Abstract
Curcumin (CUR), a natural phenolic compound, has been increasingly investigated in several malignancies due to its safe profile and ability to affect a wide range of oncogenic targets. With the ability to affect metastasis, apoptosis, and angiogenesis in colorectal cancer (CRC) and its tolerability at high doses, CUR is an attractive target for study. However, poor bioavailability and unfavorable pharmacokinetics and pharmacodynamics have hampered CUR’s efficacy in clinical trials. Development of its derivatives and alternative delivery methods have shown the potential to overcome its inherent bioavailability issues. Recent analyses of various derivatives and nanoparticle encapsulation of CUR have demonstrated increased effectiveness in CRC studies. A major advantage of CUR has been its synergistic effects when used in combination with various chemotherapeutic agents. CUR offers a unique treatment option in terms of patient safety and its ability to be used in combination with current treatments for CRC. Further development of its derivatives and alternative delivery options offer potential new avenues of treatment that could outperform previous efforts to establish CUR as a CRC therapy.
Keywords: curcumin, curcumin derivatives, colon cancer, clinical trials, combination treatment
I. INTRODUCTION
Colorectal cancer (CRC) comprises the fourth and second most common malignancy in terms of incidence and deaths, respectively, in the United States.1 The global burden of CRC is predicted to rise to 2.2 million new cases with 1.1 million deaths annually in the next decade.2 The CRC incidence has remained relatively the same over the last few decades despite increased screening and awareness of CRC. This issue could be due to the conundrum of increases in cases for younger patients (ages 20–49), whereas traditionally CRC has been thought to mainly affect older populations usually 50 years of age and above.3 Current CRC therapies consist of various surgical techniques, including polypectomy, mucosal resection, partial colectomy, and lymph node removal followed by radiation, and chemotherapy, including the FOLFOX (combination of 5-fluorouracil, oxaliplatin, and folinic acid).4 Some newer immunotherapies and more targeted drug therapies, including bevacizumab and cetuximab, are available as well but are typically reserved for more advanced CRC cases. CRC carries a 5-year survival rate around 60–70%; however, in patients with distant metastasis, this drops to around 14–15%.5
The majority of CRC cases is carcinomas, namely adenocarcinomas, however, gastrointestinal stromal tumors (GISTs), carcinoid tumors, sarcomas, and lymphomas are also considered as types of CRC depending on the malignancy’s origin. CRC has strong links to genetic risk and environmental factors. Lifestyle factors (e.g., diet and exercise) and comorbid conditions (e.g., inflammatory bowel disease, diabetes mellitus) have demonstrated a well-established role in multiple cancers including CRC.7 However, CRC has also been linked to unique genetic dispositions. Inherited syndromes such as the Lynch syndrome, familial adenomatous polyposis (FAP), and hereditary non-polyposis colorectal cancer (HNPCC) are most common attributing to around 5% of all CRC cases.6 Diseases such as inflammatory bowel disease (IBD), Crohn’s disease, and diabetes mellitus are also well-known risk factors for the development of CRC.7 Certain mutations are also closely related to CRC oncogenesis and progression. The most common driver mutations include KRAS, BRAF, APC, SMAD4, TP53, and PIK3CA.8 The accumulation of these gene mutations not only results in increased CRC oncogenic processes, but also confers therapy resistance, late tumor recurrence, and worse overall prognosis for CRC patients.9
Natural products and their variants have been utilized as treatments for various diseases, and many have become models for drug designs as well. Cucurmin (CUR) is a phenolic compound and the active metabolite in Curcuma longa (turmeric), which has been used in Ayurvedic medicine as a treatment for inflammatory disorders.10 Similar to other phenolic compounds, CUR has inherent antioxidant abilities and serves as a free radical scavenger.11 Recently, CUR has been shown to have anti-neoplastic activity in a variety of malignancies through modulation of multiple pathways and oncogenic targets.12-14 In glioblastoma, CUR decreases Skp2, an oncoprotein that degrades multiple key cyclin-dependent kinase (CDK) inhibitors, resulting in upregulation of p57 and a downregulation of pAkt in U251 and SNB19 cell lines.15 In prostate cancer, CUR represses H3K4me3 cells through epigenetic regulation via inhibition of the JNK pathways.16 CUR has also shown the ability to induce apoptosis and cell cycle arrest in medulloblastoma via reduced histone deacetylase (HDAC) 4 activity and increased tubulin acetylation.17 The same effects were mirrored in vivo in a medulloblastoma Smo/Smo transgenic mouse model, where CUR decreased tumor growth and improved survival.17 Despite successes at the in vitro and in vivo levels in several cancers, CUR’s bioavailability has been a challenge to its translational applications. This low bioavailability is attributed to its insolubility in water and rapid conversion to inactive metabolites, resulting in poor pharmacokinetics (PK) and pharmacodynamics (PD), thus limiting its uses as an effective medication.
II. CURCUMIN (CUR) THERAPEUTIC APPLICATIONS IN CANCER
Despite its low bioavailability, CUR has been shown to be safe in high doses and used in multiple clinical trials for a variety of malignancies.18 A clinical trial for combination CUR treatments in advanced breast cancer opened in 2017 to assess paclitaxel and CUR combination therapy (NCT03072992). CUR (300 mg) was administered intravenously (IV) in combination with paclitaxel (80 mg/m2) once a week for 12 weeks. The control group received IV administration of placebo in combination with paclitaxel (80 mg/m2) given on the same weekly interval for 12 weeks. Enrollment consisted of 150 patients with 93 completing all study visits. The overall response rate (ORR), in terms of complete or partial tumor reduction, was significantly higher in the CUR group (61.3%) compared to placebo (38.5%). The authors also saw a better response to CUR treatment in patients with HR+/HER2− breast cancer and noted that CUR had a slight benefit in relieving fatigue in the treatment groups.14 Another small clinical trial in castration-resistant prostate cancer (CRPC) investigated CUR administration in combination with docetaxel (DOC) and prednisone (PRED) with promising results. Thirty patients received DOC/PRED for six cycles in combination with oral CUR (6,000 mg/day) at day −4 to day +2 of DOC. The dose was determined from a phase I dose escalation study showing a maximum tolerable dose of 8000 mg/day.19 Twenty patients were 100% compliant with CUR treatment, and the authors noted that the dosage and regimen were well tolerated with no adverse events correlated with the CUR treatment. There was a tumor and prostate specific antigen (PSA) response in 40% and 59% of patients, respectively.20 It was a single-arm clinical trial with all patients receiving CUR and DOC/PRED treatments.
Outside of the direct effects on tumor growth and proliferation, clinical trials involving CUR’s anti-inflammatory and immunomodulatory effects in the context of endometrial cancer have been undertaken (NCT02017353). The authors of the study utilized a lecithin-based delivery system of CUR in the hope of improving CUR plasma concentration. Seven patients were given CUR (2 g/day) orally for two weeks. Non-significant effects were seen on inflammatory markers, but some slight immunomodulatory actions were noted. The CUR treatment downregulated MHC expression in leukocytes and ICOS expression in CD8+ T cells but upregulated CD69 levels on CD16-NK cells. Some of their findings were in contradiction with previous studies, and the authors noted that this could have been due to the low number of study participants.21 Multiple clinical trials in a variety of cancers have shown CUR’s effectiveness as a monotherapy or alongside chemotherapeutics or radiation as an adjuvant to decrease side effects of traditional cancer treatments (Table 1).
TABLE 1:
National clinical trials (NCTs) utilizing CUR
| NCT number | Title | Status | Intervention | Characteristics |
|---|---|---|---|---|
| NCT03072992 | Curcumin in Combination with Chemotherapy in Advanced Breast Cancer | Completed | Curcumin (CUC-01) vs. Placebo, in combination with Paclitaxel | Interventional Phase 2 |
| NCT01160302 | An Exploratory Biomarker Trial of the Food Substances Curcumin C3 Complex® in Subjects with Newly Diagnosed Head and Neck Squamous Cell Carcinoma | Completed | Microgranular Curcumin C3 Complex | Interventional Early Phase 1 |
| NCT02017353 | Effect of Curcumin Addition to Standard Treatment on Tumour-induced Inflammation in Endometrial Carcinoma | Completed | Dietary Supplement: Curcuphyt | Interventional Phase 2 |
| N/A | The New Combination Docetaxel, Prednisone, and Curcumin in Patients with Castration-Resistant Prostate Cancer: A Pilot Phase II Study | Completed | Docetaxel, Prednisone or Prenisolone, Curcumin | Interventional Phase 2 |
| NCT01712542 | Measurement of Intratumoral Concentration of the Nontoxic Natural Compound Curcumin in Glioblastoma Patients | Completed | Curcumin | Observational |
| NCT01740323 | Meriva for Treatment-Induced Inflammation and Fatigue in Women with Breast Cancer | Completed | Meriva (Curcumin) | Interventional Phase 2 |
| NCT02556632 | Effectiveness of Prophylactic Topical Agents for Radiation Dermatitis | Completed | HPR Plus or Curcumin | Interventional Phase 2 |
III. CUR AND CRC
Poor prognosis for late-stage CRC and hope for better patient outcomes have pushed studies for alternative treatments that offer better outcomes with lower side effects. CUR has been investigated both as a monotherapy and in combination with other treatments for CRC in multifaceted in vivo and in vitro studies. Antineoplastic properties of CUR are related to its effects on modulation of cytokines, growth factors, and signaling pathways involved in oncogenesis.
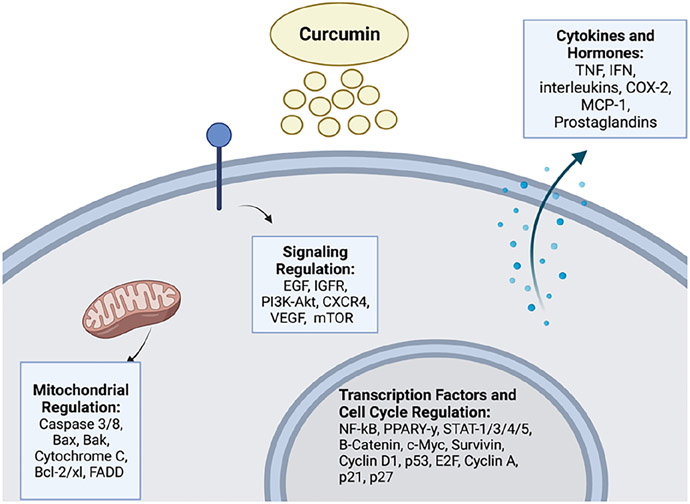
Tong et al. showed the ability of CUR to suppress CRC cell invasion via the AMPK-induced inhibition of NF-κB, urokinase-type plasminogen activator (uPA), and MMP9. Human colon cancer cells SW480 and LoVo exhibited decreased cell viability and invasion upon CUR treatment elucidated by MTT assays and transwell assays, respectively. uPA and MMP9 expressions were decreased after CUR treatment, and NF-κB DNA binding activity was decreased in a concentration-dependent manner through ELISA analyses.22 Along this line, NF-κB translocation has been shown to be inhibited by CUR and CUR synthetic analog intervention in CRC, leading to G0/G1 cell cycle arrest and downregulation of thymidylate synthase in HCT116 and HT-29 cells.23 Yu et al. reported effects of LncRNA pathways on CUR treatment effectiveness in CRC cell lines, and NBR2 (long noncoding RNA neighbor of BRCA1 lncRNA2) was shown to mediate CUR’s effects on CRC cell lines by activating AMPK and inactivating the mTOR signaling pathway.24 CUR inhibition of rat CRC carcinogenesis through peroxisome proliferator-activated receptor gamma (PPAR-y) activation was shown by Liu et al.25 A group of 1, 2-dimethylhydrazine (DMH) induced CRC rats showed decreased oncogenesis upon CUR intervention, and PPAR-y expression in colon mucosal tissue was significantly decreased in a concentration-dependent manner.25 CUR has been shown to have a wide range of other proteins and signaling pathway interactions expanding avenues for CRC treatment (Fig. 1).
FIG. 1:
General effects of CUR in colorectal cancer. CUR exerts its effects through multiple signaling pathways leading to the regulation of mitochondrial and cell cycle proteins, transcription factors, and may also affect cytokines and hormonal activities (image was created with https://biorender.com/).
Besides late term metastasis in CRC patients, a major challenge for efficacious treatment is resistance to cytotoxic chemotherapy. Chemo-surviving cells can often lead to relapse and proliferation of non-responding malignant cells. Resistance to a single drug can also lead to the development of multidrug resistance (MDR). This chemoresistance can develop over the time of the cancer, resulting in increasing chemotherapy failure at recurrent and later stages of CRC. The development of genotypic and phenotypic heterogeneities result in the ability of CRC to resist external inhibitory signaling via multiple diverse mechanisms. This resistance to treatment stems from CRC cell modulation of signaling pathways like the Wnt/B-catenin, PI3K/Akt, and MAPK pathways. The regulation of these pathways enhances cell proliferation, induces metabolic modifications that increases glycolysis and inhibits the pentose phosphate pathway (PPP), and can result in aberrant DNA repair and increased autophagy, enhancing chemoresistance.26-28 The MDR-associated protein 1 (MRP1), sequestration of chemotherapeutic drugs, and the presence of ABC transporters resulting in augmented drug efflux can additionally lead to increased resistance in treatment.29,30 CUR has demonstrated the ability to reverse CRC drug resistance when used in combination with traditional chemotherapeutics. When combined with both oxaliplatin and 5-FU, two treatments commonly associated with resistance in CRC, CUR has highlighted the ability to rescue chemoresistance both in vitro and in vivo. Combination of CUR with oxaliplatin was shown to reverse resistance in multiple CRC cell lines through modulation of the CXC-chemokine/NF-κB signaling pathways.31 More recently, CUR has also been shown to reverse oxaliplatin resistance in CRC through dampening of the TGF-b/Smad signaling pathway.32 Furthermore, CUR has been shown to sensitize CRC cells to 5-FU treatment and rescue resistance through inhibition of the Src protein kinase and NF-κB signaling pathways.33,34 Separate groups have also attributed CUR’s reversal of 5-FU resistance in CRC cells to the regulation of the TET1-NKD-Wnt signaling and downregulation of heat shock protein 27 and P-glycoprotein.35,36
IV. CLINICAL TRIALS OF CUR FOR CRC
CUR has demonstrated varying degrees of success in CRC clinical trials. NCT01490996 was a phase II clinical trial involving 28 patients with metastatic CRC receiving either FOLFOX and bevacizumab or 2g oral CUR + FOLFOX and bevacizumab (CU-FOX). The main goals of the study were to observe safety, curcuminoid levels, and the C-X-C motif chemokine ligand 1 (CXCL1). In a separate explant model study, CUR response was found to be correlated with greater tissue expression of CXCL1.37 The addition of CUR to the regimen was noted to be safe and tolerable, and there was no significant difference between the groups in terms of neurotoxicity. CUR also had no significant alteration of CXCL1 expression over time.38 Clinical trial NCT00365209 studied oral CUR delivery (2g group and 4g group) for 30 days in patients with subclinical neoplastic lesions. 21 patients and 19 patients completed the study in the 2g and 4g groups respectively. Serious adverse events occurred in a single patient from the 4g groups, but the CUR regimens were generally well tolerated. The study also intended to measure prostaglandin E2 (PGE2) and 5-hydroxy-eicosatetraenoic acid (5-HETE) with the anticipation of seeing CUR’s ability to modulate these two factors. Other CUR clinical trials have been completed and are detailed in Table 2.
TABLE 2:
NCTs in CRC utilizing curcumin
| NCT Number | Title | Status | Intervention | Characteristics |
|---|---|---|---|---|
| NCT01333917 | Curcumin Chemoprevention of Colorectal Neoplasia | Completed | Curcumin C3 tablet | Interventional Phase 1 |
| NCTO1490996 | A Phase I/IIa Study Combining Curcumin (Curcumin C3-Complex, Sabinsa) with Standard Care FOLFOX Chemotherapy in Patients with Inoperable Colorectal Cancer | Completed | Curcumin C3, FOLFOX | Interventional Phase 1/2 |
| N/A | Adjuvant Therapy with Bioavailability-Boosted Curcuminoids Suppresses Systemic Inflammation and Improves Quality of Life in Patients with Solid Tumors: A Randomized Double-Blind Placebo-Controlled Trial | Completed | Meriva (Curcumin) | Double Blind Randomized Placebo Controlled |
| NCT00365209 | Phase IIA Trial of Curcumin Among Patients with Prevalent Subclinical Neoplastic Lesions (Aberrant Crypt Foci) | Completed | Oral Curcumin | Interventional Phase 2 |
V. CUR DERIVATIVES TESTED FOR CRC
Poor bioavailability, low aqueous solubility, and poor cellular uptake are major obstacles in the utilization of CUR for CRC. Low plasma concentrations and higher required dosages to maintain effective CUR treatments have led to the development of CUR derivatives and alternative treatment delivery systems. The ability to create derivatives based on the original CUR structure has revealed new targets that can be exploited in CRC, and multiple potential modifications of CUR’s chemical structure have been identified (Fig. 2). Targeting different CRC mutations and signaling pathways has given insight into CRC oncogenesis but also has created actionable targets for CUR treatment.
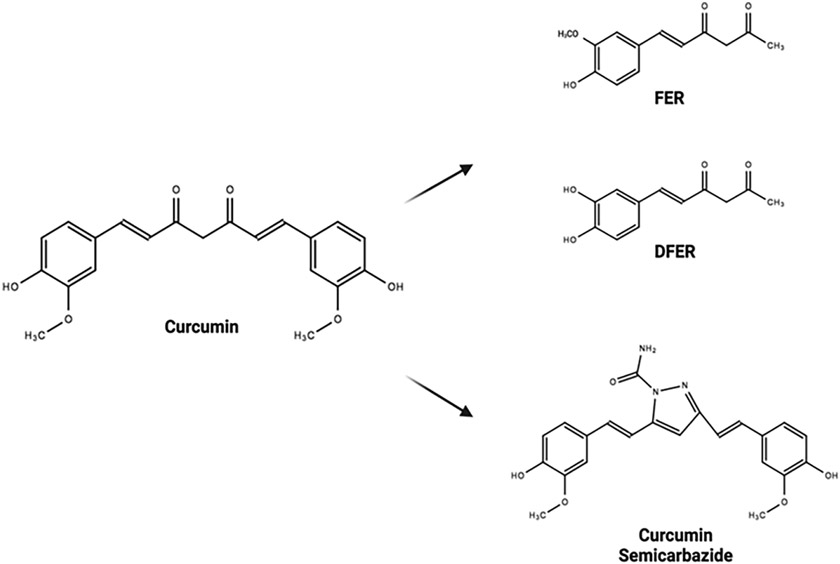
FIG. 2:
Various structural derivatives of CUR have been developed and have shown improvements over base CUR in CRC. Feruloylacetone (FER)/demethoxy-feruloylacetone (DFER), natural degradants of CUR, showed increased apoptosis in CRC cell lines.40 CUR semicarbazide was the most viable synthetic analog created with the purpose of stabilizing the base CUR structure.39 Both variations are examples of modifications on CUR’s chemical structure leading to a drug with a more favorable drug profile (chemical structures were drawn in ChemSpider; image was created with https://biorender.com/).
Active ligand docking has provided the ability to evaluate multiple CUR analogs prior to in vitro studies. In silico studies by Rodrigues et al., a semicarbazide derivative of CUR was discovered to have an IC50 roughly sevenfold lower compared to CUR in CRC cell lines. Stabilizing the diketo site of CUR, a source of instability for the molecule, through ring cyclization led to the screening of seven separate ligands. The most effective compound, CUR semicarbazide, was found to dock with high affinity against the Ab1-kinase protein, suggesting a potential mechanism of action for this analog in its ability to effectively kill CRC cell lines. However, a comparison with doxorubicin (Dox) showed the derivative to be slightly less effective than Dox, but still much more effective than CUR alone.39 Chou et al. investigated feruloylacetone (FER), a natural degradant of CUR, and demethoxy-feruloylacetone (DFER) in CRC cells. DFER was used to compare the difference at the methoxy group in FER, and both were found to inhibit proliferation in HCT116 cells. Suppression of mTOR/STAT3 signaling was noted with FER significantly inhibiting STAT3 phosphorylation and protein levels. Arrest of HCT116 cells at the G2/M phase was seen via p53/p21 activation and cyclin B upregulation. The effectiveness of both compounds was demonstrated in CRC cells; however, FER exhibited an increased apoptotic effect, possibly due to the methoxy group presence on the aromatic ring.40
MS13, a diarylpentaoid CUR analog, was found to inhibit SW480 and SW620 cells with greater cytotoxic effects when compared to CUR alone at 48 and 72 hours. Both cell lines showed apoptotic morphological features through a fluorescent microscopic analysis. Caspase 3 and Bcl-2 were increased and decreased, respectively, indicative of increased apoptotic activity in both cell lines.41 Another chemically synthesized analog of CUR, DMCH, was synthesized through manipulation of methoxy groups in the CUR’s base structure. Rahim et al. evaluated DMCH and its effectiveness against HT29 and SW620 cells. IC50 values for DMCH were between 2–3 times less than that of base CUR at multiple time points in both CRC cell lines. Annexin V/FITC and JC-1 analyses confirmed an increase in early and late apoptosis and changes in mitochondrial membrane potential after DMCH treatment and cell cycle accumulation was observed at sub-G0/G1 phase. DMCH also showed the ability to regulate multiple apoptosis-related genes and proteins, including Bax and Bad, Livin proteins, Bclx, pro-caspase 3, SMAC/Diablo proteins, and cytochrome C.42
The use of molecular dynamics and docking simulations have substantially increased the capability of investigators to observe multiple structural modifications at high throughput, allowing for increased stability of the CUR structure. These studies coupled with traditional manipulation of CUR’s structure and continued efforts to address the poor bioavailability of CUR will determine if effective analogs will be able to move through clinical trials more successfully than previous attempts with standard CUR.
VI. ALTERNATIVE DELIVERY SYSTEMS
Alternative delivery systems including nanoparticle (NP) formulations are allowing the ability of previously ineffective treatments to be evaluated at a more refined level. Free drug comparison studies to NP encapsulated drugs have shown efficacy in a variety of cancers.43-45 NPs and other novel forms of drug delivery have seemingly been able to overcome some of the bioavailability issues seen with traditional CUR treatments. However, the formulation of NPs is a multifaceted process that requires consideration of multiple factors, including size and surface charge of the NP and how effective the drug loading is for that particular NP. Defining these characteristics is the first step in developing effective NPs for CUR treatment in CRC. Kulbacka et al. loaded multiple poly (D, U-lactide) (PUA) nanocarriers with CUR and tested NPs with varying surface charges. The group also observed the effects of electroporation combined with their CUR NPs on LoVo, CHO-K1, and L6 cells. Electroporation or electro permeabilization, the delivery of electric stimulation to a specific site, disturbs cells in order to alter the permeability of the cell membrane potentially allowing drugs, chemicals, etc. to be introduced to the cell more easily. Kulbacka et al. reported that electroporation enhanced both free and encapsulated CUR delivery. Anionic nanocarriers that were stabilized with C12 surfactant gave the highest photodynamic potential, while cationic NPs allowed for the most efficient delivery of CUR in CHO-K1 and LoVo cells. Their nano systems had significant effects against cell viability depending on charge and degree of electroporation, but mostly outperformed free CUR in all cell lines and showed an increase in ROS confirmed by DCF assays.46
Other NP formulations including polysaccharide-zein composites have been studied for the purpose of enhancing uptake and oral bioavailability of CUR. Zein, a protein in corn, is a macromolecule that has the ability to self-assemble into NPs and is highly amphipathic. Further coating zein with polysaccharides has increased its movement through the GI tract, allowing for the potential effective loading of hydrophobic bioactive compounds.47 Liu et al. were able to formulate composite CUR NPs that cross linked polysaccharides, either gum Arabic, hyaluronic acid (HA), or pectin with zein. Each polysaccharide had distinct effects on the physicochemical properties of the NP, including stability, particle size, and encapsulation efficiency. However, of the three, HA-Zein-CUR had the most promising PK and distribution profile against multiple CRC cell lines, allowing for decreased IC50 values, low release of CUR in simulated GI digestion, and the highest amount of cellular uptake compared to free CUR treatment. These findings were corroborated in a xenograft nude mouse model where HA-Zein-CUR had higher plasma concentration and a longer mean residence time, indicating longer staying time and slower elimination of the HA NP formulation. Additionally, CUR concentration was significantly higher in tumor tissue of the mice after HA-Zein-CUR administration when compared to free CUR treatment.48 Other attempts at nanoparticle encapsulation and enhanced delivery methods of CUR for the treatment of CRC have been studied with most showing increases in efficacy compared to that of base CUR.49-51
The development of NP formulations and structural modifications of CUR to create effective PK/PD profiles for CRC treatment have allowed great strides in side-stepping the issue of low bioavailability. The potential for combining the derivatives mentioned above with NP encapsulation or other chemotherapeutic and non-traditional treatments for CRC could lead to the development of effective and more targeted treatments with better outcomes and potentially better quality of life for patients.
VII. CUR DERIVATIVES AND COMBINATION TREATMENTS
Current chemotherapeutic treatments are mainly given in combinations of several drugs to attack malignancies from diverse directions. Investigating potentially synergistic or additive drugs allows researchers to find improvements over current treatment options and evaluate the usefulness of novel drugs. The other reason for studying drug combinations is to reduce the dosages of toxic drugs used. Using minimal effective concentrations of drugs leads to potentially lower side effects in patients undergoing treatment. FOLFOX is a prime example of the efficacy of combination treatments currently used in CRC treatment. FOLFOX has been a mainstay of CRC treatment since the early 2000’s, offering better results compared to 5-FU treatment.52
Increasing the cytotoxic capabilities of CUR by using it in combination with other chemotherapeutics and toward other investigational targets allows for lowering drug dosages and improving patient tolerability. CUR and FOLFOX combination has been shown to effectively modulate multiple targets in CRC, including CRC stem cells (CSCs), EGFR and IGF-1R, and its efficacy has been shown in a clinical trial NCT01490996 (Table 2).53-56 CUR’s ability to combat chemoresistance has already been discussed, and its use in combination with dasatinib, a Src and Ab1 kinase inhibitor, has also been shown to further eliminate chemoresistant CRC cells. Nautiyal et al. demonstrated the effectiveness of CUR and dasatinib combination against chemo-resistant HCT116 and HT29 cells. The combination treatment decreased CSC bioThwe markers CD133, CD44, CD166, and aldolase dehydrogenase (ALDH). The treatment also allowed for decreased toxicity of dasatinib, shown through dose reduction indices and was effective at inhibiting colonosphere formation.57 Decitabine (DAC), a DNA methyltransferase, has been shown to elicit drug resistance leading to tumor recurrence.58 Previous research has shown an interaction between deoxycytidine kinase (dCK) affecting the cellular uptake of decitabine in HCT116 cells, possibly attributing to the resistance to decitabine treatment. Hisokawa et al. showed the ability of azacytidine (AC) and CUR to enhance cytotoxicity in decitabine-resistant CRC cells. AC exerted a degree of DNA demethylation, and CUR showed a synergistic effect with AC. The authors recommended it be further examined as an alternative to counter DAC resistance mostly due to the combination’s ability to attenuate dCK.59
CUR has also been used in combination with other non-standard chemotherapeutic options in order to find alternative safer treatments. Tolfenamic acid (TA), a non-steroidal anti-inflammatory drug (NSAID), has been used in a variety of malignancies and has recently been investigated in combination with CUR.60-62 TA in combination with CUR has shown a synergistic response in CRC cell lines, such as inhibited CRC cell line growth, induced loss of mitochondrial membrane potential, increased c-PARP and caspase activity, downregulated survivin and Sp1, and increased ROS.63 CUR combination with other dietary agents including oligomeric proanthocyanidins (OPCs) and resveratrol have also shown efficacy as anti-carcinogenic treatments through regulation of DNA replication, cell cycle, and various other cancer related pathways in CRC cell lines.64-66
VIII. CONCLUSIONS, FUTURE DIRECTIONS
The improvement in CRC treatment is crucial to address the poor 5-year survival rates and treatment success in recurrent and metastatic cases. The expansion of chemotherapeutic intervention through the study of novel drugs is vital for the progression of cancer treatment. CUR is a natural and safe alternative drug that is used alone and in combination with other drugs with hopes of improving treatment in multiple malignancies. CUR has the ability to modulate a wide range of targets in CRC oncogenesis, proliferation and metastasis. The main limiting factor for CUR usage is its poor bioavailability and lackluster PK/PD profile. The advantages of CUR are high tolerance of patients at relatively high doses and its ability to be incorporated into current chemotherapeutic regimen resulting in increased treatment efficacy. In order to increase the clinical applications for CRC treatment, the development of derivatives and various delivery methods are being investigated. Alteration of CUR structure and implementation of NP encapsulation in order to increase stability and bolster plasma concentration have been successfully implemented in multiple in vitro and in vivo studies. Continued evaluation of safety, development of derivatives and efficient delivery methods, and combinations with other drugs will show the true clinical capabilities of CUR.
ACKNOWLEDGMENTS
CL and RB were supported by the Cancer Prevention and Research Institute of Texas (CPRIT) through Grant (#RP210046). RB was also supported by the National Institutes of Health (NIH) Grants (NIH/NCI # P20CA233355; NIH/NIMHD # U54 MD006882; NIH/NIMHD #: 1S21 MD012472). The content in this article is the responsibility of the authors and not necessarily represent the official views of CPRIT or NIH.
ABBREVIATIONS:
- CDK
cyclin-dependent kinase
- CRC
colorectal cancer
- CRPC
castration-resistant prostate cancer
- CU-FOX
curcumin + FOLFOX (5-fluorouracil, oxaliplatin, and folinic acid) and bevacizumab
- CUR
curcumin
- CXCL1
C-X-C motif chemokine ligand 1
- DFER
demethoxy-feruloylacetone
- DMH
1, 2-dimethylhydrazine
- FAP
familial adenomatous polyposis
- FER
feruloylacetone
- FOLFOX
5-fluorouracil, oxaliplatin, and folinic acid
- GISTs
gastrointestinal stromal tumors
- HA
hyaluronic acid
- HDAC
histone deacetylase
- HNPCC
hereditary non-polyposis colorectal cancer
- IBD
inflammatory bowel disease
- IV
intravenously
- MDR
multidrug resistance
- MRP1
multidrug resistance associated protein 1
- NCT
national clinical trial
- NP
nanoparticle
- ORR
overall response rate
- PD
pharmacodynamics
- PK
pharmacokinetics
- PGE2
prostaglandin E2
- PLA
poly (D, L-lactide)
- PPAR-y
peroxisome proliferator-activated receptor gamma
- PPP
pentose phosphate pathway
- uPA
urokinase-type plasminogen activator
- 5-HETE
hydroxy-eicosatetraenoic acid
REFERENCES
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7–33. [DOI] [PubMed] [Google Scholar]
- 2.Arnold M, Sierra MS, Laversamie M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66(4):683–91. [DOI] [PubMed] [Google Scholar]
- 3.Willyard C. The colon cancer conundrum. Nature. In press 2021. [DOI] [PubMed] [Google Scholar]
- 4.Chan GHJ, Chee CE. Making sense of adjuvant chemotherapy in colorectal cancer. J Gastrointest Oncol 2019;10(6):1183–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mattiuzzi C, Sanchis-Gomar F, Lippi G. Concise update on colorectal cancer epidemiology. Ami Transl Med. 2019;7(21):609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Snyder C, Hampel H. Hereditary colorectal cancer syndromes. Semin Oncol Nurs. 2019;35(1):58–78. [DOI] [PubMed] [Google Scholar]
- 7.Lotfollahzadeh S, Recio-Boiles A, Cagir B. Colon Cancer. 2022. Jul 10. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2022. [PubMed] [Google Scholar]
- 8.Raskov H, Søby JH, Troelsen J, Bojesen RD, Gögenur I. Driver gene mutations and epigenetics in colorectal cancer. ANN Surg. 2020;271(l):75–85. [DOI] [PubMed] [Google Scholar]
- 9.Hammond WA, Swaika A, Mody K. Pharmacologic resistance in colorectal cancer: A review. Ther Adv Med Oncol. 2016;8(1):57–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jurenka JS. Anti-inflammatory properties of curcumin, a major constituent of Curcuma longa: A review of preclinical and clinical research. Altern Med Rev. 2009;14(2):141–53. [PubMed] [Google Scholar]
- 11.Ak T, Gülçin I. Antioxidant and radical scavenging properties of curcumin. Chem Biol Interact. 2008;174(1):27–37. [DOI] [PubMed] [Google Scholar]
- 12.Lelli D, Pedone C, Majeed M, Sahebkar A. Curcumin and lung cancer: The role of microRNAs. Curr Pharm Des. 2017;23(23):3440–4. [DOI] [PubMed] [Google Scholar]
- 13.Zhao W, Zhou X, Qi G, Guo Y. Curcumin suppressed the prostate cancer by inhibiting JNK pathways via epigenetic regulation. J Biochem Mol Toxicol. 2018;32(5):e22049. [DOI] [PubMed] [Google Scholar]
- 14.Saghatelyan T, Tananyan A, Janoyan N, Tadevosyan A, Petrosyan H, Hovhannisyan A, Hayrapetyan L, Arustamyan M, Arnhold J, Rotmann AR, Hovhannisyan A, Panossian A. Efficacy and safety of curcumin in combination with paclitaxel in patients with advanced, metastatic breast cancer: A comparative, randomized, double-blind, placebo-controlled clinical trial. Phytomedicine. 2020;70:153218. [DOI] [PubMed] [Google Scholar]
- 15.Wang L, Ye X, Cai X, Su J, Ma R, Yin X, Zhou X, Li H, Wang Z. Curcumin suppresses cell growth and invasion and induces apoptosis by down-regulation of Skp2 pathway in glioma cells. Oncotarget. 2015;6(20):18027–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao W, Zhou X, Qi G, Guo Y. Curcumin suppressed the prostate cancer by inhibiting JNK pathways via epigenetic regulation. J Biochem Mol Toxicol. 2018;32(5):e22049. [DOI] [PubMed] [Google Scholar]
- 17.Lee SJ, Krauthauser C, Maduskuie V, Fawcett PT, Olson JM, Rajasekaran SA. Curcumin-induced HDAC inhibition and attenuation of medulloblastoma growth in vitro and in vivo. BMC Cancer. 2011;11:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dei Cas M, Ghidoni R. Dietary curcumin: Correlation between bioavailability and health potential. Nutrients. 2019;11(9):2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mahammedi H, Planchat E, Pouget M, Durando X, Curé H, Guy L, Van-Praagh I, Savareux L, Atger M, Bayet-Robert M, Gadea E, Abrial C, Thivat E, Chollet P, Eymard JC. The new combination docetaxel, prednisone and curcumin in patients with castration-resistant prostate cancer: A pilot phase II study. Oncology. 2016;90(2):69–78. [DOI] [PubMed] [Google Scholar]
- 20.Bayet-Robert M, Kwiatkowski F, Leheurteur M, Gachon F, Planchat E, Abrial C, Mouret-Reynier MA, Durando X, Barthomeuf C, Chollet P. Phase I dose escalation trial of docetaxel plus curcumin in patients with advanced and metastatic breast cancer. Cancer Biol Ther. 2010;9(1):8–14. [DOI] [PubMed] [Google Scholar]
- 21.Tuyaerts S, Rombauts K, Everaert T, Van Nuffel AMT, Amant F. A phase 2 study to assess the immunomodulatory capacity of a lecithin-based delivery system of curcumin in endometrial cancer. Front Nutr. 2019;5:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tong W, Wang Q, Sun D, Suo J. Curcumin suppresses colon cancer cell invasion via AMPK-induced inhibition of NF-κB, uPA activator and MMP9. Oncol Lett. 2016;12(5):4139–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rajitha B, Belalcazar A, Nagaraju GP, Shaib WL, Snyder JP, Shoji M, Pattnaik S, Alam A, El-Rayes BF. Inhibition of NF-κB translocation by curcumin analogs induces G0/G1 arrest and downregulates thymidylate synthase in clorectal cancer. Cancer Lett. 2016;373(2):227–33. [DOI] [PubMed] [Google Scholar]
- 24.Yu H, Xie Y, Zhou Z, Wu Z, Dai X, Xu B. Curcumin regulates the progression of colorectal cancer via LncRNA NBR2/AMPK pathway. Technol Cancer Res Treat. 2019;18:1533033819870781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu LB, Duan CN, Ma ZY, Xu G. Curcumin inhibited rat colorectal carcinogenesis by activating PPAR-γ: An experimental study. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2015;35(4):471–5 (in Chinese). [PubMed] [Google Scholar]
- 26.Chikazawa N, Tanaka H, Tasaka T, Nakamura M, Tanaka M, Onishi H, Katano M. Inhibition of Wnt signaling pathway decreases chemotherapy-resistant side-population colon cancer cells. Anticancer Res. 2010;30(6):2041–8. [PubMed] [Google Scholar]
- 27.Kumar V, Vashishta M, Kong L, Wu X, Lu JJ, Guha C, Dwarakanath BS. The role of Notch, Hedgehog, and Wnt signaling pathways in the resistance of tumors to anticancer therapies. Front Cell Dev Biol. 2021;9:650722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cai B-Q, Chen W-M, Zhao J, Hou W, Tang J-C. Nrf3 promotes 5-FU resistance in colorectal cancer cells via the NF-κB/BCL-2 signaling pathway in vitro and in vivo. J Oncol. 2021;2021:9355555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cao D, Qin S, Mu Y, Zhong M. The role of MRP1 in the multidrug resistance of colorectal cancer. Oncol Lett. 2017;13(4):2471–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Po A, Citarella A, Catanzaro G, Besharat ZM, Trocchianesi S, Gianno F, Sabato C, Moretti M, De Smaele E, Vacca A, Fiori ME, Ferretti E. Hedgehog-GLI signalling promotes chemoresistance through the regulation of ABC transporters in colorectal cancer cells. Sci Rep. 2020;10(1):13988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ruiz de Porras V, Bystrup S, Martínez-Cardús A, Pluvinet R, Sumoy L, Howells L, James MI, Iwuji C, Manzano JL, Layos L, Bugés C, Abad A, Martínez-Balibrea E. Curcumin mediates oxaliplatin-acquired resistance reversion in colorectal cancer cell lines through modulation of CXC-Chemokine/NF-κB signalling pathway. Sci Rep. 2016;6(1):24675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yin J, Wang L, Wang Y, Shen H, Wang X, Wu L. Curcumin reverses oxaliplatin resistance in human colorectal cancer via regulation of TGF-β/Smad2/3 signaling pathway. Onco Targets Ther. 2019;12:3893–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shakibaei M, Mobasheri A, Lueders C, Busch F, Shayan P, Goel A. Curcumin enhances the effect of chemotherapy against colorectal cancer cells by inhibition of NF-κB and Src protein kinase signaling pathways. PLoS One. 2013;8(2):e57218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shakibaei M, Buhrmann C, Kraehe P, Shayan P, Lueders C, Goel A. Curcumin chemosensitizes 5-fluorouracil resistant MMR-deficient human colon cancer cells in high density cultures. PLoS One. 2014;9(l):e85397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.He WT, Zhu YH, Zhang T, Abulimiti P, Zeng FY, Zhang LP, Luo LJ, Xie XM, Zhang HL. Curcumin reverses 5-fluorouracil resistance by promoting human colon cancer HCT-8/5-FU cell apoptosis and down-regulating heat shock protein 27 and P-glycoprotein. Chin J Integr Med. 2019;25(6):416–24. [DOI] [PubMed] [Google Scholar]
- 36.Lu Y, Zhang R, Zhang X, Zhang B, Yao Q. Curcumin may reverse 5-fluorouracil resistance on colonic cancer cells by regulating TET1-NKD-Wnt signal pathway to inhibit the EMT progress. Biomed Pharmacother. 2020;129:110381. [DOI] [PubMed] [Google Scholar]
- 37.Ruiz de Porras V, Bystrup S, Martínez-Cardús A, Pluvinet R, Sumoy L, Howells L, James MI, Iwuji C, Manzano JL, Layos L, Bugés C, Abad A, Martínez-Balibrea E. Curcumin mediates oxaliplatin-acquired resistance reversion in colorectal cancer cell lines through modulation of CXC-Chemokine/NF-κB signalling pathway. Sci Rep. 2016;6:24675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Howells LM, Iwuji COO, Irving GRB, Barber S, Walter H, Sidat Z, Griffin-Teall N, Singh R, Foreman N, Patel SR, Morgan B, Steward WP, Gescher A, Thomas AL, Brown K. Curcumin combined with FOLFOX chemotherapy is safe and tolerable in patients with metastatic colorectal cancer in a randomized phase IIa trial. J Nutr. 2019;149(7):1133–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rodrigues FC, Hari G, Pai KSR, Suresh A, Nayak UY, Anilkumar NV, Thakur G. Molecular modeling piloted analysis for semicarbazone derivative of curcumin as a potent Abl-kinase inhibitor targeting colon cancer. 3 Biotech. 2021;11(12):506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chou YT, Koh YC, Nagabhushanam K, Ho CT, Pan MH. A natural degradant of curcumin, feruloylacetone inhibits cell proliferation via inducing cell cycle arrest and a mitochondrial apoptotic pathway in HCT116 colon cancer cells. Molecules. 2021;26(16):4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ismail NI, Othman I, Abas F, N HL, Naidu R. The curcumin analogue, MS13 (1,5-Bis(4-hydroxy-3- methoxy-phenyl)-1,4-pentadiene-3-one), Inhibits cell proliferation and induces apoptosis in primary and metastatic human colon cancer cells. Molecules. 2020;25(17):3798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rahim NFC, Hussin Y, Aziz MNM, Mohamad NE, Yeap SK, Masarudin MJ, Abdullah R, Akhtar MN, Alitheen NB. Cytotoxicity and apoptosis effects of curcumin analogue (2E,6E)-2,6-Bis(2,3-dimethoxybenzylidine) cyclohexanone (DMCH) on human colon cancer cells HT29 and SW620 in vitro. Molecules. 2021;26(5):1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aghebati-Maleki A, Dolati S, Ahmadi M, Baghbanzhadeh A, Asadi M, Fotouhi A, Yousefi M, Aghebati-Maleki L. Nanoparticles and cancer therapy: Perspectives for application of nanoparticles in the treatment of cancers. J Cell Physiol. 2020;235(3):1962–72. [DOI] [PubMed] [Google Scholar]
- 44.Awasthi R, Roseblade A, Hansbro PM, Rathbone MJ, Dua K, Bebawy M. Nanoparticles in cancer treatment: Opportunities and obstacles. Curr Drug Targets. 2018;19(14):1696–709. [DOI] [PubMed] [Google Scholar]
- 45.Gavas S, Quazi S, Karpiński TM. Nanoparticles for cancer therapy: Current progress and challenges. Nanoscale Res Lett. 2021;16(1):173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kulbacka J, Wilk KA, Bazylińska U, Dubińska-Magiera M, Potoczek S, Saczko J. Curcumin loaded nanocarriers with varying charges augmented with electroporation designed for colon cancer therapy. Int J Mol Sci. 2022;23(3):1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tapia-Hernández JA, Rodríguez-Felix F, Juárez-Onofre JE, Ruiz-Cruz S, Robles-García MA, Borboa-Flores J, Wong-Corral FJ, Cinco-Moroyoqui FJ, Castro-Enríquez DD, Del-Toro-Sánchez CL. Zein-polysaccharide nanoparticles as matrices for antioxidant compounds: A strategy for prevention of chronic degenerative diseases. Food Res Int. 2018;111:451–71. [DOI] [PubMed] [Google Scholar]
- 48.Liu L, Yang S, Chen F, Cheng K-W. Polysaccharide-zein composite nanoparticles for enhancing cellular uptake and oral bioavailability of curcumin: Characterization, anti-colorectal cancer effect, and pharmacokinetics. Front Nutr. 2022;9:846282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hassani A, Mahmood S, Enezei HH, Hussain SA, Hamad HA, Aldoghachi AF, Hagar A, Doolaanea AA, Ibrahim WN. Formulation, characterization and biological activity screening of sodium alginate-gum arabic nanoparticles loaded with curcumin. Molecules. 2020;25(9):2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Abedi F, Davaran S, Hekmati M, Akbarzadeh A, Baradaran B, Moghaddam SV. An improved method in fabrication of smart dual-responsive nanogels for controlled release of doxorubicin and curcumin in HT-29 colon cancer cells. J Nanobiotechnology. 2021;19(1):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.O’Connor NA, Einbond LS, Redenti S, Sauane M, Jitianu A. A self-degradable curcumin polymer with anti-cancer activity. J Appl Polym Sci. 2018;135(47):46867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gustavsson B, Carlsson G, Machover D, Petrelli N, Roth A, Schmoll HJ, Tveit KM, Gibson F. A review of the evolution of systemic chemotherapy in the management of colorectal cancer. Clin Colorectal Cancer. 2015;14(1):1–10. [DOI] [PubMed] [Google Scholar]
- 53.Howells LM, Iwuji COO, Irving GRB, Barber S, Walter H, Sidat Z, Griffin-Teall N, Singh R, Foreman N, Patel SR, Morgan B, Steward WP, Gescher A, Thomas AL, Brown K. Curcumin combined with FOLFOX chemotherapy is safe and tolerable in patients with metastatic colorectal cancer in a randomized phase IIa trial. J Nutr. 2019;149(7):1133–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Irving GR, Iwuji CO, Morgan B, Berry DP, Steward WP, Thomas A, Brown K, Howells LM. Combining curcumin (C3-complex, Sabinsa) with standard care FOLFOX chemotherapy in patients with inoperable colorectal cancer (CUFOX): Study protocol for a randomised control trial. Trials. 2015;16(1):110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.James MI, Iwuji C, Irving G, Karmokar A, Higgins JA, Griffin-Teal N, Thomas A, Greaves P, Cai H, Patel SR, Morgan B, Dennison A, Metcalfe M, Garcea G, Lloyd DM, Berry DP, Steward WP, Howells LM, Brown K. Curcumin inhibits cancer stem cell phenotypes in ex vivo models of colorectal liver metastases, and is clinically safe and tolerable in combination with FOLFOX chemotherapy. Cancer Lett. 2015;364(2):135–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Patel BB, Sengupta R, Qazi S, Vachhani H, Yu Y, Rishi AK, Majumdar AP. Curcumin enhances the effects of 5-fluorouracil and oxaliplatin in mediating growth inhibition of colon cancer cells by modulating EGFR and IGF-1R. Int J Cancer. 2008;122(2):267–73. [DOI] [PubMed] [Google Scholar]
- 57.Nautiyal J, Kanwar SS, Yu Y, Majumdar AP. Combination of dasatinib and curcumin eliminates chemo-resistant colon cancer cells. J Mol Signal. 2011;6:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hosokawa M, Tanaka S, Ueda K, Iwakawa S, Ogawara KI. Decitabine exerted synergistic effects with oxaliplatin in colorectal cancer cells with intrinsic resistance to decitabine. Biochem Biophys Res Commun. 2019;509(1):249–54. [DOI] [PubMed] [Google Scholar]
- 59.Hosokawa M, Seiki R, Iwakawa S, Ogawara K-i. Combination of azacytidine and curcumin is a potential alternative in decitabine-resistant colorectal cancer cells with attenuated deoxycytidine kinase. Biochem Biophys Res Commun. 2021;578:157–62. [DOI] [PubMed] [Google Scholar]
- 60.Basha R, Connelly SF, Sankpal UT, Nagaraju GP, Patel H, Vishwanatha JK, Shelake S, Tabor-Simecka L, Shoji M, Simecka JW, El-Rayes B. Small molecule tolfenamic acid and dietary spice curcumin treatment enhances antiproliferative effect in pancreatic cancer cells via suppressing Sp1, disrupting NF-κB translocation to nucleus and cell cycle phase distribution. J Nutr Biochem. 2016;31:77–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhou W, Liu Q, Zang X, Hu M, Yue Y, Wang Y, Lv C, Du Z. Combination use of tolfenamic acid with curcumin improves anti-inflammatory activity and reduces toxicity in mice. J Food Biochem. 2020;44(6):e13240. [DOI] [PubMed] [Google Scholar]
- 62.Selvam C, Prabu SL, Jordan BC, Purushothaman Y, Uma-maheswari A, Hosseini Zare MS, Thilagavathi R. Molecular mechanisms of curcumin and its analogs in colon cancer prevention and treatment. Life Sci. 2019;239:117032. [DOI] [PubMed] [Google Scholar]
- 63.Sankpal UT, Nagaraju GP, Gottipolu SR, Hurtado M, Jordan CG, Simecka JW, Shoji M, El-Rayes B, Basha R. Combination of tolfenamic acid and curcumin induces colon cancer cell growth inhibition through modulating specific transcription factors and reactive oxygen species. Oncotarget. 2016;7(3):3186–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ravindranathan P, Pasham D, Balaji U, Cardenas J, Gu J, Toden S, Goel A. A combination of curcumin and oligomeric proanthocyanidins offer superior anti-tumorigenic properties in colorectal cancer. Sci Rep. 2018;8(1):13869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Majumdar AP, Banerjee S, Nautiyal J, Patel BB, Patel V, Du J, Yu Y, Elliott AA, Levi E, Sarkar FH. Curcumin synergizes with resveratrol to inhibit colon cancer. Nutr Cancer. 2009;61(4):544–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kuo IM, Lee JJ, Wang YS, Chiang HC, Huang CC, Hsieh PJ, Han W, Ke CH, Liao ATC, Lin CS. Potential enhancement of host immunity and anti-tumor efficacy of nanoscale curcumin and resveratrol in colorectal cancers by modulated electro-hyperthermia. BMC Cancer. 2020;20(1):603. [DOI] [PMC free article] [PubMed] [Google Scholar]