Abstract
Background
The appropriate administration regimen of polymyxin B is yet controversial. The present study aimed to explore the optimal dose of polymyxin B under therapeutic drug monitoring (TDM) guidance.
Methods
In China’s Henan province, 26 hospitals participated in a randomized controlled trial. We included patients with sepsis caused by carbapenem-resistant Gram-negative bacteria (CR-GNB) susceptible to polymyxin B. The patients were randomly divided into a high-dose (HD) group or a low-dose (LD) group and received 150 mg loading dose, 75 mg every 12 h and 100 mg loading dose, 50 mg every 12 h, respectively. TDM was employed to determine if the dose of polymyxin B needs adjustment based on the area under the concentration–time curve across 24 h at a steady state (ssAUC0–24) of 50–100 mg h/L. The primary outcome was the 14-day clinical response, and the secondary outcomes included 28- and 14-day mortality.
Results
This trial included 311 patients, with 152 assigned to the HD group and 159 assigned to the LD group. Intention-to-treat analysis showed that the 14-day clinical response was non-significant (p = 0.527): 95/152 (62.5%) in the HD group and 95/159 (59.7%) in the LD group. Kaplan–Meier’s 180-day survival curve showed survival advantage in the HD group than in the LD group (p = 0.037). More patients achieved the target ssAUC0–24 in the HD than in the LD group (63.8% vs. 38.9%; p = 0.005) and in the septic shock subgroup compared to all subjects (HD group: 71.4% vs. 63.8%, p = 0.037; LD group: 58.3% vs. 38.9%, p = 0.0005). Also, the target AUC compliance was not correlated with clinical outcomes but with acute kidney injury (AKI) (p = 0.019). Adverse events did not differ between the HD and LD groups.
Conclusion
A fixed polymyxin B loading dose of 150 mg and a maintenance dose of 75 mg every 12 h was safe for patients with sepsis caused by CR-GNB and improves long-term survival. The increased AUC was associated with increased incidence of AKI, and TDM results were valued to prevent AKI.
Trial registration Trial registration ClinicalTrials.gov: ChiCTR2100043208, Registration date: January 26, 2021.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13054-023-04522-6.
Keyword: Polymyxin B, Therapeutic drug monitoring, Carbapenem-resistant gram-negative bacteria, Severe infection, Optimization dose
Background
The increasing prevalence of multi-drug-resistant bacteria causes high attributable mortality, which is partially due to a lack of effective therapeutic drugs [1, 2]. Carbapenem-resistant Gram-negative bacteria (CR-GNB), also known as carbapenem-resistant organisms (CRO), including carbapenem-resistant Enterobacteriaceae (CRE), carbapenem-resistant Acinetobacter baumannii (CRAB), and carbapenem-resistant Pseudomonas aeruginosa (CRPA), are the main contributors of infectious diseases caused by multi-drug-resistant bacteria [3]. Although several new β-lactam antibiotics and β-lactamase inhibitors against CR-GNB with fewer side effects have been identified, many of them are not sold in China. In this event, polymyxin B, an old drug approved in the late 1950s and abandoned in the 1970s due to its toxicity, has been reintroduced to treat CR-GNB-caused infections [4, 5]. Polymyxins have a narrow therapeutic window and cause significant nephrotoxicity, which hinders their clinical application. Clinical guidelines recommend that the dosage of polymyxin B should be based on total body weight (TBW). A loading dose of 2.0–2.5 mg/kg (equivalent to 20,000–25,000 IU/kg) and a maintenance dose of 1.25–1.5 mg/kg (equivalent to 12,500–15,000 IU/kg) every 12 h may be appropriate, which is expected to achieve an area under the concentration–time curve across 24 h at a steady state (ssAUC0–24) target of 50–100 mg h/L (corresponding to average steady-state plasma concentration [Css, avg] of 2–4 mg/L) [6]. Nevertheless, the most appropriate administration regimen for polymyxin B is controversial [7], and the therapeutic drug monitoring (TDM) of polymyxin B has not been widely used in clinics. Currently, there is little evidence that reaching a target therapeutic window of 50–100 mg h/L improves efficacy. In addition, weight-based strategies are being challenged by studies on the population pharmacokinetics (PK) of polymyxin B [8, 9].
Polymyxin B is the most commonly used polymyxin in China. However, the clinical regimen is yet to be standardized. Some studies have shown that the dosage of polymyxin B is lower than recommended in the guidelines and that doctors prefer to use a fixed daily dose of 50 mg or 75 mg every 12 h, which might not be according to TBW but it can be implemented easily and avoids wastage. Additionally, some patients did not receive the polymyxin B loading dose [10, 11]. To the best of our knowledge, prolonged exposure to low-concentration antibiotics may lead to bacterial resistance, and without employing the appropriate loading dose of polymyxin B, optimal plasma exposure cannot be achieved quickly [12], leading to treatment failure.
In this study, we conducted a randomized trial to explore the appropriate clinical administration regimen of polymyxin B for the treatment of severe infections caused by CR-GNB. Also, TDM was used to explain the polymyxin B dose–response correlation and determine the optimal dosage.
Methods
Study design and patients
This is an open, multicenter, randomized, controlled study conducted according to the principles of Helsinki Declaration and was approved by the ethics committees of the participating hospitals. Written informed consent was obtained from the patients before they were randomly assigned to various groups. When patients and had no capabilities due to consciousness disorders, sedative states, intense weakness, their families signed the informed consent, and the patient was required to sign a new informed consent if he/she regained capabilities during the trial.
A total of 26 hospitals in Henan province of China and all patients admitted from January 2021–2022 participated in this study. The inclusion criteria were as follows: (1) age 18–75 years; (2) suffered from severe infections caused by CR-GNB susceptible to polymyxin B (minimal inhibitory concentration [MIC] ≤ 2 mg/L by microdilution broth method) [13]; (3) clinical diagnosis of sepsis or septic shock as defined by sepsis 3.0 [14]; (4) infections included bacteremia, pneumonia, intraabdominal infection, skin and soft tissue infection, and central nervous system infection. This study excluded patients who had received polymyxin B treatment previously, pregnant or lactating women, patients with known polymyxin B allergies, and those enrolled in other trials.
Randomization and masking
All patients meeting the inclusion criteria were divided into a high initial dose group or a low initial dose group according to age and infection sites in a randomized block design; the randomization was computer-generated, and the block size was set at 6. If a subject was considered eligible for enrollment, we queried the random information corresponding to the group number: the letter “A” for a high initial dose and the letter “B” for a low initial dose. Thus, the subjects were randomized equally into two groups. Participants and doctors were not blinded to the randomization, and the primary outcome was decided by two researchers who were unaware of the treatment arm.
Procedures
The time from the diagnosis of CR-GNB infection to entering the trial and receiving polymyxin B treatment should not exceed 48 h. In the high-dose (HD) group, patients received 150 mg of polymyxin B (Shanghai First Biochemical Pharmaceutical Co., Ltd.) intravenously as a loading dose and 75 mg every 12 h as an initial maintenance dose, while in the low-dose (LD) group, patients received 100 mg of polymyxin B intravenously as an initial loading dose and 50 mg every 12 h as an initial maintenance dose. For TDM, blood samples were withdrawn from all dosages during the second and seventh dosages. Two blood samples were collected immediately before the infusion (2 mL, C0h) and 2 h (2 mL, C2h) after the beginning of the infusion for measurements.
Before analysis, the supernatant collected from blood samples was stored at − 80 °C. The plasma concentrations of polymyxin B, B1 and B2, were determined using a validated ultra-performance liquid chromatography-tandem mass spectrometry in our hospital laboratory, and the plasma concentration of polymyxin B was the sum of the above two polypeptides [15].
The limited sampling strategies of AUC0–24 were investigated using Bayesian and linear regression analyses based on previously published population PK model using Phoenix® NLME software (v8.3, Pharsight, Mountain View, CA, USA). The polymyxin B AUC0–24 was calculated using the following equation: AUC0–24 = 2 × (− 0.673 + 6.084 × C0h + 6.230 × C2h) [16]. Using this method, we determined the ssAUC0–24 of polymyxin B that reached a steady-state plasma level after the seventh dosage (on day 4).
If the ssAUC0–24 reached the target of 50–100 mg·h/L, we maintained the current dosage unchanged, but if not, the maintenance dose was increased or decreased by 25 mg every time on the day or the next day and plasma concentration of polymyxin B was rechecked after four dose administration, until the daily dose of polymyxin B exceeded the range of 50–200 mg or the ssAUC0-24 reached the 50–100 mg·h/L window, new blood samples were collected. The dose of polymyxin B does not need to be adjusted according to renal function [12, 17, 18]. Patients were administered polymyxin B for at least 7 days or until either discharge or death. Except for polymyxin B, doctors could choose one or two anti-infective agents for combination therapy (i.e., β-lactam, aminoglycosides, cephalosporin, quinolone, oxazolidinone, minocycline, fosfomycin), according to bacterial drug resistance. Patients with clinical signs of infection were sampled every 72 h until two consecutive negative results were obtained.
Outcomes
The primary outcome of this analysis was the 14-day clinical response rate, which was defined based on survival [19]: hemodynamic stability in patients with shock (mean blood pressure > 65 mmHg without vasopressor support), the improved or stable ratio of arterial partial pressure of oxygen/fraction of inspired oxygen (PaO2/FiO2) for patients with pneumonia, microbiological cure for patients with bacteremia (no growth in the blood of index isolate on day 14), and improved or stable Sequential Organ Failure Assessment (SOFA) score. (Baseline SOFA score ≥ 3 was improved by at least 30%, and for baseline SOFA < 3 the score remained the same or decreased.) Patients who fit the above description were assessed for clinical responses. The secondary outcomes included 28-day mortality, 14-day mortality, bacterial clearance rate, ventilated-free days at 28 days, length of stay in the intensive care unit, total in-hospital stay, superinfections, and adverse events.
Statistical analysis
The primary analysis was based on intention-to-treat. Patients who survived for > 72 h after randomization were included in the per-protocol analysis. Considering the impact of population characteristics on outcomes, we conducted subgroup analysis in populations with septic shock or with different infection sites and bacteria.
Statistical analysis was conducted using SAS 9.4. The continuous variable data were subjected to Kolmogorov–Smirnov test to determine whether they adhered to normal distribution. Mean value (SD) or quartile (upper quartile Q1, median Q2, lower quartile Q3) was used to describe the baseline characteristics of continuous variables, and the percentage was used for descriptive analysis of counting variables.
The primary endpoints of patients lost to follow-up were deemed ineffective. A log-rank test was used to compare time-to-event endpoints, and patients without an event were censored up to the date last known to be at risk; also, Kaplan–Meier estimation was carried out. A chi-square test was used to examine the binary endpoints. For the risk difference and relative risk, Wald and Newcombe confidence intervals were offered, respectively. Continuous endpoints were analyzed with a two-independent sample t test or the Wilcoxon rank test. All the reported p values were two-sided.
Based on the primary outcomes, we calculated a sample size of 311 that could achieve 80% power to detect a relative risk of 1.260 at a significance level of 0.05, and the 14-day clinical response rate in the low-dose group was assumed to be 58.0%.
Results
Patients characteristics
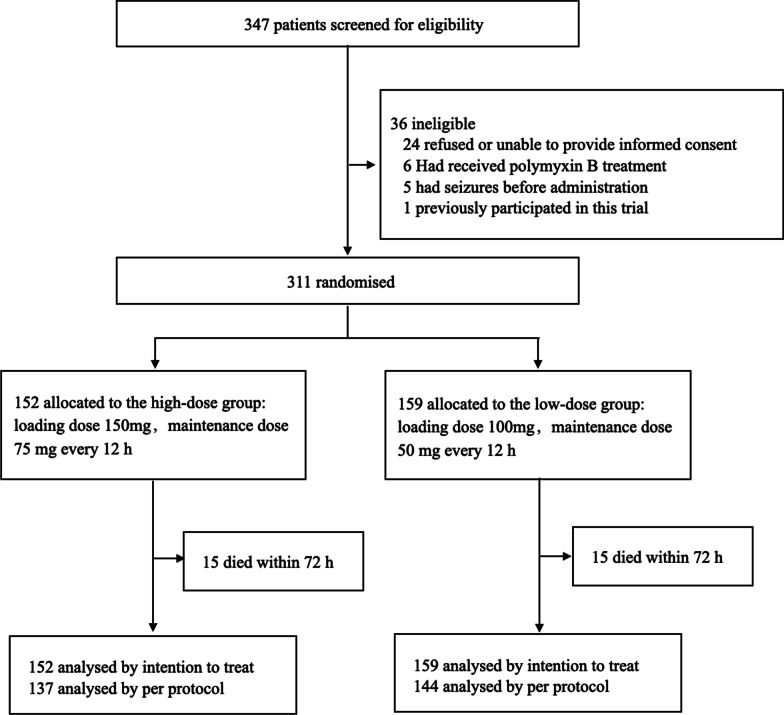
A total of 347 patients were screened between January 2021 and 2022, and of them, 36 were excluded. Finally, the trial comprised 311 patients, of whom 152 were randomly assigned to the HD group and 159 to the LD group (Fig. 1). The majority of patients (74%, 230/311) were males, 113 out of 311 (36.3%) patients had recently undergone trauma or surgery, and 241 out of 311 (77.5%) patients presented one or more comorbidities. The most common infection sites were lung (244/311, 78.5%) and blood (68/311, 21.9%), and the most common pathogens were CRAB (162/311; 52.1%) and CRE (145/311; 46.6%). Almost all the patients (300/311; 96.5%) received double and triple combinations of anti-infection therapy based on polymyxin B, of which carbapenems were the most common combination agents (167/311, 53.7%). The total 28-day mortality was 32.2% (107/311), and the total 180-day mortality was 75.3% (232/308).
Fig. 1.
Trial profile
Polymyxin B was administered at a lower dose in 159 patients than in 152 patients who received a higher dose. All of them received a loading dose; the HD group received a loading dose of 2.14 (interquartile range [IQR] 2, 2.5) mg/kg and a maintenance dose of 2.14 (IQR 2, 2.5) mg/kg/day, and the LD group received a loading dose of 1.54 (IQR 1.41, 1.75) mg/kg and a maintenance dose of 1.54 (IQR 1.41, 1.75) mg/kg/day. The median age of both groups was 58, and the average acute physiology and chronic health assessment (APACHE II) score were 18.7 in the HD group and 18.4 in the LD group. TBWs ranged from 45 to 99 kg (68.5 [IQR 60, 75]) in 150 patients in the HD group, and 2 patients were extremely obese at 120 kg and 180 kg, respectively. On the other hand, TBWs ranged from 40 to 100 kg (65.0 [IQR 57.0, 71.0]) in 159 patients in the LD group. The two groups were similar in most baseline characteristics and severity but not in TBW (70.0 [IQR 60.0, 75.0] vs. 65.0 [IQR 57.0, 71.0]; p = 0.006), which might be because gender was not considered during randomization (31 out of 152 [20.4%] in the HD group vs. 50 out of 159 [31.4%] in the LD group; p = 0.026]. This led to the inclusion of more female patients in the LD group, and the low weight of female patients resulted in the lower weight of patients in the LD group (Table 1).
Table 1.
Patient and infection characteristics
| Demographics and background | HD group (n = 152) | LD group (n = 159) | p value |
|---|---|---|---|
| Age, years, Q2 (Q1, Q3) | 58 (48, 66) | 58 (50, 68) | 0.360 |
| Gender | |||
| Male, % | 121 (79.6) | 109 (68.6) | 0.026 |
| Female, % | 31 (20.4) | 50 (31.4) | |
| BMI, kg/m2, Q2 (Q1, Q3) | 23.6 (21.6, 25.4) | 22.9 (20.9, 24.5) | 0.010 |
| Weight, kg, Q2 (Q1, Q3) | 70.0 (60.0, 75.0) | 65.0 (57.0, 71.0) | 0.006 |
| Comorbidity, % | |||
| Chronic heart disease | 68 (44.7) | 69 (43.4) | 0.812 |
| Chronic lung disease | 14 (9.2) | 15 (9.4) | 0.946 |
| Central nervous system diseases | 67 (44.1) | 55 (34.6) | 0.087 |
| Chronic kidney disease | 7 (4.6) | 5 (3.1) | 0.504 |
| Chronic liver disease | 9 (5.9) | 4 (2.5) | 0.134 |
| Diabetes | 31 (20.4) | 36 (22.6) | 0.630 |
| Solid malignancy | 6 (3.9) | 10 (6.3) | 0.350 |
| Hematological malignancy | 0 (0.0) | 2 (1.3) | 0.499 |
| Immune suppressive therapy | 15 (9.9) | 8 (5.0) | 0.103 |
| Other | 9 (5.9) | 7 (4.4) | 0.545 |
| Recent trauma or surgery | 54 (35.5) | 59 (37.1) | 0.772 |
| Infection site, % | |||
| Pulmonary infection | 122 (80.3) | 122 (76.7) | 0.449 |
| Bloodstream infection | 31 (20.4) | 37 (23.3) | 0.540 |
| Abdominal infection | 13 (8.6) | 14 (8.8) | 0.937 |
| Intracranial infection | 6 (3.9) | 12 (7.5) | 0.174 |
| Skin and soft tissue infection | 4 (2.6) | 9 (5.7) | 0.182 |
| Other | 9 (5.9) | 6 (3.8) | 0.377 |
| Single-site infection | 123 (80.9) | 119 (74.8) | 0.197 |
| Two-site infection | 21 (13.8) | 34 (21.4) | 0.080 |
| Multi-site infection | 8 (5.3) | 6 (3.8) | 0.527 |
| Pathogen, % | |||
| CRE | 66 (43.4) | 79 (49.7) | 0.268 |
| CRAB | 80 (52.6) | 82 (51.6) | 0.852 |
| CRPA | 20 (13.2) | 19 (11.9) | 0.748 |
| Other | 2 (1.3) | 3 (1.9) | 1.000 |
| Single-bacterial infection | 135 (88.8) | 137 (86.2) | 0.480 |
| Multi-bacterial infection | 17 (11.2) | 22 (13.8) | 0.480 |
| Mechanical ventilation (invasive), % | 108 (71.1) | 114 (71.7) | 0.721 |
| Hemodialysis, % | 20 (13.2) | 19 (11.9) | 0.677 |
| Temperature, °C, Q2 (Q1, Q3) | 37.3 (36.8, 38.0) | 37.4 (36.8, 38.2) | 0.513 |
| Mean artery pressure, mm Hg, M ± SD | 86.8 ± 15.5 | 85.8 ± 15.4 | 0.579 |
| Hemodynamic support, % | 25 (16.4) | 28 (17.6) | 0.785 |
| PaO2/FiO2, M ± SD | 227.7 ± 94.9 | 233.4 ± 93.5 | 0.601 |
| Scr, mg/dL, Q2 (Q1, Q3) | 59.4 (40.0, 99.8) | 62.5 (44.5, 97.9) | 0.323 |
| Albumin, g/dL, Q2 (Q1, Q3) | 31.3 (28.0, 33.9) | 31.3 (27.5, 37.0) | 0.461 |
| C-reactive protein, mg/L, Q2 (Q1, Q3) | 75.2 (41.9, 141.0) | 85.2 (38.0, 161.5) | 0.399 |
| D-dimer, Q2 (Q1, Q3) | 3.7 (1.4,6.3) | 2.8 (1.3, 5.9) | 0.456 |
| Lactic acid, mmol/L, Q2 (Q1, Q3) | 1.4 (1.0,2.0) | 1.6 (1.2, 2.1) | 0.169 |
| White blood cells, 109/L, Q2 (Q1, Q3) | 11.3 (8.0, 15.7) | 11.4 (8.1, 15.1) | 0.856 |
| Platelet, 109/L, Q2 (Q1, Q3) | 168.5 (85.5, 289.8) | 151.0 (79.0, 244.0) | 0.244 |
| Anti-infection therapy, % | |||
| Polymyxin B monotherapy | 6 (3.9) | 5 (3.1) | 0.702 |
| Polymyxin B + carbapenem | 44 (28.9) | 47 (29.6) | 0.906 |
| Polymyxin B + tigecycline | 13 (8.6) | 18 (11.3) | 0.415 |
| Polymyxin B + β-lactam | 27 (17.8) | 24 (15.1) | 0.525 |
| Polymyxin B + ceftazidime avibactam | 2 (1.3) | 7 (4.4) | 0.174 |
| Polymyxin B + X | 5 (3.3) | 6 (3.8) | 0.817 |
| Polymyxin B + carbapenem + tegacyclin | 21 (13.8) | 25 (15.7) | 0.636 |
| Polymyxin B + tegacyclin + X | 14 (9.2) | 10 (6.3) | 0.335 |
| Polymyxin B + β-lactam + X | 3 (2.0) | 3 (1.9) | 1.000 |
| Polymyxin B + carbapenem + X | 17 (11.2) | 13 (8.2) | 0.369 |
| GCS, Q2 (Q1, Q3) | 9.0 (6.0, 13.8) | 8.0 (5.0, 14.0) | 0.771 |
| APACHE II score, M ± SD | 19.0 ± 7.6 | 18.8 ± 6.9 | 0.819 |
| SOFA score, Q2 (Q1, Q3) | 7.0 (5.0, 10.0) | 8.0 (5.0, 10.0) | 0.774 |
Data are mean (SD), n (%), or median (IQR). HD: high dose. LD: low dose. BMI = body mass index. CRE = carbapenem-resistant Enterobacteriaceae. CRAB = carbapenem-resistant Acinetobacter baumannii. CRPA = carbapenem-resistant Pseudomonas aeruginosa. Scr: serum creatinine. GCS = Glasgow Coma Scale. APACHE = Acute Physiology and Chronic Health Evaluation. SOFA = Sequential Organ Failure Assessment. X = β-lactam / aminoglycosides / cephalosporin / quinolone / oxazolidinone / minocycline / fosfomycin
Intention-to-treat analysis
No significant difference was observed in the 14-day clinical response between the HD and LD groups in the intention-to-treat analysis. 95/152 (62.5%) patients in the HD group and 95/159 (59.7%) patients in the LD group met the criteria for clinical response (risk difference [RD], 2.75%; relative risk [RR]: 1.046; 95% confidence interval [CI]: 0.876–1.249; p = 0.619) (Table 2).
Table 2.
Outcomes for intention-to-treat population
| Outcomes | HD group (n = 152) | LD group (n = 159) | RR or HR (95% CI) | RD (95% CI) | p value |
|---|---|---|---|---|---|
| 14-day response, % | 62.5 (95/152) | 59.7 (95/159) | 1.046 (0.876, 1.249) | 2.75 (− 8.08, 13.58) | 0.619 |
| Mortality | |||||
| 14-day mortality, % | 23.7 (36/152) | 27.8 (44/159) | 0.856 (0.585, 1.252) | − 3.99 (− 13.57, 5.74) | 0.421 |
| 28-day mortality,% | 30.9 (47/152) | 37.7 (60/159) | 0.819 (0.601, 1.118) | − 6.81 (− 17.12, 3.73) | 0.206 |
| 72-h mortality, % | 9.9 (15/152) | 9.4 (15/159) | 1.046 (0.530, 2.066) | 0.43 (− 6.13, 7.00) | 0.897 |
| Disposition at day 28 | |||||
| Dead, % | 30.9 (47/152) | 37.7 (60/159) | 0.819 (0.601,1.118) | − 6.81 (− 17.12, 3.73) | 0.250 |
| Alive, still in the ICU, % | 25 (38/152) | 30.2 (48/159) | 0.828 (0.576,1.190) | − 5.19 (− 15.10,4.73) | 0.306 |
| Alive, discharged home, % | 16.4 (25/152) | 9.4 (15/159) | 1.743 (0.956,3.175) | 7.01 (− 0.42,14.45) | 0.065 |
| Alive, palliative care, % | 2.6 (4/152) | 1.9 (3/159) | 1.395 (0.163,3.151) | 0.74 (− 2.56,4.05) | 0.718 |
| Alive, still hospitalized, % | 25 (38/152) | 21.4 (34/159) | 0.872 (0.317,1.721) | 3.62 (− 5.76,13.00) | 0.506 |
| Bacterial clearance* | |||||
| Microbiological cure, % | 35.2 (50/142) | 33.3 (48/144) | 1.056 (0.766, 1.456) | 1.88 (− 9.03, 12.74) | 0.738 |
| Bacterial persistence, % | 56.3 (80/142) | 55.6 (80/144) | 1.014 (0.826, 1.246) | 0.78 (− 10.59, 12.13) | 0.894 |
| Superinfection, % | 11.3 (16/142) | 12.5 (18/144) | 0.901 (0.479, 1.696) | − 1.23 (− 8.89, 6.44) | 0.748 |
| Time-to-event data | |||||
| VFD#, Q2 (Q1, Q3) | 14 (6, 21) | 15 (6, 22) | 1.064 (0.831, 1.362) | Not Applicable | 0.613 |
| ICU-days†, Q2 (Q1, Q3) | 21 (15, 31) | 21 (13, 30) | 0.924 (0.738, 1.158) | Not Applicable | 0.495 |
| Hospital-days‡, Q2 (Q1, Q3) | 30 (19, 42) | 28 (17, 45) | 0.943 (0.752, 1.182) | Not Applicable | 0.611 |
Data are n (%) or median (IQR). n: sample size. HD: high dose. LD: low dose. RR: relative risk. HR: hazard ratio. RD: risk difference. *: Superinfection and original Bacterial persistence co-exist in 6 patients, so the sum of the parts of bacterial clearance is greater than 100% #: Ventilated-free days at 28 days. †: Length of ICU stay. ‡: Length of hospital stay
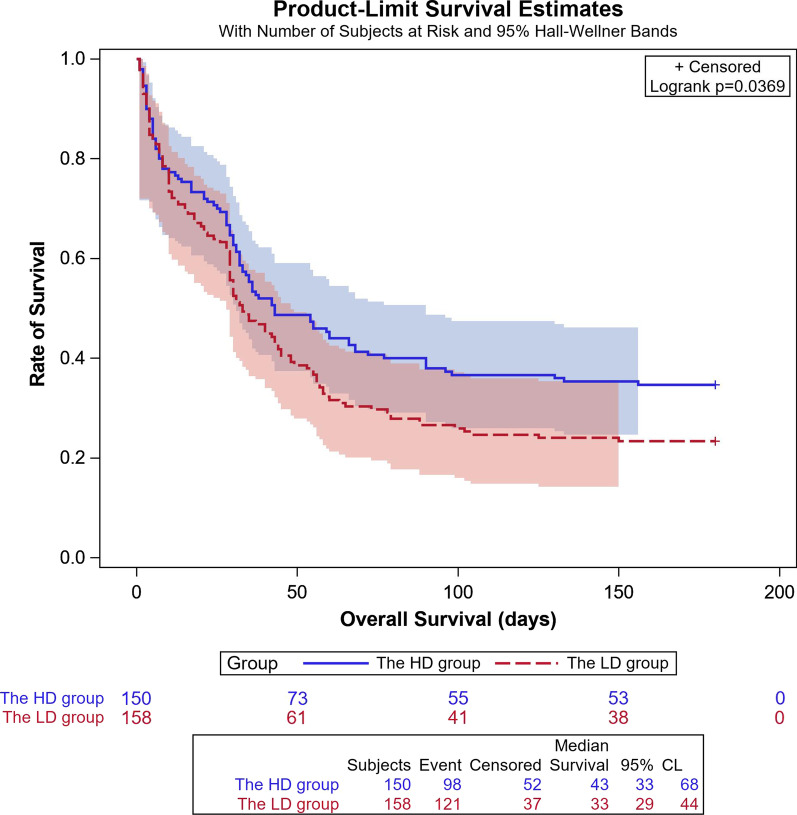
Also, no significant difference was detected in all-cause mortality at 14 or 28 days after randomization. The 14-day mortality rate of the HD and LD groups was 23.7% (36/152) and 27.7% (44/159), respectively, and the RD was − 3.99% (95% CI − 13.57% to 5.74%). The 28-day mortality rate was 30.9% (47/152) and 37.7% (60/159) in the HD and LD groups, respectively (RD: − 6.8%, 95% CI − 17.12% to 3.73%). Subsequently, 25 (16.4%) patients in the HD group and 15 (9.4%) patients in the LD group were discharged from the hospital on day 28 after randomization (Table 2). A prolonged follow-up of 180 days reported 34.7% (52/150) survival rate in the HD group and 23.4% (37/158) in the LD group, and there was an obvious survival advantage in patients who received a high dose of polymyxin B; the Kaplan–Meier’s survival curve of 180 days showed that the hazard ratio (HR) was 0.754 (95% CI 0.577–0.984; p = 0.037) (Fig. 2).
Fig. 2.
Kaplan–Meier’s survival curve of 180 days
TDM
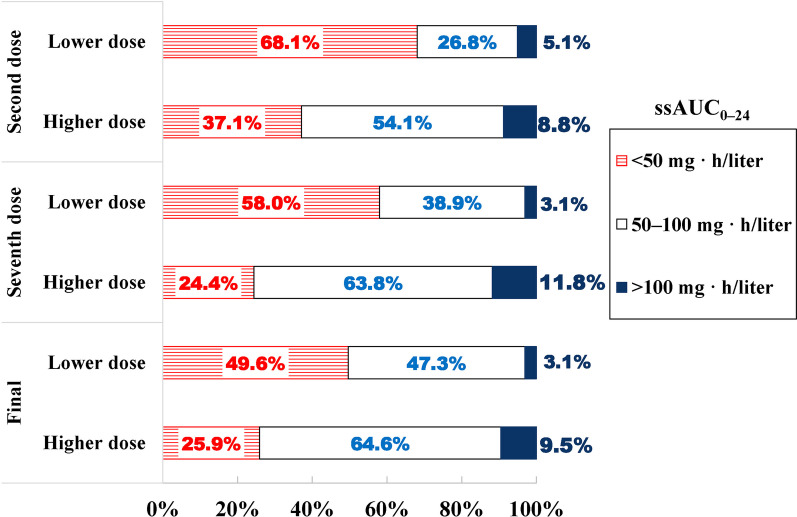
The ssAUC0–24 achieving rate (50–100 mg·h/L) of the HD group was significantly higher than that of LD group (63.8%, 81/127 vs. 38.9%, 51/131; RR, 1.638; 95% CI 1.274–2.106; p < 0.001) after the seventh dosage. Moreover, more patients exceeded 100 mg·h/L in the HD group than in the LD group (11.8, 15/127 vs. 5.1, 8/157; RR, 1.724; 95% CI 0.736–4.040; p = 0.007). Patients who did not hit the ssAUC0-24 target had their dosage adjusted, and finally, the ssAUC0–24 achieving a rate of 64.6% (82/127) in the HD group was significantly different from 47.3% (62/131) in the LD group (RR, 1.364; 95% CI 1.093–1.703, p = 0.005) (Table 3 and Fig. 3).
Table 3.
The target AUC compliance of polymyxin B
| HD group n, % |
LD group n, % |
RR (95% CI) | RD (95% CI) | p value | |
|---|---|---|---|---|---|
| ssAUC0–24 between 50–100 mg · h/L at the second dose, % | 54.1 (80/148) | 26.8 (42/157) | 2.021 (1.499, 2.723) | 27.30 (16.37, 37.35) | < 0.001 |
| ssAUC0–24 > 100 mg · h/L at the second dose, % | 8.8 (13/148) | 5.1 (8/157) | 1.724 (0.736, 4.040) | 3.69 (− 2.17, 9.88) | 0.204 |
| ssAUC0–24 between 50 and100 mg · h/L at the seventh dose, % | 63.8 (81/127) | 38.9 (51/131) | 1.638 (1.274, 2.106) | 24.85 (12.68, 35.99) | < 0.001 |
| ssAUC0–24 > 100 mg · h/L at the seven dose, % | 11.8 (15/127) | 3.1 (4/131) | 3.868 (1.319, 11.340) | 8.76 (2.35, 15.77) | 0.007 |
| ssAUC0–24 between 50–100 mg · h/L at final, % | 64.6 (82/127) | 47.3 (62/131) | 1.364 (1.093, 1.703) | 17.24 (5.12, 28.65) | 0.005 |
| ssAUC0–24 > 100 mg · h/L at final, % | 9.5 (12/127) | 3.1 (4/131) | 3.095 (1.025, 9.343) | 6.40 (0.37, 13.00) | 0.033 |
n: sample size. HD: high dose. LD: low dose. RR: risk ratio. RD: risk difference
Fig. 3.
The target AUC compliance of polymyxin B
Among the 258 patients who reported the ssAUC0–24 after the seventh dosage, 150 required dose adjustments due to failure to reach the target AUC. However, the plasma concentration of polymyxin B was rechecked in only 48 (32%) patients, while in the remaining 102 patients, we were unable to adjust the dosage or recheck for the progression of the disease, death, transfer out of the ICU, or discharge from the hospital. Subsequently, 18/48 (37.5%) patients did not reach the target AUC after the first dose adjustment. Among these, 7 patients underwent a second dose adjustment.
Subgroup analysis
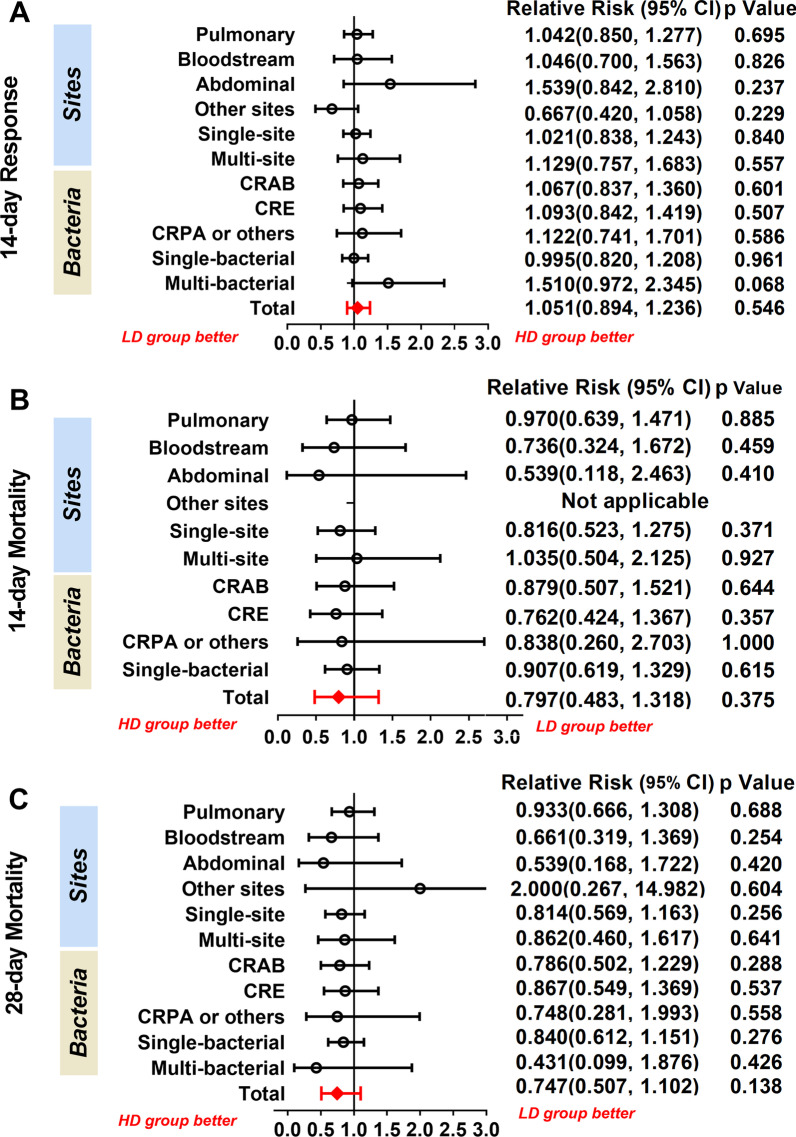
A total of 137 participants in the HD group and 144 participants in the LD group who survived for more than 72 h constituted the per-protocol population. The results are similar to those obtained in the intention-to-treat analysis; no difference was observed in 14-day clinical response, 14-day mortality, and 28-day mortality (Additional file 1: Table S1). Also, the infection sites and pathogens were not related to clinical outcomes (Fig. 4 and Additional file 1: Table S2).
Fig. 4.
Forest plots for subgroup analysis of infection sites and bacteria. CRE = carbapenem-resistant Enterobacteriaceae. CRAB = carbapenem-resistant Acinetobacter baumannii. CRPA = carbapenem-resistant Pseudomonas aeruginosa. Results for subgroup infected at other sites and subgroup infected with multiple bacteria are not applicable since a denominator value of zero is illegal
Subgroup analysis of participants with septic shock showed that the primary and secondary outcomes did not differ markedly (Table 4). 15/21 (71.4%) patients in the HD septic shock subgroup and 14/24 (58.3%) patients in the LD septic shock subgroup reached the ssAUC0-24 standard, which was significantly higher than that in all subjects (HD group: 71.4% vs. 63.8%, RR: 1.11; 95% CI 0.593–1.627, p = 0.037; LD: 58.3% vs. 38.9%, RR: 1.498; 95% CI 0.810–2.186, p = 0.0005).
Table 4.
Subgroup analysis results for patients with septic shock
| Outcomes | HD group (n = 25) |
LD group (n = 28) |
RR or HR (95% CI) |
RD (95% CI) | p value |
|---|---|---|---|---|---|
| 14-day response, % | 48.0 (12/25) | 50.0 (14/28) | 0.960 (0.553, 1.666) | − 2.0 (− 27.0, 23.4) | 0.884 |
| Mortality | |||||
| 14-day mortality, % | 24.0 (6/25) | 32.1 (9/28) | 0.747 (0.309, 1.802) | − 8.1 (− 30.5, 15.9) | 0.511 |
| 28-day mortality, % | 40.0 (10/25) | 53.6 (15/28) | 0.747 (0.413, 1.348) | − 13.6 (− 37.3, 12.6) | 0.323 |
| Bacterial Clearance* | |||||
| Microbiological cure, % | 34.8 (8/23) | 32.0 (8/25) | 1.087 (0.489, 2.419) | − 2.8 (22.5, − 27.9) | 0.838 |
| Bacterial persistence, % | 65.2 (15/23) | 52.0 (13/25) | 1.254 (0.776, 2.028) | 13.2 (− 13.9, 37.7) | 0.354 |
| Superinfection, % | 8.7 (2/23) | 16.0 (4/25) | 0.544 (0.110, 2.692) | − 7.3 (− 27.0, 13.2) | 0.445 |
| Time-to-event data | |||||
| VFD#, Q2(Q1, Q3) | 10 (0, 22) | 14 (8, 18) | 0.947 (0.536, 1.674) | Not Applicable | 0.798 |
| ICU-days†, Q2(Q1, Q3) | 23 (15, 35) | 22 (12, 26) | 0.726 (0.416, 1.267) | Not Applicable | 0.271 |
| Hospital-days‡, Q2(Q1, Q3) | 28 (20, 39) | 28 (16, 46) | 1.112 (0.640, 1.930) | Not Applicable | 0.725 |
| OS, Q2(Q1, Q3) | 31 (17, 60) | 25 (10, 48) | 0.768 (0.434, 1.359) | Not Applicable | 0.369 |
| ssAUC0–24 at the seventh dose | |||||
| 50–100 mg · h/L, % | 71.4 (15/21) | 58.3 (14/24) | 1.225 (0.794, 1.888) | 13.1 (− 14.4, 37.6) | 0.360 |
| > 100 mg · h/L, % | 14.3 (3/21) | 0.0 (0/24) | Not Applicable | 14.3 (− 2.4, 34.6) | 0.055 |
| Adverse events | |||||
| AE, % | 52.0 (13/25) | 50.0 (14/28) | 1.04 (0.613, 1.764) | 2.0 (− 23.4, 27.0) | 0.884 |
| AKI, % | 16.0 (4/25) | 21.4 (6/28) | 0.747 (0.238, 2.345) | − 5.4 (− 25.9, 16.3) | 0.614 |
| KIDGO Stage 1 | 50.0 (2/4) | 50.0 (3/6) | 1.000 (0.282, 3.544) | 0.0 (46.9, 46.9) | 1.000 |
| KIDGO Stage 2 | 25.0 (1/4) | 33.3 (2/6) | 0.750 (0.098, 5.768) | − 8.3 (− 50.3, 42.5) | 1.000 |
| KIDGO Stage 3 | 25.0 (1/4) | 16.7 (1/6) | 1.500 (0.127, 17.667) | 8.3 (−36.3, 55.3) | 1.000 |
Data are n (%) or median (IQR). n: sample size. HD: high dose. LD: low dose. RR: relative risk. HR: hazard ratio. RD: Risk Difference. *: Superinfection and original Bacterial persistence co-exist in 2 patients, so the sum of the parts of bacterial clearance is greater than 100% #: Ventilated-free days at 28 days. †: Length of ICU stay. ‡: Length of hospital stay. OS: overall survival. AE: adverse events. AKI: acute kidney injury. KDIGO: Kidney Disease Improving Global Outcomes
Sensitivity analysis
Since convective solute removal used in hemofiltration (for example, CVVH) significantly reduces the plasma concentration of polymyxin B [20], statistical analysis on the patients without CRRT or without CVVH did not detect any significant difference (Additional file 1: Tables S3 and S4).
The target AUC compliance rate had no correlation with the clinical outcomes (Additional file 1: Table S5). No difference was detected in both primary and secondary outcomes, but the incidence of acute renal injury (AKI) varies significantly between the groups (p = 0.019); it was 14.0% (15/107) in patients with ssAUC0-24 < 50 mg ·h/L, 26.5% (35/132) with ssAUC0-24 in 50–100 mg ·h/L, and 36.8% (7/19) with ssAUC0-24 exceeding 100 mg ·h/L. We also attempted to analyze the mortality in patients with septic shock, and the results showed that the 28-day mortality was 61.5% in patients with an AUC < 50 mg ·h/L and 34.5% in patients with an AUC between 50 and 100 mg h/L, and there is no difference between them (p = 0.248) (Additional file 1: Table S6).
The comparison of baseline characteristics between sepsis patients with and without shock showed that the level of serum creatinine (Scr) and lactate was significantly higher in patients with septic shock compared to sepsis patients without shock (Additional file 1: Table S7). The mean level of Scr was 116.7 ± 87.1 mg/dL in septic shock patients and 86.3 ± 86.3 mg/ dL in sepsis patients without shock (p = 0.001). The mean level of lactate was 4.5 ± 15.6 mmol/L in septic shock patients and 2.3 ± 8.7 mmol/L in sepsis patients without shock (p = 0.001).
Adverse events
The frequency of total adverse events and individual adverse events did not differ significantly between the HD and LD groups; the two groups reported 48.7 (74/152) and 43.4 (69/159) total adverse events, respectively (RR 0.891; 95% CI 0.70–1.134; p = 0.350). AKI is the most frequent adverse event, occurring in 30/152 (19.7%) and 32/159 (20.1%) of the HD and LD groups, respectively. According to the Kidney Disease Improving Global Outcomes (KDIGO) guidelines, the majority of patients had stage 1 [17/30 (56.7%) vs. 21/31 (67.7%)] and stage 2 [10/30 (33.3%) vs. 8/31 (25.8%)] AKI [21]. In addition, diarrhea [28/152 (18.4%) vs. 29/159 (18.2%)], pigmentation [24/152 (15.8%) vs. 20/159 (12.6%)], and superinfection [17/152 (11.2%) vs. 20/159 (12.6%)] were common adverse events in the two groups (Table 5), while no relevant adverse event was detected after discontinuation of treatment.
Table 5.
The adverse events
| HD group (n = 152, %) | LD group (n = 159, %) | RR (95% CI) | p value | |
|---|---|---|---|---|
| AE | 48.7 (74/152) | 43.4 (69/159) | 0.891 (0.700,1.134) | 0.350 |
| AKI | 19.7 (30/152) | 20.1 (32/159) | 1.020 (0.653,1.592) | 0.932 |
| KIDGO Stage 1 | 56.7 (17/30) | 67.7 (21/31) | 1.195 (0.804,1.777) | 0.372 |
| KIDGO Stage 2 | 33.3 (10/30) | 25.8 (8/31) | 0.774 (0.354,1.693) | 0.519 |
| KIDGO Stage 3 | 10.0 (3/30) | 6.5 (2/31) | 0.645 (0.116,3.593) | 0.614 |
| Diarrhea | 18.4 (28/152) | 18.2 (29/159) | 0.999 (0.619,1.583) | 0.967 |
| Pigmentation | 15.8 (24/152) | 12.6 (20/159) | 0.797 (0.460,1.381) | 0.417 |
| Superinfection | 11.2 (17/152) | 12.6 (20/159) | 1.125 (0.613,2.064) | 0.704 |
| Coagulation abnormalities | 6.6 (10/152) | 5.7 (9/159) | 0.860 (0.359,2.059) | 0.735 |
| Paresthesia | 0.7 (1/152) | 0.0 (0/159) | Not Applicable | 0.489 |
Data are n (%). n: sample size. HD: high dose. LD: low dose. RR: risk ratio. AE: adverse events. AKI: acute kidney injury. KDIGO: Kidney Disease Improving Global Outcomes. The RR for paresthesia is not applicable since a numerator value of zero is illegal
Discussion
The clinical and microbiological outcomes of different doses of polymyxin B in the treatment of sepsis caused by CR-GNB were similar, although more patients in the HD group (63.8%) achieved a ssAUC0–24 target of 50–100 mg·h/L after the seventh dose compared to the LD group (38.9%) (p < 0.001). Irrespective of high dose [loading dose: 150 mg (equivalent to 1,500,000 IU), maintenance dose: 75 mg every 12 h] or a low (loading dose: 100 mg, maintenance dose: 50 mg every 12 h) initial therapeutic dose, there is no significant difference in 14-day response of the patients. The 28-day mortality rate of the HD group (30.9%) was 6.8% lower than that of the LD group (37.7%), but the difference was not statistically significant. After prolonged follow-up, we found that the 180-day survival in the HD group was higher than that in the LD group. This result could be attributed to prolonged illness and increased long-term mortality due to low exposure dose of antibiotics. However, we also observed that the 180-day survival of both groups was extremely low. This phenomenon could be related to the critical condition and numerous complications of patients infected with CR-GNB. The most frequent adverse event is AKI, followed by superinfection, pigmentation, and diarrhea. The frequency of adverse events did not differ significantly between the two groups.
This is the first randomized controlled trial comparing different polymyxin B doses in the treatment of severe CR-GNB infections under TDM. Based on the results of a published murine thigh infection model study, when the MIC of polymyxin B to CR-GNB is ≤ 2 mg/L, the lower bound of the target window is estimated to be a ssAUC0–24 of 50 mg h/L, and the upper limit of the therapeutic window for polymyxin B is estimated to be a ssAUC0–24 of 100 mg ·h/L [9]. The current guidelines recommend a loading dose of 2.0–2.5 mg/kg (equivalent to 20,000–25,000 IU/kg) and a maintenance dose of 1.25–1.5 mg/kg (equivalent to 12,500–15,000 IU/kg) every 12 h for polymyxin B based on TBW [6]. However, no appropriate dosing regimen has been deduced for polymyxin B. According to the guidelines for the administration of a standard dose of polymyxin B, Monte Carlo simulations showed that only 71% of simulated subjects achieved a ssAUC0–24 target of 50–100 mg·h/L [9]. Thus, an appropriate initial dose and a rapid method of TDM would be required to improve the efficacy of polymyxin B.
In the current study, only a minority of patients (32%) who failed to achieve the target AUC received dosage adjustments and rechecked plasma concentration of polymyxin B. Such a finding indicated that TDM could not be carried out smoothly in clinical practice because it is time-consuming and requires multiple blood samples at different time points to determine whether ssAUC0–24 meets the standard value. Also, more than one-third of patients failed to achieve the target AUC after the first dose adjustment, suggesting that the method of dose adjustment with a 25 mg increase or decrease may be simple to use but insufficient to achieve the target AUC rapidly. Dose adjustment may be depends on the difference between the measured and target AUC, prompting us to explore suitable dosage adjustment strategies using the Bayesian approach in the future.
Interestingly, a higher proportion of patients reached the ssAUC0-24 standard of 50–100 mg ·h/L in the septic shock subgroup compared to the total group. Hence, we compared some baseline characteristics between sepsis patients with and without shock to explain why target AUC compliance rates were high in more severe patients, and the results showed that the level of Scr was significantly higher in patients with septic shock and the plasma concentration of polymyxin B appeared to increase with increasing Scr; a similar phenomenon was described in previous studies [22, 23].
Data from our prospective study indicated there was no correlation between whether ssAUC0-24 met the criteria and clinical outcomes; however, the 28-day mortality in septic shock patients with a ssAUC0-24 of polymyxin B between 50 and 100 mg ·h/L showed a decreasing trend, although not statistically significant due to limited sample size; also, AKI increased with increasing AUC, which is consistent with our previous findings in a real-world cohort of patients [24]. Septic shock is a severe subtype of sepsis with high mortality [25]. Briefly, critically ill patients may experience increased plasma concentrations of polymyxin B due to renal dysfunction, but the severity of the disease has a greater impact on outcomes than the benefit of increased AUC.
The most accurate PK/PD index for colistin is the ratio of the area under the unbound concentration–time curve to the MIC (fAUC/MIC), which is also applicable to polymyxin B. In murine thigh infection models, the fAUC/MIC value for 2-log bacterial killing was approximately 20 [26]. In order to reach a fAUC/MIC value of 20, the Monte Carlo simulations showed that a daily dose of 3 mg/kg/day should be considered for severe infections caused by CR-GNB with polymyxin B MIC of ≤ 2 mg/L [12]. The high doses of polymyxin B are frequently constrained in clinical practice due to nephrotoxicity. This study reported the incidence of AKI as 19.7% (30/152) and 20.1% (32/159) in the HD and LD groups, respectively, and more than half of them had mild renal toxicity (Class I of KDIGO classification). Such AKI incidence seems acceptable because severe infection can also lead to renal impairment.
Some studies speculated that the total dose of polymyxin B is highly related to both efficacy and toxicity, irrespective of patient weight [27]. In a retrospective cohort study, the polymyxin B dose of ≥ 200 mg/day (corresponding to 2.5–3 mg/kg/day in patients weighing 80–65 kg) was independently associated with low hospital mortality, although 119 (50.6%) presented some degree of renal impairment during therapy. The findings speculated that the survival benefits of high doses of drugs outweighed the risk of nephrotoxicity [28]. Another cohort study of 58 patients with sepsis who received a high-dose polymyxin B (median daily dose of 3.2 mg/kg/day) for ≥ 72 h showed promising mortality rates; among them, 25 (58.1%) patients developed AKI [29]. Therefore, regimens containing > 3 mg/kg/day of polymyxin B should not be recommended due to a lack of clinical data on safety. On the other hand, reducing the daily dosage of polymyxin B might weaken the efficacy of antibiotic therapy. A retrospective cohort study showed that polymyxin B dosages of < 1.3 mg/kg/day were associated with 30-day mortality in patients with renal impairment [30]. Xiao et al. collected data from 10,066 Gram-negative organisms isolated from patients with bloodstream infections (BSIs) to optimize the balance between efficacy and toxicity in different populations. Fixed and weight-based polymyxin B maintenance dose was simulated using Monte Carlo method. The results showed that the appropriate loading dose is 2.5 mg/kg of polymyxin B regardless of renal function, followed by a fixed maintenance dose of 60 mg every 12 h in patients with impaired renal function and 1.25 mg/kg every 12 h in patients with normal renal function [31]. However, these simulated data have not yet been substantiated.
Our previous real-world study of 100 patients with CR-GNB infections treated with polymyxin B found that the 28-day mortality was 40%, and about 50% of patients in the study were administered a fixed daily dose of 100 mg of polymyxin B [11]. Another retrospective study from China involving 268 similar patients claimed that the clinical efficacy rate was 36.57%, and the all-cause mortality rate was 33.96%, in this study, only 110/268 (41.04%) patients were administered a loading dose, and after calculation based on TBW, the median loading dose was 1.01 mg/kg and the median maintenance dose was 0.85 mg/kg [10]. In our current study, the total 28-day mortality was 32.2% (107/311), with 30.9% (47/152) in the HD group and 37.7% (60/159) in the LD group. Compared to the above study, our regimens of polymyxin B showed promise for the 28-day mortality, especially in patients who received a loading dose of 150 mg and a maintenance dose of 75 mg every 12 h. This phenomenon could be partially attributed to the fact that every patient in our study received a loading dose of polymyxin B, which helped in achieving optimal plasma exposure at the earliest [12].
Nevertheless, the present trial has several limitations, which might explain the lack of clinical difference between the two groups treated with different doses of polymyxin B for severe CR-GNB infection. First, there was sample shortage and selection bias. We may be able to make some inferences if the sample size is increased because a 7% difference was detected in the 28-day mortality between the two groups. Moreover, patients who received high doses of polymyxin B had elevated body weight which could have affected the results. Next, the dosage of polymyxin B in both groups was lower than the standard recommended dosage; the higher initial dose group received a mean maintenance dose of 2.14 (IQR 2, 2.5) mg/kg/day, and the lower initial dose group received a mean maintenance dose of 1.54 (IQR 1.41, 1.75) mg/kg/day, and only 64.6% and 47.3% achieved ssAUC0–24 values within the target therapeutic window of 50–100 mg·h/L. Due to unscientific usage, the therapeutic effects may not be satisfactory in the two groups. Finally, the recommended PK/PD exposure targets should be applied to polymyxin B monotherapy. However, in the current study, almost all patients received combination therapy with one or two anti-infective agents in addition to polymyxin B, which might affect the final clinical outcomes. We did not limit the combination therapy with polymyxin B because this is still a mainstream treatment pattern in China. A recent study showed that combination therapy with colistin and meropenem was not superior to colistin monotherapy for the treatment of pneumonia or BSI caused by drug-resistant (XDR) Acinetobacter baumannii, XDR Pseudomonas aeruginosa, and CRE [32]. Additional studies are warranted to fully understand the role of polymyxin B combination therapy.
Patients with CR-GNB infections have various diseases and poor physical condition, which are likely to affect clinical outcomes. Accumulating evidence showed that polymyxin B has differential efficacy according to types [33] and sites [26] of CR-GNB infections. Thus, in the future, randomized controlled trials will be required for various patient subgroups and anti-infection strategies.
Conclusions
For patients with sepsis caused by CR-GNB, a fixed polymyxin B loading dose of 150 mg followed by a maintenance dose of 75 mg every 12 h was safe, with better 180-day survival compared to patients receiving a lower dose. The target AUC compliance of polymyxin B was not correlated with clinical outcomes, but increased AUC was associated with increased incidence of AKI. Therefore, the TDM should be valued to prevent AKI in sepsis patients.
Supplementary Information
Additional file 1: Table S1. Outcomes for per protocolpopulation. Table S2. Subgroup analyses of infection site and bacteria. Table S3. Subgroup analysis results for patients without CRRT. Table S4. Subgroup analysis results for patients without CVVH. Table S5. Association between the target AUC compliance and clinical outcomes. Table S6. Association between the target AUC compliance and clinical outcomes in septic shock patients. Table S7. Baseline characteristics for sepsis patients with and without shock.
Acknowledgements
The authors would like to thank Xiaoguang Duan, Haixu Wang, Yonggang Luo, Yu Fang, Wang Miao, Chao Lan (The First Affiliated Hospital of Zhengzhou University), Yongmei Zhang (First Affiliated Hospital, Henan University of Science and Technology), Xisheng Zheng, Qiang Dang (Nanyang Central Hospital), Xianfa Jiao, Jinguang Jia (Zhengzhou People’s Hospital), Yanhua Shan (Zhumadian Central Hospital), Xianrong Song (Henan Provincial Chest hospital), Dongpu Ma, Lanjuan Xu (Zhengzhou Central Hospital), Jian Chen, Huaqiang Wang (Xuchang Central Hospital), Fengling Ju (Nanyang Second General Hospital), Xiaohui Li, Suping Guo (Fuwai Central China Cardiovascular Hospital), Yonghui Fan (Central Hospital of Pingmei Shenma Group), Dezhi Liu, Yongsheng Xing, Yun Fu, Weidong Guo (Xinxiang Central Hospital), Tiancai Wang (Nanshi Hospital of Nanyang), Jun Wang (Zhangzhongjing Hospital of Nanyang), Xiaoye Jin (Kaifeng People’s Hospital), Zhengrong Mao (The First Affiliated Hospital of Henan CM), Changying Guo, Lin Guo (The Seventh People’s Hospital of Zhengzhou) for their support with data collection, thank Peile Wang (The First Affiliated Hospital of Zhengzhou University) for the work of therapy drug monitoring, thank Clement Yaw Effah (The First Affiliated Hospital of Zhengzhou University) for modification and polish the language of this paper.
Author contributions
TS, JY, PC, and SL were involved in the research concept and design. SL, SQ, HS, MF, LX, HL, YG, ZZ, SZ, YD, and YL contributed to the data acquisition. SL and YW have reviewed and take responsibility for all the data. SL, YW, and PC performed the analysis and interpretation of the data. SL contributed to the writing of the first draft. TS, PC, and JY reviewed this article and made necessary changes to improve it. PC and JY adjudicated the primary outcome. All authors reviewed the final manuscript. All authors read and approved the final manuscript.
Funding
This study was supported by the United Fund of National Natural Science Foundation of China (Grant No. U2004110); The National Natural Science Foundation of China (Grant No. 82172129); The central government guides local science and technology development funds (Grant No. Z20221343037); and Medical Science and Technology Tackling Plan Provincial and Ministerial Major Projects of Henan Province (Grant No. SBGJ202101015).
Availability of data and materials
The data supporting the findings of the article are available on request by contacting the corresponding author.
Declarations
Ethics approval and consent for participate
This study was approved by the Human Research Ethics Committee of The First Affiliated Hospital of Zhengzhou University (2020-KY-0318-001, 01/26/2021) and was conducted in accordance with the Declaration of Helsinki and relevant clinical research regulations in China. Written informed consent was obtained from patients or patients’ legally authorized representatives.
Consent for publication
All authors approved the manuscript for submission.
Competing interests
None of the authors has any conflict of interest to declare.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Shaohua Liu and Ying Wu contributed equally to this work.
References
- 1.Spellberg B, Bartlett JG, Gilbert DN. The future of antibiotics and resistance. N Engl J Med. 2013;368(4):299–302. doi: 10.1056/NEJMp1215093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tacconelli E, Carrara E, Savoldi A, Harbarth S, Mendelson M, Monnet DL, Pulcini C, Kahlmeter G, Kluytmans J, Carmeli Y, et al. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis. 2018;18(3):318–327. doi: 10.1016/s1473-3099(17)30753-3. [DOI] [PubMed] [Google Scholar]
- 3.Paul M, Carrara E, Retamar P, Tängdén T, Bitterman R, Bonomo RA, de Waele J, Daikos GL, Akova M, Harbarth S, et al. European society of clinical microbiology and infectious diseases (ESCMID) guidelines for the treatment of infections caused by multidrug-resistant Gram-negative bacilli (endorsed by European society of intensive care medicine) Clin Microbiol Infect. 2022;28(4):521–547. doi: 10.1016/j.cmi.2021.11.025. [DOI] [PubMed] [Google Scholar]
- 4.Zavascki AP, Goldani LZ, Li J, Nation RL. Polymyxin B for the treatment of multidrug-resistant pathogens: a critical review. J Antimicrob Chemother. 2007;60(6):1206–1215. doi: 10.1093/jac/dkm357. [DOI] [PubMed] [Google Scholar]
- 5.Landman D, Georgescu C, Martin DA, Quale J. Polymyxins revisited. Clin Microbiol Rev. 2008;21(3):449–465. doi: 10.1128/CMR.00006-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsuji BT, Pogue JM, Zavascki AP, Paul M, Daikos GL, Forrest A, Giacobbe DR, Viscoli C, Giamarellou H, Karaiskos I, et al. International consensus guidelines for the optimal use of the polymyxins: endorsed by the American college of clinical pharmacy (ACCP), European society of clinical microbiology and infectious diseases (ESCMID), infectious diseases society of America (IDSA), international society for anti-infective pharmacology (ISAP), society of critical care medicine (SCCM), and society of infectious diseases pharmacists (SIDP) Pharmacotherapy. 2019;39(1):10–39. doi: 10.1002/phar.2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Onufrak NJ, Rao GG, Forrest A, Pogue JM, Scheetz MH, Nation RL, Li J, Kaye KS. Critical need for clarity in polymyxin B dosing. Antimicrob Agents Chemother. 2017 doi: 10.1128/AAC.00208-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kubin CJ, Nelson BC, Miglis C, Scheetz MH, Rhodes NJ, Avedissian SN, Cremers S, Yin MT. Population pharmacokinetics of intravenous polymyxin B from clinical samples. Antimicrob Agents Chemother. 2018 doi: 10.1128/aac.01493-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lakota EA, Landersdorfer CB, Nation RL, Li J, Kaye KS, Rao GG, Forrest A. Personalizing polymyxin B dosing using an adaptive feedback control algorithm. Antimicrob Agents Chemother. 2018 doi: 10.1128/AAC.00483-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qu J, Qi TT, Qu Q, Long WM, Chen Y, Luo Y, Wang Y. Polymyxin B-based regimens for patients infected with carbapenem-resistant gram-negative bacteria: clinical and microbiological efficacy, mortality, and safety. Infect Drug Resist. 2022;15:1205–1218. doi: 10.2147/IDR.S357746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang X, Qi S, Duan X, Han B, Zhang S, Liu S, Wang H, Zhang H, Sun T. Clinical outcomes and safety of polymyxin B in the treatment of carbapenem-resistant Gram-negative bacterial infections: a real-world multicenter study. J Transl Med. 2021;19(1):431. doi: 10.1186/s12967-021-03111-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sandri AM, Landersdorfer CB, Jacob J, Boniatti MM, Dalarosa MG, Falci DR, Behle TF, Bordinhao RC, Wang J, Forrest A, et al. Population pharmacokinetics of intravenous polymyxin B in critically ill patients: implications for selection of dosage regimens. Clin Infect Dis. 2013;57(4):524–531. doi: 10.1093/cid/cit334. [DOI] [PubMed] [Google Scholar]
- 13.Richter SS, Karichu J, Otiso J, Van Heule H, Keller G, Cober E, Rojas LJ, Hujer AM, Hujer KM, Marshall S, et al. Evaluation of Sensititre Broth Microdilution Plate for determining the susceptibility of carbapenem-resistant Klebsiella pneumoniae to polymyxins. Diagn Microbiol Infect Dis. 2018;91(1):89–92. doi: 10.1016/j.diagmicrobio.2018.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, Kumar A, Sevransky JE, Sprung CL, Nunnally ME, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43(3):304–377. doi: 10.1007/s00134-017-4683-6. [DOI] [PubMed] [Google Scholar]
- 15.Wang P, Zhang Q, Qin Z, Xing H, Xu M, Pei H, Yang J, Zhang X. A simple and robust liquid chromatography with tandem mass spectrometry analytical method for therapeutic drug monitoring of plasma and cerebrospinal fluid polymyxin B1 and B2. Ther Drug Monit. 2020;42(5):716–723. doi: 10.1097/ftd.0000000000000754. [DOI] [PubMed] [Google Scholar]
- 16.Wang P, Zhang Q, Zhu Z, Feng M, Sun T, Yang J, Zhang X. Population pharmacokinetics and limited sampling strategy for therapeutic drug monitoring of polymyxin B in Chinese patients with multidrug-resistant gram-negative bacterial infections. Front Pharmacol. 2020;11:829. doi: 10.3389/fphar.2020.00829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zavascki AP, Goldani LZ, Cao G, Superti SV, Lutz L, Barth AL, Ramos F, Boniatti MM, Nation RL, Li J. Pharmacokinetics of intravenous polymyxin B in critically ill patients. Clin Infect Dis. 2008;47(10):1298–1304. doi: 10.1086/592577. [DOI] [PubMed] [Google Scholar]
- 18.Thamlikitkul V, Dubrovskaya Y, Manchandani P, Ngamprasertchai T, Boonyasiri A, Babic JT, Tam VH. Dosing and pharmacokinetics of polymyxin B in patients with renal insufficiency. Antimicrob Agents Chemother. 2017 doi: 10.1128/AAC.01337-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paul M, Daikos GL, Durante-Mangoni E, Yahav D, Carmeli Y, Benattar YD, Skiada A, Andini R, Eliakim-Raz N, Nutman A, et al. Colistin alone versus colistin plus meropenem for treatment of severe infections caused by carbapenem-resistant Gram-negative bacteria: an open-label, randomised controlled trial. Lancet Infect Dis. 2018;18(4):391–400. doi: 10.1016/s1473-3099(18)30099-9. [DOI] [PubMed] [Google Scholar]
- 20.Wang P, Xing H, Zhang F, Liu S, Lu Y, Zhang X, Yang J, Sun T. Population pharmacokinetics of polymyxin B in critically ill patients receiving continuous venovenous haemofiltration. Int J Antimicrob Agents. 2022;60(1):106599. doi: 10.1016/j.ijantimicag.2022.106599. [DOI] [PubMed] [Google Scholar]
- 21.Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120(4):c179–184. doi: 10.1159/000339789. [DOI] [PubMed] [Google Scholar]
- 22.Yu XB, Jiao Z, Zhang CH, Dai Y, Zhou ZY, Han L, Wen X, Sheng CC, Lin GY, Pan JY. Population pharmacokinetic and optimization of polymyxin B dosing in adult patients with various renal functions. Br J Clin Pharmacol. 2021;87(4):1869–1877. doi: 10.1111/bcp.14576. [DOI] [PubMed] [Google Scholar]
- 23.Ye Q, Wang Q, Chen W, Zhang R, Chen Z, Li P, Zhang X, Zhan Q, Wang C. The population pharmacokinetics and dose optimization of polymyxin B in critically ill patients with or without extracorporeal membrane oxygenation. J Clin Pharm Ther. 2022;47(10):1608–1618. doi: 10.1111/jcpt.13711. [DOI] [PubMed] [Google Scholar]
- 24.Yang J, Liu S, Lu J, Sun T, Wang P, Zhang X. An area under the concentration-time curve threshold as a predictor of efficacy and nephrotoxicity for individualizing polymyxin B dosing in patients with carbapenem-resistant gram-negative bacteria. Crit Care. 2022;26(1):320. doi: 10.1186/s13054-022-04195-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Evans L, Rhodes A, Alhazzani W, Antonelli M, Coopersmith CM, French C, Machado FR, McIntyre L, Ostermann M, Prescott HC, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021;47(11):1181–1247. doi: 10.1007/s00134-021-06506-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dudhani RV, Turnidge JD, Nation RL, Li J. fAUC/MIC is the most predictive pharmacokinetic/pharmacodynamic index of colistin against Acinetobacter baumannii in murine thigh and lung infection models. J Antimicrob Chemother. 2010;65(9):1984–1990. doi: 10.1093/jac/dkq226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rigatto MH, Behle TF, Falci DR, Freitas T, Lopes NT, Nunes M, Costa LW, Zavascki AP. Risk factors for acute kidney injury (AKI) in patients treated with polymyxin B and influence of AKI on mortality: a multicentre prospective cohort study. J Antimicrob Chemother. 2015;70(5):1552–1557. doi: 10.1093/jac/dku561. [DOI] [PubMed] [Google Scholar]
- 28.Elias LS, Konzen D, Krebs JM, Zavascki AP. The impact of polymyxin B dosage on in-hospital mortality of patients treated with this antibiotic. J Antimicrob Chemother. 2010;65(10):2231–2237. doi: 10.1093/jac/dkq285. [DOI] [PubMed] [Google Scholar]
- 29.Cai Y, Leck H, Tan RW, Teo JQ, Lim TP, Lee W, Chlebicki MP, Kwa AL. Clinical experience with high-dose polymyxin B against carbapenem-resistant gram-negative bacterial infections-a cohort study. Antibiotics. 2020 doi: 10.3390/antibiotics9080451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nelson BC, Eiras DP, Gomez-Simmonds A, Loo AS, Satlin MJ, Jenkins SG, Whittier S, Calfee DP, Furuya EY, Kubin CJ. Clinical outcomes associated with polymyxin B dose in patients with bloodstream infections due to carbapenem-resistant Gram-negative rods. Antimicrob Agents Chemother. 2015;59(11):7000–7006. doi: 10.1128/AAC.00844-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu X, Huang C, Wang H, Ji J, Ying C, Xiao Y. Optimal empiric polymyxin B treatment of patients infected with gram-negative organisms detected using a blood antimicrobial surveillance network in China. Drug Des Devel Ther. 2021;15:2593–2603. doi: 10.2147/DDDT.S313714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaye Keith S, Marchaim D, Thamlikitkul V, Carmeli Y, Chiu C-H, Daikos G, Dhar S, Durante-Mangoni E, Gikas A, Kotanidou A, et al. Colistin monotherapy versus combination therapy for carbapenem-resistant organisms. NEJM Evidence. 2022;2(1):2200131. doi: 10.1056/EVIDoa2200131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lu Q, Zhu HH, Li GH, Qi TT, Ye LJ, Teng XQ, Qu Q, He GF, Qu J. A comparative study of the microbiological efficacy of polymyxin B on different carbapenem-resistant gram-negative bacteria infections. Front Med. 2021;8:620885. doi: 10.3389/fmed.2021.620885. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Outcomes for per protocolpopulation. Table S2. Subgroup analyses of infection site and bacteria. Table S3. Subgroup analysis results for patients without CRRT. Table S4. Subgroup analysis results for patients without CVVH. Table S5. Association between the target AUC compliance and clinical outcomes. Table S6. Association between the target AUC compliance and clinical outcomes in septic shock patients. Table S7. Baseline characteristics for sepsis patients with and without shock.
Data Availability Statement
The data supporting the findings of the article are available on request by contacting the corresponding author.