , Mioc, A. , Prodea, A. , Racoviceanu, R. , Ghiulai, R. , Cristea, A. , Căruntu, F. , & Șoica, C. (2022). Recent advances regarding the molecular mechanisms of triterpenic acids: A review (part I). International Journal of Molecular Sciences, 23(14), 7740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misbah Bhatti, I. A. , Zia, K. M. , Bhatti, H. N. , & Shahid, M. (2021). Synthesis, biological efficiency evaluation and application of sodium alginate‐based polyurethane dispersions using cycloaliphatic isocyanate, as antibacterial textile coating. Journal of Industrial Textiles, 50(10), 1625–1642. [Google Scholar]
- Mou, L. , Liao, L. , Zhang, Y. , Ming, D. , & Jiang, J. (2021). Ursolic acid ameliorates Nthy‐ori 3‐1 cells injury induced by IL‐1β through limiting MALAT1/miR‐206/PTGS1 ceRNA network and NF‐κB signaling pathway. Psychopharmacology, 238(4), 1141–1156. [DOI] [PubMed] [Google Scholar]
- Munir, H. , Sarfraz, R. , Hussain, A. , Shahid, M. , & Sultana, B. (2014). Antioxidant and antimicrobial activities of different solvent extracts of Asphodilus tenifolius . Oxidation Communications, 37(3), 741–754. [Google Scholar]
- Munir, H. , Shahid, M. , Anjum, F. , & Mudgil, D. (2016). Structural, thermal and rheological characterization of modified Dalbergia sissoo gum—A medicinal gum. International Journal of Biological Macromolecules, 84, 236–245. [DOI] [PubMed] [Google Scholar]
- Munir, H. , Shahid, M. , Subhani, Z. , Afzal, M. , & Pirzada, T. (2015). Antimicrobial and antioxidant activities of hydrolysed and modified gum Acacia modesta and Dalbergia sissoo . Oxidation Communications, 38(4), 1632–1644. [Google Scholar]
- Munir, M. , Shahid, M. , Munir, H. , Javaid, S. , Sharif, S. , & Ahmed, E. (2017). Xanthan gum biochemical profiling, antioxidant, antibacterial, biofilm inhibition and mutagenic potential. Current Science, 114, 1903–1913. [Google Scholar]
- Nafees, M. , Jaskani, M. J. , Ahmad, S. , Shahid, M. , Malik, Z. , & Jamil, M. (2017). Biochemical diversity in wild and cultivated pomegranate (Punica granatum L.) in Pakistan. The Journal of Horticultural Science and Biotechnology, 92(2), 199–205. [Google Scholar]
- Naqvi, S. A. , Khan, M. , Shahid, M. , Jaskani, M. , Khan, I. A. , Zuber, M. , & Zia, K. M. (2011). Biochemical profiling of mucilage extracted from seeds of different citrus rootstocks. Carbohydrate Polymers, 83(2), 623–628. [Google Scholar]
- Naseem, Z. , Zahid, M. , Hanif, M. A. , & Shahid, M. (2020). Environmentally friendly extraction of bioactive compounds from Mentha arvensis using deep eutectic solvent as green extraction media. Polish Journal of Environmental Studies, 29(5), 3749–3757. [Google Scholar]
- Naz, S. , Hanif, M. , Bhatti, H. , & Shahid, M. (2016). Partition, fractionation, antioxidant potential and phenolics profiling of Cannabis sativa growing in Pakistan. Oxidation Communications, 39(4), 2946–2960. [Google Scholar]
- Nguyen, L. T. H. , Nguyen, U. T. , Kim, Y.‐H. , Shin, H.‐M. , & Yang, I.‐J. (2019). Astragali radix and its compound formononetin ameliorate diesel particulate matter‐induced skin barrier disruption by regulation of keratinocyte proliferation and apoptosis. Journal of Ethnopharmacology, 228, 132–141. [DOI] [PubMed] [Google Scholar]
- Nighat, F. , Mushtaq, Z. , Maqsood, M. , Shahid, M. , Hanif, M. A. , & Jamil, A. (2020). Cytotoxic, a‐amylase inhibitory and thrombolytic activities of organic and aqueous extracts of Bacillus clausii KP10. Pakistan Journal of Pharmaceutical Sciences, 33(1), 135–139. [PubMed] [Google Scholar]
- Noor, N. , Sarfraz, R. A. , Ali, S. , & Shahid, M. (2014). Antitumour and antioxidant potential of some selected Pakistani honeys. Food Chemistry, 143, 362–366. [DOI] [PubMed] [Google Scholar]
- Noreen, A. , Hussain, F. , & Shahid, M. (2020). Insights on the antioxidant, antidiabetic, antiamnesic, cytotoxic, thrombolytic and antibiofilm activities of Stevia rebaudiana leaves. Progress in Nutrition, 22(3), 1–9. [Google Scholar]
- Noreen, A. , Zia, K. M. , Tabasum, S. , Aftab, W. , Shahid, M. , & Zuber, M. (2020). Structural elucidation and biological aptitude of modified hydroxyethylcellulose‐polydimethyl siloxane based polyurethanes. International Journal of Biological Macromolecules, 150, 426–440. [DOI] [PubMed] [Google Scholar]
- Okpuzor, J. , Adebesin, O. , Ogbunugafor, H. , & Amadi, I. (2021). The potential of medicinal plants in sickle cell disease control: A review. International Journal of Biomedical and Health Sciences, 4(2), 1–10. [Google Scholar]
- Omata, M. , Matsui, N. , Inomata, N. , & Ohno, T. (1997). Protective effects of polysaccharide fucoidin on myocardial ischemia‐reperfusion injury in rats. Journal of Cardiovascular Pharmacology, 30(6), 717–724. [DOI] [PubMed] [Google Scholar]
- Özyürek, M. , Güçlü, K. , Tütem, E. , Başkan, K. S. , Erçağ, E. , Çelik, S. E. , Baki, S. , Yıldız, L. , Karaman, Ş. , & Apak, R. (2011). A comprehensive review of CUPRAC methodology. Analytical Methods, 3(11), 2439–2453. [Google Scholar]
- Pal, H. C. , Sharma, S. , Strickland, L. R. , Agarwal, J. , Athar, M. , Elmets, C. A. , & Afaq, F. (2013). Delphinidin reduces cell proliferation and induces apoptosis of non‐small‐cell lung cancer cells by targeting EGFR/VEGFR2 signaling pathways. PLoS One, 8(10), e77270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panigrahy, S. K. , Bhatt, R. , & Kumar, A. (2021). Targeting type II diabetes with plant terpenes: The new and promising antidiabetic therapeutics. Biologia, 76(1), 241–254. [Google Scholar]
- Patten, G. S. , Abeywardena, M. Y. , & Bennett, L. E. (2016). Inhibition of angiotensin converting enzyme, angiotensin II receptor blocking, and blood pressure lowering bioactivity across plant families. Critical Reviews in Food Science and Nutrition, 56(2), 181–214. [DOI] [PubMed] [Google Scholar]
- Pramila, K. , & Julius, A. (2019). In vitro antioxidant effect of green tea polyphenol epigallocatechin‐3‐Gallate (EGCG) in protecting cardiovascular diseases. Research Journal of Pharmacy and Technology, 12(3), 1265–1267. [Google Scholar]
- Przybylska, S. , & Tokarczyk, G. (2022). Lycopene in the prevention of cardiovascular diseases. International Journal of Molecular Sciences, 23(4), 1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qasim, N. , Shahid, M. , Yousaf, F. , Riaz, M. , Anjum, F. , Faryad, M. A. , & Shabbir, R. (2020). Therapeutic potential of selected varieties of Phoenix dactylifera L. against microbial biofilm and free radical damage to DNA. Dose‐Response, 18(4), 1559325820962609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qazi, A. , Nazir, M. , Shahid, M. , Butt, S. , & Basit, M. A. (2021). Facile development of hybrid bulk‐nanostructured SnSe/SnS for antibacterial activity with negligible cytotoxicity. Journal of Cluster Science, 32(3), 665–672. [Google Scholar]
- Quideau, S. , Deffieux, D. , Douat‐Casassus, C. , & Pouységu, L. (2011). Plant polyphenols: Chemical properties, biological activities, and synthesis. Angewandte Chemie International Edition, 50(3), 586–621. [DOI] [PubMed] [Google Scholar]
- Radha, R. , Chauhan, P. , Puri, S. , Thakur, M. , Rathour, S. , Sharma, A. , & Pundir, A. (2021). A study of wild medicinal plants used in Nargu wildlife sanctuary of district Mandi in Himachal Pradesh, India. Journal of Applied Pharmaceutical Science, 11, 135–144. [Google Scholar]
- Rahman, M. M. , Rahaman, M. S. , Islam, M. R. , Rahman, F. , Mithi, F. M. , Alqahtani, T. , Almikhlafi, M. A. , Alghamdi, S. Q. , Alruwaili, A. S. , Hossain, M. S. , Ahmed, M. , Das, R. , Emran, T. , & Hossain, M. S. (2021). Role of phenolic compounds in human disease: Current knowledge and future prospects. Molecules, 27(1), 233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos, S. (2008). Cancer chemoprevention and chemotherapy: Dietary polyphenols and signalling pathways. Molecular Nutrition & Food Research, 52(5), 507–526. [DOI] [PubMed] [Google Scholar]
- Rasgele, P. G. , & Dulger, G. (2021). Chemical compositions and antimutagenic effects of ethanolic extracts of Stachys thirkei and Stachys annua subsp. annua using the Ames assay. Pharmaceutical Chemistry Journal, 54(12), 1255–1262. [Google Scholar]
- Rasool, N. , Afzal, S. , Riaz, M. , Rashid, U. , Rizwan, K. , Zubair, M. , Ali, S. , & Shahid, M. (2013). Evaluation of antioxidant activity, cytotoxic studies and GC‐MS profiling of Matthiola incana (stock flower). Legume Research‐An International Journal, 36(1), 21–32. [Google Scholar]
- Rehman, F.‐U. , Adeel, S. , Shahid, M. , Bhatti, I. A. , Nasir, F. , Akhtar, N. , & Ahmad, Z. (2013). Dyeing of γ‐irradiated cotton with natural flavonoid dye extracted from irradiated onion shells (Allium cepa) powder. Radiation Physics and Chemistry, 92, 71–75. [Google Scholar]
- Riaz, M. , Rasool, N. , Bukhari, I. , Zubair, M. , Shahid, M. , Bokhari, T. , Gull, Y. , Rizwan , Iqbal, M. , & Zia‐Ul‐Haq, M. (2013). Antioxidant studies using sunflower oil as oxidant substrate and protective assay by Antirrhinum majus . Oxidation Communications, 36(1), 272–282. [Google Scholar]
- Riaz, M. , Rasool, N. , Bukhari, I. H. , Shahid, M. , Zahoor, A. F. , Gilani, M. A. , & Zubair, M. (2012). Antioxidant, antimicrobial and cytotoxicity studies of Russelia equisetiformis . African Journal of Microbiology Research, 6(27), 5700–5707. [Google Scholar]
- Riaz, M. , Rasool, N. , Bukhari, I. H. , Shahid, M. , Zubair, M. , Rizwan, K. , & Rashid, U. (2012). In vitro antimicrobial, antioxidant, cytotoxicity and GC‐MS analysis of Mazus goodenifolius . Molecules, 17(12), 14275–14287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riaz, T. , Abbasi, M. A. , Shazadi, T. , & Shahid, M. (2019). Assessment of Fumaria indica, Dicliptera bupleuroides and Curcuma zedoaria for their antimicrobial and hemolytic effects. Pakistan Journal of Pharmaceutical Sciences, 32(2), 697–702. [PubMed] [Google Scholar]
- Riaz, T. , Abbasi, M. A. , Siddiqui, S. Z. , Shahid, M. , Fatima, H. , Ashraf, M. , Fatima, H. , Ashraf, M. , Ejaz, S. A. , Rasool, Z. G. , & Abbasi, G. H. (2017). Enzyme inhibitory, antifungal, antibacterial and hemolytic potential of various fractions of Colebrookia oppositifolia . Pakistan Journal of Pharmaceutical Sciences, 30(1), 155–162. [PubMed] [Google Scholar]
- Riaz, T. , Zeeshan, R. , Zarif, F. , Ilyas, K. , Muhammad, N. , Safi, S. Z. , Rahim, A. , Rizvi, S. A. A. , & Rehman, I. U. (2018). FTIR analysis of natural and synthetic collagen. Applied Spectroscopy Reviews, 53(9), 703–746. [Google Scholar]
- Rizwan, K. , Zubair, M. , Rasool, N. , Ali, S. , Zahoor, A. F. , Rana, U. A. , Rahim, A. , Rizvi, S. A. A. , & Jaafar, H. Z. (2014). Regioselective synthesis of 2‐(bromomethyl)‐5‐aryl‐thiophene derivatives via palladium (0) catalyzed suzuki cross‐coupling reactions: as antithrombotic and haemolytically active molecules. Chemistry Central Journal, 8(1), 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha, F. S. , Gomes, A. J. , Lunardi, C. N. , Kaliaguine, S. , & Patience, G. S. (2018). Experimental methods in chemical engineering: Ultraviolet visible spectroscopy—UV‐vis. The Canadian Journal of Chemical Engineering, 96(12), 2512–2517. [Google Scholar]
- Romano, B. , Lucariello, G. , & Capasso, R. (2021). Topical collection “Pharmacology of medicinal plants”: multidisciplinary. Biomolecules, 11(1), 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubab, K. , Abbasi, M. A. , Siddiqui, S. Z. , Ashraf, M. , Shaukat, A. , Ahmad, I. , Hassan, S. , Lodhi, M. A. , Ghufran, M. , Shahid, M. , & Shahid, M. (2016). S‐alkylated/aralkylated 2‐(1H‐indol‐3‐yl‐methyl)‐1, 3, 4‐oxadiazole‐5‐thiol derivatives. 2. Anti‐bacterial, enzymeinhibitory and hemolytic activities. Tropical Journal of Pharmaceutical Research, 15(7), 1525–1533. [Google Scholar]
- Rubab, K. , Abbasi, M. A. , Siddiqui, S. Z. , Ashraf, M. , Shaukat, A. , Ahmad, I. , Lodhi, M. A. , Khan, F. A. , Shahid, M. , & Akhtar, M. N. (2015). Convergent synthesis of new N‐substituted 2‐5‐1H‐indol‐3‐ylmethyl‐1, 3, 4‐oxadiazol‐2‐yl sulfanyl acetamides as suitable therapeutic agents. Brazilian Journal of Pharmaceutical Sciences, 51, 931–947. [Google Scholar]
- Sadaf, S. , Shahid, M. , & Iqbal, Z. (2009). Chemical composition, antioxidant and antimicrobial activities of Citrus jambhiri Lush and Citrus reticulata Blanco essential oils. Journal of the Chemical Society of Pakistan, 31(5), 838–844. [Google Scholar]
- Safdar, M. , Naqvi, S. A. , Anjum, F. , Pasha, I. , Shahid, M. , Jaskani, M. J. , Khan, I. A. , & Aadil, R. M. (2021). Microbial biofilm inhibition, antioxidants and chemical fingerprints of Afghani pomegranate peel extract documented by GC‐MS and FTIR. Journal of Food Processing and Preservation, 45(7), e15657. [Google Scholar]
- Sahu, P. K. , Ramisetti, N. R. , Cecchi, T. , Swain, S. , Patro, C. S. , & Panda, J. (2018). An overview of experimental designs in HPLC method development and validation. Journal of Pharmaceutical and Biomedical Analysis, 147, 590–611. [DOI] [PubMed] [Google Scholar]
- Saini, A. , Pandey, A. , Sharma, S. , Suradkar, U. S. , Ambedkar, Y. R. , Meena, P. , Raman, R. , & Gurjar, A. S. (2020). Assessment of antioxidant activity of rosemary (Rosmarinus officinalis) leaves extract. Journal of Pharmacognosy and Phytochemistry, 9, 14–17. [Google Scholar]
- Sajid, A. , Sarfraz, R. A. , Hanif, M. A. , & Shahid, M. (2016). Evaluation of chemical composition and biological activities of Citrus pseudolimon and Citrus grandis peel essential oils. Journal of the Chemical Society of Pakistan, 38(2), 1–8. [Google Scholar]
- Sari, N. , Katanasaka, Y. , Honda, H. , Miyazaki, Y. , Sunagawa, Y. , Funamoto, M. , Shimizu, K. , Shimizu, S. , Wada, H. , Hasegawa, K. , & Morimoto, T. (2020). Cacao bean polyphenols inhibit cardiac hypertrophy and systolic dysfunction in pressure overload‐induced heart failure model mice. Planta Medica, 86(17), 1304–1312. [DOI] [PubMed] [Google Scholar]
- Shah, A. A. , Shahid, M. , Fatima, H. , Iftikhar, S. , & Saleem, R. S. Z. (2019). Synthesis of new S‐substituted derivatives of 5‐[3‐(1H‐indol‐3‐yl) propyl]‐1, 3, 4‐oxadiazol‐2‐ylhydrosulfide as suitable antibacterial and anticancer agents with moderate cytotoxicity. Pakistan Journal of Pharmaceutical Sciences, 32(6), 2585–2597. [PubMed] [Google Scholar]
- Shahid, M. , Anjum, F. , Iqbal, Y. , Khan, S. G. , & Pirzada, T. (2020). Modification of date palm mucilage and evaluation of their nutraceutical potential. Pakistan Journal of Agricultural Sciences, 57(2), 401–411. [Google Scholar]
- Shahid, M. , Bukhari, S. A. , Gul, Y. , Munir, H. , Anjum, F. , Zuber, M. , Jamil, T. , & Zia, K. M. (2013). Graft polymerization of guar gum with acryl amide irradiated by microwaves for colonic drug delivery. International Journal of Biological Macromolecules, 62, 172–179. [DOI] [PubMed] [Google Scholar]
- Shahid, M. , Khan, M. , Hameed, A. , Ashraf, M. , & Jamil, A. (2012). Antioxidant enzymes and inorganic elements in seeds and leaves of four potential medicinal plants from Pakistan. Agrochimica, 56(6), 281–291. [Google Scholar]
- Shahid, M. , Naureen, I. , Riaz, M. , Anjum, F. , Fatima, H. , & Rafiq, M. A. (2021). Biofilm inhibition and antibacterial potential of different varieties of garlic (Allium sativum) against sinusitis isolates. Dose‐Response, 19(4), 15593258211050491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahid, M. , Riaz, M. , Talpur, M. , & Pirzada, T. (2016). Phytopharmacology of Tribulus terrestris . Journal of Biological Regulators and Homeostatic Agents, 30(3), 785–788. [PubMed] [Google Scholar]
- Shahid, M. , Tayyab, M. , Naz, F. , Jamil, A. , Ashraf, M. , & Gilani, A. H. (2008). Activity‐guided isolation of a novel protein from Croton tiglium with antifungal and antibacterial activities. Phytotherapy Research, 22(12), 1646–1649. [DOI] [PubMed] [Google Scholar]
- Shahzadi, T. , Riaz, T. , Abbasi, M. A. , Mazhar, F. , Shahid, M. , & Ajaib, M. (2018). Wendlandia exserta: A pertinent source of antioxidant and antimicrobial agent. Turkish Journal of Biochemistry, 43(4), 456–463. [Google Scholar]
- Shahzadi, T. , Zaib, M. , Riaz, T. , Shehzadi, S. , Abbasi, M. A. , & Shahid, M. (2019). Synthesis of eco‐friendly cobalt nanoparticles using Celosia argentea plant extract and their efficacy studies as antioxidant, antibacterial, hemolytic and catalytical agent. Arabian Journal for Science and Engineering, 44(7), 6435–6444. [Google Scholar]
- Shang, A. , Gan, R.‐Y. , Xu, X.‐Y. , Mao, Q.‐Q. , Zhang, P.‐Z. , & Li, H.‐B. (2021). Effects and mechanisms of edible and medicinal plants on obesity: An updated review. Critical Reviews in Food Science and Nutrition, 61(12), 2061–2077. [DOI] [PubMed] [Google Scholar]
- Sharif, S. , Atta, A. , Huma, T. , Shah, A. A. , Afzal, G. , Rashid, S. , Shahid, M. , & Mustafa, G. (2018). Anticancer, antithrombotic, antityrosinase, and anti‐α‐glucosidase activities of selected wild and commercial mushrooms from Pakistan. Food Science & Nutrition, 6(8), 2170–2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen, T. , Han, X.‐Z. , Wang, X.‐N. , Fan, P.‐H. , Ren, D.‐M. , & Lou, H.‐X. (2017). Protective effects of dietary polyphenols in human diseases and mechanisms of action. In Al‐Gubory K. & Laher I. (Eds.), Nutritional antioxidant therapies: Treatments and perspectives (pp. 307–345). Springer. [Google Scholar]
- Shi, H. , Shi, A. , Dong, L. , Lu, X. , Wang, Y. , Zhao, J. , Dai, F. , & Guo, X. (2016). Chlorogenic acid protects against liver fibrosis in vivo and in vitro through inhibition of oxidative stress. Clinical Nutrition, 35(6), 1366–1373. [DOI] [PubMed] [Google Scholar]
- Shi, Y.‐J. , Sun, L.‐L. , Ji, X. , Shi, R. , Xu, F. , & Gu, J.‐H. (2021). Neuroprotective effects of oleanolic acid against cerebral ischemia‐reperfusion injury in mice. Experimental Neurology, 343, 113785. [DOI] [PubMed] [Google Scholar]
- Shimizu, M. , Shirakami, Y. , Sakai, H. , Tatebe, H. , Nakagawa, T. , Hara, Y. , Weinstein, I. B. , & Moriwaki, H. (2008). EGCG inhibits activation of the insulin‐like growth factor (IGF)/IGF‐1 receptor axis in human hepatocellular carcinoma cells. Cancer Letters, 262(1), 10–18. [DOI] [PubMed] [Google Scholar]
- Singh, A. , & Easwari, T. (2021). Recent advances in psoriasis therapy: Trends and future prospects. Current Drug Targets, 22(15), 1760–1771. [DOI] [PubMed] [Google Scholar]
- Singh, A. K. , Singh, S. K. , Nandi, M. K. , Mishra, G. , Maurya, A. , Rai, A. , Rai, G. K. , Awasthi, R. , Sharma, B. , & Kulkarni, G. T. (2019). Berberine: A plant‐derived alkaloid with therapeutic potential to combat Alzheimer's disease. Central Nervous System Agents in Medicinal Chemistry, 19(3), 154–170. [DOI] [PubMed] [Google Scholar]
- Sohaib, M. , Anjum, F. M. , Sahar, A. , Arshad, M. S. , Rahman, U. U. , Imran, A. , & Hussain, S. (2017). Antioxidant proteins and peptides to enhance the oxidative stability of meat and meat products: A comprehensive review. International Journal of Food Properties, 20(11), 2581–2593. [Google Scholar]
- Sohaib, M. , Butt, M. S. , Shabbir, M. A. , & Shahid, M. (2015). Lipid stability, antioxidant potential and fatty acid composition of broilers breast meat as influenced by quercetin in combination with α‐tocopherol enriched diets. Lipids in Health and Disease, 14(1), 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soulimani, R. , Dicko, A. , Baudelaire, E. , & Bouayed, J. (2021). Increased anti‐inflammatory activity and enhanced phytochemical concentrations in superfine powders obtained by controlled differential sieving process from four medicinal plants. International Journal for Vitamin and Nutrition Research. 10.1024/0300-9831/a000739 [DOI] [PubMed] [Google Scholar]
- Sridhar, K. , & Charles, A. L. (2019). In vitro antioxidant activity of Kyoho grape extracts in DPPH and ABTS assays: Estimation methods for EC50 using advanced statistical programs. Food Chemistry, 275, 41–49. [DOI] [PubMed] [Google Scholar]
- Stefanska, B. (2012). Curcumin ameliorates hepatic fibrosis in type 2 diabetes mellitus–insights into its mechanisms of action. British Journal of Pharmacology, 166(8), 2209–2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultana, S. , Munir, N. , Mahmood, Z. , Riaz, M. , Akram, M. , Rebezov, M. , Kuderinova, N. , Moldabayeva, Z. , Shariati, M. A. , Rauf, A. , & Rauf, A. (2021). Molecular targets for the management of cancer using Curcuma longa Linn. Phytoconstituents: A review. Biomedicine & Pharmacotherapy, 135, 111078. [DOI] [PubMed] [Google Scholar]
- Sur, S. , Pal, D. , Roy, R. , Barua, A. , Roy, A. , Saha, P. , & Panda, C. K. (2016). Tea polyphenols EGCG and TF restrict tongue and liver carcinogenesis simultaneously induced by N‐nitrosodiethylamine in mice. Toxicology and Applied Pharmacology, 300, 34–46. [DOI] [PubMed] [Google Scholar]
- Swanson, K. V. , Deng, M. , & Ting, J. P.‐Y. (2019). The NLRP3 inflammasome: Molecular activation and regulation to therapeutics. Nature Reviews Immunology, 19(8), 477–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swargiary, A. , Verma, A. K. , Singh, S. , Roy, M. K. , & Daimari, M. (2021). Antioxidant and antiproliferative activity of selected medicinal plants of lower assam, India: An in vitro and in silico study. Anti‐Cancer Agents in Medicinal Chemistry, 21(2), 267–277. [DOI] [PubMed] [Google Scholar]
- Tang, B. , Chen, G.‐X. , Liang, M.‐Y. , Yao, J.‐P. , & Wu, Z.‐K. (2015). Ellagic acid prevents monocrotaline‐induced pulmonary artery hypertension via inhibiting NLRP3 inflammasome activation in rats. International Journal of Cardiology, 180, 134–141. [DOI] [PubMed] [Google Scholar]
- Tanweer, S. , Shehzad, A. , Butt, M. S. , & Shahid, M. (2018). Probing the therapeutical potential of conventional and supercritical fluid extract of Zingiber officinale to mitigate ulcer, inflammation, hepatotoxicity and nephron toxicity. Progress in Nutrition, 20, 255–278. [Google Scholar]
- Ullah, A. A. , Munir, H. , & Shahid, M. (2019). Synthesis of Bombax malabaricum gum based silver and zinc nanoparticles and their application in controlled drug delivery. Materials Research Express, 6(11), 115414. [Google Scholar]
- Ullah, Q. (2020). Separation and analysis of heavy metal ions by thin‐layer chromatography (TLC)—A mini‐review (2000–2019). JPC–Journal of Planar Chromatography–Modern TLC, 33(4), 329–340. [Google Scholar]
- Vinatoru, M. (2001). An overview of the ultrasonically assisted extraction of bioactive principles from herbs. Ultrasonics Sonochemistry, 8(3), 303–313. [DOI] [PubMed] [Google Scholar]
- Wang, D. , Lao, L. , Pang, X. , Qiao, Q. , Pang, L. , Feng, Z. , Bai, F. , Sun, X. , Lin, X. , & Wei, J. (2018). Asiatic acid from Potentilla chinensis alleviates non‐alcoholic fatty liver by regulating endoplasmic reticulum stress and lipid metabolism. International Immunopharmacology, 65, 256–267. [DOI] [PubMed] [Google Scholar]
- Wang, F. , Gao, Q. , Yang, J. , Wang, C. , Cao, J. , Sun, J. , Fan, Z. , & Fu, L. (2020). Artemisinin suppresses myocardial ischemia–reperfusion injury via NLRP3 inflammasome mechanism. Molecular and Cellular Biochemistry, 474(1), 171–180. [DOI] [PubMed] [Google Scholar]
- Wang, F. , Wang, Y.‐H. , Wang, J.‐J. , Xu, H.‐L. , & Wang, C.‐M. (2016). Eriodictyol‐induced anti‐cancer and apoptotic effects in human hepatocellular carcinoma cells are associated with cell cycle arrest and modulation of apoptosis‐related proteins. Bangladesh Journal of Pharmacology, 11(2), 285–291. [Google Scholar]
- Wang, X. , Zhang, Z.‐F. , Zheng, G.‐H. , Wang, A.‐M. , Sun, C.‐H. , Qin, S.‐P. , Zhuang, J. , Lu, J. , Ma, D.‐F. , & Zheng, Y.‐L. (2017). The inhibitory effects of purple sweet potato color on hepatic inflammation is associated with restoration of NAD+ levels and attenuation of NLRP3 inflammasome activation in high‐fat‐diet‐treated mice. Molecules, 22(8), 1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, X.‐Y. , Dong, W.‐P. , Bi, S.‐H. , Pan, Z.‐G. , Yu, H. , Wang, X.‐W. , Ma, T. , Wang, J. , & Zhang, W. D. (2013). Protective effects of osthole against myocardial ischemia/reperfusion injury in rats. International Journal of Molecular Medicine, 32(2), 365–372. [DOI] [PubMed] [Google Scholar]
- Wang, Y. , Che, J. , Zhao, H. , Tang, J. , & Shi, G. (2018). Platycodin D inhibits oxidative stress and apoptosis in H9c2 cardiomyocytes following hypoxia/reoxygenation injury. Biochemical and Biophysical Research Communications, 503(4), 3219–3224. [DOI] [PubMed] [Google Scholar]
- Waseem, M. , Naqvi, S. A. , Haider, M. S. , Shahid, M. , Jaskani, M. J. , Khan, I. A. , & Abbas, H. (2021). Antioxidant activity, sugar quantification, phytochemical and physical profiling of apricot varieties of Chitral and Gilgit‐Pakistan. Pakistan Journal of Botany, 53, 1–9. [Google Scholar]
- Wei, L. , Chen, Q. , Guo, A. , Fan, J. , Wang, R. , & Zhang, H. (2018). Asiatic acid attenuates CCl4‐induced liver fibrosis in rats by regulating the PI3K/AKT/mTOR and Bcl‐2/Bax signaling pathways. International Immunopharmacology, 60, 1–8. [DOI] [PubMed] [Google Scholar]
- Yadav, A. , & Mohite, S. (2020). Screening of in‐vitro anti‐inflammatory and antibacterial assay of Malvastrum coromandelianum . International Journal of Pharma Sciences and Research, 11(4), 68–70. [Google Scholar]
- Yan, Y. , Liu, N. , Hou, N. , Dong, L. , & Li, J. (2017). Chlorogenic acid inhibits hepatocellular carcinoma in vitro and in vivo. The Journal of Nutritional Biochemistry, 46, 68–73. [DOI] [PubMed] [Google Scholar]
- Yazarlu, O. , Iranshahi, M. , Kashani, H. R. K. , Reshadat, S. , Habtemariam, S. , Iranshahy, M. , & Hasanpour, M. (2021). Perspective on the application of medicinal plants and natural products in wound healing: A mechanistic review. Pharmacological Research, 105841, 105841. [DOI] [PubMed] [Google Scholar]
- Yeh, M.‐H. , Kao, S.‐T. , Hung, C.‐M. , Liu, C.‐J. , Lee, K.‐H. , & Yeh, C.‐C. (2009). Hesperidin inhibited acetaldehyde‐induced matrix metalloproteinase‐9 gene expression in human hepatocellular carcinoma cells. Toxicology Letters, 184(3), 204–210. [DOI] [PubMed] [Google Scholar]
- Yen, H.‐R. , Liu, C.‐J. , & Yeh, C.‐C. (2015). Naringenin suppresses TPA‐induced tumor invasion by suppressing multiple signal transduction pathways in human hepatocellular carcinoma cells. Chemico‐Biological Interactions, 235, 1–9. [DOI] [PubMed] [Google Scholar]
- Yi, C. , Song, M. , Sun, L. , Si, L. , Yu, D. , Li, B. , Lu, P. , Wang, W. , & Wang, X. (2022). Asiatic acid alleviates myocardial ischemia‐reperfusion injury by inhibiting the ROS‐mediated mitochondria‐dependent apoptosis pathway. Oxidative Medicine and Cellular Longevity, 2022, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousaf, F. , Shahid, M. , Riaz, M. , Atta, A. , & Fatima, H. (2017). Immunomodulatory potential of Anacyclus pyrethrum (L.) and Mucuna pruriens (L.) in male albino rats. Journal of Biological Regulators & Homeostatic Agents, 31(2), 425–429. [PubMed] [Google Scholar]
- Yu, H.‐B. , Zhang, H.‐F. , Zhang, X. , Li, D.‐Y. , Xue, H.‐Z. , Pan, C.‐E. , & Zhao, S.‐H. (2010). Resveratrol inhibits VEGF expression of human hepatocellular carcinoma cells through a NF‐kappa B‐mediated mechanism. Hepato‐Gastroenterology, 57(102–103), 1241–1246. [PubMed] [Google Scholar]
- Yuyun, X. , Xi, C. , Qing, Y. , Lin, X. , Ke, R. , & Bingwei, S. (2018). Asiatic acid attenuates lipopolysaccharide‐induced injury by suppressing activation of the notch signaling pathway. Oncotarget, 9(19), 15036–15046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahid, M. , Bashir, M. , Shahid, M. , & Khan, B. (2016). Antioxidant, hemolytic, biofilm inhibition and toxicity evaluation of some selected plant origin pesticides for predatory mite Neoseiulus barkeri (Acari: Phytoseiidae). Oxidation Communications, 39(2), 1346–1360. [Google Scholar]
- Zapf, M. A. , Kothari, A. N. , Weber, C. E. , Arffa, M. L. , Wai, P. Y. , Driver, J. , Gupta, G. N. , Kuo, P. C. , & Mi, Z. (2015). Green tea component epigallocatechin‐3‐gallate decreases expression of osteopontin via a decrease in mRNA half‐life in cell lines of metastatic hepatocellular carcinoma. Surgery, 158(4), 1039–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidali, E. , Mardani Korrani, H. , Alizadeh, Y. , & Kamari, F. (2021). Ethnopharmacological survey of medicinal plants in semi‐arid rangeland in western Iran. Central Asian Journal of Plant Science Innovation, 1(1), 46–55. [Google Scholar]
- Zhang, H. , Sun, Q. , Xu, T. , Hong, L. , Fu, R. , Wu, J. , & Ding, J. (2016). Resveratrol attenuates the progress of liver fibrosis via the Akt/nuclear factor‐κB pathways. Molecular Medicine Reports, 13(1), 224–230. [DOI] [PubMed] [Google Scholar]
- Zhang, J. , Song, J. , Wu, D. , Wang, J. , & Dong, W. (2015). Hesperetin induces the apoptosis of hepatocellular carcinoma cells via mitochondrial pathway mediated by the increased intracellular reactive oxygen species, ATP and calcium. Medical Oncology, 32(4), 1–11. [DOI] [PubMed] [Google Scholar]
- Zhang, F. , Thakur, K. , Hu, F. , Zhang, J.‐G. , & Wei, Z.‐J. (2017). 10‐Gingerol, a phytochemical derivative from “Tongling white ginger”, inhibits cervical cancer: Insights into the molecular mechanism and inhibitory targets. Journal of Agricultural and Food Chemistry, 65(10), 2089–2099. [DOI] [PubMed] [Google Scholar]
- Zhu, N. , Li, J. , Li, Y. , Zhang, Y. , Du, Q. , Hao, P. , Li, J. , Cao, X. , & Li, L. (2020). Berberine protects against simulated ischemia/reperfusion injury‐induced H9C2 cardiomyocytes apoptosis in vitro and myocardial ischemia/reperfusion‐induced apoptosis in vivo by regulating the mitophagy‐mediated HIF‐1α/BNIP3 pathway. Frontiers in Pharmacology, 11, 367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zia, K. M. , Zuber, M. , Rizwan, A. , Jamil, T. , Tabasum, S. , & Shahid, M. (2012). Modification of cellulosic fabric using polyvinyl alcohol—Part‐I: Physicochemical properties. Carbohydrate Polymers, 87(3), 2063–2067. [DOI] [PubMed] [Google Scholar]
- Zuo, Y.‐H. , Liu, Y.‐B. , Cheng, C.‐S. , Yang, Y.‐P. , Xie, Y. , Luo, P. , Wang, W. , & Zhou, H. (2020). Isovaleroylbinankadsurin A ameliorates cardiac ischemia/reperfusion injury through activating GR dependent RISK signaling. Pharmacological Research, 158, 104897. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable—no new data are generated and this review article describes entirely the published literature.