Abstract
Over the past century, human health has been enhanced by antimicrobial development. Following the deployment of the first antibiotics in the 1940s, bacterial resistance emerged and has increasingly outmaneuvered even the most promising antimicrobial agents. Accordingly, increased interest has been placed on alternative methods to circumvent antimicrobial resistance evolution. In the enclosed short review, we discuss the antimicrobial properties of human breast milk with a special emphasis on human milk oligosaccharides (HMOs). We recount studies across gram-negative and gram-positive pathogens, highlighting the usage of HMOs in promoting human health and wellness.
1. Introduction.
Human breast milk is recommended as the sole nutritional source for infants during the first six months of life. As the neonate ages, the World Health Organization (WHO) advises breast milk supplemented with solid foods until two years of age. The American Academy of Pediatrics (AAP), the U.S. Department of Health and Human Services, and the U.S. Centers for Disease Control (CDC) all echo the importance of human milk as the cornerstone of infant nutrition. Interestingly, global breastfeeding rates do not mirror these recommendations, with only 40% of infants under 6 months receiving breastmilk as their exclusive source of nutrition [1]. Several factors contribute to a communities observed breastfeeding rate. These include inadequate support for new mothers, maternal health complications that prevent lactation or safe breastfeeding, and metabolic complications in the neonate [2]. Ultimately, while safe and nutritious, infant formula does not mirror the composition of breast milk and is unable to deliver its therapeutic profile. Human milk contains several immunomodulatory, antimicrobial, and anti-inflammatory molecules. Leveraging these molecules, human milk reduces infant morbidity, facilitates cognitive and psychomotor development, and guides the establishment of the microbiome [3]. Indeed, human milk contains hundreds of species of bacteria, and their colonization is modulated by human milk. Overall, it is well established that, in the absence of human milk, infants face increased susceptibility to health issues ranging from asthma to allergies.
2. Human Milk Oligosaccharides
Human milk oligosaccharides (HMOs) are the third largest macromolecular component of human milk after lactose and fat. Structurally, HMOs consist of five pyranoses: D-galactose, D-glucose, D-N-acetylglucosamine (GlcNAc), L-fucose, and N-acetylneuraminic acid (sialic acid) (Figure 1).[4] Biosynthetically, HMOs follow a blueprint wherein every oligosaccharide contains lactose at its reducing end. Elongation occurs via addition of N-acetyllactosamine or lacto-N-biose (termination residue) (Figure 1).[5] To date, ca. 200 unique HMOs have been characterized, incorporating between 3–22 monosaccharides. HMO composition varies from mother to mother and changes over the course of lactation [6]. Fucosylation and sialylation patterns are governed by the mother’s secretor status and Lewis blood group (Le). People with an active Se gene locus coding for the α1–2-fucoslyltransferase FUT2 are designated Secretors. Secretor mothers produce milk abundant in α1–2-fucosylated HMOs. Non-secretors lack an active FUT2 enzyme and, consequently, their milk is populated with non-α1–2-fucosylated HMOs. Mothers with activate α1–3/4-fucoslyltranferase FUT3 enzyme are Le positive and have a milk composition favoring α1–4 fucosylation [7]. Total concentrations of HMOs in human milk range from 5 to 25 g/L over the course of lactation [8].
Figure 1:

HMO architecture with representative HMOs. A. The five monosaccharide units with their corresponding symbols. B. Structural diagram of HMO biosynthesis. C. Selected non-fucosylated neutral HMOs. D. Selected fucosylated HMOs. E. Selected sialylated HMOs.
3. Epidemiology of HMOs
The composition of human milk changes frequently to meet a baby’s physiological needs. The most dynamic period occurs during the first week of life [9]. Colostrum (the first milk) is nearly non-nutritious and is primarily used to establish immunity [10]. Colostrum is produced during the first five to seven days, at which time the transition to mature human milk begins. By week four, the neonatal consists of mature breast milk [10].
During this early period, HMO concentration is at its peak. Though a mother will spend 300 to 500 calories per day producing HMOs, they are not metabolized. Rather, HMOs are used as a carbon source for commensal bacteria, providing an evolutionary advantage over potential pathogens in the gut [11]. Indeed, a recent study from the AAP revealed that breastfed infants were better protected against respiratory and gastrointestinal infections. They also showed enhanced protection against inflammatory diseases such as asthma and diabetes compared to formula-fed children [12] [13].
4. HMOs inhibit bacterial growth
If a child is delivered vaginally, the vagina is the first source of gut inoculation. Human milk is the second source and is instrumental in developing the infant gut microbiota [14]. The flora of non-breastfed infants differs from those who receive breastmilk as their sole nutrition source (Figure 2). For example, formula fed babies exhibit a high level of diversity in their microbiota, while children who are breastfed have microbiotas that are dominated early on by Bifidobacterium, a known probiotic [15]. At the later stages of lactation, the microbiome is dominated by Bacteroides – a compositional change governed by modification of HMOs. Overall, the human milk microbiome reduces infection through several mechanisms (Figure 2). First, commensal species metabolize HMOs - a competitive advantage over pathogens who generally do not possess the hydrolases needed to consume HMOs. For example, Lactobacilli have been implicated in inhibition of growth and adhesion for Escherichia coli and Pseudomonas species [16]. Secondly, HMOs are antiadhesive antimicrobial agents that prevent pathogenic growth [17] [18]. Our team, in addition to others, has investigated the ability of HMOs to promote commensal bacteria colonization and inhibit the growth of pathogenic bacteria in the gut. In the following section, we describe some of these efforts – particularly those involving pathogens important to infant health and wellness.
Figure 2:
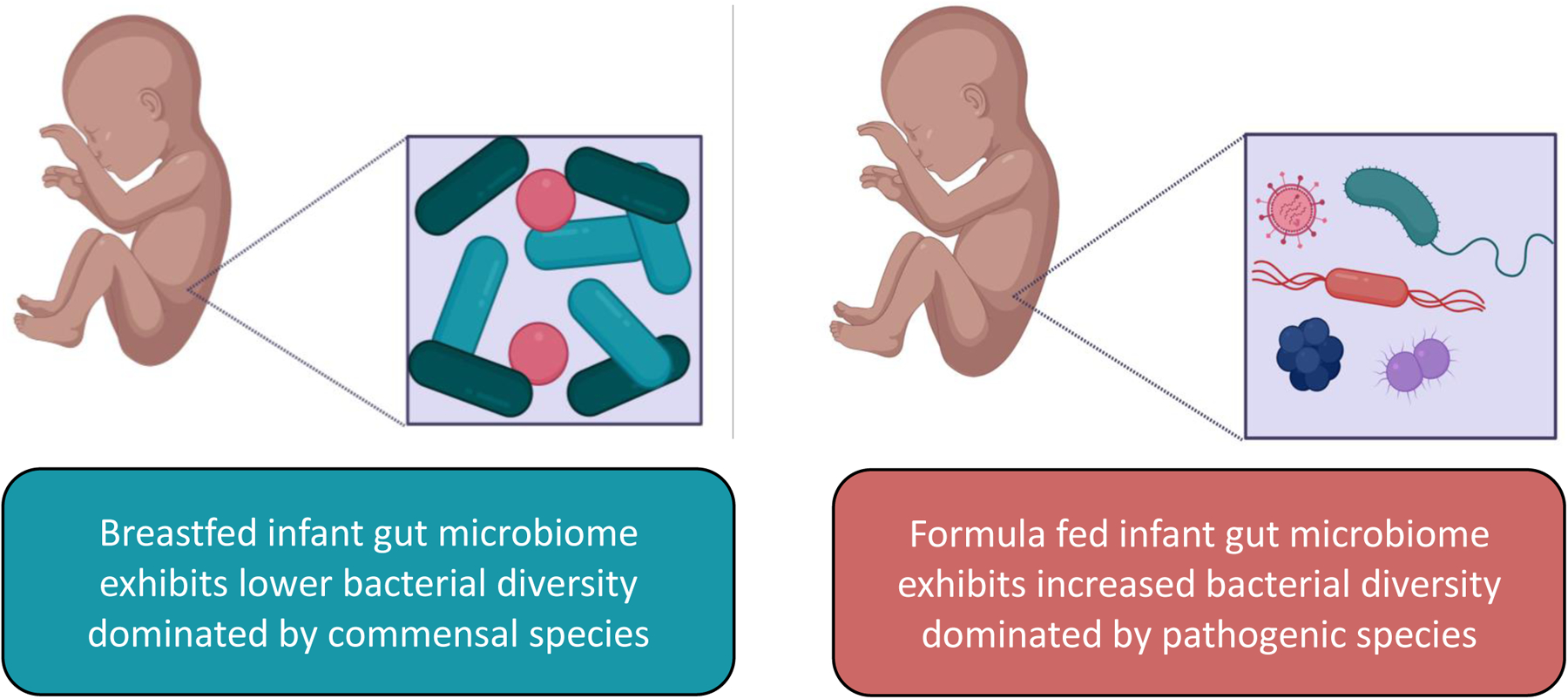
Gut microbiome differences between breastfed and formula fed infants.
Streptococcus agalactiae
Streptococcus agalactiae (Group B Streptococcus, GBS) is a gram-positive pathogen that colonizes the vaginal tract of ca. 15–30% of healthy women [19]. This bacterium can be transmitted vertically and is responsible for neonatal sepsis and meningitis [20]. To prevent transmission during labor and delivery, western countries employ a strategy of prenatal maternal screening for GBS colonization, followed by targeted antibiotic prophylaxis. Though this approach has reduced the rates of early onset GBS disease, there is no preventative or curative treatment for late onset infection which occurs after the first week of life [20]. Additionally, early onset disease has not decreased in low and middle- income countries [20].
As an alternative to antibiotics, our lab has characterized the antimicrobial activity of HMOs against GBS [21] [11]. In related work, Bode and colleagues have also detailed inhibition of GBS by HMOs [19]. Data from Patras and co-workers also supports the antimicrobial activity of HMOs against GBS, but elegantly denotes that treatment with heterogenous HMOs does not alter the composition of the vaginal microbiota in the presence or absence of a GBS infection [22]. Further publications have also outlined the in vivo utility of topical HMO treatment in a murine GBS model reducing GBS vaginal carriage [22].
Clostridiodes difficile
Clostridiodes difficile is a gram-positive, spore-forming bacteria that causes C. difficile infection (CDI). CDI is a common nosocomial infection triggered by dysbiosis of the microbiota after antibiotic use [23]. While symptoms and severity of infection range, invasive infections cause life-threatening colitis and recurrence is common. Patients experience recurrence rates increasing from 25% to over 65% after initial infections, and subsequent first and second recurrences [24]. Successful therapeutic interventions include intestinal microbiota transplantation (IMT), which showcases the importance of the host microbiome. Because of the well-established connection between microbiome modulation and HMOs, investigations to characterize whether HMOs regulate gut dysbiosis and inhibit C. difficile growth have been initiated. A study by Bajic and coworkers showed that HMOs possess antimicrobial activity (greater than a 4 log CFU/mL reduction) against C. difficle [23]. While this study did not provide mechanistic insight, the authors noted the production of short chain fatty acid and secondary bile acids as working hypotheses to explain how HMOs modulate dysbiosis [23].
5. HMOs inhibit biofilm formation
When discussing pathogenic bacteria, it is important to underscore the mechanisms by which pathogenicity is conferred. A key factor in bacterial virulence is the ability of these microbes to exist in a biofilm state [25]. Bacterial biofilms are aggregates of cells encased in a rigid and dynamic extracellular matrix. Biofilms help microbes cooperate and coordinate within a community [26]. Biofilms are known to adhere to both biotic and abiotic surfaces and through a variety of mechanisms confer antimicrobial, host immune, and environmental defense processes [18]. The extracellular matrix housing the cells provides protection from antibiotic pressures, thus giving rise to antibiotic tolerance, a phenomenon often linked to the development of resistance [27]. The imminent threat of antimicrobial resistance has created a need for tools to disrupt bacterial behaviors that favor resistance evolution. HMOs have historically been classified by their anti-adhesive properties, often acting as receptor decoys to block pathogen attachment to mucosal surfaces [20]. In this section, we describe outlined the utility of HMOs as biofilm disruptors.
Staphylococcus aureus
Staphylococcus aureus is a gram-positive bacterium that colonizes the nasopharynx and skin of roughly 30% of the population [28], with 60% of the population transiently colonized [29]. This opportunistic pathogen typically presents asymptomatically, but once pathogenicity is initiated it is responsible for a range of clinical manifestations including skin infections, catheter-associated infections, toxic shock syndrome, pneumonia, and bloodstream infections [30]. Additionally, S. aureus is the most common causative agent in surgery-associated infections, bacteremia, and sepsis [30]. Historically, S. aureus has been of particular interest due to increasing instances of antibiotic resistance. In 1942, the first penicillin-resistant strain was discovered [30]. From there, other strains have evolved resistance to methicillin and vancomycin. The true health threat lies with these resistant strains.
Antimicrobial resistance is often conferred in bacterial populations through horizontal gene transfer - the transfer of genetic material from one bacterial cell to another. Horizontal gene transfer is facilitated by the proximity indicative of bacterial biofilms. It has been shown that S. aureus is a robust biofilm producer and is the most common cause of medical device related infection (DRI) [29]. Our lab has extensively studied both the antimicrobial and antiadhesive properties of HMOs. We have reported the utility of HMOs in disrupting bacterial adherence to surfaces, both biotic and abiotic.
In a seminal study, we reported that HMOs can significantly inhibit S. aureus biofilms [29]. Additionally, synthetically modified HMOs featuring an amine at the anomeric carbon showed increased reductions in biofilm production against S. aureus [31]. In a parallel study out of the Makarewicz laboratory, significant reductions in cell viability within mature biofilms of S. aureus were observed, indicating cell death within established biofilms [32]. Taken together, while bacterial growth was not inhibited in either case, significant reductions in biofilm were observed leading to opportunities of applying HMOs in combination therapies with antibiotics.
Streptococcus agalactiae
As outlined in previous sections, GBS colonizes the maternal urogenital tract, often causing invasive infections in children. It is well understood that GBS is an excellent biofilm producer which contributes to its pathogenicity. We have shown that while heterogeneous mixtures of HMOs (isolated directly from human milk) possess potent antibiofilm properties, single HMOs are void of antibiofilm activity [21]. Serendipitously, we observed impressive antibiofilm activity with amine modified HMOs. In total we modified 4 single-entity HMOs to their amino-sugar counterparts. These amino sugars include 6’-sialyllactose, 3-fucosyllactose, lacto-N-tetraose, and 2’-fucosyllactose (the most common HMO) [30]. We initially hypothesized that a positively charged HMO could act by destabilizing biofilms through surfactant action [31]. This hypothesis is based on observations that the biofilm matrix is stabilized via anionic interactions with charged extracellular DNA and polymeric sugars, which is destabilized by cationic interactions [33]. We do recognize, however, that a positively charged species on an anomeric carbon is likely short lived and other mechanisms of action may be at play.
Escherichia coli
Breastfeeding reduces the instance of enteric bacterial infections compared to infant formula [34]. Enteropathogenic Escherichia coli (EPEC) is a group of Escherichia coli (E. coli) serotypes that were the focus of a 1940s and 1950s study of epidemic and sporadic infant diarrhea [35]. EPEC is a known contaminant of food and water sources, often leading to serious diarrheal illness with high mortality in infants, particularly in developing countries [36]. Classified as an attaching/effacing (A/E) pathogen, EPEC adheres to the intestinal epithelium leading to the formation of distinct colonies. Bode and colleagues investigated the antiadhesive properties of HMOs against EPEC. In this study they found that in vitro supplementation of HMOs into cell culture media significantly reduced cellular attachment of EPEC cells in a dose-dependent manner [34]. Further, they looked at the physiological importance of the prevention of adhesion, where newborn mice were given HMOs and then infected with EPEC. The results indicated a significant reduction in EPEC colonization in the treated mice compared to the untreated control [34]. These findings indicate the antiadhesive properties of HMOs extend beyond the disruption of bacterial biofilms impacting direct cellular attachment.
Acinetobacter baumannii
Acinetobacter baumannii (A. baumannii) is a gram-negative bacterium that is widely known for its multidrug resistance [18]. A. baumannii causes severe infection and is classified as an urgent health threat [37]. Known for its survival on hospital equipment, A. baumannii infections are commonly acquired in intensive care units where patients are already immunocompromised [27]. These infections have a wide variety of manifestations including urinary tract infections, pneumonia, sepsis, and various dermal infections [38]. Of these, the infection seen most often from A. baumannii results in ventilator acquired pneumonia (VAP). These infections are acquired from A. baumannii biofilms on ventilator equipment that get introduced to the patients’ respiratory system during intubation. In intubated patients these infections have a 25% mortality rate, which increases to 50% when the patient is receiving vasopressors [39]. With increasing instances of multi and pan-drug resistance, A. baumannii poses a unique challenge to clinicians.
As such, investigations into alternative treatment options for this bacterium are a high priority. Our lab has assessed the utility of HMOs on this invasive pathogen in a clinical strain survey of A. baumannii isolates from various disease presentations and anatomical sites. Here, we reported that across isolation sites and disease manifestations HMOs disrupt bacterial biofilm formation in A. baumannii [18]. We observed statistically significant reductions in biofilm formation when supplemented with HMOs for almost all isolates tested and corroborated these findings visually using scanning electron microscopy[18] (Figure 3). This study underscores the antiadhesive properties of these diverse sugars.
Figure 3:
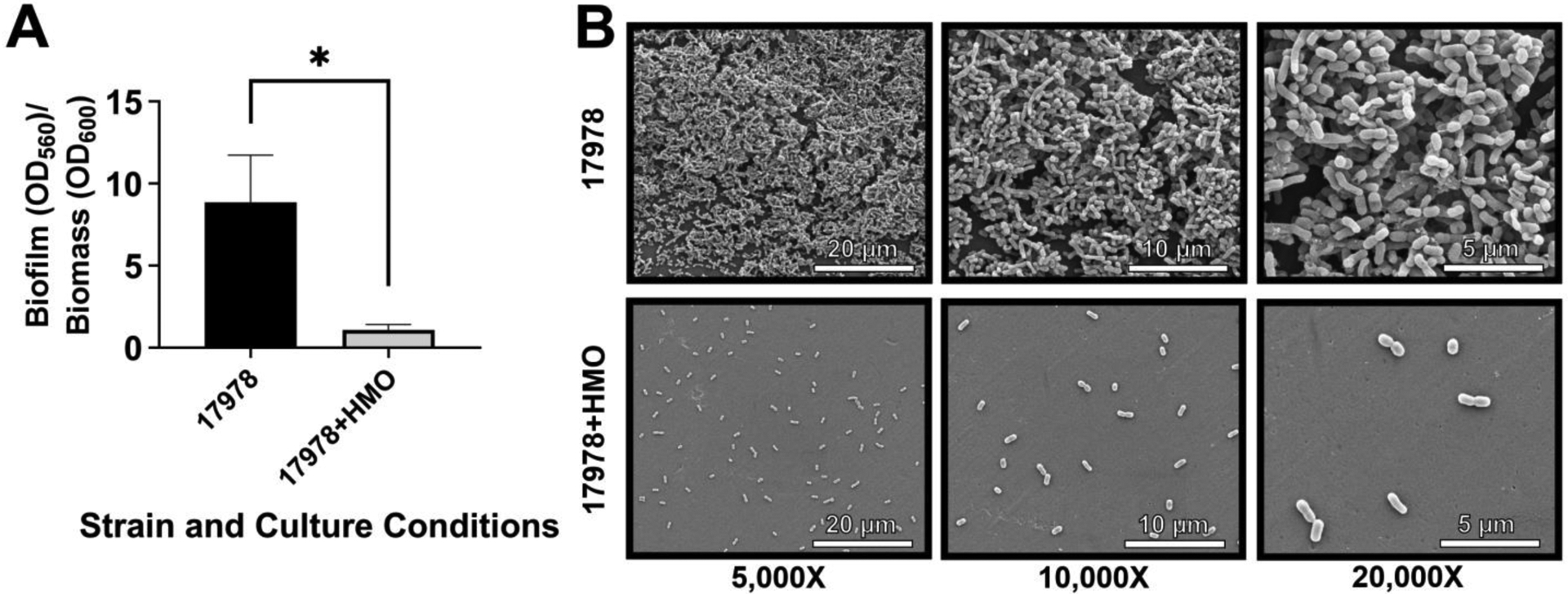
Analysis of the effect of 2.5 mg/mL of HMOs on biofilm formation by A. baumannii 17978. A. Quantitative analysis of biofilm inhibition in the presence of 2.5 mg/mL of HMOs. B. High resolution FEG-SEM at 5 000x, 10 000x, and 20 000x magnification.
7. Frontier Work
HMOs exhibit powerful antimicrobial and antibiofilm activities against both gram-negative and gram-positive bacteria, including three ESKAPE pathogens, Escherichia coli, Staphylococcus aureus and Acinetobacter baumannii. These pathogens are grouped based on their multidrug resistance profiles, an ever-growing problem in the healthcare system. With antimicrobial resistance becoming an urgent threat, many groups have looked to HMOs and other human-derived glycosides as alternative therapeutic strategies. In a first-generation study out of our group, we investigated the ability of HMOs to act as antibiotic adjuvants and potentiate the activity of antibiotics otherwise useless against various multidrug resistant bacteria. We found that these breast milk molecules indeed do decrease the minimum inhibitory concentration of many antibiotics up to 32-fold [40]. We recently expanded our study to report the impressive ability of HMOs to potentiate trimethoprim, an antifolate antibiotic, resulting in a 512-fold decrease in minimum inhibitory concentration compared to the antibiotic alone [41].
Recent publications out of the Bode lab have analyzed the link between microbiological, immunological, and HMO profiles in mothers with lactational mastitis. In this study, they interrogated the relationship between microbial and immunological health and HMOs and concluded the utility of using human milk profiles, specifically HMO profiles as potential biomarkers for disease [42]. The implications of these initial experiments highlight the impressive utility of HMOs in combatting antimicrobial resistance. Further, they give insight into the mechanisms by which these sugars work, providing excellent opportunities for further investigations.
References
- 1.Jiang H, Gallier S, Feng L, Han J, Liu W: Development of the digestive system in early infancy and nutritional management of digestive problems in breastfed and formula-fed infants. Food Funct 2022, 13:1062–1077. [DOI] [PubMed] [Google Scholar]
- 2.Ogbuanu CA, Probst J, Laditka SB, Liu J, Baek J, Glover S: Reasons why women do not initiate breastfeeding: A southeastern state study. Womens Health Issues 2009, 19:268–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stuebe A: The risks of not breastfeeding for mothers and infants. Rev Obstet Gynecol 2009, 2:222–231. [PMC free article] [PubMed] [Google Scholar]
- 4.Chambers SA, Townsend SD: Like mother, like microbe: human milk oligosaccharide mediated microbiome symbiosis. Biochem Soc Trans 2020, 48:1139–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bode L: Human milk oligosaccharides: every baby needs a sugar mama. Glycobiology 2012, 22:1147–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Townsend SD: Human Milk Oligosaccharides: Defense Against Pathogens. Breastfeed Med 2019, 14:S-5–S-6. [DOI] [PubMed] [Google Scholar]
- 7.Thurl S, Munzert M, Henker J, Boehm G, Müller-Werner B, Jelinek J, Stahl B: Variation of human milk oligosaccharides in relation to milk groups and lactational periods. Br J Nutr 2010, 104:1261–1271. [DOI] [PubMed] [Google Scholar]
- 8.Elwakiel M, Hageman JA, Wang W, Szeto IM, van Goudoever JB, Hettinga KA, Schols HA: Human Milk Oligosaccharides in Colostrum and Mature Milk of Chinese Mothers: Lewis Positive Secretor Subgroups. J Agric Food Chem 2018, 66:7036–7043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moore RE, Townsend SD: Temporal development of the infant gut microbiome. Open Biol 2019, 9:190128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ballard O, Morrow AL: Human milk composition: nutrients and bioactive factors. Pediatr Clin North Am 2013, 60:49–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Craft KM, Thomas HC, Townsend SD: Interrogation of Human Milk Oligosaccharide Fucosylation Patterns for Antimicrobial and Antibiofilm Trends in Group B Streptococcus. ACS Infect Dis 2018, 4:1755–1765. [DOI] [PubMed] [Google Scholar]
- 12.BREASTFEEDING SON, Eidelman AI, Schanler RJ, Johnston M, Landers S, Noble L, Szucs K, Viehmann L: Breastfeeding and the Use of Human Milk. Pediatrics 2012, 129:e827–e841. [DOI] [PubMed] [Google Scholar]
- 13.Xu L, Lochhead P, Ko Y, Claggett B, Leong RW, Ananthakrishnan AN: Systematic review with meta-analysis: breastfeeding and the risk of Crohn’s disease and ulcerative colitis. Aliment Pharmacol Ther 2017, 46:780–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gritz EC, Bhandari V: The human neonatal gut microbiome: a brief review. Front Pediatr 2015, 3:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Le Doare K, Holder B, Bassett A, Pannaraj PS: Mother’s Milk: A Purposeful Contribution to the Development of the Infant Microbiota and Immunity. Front Immunol 2018, 9:361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jara S, Sánchez M, Vera R, Cofré J, Castro E: The inhibitory activity of Lactobacillus spp. isolated from breast milk on gastrointestinal pathogenic bacteria of nosocomial origin. Anaerobe 2011, 17: 474–477. [DOI] [PubMed] [Google Scholar]
- 17.Lu J, Francis JD, Guevara MA, Moore RE, Chambers SA, Doster RS, Eastman AJ, Rogers LM, Noble KN, Manning SD, et al. : Antibacterial and Anti-biofilm Activity of the Human Breast Milk Glycoprotein Lactoferrin against Group B Streptococcus. ChemBioChem 2021, 22:2124–2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spicer SK, Moore RE, Lu J, Guevara MA, Marshall DR, Manning SD, Damo SM, Townsend SD, Gaddy JA: Antibiofilm Activity of Human Milk Oligosaccharides against Multidrug Resistant and Susceptible Isolates of Acinetobacter baumannii. ACS Infect Dis 2021, 7:3254–3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin AE, Autran CA, Szyszka A, Escajadillo T, Huang M, Godula K, Prudden AR, Boons G-J, Lewis AL, Doran KS, et al. : Human milk oligosaccharides inhibit growth of group B Streptococcus. J Biol Chem 2017, 292:11243–11249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ackerman DL, Doster RS, Weitkamp J-H, Aronoff DM, Gaddy JA, Townsend SD: Human Milk Oligosaccharides Exhibit Antimicrobial and Antibiofilm Properties against Group B Streptococcus. ACS Infect Dis 2017, 3:595–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ackerman DL, Craft KM, Doster RS, Weitkamp J-H, Aronoff DM, Gaddy JA, Townsend SD: Antimicrobial and Antibiofilm Activity of Human Milk Oligosaccharides against Streptococcus agalactiae, Staphylococcus aureus, and Acinetobacter baumannii. ACS Infect Dis 2018, 4:315–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mejia ME, Ottinger S, Vrbanac A, Babu P, Zulk JJ, Moorshead D, Bode L, Nizet V, Patras KA: Human Milk Oligosaccharides Reduce Murine Group B Streptococcus Vaginal Colonization with Minimal Impact on the Vaginal Microbiota. mSphere 2022, 7:e0088521–e0088521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vigsnaes LK, Ghyselinck J, Van den Abbeele P, McConnell B, Moens F, Marzorati M, Bajic D: 2’FL and LNnT Exert Antipathogenic Effects against C. difficile ATCC 9689 In Vitro, Coinciding with Increased Levels of Bifidobacteriaceae and/or Secondary Bile Acids. Pathog (Basel, Switzerland) 2021, 10:927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kelly CP: Can we identify patients at high risk of recurrent Clostridium difficile infection? Clin Microbiol Infect 2012, 18:21–27. [DOI] [PubMed] [Google Scholar]
- 25.Chambers SA, Gaddy JA, Townsend SD: Synthetic Ellagic Acid Glycosides Inhibit Early Stage Adhesion of Streptococcus agalactiae Biofilms as Observed by Scanning Electron Microscopy. Chemistry 2020, 26:9923–9928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stewart PS, William Costerton J: Antibiotic resistance of bacteria in biofilms. Lancet 2001, 358:135–138. [DOI] [PubMed] [Google Scholar]
- 27.Avery TM, Boone RL, Lu J, Spicer SK, Guevara MA, Moore RE, Chambers SA, Manning SD, Dent L, Marshall D, et al. : Analysis of Antimicrobial and Antibiofilm Activity of Human Milk Lactoferrin Compared to Bovine Lactoferrin against Multidrug Resistant and Susceptible Acinetobacter baumannii Clinical Isolates. ACS Infect Dis 2021, 7:2116–2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wójcik-Bojek U, Różalska B, Sadowska B: Staphylococcus aureus-A Known Opponent against Host Defense Mechanisms and Vaccine Development-Do We Still Have a Chance to Win? Int J Mol Sci 2022, 23:948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Craft KM, Nguyen JM, Berg LJ, Townsend SD: Methicillin-resistant Staphylococcus aureus (MRSA): antibiotic-resistance and the biofilm phenotype. Medchemcomm 2019, 10:1231–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sanchini A: Recent Developments in Phenotypic and Molecular Diagnostic Methods for Antimicrobial Resistance Detection in Staphylococcus aureus: A Narrative Review. Diagnostics (Basel, Switzerland) 2022, 12:208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moore RE, Craft KM, Xu LL, Chambers SA, Nguyen JM, Marion KC, Gaddy JA, Townsend SD: Leveraging Stereoelectronic Effects in Biofilm Eradication: Synthetic β-Amino Human Milk Oligosaccharides Impede Microbial Adhesion As Observed by Scanning Electron Microscopy. J Org Chem 2020, 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jarzynka S, Spott R, Tchatchiashvili T, Ueberschaar N, Martinet MG, Strom K, Kryczka T, Wesołowska A, Pletz MW, Olędzka G, et al. : Human Milk Oligosaccharides Exhibit Biofilm Eradication Activity Against Matured Biofilms Formed by Different Pathogen Species. Front Microbiol 2022, 12:794441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roy R, Tiwari M, Donelli G, Tiwari V: Strategies for combating bacterial biofilms: A focus on anti-biofilm agents and their mechanisms of action. Virulence 2018, 9:522–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Manthey CF, Autran CA, Eckmann L, Bode L: Human milk oligosaccharides protect against enteropathogenic Escherichia coli attachment in vitro and EPEC colonization in suckling mice. J Pediatr Gastroenterol Nutr 2014, 58:165–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Frankel G, Phillips AD, Rosenshine I, Dougan G, Kaper JB, Knutton S: Enteropathogenic and enterohaemorrhagic Escherichia coli : more subversive elements. Mol Microbiol 1998, 30:911–921. [DOI] [PubMed] [Google Scholar]
- 36.Bryce J, Boschi-Pinto C, Shibuya K, Black RE: WHO estimates of the causes of death in children. Lancet 2005, 365:1147–1152. [DOI] [PubMed] [Google Scholar]
- 37.Wong D, Nielsen TB, Bonomo RA, Pantapalangkoor P, Luna B, Spellberg B: Clinical and Pathophysiological Overview of Acinetobacter Infections: a Century of Challenges. Clin Microbiol Rev 2017, 30:409–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Said D, Willrich N, Ayobami O, Noll I, Eckmanns T, Markwart R: The epidemiology of carbapenem resistance in Acinetobacter baumannii complex in Germany (2014–2018): an analysis of data from the national Antimicrobial Resistance Surveillance system. Antimicrob Resist Infect Control 2021, 10:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Colquhoun JM, Rather PN: Insights Into Mechanisms of Biofilm Formation in Acinetobacter baumannii and Implications for Uropathogenesis. Front Cell Infect Microbiol 2020, 10:253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Craft KM, Gaddy JA, Townsend SD: Human Milk Oligosaccharides (HMOs) Sensitize Group B Streptococcus to Clindamycin, Erythromycin, Gentamicin, and Minocycline on a Strain Specific Basis. ACS Chem Biol 2018, 13:2020–2026. [DOI] [PubMed] [Google Scholar]
- 41.Chambers SA, Moore RE, Craft KM, Thomas HC, Das R, Manning SD, Codreanu SG, Sherrod SD, Aronoff DM, McLean JA, et al. : A Solution to Antifolate Resistance in Group B Streptococcus: Untargeted Metabolomics Identifies Human Milk Oligosaccharide-Induced Perturbations That Result in Potentiation of Trimethoprim. MBio 2020, 11:e00076–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Castro I, García-Carral C, Furst A, Khwajazada S, García J, Arroyo R, Ruiz L, Rodríguez JM, Bode L, Fernández L: Interactions between human milk oligosaccharides, microbiota and immune factors in milk of women with and without mastitis. Sci Rep 2022, 12:1367. [DOI] [PMC free article] [PubMed] [Google Scholar]


