Abstract
Background:
Helicobacter pylori (H. pylori) is a group 1 carcinogen and the etiological agent of gastric diseases such as gastritis, ulcers, and gastric cancer. It infects approximately half of the world’s population. Risk factors associated with H. pylori infection include socioeconomic status, lifestyle, and diet.
Objectives:
This study aimed to evaluate the association between eating habits and H. pylori infection in patients from a reference hospital in Central Brazil.
Design:
This cross-sectional study included 156 patients from 2019 to 2022.
Methods:
Data were collected using a structured questionnaire on sociodemographic and lifestyle characteristics and a validated food frequency questionnaire. The H. pylori infection status (positive versus negative) was determined using the histopathological method. After grams/day, foods were stratified into tertiles of consumption (low, medium, and high). Simple and multiple binary logistic regression models were used in the analysis of odds ratios (ORs) and their respective 95% confidence intervals (CIs), with a 5% significance level.
Results:
The prevalence of H. pylori infection was 44.2% (69/156 patients). Infected individuals had a mean age of 49.6 ± 14.6 years; 40.6% were men, 34.8% were aged 60 years or older, 42.0% were unmarried, 7.2% had higher education, 72.5% were non-white, and 30.4% were obese. In the H. pylori-positive group, 55.1% were alcohol drinkers and 42.0% were smokers. The results of multiple analyses showed that the chance of H. pylori infection was higher among male participants (OR = 2.25; CI = 1.09–4.68) and individuals with obesity (OR = 2.68; CI = 1.10–6.51). Participants with moderate consumption of refined grains (bread, cookies, cakes, breakfast cereal) (OR = 2.41; CI = 1.04–5.62) and fruits (OR = 2.53; CI = 1.08–5.94) were more likely to be infected.
Conclusion:
In this study, male sex, obesity, and the consumption of refined grains and fruits were positively associated with H. pylori infection. Further research is needed to investigate this association and elucidate the underlying mechanisms.
Keywords: dietary habits, Helicobacter pylori, Midwest Brazil, risk factors
Background
Helicobacter pylori (H. pylori) is one of the most prevalent human pathogens, affecting half of the world’s population. Prevalence can vary significantly by geographic region and is the highest in developing countries. 1 The prevalence of H. pylori infection in Latin America and the Caribbean is estimated to be 69.26% in adults. 2 Brazil is the largest country in Latin America and has regional differences in the quality of life and health. This reflects a great variation in the prevalence of H. pylori infection, which is higher in the north than in the south of the country.3,4 In the Midwest region, a prevalence of 69.2% and 61.1% has been reported.5,6
Chronic infection caused by H. pylori can lead to gastrointestinal diseases such as gastritis, peptic ulcer, mucosa-associated lymphoid tissue lymphoma, and gastric adenocarcinoma (GAd). 7 Since 1994, H. pylori has been recognized as a group 1 carcinogen by the International Agency for Research on Cancer. 8 In addition, H. pylori-related extragastric manifestations have been reported in the scientific literature. Although for several of these supposed associations, the potential pathogenic mechanism remains obscure, the eradication of H. pylori is already a well-established therapeutic alternative for iron deficiency anemia, primary immune thrombocytopenia, and vitamin B12 deficiency.9–11
The main known risk factors for H. pylori infection include conditions related to low socioeconomic status such as poor sanitation, inadequate hygiene practices, overcrowding, and intrafamily grouping. In addition, diet is an important risk factor for infection. 12
Frequent consumption of fresh fruits and vegetables has been associated with protection against H. pylori infection. 13 In addition, a diet consisting of a high intake of whole grains, roots and tubers, vegetables, mushrooms, various beans, vegetable oils, nuts, and seeds is associated with a decreased risk of infection. 14 These associations may be attributed to the properties of some compounds present in these foods such as vitamin C, polyphenols, and flavonoids. These compounds may protect the gastric mucosa and inhibit the colonization of bacteria. 15
In contrast, a diet high in carbohydrates, sweets, sausages, hamburgers, mayonnaise, and soft drinks was positively associated with H. pylori infection.16,17 Furthermore, a dietary pattern characterized by a high consumption of refined grains, pickled vegetables, bacon, salted fish, salted pickled eggs, processed and cooked meat, wine, and tea is associated with an increased risk of the infection. 14
Brazil, especially in the Midwest region, has a high prevalence of H. pylori infection. Since the presence of this infection can be influenced by diet, it is important to know the foods that act as protective factors as well as those that may favor infection. However, data on the association between dietary habits and H. pylori infection in this region are limited. Therefore, this study aimed to evaluate the association between eating habits and H. pylori infection in patients admitted to a reference hospital in the Brazilian Midwest.
Methods
Study design and participants
This was a cross-sectional study conducted from March 2019 to February 2022 at a referral hospital located in Central Brazil. Participants were recruited from the Department of Digestive Tracts.
Dyspeptic patients of both sexes aged between 18 and 75 years who underwent upper digestive endoscopy were included. Individuals who did not have an available histopathology report, those with cancer, or those without physical and/or psychological conditions to participate in the study were excluded.
Ethical aspects
The reporting of this study conforms to the STROBE statement. 18
Data collection and evaluation
Data were collected using structured questionnaires completed during an interview and transferred online to the Research Electronic Data Capture (REDCap™) EPGC version 7.4.4.
A food frequency questionnaire (FFQ), validated in Brazilian populations with patients treated for colorectal cancer and patients from a case–control study of GAd, was administered during the interview.19,20 The FFQ contained more than 120 food items, and each question was divided into three categories: (1) consumption frequency ranging from 1 to 10; (2) the unit of consumption (daily, weekly, monthly, or yearly); and (3) the size of the portion eaten (small, medium, or large) which were represented in grams or milliliters and their equivalents in home measures, for example teacups, cups, tablespoons, slices, among others. The responses were converted to food intake (grams per day or milliliters per day if applicable). The foods were selected and categorized according to Table 1, based on similar characteristics and nutritional content.
Table 1.
Food groups analyzed between 2019 and 2022 in Goiania, Goiás, Brazil.
| Refined grains | French bread and flatbread; cookies without filling (sweet and salty); filled cookies, wafer, and buttery; simple cake; filled cake; breakfast cereal |
| Milk and dairy products | Milk; yogurt (natural, with fruit or diet); mozzarella cheese, queijo prato, parmesan and provolone; cheese mines, ricotta; Fruit smoothie |
| Fruit | Orange and tangerine, banana, apple and pear, papaya, melon and watermelon, pineapple, mango, avocado, guava, kaki, grape, berries (strawberry, blackberry, raspberry, blueberry), cupuaçu, bacuri, pupunha, graviola |
| Cereals and legumes | Beans (carioca, purple, black, green), Feijoada and Tropeiro’s beans, lentil, white rice cooked with oil and seasonings, brown rice cooked with oil and seasonings, oat |
| Vegetables | Lettuce, spinach and endive, watercress and rocket, tomato, kale, cabbage, cauliflower and broccoli, cariru, jambu, carrot, eggplant, beetroot, chayote, pumpkin, cucumber, zucchini, onion |
| Salted and processed meats | Beef jerky and sun-dried meat; bacon; sausages; processed meats (ham, mortadella, salami); nuggets; meatballs, hamburgers; canned meat; salted/dried shrimp; hot dog, sandwich, and hamburger |
| Red meat and offal | Roasted beef/cooked/stewed meat, fried meat, meat with vegetables, pork (loin, beef), offal (beef or chicken), barbecue |
| White meats | Roasted/boiled/stewed chicken, fried chicken, roasted/boiled/stewed fish, fried fish, pirarucu and other salted fish |
| Sweets | Candies and lollipops; whipped cream, coconut milk and condensed milk; chocolate and bonbons; fruit candy; gelatin; confectionary sweets (pies, puddings, mousses); ice cream; chocolate powder |
| Sugar-sweetened beverages | Industrialized juice, natural juice, soda, soy-based beverage, cupuaçu natural juice, bacuri natural juice, graviola natural juice |
| Hot beverages | Coffee, tea |
| Alcoholic beverages | Beer, wine, caipirinha and distilled drinks |
During the interviews, a questionnaire regarding sociodemographic data and lifestyle habits was administered. In this study, data were collected regarding body mass index (BMI), age, marital status, ethnicity, level of education, and alcohol and tobacco consumption.
BMI was categorized according to the World Health Organization guidelines, which is calculated by dividing the weight (kg) by the square of the height (m). The adult population was categorized into underweight (>18.5 kg/m2), normal weight (18.5–24.9 kg/m2), overweight (25.0–29.9 kg/m2), and obese (⩾30.0 kg/m2); and the elderly population (60 years and older) into underweight (<23 kg/m2), normal weight (23–27.9 kg/m2), overweight (28–29.9 kg/m2), and obese (⩾30 kg/m2).21,22
Marital status was categorized into married and unmarried (single, widowed, divorced/separated). 23 For ethnicity, two categories were created: white and non-white (black, brown). 24 Educational attainment was categorized into <12 years (illiterate, less than 5 years, 6–8 years, and 9–11 years), 12 years (high school), and ⩾13 years (undergraduate and graduate). 23
Diagnosis of H. pylori infection
H. pylori status (positive versus negative) was determined by histopathological examination, which is considered the gold standard, with a sensitivity and specificity of approximately 94%. 25 Reports of the histopathological examinations performed at the Department of Digestive Tract were accessed. The examinations were carried out using biopsies of the gastric body and antrum stained with hematoxylin–eosin and Giemsa staining methods.
Statistical analyses
The variables are described using absolute and relative frequencies, and measures of central tendency and dispersion. After classification in grams per day, foods were stratified into tertiles of consumption (low, medium, and high). The chi-square test or Fisher’s exact test was used for association analysis of categorical variables when appropriate and Student’s t-test for continuous variables.
Simple and multiple binary logistic regression models were used to analyze odds ratios (ORs) and their respective 95% confidence intervals (CI). In the construction of the multiple model, simple regression analysis were conducted employing a p value of ⩽0.20 as an entry criterion in the modelling process. A stepwise forward methodology was used to select the most appropriate model. The Hosmer–Lemeshow test was used to verify the adequacy of the model.
The significance level adopted was 5%. All statistical analysis were performed using IBM Statistical Package for the Social Science (IBM SPSS) version 25.0.
Results
A total of 218 individuals were recruited; however, 62 individuals were excluded because they did not meet the eligibility criteria. After exclusion, 156 participants were eligible (Figure 1).
Figure 1.
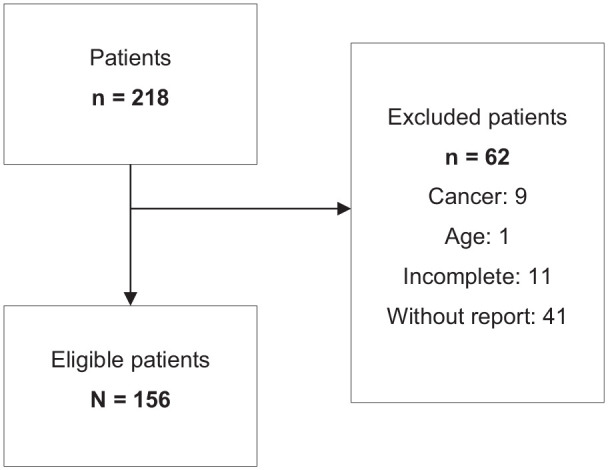
Flowchart of participant eligibility for the study conducted during the period 2019–2022, in Goiania, Goiás, Brazil.
In this study, the prevalence of H. pylori infection was 44.2% (69/156). Infected individuals had a mean age of 49.6 ± 14.6 years; 40.6% were men, 34.8% were 60 years or older, 42.0% were unmarried, 7.2% had higher education, 72.5% were non-white, and 30.4% were obese (Table 2).
Table 2.
Sociodemographic characteristics and lifestyle habits of the study participants from 2019 to 2022 in Goiania, Goiás, Brazil.
| Characteristics |
H. pylori positive n = 69 (44.2%) |
H. pylori negative n = 87 (55.8%) |
p Value a |
|---|---|---|---|
| Sex | |||
| Female | 41 (59.4) | 63 (72.4) | 0.124 |
| Male | 28 (40.6) | 24 (27.6) | |
| Age (years) | |||
| ⩽45 | 27 (39.1) | 37 (42.5) | 0.721 |
| 46–59 | 18 (26.1) | 25 (28.7) | |
| ⩾60 | 24 (34.8) | 25 (28.7) | |
| Marital status | |||
| Married | 40 (58.0) | 52 (59.8) | 0.950 |
| Not married | 29 (42.0) | 35 (40.2) | |
| Level of education | |||
| <12 years | 40 (58.0) | 52 (59.8) | 0.937 |
| 12 years | 24 (34.8) | 28 (32.2) | |
| ⩾13 years | 5 (7.2) | 7 (8.0) | |
| Ethnicity | |||
| White | 19 (27.5) | 21 (24.1) | 0.766 |
| Non-white | 50 (72.5) | 66 (75.9) | |
| BMI (kg/m2) | |||
| Underweight and eutrophic | 28 (40.6) | 42 (48.3) | 0.152 |
| Overweight | 20 (29.0) | 30 (34.5) | |
| Obesity | 21 (30.4) | 15 (17.2) | |
| Alcohol | |||
| Yes | 38 (55.1) | 39 (44.8) | 0.267 |
| No | 31 (44.9) | 48 (55.2) | |
| Tobacco | |||
| Yes | 29 (42.0) | 33 (37.9) | 0.723 |
| No | 40 (58.0) | 54 (62.1) | |
Pearson’s chi-square test.
BMI, body mass index.
Non-infected individuals had a mean age of 48.2 ± 15.7 years; 27.6% were men, 28.7% were 60 years or older, 40.2% were unmarried, 8.0% had higher education, 75.9% were non-white, and 17.2% were obese. There was no significant difference between the positive and negative groups for bacterial infection regarding the sociodemographic variables evaluated (Table 2).
In the H. pylori-positive group, 55.1% were alcohol drinkers and 42.0% were smokers. In the negative group, the frequencies of individuals who consumed alcohol and tobacco were 44.8% and 37.9%, respectively. There was no difference in lifestyle habits between the groups (Table 2).
Regarding food consumption, there was no difference between infected and non-infected individuals for the intake of the food groups refined grains, milk and dairy products, fruits, cereals and legumes, vegetables, sweets, salted and processed meats, red meats and offal, white meats, sugar-sweetened beverages, hot beverages, and alcoholic beverages (Table 3).
Table 3.
Dietary habits of the study participants from 2019 to 2022 in Goiania, Goiás, Brazil.
| Food groups |
H. pylori positive n = 69 (44.2%) |
H. pylori negative n = 87 (55.8%) |
p Value |
|---|---|---|---|
| Refined grains (g/day) | |||
| ⩽43.04 | 17 (24.6) | 35 (40.2) | 0.089 a |
| 43.05–83.87 | 28 (40.6) | 24 (27.6) | |
| ⩾83.88 | 24 (34.8) | 28 (32.2) | |
| Milk and dairy products (g/day) | |||
| ⩽37.25 | 19 (27.5) | 33 (37.9) | 0.363 a |
| 37.26–132.52 | 26 (37.7) | 26 (29.9) | |
| ⩾132.53 | 24 (34.8) | 28 (32.2) | |
| Fruit (g/day) | |||
| ⩽105.97 | 19 (27.5) | 33 (37.9) | 0.056 a |
| 105.98–235.18 | 30 (43.5) | 22 (25.3) | |
| ⩾235.19 | 20 (29.0) | 32 (36.8) | |
| Cereals and legumes (g/day) | |||
| ⩽240.30 | 25 (36.2) | 27 (31.0) | 0.679 a |
| 240.31–426.58 | 20 (29.0) | 24 (27.6) | |
| ⩾426.59 | 24 (34.8) | 36 (41.4) | |
| Vegetables (g/day) | |||
| ⩽78.24 | 23 (33.3) | 28 (32.2) | 0.985 a |
| 78.25–132.47 | 23 (33.3) | 29 (33.3) | |
| ⩾132.48 | 23 (33.3) | 30 (34.5) | |
| Sweets (g/day) | |||
| ⩽13.62 | 22 (31.9) | 29 (33.3) | 0.658 a |
| 13.63–47.87 | 26 (37.7) | 27 (31.0) | |
| ⩾47.88 | 21 (30.4) | 31 (35.6) | |
| Salted and processed meats (g/day) | |||
| ⩽8.57 | 21 (30.4) | 33 (37.9) | 0.607 a |
| 8.58–39.28 | 23 (33.3) | 27 (31.0) | |
| ⩾39.29 | 25 (36.2) | 27 (31.0) | |
| Red meats and offal (g/day) | |||
| ⩽78.01 | 25 (36.2) | 27 (31.0) | 0.732 a |
| 78.02–132.29 | 23 (33.3) | 29 (33.3) | |
| ⩾132.30 | 21 (30.4) | 31 (35.6) | |
| White meats (g/day) | |||
| ⩽24.17 | 20 (29.0) | 32 (36.8) | 0.227 a |
| 24.18–57.86 | 28 (40.6) | 24 (27.6) | |
| ⩾57.87 | 21 (30.4) | 31 (35.6) | |
| Sugar-sweetened beverages (ml/day) | |||
| ⩽102.86 | 22 (31.9) | 33 (37.9) | 0.646 a |
| 102.87–274.29 | 22 (31.9) | 28 (32.2) | |
| ⩾274.30 | 25 (36.2) | 26 (29.9) | |
| Hot beverages (ml/day) | |||
| ⩽98.57 | 22 (31.9) | 31 (35.6) | 0.780 a |
| 98.58–229.74 | 22 (31.9) | 29 (33.3) | |
| ⩾229.75 | 25 (36.2) | 27 (31.0) | |
| Alcoholic beverages (ml/day) | |||
| 0 | 57 (65.5) | 40 (58.0) | 0.339 b |
| 0.01–3.83 | 5 (5.7) | 2 (2.9) | |
| ⩾3.84 | 25 (28.7) | 27 (39.1) | |
Pearson’s chi-square test.
Fisher’s exact test.
In the univariate analysis, sociodemographic and lifestyle variables were not associated with the presence of H. pylori infection (Table 4).
Table 4.
Univariate logistic regression analysis of H. pylori infection, sociodemographic characteristics, and lifestyle habits from 2019 to 2022 in Goiania, Goiás, Brazil.
| Characteristics | Univariate OR (95% CI) |
p Value |
|---|---|---|
| Sex | ||
| Female | 1 | |
| Male | 1.79 (0.91–3.51) | 0.089 |
| Age (years) | ||
| ⩽45 | 1 | |
| 46–59 | 0.98 (0.45–2.15) | 0.973 |
| ⩾60 | 1.31 (0.62–2.78) | 0.472 |
| Marital status | ||
| Not married | 1 | |
| Married | 0.92 (0.48–1.76) | 0.821 |
| Level of education | ||
| <12 years | 1 | |
| 12 years | 1.11 (0.56–2.20) | 0.756 |
| ⩾13 years | 0.92 (0.27–3.14) | 0.905 |
| Ethnicity | ||
| White | 1 | |
| Non-white | 0.83 (0.40–1.72) | 0.629 |
| BMI (kg/m2) | ||
| Underweight and eutrophic | 1 | |
| Overweight | 1.00 (0.47–2.09) | 1.000 |
| Obesity | 2.10 (0.92–4.75) | 0.075 |
| Alcohol | ||
| No | 1 | |
| Yes | 1.50 (0.79–2.84) | 0.205 |
| Tobacco | ||
| No | 1 | |
| Yes | 1.18 (0.62–2.26) | 0.604 |
BMI, body mass index; CI, confidence interval; OR, odds ratio.
Regarding eating habits, in the univariate analysis, individuals with a moderate consumption of refined grains had a higher chance of being infected with H. pylori (OR = 2.40; 95% CI = 1.08–5.32). Patients with moderate fruit consumption also had a higher chance of being infected with H. pylori (OR = 2.36; 95% CI = 1.07–5.20). The consumption of milk and dairy products, cereals and legumes, vegetables and legumes, sweets, salted and processed meats, red meats and offal, white meats, sugar-sweetened beverages, hot beverages, and alcoholic beverages was not associated with H. pylori infection (Table 5).
Table 5.
Univariate logistic regression analysis of H. pylori infection and eating habits from 2019 to 2022 in Goiania, Goiás, Brazil.
| Food groups | Univariate OR (95% CI) |
p Value |
|---|---|---|
| Refined grains (g/day) | ||
| ⩽43.04 | 1 | |
| 43.05–83.87 | 2.40 (1.08–5.32) | 0.031 |
| ⩾83.88 | 1.76 (0.79–3.91) | 0.162 |
| Milk and dairy products (g/day) | ||
| ⩽37.25 | 1 | |
| 37.26–132.52 | 1.73 (0.79–3.80) | 0.167 |
| ⩾132.53 | 1.48 (0.67–3.26) | 0.320 |
| Fruit (g/day) | ||
| ⩽105.97 | 1 | |
| 105.98–235.18 | 2.36 (1.07–5.20) | 0.032 |
| ⩾235.19 | 1.08 (0.49–2.40) | 0.840 |
| Cereals and legumes (g/day) | ||
| ⩽240.30 | 1 | |
| 240.31–426.58 | 0.90 (0.40–2.01) | 0.798 |
| ⩾426.59 | 0.72 (0.34–1.52) | 0.391 |
| Vegetables (g/day) | ||
| ⩽78.24 | 1 | |
| 78.25–132.47 | 0.96 (0.44–2.10) | 0.929 |
| ⩾132.48 | 0.93 (0.43–2.02) | 0.861 |
| Sweets (g/day) | ||
| ⩽13.6 | 1 | |
| 13.63–47.87 | 1.26 (0.58–2.74) | 0.545 |
| ⩾47.88 | 0.89 (0.40–1.95) | 0.777 |
| Salted and processed meats (g/day) | ||
| ⩽8.57 | 1 | |
| 8.58–39.28 | 1.33 (0.61–2.92) | 0.464 |
| ⩾39.29 | 1.45 (0.67–3.14) | 0.341 |
| Red meats and offal (g/day) | ||
| ⩽78.01 | 1 | |
| 78.02–132.29 | 0.85 (0.39–1.85) | 0.694 |
| ⩾132.30 | 0.73 (0.33–1.59) | 0.430 |
| White meats (g/day) | ||
| ⩽24.17 | 1 | |
| 24.18–57.86 | 1.86 (0.85–4.07) | 0.117 |
| ⩾57.87 | 1.08 (0.49–2.38) | 0.841 |
| Sugar-sweetened beverages (ml/day) | ||
| ⩽102.86 | 1 | |
| 102.87–274.29 | 1.17 (0.54–2.56) | 0.678 |
| ⩾274.30 | 1.44 (0.66–3.11) | 0.351 |
| Hot beverages (ml/day) | ||
| ⩽98.57 | 1 | |
| 98.58–229.74 | 1.06 (0.49–2.32) | 0.867 |
| ⩾229.75 | 1.30 (0.60–2.82) | 0.499 |
| Alcoholic beverages (ml/day) | ||
| 0 | 1 | |
| 0.01–3.83 | 0.57 (0.10–3.08) | 0.514 |
| ⩾3.84 | 1.53 (0.78–3.03) | 0.212 |
CI, confidence interval; OR, odds ratio.
Bold values are statistically significant.
In the multiple logistic regression analysis, male individuals had a higher chance of being infected with H. pylori (OR = 2.25; 95% CI = 1.08–4.67). Participants with obesity also had a higher chance of being infected (OR = 2.67; 95% CI = 1.10–6.51), compared to underweight and eutrophic individuals. Regarding dietary habits, participants with moderate consumption of refined grains had a higher chance of being infected with H. pylori (OR = 2.41; 95% CI = 1.03–5.617). Individuals with moderate fruit consumption also had a higher chance of being infected with H. pylori (OR = 2.53; 95% CI = 1.08–5.93) (Table 6).
Table 6.
Multiple logistic regression analysis of H. pylori infection, sociodemographic characteristics and eating habits from 2019 to 2022 in Goiania, Goiás, Brazil.
| Variables | Adjusted OR (95% CI) |
p Value |
|---|---|---|
| Sex | ||
| Female | 1 | |
| Male | 2.25 (1.08–4.67) | 0.029 |
| BMI (kg/m2) | ||
| Underweight and eutrophic | 1 | |
| Overweight | 1.10 (0.50–2.42) | 0.813 |
| Obesity | 2.67 (1.10–6.51) | 0.030 |
| Refined grains (g/day) | ||
| ⩽43.04 | 1 | |
| 43.05–83.87 | 2.41 (1.03–5.61) | 0.041 |
| ⩾83.88 | 1.49 (0.64–3.46) | 0.351 |
| Fruit (g/day) | ||
| ⩽105.97 | 1 | |
| 105.98–235.18 | 2.53 (1.08–5.93) | 0.032 |
| ⩾235.19 | 1.17 (0.48–2.81) | 0.721 |
BMI, body mass index; CI, confidence interval; OR, odds ratio.
Bold values are statistically significant.
Discussion
In this study, the prevalence of H. pylori infection was 44.2%. This value is considered high when compared with data from developed countries such as Switzerland and Australia, which have prevalence rates of 18.9% and 24.6%, respectively. 26 A recent meta-analysis reported a prevalence of 69.26% in adults in Latin America and the Caribbean. 2 In Brazil, the prevalence is heterogeneous in different regions, with proportions of 31.7%, 61.1%, and 91% in the Southeast, Midwest, and North regions, respectively.3,4,6 The prevalence of infection depends on different factors, and it is necessary to consider that these values may have been influenced by the living conditions of the population, different diagnostic methods, genetic characteristics of patients and bacteria, and lifestyle habits, among others.
Male individuals were more likely to be infected with H. pylori. Some studies have found no relationship between sex and infection.1,2 However, the results of this study corroborate a robust meta-analysis that showed a male predominance in the prevalence of H. pylori in adult populations from all continents. 27 Differences in sex in the prevalence of H. pylori infection among adults may be explained by distinct exposure to environmental factors such as smoking, which has been associated in the literature with an increased risk of H. pylori infection. 28 It has also been hypothesized that physiological differences, especially sex hormones, may affect immunity and inflammatory responses to H. pylori differently in males and females. These hormones may interfere directly or indirectly with cell receptors, thereby altering the immune response. 29
In this study, obesity was found to increase the risk of H. pylori infection. However, data on the association between this infection and obesity are conflicting. Some studies have suggested an inverse association between H. pylori prevalence and obesity. 30 Consistent with the findings of the present study, meta-analyses have indicated a positive correlation between the risk of H. pylori infection and prevalence of obesity.31,32 Furthermore, a cohort study demonstrated that H. pylori infection was positively associated with increased BMI regardless of socioeconomic status and other confounding factors. 33
The mechanisms involved in the relationship between H. pylori infection and obesity are not well established. However, it has been suggested that regulation of the gastrointestinal hormones ghrelin and leptin may be involved. Individuals positive for H. pylori have lower plasma leptin levels. The reduction in this hormone delays the feeling of satiety, favoring obesity. 34 Low ghrelin levels have also been identified in H. pylori-infected obese patients. The reduced production of this hormone may be the result of atrophic gastritis. 35 Another mechanism that may contribute to the development of obesity in H. pylori-infected individuals is increased insulin resistance identified in these patients. 36 In addition, impaired immune function of the gastrointestinal tract in the obese population may favor H. pylori survival. 37 Few studies have investigated the frequency of H. pylori virulence factors in the obese patients. A recent observational study revealed a high prevalence of vacA and cagE followed by cagA, dupA, iceA1, oipA, and babA2 genotypes in H. pylori strains isolated from obese patients diagnosed with gastric ulcer, duodenal ulcer, and gastric cancer. 38
Moderate consumption of the ‘refined grains’ food group, characterized by breads, cakes, cookies, and breakfast cereal, was positively associated with H. pylori infection. These foods are rich in carbohydrates with a high glycemic index and are present in the diet of Brazilians. 39 Different studies have also shown a positive relationship between the intake of breads, refined grain products, and carbohydrates in the diet and the risk of H. pylori infection.16,17,40 In addition, Sohouli et al. showed that a high glycemic load and a high glycemic index in the diet significantly increased the risk of H. pylori infection. 40
The intake of carbohydrates and acquisition of H. pylori infection may be related to the effect of these compounds on blood glucose levels. High blood glucose levels can lead to the development of enteric neuropathy, increasing the production of pro-inflammatory cytokines, and eventually causing neurodegeneration. Consequently, delayed gastric emptying, decreased acid secretion, and increased bacterial colonization favor H. pylori infection. 41 In addition, several recent studies have shown an association between H. pylori infection and metabolic syndrome. 36 Other studies have reported a higher prevalence of this infection in individuals with diabetes. 42 Considering that carbohydrate intake is associated with metabolic syndrome and diabetes, these findings reinforce a possible link between carbohydrates and H. pylori infection. 43
Moderate fruit consumption was positively associated with H. pylori infection. This result contrasts with studies demonstrating that fruit consumption has a protective effect against this bacterium. 13 However, it is worth considering that the consumption of raw fruits and vegetables, often irrigated by water contaminated by feces, has been associated with H. pylori transmission.44,45 Bacteria can form a biofilm, which favors the persistence of microorganisms in the environment for long periods. Moreover, the cells in biofilms are highly resistant to elimination by sanitizing agents. Thus, raw vegetables can potentially serve as vehicles for the transmission of this pathogen. 46
Food can also be contaminated with H. pylori owing to poor hygiene practices during processing. Food handling can contribute to contamination via hands, nails, and oral and nasal secretions. Previous studies have shown that some types of raw ready-to-eat foods, such as fruit salad, can be sources of resistant and virulent H. pylori strains. 47
Some limitations of this study should be considered when interpreting these results. The sample comprised individuals who sought health care; these patients are probably more likely to have been diagnosed with H. pylori and thus changed their eating habits. Owing to the nature of the self-reported questionnaire, there is information and memory bias, and the amount of food intake may not be accurate. Another limiting factor was the lack of information regarding the previous treatment of the infection. Finally, the cross-sectional design precludes inference of causality. However, this study has several strengths. This is the first study to examine the association between H. pylori infection and dietary habits in this region. A previously validated FFQ (food frequency questionnaire) was used to assess dietary intake.19,20 In addition, the method used to assess the presence of infection is considered the gold standard. 25
Conclusion
The prevalence of H. pylori infections was 44.2%. Males and obese individuals are more likely to be infected with H. pylori. The findings of this study indicated that dietary habits influence the presence of H. pylori infection. Consumption of refined grains increases the chance of infection, which may reinforce the role of H. pylori in metabolic diseases. Interestingly, fruit consumption also had a positive association with the presence of bacteria, probably because of its role as a vehicle for transmission of this pathogen. Further research is required to elucidate the mechanisms underlying the associations identified in this study.
Acknowledgments
None.
Footnotes
ORCID iD: Mônica Santiago Barbosa  https://orcid.org/0000-0001-6964-5219
https://orcid.org/0000-0001-6964-5219
Contributor Information
Giovana Alice Sampaio Soares, Núcleo de Estudo da Helicobacter pylori, Department of Biosciences and Biotechnology, Institute of Tropical Pathology and Public Health, Federal University of Goiás, Goiânia, GO, Brazil.
Felipe Augusto de Sousa Moraes, Núcleo de Estudo da Helicobacter pylori, Department of Biosciences and Biotechnology, Institute of Tropical Pathology and Public Health, Federal University of Goiás, Goiânia, GO, Brazil.
Amanda Ferreira Paes Landim Ramos, Núcleo de Estudo da Helicobacter pylori, Department of Biosciences and Biotechnology, Institute of Tropical Pathology and Public Health, Federal University of Goiás, Goiânia, GO, Brazil.
Silvana Barbosa Santiago, Núcleo de Estudo da Helicobacter pylori, Department of Biosciences and Biotechnology, Institute of Tropical Pathology and Public Health, Federal University of Goiás, Goiânia, GO, Brazil.
Janaina Naiara Germano, A.C. Camargo Cancer Center, São Paulo, SP, Brazil.
Gisele Aparecida Fernandes, A.C. Camargo Cancer Center, São Paulo, SP, Brazil.
Maria Paula Curado, A.C. Camargo Cancer Center, São Paulo, SP, Brazil.
Mônica Santiago Barbosa, Núcleo de Estudo da Helicobacter pylori, Department of Biosciences and Biotechnology, Institute of Tropical Pathology and Public Health, Federal University of Goiás, St. 235 Setor Leste Universitario, Goiânia, GO 74605-050, Brazil.
Declarations
Ethics approval and consent to participate: This study was approved by the Research Ethics Committees of Fundação Antônio Prudente – A.C. Camargo Cancer Center, consolidated opinion 3.174.666 (CAAE:53166915.9.1001.5432), and Hospital das Clínicas da Universidade Federal de Goiás, consolidated opinion 3.005.621 (CAAE:53166915.9.3003.5078). Written informed consent was obtained from all individuals who agreed to participate in the study.
Consent for publication: Written informed consent was obtained from all individuals who agreed to participate in the study.
Author contribution(s): Giovana Alice Sampaio Soares: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Resources; Visualization; Writing – original draft; Writing – review & editing.
Felipe Augusto de Sousa Moraes: Data curation; Investigation; Methodology; Resources; Writing – review & editing.
Amanda Ferreira Paes Landim Ramos: Investigation; Methodology; Resources; Writing – review & editing.
Silvana Barbosa Santiago: Investigation; Methodology; Resources; Writing – review & editing.
Janaina Naiara Germano: Data curation; Formal analysis; Methodology; Resources; Software; Validation; Writing – review & editing.
Gisele Aparecida Fernandes: Supervision; Validation; Writing – review & editing.
Maria Paula Curado: Conceptualization; Funding acquisition; Investigation; Methodology; Project administration; Writing – review & editing.
Mônica Santiago Barbosa: Conceptualization; Investigation; Methodology; Project administration; Supervision; Validation; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Fundação de Amparo à Pesquisa do Estado de São Paulo [grant number 2014/26897-0].
The authors declare that there is no conflict of interest.
Availability of data and materials: Not applicable.
References
- 1.Zamani M, Abramabad F, Zamani V, et al. Systematic review with meta-analysis: the worldwide prevalence of Helicobacter pylori infection. Aliment Pharmacol Ther 2018; 47: 868–876. [DOI] [PubMed] [Google Scholar]
- 2.Curado MP, de Oliveira MM, de Araújo Fagundes M.Prevalence of Helicobacter pylori infection in Latin America and the Caribbean populations: a systematic review and meta-analysis. Cancer Epidemiol 2019; 60: 141–148. [DOI] [PubMed] [Google Scholar]
- 3.Vinagre ID, Queiroz AL, Silva Júnior MR, et al. Helicobacter pylori infection in patients with different gastrointestinal diseases from northern Brazil. Arq Gastroenterol 2015; 52: 266–271. [DOI] [PubMed] [Google Scholar]
- 4.Rodrigues MF, Guerra MR, Alvarenga AVR, et al. Helicobacter pylori infection and gastric cancer precursor lesions: prevalence and associated factors in a reference laboratory in southeastern Brazil. Arq Gastroenterol 2019; 56: 419–424. [DOI] [PubMed] [Google Scholar]
- 5.Barbosa MS, Ramos AFPL, Silva LL de L, et al. Helicobacter pylori infection and risk factors in the development of gastroduodenal diseases in a population from the Central-west region of Brazil. Sapiencia 2019; 8: 181–196. [Google Scholar]
- 6.Borges SS, Ramos AFPL, Moraes Filho AV, et al. [Article partial retraction] Prevalence of Helicobacter pylori infection in dyspeptic patients and its association with clinical risk factors for developing gastric adenocarcinoma. Arq Gastroenterol 2019; 56: 66–70. Erratum in: Arq Gastroenterol 2019; 56: 110. [DOI] [PubMed] [Google Scholar]
- 7.de Brito BB, da Silva FAF, Soares AS, et al. Pathogenesis and clinical management of Helicobacter pylori gastric infection. World J Gastroenterol 2019; 25: 5578–5589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schistosomes, liver flukes and Helicobacter pylori. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Lyon, 7–14 June 1994. IARC Monogr Eval Carcinog Risks Hum 1994; 61: 1–241. https://pubmed.ncbi.nlm.nih.gov/7715068/ [PMC free article] [PubMed] [Google Scholar]
- 9.Gravina AG, Zagari RM, De Musis C, et al. Helicobacter pylori and extragastric diseases: a review. World J Gastroenterol 2018; 24: 3204–3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Franceschi F, Covino M, Roubaud Baudron C.Review: Helicobacter pylori and extragastric diseases. Helicobacter 2019; 24: e12636. [DOI] [PubMed] [Google Scholar]
- 11.Malfertheiner P, Megraud F, O’Morain CA, et al. Management of Helicobacter pylori infection-the Maastricht V/Florence Consensus Report. Gut 2017; 66: 6–30. [DOI] [PubMed] [Google Scholar]
- 12.Sjomina O, Pavlova J, Niv Y, et al. Epidemiology of Helicobacter pylori infection. Helicobacter 2018; 23: e12514. [DOI] [PubMed] [Google Scholar]
- 13.Zhang F, Pu K, Wu Z, et al. Prevalence and associated risk factors of Helicobacter pylori infection in the Wuwei cohort of north-western China. Trop Med Int Health 2021; 26: 290–300. [DOI] [PubMed] [Google Scholar]
- 14.Shu L, Zheng PF, Zhang XY, et al. Dietary patterns and Helicobacter pylori infection in a group of Chinese adults ages between 45 and 59 years old: an observational study. Medicine 2019; 98: e14113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ullah H, Di Minno A, Santarcangelo C, et al. Vegetable extracts and nutrients useful in the recovery from Helicobacter pylori infection: a systematic review on clinical trials. Molecules 2021; 26: 2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mard SA, Khadem Haghighian H, Sebghatulahi V, et al. Dietary factors in relation to Helicobacter pylori infection. Gastroenterol Res Pract 2014; 2014: 826910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xia Y, Meng G, Zhang Q, et al. Dietary patterns are associated with Helicobacter pylori infection in Chinese adults: a cross-sectional study. Sci Rep 2016; 6: 32334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Von Elm E, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. PLOS Med 2007; 4: e296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lameza MMS. Validation of a food frequency questionnaire for patients treated for colorectal cancer . Master’s Thesis, Fundação Antônio Prudente, São Paulo, Brazil, 2010. [Google Scholar]
- 20.Peres SV, Silva DRM, Coimbra FJF, et al. Consumption of processed and ultra-processed foods by patients with stomach adenocarcinoma: a multicentric case-control study in the Amazon and southeast regions of Brazil. Cancer Causes Control 2022; 33: 889–898. [DOI] [PubMed] [Google Scholar]
- 21. Obesity: preventing and managing the global epidemic. Report of a WHO consultation . World Health Organ Technical Report Series; 2000; 894: 1–253. https://pubmed.ncbi.nlm.nih.gov/11234459/ [PubMed] [Google Scholar]
- 22.Multicenter survey aging, health and wellbeing in Latin America and the Caribbean (SABE): preliminary report. Meeting of the Advisory Committee on Health Research, 36. Pan American Health Organization, 9–11July2001. [Google Scholar]
- 23.Brazilian Institute of Geography and Statistics. National survey by household sample: summary of indicators 2015. Rio de Janeiro: IBGE, 2016. [Google Scholar]
- 24.Brazilian Institute of Geography and Statistics. National health survey 2013: perception of health status, lifestyles, and chronic diseases - Brazil, major regions and federation units. Rio de Janeiro: IBGE, 2014. [Google Scholar]
- 25.Abadi ATB. Diagnosis of Helicobacter pylori using invasive and noninvasive approaches. J Pathog 2018; 2018: 9064952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hooi JKY, Lai WY, Ng WK, et al. Global prevalence of Helicobacter pylori infection: systematic review and meta-analysis. Gastroenterology 2017; 153: 420–429. [DOI] [PubMed] [Google Scholar]
- 27.Ibrahim A, Morais S, Ferro A, et al. Sex-differences in the prevalence of Helicobacter pylori infection in pediatric and adult populations: systematic review and meta-analysis of 244 studies. Dig Liver Dis 2017; 49: 742–749. [DOI] [PubMed] [Google Scholar]
- 28.Monno R, De Laurentiis V, Trerotoli P, et al. Helicobacter pylori infection: association with dietary habits and socioeconomic conditions. Clin Res Hepatol Gastroenterol 2019; 43: 603–607. [DOI] [PubMed] [Google Scholar]
- 29.Taneja V.Sexual dimorphism, aging and immunity. Vitam Horm 2021; 115: 367–399. [DOI] [PubMed] [Google Scholar]
- 30.Lender N, Talley NJ, Enck P, et al. Review article: associations between Helicobacter pylori and obesity–an ecological study. Aliment Pharmacol Ther 2014; 40: 24–31. [DOI] [PubMed] [Google Scholar]
- 31.Xu X, Li W, Qin L, et al. Relationship between Helicobacter pylori infection and obesity in Chinese adults: a systematic review with meta-analysis. PLoS One 2019; 14: e0221076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baradaran A, Dehghanbanadaki H, Naderpour S, et al. The association between Helicobacter pylori and obesity: a systematic review and meta-analysis of case-control studies. Clin Diabetes Endocrinol 202110;7: 15–. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suki M, Leibovici Weissman Y, Boltin D, et al. Helicobacter pylori infection is positively associated with an increased BMI, irrespective of socioeconomic status and other confounders: a cohort study. Eur J Gastroenterol Hepatol 2018; 30: 143–148. [DOI] [PubMed] [Google Scholar]
- 34.Romo-González C, Mendoza E, Mera RM, et al. Helicobacter pylori infection and serum leptin, obestatin, and ghrelin levels in Mexican schoolchildren. Pediatr Res 2017; 82: 607–613. [DOI] [PubMed] [Google Scholar]
- 35.Nweneka CV, Prentice AM.Helicobacter pylori infection and circulating ghrelin levels - a systematic review. BMC Gastroenterol 2011; 11: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Azami M, Baradaran HR, Dehghanbanadaki H, et al. Association of Helicobacter pylori infection with the risk of metabolic syndrome and insulin resistance: an updated systematic review and meta-analysis. Diabetol Metab Syndr 2021; 13: 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arslan E, Atilgan H, Yavaşoğlu I.The prevalence of Helicobacter pylori in obese subjects. Eur J Intern Med 2009; 20: 695–697. [DOI] [PubMed] [Google Scholar]
- 38.Farsimadan M, Heravi FS, Emamvirdizadeh A, et al. Evaluation of Helicobacter pylori genotypes in obese patients with gastric ulcer, duodenal ulcer, and gastric cancer: an observational study. Dig Dis 2022; 40: 355–361. [DOI] [PubMed] [Google Scholar]
- 39.Brazilian Institute of Geography and Statistics. Family budget survey 2017–2018: analysis of food security in Brazil. Rio de Janeiro: IBGE, 2020. [Google Scholar]
- 40.Sohouli MH, Haghshenas N, Pouladi F, et al. Association between glycemic index and Helicobacter pylori infection risk among adults: a case-control study. Nutrition 2021; 83: 111069. [DOI] [PubMed] [Google Scholar]
- 41.Brown CT, Davis-Richardson AG, Giongo A, et al. Gut microbiome metagenomics analysis suggests a functional model for the development of autoimmunity for type 1 diabetes. PLoS One 2011; 6: e25792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou X, Zhang C, Wu J, et al. Association between Helicobacter pylori infection and diabetes mellitus: a meta-analysis of observational studies. Diabetes Res Clin Pract 2013; 99: 200–208. [DOI] [PubMed] [Google Scholar]
- 43.Schwingshackl L, Hoffmann G, Lampousi AM, et al. Food groups and risk of type 2 diabetes mellitus: a systematic review and meta-analysis of prospective studies. Eur J Epidemiol 2017; 32: 363–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Atapoor S, Safarpoor Dehkordi F, Rahimi E.Detection of Helicobacter pylori in various types of vegetables and salads. Jundishapur J Microbiol 2014; 7: e10013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yahaghi E, Khamesipour F, Mashayekhi F, et al. Helicobacter pylori in vegetables and salads: genotyping and antimicrobial resistance properties. Biomed Res Int 2014; 2014: 757941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ng CG, Loke MF, Goh KL, et al. Biofilm formation enhances Helicobacter pylori survivability in vegetables. Food Microbiol 2017; 62: 68–76. [DOI] [PubMed] [Google Scholar]
- 47.Hemmatinezhad B, Momtaz H, Rahimi E.VacA, cagA, iceA and oipA genotypes status and antimicrobial resistance properties of Helicobacter pylori isolated from various types of ready to eat foods. Ann Clin Microbiol Antimicrob 2016; 15: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]


