Abstract
Study Objectives
There is strong evidence that sleep disturbances are an independent risk factor for the development of chronic pain conditions. The mechanisms underlying this association, however, are still not well understood. We examined the effect of experimental sleep disturbances (ESDs) on three pathways involved in pain initiation/resolution: (1) the central pain-inhibitory pathway, (2) the cyclooxygenase (COX) pathway, and (3) the endocannabinoid (eCB) pathway.
Methods
Twenty-four healthy participants (50% females) underwent two 19-day long in-laboratory protocols in randomized order: (1) an ESD protocol consisting of repeated nights of short and disrupted sleep with intermittent recovery sleep; and (2) a sleep control protocol consisting of nights with an 8-hour sleep opportunity. Pain inhibition (conditioned pain modulation, habituation to repeated pain), COX-2 expression at monocyte level (lipopolysaccharide [LPS]-stimulated and spontaneous), and eCBs (arachidonoylethanolamine, 2-arachidonoylglycerol, docosahexaenoylethanolamide [DHEA], eicosapentaenoylethanolamide, docosatetraenoylethanolamide) were measured every other day throughout the protocol.
Results
The central pain-inhibitory pathway was compromised by sleep disturbances in females, but not in males (p < 0.05 condition × sex effect). The COX-2 pathway (LPS-stimulated) was activated by sleep disturbances (p < 0.05 condition effect), and this effect was exclusively driven by males (p < 0.05 condition × sex effect). With respect to the eCB pathway, DHEA was higher (p < 0.05 condition effect) in the sleep disturbance compared to the control condition, without sex-differential effects on any eCBs.
Conclusions
These findings suggest that central pain-inhibitory and COX mechanisms through which sleep disturbances may contribute to chronic pain risk are sex specific, implicating the need for sex-differential therapeutic targets to effectively reduce chronic pain associated with sleep disturbances in both sexes.
Clinical Trials Registration
NCT02484742: Pain Sensitization and Habituation in a Model of Experimentally-induced Insomnia Symptoms. https://clinicaltrials.gov/ct2/show/NCT02484742.
Keywords: sleep disturbance, pain mechanisms, central pain modulation, cyclooxygenase, endocannabinoids, sex differences
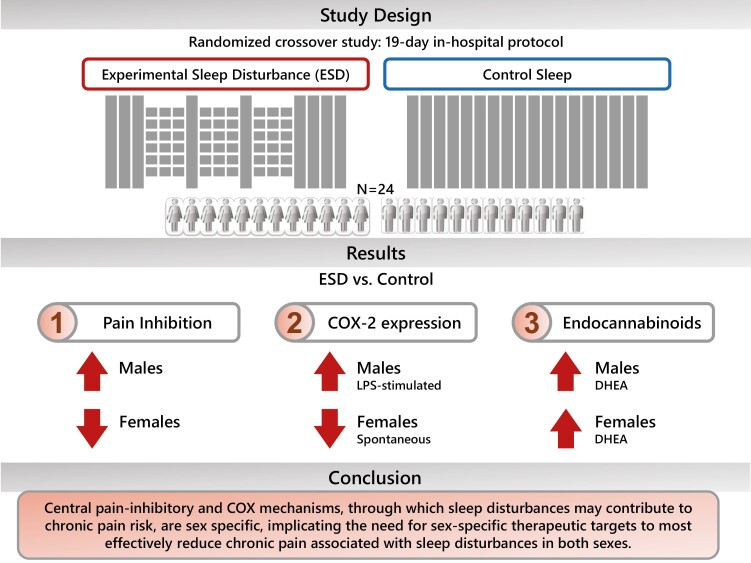
Graphical abstract
Graphical Abstract.
Statement of Significance.
Sleep disturbances are not only highly comorbid with chronic pain conditions, but they are also increasingly recognized as a strong risk factor for the development and nonresolution of chronic pain conditions, such as headache disorders, back pain, or widespread chronic pain. Mechanistic knowledge is urgently needed for the development of novel and targeted therapies. We here show that experimental prolonged sleep disturbances compromise pathways involved in pain initiation/resolution in a sex-dependent manner, with females predominantly responding with a deficit in the central pain-inhibitory pathway, while males predominantly responded with an activation of the inflammatory cyclooxygenase pathway to sleep disturbances. These findings suggest the need of sex-differential therapeutic targets to effectively reduce chronic pain associated with sleep disturbances in both sexes.
Introduction
About 20% of individuals in the United States and worldwide suffer from chronic pain conditions [1], with more than 70% of these individuals also suffering from comorbid sleep disturbances [2, 3]. There is increasing evidence from prospective longitudinal cohort studies showing that sleep disturbances predict increased risk of developing a chronic pain condition [4–8]. These findings highlight the need to identify causal pain pathways by which sleep disturbances contribute to chronic pain development.
Several mechanisms have been suggested by which sleep disturbances may contribute to increased chronic pain risk [9]. This investigation focused on (1) the central pain-modulatory pathways, (2) the inflammatory cyclooxygenase (COX) pathway, and (3) the endocannabinoid (eCB) pathway. The central pain-modulatory pathways (1) consist of descending pathways projecting from various cortical structures to the dorsal horn of the spinal cord, where the transmission of nociceptive information can be modulated (either inhibited or facilitated) [10]. Dysfunctions in these pathways have been observed in various chronic pain conditions and appear to determine whether pain becomes chronic [11, 12]. Pain-inhibitory circuits, in particular, have been found to be compromised in response to experimental sleep restriction or sleep disruption in humans [13–16], suggesting a mechanism by which short or disrupted sleep increase risk for chronic pain over time. Another potential mechanism underlying the contribution of sleep disturbances to pain is the COX pathway (2). COX-2 is the enzyme that converts omega-6 arachidonic fatty acid to prostaglandin (PG) H2, which then is converted to inflammatory PGs, such as PGE2. Inflammatory PGs sensitize peripheral nociceptive neurons and pain transmission neurons in the central nervous system, thereby increasing their responsiveness to nociceptive input [17]. Inhibition of COX enzymes is the main mechanism of action of many nonsteroidal anti-inflammatory drugs (NSAIDs) [18]. To our knowledge, COX-2 has not been assessed in the context of short or disturbed sleep in humans, despite its central role in pain induction and persistence. A third potential mechanistic candidate underlying the pain promoting effect of sleep disturbances is the eCB pathway (3). ECBs, such as the most studied ligands 2-arachidonoylglycerol (2-AG) and arachidonoylethanolamine (anandamide, AEA), are lipid mediators that exert analgesic properties in various diseases associated with chronic pain, but appear to have no analgesic effect in acute pain conditions [19]. ECB system activation through cannabinoid drugs suggests that they alter the affective component of pain (making pain less unpleasant), but not the sensory (nociceptive) component [20]. Investigations of eCBs in the context of short or disrupted sleep are scarce, but those that exist suggest that acute, short-term sleep restriction in healthy individuals activates the eCB pathway, as indicated by increased circulating levels of 2-AG [21, 22]. The effect of prolonged sleep disturbances on eCB lipids is currently unknown.
The goal of the current study was to investigate potential mechanisms through which sleep disturbance contributes to increased pain risk, focusing on (1) the central pain-inhibitory pathway, (2) the COX pathway, and (3) the eCB pathway. We utilized an experimental model mimicking sleep disturbance patterns characterized by short and disrupted sleep. Such patterns are common in individuals with chronic pain conditions [2] and also frequently found in the general population [23]. Given that most pain disorders and insomnia disorder are more common in females [24, 25], we further explored sex differences in the response of these pain pathways to sleep disturbances.
Methods
Participants
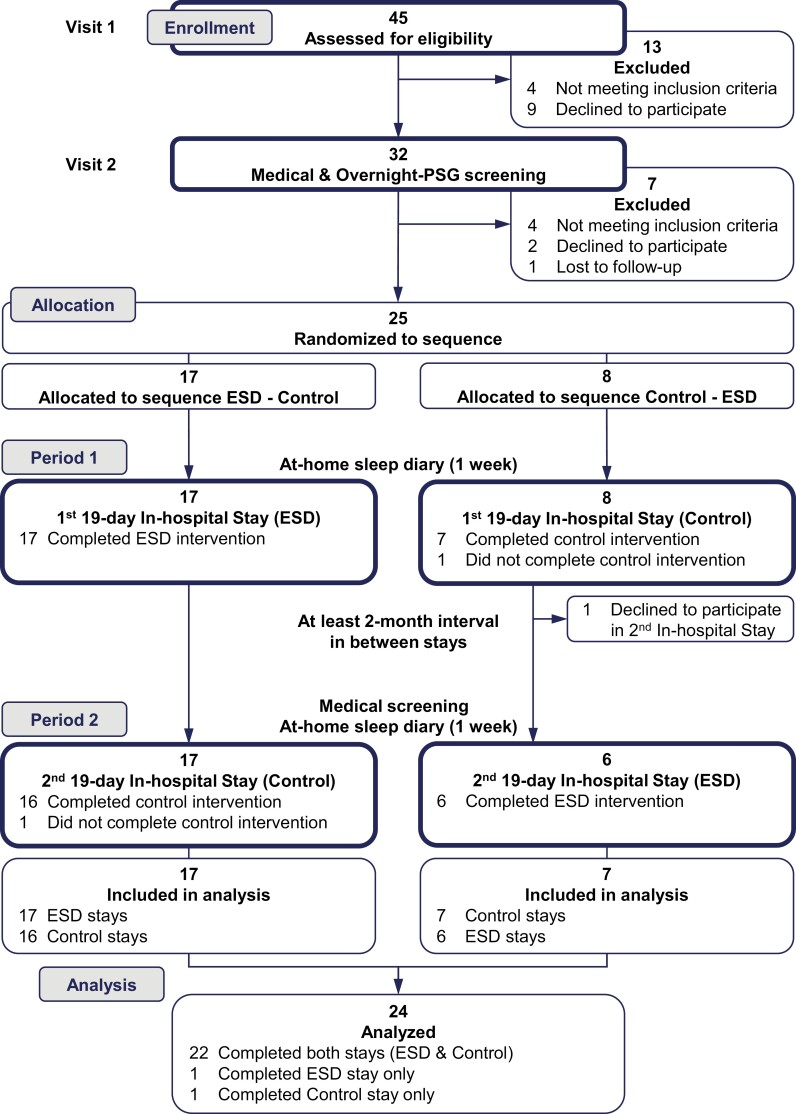
This study was approved by the Institutional Review Board for the Protection of Human Subjects at the Beth Israel Deaconess Medical Center (BIDMC) in Boston, MA, and registered at clinicaltrials.gov (NCT02484742). Participants were recruited via community and website advertisements. Twenty-four healthy young females and males were included in the analysis (see CONSORT flow diagram Figure 1). Twenty-two participants completed the first and second in-hospital protocols (see study protocol below); two participants did not complete the second in-hospital protocol due to work/family requirements or difficulties in following study procedures. With respect to occupational status, 22 of the enrolled participants were freelance or self-employed workers or were able to work remotely, two participants were college students.
Figure 1.
CONSORT flow diagram.
Inclusion criteria were age between 18 and 45 years, a body mass index between 18.5 and 30 kg/m2, habitual nightly sleep duration between 7 and 9 hours (verified by sleep diary data collected over 7 days), habitual time of sleep onset within 1 hour of the study bedtime of 2300 hours (to ensure entrainment), blood chemistry levels within the normal range (including white blood cell and differential blood cell counts, T-cell subsets, thyroid hormones, glucose, insulin, creatinine, liver enzymes, erythrocyte sedimentation rate), and negative urine toxicology. Female participants were eligible if they had regular menstrual cycles and no significant discomfort during premenses/menses. Exclusion criteria included presence or history of medical or psychiatric disorders (determined by diagnostic interview, physician’s medical history, and physical examination), sleep disorders (based on questionnaires and in-hospital polysomnography), pregnant or nursing status, regular medication use other than hormonal contraceptives, NSAID use in the 2 weeks prior to the in-hospital stays, and donation of blood or platelets 3 months prior to (or in-between) in-hospital stays. Blood tests and urine toxicology screening were repeated prior to the second 19-day in-hospital stay to ensure values remained in the normal range.
Study protocol
Model of experimental sleep disturbance
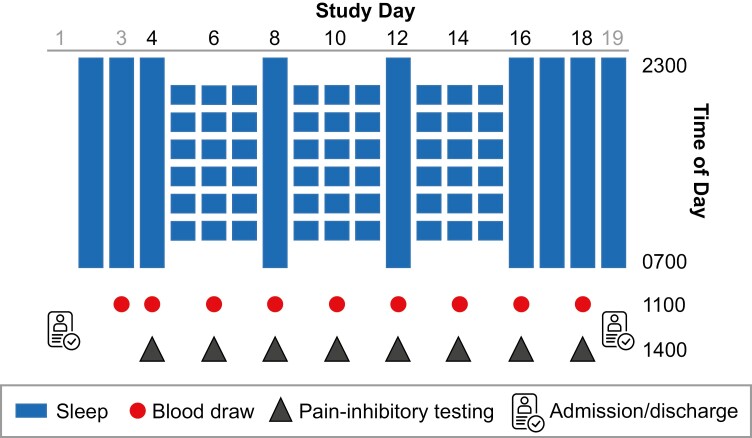
This study employed an intraindividual balanced 2 × 19-day (18 nights) in-hospital protocol where each participant completed the experimental sleep disturbance (ESD) and the control sleep condition. The first two nights of each 19-day in-hospital stay were adaptation nights and the third night served as the baseline night, with a sleep opportunity of 8 hours (2300–0700 hours) on all three nights. In the ESD condition, disturbed sleep was induced during the next three nights, where sleep was both shortened and disrupted resulting in a sleep opportunity of 4 hours. These three nights were followed by one night of recovery sleep with an undisturbed sleep opportunity of 8 hours. This cycle of three nights of disturbed sleep followed by one night of recovery sleep was repeated two more times. This temporal patterning of disturbed with intermittent undisturbed sleep nights was chosen to mimic sleep patterns observed in individuals with sleep disturbances (i.e. after one to three nights of poor sleep, a better night of sleep follows, likely resulting from a buildup of homeostatic sleep pressure during the previous nights of disrupted sleep) [26]. After the third ESD cycle, participants had three additional recovery nights each with an 8-hour sleep opportunity (see Figure 2 for the full ESD protocol). In the control sleep condition, participants received an 8-hour sleep opportunity on all nights of the protocol.
Figure 2.
Study protocol. Depicted is the ESD condition. The sleep control condition consisted of 19 days (18 nights) with a sleep opportunity of 8 hours per night.
During each of the ESD nights, the timing of sleep onset was delayed by 1 hour (from 2300 to 0000 hours). This was followed by a 6-hour interval (between 0000 and 0600 hours) where sleep was interrupted by 20-minute awakenings every hour, resulting in a 4-hour sleep opportunity and 2 hours of induced wake time. Lastly, sleep offset was advanced by 1 hour (from 0700 to 0600 hours). This combination of frequent and prolonged awakenings across the nighttime period together with a delayed sleep onset time and advanced morning awakening time is common in chronic pain disorders.
To implement nighttime awakenings, the research nurse entered the room, turned on the light to less than 20 lux, and woke up the participant by calling their name. During the 20-minute awakenings at night, participants interacted with the attending research assistant while staying in bed in a semi-recumbent position until the next sleep opportunity began.
The two in-hospital conditions were separated by an interval of at least 2 months in order to allow recovery from blood sampling and from potential residual effects related to exposure to ESD. During the 19-day in-hospital stays, participants had 8 days of intensive monitoring (second baseline day, every second sleep disturbance day and every recovery day of each of the three cycles, and the third recovery day at the end of the protocol, see Figure 2). Measurements during the intensive monitoring days included assessment of central pain modulation, blood sampling, and polysomnographic (PSG) recordings. In addition, blood samples for eCB pathway measurements were collected on the first baseline day. The PSG findings have been reported in [27].
Research environment
Throughout both in-hospital stays, participants stayed in a private room at the Clinical Research Center at BIDMC. Ambient room temperature was based on the individually tailored comfort level and kept stable throughout all study days. Participants were maintained on a balanced diet (NA+ and K+ controlled) and regimented fluid intake (no caffeine) in order to prevent changes in body weight/composition throughout the study. Meals and fluids were served at standardized times (0730 hours breakfast, 1230 hours lunch, 1830 hours dinner, 2050 hours snack).
To prevent sedentary conditions and maintain constant activity levels, the attending research assistant took participants on a 10- to 15-minute walk within the Clinical Research Center or outside on hospital property every 2–3 hours throughout the waking periods of the protocol (except during induced wake periods at night). Participants were encouraged to follow their prestudy exercise habits by visiting the hospital gym on the nonintensive monitoring days. Participants could have visitors during daytime periods and had access to internet and phone, in order to minimize disruptions to their social networks.
Blood collection
Blood was collected by the study nurse at the 1100 hours time point on each of the intensive monitoring days and used to measure COX and eCB pathway activation. Participants refrained from food and fluid intake for 60 minutes, and remained in a seated position for 15 minutes, prior to blood collection. Blood was taken through direct venipuncture on the arm not used for pain testing procedures. Sodium heparinized whole blood was immediately processed for cell stimulation to investigate COX expression and extracted EDTA plasma was stored at −80°C to assess eCBs.
Assessment of pain pathways
Central pain-inhibitory pathway.
Two testing paradigms were used:
(a) Conditioned pain modulation (CPM)
CPM is based on the “pain-inhibits-pain” model, in which a painful conditioning stimulus exerts an analgesic effect on a painful test stimulus applied to another body site. For this study, the noxious conditioning stimulus was the immersion of the foot in hot water and the test stimulus was a sequence of 10 heat pulses applied to the contralateral arm and leg, respectively, with an inter-pulse interval was 2.5 seconds and a pulse duration of 0.5 seconds. Heat pulses were delivered using a thermode attached to the volar forearm (TSA-II NeuroSensory Analyzer, Medoc, Minneapolis, MS). The temperature of the heat pulses was individually tailored by determining the maximal tolerable temperature for each participant (for details, see [28]). The maximal tolerable temperature was then reduced by 1.5°C, in order to prevent participants needing to end the CPM testing protocol before completion because of a potential pain sensitization effect following sleep disturbance. The conditioning stimulus consisted of the immersion of the contralateral foot into a hot water bath (Techne water baths, Bibby Scientific US, Burlington, NJ). The temperature of the water bath was maintained at 47°C with a clip-on Tempette thermoregulator (TE-10D, Bibby Scientific US, Burlington, NJ). Shortly before foot immersion, the thermoregulator was removed from the water to comply with hospital safety regulations. Potential changes of water temperature during foot immersion was recorded with a traceable thermometer (Control Company, Friendswood, TX) attached to the water bath. After 20 seconds of foot immersion, the test stimulus (heat-pulse sequence) was applied to the forearm or leg, respectively, and the participant was prompted to rate the pain intensity of the 1st, 4th, 7th, and 10th heat pulse using visual analog scales. The heat-pulse sequence was further applied in presence of a neutral stimulus (i.e. foot wrapped in towel), serving as a control condition. Four CPM trials were conducted in total (i.e. two on the forearm with random exposure to the conditioning and neutral stimulus, respectively, followed by two trials on the leg, again with exposure to the conditioning and neutral stimulus in random order). There was a 10-minute long rest period between each of the four trials, following CPM practice recommendations [29]. During the rest periods, the thermode delivering the heat pulses was systematically moved from the distal to proximal sites along the C8-T1 dermatomes of the forearm, and along L5-S1 for the leg. The CPM magnitude was calculated as the pain intensity ratings of the test stimulus (heat-pulse sequence) in the presence of the conditioning stimulus (painfully hot water) minus the pain ratings of the test stimulus in the presence of the neutral stimulus (foot wrapped in towel). Area under the curve for the pain intensity ratings of the heat pulses was calculated to reflect CPM magnitude. Lower values indicated more efficient pain inhibition.
(b) Habituation to repeated pain (HRP)
The ability to habituate to the repeated exposure to a painful (nonthreatening) stimulus reflects the efficiency of pain-inhibitory pathways [30]. HRP in this protocol was assessed using the repeated administration of the cold pressor test on the intensive recording days. For each HRP test, participants were asked to insert their hand in a temperature-controlled water bath (Techne water baths, Bibby Scientific US, Burlington, NJ), kept at 3°C, and instructed to leave their hand immersed in the cold water bath until the pain became intolerable. Participants rated the intensity of the pain prior to hand immersion into the cold water bath, and at time of hand removal, using visual analog scales. The degree of HRP was calculated as the change in pain tolerance (seconds) across the repeated cold pain exposure across the intensive recording days of the study. The greater the increase in pain tolerance upon repeated noxious cold stimulation, the higher the ability to habituate to pain, indicating more efficient pain inhibition.
(2) COX pathway
Intracellular COX-2 expression in monocytes was investigated in lipopolysaccharide (LPS)-stimulated and unstimulated blood samples drawn at 1100 hours on the intensive monitoring days. Whole blood was stimulated with LPS from Escherichia coli O127:B8 (100 pg/mL, L3137, Sigma-Aldrich) or left unstimulated, brefeldin A (10 µg/mL, B5936, Sigma-Aldrich) was added, and the samples were incubated for 4 hours at 37°C in a 5% CO2 atmosphere. Following fixation and permeabilization (IntraPrep Permeabilization Reagent, A07803, Beckman Coulter), fluorescence-conjugated antibodies were added (CD45 KrO, CD14 APC [both Beckman Coulter], and COX-1 FITC/COX-2 PE antibody cocktail [BD Biosciences]), and the samples were incubated for 15 minutes at room temperature in the dark. Then, the samples were washed with phosphate-buffered saline solution (PBS 1X, Sigma Aldrich), resuspended in PBS containing 0.5% formaldehyde and stored at 4°C in the dark until flow cytometric measurement (Gallios, Beckman Coulter, Flow Cytometry Core at BIDMC). Preparations were analyzed within 24 hours and 100 000 events were acquired per sample. Percentage of COX-2-positive monocytes (LPS-stimulated and unstimulated) was quantified using Kaluza Analysis Software (Beckman Coulter).
(3) eCB pathway
The following eCBs were measured in plasma of the 1100 hours blood draw on four of the intensive monitoring days, day 3 (first baseline), day 14 (last sleep disturbance cycle), days 16 and 18 (after first and third night of recovery sleep): The omega-6 derivatives AEA (anandamide), 2-AG, and docosatetraenoylethanolamide (DTEA), and the omega-3 derivatives docosahexaenoylethanolamide (DHEA) and eicosapentaenoylethanolamide (EPEA). Values were assessed by the contract research company Ambiotis SAS, France, using a liquid chromatography–mass spectrometry (LC–MS/MS) platform (for method details see [31]). For 2-AG, 12.2% of values were below the limit of quantification (LOQ) but above the limit of detection (LOD) and were included in statistical analyses; 13.3% of values were below the LOD and were set to half the LOD.
Statistical analysis
The main analysis employed generalized linear mixed models (GLMM, SPSS 28) with condition (ESD vs. control sleep [CONTROL]), study day (representing the intensive monitoring days), and sex (females vs. males) as well as their interactions as fixed factors. Participant’s ID was included as random factor. To control for baseline differences at the beginning of the protocol between conditions, all GLMM analyses were performed on delta values (i.e. values in response to sleep disturbances or control sleep minus baseline). This approach was preferred to the approach of using baseline as a covariate because of potential differences not only in the factor condition but also in the factor sex. Satterthwaite’s approximation was used to calculate denominator degrees of freedom. Robust estimation was used in order to protect against potential violations of model assumptions, i.e. homogeneity and normal distribution. Homogeneity of variances and normality were assessed by plotting residuals against predicted values as well as plotting histograms of residuals, respectively. Significant interaction effects with study day were considered appropriate for follow-up with pairwise comparisons at single days. Data are presented as estimated marginal means (EMMs) ± standard errors (SEMs). The level of significance was set to an alpha value of rejection of p < 0.05 for main and interaction effects. Power was estimated in the planning phase of this study and aimed at detecting differences between groups of ESD and CONTROL with 80% power using an alpha value of p < 0.05. The study was not powered for the detection of sex differences.
Results
Participant characteristics are shown in Table 1.
Table 1.
Participant characteristics at baseline (day 1 of stay 1)
| All* (N = 24) |
Female (n = 12) |
Male (n = 12) |
|
|---|---|---|---|
| Age (years, mean ± SEM) | 28.0 ± 1.2 | 26.7 ± 1.4 | 29.4 ± 1.9 |
| BMI (kg/m2, mean ± SEM) | 23.9 ± 0.7 | 24.2 ± 1.2 | 23.5 ± 0.8 |
| Follicular/luteal menstrual cycle phase at study day 1 of conditions ESD and control sleep† | ESD: 4/8 Control: 4/7 |
||
| Hormonal contraceptive use | 2 | ||
| Race (N) | |||
| Black/African American | 10 | 5 | 5 |
| White | 10 | 5 | 5 |
| Asian | 1 | 0 | 1 |
| Multiracial‡ | 1 | 1 | 0 |
| Other§ | 1 | 1 | 0 |
| Not provided | 1 | 0 | 1 |
| Ethnicity (N) | |||
| Hispanic | 6 | 3 | 3 |
| Non-Hispanic | 14 | 8 | 6 |
| Not provided | 4 | 1 | 3 |
BMI, body mass index.
*Twenty-two participants completed both conditions (ESD condition and control sleep condition), 2 participants completed 1 stay only.
†Two females did not have regular menstrual cycles; one due to an intrauterine device.
‡Reported as “mixed Black and Alaska Native.”
§Reported as “Peruvian.”
Pain pathways
(1) Central pain-inhibitory pathway
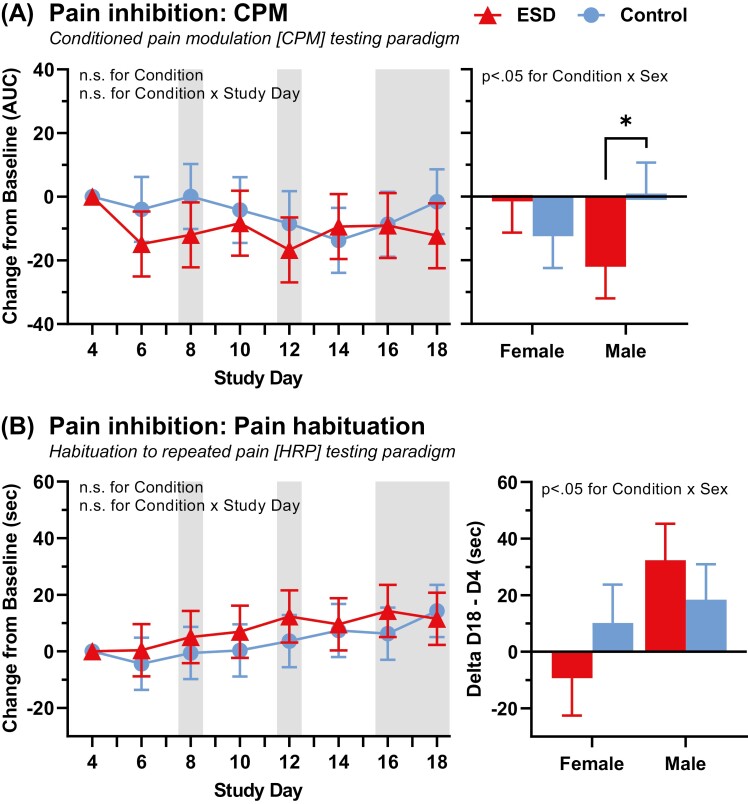
CPM responses were averaged across the two body testing sites (forearm and leg), since there was no main or interaction effect for testing site (leg vs. arm) with condition (p > 0.05). CPM did not differ between the ESD and control sleep condition in the combined group of females and males (p > 0.05 for condition and condition × day effect, respectively, Figure 3, A). There was a sex-differential effect (p < 0.05 for condition × sex effect), indicating an impairment in CPM (i.e. less pain-inhibitory capacity) in the ESD condition in females and an improvement of CPM in the ESD condition in males when compared to the sleep control condition.
Figure 3.
Change in pain-inhibitory pathway in response to ESD and modulation by sex. (A) CPM testing paradigm (N = 24). Lower values indicate better pain-inhibitory capacity. (B) HRP testing paradigm (N = 23). Greater pain tolerance indicates better habituation, thus greater pain-inhibitory capacity. Presented are EMM ± SEM from GLMM analysis performed on delta values (values minus baseline). Study day 4 is the baseline day. Gray shaded areas depict sleep recovery periods. AUC, area under the curve; sec, seconds. *p < 0.05 for pairwise comparison between conditions.
HRP is depicted in Figure 3, B. As expected, participants habituated to cold pain across testing days in the control sleep condition, as indicated by an increase of cold pain tolerance by 14.2 ± 8.7 seconds from baseline to day 18. The increase in cold pain tolerance in the ESD condition from baseline to day 18 was 11.5 ± 8.6 seconds, and did not differ from the control condition in the combined group of females and males (p > 0.05 for condition × study day effect). There was a sex-differential effect (condition × sex interaction (p < 0.05), indicating that females were less able to habituate to pain in the ESD condition while males showed the opposite effect and were more able to habituate to pain in the ESD compared to the sleep control condition. This indicates greater pain-inhibitory capacity in males and impaired pain-inhibitory capacity in females when exposed to sleep disturbance.
(2) COX pathway
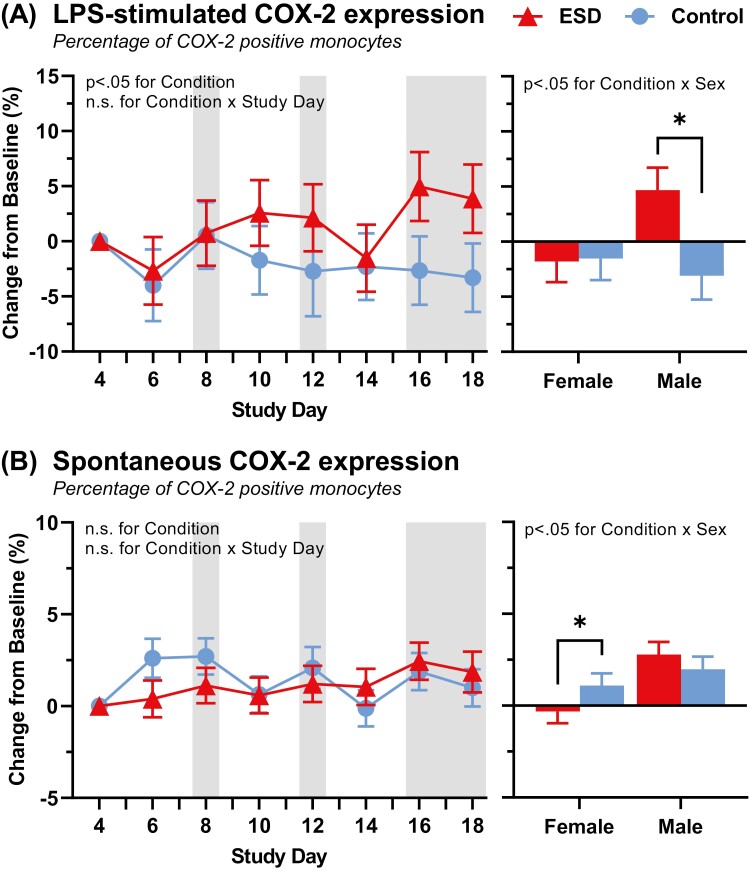
LPS-stimulated COX-2 expression in monocytes was higher in the ESD compared to the sleep control condition (p < 0.05 for condition × study day effect, Figure 4, A). The condition × sex effect (p < 0.05) indicated that the LPS-stimulated COX-2 expression was higher in males in the ESD compared to the sleep control condition, while in females, there was no difference between conditions.
Figure 4.
Change in COX-2 pathway in response to ESD and modulation by sex. (A) COX-2 expression in LPS-stimulated monocytes. (B) Spontaneous COX-2 expression in unstimulated monocytes. COX-2 expression in monocytes is defined as the portion of COX-2-positive monocytes among all monocytes. Presented are EMM ± SEM from GLMM analysis performed on delta values (values minus baseline). Study day 4 is the baseline day. Gray shaded areas depict sleep recovery periods. N = 24, *p < 0.05 for pairwise comparisons between conditions.
Spontaneous COX-2 expression in monocytes did not differ between the ESD and the sleep control condition (p > 0.05 for condition effect, Figure 4, B). The condition × sex effect (p < 0.05) indicated that males expressed slightly more and females less spontaneous COX-2 in the ESD compared to the sleep control condition.
(3) eCB pathway
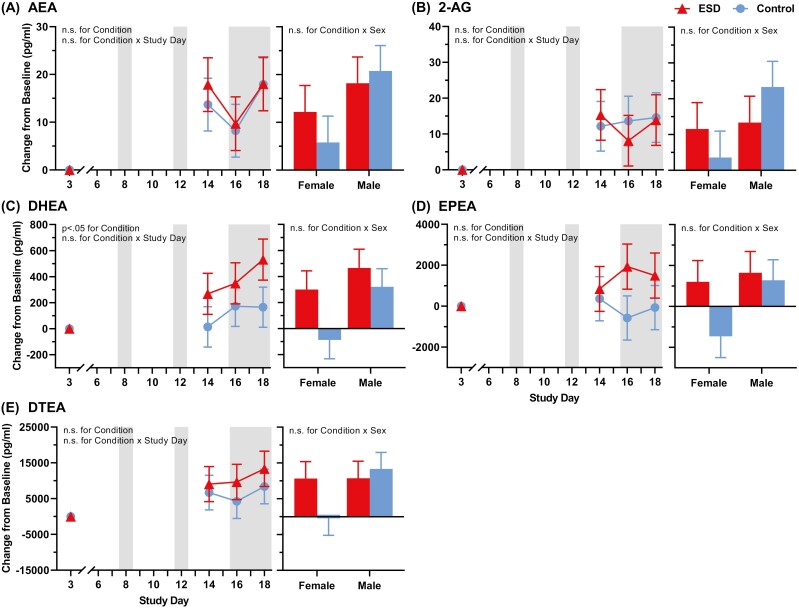
AEA, 2-AG, and DTEA did not differ between the ESD and sleep control condition (Figure 5, A, B, and E). DHEA was higher in the ESD compared to the sleep control condition (p< 0.05 for condition effect, Figure 5, C), and EPEA was higher in the ESD compared to the sleep control condition (p < 0.07 for condition effect, Figure 5, D). For both DHEA and EPEA, elevations continued into the sleep recovery period at the end of the protocol. None of the eCBs showed a sex-differential effect (all p > 0.05 condition × sex effect).
Figure 5.
Change in eCB pathway in response to ESD and modulation by sex. (A) AEA (anandamide), (B) 2-AG, (C) DHEA, (D) EPEA, and (E) DTEA. Presented are EMM ± SEM from GLMM analysis performed on delta values (values minus baseline). Study day 3 is the baseline day. Gray shaded areas depict sleep recovery periods. N = 24.
Discussion
This study examined the effects of prolonged experimental sleep disturbance on three pathways that are known to play a role in pain initiation and resolution: (1) the central pain-inhibitory pathway, (2) the COX pathway, and (3) the eCB pathway. Overall, the central pain-inhibitory pathway was not affected by experimental sleep disturbances in the combined group of females and males; the COX-2 pathway was activated as indicated by higher LPS-stimulated intracellular expression of COX-2 in monocytes in response to experimental sleep disturbances; and the eCB pathway showed activation of DHEA by sleep disturbances, without significant changes in the eCBs AEA, 2-AG, EPEA, and DTEA. The most striking finding of this investigation was that experimental sleep disturbances affected the central pain-inhibitory and the COX pathway in a sex-dependent manner. In females, a weakening of the pain-inhibitory pathway of the central nervous system in response to sleep disturbances was indicated by a decrease in the capacity to inhibit pain. Males showed the opposite effect, specifically, they demonstrated a stronger pain-inhibitory response to sleep disturbances. With respect to the inflammatory COX pathway, males showed a stronger stimulated COX-2 response to sleep disturbances, while females showed a reduced unstimulated COX-2 response. Sex differences were not observed for the eCB pathway. These findings provide preliminary evidence that sleep disturbances alter the central pain-inhibitory and the COX pathway is a sex-specific manner. If the sexual dimorphic alterations in pain pathways that were observed in the current study are replicated in larger studies, it would suggest the need for sex-specific therapeutics to effectively and equally prevent or alleviate pain.
Alterations of pain pathways across the repeated exposure to sleep disturbance with intermittent recovery sleep
The current study is among the first to model prolonged exposure to sleep disturbances in the experimental setting. This was accomplished by mimicking frequent and prolonged awakenings throughout the sleep period in combination with delayed sleep onset times and advanced awakening times in the morning, as are common in individuals with chronic pain conditions. Such prolonged exposure to experimental sleep disruption helps us better understand to which degree biological systems adapt to periods of disrupted sleep, and to which degree they can recover during intermittent good sleep [32]. Overall, we did not observe adaptive responses for any of the pain-inhibitory, COX, or eCB measures in the form of a progressive response decrease or increase over the course of the sleep disturbance challenge. We further did not observe complete normalization of some of the measures following recovery sleep. For instance, the eCB DHEA continued to be enhanced in the sleep disturbance condition after three nights of recovery sleep. With respect to the LPS-stimulated COX-2 expression by monocytes, values appear to increase during the final recovery period at end of protocol. These findings suggest a delay in the impact of sleep disturbance on some pathways. Incomplete normalization of immune responses following recovery from sleep loss has similarly been observed in limited human studies [33–35]. In animals, extended sleep loss or fragmentation affects select populations of neurons and glia cells that do not readily reverse even with extended recovery sleep [36]. These long lasting effects of sleep disturbance further emphasize the importance of adequate sleep in maintaining optimal physiological functioning and, consequently, health.
The central pain-inhibitory pathway in the current study was assessed by using two testing paradigms, the CPM test and the HRP test. The impairment of descending inhibitory modulation can contribute to the manifestation of chronic pain states and can affect treatment outcomes. For example, in patients with a systemic inflammatory condition, such as rheumatoid arthritis, drug treatment responses are poor if central pain inhibition, as assessed with the CPM test, is impaired [37–39]. The current findings show that experimental sleep disturbance did not affect pain inhibition in the combined group of females and males. However, for both testing paradigms (CPM and HRP), a sex-differential effect was observed, indicating impaired pain inhibition in females and improved pain inhibition in males in response to sleep disturbances. A few other studies have suggested a sex-differential effect of sleep disturbances on pain inhibition. Two nights of experimental sleep disturbance in females have been reported to reduce CPM [15], as did a single night of total sleep deprivation in females, but not in males [40]. With respect to more chronic naturally occurring forms of sleep disturbance, we previously reported a profound deficiency in the capacity to inhibit pain using the same CPM test in a sample consisting mostly of females with insomnia disorder, compared to healthy sleepers [28]. A weaker CPM effect has been reported in a mixed-sex sample following a night of total sleep deprivation in healthy participants [41]. Interestingly, a stronger, rather than weaker, inhibitory CPM effect has been also found in a mixed-sex sample of healthy participants following two nights of sleep restriction [42], which is consistent with the stronger inhibitory CPM effect observed in the current study in males only. However, analysis was not stratified by sex in this study. Similarly, HRP using ischemia-induced deep muscle pain has been found reduced in females in response to two nights of experimental sleep disturbance, suggesting less efficient pain inhibition (males were not studied [13]). With respect to the effect of recovery sleep on the pain-inhibitory pathway, the current results show that neither intermittent nor extended recovery sleep at the end of the protocol improved pain-inhibitory efficacy in females, suggesting that the impact of sleep disturbance on this pathway is longer lasting. Similarly, the study by Simpson et al. suggested a longer lasting effect of prolonged sleep restriction on HRP, as indicated by the persistence of poor HRP following several nights of full sleep [14]. Deficits in HRP have been reported in clinical pain populations, including disorders of migraine, low back pain, or fibromyalgia (reviewed in [43]), and have been found to be the best predictor of neuropathic pain emergence in patients with spinal cord injury [44]. To conclude, the current findings of a sex-differential effect on central pain-inhibitory pathways suggest that these pathways may play a mechanistic role in the association between sleep disturbance and pain in females but not in males.
COX pathway
To our knowledge, this is the first study investigating the COX pathway in response to sleep disturbance in humans. Inhibition of COX enzymes is the mechanism of action of NSAIDs used in the treatment of acute and chronic pain [45]. We found that COX-2 expression by monocytes following in vitro stimulation with LPS was overall greater when exposed to sleep disturbances compared to control sleep. This effect was sex specific, such that only males showed higher stimulated COX-2 expression. This finding suggests that in the context of a LPS-challenge, sleep disturbance may contribute to pain and inflammation by activating the COX-2 pathway in males, but not in females. This may mean that sleep disturbance potentially contributes to more severe manifestations of acute infections in males, an effect that has been reported, for instance, for the infection with SARS-CoV-2 [46]. In contrast to LPS-stimulated COX-2 expression, unstimulated (i.e. in the absence of a pathogenic challenge) COX-2 expression in monocytes was lower in females and slightly higher in males in response to sleep disturbances compared to control sleep. The observed suppression of spontaneous COX-2 expression during the ESD protocol in females could be explained by increased activation of the major stress system, manifested by elevated morning cortisol levels in these female participants, as reported before [27]. Cortisol is known to suppress COX-2 expression in monocytes [47] and cortisol levels have been found to be higher in females compared to males in response to sleep disturbance [27], which is fitting with the observed stronger suppression of spontaneous COX-2 in females compared to males. A similar sex-differential effect of the inflammatory response to experimental sleep disturbances was observed for the stimulated production of IL-6 [27], which corroborates sex differences observed here for the COX-2 production. To conclude, the current findings suggest that activation of the COX-2 pathway may underlie the association between sleep disturbances and pain, and this effect appears to be specific to males.
ECB pathway
Two out of the five assessed eCBs, DHEA and EPEA, showed an increase during the last cycle of sleep disturbance when compared to control sleep, which was significant for DHEA. These two eCBs showed a continued elevation after initiation of recovery sleep, suggesting a longer lasting effect of sleep disturbances. Studies on acute sleep restriction over three nights reported higher peak afternoon concentrations of 2-AG [22] and higher 2-AG levels in the morning [21], contrasting with the current study that did not find an effect of sleep disturbances on 2-AG when measured in the late morning. ECBs act as anti-inflammatory and analgesic lipid mediators [48], and exogenous CBs have received attention in the treatment of sleep disturbances and in particular in the treatment of pain. In light of the current findings, the assessed eCBs may not be involved in the pain promoting effects of sleep disturbances, given that they do not change or are increased following sleep disturbances. However, they may be responsible for other physiological changes following sleep disturbances, such as changes in appetite [49]. In addition, other eCBs not assessed here may play a potential role in the association between sleep and pain.
Limitations
Although our study provides a basis for further investigation into how the mechanisms linking sleep disturbances and chronic pain risk may differ between females and males, it is important to note that this study was statistically powered for detecting a difference between the sleep disturbance and sleep control condition, but not for the detection of sex-differential effects. The current findings on sex-differential effects are therefore preliminary and need replication in adequately powered studies. Such larger studies will also allow the investigation of menstrual cycle influences on sleep and pain regulation, which could not be performed in the current study due to the small sample size of female and male participants.
Conclusions
In summary, experimental sleep disturbances with intermittent recovery sleep over a period of more than 2 weeks affect pain pathways in a sex-dependent manner. Specifically, an impairment in the central pain-inhibitory pathway was observed only in females, while an activation of the inflammatory COX pathway was observed only in males. If follow-up investigations replicate the current findings in larger samples, females and males likely need different therapeutic approaches to prevent pain development and resolve pain associated with sleep disturbance. Given that treatments for sleep disturbances are often associated with only moderate clinical sleep improvements [50], there is a critical need to address pain associated with sleep disturbances from alternate mechanistic pathways.
Contributor Information
Monika Haack, Harvard Medical School, Boston, MA, USA; Department of Neurology, Beth Israel Deaconess Medical Center, Boston, MA, USA.
Larissa C Engert, Harvard Medical School, Boston, MA, USA; Department of Neurology, Beth Israel Deaconess Medical Center, Boston, MA, USA.
Luciana Besedovsky, Harvard Medical School, Boston, MA, USA; Institute of Medical Psychology, Ludwig-Maximilians-Universität München, Munich, Germany.
Michael R Goldstein, Harvard Medical School, Boston, MA, USA.
Jaime K Devine, Institutes for Behavior Resources, Inc., Baltimore, MD, USA.
Rammy Dang, Department of Neurology, Beth Israel Deaconess Medical Center, Boston, MA, USA.
Keeyon Olia, Department of Neurology, Beth Israel Deaconess Medical Center, Boston, MA, USA.
Victoria Molina, Department of Neurology, Beth Israel Deaconess Medical Center, Boston, MA, USA.
Suzanne M Bertisch, Harvard Medical School, Boston, MA, USA; Division of Sleep and Circadian Disorders, Department of Medicine, Brigham and Women’s Hospital, Boston, MA, USA.
Navil Sethna, Harvard Medical School, Boston, MA, USA; Department of Anesthesia and Perioperative Medicine, Children’s Hospital Boston, Boston, MA, USA.
Norah Simpson, Stanford Sleep Heath & Insomnia Program, Department of Psychiatry and Behavioral Sciences, Stanford University School of Medicine, Palo Alto, CA, USA.
Funding
This work was supported by grants NIH/NINDS R01 NS091177 to MH, DFG EN1291/1-1 to LCE, DFG BE6319/1-1 to LB, 5T32HL007901-22 to MRG, NIH/NCRR UL1 RR02758 and M01-RR-01032 to the Harvard Clinical and Translational Science Center.
Disclosure Statement
None declared.
References
- 1. Dahlhamer J, et al. Prevalence of chronic pain and high-impact chronic pain among adults—United States, 2016. MMWR Morb Mortal Wkly Rep. 2018;67(36):1001–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mathias JL, et al. Sleep disturbances and sleep disorders in adults living with chronic pain: a meta-analysis. Sleep Med. 2018;52:198–210. [DOI] [PubMed] [Google Scholar]
- 3. Sun Y, et al. Prevalence of sleep disturbances in patients with chronic non-cancer pain: a systematic review and meta-analysis. Sleep Med Rev. 2021;57:101467. [DOI] [PubMed] [Google Scholar]
- 4. Afolalu EF, et al. Effects of sleep changes on pain-related health outcomes in the general population: a systematic review of longitudinal studies with exploratory meta-analysis. Sleep Med Rev. 2018;39:82–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bonvanie IJ, et al. Sleep problems and pain: a longitudinal cohort study in emerging adults. Pain. 2016;157(4):957–963. [DOI] [PubMed] [Google Scholar]
- 6. Chen T-Y, et al. Longitudinal relationship between sleep deficiency and pain symptoms among community-dwelling older adults in Japan and Singapore. Sleep. 2019;42(2). doi: 10.1093/sleep/zsy219 [DOI] [PubMed] [Google Scholar]
- 7. Kok VC, et al. Risk of autoimmune disease in adults with chronic insomnia requiring sleep-inducing pills: a population-based longitudinal study. J Gen Intern Med. 2016;31(9):1019–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sivertsen B, et al. Insomnia as a risk factor for ill health: results from the large population-based prospective HUNT Study in Norway. J Sleep Res. 2014;23(2):124–132. [DOI] [PubMed] [Google Scholar]
- 9. Haack M, et al. Sleep deficiency and chronic pain: potential underlying mechanisms and clinical implications. Neuropsychopharmacology. 2020;45(1):205–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ossipov MH, et al. Central modulation of pain. J Clin Invest. 2010;120(11):3779–3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ossipov MH, et al. Descending pain modulation and chronification of pain. Curr Opin Support Palliat Care. 2014;8(2):143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vaegter HB, et al. Preoperative hypoalgesia after cold pressor test and aerobic exercise is associated with pain relief 6 months after total knee replacement. Clin J Pain. 2017;33(6):475–484. [DOI] [PubMed] [Google Scholar]
- 13. Iacovides S, et al. Sleep fragmentation hypersensitizes healthy young women to deep and superficial experimental pain. J Orofac Pain. 2017;18(7):844–854. [DOI] [PubMed] [Google Scholar]
- 14. Simpson NS, et al. Chronic exposure to insufficient sleep alters processes of pain habituation and sensitization. Pain. 2018;159(1):33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Smith MT, et al. The effects of sleep deprivation on pain inhibition and spontaneous pain in women. Sleep. 2007;30(4):494–505. doi: 10.1093/sleep/30.4.494 [DOI] [PubMed] [Google Scholar]
- 16. Smith MT, et al. Sex differences in measures of central sensitization and pain sensitivity to experimental sleep disruption: implications for sex differences in chronic pain. Sleep. 2019;42(2). doi: 10.1093/sleep/zsy209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pinho-Ribeiro FA, et al. Nociceptor sensory neuron-immune interactions in pain and inflammation. Trends Immunol. 2017;38(1):5–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Burian M, et al. COX-dependent mechanisms involved in the antinociceptive action of NSAIDS at central and peripheral sites. Pharmacol Ther. 2005;107(2):139–154. [DOI] [PubMed] [Google Scholar]
- 19. Donvito G, et al. The endogenous cannabinoid system: a budding source of targets for treating inflammatory and neuropathic pain. Neuropsychopharmacology. 2018;43(1):52–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. De Vita MJ, et al. Association of cannabinoid administration with experimental pain in healthy adults: a systematic review and meta-analysis. JAMA Psychiatry. 2018;75(11):1118–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cedernaes J, et al. Sleep restriction alters plasma endocannabinoids concentrations before but not after exercise in humans. Psychoneuroendocrinology. 2016;74:258–268. [DOI] [PubMed] [Google Scholar]
- 22. Hanlon EC, et al. Sleep restriction enhances the daily rhythm of circulating levels of endocannabinoid 2-arachidonoylglycerol. Sleep. 2016;39(3):653–664. doi: 10.5665/sleep.5546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zeng L-N, et al. Gender difference in the prevalence of insomnia: a meta-analysis of observational studies. Front Psychiatry. 2020;11:577429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mogil JS. Sex differences in pain and pain inhibition: multiple explanations of a controversial phenomenon. Nat Rev Neurosci. 2012;13(12):859–866. [DOI] [PubMed] [Google Scholar]
- 25. Pengo MF, et al. Sleep in women across the life span. Chest. 2018;154(1):196–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Perlis ML, et al. The incidence and temporal patterning of insomnia: a pilot study. J Sleep Res. 2010;19(1):31–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Besedovsky L, et al. Differential effects of an experimental model of prolonged sleep disturbance on inflammation in healthy females and males. PNAS Nexus. 2022;1(1):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Haack M, et al. Pain sensitivity and modulation in primary insomnia. Eur J Pain. 2012;16(4):522–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yarnitsky D. Conditioned pain modulation (the diffuse noxious inhibitory control-like effect): its relevance for acute and chronic pain states. Curr Opin Anesthesiol. 2010;23(5):611–615. [DOI] [PubMed] [Google Scholar]
- 30. Bingel U, et al. Habituation to painful stimulation involves the antinociceptive system. Pain. 2007;131(1–2):21–30. [DOI] [PubMed] [Google Scholar]
- 31. Le Faouder P, et al. LC-MS/MS method for rapid and concomitant quantification of pro-inflammatory and pro-resolving polyunsaturated fatty acid metabolites. J Chromatogr B Analyt Technol Biomed Life Sci. 2013;932:123–133. [DOI] [PubMed] [Google Scholar]
- 32. Grissom N, et al. Habituation to repeated stress: get used to it. Neurobiol Learn Mem. 2009;92(2):215–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lasselin J, et al. Effect of long-term sleep restriction and subsequent recovery sleep on the diurnal rhythms of white blood cell subpopulations. Brain Behav Immun. 2015;47:93–99. [DOI] [PubMed] [Google Scholar]
- 34. Simpson NS, et al. Repeating patterns of sleep restriction and recovery: do we get used to it? Brain Behav Immun. 2016;58:142–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. van Leeuwen WMA, et al. Sleep restriction increases the risk of developing cardiovascular diseases by augmenting proinflammatory responses through IL-17 and CRP. PLoS One. 2009;4(2):e4589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Owen JE, et al. Impact of sleep disturbances on neurodegeneration: insight from studies in animal models. Neurobiol Dis. 2020;139:104820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Edwards RR, et al. Variability in conditioned pain modulation predicts response to NSAID treatment in patients with knee osteoarthritis. BMC Musculoskelet Disord. 2016;17:284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Heisler AC, et al. Association of dysregulated central pain processing and response to disease-modifying antirheumatic drug therapy in rheumatoid arthritis. Arthritis Rheumatol. 2020;72:2017–2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Petersen KK, et al. Pain inhibitory mechanisms and response to weak analgesics in patients with knee osteoarthritis. Eur J Pain. 2019;23(10):1904–1912. [DOI] [PubMed] [Google Scholar]
- 40. Eichhorn N, et al. The role of sex in sleep deprivation related changes of nociception and conditioned pain modulation. Neuroscience. 2018;387:191–200. [DOI] [PubMed] [Google Scholar]
- 41. Staffe AT, et al. Total sleep deprivation increases pain sensitivity, impairs conditioned pain modulation and facilitates temporal summation of pain in healthy participants. PLoS One. 2019;14(12):e0225849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Matre D, et al. Conditioned pain modulation is not decreased after partial sleep restriction. Eur J Pain. 2016;20(3):408–416. [DOI] [PubMed] [Google Scholar]
- 43. De Paepe AL, et al. Habituation to pain: a motivational-ethological perspective. Pain. 2019;160(8):1693–1697. [DOI] [PubMed] [Google Scholar]
- 44. Gruener H, et al. Biomarkers for predicting central neuropathic pain occurrence and severity after spinal cord injury: results of a long-term longitudinal study. Pain. 2020;161(3):545–556. [DOI] [PubMed] [Google Scholar]
- 45. FitzGerald GA. COX-2 and beyond: approaches to prostaglandin inhibition in human disease. Nat Rev Drug Discov. 2003;2(11):879–890. [DOI] [PubMed] [Google Scholar]
- 46. Bunders MJ, et al. Implications of sex differences in immunity for SARS-CoV-2 pathogenesis and design of therapeutic interventions. Immunity. 2020;53(3):487–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Santini G, et al. The human pharmacology of monocyte cyclooxygenase 2 inhibition by cortisol and synthetic glucocorticoids. Clin Pharmacol Ther. 2001;70(5):475–483. [DOI] [PubMed] [Google Scholar]
- 48. McDougle DR, et al. Anti-inflammatory ω-3 endocannabinoid epoxides. Proc Natl Acad Sci U S A. 2017;114(30):E6034–E6043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zhu B, et al. Effects of sleep restriction on metabolism-related parameters in healthy adults: a comprehensive review and meta-analysis of randomized controlled trials. Sleep Med Rev. 2019;45:18–30. [DOI] [PubMed] [Google Scholar]
- 50. van Someren EJW. Brain mechanisms of insomnia: new perspectives on causes and consequences. Physiol Rev. 2021;101(3):995–1046. [DOI] [PubMed] [Google Scholar]