Abstract
The mandate of the current investigation was to elucidate the therapeutic and antioxidant perspective of black tea. Purposely, black tea compositional analysis followed by polyphenol extraction and antioxidant characterization was done. Moreover, the theaflavin from black tea extract was also isolated through the solvent partition method. Lastly, the neuroprotective effect of isolated theaflavin was assessed through a bio‐efficacy trial. The outcomes delineated that black tea showed promising nutritional composition with special reference to protein and fiber. Among the extraction solvent, ethanol performed better as compared to methanol and water likewise, higher extraction was noticed at 60 min followed by 90 and 30 min. All the extracts indicated antioxidant activity reflected through significant DPPH, TPC, FRAP, and beta carotene as‐69.13 ± 3.00, 1148.92 ± 14.01, 752.44 ± 10.30, and 65.74 ± 3.28, respectively. However, isolated theaflavin exhibited higher antioxidant capacity as‐737.74 ± 12.55, 82.60 ± 2.33, and 853.77 ± 9.55, for TPC, DPPH, and FRAP, respectively, as compared to extracts. In 15 days' efficacy was physically induced with sciatic nerve injury h sciatic nerve injury physically and treated with isolated theaflavin. A total of 12 healthy albino mice were randomly assigned to either the control (n = 6) or theaflavin (5.0 mg/kg (n = 6)) groups. In these groups, behavioral tests were used to assess and compare enhanced functional recovery as well as skeletal muscle mass measurement. Serum samples included oxidative stress markers. In theaflavin leaves, behavioral tests revealed a statistically significant (p < .001) improvement in sensorimotor function restoration, muscle mass restoration, a substantial decrease in TOS, a significant increase in TAC, and enhanced antioxidative enzyme activity. Considering the above‐mentioned therapeutic perspectives of theaflavin, the current research was planned to optimize the isolation of theaflavin from black tea and probed for their neuroprotective effect in mice models.
Keywords: inflammatory response, neuroprotective effect, oxidative stress, theaflavin
The mandate of the current investigation was to elucidate the therapeutic and antioxidant perspective of black tea. Purposely, black tea compositional analysis followed by polyphenol extraction and antioxidant characterization was done. Moreover, the theaflavin from black tea extract was also isolated through the solvent partition method. Lastly, the neuroprotective effect of isolated theaflavin was assessed through a bioefficacy trial. The outcomes delineated that black tea showed promising nutritional composition with special reference to protein and fiber. Among the extraction solvent, ethanol performed better as compared to methanol and water likewise, higher extraction was noticed at 60 min followed by 90 and 30 min.
1. INTRODUCTION
Globally, oxidative stress caused numerous health disparities, directly and indirectly, owing to its adverse effect on human metabolism (Afzaal et al., 2023; Maqbool et al., 2023). Among the various curative strategies, polyphenol‐based dietary interventions are in limelight due to their capacity to normalize the imbalance between antioxidants and free radicals thus curtailing the menace of oxidative stress. Polyphenols are classified into four primary groups: flavonoids, lignans, stilbenes, and phenolic acids. Many in vivo and in vitro investigations have been conducted to assess their health consequences. They play a crucial function in defending the body against external stressors and removing reactive oxygen species (ROS), which are the root of many disorders (Islam et al., 2023). Polyphenols, which could be present in tea, cocoa, fruits, and vegetables, have the power to improve human health (Rana et al., 2022). Polyphenols have antioxidant, anti‐inflammatory, anti‐apoptotic, anticarcinogenic, and antibacterial properties and might be employed in medications, cosmetics, nutraceuticals, and food (Rajha et al., 2022). In humans, tea and coffee contain polyphenolic compounds that have antioxidant and neuroprotective properties (LIczbiński & Bukowska, 2022). They can also boost conception and help with Covid‐19 treatment. Because the brain has fewer antioxidant qualities than other organs, large levels of ROS appear to be more common, raising the risk of neurodegenerative diseases including Alzheimer's and Parkinson's (LIczbiński & Bukowska, 2022).
Tea is among the world's most nutritious beverages, and its aroma is an important component of the tea flavor profile as well as a significant predictor of tea quality (Li et al., 2013). The most common variety of tea is black tea, which accounts for 78% of global production (Chung, 2009; Imran et al., 2019). Tea consumption has been linked to oxidation, anti‐inflammation, and cancer prevention (de Majia et al., 2009), cardiovascular preventative medicine, and other health advantages (Stangl et al., 2007). Coffee has traditionally been linked to improved information, including brain clarity, focus, and relaxation. Several studies have discovered that the nutritional components of tea, notably caffeine, and the amino acid L‐theanine, change brain activity and have a positive impact on mental states such as emotional wellness and cognitive performance (Bryan, 2008). Parkinson's disease and Alzheimer's disease became two of the major neurodegenerative conditions worldwide (Mazumder & Choudhury, 2019; Zhou et al., 2019). Parkinson's disease affects 1–2% of the aging population, and the number of cases increases each year (Tysnes & Storstein, 2017). Alzheimer's disease impacted 35–40% of individuals over the age of 80, and its prevalence was rapidly increasing as the country's population aged (Chen et al., 2018; Mazumder & Choudhury, 2019). Tea may reduce the mortality rate of Parkinson's and Alzheimer's disease, as well as the risk of suicide, by reducing free radical precursors and inflammatory processes, changing variations, regulating the HPA axis and serotonin levels, and using metal‐chelating chemicals (Chen et al., 2018; Dong et al., 2015; Zhu et al., 2012). According to psychological studies, theaflavins protect the brain from long‐term damage (Anandhan et al., 2013). Theaflavins improved the behavior problem in an open area, rotary, and suspension tests (Anandhan et al., 2012). One study of 278 Parkinson's disease patients discovered that drinking more than three cups of tea each day could postpone the disease by 7.7 years (Kandinov et al., 2009). Pesticides and metal ions can cause peroxidation in the brain, which can cause dopamine cell death in Parkinson's disease patients (Chin‐Chan et al., 2015; Maturana et al., 2015). In the treatment of Parkinson's disease, tea has been proven to have neuroprotective qualities (Aquilano et al., 2008; Deb et al., 2019). Microglial cell activation was assumed to be important in selective neuron destruction in degenerative illnesses like Parkinson's disease (Li et al., 2004). Theaflavin from black tea (10 mg/kg) reduced the MPTP‐induced release of proinflammatory cytokines such as IL‐1β, IL‐6, TNF‐α, and IL‐6 in the striatum, showing that theaflavin's survival benefit is due to part to lower MPTP‐related neuron activation (Anandhan et al., 2013; Lagha & Grenier, 2016). Black tea extract upped the number of remaining tyrosine carbonic anhydrase immunoreactive neurons, the amount of tyrosine carbonic anhydrase protein, and the synthesis of tyrosine hydroxylase mRNA in the substantia nigra, all of which resulted in neuronal cell preservation (Chaturvedi et al., 2006). Theaflavin can diminish probenecid‐induced mortality by reducing apoptotic markers such as caspase‐3,8,9 (Anandhan et al., 2012). In an analysis of 26 epidemiological studies, tea drinking was significantly associated with a decreased frequency of impairments (Ma et al., 2016). Nevertheless, a study of 9375 Chinese people found that drinking black tea improved mental function but not green tea (Shen et al., 2015). Tea has been demonstrated to be a good source of ace inhibitors, which may help in Alzheimer's disease therapy (Baranowska‐Wojcik et al., 2020). Moreover, in Alzheimer's disease rats induced by aluminum chloride, black tea extract significantly improved cognitive deficits while decreasing the activity of (AChE) acetylcholinesterase (Mathiyazahan et al., 2015). Considering the above‐mentioned therapeutic perspectives of theaflavin, the current research was planned to optimize the isolation of theaflavin from black tea and probe for its neuroprotective effect in mice models.
2. MATERIALS AND METHODOLOGY
The present research was carried out in the nutritional laboratory of the department of food science, whereas an efficacy trial was conducted in the department of physiology. Black tea is produced in Faisalabad. The reagents and standards were supplied by Merck and Sigma‐Aldrich. Mice will be housed in the GCUF Physiology Department's Animal Room for the efficacy test. After inducing the sciatic nerve mechanical crush, two rat groups were created. The control group received regular rodent chow, whereas the other group received theaflavin extract mixed chow. The utility of behavioral analysis will be assessed.
The therapeutic potential of the examined medications against sciatic nerve injury was assessed using an efficacy trial. For this purpose, 12 mice were housed in the Animal House of the Department of physiology at GCUF, Faisalabad. The GCUF Departmental Ethical Committee accepted the procedure for this biological experiment, which satisfied ERC NO 2121 worldwide standards. For 2 weeks, the mice were fed a basic meal to help them adjust to their new surroundings. During the experiment, the temperature (22°C) and relative humidity (55%) will be monitored, as well as a 12‐h light–dark cycle.
3. THE CHARACTERISTICS OF BLACK TEA
At first, black tea was tested for configurational characteristics such as causal test, nutrient biomarker, nutrient characteristics, toxic metabolites, and total phenolic excavation.
4. EVALUATION OF CONSTITUENTS
Black tea tests were performed in triplicate for compositional analysis on a dry‐weight basis.
5. POLYPHENOL EXTRACTION
Tea polyphenols were derived in three different time intervals of 30, 60, and 90 min at a safe temperature of 60°C that used water, methanol, and ethanol process. After that, the sample was obtained through a muslin cloth, and the fluids were retrieved by rotary and freeze‐drying. Table 1 shows the method for extraction.
TABLE 1.
Procedure used to calculate extraction yield.
| Treatment | Solvent | Time (minutes) |
|---|---|---|
| T1 | Water (100%) | 30 |
| T2 | Methanol (%) | 60 |
| T3 | Ethanol (%) | 90 |
| T4 | Water (%) | 30 |
| T5 | Methanol (%) | 60 |
| T6 | Ethanol (%) | 90 |
| T7 | Water (100%) | 30 |
| T8 | Methanol (100%) | 60 |
| T9 | Ethanol (100%) | 90 |
6. ISOLATION OF THEAFLAVIN
It will be carried out at a 1:6 ratio at 30‐, 60‐, and 90‐min intervals, water will be used to extract theaflavin, and theaflavin will be separated using the solvent partition technique (Xie et al., 1993). With exception of liquid extracts, all extracts will be concentrated in boiling water, sifted, and dissolvable partitioned with chloroform, ethyl acetate, and butanol. Once the theaflavin‐rich fractions are separated, freeze‐drying (CHIRST, Alpha 1–4 LD plus, Germany) would be used.
7. BIOLOGICAL TEST
Theaflavin's therapeutic efficacy in the treatment of sciatic nerve injury was to be evaluated through an efficacy study. Male albino mice weighing 25–35 g were purchased from the Department of Physiology at Government College University Faisalabad's animal treatment facility. The creatures were kept in cages, one per cage (plastic rodent cage). Housing requirements such as room temperature of 23–27°C, supply of a 12‐h light and 12‐h dark cycle, sufficient moisture, and ad libitum delivery of diet and drinking water were maintained during the acclimatization period (1 week) and the whole experiment. All behavioral observations and experiments were conducted throughout the day.
8. BEHAVIORAL ANALYSES
8.1. Nociceptive analysis
The pinprick test is another method for assessing sensory capacity recovery after nerve damage. It was carried out by the procedure outlined by (Chen et al., 2018).
8.2. Walking track analysis
The sciatic functional index (SFI) is a numerical method for evaluating the motor functions of mice. The restoration of motor functions following sciatic nerve crush was investigated by calculating the SFI based on walking track analysis, as stated in previous studies by Komirishetty et al. (2016).
8.3. Grip strength analysis
Resistance training testing is an efficient method of assessing motor function recovery after a sciatic crush injury. The trying to comprehend the strength of both hind limbs (upper distal phalanx and caudal to the affected site) was evaluated for each mouse using a grip trimeter (Bioseb, Chaville, France). Its final result was calculated using the average of three readings taken at 1–2 min intervals (Hussain et al., 2013; Razzaq et al., 2020).
8.4. Muscle weight analysis
Muscular atrophy occurs when the connection between the muscle and the nerve is disrupted as a result of an injury, resulting in muscle mass. As a result, muscle mass evaluation provides a method for determining the degree of muscular atrophy, which is a major barrier to functional status. The soleus and tibialis anterior (TA) muscles were removed and weighed from both rear legs (upper extremity and contralateral to the affected site). In the same animals, the muscular proportion was computed by dividing the mass of the contralateral muscular even by the weight of the ipsilateral lean muscle. To assess operational retrieval, the mean ratio was calculated for each group and compared across groups (Hagstrom et al., 2004).
8.5. Random glycemic level
The random glycemic data were measured throughout nerve impingement initiation to investigate the role of glucose in enraging neurotic episodes in the nervous injured area. Hyperglycemia inhibits its healing process after injuries. In both groups, the glucose level was evaluated by depositing a sample of spider tail blood on a blood sugar meter strip and measuring it with an Accu‐Check glucometer, as previously stated (Asmat et al., 2016; Razzaq et al., 2020).
9. BIOCHEMICAL ANALYSES
9.1. Total antioxidant capacity
A body system's antioxidant activities are defined as its ability to confront free radicals produced as a result of various pathogenetic processes that occur in the body. An ideal antioxidant‐bearing capacity improves the body's ability to resist various ailments (Erel, 2004).
9.2. Total oxidant status
The degree of Total oxidant status (TOS) within a living system is reported to be related to the stage of oxidative stress. This test determines the overall state of oxidative stress. This was achieved by utilizing the protocol outlined in previous studies (Aziz et al., 2019; Erel, 2005).
10. STATISTICAL ANALYSIS
SAS was utilized to evaluate the data gathered throughout this investigation (version 9.1; Cary, NC). A two‐way ANOVA was used to assess the effect of extreme action period and dissolvable on polyphenol isolation (ANOVA). Furthermore, in the efficacy study, ANOVA and LSD were used to assess the effectiveness of therapies using Graph Pad prism 8.4.2.
11. RESULTS AND DISCUSSION
Proximate analysis is a critical factor in determining raw material quality. Black tea (dry weight basis) was subjected to different quality traits assessment and revealed moisture, crude fat, crude protein, crude fiber, ash, NFE, and total alkaloids as 7.02 ± 0.21, 4.60 ± 0.20, 15.13 ± 0.70, 15.30 ± 0.76, 4.87 ± 0.22, 53.15 ± 2.01, and 2.51 ± 0.08, respectively (Table 2).
TABLE 2.
Characteristics of black tea analysis.
| Proximate analysis of black tea | |
|---|---|
| Parameters | Quantity |
| Moisture (%) | 7.02 ± 0.21 |
| Ash (%) | 4.87 ± 0.22 |
| Crude fat (%) | 4.60 ± 0.20 |
| Crude fiber (%) | 15.30 ± 0.76 |
| Crude protein (%) | 15.13 ± 0.70 |
| NFE | 53.15 ± 2.01 |
| Total alkaloids | 2.51 ± 0.08 |
Note: Values are expressed as means ± standard deviation (n = 3).
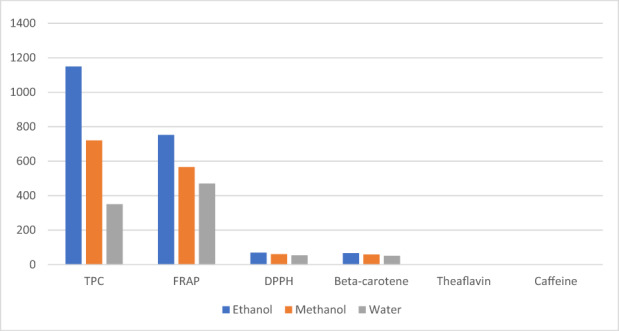
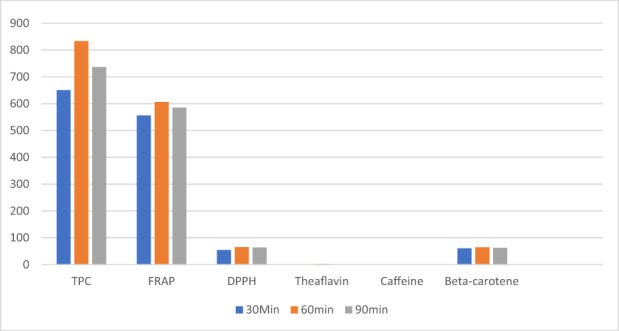
The findings of the current investigation regarding the proximate profile are in line with the earlier findings of Aregbesola et al. (2018) determined moisture, ash, protein, and fiber levels in black tea samples as 5.94, 5.46, 11.03, and 14.25, respectively. Earlier, Modupe et al. (2013) determined moisture, ash, crude fat, crude protein, and nitrogen levels in black tea were 8.10, 9.59, 3.25, 17.78, and 2.83%, respectively. According to Ramdani et al. (2018), green tea alkaloids (31.5%) are significantly higher than black tea alkaloids (28.7%). The alkaloids result was statistically significant when compared with the previous findings of Erol et al. (2010), who discovered total alkaloids ranging from 25.97 to 26.26 mg/g in various Turkish black tea samples. The antioxidant properties of black tea extracts are affected by the solvent used and the time of extraction. Increasing the extraction time from 30 to 60 min increased antioxidant activity in all extracts; however, at 90 min, antioxidant activity decreased. The ethanolic extract significantly outperforms the water and methanolic extracts shown in Figures 1, 2, 3.
FIGURE 1.
Effect of solvent on black tea extract.
FIGURE 2.
Potential of time on black tea extract.
FIGURE 3.
Extraction yield of theaflavin.
The means for solvents regarding antioxidants indices (Figure 3) showed the highest values for an ethanolic extract for TPC (1148.92 ± 14.01a), FRAP (752.44 ± 10.30), DPPH (69.13 ± 3.00) ß‐carotene (65.74 ± 3.28), theaflavin (2.52 ± 0.12), and caffeine (1.88 ± 0.06), respectively, as compared to the methanol and water. The methanolic extract performs better than water as 720.41 ± 10.23, 565.78 ± 11.01, 60.37 ± 2.00, 58.87 ± 2.94, 2.04 ± 0.10, and 1.70 ± 0.07, respectively, for these traits. Likewise, the lowest values were recorded in water extract 350.02 ± 5.12, 469.78 ± 10.50, 53.27 ± 3.10, 50.27 ± 3.80, 1.22 ± 0.01, and 1.55 ± 0.08, respectively.
Similarly, time (Figure 4) also showed good performance at 60 min for TPC (833.30 ± 20.1a mg/100 g GAE), FRAP (605.89 ± 15.904a μmol Fe2+/g), DPPH (65.30 ± 3.00a %), ß‐carotene (64.24 ± 3.60a %), theaflavin (2.00 ± 0.13a %), except for caffeine (1.67 ± 0.04a %). However, the extracts at 90 min showed maximum caffeine (1.74 ± 0.05b %). Likewise, the extracts at 30 min showed minimum values for TPC, FRAP, DPPH, ß‐carotene, theaflavin, catechins, and caffeine by 650.44 ± 15.01c mg/100 g GAE, 556.00 ± 13.01c μmol Fe2+/g, 59.69 ± 2.30%, 60.30 ± 2.80c, 1.69 ± 0.04c, and 1.63 ± 0.01, respectively.
FIGURE 4.
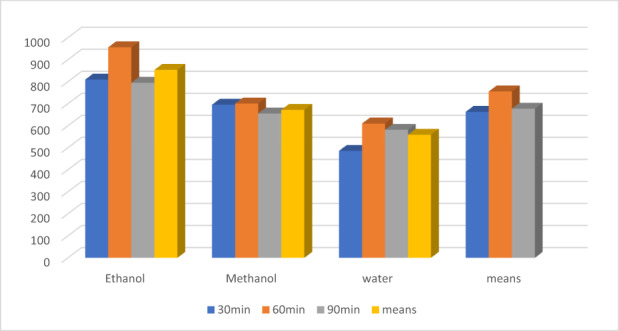
FRAP (theaflavin) μmol Fe2+/g.
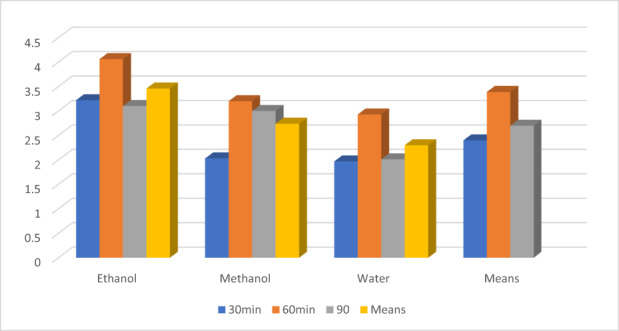
Extraction efficiency was influenced by time and the maximum yield for theaflavin was obtained at 60 min 3.39 ± 0.17 g/100 g, while, minimum at 30 min 2.40 ± 0.12, respectively. However, at 90 min, the values were 2.71 ± 0.14 for theaflavin (Figure 5).
FIGURE 5.
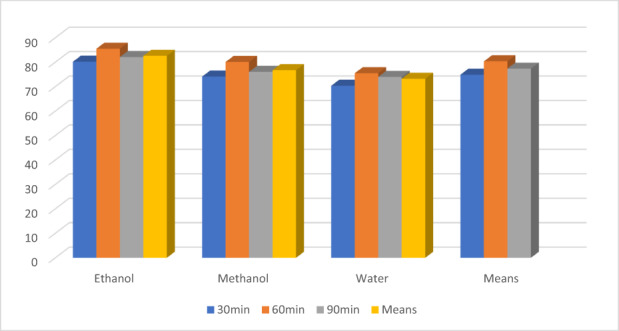
DPPH activity (theaflavin)%.
Yang et al. (2008) showed that 1.51 g/50 g of theaflavin in black tea samples which is comparable with theaflavin yield results. They used ethyl alcohol as an extraction fluid during the experiment. The difference in extraction yields in this test is due to the incorrect assumption that extraction times were 40 min rather than 60 min. Tea polyphenols recovered 75–80% of their isolation, according to Sharma and Zhou (2011).
The theaflavin showed high value for FRAP in ethanol than for methanol and water. The FRAP values of theaflavin in ethanolic, methanolic, and water extract were 853.77 ± 9.55, 672.11 ± 12.33, and 559.19 ± 11.22 μmol Fe2+/g, shown in Figure 4, respectively. Similarly, for time intervals, theaflavin showed higher FRAP activity at 30 min (663 ± 10.33 μmol Fe2+/g), 60 min (755.86 ± 8.33 μmol Fe2+/g), and 90 min (677.55 ± 9.34 μmol Fe2+/g). Likewise, the statistical analysis showed the momentous effect of time and solvents for DPPH. Figure 5 showed the highest values for DPPH in theaflavin in ethanolic extract 82.60 ± 2.33%, as compared to the methanol 76.73 ± 3.23%, and water 73.23 ± 2.63%, correspondingly. Time also showed the highest DPPH activity of theaflavin was 80.36 ± 4.00% at 60 min, while 77.36 ± 3.35 and 74.89 ± 3.62% at 90 min and 30 min, respectively.
Furthermore, statistical analysis elucidated the significant effect of solvent and time for the ß‐carotene activity of theaflavin presented in Figure 6. Similarly, ethanolic extract showed maximum activity of 66.74 ± 3.22% than methanol 62.20 ± 2.88% and water 59.27 ± 2.55%. Time also showed that 60‐min extraction time resulted in higher ß‐carotene activity 66.24 ± 2.15% in comparison with 90 and 30 min by 63.00 ± 3.00 and 58.97 ± 2.44%, respectively.
FIGURE 6.
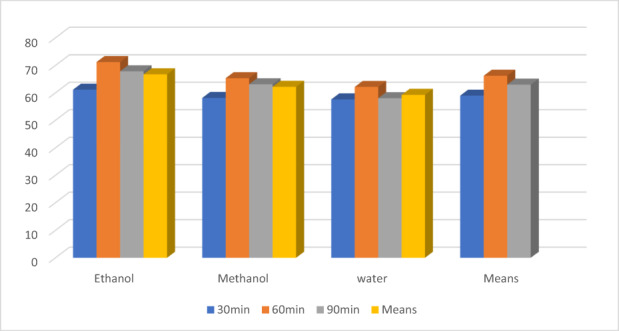
ß‐carotene (theaflavin)%.
The statistical analysis shown imparted a significant effect of solvents and time on the yield of TPC. Theaflavin showed TPC values maximum for ethanol as compared to methanol and water. Figure 7 showed higher activity for theaflavin. The TPC values in ethanolic were 737.74 ± 12.55, followed by methanol and ethanol 735.08 ± 10.22 and 732.77 ± 9.99 mg/100 g GAE, correspondingly. However, for time intervals, theaflavin showed higher TPC activity at 60 min (827.99 ± 15.55 mg/100 g GAE). While at 30 min, theaflavin showed minimum value (645.10 ± 12.44 mg/100 g GAE), and at 90 min, theaflavin showed732.55 ± 8.55 mg/100 g GAE.
FIGURE 7.
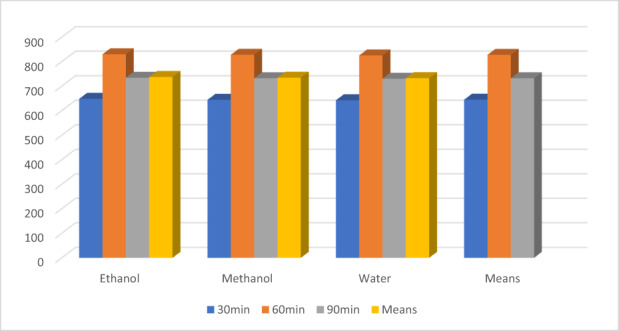
TPC mg/100 g GAE.
The statistical analysis shown elucidated a significant effect of solvents and time on the yield of Caffeine. The value for Caffeine% activity of theaflavin showed a maximum in ethanol, while the minimum for water is shown in Figure 8. In theaflavin, ethanolic extract showed maximum activity of 1.67 ± 0.07% than methanol 1.66 ± 0.06% and water 1.64 ± 0.05%. Furthermore, 90‐min extraction time resulted in higher Caffeine activity by 1.71 ± 0.04% in comparison with 60 and 30 min by 1.65 ± 0.02 and 1.61 ± 0.01%, respectively. Initially, Sun et al. (2012) investigated the DPPH free radical scavenging capacity of an Assam black tea extract (ASTE) and theaflavin combination in vitro, and their results are in resemble to the most recent DPPH free radical scavenging activity findings (TFSM). They discovered that TFSM has a higher DPPH value than ASTE (60–97%) (40–82%). Initially, Friedman et al. (2006) created bioactive fragments with aqueous ethanol and water at different solvent‐to‐water ratios of 60%, 70%, and 80%. TF1, TF2, TF2B, and TF3 levels in ethanolic extract ranged from 1.7 to 4.4, 3.6 to 5.8, 0.4 to 1.8, and 1.6 to 5.12 mg/g, respectively. The affiliated subsets in water extract ranged from 0.1 to 1.1, 1.2 to 2.1, 0.2 to 1.10, and 1.1 to 3.21 mg/g. According to the most recent research, ethanol and methanol produce higher polyphenolic yields of tea catechins than water, which support the findings on the effect of various solvents on tea polyphenol extraction (Bastos et al., 2007). The study's findings are aligned with those of another research study conducted by (Chen et al., 2012).
FIGURE 8.
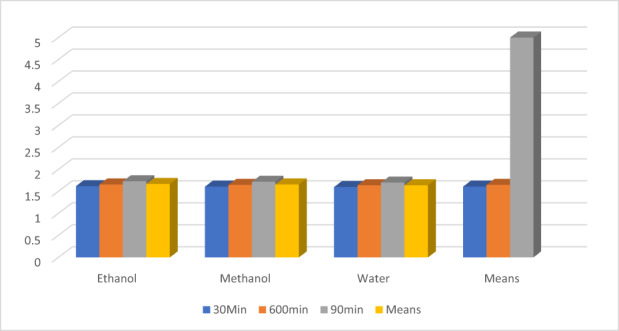
Caffeine %.
12. BIOLOGICAL TRAILS
Statistical analysis elucidated the significant effect of treatment and study interval on the pinprick test of nerve injury in mice. Similarly, Figure 9 shows that the sensory threshold was assessed using pinprick analysis, and the treatment group produced significantly higher scores in response to the pinprick stimulus. On day 7, the 5.0 mg/kg group appeared to be significantly (p = .01) effective, and on day 10, it appeared to be highly effective (p = .0001). As a result, statistically significant improvements in outcomes, as shown in Figure 9, were observed. These findings support theaflavin's ability to lower sensory threshold following sciatic nerve injury.
FIGURE 9.
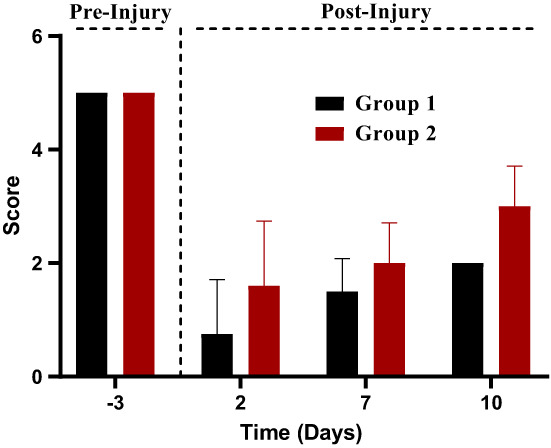
Paw withdrawal latency.
Despite technological advancement, humanity has been plagued by a wide range of diseases. Regulating and tracking cases of life‐long disability or biological reliance caused by nerve damage caused by various types of crashes, most notably roadside motor vehicle crashes, gunfire, and sudden falls, is one of the most difficult issues in the field of health research (Kouyoumdjian et al., 2017). Unfortunately, these incidents are one of the leading causes of PNI development, especially in over‐packed, stunted countries like Pakistan, where the transportation system is already overburdened (Mushtaq et al., 2021). The pinprick test was used to measure sensory function recovery after sciatic nerve injury. This memory test tests mice's sensory threshold retrieval and our results are in line with his findings (Deuis et al., 2017). One of these critical features is oxidative stress, which greatly contributes to the pathophysiological processes that occur at the injury site (Wang et al., 2015). The reaction spirals which include mitochondrial disorders, dementia, neuronal damage, and apoptosis (Hussain et al., 2020) induce systemic nerve damage, produce oxidants that aggravate the harm, and delay diagnosis (Al‐Nimer et al., 2012; Areti et al., 2014). When compared with the control group, the treatment group demonstrated substantial improvement in motor functions in terms of SFI measurement and grip strength force (percent of original force) (Figure 10). According to SFI results, animals in the treatment group showed a considerably better walking pattern. In this study, the 5.0 mg/kg groups were notably efficient at normalizing SFI levels on day 6 (p = .001) and day 9 (p = .006) after injury. These data suggest that theaflavin can hasten motor functional recovery.
FIGURE 10.
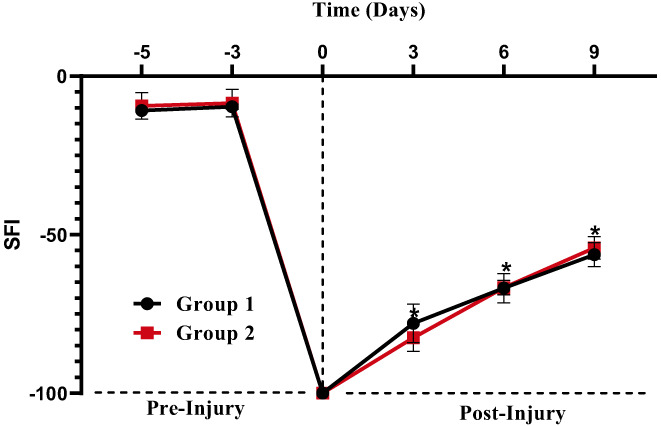
Sciatic functional index.
When compared with the control group, all treatment groups improved significantly in terms of grip strength force (percent of beginning force) shown above in (Figure 11). When compared with controls on day 6 post‐injury, treatment groups showed a significant difference (rapid restoration of the gripping ability of injured paw to hold the wire of grip strength meter), with the 5.00 mg/kg group revealing to be suggestively advantageous (p = .001). In the following periods, the treatment group had significantly greater gripping strength disparities (p = .001) than the control group (days 7 and 8 post‐injury). The 5 mg/kg group, on the other hand, demonstrated significant changes on day 8 following injury (p = .02). Our results are in line with the findings of (Rasul et al., 2019), for motor functions and retrieval in case of peripheral nerve injury. Their results indicate that the treated group acquires motor functions earlier as compared to Normal control group. During the whole experiment, the comparison of body mass % among all groups was similarly determined to be non‐significant (p = .956) (Figure 12).
FIGURE 11.
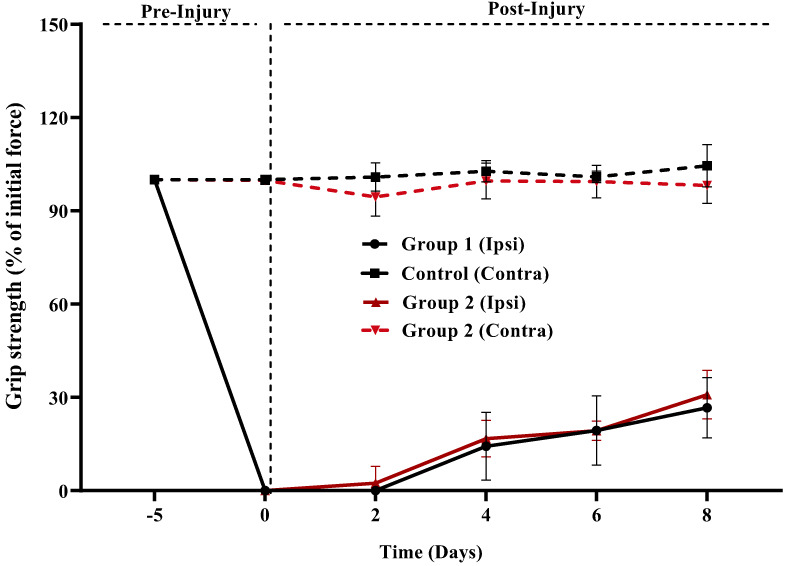
GRIP strength.
FIGURE 12.
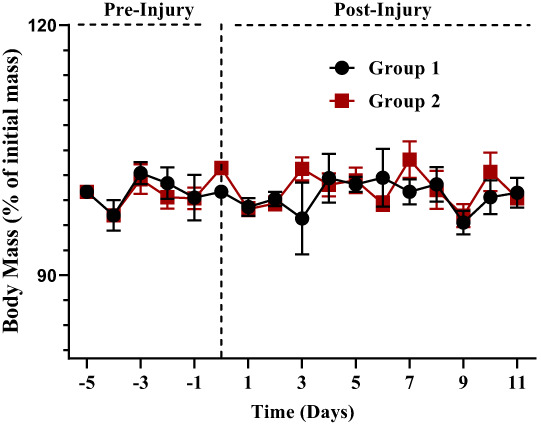
Body mass.
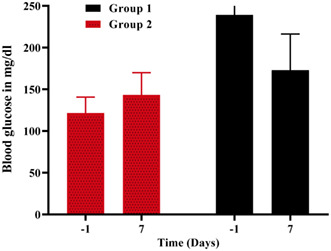
The statistically significant drop in glycemia following theaflavin‐containing diet consumption illustrates the hypoglycemic influence of this regimen. In this context, the glycemic levels of all groups were evaluated and compared before and after injury induction (Figure 13). It was revealed that all treatment groups kept their glucose levels regular. However, mice fed regular chow had somewhat higher glucose levels (control group). At 5.00 mg/kg, theaflavin significantly lowered glycemic levels (p = .002).
FIGURE 13.
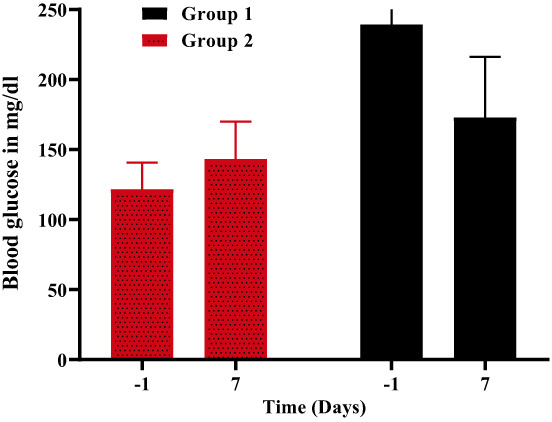
Glycemic level.
Total antioxidant capacity (TAC) and TOS levels in animal blood samples were measured following decapitation to best explain the mechanisms involved in enhanced functional recovery, and the results demonstrated that theaflavin leaves had a highly significant influence on reducing oxidative stress (i.e., improved TAC values and lowered TOS values). TAC levels increased significantly (p.0001) in all treated groups as compared to the control (Figure 14), confirming theaflavin's outstanding antioxidant activity. The number of free radicals increases when the sciatic nerve is injured, raising the TOS level. TOS values in all treated groups were significantly lower (p.0001) than in the control group (Figure 15). However, these results are in line with the findings of (Rasul et al., 2019).
FIGURE 14.
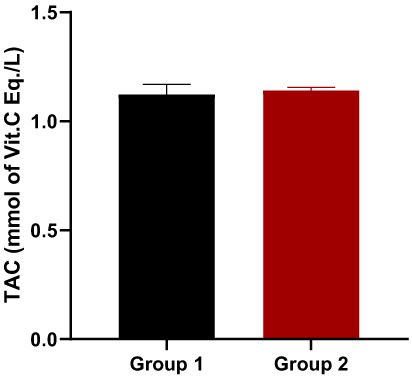
Total antioxidant capacity level.
FIGURE 15.
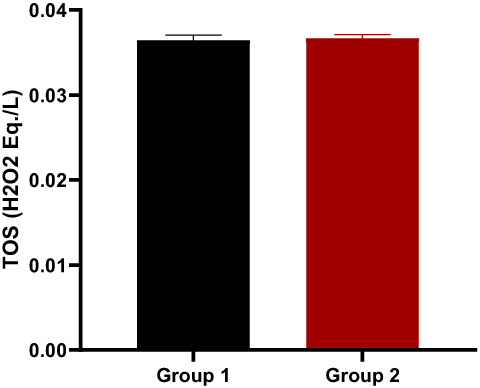
Total oxidant status level.
13. CONCLUSION AND RECOMMENDATIONS
It is envisaged from the current findings that black tea effectively alleviates oxidative stress symptoms. Black tea is selected due to its high phenolic content and high antioxidant activity based on the DPPH, FRAP, and TPC. The results showed that black tea extracts performed better with ethanol solvent than with water or methanol. Similarly, antioxidant indices of isolated theaflavin imparted significant activity with ethanol at 60 min. In light of all of the above, the study concluded that theaflavin not only expedited functional sciatic nerve recovery, as seen by behavioral parameter data but also effectively combats oxidative stress. However, there is a dire need to investigate theaflavin for these health‐promoting effects on a molecular and genetic level. No doubt, this research provides a basic footstep toward the neuroprotective effect of theaflavin however, mechanistic elucidation on cellular and molecular levels is still lacking to unveil its application at a large level. It is recommended that human‐based clinical should be carried out in the future.
FUNDING INFORMATION
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no conflict of interest.
ETHICS STATEMENT
This article does not contain any studies with human participants or animals performed by any of the authors.
CONSENT TO PARTICIPATE
Corresponding and all the co‐authors are willing to participate in this manuscript.
CONSENT FOR PUBLICATION
All authors are willing for publication of this manuscript.
ACKNOWLEDGMENTS
The authors are thankful to the Government College University for providing literature collection facilities, which helped in the study.
Ahmad, A. , Nosheen, F. , Arshad, M. U. , Saeed, F. , Afzaal, M. , Islam, F. , Imran, A. , Noreen, R. , Amer Ali, Y. , & Shah, M. A. (2023). Isolation and antioxidant characterization of theaflavin for neuroprotective effect in mice model. Food Science & Nutrition, 11, 3485–3496. 10.1002/fsn3.3337
Contributor Information
Fakhar Islam, Email: fakhar.ft440@gmail.com.
Ali Imran, Email: aliimran.ft@gmail.com.
Yuosra Amer Ali, Email: yuosra_amer@uomosul.edu.iq.
DATA AVAILABILITY STATEMENT
Even though adequate data have been given in the form of tables and figures, all authors declare that if more data are required, then the data will be provided on a request basis.
REFERENCES
- Afzaal, M. , Saeed, F. , Ateeq, H. , Akhtar, M. N. , Imran, A. , Ahmed, A. , Aamir, M. , Islam, F. , Yasmin, I. , Shah, Y. A. , Hussain, M. , Hameed, A. , Kumar, R. , & Awuchi, C. G. (2023). Probiotics encapsulated gastroprotective cross‐linked microgels: Enhanced viability under stressed conditions with dried apple carrier. Food Science & Nutrition, 11(2), 817–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al‐Nimer, M. , Al‐Ani, F. , & Ali, F. (2012). Role of nitrosative and oxidative stress in neuropathy in patients with type 2 diabetes mellitus. Journal of Neurosciences in Rural Practice, 3(1), 41–44. 10.4103/0976-3147.91932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anandhan, A. , Essa, M. M. , & Manivasagam, T. (2013). Therapeutic attenuation of Neuroinflammation and apoptosis by black tea Theaflavin in chronic MPTP/probenecid model of Parkinson's disease. Neurotoxicity Research, 23(2), 166–173. 10.1007/s12640-012-9332-9 [DOI] [PubMed] [Google Scholar]
- Anandhan, A. , Tamilselvam, K. , Radhiga, T. , Rao, S. , Essa, M. M. , & Manivasagam, T. (2012). Theaflavin, a black tea polyphenol, protects nigral dopaminergic neurons against chronic MPTP/probenecid induced Parkinson's disease. Brain Research, 1433, 104–113. [DOI] [PubMed] [Google Scholar]
- Aquilano, K. , Baldelli, S. , Rotilio, G. , & Ciriolo, M. R. (2008). Role of nitric oxide synthases in Parkinson's disease: A review on the antioxidant and anti‐inflammatory activity of polyphenols. Neurochemical Research, 33(12), 2416–2426. 10.1007/s11064-008-9697-6 [DOI] [PubMed] [Google Scholar]
- Aregbesola, O. A. , Faborode, M. O. , & Hounkanrin, B. A. (2018). Studies on black tea production from fresh roselle calyxes. International Food Research Journal, 25(1), 310–313. [Google Scholar]
- Areti, A. , Yerra, V. G. , Naidu, V. , & Kumar, A. (2014). Oxidative stress and nerve damage: Role in chemotherapy induced peripheral neuropathy. Redox Biology, 2, 289–295. 10.1016/j.redox.2014.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asmat, U. , Abad, K. , & Ismail, K. (2016). Diabetes mellitus and oxidative stress—A concise review. Saudi Pharmaceutical Journal, 24, 547–553. 10.1016/j.jsps.2015.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aziz, N. , Rasul, A. , Malik, S. A. , Anwar, H. , Imran, A. , Razzaq, A. , & Hussain, G. (2019). Supplementation of Cannabis sativa L. leaf powder accelerates functional recovery and ameliorates haemoglobin level following an induced injury to sciatic nerve in mouse model. Pakistan Journal of Pharmaceutical Sciences, 32(2), 785–792. [PubMed] [Google Scholar]
- Baranowska‐Wojcik, E. , Szwajgier, D. , & Winiarska‐Mieczan, A. (2020). Regardless of the brewing conditions, various types of tea are a source of acetylcholinesterase inhibitors. Nutrients, 12(3), 709. 10.3390/nu12030709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastos, D. H. , Saldanha, L. A. , Catharino, R. R. , Sawaya, A. , Cunha, I. B. , Carvalho, P. O. , & Eberlin, M. N. (2007). Phenolic antioxidants identified by ESI‐MS from yerba mate (Ilex paraguariensis) and green tea (Camelia sinensis) extracts. Molecules, 12(3), 423–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan, J. (2008). Psychological effects of dietary components of tea: Caffeine and L‐theanine. Nutrition Reviews, 66, 82–90. [DOI] [PubMed] [Google Scholar]
- Chaturvedi, R. K. , Shukla, S. , Seth, K. , Chauhan, S. , Sinha, C. , Shukla, Y. , & Agrawal, A. K. (2006). Neuroprotective and Neurorescue effect of black tea extract in 6‐hydroxydopamine‐lesioned rat model of Parkinson's disease. Neurobiology of Disease, 22(2), 421–434. 10.1016/j.nbd.2005.12.008 [DOI] [PubMed] [Google Scholar]
- Chen, H. , Zhang, Y. , Lu, X. , & Qu, Z. (2012). Comparative studies on the physicochemical and antioxidant properties of different tea extracts. Journal of Food Science and Technology, 49(3), 356–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, S. Q. , Wang, Z. S. , Ma, Y. X. , Zhang, W. , Lu, J. L. , Liang, Y. R. , & Zheng, X. Q. (2018). Neuroprotective effects and mechanisms of tea bioactive components in neurodegenerative diseases. Molecules, 23(3), 512. 10.3390/molecules23030512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin‐Chan, M. , Navarro‐Yepes, J. , & Quintanilla‐Vega, B. (2015). Environmental pollutants as risk factors for neurodegenerative disorders: Alzheimer and Parkinson diseases. Frontiers in Cellular Neuroscience, 9, 124. 10.3389/fncel.2015.00124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung, S. Y. (2009). Cancer prevention by tea: Animal studies, molecular mechanisms and human relevance. Nature Reviews. Cancer, 9(6), 429–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Mejia, E. G. , Ramirez‐Mares, M. V. , & Puangpraphant, S. (2009). Bioactive components of tea: Cancer, inflammation and behavior. Brain, Behavior, and Immunity, 23(6), 721–731. [DOI] [PubMed] [Google Scholar]
- Deb, S. , Dutta, A. , Phukan, B. C. , Manivasagam, T. , Thenmozhi, A. J. , Bhattacharya, P. , Paul, R. , & Borah, A. (2019). Neuroprotective attributes of L‐theanine, a bioactive amino acid of tea, and its potential role in Parkinson's disease therapeutics. Neurochemistry International, 129, 104478. 10.1016/j.neuint.2019.104478 [DOI] [PubMed] [Google Scholar]
- Deuis, J. R. , Dvorakova, L. S. , & Vetter, I. (2017). Methods used to evaluate pain behaviors in rodents. Frontiers in Molecular Neuroscience, 10, 284. 10.3389/fnmol.2017.00284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong, X. X. , Yang, C. , Cao, S. H. , Gan, Y. , Sun, H. L. , Gong, Y. H. , Yang, H. J. , Yin, X. X. , & Lu, Z. X. (2015). Tea consumption and the risk of depression: A meta‐analysis of observational studies. The Australian and New Zealand Journal of Psychiatry, 49(4), 334–345. 10.1177/0004867414567759 [DOI] [PubMed] [Google Scholar]
- Erel, O. (2004). A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clinical Biochemistry, 37(4), 277–285. 10.1016/j.clinbiochem.2003.11.015 [DOI] [PubMed] [Google Scholar]
- Erel, O. (2005). A new automated colorimetric method for measuring total oxidant status. Clinical Biochemistry, 38(12), 1103–11111. 10.1016/j.clinbiochem.2005.08.008 [DOI] [PubMed] [Google Scholar]
- Erol, N. T. , Sarı, F. , & Velioglu, Y. S. (2010). Polyphenols, alkaloids and antioxidant activity of different grades Turkish black tea. Gida, 35(3), 161–168. [Google Scholar]
- Friedman, M. , Levin, C. E. , Choi, S. H. , Kozukue, E. , & Kozukue, N. (2006). HPLC analysis of catechins, theaflavins, and alkaloids in commercial teas and green tea dietary supplements: Comparison of water and 80% ethanol/water extracts. Journal of Food Science, 71(6), C328–C337. [Google Scholar]
- Hagstrom, J. E. , Hegge, J. , Zhang, G. , Noble, M. , Budker, V. , Lewis, D. L. , Herweijer, H. , & Wolff, J. A. (2004). A facile nonviral method for delivering genes and siRNAs to skeletal muscle of mammalian limbs. Molecular Therapy, 10(2), 386–398. [DOI] [PubMed] [Google Scholar]
- Hussain, G. , Schmitt, F. , Henriques, A. , Lequeu, T. , Rene, F. , Bindler, F. , Dirrig‐Grosch, S. , Oudart, H. , Palamiuc, L. , Metz‐Boutigue, M.‐H. , Dupuis, L. , Marchioni, E. , Gonzalez De Aguilar, J.‐L. , & Loeffler, J.‐ P. (2013). Systemic down‐regulation of delta‐9 desaturase promotes muscle oxidative metabolism and accelerates muscle function recovery following nerve injury. PLoS One, 8(6), e64525. 10.1371/journal.pone.0064525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain, G. , Wang, J. , Rasul, A. , Anwar, H. , Qasim, M. , Zafar, S. , Aziz, N. , Razzaq, A. , Hussain, R. , de Aguilar, J.‐L. , & Sun, T. (2020). Current status of therapeutic approaches against peripheral nerve injuries: A detailed story from injury to recovery. International Journal of Biological Sciences, 16, 116–134. 10.7150/ijbs.35653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imran, A. , Butt, M. S. , Xiao, H. , Imran, M. , Rauf, A. , Mubarak, M. S. , & Ramadan, M. F. (2019). Inhibitory effect of black tea (Camellia sinensis) theaflavins and thearubigins against HCT 116 colon cancer cells and HT 460 lung cancer cells. Journal of Food Biochemistry, 43(5), e12822. [DOI] [PubMed] [Google Scholar]
- Islam, F. , Amer Ali, Y. , Imran, A. , Afzaal, M. , Zahra, S. M. , Fatima, M. , Saeed, F. , Usman, I. , Shehzadi, U. , Mehta, S. , & Shah, M. A. (2023). Vegetable proteins as encapsulating agents: Recent updates and future perspectives. Food Science & Nutrition, 11(3), 1–13. 10.1002/fsn3.3234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandinov, B. , Giladi, N. , & Korczyn, A. D. (2009). Smoking and tea consumption delay onset of Parkinson's disease. Parkinsonism & Related Disorders, 15(1), 41–46. 10.1016/j.parkreldis.2008.02.011 [DOI] [PubMed] [Google Scholar]
- Komirishetty, P. , Areti, A. , Yerra, V. G. , Pk, R. , Sharma, S. S. , Gogoi, R. , Sistla, R. , & Kumar, A. (2016). PARP inhibition attenuates neuroinflammation and oxidative stress in chronic constriction injury‐induced peripheral neuropathy. Life Sciences, 150, 50–60. 10.1016/j.lfs.2016.02.085 [DOI] [PubMed] [Google Scholar]
- Kouyoumdjian, J. , Graç, C. , & Ferreira, V. M. (2017). Peripheral nerve injuries: A retrospective survey of 1124 cases. Neurology India, 65(3), 551–555. 10.4103/neuroindia.NI_987_16 [DOI] [PubMed] [Google Scholar]
- Lagha, A. B. , & Grenier, D. (2016). Tea polyphenols inhibit the activation of NF‐κB and the secretion of cytokines and matrix metalloproteinases by macrophages stimulated with Fusobacterium nucleatum . Scientific Reports, 6(1), 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, R. , Huang, Y. G. , Fang, D. , & Le, W. D. (2004). (−)‐epigallocatechin Gallate inhibits lipopolysaccharide‐induced microglial activation and protects against inflammation‐mediated dopaminergic neuronal injury. Journal of Neuroscience Research, 78(5), 723–731. 10.1002/jnr.20315 [DOI] [PubMed] [Google Scholar]
- Li, S. , Lo, C. Y. , Pan, M. H. , Lai, C. S. , & Ho, C. T. (2013). Black tea: Chemical analysis and stability. Food & Function, 4, 10–18. [DOI] [PubMed] [Google Scholar]
- LIczbiński, P. , & Bukowska, B. (2022). Tea and coffee polyphenols and their biological properties based on the latest in vitro investigations. Industrial Crops and Products, 175, 114265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, Q. P. , Huang, C. , Cui, Q. Y. , Yang, D. J. , Sun, K. , Chen, X. , & Li, X. H. (2016). Meta‐analysis of the association between tea intake and the risk of cognitive disorders. PLoS One, 11(11), e165861. 10.1371/journal.pone.0165861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maqbool, J. , Anwar, H. , Rasul, A. , Imran, A. , Saadullah, M. , Malik, S. A. , Shabbir, A. , Akram, R. , Sajid, F. , Zafar, S. , Saeed, S. , Akram, M. N. , Islam, F. , Hussain, G. , & Islam, S. (2023). Comparative evaluation of ethyl acetate and n‐Hexane extracts of Cannabis sativa L. leaves for muscle function restoration after peripheral nerve lesion. Food Science & Nutrition, 11(3), 1–9. 10.1002/fsn3.3255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathiyazahan, D. B. , Thenmozhi, A. J. , & Manivasagam, T. (2015). Protective effect of black tea extract against Aluminium chloride‐induced Alzheimer's disease in rats: A Behavioural, Biochemical and molecular approach. Journal of Functional Foods, 16, 423–435. 10.1016/j.jff.2015.05.001 [DOI] [Google Scholar]
- Maturana, M. G. V. , Pinheiro, A. S. , De Souza, T. L. F. , & Follmer, C. (2015). Unveiling the role of the pesticides Paraquat and rotenone on alpha‐synuclein fibrillation in vitro. Neurotoxicology, 46, 35–43. 10.1016/j.neuro.2014.11.006 [DOI] [PubMed] [Google Scholar]
- Mazumder, M. K. , & Choudhury, S. (2019). Tea polyphenols as multi‐target therapeutics for Alzheimer's disease: An in silico study. Medical Hypotheses, 125, 94–99. 10.1016/j.mehy.2019.02.035 [DOI] [PubMed] [Google Scholar]
- Modupe, O. D. , Samuel, O. O. , & Godwin, O. O. (2013). Trace metal concentrations in some tea leaves consumed in Ibadan, Nigeria. African Journal of Agricultural Research, 8(46), 5771–5775. [Google Scholar]
- Mushtaq, S. , Hina, S. , Maqbool, H. , Ahmed, A. , Nazim, M. , Hussain, E. , Mussab, R. M. , & Kumar, B. (2021). Frequency of peripheral nerve injury in trauma in emergency settings. Cureus, 13(3), e14195. 10.7759/cureus.14195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajha, H. N. , Paule, A. , Aragonès, G. , Barbosa, M. , Caddeo, C. , Debs, E. , … Edeas, M. (2022). Recent advances in research on polyphenols: Effects on microbiota, metabolism, and health. Molecular Nutrition & Food Research, 66(1), 2100670. [DOI] [PubMed] [Google Scholar]
- Ramdani, D. , Chaudhry, A. S. , & Seal, C. J. (2018). Alkaloid and polyphenol analysis by HPLC in green and black tea powders and their potential use as additives in ruminant diets. In AIP conference proceedings 1927, 1, 030008. AIP Publishing LLC. [Google Scholar]
- Rana, A. , Samtiya, M. , Dhewa, T. , Mishra, V. , & Aluko, R. E. (2022). Health benefits of polyphenols: A concise review. Journal of Food Biochemistry, 46(10), e14264. [DOI] [PubMed] [Google Scholar]
- Rasul, A. , Al‐Shawi, A. A. , Malik, S. A. , Anwar, H. , Rasool, B. , Razzaq, A. , Aziz, N. , Kamran, S. A. S. , Sarfraz, I. , Shabbir, A. , Selamoglu, Z. , & Hussain, G. (2019). Neurada procumbens promotes functions regain in a mouse model of mechanically induced sciatic nerve injury. Pakistan Journal of Pharmaceutical Sciences, 32(4), 1761–1766. [PubMed] [Google Scholar]
- Razzaq, A. , Ahmad, S. , Farhan, M. , Ali, S. , Rasul, A. , Qasim, M. , Zafar, S. , Kamran, S. K. S. , Maqbool, J. , Imran, M. , Hussain, G. , & Hussain, M. (2020). Moringa oleifera Lam. Ameliorates the muscles function recovery following an induced insult to the sciatic nerve in a mouse model. Food Science & Nutrition, 8, 1–8. 10.1002/fsn3.1620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma, A. , & Zhou, W. (2011). A stability study of green tea catechins during the biscuit making process. Food Chemistry, 126, 568–573. [Google Scholar]
- Shen, W. , Xiao, Y. , Ying, X. , Li, S. , Zhai, Y. , Shang, X. , Li, F. , Wang, X. , He, F. , & Lin, J. (2015). Tea consumption and cognitive impairment: a cross‐sectional study among Chinese elderly. PLoS One, 10(9), e0137781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stangl, V. , Dreger, H. , Stangl, K. , & Lorenz, M. (2007). Molecular targets of tea polyphenols in the cardiovascular system. Cardiovascular Research, 73, 348–358. 10.1016/j.cardiores.2006.08.022 [DOI] [PubMed] [Google Scholar]
- Sun, S. , Pan, S. , Ling, C. , Miao, A. , Pang, S. , Lai, Z. , Chen, D. , & Zhao, C. (2012). Free radical scavenging abilities in vitro and antioxidant activities in vivo of black tea and its main polyphenols. Journal of Medicinal Plants Research, 6(1), 114–121. [Google Scholar]
- Tysnes, O. B. , & Storstein, A. (2017). Epidemiology of Parkinson's disease. Journal of Neural Transmission, 124(8), 901–905. 10.1007/s00702-017-1686-y [DOI] [PubMed] [Google Scholar]
- Wang, H. , Ding, X.‐G. , Li, S.‐W. , Zheng, H. , Zheng, X.‐M. , Navin, S. , Li, L. U. , & Wang, X.‐H. (2015). Role of oxidative stress in surgical cavernous nerve injury in a rat model. Journal of Neuroscience Research, 93(6), 922–929. 10.1002/jnr.23545 [DOI] [PubMed] [Google Scholar]
- Xie, B. , Shi, H. , Chen, Q. , Ho, C.T. (1993). Antioxidant properties of fractions and polyphenol constituents from green, oolong and black teas. Proceedings of the National Science Council, Republic of China, 17(2): 77‐84. [PubMed] [Google Scholar]
- Yang, Z. , Jie, G. , Dong, F. , Xu, Y. , Watanabe, N. , & Tu, Y. (2008). Radical‐scavenging abilities and antioxidant properties of theaflavins and their gallate esters in H2O2‐mediated oxidative damage system in the HPF‐1 cells. Toxicology In Vitro, 22(5), 1250–1256. [DOI] [PubMed] [Google Scholar]
- Zhou, Z. D. , Xie, S. P. , Saw, W. T. , Ho, P. G. H. , Wang, H. , Lei, Z. , Yi, Z. , & Tan, E. K. (2019). The therapeutic implications of tea polyphenols against dopamine (DA) neuron degeneration in Parkinson's disease (PD). Cells, 8(8), 911. 10.3390/cells8080911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, W. L. , Shi, H. S. , Wei, Y. M. , Wang, S. J. , Sun, C. Y. , Ding, Z. B. , & Lu, L. (2012). Green tea polyphenols produce antidepressant‐like effects in adult mice. Pharmacological Research, 65(1), 74–80. 10.1016/j.phrs.2011.09.007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Even though adequate data have been given in the form of tables and figures, all authors declare that if more data are required, then the data will be provided on a request basis.


