Abstract
Background
Shared decision making (SDM) has long been advocated as the preferred way for physicians and men with prostate cancer to make treatment decisions. However, the implementation of formal SDM programs in routine care remains limited, and implementation outcomes for disadvantaged populations are especially poorly described. We describe the implementation outcomes between academic and county health care settings.
Methods
We administered a decision aid (DA) for men with localized prostate cancer at an academic center and across a county health care system. Our implementation was guided by the Consolidated Framework for Implementation Research and the Reach, Effectiveness, Adoption, Implementation, and Maintenance framework. We assessed the effectiveness of the DA through a postappointment patient survey.
Results
Sites differed by patient demographic/clinical characteristics. Reach (DA invitation rate) was similar and insensitive to implementation strategies at the academic center and county (66% v. 60%, P = 0.37). Fidelity (DA completion rate) was also similar at the academic center and county (77% v. 80%, P = 0.74). DA effectiveness was similar between sites, except for higher academic center ratings for net promoter for the doctor (77% v. 37%, P = 0.01) and the health care system (77% v. 35%, P = 0.006) and greater satisfaction with manner of care (medians 100 v. 87.5, P = 0.04). Implementation strategies (e.g., faxing of patients’ records and meeting patients in the clinic to complete the DA) represented substantial practice changes at both sites. The completion rate increased following the onset of reminder calls at the academic center and the creation of a Spanish module at the county.
Conclusions
Successful DA implementation efforts should focus on patient engagement and access. SDM may broadly benefit patients and health care systems regardless of patient demographic/clinical characteristics.
Keywords: decision aids, electronic decision aids, implementation, prostate cancer, shared decision making, vulnerable populations
Introduction
Shared decision making (SDM) has come to represent a paradigm of patient-centered, evidence-based care in which both physician and patient work together to decide on a preferred treatment.1,2 Specifically, SDM occurs when a patient’s values and preferences are identified and integrated with evidence to inform treatment choice and achieve personal goals.3,4 In practice, this often occurs through the use of decision aids (DAs), electronic or paper tools intended to foster the uptake and implementation of SDM. 5 Much evidence supports the benefits of SDM in increasing knowledge, clarifying values, and decreasing decisional conflict for patients.5,6 SDM has been heralded at the highest levels of health policy, including in the Affordable Care Act.7,8 But despite this promise, widespread implementation of SDM has lagged in routine care.9–12
Prostate cancer treatment is an especially suitable avenue for SDM. First, prostate cancer treatment is preference sensitive. 13 Treatments for prostate cancer, including prostatectomy, brachytherapy, and external-beam radiotherapy, are similarly efficacious, but each brings distinct quality-of-life changes.14,15 Therefore, individual patient preferences are critical in informing optimal treatment decisions for men with this diagnosis. Furthermore, men with low-risk prostate cancer are at risk of overtreatment with surgery or radiotherapy. 16 This evidence has led the American Urological Association, American Society for Radiation Oncology, and Society of Urologic Oncology to jointly endorse SDM in caring for patients with clinically localized prostate cancer. 17 However, broad implementation efforts that can succeed in multiple care settings and that are scalable remain limited.
One question regarding SDM is whether minority and nonminority patients experience DAs differently. Studies have shown that minorities tend to receive care in resource-constrained public and safety net hospitals.18–20 Racial and ethnic minorities are known to have higher rates of incidence and death from chronic diseases such as cancer, heart disease, and diabetes. 21 In addition, minority populations experience less access to health care resources and, on average, have lower rates of health literacy.22–24 Life expectancy is also shorter in minority and disadvantaged populations. For prostate cancer specifically, black men have higher rates of disease incidence and mortality when compared with white men.25,26 In conjunction with communication barriers between minority patients and nonminority physicians, these factors may lead to differences in the SDM process between minority and nonminority patients.27,28
Previous studies about SDM in minority populations suggest that electronic DAs increase knowledge, decrease decisional conflict, and increase satisfaction with the decision-making process for minority patients.29–33 Similar benefits can be found for other forms of DAs.34,35 However, few studies about DAs have directly compared the effects on minority versus nonminority populations, and access to electronic platforms in disadvantaged populations varies widely. Evidence is also sparse on DAs for prostate cancer treatment, as opposed to prostate cancer screening or other health conditions. Finally, a recent study shows that culturally targeted DAs are no better than generic versions in increasing decisional quality, bringing into question whether there are any significant differences between minority and nonminority patients with regard to the implementation of SDM DAs. 36
We conducted an implementation study to assess strategies leading to successful implementation of a previously described DA for prostate cancer (WiserCare) in 2 distinct care settings. 37 We administered the DA to patients at an academic medical center and across a public (county) health care system and then measured decisional quality through a postappointment questionnaire to verify efficacy. We hypothesized a priori that 1) implementation science methods would identify different implementation facilitators between the different sites and patient populations, 2) the use of DAs would lead to practice patterns changes, and 3) patients using DAs would have high treatment satisfaction and low decisional conflict.
Methods
Participant Recruitment
Eligible patients were seen at an academic medical center (UCLA Ronald Reagan Medical Center) and a county hospital (Olive View Medical Center) from March 2019 to March 2020. To determine patients’ eligibility, men with newly diagnosed prostate cancer in both settings were prospectively screened for appropriateness. Inclusion criteria included prostate-specific antigen (PSA) level lower than 20 ng/mL, no evidence of metastatic disease, incident prostate cancer without prior treatment (including on active surveillance), no diagnosis of dementia, and ability to read English or Spanish (eligible minority patients were predominantly Hispanic or Latino). Men with any Gleason score were eligible if they met the above criteria. Patients at the county hospital were patients of that clinic or referred from geographically distinct Urology departments across the Los Angeles County Department of Health Services (LAC-DHS). LAC-DHS determined that all men with incident disease should be given access to this quality improvement project and so arranged for transportation. LAC-DHS is the second largest public health system in the country and includes 4 hospitals and 20 ambulatory clinics and supports several hundred community partner clinics.
Implementation Strategy
In implementing our DA, we used the Consolidated Framework for Implementation Research (CFIR) to identify potential contextual factors leading to implementation success and applied the Reach, Effectiveness, Adoption, Implementation, and Maintenance (RE-AIM) framework to assess implementation outcomes.38–40 Using both frameworks allowed for practical evaluation of implementation outcomes (RE-AIM) and understanding of the contextual factors influencing those outcomes (CFIR). 41
The CFIR assesses constructs in 5 domains: Intervention Characteristic, Outer Setting, Inner Setting, Characteristics of Individuals, and Process. 38 We assessed these domains at the participating sites using the Model for Understanding Success in Quality (MUSIQ) scores. 42 The 6 domains of MUSIQ represent organizational capacities that generally align with CFIR constructs: QI Team (9–63 total), Microsystem (4–28 total), QI Support (2–14 total), Organization (6–42 total), Environment (2–14 total), and Other (1–7 total), with a higher total score on the 24 items indicating a higher likelihood of successful implementation. 42 MUSIQ is a practical, organizational self-assessment for anticipating and mitigating potential implementation barriers.
We used the RE-AIM framework to evaluate implementation of our DA. The RE-AIM framework evaluates an intervention’s potential for dissemination and impact on public health through 5 key criteria: Reach, Effectiveness, Adoption, Implementation, and Maintenance. 39 We operationalized the reach of our DA as the invitation rate (the number of patients identified and invited divided by the eligible number of patients) and the effectiveness of the DA as the completion rate (fidelity) and its impact on clinical effectiveness measures. RE-AIM has been successfully used to incorporate clinical and community-based interventions into real-world practice. 40
Patient DA
Eligible patients were contacted via telephone and offered the DA (WiserCare) before their clinic visit. WiserCare is an internet-based DA that integrates high-quality evidence with patient preferences and clinical data and has been validated in several different settings.37,43 Amenable patients at both sites were emailed a secure link to access the DA. For patients at the county without appropriate technology at home, a staff research associate completed the DA with them in clinic before their appointment. The DA educated patients about different treatment options for prostate cancer and used conjoint analysis to explore treatment goals and personal preferences. Through real-time decisional analysis in its online interface, the DA quantified the “expected value” of evidence-based treatment options for prostate cancer, using preference values as weights in the analysis. Upon completion, the patient received an electronic report detailing their clinical data (e.g., PSA, Gleason score, and clinical T stage), their personal values and goals in order of importance, and potential treatment options ranked from highest to lowest “expected value.” The report included additional educational information on each listed treatment option, including the associated risks and benefits. A copy of this 1-page report was emailed to the patient’s physician before their appointment. A double-back Spanish-translated version of the DA was available at the county, but the DA was otherwise equivalent at both sites. 44
Postappointment Questionnaire
We evaluated the DA’s effectiveness through a postappointment questionnaire. After their visit, patients completed the questionnaire in the clinic or at home by themselves. Responses were recorded on the same online platform used to implement the DA, and questions measured the following:
Control Preferences Scale. The Control Preferences Scale is an instrument widely used in decision-making research to assess patient preferences for their involvement in decision making regarding their health. 45 The scale ranges from less to more personal involvement.
Decisional Conflict. The validated Decisional Conflict Scale measures evidence of decisional conflict surrounding treatment choice. 45 Patients responded “yes,”“probably yes,”“unsure,”“probably no,” and “no” to 16 items to calculate a score for 5 domains: informed, values/clarity, support, uncertainty, and effective decision. These domains measure uncertainty in choosing between options, with modifiable factors, such as feeling uninformed, effectiveness of decision making, and satisfaction with choice, contributing to uncertainty. The total score ranges from 0 to 100, with higher scores corresponding to more decisional conflict.
Shared Decision Making. The validated Shared Decision Making Questionnaire (SDM-Q-9) assesses patient perception that SDM occurred between themselves and their clinician during the appointment and is measured on a scale between 0 and 45, with higher scores indicating more SDM. 46
Clinician and Institution Satisfaction/Loyalty. Net promoter evaluates a patient’s likelihood of referring a friend to the provider from whom they received care and to the institution where they received care. Patients responded “very likely,”“likely,”“neither likely nor unlikely,”“unlikely,” or “very unlikely” on how likely they would be to make the referrals. Responses were categorized as “detractor” (“neither likely nor unlikely,”“unlikely,” and “very unlikely”), “passive” (“likely”), and “promoter (“very likely”). The net promoter score is calculated by subtracting the group percentage of “detractors” from the group percentage of “promoters.” A higher net promoter score indicates greater endorsement of the doctor or institution.
Data Abstraction
Patient treatment preferences were assessed through the DA. Statistical process control and Pareto charts were generated monthly from a REDCap database to track DA reach (how many men eligible to receive the DA receive it) and fidelity (how many men who receive the DA complete it). DA and postappointment questionnaire data were stored and downloadable from the DA website.
Statistical Analysis
Statistical control charts display data over time to understand and improve the behavior of a system.47,48 Implementation measures were examined in real time to identify potential change associated with implementation strategies. We performed univariate analysis on implementation characteristics and measures from the postappointment questionnaire between the 2 hospital sites using a chi-square test (or Fisher’s exact test) for categorical variables and Wilcoxon rank-sum test for continuous but nonnormally distributed variables. All tests were 2 sided with an α set at 0.05 for statistical significance. All statistical analyses were performed using SAS 9.4.
Human Subjects Approval
This study was approved under a QI waiver by the Institutional Review Boards at each of the participating sites.
Results
Implementation Differences between Sites
Table 1 summarizes overall implementation outcomes at both sites. The MUSIQ score was high at the academic center and county (144.4/168 v. 157/168), indicating a substantial likelihood of success for our intervention. MUSIQ results identified potential challenges prior to implementation (e.g., concerns from stakeholders that DA would slow clinic) and formed the basis of later strategies after challenges arose (e.g., integration of the DA into the electronic medical record).
Table 1.
Implementation of the Decision Aid by Site (N = 1553)
| Academic Center (n = 1213) | LAC-DHS (n = 340) | P Value | |
|---|---|---|---|
| MUSIQ score a | |||
| Environment | 12/14 | 8/14 | |
| Organization | 33.75/42 | 42/42 | |
| QI support | 14/34 | 12/34 | |
| QI team | 59.67/63 | 63/63 | |
| Microsystem | 24/28 | 25/28 | |
| Other | 1/7 | 7/7 | |
| Total | 144.4/168 | 157/168 | |
| Implementation | |||
| Eligible (among screened) | 348 (29%) | 73 (21%) | 0.008 |
| Reach | |||
| Invited (among eligible) | 229 (66%) | 44 (60%) | 0.36 |
| Fidelity | |||
| Completed (among invited) | 177 (77%) | 35 (80%) | 0.74 |
| Post-MD survey completed (among DA completed) | 78 (44%) | 21 (60%) | 0.31 |
DA, decision aid; LAC-DHS, Los Angeles County Department of Health Services; MUSIQ, Model for Understanding Success in Quality.
Higher scores indicate more favorable contextual factors and a higher likelihood of QI success.
There was a greater percentage of patients eligible for the DA at the academic center than at the county (29% v. 21%, P = 0.008). To corroborate putative differences between the academic center and the county, we compared patients’ demographic and clinical characteristics by site. Among invited patients, there was a higher percentage of non-white men at the county versus the academic center (83% v. 45%, P < 0.001) and significant differences in clinical characteristics, including PSA, Gleason grade group, and T stage, between men invited to complete the DA at each site (Table 2). There were no significant differences in demographic or clinical characteristics between nonrespondents and respondents to the DA at each site (Supplementary Table S1). A similar trend was observed for nonrespondents and respondents to the postappointment questionnaire, except for a higher percentage of Gleason grade groups 1–2 among nonrespondents at the academic center (Supplementary Table S2).
Table 2.
Patient Characteristics among Those Who Were Invited to Complete the Decision Aid (n = 277)
| Total (n = 277) | Academic Center (n = 219) | LAC-DHS (n = 58) | P Value | |
|---|---|---|---|---|
| Age, median (IQR) | 63 (58, 68) | 64 (58, 69) | 61 (49, 64) | <0.001 a |
| Race/ethnicity | <0.001 b | |||
| White | 118 (46%) | 108 (55%) | 10 (17%) | |
| Black | 27 (11%) | 18 (9%) | 9 (16%) | |
| Asian | 22 (9%) | 17 (9%) | 5 (8%) | |
| Hispanic/Latino | 26 (10%) | 2 (1%) | 24 (41%) | |
| Mixed | 3 (1%) | 2 (1%) | 1 (2%) | |
| Other | 58 (23%) | 49 (25%) | 9 (16%) | |
| PSA, median (IQR) | 6.5 (4.6, 9.4) | 6.3 (4.6, 8.7) | 7.7 (4.8, 13.1) | 0.049 a |
| Gleason grade group | <0.001 b | |||
| 1 | 80 (30%) | 46 (22%) | 34 (63%) | |
| 2 | 99 (38%) | 87 (41%) | 12 (22%) | |
| 3 | 51 (19%) | 50 (24%) | 1 (2%) | |
| 4 | 17 (6%) | 13 (6%) | 4 (7%) | |
| 5 | 18 (7%) | 15 (7%) | 3 (6%) | |
| Clinical T stage | 0.03 b | |||
| T1 | 239 (94%) | 181 (92%) | 58 (100%) | |
| T2 | 16 (6%) | 16 (8%) | 0 (0%) |
IQR, interquartile range; LAC-DHS, Los Angeles County Department of Health Services; PSA, prostate-specific antigen.
Fisher’s exact test.
Wilcoxon rank-sum test.
Reach
Reach
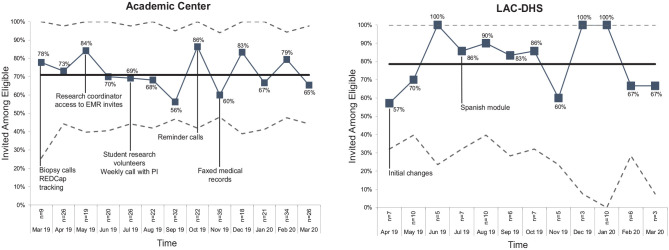
Among eligible patients, similar percentages were invited to complete the DA at the academic center and at the county (66% v. 60%, P = 0.37) (Figure 1). At the medical center, the reasons why patients were not invited included not responding to telephone calls (44%) and declining to participate (20%) (Supplementary Figure S1). At the county, most of the noninvites were primarily due to logistical barriers (e.g., appointment scheduling with no study coordinator available) or patients declining to participate (33%). The percentage not invited remained near baseline after the introduction of new strategies at both sites.
Figure 1.
Reach of the decision aid measured as the percentage invited among eligible patients by site.
Effectiveness
The effectiveness of our DA was captured by its fidelity (operationalized as the DA completion rate once invited) and impact on clinical effectiveness measures. Importantly, our DA was shown in previous research to improve decisional quality, so our reporting of its clinical effectiveness is primarily confirmatory.37,43
Fidelity
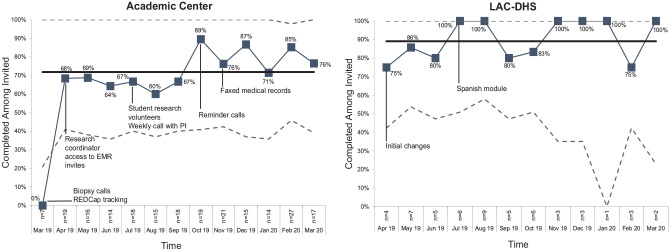
The overall DA completion rate for invited patients was similar at the academic center and the county (77% v. 80%, P = 0.74). Notably, Figure 2 shows that the completion rate at the academic center rose from 62% to 81% beginning October 2019, coinciding with the onset of reminder calls. A similar trend was noted at the county, from 81% to 92%, after the introduction of a translated Spanish-version of the DA in July 2019 (Figure 2). The average time patients spent on the DA was 23 min at the academic center and 38 min at LAC-DHS (the research coordinator met with most patients in the clinic for DA completion at LAC-DHS.)
Figure 2.
Fidelity of the decision aid measured as the percentage completed among invited patients by site.
Table 3 summarizes postappointment questionnaire responses from patients at both sites. Of the 212 patients who completed the DA at both sites, 78 of 177 (44%) responded to the postappointment questionnaire at the academic medical center and 21 of 35 (60%) responded at the county. Most patients had seen 1 to 2 doctors (58%), with most seeing both urologists and radiation oncologists (64%). Patients at both sites favored active treatment (radical prostatectomy/radiotherapy/focal therapy) before (46%) and after (56%) seeing their doctor. There were no statistically significant differences between the distributions of the number of doctors seen or treatment preferences (Table 3).
Table 3.
Clinical Effectiveness Measures among Those Who Completed the Decision Aid (n = 99)
| Academic Center (n = 78) | LAC-DHS (n = 21) | P Value | |
|---|---|---|---|
| Total no. of doctors seen after appointment | 0.98 a | ||
| 0 | 4 (5%) | 1 (5%) | |
| 1 | 27 (34%) | 7 (33%) | |
| 2 | 17 (22%) | 6 (29%) | |
| 3 | 14 (18%) | 3 (14%) | |
| 4–10 | 16 (21%) | 4 (19%) | |
| Types of doctors seen after appointment | 0.07 a | ||
| Urologist | 27 (38%) | 3 (15%) | |
| Radiation oncologist | 3 (4%) | 0 (0%) | |
| Both | 41 (58%) | 17 (85%) | |
| Primary treatment choice (before seeing the doctor) | 0.90 | ||
| Active surveillance | 15 (21%) | 5 (24%) | |
| Active treatment (RP/RT/FT) | 32 (45%) | 10 (48%) | |
| Unsure | 24 (34%) | 6 (28%) | |
| Primary treatment choice (after seeing the doctor) | 0.70 a | ||
| Active surveillance | 13 (19%) | 6 (29%) | |
| Active treatment (RP/RT/FT) | 39 (57%) | 11 (52%) | |
| Unsure | 16 (24%) | 4 (19%) | |
| Net promoter, doctor | 0.01 a | ||
| Promoter | 58 (81%) | 10 (40%) | |
| Passive | 11 (15%) | 7 (35%) | |
| Detractor | 3 (4%) | 3 (15%) | |
| NPS (promoter-detractor) b | 77% | 37% | |
| Net promoter, health care | 0.006 a | ||
| Promoter | 58 (81%) | 9 (45%) | |
| Passive | 11 (15%) | 9 (45%) | |
| Detractor | 3 (4%) | 2 (10%) | |
| NPS (promoter-detractor) b | 77% | 35% | |
| Control Preference Scale | 0.25 a | ||
| My doctor should make the decision for me | 0 (0%) | 1 (5%) | |
| My doctor should make the decision for me after considering my opinions and values | 1 (1%) | 1 (5%) | |
| My doctor and I make the decision together | 29 (40%) | 8 (40%) | |
| I make the decision after considering the doctor’s opinions and advice | 42 (58%) | 10 (50%) | |
| I make the decision based on what I believe alone | 1 (1%) | 0 (0%) | |
| Decisional Conflict, median (IQR) c | |||
| Uncertainty | 25 (0, 50) | 12.5 (0, 41.7) | 0.87 d |
| Informed | 0 (0, 25) | 4.2 (0, 41.6) | 0.28 d |
| Values/clarity | 0 (0, 25) | 16.7 (0, 37.5) | 0.10 d |
| Support | 8.3 (0, 25) | 8.3 (0, 37.5) | 0.31 d |
| Effective decision | 3.1 (0, 25) | 0 (0, 12.5) | 0.19 d |
| Total | 7 (0, 25) | 13.3 (3.9, 29.7) | 0.41 d |
| Satisfaction, median (IQR) e | |||
| Care outcome | 85 (75, 100) | 80 (70, 97.5) | 0.23 d |
| Manner | 100 (85, 100) | 87.5 (72.5, 100) | 0.04 d |
| Information | 100 (75, 100) | 75 (70.8, 100) | 0.16 d |
| Access | 83.3 (66.7, 100) | 75 (66.7, 100) | 0.59 d |
| Total | 90.6 (78.1, 98.4) | 82.8 (68.8, 98.4) | 0.23 d |
| Shared decision making, median (IQR) f | 36 (30, 44) | 42 (32, 45) | 0.39 d |
FT, focal therapy; IQR, interquartile range; LAC-DHS, Los Angeles County Department of Health Services; NPS, net promoter score; RP, radical prostatectomy; RT, radiotherapy.
Fisher’s exact text.
Net promoter is calculated by subtracting the percentage of detractors from the percentage of promoters.
Scores range from 0 (no decisional conflict) to 100 (extremely high decisional conflict).
Wilcoxon rank-sum test.
Scores range from 0 (extremely dissatisfied) to 100 (extremely satisfied).
Scores range from 0 (less shared decision making) to 45 (more shared decision making).
Net promoter
Net promoter scores for the doctor and health care institution were higher at the academic center than at the county (77% v. 37%, P = 0.01; 77% v. 35%, P = 0.006, respectively). This likely reflects institutional differences between each site rather than differences in effects of the DA itself.
Control Preference Scale
An overwhelming majority of patients at the academic center and county indicated “My doctor and I make the decision together” or “I make the decision after considering the doctor’s opinions and advice” (98% v. 90%, P = 0.25).
Decisional Conflict
Overall, there was very low decisional conflict in patients after using the DA and being counseled at the academic center and the county. The median total decisional conflict was 7 (interquartile range [IQR] 0, 25) at the academic center and 13.3 (IQR 3.9, 29.7) at the county. We found no statistically significant differences across total or other decisional conflict submeasures between the 2 sites.
Patient Satisfaction
Patient satisfaction was generally high at both sites. The median total satisfaction with cancer care was 90.6 (IQR 78.1, 98.4) at the academic medical center and 82.8 (IQR 68.8, 98.4) at the county. Notably, there was a significantly higher satisfaction with the manner of care at the academic center as compared with the county (P = 0.04).
Shared Decision Making
SDM was high in both groups with median scores of 36 (IQR 30, 44) at the academic center and 42 (IQR 32, 45) at the county. This did not differ between the 2 sites (P = 0.39).
Adoption
Physicians at the academic center and county received information about the DA’s design and goals to encourage adoption. Overall, 12 of 12 (100%) physicians at our 2 study sites participated in DA implementation.
Implementation
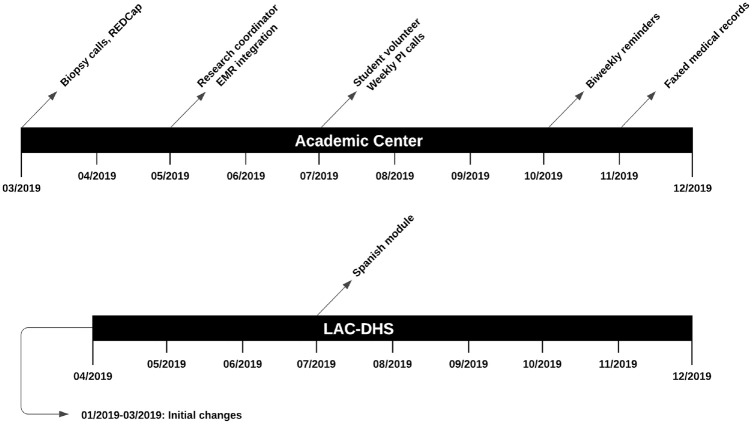
Throughout implementation of the DA, we employed a variety of implementation strategies to increase reach and fidelity (Figure 3). We describe these strategies below, following recommended reporting guidelines in the implementation science literature.49,50
Figure 3.
Timeline of strategies to increase reach and fidelity of the decision aid.
- 1. Academic Medical Center
- • Development and organization of quality monitoring systems: patients’ eligibility and enrollment status were monitored through REDCap starting March 2019. A full-time staff research coordinator conducted chart reviews for patients with an appointment scheduled within 1 mo. Chart review was conducted twice weekly to input relevant data into REDCap. Implementation outcomes targeted included DA reach, fidelity, and maintenance, as this strategy enabled organized tracking of patients and real-time identification of implementation barriers. For example, one barrier to DA reach was that patients in one practice were not notified of biopsy results before their consultation. After discussion with the physician, his team began notifying patients of biopsy results before appointments.
- • Change records systems: DA results were integrated into patients’ electronic medical records (EMRs) starting May 2019. The staff research coordinator generated EMR invitations, which autopopulated DA results in the patient’s chart, as additional patients received the DA. The targeted implementation outcome was the DA’s clinical effectiveness, as results of the DA were visible to the patients’ physicians for review before the appointment.
- • Organize implementation team meetings: frequency of implementation team meetings increased from monthly to weekly in July 2019. During these meetings, the study team evaluated implementation progress, identified barriers, and proposed relevant new strategies. All implementation outcomes were targeted by this strategy, as new strategies arising from discussions enhanced overall implementation efforts. A student volunteer assisted with executing these strategies.
- • Intervene with patients to enhance fidelity: we began reminding patients to complete the DA in October 2019. To integrate into the existing workflow, reminders were sent out by the research coordinator following twice-weekly chart review through phone calls to patients who had not yet completed the DA. This targeted the fidelity of our DA as it encouraged completion and provided troubleshooting for technical or logistical issues faced by the patient.
- • Facilitate relay of clinical data to the implementation team: in November 2019, our team began requesting faxed medical records from patients without existing records in our EMR. The staff research coordinator identified these patients through weekly chart review and provided a confidential fax number through phone calls or voicemail. The implementation target of this strategy was our DA’s reach, as we were unable to administer it to patients without first confirming eligibility in the EMR.
- 2. Los Angeles County Department of Health Services
- • Revise professional roles: from January to March 2019, professional roles were revised in preparation for DA implementation: attending called patients with biopsy results before visit, clinic administrators arranged free transportation for eligible patients, research coordinator offered DA to patients on active surveillance after participating physicians became familiar with its use, and research coordinator met patients in clinic to assist with DA completion. These strategies targeted reach and fidelity of the DA by mitigating technological, cultural, and socioeconomic barriers.
- • Translated DA: in July 2019, a Spanish-translated version of the DA, created by the implementation team, was approved by the Institutional Review Board and made available to patients. Patients whose primary language was Spanish were identified during chart review and subsequently on REDCap; the research coordinator administered the Spanish-translated DA electronically via email or in-person with patients before clinic. Implementation outcomes targeted were reach, fidelity, and clinical effectiveness, as we identified a higher proportion of Spanish-speaking patients at the county facility.
Maintenance
Our team continues to provide this DA for prostate cancer patients at the original sites in an effort to increase the number of patients reached and observe longitudinal trends in response to our implementation strategies. One site (UCLA) has committed an enterprise license for the DA software. We believe the successful implementation of our DA across both academic and county settings demonstrates its long-term suitability for a variety of settings.
Discussion
Our study demonstrates the successful yet differing implementation of a DA broadly across 2 care settings. We implemented the DA at an academic center and a public facility whose patients with prostate cancer differ significantly in terms of demographic and clinical characteristics. The reach of our DA was stable at both sites and nonresponsive to new implementation strategies. However, the fidelity of our DA was sensitive to implementation strategies, as the completion rate increased following the onset of reminder calls and creation of a Spanish-translated version. Clinical effectiveness measures were favorable with low decisional conflict and high treatment satisfaction and were not site dependent, except for net promoter scores and satisfaction with the manner of care—both of which were higher at the academic center. These findings are of particular relevance to other institutions seeking to implement SDM in a consistent and scalable manner considering its infrequent implementation in routine care.9–12
A number of interpretations may explain our findings. The stable invitation rate regardless of implementation strategies suggests that the reach of our DA is not a substantial implementation barrier and may represent an upper threshold that reflects patient preference. Identification of eligible patients and subsequent delivery of the DA proceeds efficaciously within an established system. However, the increase in completion rate following the onset of reminder phone calls and creation of a Spanish version of the DA reiterates the importance of patient-centered efforts to connect with patients and facilitate access and shows that implementation efforts necessarily differ in different systems and for disparate populations. Indeed, much of our efforts conducting this study centered around engaging patients and providing access to mitigate logistical and social barriers at the academic center and county, efforts that were necessary to ensure implementation fidelity. It is compelling that both sites were similar with regard to DA benefits and implementation measures despite substantial differences in patient demographics and practice settings. Although other studies have demonstrated these benefits in different environments, ours is the first to directly compare between 2 differing care settings.5,6,29–33
In addition to these findings, particularly striking are the practice changes that accompanied implementation of our DA, leading to a suite of ancillary benefits for patients. By virtue of increased contact for study implementation, patients at the academic center received earlier notification of their biopsy results, review of outside medical records by research staff, and remote support for relevant technical and logistical issues. These benefits were particularly salient at the county. Because of challenges specific to the patient population, patients were met in person for DA completion, provided free transportation to the clinic when eligible, and offered a Spanish version of the module to account for language barriers. These patients also benefited from earlier biopsy notifications, access to the DA regardless of point of entry into LAC-DHS, and freedom in choosing where to pursue subsequent treatment. Although these benefits were not primarily targeted by our DA, they highlight the value of implementing SDM in a broader context in driving institutional and organizational level improvements. 51
Implementation science focuses on methods to promote the uptake of research findings into routine care. 52 A number of implementation studies about SDM aids have highlighted similar strategies to successfully increase the reach and efficacy of different DAs.53,54 Notably, a continuing theme in DA implementation is overcoming obstacles related to workflow integration and buy-in from leadership and physicians. 55 Although our iterative research processes and support from leadership at both institutions mitigated many of these obstacles, broadly implementing SDM aids in settings without comparable resources, and staffing remains challenging. Recognizing this, the National Quality Forum recently published guidelines on 6 principles for success in their “SDM playbook”: promote leadership and culture; enhance patient education and engagement; provide health care team knowledge and training; take concrete actions; track, monitor, and report; and establish accountability. 56
Some considerations may limit the interpretation of our findings. First, the number of patients enrolled at the county was substantially fewer than that at the academic center because of differences in patient populations and health care systems. The screening and enrollment procedures also differed to adapt to unique challenges at each site. Therefore, direct comparisons with regard to clinical effectiveness measures should be interpreted with caution. However, the primary goal of our study was to successfully implement our DA at disparate sites and not to compare effects on clinical measures, which have been shown in the literature. In addition, it is important to note that substantial efforts in the form of practice changes and institutional collaborations were required to successfully implement our DA. These efforts may be prohibitive at other institutions considering limited resources (e.g., lack of staffing for meeting patients in person, organizing free transportation) as well as institutional barriers (staffing resources required to confirm receipt of outside records). Regarding the latter item, many institutions already attempt to do this, as it results in fewer “wasted” clinic visits for patients because of the lack of complete clinical information for counseling. Finally, time-dependent measures in our study such as decisional conflict should be interpreted cautiously, as these represent a snapshot of the patient’s feelings, which may change with additional time after the appointment.
Further research is needed to identify consistently successful strategies in the dissemination and implementation of SDM aids across multiple settings. Barriers to implementation, such as reasons for not responding to DAs, should be identified and explicitly targeted by implementation strategies. Given its benefits, an interesting question is how to promote SDM broadly in the US health care system without excessively burdening clinicians. For example, compensating providers for practicing SDM may be an effective strategy considering implementation barriers. Successful strategies identified in the literature should be translated into policies that reward institutions and physicians for practicing SDM and employing DAs in their own practice. Implementation science methods provide a mechanism for identifying these strategies and translating research findings into impactful practice changes.
Conclusion
Implementation of SDM aids remains uncommon in routine practice. Our findings suggest that successful implementation in disparate care settings is achievable and may improve patients’ decisional quality and satisfaction with their care. Other institutions and clinicians seeking to emulate our work should focus on improving accessibility and engaging with patients prior to their appointment to reach the greatest number of individuals and ensure proper utilization of DAs. Although a great deal of work remains to be done until appropriate application of SDM is widespread, its potential benefits and feasibility of implementation in routine care are already evident.
Supplemental Material
Supplemental material, sj-docx-1-mdm-10.1177_0272989X20982533 for Differences in Implementation Outcomes of a Shared Decision-Making Program for Men with Prostate Cancer between an Academic Medical Center and County Health Care System by Kevin D. Li, Christopher S. Saigal, Megha D. Tandel, Lorna Kwan, Moira Inkelas, Dana L. Alden, Stanley K. Frencher, Kiran Gollapudi, Jeremy Blumberg, Jamal Nabhani and Jonathan Bergman in Medical Decision Making
Supplemental material, sj-docx-2-mdm-10.1177_0272989X20982533 for Differences in Implementation Outcomes of a Shared Decision-Making Program for Men with Prostate Cancer between an Academic Medical Center and County Health Care System by Kevin D. Li, Christopher S. Saigal, Megha D. Tandel, Lorna Kwan, Moira Inkelas, Dana L. Alden, Stanley K. Frencher, Kiran Gollapudi, Jeremy Blumberg, Jamal Nabhani and Jonathan Bergman in Medical Decision Making
Supplemental material, sj-docx-3-mdm-10.1177_0272989X20982533 for Differences in Implementation Outcomes of a Shared Decision-Making Program for Men with Prostate Cancer between an Academic Medical Center and County Health Care System by Kevin D. Li, Christopher S. Saigal, Megha D. Tandel, Lorna Kwan, Moira Inkelas, Dana L. Alden, Stanley K. Frencher, Kiran Gollapudi, Jeremy Blumberg, Jamal Nabhani and Jonathan Bergman in Medical Decision Making
Supplemental material, sj-docx-4-mdm-10.1177_0272989X20982533 for Differences in Implementation Outcomes of a Shared Decision-Making Program for Men with Prostate Cancer between an Academic Medical Center and County Health Care System by Kevin D. Li, Christopher S. Saigal, Megha D. Tandel, Lorna Kwan, Moira Inkelas, Dana L. Alden, Stanley K. Frencher, Kiran Gollapudi, Jeremy Blumberg, Jamal Nabhani and Jonathan Bergman in Medical Decision Making
Acknowledgments
This work was supported by our collaborators across LAC-DHS who helped refer patients to receive the DA and played a large role in making our SDM program a success. We would also like to thank Sophia Monica Soni, Hal Yee, and the rest of the team at LAC-DHS who helped build and support a health care system of choice in the safety net.
Footnotes
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Christopher Saigal is on the board of directors for WiserCare. All other coauthors have no conflicts of interest.
The authors received no financial support for the research, authorship, and/or publication of this article.
Disclosures: Christopher Saigal is on the board of directors for WiserCare. The principal investigator and all other coauthors have no conflicts of interest.
ORCID iD: Kevin D. Li  https://orcid.org/0000-0003-0550-0250
https://orcid.org/0000-0003-0550-0250
Supplemental Material: Supplementary material for this article is available on the Medical Decision Making Web site at http://journals.sagepub.com/home/mdm.
Contributor Information
Kevin D. Li, Department of Urology, University of California Los Angeles, David Geffen School of Medicine, Los Angeles, CA, USA; University of California San Francisco, School of Medicine, San Francisco, CA, USA.
Christopher S. Saigal, Department of Urology, University of California Los Angeles, David Geffen School of Medicine, Los Angeles, CA, USA
Megha D. Tandel, Department of Urology, University of California Los Angeles, David Geffen School of Medicine, Los Angeles, CA, USA
Lorna Kwan, Department of Urology, University of California Los Angeles, David Geffen School of Medicine, Los Angeles, CA, USA.
Moira Inkelas, University of California Los Angeles, Jonathan and Karin Fielding School of Public Health, Los Angeles, CA, USA.
Dana L. Alden, University of Hawai’i at Manoa Shidler College of Business, Marketing, Honolulu, HI, USA
Stanley K. Frencher, Department of Urology, University of California Los Angeles, David Geffen School of Medicine, Los Angeles, CA, USA Los Angeles County Department of Health Services, Los Angeles, CA, USA.
Kiran Gollapudi, Department of Urology, University of California Los Angeles, David Geffen School of Medicine, Los Angeles, CA, USA; Los Angeles County Department of Health Services, Los Angeles, CA, USA.
Jeremy Blumberg, Department of Urology, University of California Los Angeles, David Geffen School of Medicine, Los Angeles, CA, USA; Los Angeles County Department of Health Services, Los Angeles, CA, USA.
Jamal Nabhani, Los Angeles County Department of Health Services, Los Angeles, CA, USA; USC Keck School of Medicine, Department of Urology, Los Angeles, CA, USA.
Jonathan Bergman, Department of Urology, University of California Los Angeles, David Geffen School of Medicine, Los Angeles, CA, USA; Los Angeles County Department of Health Services, Los Angeles, CA, USA; VA Greater Los Angeles Healthcare System, Los Angeles, CA, USA.
References
- 1.Charles C, Gafni A, Whelan T. Shared decision-making in the medical encounter: what does it mean? (or it takes at least two to tango). Soc Sci Med. 1997;44(5):681–92. [DOI] [PubMed] [Google Scholar]
- 2.Barry MJ, Edgman-Levitan S. Shared decision making—pinnacle of patient-centered care. N Engl J Med. 2012;366(9):780–1. Available from: 10.1056/NEJMp1109283 [DOI] [PubMed] [Google Scholar]
- 3.Elwyn G, Edwards A, Kinnersley P. Shared decision-making in primary care: the neglected second half of the consultation. Br J Gen Pract. 1999;49(443):477–82. Available from: https://www.ncbi.nlm.nih.gov/pubmed/10562751 [PMC free article] [PubMed] [Google Scholar]
- 4.Towle A, Godolphin W. Framework for teaching and learning informed shared decision making. BMJ. 1999;319(7212):766–71. Available from: 10.1136/bmj.319.7212.766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stacey D, Légaré F, Lewis K, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2017;4:CD001431. Available from: 10.1002/14651858.CD001431.pub5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Riikonen JM, Guyatt GH, Kilpeläinen TP, et al. Decision aids for prostate cancer screening choice: a systematic review and meta-analysis. JAMA Intern Med. 2019;179(8):1072–82. Available from: 10.1001/jamainternmed.2019.0763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stacey D, Légaré F, Lewis KB. Patient decision aids to engage adults in treatment or screening decisions. JAMA. 2017;318(7):657–8. Available from: 10.1001/jama.2017.10289 [DOI] [PubMed] [Google Scholar]
- 8.Affordable Care Act. Patient protection and affordable care act. Public Law. 2010;111–48. Available from: https://www.connectthedotsusa.com/wp-content/uploads/2019/06/ACAvNIMASlidesScript_6_3_19.pdf
- 9.Charles C, Gafni A, Whelan T. Self-reported use of shared decision-making among breast cancer specialists and perceived barriers and facilitators to implementing this approach. Health Expect. 2004;7(4):338–48. Available from: https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1369-7625.2004.00299.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fowler FJ, Jr, Gerstein BS, Barry MJ. How patient centered are medical decisions? Results of a national survey. JAMA Intern Med. 2013;173(13):1215–21. Available from: 10.1001/jamainternmed.2013.6172 [DOI] [PubMed] [Google Scholar]
- 11.Stiggelbout AM, Van der Weijden T, De Wit MPT, et al. Shared decision making: really putting patients at the centre of healthcare. BMJ. 2012;344:e256. Available from: 10.1136/bmj.e256 [DOI] [PubMed] [Google Scholar]
- 12.Vogel BA, Helmes AW, Hasenburg A. Concordance between patients’ desired and actual decision-making roles in breast cancer care. Psycho-Oncology. 2008;17(2):182–9. Available from: 10.1002/pon.1215 [DOI] [PubMed] [Google Scholar]
- 13.de Bekker-Grob EW, Bliemer MCJ, Donkers B, et al. Patients’ and urologists’ preferences for prostate cancer treatment: a discrete choice experiment. Br J Cancer. 2013;109(3):633–40. Available from: 10.1038/bjc.2013.370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamdy FC, Donovan JL, Lane JA, et al. 10-year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med. 2016;375(15):1415–24. Available from: 10.1056/NEJMoa1606220 [DOI] [PubMed] [Google Scholar]
- 15.Sanda MG, Dunn RL, Michalski J, et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. N Engl J Med. 2008;358(12):1250–61. Available from: 10.1056/NEJMoa074311 [DOI] [PubMed] [Google Scholar]
- 16.Wilt TJ, Jones KM, Barry MJ, et al. Follow-up of prostatectomy versus observation for early prostate cancer. N Engl J Med.;377(2):132–42. Available from: 10.1056/NEJMoa1615869 [DOI] [PubMed] [Google Scholar]
- 17.Sanda MG, Cadeddu JA, Kirkby E, et al. Clinically localized prostate cancer: AUA/ASTRO/SUO guideline. Part I: risk stratification, shared decision making, and care options. J Urol. 2018;199(3):683–90. Available from: 10.1016/j.juro.2017.11.095 [DOI] [PubMed] [Google Scholar]
- 18.Hussain-Gambles M, Atkin K, Leese B. Why ethnic minority groups are under-represented in clinical trials: a review of the literature. Health Soc Care Commun. 2004;12(5):382–8. Available from: 10.1111/j.1365-2524.2004.00507.x [DOI] [PubMed] [Google Scholar]
- 19.Hasnain-Wynia R, Baker DW, Nerenz D, et al. Disparities in health care are driven by where minority patients seek care: examination of the hospital quality alliance measures. Arch Intern Med. 2007;167(12):1233–9. Available from: 10.1001/archinte.167.12.1233 [DOI] [PubMed] [Google Scholar]
- 20.Institute of Medicine, Committee on Cancer Research among Minorities and the Medically Underserved. The Unequal Burden of Cancer: An Assessment of NIH Research and Programs for Ethnic Minorities and the Medically Underserved. Washington (DC): National Academies Press; 1999. Available from: https://play.google.com/store/books/details?id=v1ubAgAAQBAJ [PubMed] [Google Scholar]
- 21.Nelson A. Unequal treatment: confronting racial and ethnic disparities in health care. J Natl Med Assoc. 2002;94(8):666–8. Available from: https://www.ncbi.nlm.nih.gov/pubmed/12152921 [PMC free article] [PubMed] [Google Scholar]
- 22.Purnell TS, Calhoun EA, Golden SH, et al. Achieving health equity: closing the gaps in health care disparities, interventions, and research. Health Aff. 2016;35(8):1410–5. Available from: 10.1377/hlthaff.2016.0158 [DOI] [PubMed] [Google Scholar]
- 23.Christy SM, Gwede CK, Sutton SK, et al. Health literacy among medically underserved: the role of demographic factors, social influence, and religious beliefs. J Health Commun. 2017;22(11):923–31. Available from: 10.1080/10810730.2017.1377322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Becerra BJ, Arias D, Becerra MB. Low health literacy among immigrant Hispanics. J Racial Ethn Health Disparities. 2017;4(3):480–3. Available from: 10.1007/s40615-016-0249-5 [DOI] [PubMed] [Google Scholar]
- 25.Schwartz K, Powell IJ, Underwood W, III, George J, Yee C, Banerjee M. Interplay of race, socioeconomic status, and treatment on survival of patients with prostate cancer. Urology. 2009;74(6):1296–302. Available from: 10.1016/j.urology.2009.02.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tyson MD, II, Castle EP. Racial disparities in survival for patients with clinically localized prostate cancer adjusted for treatment effects. Mayo Clin Proc. 2014;89(3):300–7. Available from: 10.1016/j.mayocp.2013.11.001 [DOI] [PubMed] [Google Scholar]
- 27.Schouten BC, Meeuwesen L. Cultural differences in medical communication: a review of the literature. Patient Educ Couns. 2006;64(1-3):21–34. Available from: 10.1016/j.pec.2005.11.014 [DOI] [PubMed] [Google Scholar]
- 28.Johnson RL, Roter D, Powe NR, Cooper LA. Patient race/ethnicity and quality of patient–physician communication during medical visits. Am J Public Health. 2004;94(12):2084–90. Available from: 10.2105/AJPH.94.12.2084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller DP, Spangler JG, Doug Case L, Goff DC, Singh S, Pignone MP. Effectiveness of a web-based colorectal cancer screening patient decision aid: a randomized controlled trial in a mixed-literacy population. Am J Prev Med. 2011;40(6):608–15. Available from: http://www.ajpmonline.org/article/S074937971100136X/abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Volk RJ, Jibaja-Weiss ML, Hawley ST, et al. Entertainment education for prostate cancer screening: a randomized trial among primary care patients with low health literacy. Patient Educ Couns. 2008;73(3):482–9. Available from: 10.1016/j.pec.2008.07.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taylor KL, Davis JL, III, Turner RO, et al. Educating African American men about the prostate cancer screening dilemma: a randomized intervention. Cancer Epidemiol Biomarkers Prev. 2006;15(11):2179–88. Available from: 10.1158/1055-9965.EPI-05-0417 [DOI] [PubMed] [Google Scholar]
- 32.Schroy PC, III, Emmons K, Peters E, et al. The impact of a novel computer-based decision aid on shared decision making for colorectal cancer screening: a randomized trial. Med Decis Making. 2011;31(1):93–107. Available from: 10.1177/0272989X10369007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ellison GL, Weinrich SP, Lou M, Xu H, Powell IJ, Baquet CR. A randomized trial comparing web-based decision aids on prostate cancer knowledge for African-American men. J Natl Med Assoc. 2008;100(10):1139–45. Available from: 10.1016/s0027-9684(15)31481-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Durand M-A, Carpenter L, Dolan H, et al. Do interventions designed to support shared decision-making reduce health inequalities? A systematic review and meta-analysis. PLoS One. 2014;9(4):e94670. Available from: 10.1371/journal.pone.0094670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nathan AG, Marshall IM, Cooper JM, Huang ES. Use of decision aids with minority patients: a systematic review. J Gen Intern Med. 2016;31(6):663–76. Available from: 10.1007/s11606-016-3609-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alden DL, Friend J, Fraenkel L, Jibaja-Weiss M. The effects of culturally targeted patient decision aids on medical consultation preparation for Hispanic women in the U.S.: results from four randomized experiments. Soc Sci Med. 2018;212:17–25. Available from: http://www.sciencedirect.com/science/article/pii/S0277953618303381 [DOI] [PubMed] [Google Scholar]
- 37.Peña A, Qian Z, Lambrechts S, et al. Evaluation of implementation outcomes after initiation of a shared decision-making program for men with prostate cancer. Urology. 2019;132:94–100. Available from: 10.1016/j.urology.2019.06.032 [DOI] [PubMed] [Google Scholar]
- 38.Damschroder LJ, Aron DC, Keith RE, Kirsh SR, Alexander JA, Lowery JC. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci. 2009;4:50. Available from: 10.1186/1748-5908-4-50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Glasgow RE, Harden SM, Gaglio B, et al. RE-AIM planning and evaluation framework: adapting to new science and practice with a 20-year review. Front Public Health. 2019;7:64. Available from: 10.3389/fpubh.2019.00064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kwan BM, McGinnes HL, Ory MG, Estabrooks PA, Waxmonsky JA, Glasgow RE. RE-AIM in the real world: use of the RE-AIM framework for program planning and evaluation in clinical and community settings. Front Public Health. 2019;7:345. Available from: 10.3389/fpubh.2019.00345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.King DK, Shoup JA, Raebel MA, et al. Planning for implementation success using RE-AIM and CFIR frameworks: a qualitative study. Front Public Health. 2020;8:59. Available from: 10.3389/fpubh.2020.00059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reed JE, Kaplan HC, Ismail SA. A new typology for understanding context: qualitative exploration of the model for understanding success in quality (MUSIQ). BMC Health Serv Res. 2018;18(1):584. Available from: 10.1186/s12913-018-3348-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Johnson DC, Mueller DE, Deal AM, et al. Integrating patient preference into treatment decisions for men with prostate cancer at the point of care. J Urol. 2016;196(6):1640–4. Available from: 10.1016/j.juro.2016.06.082 [DOI] [PubMed] [Google Scholar]
- 44.Brislin RW. Back-translation for cross-cultural research. J Cross Cult Psychol. 1970;1(3):185–216. Available from: 10.1177/135910457000100301 [DOI] [Google Scholar]
- 45.Degner LF, Sloan JA, Venkatesh P. The control preferences scale. Can J Nurs Res. 1997;29(3):21–43. Available from: https://www.ncbi.nlm.nih.gov/pubmed/9505581 [PubMed] [Google Scholar]
- 46.Simon D, Schorr G, Wirtz M, et al. Development and first validation of the shared decision-making questionnaire (SDM-Q). Patient Educ Couns. 2006;63(3):319–27. Available from: 10.1016/j.pec.2006.04.012 [DOI] [PubMed] [Google Scholar]
- 47.Benneyan JC, Lloyd RC, Plsek PE. Statistical process control as a tool for research and healthcare improvement. Qual Saf Health Care. 2003;12(6):458–64. Available from: 10.1136/qhc.12.6.458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Provost LP, Murray S. The Health Care Data Guide: Learning from Data for Improvement. New York: John Wiley & Sons; 2011. Available from: https://play.google.com/store/books/details?id=5JscBgAAQBAJ [Google Scholar]
- 49.Proctor EK, Powell BJ, McMillen JC. Implementation strategies: recommendations for specifying and reporting. Implement Sci. 2013;8:139. Available from: 10.1186/1748-5908-8-139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Powell BJ, McMillen JC, Proctor EK, et al. A compilation of strategies for implementing clinical innovations in health and mental health. Med Care Res Rev. 2012;69(2):123–57. Available from: 10.1177/1077558711430690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Elwyn G, Frosch DL, Kobrin S. Implementing shared decision-making: consider all the consequences. Implement Sci. 2016;11:114. Available from: 10.1186/s13012-016-0480-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bauer MS, Damschroder L, Hagedorn H, Smith J, Kilbourne AM. An introduction to implementation science for the non-specialist. BMC Psychol. 2015;3:32. Available from: 10.1186/s40359-015-0089-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Belkora JK, Teng A, Volz S, Loth MK, Esserman LJ. Expanding the reach of decision and communication aids in a breast care center: a quality improvement study. Patient Educ Couns. 2011;83(2):234–9. Available from: 10.1016/j.pec.2010.07.003 [DOI] [PubMed] [Google Scholar]
- 54.Lewis CL, Pignone MP. Promoting informed decision-making in a primary care practice by implementing decision aids. N C Med J. 2009;70(2):136–9. Available from: https://www.ncbi.nlm.nih.gov/pubmed/19489371 [PMC free article] [PubMed] [Google Scholar]
- 55.Uy V, May SG, Tietbohl C, Frosch DL. Barriers and facilitators to routine distribution of patient decision support interventions: a preliminary study in community-based primary care settings. Health Expect. 2014;17(3):353–64. Available from: 10.1111/j.1369-7625.2011.00760.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.National Quality Forum. National Quality Partners Playbook™: Shared Decision Making in Healthcare. Washington (DC): National Quality Forum; 2018. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-mdm-10.1177_0272989X20982533 for Differences in Implementation Outcomes of a Shared Decision-Making Program for Men with Prostate Cancer between an Academic Medical Center and County Health Care System by Kevin D. Li, Christopher S. Saigal, Megha D. Tandel, Lorna Kwan, Moira Inkelas, Dana L. Alden, Stanley K. Frencher, Kiran Gollapudi, Jeremy Blumberg, Jamal Nabhani and Jonathan Bergman in Medical Decision Making
Supplemental material, sj-docx-2-mdm-10.1177_0272989X20982533 for Differences in Implementation Outcomes of a Shared Decision-Making Program for Men with Prostate Cancer between an Academic Medical Center and County Health Care System by Kevin D. Li, Christopher S. Saigal, Megha D. Tandel, Lorna Kwan, Moira Inkelas, Dana L. Alden, Stanley K. Frencher, Kiran Gollapudi, Jeremy Blumberg, Jamal Nabhani and Jonathan Bergman in Medical Decision Making
Supplemental material, sj-docx-3-mdm-10.1177_0272989X20982533 for Differences in Implementation Outcomes of a Shared Decision-Making Program for Men with Prostate Cancer between an Academic Medical Center and County Health Care System by Kevin D. Li, Christopher S. Saigal, Megha D. Tandel, Lorna Kwan, Moira Inkelas, Dana L. Alden, Stanley K. Frencher, Kiran Gollapudi, Jeremy Blumberg, Jamal Nabhani and Jonathan Bergman in Medical Decision Making
Supplemental material, sj-docx-4-mdm-10.1177_0272989X20982533 for Differences in Implementation Outcomes of a Shared Decision-Making Program for Men with Prostate Cancer between an Academic Medical Center and County Health Care System by Kevin D. Li, Christopher S. Saigal, Megha D. Tandel, Lorna Kwan, Moira Inkelas, Dana L. Alden, Stanley K. Frencher, Kiran Gollapudi, Jeremy Blumberg, Jamal Nabhani and Jonathan Bergman in Medical Decision Making