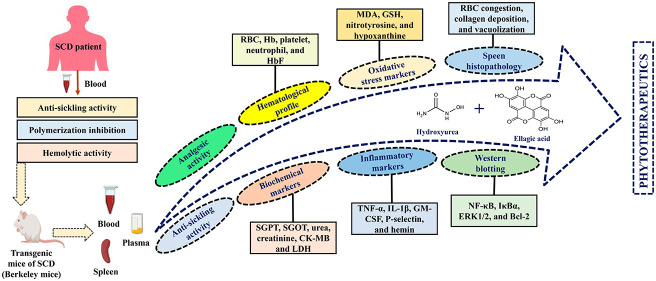
Abstract
The use of adjuvant therapy is an attractive approach to manage sickle cell disease (SCD) symptomatically. The present study aimed to investigate the potential of ellagic acid as an adjuvant therapy with hydroxyurea (HU), a key drug for SCD with myelosuppressive toxic effects. A panel of experiments was performed using SCD patient’s blood (ex vivo) and transgenic mice model of SCD (in vivo). Ellagic acid exhibited the following beneficial pharmacological actions: (a) potent anti-sickling, polymerization inhibitory, and inherent non-hemolytic activity; (b) pronounced action to abrogate HU-induced neutropenia and to improve key hematological parameters during SCD (RBC, Hb, platelet levels); (c) considerable action to foster vascular tone (L-proline); (d) marked attenuating effect against oxidative stress (nitrotyrosine, hypoxanthine, MDA, GSH); (e) substantial inhibitory role against inflammation (analgesic activity and regulation of hemin, TNF-α, IL-1β, NF-κB/IκBα); (f) remarkable outcome of declining vaso-occlusive crisis (P-selectin, ERK1/2); (g) notable shielding deed against elevated biochemical marker for organ toxicity (creatinine); (h) noticeably prevented histopathological alterations of the spleen. Additionally, the pharmacokinetic study results of HU in the presence and absence of ellagic acid using a mouse model demonstrate that ellagic acid could be safely co-administered with HU. Overall findings suggest that ellagic acid is a promising candidate for adjuvant therapy in SCD based on its own significant ability against SCD and potentiating capability of HU action via targeting improvement at the various stages of pathophysiological complications during SCD and minimizing HU-induced toxicological manifestations.
Keywords: sickle cell disease, ellagic acid, anti-sickling agent, hydroxyurea, myelosuppression, adjuvant therapy
Introduction
Sickle cell disease (SCD) is congenital hemolytic anemia that arises due to mutation in the β-globin subunit of hemoglobin (Hb), which directs sickle Hb (HbS) production. Under hypoxic conditions, the polymerization of HbS leads to the sickling of red blood cells (RBC). Sickled RBC undergoes hemolysis that triggers vaso-occlusion, ischemia, and pain crises, which are the main pathological features of SCD.1 This complex disease phenomenon includes severe symptoms such as extreme back pain, acute chest syndrome, cerebral infraction, vascular necrosis, pulmonary hypertension, nephropathy, etc.2 Hydroxyurea (HU) is the first-ever approved drug (1998) to curb the manifestation of SCD. It increases fetal Hb (HbF) production, which reduces the severe consequences of HbS polymerization.3 As HU has been initially approved as an anti-cancer drug, its therapy is associated with significant adverse effects like myelosuppression.4
Under these circumstances, recent studies are ongoing to discover new drug modalities and adjuvant/supplementation therapy to counteract the signs/symptoms and, subsequently, improve the survival of SCD patients. In this pursuit, the main pathophysiological targets of SCD that have been explored globally by using drugs/natural products/phytochemicals/botanical drugs are: (a) decline in sickling behavior, (b) reduction in oxidative stress; (c) enhancement in HbF production, (d) lessening of platelet aggregation, (e) lowering of adhesion behavior, (f) reduction in inflammation (ClinicalTrials.gov). In this context, crizanlizumab (2019) and voxelotor (2019) are the two recently approved drugs by the United States Food and Drug Administration (USFDA) to restrict vaso-occlusive crises via blocking P-selectin function and prevent RBC sickling, respectively.5 Moreover, L-glutamine (2017) has also been approved by USFDA as a supplementation therapy to minimize the signs/symptoms of SCD by reducing oxidative stress.6
In the present study, we hypothesized that ellagic acid (EA) might be helpful to combat HU-induced myelosuppression and could counteract symptoms of SCD patients. The main reasons for choosing EA in the present study are: (a) EA is a biologically active phytoconstituent that is predominantly present in a wide range of fruits,7 and (b) EA is enlisted in the generally recognized as safe (GRAS) substances by USFDA for human consumption.
To date, no report is available for any role of EA alone and in combination with HU to symptomatically manage SCD. Hence, we aimed to explore the same under the purview of improvement in the pathophysiology of SCD using ex vivo (SCD patient’s blood) and in vivo (transgenic mice model of SCD) experiments.
Results
EA Displayed Anti-Sickling Action
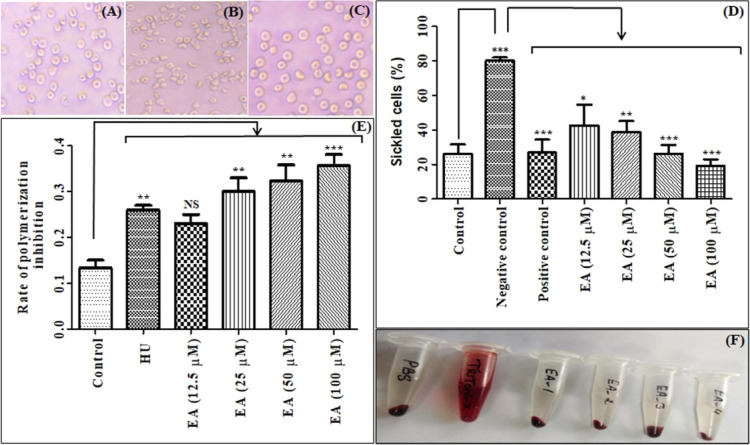
EA was tested for anti-sickling activity using SCD patients’ blood (Figures 1A–D). p-Hydroxybenzoic acid as a positive control exhibited significant sickling inhibition corroborating reported findings.8 EA (12.5–100 μM) displayed a pronounced effect on sickling inhibition.
Figure 1.
Representative images of anti-sickling activity: control blood (A); control blood after treatment with the inducing agent (negative control) (B); control blood after treatment with EA and subsequently with the inducing agent (C); effect of EA on the anti-sickling activity (D) and the rate of polymerization inhibition (E); representative images for the effect of EA on hemolysis (F) (EA_1, EA_2, EA_3, and EA_4 represent the concentration of EA at 0.01, 0.05, 0.1, and 1 mg/mL, respectively). Data are presented as mean ± SEM (n = 3). p < 0.05/0.01/0.001 denotes statistically significant (*/**/***). NS denotes not statistically significant.
Influence of EA on polymerization inhibition was explored using SCD patients’ blood (Figure 1E). HU showed considerable polymerization inhibition, which aligns with the previous reports.8 EA (25–100 μM) significantly inhibited the polymerization of HbS.
To check any hemolytic effect of EA, results displayed that it had a negligible impact on hemolysis (≤2%) even at a high concentration (Figure 1F).
The effect of EA on anti-sickling activity was also investigated in the presence of HU using mutant/sickle mice. Mutant mice exhibited considerable sickling of RBC compared to the wild type. Treatment with HU significantly inhibited sickling compared to the mutant group (Figure S1), corroborating the earlier findings.9 In combination with HU, EA significantly reduced the sickling of RBC compared to HU alone (100–200 mg/kg)/mutant group (50–200 mg/kg).
EA Abrogated Hematological Profile
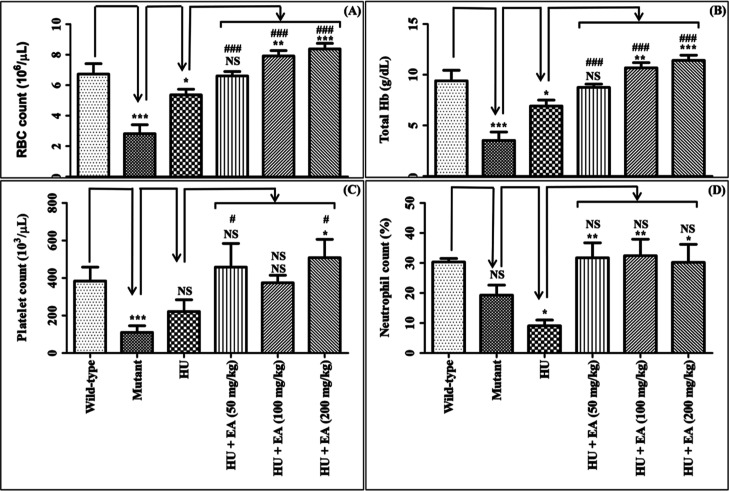
Compared to the wild type, we observed a considerable decline in RBC, Hb, and platelet, except the neutrophil count in the mutant group (Figures 2A–D). HU treatment led to a reduction in all the above-mentioned parameters compared to the wild type, demonstrating its myelosuppressive effect. Administration of HU exhibited a significant improvement in RBC and Hb levels compared to the mutant group. Concomitant treatment of EA (100–200 mg/kg) with HU considerably enhanced the same parameters compared to HU.
Figure 2.
Effect of EA upon concomitant treatment with HU on the following parameters: RBC (A); Hb (B); platelet (C); neutrophil (D). Data are expressed as mean ± SEM (n = 5). p < 0.05/0.01/0.001 denotes statistically significant (*/**/*** or #/##/###). *Wild-type vs mutant/mutant vs HU/HU vs HU + EA. #Mutant vs HU + EA. NS denotes not statistically significant.
Although HU lacked any effect on platelet count compared to the mutant group, EA treatment (200 mg/kg) with HU showed notable improvement of platelet count compared to HU alone. Conversely, HU treatment caused a significant reduction in the neutrophil count. However, simultaneous administration of EA (50–200 mg/kg) with HU substantially normalized the neutrophil level.
HbF level was decreased in mutant mice compared to the wild type (Figure S2) in statistically insignificant manner. Compared to the mutant group, HU treatment significantly increased the HbF level, corroborating with the reported literature.10 EA treatment with HU exhibited a negligible effect on HbF levels compared to HU alone. However, concomitant administration of EA with HU boosted the HbF level compared to the mutant group.
EA Fostered the Vascular Tone
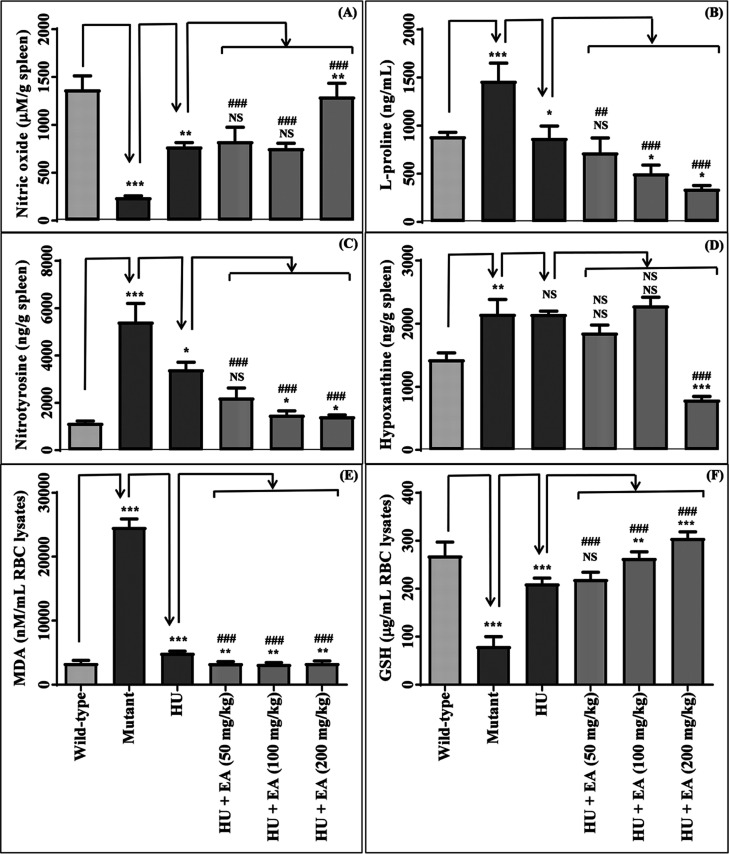
A significant decline in the nitric oxide levels was observed in the mutant group compared to the wild type (Figure 3A), and results align with the reported literature.4,11 HU treatment was associated with a notable improvement in nitric oxide levels compared to the mutant group, corroborating observed effects in SCD patients.4,12 EA treatment exhibited a negligible effect on nitric oxide levels except at 200 mg/kg compared to HU alone. However, EA at all the experimental doses with HU substantially enhanced the nitric oxide levels compared to the mutant group.
Figure 3.
Effect of EA upon concomitant treatment with HU on the following parameters: nitric oxide (A); L-proline (B); nitrotyrosine (C); hypoxanthine (D); MDA (E); GSH (F). Data are expressed as mean ± SEM (n = 5). p < 0.05/0.01/0.001 denotes statistically significant (*/**/*** or #/##/###). *Wild-type vs mutant/mutant vs HU/HU vs HU + EA. #Mutant vs HU + EA. NS denotes not statistically significant.
L-Arginine level was reduced in the mutant group compared to the wild-type group (Figure S3) and improved upon HU treatment that lacks statistical significance. EA, in combination with HU, had a negligible effect on the L-arginine levels compared to HU alone but notably enhanced by EA (200 mg/kg) in combination with HU compared to the mutant group.
L-Proline level was markedly increased in the mutant group and declined upon the treatment of HU (Figure 3B), corroborating with the reported literature.13 Concomitant administration of EA (100–200 mg/kg) significantly reduced the L-proline level compared to HU alone. Compared to the mutant group, EA at entire dose levels considerably improved the L-proline levels.
EA Attenuated the Oxidative Stress
A significant increase in the nitrotyrosine level of the spleen tissue was observed for the mutant group compared to the wild type (Figure 3C), corroborating with the reported literature.14 We noticed a notable reduction in the nitrotyrosine level upon the treatment of HU compared to the mutant group. EA (100–200 mg/kg) in combination with HU remarkably reduced the nitrotyrosine level compared to HU alone. In comparison to the mutant group, nitrotyrosine levels substantially declined upon concomitant administration of EA at all doses with HU.
A significant increase in the hypoxanthine level was observed in the mutant group compared to the wild type (Figure 3D). HU treatment showed a negligible effect on hypoxanthine levels compared to the mutant group. Co-administration of EA (200 mg/kg) with HU significantly reduced the hypoxanthine level in spleen tissue compared to the HU alone/mutant group.
Malondialdehyde (MDA) level in RBC lysates/spleen tissue was significantly elevated in the mutant group compared to the wild type (Figures 3E and S4) and was notably declined upon treatment with HU. HU is reported to decrease MDA levels in SCD patients.15 Although the treatment of EA in combination with HU remarkably declined the MDA level in spleen tissue at only 200 mg/kg compared to HU alone, it significantly dropped at entire dose levels in the case of the RBC lysate. Moreover, EA at all dose levels improved the MDA level in both RBC lysates and spleen tissue homogenate compared to the mutant group.
Glutathione (GSH) level was markedly reduced in RBC lysate/spleen tissue in the mutant group compared to the wild type (Figures 3F and S4). The same was significantly enhanced upon HU treatment compared to the mutant group, which aligns with the HU effects in SCD patients.15 Concomitant administration of EA (100–200 mg/kg) with HU further improved the GSH level in only the RBC lysate compared to HU alone. However, EA at entire dose levels upon concomitant treatment with HU significantly augmented the GSH level in RBC lysate/spleen tissue compared to the mutant group.
EA Inhibited Inflammation
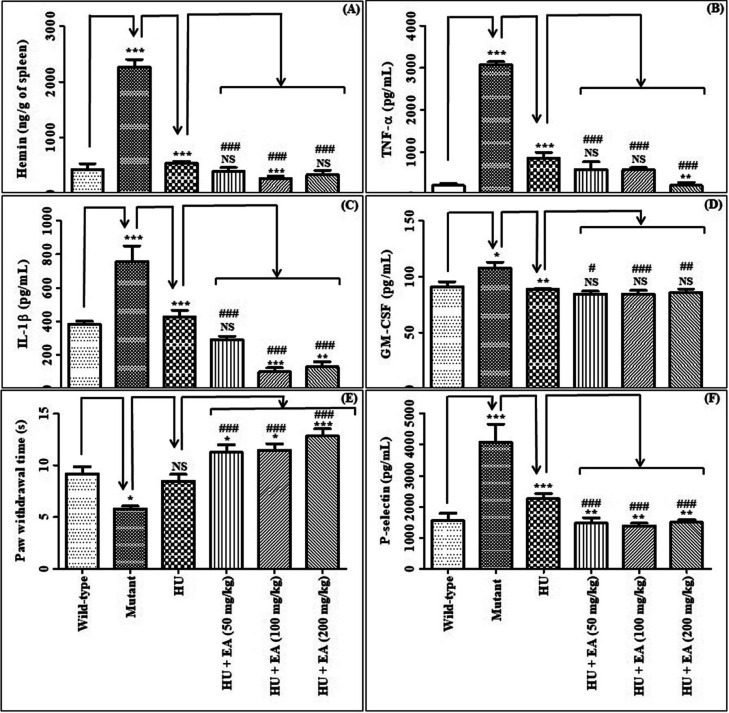
Hemin, TNF-α, and IL-1β were significantly elevated in the mutant group and remarkably reduced upon HU treatment (Figures 4A–C). Results corroborate with the reported literature.2 EA treatment with HU substantially declined hemin (100 mg/kg), TNF-α (200 mg/kg), and IL-1β (100–200 mg/kg) levels compared to HU alone. Compared to the mutant group, all these levels were remarkably reduced upon concurrent EA and HU treatment.
Figure 4.
Effect of EA upon concomitant treatment with HU on the following parameters: hemin (A); TNF-α (B); IL-1β (C); GM-CSF (D); paw withdrawal time (E); P-selectin (F). Data are expressed as mean ± SEM (n = 5). p < 0.05/0.01/0.001 denotes statistically significant (*/**/*** or #/##/###). *Wild-type vs mutant/mutant vs HU/HU vs HU + EA. #mutant vs HU + EA. NS denotes not statistically significant.
A significant increase in granulocyte-macrophage colony-stimulating factor (GM–CSF) level was observed in the mutant group compared to the wild type (Figure 4D). HU treatment led to a considerable decrease in the GM–CSF level compared to the mutant group, corroborating the previous findings.16 Concomitant administration of EA with HU exhibited a negligible effect on GM–CSF levels in comparison to HU alone. However, the GM–CSF level substantially declined upon concurrent treatment of EA at all doses in the presence of HU compared to the mutant group.
Mutant mice exhibited a notable decrease in the paw withdrawal time compared to the wild type (Figure 4E). HU treatment did not significantly affect the paw withdrawal time compared to the mutant group. Concomitant administration of EA at entire doses in combination with HU considerably increased the paw withdrawal time compared to the HU alone/mutant group.
Protein expression of nuclear factor-kappa B (NF-κB)/inhibitor of kappa B alpha (IκBα) was significantly elevated in the mutant mice compared to the wild type (Figure S5), which are in line with the reported literature.17 HU displayed a negligible effect on these proteins compared to the mutant group. EA (200 mg/kg) treatment with HU considerably down-regulated the NF-κB/IκBα levels compared to HU alone. EA (100–200 mg/kg) in combination with HU notably reduced the NF-κB/IκBα levels compared to the mutant group.
EA Declined the Vaso-occlusion Crisis
A significant increase in the P-selectin level in the mutant group was observed compared to the wild type (Figure 4F). HU treatment significantly reduced the P-selectin level compared to the mutant group. EA (50–200 mg/kg) treatment with HU remarkably reduced the P-selectin level compared to the HU alone/mutant group.
Protein expression of extracellular signal-regulated kinase 1/2 (ERK1/2) was significantly enhanced in the mutant group compared to the wild type (Figure S5). Treatment of HU reduced the ERK1/2 expression compared to the mutant group, which is in line with the reported literature.11 EA (200 mg/kg) with HU significantly reduced the ERK1/2 level compared to HU alone. Additionally, the ERK1/2 level remarkably declined at all doses of EA with HU compared to the mutant group.
EA Prevented Histopathological Changes in the Spleen
Histopathological examination of spleen tissue was done to get better insights into the pathological changes of spleen (Figures S6 and S7). There was a significant increase in RBC congestion, collagen deposition, and vacuolization of histiocytes in the mutant group compared to the wild type. Administration of HU significantly alleviated all the parameters compared to the mutant group, which aligns with the reported literature.18 EA at all dose levels aided to the preventive effect of HU on the spleen architecture.
EA Unaffected the Pharmacokinetics of HU
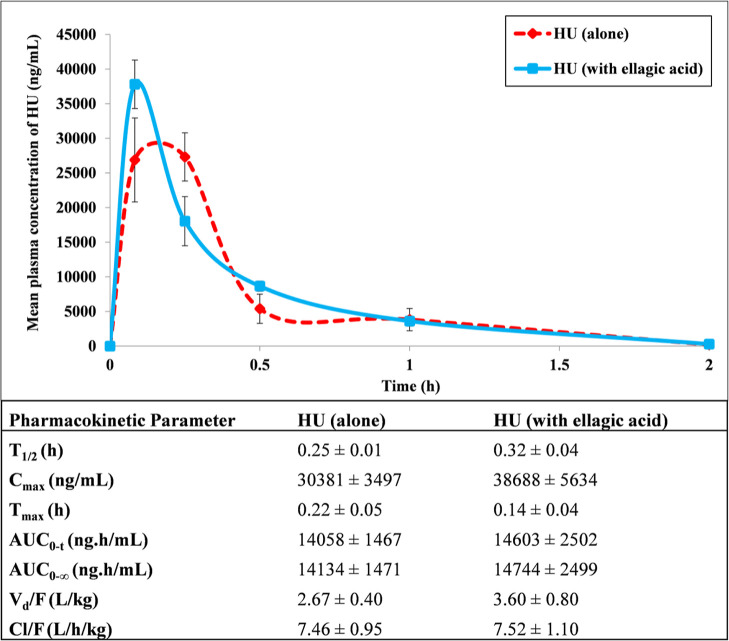
We evaluated the effect of EA on the pharmacokinetics of HU. The plasma concentration versus time profile and pharmacokinetic parameters are represented in Figure 5. EA did not alter any pharmacokinetic parameters of HU upon the simultaneous administration of HU with EA.
Figure 5.
Mean plasma concentration vs time profiles and main pharmacokinetic parameters of HU after oral administration alone as well as in the presence of EA in mice. Data are expressed as mean ± SEM (n = 5).
Discussion
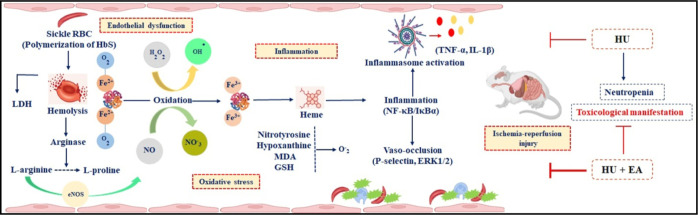
In the quest to explore the potential of EA as an adjuvant therapy for the symptomatic management of SCD, we elucidated the role of EA alone and in combination with a widely prescribed drug for SCD like HU (Figure 6) using SCD patients’ blood (ex vivo) and transgenic mice model of SCD (in vivo).
Figure 6.
Pictorial presentation for the beneficial action of EA on the pathophysiological targets of SCD.
In SCD, polymerization of HbS results in sickling of RBC, which leads to hemolysis. Hemolysis is a hallmark of SCD and the origin of vaso-occlusion, inflammation, and oxidative stress situations.4,19 Therefore, any candidate having anti-sickling activity, including polymerization inhibition, can restrict the downstream effect of sickling. Voxelotor is the recent USFDA-approved drug to prevent RBC sickling. In the present study, EA is found to be a potent anti-sickling compound, regardless of using blood from SCD patients’ or Berkeley mice. Additionally, the proposed adjuvant therapy should be devoid of inherent hemolytic activity. Current study results suggest that EA lacked any effect to trigger further hemolysis in SCD. HU is an anti-cancer agent, and its treatment is associated with the reduced expression of B-cell lymphoma 2 (Bcl-2), which is related to apoptosis.20 We observed minimal impact of EA to limit the risk of HU treatment linked apoptosis in SCD (Figure S5). Treatment of HU is associated with an increase in γ-globin gene expression, thereby increasing HbF production that helps to prevent RBC’s sickling.21 EA displayed a minor role in the γ-globin gene to potentiate HU action.
Hemolysis in SCD patients causes a decline in the RBC count and Hb level. EvenFlo is reported to increase the Hb level during clinical investigation in SCD patients.22 Moreover, SCD patients experience life-threatening thrombocytopenia during a prolonged vaso-occlusive crisis. Conversely, HU treatment in SCD patients reduces the severity of the disease, but its chronic treatment causes myelosuppression including neutropenia.4 Based on the current study results on RBC, Hb, platelet, and neutrophil levels, EA could be beneficial in ameliorating the hematological profile to limit the onset of pain crises in SCD.
Intravascular hemolysis releases free heme and free radicals that diminish arginine and nitric oxide levels. Changes in the normal equilibrium of arginine and its catabolic by-products like ornithine, citrulline, and proline have been associated with pulmonary fibrosis. Supplementation therapy of L-arginine is under clinical exploration for SCD patients to improve the nitric oxide level.23 Based on our current results on the improvement of nitric oxide, L-arginine, and L-proline levels, EA treatment with HU could be beneficial to improve the vascular tone and minimize pulmonary fibrosis, aiding to combat pathophysiological complications of SCD.
SCD is characterized by producing reactive oxygen species (ROS), resulting in oxidative stress and endothelial dysfunction. L-Glutamine has been recently approved by the USFDA to reduce oxidative stress.6 Gum Arabic is under clinical exploration to improve anti-oxidant defense in SCD patients.4 Nitrotyrosine is a marker for nitro-oxidative stress, and our experimental results illustrate that EA could potentiate the HU action to reduce the nitrotyrosine level. Ippoushi et al. reported similar results for EA to prevent oxidative damage.24 Hypoxanthine is metabolized by xanthine oxidase, which contributes to oxidative stress in SCD, leading to multi-organ dysfunction.25,26 Febuxostat, a xanthine oxidoreductase inhibitor, is reported to improve endothelial dysfunction in sickle mice.27 In the present study, we observed the influence of EA with HU to reduce the hypoxanthine level. MDA is produced due to lipid peroxidation and can serve as a marker for oxidative stress.28 Our results suggest that in the presence of HU, EA could restrict the SCD-mediated elevation of MDA. GSH neutralizes ROS and protects cells from oxidative stress-related cell damage.28 Like the results of MDA, we also observed a similar line of effect by EA with HU on GSH. EA is also reported to reduce MDA and GSH levels in cisplatin-induced nephrotoxicity.29 Thus, the adjuvant therapy of EA with HU could be beneficial to prevent the exacerbation of oxidative burden in SCD.
Hemolysis causes hemin release into the extracellular space. Hemin activates the adhesion molecules and their adherence to endothelial cells during the active phase of SCD. The increased oxidative burden in SCD patients due to excess quantities of cell-free Hb and heme triggers ROS formation, thereby activating the neutrophil molecules, which can capture circulating sickle RBC and hinder blood flow.30 Deferasirox and deferoxamine are reported to decline the iron burden in SCD patients.31 Moreover, curcumin is under preclinical investigation to reduce iron overload.32 In SCD, sickling of RBC leads to vaso-occlusion, followed by the production of TNF-α and IL-1β, resulting in vasculopathy.33 A TNF-α blocker (etanercept) and leukotrienes inhibitor (zileuton) are under investigation to treat SCD.4 Thus, a candidate with the ability to minimize the hemin level and anti-inflammatory activity can assist in decreasing the severity of SCD. Current results reveal that EA could check the inflammatory conditions to prevent the complications of SCD.
GM-CSF is a hematopoietic cytokine that prevents TFr-1 hematopoietic cells to produce γ-globin during erythropoietin-stimulated differentiation. GM–CSF also contributes to leukocytosis in SCD patients.34 Although EA is reported to reduce the GM–CSF production in LnCaP cells,35 our current results indicate that EA had a negligible effect on potentiating the HU effect on regulating GM–CSF. Pain crises during SCD are the most common reason for patients to seek medical assistance. SCD patients are generally prescribed acetaminophen with or without codeine or oxycodone, depending on the severity of their pain.36 Our present results reveal that EA with HU could aid to counteract nociceptive pain in SCD.
Activation of NF-κB involves the phosphorylation of IκBα in the cytoplasm, followed by the subsequent translocation of NF-κB to the nucleus, thereby activating inflammatory genes, which are responsible for the production of cytokines and chemokines.37 Heme, which is released upon hemolysis, triggers the activation of NF-κB. Sulfasalazine is an anti-inflammatory drug that can inhibit the transcription of NF-κB and interfere with the activation of endothelial cells in sickle mice.17 The present results of NF-κB/IκBα expression are in line with the results of TNF-α, IL-1β, and hemin levels. Hence, NF-κB/IκBα-mediated regulation of inflammatory pathways by EA could be helpful to attenuate the inflammatory burden of SCD.
In SCD, enhanced expression of P-selectin on the activated endothelium and platelets leads to the progression of vaso-occlusive crises. Crizanlizumab has been recently approved by USFDA to inhibit the action of P-selectin.38 Current results reveal that EA could augment the action of HU to trim down the P-selectin level. Alves et al. reported similar results for EA in reducing the expression of P-selectin during inflammation.39 ERK1/2 is aberrantly active in sickle RBC. ERK1/2 activation mediates the adhesion of sickle cells to the vascular endothelium. Inhibition of ERK1/2 in activated neutrophils reduces the expression of adhesion molecules.40 The effect of EA on ERK1/2 expression is in line with the results of hemin and P-selectin levels. Thus, EA could check both P-selectin and ERK1/2 status (P-selectin > ERK1/2), which can be useful to retard the vaso-occlusion crises in SCD patients.
SCD is associated with splenomegaly and spleen dysfunction in pediatric patients.17 Results of histopathological examination illustrate that EA, in combination with HU, could exhibit a better protective effect against spleen pathophysiological alterations during SCD. SCD is associated with liver ischemia41 and kidney impairment.42 The elevated levels of urea and creatinine in SCD and the effect of HU on these levels are in line with the reported literature.3,43 Current results indicate that EA had minimal effect for any further improvement in the HU effect on serum glutamic pyruvic transaminase (SGPT), serum glutamic oxaloacetic transaminase (SGOT), and urea levels (Figure S8). Conversely, EA displayed prominent action to trim down the augmented level of creatinine in SCD (Figure S8). In SCD, chronic kidney failure can lead to progressive myocardial damage, and creatine kinase–myocardial band (CK–MB) is reported to increase during SCD.44 We observed the effect of HU on preventing the elevation of CK–MB levels in SCD, and the results corroborate the previous findings.45 EA did not aid in any CK–MB lowering effect by HU (Figure S8). Lactate dehydrogenase (LDH) is a direct marker of hemolysis and is elevated during SCD.21 In the present study, HU restricted the enhanced LDH level due to SCD, and the results are in line with the reported literature.46 EA treatment with HU exhibited a minimal effect compared to HU alone (Figure S8). Results demonstrate that EA in combination with HU did not exacerbate but could restrict the elevation of hepatic, renal, and cardiac injury markers associated with disease pathophysiology, thereby exerting improvement in the survival of SCD patients.
Adjuvant therapy should be devoid of unintended pharmacokinetic interaction to circumvent any drug interaction that can cause an increase or decrease in plasma exposure, leading to the precipitation of toxic effects of drugs or therapeutic failure of drugs, respectively.47,48 Lack of any pharmacokinetic interaction between HU (prescribed drug) with EA (adjuvant therapy) was observed in the present study. Hence, our current results dictate safe co-administration of HU with EA for desired pharmacological effects.
Conclusions
The potential of EA was investigated under the purview of adjuvant therapy in SCD using a battery of ex vivo and in vivo models. Based on the experimental findings, the specific advantageous pharmacological actions are concisely stated as follows: (a) EA has its own pronounced action to combat the disease pathophysiology of SCD, (b) EA can potentiate the HU action to counteract the disease pathophysiology of SCD, (c) EA can prevent HU-induced myelosuppressive effects, and (d) EA can be safely co-administered with HU. EA is found to be a promising candidate for supplementation therapy with HU via targeting various stages of pathophysiological conditions in SCD to prevent finally ischemia-reperfusion injury/organ damage. Results insinuate further exploration of EA in combination with HU at the clinical level.
Materials and Methods
EA was purchased from a commercial source (Sigma-Aldrich). The chromatographic purity of EA was assessed in-house and found to be >99% (Figure S9). The ex vivo studies were carried out using SCD patient’s blood (donor). The in vivo experimentations were performed in the Berkeley mice model. Detailed materials and methods are represented in the “Supporting Information”.
Acknowledgments
A.G., D.K., and M.B. are thankful to DST/CSIR/UGC: RGNF (New Delhi, India) for providing their research fellowship. Authors are grateful to Dr. Sanjeev K. Digra for necessary support to execute the present research work. IIIM publication number: CSIR-IIIM/IPR/00489.
Glossary
Abbreviations
- SCD
sickle cell disease
- Hb
hemoglobin
- HbS
sickle hemoglobin
- RBC
red blood cells
- HU
hydroxyurea
- HbF
fetal hemoglobin
- USFDA
United States Food and Drug administration
- GRAS
generally recognized as safe
- MDA
malondialdehyde
- GSH
glutathione
- GM–CSF
granulocyte-macrophage colony-stimulating factor
- NF-κB
nuclear factor-kappa B
- IκBα
inhibitor of kappa B alpha
- ERK1/2
extracellular signal-regulated kinase 1/2
- Bcl-2
B-cell lymphoma 2
- ROS
reactive oxygen species
- SGPT
serum glutamic pyruvic transaminase
- SGOT
serum glutamic oxaloacetic transaminase
- CK–MB
creatine kinase–myocardial band
- LDH
lactate dehydrogenase
- Cmax
maximum plasma concentration
- Tmax
time to reach Cmax
- AUC0–t
area under the curve for plasma concentration from zero to the last measurable plasma sample time
- AUC0–∞
area under the curve for plasma concentration from zero to infinity
- Vd/F
volume of distribution after oral administration
- Cl/F
clearance after oral administration
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsptsci.3c00026.
Detailed information on materials and methods; anti-sickling activity data in mice model; effect on HbF, L-argenine, MDA (spleen), and GSH (spleen); protein expression data; histopathological data; and biochemical data (PDF)
Author Contributions
A.G. performed the polymerization assay, hemolysis activity, all animal experimentations, LC–MS/MS analysis, data evaluation, and writing—original draft; D.K.: anti-sickling activity, western blotting; R.P.: identification of SCD mice; M.B.: analgesic activity, hematological and biochemical parameter analysis; S.D.S.: resources for funding. A.K.: supervision of anti-sickling activity, western blotting; U.N.: conceptualization, planning and supervision of animal experimentations, writing—review and editing.
Research support was obtained from the Sickle Cell Mission Project (HCP08 and HCP23) by the Council of Scientific and Industrial Research (New Delhi, India).
The authors declare no competing financial interest.
This paper was published online on May 11, 2023 with an error to the TOC. The corrected version was reposted May 12, 2023.
Supplementary Material
References
- Williams T. N.; Thein S. L. Sickle cell anemia and its phenotypes. Annu. Rev. Genom. Hum. Genet. 2018, 19, 113–147. 10.1146/annurev-genom-083117-021320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conran N.; Belcher J. D. Inflammation in sickle cell disease. Clin. Hemorheol. Microcirc. 2018, 68, 263–299. 10.3233/CH-189012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barma S. K.; Dash M. R.; Samal S. R.; Sethy G.; Panigrahi P. Effect of hydroxyurea on clinical and haematological profile of children with sickle cell anaemia. Int. J. Res. Rev. 2020, 7, 493–499. [Google Scholar]
- Kapoor S.; Little J. A.; Pecker L. H.. Advances in the treatment of sickle cell disease. In Mayo Clinic Proceedings; Elsevier, 2018, pp 1810–1824. [DOI] [PubMed] [Google Scholar]
- Leibovitch J. N.; Tambe A. V.; Cimpeanu E.; Poplawska M.; Jafri F.; Dutta D.; Lim S. H. l-glutamine, crizanlizumab, voxelotor, and cell-based therapy for adult sickle cell disease: Hype or hope?. Blood Rev. 2022, 53, 100925. 10.1016/j.blre.2021.100925. [DOI] [PubMed] [Google Scholar]
- Niihara Y.; Miller S. T.; Kanter J.; Lanzkron S.; Smith W. R.; Hsu L. L.; Gordeuk V. R.; Viswanathan K.; Sarnaik S.; Osunkwo I.; et al. A phase 3 trial of l-glutamine in sickle cell disease. N. Engl. J. Med. 2018, 379, 226–235. 10.1056/NEJMoa1715971. [DOI] [PubMed] [Google Scholar]
- Aguilera-Carbo A.; Augur C.; Prado-Barragan L.; Aguilar C.; Favela-Torres E. Extraction and analysis of ellagic acid from novel complex sources. Chem. Pap. 2008, 62, 440–444. 10.2478/s11696-008-0042-y. [DOI] [Google Scholar]
- Gour A.; Kour D.; Dogra A.; Manhas D.; Wazir P.; Digra S. K.; Kumar A.; Nandi U. Epicatechin exerts dual action to shield sickling and hydroxyurea-induced myelosuppression: Implication in sickle cell anemia management. Toxicol. Appl. Pharmacol. 2022, 449, 116113. 10.1016/j.taap.2022.116113. [DOI] [PubMed] [Google Scholar]
- Goldberg M. A.; Brugnara C.; Dover G. J.; Schapira L.; Charache S.; Bunn H. F. Treatment of sickle cell anemia with hydroxyurea and erythropoietin. N. Engl. J. Med. 1990, 323, 366–372. 10.1056/NEJM199008093230602. [DOI] [PubMed] [Google Scholar]
- Lebensburger J. D.; Pestina T. I.; Boyd K.; Ware R. E.; Persons D. A. Hydroxyurea therapy requires HbF induction for clinical benefit in a sickle cell mouse model. Haematologica 2009, 114, 817. 10.1182/blood.V114.22.817.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J.-I.; Choi H.-S.; Jeong J.-S.; Han J.-Y.; Kim I.-H. Involvement of p38 kinase in hydroxyurea-induced differentiation of K562 cells. Cell Growth Differ. 2001, 12, 481–486. [PubMed] [Google Scholar]
- Morris C. R.; Vichinsky E. P.; van Warmerdam J.; Machado L.; Kepka-Lenhart D.; Morris S. M. Jr; Kuypers F. A. Hydroxyurea and arginine therapy: impact on nitric oxide production in sickle cell disease. J. Pediatr. Hematol. Oncol. 2003, 25, 629–634. 10.1097/00043426-200308000-00008. [DOI] [PubMed] [Google Scholar]
- Morris C. R.; Kato G. J.; Poljakovic M.; Wang X.; Blackwelder W. C.; Sachdev V.; Hazen S. L.; Vichinsky E. P.; Morris S. M.; Gladwin M. T. Dysregulated arginine metabolism, hemolysis-associated pulmonary hypertension, and mortality in sickle cell disease. JAMA 2005, 294, 81–90. 10.1001/jama.294.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bank N.; Kiroycheva M.; Ahmed F.; Anthony G. M.; Fabry M. E.; Nagel R. L.; Singhal P. C. Peroxynitrite formation and apoptosis in transgenic sickle cell mouse kidneys. Kidney Int. 1998, 54, 1520–1528. 10.1046/j.1523-1755.1998.00148.x. [DOI] [PubMed] [Google Scholar]
- Saker H. D.; Jewad A. M.; Hussein M. G. Effects of hydroxyurea on the oxidative stress and markers of renal tissue damage in patients with sickle cell disease of adults in basra. Biochem. Cell. Arch. 2020, 20, 3927–3931. [Google Scholar]
- Ikuta T.; Adekile A. D.; Gutsaeva D. R.; Parkerson J. B.; Yerigenahally S. D.; Clair B.; Kutlar A.; Odo N.; Head C. A. The proinflammatory cytokine GM-CSF downregulates fetal hemoglobin expression by attenuating the cAMP-dependent pathway in sickle cell disease. Blood Cells Mol. Dis. 2011, 47, 235–242. 10.1016/j.bcmd.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Franceschi L. Pathophisiology of sickle cell disease and new drugs for the treatment. Mediterr. J. Hematol. Infect. Dis. 2009, 1, e2009024 10.4084/MJHID.2009.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tshilolo L.; Tomlinson G. A.; McGann P. T.; Latham T. S.; Olupot-Olupot P.; Santos B.; Aygun B.; Stuber S.; Lane A.; Williams T. N.; et al. Splenomegaly in Children with Sickle Cell Anemia Receiving Hydroxyurea in Sub-Saharan Africa. Blood 2019, 134, 993. 10.1182/blood-2019-129937.31537539 [DOI] [Google Scholar]
- Ferrone F. A. The delay time in sickle cell disease after 40 years: A paradigm assessed. Am. J. Hematol. 2015, 90, 438–445. 10.1002/ajh.23958. [DOI] [PubMed] [Google Scholar]
- Domen J.; Gandy K. L.; Weissman I. L. Systemic overexpression of BCL-2 in the hematopoietic system protects transgenic mice from the consequences of lethal irradiation. Blood 1998, 91, 2272–2282. 10.1182/blood.V91.7.2272. [DOI] [PubMed] [Google Scholar]
- Agrawal R. K.; Patel R. K.; Shah V.; Nainiwal L.; Trivedi B. Hydroxyurea in sickle cell disease: drug review. Indian J. Hematol. Blood Transfus. 2014, 30, 91–96. 10.1007/s12288-013-0261-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muga R.; Ajwang A.; Ouma J.; Ojigo J.; Otieno J.; Okoth P.; Wafula C.; Ajwang S.; Ogolla D.; Hollist A.; et al. Efficacy of the Nutritional Supplement, EvenFlo, in the Management of Sickle Cell Disease: A Randomized Controlled Trial. Nurs. Health Sci. 2020, 3, 35–45. 10.55481/2578-3750.1058. [DOI] [Google Scholar]
- Morris C. R.; Kuypers F. A.; Lavrisha L.; Ansari M.; Sweeters N.; Stewart M.; Gildengorin G.; Neumayr L.; Vichinsky E. P. A randomized, placebo-controlled trial of arginine therapy for the treatment of children with sickle cell disease hospitalized with vaso-occlusive pain episodes. Haematologica 2013, 98, 1375. 10.3324/haematol.2013.086637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ippoushi K.; Takeuchi A.; Azuma K. Prevention of peroxynitrite-induced oxidation and nitration reactions by ellagic acid. Food Chem. 2009, 112, 185–188. 10.1016/j.foodchem.2008.05.057. [DOI] [Google Scholar]
- Domowicz D. A. L.; Welsby I.; Esther C. R. Jr; Zhu H.; Marek R. D.; Lee G.; Shah N.; Poisson J. L.; McMahon T. J. Effects of repleting organic phosphates in banked erythrocytes on plasma metabolites and vasoactive mediators after red cell exchange transfusion in sickle cell disease. Blood Transfus. 2020, 18, 200. 10.2450/2020.0237-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupesiz A.; Celmeli G.; Dogan S.; Antmen B.; Aslan M. The effect of hemolysis on plasma oxidation and nitration in patients with sickle cell disease. Free Radic. Res. 2012, 46, 883–890. 10.3109/10715762.2012.686037. [DOI] [PubMed] [Google Scholar]
- Valverde Y.; Benson B.; Gupta M.; Gupta K. Spinal glial activation and oxidative stress are alleviated by treatment with curcumin or coenzyme Q in sickle mice. Haematologica 2016, 101, e44 10.3324/haematol.2015.137489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padurariu M.; Ciobica A.; Hritcu L.; Stoica B.; Bild W.; Stefanescu C. Changes of some oxidative stress markers in the serum of patients with mild cognitive impairment and Alzheimer’s disease. Neurosci. Lett. 2010, 469, 6–10. 10.1016/j.neulet.2009.11.033. [DOI] [PubMed] [Google Scholar]
- Ateşşahín A.; Çeríbaşi A. O.; Yuce A.; Bulmus Ö.; Çikim G. Role of ellagic acid against cisplatin-induced nephrotoxicity and oxidative stress in rats. Basic Clin. Pharmacol. Toxicol. 2006, 100, 121–126. 10.1111/j.1742-7843.2006.00015.x. [DOI] [PubMed] [Google Scholar]
- Chen G.; Zhang D.; Fuchs T. A.; Manwani D.; Wagner D. D.; Frenette P. S. Heme-induced neutrophil extracellular traps contribute to the pathogenesis of sickle cell disease. Blood 2014, 123, 3818–3827. 10.1182/blood-2013-10-529982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vichinsky E.; Onyekwere O.; Porter J.; Swerdlow P.; Eckman J.; Lane P.; Files B.; Hassell K.; Kelly P.; Wilson F.; et al. A randomised comparison of deferasirox versus deferoxamine for the treatment of transfusional iron overload in sickle cell disease. Br. J. Haematol. 2007, 136, 501–508. 10.1111/j.1365-2141.2006.06455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badria F. A.; Ibrahim A. S.; Badria A. F.; Elmarakby A. A. Correction: Curcumin attenuates iron accumulation and oxidative stress in the liver and spleen of chronic iron-overloaded rats. PLoS One 2015, 10, e0134156 10.1371/journal.pone.0134156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wun T. The role of inflammation and leukocytes in the pathogenesis of sickle cell disease. Hematology 2000, 5, 403–412. 10.1080/10245332.2000.11746536. [DOI] [PubMed] [Google Scholar]
- Conran N.; Ikuta T.; Saad S. T.; Costa F. F. Increased GM-CSF Levels in Sickle Cell Disease Are Associated with Increased Leukocyte Counts and Are Reversed by Hydroxyurea. Am. J. Hematol. 2004, 104, 3573. 10.1182/blood.V104.11.3573.3573. [DOI] [Google Scholar]
- Vanella L.; Di Giacomo C.; Acquaviva R.; Barbagallo I.; Li Volti G.; Cardile V.; Abraham N. G.; Sorrenti V. Effects of ellagic acid on angiogenic factors in prostate cancer cells. Cancers 2013, 5, 726–738. 10.3390/cancers5020726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yale S. H.; Nagib N.; Guthrie T. Approach to vaso-occlussive crisis in adults with sickle cell disease. Am. Fam. Physician 2000, 61, 1349–1356. [PubMed] [Google Scholar]
- Baeuerle P. A.; Baichwal V. R. NF-kB as a frequent target for immunosuppressive and anti-inflammatory molecules. Adv. Immunol. 1997, 65, 111–138. [PubMed] [Google Scholar]
- Karki N. R.; Kutlar A. P-selectin blockade in the treatment of painful vaso-occlusive crises in sickle cell disease: a spotlight on crizanlizumab. J. Pain Res. 2021, 14, 849–856. 10.2147/JPR.S278285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves C. d. F.; Angeli G. N.; Favarin D. C.; de Andrade E. L.; Chica J. E. L.; Faccioli L. H.; da Silva P. R.; de Paula Rogerio A. The effects of proresolution of ellagic acid in an experimental model of allergic airway inflammation. Mediat. Inflamm. 2013, 2013, 1. 10.1155/2013/863198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahima Z. MEK1/2 as a therapeutic target in sickle cell disease. Int. J. Blood Res. Disord. 2019, 6, 38. 10.23937/2469-5696/1410038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah R.; Taborda C.; Chawla S. Acute and chronic hepatobiliary manifestations of sickle cell disease: A review. World J. Gastrointest. Pathophysiol. 2017, 8, 108. 10.4291/wjgp.v8.i3.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patra P.; Lakkakula B. K. S.; Verma H.; Choubey M.; Patra S.; Khodiar P. Assessment of renal function in Indian patients with sickle cell disease. Saudi J. Kidney Dis. Transplant. 2017, 28, 524. 10.4103/1319-2442.206440. [DOI] [PubMed] [Google Scholar]
- Madhuri M.; Manoj P.; Rajkumari D.; Raju A. G. Study on serum electrolytes in sickle cell disease patients on hydroxyurea therapy and non-hydroxyurea therapy. Int. J. Contemp. Med. Res. 2019, 6, L1–L4. 10.21276/ijcmr.2019.6.12.41. [DOI] [Google Scholar]
- Antwi-Boasiako C.; Asare M. M.; Baba I.; Doku A.; Adutwum-Ofosu K.; Hayfron-Benjamin C.; Asare C. P.; Aryee R.; Dankwah G. B.; Ahenkorah J. Association between pulmonary function and cardiac enzymes in sickle cell disease. Am. J. Blood Res. 2021, 11, 199. [PMC free article] [PubMed] [Google Scholar]
- Raj A. B.; Condurache T.; Bertolone S.; Williams D.; Lorenz D.; Sobczyk W. Quantitative assessment of ventricular function in sickle cell disease: Effect of long-term erythrocytapheresis. Blood Cancer 2005, 45, 976–981. 10.1002/pbc.20521. [DOI] [PubMed] [Google Scholar]
- Ballas S. K. Lactate dehydrogenase and hemolysis in sickle cell disease. J. Am. Soc. Hematology. 2013, 121, 243–244. 10.1182/blood-2012-10-462135. [DOI] [PubMed] [Google Scholar]
- Dogra A.; Bhatt S.; Magotra A.; Sharma A.; Kotwal P.; Gour A.; Wazir P.; Singh G.; Nandi U. Intervention of curcumin on oral pharmacokinetics of daclatasvir in rat: A possible risk for long-term use. Phytother Res. 2018, 32, 1967–1974. 10.1002/ptr.6123. [DOI] [PubMed] [Google Scholar]
- Kotwal P.; Dogra A.; Sharma A.; Bhatt S.; Gour A.; Sharma S.; Wazir P.; Singh P. P.; Kumar A.; Nandi U. Effect of natural phenolics on pharmacokinetic modulation of bedaquiline in rat to assess the likelihood of potential food–drug interaction. J. Agric. Food Chem. 2020, 68, 1257–1265. 10.1021/acs.jafc.9b06529. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.