Abstract
Background
Regional anesthesia appears to reduce cancer recurrence, but the optimal anesthesia modality for non-muscle invasive bladder cancer (NMIBC) were still under debate. Therefore, we sought to assess the effect of regional and GA only upon the recurrence and long-term prognosis of NMIBC through this meta-analysis.
Methods
We performed an extensive literature search of PubMed, Embase, Web of Science, the Cochrane Library and China National Knowledge Infrastructure (up to October 30, 2022) to identify eligible articles on the possible impact of different anesthetic modalities for the recurrence rate of NMIBC.
Results
Eight studies comprising 3764 participants, including 2117 subjects with RA and 1647 with GA, were finally enrolled. Cancer recurrence rate was significantly lower in subjects with RA than those with GA (RR 0.84, 95%CI 0.72–0.98, P = 0.03). We didn’t detect the differences between GA and RA in the time of recurrence (SMD 2.07, 95% CI -0.49–4.63, P = 0.11) and cancer progression (RR 1.14, 95%CI 0.71–1.84, P = 0.59). Results from subgroup analysis demonstrated that spinal anesthesia could significantly decrease the incidence of cancer recurrence in comparison with general anesthesia (RR 0.80, 95%CI 0.72–0.88, P < 0.001) and high-risk NMIBC patients who received RA tended to have less recurrence (HR 0.55, 95%CI 0.39–0.79, P = 0.001) than those receiving GA.
Conclusions
RA, especially spinal anesthesia, may be effective in reducing the recurrence rate after transurethral resection of NMIBC. More prospective experimental and clinical studies are needed to validate our findings.
Trial registration
INPLASY registration INPLASY2022110097.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12871-023-02136-7.
Keywords: General anesthesia, Regional anesthesia, Bladder cancer, Recurrence, Meta-analysis
Introduction
As the ninth most common cancer in the world [1], bladder cancer (BC) is the most common malignant tumor of the urinary system at present, with an estimated 550 000 new cases and 200 000 deaths worldwide in 2019 [2]. Non-muscle invasive bladder cancer (NMIBC) is the most common type of BC, accounting for about 75% of all cases in patients with BC [3]. For those with NMIBC, transurethral resection of bladder tumor (TURBT) is considered to be a prior treatment. Following TURBT surgery, the doctor will determine whether subsequent continuation of adjuvant treatment is necessary based on the histological origin of the BC and the TNM stage [4]. Recurrence and progression of NMIBC is common, with 50–70% of patients experiencing at least one recurrence within 5 years [3].
Perioperative factors, including surgical stress, blood transfusions, inhaled anesthetics, opioids, and hypothermia, all contribute to cancer recurrence through immunosuppression or cancer promotion [5–9]. The application of regional anesthesia (RA) has demonstrated the potential to suppress surgical stress as well as reduce opioid use, thereby possibly decreasing the occurrence of cancer recurrence [10, 11]. A retrospective study suggested for the first time that the use of paravertebral anesthesia may improve recurrence-free survival for patients with breast cancer [12]. Unlike other malignancies, types of anesthesia adopted during TURBT seemed to not influence the recurrence rate of BC [13], and some recent studies supported this conclusion [14, 15]. However, some researchers have linked the use of spinal anesthesia to a lower rate of recurrence of NMIBC [16–18].
Recently, there have been many studies [14–21] exploring the association between anesthesia methods and recurrence of the NMIBC after TURBT. However, the inconsistent conclusions and limited subjects of these articles reduce their credibility of the evidence. A previous study comparing the impact of RA only with general anesthesia (GA) upon cancer recurrence included only three researches of BC and significant heterogeneity was detected among the selected articles in assessing the final results [22]. The optimal modality of anesthesia for NMIBC remains controversial. Therefore, we sought to compare and assess the effect of regional and GA only upon the recurrence and long-term prognosis of NMIBC through this more precise meta-analysis.
Methods
This systematic review and meta-analysis was reported in accordance with the Cochrane Handbook for Systematic Reviews and Interventions and the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) [23]. We registered our systematic review protocol in the INPLASY (INPLASY2022110097) on November 20,2022.
Literature search strategy
We performed an extensive literature search on the PubMed, Embase, Web of Science, the Cochrane Library and China National Knowledge Infrastructure (up to October 30, 2022) to identify eligible articles on the possible impact of different anesthetic modalities for the recurrence rate of NMIBC. We adopted a search pattern of subject terms combined with their respective free terms. Among the subject terms were “urinary bladder neoplasms”, “regional anesthesia” and “general anesthesia”. The languages of the included studies were English and Chinese. Other possible studies were collected through searching the references of the enrolled literature.
Eligibility and exclusion criteria
Enrolled studies met these criteria: (1) The study subjects were diagnosed with NMIBC and received TURBT with RA or GA; (2) Subjects in the intervention group underwent RA only during surgery; (3) Subjects in the control group underwent GA during surgery; (4) Studies compared cancer recurrence, time to recurrence, progression as well as survival rates between the two groups. (5) The type of literature was a cohort study or a randomized clinical trial. The main exclusion criteria were: (1) other types of cancers; (2) patients with combined multiple anesthetic modalities; (3) no comparison of cancer recurrence rates between the control and intervention groups after surgery; (4) Letters, reviews, comments, author responses and case report studies.
Data collection
The results of the database searches were conducted by two researchers independently and data was collected from every of the eligible articles. When disagreements arose, a third reviewer would join the discussion and reach agreement. Features of the literatures were collected (including first author’s name, publication year, country, ethnicity, study types and number of subjects), basic patient information (including clinicopathological stage, ASA score, mode of anesthesia).
Bias and methodological quality assessment
We assessed eligible cohort studies for risk of bias and quality assessment utilizing the Newcastle–Ottawa Scale (NOS) [24]. The scale evaluates the literature in terms of the appropriateness of study population selection, comparability between groups, and clarity and adequacy of exposures and outcomes. High quality studies generally require a score of more than 7 on the scale.
Statistical analysis
We applied relative risks (RR) and hazard ratios (HR) to compare the different anesthetic modalities in relation to the recurrence rate, progression and overall survival of NMIBC. Standardized mean differences (SMD) were adopted to compare the differences in time to NMIBC recurrence between the two groups. We applied their 95% confidence intervals (95%CI) for final comparison of the pooled results. We also adopted random or fixed effects models to calculate RR, HR, and SMD. Heterogeneity among different studies was detected through the I2 as well as Chi-squared test results. Fixed effect model (FEM) was used when P > 0.05 for Chi-squared test or I2 < 50% [25]; Alternatively, random effect model (REM) [26] was applied. Besides, different types of RA and the risk of BC were adopted for subgroup analyses to explore their possible influence on the pooled outcomes. We also used sensitivity analyses to evaluate the robustness and reliability of the outcomes by removing every enrolled literature in turn. Begg's test as well as Egger's test was adopted to measure publication bias. We conducted our meta-analysis with Stata software (version 12.0) and Review Manager (version 5.3, Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014).
Results
Literature selection and characteristics of the enrolled articles
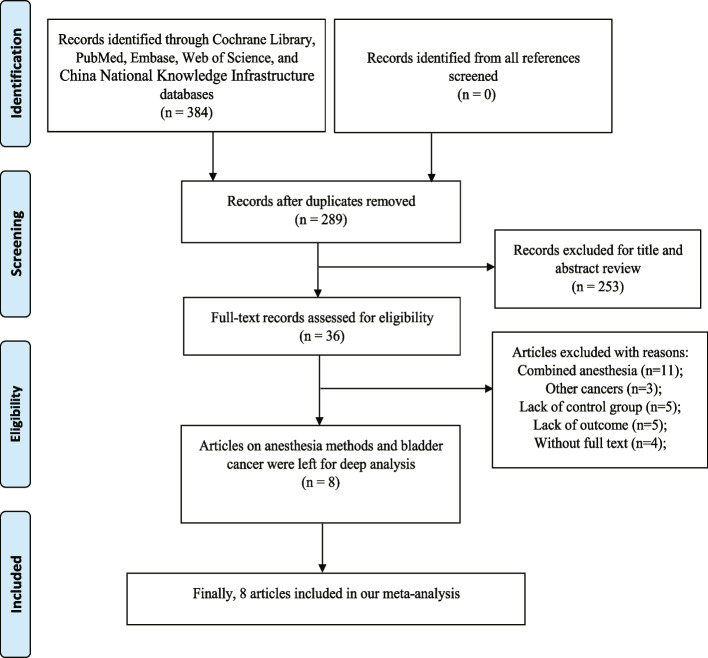
We collected 384 records based on the initial search strategy. After removing duplicate articles, 289 documents were retained. By scanning the title or abstract of potential articles, 253 studies were excluded and 36 were eventually included for reading in full. According to our meta-analysis inclusion and exclusion criteria, eight studies comprising 3764 participants, including 2117 patients with RA and 1647 patients with GA, were finally enrolled. The process of literature search and study enrollment was displayed in Fig. 1. All the literatures were retrospective cohort studies. Seven articles were single-center, while one [18] was multicenter. The specific methods of RA included spinal anesthesia and epidural anesthesia, and the anesthetics used included lidocaine, ropivacaine, bupivacaine and midazolam. Total intravenous anesthesia and inhalational anesthesia were regarded as GA. Anesthetics used included propofol remifentanil, fentanyl, sevoflurane, nitrous oxide, etomidate, vecuronium, rocuronium, cis-atracurium, thiopental and atracurium. The features of the enrolled studies are present in Table 1.
Fig. 1.
Flow chart of the process of literature search and study enrollment
Table 1.
Main clinical characteristics of the included studies
| First Author | Year | Country | Stage | Study design | Total | RA | GA | Follow-up | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Agents | Number | Age | Gender(M/F) | Agents | Number | Age | Gender(M/F) | (months) | ||||||
| Cheng Luo | 2020 | China | NMIBC | Retrospective single center | 302 | Marcaine | 182 | 60a | 141/41 | Propofol, Remifentanil Sevoflurane | 120 | 60a | 98/22 | 33.5 |
| Dale Jang | 2016 | Korea | T1-T2 | Retrospective single center | 161 | Bupivacaine, Lidocaine | 137 | 62.4 ± 10.8 | 107/30 | Propofol, Etomidate Vecuronium, Rocuronium | 24 | 67.5 ± 9.0 | 17/7 | 60 |
| Ruifeng Xue | 2022 | China | NMIBC | Retrospective single center | 528 | Bupivacaine, Lidocaine, Ropivacaine | 264 | 56.62 ± 13.809 | 239/25 | Cis-atracurium, Fentanyl, Propofol, Remifentanil, Sevoflurane | 264 | 58.54 ± 11.968 | 229/35 | 36 |
| Sang Won Lee | 2022 | Korea | NMIBC | Retrospective single center | 1164 | NA | 582 | 66.75 ± 6.09 | 460/122 | NA | 582 | 66.50 ± 6.06 | 460/122 | 52.6 |
| Tingting Wang | 2019 | China | NMIBC | Retrospective single center | 388 | Bupivacaine Midazolam | 136 | 69.27 ± 13.00 | 207/45 | Propofol, Remifentanil, Rocuronium | 252 | 62.09 ± 9.00 | 100/36 | 35.6 |
| Woo-Jong Choi | 2017 | Korea | NMIBC | Retrospective single center | 690 | Bupivacaine, Midazolam | 534 | 62 ± 12 | 436/98 | Sevoflurane, Thiopental, Rocuronium, Atracurium, Nitrous oxide | 156 | 61 ± 13 | 124/32 | 35 |
| Yuri Koumpan | 2018 | Canada | NMIBC | Retrospective single center | 231 | NA | 135 | 71.72 ± 10.57 | 109/26 | NA | 96 | 65.45 ± 10.86 | 77/19 | 65 |
| Yuto Baba | 2021 | Japan | NMIBC | Retrospective multicenter | 300 | Bupivacaine | 147 | 75b | 115/32 | Fentanyl, Propofol, Sevoflurane | 153 | 75b | 129/24 | 46.5 |
NMIBC non-muscle invasive bladder cancer, RA regional anesthesia, GA general anesthesia, M/F male/female, NA not available
aRA: ≥ 60:114, < 60:68; GA: ≥ 60:80, < 60:40; bRA: ≥ 75:37, < 75:110;GA: ≥ 75:64, < 75: 89
Bias and quality assessment
The bias and quality assessment of the eight enrolled articles is demonstrated in Supplemental Table 1. All the eligible studies in our meta-analysis were well-represented and exposed. Three studies [14, 15, 17] were propensity matched, and the comparability was good. However, four studies [16, 18–20] have significant differences in the age of patients receiving RA and GA, which may induce confounding bias and thus score poorly on comparability. The mean follow-up time was around three years in all included studies. Overall, all eight included studies were of high quality.
Meta-analysis results
Cancer recurrence rate
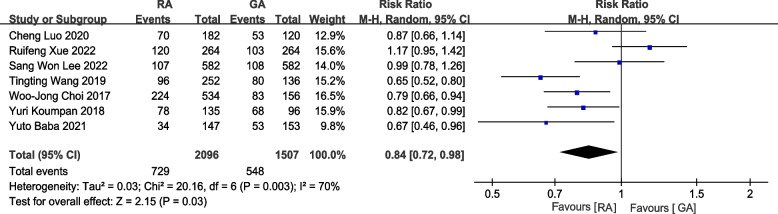
Seven studies with 3603 patients reported postoperative cancer recurrence rates [14–18, 20, 21]. The pooled results indicated that the postoperative cancer recurrence rate was significantly lower among the subjects with RA than those who underwent GA. (RR = 0.84,95%CI = 0.72–0.98, P = 0.03, Fig. 2).
Fig. 2.
Forest plot of cancer recurrence rate (RA vs GA)
Time to recurrence
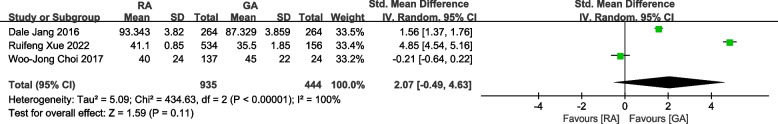
Three studies, including 1379 patients, reported time to recurrence [14, 17, 19]. The duration of cancer recurrence after transurethral resection of BC did not differ significantly among subjects with RA or GA (SMD = 2.07, 95% CI = -0.49–4.63, P = 0.11, Fig. 3).
Fig. 3.
Forest plot of time to recurrence (RA vs GA)
Progression
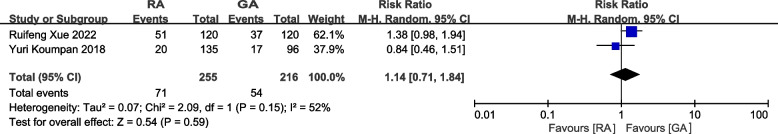
Two studies, comprising 300 subjects, compared cancer progression between two groups [14, 16]. No significant association was found between RA or GA and the progression of NMIBC (RR = 1.14, 95%CI = 0.71–1.84, P = 0.59, Fig. 4).
Fig. 4.
Forest plot of progression (RA vs GA)
Subgroup analysis of type of RA, GA and risk of NMIBC
When subgroup analyses [14–18, 20] were performed based on the specific types of RA [14–18, 20], we found that spinal anesthesia significantly reduced the recurrence of NMIBC (RR = 0.80, 95%CI = 0.72–0.88, P < 0.001, Supplemental Fig. 1). In contrast, there was no significant advantage in cancer recurrence for patients with epidural anesthesia in comparison with those who received GA (RR = 1.17, 95%CI = 0.95–1.42, P = 0.14, Supplemental Fig. 1). When the different types of general anesthesia are further considered, RA has obvious advantages in reducing the recurrence rate of NMIBC, compared with both inhalational anesthesia (RR = 0.78, 95%CI = 0.68–0.90, P = 0.0007, Supplemental Fig. 2) and total intravenous anesthesia (RR = 0.65, 95%CI = 0.52–0.80, P < 0.0001, Supplemental Fig. 2). We also conducted the subgroup analysis based on risk stratification of NMIBC [15, 16, 18], Similarly, the adoption of RA may lead to less recurrence in high-risk NMIBC (HR = 0.55, 95%CI = 0.39–0.79, P = 0.001, Supplemental Fig. 3). However, different anesthetic modalities had no obvious impact on the recurrence of low-risk NMIBC (HR = 0.95, 95%CI = 0.58–1.53, P = 0.82, Supplemental Fig. 3).
Sensitivity analyses and publication bias detection
We applied the sensitivity analysis to evaluate the stability of all aggregated outcomes by sequentially excluding every single article. As it could conclude from Supplemental Fig. 4, the overall results of our literature were stable, with no individual study obviously altering the final results. Egger's test and Begg's test were applied to monitor for possible publication bias among the enrolled studies. The results detected no publication bias, which further supported the credibility of the meta-analysis. (P > 0.05, Supplemental Fig. 5).
Discussion
Patients with NMIBC who received RA tended to be less likely to present with recurrence than those with GA. Notably, there was no obvious effect of the two anesthesia methods on the time to recurrence, survival and progression rates of subjects with NMIBC. A further subgroup analysis found that spinal anesthesia was more effective than epidural anesthesia in reducing cancer recurrence rate in comparison with GA. Interestingly, we also discovered that RA significantly decreased the recurrence of NMIBC, whether compared with total intravenous anesthesia or inhalational anesthesia. Similarly, cancer recurrence in high-risk NMIBC was significantly reduced with RA. In contrast, recurrence in low-risk patients was not found to be related to the types of anesthesia.
GA is performed with intravenous anesthetics, inhaled anesthetics, or a combination of the two. Volatile anesthetics have been proven to suppress the immune system and promote inflammatory responses, and therefore affects the survival of tumor cells, including by regulating gene expression in immune cells [27, 28]. For example, sevoflurane inhibits the function of T lymphocytes as well as increases the production of hypoxia-inducible factor (HIF) and insulin-like growth factor, thereby promoting cancer growth [29–31]. On the contrary, natural killer cells are activated and cancer growth is reduced owing to the regional anesthetics, such as lidocaine [29]. Surgical stress also inhibited NK cell activity [32] and increases the production of some of the ILs associated with cancer progression, among which the elevated level of IL-6 promotes the angiogenesis and progression of BC [33] and IL-8 is associated with tumor recurrence [34]. Regional anesthetics have been shown to reduce surgical pressure, and a diminished stress response could also decrease the occurrence of immunosuppression [10, 35]. Moreover, high-risk NMIBC has a longer operation time and tremendous surgical pressure. Thus, this may explain the advantage of RA in decreasing the recurrence of high-risk NMIBC. RA also reduces the dose of opioids used. Opioids can prevent natural killer cells from functioning properly and can also inhibit immune pathways of immunoglobulin secretion and cytokine release, therefore promoting the recurrence of tumors [36].
Among patients with muscle-invasive bladder cancer, spinal anesthesia combined with GA achieved opioid-sparing results without significant improvement in final oncological outcomes [37]. Some population-based analyses also found that using epidural anesthesia for radical cystectomy did not improve cancer-specific or overall survival [38, 39]. Interestingly, when sufentanil was adopted in epidural anesthesia, a higher recurrence rate and shorter disease-free survival was found among BC patients undergoing radical surgery. This may be the result of the sufentanil itself or the adoption of this drug which in turn increases the total amount of morphine equivalents absorbed by the patients, given the immunosuppressive effects present with opioids [40]. However, these studies all used a combination of RA and GA. This may be why RA does not seem to improve outcomes in cancer patients: RA applied in addition to GA is insufficient to counteract the adverse effects of GA. In addition, it should also be considered that the malignant degree of MIBC is significantly higher. Compared with pathological stage, lymph node invasion and neoadjuvant chemotherapy, the influence of an anesthesia regimen on patients with MIBC may be insignificant.
When the types of regional anesthesia used was further considered, spinal anesthesia during surgery in patients with NMIBC is effective in reducing the recurrence rate. Spinal anesthesia suppresses cancer growth by inhibiting sodium channels, which forces cancer cells to re-express voltage-gated sodium channels and reduces metastatic cells activity [41]. In contrast, one cohort study [14] showed that the adoption of epidural anesthesia failed to obviously reduce cancer recurrence. The studies [38, 39] mentioned above focusing on MIBC also used epidural anesthesia and did not significantly improve the prognosis of patients. It has even been claimed that the combination of epidural anesthesia reduces the disease-free recurrence rate and cancer-specific mortality of patients. In addition, different types of GA also seem to influence postoperative patient outcomes. Compared with intravenous anesthesia, inhalational anesthesia for radical resection promotes the potential of distant recurrence as well as favors a higher tumor pathological stage [42]. Similarly, some studies also confirmed longer disease-free survival in subjects that received propofol than those with sevoflurane and opioids [43, 44]. This could be explained by the reason that propofol does not inhibit natural killer cell function but inhibits cancer cells from spreading, invading, or surviving [44, 45].
According to a prospective clinical study, epidural anesthesia during surgery does not improve their disease-free survival [46]. Similarly, a meta-analysis that included six prospective clinical trials came to the conclusion that the combination of RA and GA did not decrease cancer recurrence after tumor resection [47]. The anesthesia approach for malignant tumor surgery is still controversial, and more randomized clinical trials should be conducted to find and explore the best anesthetic methods for different types of cancers. Unlike the results of the previous meta-analysis [48, 49], this article failed to detect a relationship between RA and cancer progression rates. This could be due to the low progression rate of early BC [4], and the influence of anesthesia methods on the prognosis of BC is more reflected in the recurrence rate of cancer.
Our study is the first meta-analysis of anesthesia methods for NMIBC. The included studies used only RA or GA, excluding the impact of combined RA and GA application on outcomes. However, although we conducted as thorough a literature search as possible, all the enrolled researches were retrospective studies, which reduced the credibility of the conclusions to some extent. In addition, some studies were not propensity-matched, which may also cause confounding bias. Although significant heterogeneity was detected in the included studies, heterogeneity was reduced after subgroup analysis. However, reduced heterogeneity of high-risk patients did not seem to exclude the effect of chance due to the limited inclusion of literature. Therefore, large prospective clinical studies and meta-analyses are required to further explore these questions.
Conclusion
RA, especially spinal anesthesia, may be effective in reducing the recurrence rate after transurethral resection of NMIBC. More prospective experimental and clinical studies are needed to validate our findings.
Supplementary Information
Additional file 1: Supplemental Table 1. The risk assessmentof the included studies.
Additional file 2: Supplemental Fig 1. Subgroup analysis based on types of RA (epiduralanesthesia/spinal anesthesia : GA).
Additional file 3: Supplemental Fig 2. Subgroup analysis based on types of GA (RA :inhalational anesthesia/total intravenous anesthesia).
Additional file 4: Supplemental Fig 3. Subgroupanalysis based on the risk of NMIBC.
Additional file 5: Supplemental Fig 4. Sensitivity analysis of anesthesia type.
Additional file 6: Supplemental Fig 5. Begg’sfunnel plot for publication bias test under cancer recurrence rate (RA vs. GA).
Acknowledgements
Not applicable.
Abbreviations
- BC
Bladder cancer
- CI
Confidence interval
- GA
General anesthesia
- HR
Hazard ratio
- NMIBC
Non-muscle invasive bladder cancer
- TURBT
Transurethral resection of bladder tumor
- RA
Regional anesthesia
- RR
Relative risk
- SMD
Standardized mean difference
Authors’ contributions
Yulong Wang and Yuxuan Song conceived and designed the study; Yulong Wang, Yuxuan Song, and Caipeng Qin searched the literature, extracted data from the collected literature, and analyzed the data; Yulong Wang and Yuxuan Song wrote the manuscript; Chunlong Zhang, Yiqing Du and Tao Xu revised the manuscript; All authors read and approved the final manuscript.
Funding
This research was supported by National Key Research and Development Program of China (2018YFA0902802), National Natural Science Foundation of China (82271877), and National Natural Science Foundation of China (82071777).
Availability of data and materials
The datasets generated and analyzed during the current study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yulong Wang and Yuxuan Song contributed equally to this work.
References
- 1.Dobruch J, Daneshmand S, Fisch M, Lotan Y, Noon AP, Resnick MJ, Shariat SF, Zlotta AR, Boorjian SA. Gender and bladder cancer: a collaborative review of etiology, biology, and outcomes. Eur Urol. 2016;69(2):300–310. doi: 10.1016/j.eururo.2015.08.037. [DOI] [PubMed] [Google Scholar]
- 2.Richters A, Aben KKH, Kiemeney L. The global burden of urinary bladder cancer: an update. World J Urol. 2020;38(8):1895–1904. doi: 10.1007/s00345-019-02984-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kamat AM, Hahn NM, Efstathiou JA, Lerner SP, Malmström PU, Choi W, Guo CC, Lotan Y, Kassouf W. Bladder cancer. Lancet. 2016;388(10061):2796–2810. doi: 10.1016/S0140-6736(16)30512-8. [DOI] [PubMed] [Google Scholar]
- 4.Babjuk M, Burger M, Capoun O, Cohen D, Compérat EM, Dominguez Escrig JL, Gontero P, Liedberg F, Masson-Lecomte A, Mostafid AH, et al. European association of urology guidelines on non-muscle-invasive bladder cancer (Ta, T1, and Carcinoma in Situ) Eur Urol. 2022;81(1):75–94. doi: 10.1016/j.eururo.2021.08.010. [DOI] [PubMed] [Google Scholar]
- 5.Sacerdote P, Bianchi M, Gaspani L, Manfredi B, Maucione A, Terno G, Ammatuna M, Panerai AE. The effects of tramadol and morphine on immune responses and pain after surgery in cancer patients. Anesth Analg. 2000;90(6):1411–1414. doi: 10.1097/00000539-200006000-00028. [DOI] [PubMed] [Google Scholar]
- 6.Weber RS, Jabbour N, Martin RC., 2nd Anemia and transfusions in patients undergoing surgery for cancer. Ann Surg Oncol. 2008;15(1):34–45. doi: 10.1245/s10434-007-9502-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beilin B, Shavit Y, Razumovsky J, Wolloch Y, Zeidel A, Bessler H. Effects of mild perioperative hypothermia on cellular immune responses. Anesthesiology. 1998;89(5):1133–1140. doi: 10.1097/00000542-199811000-00013. [DOI] [PubMed] [Google Scholar]
- 8.Snyder GL, Greenberg S. Effect of anaesthetic technique and other perioperative factors on cancer recurrence. Br J Anaesth. 2010;105(2):106–115. doi: 10.1093/bja/aeq164. [DOI] [PubMed] [Google Scholar]
- 9.Kim R. Anesthetic technique and cancer recurrence in oncologic surgery: unraveling the puzzle. Cancer Metastasis Rev. 2017;36(1):159–177. doi: 10.1007/s10555-016-9647-8. [DOI] [PubMed] [Google Scholar]
- 10.O'Riain SC, Buggy DJ, Kerin MJ, Watson RWG, Moriarty DC. Inhibition of the stress response to breast cancer surgery by regional anesthesia and analgesia does not affect vascular endothelial growth factor and prostaglandin E2. Anesth Analg. 2005;100(1):244–249. doi: 10.1213/01.ANE.0000143336.37946.7D. [DOI] [PubMed] [Google Scholar]
- 11.Cakmakkaya OS, Kolodzie K, Apfel CC, Pace NL: Anaesthetic techniques for risk of malignant tumour recurrence. Cochrane Database Syst Rev 2014(11):Cd008877. [DOI] [PMC free article] [PubMed]
- 12.Exadaktylos AK, Buggy DJ, Moriarty DC, Mascha E, Sessler DI. Can anesthetic technique for primary breast cancer surgery affect recurrence or metastasis? Anesthesiology. 2006;105(4):660–664. doi: 10.1097/00000542-200610000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.RiChard J, Lipsky M, Whalen M, Motamedinia P, Finkelstein J, Hruby G, DeCastro G, Curry S, Benson MC, McKiernan JM: The impact of regional anesthesia on bladder cancer outcomes. J Clin Oncol 2012, 30(15).
- 14.Xue R, Zhao C, Chen D, Wang P, Xing W, Zeng W, Li Q. Potential influence of anaesthesia techniques on the recurrence and progression after resection of non-muscle-invasive bladder cancer: a propensity score-matched analysis. BMC Anesthesiol. 2022;22(1):263. doi: 10.1186/s12871-022-01802-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee SW, Tae BS, Choi YJ, Yoon SM, Lee YS, Kim JH, Shin HW, Park JY, Bae JH. A Comparison of the Anesthetic Methods for Recurrence Rates of Bladder Cancer after Transurethral Resection of Bladder Tumors Using National Health Insurance Claims Data of South Korea. J Clin Med . 2022;11(4):1143. doi: 10.3390/jcm11041143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koumpan Y, Jaeger M, Mizubuti GB, Tanzola R, Jain K, Hosier G, Hopman W, Siemens DR. Spinal anesthesia is associated with lower recurrence rates after resection of nonmuscle invasive bladder cancer. J Urol. 2018;199(4):940–946. doi: 10.1016/j.juro.2017.11.064. [DOI] [PubMed] [Google Scholar]
- 17.Choi WJ, Baek S, Joo EY, Yoon SH, Kim E, Hong B, Hwang JH, Kim YK. Comparison of the effect of spinal anesthesia and general anesthesia on 5-year tumor recurrence rates after transurethral resection of bladder tumors. Oncotarget. 2017;8(50):87667–87674. doi: 10.18632/oncotarget.21034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baba Y, Kikuchi E, Shigeta K, Ogihara K, Matsushima M, Nishimoto Y, Murata Y, Asakura H, Oyama M, Mizuno R, et al. Effects of transurethral resection under general anesthesia on tumor recurrence in non-muscle invasive bladder cancer. Int J Clin Oncol. 2021;26(11):2094–2103. doi: 10.1007/s10147-021-02000-z. [DOI] [PubMed] [Google Scholar]
- 19.Jang D, Lim CS, Shin YS, Ko YK, Park SI, Song SH, Kim BJ. A comparison of regional and general anesthesia effects on 5 year survival and cancer recurrence after transurethral resection of the bladder tumor: a retrospective analysis. BMC Anesthesiol. 2016;16:16. doi: 10.1186/s12871-016-0181-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang T, Yi L, Chen Y. Comparison of the effects of two anesthesia methods on recurrence of non-muscular invasive bladder cancer Journal of Youjiang Medical University for Nationalities. 2019;41(04):429–432. [Google Scholar]
- 21.Luo C, Baihetiya A, Wang W. Qianjin Li. Yujie Wang: Effects of different anesthesia approaches on postoperative prognosis of patients with non-muscle invasive bladder cancer undergoing transurethral resection of bladder tumor Guangxi Medical Journal. 2020;42(20):2620–2624. [Google Scholar]
- 22.Ang E, Ng KT, Lee ZX, Ti LK, Chaw SH, Wang CY. Effect of regional anaesthesia only versus general anaesthesia on cancer recurrence rate: a systematic review and meta-analysis with trial sequential analysis. J Clin Anesth. 2020;67:110023. doi: 10.1016/j.jclinane.2020.110023. [DOI] [PubMed] [Google Scholar]
- 23.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62(10):e1–34. doi: 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 24.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 25.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22(4):719–748. [PubMed] [Google Scholar]
- 26.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 27.Yuki K, Eckenhoff RG. Mechanisms of the immunological effects of volatile anesthetics: a review. Anesth Analg. 2016;123(2):326–335. doi: 10.1213/ANE.0000000000001403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Inada T, Yamanouchi Y, Jomura S, Sakamoto S, Takahashi M, Kambara T, Shingu K. Effect of propofol and isoflurane anaesthesia on the immune response to surgery. Anaesthesia. 2004;59(10):954–959. doi: 10.1111/j.1365-2044.2004.03837.x. [DOI] [PubMed] [Google Scholar]
- 29.Kim R. Effects of surgery and anesthetic choice on immunosuppression and cancer recurrence. J Transl Med. 2018;16(1):8. doi: 10.1186/s12967-018-1389-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shi QY, Zhang SJ, Liu L, Chen QS, Yu LN, Zhang FJ, Yan M. Sevoflurane promotes the expansion of glioma stem cells through activation of hypoxia-inducible factors in vitro. Br J Anaesth. 2015;114(5):825–830. doi: 10.1093/bja/aeu402. [DOI] [PubMed] [Google Scholar]
- 31.Benzonana LL, Perry NJ, Watts HR, Yang B, Perry IA, Coombes C, Takata M, Ma D. Isoflurane, a commonly used volatile anesthetic, enhances renal cancer growth and malignant potential via the hypoxia-inducible factor cellular signaling pathway in vitro. Anesthesiology. 2013;119(3):593–605. doi: 10.1097/ALN.0b013e31829e47fd. [DOI] [PubMed] [Google Scholar]
- 32.Bar-Yosef S, Melamed R, Page GG, Shakhar G, Shakhar K, Ben-Eliyahu S. Attenuation of the tumor-promoting effect of surgery by spinal blockade in rats. Anesthesiology. 2001;94(6):1066–1073. doi: 10.1097/00000542-200106000-00022. [DOI] [PubMed] [Google Scholar]
- 33.Chen MF, Lin PY, Wu CF, Chen WC, Wu CT. Correction: IL-6 expression regulates tumorigenicity and correlates with prognosis in bladder cancer. PLoS ONE. 2016;11(5):e0155774. doi: 10.1371/journal.pone.0155774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reis ST, Leite KR, Piovesan LF, Pontes-Junior J, Viana NI, Abe DK, Crippa A, Moura CM, Adonias SP, Srougi M, et al. Increased expression of MMP-9 and IL-8 are correlated with poor prognosis of Bladder Cancer. BMC Urol. 2012;12:18. doi: 10.1186/1471-2490-12-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tønnesen E, Wahlgreen C. Influence of extradural and general anaesthesia on natural killer cell activity and lymphocyte subpopulations in patients undergoing hysterectomy. Br J Anaesth. 1988;60(5):500–507. doi: 10.1093/bja/60.5.500. [DOI] [PubMed] [Google Scholar]
- 36.Gupta K, Kshirsagar S, Chang L, Schwartz R, Law PY, Yee D, Hebbel RP. Morphine stimulates angiogenesis by activating proangiogenic and survival-promoting signaling and promotes breast tumor growth. Cancer Res. 2002;62(15):4491–4498. [PubMed] [Google Scholar]
- 37.Weingarten TN, Taccolini AM, Ahle ST, Dietz KR, Dowd SS, Frank I, Boorjian SA, Thapa P, Hanson AC, Schroeder DR, et al. Perioperative management and oncological outcomes following radical cystectomy for bladder cancer: a matched retrospective cohort study. Can J Anesth. 2016;63(5):584–595. doi: 10.1007/s12630-016-0599-9. [DOI] [PubMed] [Google Scholar]
- 38.Miller BL, Abel EJ, Allen G, Schumacher JR, Jarrard D, Downs T, Richards KA. Trends in epidural anesthesia use at the time of radical cystectomy and its association with perioperative and survival outcomes: a population-based analysis. Am J Clin Exp Urol. 2020;8(1):28–37. [PMC free article] [PubMed] [Google Scholar]
- 39.Christopher Doiron R, Jaeger M, Booth CM, Wei X, Robert Siemens D. Is there a measurable association of epidural use at cystectomy and postoperative outcomes? A population-based study. Can Urol Assoc J. 2016;10(9–10):321–327. doi: 10.5489/cuaj.3856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chipollini J, Alford B, Boulware DC, Forget P, Gilbert SM, Lockhart JL, Pow-Sang JM, Sexton WJ, Spiess PE, Poch MA, et al. Epidural anesthesia and cancer outcomes in bladder cancer patients: is it the technique or the medication? A matched-cohort analysis from a tertiary referral center. BMC Anesthesiol. 2018;18(1):157. doi: 10.1186/s12871-018-0622-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fraser SP, Ozerlat-Gunduz I, Brackenbury WJ, Fitzgerald EM, Campbell TM, Coombes RC, Djamgoz MB. Regulation of voltage-gated sodium channel expression in cancer: hormones, growth factors and auto-regulation. Philos Trans R Soc Lond B Biol Sci. 2014;369(1638):20130105. doi: 10.1098/rstb.2013.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pfail JL, Katims AB, Gul Z, Rosenzweig SJ, Razdan S, Nathaniel S, Martini A, Mehrazin R, Wiklund PN, Loftus K, et al. Can anesthetics affect bladder cancer recurrence? Total intravenous versus volatile anesthesia in patients undergoing robot-assisted radical cystectomy: a single institution retrospective analysis. Urol Oncol. 2021;39(4):233.e231–233.e238. doi: 10.1016/j.urolonc.2020.08.024. [DOI] [PubMed] [Google Scholar]
- 43.Guerrero Orriach JL, Raigon Ponferrada A, Malo Manso A, Herrera Imbroda B, Escalona Belmonte JJ, Ramirez Aliaga M, Ramirez Fernandez A, Diaz Crespo J, Soriano Perez AM, Fontaneda Heredia A, et al. Anesthesia in combination with propofol increases disease-free survival in bladder cancer patients who undergo radical tumor cystectomy as compared to inhalational anesthetics and opiate-based analgesia. Oncology. 2020;98(3):161–167. doi: 10.1159/000504807. [DOI] [PubMed] [Google Scholar]
- 44.Lee JH, Kang SH, Kim Y, Kim HA, Kim BS. Effects of propofol-based total intravenous anesthesia on recurrence and overall survival in patients after modified radical mastectomy: a retrospective study. Korean J Anesthesiol. 2016;69(2):126–132. doi: 10.4097/kjae.2016.69.2.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Melamed R, Bar-Yosef S, Shakhar G, Shakhar K, Ben-Eliyahu S. Suppression of natural killer cell activity and promotion of tumor metastasis by ketamine, thiopental, and halothane, but not by propofol: mediating mechanisms and prophylactic measures. Anesth Analg. 2003;97(5):1331–1339. doi: 10.1213/01.ANE.0000082995.44040.07. [DOI] [PubMed] [Google Scholar]
- 46.Myles PS, Peyton P, Silbert B, Hunt J, Rigg JR, Sessler DI. Perioperative epidural analgesia for major abdominal surgery for cancer and recurrence-free survival: randomised trial. BMJ. 2011;342:d1491. doi: 10.1136/bmj.d1491. [DOI] [PubMed] [Google Scholar]
- 47.Lee ZX, Ng KT, Ang E, Wang CY, Binti S., II Effect of perioperative regional anesthesia on cancer recurrence: a meta-analysis of randomized controlled trials. Int J Surg. 2020;82:192–199. doi: 10.1016/j.ijsu.2020.08.034. [DOI] [PubMed] [Google Scholar]
- 48.Weng M, Chen W, Hou W, Li L, Ding M, Miao C. The effect of neuraxial anesthesia on cancer recurrence and survival after cancer surgery: an updated meta-analysis. Oncotarget. 2016;7(12):15262–15273. doi: 10.18632/oncotarget.7683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee BM, Singh Ghotra V, Karam JA, Hernandez M, Pratt G, Cata JP. Regional anesthesia/analgesia and the risk of cancer recurrence and mortality after prostatectomy: a meta-analysis. Pain Manag. 2015;5(5):387–395. doi: 10.2217/pmt.15.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Supplemental Table 1. The risk assessmentof the included studies.
Additional file 2: Supplemental Fig 1. Subgroup analysis based on types of RA (epiduralanesthesia/spinal anesthesia : GA).
Additional file 3: Supplemental Fig 2. Subgroup analysis based on types of GA (RA :inhalational anesthesia/total intravenous anesthesia).
Additional file 4: Supplemental Fig 3. Subgroupanalysis based on the risk of NMIBC.
Additional file 5: Supplemental Fig 4. Sensitivity analysis of anesthesia type.
Additional file 6: Supplemental Fig 5. Begg’sfunnel plot for publication bias test under cancer recurrence rate (RA vs. GA).
Data Availability Statement
The datasets generated and analyzed during the current study are available from the corresponding author upon reasonable request.