Abstract
Aims:
This study aimed to determine the association of stress and salivary cortisol levels in the adult Indian population with and without temporomandibular disorder (TMD) and to validate it with bite force.
Settings and Design:
The present study had an observational, case–control study design.
Materials and Methods:
This study sample comprised two groups of 25 cases and 25 controls between 18 and 45 years of age. Diagnostic criteria-TMD questionnaire Axis I was used to assess TMD classification, the TMD Disability Index and modified Perceived Stress Scale (PSS) questionnaires were filled, and salivary cortisol levels were measured using electrochemiluminescence immunoassay (ECLIA). Bite force analysis was performed using a portable load indicator.
Statistical Analysis Used:
To characterize and analyze the study variables, means, standard deviations, Mann–Whitney U-test, and logistic regression were employed (STATA 14.2 [Texas, USA]). Shapiro–Wilk test was used to test the normality of the data. P < 0.05 was considered statistically significant (95% power).
Results:
Female gender was proportionately higher in both the groups (P = 0.508), TMD Disability Index was significantly higher for cases (P < 0.001), TMD cases perceived higher stress levels (P = 0.011), there was no statistically significant difference in salivary cortisol level between cases and controls (P = 0.648), and the median bite force was lower for cases (P = 0.0007).
Conclusions:
This study concluded that the chance of developing TMD increased with age. An increase in the TMD Disability Index score and modified PSS scores; and a decrease in the bite force increased the likelihood of TMD. Modified PSS score was negatively correlated with salivary cortisol concentrations, indicating a two-way response to TMD symptoms.
Keywords: Bite force, case–control study, immunoassay, psychological stress, temporomandibular disorders
INTRODUCTION
“Temporomandibular disorders” (TMDs) refer to a wide range of musculoskeletal conditions that impact the temporomandibular joints (TMJs) and the overall stomatognathic system. TMD is the second most typical musculoskeletal disorder that compromises the quality of life. Epidemiological studies have highlighted a significant prevalence of TMD in the adult (5%–60% of the population) with a fourfold higher prevalence in women, especially between the ages of 18 and 45 years.[1]
TMD showed the highest prevalence of 28.38% in Puducherry population. TMDs have recently begun to exhibit a higher incidence percentage in young adults which might be explained by the high levels of stress that youngsters experience in the extraordinary post-COVID situation.
The literature search has so far linked stress to TMD; however, the adult Indian population (particularly Puducherry residents) had not been studied for this association with bite force. Stress is a potential predisposing factor that induces increased masticatory muscle activity, which can be measured using salivary biomarkers, such as cortisol, and confirmed by a bite force analysis. Thus, the objective of this study was to determine the association of stress and salivary cortisol levels in the adult Indian population with and without TMD and confirm it with bite force.
MATERIALS AND METHODS
Participants
Fifty subjects aged between 18 and 45 years were selected for this study, with 25 placed in the TMD/case group and 25 participants placed in the control group. Subjects were screened over a period of 12 months from November 2021 to September 2022 from the outpatient registry of our institute. The Research and Ethical Committee on Human Research of our institute approved this study on October 18 and October 25, 2021, with the following permission number: 290/MGPGIDS/Aca-I/2021–22/1692.
Study design
A case–control study was chosen as the study design, as it is comparatively faster and more economical.[2] The clinical and biochemical evaluation of all the cases and controls was conducted in the same way.
Study settings
Center of research
This study was conducted at the Department of Prosthodontics.
Study population
The adult Indian population of Puducherry were enrolled in the study.
Study duration
The study duration was 12 months.
Sampling strategy
A simple random sampling method was used to obtain the required sample size.
Sample size
For comparing between independent sample groups, the sample size was calculated using G*Power software (Version 3.1.9.4) Mac OS X and windows XP/ vista/7/8. The number of subjects per group will be n = 15 for each group using a maximum significance level of 0.05 and a maximum test power of 95%.[3] Expecting a 30% dropout and wide inter-examiner bias in a case–control study, we decided to include a slightly bigger sample of 50 samples (25 cases and 25 controls).
Samples involved
After obtaining informed consent, willing dentulous and partially edentulous dental students (only undergraduates) or outpatients between the ages of 18 and 45 years who attended the outpatient registry were chosen with an unequal distribution of men and women.
Selection criteria
Inclusion criteria
Subjects who agreed to take part in the study were included
Dentate or partially edentate students (18–25 years) and patients attending the outpatient department (18–45 years)] from both genders with TMD were included in the case group
Dentate or partially edentate students (18–25 years) and patients attending the outpatient department (18–45 years) from both genders without TMD were included in the control group.
At least two symptoms (clicking, deviation/deflection, etc.) or a single painful symptom was required for inclusion.
Exclusion criteria
Subjects who were not willing to participate in the study
Subjects under the medications that affect salivary cortisol (eg. corticosteroids, antidepressant medications, and hormone supplements)
Subjects with a history of TMJ trauma or orofacial infections
Subjects undergoing TMJ splint therapy or orthodontic treatment and
Subjects with distal extension partially edentulous situations were excluded from the study.
After the Ethical and Research Committee approval, the participants were given ample time to read the consent form (with Patient Information Sheet) themselves thoroughly and to sign it. The study participants were made to fill out two questionnaires, the TMD Disability Index (TDI) and the modified Perceived Stress Scale (PSS), and then underwent sampling of 5 mL of saliva followed by a bite force analysis using a custom-designed portable load indicator.
TMD Disability Index (TDI)
TDI is a tool that includes ten questions regarding TMD disability, and each question is assigned a score of 0–4. The final rating of each research questionnaire is expressed as a percentage (%) of disability.
Psychosocial stress assessment
Modified Perceived Stress Scale
PSS was originally proposed by Cohen et al. and Williamson. It was modified with the help of a psychotherapist to suit Indian standards.[4] The score of the respondent’s stress perception ranged from 0 to 40, with higher numbers indicating higher levels of stress. People were asked to fill out questionnaires about the uncontrollable situations that stressed them out in the past year using a 5-point Likert scale: 0 = never, 1 = almost never, 2 = sometimes, 3 = fairly often, and 4 = very often.
The PSS is an instrument with good reliability (Cronbach’s alpha score of 0.84 or greater), construct validity, and significant association with other aspects of stress assessment. It has a reverse scoring for four positively stated items (items no: 4, 5, 8, and 10).
Saliva sample collection
The passive drool method was used to collect 5 ml of unstimulated saliva from 25 TMD sufferers and 25 controls in a sterile disposable plastic tube. Two hours before sample collection, participants in the study were instructed not to brush their teeth, drink or eat anything, or smoke. Between 10:00 a. m. and 12:00 p. m., saliva samples were collected.[5] Automated electrochemiluminescence assay (ECLIA) was performed to measure the salivary cortisol concentration by qualified professionals at Anderson Diagnostics and Labs in Nungambakkam, Chennai.
Maximum voluntary bite force
The maximum voluntary bite force was measured using a custom-made portable load indicator developed specifically for oral situations. It was an electronic bite force analyzer with a biosensor for intraoral pressure and a digital display monitor. Study participants were given a biosensor (with a sterile, single-use sleeve) instructed to be bite on as firmly as possible, which recorded the bite force in the permanent first molars on the right and left. The final outcome was achieved by repeating the process three times, with a 2-min break between each repetition. The data quality was managed by two examiners entering the data, and all the data entry was constantly monitored by the co-investigators periodically.
Ethical and human participant protection considerations
The Research and Ethical Committee on Human Research of our institute approved this study on October 18 and October 25, 2021, with the following permission number: 290/MGPGIDS/Aca-I/2021–22/1692. In order to uphold the principles of anonymity, decency, beneficence, nonmaleficence, and justice, any information acquired that may be used to identify the patient was kept secret. All the procedures have been performed as per the ethical guidelines laid down by the Declaration of Helsinki (2013) after obtaining informed consent. Furthermore, the research protocol was strictly adhered to.
Statistical analysis
Mean with standard deviation (if the data follow normal distribution), median with range (if the data not follow normal distribution), and frequency with percentage were used to describe the summary information about data. Shapiro–Wilk test was used to test the normality of the data. Student’s t-test/nonparametric Mann–Whitney U-test was used to test the difference of continuous variables between the groups. Logistic regression analysis was used to find out the risk factors associated with the groups. P < 0.05 was considered statistically significant (95% confidence interval with degree of freedom = 28). All statistical analysis was done by STATA 14.2 (Texas, USA).[6]
RESULTS
Demographic data
In the current study, the mean age of all the cases was 25.72 years (range: 21–35 years). The mean age in the control group was 22.24 years (range: 19–24 years). Age was significantly higher in the case group than the control group (P = 0.0003, using t-test [Table 1].
Table 1.
Mean distribution of various parameters (age, gender, bite force, modified Perceived Stress Scale, Temporomandibular Disorder Disability Index, and salivary cortisol) between cases and controls
| Variable | Case (n=25) | Control (n=25) | Total (n=50) | P |
|---|---|---|---|---|
| Age* | ||||
| Mean±SD | 25.72±4.36 | 22.24±0.97 | 23.98±3.59 | 0.0003 |
| Minimum–maximum | 21–35 | 19–24 | 19–35 | |
| Gender | ||||
| Male | 7 (28.0) | 5 (20.0) | 12 (24.0) | 0.508 |
| Female | 18 (72.0) | 20 (80.0) | 38 (76.0) | |
| TMD** | ||||
| Mean±SD | 17.00±14.09 | 2.10±2.57 | 9.55±12.53 | <0.001 |
| Median | 12.5 | 2.5 | 5 | |
| Minimum–maximum | 0.00–62.50 | 0–10 | 0–62.5 | |
| PSS index* | ||||
| Mean±SD | 21.00±4.31 | 18.04±3.54 | 19.52±4.18 | 0.011 |
| Minimum–maximum | 12–30 | 13–29 | 12–30 | |
| Salivary cortisol** | ||||
| Mean±SD | 0.270±0.164 | 0.241±0.114 | 0.255±0.141 | 0.648 |
| Median | 0.239 | 0.254 | 0.251 | |
| Minimum–maximum | 0.013–0.713 | 0.055–0.540 | 0.013–0.713 | |
| Bite force (right)** | ||||
| Mean±SD | 147.35±117.54 | 192.15±75.90 | 169.75±100.50 | 0.009 |
| Median | 106.70 | 183.10 | 147.38 | |
| Minimum–maximum | 20.63–555.23 | 71.13–379.7 | 20.63–555.23 | |
| Bite force (left)** | ||||
| Mean±SD | 163.52±148.40 | 204.03±118.87 | 183.78±134.63 | 0.068 |
| Median | 132.83 | 177.30 | 149.10 | |
| Minimum–maximum | 9.30–704.8 | 54.50–601.10 | 9.30–704.80 | |
| Bite force** | ||||
| Mean±SD | 127.10±114.47 | 198.09±93.57 | 162.60±109.51 | 0.0007 |
| Median | 100.26 | 163.33 | 134.78 | |
| Minimum–maximum | 9.30–555.23 | 68.13–490.40 | 9.30–555.23 |
*t-test, **Mann–Whitney’s test. SD: Standard deviation, PSS: Perceived Stress Scale, TMD: Temporomandibular disorder, TDI: TMD Disability Index
Samples comprised both males (12 ± 24.0) and females (38 ± 76.0) in an unequal proportion. The distribution of males and females in cases was 7.0 ± 28.0 and 18 ± 72.0, respectively [Table 1]. Female gender was proportionately higher in both the groups, and it was showing a statistically significant difference (P = 0.508, using Chi-square test). The results of the current research indicated that women had a higher risk of TMD than men (females 76% and males 24%).
Temporomandibular Disorder Disability Index (TDI) scores
According to Table 1, the mean TMD Disability Index for cases was 17.00 ± 14.09, which was significantly higher than the 9.55 ± 12.53 for controls (P < 0.001). Furthermore, the Mann–Whitney U-test, with P < 0.001, demonstrated that the median TMD was substantially greater in the case group compared to the control group [Graph 1].
Graph 1.

Distribution of TMD Disability Index [TDI] values between cases and controls. TMD: Temporomandibular disorder
Modified Perceived Stress Scale scores
In the present study, TMD cases perceived statistically significant higher stress levels (21.00 ± 4.31) than controls (18.04 ± 3.54) (P = 0.011, t-test) [Table 1 and Graph 2].
Graph 2.

Distribution of PSS values between cases and controls. PSS: Perceived Stress Scale
Salivary cortisol estimation
Table 1 demonstrates that there was no statistical difference between the mean levels of salivary cortisol in the cases (0.270 ± 0.164) and the controls (0.241 ± 0.114). Furthermore, the median salivary cortisol did not show any statistically significant difference between both the groups (P = 0.648, Mann–Whitney U-test) [Table 1 and Graph 3]. In fact, there was a negative correlation between salivary cortisol and psychological stress in the case group, and this association was not strong.
Graph 3.
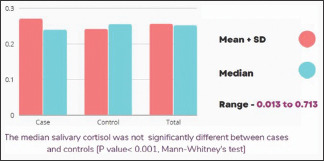
Distribution of salivary cortisol values between cases and controls
Bite force analysis
When compared to the controls, the maximum bite force of cases on both the sides was significantly decreased. The median bite force in affected side of cases (127.10 ± 114.47) was significantly lower than the control group (198.09 ± 93.57) (P = 0.0007, Mann–Whitney U-test) [Table 1] [Graph 4], indicating that TMD is associated with a decrease in the bite force.
Graph 4.

Distribution of bite force between cases and controls in affected and unaffected sides
Diagnostic criteria for TMD (DC/TMD) history questionnaire and clinical assessment data were used to derive Axis I. According to Table 2, the most frequent TMD was anterior disc displacement with reduction (ADDwR), which was found in 52.0% of patients. This was followed by ADDwR with intermittent locking and myalgia (each 20.0% of cases) and ADDwR + intermittent locking + localized myalgia (each 8% of cases).
Table 2.
Prevalence of various types of temporomandibular disorder
| TMD types | n (%) |
|---|---|
| ADDwR | 13 (52.0) |
| ADDwR + IL | 5 (20.0) |
| ADDwR + IL + localized myalgia | 2 (8.0) |
| TMJ myalgia | 5 (20.0) |
| Total | 25 (100.0) |
TMJ: Temporomandibular joints, TMD: Temporomandibular disorder, ADDwR: Anterior disc displacement with reduction, IL: Intermittent locking
Logistic regression analysis was used to find out factors associated with risk factors of disease. Higher age (odds ratio = 1.98, P = 0.066), biting force ≤ 100 (odds ratio = 6.61, P = 0.117), and TMD index values more than 2.5 (odds ratio = 25.76, P = 0.001) were significantly associated with the TMD case group [Table 3].
Table 3.
Risk factors associated with cases
| Variable | Case (n=25) | Control (n=25) | OR (95% CI) | P |
|---|---|---|---|---|
| Age | ||||
| Mean±SD | 25.72±4.36 | 22.24±0.97 | 1.98 (0.96–4.11) | 0.066 |
| Minimum–maximum | 21–35 | 19–24 | ||
| TMD | ||||
| ≤2.5 | 3 (12.0) | 21 (84.0) | 1.00 | - |
| >2.5 | 22 (88.0) | 4 (16.0) | 25.76 (3.68–180.54) | 0.001 |
| Bite force | ||||
| >100 | 13 (52.0) | 23 (92.0) | 1.00 | - |
| ≤100 | 12 (48.0) | 2 (8.0) | 6.61 (0.62–69.99) | 0.117 |
OR: Odds ratio, CI: Confidence interval, SD: Standard deviation, TMD: Temporomandibular disorder
| S.No | Name | Role |
|---|---|---|
| 1. | TMJ Consultancy Services, Bhopal (Madhya Pradesh), India, and DARSN Academy for Maxillofacial Education and Research, DAMER, India | Dr. Prem Narayan Sharma and Hari Shankar Bhargava International Research Grant for Temporomandibular Joint Disorders |
| 2. | Dr. S.P.K. Kennedy Babu M.D.S., | Dean, M.G.P.G.I.D.S., Puducherry |
| 3. | Mr.B. Vijayakumar Technical assistant (Statistics) ICMR-Vector Control Research Centre Indian Council of Medical Research Puducherry -605009 | Statistical assistance |
DISCUSSION
Stress can lead to positive and negative outcomes, such as motivation and improved task performance, but can also cause anxiety, depression, melancholy, and social dysfunction. 25 TMD cases and 25 healthy controls had their salivary cortisol and psychological stress levels examined to prove that psychological stress plays a significant role in the development and maintenance of TMD.[7]
DC-TMD Axis I questionnaire was used to diagnose TMD. The participants completed 2 questionnaires – a modified 10-item PSS questionnaire and TMD Disability Index questionnaire. The results of this study revealed that TMD is more likely to develop with advancing age. It is more common in females than in males. Nadendla et al. included more women than men, and the participants’ mean age was greater (29.2 years) than it was in the latter (23.98 years).[8] An increase in TMD Disability Index score >2.5, modified PSS scores, and bite force <100 Newton indicates a higher likelihood of TMD. This is in accordance with a study by Johnston et al.[9]
According to the present study, the mean salivary cortisol level of the case group was marginally higher than those of the control group. It was unexpected to see the TMD cases to have a low cortisol response. The low cortisol response in the case group and its potently inverse relationship with modified PSS value implied that each patient responded to stress in a different manner. Some subjects with stress had increased salivary cortisol concentration. Some subjects with stress had decreased salivary cortisol concentration (due to deficient cortisol awakening response).
Thus, salivary cortisol elicited a two-way response to chronic stress and TMD symptoms in the present study. This is in accordance with the investigations of Jones et al., who found that patients with TMDs appeared to be more stressed than controls but did not discover any significant variation in salivary cortisol levels.[10] This was also supported by the findings of Salameh et al., Crnkovic et al., and Mirzaei et al.[5,11,12] Contrary to the results of the present investigation, studies by Nadendla et al., Goyal et al., and Lu et al. found a significant positive correlation between salivary cortisol levels in both the case and control groups.[8,11,12,13,14] According to Delboni, 2007, and Di Laccio et al., every person had a varied threshold for stress.[15,16]
In the current study, the case group’s mean maximal biting force was significantly lower than that of the control, which conforms to the study by Koc et al.[17] However, according to Pizolato et al., the bite force was lower in women with TMD whereas there was a rise in the bite force in men with TMD.[18] According to earlier investigations, patients with TMD have a significantly lower bite force, which might be due to the articular pain.[19]
CONCLUSIONS
A weak negative correlation was demonstrated between self-reported modified perceived stress score (chronic stress) and salivary cortisol concentrations
Thus, salivary cortisol elicited a two-way response to TMD symptoms
This might be because of the biologic changes brought about by TMD-induced changes reducing the adaptive cortisol response to chronic psychological stressors
Low cortisol-secreting cases would have responded to the stress in the same way as that of the controls. This might be attributed to the way they had handled their stress (coping mechanism)
This study concluded that the etiopathogenesis of TMD was significantly influenced by psychosocial stress. When compared to men, women have a higher risk of TMD. This study concluded that the chance of developing TMD increased with age. An increase in the TMD Disability Index score and modified PSS scores; and a decrease in the bite force increased the likelihood of TMD. Additionally, it may be concluded that salivary cortisol levels may not necessarily indicate psychological stress.
Recommendations
The patient’s coping strategies might be vital in the control of stress, the regulation of the hypothalamus–pituitary–adrenal axis, and the prevention of TMJ damage. TMD sufferers should be taught about the coping strategies to combat stress.[20]
Future scope/clinical significance
The biopsychosocial model of TMD has emerged, recognizing a multifaceted etiology. The two-way response demonstrated by the salivary cortisol in the present study depends on the individual’s stress perception and coping mechanism. In future studies, it would be interesting to collect saliva samples at several time points to compare their diurnal rhythm.
Financial support and sponsorship
“The project is supported by the research grant from TMJ Consultancy Services, Bhopal (Madhya Pradesh), India, and DARSN Academy for Maxillofacial Education and Research, DAMER, India.”
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
| S.No | Name | Role |
|---|---|---|
| 1. | TMJ Consultancy Services, Bhopal (Madhya Pradesh), India, and DARSN Academy for Maxillofacial Education and Research, DAMER, India | Dr. Prem Narayan Sharma and Hari Shankar Bhargava International Research Grant for Temporomandibular Joint Disorders |
| 2. | Dr. S.P.K. Kennedy Babu M.D.S., | Dean, M.G.P.G.I.D.S., Puducherry |
| 3. | Mr.B. Vijayakumar Technical assistant (Statistics) ICMR-Vector Control Research Centre Indian Council of Medical Research Puducherry -605009 | Statistical assistance |
REFERENCES
- 1.Gupta AK, Gupta R, Gill S. Effectiveness of Vitamin D along with Splint therapy in the Vit D deficient patients with Temporomandibular disorder-A Randomized, double-blind, placebo-controlled clinical trial. J Indian Prosthodont Soc. 2022;22:65–73. doi: 10.4103/jips.jips_334_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Setia MS. Methodology series module 2:Case-control Studies. Indian J Dermatol. 2016;61:146–51. doi: 10.4103/0019-5154.177773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salgueiro MD, Bortoletto CC, Horliana AC, Mota AC, Motta LJ, Motta PB, et al. Evaluation of muscle activity, bite force and salivary cortisol in children with bruxism before and after low level laser applied to acupoints:Study protocol for a randomised controlled trial. BMC Complement Altern Med. 2017;17:391. doi: 10.1186/s12906-017-1905-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen S, Williamson G. Perceived Stress in a Probability Sample of the United States. In: Spacapan S, Oskamp S, editors. The Social Psychology of Health:Claremont Symposium on Applied Social Psychology. Newbury Park, CA: Sage; 1988. pp. 31–67. [Google Scholar]
- 5.Salameh E, Alshaarani F, Hamed HA, Nassar JA. Investigation of the relationship between psychosocial stress and temporomandibular disorder in adults by measuring salivary cortisol concentration:A case-control study. J Indian Prosthodont Soc. 2015;15:148–52. doi: 10.4103/0972-4052.158075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Staniszewski K, Lygre H, Bifulco E, Kvinnsland S, Willassen L, Helgeland E, et al. Temporomandibular Disorders Related to Stress and HPA-Axis Regulation. Pain Res Manag. 2018;2018:7020751. doi: 10.1155/2018/7020751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Korszun A, Young EA, Singer K, Carlson NE, Brown MB, Crofford L. Basal circadian cortisol secretion in women with temporomandibular disorders. J Dent Res. 2002;81:279–83. doi: 10.1177/154405910208100411. [DOI] [PubMed] [Google Scholar]
- 8.Nadendla LK, Meduri V, Paramkusam G, Pachava KR. Evaluation of salivary cortisol and anxiety levels in myofascial pain dysfunction syndrome. Korean J Pain. 2014;27:30–4. doi: 10.3344/kjp.2014.27.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnston K, Bird L, Bright P. Temporomandibular joint effusion and its relationship with perceived disability assessed using musculoskeletal ultrasound and a patient-reported disability index. Ultrasound. 2015;23:90–6. doi: 10.1177/1742271X14568608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jones DA, Rollman GB, Brooke RI. The cortisol response to psychological stress in temporomandibular dysfunction. Pain. 1997;72:171–82. doi: 10.1016/s0304-3959(97)00035-3. [DOI] [PubMed] [Google Scholar]
- 11.Crnković D, Peco M, Gelo J. Correlation between salivary biochemical stress indicators and psychological indicators. Acta Clin Croat. 2018;57:316–26. doi: 10.20471/acc.2018.57.02.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mirzaei M, Zarabadipour M, Mirzadeh M. Evaluation the relationship between psychological profile and salivary cortisol in patients with recurrent aphthous stomatitis. Dent Res J (Isfahan) 2021;18:50. [PMC free article] [PubMed] [Google Scholar]
- 13.Goyal G, Gupta D, Pallagatti S. Salivary cortisol could be a promising tool in the diagnosis of temporomandibular disorders associated with psychological factors. J Indian Acad Oral Med Radiol. 2020;32:354–9. [Google Scholar]
- 14.Lu L, Yang B, Li M, Bao B. Salivary cortisol levels and temporomandibular disorders –A systematic review and meta-analysis of 13 case-control studies. Trop J Pharm Res. 2022;21:1341–9. [Google Scholar]
- 15.Di Laccio KJ, Colato AS, Dorneles GP, Peres A. Assessment of levels of salivary cortisol and stress in patients with signs and symptoms of temporomandibular joint disorders. Int J Health Sci. 2014;2:59–72. [Google Scholar]
- 16.Rondó PH, Vaz AJ, Moraes F, Tomkins A. The relationship between salivary cortisol concentrations and anxiety in adolescent and non-adolescent pregnant women. Braz J Med Biol Res. 2004;37:1403–9. doi: 10.1590/s0100-879x2004000900016. [DOI] [PubMed] [Google Scholar]
- 17.Koc D, Dogan A, Bek B. Bite force and influential factors on bite force measurements:A literature review. Eur J Dent. 2010;4:223–32. [PMC free article] [PubMed] [Google Scholar]
- 18.Pizolato RA, Gavião MB, Berretin-Felix G, Sampaio AC, Trindade Junior AS. Maximal bite force in young adults with temporomandibular disorders and bruxism. Braz Oral Res. 2007;21:278–83. doi: 10.1590/s1806-83242007000300015. [DOI] [PubMed] [Google Scholar]
- 19.Kogawa EM, Calderon PS, Lauris JR, Araujo CR, Conti PC. Evaluation of maximal bite force in temporomandibular disorders patients. J Oral Rehabil. 2006;33:559–65. doi: 10.1111/j.1365-2842.2006.01619.x. [DOI] [PubMed] [Google Scholar]
- 20.Špiljak B, Vilibić M, Glavina A, Crnković M, Šešerko A, Lugović-Mihić L. A Review of psychological stress among students and its assessment using salivary biomarkers. Behav Sci (Basel) 2022;12:400. doi: 10.3390/bs12100400. [DOI] [PMC free article] [PubMed] [Google Scholar]


