Abstract
Background:
Cisgender women have been underrepresented in antibiotic-resistant gonorrhea (ARGC) surveillance systems. Three of 8 project sites (City of Milwaukee [MIL], Guilford County [GRB], Denver County [DEN]), funded under the Centers for Disease Control and Prevention’s Strengthening the US Response to Resistant Gonorrhea (SURRG), focused efforts to better include cisgender women in ARGC surveillance.
Methods:
MIL, GRB, and DEN partnered with diverse health care settings and developed gonorrhea culture criteria to facilitate urogenital specimen collection in cisgender women and men. Regional laboratories within the Antibiotic Resistance Laboratory Network performed agar dilution antibiotic susceptibility testing (AST) of gonococcal isolates. Data from 2018 and 2019 were analyzed.
Results:
In SURRG, 90.5% (11,464 of 12,667) of the cisgender women from whom urogenital culture specimens were collected were from MIL, GRB, and DEN. Of women in SURRG whose gonococcal isolates underwent AST, 70% were from these 3 sites. In these 3 sites, a substantial proportion of cisgender women with positive urogenital cultures and AST were from health care settings other than sexually transmitted disease (STD) clinics (non-STD clinics; MIL, 56.0%; GRB, 80.4%; and DEN, 23.5%). Isolates with AST were obtained from 5.1%, 10.2%, and 2.4% of all diagnosed gonorrhea cases among cisgender women in MIL, GRB, and DEN, respectively, and were more often susceptible to all antibiotics than those from cisgender men from each of these sites.
Conclusions:
With focused efforts and partnerships with non-STD clinics, 3 SURRG sites were able to include robust ARGC surveillance from cisgender women. These findings may guide further efforts to improve gender equity in ARGC surveillance.
In 2019, the United States reported more than 616,000 cases of Neisseria gonorrhoeae (GC), a 56% increase since 2015.1 From 2015 to 2019, rates increased in both men and women. The threat of gonorrhea is further heightened by evolving antimicrobial resistance (AMR) and shrinking options for treatment. In 1986, the Centers for Disease Control and Prevention (CDC) initiated the Gonococcal Isolate Surveillance Project (GISP), a sentinel surveillance system to monitor gonococcal antimicrobial susceptibility and guide gonococcal treatment. Although GISP has been successful at identifying long-term trends and informing treatment guidelines, it relies on systematic collection of urogenital specimens from cisgender men and may not fully represent the spectrum of gonococcal AMR across all genders and anatomical sites. To achieve surveillance of antibiotic-resistant gonorrhea (ARGC) with robust representativeness, evaluation of gonococcal isolates should be conducted in a sufficient proportion of cases, and isolates should represent the morbidity of disease by geography, race/ethnicity, age, gender identity, sexual orientation, and anatomical site of infection.2 However, in the United States and globally, there are limited data on gonococcal AMR rates in women.3–5 In the United States, women represent approximately 40% of reported GC cases, but to date, reported GISP data have only included gonococcal isolates obtained from men.1
Sex at birth and sexual orientation play a role in the prevalence of gonococcal disease and associated morbidity.1 Limited data suggest that sex at birth may play a role in the prevalence of ARGC. Expression of some gonococcal AMR-associated genes such as the mtrCDE-encoded efflux pump is linked to environmental conditions such as availability of free iron.6 High iron environments in the female urogenital tract and the formation of biofilms during infection in cisgender women may influence AMR.6–8 Data suggest that nearly 14% of gonococcal genes are differentially expressed when compared during growth in the cisgender male and female urogenital tracts including genes associated with AMR.9
Surveillance for AMR in penile urethral gonococcal infection is facilitated by higher rates of urogenital GC among cisgender men than among women, the increased likelihood of symptomatic disease during urethral as compared with endocervical infection, and a greater proportion of cisgender male GC diagnoses (10.4%) occurring in sexually transmitted disease (STD) clinics compared with the proportion of GC diagnoses in cisgender women (5.9%).1 N. gonorrhoeae culture and access to penile urethral Gram stain for point-of-care diagnosis is more readily available in STD clinics, which allows for same visit collection of GC diagnostic and AMR surveillance samples. In addition, sample collection at the male urethra for GC culture is quick, requires low technical expertise, and supports surveillance of gonococcal resistance trends in cisgender men who have sex with men (MSM) and heterosexual sexual networks. The GISP leverages these features of urogenital infection in cisgender men to create a systematic and standardized ARGC surveillance system based in STD clinics across the United States.
Conversely, systematic and representative gonococcal AMR surveillance in cisgender women is limited by lower GC rates among women than among men, the high proportion of asymptomatic disease at the endocervix, and requirements for speculum examination for endocervical swab collection for GC culture. Furthermore, STD care for cisgender women is decentralized across varied health care facilities that have limited access to GC culture.1,10 Another substantial barrier to ARGC surveillance in cisgender women is the overall poor sensitivity of endocervical/vaginal GC culture in cases with vaginal or urine positive nucleic acid amplification tests (NAATs; symptomatic, 58.9%; asymptomatic, 55.3%) compared with male urethral GC culture (symptomatic, 92.2%; asymptomatic, 78.7%).11,12 Finally, the sensitivity of endocervical culture declines as time between culture collection and positive NAAT collection increases, making limited availability of point-of-care diagnostics yet another hurdle to establishing effective AMR surveillance systems that include cisgender women.11 Together, these factors limit the options for systematic and predictable GC culture collection in cisgender women across the United States and prevent robust comparisons of AMR data across jurisdictions or between genders.
In 2016, the CDC initiated the Strengthening the US Response to Resistant Gonorrhea (SURRG) project to bolster local rapid detection and response capacity, with goals that included collection of samples in patients of all genders and sexual orientations from STD clinics and other health care settings providing sexual health services (non-STD clinics). Such non-STD clinics included family planning clinics, women’s health clinics (WHCs), clinics providing HIV care, urgent and emergency care settings, and adolescent care clinics. The SURRG project aims to represent approximately 15% of GC morbidity in funded jurisdictions while advancing the capacity for local antimicrobial susceptibility testing (AST) and GC-focused partner services. Rather than GISP’s focus on systematic gonococcal AMR surveillance of susceptibility trends to guide treatment recommendations, SURRG focuses on surveillance for rapid detection and response and has the potential to address surveillance gaps, gender bias, and the possibility of gender-based differences in gonococcal AMR. In 2018 and 2019, 3 SURRG sites accounted for the majority of cisgender women with GC isolates with AST in SURRG. This article will discuss methods undertaken by the SURRG projects in the city of Milwaukee, Wisconsin (MIL); Guilford County, North Carolina (GRB); and Denver County, Colorado (DEN) to identify and address barriers to equitable ARGC surveillance in cisgender women. The analysis will compare demographic characteristics and AMR results of urogenital cultures in cisgender women and cisgender men for these SURRG sites.
METHODS
Participating Clinics
In 2018 and 2019, 3 of 8 SURRG sites (MIL, GRB, and DEN) focused project efforts to increase representation of cisgender women in ARGC surveillance. These 3 SURRG sites recruited clinics with elevated GC morbidity and relatively high numbers of GC diagnoses among cisgender women. MIL included 3 reproductive health/family planning clinics and a categorical STD clinic. GRB included 1 women’s health hospital and a WHC, 2 emergency departments, and 2 categorical STD clinics with dedicated teen clinics. In DEN, the clinics with a focus on cisgender women included one WHC, an urgent care clinic, and a categorical STD clinic. Other MIL and DEN SURRG non-STD clinics included an LGTBQ sexual health clinic, an infectious disease clinic, and an HIV primary care clinic, which contributed minimally to collection of GC isolates from cisgender women, demonstrating the critical importance of surveillance site selection to representation of cisgender women and alignment to local morbidity.
Specimen Collection
GRB and DEN engaged with emergency departments and urgent care sites to implement broad, inclusive GC culture criteria that recommended specimen culture collection from all cisgender women with any exposure to a partner with an STD in GRB and from any women undergoing a speculum examination in DEN. Broad culture criteria can result in lower culture recovery. However, in these emergency departments and urgent care sites, which often administered empiric syndromic therapy, more selective culture criteria (e.g., that entailed collecting specimens for culture days after a GC NAAT tested positive) would have likely resulted in missed opportunities to obtain specimens for culture. Same-day collection of specimens for culture and NAAT during the speculum examination also likely improved culture sensitivity.11 Other more specific criteria were also used to guide GC culture collection in cisgender women in these sites, such as positive GC test results or in GRB findings of cervicitis or pelvic inflammatory disease on exam.
In reproductive health clinics, WHCs, and STD clinics, many cisgender female patients underwent screening for asymptomatic GC. For these clinics, high-yield criteria for culture collection were based on positive results of NAATs, and specimens for culture were collected from women returning for treatment. In addition, these clinics used moderate yield criteria for endocervical culture collection based on a patient’s exposure to a partner with GC or other STD, clinical symptoms such as pelvic pain or discharge, or clinical examination findings such as cervicitis or pelvic inflammatory disease. Non-STD clinic criteria for urethral culture in cisgender men varied by SURRG site but included indications such as symptoms of urethritis or epididymitis, presence of urethral discharge, exposure to a partner with GC or another STD, and a positive GC NAAT result. In STD clinics, urethral GC culture was also obtained if a urethral swab Gram stain was compatible with GC.
Clinic staff reminders for GC culture specimen collection included signage, posted protocols, and electronic health record triggers. In some clinics, including the STD clinic and WHC in DEN, workflows were altered to allow providers to collect endocervical culture specimens during visits that were classically only treatment visits. Each SURRG site used quality assessments for adherence to culture criteria or analysis of culture recovery to drive quality improvement initiatives for GC culture collection at STD and non-STD clinics.
Laboratory Services
Providers’ specimen collection methodology varied among the sites. In MIL, providers in the STD clinic used InTray GC (BioMed Diagnostics, San Jose, CA) with onsite incubators; non-STD clinics used ESwab (Copan Diagnostics, Murrieta, CA). In GRB, providers in the STD clinics performed direct inoculation of modified Thayer-Martin plates; those in non-STD clinics used InTray GC. All GRB clinics had onsite incubators. Providers in all DEN clinics participating in SURRG used direct provider inoculation of Jembec plates with onsite incubators.
All culture specimens with GC growth were evaluated locally for ceftriaxone (CRO), cefixime (CFX), and azithromycin (AZM) susceptibility by Etest (bioMeriéux, Marcy l’Etoile, France). Agar dilution AST for penicillin (PCN), ciprofloxacin (CIPRO), gentamicin, CRO, CFX, tetracycline (TET), and AZM was performed at the designated Antibiotic Resistance Laboratory Network regional laboratory (Texas Department of State Health Services for DEN and MIL; Tennessee Department of Health for GRB). Agar dilution antimicrobial susceptibility data were included in this analysis. Clinical and Laboratory Standards Institute criteria were used to define GC isolates as susceptible to AZM for minimum inhibitory concentration (MIC) ≤1.0 μg/mL.13 For SURRG, GC isolates with AZM MIC ≥2.0 μg/mL were designated nonsusceptible. The Clinical and Laboratory Standards Institute breakpoint for CRO and CFX susceptibility is MIC ≤0.25 μg/mL. For SURRG, to improve detection of emerging resistance, lower thresholds were used to designate reduced susceptibility for CRO and CFX (CRO MIC ≥0.125 μg/mL; CFX MIC ≥0.25 μg/mL). Clinical and Laboratory Standards Institute gonococcal resistance breakpoints were used for TET (MIC ≥2.0 μg/mL), CIPRO (MIC ≥1.0 μg/mL), and PCN (MIC ≥2.0 μg/mL).
Data Management and Analysis
The first full year of SURRG culture specimen and data collection activities occurred in 2018. Demographic data were collected using electronic health records and provider-completed order forms and reported to the CDC as deidentified line-listed data. SURRG within jurisdiction and out of jurisdiction designations were made by self-reported patient address. We defined cisgender females as patients who self-identified as female, were assigned female at birth, and had endocervical or vaginal specimens collected for culture. We defined cisgender males as patients who self-identified as male, were assigned male at birth, and had urethral specimens collected for culture. Patients self-reported sex of sex partners. Laboratory agar dilution data were reported from participating regional laboratories. Jurisdictional GC case report data were extracted from Wisconsin Department of Health Services, North Carolina Division of Public Health, and Colorado Department of Public Health and Environment STD surveillance systems. For this article, we analyzed results from urogenital culture specimens collected for GC among cisgender men and women in 2018 to 2019. More than one urogenital specimen may have been collected for GC culture from some patients at different episodes of GC infection over the study period. Results of extragenital GC cultures were excluded from this analysis to reduce heterogeneity in comparisons and because of overall low numbers of cisgender women with SURRG GC isolates with AST from extragenital sites. We examined epidemiological characteristics of cisgender patients in MIL, GRB, and DEN who had urogenital culture specimens collected and whose isolates underwent AST. To better understand the representation of GC morbidity in each jurisdiction, we calculated the percentage of reported GC cases that were evaluated with GC culture and AST in patients residing within the project jurisdiction (i.e., were “in-jurisdiction”) in 2018 to 2019. Frequencies and percentages were calculated using SAS 9.4 (SAS Institute Inc., Cary, NC), and tests of significance were not conducted for this descriptive data.
Human Subjects Protection
The institutional review board of the CDC determined the SURRG project to be a public health activity and not human subject research.
RESULTS
Overall SURRG Data, 8 Sites
In 2018 and 2019, a total of 26,898 cisgender persons (14,231 men and 12,667 women) had urogenital specimens collected for GC culture in SURRG. Among the 26,898, 7498 (27.9%) (6668 men and 830 women) had positive GC cultures that underwent AST. Across all SURRG sites, most (90.5%; n = 11,464 of 12,667) of the cisgender women with urogenital specimens collected for GC culture and the majority (70%; n = 581 of 830) of cisgender women with positive urogenital GC isolates that underwent AST were from MIL, GRB, or DEN (Table 1). Cisgender women represented a higher proportion of all cisgender patients with positive urogenital GC cultures for each of the 3 sites (MIL, 21.8%; GRB, 29.9%; DEN, 12.1%) than for the other 5 SURRG sites combined (5.3%; results not shown).
TABLE 1.
Demographic Characteristics of Patients With Urogenital Specimen Collected for GC Culture and Patients With Positive Urogenital Culture With Antimicrobial Susceptibility Testing, by Site, Strengthening the US Response to Resistant Gonorrhea (SURRG), 2018 to 2019
| City of Milwaukee, WI |
Guilford County, NC |
Denver County, CO |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patients With Urogenital Culture* |
Patients With Positive Urogenital GC Culture* With AST |
Patients With Urogenital Culture* |
Patients With Positive Urogenital GC Culture* With AST |
Patients With Urogenital Culture* |
Patients With Positive Urogenital GC Culture* With AST |
|||||||
| Characteristic | n | % | n | % | n | % | n | % | n | % | n | % |
| Total | 3854 | 992 | 11,006 | 837 | 2445 | 951 | ||||||
| Cisgender female | 2096 | 54.39 | 216 | 21.77 | 8089 | 73.50 | 250 | 29.87 | 1279 | 52.31 | 115 | 12.09 |
| Cisgender male | 1758 | 45.61 | 776 | 78.23 | 2917 | 26.50 | 587 | 70.13 | 1166 | 47.69 | 836 | 87.91 |
| Clinic type | ||||||||||||
| Cisgender female† | ||||||||||||
| STD clinic | 649 | 30.96 | 95 | 43.98 | 342 | 4.23 | 49 | 19.60 | 1006 | 78.66 | 88 | 76.52 |
| Non-STD clinic‡ | 1447 | 69.04 | 121 | 56.02 | 7747 | 95.77 | 201 | 80.40 | 273 | 21.34 | 27 | 23.48 |
| Cisgender male† | ||||||||||||
| STD clinic | 1401 | 79.69 | 629 | 81.06 | 2462 | 84.40 | 471 | 80.24 | 1086 | 93.14 | 800 | 95.69 |
| Non-STD clinic‡ | 357 | 20.30 | 147 | 18.94 | 455 | 15.60 | 116 | 19.76 | 80 | 6.86 | 36 | 4.31 |
| Age, median (IQR), y | ||||||||||||
| Cisgender female† | 22 | (20–25) | 22 | (19–26) | 26 | (22–33) | 24 | (20–28) | 26 | (22–33) | 24 | (20–30) |
| Cisgender male† | 28 | (24–36) | 29 | (25–37) | 27 | (23–34) | 26 | (22–32) | 30 | (25–36) | 30 | (25–36.5) |
| Gender of sex partners among cisgender males†,§ | ||||||||||||
| MSW | 1418 | 80.66 | 674 | 86.86 | 2120 | 72.68 | 422 | 71.89 | 583 | 50.00 | 455 | 54.43 |
| MSM | 223 | 12.68 | 65 | 8.38 | 404 | 13.85 | 81 | 13.80 | 441 | 37.82 | 300 | 35.89 |
| MSMW | 43 | 2.45 | 12 | 1.55 | 63 | 2.16 | 14 | 2.39 | 61 | 5.23 | 47 | 5.62 |
| Unknown | 74 | 4.21 | 25 | 3.22 | 330 | 11.3 | 70 | 11.93 | 81 | 6.95 | 34 | 4.07 |
More than one urogenital specimen may have been collected for Neisseria gonorrhoeae culture from some patients.
We defined cisgender females as patients who self-identified as female, were assigned female at birth, and had endocervical or vaginal specimens collected for culture. We defined cisgender males as patients who self-identified as male, were assigned male at birth, and had urethral specimens collected for culture.
Non-STD clinics included the following: emergency departments, urgent care centers, infectious disease practices, LGBTQ-focused health centers, HIV care sites, federally qualified health centers, reproductive health clinics, and women’s hospital and health clinics.
Patient self-reported gender of sex partners in the previous 3 months among cisgender males.
AST indicates antimicrobial susceptibility testing; CO, Colorado; GC, Neisseria gonorrhoeae; IQR, interquartile range; NC, North Carolina; MSM, men who have sex with men; MSMW, men who have sex with men and women; MSW, men who have sex with women; STD, sexually transmitted disease; WI, Wisconsin.
City of Milwaukee, Greensboro County, and Denver County SURRG Data
The vast majority of included cisgender female patients in MIL (69%) and GRB (95.8%) had specimens collected in non-STD clinics (Table 1). In DEN, nearly 80% of the included cisgender female patients had urogenital culture specimens collected in the STD clinic, but non-STD clinics contributed a sizable proportion of female patients with urogenital culture specimens (21.3%). The percentage of cisgender women whose urogenital GC cultures were positive was generally lower in non-STD clinics (MIL, 8.4%; GRB, 2.6%; DEN, 9.9%) than in STD clinics (MIL, 14.6%; GRB, 14.3%; DEN, 8.7%; Table 1). Despite the lower positivity in non-STD clinics, a substantial proportion of cisgender women with positive urogenital GC cultures and whose isolates underwent AST were from non-STD clinics (MIL, 56.0%; GRB, 80.4%; and DEN, 23.5%; Table 1).
Overall, across these 3 SURRG sites, 71.6% of women were Black, 12.2% were White, 12.1% were Hispanic, and 1.0% were of another or unknown race/ethnicity (results not shown). Although age varied slightly across the 3 jurisdictions, the median age of cisgender women with positive urogenital GC cultures and AST was 23 years (interquartile range, 20–27 years).
Among cisgender women whose GC isolates underwent AST, most resided within the funded project jurisdiction (i.e., in-jurisdiction) in MIL (98.6%) and GRB (78.0%), but less than half were in-jurisdiction in DEN (40.9%). In DEN, cisgender women with GC isolates with AST frequently resided in other surrounding Denver Metro counties. Positive urogenital GC cultures with AST were obtained from 15.8%, 13.8%, and 11.5% of jurisdictional GC cases in men in MIL, GRB, and DEN, respectively, and 5.1%, 10.2%, and 2.4% of the jurisdictional cases in women in MIL, GRB, and DEN, respectively (Table 2).
TABLE 2.
In-Jurisdiction Gonorrhea Surveillance Cases and Strengthening the US Response to Resistant Gonorrhea (SURRG) Gonorrhea Cases With Urogenital Culture and Antimicrobial Susceptibility Testing, by Site and Demographic Characteristics, 2018 to 2019
| City of Milwaukee, WI |
Guilford County, NC |
Denver County, CO |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Surveillance GC Cases* |
SURRG In-Jurisdiction†,‡ Urogenital GC Cases With AST |
Surveillance GC Cases* |
SURRG In-Jurisdiction†,‡,§ Urogenital GC Cases With AST |
Surveillance GC Cases* |
SURRG In-Jurisdiction†,‡ Urogenital GC Cases With AST |
||||
| Characteristic | n | n | % of Surveillance Cases | n | n | % of Surveillance Cases | n | n | % of Surveillance Cases |
| Total | 8888 | 953 | 10.7 | 4210 | 513 | 12.2 | 5940 | 506 | 8.5 |
| Sex¶ | |||||||||
| Female | 4194 | 213 | 5.1 | 1913 | 195 | 10.2 | 1939 | 47 | 2.4 |
| Male | 4691 | 740 | 15.8 | 2297 | 318 | 13.8 | 4001 | 459 | 11.5 |
| Gender of sex | |||||||||
| partners among males∥ | |||||||||
| MSW | 1132 | 648 | 57.2 | NA | 196 | NA | NA | 227 | NA |
| MSM | 406 | 59 | 14.5 | NA | 46 | NA | NA | 188 | NA |
| MSMW | 65 | 10 | 15.4 | NA | 8 | NA | NA | 20 | NA |
| Unknown | 3088 | 23 | 0.7 | 2297 | 68 | 3.0 | 4001 | 24 | 0.6 |
| Race/Ethnicity | |||||||||
| White | 520 | 29 | 5.6 | 343 | 38 | 11.1 | 1831 | 179 | 9.8 |
| Black | 5883 | 854 | 14.5 | 3067 | 452 | 14.7 | 1011 | 142 | 14.0 |
| Hispanic | 607 | 42 | 6.9 | 85 | 15 | 17.6 | 1778 | 162 | 9.1 |
| Asian/NHOPI | 72 | 9 | 12.5 | 15 | 0 | 0 | 81 | 7 | 8.6 |
| American Indian/Alaskan Native | 38 | 0 | 0.0 | 6 | 1 | 16.7 | 59 | 2 | 3.4 |
| Other | 301 | 124 | |||||||
| Multirace | 35 | 10 | 28.6 | 38 | 5 | 13.2 | NA | 8 | NA |
| Unknown | 820 | 1 | 0.1 | 656 | 2 | 0.3 | 1056 | 6 | 0.6 |
| Age, y | |||||||||
| 12–19 | 2194 | 156 | 7.1 | 691 | 78 | 11.3 | 804 | 29 | 3.6 |
| 20–24 | 2318 | 238 | 10.3 | 1473 | 150 | 10.2 | 1132 | 94 | 8.3 |
| 25–29 | 1768 | 206 | 11.7 | 954 | 131 | 13.7 | 1354 | 141 | 10.4 |
| 30–34 | 974 | 134 | 13.8 | 463 | 72 | 15.6 | 1034 | 94 | 9.1 |
| 35–44 | 913 | 110 | 12.0 | 367 | 51 | 13.9 | 999 | 68 | 6.8 |
| >45 | 699 | 109 | 15.6 | 261 | 31 | 11.9 | 617 | 80 | 13.0 |
Jurisdiction surveillance gonorrhea data were provided by Wisconsin Department of Health Services, North Carolina Division of Public Health, and Colorado Department of Public Health and Environment STD surveillance systems.
Patient resides in the funded SURRG jurisdiction.
SURRG jurisdiction status determined using patient zip code of residence.
Jurisdiction status could not be determined for 300 Guilford County patients with positive urogenital GC cultures with AST because of missing data.
Data defining cisgender or transgender are not widely available in jurisdictional gonorrhea surveillance data. SURRG patients self-identified gender categories.
Patient self-reported gender of sex partners in the previous 3 months among males.
AST indicates antimicrobial susceptibility testing; CO, Colorado; NC, North Carolina; GC, Neisseria gonorrhoeae; MSM, men who have sex with men; MSMW, men who have sex with men and women; MSW, men who have sex with women; NA, not available; NHOPI, Native Hawaiian and other Pacific Islander; SURRG, Strengthening the US Response to Resistant Gonorrhea; WI, Wisconsin.
Antimicrobial susceptibility of urogenital GC isolates from cisgender women varied considerably between the 3 sites. Azithromycin nonsusceptibility ranged from 0.4% in GRB to 6.9% in MIL. Tetracycline resistance ranged from 14.2% in GRB to 41.4% in DEN. Penicillin resistance ranged from 7.8% in MIL to 12.6% in DEN, and CIPRO resistance ranged from 15.1% in MIL to 26.1% in DEN (Fig. 1). Across all 3 sites, cisgender women more frequently had urogenital GC isolates that were susceptible to all antibiotics (AZM, TET, CIPRO, CRO, CFX, and PCN; MIL, 58.3%; GRB, 72%; DEN, 51.4%) than did cisgender MSM only or both men and women (MSM/MSMW; MIL, 40.3%; GRB, 54.7%; DEN, 37.8%) and cisgender men who have sex with women only (MSW; MIL, 55.5%; GRB, 68.2%; DEN, 47.2%). Overall, across the 3 sites, the proportion of GC isolates with AMR from cisgender women seemed to align more closely to those seen in MSW than MSM/MSMW. For all 3 sites and in all genders, cephalosporin (CRO and CFX) reduced susceptibility was low (<0.5%).
Figure 1.
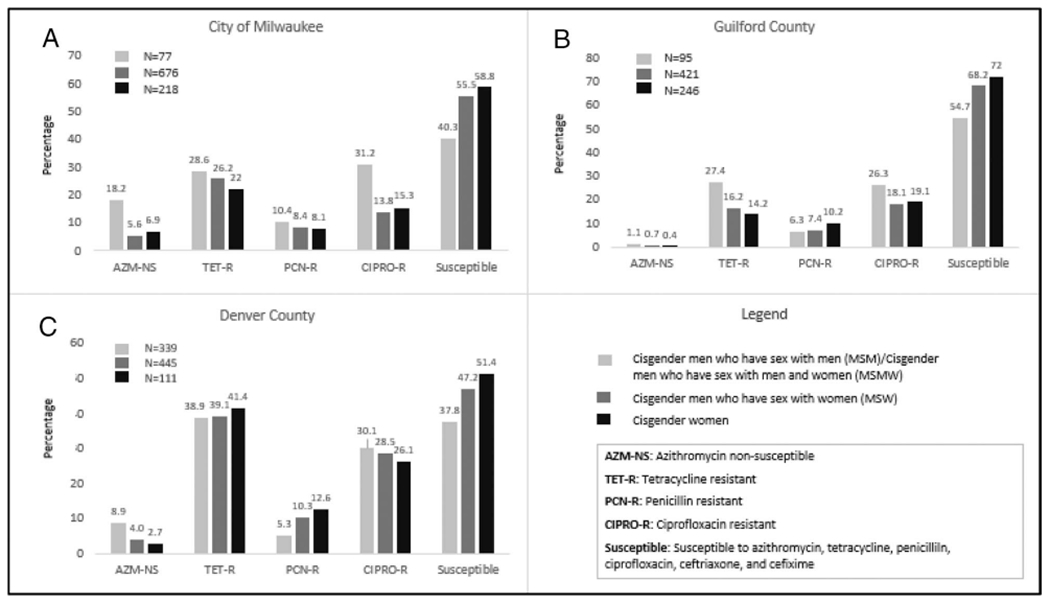
Percent of urogenital Neisseria gonorrhoeae isolates with reduced antimicrobial susceptibility by site, gender, and gender of sex partner among cisgender males and females, SURRG, 2018 to 2019. AZM nonsusceptible (NS) MIC ≥2.0 μg/mL, TET-R MIC ≥2.0 μg/mL, CIPRO-R MIC ≥1.0 μg/mL, and PCN-R MIC≥2.0 μg/mL. Ceftriaxone and cefixime resistance is not included in the figure because for all 3 SURRG sites and in all genders, cephalosporin (ceftriaxone and cefixime) reduced susceptibility was low (<0.5%).
DISCUSSION
Surveillance for ARGC is critical for rapid detection and response and development of effective GC treatment guidelines. In addition, AST and genomic surveillance has the potential to inform our understanding of the influence of anatomical sites on acquisition and expression of genes conferring AMR and potential differences in antimicrobial susceptibility based on gender, sexual orientation, and geographic location. However, in the United States and globally, surveillance of gonococcal AMR has historically been focused on urethral GC in cisgender men, and less than 20% of GC isolates evaluated for AMR by the Euro-Gonococcal Antimicrobial Surveillance Program in 2018 and the Australian Gonococcal Surveillance Programme in 2019 were collected from cisgender women.3,4 The CDC SURRG project aims to promote more diverse and robust ARGC surveillance aligned to national morbidity and to develop an infrastructure for rapid identification of ARGC with outbreak response.
Experience from the SURRG projects in MIL, GRB, and DEN demonstrates that incorporation of diverse health care sites with a clear track record for serving cisgender women with gonococcal infection can substantially contribute to expanded ARGC surveillance. However, as outlined, surveillance activities in urgent and emergency care sites may require broad and nonspecific urogenital culture criteria in cisgender women with low overall urogenital culture recovery and considerable additional staff time in specimen collection and culture processing. The increased proportion of GC isolates that were fully susceptible to antibiotics in cisgender women compared with cisgender men in MIL, GRB, and DEN highlights the potential importance of expanded ARGC surveillance in cisgender women and aligns with European surveillance reports showing some discordance in AST between GC isolates collected from heterosexual males and females.4 Our data also raise further interest in the potential clinical implications of limited published data suggesting that gender and urogenital site of infection may influence prevalence and expression of GC AMR determinants.6,9 Although systematic ARGC surveillance in cisgender women remains challenging because of the high proportion of asymptomatic infection, decentralized care with poor access to GC culture, and low sensitivity of endocervical/vaginal cultures, experience in these 3 SURRG sites demonstrates that focused efforts can improve morbidity aligned ARGC surveillance in cisgender women and may be useful for AMR rapid detection and response activities.
Novel approaches and workflows may further improve collection of urogenital GC cultures from cisgender women in the future. Both MIL and GRB have validated self-collection of vaginal GC culture swabs to increase culture collection by eliminating the need for speculum evaluation, decreasing expertise required for specimen collection, and increasing patient convenience and comfort. This approach has been described by SURRG sites for pharyngeal, rectal, vaginal/endocervical, and urine/urethral gonococcal cultures, with results demonstrating similar sensitivity of patient and provider-collected cultures at these anatomic sites.14 However, more comparative data are needed to identify the best anatomical sample, collection process, culture media, swabs, and transportation and storage processes to optimize culture sensitivity and to determine the most cost-effective approach. In addition, use of point-of-care GC NAATs could facilitate standard same-day GC culture collection using simplified and focused culture criteria based on NAAT results. Collecting GC culture specimens on the same day as positive NAAT specimens could improve culture sensitivity, decrease staff and laboratory time spent on processing of cultures collected on patients with negative GC NAATS, shorten time to identification of patients with ARGC, and possibly facilitate more rapid initiation and coordination of public health responses to patients with ARGC. Monitoring and assessing these best practices and associated costs of surveillance systems will be a key to building sustainable and data-informed ARGC surveillance programs in the United States and globally.
Although robust culture-based surveillance of ARGC in cisgender women remains challenging, whole-genome sequencing and molecular identification of AMR determinants from remnant NAATs represent exciting options that could augment our knowledge about ARGC prevalence and specific mechanisms of AMR in cisgender women. These techniques have already been used to assess outbreak control in a local population after identification of a case of ARGC by culture and AST and if expanded could allow for broad-based representative surveillance across all genders.15,16 However, to date, these tools have not been routinely used for the purposes of general nationwide or jurisdictional surveillance, and because of GC’s propensity for rapid development of new resistance mutations, culture and AST will necessarily remain a cornerstone of surveillance that could be strengthened by these culture-independent molecular techniques.
There are several limitations to this analysis. Observed differences across sites must be interpreted carefully in light of different types of non-STD clinics, culture criteria, and culture collection and laboratory processing protocols. Patients attending different non-STD clinic types may differ by multiple characteristics including age, race/ethnicity, insurance status, and sexual networks that could impact the prevalence of GC and therefore culture recovery as well as the presence of gonococcal AMR. Similarly, our ability to detect and compare any differences in the prevalence of ARGC in cisgender women and cisgender men may be affected by other differences between these genders associated with GC urogenital symptoms and the type of health care clinics (such as STD or emergency department) at which they are seeking care. Differences in STD clinic patient population between jurisdictions also can affect the relative impact that non-STD clinics may make in ARGC surveillance for cisgender women. Finally, rigorous quantitative comparisons of results from different types of non-STD clinics and different culture criteria, specimen collection, and culture processes have not been performed. More rigorous evaluation of best practices could further guide work for equitable, large-scale expansion of ARGC surveillance activities in cisgender women.
The CDC’s SURRG project has broadened the US approach to ARGC surveillance and built capacity not only for rapid identification and public health response but also for identification of differences in ARGC prevalence by gender. SURRG’s focus on representation of jurisdictional morbidity has led to innovative partnerships in surveillance with nontraditional clinical care sites, novel workflows, and the development of a variety of culture techniques that have expanded ARGC surveillance in cisgender women and demonstrated some differences in antimicrobial susceptibility (although these results should be interpreted with caution because of differences in culture collection criteria used across SURRG sites and symptomatic urogenital infections by sex). Continued intentional and thoughtful expansion of GC culture with AST and molecular surveillance techniques will allow for further assessment of the impact of gender and other demographic and geographic factors on ARGC prevalence and transmission and will allow for the development of surveillance and rapid response systems that more equitably represent national GC morbidity.
Acknowledgments:
The authors thank Dhana Shrestha from the Wisconsin Division of Public Health and Kaitlyn Probst from Colorado Department of Public Health and Environment for providing jurisdictional demographic data for gonorrhea cases in 2018 and 2019.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Conflict of Interest and Sources of Funding:
The authors have no conflicts of interest to disclose. Funding for the Strengthening the US Response to Resistant Gonorrhea activities described in this article was supported by federal Antibiotic Resistance Initiative funding and administered through the US Centers for Disease Control and Prevention’s Epidemiology and Laboratory Capacity for the Prevention and Control of Infectious Diseases Cooperative Agreement (CK19-1904).
REFERENCES
- 1.Centers for Disease Control and Prevention. Sexually Transmitted Disease Surveillance 2019. Atlanta, GA: US Department of Health and Human Services, CDC, 2021. Available at: https://www.cdc.gov/std/statistics/2019/default.htm. Accessed April 22, 2021. [Google Scholar]
- 2.Kirkcaldy RD, Schlanger K, Papp JR, et al. Considerations for strengthening surveillance of Neisseria gonorrhoeae antimicrobial resistance and interpreting surveillance data. Sex Transm Dis 2017; 44:154–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lahra MM, Shoushtari M, Robert George CR, et al. Australian Gonococcal Surveillance Programme, Annual Report, 2019. Canberra, Australia: Communicable Diseases Intelligence, 2020:44. Available at: https://www1.health.gov.au/internet/main/publishing.nsf/content/8FA6078276359430CA257BF0001A4C42/$File/australian_gonococcal_surveillance_programme_annual_report_2019.pdf. Accessed October 7, 2021. [DOI] [PubMed] [Google Scholar]
- 4.Jacobsson S, Unemo M, Day M, et al. , European Centre for Disease Prevention and Control. Gonococcal Antimicrobial Susceptibility Surveillance in Europe—Results Summary 2018. Stockholm, Sweden: ECDC. May 25, 2020. Available at: https://wwwecdceuropaeu/sites/default/files/documents/gonococcal-antimicrobial-susceptibility-Euro-GASP-2018pdf. Accessed April 22, 2021. [Google Scholar]
- 5.Public Health Agency of Canada. Report on the enhanced Surveillance of Antimicrobial-Resistant Gonorrhea: Results From 2015–2017. Ottawa, Canada: Centre for Communicable Diseases and Infection Control, Infectious Disease Prevention and Control Branch, Public Health Agency of Canada, 2021. Available at: https://wwwcanadaca/en/public-health/services/publications/diseases-conditions/2015-2017-report-enhanced-surveillance-antimicrobial-resistant-gonorrheahtml#a54. Accessed May 6, 2021. [Google Scholar]
- 6.Mercante AD, Jackson L, Johnson PJT, et al. MpeR regulates the mtr efflux locus in Neisseria gonorrhoeae and modulates antimicrobial resistance by an iron-responsive mechanism. Antimicrob Agents Chemother 2012; 56:1491–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Falsetta ML, Steichen CT, McEwan AG, et al. The composition and metabolic phenotype of Neisseria gonorrhoeae biofilms. Front Microbiol 2011; 2:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goytia M, Dhulipala VL, Shafer WM. Spermine impairs biofilm formation by Neisseria gonorrhoeae. FEMS Microbiol Lett 2013; 343:64–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nudel K, McClure R, Moreau M, et al. Transcriptome analysis of Neisseria gonorrhoeae during natural infection reveals differential expression of antibiotic resistance determinants between men and women. mSphere 2018; 3:e00312–e00318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention. Sexually Transmitted Disease Surveillance 2018. Atlanta, GA: US Department of Health and Human Services, CDC, 2019. Available at: https://www.cdc.gov/std/stats18/default.htm. Accessed April 22, 2021. [Google Scholar]
- 11.Nash EE, Pham CD, Raphael B, et al. Impact of anatomic site, specimen collection timing, and patient symptom status on Neisseria gonorrhoeae culture recovery. Sex Transm Dis 2021; 48(12S):S151–S156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harryman L, Scofield S, Macleod J, et al. Comparative performance of culture using swabs transported in Amies medium and the Aptima Combo 2 nucleic acid amplification test in detection of Neisseria gonorrhoeae from genital and extra-genital sites: A retrospective study. Sex Transm Infect 2012; 88:27–31. [DOI] [PubMed] [Google Scholar]
- 13.Clinical and Laboratory Standards Institute. M100: Performance Standards for Antimicrobial Susceptibility Testing. 30th ed. Wayne, PA: Clinical and Laboratory Standards Institute, 2020. Available at: http://em100.edaptivedocs.net/GetDoc.aspx?doc=CLSI%20M100%20ED31:2021&scope=user. Accessed April 22, 2021. [Google Scholar]
- 14.Barbee LA, Golden MR, Thibault CS, et al. Performance of patient-collected specimens for Neisseria gonorrhoeae culture. Clin Infect Dis 2020; ciaa1089. Available at: 10.1093/cid/ciaa1089. Accessed October 7, 2021. [DOI] [PubMed]
- 15.Picker MA, Knoblock RJ, Hansen H, et al. Notes from the field: First case in the United States of Neisseria gonorrhoeae harboring emerging mosaic penA60 allele, conferring reduced susceptibility to cefixime and ceftriaxone. MMWR Morb Mortal Wkly Rep 2020; 69: 1876–1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berenger BM, Demczuk W, Gratrix J, et al. Genetic characterization and enhanced surveillance of ceftriaxone-resistant Neisseria gonorrhoeae strain, Alberta, Canada, 2018. Emerg Infect Dis 2019; 25: 1660–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]


