Abstract

The continuing emergence of antibiotic-resistant microbes highlights the need for the identification of new chemotypes with antimicrobial activity. One of the most prolific sources of antimicrobial molecules has been the systematic screening of natural product samples. The National Institute of Allergy and Infectious Diseases and the National Cancer Institute here report a large screen of 326,656 partially purified natural product fractions against a panel of four microbial pathogens, resulting in the identification of >3000 fractions with antifungal and/or antibacterial activity. A small sample of these active fractions was further purified and the chemical structures responsible for the antimicrobial activity were elucidated. The proof-of-concept study identified many different chemotypes, several of which have not previously been reported to have antimicrobial activity. The results show that there remain many unidentified antibiotic compounds from nature.
Keywords: microbial pathogens, antibiotic, antimicrobial, antifungal, NPNPD prefractionated library, high-throughput screening, natural products
The constant battle between rapidly mutating microorganisms and antibiotic therapies we use against them has led to the evolution of microbes with significant resistance or tolerance to most, if not all, known classes of antibiotics.1 The fast development of resistant microbes has been ascribed to the overuse of antibiotics in human and veterinary fields,2 agriculture,3 and the acquisition of plasmid-encoded resistance genes.4 The current level of antibiotic-resistant microbes has had a significant impact on human health with an estimated ∼35,000 deaths in the United States in 2019 alone.5 The increasing importance of this area of research to human health is further evidenced by an average of ∼10,000 papers per year published in the PubMed database on antibiotic resistance over the last 10 years. Despite this renewed emphasis on research, recent drug approvals for antibiotic therapy have not kept up with the growing need for new classes of antibiotic drugs.6,7 Though resistance is a concern for all pathogenic microbes, with recent estimates of 1.27 million deaths associated with antibiotic-resistant microbes worldwide,8 several specific organisms have become increasingly worrisome, such as those named ESKAPE pathogens (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter species).9 These, as well as pathogenic fungi and many other infectious microbes, continue to demand attention and efforts to develop new antimicrobial agents.
In seeking ways to engage the public and encourage further research into identifying potential antibiotic compounds, the National Institute of Allergy and Infectious Diseases (NIAID) has initiated the screening of agents against resistant microbes, including ESKAPE pathogens, and other pathogens of concern, including Escherichia coli and Candida albicans.10,11 To expand the potential chemical diversity with identified antimicrobial activity, NIAID has partnered with the National Cancer Institute (NCI) in a high throughput screen of a large publicly available library of natural product samples. The NCI recently initiated the NCI Program for Natural Product Discovery (NPNPD) to help reinvigorate drug discovery research in natural products.12 As an initial aim, the NPNPD has been producing and releasing prefractionated natural product samples to the public for screening against all disease states. The Division of Microbiology and Infectious Diseases (DMID) within NIAID has a long-standing program designed to effectuate the discovery of new antimicrobial compounds. The DMID and the Natural Products Branch of the NCI here report the results of a high throughput screen of ∼326,000 natural product fractions against three bacterial and one fungal pathogen species: S. aureus, E. coli (2 strains, including an efflux mutant), and C. albicans, which is summarized in Figure 1. The screen resulted in the identification of 3067 confirmed active fractions from 1977 distinct source organisms. Importantly, the complete list of these active fractions is being made available to the public to encourage further research into the identification of new antimicrobial agents. As an initial proof of principle, a chemical evaluation of 75 of these fractions from diverse source organisms is also reported here, which resulted in the identification of new antibacterial and antifungal compounds. Several of these structures, including natural products that we describe here for the first time, have not been previously reported to have antimicrobial activity.
Figure 1.
Overview of the screening workflow. The prefractionated library comprising 326,656 samples was screened against four microbial pathogen strains. Initial screening was done at a single point concentration of 10 mg/L and the hits confirmed in 8-point dose response. 75 hit fractions were then selected for a proof-of-concept study where the active principles were identified and select pure compounds assessed against a panel of bacterial and fungal pathogenic strains.
Results and Discussion
High Throughput Screening
At the time of this study, the NPNPD prefractionated extract library,12,13 sourced from the NCI’s Natural Products Extract Repository collection, was comprised of 326,656 partially purified natural product samples. With a total of 3454 plant, 2027 marine, 104 fungal, and 94 algal genera represented, this library is one of the largest and most diverse public collections of natural product samples available for high throughput screening (HTS). The NPNPD prefractionated library was tested against four microbial strains, namely, S. aureus ATCC 29213, efflux competent E. coli BW25113 (wild-type), tolC efflux deficient E. coli JW5503-1, and C. albicans ATCC 90028 in an in vitro growth inhibition screen. The strains were chosen to represent some of the most common microbial pathogens that cause a significant threat to human health. S. aureus is a Gram-positive bacterial human pathogen that causes bacteremia and skin and soft tissue infections and effective treatment of methicillin-resistant S. aureus (MRSA) is of particular concern.14E. coli was included as an example of a Gram-negative pathogenic bacterium responsible for urinary tract infections and other serious human infections.15 Due to efficient efflux pumps that can transport many compounds outside of the cell, E. coli has shown resistance to many antimicrobial drugs, including β-lactams, fluoroquinolones, and aminoglycosides.15 We, therefore, included two different E. coli strains—an efflux competent strain and a congenic strain lacking the tolC gene associated with multidrug resistance in E. coli.16 The final microbial target was a C. albicans strain, which is a fungal pathogen of increasing concern which exhibits various resistance mechanisms.11 All four of these target strains can be obtained by outside researchers for follow-up works—the E. coli strains are available from the Coli Genetic Stock Center while the S. aureus and C. albicans strains are available at the American Type Culture Collection.
NPNPD fraction library screening was carried out in a 384-well plate format at a single point concentration of 10 mg/L with ≥2 replicates per well (Figure 1). Two criteria were used for hit selection in the primary screen: (1) any well that led to a % inhibition value 4 standard deviations ≥ the mean value for ≥50% of the replicates; or (2) any well not identified by hit criterion 1 that displayed a % inhibition value 3 standard deviations ≥ the mean value for ≥2 replicates. The purpose of these generous cutoff values was to select as many potential hits as possible in the subsequent phase of hit confirmation and dose–response testing.
Z′ scores of ≥0.7 across the entire series of screening plates for all 4 strains were observed. Figure 2A–D depicts the overall screening results in three dimensions for each of the microbes showing the reproducibility of the replicates and graphically indicating with right hand peaks, the initial actives identified from the NPNPD fraction library.
Figure 2.
Three-dimensional views of the single-point screening results of the NPNPD prefractionated library against (A) C. albicans, (B) S. aureus, (C) E. coli (wild type), and (D) E. coli (tolC). Graphs show the general reproducibility of the two tested replicates for each sample. Each fraction in the library was screened in duplicate at 10 mg/L against each of the four strains, and the resulting % inhibition data were plotted on the X and Y axes of each panel. A small percentage of fractions were tested 3–4 times on an ad hoc basis if poor reproducibility was observed with the first 2 replicates, with additional data not displayed here. The Z axis reflects the number of fractions in each % inhibition bin. The fractions that were identified as putative hits are color coded in each panel.
These hit criteria were used to select active fractions to move forward into dose–response confirmation. The resulting number of fractions categorized as hits against any of the four strains are presented in Table 1. Overall, the single point HTS hit rate in total for the four microbes was 2.9%, the majority of which was due to activity against C. albicans (1.6% hit rate), followed by tolC efflux-deficient E. coli (0.7% hit rate), S. aureus (0.6% hit rate), and the wild-type efflux competent E. coli (0.4% hit rate). Many antimicrobial compounds are substrates for tolC-dependent efflux pump(s) in E. coli(17) and, therefore, exhibit greater antimicrobial activity against tolC strains. The similarity in the E. coli (wild type) and E. coli (tolC) hit rates suggested that many of the apparent wild-type selective hits were spurious, and this was confirmed in follow-up testing. Confirmation of single-point hits was then done in eight-point dose–response at a concentration of 0.08 to 10 mg/L. An example of the data generated for six representative active fractions is shown in Figure 3. The dose–response step in the workflow enabled hit confirmation, relative potency, and selectivity data to be acquired for each sample, which was important for the subsequent prioritization of select fractions for active compound isolation and structure elucidation efforts.
Table 1. Number of Prefractionated Natural Product High Throughput Screening Hits by Microbial Straina.
| strain | number of hit fractions in single-point HTSb | number of hit fractions in dose–response HTSb |
|---|---|---|
| any | 9524 | 3067 |
| C. albicans | 5084 | 2590 |
| E. coli (wild-type) | 1467 | 140 |
| E. coli (tolC) | 2347 | 682 |
| S. aureus | 1951 | 734 |
Out of a total of 326,656 natural product samples that were tested.
Some fractions were hits against >1 strain.
Figure 3.
Percent inhibition graphs from a representative selection of six active natural product fractions. (A) M19655_6 sourced from the ascidian Eudistoma reginum inhibited all 4 microbial strains; (B,C) M20753_4 sourced from the sponge Clathrina darwinii and M16187_5 sourced from the sponge Leucetta chagosensis selectively inhibited Clathrina albicans and compounds shown in Scheme 1 were determined to be responsible for this activity; and (D) M9231_7 sourced from the sponge from the family Petrosiidae selectively inhibited the E. colitolC strain. (E,F) M25263_2 and M25263_6, sourced from the sponge Petrosia sp., primarily inhibited S. aureus and compounds shown in Scheme 2 were determined to be responsible for this activity. The inhibition data for each fraction was curve-fit in GraphPad Prism using a sigmoidal dose-response model to generate IC50 and R2 values.
The resulting confirmation screen hit rates are presented in Table 1. The criteria used to classify a hit were any fraction with: (1) ≥1 replicate that displayed an IC50 ≤ 7.5 mg/L and an R2 value ≥ 0.8 or (2) an IC50 ≤ 0.1 mg/L without regard to the corresponding R2 value. Hence, the overall hit rate for all 4 organisms was reduced to 0.9%, with C. albicans again the dominant sensitive microbe (0.79% hit rate), followed by S. aureus (0.22% hit rate), tolC efflux deficient E. coli (0.21% hit rate), and the wild-type efflux competent E. coli (0.04% hit rate).
Figure 4 shows a violin plot rendering of the IC50 values of the dose response data on all samples classified as hits in the primary screening of natural product fractions. The plot depicts the distribution and density of the data with the median and interquartile ranges in the center of the violin, and the lower and upper adjacent values as well as outliers shown away from the center. The density of the data is depicted as horizontal ranges of the violin. Activity against C. albicans displayed an IC50 range from 13.5 to 0.06 mg/L, S. aureus hits showed a range from 10.8 to 0.06 mg/L, efflux deficient tolCE. coli IC50 values showed a range from 10.5 to 0.06 mg/L while the smallest data set was from the wild-type E. coli strain, which showed an IC50 range from 9.9 to 0.3 mg/L. The complete list of all active fractions and their activities is now publicly available on https://wiki.nci.nih.gov/display/NCIDTPdata/NPNPD+NIAID+Screen and an example of the format the results are presented in is given in Supporting Information, Figure S1.
Figure 4.

Violin plot of the IC50 values of hit fractions in dose-response HTS. The plot was built using median IC50 values and omitted any crude extracts that were tested. On the box plots the following values are depicted: median, first and third quartile, lower and upper adjacent range, and outside points. Plot summary statistics are presented in Supporting Information, Table S1.
Chemical Analysis
For proof-of-concept of the current study, we selected a very small representative subset of 75 fractions that showed antimicrobial activity against 1 or more of the four strains tested in the HTS assay. In addition, we used previous data from a cell cytotoxicity screen to select fractions that also showed a lack of toxicity against a mammalian cell line. Finally, we attempted to sample a broad distribution of taxonomic diversity of source material in the 75 selected fractions for study. Selection criteria were not designed to pick the best or most selective active fractions but were instead chosen to be representative of the diverse activities identified (both selective and non-selective). In addition, we evaluated available database information on known antimicrobial activity associated with each relevant taxonomic family found in previous publications (for a full list of selection criteria, see Supporting Information, Figure S2). This initial proof-of-concept study was intentionally limited in scope such that a significant number of the potentially most-interesting active fractions remain open to extramural researchers for investigation.
The NPNPD automated natural product isolation procedure for further sub-fractionating each active fraction involved separating 1 mg of the active fraction mixture on a semi-preparative HPLC, collecting subfractions in timed increments.18 Here, 75 identified active fractions were each subdivided into 22 subfractions which resulted in 1650 subfractions that were submitted for single-point testing against all four microbial pathogens (Figure 1; results shown in Supporting Information, Figure S3). Approximately 80% of the expected antimicrobial activities for the 75 hit fractions were further narrowed to ≥1 subfraction after the antimicrobial data were analyzed. Following the identification of active subfractions, the chromatographic procedure was repeated, active subfractions were collected and analyzed by LC/MS, NMR, and FTIR to gather initial chemical composition data on the active molecules.18 This step, done on a 1 mg scale, provided an opportunity to gain insights into the active principle chemotype without undertaking large-scale natural product isolation, and was used to guide project prioritization. Using this methodology, we were able to assign chemotypes to ∼70% of the fractions that retained activity following second stage HPLC (Supporting Information, Table S2). Figure 5 shows representative examples of the active principles identified from a marine sponge Jaspis splendens, the plant Bombax rhodognaphalon, and the fungus Coemansia pectinata. As all three of these chemotypes, namely jaspamide, gnemonols, and gliotoxins, are known to have antimicrobial activity,19−21 they were de-prioritized for further isolation work. This enabled us to focus on fractions potentially containing new natural products with antimicrobial activity. Below, we present the results of two projects: the isolation of 2-amino imidazole alkaloids with selective antifungal activity, and the isolation of pyridoacridine alkaloids with selective antibacterial activity.
Figure 5.
Examples of natural products identified from the small-scale dereplication study of the active fractions. The analysis was done by injecting 1 mg of the active fraction on a semi-preparative HPLC and collecting NMR, high-resolution mass spectrometry, and FTIR data on the active subfractions. An example of the analytical methodology and spectral fingerprints used to identify jaspamide is given in Supporting Information, Figure S4.
Antifungal Activity of 2-Amino Imidazole-Containing Natural Products
Bioassay and spectroscopically guided isolation of the active principles from the calcareous sponges Leucetta chagosensis (NSC #C16187) and Clathrina darwinii (NSC #C20753) yielded the known 2-amino imidazole alkaloids naamidines A (1) and D (2)22 as well as the recently reported naamidine K (3)23 (Scheme 1). Natural products containing a 2-amino imidazole group have previously been reported from both Lucetta and Clathrina species sponges,24 and the naamidine series, namely, naamidines A-K,23,25−27 and isonaamidine C28 display different combinations of methyl substitutions on the 2-amino imidazole core, the two benzyl groups, and the hydantoin substituent. There are very few reported activities of naamidine-type 2-amino imidazole alkaloids in the literature. Naamidine A (1) was found to be an antagonist of the epidermal growth factor (EGF) receptor signaling pathway and showed modest activity in an EGF-dependent A431 athymic mice xenograft model,28 while naamidines A (1), B, and H and a series of their semi-synthetic derivatives were found to have activity against a panel of phytopathogenic fungi.29
Scheme 1. Structures of the Isolated 2-Amino Imidazole Alkaloids.
In our antimicrobial panel, compounds 1–3 showed selective activity against C. albicans ATCC 90028 at concentrations of 0.08 mg/L (0.18 μM), 1.25 mg/L (2.89 μM), and 0.16 mg/L (0.39 μM), respectively, while activity against all of the bacterial strains tested was not detected at concentrations up to 20 mg/L (Table 2). Strikingly, the three compounds retained activity against azole- and echinocandin-resistant strains of C. albicans, as well as four other pathogenic Candida species and an Aspergillus fumigatus fungal isolate. We conclude that 1–3 may represent broad-spectrum antifungal compounds. Interestingly, while compounds 1–3 do possess a modified azole moiety common in many antifungal drugs, they displayed potent low micromolar activity against the azole-resistant strain of C. albicans, suggesting the naamidine scaffold may be an interesting starting point for the development of new antifungal drugs.
Table 2. Antifungal Activity of Compounds 1–3a.
| compound | Mw | E. coli BW25113 (WT) | E. coli JW5503-1 (tolC) | S. aureus ATCC 29213 | C. albicans ATCC 90028 | C. albicans #1186858 (azole R) | C. albicans #1181444 (echinocandin R) | C. kruse ATCC 6258 | C. parapsilosis ATCC 22019 | C. tropicalis ATCC 13803 | C. glabrata ATCC MYA-2950 | A. fumigatus ATCC MYA-3626 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 433.2 | >20 | >20 | >20 | 0.08 | 0.16 | 0.08 | 0.16 | 0.16 | 0.08 | 0.08 | 0.16 |
| 2 | 433.2 | >20 | >20 | >20 | 1.25 | 2.5 | 1.25 | 1.25 | 1.25 | 1.25 | 1.25 | 2.5 |
| 3 | 405.4 | >20 | >20 | >20 | 0.16 | 0.3 | 0.16 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 |
| fluconazole | 306.3 | >64 | >64 | >64 | 0.25 | >64 | 0.5 | 32 | 2 | 0.5 | 8 | >64 |
| micafungin | 1270.3 | >4 | >4 | >4 | 0.03 | 0.03 | 1 | 0.12 | 0.5 | 0.06 | 0.02 | 0.06 |
MIC values are presented in mg/L. Strains tested were efflux-competent WT E. coli BW25113, tolC efflux deficient E. coli JW5503-1, S. aureus ATCC 29213, C. albicans ATCC 90028, azole-resistant C. albicans isolate #1186858 (JMI Laboratories), echinocandin-resistant C. albicans isolate #1181444 (JMI Laboratories), C. krusei ATCC 6258, C. parapsilosis ATCC 22019, C. tropicalis ATCC 13803, C. glabrata ATCC MYA-2950, and A. fumigatus ATCC MYA-3626. Fluconazole and micafungin were run as controls.
Antibacterial Activity of Pyridoacridine Alkaloid Natural Products
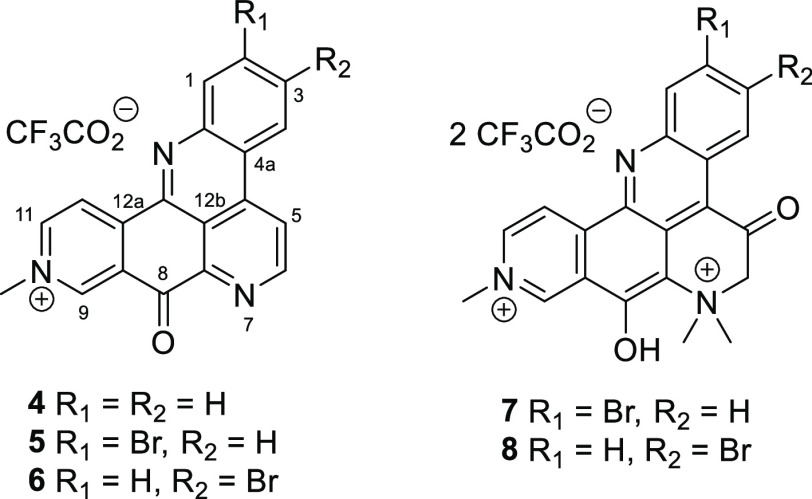
Bioassay and spectroscopically guided isolation of the active principles from the sponge Petrosia sp. (NSC #C25263) yielded five pyridoacridine-type natural products: the known compound deoxyamphimedine (4);30 two new analogues, 2-bromodeoxyamphimedine (5), and 3-bromodeoxyamphimedine (6); as well as two other known natural products in the pentacyclic amphimedine series petrosamine (7)31 and petrosamine B (8)32 (Scheme 2).
Scheme 2. Structures of the Isolated Pyridoacridine Alkaloids.
Over one hundred tetra- and penta-cyclic pyridoacridine natural products have so far been reported from marine organisms.33,34 Deoxyamphimedine analogues are relatively uncommon, with compounds 5 and 6 only the fifth and sixth examples, and the first brominated compounds in the series35 (for full structural elucidation of the new natural products 5 and 6, see Supporting Information, Figure S5 and the accompanying text). When tested against a panel of fungal and bacterial pathogens, the three fully aromatized natural products showed potent antibacterial activity, with MIC values against the S. aureus ATCC 29213 strain of 2.5 mg/L (6.08 μM), 0.3 mg/L (0.61 μM), and 0.6 mg/L (1.22 μM) for 4, 5, and 6, respectively, while 7 and 8 were inactive at the highest concentrations tested (Table 3). Bromination of the deoxyamphimedine scaffold was significant as compound 5 was almost an order of magnitude more active than the non-brominated 4 against S. aureus ATCC 29213. Notably, compounds 4, 5, and 6 were also active against an MRSA/MDR S. aureus isolate, an Enterococcus faecalis strain, and an E. faecium strain in the test panel. Against E. coli, compounds 4, 5, and 6 exhibited antimicrobial activity against the tolC strain but not the wild-type strain, which suggests that they are efflux substrates. Compounds 4 and 5 exhibited weaker MIC activity against the A. baumannii strain and only 4 retained activity against the efflux-deficient P. aeruginosa strain. Thus, we conclude that the pyridoacridine alkaloids exhibit broad-spectrum antibacterial but not antifungal activity in vitro.
Table 3. Antibacterial Activity of Compounds 4–8a.
| compound | Mw | E. coli BW25113 (WT) | E. coli JW5503-1 (tolC) | S. aureus ATCC 29213 | S. aureus #1180973 (MRSA/MDR) | E. faecalis ATCC 29212 | E. faecium ATCC 700221 | A. baumannii #1186154 | P. aeruginosa PAM1626 (efflux mutant) | C. albicans ATCC 90028 |
|---|---|---|---|---|---|---|---|---|---|---|
| 4 | 411.3 | >20 | 2.5 | 2.5 | 2.5 | 20 | 20 | 10 | 20 | >10 |
| 5 | 490.2 | >20 | 10 | 0.3 | 0.3 | 2.5 | 2.5 | 20 | >20 | >10 |
| 6 | 490.2 | >20 | 10 | 0.6 | 1.3 | 5 | 5 | >20 | >20 | >10 |
| 7 | 650.3 | >20 | >20 | >20 | >20 | >20 | >20 | >20 | >20 | >10 |
| 8 | 650.3 | >20 | >20 | >20 | >20 | >20 | >20 | >20 | >20 | >10 |
| ampicillin | 349.4 | 4 | 2 | 2 | >16 | 2 | >16 | 4 | >16 | >16 |
| levofloxacin | 361.4 | 0.03 | 0.008 | 0.25 | >2 | 1 | >2 | 0.015 | 0.015 | >2 |
MIC values are presented in mg/L. Strains tested were efflux-competent WT E. coli BW25113, tolC efflux-deficient E. coli JW5503-1, S. aureus ATCC 29213, MRSA and multidrug-resistant (MDR) S. aureus isolate #1180973 (JMI Laboratories), E. faecalis ATCC 29212, E. faecium ATCC 700221, A. baumannii isolate #1186154 (JMI Laboratories), efflux-deficient P. aeruginosa PAM1626,36 and C. albicans ATCC 90028. Ampicillin and levofloxacin were run as controls.
Conclusions
Small molecules sourced from plant, marine invertebrate, and microbial biota have often been a significant source of drugs and drug leads. In a review of natural products as sources of new drugs from 1981 to 2019, natural products and their derivatives have contributed 55% of new antibacterial therapeutics entering the clinic.37 In this report, a high throughput screen of 326,656 samples from the NPNPD natural product fraction library was undertaken against a panel of four bacterial and fungal human pathogens, and our results confirmed the utility of natural product samples in antimicrobial drug discovery. As the screen included both the initial crude extract and seven semi-purified fractions, we were able to determine that in ∼75% of the instances where a fraction showed antimicrobial activity, the corresponding crude extract did not. This further demonstrated the value in screening prefractionated natural product samples as opposed to only crude extracts.18,38−40 In the primary high-throughput assay, >3000 fractions displayed antimicrobial activity against at least one of the four target pathogenic microbes at concentrations <10 mg/L. We then selected 75 hit fractions (diverse both by activity profile and taxonomic designation) and conducted a proof-of-concept study demonstrating a fast and efficient workflow for modern bioassay-guided isolation of biologically active compounds from natural product libraries. Examples of potent and selective antibacterial and antifungal natural products, including new natural products, were isolated from the screening hits, thereby validating our overall strategy. However, the majority of potential new discoveries that could result from this HTS program remain open to extramural researchers. We speculate that the chemical matter that we have identified may include attractive, novel scaffolds for antimicrobial drug discovery and potential development. In an effort to maximize value and enable further research into the results of this screening effort, NIAID and the NCI are sharing data from this screen with the broader research community, available at https://wiki.nci.nih.gov/display/NCIDTPdata/NPNPD+NIAID+Screen.
Methods
Strains and Isolates
S. aureus ATCC 29213, C. albicans ATCC 90028, E. faecalis ATCC 29212, E. faecium ATCC 700221, Candida krusei ATCC 6258, Candida parapsilosis ATCC 22019, Candida tropicalis ATCC 13803, Candida glabrata ATCC MYA-2950, and A. fumigatus ATCC MYA-3626 were obtained from the American Type Culture Collection (Manassas, VA). E. coli BW25113 (wild-type) and JW5503-1 (tolC derivative of BW25113) were obtained from the Coli Genetic Stock Center (New Haven, CT). S. aureus #1180973 (MRSA/MDR), A. baumannii #1186154, C. albicans #1186858 (azole resistant), and C. albicans #1181444 (echinocandin resistant) were obtained from the JMI Laboratories SENTRY strain collection.41P. aeruginosa (ΔmexAB-oprM::Cm ΔmexCD-oprJ::Gm ΔmexEF-oprN::ΩHg) PAM1626 is a derivative of PAO1 and was the kind gift of O. Lomovskaya.36
Growth of Microbial Strains
Blood agar plates were used to propagate bacterial strains. Sabouraud dextrose agar plates were used to propagate fungal strains. Cation-adjusted Mueller–Hinton broth (CAMHB) was used for liquid-phase growth of the bacterial strains. The RPMI 1640 medium was used for liquid-phase growth of the fungal strains. These media were chosen because they are used in reference-method MIC testing according to Clinical and Laboratory Standards Institute (CLSI) methods. Plates and broth cultures were grown at 35 °C with an ambient atmosphere.
Plates
Black 384-well plates were used for the HTS (Corning nonbinding surface 384-well microplates [product #3575] or Greiner non-binding polystyrene microplates [product #781900]). 96-well untreated, polystyrene plates were used for MIC testing. Standard plate cover seals were used for test plate storage at −80 °C. Universal lids were used to cover the plates during screening.
High Throughput Screening
Liquid handling for 384-well plates utilized a Biomek FX robot with a 96-channel head. Growth media, bacterial inocula, and alamarBlue were added to the 384-well plates using a Multidrop Combi Reagent Dispenser (Thermo Fisher Scientific). The prefractionated natural product compound library was obtained from the National Cancer Institute. This library was composed of 326,656 compound fractions stored in the wells of 928 384-well plates (Figure 1). These compounds were plated (excluding the first 2 columns) at a final concentration of 5 mg/mL (10 μg in a volume of 2 μL of dimethyl sulfoxide [DMSO]). A single plate type was used to test both the bacterial and yeast strains. Several positive control wells containing a bacterial (ampicillin, levofloxacin, and linezolid) or fungal (amphotericin B) inhibitor were present on each plate at concentrations above their respective MIC values. The mean fluorescence value from these positive inhibition control wells was used as the background fluorescence value for the assay to determine the minimum growth. Several negative inhibition control wells (i.e., dimethyl sulfoxide [DMSO] only) were present on each plate to measure the level of maximal growth. The mean fluorescence value from these wells corresponded to the highest possible fluorescence value for the assay and determined the maximum growth. Amphotericin B and levofloxacin or ampicillin dose responses also were present on each plate for quality control purposes. The fluorescence data from the dilution series were curve-fit to calculate IC50 values that could be compared from plate-to-plate and run-to-run. Upon the addition of 25 μL of inoculum to each well, the final compound screening concentration for NPNPD fractions was 10 mg/L. For each strain, test cultures were prepared by inoculating 1× CAMHB or RPMI 1640 broth medium in a sterile tube with 1 or more purified colonies from an agar plate. Each culture was incubated overnight with shaking at 35 °C. The turbidity of each culture was measured using a spectrophotometer or turbidity meter. Each overnight culture was diluted into fresh sterile 2× CAMHB or 2× RPMI 1640 medium, as appropriate, to ∼106 colony forming units (cfu)/mL for bacterial species and ∼103 cfu/mL for the C. albicans strain. Upon their addition to the screening daughter plates, these inoculum densities corresponded to those densities prescribed for CLSI reference-method 96-well MIC test plates. The incubation times for testing in the 384-well format were ∼18 h for the bacterial strains and 24 h for C. albicans. Growth of the 4 strains was monitored using the metabolic indicator dye, alamarBlue (BioRad; catalogue #BUF012B). After plates were incubated overnight, 5 μL of alamarBlue reagent (i.e., ∼10% final volume) was added to each test well and the plates were returned to 35 °C. Bacterial and fungal cells were incubated with alamarBlue for 1 or 3 h, respectively. Cell growth was measured fluorometrically using a Tecan Spark plate reader equipped with a plate stacker, a 560 nm excitation filter, and a 590 nm emission filter. The gain was automatically optimized for each plate. Using the mean positive and negative control-well data from each test plate, the fluorescent signal in each well was converted to a percent inhibition (% I) value according to the following equation
The equation developed by Zhang et al.42 was used to calculate the Z′ value for each test plate. Most strain and well combinations were screened in duplicate. Some plates were screened in triplicate or quadruplicate when greatly discordant replicate data were obtained for a single microbial strain. A dedicated Microsoft SQL Server database created for the study was used to support storage, analysis, and report generation of the HTS data. These data were analyzed using custom-built Excel Visual Basic for Applications and Python v3.9 scripts.
Dose–Response Testing of Initial Hits
In total, 9524 wells based on single-point growth inhibition results were selected for dose responses. The contents of each hit well of the pre-fractionated natural product compound library master plates were cherry-picked and then serially diluted as an 8-point dose response across a high-dose–response (HDR) and low-dose–response (LDR) plate. Prior to the cherry-picking and dose–response generation, 5 μL of the control compounds were transferred to every daughter plate using an Agilent Bravo robot. This step excluded the ampicillin and amphotericin B dose–response controls, which were added later and diluted concurrently with the library compounds. During the cherry-picking process for a set of 60 hits, each hit well was diluted 50-fold by the addition of 98 μL of 18% DMSO. This dilution generated an intermediate well at a top concentration (100 mg/L in 20% DMSO) of the selected samples. Each hit then was serially diluted a total of 7 times across both dose–response plates. These master dose–response plates were used to dispense material into daughter plates. This process resulted in 10 identical daughter plates with 5 μL final volume of hit material starting at a top concentration of 100 mg/L in 20% DMSO. After the addition of 45 μL of inoculum to each well, the final dose–response test range was 10 to 0.08 mg/L in 2% DMSO. These plates were tested in duplicate growth inhibition assays against S. aureus ATCC 29213, E. coli BW25113, E. coli JW5503-1, and C. albicans ATCC 90028. Growth media, bacterial inocula, and alamarBlue were added to the 384-well plates using a Multidrop Combi Reagent Dispenser (Thermo Fisher Scientific). The plates were incubated, read, and interpreted as described above.
Confirmation of Antimicrobial Activity from Purified Subfractions
Based on results of the HTS campaign, the NCI selected 75 fractions from the NPNPD prefractionated library for further chemistry efforts (Figure 1). Each of these fractions was further separated into 22 subfractions. All subfractions were aliquoted and dried in 96-well deep-well plates. Exact amount of material in each subfraction was not weighed but assumed to be a nominal mass of 45 μg based on 1 mg of fraction material divided among the 22 subfractions. Additionally, NCI provided 2 separate plates where 100 μg of the 75 parent fractions had been plated to serve as controls. Wells containing ∼40 μg of purified subfractions or ∼100 μg of parent fractions were solvated in 80 or 200 μL of DMSO, respectively, to a concentration of ∼500 μg/mL. An intermediate dilution plate was created by combining 40 μL of solvated extract with 60 μL of H2O. Intermediate dilutions (5 μL at ∼200 μg/mL) were spotted into 96-well daughter plates. Finally, 100 μL of growth media and microbial inocula were added to the 96-well plates. Thus, the final compound test concentration was a nominal 10 mg/L, and the final DMSO concentration in each well was 2% (v/v). The plates were incubated and interpreted as described above, except that growth was evaluated by OD600 measurements rather than alamarBlue fluorescence. Importantly, OD600 measurements represent a second, independent readout format and confirmation by this method provides additional confidence that bona fide antimicrobial activity was observed.
MIC Testing
Purified compounds (m = 0.2 mg) were solvated in 200 μL of DMSO prior to the addition of 300 μL of H2O, resulting in an intermediate concentration of 400 μg/mL. Solvated compounds were transferred (200 μL) to column 1 of a 96-well dilution plate. Using liquid handling robots, 40% DMSO in water was added to the remainder of the dilution plate (columns 2–12). Compounds were then serially diluted across the plate (2-fold dilutions), prior to the transfer of 5 μL into daughter plates. Finally, 100 μL of growth media and bacterial inocula or 200 μL of growth media and fungal inocula were added to the 96-well plates using a Multidrop Combi Reagent Dispenser (Thermo Fisher Scientific). The final concentrations of the highest dilutions tested were 20 mg/L for bacteria and 10 mg/L for fungal isolates. These daughter plates were used to measure MIC values against several bacterial and fungal strains. These included the 4 strains used in the HTS plus several supplementary strains from other species to better interrogate the activity spectrum of the purified compounds. The MIC assays were conducted according to reference CLSI methodology described in the M07Ed11 and M100Ed32 documents for bacterial testing, the M27Ed4 and M27M44S-Ed3 documents for yeast testing, and M38Ed3 and M38M51S-Ed3 for mold testing. The testing media were cation-adjusted Mueller–Hinton broth for bacteria and RPMI 1640 for fungi. Where applicable, MIC values for ampicillin, levofloxacin, fluconazole, and micafungin were within quality control ranges prescribed by CLSI.43,44
General Chemical Experimental Procedures
IR spectra were recorded as a dry film on a Bruker Alpha II spectrometer or a Bruker Invenio S spectrometer equipped with an HTS-XT accessory. UV spectra were recorded as methanol solutions on a Varian Cary 50-Bio UV/vis spectrophotometer. NMR spectra were recorded at 25 °C on a 600 MHz Bruker Avance III HD spectrometer, equipped with a triple resonance 5 mm CPP TCI cryo-probe, and operating at a frequency of 600.0 MHz for the 1H nucleus and 150.9 MHz for the 13C nucleus. All 2D NMR experiments were acquired with non-uniform sampling (NUS) set to 40% (for 1H–1H detected experiments) or 35% (for 1H–13C detected experiments). 1H and 13C NMR chemical shifts were referenced to the solvent peak for DMSO-d6 at δH 2.50 δC 39.50. NMR FID processing and data interpretation was done using MestReNova software, version 14.2. High-resolution mass spectra were recorded on an Agilent 6545 Accurate-Mass Q-TOF LC/MS system (1290 Infinity II) equipped with a dual AJS ESI source. Semi-preparative scale HPLC purification was performed with either a Gilson HPLC purification system equipped with a GX-281 liquid handler, a 322-binary pump, and a 172-photodiode array detector or a Waters Prep LC system, equipped with a Delta 600 pump and a 996-photodiode array detector. All solvents used for chromatography, UV, and MS were HPLC grade, and the H2O was Millipore Milli-Q PF filtered.
L. chagosensis—Collection, Extraction, and Isolation
The sponge L. chagosensis was collected by SCUBA at a depth of 7 m in Fiji in Oct 1996 under contract through the Coral Reef Research Foundation for the National Cancer Institute. The specimen was taxonomically identified by Michelle Kelly, and a voucher specimen (0CDN4189) was deposited at the Smithsonian Institution. The sponge (wet weight 247.76 g) was extracted in water, followed by a MeOH/DCM overnight soak according to the National Cancer Institute’s standard marine extraction procedure detailed in McCloud45 to give the organic solvent extract C16187 (2.62 g). A portion of the organic extract (1 g) was pre-fractionated on C8 SPE (8 g) eluting sequentially with: water/methanol (95:5) to yield 225.1 mg of fraction 1, water/methanol (80:20) to yield 16.0 mg of fraction 2, water/methanol (60:40) to yield 9.8 mg of fraction 3, water/methanol (40:60) to yield 20.5 mg of fraction 4, water/methanol (20:80) to yield 116.0 mg of fraction 5, methanol to yield 137.0 mg of fraction 6, and methanol/acetonitrile (50:50) to yield 74.5 mg of fraction 7. Fraction 5 was purified on C18 semi-preparative HPLC using a Phenomenex Onyx C18 HPLC column [100 × 10 mm] at a flow rate of 3.8 mL/min with the following conditions: an initial isocratic hold at water/acetonitrile [0.1% formic acid] (30:70) for 1.5 min, followed by a linear gradient to acetonitrile [0.1% formic acid] over 7.5 min, an isocratic hold at acetonitrile [0.1% formic acid] for 5 min. Collection was performed in 30 s increments between 1.5 and 12.5 min (22 total fractions). Fraction 9 (23.0 mg) enriched in compound 1 was further purified on the same column and flow rate with a modified gradient that included an initial isocratic hold at water/acetonitrile [0.1% formic acid] (30:70) for 1.5 min, followed by a linear gradient to water/acetonitrile [0.1% formic acid] (75:25) over 7.5 min, and an isocratic hold at water/acetonitrile [0.1% formic acid] (75:25) for 5 min. Collection was performed in 30 s increments between 1.5 and 12.5 min (22 total fractions). Fraction 12 yielded 1 12.4 mg (1.24% crude organic extract weight).
Naamidine A (1)
Yellow powder; NMR spectroscopic data in agreement to those previously reported;22 high-resolution electrospray ionization mass spectrometry (HRESIMS) m/z: [M + H]+ 434.18240 (calcd for C23H24N5O4+, 434.18228).
C. darwinii—Collection, Extraction, and Isolation
The sponge C. darwinii cf. was collected by SCUBA at a depth of 10 m in Palau in Sept 2000 under contract through the Coral Reef Research Foundation for the National Cancer Institute. The specimen was taxonomically identified by Michelle Kelly, and a voucher specimen (0CDN7077) was deposited at the Smithsonian Institution. The sponge (wet weight 25.53 g) was extracted in water, followed by a MeOH/DCM overnight soak according to the National Cancer Institute’s standard marine extraction procedure detailed in McCloud45 to give the organic solvent extract C20753 (0.71 g). A portion of the organic extract (350 mg) was pre-fractionated on C8 SPE (4 g) eluting sequentially with: water/methanol (95:5) to yield 52.7 mg of fraction 1, water/methanol (80:20) to yield 11.8 mg of fraction 2, water/methanol (60:40) to yield 4.7 mg of fraction 3, water/methanol (40:60) to yield 5.0 mg of fraction 4, water/methanol (20:80) to yield 19.2 mg of fraction 5, methanol to yield 52.5 mg of fraction 6, and methanol/acetonitrile (50:50) to yield 25.8 mg of fraction 7. Combined fractions 3–7 (100 mg) were further purified on C8 preparative HPLC using a Phenomenex Kinetex C8 [5 μm, 150 × 21.2 mm] column at a flow rate of 15 mL/min eluting with a steep gradient from water/acetonitrile [0.1% formic acid] (95:5) to acetonitrile [0.1% formic acid] over 60 min. The conditions were isocratic at water/acetonitrile [0.1% formic acid] (95:5) for 5 min followed by a gradient from water/acetonitrile [0.1% formic acid] (95:5) to acetonitrile [0.1% formic acid] over 45 min, followed by isocratic acetonitrile [0.1% formic acid] conditions over 10 min. Collection was performed in 1 min increments. Fraction 17 yielded 3 9.0 mg (2.4% crude organic extract weight). Fraction 29 enriched in compound 2 was further purified on C18 preparative HPLC using a Phenomenex Kinetex C18 [5 μm, 250 × 10 mm] column at a flow rate of 6 mL/min eluting with a steep gradient from water/acetonitrile [0.1% formic acid] (98:2) to water/acetonitrile [0.1% formic acid] (60:40) over 48 min. The conditions were isocratic at water/acetonitrile [0.1% formic acid] (98:2) for 5 min followed by a gradient from water/acetonitrile [0.1% formic acid] (98:2) to water/acetonitrile [0.1% formic acid] (60:40) over 35 min, followed by isocratic water/acetonitrile [0.1% formic acid] (60:40) conditions over 8 min. Collection was performed in 20 s increments. Fraction 129–134 yielded 2 1.1 mg (0.3% crude organic extract weight).
Naamidne D (2)
Yellow powder; NMR spectroscopic data in agreement to those previously reported;22 HRESIMS m/z: [M + H]+ 434.18266 (calcd for C23H24N5O4+, 434.18228).
Naamidine K (3)
Yellow powder; NMR spectroscopic data in agreement to those previously reported;23 HRESIMS m/z: [M + H]+ 406.15109 (calcd for C21H20N5O4+, 406.15098).
Petrosia sp. 1647—Collection, Extraction, and Isolation
The sponge Petrosia sp. 1647 was collected by SCUBA at a depth of 9 m in April 2003 in Malaysia under contract through the Coral Reef Research Foundation for the National Cancer Institute. The specimen was taxonomically identified by Michelle Kelly, and a voucher specimen (0M9I5715) was deposited at the Smithsonian Institution. The sponge (wet weight 178.18 g) was extracted in water, followed by a MeOH/DCM overnight soak according to the National Cancer Institute’s standard marine extraction procedure detailed in McCloud45 to give the organic solvent extract C25263 (4.83 g). A portion of the organic extract (1.0 g) was pre-fractionated on C8 SPE (8 g) eluting sequentially with: water/methanol (95:5) to yield 22.7 mg of fraction 1, water/methanol (80:20) to yield 44.8 mg of fraction 2, water/methanol (60:40) to yield 74.1 mg of fraction 3, water/methanol (40:60) to yield 36.8 mg of fraction 4, water/methanol (20:80) to yield 31.1 mg of fraction 5, methanol to yield 32.4 mg of fraction 6, and methanol/acetonitrile (50:50) to yield 38.0 mg of fraction 7. Fraction 2 was purified on Biphenyl preparative HPLC using a Biphenyl [5 μm, 150 × 21.2 mm] column at a flow rate of 15 mL/min eluting with a steep gradient from water [0.1% trifluoroacetic acid]/acetonitrile [0.1% formic acid] (95:5) to water [0.1% trifluoroacetic acid]/acetonitrile [0.1% formic acid] (50:50) over 50 min. The conditions were isocratic at water [0.1% trifluoroacetic acid]/acetonitrile [0.1% formic acid] (95:5) for 5 min followed by a gradient from water [0.1% trifluoroacetic acid]/acetonitrile [0.1% formic acid] (95:5) to water [0.1% trifluoroacetic acid]/acetonitrile [0.1% formic acid] (50:50) over 50 min, followed by a gradient from water/acetonitrile [0.1% formic acid] (50:50) to acetonitrile [0.1% formic acid] over 10 min. Collection was performed in 1 min increments. Fraction 24 yielded deoxyamphimedine (4) 0.1 mg (0.01% crude organic extract weight). Fractions 34 and 38 enriched in 2-bromo deoxyamphimedine (5) and 3-bromo deoxyamphimedine (6) were further purified on C18 preparative HPLC using a Phenomenex Kinetex C18 [5 μm, 250 × 10 mm] column at a flow rate of 6 mL/min eluting with isocratic conditions water [0.1% trifluoroacetic acid]/acetonitrile [0.1% formic acid] (25:75) over 30 min. The conditions were a gradient from water/acetonitrile [0.1% formic acid] (90:10) to water [0.1% trifluoroacetic acid]/acetonitrile [0.1% formic acid] (25:75) over 10 min followed by isocratic conditions water [0.1% trifluoroacetic acid]/acetonitrile [0.1% formic acid] (25:75) over 30 min, followed by a gradient from water/acetonitrile [0.1% formic acid] (25:75) to acetonitrile [0.1% formic acid] over 10 min. Collection was performed in 20 s increments. Fraction 79 yielded 2-bromo deoxyamphimedine (5) 0.4 mg (0.04% crude organic extract weight) and fraction 75 yielded in 3-bromo deoxyamphimedine (6) 0.3 mg (0.03% crude organic extract weight). Fraction 40 enriched in petrosamine (7) and petrosamine B (8) was further purified on C18 preparative HPLC using a Phenomenex Kinetex C18 [5 μm, 250 × 10 mm] column at a flow rate of 6 mL/min eluting with a steep gradient from water [0.1% trifluoroacetic acid]/acetonitrile [0.1% formic acid] (90:10) to water [0.1% trifluoroacetic acid]/acetonitrile [0.1% formic acid] (70:30) over 50 min. The conditions were isocratic at water/acetonitrile [0.1% formic acid] (90:10) for 5 min followed by a gradient from water/acetonitrile [0.1% formic acid] (90:10) to water [0.1% trifluoroacetic acid]/acetonitrile [0.1% formic acid] (70:30) over 50 min, followed by a gradient from water/acetonitrile [0.1% formic acid] (70:30) to acetonitrile [0.1% formic acid] over 10 min. Collection was performed in 20 s increments. Fractions 24–25 yielded petrosamine (7) 0.5 mg (0.05% crude organic extract weight) and Fraction 19 yielded petrosamine B (8) 0.2 mg (0.02% crude organic extract weight).
Deoxyamphimedine (4)
Yellow amorphous solid; NMR spectroscopic data in agreement to those previously reported;30,46 HRESIMS m/z: [M]+ 298.09809 (calcd for C19H12N3O+, 298.09749).
2-Bromodeoxyamphimedine (5)
Yellow amorphous solid; UV (MeOH) λmax (log ε) 380 (1.46) nm; IR (film) 3420 (broad), 1679, 1202, 1181, 1127, 801, 720 cm–1; 1H NMR (600 MHz, methanol-d4): δH 9.91 (1H, s, H-9), 9.47 (1H, d, J = 6.5 Hz, H-12), 9.38 (1H, d, J = 5.7 Hz, H-6), 9.30 (1H, d, J = 6.5 Hz, H-11), 9.15 (1H, d, J = 5.7 Hz, H-5), 8.93 (1H, d, J = 8.7 Hz, H-4), 8.75 (1H, d, J = 1.8 Hz, H-1), 8.21 (1H, dd, J = 1.8, 8.7 Hz, H-3), 4.65 (3H, s, H-14); 13C NMR (151 MHz, methanol-d4): δC 179.2 (C-8), 151.6 (C-6), 149.5 (C-12a), 149.2 (C-11), 147.9 (C-9), 147.4 (C-13), 147.4 (C-7a), 147.0 (C-12b), 139.6 (C-4b), 135. 7 (C-3), 134.9 (C-1), 131.8 (C-8a), 127.8 (C-2), 126.9 (C-4), 124.7 (C-12),123.3 (C-4a), 123.3 (C-5), 121.0 (C-12c), 49.0 (C-14); HRESIMS m/z: [M]+ 376.00952 (calcd for C19H11BrN3O+, 376.00800).
3-Bromodeoxyamphimedine (6)
Yellow amorphous solid; UV (MeOH) λmax (log ε) 400 (1.60) nm; IR (film) 3422 (broad), 1691, 1203, 1175, 1125, 799, 719 cm–1; 1H NMR (600 MHz, methanol-d4): δH 9.90 (1H, s, H-9), 9.46 (1H, d, J = 6.7 Hz, H-12), 9.38 (1H, d, J = 5.7 Hz, H-6), 9.29 (1H, d, J = 6.7 Hz, H-11), 9.24 (1H, d, J = 2.1 Hz, H-4), 9.17 (1H, d, J = 5.7 Hz, H-5), 8.43 (1H, d, J = 8.7 Hz, H-1), 8.27 (1H, dd, J = 2.1, 8.7 Hz, H-2), 4.64 (3H, s, H-14); 13C NMR (151 MHz, methanol-d4): δC 179.2 (C-8), 151.6 (C-6), 149.3 (C-11), 149.3 (C-12a), 147.9 (C-7a), 147.3 (C-9), 146.1 (C-12b), 145.4 (C-13), 138.9 (C-4b), 137.2 (C-2), 134.7 (C-1), 131.7 (C-8a), 128.3 (C-4),127.4 (C-3), 125.8 (C-4a), 124.5 (C-12), 123.1 (C-5), 121.2 (C-12c), 49.0 (C-14); HRESIMS m/z: [M]+ 376.00939 (calcd for C19H11BrN3O+, 376.00800).
Petrosamine (7)
Blue amorphous solid; NMR spectroscopic data in agreement to those previously reported;31 HRESIMS m/z: [M]+ 422.05040 (calcd for C21H17BrN3O2+, 422.04987).
Petrosamine B (8)
Blue amorphous solid; NMR spectroscopic data in agreement to those previously reported;32 HRESIMS m/z: [M]+ 422.05033 (calcd for C21H17BrN3O2+, 422.04987).
Acknowledgments
We thank Drs. Erin Zeituni and Joseph Campbell from the National Institute of Allergy and Infectious Diseases for helpful discussions. This project has been funded in whole or in part with Federal funds from the Division of Microbiology and Infectious Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, under Contract no. 75N93019D00011/75N93019F00210.This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under contract HHSN261200800001E and by the National Cancer Institute’s Cancer MoonshotSM NCI Program for Natural Products Discovery-Cure (ZIA BC 011854). The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does the mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Glossary
Abbreviations
- ESKAPE
Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter species
- NIAID
National Institute of Allergy and Infectious Diseases
- NCI
National Cancer Institute
- NPNPD
NCI Program for Natural Product Discovery
- DMID
Division of Microbiology and Infectious Diseases
- HTS
high throughput screening
- MRSA
methicillin-resistant S. aureus
- HPLC
high-performance liquid chromatography
- LC/MS
liquid chromatography mass spectrometry
- NMR
nuclear magnetic resonance
- FTIR
Fourier-transform infrared
- ATCC
American Type Culture Collection
- Mw
molecular weight
- R
resistant
- MDR
multidrug resistant
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsinfecdis.3c00067.
Structure elucidation for compounds 5 and 6, format of screening data, summary statistics, selection criteria, screening results, description of de-replication methodology, H NMR, C NMR, H–C HMBC correlations, H–C COSY NMR, and H–C HSQC spectra for compounds 1−6 (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Levin-Reisman I.; Brauner A.; Ronin I.; Balaban N. Q. Epistasis between antibiotic tolerance, persistence, and resistance mutations. Proc. Natl. Acad. Sci. U.S.A. 2019, 116, 14734–14739. 10.1073/pnas.1906169116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y.; Cheng D.; Xie J.; Zhang Y.; Wan Y.; Zhang Y.; Shi X. Impacts of farmland application of antibiotic-contaminated manures on the occurrence of antibiotic residues and antibiotic resistance genes in soil: A meta-analysis study. Chemosphere 2022, 300, 134529. 10.1016/j.chemosphere.2022.134529. [DOI] [PubMed] [Google Scholar]
- Reardon S. Antibiotic use in farming set to soar despite drug-resistance fears. Nature 2023, 614, 397. 10.1038/d41586-023-00284-x. [DOI] [PubMed] [Google Scholar]
- Bennett P. M. Plasmid encoded antibiotic resistance: acquisition and transfer of antibiotic resistance genes in bacteria. Br. J. Pharmacol. 2008, 153, S347–S357. 10.1038/sj.bjp.0707607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention . Antibiotic Resistance Threats in the United States, 2019.
- Martens E.; Demain A. L. The antibiotic resistance crisis, with a focus on the United States. J. Antibiot. 2017, 70, 520–526. 10.1038/ja.2017.30. [DOI] [PubMed] [Google Scholar]
- Chahine E. B.; Dougherty J. A.; Thornby K.-A.; Guirguis E. H. Antibiotic Approvals in the Last Decade: Are We Keeping Up With Resistance?. Ann. Pharmacother. 2022, 56, 441–462. 10.1177/10600280211031390. [DOI] [PubMed] [Google Scholar]
- Murray C. J. L.; Ikuta K. S.; Sharara F.; Swetschinski L.; Robles Aguilar G.; Gray A.; Han C.; Bisignano C.; Rao P.; Wool E.; et al. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet 2022, 399, 629–655. 10.1016/s0140-6736(21)02724-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice L. B. Federal Funding for the Study of Antimicrobial Resistance in Nosocomial Pathogens: No ESKAPE. J. Infect. Dis. 2008, 197, 1079–1081. 10.1086/533452. [DOI] [PubMed] [Google Scholar]
- In Vitro Assessment for Antimicrobial Activity Program. https://www.niaid.nih.gov/research/vitro-assessment-antimicrobial-activity (accessed Jan, 2023).
- Perlin D. S.; Rautemaa-Richardson R.; Alastruey-Izquierdo A. The global problem of antifungal resistance: prevalence, mechanisms, and management. Lancet Infect. Dis. 2017, 17, e383–e392. 10.1016/s1473-3099(17)30316-x. [DOI] [PubMed] [Google Scholar]
- Thornburg C. C.; Britt J. R.; Evans J. R.; Akee R. K.; Whitt J. A.; Trinh S. K.; Harris M. J.; Thompson J. R.; Ewing T. L.; Shipley S. M.; Grothaus P. G.; Newman D. J.; Schneider J. P.; Grkovic T.; O’Keefe B. R. NCI Program for Natural Product Discovery: A Publicly-Accessible Library of Natural Product Fractions for High-Throughput Screening. ACS Chem. Biol. 2018, 13, 2484–2497. 10.1021/acschembio.8b00389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Program for Natural Products Discovery Prefractionated Library. https://dtp.cancer.gov/organization/npb/npnpd_prefractionated_library.htm (accessed Jan, 2023).
- David M. Z.; Daum R. S. Community-associated methicillin-resistant Staphylococcus aureus: epidemiology and clinical consequences of an emerging epidemic. Clin. Microbiol. Rev. 2010, 23, 616–687. 10.1128/cmr.00081-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirel L.; Madec J.-Y.; Lupo A.; Schink A.-K.; Kieffer N.; Nordmann P.; Schwarz S., Antimicrobial resistance in Escherichia coli. Microbiol. Spectrum 2018, 6 (4). 10.1128/microbiolspec.arba-0026-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba T.; Ara T.; Hasegawa M.; Takai Y.; Okumura Y.; Baba M.; Datsenko K. A.; Tomita M.; Wanner B. L.; Mori H. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol. Syst. Biol. 2006, 2, 2006.0008. 10.1038/msb4100050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulavik M. C.; Houseweart C.; Cramer C.; Jiwani N.; Murgolo N.; Greene J.; DiDomenico B.; Shaw K. J.; Miller G. H.; Hare R.; Shimer G. Antibiotic susceptibility profiles of Escherichia coli strains lacking multidrug efflux pump genes. Antimicrob. Agents Chemother. 2001, 45, 1126–1136. 10.1128/aac.45.4.1126-1136.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grkovic T.; Akee R. K.; Thornburg C. C.; Trinh S. K.; Britt J. R.; Harris M. J.; Evans J. R.; Kang U.; Ensel S.; Henrich C. J.; Gustafson K. R.; Schneider J. P.; O’Keefe B. R. National Cancer Institute (NCI) Program for Natural Products Discovery: Rapid Isolation and Identification of Biologically Active Natural Products from the NCI Prefractionated Library. ACS Chem. Biol. 2020, 15, 1104–1114. 10.1021/acschembio.0c00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabriskie T. M.; Klocke J. A.; Ireland C. M.; Marcus A. H.; Molinski T. F.; Faulkner D. J.; Xu C.; Clardy J. Jaspamide, a modified peptide from a Jaspis sponge, with insecticidal and antifungal activity. J. Am. Chem. Soc. 1986, 108, 3123–3124. 10.1021/ja00271a062. [DOI] [Google Scholar]
- Kato E.; Tokunaga Y.; Sakan F. Stilbenoids Isolated from the Seeds of Melinjo (Gnetum gnemon L.) and Their Biological Activity. J. Agric. Food Chem. 2009, 57, 2544–2549. 10.1021/jf803077p. [DOI] [PubMed] [Google Scholar]
- Johnson J. R.; Bruce W. F.; Dutcher J. D. Gliotoxin, The Antibiotic Principle of Gliocladium fimbriatum. I. Production, Physical and Biological Properties1. J. Am. Chem. Soc. 1943, 65, 2005–2009. 10.1021/ja01250a051. [DOI] [Google Scholar]
- Carmelya S.; Ilanb M.; Kashmana Y. 2-Aminoimidazole alkaloids from the marine sponge Leucetta chagosensis. Tetrahedron 1989, 45, 2193–2200. 10.1016/s0040-4020(01)80079-x. [DOI] [Google Scholar]
- Campos P.-E.; Herbette G.; Fougère L.; Clerc P.; Tintillier F.; de Voogd N. J.; Le Goff G.; Ouazzani J.; Gauvin-Bialecki A. An Aminopyrimidone and Aminoimidazoles Alkaloids from the Rodrigues Calcareous Marine Sponge Ernsta naturalis. Mar. Drugs 2022, 20, 637. 10.3390/md20100637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koswatta P. B.; Lovely C. J. Structure and synthesis of 2-aminoimidazole alkaloids from Leucetta and Clathrina sponges. Nat. Prod. Rep. 2011, 28, 511–528. 10.1039/c0np00001a. [DOI] [PubMed] [Google Scholar]
- Carroll A. R.; Bowden B. F.; Coll J. C. New imidazole alkaloids from the sponge Leucetta sp. and the associated predatory nudibranch Notodoris gardineri. Aust. J. Chem. 1993, 46, 1229–1234. 10.1071/ch9931229. [DOI] [Google Scholar]
- Tsukamoto S.; Kawabata T.; Kato H.; Ohta T.; Rotinsulu H.; Mangindaan R. E. P.; van Soest R. W. M.; Ukai K.; Kobayashi H.; Namikoshi M. Naamidines H and I, Cytotoxic Imidazole Alkaloids from the Indonesian Marine Sponge Leucetta chagosensis. J. Nat. Prod. 2007, 70, 1658–1660. 10.1021/np070246i. [DOI] [PubMed] [Google Scholar]
- Gong K.-K.; Tang X.-L.; Liu Y.-S.; Li P.-L.; Li G.-Q. Imidazole alkaloids from the South China Sea sponge Pericharax heteroraphis and their cytotoxic and antiviral activities. Molecules 2016, 21, 150. 10.3390/molecules21020150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copp B. R.; Fairchild C. R.; Cornell L.; Casazza A. M.; Robinson S.; Ireland C. M. Naamidine A Is an Antagonist of the Epidermal Growth Factor Receptor and an in Vivo Active Antitumor Agent. J. Med. Chem. 1998, 41, 3909–3911. 10.1021/jm980294n. [DOI] [PubMed] [Google Scholar]
- Guo P.; Li G.; Liu Y.; Lu A.; Wang Z.; Wang Q. Naamines and Naamidines as Novel Agents against a Plant Virus and Phytopathogenic Fungi. Mar. Drugs 2018, 16, 311. 10.3390/md16090311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasdemir D.; Marshall K. M.; Mangalindan G. C.; Concepción G. P.; Barrows L. R.; Harper M. K.; Ireland C. M. Deoxyamphimedine, a New Pyridoacridine Alkaloid from Two Tropical Xestospongia Sponges. J. Org. Chem. 2001, 66, 3246–3248. 10.1021/jo010153k. [DOI] [PubMed] [Google Scholar]
- Molinski T. F.; Fahy E.; Faulkner D. J.; Van Duyne G. D.; Clardy J. Petrosamine, a novel pigment from the marine sponge Petrosia sp. J. Org. Chem. 1988, 53, 1340–1341. 10.1021/jo00241a049. [DOI] [Google Scholar]
- Carroll A. R.; Ngo A.; Quinn R. J.; Redburn J.; Hooper J. N. A. Petrosamine B, an Inhibitor of the Helicobacter pylori Enzyme Aspartyl Semialdehyde Dehydrogenase from the Australian Sponge Oceanapia sp. J. Nat. Prod. 2005, 68, 804–806. 10.1021/np049595s. [DOI] [PubMed] [Google Scholar]
- Marshall K. M.; Barrows L. R. Biological activities of pyridoacridines. Nat. Prod. Rep. 2004, 21, 731–751. 10.1039/b401662a. [DOI] [PubMed] [Google Scholar]
- Joule J. A.; Alvarez M. Pyridoacridines in the 21st Century. Eur. J. Org. Chem. 2019, 2019, 5043–5072. 10.1002/ejoc.201900401. [DOI] [Google Scholar]
- MarineLit: A Database of the Marine Natural Products Literature. http://pubs.rsc.org/marinelit/ (acessed January 2023).
- Lomovskaya O.; Lee A.; Hoshino K.; Ishida H.; Mistry A.; Warren M. S.; Boyer E.; Chamberland S.; Lee V. J. Use of a genetic approach to evaluate the consequences of inhibition of efflux pumps in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 1999, 43, 1340–1346. 10.1128/aac.43.6.1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman D. J.; Cragg G. M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. 10.1021/acs.jnatprod.9b01285. [DOI] [PubMed] [Google Scholar]
- Appleton D. R.; Buss A. D.; Butler M. S. A simple method for high-throughput extract prefractionation for biological screening. Chimia 2007, 61, 327–331. 10.2533/chimia.2007.327. [DOI] [Google Scholar]
- Camp D.; Davis R. A.; Campitelli M.; Ebdon J.; Quinn R. J. Drug-like Properties: Guiding Principles for the Design of Natural Product Libraries. J. Nat. Prod. 2012, 75, 72–81. 10.1021/np200687v. [DOI] [PubMed] [Google Scholar]
- Carter G. T. Natural products and Pharma 2011: Strategic changes spur new opportunities. Nat. Prod. Rep. 2011, 28, 1783–1789. 10.1039/c1np00033k. [DOI] [PubMed] [Google Scholar]
- Fuhrmeister A. S.; Jones R. N. The Importance of Antimicrobial Resistance Monitoring Worldwide and the Origins of SENTRY Antimicrobial Surveillance Program. Open Forum Infect. Dis. 2019, 6, S1–S4. 10.1093/ofid/ofy346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J.-H.; Chung T. D. Y.; Oldenburg K. R. A Simple Statistical Parameter for Use in Evaluation and Validation of High Throughput Screening Assays. J. Biomol. Screening 1999, 4, 67–73. 10.1177/108705719900400206. [DOI] [PubMed] [Google Scholar]
- Clinical and Laboratory Standards Institute . M100 Performance Standards for Antimicrobial Susceptibility Testing, 32nd ed.; Clinical and Laboratory Standards Institute: Wayne, PA, 2022. [Google Scholar]
- Clinical and Laboratory Standards Institute . M27M44S Performance Standards for Antifungal Susceptibility Testing of Yeasts, 3rd ed.; Clinical and Laboratory Standards Institute: Wayne, PA, 2022. [Google Scholar]
- McCloud T. G. High throughput extraction of plant, marine and fungal specimens for preservation of biologically active molecules. Molecules 2010, 15, 4526–4563. 10.3390/molecules15074526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nukoolkarn V. S.; Saen-oon S.; Rungrotmongkol T.; Hannongbua S.; Ingkaninan K.; Suwanborirux K. Petrosamine, a potent anticholinesterase pyridoacridine alkaloid from a Thai marine sponge Petrosia n. sp. Bioorg. Med. Chem. 2008, 16, 6560–6567. 10.1016/j.bmc.2008.05.027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.