Abstract
ENPP1 (ecto-nucleotide pyrophosphatase/phosphodiesterase) participates in the hydrolysis of different purine nucleotides in an array of physiologic processes. However, ENPP1 is frequently overexpressed in local relapses and tumor metastases, which are associated with poor prognosis and survival in a range of solid tumors. ENPP1 promotes an immunosuppressive tumor microenvironment (TME) by tilting the balance of ATP/adenosine (Ado) in conjunction with other components (CD38, CD39/ENTPD1, and CD73/NT5E). Moreover, ENPP1 intersects with the stimulator of interferon genes (STING), impairing its robust immune response through the hydrolysis of the effector 2´,3´-cyclic GMP–AMP. Thus, ENPP1 blockade emerges as a unique target eliciting immune remodeling and leveraging the STING pathway. Several ENPP1 inhibitors have shown an immunostimulatory effect, and their combination with other therapeutic modalities, such as immune-checkpoint blockade, STING activation, DNA damage response (DDR) inhibitors, and radiotherapy (RT), represents a promising avenue to boost antitumor–immune responses and to improve current clinical outcomes in several tumors. This comprehensive review summarizes the current state of the art and opens new perspectives for novel treatment strategies.
Introduction
An exceptionally wide variety of physiologic processes is triggered by purinergic signaling that is the extracellular action of purines (ATP, ADP, and Ado) and pyrimidines [uridine-5´-triphosphate (UTP) and uridine-5´diphosphate (UDP)]. These include cell proliferation, migration, apoptosis, platelet aggregation (1, 2), and muscle contraction, as well as regulating hypoxia and ischemia in tissues (3, 4).
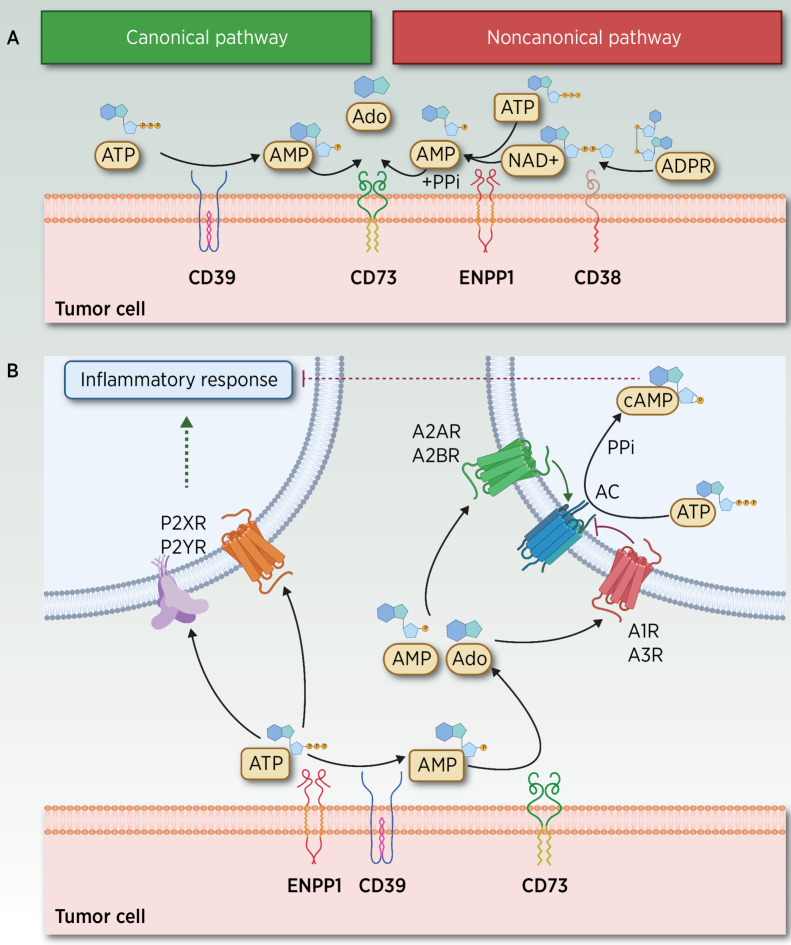
Purine homeostasis entails the concerted activity of the mechanisms of nucleotide and nucleoside release, their extracellular metabolism mediated by several transmembrane ectoenzymes (CD38, CD39/ENTPD1, CD73/NT5E, and ENPP1; Fig. 1A), the intracellular signaling pathways elicited upon binding of purine metabolites to different receptors in target cells (Fig. 1B) and their cross-talk with other intracellular cascades.
Figure 1.
ATP–Ado extracellular metabolism and elicited signaling through adenosinergic receptors. A, Canonical and noncanonical extracellular receptors contributing to the hydrolysis of ATP and other metabolites. In the TME, two different yet partially overlapping pathways mediate the hydrolysis of ATP to adenosine (Ado). In the noncanonical pathway, Ado can be released by CD38 (72), a cell-surface enzyme that functions as an adhesion molecule and as an ectoenzyme, expressed in T cells, neutrophils, lymphocytes and monocytes, and macrophages under inflammatory conditions. CD38 hydrolyzes nicotinamide-adenine dinucleotide (NAD+) to ADP-ribose (ADPR), which is subsequently metabolized to AMP by ENPP1, whereas AMP is further dephosphorylated by CD73 to Ado and PPi (73, 74). ENPP1 is a Ca2+ and Zn2+–dependent enzyme comprising two identical disulfide bonded subunits (75) involved in the regulation of hormonal (39), neurologic, immunologic, and hematologic functions (4, 76, 77) as well as in atheromatous plaque calcification (78). ENPP1 hydrolyzes its most suitable substrate ATP to AMP, and the catalysis of GTP to GMP while releasing PPi. Other substrates include dinucleotides, mainly cGAMP, diadenosine tetraphosphate, and other poor substrates (UTP and cAMP). The product AMP is subsequently dephosphorylated by CD73 (Ecto-5´-nucleotidase or NT5E) to Pi and Ado. In addition to the action of nucleotidases, different membrane transporters, which belong to SCL28 and SCL29 families, also regulate the extracellular bioavailability of Ado (6). In the canonical pathway, transmembrane or soluble ectoenzyme CD39, which converts nicotinamide-adenine dinucleotide phosphate (NADP) into NAD+, also hydrolyzes extracellular ATP to AMP (79), a catalytic activity similar to that exerted by ENPP1. CD39 is expressed in the vasculature, in malignant cells, and in several immune subpopulations, including M2-like tumor-associated macrophages (TAM), T regulatory cells (Tregs), dendritic cells (DC), natural killers (NK), monocytes, and B cells. CD39 is expressed in MDSC and Tregs, and it is probably high on tumor-specific clonally expanded Tregs (80, 81) events associated with their immunosuppressive role in T-cell function (82). Ado results from the subsequent CD73-mediated degradation of AMP. Thus, CD73 bridges canonical and noncanonical pathways, a role currently exploited by a targeted therapy (83). B, ATP, AMP, and Ado signaling through adenosinergic receptors. ATP and Ado, together with other related metabolites, signal through purinergic receptors divided into two major families: Ado P1 receptors (A1R/ADORA1, A2AR/ADORA2A, A2BR/ADORA2B, and A3R/ADORA3), whose agonists include AMP and Ado, and P2 receptors, which comprise a family of P2X ionotropic receptors (P2X1–7) stimulated by ATP, and P2Y, which are G protein–coupled metabotropic receptors (P2Y 1, 2, 4, 6, 11–14) activated by nucleotides such as ATP, ADP, UTP, and UDP (84). Under hypoxic conditions, extracellular released Ado levels are further amplified by stimulated CD39–CD73, and by decreased Ado kinase activity, leading to diminished Ado degradation (73). This accumulation of AMP and Ado metabolites stimulates P1 G-protein–coupled A2AR at nanomolar range (also A1R and A3R) and A2BR Ado P1 receptors (at micromolar levels) to elicit intracellular cyclic AMP signaling, resulting in decreased production of proinflammatory cytokines and increased synthesis of anti-inflammatory cytokines (40, 85). AC, adenylyl cyclase; PPi, pyrophosphate. (Adapted from an image created with BioRender.com.)
Within this complexity, ENPP1 (CD203a/PC-1), which belongs to the family of ENPP ectonucleotidases (ENPP 1–7), is a type II transmembrane glycoprotein, also located on the endoplasmic reticulum lumen (5). ENPP1 constitutes a major purinergic signaling regulator of extracellular ATP and GTP levels that are hydrolyzed to AMP and GMP while releasing inorganic pyrophosphate (PPi). The product AMP is subsequently dephosphorylated by CD73 (Ecto-5´-nucleotidase or NT5E) to inorganic phosphate (Pi) and Ado. In addition, different membrane transporters that belong to SCL28 and SCL29 families also regulate the extracellular bioavailability of Ado (6).
ENPP1 is highly expressed in the osteochondral compartment where it displays homeostatic functions in regulating physiologic mineralization by regulating the balance between Pi, a substrate of mineral deposition, and PPi, an inhibitor of mineralization. ENPP1 deficiency has been linked to bone abnormalities (7, 8).
In tumors, ATP, AMP, and Ado play a key role in modulating immune responses. ENPP1 arises at the interphase of tumor–host immune interactions tilting the balance from the proinflammatory ATP toward Ado with an opposite anti-inflammatory role. ATP and Ado, together with other related metabolites, signal through purinergic receptors expressed in both tumor and host–immune cells (Fig. 1B). This array of receptors with different expression, selectivity, and affinity confers a unique fine-tuned modulation of extracellular nucleotide levels that strongly regulate tumor progression.
Emerging Roles of ENPP1
ENPP1 gene is located in the 6q22–q23 locus, a region commonly amplified in many tumors, including neural brain and breast cancers (9). High ENPP1 expression levels are also detected in many solid tumors including ovarian (10), breast (11), glioblastoma (9), and NSCLC (12) among others. Its overexpression has been linked to a more aggressive clinical course associated with poor prognosis through the induction of EMT phenotype, the acquisition of stem cell–like features and the favoring of prometastatic traits (11, 12).
ENPP1 levels increase during various stages of tumorigenesis in clinical specimens. For instance, ENPP1 expression was significantly increased in 85% of patients with high-grade ovarian serous carcinoma, compared with ∼1% in benign serous cystadenoma, suggesting a putative role in malignant tumor progression. Moreover, the higher the FIGO stage, the higher the ENPP1 levels that were detected with poorer cell differentiation (10). ENPP1 levels were also significantly elevated in human primary breast tumors relative to the normal mammary epithelium, with the highest levels observed in skeletal metastases (13). Similarly, in different animal models of lung and breast cancer, ENPP1 levels were elevated in metastatic cells as compared with the primary tumor (13, 14).
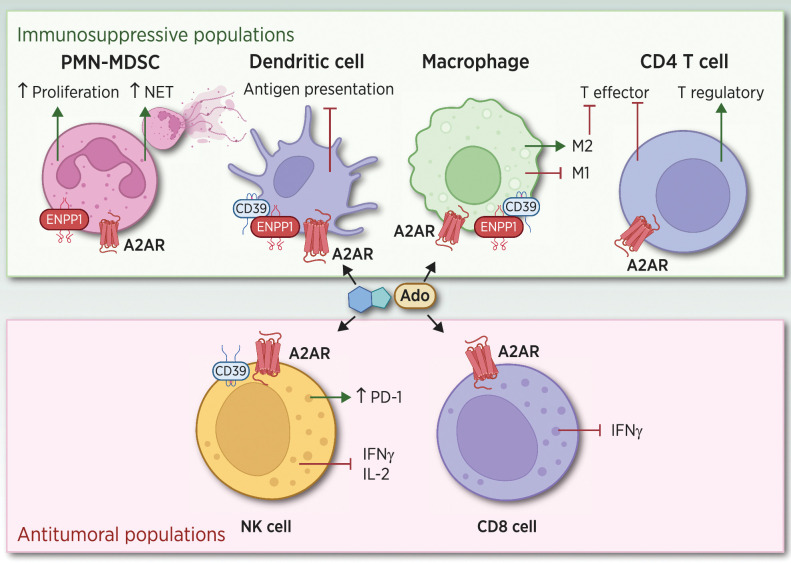
Besides tumor expression, ENPP1 is also highly expressed in several immune cells, including neutrophils and M2 macrophages promoting tumor progression, whereas low levels of expression were detected in natural killers (NK), DC, monocytes, T and B cells (15, 16). ENPP1 activity in concert with CD73 (NT5E) leads to the accumulation of Ado-promoting tumor–immune remodeling and robust immunosuppression (ref. 17; Fig. 2).
Figure 2.
Effects of Ado signaling in different immune subpopulations. ENPP1 in conjunction with CD39 and CD73 promotes the accumulation of Ado in the TME, which signals through A2AR expressed in host immune cell populations. Tumor cells with high ENPP1 promote strong tumor–immune remodeling by inducing a PMN-MDSC chemotaxis, diminishing DC infiltration, increasing M2 macrophages, and promoting CD4 regulatory T cells. In addition, the increase in Ado leads to a diminished NK and CD8 T cytotoxic activity, promoting a strong immunosuppressive milieu preventing an efficient antitumor–immune attack. (Adapted from an image created with BioRender.com.)
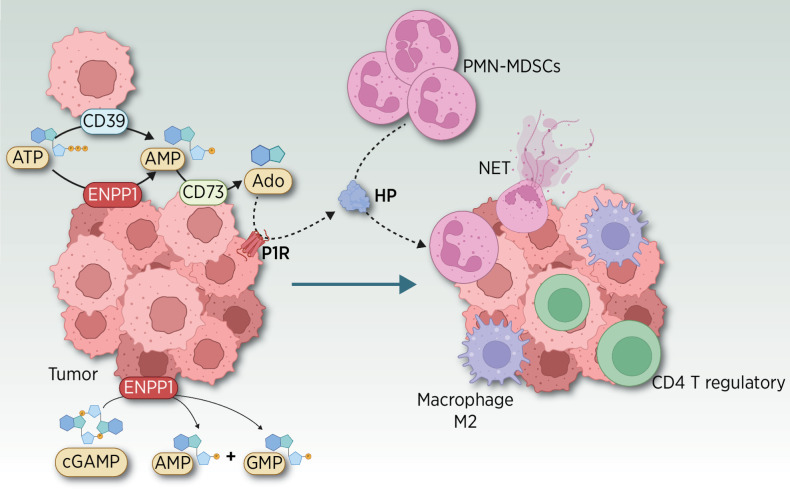
In breast cancer models, ENPP1 promotes the chemotactic infiltration of polymorphonuclear myeloid-derived suppressor cells (PMN-MDSC; ref. 18) and the inhibition of tumor-infiltrating cytotoxic T cells (19) impeding the antitumor–immune attack. This myeloid chemoattraction is elicited by the ENPP1-mediated tumor release of haptoglobin (HP), a proinflammatory acute phase reactant (20, 21) that also acts as an inducer of neutrophil extracellular traps formation (ref. 22; Fig. 3). Thus, inhibition of ENPP1 could revert the immunosuppressive landscape, impairing tumor progression.
Figure 3.
Emerging role of ENPP1 in tumor cells eliciting a strong immune remodeling. ENPP1 promotes the chemotactic infiltration of PMN-MDSC (18), M2 macrophages, CD4 T regulatory cells, and the inhibition of tumor-infiltrating cytotoxic T and NK cells (19), impeding the antitumor immune attack in preclinical models (33). This myeloid chemoattraction is elicited by the ENPP1-mediated tumor release of haptoglobin (HP), a proinflammatory acute-phase reactant (20) in a manner similar to what has been shown for other chemoattractants, such as IL8 (21). AMP and Ado signaling through P1 receptors (P1R) triggers the release of HP in the TME. HP plays an unexpected double role as a chemoattractant of MDSC and as an inducer of neutrophil extracellular traps (NET), extruded DNA meshes associated with cytotoxic enzymes that promote tumor progression (22). Besides their classical role against bacteria, NETs play different roles either in situ or at distant sites (22, 86), promoting the trapping of circulating tumor cells (CTC) in the resected tumor bed, in preventing antitumor immune cytotoxicity, their role in priming the premetastatic niche (87), and their involvement in boosting metastasis (88). (Adapted from an image created with BioRender.com.)
ENPP1 Activity Intersects with the STING Pathway
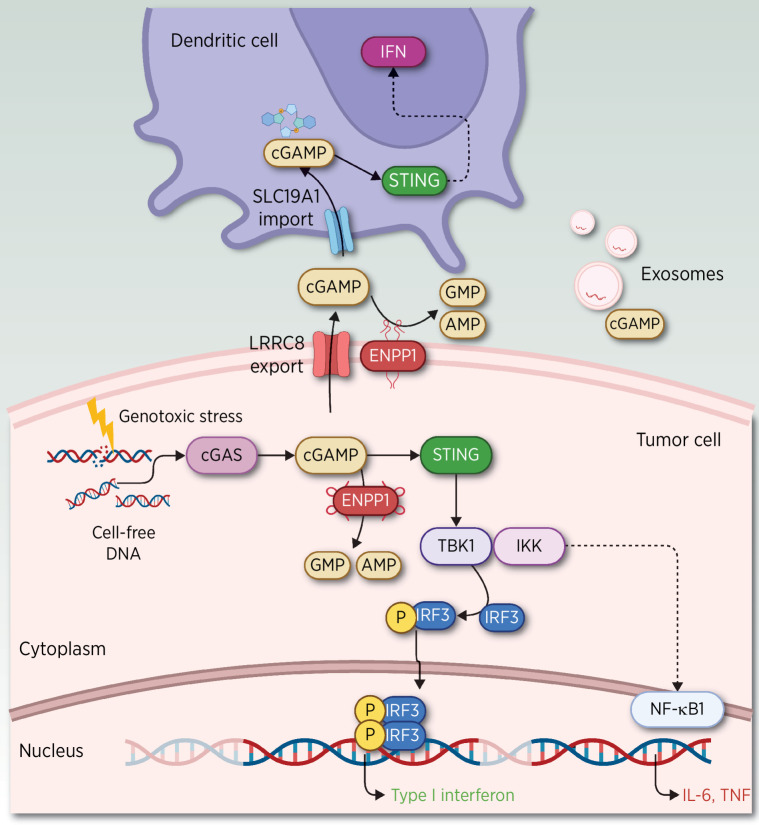
Besides its role in immunosuppression, upregulation of ENPP1 is one of the mechanisms artfully co-opted by neoplastic cells as a resistance mechanism to impair stimulator of interferon genes (STING) activation, a major DNA-sensing antiviral pathway (23) and antitumor defense mechanism (ref. 24; reviewed elsewhere; refs. 25, 26; Fig. 4).
Figure 4.
ENPP1 intersects with the STING pathway. A variety of stimuli in the TME trigger the increase in cGAS (89), including the presence of ATP, GTP, and double-strand (ds) DNA generated by damage-associated molecular patterns (DAMP), pathogen-associated molecular patterns (PAMP), apoptotic cells (a common event in solid tumors), DNA breakage induced by radiotherapy (RT), or free cellular DNA as a consequence of chromosomal instability (28). The synthesis of cGAS leads to the production of cGAMP. cGAMP binds and activates STING located in the endoplasmic reticulum, inducing the transcription of interferon (IFN) genes and other cytokines (60). Intracellular cGAMP dominantly produced by cancer cells also spreads through gap junctions (90–92) to neighboring contacting cells and to the extracellular milieu in released exosomes (93) or through widely expressed cGAMP transporters, such as heteromeric channels, better known as volume-regulated anion channels (VRAC) of the LRRC8 family. Moreover, cGAMP accumulated in the extracellular space is rapidly degraded by transmembrane expression of ENPP1 in tumor or stromal cells. ENPP1 is also expressed in the lumen of the endoplasmic reticulum, which hydrolyzes cGAMP, preventing STING activation. Alternatively, extracellular accumulation of cGAMP can also be transported through the SLC19A1 importer to the cytosol of host immune cells (94, 95), where it is then sensed by STING. Activated STING through TANK-binding kinase 1 (TBK1) and IkB kinase (IKK) induces interferon regulatory factor 3 (IRF3) phosphorylation, resulting in the transcription of type I interferon genes. Activation of STING leads to increased immune infiltration of DC, in particular cross-presenting cDC1 and cytotoxic T-cell activation (29, 96). DC attracted to capture dsDNA will induce IFNβ secretion and will in turn activate CD8α+ CD11c+ cells (97) with the release of proinflammatory cytokines (CXCL9, CXCL10, and CCL5; ref. 98). Chronic activation of the STING pathway leads to the suppression of type I IFN production and upregulation of an alternative downstream NF-κB signaling that elicits a malignant phenotype and a prometastatic program. One of the IFN-stimulated genes is indoleamine-2,3-dioxygenase-1 (IDO) released from tumors, which promotes activation of CD4+ regulatory T cells and suppresses T-helper and effector functions (99, 100). In tumors with low antigenicity, IDO induces immune tolerance by inducing TGFβ in DC, whereas in tumors with high antigenicity, antitumor responses prevail (100–102). (Adapted from an image created with BioRender.com.)
Chromosomal instability, a hallmark of cancer, is often associated with tumor progression, metastasis, and therapeutic resistance. It is a major source of cytosolic double-strand DNA sensed by cGAS (cyclic GMP–AMP synthase), which upon binding to its substrate catalyzes the formation of cyclic dinucleotide GMP–AMP (cGAMP; ref. 27) and elicits STING pathway stimulation, which activates the transcription of interferon genes and other cytokines. This tumor cell–autonomous activation is often circumvented through silencing interferon (IFN) signaling, which allows tumor progression and tumor cell dissemination (28).
Yet, cGAMP is also exported to the extracellular space where it acts in a non–cell-autonomous manner stimulating STING in host cells of the tumor microenvironment (TME). However, this STING induction is often evaded by the tumor or stromal ENPP1 transmembrane expression, which promotes the rapid degradation of exported cGAMP, which allows tumor escaping from immunosurveillance (13, 29). In contrast, inhibition of ENPP1 leads to the accumulation of cGAMP and the subsequent cGAS–STING activation, which enhances innate immune responses by inducing the production of cytokines such as IFNγ and by activating dendritic cells (DC) with antitumor–immune response.
Thus, targeting ENPP1 represents a novel opportunity to boost the cGAS–STING–IFN pathway that could be exploited in novel therapeutic approaches to immunotherapy. Furthermore, therapies that help generate cytosolic DNA such as DNA damage inhibitors, etoposide, or radiation can trigger STING activation and could synergize with ENPP1 inhibition. Of note, a delicate adjustment of STING levels may be required to achieve an optimal clinical benefit because chronic cGAS–STING engagement could lead to tumor promoter functions (30).
Exploiting ENPP1 in Cancer Therapy
Because ENPP1 is at the crossroads of several pathways, its pharmacologic blockade emerges as a promising therapeutic option in a combinatorial setting with other therapeutic strategies in a large variety of tumors (31). Inhibition of other components of the purinergic axis could be required to achieve a more salient effect. For instance, concurrent blockade of A2AR/CD73/CD39 could more efficiently abrogate the purinergic signaling, not only in the tumor but also in infiltrating immune subpopulations. Furthermore, ENPP1 inhibition could also boost the effects of STING agonists with the potential benefit of eliciting strong immune remodeling with DC activation. In addition to ENPP1 abrogation, the concomitant inhibition of purinergic pathways as well as the potential benefit of other modalities such as RT could bring about substantial benefits in the clinical setting.
A range of specific small-molecule inhibitors targeting ENPP1 have been tested in preclinical models. The ENPP1 inhibitor STF-1623/CM-3163 (Angarus) is a cell-impermeable, nontoxic specific inhibitor, which acts by chelating Zn2+ (32). Its lack of permeability presumably prevents toxic events with high specificity while keeping its strong STING activation. The inclusion of this ENPP1 inhibitor resulted in a decreased rate of locoregional failure of breast cancer models treated with surgery and radiation. This finding reveals not only a delay in the occurrence of locoregional failure but a net reduction in the number of failures, which may translate in a substantial benefit in the clinical setting (33). Analysis of the immune landscape posttreatment showed a decrease in MDSCs and a diminished tumor-associated macrophages (TAM) infiltration (specifically M2-polarized) as well as an enhanced antigen presentation observed by an increased DC, CD8+ T cells, and NK cells. Similarly, silencing ENPP1 decreased lung metastasis formation in preclinical models (13).
Other inhibitors such as AVA-NP-695 (Avammune) display cellular permeability, oral bioavailability, and show a potent effect at nmol/L doses as monotherapy, decreasing tumor volume and showing an advantage of the oral administration in breast cancer models (34). Another orally administered ENPP1 inhibitor, TXN10128, shows synergistic growth inhibition with anti–PD-L1 in a preclinical colon cancer model with increased tumor-infiltrating lymphocytes (35).
Another broad ectonucleotidase inhibitor POM1 (Sodium polyoxotungstate) lacks specificity as it inhibits CD39 (ENTPD1, ectonucleoside triphosphate diphosphohydrolase-1) and ENPP1 at high doses. Blockade of CD39 improves antitumor immunity and decreases the metastatic burden (36).
A recently reported ENPP1 inhibitor, ZX-8177, shows marked tumor growth inhibition when used alone or in combination with anti–PD-L1, with strong immune remodeling in a colon murine model (37).
RBS2418 (Riboscience) is a potent and selective small-molecule inhibitor of ENPP1 that can be delivered orally. RBS2418 as monotherapy can potentially have an activating effect on the antitumor innate immune response and leads to antitumor responses in adult subjects with advanced or metastatic tumors. Dose escalation showed no associated toxicities. Although only one clinical trial is ongoing, prospective trials could be anticipated with different ENPP1 inhibitors combined with other therapies (Supplementary Table S1).
Recently, high-affinity and specific anti-ENPP1 antibodies and derived antibody-drug conjugates, IgG-based specific T-cell engagers, and CAR T-cells have shown potent killing activities in ENPP1-expressing cells (38).
Strategies Leveraging the Purinergic Axis
ENPP1 inhibition can be partially mirrored by inhibiting other components of the purinergic pathway such as CD39/CD73 and/or A2AR. Strategies that decrease the Ado-mediated signaling by concomitant blockade of CD39/CD73 and A2AR (39, 40) have been developed. However, the unique effects elicited by ENPP1 inhibition on the activation of the STING pathway might represent an overt advantage when compared with the double abrogation of CD39/CD73 and A2AR. In this vein, the concurrent blockade of ENPP1 and A2AR could have more salient effects. Moreover, ENPP1 inhibition could be more advantageous in high ENPP1-expressing tumors. Other differences are related to differential expression levels of CD39/CD73 in B and T regulatory cells among others, as compared with ENPP1 expression in immune cells, which could yield subtle differences in the immune reshaping (41, 42).
Targeting CD39
Because CD39 is expressed in macrophages, neutrophils, DC, and regulatory T cells (Treg), a marked immune remodeling was observed when inhibiting CD39, either alone or in combination with anti–PD-1, which enhanced CD8+ T cells and decreased intratumor macrophages (43). Based on these findings, different phase I clinical trials are ongoing in advanced solid and hematologic tumors using anti-CD39 alone or in combination with anti–PD-1, A2AR inhibitors, and chemotherapy (Supplementary Table S2).
Targeting A2AR
P1 receptors are broadly expressed in many tumors and several immune subpopulations (ref. 44; Fig. 2) whereas different levels of CD39-CD73 and CD38-ENPP1 are found in tumors and fluctuate along tumor progression. These differences in tumor expression levels could have different kinds of impacts on therapeutic efficacy. For instance, a tumor growth delay was observed when tumor cells were implanted in CD73/A2AR double knockout mice, whereas the double pharmacologic systemic blockade of CD73 and A2AR powerfully impaired tumor growth and metastasis presumably related to the blockade of tumor-intrinsic functions (45). Ablating A2AR signaling promotes NK maturation and antitumor immunity, while decreasing tumor growth (46). Furthermore, blocking A2AR in combination with anti–PD-1 antibody achieved better antitumor–immune responses compared with single treatments in preclinical models (47). These effects were associated with improved immune cell infiltration, DC priming, and CD8+ T-cell expansion.
Based on these findings, several clinical trials targeting Ado receptors (AZD4635 or SCH58261) are currently ongoing (Supplementary Table S3). Inhibitors of A2AR are more frequently combined with ICB, or with anti-CD73/NT5E in combination with ICB in phase I and II clinical trials in solid tumors (48). The inclusion of RT or chemotherapy combined with A2AR inhibitors and ICB is also being explored.
Phase I clinical trials of A2AR inhibitors in combination with first-in-class CD38-targeting antibody, daratumumab, currently approved for the treatment of multiple myeloma, are ongoing, although the efficacy of CD38 antibody is most likely due to antibody-dependent cell-mediated cytotoxicity and not due to adenosine pathway degradation (49).
Targeting CD73/NT5E
CD73 is expressed in Treg and Breg cells of the immune compartment. CD73 is also expressed in mesenchymal stem cells, in tumor-associated stem cells, and it is highly expressed in the vast majority of solid tumors (50, 51). Because Ado accumulation is dependent on the expression and activity of CD73 in tumor cells, blocking CD73 in the TME could lead to substantial benefit impairing tumor growth, which could be more efficacious in combination with chemotherapy and ICB. Targeting CD73 (oleclumab) in combination with ICB showed better outcomes than a single ICB agent (52). Inhibition of CD73 in a preclinical model of pancreatic neuroendocrine tumors led to reduced tumor growth and metastatic potential in cancer stem cells (53). Similarly, CD73−/− mice also develop fewer lung metastases in preclinical models, suggesting that host CD73 also supports metastasis (54, 55). Impaired tumor growth mediated by reshaping the immune landscape with CD8+ T-cell infiltration was revealed in models of induced fibrosarcoma and prostate tumors after anti-CD73 treatment (54). Supported by these findings, a large number of phase I and phase II clinical trials targeting CD73 inhibition in combination with ICB (anti–PD-1, anti–PD-L1, or bispecific PD-1/CTLA-4 antibody) or chemotherapy are currently ongoing in a variety of advanced solid tumors (Supplementary Table S4).
Combinatorial Blockade of ENPP1
Inhibition of ENPP1 can also be exploited in combination to heighten the effects of immunotherapy by radiotherapy (RT), agonists of STING, and the use of ICB and DDR inhibitors.
Combination with RT
Besides cytotoxic effects induced by ionizing radiation (56–58), its potent antitumor–immune response is also triggered by the cGAS/STING pathway elicited by the tumor-derived cytoplasmic DNA sensing (59–61). In this context, the combination of RT with ENPP1 inhibition (which also indirectly stimulates STING) could increase the efficacy of current treatments (60, 62). For instance, in the preclinical triple-negative breast cancer model, combined RT and ENPP1 inhibition showed a synergistic effect (33). Moreover, equi-effective fractionated RT doses resulted in a higher immune stimulation, presumably by a dual effect on wave release of tumor antigens and on the sequential STING stimulation. However, in animal models, high dose fractions (above 12–18 Gy) attenuated the immunogenicity and abscopal effects by cytosolic DNA degradation mediated by TREX1 expression (61).
These findings could be translated to the clinical setting of triple-negative breast cancer, where event-free survival is largely determined by the residual cancer burden (RCB). After neoadjuvant treatment, 5-year event survival rates range from 93% in women achieving complete or near-complete pathologic response (RCB-0) to only 41% in RCB-III cases (63). Hence, in patients with RCB-I to III, combined RT with ENPP1 inhibition would be a reasonable treatment option because locoregional failure rates are exceedingly high in this patient subset.
RT also triggers Ado release and upregulates other Ado-generating enzymes such as CD38 (64). Likewise, RT could also be used as a combined strategy with the abrogation of CD73 and Ado signaling to improve current treatments (65).
Combination with STING agonists
The use of ENPP1 inhibitors in combination with STING agonists could have the advantage of activating DC and inducing strong immunostimulatory effects. Clinical trials of STING activators either alone or in combination with ICB are currently ongoing in advanced solid and hematologic tumors (ref. 66; Supplementary Table S5). The majority of drugs tested are administered by intratumor injection, a requirement to achieve the maximum therapeutic effect (67). However, this approach may restrict optimal tumor activity at noninjected lesions, thereby jeopardizing survival rates. A first-in-human trial with a systemic intravenous administration compound targeting STING (GSK3745417) in combination with ICB is ongoing. This drug, a di-amidobenzimidazole that outcompetes cGAMP for STING activation, shows efficacy in a syngeneic model of colon tumors (68). A similar trial with another systemic STING agonist (SNX281) is also being carried out. This compound showed tumor regression and robust antitumor activity in combination with anti–PD-1 in preclinical models (69). Although these are promising, a safety profile of STING agonists should be carefully monitored in clinical trials (Fig. 4).
Combination with ICB
Pathways elicited by ENPP1 inhibition in immune-infiltrating cells could also be exploited by the use of ICB. In preclinical models, overexpression of ENPP1 conferred breast and colon tumors resistance to ICB, whereas abrogation of ENPP1 rendered tumors responsive to ICB therapy (13). Based on these findings, ENPP1 inhibition could result in greater immune stimulation concomitantly with anti–PD-1, anti–PD-L1, or anti–CTLA-4 currently approved in the clinical setting. In progress phase Ia/Ib study of RBS2418 as monotherapy or in combination with pembrolizumab in subjects with advanced unresectable, recurrent, or metastatic disease, is harnessing ENPP1 inhibition to unleash antitumor–immune responses (70).
Combination with DDR inhibitors
The sensing of DNA breaks by STING activation, and the exploitation of this pathway by the use of ENPP1 inhibitor could also benefit the combination with DDR inhibitors such as Poly-(ADP-ribose) polymerases (PARP) inhibitors. PARP inhibitors lead to unrepair single- and double-strand breaks and the replication fork stalls, prompting repair by other mechanisms. PARP inhibitors sensitize tumor cells to treatments that induce DNA damage such as radiation. Interestingly, PARP inhibitors stimulate STING and upregulate PD-L1 in many tumors. ENPP1 can also metabolize PAR downstream of PARP in the DDR (71). Thus, concomitant inhibition of ENPP1 and PARP may boost the antitumor effects. Furthermore, it is tempting to speculate that the triple combination with anti–PD-1 or anti–PD-L1, or RT together with PARP or other DDR inhibitors, and ENPP1 inhibitors, could reach more prominent effects than single or double treatments. Future clinical trials will probably explore the efficacy and safety of these potentially promising combinations.
In summary, ENPP1 emerges as an attractive therapeutic target to enhance the effects of RT, to improve the benefit of DNA damage inhibitors, to leverage STING agonists, and to foster mechanisms of resistance emerging with the use of ICB. Based on the array of promising preclinical models, new ongoing trials should highlight the efficacy of ENPP1 abrogation alone, or most likely in combination with other modalities, to ameliorate current clinical outcomes improving local control and ultimately increasing current survival rates in a wide variety of tumors.
Supplementary Material
Clinical Trials of ENPP1 inhibitors
Clinical trials of CD39 inhibitors
Clinical trials of A2AR inhibitors
Clinical trials of CD73 inhibitors
STING-based Clinical Trials
Acknowledgments
This work was supported by FIMA the Fondo de Investigación Sanitaria-Fondo Europeo de Desarrollo Regional “Una manera de hacer Europa” to R. Martínez-Monge (PI16/01847 and PI 19/01884, PI17/00411 and PI20/00419), Foundation AECC (PRYES211377MART), and the Gobierno de Navarra (Ref. 34/2021). F. Lecanda was funded by the Cancer Research Thematic Network of the Instituto de Salud Carlos III (RTICC RD12/0036/0066), RTI2018-094507-B-100, PID2021-12638OB-100 financed by MCIN/AEI /10.13039/501100011033/ and by FEDER “Una manera de hacer Europa.” F. Lecanda was also funded by the Foundation AECC (PROYE20083LECA). This study was also supported by the Foundation for Applied Medical Research (FIMA) and CIBERONC (CB16/12/00443). We thank the editorial assistance of Ruth Breeze. We are grateful to Haritz Moreno and A. Pezonaga-Torres for their insightful comments.
The publication costs of this article were defrayed in part by the payment of publication fees. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Footnotes
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
Authors' Disclosures
No disclosures were reported.
References
- 1. Gachet C. P2 receptors, platelet function and pharmacological implications. Thromb Haemost 2008;99:466–72. [DOI] [PubMed] [Google Scholar]
- 2. Baroni M, Pizzirani C, Pinotti M, Ferrari D, Adinolfi E, Calzavarini S, et al. Stimulation of P2 (P2×7) receptors in human dendritic cells induces the release of tissue factor-bearing microparticles. FASEB J 2007;21:1926–33. [DOI] [PubMed] [Google Scholar]
- 3. Onyedibe KI, Wang M, Sintim HO. ENPP1, an old enzyme with new functions, and small molecule inhibitors: a STING in the tale of ENPP1. Molecules 2019;24:4192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Burnstock G. Pathophysiology and therapeutic potential of purinergic signaling. Pharmacol Rev 2006;58:58–86. [DOI] [PubMed] [Google Scholar]
- 5. Bischoff E, Tran-Thi TA, Decker KF. Nucleotide pyrophosphatase of rat liver. A comparative study on the enzymes solubilized and purified from plasma membrane and endoplasmic reticulum. Eur J Biochem 1975;51:353–61. [DOI] [PubMed] [Google Scholar]
- 6. Pastor-Anglada M, Perez-Torras S. Emerging roles of nucleoside transporters. Front Pharmacol 2018;9:606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ferreira CR, Ansh AJ, Nester C, O'Brien C, Stabach PR, Murtada SI, et al. Musculoskeletal comorbidities and quality of life in ENPP1-deficient adults and the response of enthesopathy to enzyme replacement therapy in murine models. J Bone Miner Res 2022;37:494–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Roberts F, Zhu D, Farquharson C, Macrae VE. ENPP1 in the regulation of mineralization and beyond. Trends Biochem Sci 2019;44:616–28. [DOI] [PubMed] [Google Scholar]
- 9. Grobben B, De Deyn PP, Slegers H. Rat C6 glioma as experimental model system for the study of glioblastoma growth and invasion. Cell Tissue Res 2002;310:257–70. [DOI] [PubMed] [Google Scholar]
- 10. Wang H, Ye F, Zhou C, Cheng Q, Chen H. High expression of ENPP1 in high-grade serous ovarian carcinoma predicts poor prognosis and as a molecular therapy target. PLoS One 2021;16:e0245733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Takahashi RU, Miyazaki H, Takeshita F, Yamamoto Y, Minoura K, Ono M, et al. Loss of microRNA-27b contributes to breast cancer stem cell generation by activating ENPP1. Nat Commun 2015;6:7318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hu M, Guo W, Liao Y, Xu D, Sun B, Song H, et al. Dysregulated ENPP1 increases the malignancy of human lung cancer by inducing epithelial-mesenchymal transition phenotypes and stem cell features. Am J Cancer Res 2019;9:134–44. [PMC free article] [PubMed] [Google Scholar]
- 13. Li J, Duran MA, Dhanota N, Chatila WK, Bettigole SE, Kwon J, et al. Metastasis and immune evasion from extracellular cGAMP hydrolysis. Cancer Discov 2021;11:1212–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lau WM, Doucet M, Stadel R, Huang D, Weber KL, Kominsky SL. Enpp1: a potential facilitator of breast cancer bone metastasis. PLoS One 2013;8:e66752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Abbasi S, Shin DM, Beaty N, Masiuk M, Chen S, Gonzalez-Garcia I, et al. Characterization of monoclonal antibodies to the plasma cell alloantigen ENPP1. Hybridoma (Larchmt) 2011;30:11–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yoon J, Wang H, Kim YC, Yoshimoto M, Abbasi S, Morse Iii HC. Plasma cell alloantigen ENPP1 is expressed by a subset of human B cells with potential regulatory functions. Immunol Cell Biol 2016;94:719–28. [DOI] [PubMed] [Google Scholar]
- 17. Antonioli L, Blandizzi C, Pacher P, Hasko G. Immunity, inflammation and cancer: a leading role for adenosine. Nat Rev Cancer 2013;13:842–57. [DOI] [PubMed] [Google Scholar]
- 18. Ryzhov S, Novitskiy SV, Goldstein AE, Biktasova A, Blackburn MR, Biaggioni I, et al. Adenosinergic regulation of the expansion and immunosuppressive activity of CD11b+Gr1+ cells. J Immunol 2011;187:6120–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hoskin DW, Butler JJ, Drapeau D, Haeryfar SM, Blay J. Adenosine acts through an A3 receptor to prevent the induction of murine anti-CD3-activated killer T cells. Int J Cancer 2002;99:386–95. [DOI] [PubMed] [Google Scholar]
- 20. Levy AP, Asleh R, Blum S, Levy NS, Miller-Lotan R, Kalet-Litman S, et al. Haptoglobin: basic and clinical aspects. Antioxid Redox Signal 2010;12:293–304. [DOI] [PubMed] [Google Scholar]
- 21. Schalper KA, Carleton M, Zhou M, Chen T, Feng Y, Huang SP, et al. Elevated serum interleukin-8 is associated with enhanced intratumor neutrophils and reduced clinical benefit of immune-checkpoint inhibitors. Nat Med 2020;26:688–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Teijeira A, Garasa S, Ochoa MC, Villalba M, Olivera I, Cirella A, et al. IL8, neutrophils, and NETs in a collusion against cancer immunity and immunotherapy. Clin Cancer Res 2021;27:2383–93. [DOI] [PubMed] [Google Scholar]
- 23. Wu J, Sun L, Chen X, Du F, Shi H, Chen C, et al. Cyclic GMP-AMP is an endogenous second messenger in innate immune signaling by cytosolic DNA. Science 2013;339:826–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Harding SM, Benci JL, Irianto J, Discher DE, Minn AJ, Greenberg RA. Mitotic progression following DNA damage enables pattern recognition within micronuclei. Nature 2017;548:466–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kwon J, Bakhoum SF. The cytosolic DNA-sensing cGAS-STING pathway in cancer. Cancer Discov 2020;10:26–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ritchie C, Carozza JA, Li L. Biochemistry, cell biology, and pathophysiology of the innate immune cGAS-cGAMP-STING pathway. Annu Rev Biochem 2022;91:599–628. [DOI] [PubMed] [Google Scholar]
- 27. Sun L, Wu J, Du F, Chen X, Chen ZJ. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science 2013;339:786–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bakhoum SF, Ngo B, Laughney AM, Cavallo JA, Murphy CJ, Ly P, et al. Chromosomal instability drives metastasis through a cytosolic DNA response. Nature 2018;553:467–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Carozza JA, Böhnert V, Nguyen KC, Skariah G, Shaw KE, Brown JA, et al. Extracellular cGAMP is a cancer-cell-produced immunotransmitter involved in radiation-induced anticancer immunity. Nature Cancer 2020;1:184–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gulen MF, Koch U, Haag SM, Schuler F, Apetoh L, Villunger A, et al. Signalling strength determines proapoptotic functions of STING. Nat Commun 2017;8:427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lee SY, Muller CE. Nucleotide pyrophosphatase/phosphodiesterase 1 (NPP1) and its inhibitors. Medchemcomm 2017;8:823–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Carozza JA, Brown JA, Bohnert V, Fernandez D, AlSaif Y, Mardjuki RE, et al. Structure-aided development of small-molecule inhibitors of ENPP1, the extracellular phosphodiesterase of the immunotransmitter cGAMP. Cell Chem Biol 2020;27:1347–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ruiz-Fernandez de Cordoba B, Moreno H, Valencia K, Perurena N, Ruedas P, Walle T, et al. Tumor ENPP1 (CD203a)/haptoglobin axis exploits myeloid-derived suppressor cells to promote post-radiotherapy local recurrence in breast cancer. Cancer Discov 2022;12:1356–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gangar M, Goyal S, Raykar D, Khurana P, Martis AM, Goswami A, et al. Design, synthesis and biological evaluation studies of novel small molecule ENPP1 inhibitors for cancer immunotherapy. Bioorg Chem 2022;119:105549. [DOI] [PubMed] [Google Scholar]
- 35. Kim S, Ali I, Yu A, Lee S, Park S, Choi J, et al. Orally available ENPP1 inhibitor, TXN10128, restores STING activation in tumor microenvironment and confers anti-tumor responses in combination with immune checkpoint blockade. Molecular Cancer Therapies 2021;20:LBA009. [Google Scholar]
- 36. Zhang H, Vijayan D, Li XY, Robson SC, Geetha N, Teng MWL, et al. The role of NK cells and CD39 in the immunological control of tumor metastases. Oncoimmunology 2019;8:e1593809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Li Y-yPeng Z, Sun S, Guo kKong D, Li X, et al. ENPP1 inhibitor ZX-8177 enhances anti-tumor activity of conventional therapies by modulating tumor microenvironment. Cancer Res 2022;82:5486. [Google Scholar]
- 38. Chu X, Baek D-S, Li W, Shyp T, Mooney B, Hines MG, et al. Human antibodies targeting ENPP1 as candidate therapeutics for cancers. Front Immunol 2023;14:1070492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Vijayan D, Young A, Teng MWL, Smyth MJ. Targeting immunosuppressive adenosine in cancer. Nat Rev Cancer 2017;17:765. [DOI] [PubMed] [Google Scholar]
- 40. Vigano S, Alatzoglou D, Irving M, Menetrier-Caux C, Caux C, Romero P, et al. Targeting adenosine in cancer immunotherapy to enhance T-cell function. Front Immunol 2019;10:925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kaku H, Cheng KF, Al-Abed Y, Rothstein TL. A novel mechanism of B cell-mediated immune suppression through CD73 expression and adenosine production. J Immunol 2014;193:5904–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A, et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med 2007;204:1257–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Li XY, Moesta AK, Xiao C, Nakamura K, Casey M, Zhang H, et al. Targeting CD39 in cancer reveals an extracellular ATP- and inflammasome-driven tumor immunity. Cancer Discov 2019;9:1754–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ohta A, Ohta A, Madasu M, Kini R, Subramanian M, Goel N, et al. A2A adenosine receptor may allow expansion of T cells lacking effector functions in extracellular adenosine-rich microenvironments. J Immunol 2009;183:5487–93. [DOI] [PubMed] [Google Scholar]
- 45. Young A, Ngiow SF, Barkauskas DS, Sult E, Hay C, Blake SJ, et al. Co-inhibition of CD73 and A2AR adenosine signaling improves anti-tumor immune responses. Cancer Cell 2016;30:391–403. [DOI] [PubMed] [Google Scholar]
- 46. Young A, Ngiow SF, Gao Y, Patch AM, Barkauskas DS, Messaoudene M, et al. A2AR adenosine signaling suppresses natural killer cell maturation in the tumor microenvironment. Cancer Res 2018;78:1003–16. [DOI] [PubMed] [Google Scholar]
- 47. Beavis PA, Milenkovski N, Henderson MA, John LB, Allard B, Loi S, et al. Adenosine receptor 2A blockade increases the efficacy of anti-PD-1 through enhanced antitumor T-cell responses. Cancer Immunol Res 2015;3:506–17. [DOI] [PubMed] [Google Scholar]
- 48. Lim EA, Bendell JC, Falchook GS, Bauer TM, Drake CG, Choe JH, et al. Phase 1a/b, open-label, multicenter study of AZD4635 (an adenosine 2A receptor antagonist) as monotherapy or combined with durvalumab, in patients with solid tumors. Clin Cancer Res 2022;28:4871–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. San-Miguel J, Avet-Loiseau H, Paiva B, Kumar S, Dimopoulos MA, Facon T, et al. Sustained minimal residual disease negativity in newly diagnosed multiple myeloma and the impact of daratumumab in MAIA and ALCYONE. Blood 2022;139:492–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Breitbach M, Kimura K, Luis TC, Fuegemann CJ, Woll PS, Hesse M, et al. In vivo labeling by CD73 marks multipotent stromal cells and highlights endothelial heterogeneity in the bone marrow niche. Cell Stem Cell 2018;22:262–76. [DOI] [PubMed] [Google Scholar]
- 51. Chen X, Shao H, Zhi Y, Xiao Q, Su C, Dong L, et al. CD73 pathway contributes to the immunosuppressive ability of mesenchymal stem cells in intraocular autoimmune responses. Stem Cells Dev 2016;25:337–46. [DOI] [PubMed] [Google Scholar]
- 52. Herbst RS, Majem M, Barlesi F, Carcereny E, Chu Q, Monnet I, et al. COAST: an open-label, phase II, multidrug platform study of durvalumab alone or in combination with oleclumab or monalizumab in patients with unresectable, stage iii non-small-cell lung cancer. J Clin Oncol 2022:JCO2200227. doi 10.1200/JCO.22.00227. [DOI] [PubMed] [Google Scholar]
- 53. Katsuta E, Tanaka S, Mogushi K, Shimada S, Akiyama Y, Aihara A, et al. CD73 as a therapeutic target for pancreatic neuroendocrine tumor stem cells. Int J Oncol 2016;48:657–69. [DOI] [PubMed] [Google Scholar]
- 54. Stagg J, Beavis PA, Divisekera U, Liu MC, Moller A, Darcy PK, et al. CD73-deficient mice are resistant to carcinogenesis. Cancer Res 2012;72:2190–6. [DOI] [PubMed] [Google Scholar]
- 55. Stagg J, Divisekera U, Duret H, Sparwasser T, Teng MW, Darcy PK, et al. CD73-deficient mice have increased antitumor immunity and are resistant to experimental metastasis. Cancer Res 2011;71:2892–900. [DOI] [PubMed] [Google Scholar]
- 56. Walle T, Martinez Monge R, Cerwenka A, Ajona D, Melero I, Lecanda F. Radiation effects on antitumor immune responses: current perspectives and challenges. Ther Adv Med Oncol 2018;10:1758834017742575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Golden EB, Pellicciotta I, Demaria S, Barcellos-Hoff MH, Formenti SC. The convergence of radiation and immunogenic cell death signaling pathways. Front Oncol 2012;2:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol 2013;14:1014–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Gupta A, Probst HC, Vuong V, Landshammer A, Muth S, Yagita H, et al. Radiotherapy promotes tumor-specific effector CD8+ T cells via dendritic cell activation. J Immunol 2012;189:558–66. [DOI] [PubMed] [Google Scholar]
- 60. Deng L, Liang H, Xu M, Yang X, Burnette B, Arina A, et al. STING-dependent cytosolic DNA sensing promotes radiation-induced type I interferon-dependent antitumor immunity in immunogenic tumors. Immunity 2014;41:843–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Vanpouille-Box C, Alard A, Aryankalayil MJ, Sarfraz Y, Diamond JM, Schneider RJ, et al. DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nat Commun 2017;8:15618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Burnette BC, Liang H, Lee Y, Chlewicki L, Khodarev NN, Weichselbaum RR, et al. The efficacy of radiotherapy relies upon induction of type i interferon-dependent innate and adaptive immunity. Cancer Res 2011;71:2488–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Symmans WF, Yau C, Chen YY, Balassanian R, Klein ME, Pusztai L, et al. Assessment of residual cancer burden and event-free survival in neoadjuvant treatment for high-risk breast cancer: an analysis of data from the I-SPY2 randomized clinical trial. JAMA Oncol 2021;7:1654–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wirsdorfer F, de Leve S, Cappuccini F, Eldh T, Meyer AV, Gau E, et al. Extracellular adenosine production by ecto-5'-nucleotidase (CD73) enhances radiation-induced lung fibrosis. Cancer Res 2016;76:3045–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. de Leve S, Wirsdorfer F, Jendrossek V. Targeting the immunomodulatory CD73/adenosine system to improve the therapeutic gain of radiotherapy. Front Immunol 2019;10:698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Meric-Bernstam F, Sweis RF, Kasper S, Hamid O, Bhatia S, Dummer R, et al. Combination of the STING agonist MIW815 and PD-1 inhibitor spartalizumab in advanced/metastatic solid tumors or lymphomas: an open-label, multicenter, phase Ib study. Clin Cancer Res 2023;29:110–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Harrington KJ, William WN, Khilnani A, Algazi AP. 972TiP Phase II study of intratumoral MK-1454 plus pembrolizumab compared with pembrolizumab monotherapy as first-line treatment for metastatic or unresectable, recurrent head and neck squamous cell carcinoma. Ann Oncol 2020;31(Supplement 4):S683. [Google Scholar]
- 68. Ramanjulu JM, Pesiridis GS, Yang J, Concha N, Singhaus R, Zhang SY, et al. Design of amidobenzimidazole STING receptor agonists with systemic activity. Nature 2018;564:439–43. [DOI] [PubMed] [Google Scholar]
- 69. Wang J, Falchook G, Nabhan S, Kulkarni M, Sandy P, Dosunmu O, et al. Trial of SNX281, A systematically delivered small molecule sting agonist in solid tumors and lymphomas J Immunother Cancer 2021;9(Suppl 2):A1–A1054. [Google Scholar]
- 70. Csiki IDA, Tuan BY, John E, O'Toole L, Seppa J, Huang N, et al. First-in-human experience using RBS2418, an oral ENPP1 inhibitor within an expanded access protocol in combination with pembrolizumab in a patient with metastatic adrenal cancer. J Clin Oncol 2022;16(suppl (June 01, 2022)):e14550. [Google Scholar]
- 71. Palazzo L, Daniels CM, Nettleship JE, Rahman N, McPherson RL, Ong SE, et al. ENPP1 processes protein ADP-ribosylation in vitro. FEBS J 2016;283:3371–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Horenstein AL, Chillemi A, Zaccarello G, Bruzzone S, Quarona V, Zito A, et al. A CD38/CD203a/CD73 ectoenzymatic pathway independent of CD39 drives a novel adenosinergic loop in human T lymphocytes. Oncoimmunology 2013;2:e26246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Allard B, Longhi MS, Robson SC, Stagg J. The ectonucleotidases CD39 and CD73: novel checkpoint inhibitor targets. Immunol Rev 2017;276:121–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Zimmermann H, Zebisch M, Strater N. Cellular function and molecular structure of ecto-nucleotidases. Purinergic Signal 2012;8:437–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Kato K, Nishimasu H, Mihara E, Ishitani R, Takagi J, Aoki J, et al. Expression, purification, crystallization and preliminary X-ray crystallographic analysis of Enpp1. Acta Crystallogr Sect F Struct Biol Cryst Commun 2012;68(Pt 7):778–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Maddux BA, Sbraccia P, Kumakura S, Sasson S, Youngren J, Fisher A, et al. Membrane glycoprotein PC-1 and insulin resistance in non-insulin-dependent diabetes mellitus. Nature 1995;373:448–51. [DOI] [PubMed] [Google Scholar]
- 77. Lomashvili KA, Narisawa S, Millan JL, O'Neill WC. Vascular calcification is dependent on plasma levels of pyrophosphate. Kidney Int 2014;85:1351–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Nitschke Y, Hartmann S, Torsello G, Horstmann R, Seifarth H, Weissen-Plenz G, et al. Expression of NPP1 is regulated during atheromatous plaque calcification. J Cell Mol Med 2011;15:220–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Conti M. Phosphodiesterases and cyclic nucleotide signaling in endocrine cells. Mol Endocrinol 2000;14:1317–27. [DOI] [PubMed] [Google Scholar]
- 80. Kortekaas KE, Santegoets SJ, Sturm G, Ehsan I, van Egmond SL, Finotello F, et al. CD39 identifies the CD4(+) tumor-specific T-cell population in human cancer. Cancer Immunol Res 2020;8:1311–21. [DOI] [PubMed] [Google Scholar]
- 81. Kidani Y, Nogami W, Yasumizu Y, Kawashima A, Tanaka A, Sonoda Y, et al. CCR8-targeted specific depletion of clonally expanded Treg cells in tumor tissues evokes potent tumor immunity with long-lasting memory. Proc Natl Acad Sci U S A 2022;119:e2114282119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Montalban Del Barrio I, Penski C, Schlahsa L, Stein RG, Diessner J, Wockel A, et al. Adenosine-generating ovarian cancer cells attract myeloid cells which differentiate into adenosine-generating tumor associated macrophages - a self-amplifying, CD39- and CD73-dependent mechanism for tumor immune escape. J Immunother Cancer 2016;4:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Ferretti E, Horenstein AL, Canzonetta C, Costa F, Morandi F. Canonical and non-canonical adenosinergic pathways. Immunol Lett 2019;205:25–30. [DOI] [PubMed] [Google Scholar]
- 84. Idzko M, Ferrari D, Riegel AK, Eltzschig HK. Extracellular nucleotide and nucleoside signaling in vascular and blood disease. Blood 2014;124:1029–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Mora-Garcia S, de Leone MJ, Yanovsky M. Time to grow: circadian regulation of growth and metabolism in photosynthetic organisms. Curr Opin Plant Biol 2017;35:84–90. [DOI] [PubMed] [Google Scholar]
- 86. de Andrea CE, Ochoa MC, Villalba-Esparza M, Teijeira A, Schalper KA, Abengozar-Muela M, et al. Heterogenous presence of neutrophil extracellular traps in human solid tumours is partially dependent on IL-8. J Pathol 2021;255:190–201. [DOI] [PubMed] [Google Scholar]
- 87. Park J, Wysocki RW, Amoozgar Z, Maiorino L, Fein MR, Jorns J, et al. Cancer cells induce metastasis-supporting neutrophil extracellular DNA traps. Sci Transl Med 2016;8:361ra138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Cools-Lartigue J, Spicer J, McDonald B, Gowing S, Chow S, Giannias B, et al. Neutrophil extracellular traps sequester circulating tumor cells and promote metastasis. J Clin Invest 2013;123:3446-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Mackenzie KJ, Carroll P, Martin CA, Murina O, Fluteau A, Simpson DJ, et al. cGAS surveillance of micronuclei links genome instability to innate immunity. Nature 2017;548:461–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Ablasser A, Schmid-Burgk JL, Hemmerling I, Horvath GL, Schmidt T, Latz E, et al. Cell intrinsic immunity spreads to bystander cells via the intercellular transfer of cGAMP. Nature 2013;503:530–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Chen Q, Boire A, Jin X, Valiente M, Er EE, Lopez-Soto A, et al. Carcinoma-astrocyte gap junctions promote brain metastasis by cGAMP transfer. Nature 2016;533:493–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Schadt L, Sparano C, Schweiger NA, Silina K, Cecconi V, Lucchiari G, et al. Cancer-cell-intrinsic cGAS expression mediates tumor immunogenicity. Cell Rep 2019;29:1236–48. [DOI] [PubMed] [Google Scholar]
- 93. Gentili M, Kowal J, Tkach M, Satoh T, Lahaye X, Conrad C, et al. Transmission of innate immune signaling by packaging of cGAMP in viral particles. Science 2015;349:1232–6. [DOI] [PubMed] [Google Scholar]
- 94. Ritchie C, Cordova AF, Hess GT, Bassik MC, Li L. SLC19A1 is an importer of the immunotransmitter cGAMP. Mol Cell 2019;75:372–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Lahey LJ, Mardjuki RE, Wen X, Hess GT, Ritchie C, Carozza JA, et al. LRRC8A:C/E heteromeric channels are ubiquitous transporters of cGAMP. Mol Cell 2020;80:578–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Marcus A, Mao AJ, Lensink-Vasan M, Wang L, Vance RE, Raulet DH. Tumor-derived cGAMP triggers a STING-mediated interferon response in non-tumor cells to activate the NK cell response. Immunity 2018;49:754–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Belz GT, Smith CM, Eichner D, Shortman K, Karupiah G, Carbone FR, et al. Cutting edge: conventional CD8 alpha+ dendritic cells are generally involved in priming CTL immunity to viruses. J Immunol 2004;172:1996–2000. [DOI] [PubMed] [Google Scholar]
- 98. Harlin H, Meng Y, Peterson AC, Zha Y, Tretiakova M, Slingluff C, et al. Chemokine expression in melanoma metastases associated with CD8+ T-cell recruitment. Cancer Res 2009;69:3077–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Lemos H, Huang L, McGaha TL, Mellor AL. Cytosolic DNA sensing via the stimulator of interferon genes adaptor: Yin and Yang of immune responses to DNA. Eur J Immunol 2014;44:2847–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Lemos H, Mohamed E, Huang L, Ou R, Pacholczyk G, Arbab AS, et al. STING promotes the growth of tumors characterized by low antigenicity via IDO activation. Cancer Res 2016;76:2076–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Huang L, Li L, Lemos H, Chandler PR, Pacholczyk G, Baban B, et al. Cutting edge: DNA sensing via the STING adaptor in myeloid dendritic cells induces potent tolerogenic responses. J Immunol 2013;191:3509–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Munn DH, Sharma MD, Lee JR, Jhaver KG, Johnson TS, Keskin DB, et al. Potential regulatory function of human dendritic cells expressing indoleamine 2,3-dioxygenase. Science 2002;297:1867–70. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Clinical Trials of ENPP1 inhibitors
Clinical trials of CD39 inhibitors
Clinical trials of A2AR inhibitors
Clinical trials of CD73 inhibitors
STING-based Clinical Trials