Abstract
There has been extensive growth in both the technical development and the clinical applications of MRI, establishing this modality as one of the most powerful diagnostic imaging tools. However, long examination and image interpretation times still limit the application of MRI, especially in emergent clinical settings. Rapid and abbreviated MRI protocols have been developed as alternatives to standard MRI, with reduced imaging times, and in some cases limited numbers of sequences, to more efficiently answer specific clinical questions. A group of rapid MRI protocols used at the authors’ institution, referred to as FAST (focused abbreviated survey techniques), are designed to include or exclude emergent or urgent conditions or screen for specific entities. These FAST protocols provide adequate diagnostic image quality with use of accelerated approaches to produce imaging studies faster than traditional methods. FAST protocols have become critical diagnostic screening tools at the authors’ institution, allowing confident and efficient confirmation or exclusion of actionable findings. The techniques commonly used to reduce imaging times, the imaging protocols used at the authors’ institution, and future directions in FAST imaging are reviewed to provide a practical and comprehensive overview of FAST MRI for practicing neuroradiologists.
©RSNA, 2023
Quiz questions for this article are available in the supplemental material.
Introduction
There has been rapid growth in MRI applications both technologically and in clinical indications, establishing this modality as one among the most powerful tools for imaging and clinical diagnosis (1,2). However, long examination times and often long image interpretation times still represent substantial barriers to the use of MRI. Rapid or abbreviated MRI protocols have emerged as alternatives to standard MRI, allowing reduced imaging times, and in some cases fewer imaging sequences, to answer specific clinical questions (3,4). Our family of rapid MRI protocols are referred to as FAST (focused abbreviated survey techniques); this acronym identifies these protocols internally and “brands” them as distinct from our traditional comprehensive protocols. The FAST designation also triggers a workflow system of expedited ordering, screening, imaging, and image interpretation.
Although our FAST MRI protocols were originally optimized for stroke evaluation, the use of these examinations has now expanded to other applications in the brain and spine, not only for emergent and urgent indications but also for targeting highly specific screening needs. Rapid MRI protocols (including our in-house FAST protocols and more generally all abbreviated MRI protocols) are designed to confidently include or exclude such actionable findings as hydrocephalus (5–7), syrinx (8), seizure (9,10), tumor (11), infection, traumatic injury (12–14), and vascular malformations, among others (15,16).
Our FAST MRI protocols have been designed to provide adequate diagnostic image quality with use of accelerated acquisition techniques, lower imaging matrices, phased-array coils, and innovative reconstruction methods to produce image studies faster than traditional methods. There is an intentional trade-off between image quality and time, with a combination of modified standard sequences on commercially available 1.5- or 3.0-T units used, sometimes in unique ways. Given the urgency implicit with stroke and other emergent neurologic conditions and our desire to limit the time spent performing screening examinations, our FAST protocols are targeted to achieve a total imaging time of less than 10 minutes (including calibration, scout, and localizer image acquisitions) and a total room time of less than 15 minutes. These time limits not only facilitate rapid treatment decisions but also permit the “add on” or “squeeze in” of emergent cases between scheduled MRI slots in a busy practice. FAST protocols have become critical diagnostic screening tools for our emergency department, allowing confident treatment disposition for positive cases and often welcome discharge for negative results.
In this article, we discuss the acquisition and reconstruction techniques most commonly used to reduce imaging times, review the imaging protocols that we use at our institution, and discuss future directions in FAST imaging, including the application of artificial intelligence. We provide an overview of FAST imaging protocols that are essential for the practicing neuroradiologist to comprehend and implement.
Acquisition and Reconstruction Techniques
General Considerations regarding FAST MRI
FAST MRI can be approached in a number of ways, but there are a few general discussion points to mention before diving into the technical details that can have a major impact on the degree of image acquisition acceleration, such as MRI system type and coil selection. Field strength is an important determinant of image quality and thus the potential image acquisition acceleration. The most common fields strengths used clinically are 1.5 T and 3.0 T (17). From a physics perspective, higher field strengths result in higher achievable signal intensity (18). This leads to increased imaging efficiency, which can be used to accelerate image acquisitions, increase spatial resolution, and/or improve the signal-to-noise ratio (SNR). This has been shown to be particularly advantageous for perfusion, vascular, spectroscopy, and functional MRI applications (17,19).
While there has been a gradual trend toward the use of 3.0-T and higher field strengths in neuroimaging, with it has come a requirement for increasing complexity to address certain technical considerations related to higher field strengths (20,21). Furthermore, some neuroimaging applications may favor lower field strengths (ie, 1.5 T and less) due to the associated decreased distortion and artifacts, decreased energy deposition, ability to image certain medical devices and implants, and overall lower cost of the system (19,22). Thus, different fields strengths may be more or less appropriate for different clinical situations. In addition, MRI systems with better gradient performance (ie, higher maximal gradient amplitudes and faster slew rates) are very desirable, particularly for rapid sequences such as diffusion and perfusion imaging. Stronger and faster gradients minimize repetition times, echo times, echo spacing, and diffusion-encoding times, allowing overall reductions in imaging times (21). For this reason, the most recent generation of commercial MRI units was developed with high-performance gradient systems in mind. Selecting an optimized coil system with a large number of coil elements also allows increased sensitivity and signal intensity uniformity, and it improves image acceleration techniques such as parallel imaging and simultaneous multislice (SMS) acquisitions (23).
Another important consideration is the choice of k-space trajectory. With most standard sequences, cartesian trajectories that acquire lines of k-space row by row are used. While this approach is the most common and relatively straightforward to implement, it results in undesirable spatially coherent (ie, readily visible) artifacts when attempting to acquire fewer data (ie, “undersampling”). Noncartesian (eg, radial and spiral) trajectories are more SNR efficient and have more favorable noise properties, which allow these trajectories to minimize motion artifacts and preserve spatial resolution while undersampling. These properties also make them well suited for the sparse sampling schemes needed for techniques such as MR fingerprinting (24), compressed sensing (25), and deep learning reconstructions (26). However, these trajectories are more complex in terms of both pulse sequence design and image reconstruction, limiting how widely they have been clinically adopted. Each institution must optimize its own choices for equipment, workflow, and patient population. Herein, we introduce a number of ways that we have adapted to create our FAST protocols.
The two primary strategies used to shorten MRI times rely on faster sampling and accelerated reconstruction. The main technical methods used in FAST imaging to accelerate image acquisition and the main applications, advantages, and disadvantages of these methods are listed in Table 1. Each method is discussed individually in the following sections.
Table 1:
Commonly Used Rapid Sampling and Reconstruction Techniques
Rapid Sampling
Echo-planar Imaging.—Echo-planar imaging is an ultrafast MRI technique that enables the acquisition of an entire image in a 10th of a second. Unlike standard gradient-echo sequences that involve the use of multiple radiofrequency pulses, the primary advantage of echo-planar imaging is that it enables multiple lines of k-space imaging data to be rapidly acquired after a single radiofrequency pulse (Fig 1). With echo-planar imaging, all lines are typically collected in a single repetition time such that an image is acquired in 20–100 msec (27). Echo-planar imaging is particularly sensitive to off-resonance and magnetic susceptibility effects, which can lead to image distortion and overall poor image quality. To reduce these distortions and allow rapid imaging, high-performance gradients are typically required (28). Distortions can be further minimized by implementing techniques that reduce the number of lines collected per acquisition with use of parallel imaging (29), multishot sequences (30), and/or spatial registration (31). Echo-planar imaging is a core technology used for diffusion and perfusion imaging. It is also the basis for a multicontrast imaging method, echo-planar imaging mix (EPIMix), which is discussed later in this article.
Figure 1.
Comparison of echo-planar versus standard MRI. Whereas standard MRI involves the use of many radiofrequency (RF) pulses to fill k-space (one per line), echo-planar imaging involves the use of a single radiofrequency pulse to collect data in k-space, thus enabling an extremely short acquisition time (<1 sec). Gx = gradient in frequency-encoding direction, Gy = gradient in phase-encoding direction, kx = coordinate in k-space in frequency-encoding direction, ky = coordinate in k-space in phase-encoding direction, Ny = number of phase encodings, TE = echo time (msec), TR = repetition time (msec).
Single-Shot Fast Spin Echo.—Single-shot fast spin echo (FSE) (SSFSE), also known as HASTE (half-fourier acquisition single-shot turbo spin echo), is a single-shot version of the FSE MRI sequence. Similar to FSE imaging, SSFSE imaging involves the use of a series of 180° pulses to generate echoes, whereas with echo-planar imaging, the gradients alone are used. However, while FSE imaging involves the use of multiple radiofrequency pulses to acquire a complete raw dataset, with SSFSE imaging, a single radiofrequency pulse is used and slightly more than half of the k-space data are acquired during a single repetition time, with the rest of the k-space recovered by using partial Fourier reconstruction (32). Consequently, similar to echo-planar imaging, SSFSE imaging enables one to collect an entire T2- or proton-density–weighted MR image after a single radiofrequency pulse (<1 second per section) (33). This method has been used for ultrafast examinations that require less than 30 seconds per series, such as in nonsedated pediatric patients undergoing repeated brain imaging for monitoring shunted hydrocephalus (5) (discussed later in this article).
SSFSE imaging is less sensitive to motion than are other T2-weighted MRI sequences, and compared with echo-planar imaging, it is much less sensitive to chemical shift and susceptibility artifacts. Because imaging is performed in a single shot, SSFSE imaging involves reductions in SNR and image contrast (34). These limitations can lead to decreased sensitivity for evidence of pediatric head trauma (eg, small intracranial and extra-axial hemorrhages); thus, use of a conventional diagnostic MRI protocol is advised (35).
SMS Acquisitions.—For SMS acquisition, a complex radiofrequency pulse is used to excite multiple section planes simultaneously, and reconstruction techniques are used to separate the sections after reception. This technique significantly accelerates the acquisition process, with little to no SNR penalty. Acceleration factors as high as 16 can be achieved in some implementations (36). In SMS acquisition, parallel imaging (discussed in the next section) is combined with radiofrequency or gradient encoding to resolve the section direction (37). Common artifacts include Nyquist-like ghosts and aliasing. Time-critical neuroimaging applications that often involve the use of SMS acquisition include functional MRI and diffusion-tensor imaging (37).
Accelerated Reconstruction
Parallel Imaging.—Parallel imaging accelerates data acquisition by exploiting the spatially varying sensitivities that are available from an array of independent receiving coils to reduce the number of required phase encodings. There are two main categories of parallel imaging techniques: sensitivity encoding (SENSE) (38) and generalized autocalibrating partially parallel acquisition (GRAPPA) (39). SENSE works with images reconstructed from individual coils. Each coil is sensitive only to signals from tissue in its vicinity. By creating a map of sensitivity profiles from all available coils (through a separate “calibration” image), aliasing artifacts from undersampling in the phase-encoding direction could be eliminated. Since SENSE requires an additional calibration image, it can be susceptible to artifacts when motion is present (40). Unlike with SENSE, with GRAPPA, a standard reconstruction is performed on undersampled k-space data for each coil. GRAPPA requires the central region of k-space to be fully sampled; then, the remaining portion of k-space is estimated. Unlike SENSE, GRAPPA cannot be applied to noncartesian imaging in a straightforward manner (41). GRAPPA is less sensitive to motion and other artifacts (42), but it provides a slightly lower acceleration level due to the full sampling of the center of k-space required.
Compressed Sensing.—Whereas parallel imaging reduces the required number of samples by exploiting the redundancy in data collected from multiple coils, compressed sensing (CS) (43,44) takes advantage of other redundancies that exist in imaging data, regardless of how the signal was received. As an example of such redundancy, MR images often can be described with fewer parameters after some mathematical transformation (eg, wavelet). The reduction in the data needed to fully describe the image is referred to as “sparsity.” CS is based on the idea that if an image can be “compressed” (ie, mathematically transformed into a representation that requires fewer data while the essential information is preserved), then the converse should also be true—that is, one can use fewer measurements to acquire the image in the first place. The more “sparse” an image is, the fewer data are required to represent the image. Many types of clinical images are sufficiently sparse to allow accelerated imaging by means of CS. In general, three-dimensional acquisitions have higher sparsity than two-dimensional acquisitions and can be accelerated in two directions to provide higher levels of acceleration. Temporally dynamic imaging (eg, dynamic susceptibility-weighted contrast-enhanced perfusion MRI) is another example in which ample sparsity can be exploited by using the CS approach. Vendors have implemented CS under various names, such as HyperSense (GE Healthcare) and Compressed SENSE (Philips).
Clinical Applications in Neuroradiology
FAST Stroke MRI Protocols with MR Angiography
Rapid imaging protocols and a streamlined workflow are critical for the diagnosis of stroke and the subsequent triage for emergent therapy, as well as for a wide range of encountered brain emergencies. Pivotal clinical trials showing improved neurologic outcomes after timely detection and endovascular treatment of large-vessel occlusions have propelled the deployment of rapid yet comprehensive imaging protocols for emergent central nervous system triage. Widely available CT, CT angiography, and often CT perfusion imaging remain the best established techniques for evaluation of suspected large-vessel occlusion in the early hours after presentation (45,46). CT remains our first-choice modality for adults who are suspected of having large-vessel occlusions at presentation. However, in many instances, MRI may be preferred, either as the first-line imaging modality or for follow-up to baseline CT (47,48). In stroke imaging, the preference for MRI is based largely on the sensitivity of diffusion-weighted imaging to acute brain ischemia, and therefore the prediction of irreversible core infarct, as well as on the ability to sensitively detect blood products and perform whole-brain perfusion-weighted imaging for comprehensive triage with MRI (48).
Since the time to treatment is critical in the setting of stroke therapy, the length of time required for traditional comprehensive stroke MRI protocols (30–45 min) is too long for urgent triage, creating a need for abbreviated protocols. In our practice, rapid MRI protocols designed to be used on 1.5-T or 3.0-T systems are preferred over CT for patients who present more than 6 hours after symptom onset or in the case of unknown symptom onset time (eg, “wake-up” stroke), patients who have had a lengthy transfer from an outside facility, pediatric patients, and patients suspected of having posterior fossa involvement, for which CT may be less sensitive or less desirable due to radiation exposure. We have introduced a suite of abbreviated MRI protocols to address the needs of other types of emergent brain triage, including not only stroke but also important stroke mimics seen in daily practice.
FAST Stroke with Contrast Protocol.—The FAST stroke with contrast MRI protocol (Tables 2, S1 [part A]) is our default choice for acute neurologic emergency MRI, including that in stroke workup, as the name of the protocol implies. This protocol involves comprehensive diffusion imaging and perfusion imaging, which are performed within the first few minutes of image acquisition. Postcontrast MRI for evaluation of tumor or infection, along with a whole-brain three-dimensional phase-contrast angiographic examination, is included. The three-dimensional phase-contrast angiographic examination is performed by using an intermediate velocity encoding of 40–50 cm/sec to screen large arteries and veins on the phase reconstructions, while magnitude imaging provides nominally T1-weighted MR images of the brain without additional imaging time.
Table 2:
FAST MRI Protocols
The field of view is extended into the neck for the sagittally acquired three-dimensional phase-contrast angiogram, which covers the cervical carotid bifurcations for key vascular screening in cases of transient ischemic attack or stroke (Fig 2). Because diffusion- and perfusion-weighted images are reordered early in the protocol, the automated perfusion color maps and mismatch calculations are obtained in parallel with the final series of the examination, such that all key information is ready as soon as imaging is complete.
Figure 2.
FAST stroke with contrast MRI in a 65-year-old woman with a history of endometrial carcinoma and strokelike symptoms. She presented to the emergency department with right leg weakness. Owing to her history of endometrial carcinoma, cerebral metastases were suspected and FAST stroke MRI with contrast was performed. (A–C) While there were no metastases, axial diffusion-weighted imaging (DWI) at b = 1000 (A), apparent diffusion coefficient (ADC) mapping (B), and FLAIR imaging (C) demonstrated a punctate recent infarct (arrowhead in A) with signs of adjacent older subcortical gliosis in the deep watershed region of the left parieto-occipital junction (arrow in B and C). (D) At three-dimensional phase-contrast angiography (3D PCA), the proximal segment of the cervical internal carotid artery has a flow gap (ellipse), suggesting critical stenosis. This finding highlights the importance of extending the field of view at 3D PCA so that it covers the cervical carotid bifurcations. (E, F) Perfusion-weighted MR images processed with RAPID software (iSchemaView) show extensive hypoperfusion of the left hemisphere (increased time-to-maximum [Tmax] ) in association with reduced cerebral blood volume (CBV) and cerebral blood flow (CBF) in the parieto-occipital region (arrows in F). These findings are compatible with old and new border zone infarcts. MTT = mean transit time.
FAST Stroke without Contrast Protocol.—The FAST stroke without contrast MRI protocol (Tables 2, S1 [part B]), the more limited FAST stroke protocol, may be indicated for adult or pediatric patients with acute neurologic deficits in whom intravenous access either is not desired or cannot be easily obtained. It facilitates a fair evaluation of the brain parenchyma, arteries, and veins with use of the MRI sequences listed in Table S1.
The FAST stroke MRI without contrast protocol is limited in that without contrast material, we are unable to detect perfusion or identify enhancing lesions. The MR angiographic images show less signal intensity and enable poorer small-vessel detection than the contrast-enhanced images in this protocol, since the T1-shortening effects of gadolinium-based contrast material are not achieved for vascular imaging. However, this remains a good technique, particularly for pediatric patients such as newborns with vein of Galen malformations and anxious young children in whom intravenous access may be difficult without sedation. Since both diffusion-weighted imaging (DWI) and T2-weighted fluid-attenuated inversion-recovery (FLAIR) imaging are included in this protocol, it allows consideration of intravenous tissue plasminogen activator treatment, with the FLAIR-DWI mismatch used in cases of stroke of unknown onset time (Fig 3) (48). T2-weighted FLAIR MRI is sensitive for subarachnoid hemorrhage, and T2*-weighted gradient-recalled-echo echo-planar imaging is sensitive for acute parenchymal hematomas and older blood products such as remote parenchymal hemorrhages, microbleeds, and amyloid-related imaging abnormalities in elderly patients. However, it should be noted that if an abnormality that needs further characterization is found, the clinical signs and symptoms persist or worsen, or there is continued clinical concern for any reason, a full-length follow-up MRI examination performed with all routine and traditional sequences should be considered. We apply these principals to all of the protocols outlined in this article.
Figure 3.
FAST stroke without contrast MRI in an 80-year-old man with expressive aphasia for approximately 4 hours. Admission CT (not shown) was negative for parenchymal infarction and large-vessel occlusion. Ischemic stroke was highly suspected clinically, and noncontrast FAST stroke MRI was performed. (A, B) Axial diffusion-weighted image (DWI) (b = 2500) (A) and apparent diffusion coefficient (ADC) map (B) show restricted diffusion (arrow) in the posterior part of the left frontal cortex. (C) Axial FLAIR image does not yet show hyperintensity (FLAIR-DWI mismatch), suggesting an acute infarct. (D) Three-dimensional phase-contrast angiographic (3D PCA) image shows no large-vessel occlusion.
FAST Brain MRI Protocols without MR Angiography
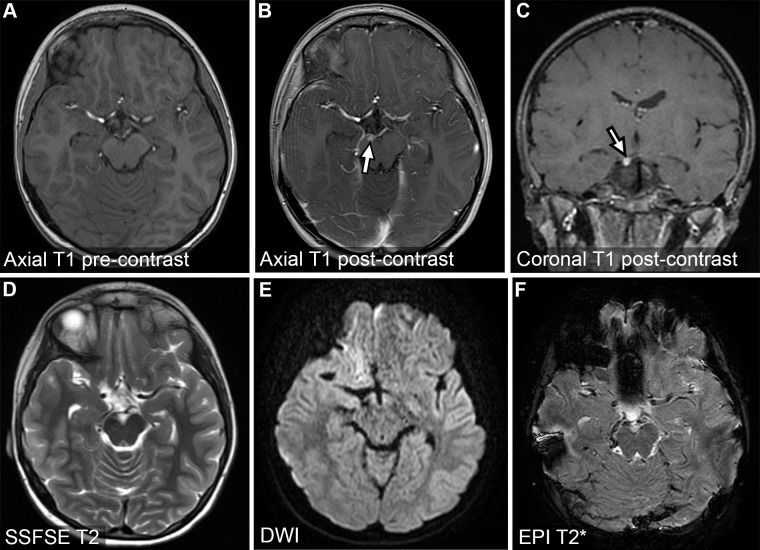
FAST Brain with Contrast Protocol.—The FAST brain with contrast MRI protocol (Tables 2, S2 [part A]) is a modified version of the FAST stroke MRI protocol but with the MR angiography–MR venography series omitted. This protocol can be used for cases in which perfusion or more robust enhancement characteristics are needed, such as those involving assessment of perfusion-diffusion mismatch or suspected tumor, demyelination, or infection in the emergent setting (Fig 4). The FAST brain with contrast protocol includes a more comprehensive postcontrast volumetric series than the FAST stroke protocol and is accelerated with use of parallel techniques and compressed sensing.
Figure 4.
FAST brain with contrast MRI in a 46-year-old patient who presented with right ptosis, migraine, and diplopia. MR images show a small enhancing lesion of the oculomotor nerve (arrow in B and C), consistent with oculomotor schwannoma. DWI = diffusion-weighted imaging, EPI = echo-planar imaging.
FAST Brain without Contrast Protocol.—The FAST brain without contrast MRI protocol (Tables 2, S2 [part B]) is similar to the FAST brain with contrast approach; however, the postcontrast sequences are omitted. This protocol may be indicated for follow-up after a comprehensive CT–CT angiography examination when there is ongoing suspicion of lacunar or brainstem infarcts, or as a more limited nonsedation protocol for traumatic brain injury, including nonaccidental trauma in infants. It has the strengths of good screening evaluation of blood products, ischemia, extra-axial collections, and ventricular morphology. In addition, it may facilitate an expedited (<5 min) imaging time for critically ill patients, including those who are unable to undergo sedation.
FAST Spine MRI Protocol without Contrast
The FAST spine MRI protocol (Tables 2, S3) was developed to assist our emergency department in triaging patients with symptoms that are diffuse or difficult to localize. It is intended as a monitored screening examination with the option to acquire tailored images of a specific spinal region (cervical, thoracic, or lumbar) as needed. Whereas a more traditional total spine MRI protocol can take up to 90 minutes, our FAST spine protocol can be completed in less than 20 minutes. Following localizer image acquisitions, sagittal T1-weighted three-dimensional FSE and sagittal two-dimensional short τ inversion-recovery (STIR) MR images of the cervical, thoracic, and lumbar regions are obtained.
The T1-weighted three-dimensional FSE sequence was selected for its capability to depict the anatomy and the volumetric acquisition that allows reformatting in any plane. A downside is the relatively longer acquisition time (3 minutes vs 2 minutes with traditional two-dimensional FSE acquisition), which can be a challenge when the patient is moving. STIR sequences allow identification of edema within the cord, osseous spinal column, or paraspinal soft tissues. The T1-weighted three-dimensional FSE and STIR sequences combined enable robust detection of disease that causes spinal cord compression that requires emergent decompression. Depending on the initial imaging review at the time of image acquisition, the decision can be made to add contrast material if findings suggest possible infection or neoplasm. If acquired, the postcontrast image is also obtained by using the same sagittal T1-weighted three-dimensional FSE sequence.
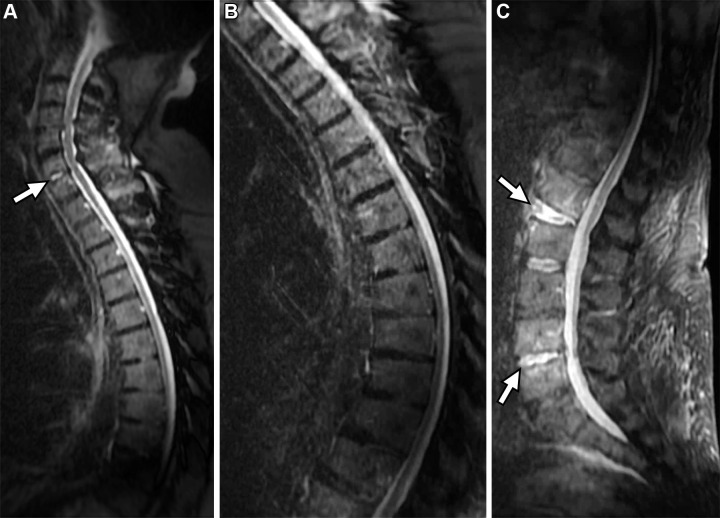
Figure 5 shows the findings in a patient who presented to the emergency department with slowly progressive myelopathic symptoms after an acute exacerbation. The FAST spine MR images showed a clear intramedullary lesion with T2 hypointensity, consistent with hemorrhage. The imaging study was converted to a comprehensive cervical spine MRI study with findings that confirmed the diagnosis of intramedullary arteriovenous malformation. This protocol also enables the diagnosis of acute osseous abnormalities, such as those that were seen in an elderly patient who presented with poorly localized back pain and was found to have a vertebral fragility fracture (Fig 6), and those in another patient (Fig 7) who presented with diffuse back pain and was found to have multifocal osteomyelitis and discitis.
Figure 5.
FAST spine MRI in a patient with slowly progressive myelopathic symptoms after an acute exacerbation. Sagittal STIR (A) and T2-weighted FSE (B) images show a heterogeneous intramedullary lesion (arrow) with associated T2 hypointensity, consistent with flow voids and blood products, and cranial and caudal T2 hyperintensity, consistent with cord edema.
Figure 6.

FAST spine MRI in a patient who presented with poorly localized back pain. Sagittal T1-weighted FSE (A) and T2-weighted STIR (B) images show a linear T1-hypointense and T2-hyperintense fluid cleft (arrowhead) within the superior aspect of the L3 vertebral body, consistent with a vertebral fragility fracture.
Figure 7.
FAST spine MRI in a patient who presented with diffuse back pain. Sagittal STIR images of the entire spine show multifocal osseous and disk hyperintensity (arrows in A and C) at the C6–C7, L1–L2, and L4–L5 spinal levels, consistent with osteomyelitis and discitis.
Pediatric “Quick” MRI Protocols without Contrast
Our “quick brain” MRI examination consists mainly of three two-dimensional SSFSE acquisitions (Tables 2, S4 [part A]), which yield sagittal, axial, and coronal whole-brain T2-weighted images within a total table time—including positioning, pre-imaging, section prescription, and the combined imaging times for all three planes—of less than 4 minutes (5). The images are highly robust in the face of substantial head motion, as is often seen with nonsedated infants and young children. As previously noted, this method has been used for ultrafast examinations that take less than 30 seconds per series, such as in nonsedated pediatric patients undergoing repeated brain imaging for monitoring shunted hydrocephalus (5), reducing the need for sedation in this population.
In addition to being useful for evaluation of hydrocephalus, the quick brain examination is well suited for rapidly evaluating fluid-filled regions, whether they are cerebrospinal fluid spaces, cystic masses, or other fluid collections (eg, arachnoid cysts, cystic neoplasms). While the quick brain examination is limited to relatively strong T2 weighting and therefore incomplete in terms of tissue characterization, it is often sufficient for preliminary evaluation of obvious structural abnormalities (eg, Chiari I malformation) (49) or surveillance of known lesions (eg, tumors, hematomas) when only a gross assessment of stability is required (Fig 8). It is important to note that the quick brain examination is not well suited for detailed characterization of soft-tissue structures, and, lacking both diffusion weighting and T2* weighting, it is relatively insensitive to ischemia, blood products, air, calcification, and implanted devices. Therefore, other accelerated or abbreviated protocols discussed elsewhere in this article should be considered for the assessment of brain parenchymal lesions. In particular, we discourage the use of the quick brain protocol in the setting of abusive head trauma, as this approach is relatively insensitive to subdural hematoma, subarachnoid hemorrhage, cortical contusion, and diffuse axonal injury. One approach to extending the capabilities of quick brain MRI to include additional tissue contrasts is to combine it with another highly accelerated protocol, such as EPIMix (discussed in the next section).
Figure 8.
Pediatric quick brain MRI in a nonsedated 16-year-old adolescent girl with neurofibromatosis type 1. Sagittal (A), axial (B), and coronal (C) SSFSE surveillance images to monitor the size of the mass and ventricles show a large optic pathway glioma (arrow) obstructing the ventricular system. A ventriculostomy catheter (arrowhead in B) is visible in the left lateral ventricle on the axial image.
The SSFSE sequence plays a major role in fetal MRI (50), in which substantial fetal motion typically is expected and motion-insensitive imaging is critical. It has also been applied to our postnatal quick spine protocol (Tables 2, S4 [part B]), which is focused on the evaluation and follow-up of syrinx, especially in cases of Chiari malformation. The pediatric quick spine MRI study includes the acquisition of axial, coronal, and sagittal T2-weighted SSFSE images from the cervical spine through the coccyx. This is expanded in cases of screening for tethered cord by adding axial and sagittal T1-weighted FSE images from the T11 spinal level through the coccyx, which is intended to help with the identification of fat in abnormal locations (Fig 9).
Figure 9.
Pediatric quick spine MRI in a patient for whom there was concern for tethered cord. (A, B) Sagittal (A) and coronal (B) T2-weighted images show a butterfly vertebral body (arrow). (C, D) Axial T2-weighted (C) and T1-weighted (D) images show an intrinsically hyperintense filum lipoma (arrowhead).
EPIMix MRI Protocol
EPIMix is a 1-minute full-brain examination that was invented by Dr Stefan Skare and colleagues at the Karolinska Institute and generously provided to us for pilot evaluation (15,51,52). It is a self-calibrating two-dimensional echo-planar imaging–based sequence that simultaneously produces T1-weighted, T2-weighted FLAIR, T2*-weighted, diffusion-weighted, and apparent diffusion coefficient images, all with built-in motion correction (52). Following an initial volunteer (with consent) phase conducted under institutional review board guidelines, we adopted this sequence for clinical use 3 years ago. We have now performed more than 3000 EPIMix examinations in adults and children, mainly as an add-on rather than a replacement for other protocols, although the potential for stand-alone use in some situations seems evident. While there are plans for eventual commercialization, the exact timeline has not been set.
EPIMix has all of the benefits and possible detractions of two-dimensional echo-planar imaging, including a very fast imaging time but also geometric distortion, signal intensity pile-up, cerebrospinal fluid pulsation artifacts, and other echo-planar imaging–based artifacts, such as those seen in the posterior fossa or around air-bone interfaces. Although the imaging acquisition, including self-calibration, is reliably completed in about 75 seconds, reconstruction times currently are on the order of several minutes, such that real-time review at the imaging console is not immediately available. The combination protocol of three-plane quick brain MRI plus EPIMix MRI, referred to as “quick mix,” can produce a comprehensive examination with excellent nondistorted depiction of morphology in the SSFSE series and multicontrast evaluation with EPIMix for most brain evaluation purposes, all in less than 2 minutes of imaging time and less than 5 minutes of room time (Fig 10). We prescribe this protocol prospectively for many pediatric examinations and allow our technologists to freely add EPIMix to examinations in which movement and time limitations are encountered.
Figure 10.
Quick mix MRI in a minimally cooperative 8-year-old child who was brought to the emergency department due to progressive cognitive decline without focal findings. Combined T2-weighted SSFSE pediatric quick brain images (A–C) and axial multicontrast (EPIMix) images (D–I), together referred to as a quick mix, show symmetric white matter changes in a tigroid pattern of radial perivenular sparing, diagnostic of previously unsuspected metachromatic leukodystrophy. The imaging examination was completed in less than 2 minutes of the total gradient time.
Future Directions
While many approaches can be used to improve image quality and shorten examination times, artificial intelligence (AI) has emerged as a key player in recent years and will likely continue to make a significant impact on the trajectory of FAST MRI. AI is one of the most rapidly growing fields of research in medical imaging, enabling substantially increased acceleration of image acquisition by improving image quality. While many approaches exist, convolutional neural networks (CNNs) are some of the most commonly used architectures for artificial intelligence (AI)–based MRI reconstructions and are designed to extract and process features from images. Using CNNs such as U-Net (an architecture for semantic segmentation) and ResNet (residual network, a deep learning model used for computer vision applications), the mapping of undersampled k-space data to artifact-free images can be learned by training the network on large collections of fully sampled k-space data (53–55).
A generalized framework for AI reconstruction with a neural network is shown in Figure 11. Deep learning reconstruction frameworks can also be trained to improve spatial resolution (super-resolution MRI) and reduce noise on the reconstructed images (56,57). While specific applications are still being explored and validated, reconstruction methods to increase spatial resolution and/or decrease noise may be particularly useful in situations in which rapid k-space sampling is necessary. These settings include pediatric and functional imaging and those in which higher spatial resolution is not possible owing to a low inherent SNR, as in perfusion imaging or low-field-strength MRI (58–60).
Figure 11.
General steps for training a neural network for artificial intelligence–based MRI reconstruction. Far left panel (green): The first step is the initial data collection and curation process. This can be the most time-consuming and manually intensive step, as it involves de-identifying, data cleaning and formatting, and labeling. Also, it is important to note that a sufficiently large dataset is required to generalize results. Second panel from left (blue): The second step involves selecting an appropriate network, which should be chosen with the required task in mind. Third panel from left (yellow): The third step involves training and validating the network, which can take hours or days. It is important to reserve a portion of the data to validate the training process. An appropriate learning rate must be chosen to avoid under- or overfitting the data. Far right panel (purple): The last step in developing a neural network involves applying the trained network to a separate dataset and ideally improving image quality.
With ever-increasing technical advancements, AI-based reconstructions have continued to improve, with several recently introduced state-of-the-art strategies—including general adversarial networks (GANs) (61), AUTOMAP (62), and recurrent neural networks (63)—showing great promise. Due to the success of AI, many major MRI vendors have incorporated deep learning reconstructions into their platforms. These reconstructions include AIR Recon DL (GE Healthcare), Deep Resolve (Siemens Healthineers), SmartSpeed (Philips), and AiCE (Canon), all of which can be applied across various anatomies and sequences. A recent study from our institution, in which AIR Recon DL was used in a T2-weighted SSFSE sequence (similar to that used in our FAST brain protocol) with an acceleration of 4 (48-sec imaging time), showed no differences in rated image quality relative to the baseline sequence (imaging time: 2 min, 24 sec) (64). Another benefit of AI is the dramatic reduction in reconstruction times, as compared with the times required for sparsity-based reconstructions (65), without compromises in robustness (66).
In light of the increasing prevalence of AI, it is relevant to discuss the shortcomings and artifacts associated with AI. For instance, synthesized full-contrast reconstructions from low-dose contrast-enhanced T1-weighted MRI sequences can show in-flow artifacts that lead to underestimations of the true contrast enhancement (67). Also, motion needs to be carefully checked on a patient-by-patient basis, as it is likely to adversely affect AI reconstructions that do not control for motion. Methods to quantify the risk, reliability, and generalizability of AI models need to be developed (68); this is particularly important for models trained on a small number of subjects and for deployment into clinical settings. There has been a collaborative effort to create open-source databases containing a large number of de-identified curated datasets with clinical data to improve the generalizability of AI over a larger population of subjects (69,70). It is anticipated that AI ultimately will be increasingly incorporated into many accelerated neuroimaging protocols that can be applied to various anatomic regions and that have thus far demonstrated impressive gains in image acquisition acceleration, spatial resolution, and noise reduction.
Conclusion
Rapid MRI protocols have made tremendous strides in shortening the long examination and image interpretation times that have previously limited the application of MRI, especially in emergent clinical settings. As an alternative to standard MRI protocols, rapid techniques provide accelerated and/or fewer sequences to answer focused clinical questions. Our FAST MRI protocols, which are designed to confidently include or exclude emergent and urgent conditions and/or screen for specific entities provide adequate diagnostic image quality while enabling examinations to be performed faster than traditional methods. In certain instances, these protocols can be essential for expediting examinations while providing the sensitive and specific diagnostic quality, without radiation exposure, that can only be achieved by using MRI. With continued technologic advancements, FAST MRI protocols will likely become more commonly used, with improving image quality and expanding clinical applications.
Acknowledgments
Acknowledgments
A special thanks to all of the other members of our University of Wisconsin neuroradiology and medical physics team, our neurology and neurosurgical clinical partners, and the MRI technologists and administration staff, who not only do an excellent job imaging patients at our institution but also help in the development and implementation of innovative ideas that move the field forward.
L.B.E. and A.P. contributed equally to this work.
Presented as an education exhibit at the 2021 RSNA Annual Meeting.
Funding.—L.B.E. supported by the Clinical and Translational Science Award (CTSA) program, through the NIH National Center for Advancing Translational Sciences (NCATS) (grants UL1TR002373 and KL2TR002374), and by the Wisconsin Alzheimer's Disease Research Center (grant P30-AG062715). A.P. supported by a postdoctoral fellowship from the Belgian American Educational Foundation (BAEF) in 2020-2021. H.A.R. supported by GE Healthcare, Bracco Diagnostics, and iSchemaView.
Disclosures of conflicts of interest.—: H.A.R. Consulting fees from Guerbet and Bayer. All other authors, the editor, and the reviewers have disclosed no relevant relationships.
Abbreviations:
- EPIMix
- echo-planar imaging mix
- FAST
- focused abbreviated survey techniques
- FSE
- fast spin echo
- GRAPPA
- generalized autocalibrating partially parallel acquisition
- SENSE
- sensitivity encoding
- SMS
- simultaneous multislice
- SNR
- signal-to-noise ratio
- SSFSE
- single-shot FSE
- STIR
- short τ inversion recovery
References
- 1. Beker K , Garces-Descovich A , Mangosing J , Cabral-Goncalves I , Hallett D , Mortele KJ . Optimizing MRI Logistics: Prospective Analysis of Performance, Efficiency, and Patient Throughput . AJR Am J Roentgenol 2017. ; 209 ( 4 ): 836 – 844 . [DOI] [PubMed] [Google Scholar]
- 2. O'Brien JJ , Stormann J , Roche K , et al . Optimizing MRI Logistics: Focused Process Improvements Can Increase Throughput in an Academic Radiology Department . AJR Am J Roentgenol 2017. ; 208 ( 2 ): W38 – W44 . [DOI] [PubMed] [Google Scholar]
- 3. Canellas R , Rosenkrantz AB , Taouli B , et al . Abbreviated MRI Protocols for the Abdomen . RadioGraphics 2019. ; 39 ( 3 ): 744 – 758 . [DOI] [PubMed] [Google Scholar]
- 4. Ramgopal S , Karim SA , Subramanian S , Furtado AD , Marin JR . Rapid brain MRI protocols reduce head computerized tomography use in the pediatric emergency department . BMC Pediatr 2020. ; 20 ( 1 ): 14 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Iskandar BJ , Sansone JM , Medow J , Rowley HA . The use of quick-brain magnetic resonance imaging in the evaluation of shunt-treated hydrocephalus . J Neurosurg 2004. ; 101 ( 2 suppl ): 147 – 151 . [DOI] [PubMed] [Google Scholar]
- 6. Kim I , Torrey SB , Milla SS , Torch MC , Tunik MG , Foltin JC . Benefits of brain magnetic resonance imaging over computed tomography in children requiring emergency evaluation of ventriculoperitoneal shunt malfunction: reducing lifetime attributable risk of cancer . Pediatr Emerg Care 2015. ; 31 ( 4 ): 239 – 242 . [DOI] [PubMed] [Google Scholar]
- 7. Patel DM , Tubbs RS , Pate G , Johnston JM Jr , Blount JP . Fast-sequence MRI studies for surveillance imaging in pediatric hydrocephalus . J Neurosurg Pediatr 2014. ; 13 ( 4 ): 440 – 447 . [DOI] [PubMed] [Google Scholar]
- 8. Gewirtz JI , Skidmore A , Smyth MD , et al . Use of fast-sequence spine MRI in pediatric patients . J Neurosurg Pediatr 2020. ; 26 ( 6 ): 676 – 681 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Grewal SS , Middlebrooks EH , Kaufmann TJ , et al . Fast gray matter acquisition T1 inversion recovery MRI to delineate the mammillothalamic tract for preoperative direct targeting of the anterior nucleus of the thalamus for deep brain stimulation in epilepsy . Neurosurg Focus 2018. ; 45 ( 2 ): E6 . [DOI] [PubMed] [Google Scholar]
- 10. Wieshmann UC , Free SL , Everitt AD , et al . Magnetic resonance imaging in epilepsy with a fast FLAIR sequence . J Neurol Neurosurg Psychiatry 1996. ; 61 ( 4 ): 357 – 361 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Loução R , Oros-Peusquens AM , Langen KJ , Ferreira HA , Shah NJ . A Fast Protocol for Multiparametric Characterisation of Diffusion in the Brain and Brain Tumours . Front Oncol 2021. ; 11 : 554205 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Flom L , Fromkin J , Panigrahy A , Tyler-Kabara E , Berger RP . Development of a screening MRI for infants at risk for abusive head trauma . Pediatr Radiol 2016. ; 46 ( 4 ): 519 – 526 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kralik SF , Yasrebi M , Supakul N , et al . Diagnostic Performance of Ultrafast Brain MRI for Evaluation of Abusive Head Trauma . AJNR Am J Neuroradiol 2017. ; 38 ( 4 ): 807 – 813 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ryan ME , Jaju A , Ciolino JD , Alden T . Rapid MRI evaluation of acute intracranial hemorrhage in pediatric head trauma . Neuroradiology 2016. ; 58 ( 8 ): 793 – 799 . [DOI] [PubMed] [Google Scholar]
- 15. Ha JY , Baek HJ , Ryu KH , et al . One-Minute Ultrafast Brain MRI With Full Basic Sequences: Can It Be a Promising Way Forward for Pediatric Neuroimaging? AJR Am J Roentgenol 2020. ; 215 ( 1 ): 198 – 205 . [DOI] [PubMed] [Google Scholar]
- 16. Khaira G , Kurz JE . Rapid Brain MRI Use in a Pediatric Emergency Department . Pediatr Neurol Briefs 2020. ; 34 ( 0 ): 21 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Moser E , Laistler E , Schmitt F , Kontaxis G . Ultra-High Field NMR and MRI: The Role of Magnet Technology to Increase Sensitivity and Specificity . Front Phys 2017. ; 5 : 33 . [Google Scholar]
- 18. Schick F , Pieper CC , Kupczyk P , et al . 1.5 vs 3 Tesla Magnetic Resonance Imaging: A Review of Favorite Clinical Applications for Both Field Strengths—Part 1 . Invest Radiol 2021. ; 56 ( 11 ): 680 – 691 . [DOI] [PubMed] [Google Scholar]
- 19. Radbruch A , Paech D , Gassenmaier S , et al . 1.5 vs 3 Tesla Magnetic Resonance Imaging: A Review of Favorite Clinical Applications for Both Field Strengths—Part 2 . Invest Radiol 2021. ; 56 ( 11 ): 692 – 704 . [DOI] [PubMed] [Google Scholar]
- 20. Alvarez-Linera J . 3T MRI: advances in brain imaging . Eur J Radiol 2008. ; 67 ( 3 ): 415 – 426 . [DOI] [PubMed] [Google Scholar]
- 21. Vachha B , Huang SY . MRI with ultrahigh field strength and high-performance gradients: challenges and opportunities for clinical neuroimaging at 7 T and beyond . Eur Radiol Exp 2021. ; 5 ( 1 ): 35 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hori M , Hagiwara A , Goto M , Wada A , Aoki S . Low-Field Magnetic Resonance Imaging: Its History and Renaissance . Invest Radiol 2021. ; 56 ( 11 ): 669 – 679 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gruber B , Froeling M , Leiner T , Klomp DWJ . RF coils: A practical guide for nonphysicists . J Magn Reson Imaging 2018. ; 48 ( 3 ): 590 – 604 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Panda A , Mehta BB , Coppo S , et al . Magnetic Resonance Fingerprinting: An Overview . Curr Opin Biomed Eng 2017. ; 3 : 56 – 66 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Feng L , Grimm R , Block KT , et al . Golden-angle radial sparse parallel MRI: combination of compressed sensing, parallel imaging, and golden-angle radial sampling for fast and flexible dynamic volumetric MRI . Magn Reson Med 2014. ; 72 ( 3 ): 707 – 717 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Terpstra ML , Maspero M , d'Agata F , et al . Deep learning-based image reconstruction and motion estimation from undersampled radial k-space for real-time MRI-guided radiotherapy . Phys Med Biol 2020. ; 65 ( 15 ): 155015 . [DOI] [PubMed] [Google Scholar]
- 27. Poustchi-Amin M , Mirowitz SA , Brown JJ , McKinstry RC , Li T . Principles and applications of echo-planar imaging: a review for the general radiologist . RadioGraphics 2001. ; 21 ( 3 ): 767 – 779 . [DOI] [PubMed] [Google Scholar]
- 28. Kang D , Jo HJ , In MH , et al . The benefit of high-performance gradients on echo planar imaging for BOLD-based resting-state functional MRI . Phys Med Biol 2020. ; 65 ( 23 ): 235024 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bammer R , Keeling SL , Augustin M , et al . Improved diffusion-weighted single-shot echo-planar imaging (EPI) in stroke using sensitivity encoding (SENSE) . Magn Reson Med 2001. ; 46 ( 3 ): 548 – 554 . [DOI] [PubMed] [Google Scholar]
- 30. McKinnon GC . Ultrafast interleaved gradient-echo-planar imaging on a standard scanner . Magn Reson Med 1993. ; 30 ( 5 ): 609 – 616 . [DOI] [PubMed] [Google Scholar]
- 31. Andersson JL , Skare S , Ashburner J . How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging . Neuroimage 2003. ; 20 ( 2 ): 870 – 888 . [DOI] [PubMed] [Google Scholar]
- 32. Semelka RC , Kelekis NL , Thomasson D , Brown MA , Laub GA . HASTE MR imaging: description of technique and preliminary results in the abdomen . J Magn Reson Imaging 1996. ; 6 ( 4 ): 698 – 699 . [DOI] [PubMed] [Google Scholar]
- 33. Patel MR , Klufas RA , Alberico RA , Edelman RR . Half-fourier acquisition single-shot turbo spin-echo (HASTE) MR: comparison with fast spin-echo MR in diseases of the brain . AJNR Am J Neuroradiol 1997. ; 18 ( 9 ): 1635 – 1640 . [PMC free article] [PubMed] [Google Scholar]
- 34. Bhosale P , Ma J , Choi H . Utility of the FIESTA pulse sequence in body oncologic imaging: review . AJR Am J Roentgenol 2009. ; 192 ( 6 suppl ): S83 – S93 ; Quiz S94–S97. [DOI] [PubMed] [Google Scholar]
- 35. Kessler BA , Goh JL , Pajer HB , et al . Rapid-sequence MRI for evaluation of pediatric traumatic brain injury: a systematic review . J Neurosurg Pediatr 2021. ; 28 ( 3 ): 278 – 286 . [DOI] [PubMed] [Google Scholar]
- 36. Chen L , Vu AT , Xu J , et al . Evaluation of highly accelerated simultaneous multi-slice EPI for fMRI . Neuroimage 2015. ; 104 : 452 – 459 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Barth M , Breuer F , Koopmans PJ , Norris DG , Poser BA . Simultaneous multislice (SMS) imaging techniques . Magn Reson Med 2016. ; 75 ( 1 ): 63 – 81 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pruessmann KP , Weiger M , Scheidegger MB , Boesiger P . SENSE: sensitivity encoding for fast MRI . Magn Reson Med 1999. ; 42 ( 5 ): 952 – 962 . [PubMed] [Google Scholar]
- 39. Griswold MA , Jakob PM , Heidemann RM , et al . Generalized autocalibrating partially parallel acquisitions (GRAPPA) . Magn Reson Med 2002. ; 47 ( 6 ): 1202 – 1210 . [DOI] [PubMed] [Google Scholar]
- 40. Faraji-Dana Z , Tam F , Chen JJ , Graham SJ . Interactions between head motion and coil sensitivity in accelerated fMRI . J Neurosci Methods 2016. ; 270 : 46 – 60 . [DOI] [PubMed] [Google Scholar]
- 41. Luo T , Noll DC , Fessler JA , Nielsen JF . A GRAPPA algorithm for arbitrary 2D/3D non-Cartesian sampling trajectories with rapid calibration . Magn Reson Med 2019. ; 82 ( 3 ): 1101 – 1112 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wild JM , Marshall H , Bock M , et al . MRI of the lung (1/3): methods . Insights Imaging 2012. ; 3 ( 4 ): 345 – 353 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Candes E , Romberg J , Tao T . Stable Signal Recovery from Incomplete and Inaccurate Measurements . Cornell University arXiv website. https://arxiv.org/abs/math/0503066. Posted March 3, 2005. Accessed October 12, 2022 .
- 44. Lustig M , Donoho DL , Santos JM , Pauly JM . Compressed Sensing MRI . IEEE Signal Process Mag 2008. ; 25 ( 2 ): 72 – 82 . [Google Scholar]
- 45. Kuner AD , Rowley HA . Should Perfusion CT and CTA Be Performed in All Patients With Suspected Stroke? Point-Yes, for Fast and Accurate Stroke Triage and Treatment . AJR Am J Roentgenol 2021. ; 217 ( 2 ): 291 – 292 . [DOI] [PubMed] [Google Scholar]
- 46. Powers WJ , Rabinstein AA , Ackerson T , et al . Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke—A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association . Stroke 2019. ; 50 ( 12 ): e344 – e418 . [DOI] [PubMed] [Google Scholar]
- 47. Nael K , Khan R , Choudhary G , et al . Six-minute magnetic resonance imaging protocol for evaluation of acute ischemic stroke: pushing the boundaries . Stroke 2014. ; 45 ( 7 ): 1985 – 1991 . [DOI] [PubMed] [Google Scholar]
- 48. Thomalla G , Simonsen CZ , Boutitie F , et al . WAKE-UP Investigators. MRI-Guided Thrombolysis for Stroke with Unknown Time of Onset . N Engl J Med 2018. ; 379 ( 7 ): 611 – 622 . [DOI] [PubMed] [Google Scholar]
- 49. Pan J , Quon JL , Johnson E , et al . Rapid-sequence brain magnetic resonance imaging for Chiari I abnormality . J Neurosurg Pediatr 2018. ; 22 ( 2 ): 158 – 164 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Saleem SN . Fetal MRI: An approach to practice—a review . J Adv Res 2014. ; 5 ( 5 ): 507 – 523 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Delgado AF , Kits A , Bystam J , et al . Diagnostic performance of a new multicontrast one-minute full brain exam (EPIMix) in neuroradiology: a prospective study . J Magn Reson Imaging 2019. ; 50 ( 6 ): 1824 – 1833 . [DOI] [PubMed] [Google Scholar]
- 52. Váša F , Hobday H , Stanyard RA , et al . Rapid processing and quantitative evaluation of multicontrast EPImix scans for adaptive multimodal imaging . Cold Spring Harbor Laboratory : bioRxiv website. https://www.biorxiv.org/content/10.1101/2021.02.12.430956v1. Posted February 14, 2021. Accessed October 12, 2022. [DOI] [PMC free article] [PubMed]
- 53. Ghodrati V , Shao J , Bydder M , et al . MR image reconstruction using deep learning: evaluation of network structure and loss functions . Quant Imaging Med Surg 2019. ; 9 ( 9 ): 1516 – 1527 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Han Y , Sunwoo L , Ye JC . k-Space Deep Learning for Accelerated MRI . IEEE Trans Med Imaging 2020. ; 39 ( 2 ): 377 – 386 . [DOI] [PubMed] [Google Scholar]
- 55. Dhengre N , Sinha S . Multiscale U-net-based accelerated magnetic resonance imaging reconstruction . Signal Image Video Process 2022. ; 16 ( 4 ): 881 – 888 . [Google Scholar]
- 56. Kidoh M , Shinoda K , Kitajima M , et al . Deep Learning Based Noise Reduction for Brain MR Imaging: Tests on Phantoms and Healthy Volunteers . Magn Reson Med Sci 2020. ; 19 ( 3 ): 195 – 206 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Rudie JD , Gleason T , Barkovich MJ , et al . Clinical Assessment of Deep Learning-based Super-Resolution for 3D Volumetric Brain MRI . Radiol Artif Intell 2022. ; 4 ( 2 ): e210059 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. de Leeuw den Bouter ML , Ippolito G , O'Reilly TPA , Remis RF , van Gijzen MB , Webb AG . Deep learning-based single image super-resolution for low-field MR brain images . Sci Rep 2022. ; 12 ( 1 ): 6362 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kim SH , Choi YH , Lee JS , et al . Deep learning reconstruction in pediatric brain MRI: comparison of image quality with conventional T2-weighted MRI . Neuroradiology 2023 Jan. ; 65 ( 1 ): 207 – 214 . [DOI] [PubMed] [Google Scholar]
- 60. Shou Q , Shao X , Wang DJJ . Super-Resolution Arterial Spin Labeling Using Slice-Dithered d Resolution and Simultaneous Multi-Slice Acquisition . Front Neurosci 2021. ; 15 : 737525 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Yuan Z , Jiang M , Wang Y , et al . SARA-GAN: Self-Attention and Relative Average Discriminator Based Generative Adversarial Networks for Fast Compressed Sensing MRI Reconstruction . Front Neuroinform 2020. ; 14 : 611666 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Zhu B , Liu JZ , Cauley SF , Rosen BR , Rosen MS . Image reconstruction by domain-transform manifold learning . Nature 2018. ; 555 ( 7697 ): 487 – 492 . [DOI] [PubMed] [Google Scholar]
- 63. Qin C , Schlemper J , Caballero J , Price AN , Hajnal JV , Rueckert D . Convolutional Recurrent Neural Networks for Dynamic MR Image Reconstruction . IEEE Trans Med Imaging 2019. ; 38 ( 1 ): 280 – 290 . [DOI] [PubMed] [Google Scholar]
- 64. Avey G , Eisenmenger L , Kim N , et al . Accelerating Neuroradiology Protocols with Deep Learning MR Image Reconstruction: Which Methods Result in the Highest Perceived Image Quality? Presented at the 2021 ISMRM Annual Meeting and Exhibition , Vancouver, BC, Canada , May 15–20, 2021 . [Google Scholar]
- 65. Sandino CM , Dixit N , Cheng JY , Vasanawala SS . Deep convolutional neural networks for accelerated dynamic magnetic resonance imaging . Presented at the 31st Conference on Neural Information Processing Systems (NIPS 2017) , Long Beach, Calif . December 4–9, 2017 . [Google Scholar]
- 66. Darestani MZ , Chaudhari AS , Heckel R . Measuring Robustness in Deep Learning Based Compressive Sensing . Cornell University arXiv website. https://arxiv.org/abs/2102.06103. Posted February 11, 2021. Accessed September 29, 2022.
- 67. Gong E , Pauly JM , Wintermark M , Zaharchuk G . Deep learning enables reduced gadolinium dose for contrast-enhanced brain MRI . J Magn Reson Imaging 2018. ; 48 ( 2 ): 330 – 340 . [DOI] [PubMed] [Google Scholar]
- 68. Edupuganti V , Mardani M , Vasanawala S , Pauly J . Uncertainty Quantification in Deep MRI Reconstruction . IEEE Trans Med Imaging 2021. ; 40 ( 1 ): 239 – 250 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Clark K , Vendt B , Smith K , et al . The Cancer Imaging Archive (TCIA): maintaining and operating a public information repository . J Digit Imaging 2013. ; 26 ( 6 ): 1045 – 1057 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Knoll F , Zbontar J , Sriram A , et al . fastMRI: A Publicly Available Raw k-Space and DICOM Dataset of Knee Images for Accelerated MR Image Reconstruction Using Machine Learning . Radiol Artif Intell 2020. ; 2 ( 1 ): e190007 . [DOI] [PMC free article] [PubMed] [Google Scholar]







![FAST stroke with contrast MRI in a 65-year-old woman with a history of endometrial carcinoma and strokelike symptoms. She presented to the emergency department with right leg weakness. Owing to her history of endometrial carcinoma, cerebral metastases were suspected and FAST stroke MRI with contrast was performed. (A–C) While there were no metastases, axial diffusion-weighted imaging (DWI) at b = 1000 (A), apparent diffusion coefficient (ADC) mapping (B), and FLAIR imaging (C) demonstrated a punctate recent infarct (arrowhead in A) with signs of adjacent older subcortical gliosis in the deep watershed region of the left parieto-occipital junction (arrow in B and C). (D) At three-dimensional phase-contrast angiography (3D PCA), the proximal segment of the cervical internal carotid artery has a flow gap (ellipse), suggesting critical stenosis. This finding highlights the importance of extending the field of view at 3D PCA so that it covers the cervical carotid bifurcations. (E, F) Perfusion-weighted MR images processed with RAPID software (iSchemaView) show extensive hypoperfusion of the left hemisphere (increased time-to-maximum [Tmax] ) in association with reduced cerebral blood volume (CBV) and cerebral blood flow (CBF) in the parieto-occipital region (arrows in F). These findings are compatible with old and new border zone infarcts. MTT = mean transit time.](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/dd82/10262597/c315cc6106cd/rg.220147.fig2.jpg)