Abstract
A major obstacle in treating opioid use disorder is the persistence of drug seeking or craving during periods of abstinence, which is believed to contribute to relapse. Dopamine neurotransmission in the mesolimbic pathway is posited to contribute to opioid reinforcement, but the processes by which dopamine influences drug seeking have not been elucidated. To examine whether opioid seeking during abstinence is associated with alterations in dopamine neurotransmission, female and male rats self-administered oxycodone under an intermittent access schedule of reinforcement. Following self-administration, rats underwent a forced abstinence period and cue-induced seeking tests were conducted to assess oxycodone seeking. One day following the final seeking test, rats were sacrificed to perform ex vivo fast scan cyclic voltammetry and western blotting in the nucleus accumbens. Rats displayed reduced dopamine uptake rate on abstinence day 2 and abstinence day 15, compared to oxycodone-naïve rats. Further, on abstinence day 15 rats had reduced phosphorylation of the dopamine transporter. Additionally, local application of oxycodone to the nucleus accumbens reduced dopamine uptake rate in oxycodone-naïve rats and in rats during oxycodone abstinence, on abstinence day 2 and abstinence day 15. These observations suggest that abstinence from oxycodone results in dysfunctional dopamine transmission, which may contribute to sustained oxycodone seeking during abstinence.
Keywords: craving, dopamine uptake, dopamine transporter, opioid, self-administration, threonine 53
Graphical Abstract
Text summary:
Intermittent access to oxycodone reduced dopamine uptake rate in the nucleus accumbens core on abstinence day 2 and day 15. Additionally, acute application of oxycodone to the nucleus accumbens core induced a robust reduction in dopamine uptake rate.
Introduction
A significant barrier for mitigating the current opioid crisis is high rates of relapse, which can occur even after extended periods of abstinence 1,2. Drug craving greatly contributes to relapse and has been shown to persist across periods of abstinence 3,4. Drug craving has been modeled in rodents, such that animals trained to self-administer opioids followed by an abstinence period exhibit prolonged drug seeking behavior, similar to humans 5–7. Unfortunately, there remain critical gaps in our understanding of the neurobiological basis for drug seeking during abstinence.
The role of mesolimbic dopamine in opioid reinforcement remains controversial 8, with studies showing that dopamine receptor antagonists or lesions of dopamine terminals in the nucleus accumbens (NAc) have little effect on opioid self-administration 9,10, while others show significant reductions in opioid self-administration using similar approaches 11–17. Nevertheless, recent work indicates that chemogenetic inhibition of ventral tegmental area (VTA) dopamine neurons significantly reduces heroin self-administration, supporting the importance of dopamine transmission in the reinforcing effects of opioids 18. Despite these potentially conflicting observations, there is a consensus that opioid administration increases dopamine transmission. Acutely, opioids increase VTA dopamine neuron activity leading to increased dopamine efflux in terminal regions, such as the NAc 18–22. Beyond these acute actions, repeated exposure to opioids leads to adaptations in both the VTA and dopamine terminal regions. For example, in the early stages of opioid abstinence VTA dopamine neurons display increased excitability and increased firing 23–25, while dopamine uptake rate is enhanced in the NAc shell 26. Interestingly, evidence in humans suggest that individuals with active opioid use disorder and during opioid abstinence have reductions in dopamine transporter levels in various brain regions, including key dopamine terminal regions such as the striatum 27–32. Despite these observations, it remains unclear to what extent alterations in dopamine transmission are present across abstinence periods.
To examine dopamine transmission in the NAc during opioid abstinence, rats were trained to self-administer the prescription opioid oxycodone followed by fast-scan cyclic voltammetry (FSCV) and western blotting after periods of abstinence. One group of rats underwent oxycodone self-administration with a single cue-induced seeking test on abstinence day (AD) 1 and rats were then sacrificed on AD2 to determine changes in dopamine transmission early in abstinence. Another group of rats underwent oxycodone self-administration with cue-induced seeking tests on both AD1 and AD14 and rats were then sacrificed on AD15 to determine changes in dopamine transmission later in abstinence. NAc tissue was obtained from both groups for western blot analysis of the dopamine transporter (DAT) and mu opioid receptor (MOR) expression. Finally, to assess if abstinence from oxycodone was associated with changes in acute dopamine responses to oxycodone in the NAc, we applied oxycodone onto brain slices and measured dopamine transmission.
Materials and Methods
Animals:
Adult female (210–260g) and male (325–430g) Long Evans rats (Envigo, Frederick, MD, USA) were maintained on a 12-hour reverse light/dark cycle (1500 lights on; 0300 lights off) with ad libitum access to food and water. After arrival, rats were given at least 7 days to acclimate to the animal facility prior to surgery. All protocols and animal procedures were conducted in accordance with National Institutes of Health Guide for the Care and Use of Laboratory Animals under supervision of the Institutional Animal Care and Use Committee at Drexel University College of Medicine.
Drugs:
Oxycodone hydrochloride, DAMGO ([d-Ala2,N-Me-Phe4,Gly5-ol]-enkephalin), naloxone hydrochloride, and CTAP (d-Phe-Cys-Tyr-d-Trp-Arg-Thr-Pen-Thr-NH2) were provided by the National Institute on Drug Abuse Drug Supply Program (Research Triangle Park, NC, USA). For self-administration experiments, oxycodone hydrochloride was dissolved in 0.9% physiological saline. For FSCV experiments, all drugs were dissolved in artificial cerebrospinal fluid (aCSF).
Intravenous catheter surgery:
Rats were anesthetized using 2.5% isoflurane and implanted with a silastic catheter (ID, 0.012 in OD, 0.025 in. Access Technologies, Skokie, IL) into the right jugular vein for intravenous delivery of oxycodone. The catheter was connected to a cannula which exited through the skin on the dorsal surface in the region of the scapulae. Ketoprofen (Patterson Veterinary, Devens, MA; 5mg/kg s.c. of 5 mg/ml) and Enrofloxacin (Norbrook, Northern Ireland; 5 mg/kg s.c. of 5 mg/ml) were provided at the time of surgery and a second dose was given 12 hrs later. In addition, antibiotic/analgesic powder (Neopredef, Kalamazoo, MI) was applied around the chest and back incisions. Rats were subsequently singly-housed and allowed to recover for 5 days prior to self-administration training. Intravenous catheters were manually flushed with Gentamicin (5 mg/kg i.v. of 5 mg/ml) in heparinized saline every day during recovery to maintain catheter patency.
Self-Administration:
All self-administration sessions took place in operant testing chambers. Within the test chamber, two levers were located 6 cm above the floor on the left and right side of the back wall. The left lever was designated active and the right lever inactive. Pressing the active lever resulted in an intravenous infusion of oxycodone. All measures were recorded using custom created Ghost Software 33. Each rat underwent one oxycodone self-administration session per day (sessions ran from 1000–1600).
Intermittent Access (IntA):
Rats were first trained to self-administer oxycodone for 6 hrs under a fixed ratio 1 schedule whereby a single active lever press initiated an intravenous injection of oxycodone (0.1 mg/kg, infused over 2.5 s - 4.5 s) paired with a cue light above the active lever and a 20-s timeout during which the levers retracted. This dose was chosen based on previous oxycodone self-administration studies 34,35. Acquisition of the behavior occurred when a rat obtained ≥ 20 infusions in two consecutive sessions, with inactive lever presses being less than 1/2 the number of active lever presses. Following acquisition, rats were switched to the IntA schedule. Rats on the IntA schedule had access to oxycodone for 5 min followed by a 25-min timeout, this 30-min trial repeated for 12 trials per session for a total of 6 hrs per day. During the 25-min timeout, levers were retracted and during the 5-min trial there was no timeout following each infusion. Active lever presses resulted in a single intravenous injection of oxycodone (0.05 mg/kg), paired with a cue light above the active lever. Rats underwent this IntA schedule for ten consecutive days. We reduced the oxycodone dose (0.1 mg/kg to 0.05 mg/kg) for the IntA portion of the studies based on pilot data from the lab indicating that reducing the dose led to robust responding during IntA sessions.
Abstinence and cue-induced drug seeking:
Following the last self-administration session, rats underwent a forced abstinence period of either 1 day or 14 days. During this phase, rats remained in their home cage (except when performing cue-induced drug seeking tests). To assess oxycodone seeking, rats performed a cue-induced drug seeking test on abstinence day AD1 and AD14, or only on AD1. The seeking test was 30 mins and conditions were similar to the IntA session; an active lever press resulted in presentation of the cue light that was previously paired with oxycodone, but there was no oxycodone delivery. Under these conditions, the number of active lever presses served as a measure of the degree of oxycodone seeking.
Ex vivo fast scan cyclic voltammetry (FSCV):
One day after the last seeking test, rats were anesthetized with 2.5% isoflurane for 5 min and subsequently decapitated. The brain was rapidly dissected and transferred to ice-cold, aCSF containing NaCl (126 mM), KCl (2.5 mM), NaH2PO4 (1.2 mM), CaCl2 (2.4 mM), MgCl2 (1.2 mM), NaHCO3 (25 mM), glucose (11 mM), and L-ascorbic acid (0.4 mM), with pH adjusted to 7.4. A vibratome was used to produce 400 µm-thick coronal sections containing the NAc core. Slices were transferred to room temperature oxygenated aCSF and left to equilibrate for at least 1 hr before being transferred into a recording chamber with aCSF (32°C). Oxycodone-naïve (naïve) rats did not receive surgery and were used as a control group throughout these studies.
A bipolar stimulating electrode was placed on the surface of the tissue in the NAc core, and a carbon fiber microelectrode was implanted between the stimulating electrode leads. Dopamine release was evoked using a single electrical pulse (400 µA, 4ms, monophasic) every 3 min. Measurements were conducted with Michaelis-Menten modelling using Demon Voltammetry and Analysis Software 36. Once baseline dopamine release was stable (3 successive stimulations within <10% variation), the slice was exposed to increasing oxycodone concentrations (0.01 µM, 0.1 µM, 1 µM, 10 µM, 100 µM). Modeling of dopamine dynamics was conducted on the final 3 collections for baseline, and each concentration of oxycodone.
Additional cohorts of rats were used to test the effect of pretreatment with either the non-specific opioid receptor antagonist naloxone or the MOR specific antagonist CTAP on the effect of oxycodone on dopamine transmission. For these experiments, naïve rats were sacrificed and prepared for FSCV as described above. Following baseline recordings, naloxone [1 µM] or CTAP [0.1 µM] were applied to the slice followed by increasing concentrations of oxycodone in addition to naloxone or CTAP (i.e., 1 µM naloxone followed by 1 µM naloxone + 0.01–100 µM oxycodone, or 0.1 µM CTAP followed by 0.1 µM CTAP + 0.01–100 µM oxycodone). To assess the effectiveness of naloxone and CTAP at attenuating oxycodone-mediated changes in dopamine uptake rate, concentrations of naloxone and CTAP were chosen based on concentration response experiments indicating that these concentrations had minimal effect on dopamine uptake on their own (Supplemental Figure 1). To assess whether MOR agonism recapitulates the effects of oxycodone on dopamine transmission, the MOR specific agonist DAMGO was applied to the slice at increasing concentrations (0.01 µM, 0.1 µM, 1 µM, 10 µM) (Supplemental Figure 2).
Western Blot Procedures:
Synaptosomes were prepared and membrane fractionation was preformed using a modification of published procedures 37. Rats were decapitated and the ventral striatum was dissected and stored at −80C until preparation. Tissue was homogenized in ice-cold lysis buffer (1000ml, 50 mM Tris-HCl, pH 7.4, 1 mM EDTA, 320 mM sucrose) with 1x protease inhibitor cocktail, 1x phosphatase inhibitor cocktail, and 1 mM PMSF. The homogenate was centrifuged at 1,000x g for 5 min at 4°C. The resulting supernatant was recentrifuged at 10,000x g for 20 min at 4°C. The resulting synaptosomal pellet was resuspended with 300ml lysis buffer for Western blot studies. Immunoblotting was performed with rabbit anti-DAT polyclonal antibody (1:1000, EMD Millipore), rabbit anti phospho-DAT polyclonal antibody (1:1000, PhosphoSolutions), rabbit anti-Mu opioid receptor polyclonal antibody (1:1000, Millipore Sigma), and peroxidase-conjugated goat anti-rabbit IgG (H1 L) (1:5000, Jackson ImmunoResearch Laboratories). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as membrane protein control and was determined with rabbit anti-GAPDH polyclonal antibody (1:5000, Thermo Fisher Scientific). Total DAT (tDAT), phosphorylated DAT at threonine-53 (pDAT), MOR, and GAPDH immunoblots were quantified by densitometry with ImageQuant LAS4000 (GE Healthcare Bio-Sciences). Data were analyzed and presented as a ratio of tDAT, pDAT, or MOR to GAPDH, as we have previously reported 38,39.
Data Analysis
All active and inactive lever presses were recorded during behavioral sessions. Inactive lever presses served as a measure of nonspecific behavior. All rats that met acquisition criteria were initially used for FSCV and a subset of these rats were used for western blotting procedures.
Analyses to detect sex differences were conducted for all behavioral and voltammetry metrics evaluated here. Sex differences analyses were not conducted for the western blot data due to low sample sizes. We did not observe any interactions between sex and measures of interest indicating that both females and males responded similarly across test conditions. Therefore, female and male data were combined as recommended by prior studies 40,41. For reference, Supplemental Table I shows the results from the analyses performed.
Statistical Analysis
Statistical analyses were conducted using GraphPad Prism 9.4.0. Specific analyses are detailed in the results section. Behavioral data were analyzed using either a one-way ANOVA or with a paired t-test. Baseline voltammetry measurements of release and uptake, response to oxycodone alone on dopamine release and uptake, and western blot data were analyzed using a one-way ANOVA. The effects of naloxone and CTAP on oxycodone’s effect on dopamine release and uptake were analyzed using a two-way ANOVA with group (oxycodone alone, naloxone, and CTAP) as the between-subjects variable and oxycodone concentration as the within-subjects variable. Likewise, the effects of oxycodone on dopamine release and uptake were analyzed using a two-way ANOVA with group (naïve, AD2, and AD15) as the between-subjects variable and oxycodone concentration as the within-subjects variable. When significant effects were detected Dunnett’s post-hoc tests were subsequently performed.
Results
Rats escalate oxycodone intake under an IntA schedule and show robust drug seeking during abstinence
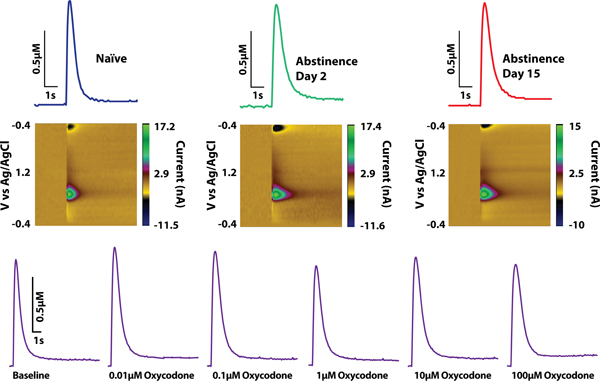
To examine the effects of oxycodone self-administration on opioid seeking during abstinence, rats self-administered oxycodone for ten days on an IntA schedule, followed by two seeking tests on AD1 and AD14 (Figure 1A). We found that rats readily acquired oxycodone self-administration (Figure 1B) and rats displayed robust oxycodone intake, which escalated across the 10-day IntA schedule as indicated by a significant non-zero slope following a linear regression (F(1,8)=12, p=0.0085; Figure 1C) 42. We also observed robust oxycodone seeking during both the AD1 and AD14 cue-induced seeking tests. However, a paired t-test revealed no differences in oxycodone seeking between AD1 and AD14 (t(11)=1.272, p=0.2298) (Figure 1D).
FIGURE 1.
Rats exhibit escalation of oxycodone intake under an IntA schedule and show exaggerated seeking behavior during abstinence. (A) Experimental timeline. (B) Days to acquire self-administration. (C) Active and inactive lever presses across the 10 days of IntA. (D) Lever presses during cue-induced seeking tests on abstinence day 1 (AD1) and abstinence day 14 (AD14). N = 13 (7 females and 6 males). Data shown are mean ± SEM. Linear regression across all days **p < 0.01.
Abstinence from oxycodone engenders reduced dopamine uptake rate in the NAc core
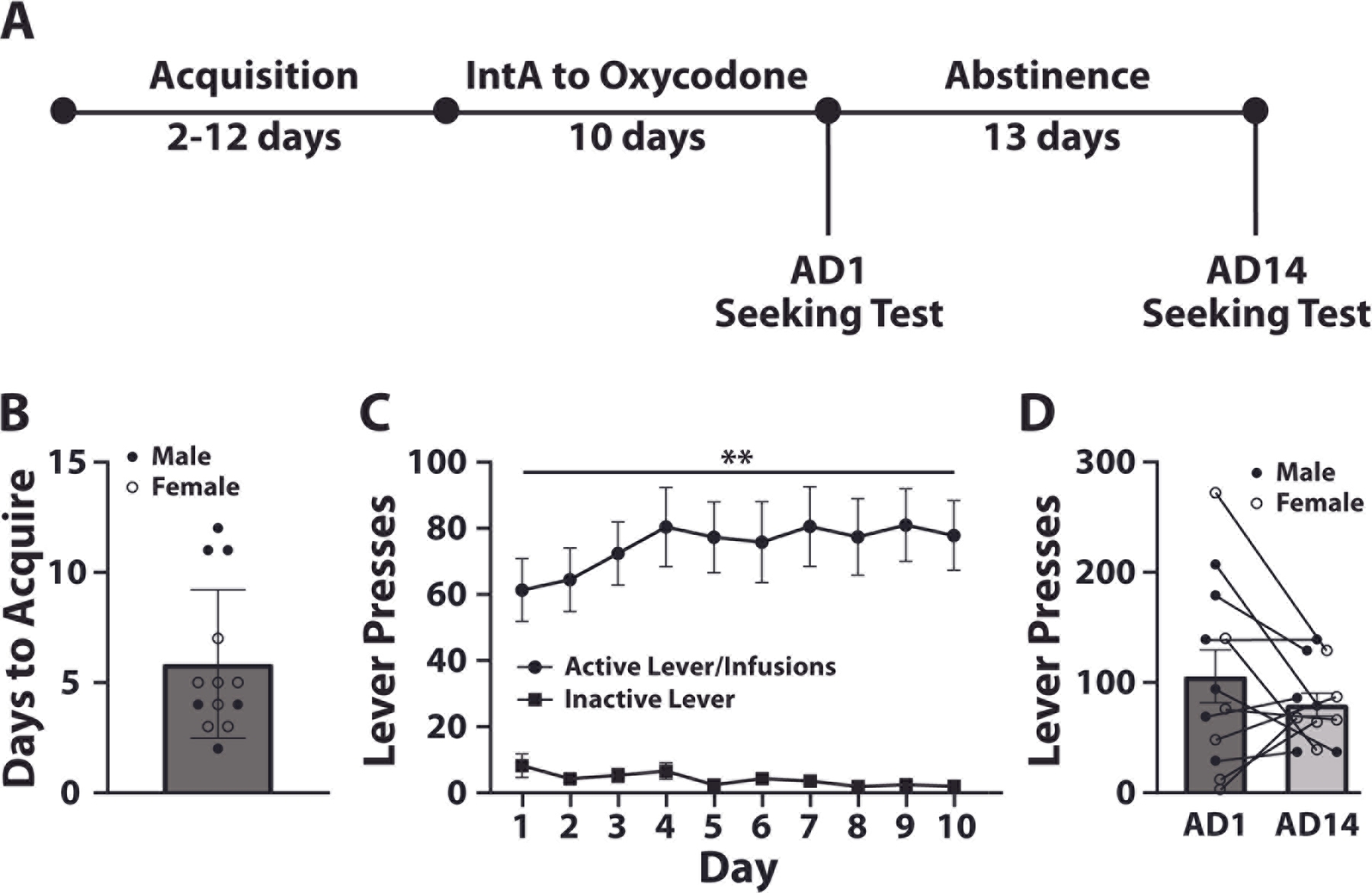
To examine whether abstinence from oxycodone leads to changes in dopamine transmission, rats underwent ten days of IntA to oxycodone and were then sacrificed on AD2 or AD15 for FSCV recordings in the NAc core (Figure 2A). We found that the oxycodone abstinence period was not associated with changes in dopamine release, but was associated with a decrease in dopamine uptake rate relative to naïve rats (Figure 2B–2D). One-way ANOVAs revealed no effect of group on dopamine release (F(2,28)=0.9262, p=0.4079), but there was a significant effect of group on dopamine uptake rate (F(2,28)=6.575, p=0.0046). Dunnett’s post-hoc analyses revealed a significant reduction in dopamine uptake on both AD2 (p=0.0075) and AD15 (p=0.0072) compared to naïve rats.
FIGURE 2.
Abstinence from IntA to oxycodone was associated with decreased dopamine uptake. (A) Experimental timeline. (B) Pseudo-color plots and example traces for Naïve, abstinence day 2 (AD2) and abstinence day 15 (AD15) groups. Arrows indicate time of stimulation (Stim). (C) Dopamine release and (D) dopamine uptake at baseline. Naïve, n = 10 (5 females and 5 males); AD2, n = 9 (4 females and 5 males); AD15, n = 12 (6 females and 6 males). Data shown are mean ± SEM. **p < 0.01 compared to Naïve.
Abstinence from oxycodone does not influence the acute effects of oxycodone on dopamine transmission
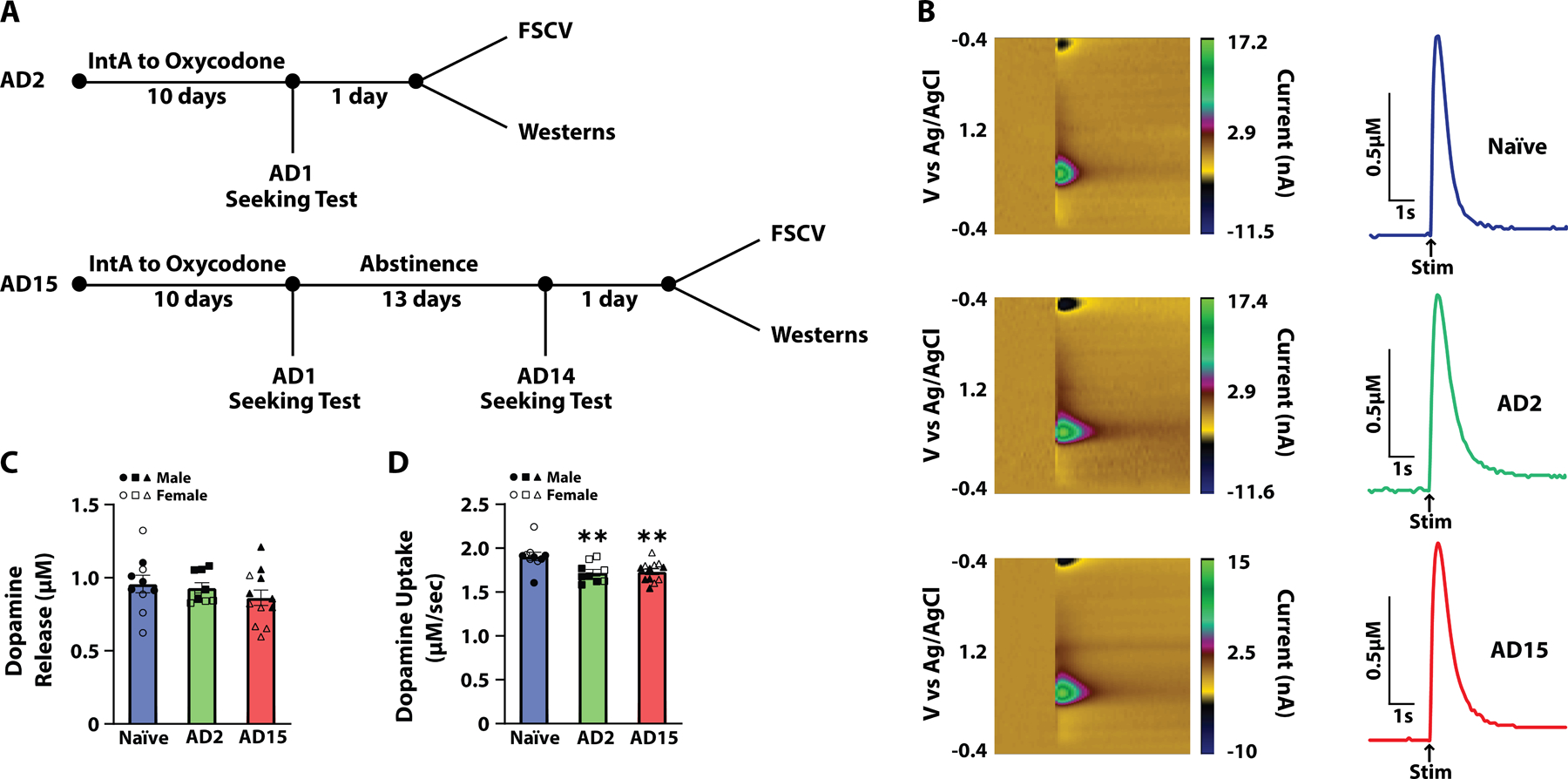
To determine whether abstinence from oxycodone alters the acute effects of oxycodone on local NAc dopamine transmission, we first established the effect of oxycodone on dopamine release and dopamine uptake in naïve rats. We found that acute application of oxycodone did not affect dopamine release but did induce a robust decrease in dopamine uptake rate. One-way ANOVAs revealed no effect of oxycodone concentration on dopamine release (F(1.659,11.86)=1.546, p=0.2511) (Figure 3B), but there was a significant effect of oxycodone concentration on dopamine uptake (F(2.573,18.01)=81.11, p<0.0001). Dunnett’s post-hoc analyses revealed a significant decrease in dopamine uptake rate at all oxycodone concentrations compared to baseline (Figure 3C).
FIGURE 3.
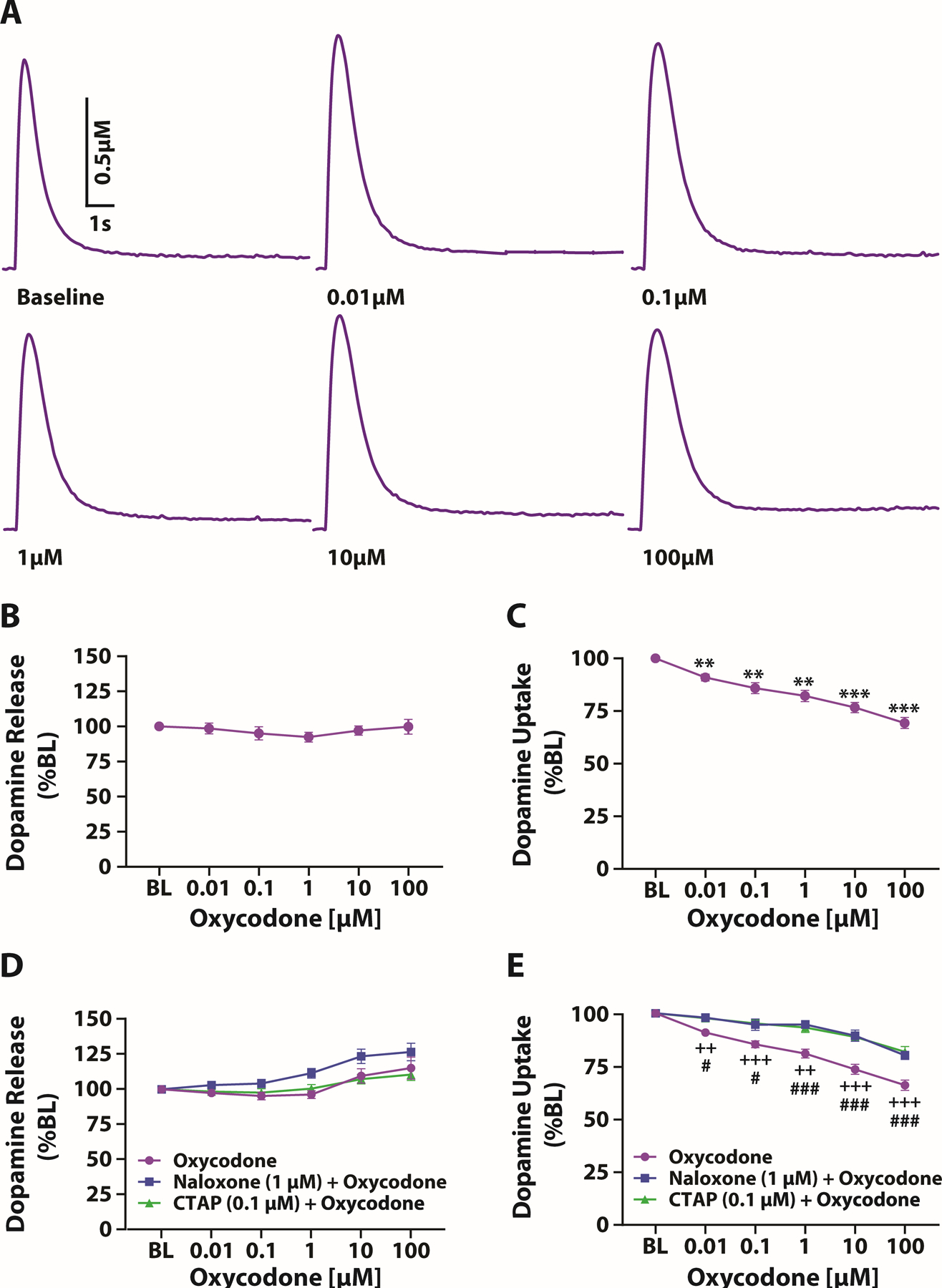
Acute oxycodone produced a concentration dependent reduction in dopamine uptake. (A) Example traces at increasing oxycodone concentrations. The effect of oxycodone application onto NAc slices on (B) dopamine release and (C) dopamine uptake; oxycodone, n = 8 (4 females and 4 males). Effect of naloxone or CTAP pretreatment on oxycodone-induced changes in (D) dopamine release and (E) dopamine uptake; oxycodone, n = 12 (6 females and 6 males); naloxone + oxycodone, n = 8 (4 females and 4 males); CTAP + oxycodone, n = 8 (4 females and 4 males). Data shown are mean ± SEM. **p < 0.01, ***p < 0.001 compared to baseline (BL); ++p < 0.01, +++p < 0.001 CTAP + oxycodone compared to oxycodone alone, # p < 0.05, ###p < 0.001 naloxone + oxycodone compared to oxycodone alone.
To confirm that the effects of oxycodone on dopamine uptake were mediated via actions on opioid receptors, slices from a separate cohort of naïve rats were pretreated with either naloxone [1 μM] or CTAP [0.1 μM]. A two-way mixed design ANOVA with treatment (oxycodone, naloxone + oxycodone, or CTAP + oxycodone) as the between-subjects variable and oxycodone concentration as the within-subjects variable revealed a significant effect of concentration on dopamine release (F(1.694,42.36)=20.29, p<0.0001), but no effect of treatment (F(2,25)=3.220, p=0.0570) and no treatment X concentration interaction (F(10,125)=1.430, p=0.1746) (Figure 3D). In contrast, we found that pretreatment with naloxone or CTAP significantly attenuated the effects of oxycodone on dopamine uptake. A two-way mixed design ANOVA with treatment as the between-subjects variable and oxycodone concentration as the within-subjects variable indicated a significant effect of concentration on dopamine uptake (F(2.996,74.91)=112.7, p<0.0001), a significant effect of treatment (F(2,25)=17.52, p<0.0001) and a significant treatment X concentration interaction (F(10,125)=6.958, p<0.0001). Dunnett’s post-hoc analyses revealed a significant difference between oxycodone alone and naloxone + oxycodone and CTAP + oxycodone at all concentrations (Figure 3E).
To determine the effect of a MOR specific agonist on dopamine release and dopamine uptake, slices from a separate cohort of naïve rats were used to test the effect of DAMGO. Similar to oxycodone, we found that application of DAMGO did not affect dopamine release, but it did induce a robust decrease in dopamine uptake rate. One-way ANOVAs revealed no effect of DAMGO concentration on dopamine release (F(1.272,6.362)=0.6646, p=0.4808), but there was a significant effect of concentration on dopamine uptake (F(1.268,6.339)=23.07, p=0.002). Dunnett’s post-hoc analyses revealed a significant decrease in dopamine uptake rate at all DAMGO concentrations compared to baseline (Supplemental Figure 2).
Given the observation that acute oxycodone reduces dopamine uptake rate on NAc slices, we examined whether abstinence from oxycodone influences the acute effect of oxycodone. We applied oxycodone to the NAc slice at increasing concentrations and measured dopamine transmission in naïve rats and in rats during oxycodone abstinence on AD2 and AD15. We found that abstinence from oxycodone did not affect dopamine responses to acute oxycodone compared to naïve rats. A two-way mixed design ANOVA with group (Naïve, AD2, vs AD15) as the between-subjects variable and oxycodone concentration as the within-subjects variable revealed a significant effect of concentration on dopamine release (F(2.249,49.49)=10.25, p=0.0001), but no effect of group (F(2,22)=0.2110, p=0.8114) and no group X concentration interaction (F(10,110)=0.8768, p=0.5571) (Figure 4A). Similarly, a two-way mixed design ANOVA with group as the between-subjects variable and oxycodone concentration as the within-subjects variable revealed a significant effect of concentration on dopamine uptake (F(2.717,59.77)=151.5, p<0.0001), but no effect of group (F(2,22)=1.219, p=0.3148) and no group X concentration interaction (F(10,110)=1.388, p=0.1951) (Figure 4B).
FIGURE 4.
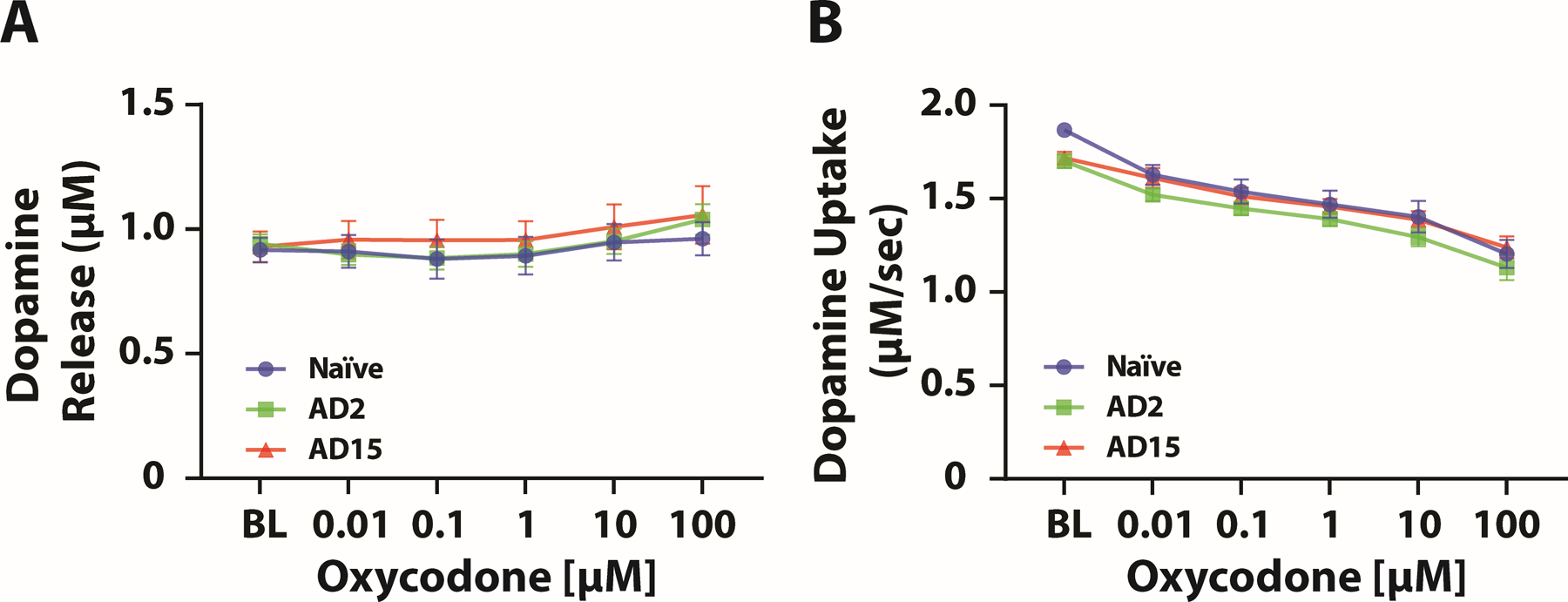
Abstinence from IntA to oxycodone did not influence the acute effects of oxycodone on dopamine transmission. The effect of oxycodone application onto NAc slices on (A) dopamine release and (B) dopamine uptake. Naïve, n = 9 (4 females and 5 males); AD2, n = 8 (3 females and 5 males); AD15, n = 8 (3 females and 5 males). Data shown are mean ± SEM.
Abstinence from oxycodone is associated with reduced phosphorylation of the DAT
To determine whether reductions in dopamine uptake rate on AD2 and AD15 were associated with changes in tDAT or pDAT expression we performed western blotting in NAc tissue in naïve rats and in rats during abstinence from oxycodone on AD2 and AD15. We found that abstinence from oxycodone did not affect tDAT expression or the ratio of pDAT/tDAT, but was associated with a reduction in pDAT expression on AD15 when compared to naïve rats. One-way ANOVAs revealed no effect of group on tDAT expression (F(2,18)=0.4439, p=0.6483) (Figure 5B) and the ratio of pDAT/tDAT (F(2,18)=3.298, p=0.0602) (Figure 5D), but there was a significant effect of group on pDAT expression (F(2,18)=3.884, p=0.0396). Dunnett’s post-hoc analyses revealed a significant reduction in pDAT in rats on AD15 compared to naïve rats (p=0.0376) (Figure 5C). To determine potential contributions of the MOR on responsivity to oxycodone, we quantified expression of the MOR in the NAc using western blotting. Consistent with the lack of difference in responsivity to oxycodone (see Figure 4), a one-way ANOVA revealed no effect of group on MOR levels (F(2,17)=0.04181, p=0.9591) (Figure 5E).
FIGURE 5.
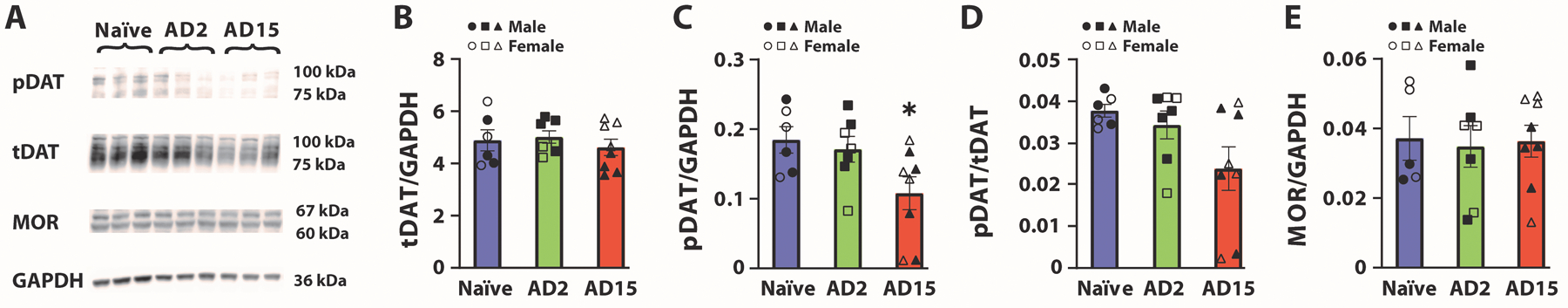
Abstinence from IntA to oxycodone was associated with reduced phosphorylated DAT at Threonine-53 (pDAT) on abstinence day (AD)15. (A) Representative blots for Naïve, AD2 and AD15 groups. (B) Total DAT (tDAT), (C) pDAT, (D) pDAT/tDAT ratio and (E) Mu opioid receptor (MOR) in the NAc. Naïve, n = 6 (3 females and 3 males); AD2, n = 7 (3 females and 4 males); AD15, n = 8 (4 females and 4 males). Data shown are mean ± SEM. *p < 0.05 compared to Naïve.
Discussion
In the current studies, we examined the effects of abstinence following oxycodone self-administration on dopamine transmission and on DAT and MOR protein expression. We found that IntA to oxycodone led to escalation of drug intake and persistent drug seeking during abstinence. Further, we found that both early (AD2) and later (AD15) in abstinence, there was a reduction in dopamine uptake rate. Using western blotting, we observed a reduction in pDAT on AD15, but not AD2. This mismatch between pDAT and uptake rate indicates that observed reductions in dopamine uptake during abstinence cannot be solely attributable to changes in pDAT expression. Additionally, we found that acute oxycodone application to the NAc induced a robust reduction in dopamine uptake rate, and this effect did not differ following abstinence from oxycodone. Consistent with this, we found no difference in MOR expression levels across groups. Taken together, these observations suggest that abstinence from oxycodone is associated with dysfunctional dopamine transmission compared to naïve rats, but acute responsivity to oxycodone on dopamine transmission remains unchanged.
IntA to oxycodone engenders escalation of intake and sustained oxycodone seeking in abstinence
Escalation of drug intake is considered a hallmark of addiction and it has been suggested that rats that escalate drug intake have greater motivation for the drug 43–45. In the current studies, we observed that rats escalated oxycodone intake across the ten days of IntA, which is consistent with previous findings using long access34,35,46,47 and IntA to opioids48. Rats trained to self-administer opioids followed by a forced abstinence period display cue-induced drug seeking. Typically rats display comparatively low drug seeking early in abstinence and seeking increases or incubates over the course of the abstinence period 5–7. In the current study, we did not observe incubation of oxycodone seeking. Rather, we observed heightened seeking early in the abstinence period that persisted for 2 weeks. This finding is surprising given considerable prior evidence indicating incubation of opioid seeking 5,49–53, including for oxycodone 34,35,54,55. One explanation for this lack of incubation may be differences in the schedule of access used. There is extensive evidence that the schedule of access engenders distinct behavioral phenotypes for cocaine self-administration, and despite being understudied, there is evidence that this may be true for opioids as well. Indeed, a recent study found that IntA to heroin resulted in maintained seeking from abstinence day 1 to abstinence day 21 with no incubation 56. Thus, in the current studies, rats may be expressing exaggerated seeking early in abstinence which remains elevated later in abstinence, resulting in the absence of incubation. Interestingly, our observation of robust oxycodone seeking both early and later in abstinence is consistent with what has been reported for human opioid craving, which is characterized by persistent opioid craving throughout abstinence and hence a lack of incubation of craving 57–59. Taken together, the current findings provide additional evidence that IntA schedules reproduce important aspects of drug use observed in humans, such as increased intake over time and persistent drug seeking.
IntA to oxycodone does not influence stimulated dopamine release
Microdialysis studies indicate reduced basal dopamine levels in the NAc during abstinence following chronic opioid exposure 60–64. In our studies, we found no changes in stimulated dopamine release at baseline during oxycodone abstinence, on AD2 or AD15, compared to naïve rats (Figure 2C). This finding is consistent with a recent study indicating that in rats on day 1 of heroin abstinence there was no change in stimulated dopamine release in the NAc shell 26. Taken together, these observations suggest that despite a reduction in basal dopamine levels in the NAc, there appears to be no deficits in dopamine release mechanisms during oxycodone abstinence.
IntA to oxycodone leads to a lasting reduction in dopamine uptake
Several studies indicate that opioids influence dopamine transmission leading to increased dopamine in the NAc following opioid administration 18,19,60,65–67. In the NAc, the primary mechanism of dopamine removal is via the DAT on dopamine terminals. However, it remains largely unknown whether repeated opioid exposure induces dopamine terminal adaptations. Imaging studies in humans examining various brain regions found that during opioid abstinence there is reduced DAT availability 27–31. In rats, it has been shown that following chronic morphine administration there are reduced DAT levels in the anterior basal forebrain 68. Consistent with those findings, we found that both early and later in abstinence there is reduced dopamine uptake in the NAc core, supporting lasting reductions in the efficiency of dopamine removal during abstinence. Further, there is evidence for a hypodopaminergic state in the NAc during opioid abstinence 60,63 which is posited to contribute to dysphoria during withdrawal 69. Therefore, a reduction in dopamine uptake rate during abstinence may reflect a compensatory response to this hypodopaminergic state, which could help to prolong dopamine availability in the extracellular space.
Contrary to our findings of reduced dopamine uptake rate during abstinence, a recent study indicated that following heroin self-administration rats have increased dopamine uptake in the NAc shell on the first day of abstinence 26. Two possible explanations for this discrepancy may involve differences in the NAc subregion where dopamine recordings were obtained, and the schedule of reinforcement used to expose rats to opioids — both of which are known to impact dopamine transmission. For example, FSCV and microdialysis studies demonstrate that morphine, oxycodone, or the MOR agonist DAMGO, lead to varying changes in dopamine levels in the NAc core versus the NAc shell 65,70,71, suggesting that these NAc subregions may have differential responses to opioids. While there are currently no published comparisons on the effects of different schedules of opioid self-administration on dopamine transmission, previous work indicates that different schedules of access for cocaine self-administration can lead to distinct dopamine adaptations. For example, long access to cocaine promotes tolerance in dopamine responses to cocaine 72,73, while IntA to cocaine leads to enhanced dopamine transporter function at baseline and sensitized dopamine responses to cocaine 73,74. Further, in addition to the schedule of access, evidence also suggests that abstinence from cocaine can also influence dopamine transmission 74,75. Future studies are needed to assess the influence of different schedules of reinforcement on the effects of opioids on dopamine uptake.
The cellular processes underlying changes in dopamine uptake are not entirely understood. Nevertheless, there is evidence that tDAT levels and modifications of the DAT influence dopamine uptake 76,77. Indeed, previous studies suggest that reduced pDAT is associated with decreased dopamine uptake 78,79. Therefore, we examined tDAT and pDAT expression in the NAc as a potential biochemical mechanism for the observed reduction in dopamine uptake following abstinence from oxycodone. We found that rats had reduced levels of pDAT on AD15 which coincides with reduced dopamine uptake at that timepoint. However, we found no changes in pDAT on AD2, despite a reduction in dopamine uptake observed at the same timepoint. This was surprising given prior evidence that pDAT expression is associated with changes in dopamine uptake rate 38. While these findings suggest that there is not a one-to-one relationship between uptake rates and pDAT expression, other modifications to the DAT may be contributing to the reduced dopamine uptake. For example, increased phosphorylation of serine-7 on the DAT has been shown to decrease dopamine uptake, while the converse is observed with decreases in phosphorylation of serine-7 80. Unfortunately, there are no available antibodies to target this phosphorylation site in the rat, therefore additional studies will be required to identify potential mechanisms mediating the effects of oxycodone abstinence on dopamine uptake rate.
Local actions of oxycodone reduce dopamine uptake in the NAc core
The widely supported mechanism through which opioids increase striatal dopamine is via disinhibition of VTA dopamine neurons through MOR-mediated inhibition of GABA neurons in the rostromedial tegmental nucleus 18,20,21,81,82. However, there is also evidence that opioids can act locally in the NAc to influence dopamine transmission, with local NAc infusion of fentanyl increasing, and MOR antagonist decreasing, dopamine levels 22,83. To initially investigate the effects of oxycodone on dopamine transmission in the NAc, we applied oxycodone directly onto NAc slices. We found that oxycodone induced a robust MOR-dependent reduction in dopamine uptake rate, which to our knowledge has not been reported previously. This observation suggests that in addition to disinhibiting dopamine neurons in the VTA, opioids may also influence striatal dopamine levels by reducing dopamine uptake rate locally.
The mechanisms by which local opioid administration alters dopamine uptake in the accumbens remain unclear. Abused opioids, such as oxycodone, act primarily at the MOR with lower affinities at the kappa and delta opioid receptors 84–86. However, dopamine terminals in the NAc lack MORs 87, suggesting that opioids must be acting to modulate dopamine uptake indirectly through local microcircuitry in the NAc. Indeed, MOR expression has been identified in both cholinergic and GABAergic interneuron populations in the NAc 88,89 and are known to influence dopamine release 90–92. However, to our knowledge there is no evidence that these interneuron populations impact dopamine uptake rate. Future studies may need to differentially examine the influence of MOR activation on cholinergic or GABAergic population to better understand the processes by which oxycodone can reduce dopamine uptake rate in the NAc.
In the current experiments, we tested if oxycodone abstinence would influence the acute effects of oxycodone on dopamine transmission and whether this was associated with alterations in MOR expression. We found that there was no difference in response to oxycodone on dopamine release and uptake between oxycodone-experienced and naïve rats. Likewise, we found no differences in MOR expression between these experimental groups. These observations are consistent with prior work indicating that opioid exposure does not affect MOR expression 26 nor MOR activity 93–95 in the NAc. Despite these findings, it is important to note that in the dorsal striatum, oxycodone self-administration has been found to decrease MOR expression 34,96, indicating that there are differential effects of opioid exposure depending on the striatal region under investigation. Given the differential effects of oxycodone exposure on MORs in the NAc versus the dorsal striatum, future studies will be needed to assess if abstinence from IntA to oxycodone influences MOR expression, DAT function or expression in other striatal regions.
Conclusions
In the current studies, we found that IntA to oxycodone engendered robust seeking behavior throughout abstinence. Further, we observed that throughout abstinence there was disrupted dopamine transmission with particularly robust reductions in dopamine uptake. This reduced dopamine uptake is present on AD2 and is maintained for two weeks, which is consistent with the lasting reduction in DAT availability observed in human imaging studies. Overall, these findings suggest that during opioid abstinence there may be lasting dysfunction in dopamine transmission which may represent a compensatory response to a hypodopaminergic state during the abstinence period. Further, these observations suggest that changes in dopamine transmission during opioid abstinence may contribute to drug seeking behavior.
Supplementary Material
Acknowledgements
We would like to thank Bethan M. O’Connor for the thoughtful discussions surrounding the conceptualization and implementation of these experiments. We also thank the NIDA drug supply program for donating the oxycodone, DAMGO, naloxone, and CTAP. This work was supported by National Institute on Drug Abuse grant R01DA039100 (RAE) and the Commonwealth Universal Research Enhancement Program (CURE; RAE).
References
- 1.Hser Y-I, Hoffman V, Grella CE, Anglin MD. A 33-year follow-up of narcotics addicts. Arch Gen Psychiatry 2001;58(5):503–508. [DOI] [PubMed] [Google Scholar]
- 2.Shah NG, Galai N, Celentano DD, Vlahov D, Strathdee SA. Longitudinal predictors of injection cessation and subsequent relapse among a cohort of injection drug users in Baltimore, MD, 1988–2000. Drug Alcohol Depend 2006;83(2):147–156. [DOI] [PubMed] [Google Scholar]
- 3.Tiffany ST, Wray JM. The clinical significance of drug craving. Ann N Y Acad Sci 2012;1248:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kakko J, Alho H, Baldacchino A, Molina R, Nava FA, Shaya G. Craving in opioid use disorder: from neurobiology to clinical practice. Front Psychiatry 2019;10:592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shalev U, Morales M, Hope B, Yap J, Shaham Y. Time-dependent changes in extinction behavior and stress-induced reinstatement of drug seeking following withdrawal from heroin in rats. Psychopharmacology 2001;156(1):98–107. [DOI] [PubMed] [Google Scholar]
- 6.Grimm JW, Hope BT, Wise RA, Shaham Y. Incubation of cocaine craving after withdrawal. Nature 2001;412(6843):141–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pickens CL, Airavaara M, Theberge F, Fanous S, Hope BT, Shaham Y. Neurobiology of the incubation of drug craving. Trends Neurosci 2011;34(8):411–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Badiani A, Belin D, Epstein D, Calu D, Shaham Y. Opiate versus psychostimulant addiction: the differences do matter. Nat Rev Neurosci 2011;12(11):685–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ettenberg A, Pettit HO, Bloom FE, Koob GF. Heroin and cocaine intravenous self-administration in rats: mediation by separate neural systems. Psychopharmacology 1982;78(3):204–209. [DOI] [PubMed] [Google Scholar]
- 10.Pettit HO, Ettenberg A, Bloom FE, Koob GF. Destruction of dopamine in the nucleus accumbens selectively attenuates cocaine but not heroin self-administration in rats. Psychopharmacology 1984;84(2):167–173. [DOI] [PubMed] [Google Scholar]
- 11.Alderson HL, Parkinson JA, Robbins TW, Everitt BJ. The effects of excitotoxic lesions of the nucleus accumbens core or shell regions on intravenous heroin self-administration in rats. Psychopharmacology 2001;153(4):455–463. [DOI] [PubMed] [Google Scholar]
- 12.Zito KA, Vickers G, Roberts DC. Disruption of cocaine and heroin self-administration following kainic acid lesions of the nucleus accumbens. Pharmacol Biochem Behav 1985;23(6):1029–1036. [DOI] [PubMed] [Google Scholar]
- 13.Dworkin SI, Guerin GF, Goeders NE, Smith JE. Kainic acid lesions of the nucleus accumbens selectively attenuate morphine self-administration. Pharmacol Biochem Behav 1988;29(1):175–181. [DOI] [PubMed] [Google Scholar]
- 14.Suto N, Wise RA, Vezina P. Dorsal as well as ventral striatal lesions affect levels of intravenous cocaine and morphine self-administration in rats. Neurosci Lett 2011;493(1–2):29–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith JE, Guerin GF, Co C, Barr TS, Lane JD. Effects of 6-OHDA lesions of the central medial nucleus accumbens on rat intravenous morphine self-administration. Pharmacol Biochem Behav 1985;23(5):843–849. [DOI] [PubMed] [Google Scholar]
- 16.Singer G, Wallace M. Effects of 6-OHDA lesions in the nucleus accumbens on the acquisition of self injection of heroin under schedule and non schedule conditions in rats. Pharmacol Biochem Behav 1984;20(5):807–809. [DOI] [PubMed] [Google Scholar]
- 17.David V, Durkin TP, Cazala P. Differential effects of the dopamine D2/D3 receptor antagonist sulpiride on self-administration of morphine into the ventral tegmental area or the nucleus accumbens. Psychopharmacology 2002;160(3):307–317. [DOI] [PubMed] [Google Scholar]
- 18.Corre J, Van Zessen R, Loureiro M, et al. Dopamine neurons projecting to medial shell of the nucleus accumbens drive heroin reinforcement. Elife 2018;7:e39945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci USA 1988;85(14):5274–5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gysling K, Wang RY. Morphine-induced activation of A10 dopamine neurons in the rat. Brain Res 1983;277(1):119–127. [DOI] [PubMed] [Google Scholar]
- 21.Jalabert M, Bourdy R, Courtin J, et al. Neuronal circuits underlying acute morphine action on dopamine neurons. Proc Natl Acad Sci USA 2011;108(39):16446–16450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoshida Y, Koide S, Hirose N, et al. Fentanyl increases dopamine release in rat nucleus accumbens: involvement of mesolimbic mu-and delta-2-opioid receptors. Neurosci 1999;92(4):1357–1365. [DOI] [PubMed] [Google Scholar]
- 23.Brown MT, Bellone C, Mameli M, et al. Drug-driven AMPA receptor redistribution mimicked by selective dopamine neuron stimulation. PLoS ONE 2010;5(12):e15870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saal D, Dong Y, Bonci A, Malenka RC. Drugs of abuse and stress trigger a common synaptic adaptation in dopamine neurons. Neuron 2003;37(4):577–582. [DOI] [PubMed] [Google Scholar]
- 25.Zhang D, Zhang H, Jin G-z, Zhang K, Zhen. Single dose of morphine produced a prolonged effect on dopamine neuron activities. Mol Pain 2008;4:1744–8069–1744–1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.George BE, Barth SH, Kuiper LB, et al. Enhanced heroin self-administration and distinct dopamine adaptations in female rats. Neuropsychopharmacology 2021:1–10. [DOI] [PMC free article] [PubMed]
- 27.Yeh TL, Chen KC, Lin S-H, et al. Availability of dopamine and serotonin transporters in opioid-dependent users—a two-isotope SPECT study. Psychopharmacology 2012;220(1):55–64. [DOI] [PubMed] [Google Scholar]
- 28.Yuan J, Liu XD, Han M, et al. Comparison of striatal dopamine transporter levels in chronic heroin‐dependent and methamphetamine‐dependent subjects. Addict Biol 2017;22(1):229–234. [DOI] [PubMed] [Google Scholar]
- 29.Shi J, Zhao L-Y, Copersino ML, et al. PET imaging of dopamine transporter and drug craving during methadone maintenance treatment and after prolonged abstinence in heroin users. Eur J Pharmacol 2008;579(1–3):160–166. [DOI] [PubMed] [Google Scholar]
- 30.Kamp F, Proebstl L, Penzel N, et al. Effects of sedative drug use on the dopamine system: a systematic review and meta-analysis of in vivo neuroimaging studies. Neuropsychopharmacology 2019;44(4):660–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu Y, Han M, Liu X, et al. Dopamine transporter availability in heroin-dependent subjects and controls: longitudinal changes during abstinence and the effects of Jitai tablets treatment. Psychopharmacology 2013;230(2):235–244. [DOI] [PubMed] [Google Scholar]
- 32.Liang CS, Ho PS, Yen CH, et al. Reduced striatal dopamine transporter density associated with working memory deficits in opioid‐dependent male subjects: a SPECT study. Addict Biol 2016;21(1):196–204. [DOI] [PubMed] [Google Scholar]
- 33.Bernosky-Smith KA, Stanger DB, Trujillo AJ, Mitchell LR, España RA, Bass CE. The GLP-1 agonist exendin-4 attenuates self-administration of sweetened fat on fixed and progressive ratio schedules of reinforcement in rats. Pharmacol Biochem Behav 2016;142:48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Blackwood CA, Hoerle R, Leary M, et al. Molecular adaptations in the rat dorsal striatum and hippocampus following abstinence-induced incubation of drug seeking after escalated oxycodone self-administration. Mol Neurobiol 2019;56(5):3603–3615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blackwood CA, Leary M, Salisbury A, McCoy MT, Cadet JL. Escalated oxycodone self-administration causes differential striatal mRNA expression of FGFs and IEGs following abstinence-associated incubation of oxycodone craving. Neurosci 2019;415:173–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yorgason JT, España RA, Jones SR. Demon voltammetry and analysis software: analysis of cocaine-induced alterations in dopamine signaling using multiple kinetic measures. J Neurosci Methods 2011;202(2):158–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dunah AW, Standaert DG. Dopamine D1 receptor-dependent trafficking of striatal NMDA glutamate receptors to the postsynaptic membrane. J Neurosci 2001;21(15):5546–5558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alonso I, Pino J, Kortagere S, Torres G, España R. Dopamine transporter function fluctuates across sleep/wake state: potential impact for addiction. Neuropsychopharmacology 2021;46(4):699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brodnik ZD, Xu W, Batra A, et al. Chemogenetic manipulation of dopamine neurons dictates cocaine potency at distal dopamine transporters. J Neurosci 2020;40(45):8767–8779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Diester CM, Banks ML, Neigh GN, Negus SS. Experimental design and analysis for consideration of sex as a biological variable. Neuropsychopharmacology 2019;44(13):2159–2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Garcia-Sifuentes Y, Maney DL. Reporting and misreporting of sex differences in the biological sciences. Elife 2021;10:e70817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gipson CD, Beckmann JS, El-Maraghi S, Marusich JA, Bardo MT. Effect of environmental enrichment on escalation of cocaine self-administration in rats. Psychopharmacology 2011;214(2):557–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ahmed SH, Koob G. Transition from moderate to excessive drug intake: change in hedonic set point. Science 1998;282(5387):298–300. [DOI] [PubMed] [Google Scholar]
- 44.Ahmed SH, Walker JR, Koob GF. Persistent increase in the motivation to take heroin in rats with a history of drug escalation. Neuropsychopharmacology 2000;22(4):413–421. [DOI] [PubMed] [Google Scholar]
- 45.Edwards S, Koob GF. Escalation of drug self-administration as a hallmark of persistent addiction liability. Behav Pharmacol 2013;24. [DOI] [PMC free article] [PubMed]
- 46.Bossert JM, Hoots JK, Fredriksson I, et al. Role of mu, but not delta or kappa, opioid receptors in context‐induced reinstatement of oxycodone seeking. Eur J Neurosci 2019;50(3):2075–2085. [DOI] [PubMed] [Google Scholar]
- 47.Wade CL, Vendruscolo LF, Schlosburg JE, Hernandez DO, Koob GF. Compulsive-like responding for opioid analgesics in rats with extended access. Neuropsychopharmacology 2015;40(2):421–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fragale JE, James MH, Aston‐Jones G. Intermittent self‐administration of fentanyl induces a multifaceted addiction state associated with persistent changes in the orexin system. Addict Biol 2021;26(3):e12946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kuntz KL, Twining RC, Baldwin AE, Vrana KE, Grigson PS. Heroin self-administration: I. Incubation of goal-directed behavior in rats. Pharmacol Biochem Behav 2008;90(3):344–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kuntz-Melcavage KL, Brucklacher RM, Grigson PS, Freeman WM, Vrana KE. Gene expression changes following extinction testing in a heroin behavioral incubation model. BMC Neurosci 2009;10(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhou W, Zhang F, Liu H, et al. Effects of training and withdrawal periods on heroin seeking induced by conditioned cue in an animal of model of relapse. Psychopharmacology 2009;203(4):677–684. [DOI] [PubMed] [Google Scholar]
- 52.Fanous S, Goldart EM, Theberge FR, Bossert JM, Shaham Y, Hope BT. Role of orbitofrontal cortex neuronal ensembles in the expression of incubation of heroin craving. J Neurosci 2012;32(34):11600–11609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Airavaara M, Pickens CL, Stern AL, et al. Endogenous GDNF in ventral tegmental area and nucleus accumbens does not play a role in the incubation of heroin craving. Addict Biol 2011;16(2):261–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fredriksson I, Applebey SV, Minier-Toribio A, Shekara A, Bossert JM, Shaham Y. Effect of the dopamine stabilizer (−)-OSU6162 on potentiated incubation of opioid craving after electric barrier-induced voluntary abstinence. Neuropsychopharmacology 2020;45(5):770–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Altshuler RD, Yang ES, Garcia KT, et al. Role of orbitofrontal cortex in incubation of oxycodone craving in male rats. Addict Biol 2021;26(2):e12927. [DOI] [PubMed] [Google Scholar]
- 56.D’Ottavio G, Reverte I, Ragozzino D, et al. Increased heroin intake and relapse vulnerability in intermittent relative to continuous self‐administration: sex differences in rats. Br J Pharmacol 2022. [DOI] [PMC free article] [PubMed]
- 57.Tsui JI, Anderson BJ, Strong DR, Stein MD. Craving and subsequent opioid use among opioid dependent patients who initiate treatment with buprenorphine. Am J Drug Alcohol Abuse 2014;40(2):163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fudala PJ, Bridge TP, Herbert S, et al. Office-based treatment of opiate addiction with a sublingual-tablet formulation of buprenorphine and naloxone. N Engl J Med 2003;349(10):949–958. [DOI] [PubMed] [Google Scholar]
- 59.Herbeck DM, Jeter KE, Cousins SJ, Abdelmaksoud R, Crèvecoeur-MacPhail D. Gender differences in treatment and clinical characteristics among patients receiving extended release naltrexone. J Addict Dis 2016;35(4):305–314. [DOI] [PubMed] [Google Scholar]
- 60.Pothos E, Rada P, Mark GP, Hoebel BG. Dopamine microdialysis in the nucleus accumbens during acute and chronic morphine, naloxone-precipitated withdrawal and clonidine treatment. Brain Res 1991;566(1):348–350. [DOI] [PubMed] [Google Scholar]
- 61.Acquas E, Carboni E, Di Chiara G. Profound depression of mesolimbic dopamine release after morphine withdrawal in dependent rats. Eur J Pharmacol 1991;193(1):133–134. [DOI] [PubMed] [Google Scholar]
- 62.Rossetti ZL, Hmaidan Y, Gessa GL. Marked inhibition of mesolimbic dopamine release: a common feature of ethanol, morphine, cocaine and amphetamine abstinence in rats. Eur J Pharmacol 1992;221(2–3):227–234. [DOI] [PubMed] [Google Scholar]
- 63.Crippens D, Robinson TE. Withdrawal from morphine or amphetamine: different effects on dopamine in the ventral-medial striatum studied with microdialysis. Brain Res 1994;650(1):56–62. [DOI] [PubMed] [Google Scholar]
- 64.Melis M, Spiga S, Diana M. The dopamine hypothesis of drug addiction: hypodopaminergic state. Int Rev Neurobiol 2005;63(10):101–154. [DOI] [PubMed] [Google Scholar]
- 65.Vander Weele CM, Porter‐Stransky KA, Mabrouk OS, et al. Rapid dopamine transmission within the nucleus accumbens: dramatic difference between morphine and oxycodone delivery. Eur J Neurosci 2014;40(7):3041–3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Spanagel R, Herz A, Shippenberg TS. The effects of opioid peptides on dopamine release in the nucleus accumbens: an in vivo microdialysis study. J Neurochem 1990;55(5):1734–1740. [DOI] [PubMed] [Google Scholar]
- 67.Leone P, Pocock D, Wise R. Morphine-dopamine interaction: ventral tegmental morphine increases nucleus accumbens dopamine release. Pharmacol Biochem Behav 1991;39(2):469–472. [DOI] [PubMed] [Google Scholar]
- 68.Simantov R Chronic morphine alters dopamine transporter density in the rat brain: possible role in the mechanism of drug addiction. Neurosci Lett 1993;163(2):121–124. [DOI] [PubMed] [Google Scholar]
- 69.Koob GF, Le Moal M. Addiction and the brain antireward system. Annu Rev Psychol 2008;59:29–53. [DOI] [PubMed] [Google Scholar]
- 70.Hipólito L, Sánchez-Catalán MJ, Zanolini I, Polache A, Granero L. Shell/core differences in mu-and delta-opioid receptor modulation of dopamine efflux in nucleus accumbens. Neuropharmacology 2008;55(2):183–189. [DOI] [PubMed] [Google Scholar]
- 71.Pontieri FE, Tanda G, Di Chiara G. Intravenous cocaine, morphine, and amphetamine preferentially increase extracellular dopamine in the” shell” as compared with the” core” of the rat nucleus accumbens. Proc Natl Acad Sci USA 1995;92(26):12304–12308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ferris MJ, Calipari ES, Mateo Y, Melchior JR, Roberts D, Jones SR. Cocaine self-administration produces pharmacodynamic tolerance: differential effects on the potency of dopamine transporter blockers, releasers, and methylphenidate. Neuropsychopharmacology 2012;37(7):1708–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Calipari ES, Ferris MJ, Zimmer BA, Roberts DC, Jones SR. Temporal pattern of cocaine intake determines tolerance vs sensitization of cocaine effects at the dopamine transporter. Neuropsychopharmacology 2013;38(12):2385–2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Calipari ES, Siciliano CA, Zimmer BA, Jones SR. Brief intermittent cocaine self-administration and abstinence sensitizes cocaine effects on the dopamine transporter and increases drug seeking. Neuropsychopharmacology 2015;40(3):728–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Alonso IP, O’Connor BM, Bryant KG, Mandalaywala RK, España RA. Incubation of cocaine craving coincides with changes in dopamine terminal neurotransmission. Addiction Neuroscience 2022:100029. [DOI] [PMC free article] [PubMed]
- 76.Mortensen OV, Amara SG. Dynamic regulation of the dopamine transporter. Eur J Pharmacol 2003;479(1–3):159–170. [DOI] [PubMed] [Google Scholar]
- 77.Vaughan RA, Foster JD. Mechanisms of dopamine transporter regulation in normal and disease states. Trends Pharmacol Sci 2013;34(9):489–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Foster JD, Yang J-W, Moritz AE, et al. Dopamine transporter phosphorylation site threonine 53 regulates substrate reuptake and amphetamine-stimulated efflux. J Biol Chem 2012;287(35):29702–29712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Foster JD, Vaughan RA. Phosphorylation mechanisms in dopamine transporter regulation. J Chem Neuroanat. 2017;83:10–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Moritz AE, Rastedt DE, Stanislowski DJ, et al. Reciprocal phosphorylation and palmitoylation control dopamine transporter kinetics. J Biol Chem 2015;290(48):29095–29105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Johnson S, North R. Opioids excite dopamine neurons by hyperpolarization of local interneurons. J Neurosci 1992;12(2):483–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Matsui A, Williams JT. Opioid-sensitive GABA inputs from rostromedial tegmental nucleus synapse onto midbrain dopamine neurons. J Neurosci 2011;31(48):17729–17735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gómez-A A, Shnitko TA, Barefoot HM, et al. Local μ-opioid receptor antagonism blunts evoked phasic dopamine release in the nucleus accumbens of rats. ACS Chem Neurosci 2018;10(4):1935–1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Peckham EM, Traynor JR. Comparison of the antinociceptive response to morphine and morphine-like compounds in male and female Sprague-Dawley rats. J Pharmacol Exp Ther 2006;316(3):1195–1201. [DOI] [PubMed] [Google Scholar]
- 85.Lalovic B, Kharasch E, Hoffer C, Risler L, Liu‐Chen LY, Shen DD. Pharmacokinetics and pharmacodynamics of oral oxycodone in healthy human subjects: role of circulating active metabolites. Clin Pharmacol Ther 2006;79(5):461–479. [DOI] [PubMed] [Google Scholar]
- 86.Yoburn BC, Shah S, Chan K, Duttaroy A, Davis T. Supersensitivity to opioid analgesics following chronic opioid antagonist treatment: relationship to receptor selectivity. Pharmacol Biochem Behav 1995;51(2–3):535–539. [DOI] [PubMed] [Google Scholar]
- 87.Trovero F, Herve D, Desban M, Glowinski J, Tassin J-P. Striatal opiate mu-receptors are not located on dopamine nerve endings in the rat. Neurosci 1990;39(2):313–321. [DOI] [PubMed] [Google Scholar]
- 88.Svingos AL, Moriwaki A, Wang JB, Uhl GR, Pickel VM. μ-Opioid receptors are localized to extrasynaptic plasma membranes of GABAergic neurons and their targets in the rat nucleus accumbens. J Neurosci 1997;17(7):2585–2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Svingos AL, Colago EE, Pickel VM. Vesicular acetylcholine transporter in the rat nucleus accumbens shell: Subcellular distribution and association with μ‐opioid receptors. Synapse 2001;40(3):184–192. [DOI] [PubMed] [Google Scholar]
- 90.Rice ME, Cragg SJ. Nicotine amplifies reward-related dopamine signals in striatum. Nat Neurosci 2004;7(6):583–584. [DOI] [PubMed] [Google Scholar]
- 91.Pitman KA, Puil E, Borgland SL. GABAB modulation of dopamine release in the nucleus accumbens core. Eur J Neurosci 2014;40(10):3472–3480. [DOI] [PubMed] [Google Scholar]
- 92.Brodnik ZD, Batra A, Oleson EB, España RA. Local GABAA receptor-mediated suppression of dopamine release within the nucleus accumbens. ACS Chem Neurosci 2018;10(4):1978–1985. [DOI] [PubMed] [Google Scholar]
- 93.Sim-Selley LJ, Selley DE, Vogt LJ, Childers SR, Martin TJ. Chronic heroin self-administration desensitizes μ opioid receptor-activated G-proteins in specific regions of rat brain. J Neurosci 2000;20(12):4555–4562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Weber R, Gomez-Flores R, Smith J, Martin T. Neuronal adaptations, neuroendocrine and immune correlates of heroin self-administration. Brain Behav Immun 2009;23(7):993–1002. [DOI] [PubMed] [Google Scholar]
- 95.Fattore L, Viganò D, Fadda P, Rubino T, Fratta W, Parolaro D. Bidirectional regulation of mu‐opioid and CB1‐cannabinoid receptor in rats self‐administering heroin or WIN 55,212‐2. Eur J Neurosci 2007;25(7):2191–2200. [DOI] [PubMed] [Google Scholar]
- 96.Blackwood CA, McCoy MT, Ladenheim B, Cadet JL. Escalated oxycodone self-administration and punishment: differential expression of opioid receptors and immediate early genes in the rat dorsal striatum and prefrontal cortex. Front Neurosci 2020;13:1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


