Abstract
Purpose: Postoperative physiotherapy in conjunction with pelvic organ prolapse (POP) surgery is still under-investigated and controversial. In this randomized controlled trial, pelvic floor muscle training (PFMT) and abdominal training were compared with a control condition (standard in-hospital treatment). Method: Eighty-one women were randomized to one of three groups. The Prolapse Quality of Life questionnaire, two-dimensional ultrasound, Pelvic Organ Prolapse Quantification System scale, the PERFECT (power, endurance, repetitions, fast contractions, every contraction timed) scheme, electromyography, Sahrmann scale, and pressure biofeedback unit (PBU) were used to measure quality of life (QOL), POP, and pelvic floor and abdominal muscle function. A mixed-model analysis of variance and the Kruskal–Wallis test was used for analysis. Results: Beneficial effects (p < 0.05) were found for the PFMT group – increased power, number of fast contractions, amount of movement, endurance, and Sahrmann and PBU measures – compared with the control group. Abdominal training led to a significant (p < 0.05) increase in bulging and discomfort, number of pelvic floor muscle contractions, and Sahrmann and PBU measures compared with the control condition; both groups showed significantly increased urinary frequency (p < 0.05). Conclusions: Postoperative physiotherapy did not have a beneficial effect on QOL or POP symptoms. PFMT and abdominal training had beneficial effects on pelvic floor muscle function and abdominal muscle measures. Additional abdominal training led to increased symptoms.
Key Words: abdominal muscles, pelvic organ prolapse, postoperative care, quality of life
Abstract
Objectif : la physiothérapie postopératoire, conjuguée à une chirurgie du prolapsus (CP), est encore sous-évaluée et controversée. Le présent essai aléatoire et contrôlé compare la rééducation périnéale et pelvienne (RPP) à l’entraînement abdominal auprès d’un groupe témoin. Méthodologie : les chercheurs ont réparti 81 femmes en trois groupes. Ils ont utilisé le questionnaire sur la qualité de vie liée au prolapsus, l’échographie bidimensionnelle, le système de classification pour quantifier le prolapsus, l’échelle PERFECT (puissance, endurance, répétitions, rapidité des contractions, durée de chaque contraction), l’électromyographie, l’échelle de Sahrmann et l’unité de rétroaction par pression (URP) pour mesurer la qualité de vie (QdV), la CP et la fonction du plancher pelvien et des muscles abdominaux. Ils ont utilisé un modèle mixte d’analyse de variance et le test de Kruskal-Wallis pour procéder à l’analyse. Résultats : les chercheurs ont constaté des effets bénéfiques (p < 0,05) dans le groupe RPP (puissance, nombre de contractions rapides, quantité de mouvements, endurance et mesures de Sahrmann et d’URP) par rapport au groupe témoin. L’entraînement abdominal a suscité une augmentation significative (p < 0,05) du gonflement et de l’inconfort, du nombre de contractions en RPP et des mesures de Sahrmann et d’URP par rapport au groupe témoin. Les deux groupes ont accru la fréquence de leurs mictions de manière significative (p < 0,05). Conclusion : la physiothérapie postopératoire n’avait pas d’effet bénéfique sur la QdV ou les symptômes de la CP. L’entraînement abdominal et la RPP avaient des effets bénéfiques sur la fonction des muscles pelviens et les mesures des muscles abdominaux. Un entraînement abdominal supplémentaire provoquait une augmentation des symptômes.
Mots-clés : : muscles abdominaux, physiothérapie périopératoire, prolapsus génital, qualité de vie
Symptomatic pelvic organ prolapse (POP) has a mean prevalence of 40%–50% in women aged 50–60 years.1 Up to 19% of women with POP may need surgery, and 30% of these women may need follow-up surgery within 2 years.2–4
The pathophysiology of POP is complex, and the health care field still does not understand some aspects; this may result in ineffective treatment choices.5 The aim of surgical management is to correct the prolapse and address the associated symptoms and dysfunction leading to decreased quality of life (QOL).6 However, surgical intervention in the more severe stages of POP does not correct the musculoskeletal impairments that may contribute to it.7 Inflammation, pain, and swelling resulting from abdominal and vaginal surgical procedures may lead to further inhibition of an already poor preoperative pelvic support mechanism.8
The changes in the integrated pelvic floor muscles (PFM) and abdominal muscle function, among other comorbidities and complications (including contextual factors), are unknown and may affect the surgical outcome.4–6 On the basis of conservative treatment outcomes, pelvic floor muscle training (PFMT) programmes can lead to improved function of the PFM and a decrease in the symptoms and signs of POP.6,9–12 The evidence for the effects of postoperative PFMT is, however, still limited and of low quality, and the effects of including light abdominal muscle training with PFMT are still controversial.6,9–12
A recent systematic review found only a few low-quality studies that had investigated perioperative physiotherapy in populations with pelvic floor dysfunction.4 The findings among these studies were inconsistent, and there was still a paucity of data. A structured PFMT programme did not indicate a consistent benefit of the outcomes after surgery. None of these studies had included abdominal muscle training in their interventions.4,13–15
We proposed an alternative hypothesis: that addressing both the PFM and the abdominal muscle groups through an integrated programme might lead to improved outcomes after surgery compared with isolated PFMT. The null hypothesis was that there would be no difference between the groups. This hypothesis was based on the assumption that if an integrated function existed, both these muscle groups might be affected in this population. Our primary aim was to determine the effect of two different postoperative physiotherapy interventions in women who had undergone pelvic floor reconstructive surgery on QOL, symptoms of POP, and PFM and abdominal muscle function over a 6-month period compared with standard care. Our secondary aim was to determine the average effect on pain and exercise adherence.
Methods
Study design
This was a double-blind, three-arm, randomized controlled clinical trial. Ethical clearance was obtained from the Ethics Committee of the Faculty of Health Sciences, University of the Free State (ECUFS No. 25/2012), and the trial was registered with the Pan-African Clinical Trials Registry (PACTR201811741131369). Informed consent and permission to conduct the study were also obtained from the participants as well as from three regional urogynecology clinics with similar socio-demographic characteristics.
The participants were assessed for eligibility by the principal investigator (CB) and the urogynecologist upon their initial visits to the clinics. The eligible participants were stratified and randomly allocated to three groups – PFMT, abdominal muscle training + PFMT, and control – using a computerized list generated by a biostatistician and kept by an independent research assistant who enrolled the participants. The participants were stratified according to age (≤ 65 y or > 65 y), muscle strength (score of ≤ 2 vs. > 2 on the Modified Oxford Scale), previous surgery, and physiotherapy received, and their outcome measures were recorded by the same researchers at baseline before surgery and at 3 months and 6 months after surgery. We, as well as the assessors, were blinded to the participants’ group allocation.
Setting
The assessment, surgery, intervention, and follow-up visits took place at two regional hospitals. All the surgery was performed by the same surgeon, who has more than 10 years’ experience and international qualifications in urogynecology.
Participants
An a priori sample size was estimated using G*Power (Version 3.1.9.7; https://www.psychologie.hhu.de/arbeitsgruppen/allgemeine-psychologie-und-arbeitspsychologie/gpower) and other calculators for an analysis of variance (ANOVA) analysis using fixed, main, and special effects and interactions and an a level of 0.05, power of 0.80, effect size of 0.5, numerator df of 16, and a SD of 3.00 (based on the change in primary outcomes). The estimate was that we needed at least 30 participants per group to account for a 20% dropout rate.13,14,16
Women who were scheduled for pelvic floor reconstructive surgery, who consented to participate in the study, and who were aged 18–75 years were included. Pregnant women, women with Stage IV POP, and women with systemic neuromusculoskeletal or psychosexual disorders were excluded.
Intervention
We conducted a pilot study with six participants who fulfilled the eligibility criteria. The same procedures were followed as for the main study, and no changes were made in methodology or outcome measures. The results of the pilot study were included in the final analysis. The data collection procedures and time frame of the main study are depicted in the CONSORT flow diagram (Figure 1).
Figure 1 .
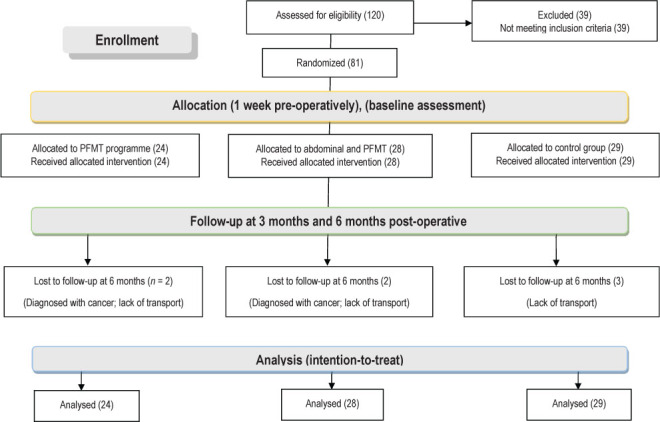
Consolidated Standards of Reporting Trials flow diagram and study outline.
Three women’s health physiotherapists with 5 years’ experience and postgraduate training in this field were independently trained to deliver the intervention according to a predetermined protocol. They were blinded to the group allocation and unaware of the content of the other protocols; each was allocated to one group to ensure blinding. The patients were unaware of the differences in treatment protocols among the groups.
The first measurements and intervention were carried out 1 week before surgery. For the two intervention groups, the intervention consisted of postoperative follow-up visits every second week (12 scheduled appointments, each 45 min long) during which the physiotherapist conducted the reassessment and instructed participants in the individual progression of exercises. Exercise prescription and progression for the intervention groups were patient specific, and dosage was adapted on the basis of the individual assessment findings every second week. The participants then exercised at home until the next follow-up visit. Their compliance and adherence were monitored weekly using a training diary, Likert scale, and telephone reminders by the PI. The control group attended only standard postoperative follow-up visits with the surgeon. These women’s outcome assessments were repeated at 3 months and 6 months (see Figure 1).
The treatment protocols for all three groups are fully described in the online Appendix.
First group: Pelvic floor muscle training
One intervention group received PFMT according to international guidelines in addition to standard in-hospital treatment procedures.17–20 Before surgery, these participants were taught how to produce the correct contractions by using vaginal palpation, ultrasound imaging, and electromyographic (EMG) biofeedback and by observing the inward movement of the perineum and a “squeeze” around the urethra, vagina, and anus.6 They were instructed to do PFMT as prescribed, five times a week, progressively increasing the number of contractions (up to 10), and increasing the holding time until 8–10 repetitions could be done for 10 seconds in the specific position (contraction:rest = 1:1).
Thereafter, they were to progress the exercises by altering the position and decreasing the rest intervals. For example, once they could complete 8–10 repetitions for 10 seconds in supine, they progressed to four-point kneeling, then sitting, and then standing, first in static positions and then with the addition of low-load limb movement. They would also progress the supine exercise by decreasing the rest intervals until the ratio of contraction:rest equaled 1:0.5. If the participants achieved all progressions before 6 months had passed, progression continued by increasing the volume of training by increasing the number of sets performed from one to three.17–20
Second group: Abdominal training + pelvic floor muscle training
The second intervention group received the same intervention as the first, as well as integrated rehabilitation of the abdominal and pelvic stabilizers. A pressure biofeedback unit (PBU) and EMG were used to help activate a correct abdominal contraction,19,21–23 and the exercises were prescribed according to evidence-based principles.19,21–23 The exercise regimen consisted of activation in isolation; co-contraction of the transversus abdominus, pelvic floor, and multifidus muscles 10 times for 10 seconds each; and progression to the sitting and then the standing positions until the same holding times were reached. Further progression included adding low-load limb movement, introducing unstable surfaces, and eventually introducing global stability and strengthening exercises.
Third group: Control
The control group received the standard in-hospital treatment and advice on lifestyle. The advice included postoperative exercises to prevent circulatory and lung complications, as well as precautionary measures regarding activities that could increase intra-abdominal pressure immediately after surgery and advice on the contraction of the PFM. Control participants were offered the same advice as the intervention groups regarding lifestyle and were instructed on performing supported nose blowing, but they were not offered regular follow-up visits and were not provided any practical instructions on or demonstrations of contraction of the PFM.
Instrumentation and outcome measures
Medical and exercise history
The urogynecologist and the PI documented demographic data and medical, gynecological, and exercise history on a self-developed questionnaire. The stage of POP was determined by the Pelvic Organ Prolapse Quantification System scale.24 This scale defines the location of six points (two on the anterior vaginal wall, two in the superior vagina, and two on the posterior vaginal wall), with reference to the plane of the hymen. The measurement was taken with a full bladder, the participant in supine on a hard plinth, and the hips and knees in maximum comfortable flexion. POP stage was allocated according to the most severe portion of the POP.24
Primary outcomes
QOL was measured by means of the Prolapse QOL questionnaire (P-QOL), which is used to assess prolapse, urinary, sexual, and defecation symptoms as well as physical, social, and emotional factors.25 The questionnaire is scored out of 100 for each domain, with higher scores indicating poorer QOL. As determined in a population with POP, the P-QOL has good internal consistency (Cronbach’s α > 0.80), interrater reliability (r > 0.5), and test–retest reliability (r = 0.872).26
The measurement of the pelvic floor and abdominal muscle function followed the same procedures as described in Brandt and Janse van Vuuren.27
A Philips HD 11 XE (Philips, Amsterdam, the Netherlands) was used for perineal ultrasound measurement of the direction, displacement, and diameter of the puborectalis muscle and levator hiatus and the thickness of the perineal body upon contraction and Valsalva manoeuvre.28,29 After the morphologic assessment, PFM strength and endurance were assessed using the PERFECT (power, endurance, repetitions, fast contractions, every contraction timed) scheme as described by Devreese and colleagues and Laycock and Jerwood.30,31 EMG was carried out using a NeuroTrac MyoPlus 2 (filter 19-375Hz; Verity Medical Ltd, Hampshire, UK) with a Periform (EMS Physio Ltd, Wantage, UK) intra-vaginal probe to determine the average activity upon contraction and endurance at 60% of the maximum voluntary contraction.32
The method described by Thompson and colleagues was used to measure the EMG activity of the internal oblique and transversus abdominus muscles,33 followed by measurement with the Stabiliser PBU (Chattanooga Group Inc., Hixson, TN). The rotational control, rotational strength, and sagittal strength of the abdominal muscles were tested on the Sahrmann scale while they were monitored with the PBU.21 The construct validity, sensitivity, specificity, test–retest, and inter- and intrarater reliability of these procedures were reported in Brandt and Janse van Vuuren.27
Secondary outcomes
The women’s adherence to exercise was measured on a four-point Likert scale ranging from 1 (not doing exercises at all) to 4 (doing exercises very regularly). The scale was used by Sluijs and colleagues.34 A woman with a score of 1 or 2 was considered non-compliant, whereas a woman with a score of 3 or 4 was considered compliant.34
Lumbar and pelvic pain were also assessed on a five-point visual faces scale. The construct validity and test–retest reliability coefficients were 0.82 and 0.70, respectively, based on the findings from a previous study investigating pain in patients with POP.35
No changes were made to the trial outcome measures after the trial commenced.
Data analysis
An intention-to-treat analysis was done using SAS (Version 9.4; SAS Institute Inc., Cary, NC) and IBM SPSS Statistics (Version 26.0; IBM Corp., Armonk, NY). Frequencies and percentages for categorical data and means and SDs or percentiles for continuous data were calculated for each group to determine the participants’ baseline characteristics. An exploratory data analysis cleaned the data and determined outliers. The groups were compared at baseline by means of a one-way ANOVA for age, BMI, and number of pregnancies and deliveries; a χ2 test for comparison of binomial measures; and a Kruskal–Wallis test for ordinal and nominal measures.
A mixed ANOVA was used to determine main effects, as well as the interactions between time (within-subjects factor) and intervention (between-groups factor) for the continuous measures. If the statistics violated the assumptions of sphericity, ANOVA statistics were estimated using the Huynh–Feldt (e > 0.75) or Greenhouse–Geisser (ε < 0.75) corrected analysis, as applicable. In the case of significant interactions between time and intervention received, the simple main effects were determined for interpretation. When no significant interactions were observed, the main effects were reported. Pairwise comparisons with the Bonferroni correction and post hoc analysis (using Tukey’s and Games–Howell tests) determined the main effects among the groups and across the three time points for each continuous measure.
The Kruskal–Wallis test was used to determine the main between-subjects effects for power, the measures using the Sahrmann scale, pain, and any symptoms experienced at each time interval. This was followed by a Mann–Whitney U-test to determine between-groups differences for the indicated significant main effects.36
The differences in compliance among the groups were determined by the Friedman test, and the Wilcoxon signed-rank test was used to determine the changes within the groups over time.
Results
Flow of participants
Enrolment stretched over a period of 24 months from January 2014 to January 2016, and data collection continued until July 2016 to ensure that we had sufficient group sizes. A total of 81 women were randomized to three groups. The two intervention groups each lost two participants to follow-up, and the control group lost three (two each in the intervention groups due to diagnosis of cancer and the three in the control group due to problems with transport; see Figure 1). The differences in sample sizes in the analysis indicate missing data. Participants were not excluded if they returned for further measurement. No adverse events were recorded as resulting from the surgery or interventions.
Participant characteristics
The one-way ANOVA did not indicate any significant differences among the groups for age, BMI, or number of pregnancies or deliveries (p > 0.05) at baseline. No differences were indicated by the χ2 test in comparing the groups with regard to participation in sports, smoking, previous surgery received, type of prolapse present, or type of surgery received during the study (Pearson χ2 test, p > 0.5). The Kruskal–Wallis test also indicated no significant differences among the groups for menopause status, stage of prolapse, or type of work (p > 0.5). Tables 1 and 2 summarize the mean values and frequencies for the participants’ demographic information and exercise and medical history.
Table 1 .
Within-Group Differences at Baseline for Continuous Demographic Variables
| Variable | Group, mean (SD)
|
Within-group differences (one-way ANOVA)
|
||||
|---|---|---|---|---|---|---|
| 1 (n = 24) | 2 (n = 28) | 3 (n = 29) | MS | F 2,78 | Significance | |
| Age, y | 60.25 (8.52) | 59.42 (8.13) | 60.27 (9.20) | 6.40 | 0.086 | 0.92 |
| BMI, kg/m2 | 30.74 (8.44) | 28.58 (5.65) | 30.79 (6.87) | 43.84 | 0.895 | 0.41 |
| No. of pregnancies | 3.48 (1.97) | 3.18 (0.94) | 3.51 (1.70) | 0.82 | 0.315 | 0.73 |
| No. of deliveries | 2.96 (1.69) | 2.96 (0.88) | 3.25 (1.50) | 0.62 | 0.296 | 0.75 |
Group 1 = pelvic floor muscle training group; Group 2 = abdominal muscle training + pelvic floor muscle training group; Group 3 = control group;
ANOVA = analysis of variance.
Table 2 .
Within-Group Differences at Baseline for Categorical Demographic Variables
| Variable | Group, no. (%)
|
Within-group difference
|
||||
|---|---|---|---|---|---|---|
| 1 (n = 24) | 2 (n = 28) | 3 (n = 29) | Pearson χ22 | Kruskal-Wallis H test | Asymptotic significance (two-sided) | |
| Post-menopausal | 16 (66.67) | 15 (53.57) | 15 (51.72) | 0.554 | – | 0.76 |
| Employment | – | – | – | 0.110 | – | 0.10 |
| Manual labour | 14 (58.33) | 18 (64.29) | 17 (58.62) | – | – | – |
| Office work | 5 (20.83) | 2 (7.14) | 9 (31.03) | – | – | – |
| Retired | 5 (20.83) | 8 (28.57) | 3 (10.34) | – | – | – |
| Participation in sports | 5 (20.83) | 7 (25.00) | 2 (6.90) | 3.566 | – | 0.17 |
| Smoking | 7 (29.17) | 5 (17.86) | 4 (13.79) | 2.055 | – | 0.36 |
| History of pelvic or abdominal surgery | 9 (37.50) | 16 (57.14) | 10 (34.48) | 3.434 | – | 0.18 |
| Stage of pelvic organ prolapse | – | – | – | – | 0.415 | 0.81 |
| Stage I | 0 | 0 | 0 | – | – | – |
| Stage II | 2 (8.33) | 4 (14.29) | 3 (10.34) | – | – | – |
| Stage III | 22 (91.67) | 24 (85.71) | 26 (89.66) | – | – | – |
| Type of pelvic organ prolapse* | ||||||
| Cystocele | 20 (83.33) | 21 (75.00) | 26 (89.66) | 1.309 | – | 0.52 |
| Rectocele | 20 (83.33) | 23 (82.14) | 21 (72.41) | 0.922 | – | 0.63 |
| Enterocele | 6 (25.00) | 3 (10.71) | 6 (20.69) | 1.805 | – | 0.41 |
| Uterocele | 2 (8.33) | 1 (3.57) | 2 (6.90) | 0.547 | – | 0.76 |
| Vault prolapse | 15 (62.50) | 20 (71.43) | 17 (58.62) | 1.059 | – | 0.59 |
| Intervention received† | ||||||
| Laparoscopic sacrocolpopexy | 4 (16.67) | 1 (3.57) | 2 (6.90) | 2.981 | – | 0.23 |
| Abdominal sacrocolpopexy | 1 (4.17) | 2 (7.14) | 0 | 2.058 | – | 0.36 |
| Anterior repair | 6 (25.00) | 8 (28.57) | 9 (31.03) | 0.236 | – | 0.89 |
| Sacrospinous fixation | 6 (25.00) | 6 (21.43) | 8 (27.59) | 0.292 | – | 0.86 |
| Rectocele plication with perineal body repair | 7 (29.17) | 12 (42.86) | 16 (55.17) | 3.621 | – | 0.16 |
| Perineo-colpo-sacrospinous fixation | 5 (20.83) | 8 (28.57) | 5 (17.24) | 1.096 | – | 0.58 |
| Vault prolift | 1 (4.17) | 0 (0.00) | 2 (6.90) | 1.920 | – | 0.38 |
| Rectopexy | 1 (4.17) | 2 (7.14) | 0 (0.00) | 2.058 | – | 0.36 |
Note: Dashes indicate not applicable.
Participants could have more than 1 type of pelvic organ prolapse.
Participants could receive more than one surgical intervention.
Group 1 = pelvic floor muscle training group; Group 2 = abdominal muscle training + pelvic floor muscle training group; Group 3 = control group.
Primary and secondary outcomes
Box’s Test for Equivalence of Covariance Matrices indicated a p-value of > 0.05.
Significant interactions between time and intervention were observed for the personal domain of the P-QOL, the thickness of the right puborectalis muscle, the amount of movement of the puborectalis muscle, the number of repetitions and fast contractions of the levator ani muscle, the measurement of the abdominal muscle function with the PBU, and the abdominal muscle activation with the EMG (Table 3). Simple main effects were interpreted for these variables. In the case of non-significant interactions (endurance measurements with EMG and PERFECT scale), main effects were interpreted.
Table 3 .
Results of Main Effects for the Interaction between Time and Intervention (N = 81)
| Measure | SS | MS | F | df | Significance |
|---|---|---|---|---|---|
| Sonar assessment, mm | |||||
| Levator hiatus at rest | 204.815 | 51.204 | 1.110 | 4, 156 | 0.35 |
| Levator hiatus with Valsalva | 212.366 | 55.232 | 0.695 | 3.845, 149.955 | 0.59* |
| Levator hiatus with contraction | 433.347 | 108.337 | 1.954 | 4, 156 | 0.11 |
| Thickness of perineal body | 89.294 | 40.354 | 1.483 | 2.213, 86.297 | 0.23† |
| Thickness of puborectalis (left) | 7.821 | 2.211 | 1.006 | 3.538, 137.975 | 0.40* |
| Thickness of puborectalis (right) | 15.222 | 4.197 | 2.642 | 3.627, 141.441 | 0.04†‡ |
| Movement of puborectalis | 601.451 | 150.363 | 5.500 | 4, 156 | 0.00‡ |
| PERFECT | |||||
| Levator ani, endurance, s | 45.985 | 11.496 | 1.631 | 4, 156 | 0.17 |
| Levator ani, repetitions | 57.828 | 14.457 | 3.253 | 4, 156 | 0.01‡ |
| Levator ani, fast contractions | 146.848 | 36.712 | 5.563 | 4, 156 | 0.00‡ |
| EMG of the pelvic floor muscles | |||||
| Levator ani, EMG with MVC, mV | 277.251 | 73.625 | 0.441 | 3.766, 146.863 | 0.77* |
| Levator ani, endurance with EMG, s | 904.283 | 226.071 | 1.256 | 4, 156 | 0.29 |
| Abdominal muscle assessment | |||||
| Abdominal muscle, EMG, mμ | 506.272 | 126.57 | 4.260 | 4, 156 | 0.00‡ |
| Abdominal muscle, PBU, mmHg | 394.819 | 98.705 | 9.759 | 4, 156 | 0.00†‡ |
| Quality of life domains | |||||
| General health | 951.295 | 237.824 | 1.346 | 4, 156 | 0.26 |
| Prolapse impact | 3,036.537 | 759.134 | 1.576 | 4, 156 | 0.19 |
| Role limitations | 1,066.525 | 290.067 | 0.397 | 3.677, 62.506 | 0.80* |
| Physical limitations | 2,130.041 | 594.682 | 0.814 | 3.582, 60.891 | 0.51* |
| Social limitations | 1292.459 | 380.455 | 0.770 | 3.397, 55.949 | 0.53* |
| Personal limitations | 8,787.782 | 2,670.144 | 3.236 | 3.291, 50.983 | 0.03†‡ |
| Emotional limitations | 2,426.307 | 653.236 | 1.007 | 3.714, 57 | 0.41* |
| Sleep disturbance | 3,051.957 | 762.989 | 1.983 | 4, 68 | 0.11 |
| Severity of prolapse | 1,288.194 | 353.138 | 1.285 | 3.648, 56.060 | 0.29* |
Huynh-Feldt corrected analysis.
Greenhouse–Geisser corrected analysis.
Statistically significant difference at p < 0.05.
SS = sum of squares; MS = mean square; PERFECT = power, endurance, repetitions, every contraction timed; EMG = electromyography; MVC = maximum voluntary contraction; PBU = pressure biofeedback unit.
Pelvic floor muscle function: Comparison between groups
The simple main effects indicated that at 6 months right puborectalis muscle thickness was significantly greater in the control group than in the abdominal muscle training + PFMT group (p = 0.004; sample mean 1.487; 95% CI: 0.276, 1.439) and the PFMT group (p = 0.00; mean 0.857; 95% CI: 0.882, 2.093), and the abdominal training + PFMT group also had significantly increased thickness compared with the PFMT group (p = 0.04; mean 0.630; 95% CI: 0.019, 1.240).
The amount of movement of the puborectalis muscle at 6 months was significantly greater in the PFMT group than in the control group (p = 0.00; mean 5.895; 95% CI: 3.269, 8.522) and the abdominal muscle training + PFMT group (p = 0.04; mean 1.581; 95% CI: 1.494, 6.790). Only the abdominal muscle training + PFMT group demonstrated significantly more repetitions of PFM contraction than the control group at 3 months (p = 0.00; mean 0.857; 95% CI: 0.091, 3.071). Both the abdominal muscle training + PFMT group (p = 0.00; mean 3.511; 95% CI: 2.232, 4.790) and the PFMT group (p = 0.00; mean 3.190; 95% CI: 1.858, 4.521) had significantly more fast contractions than the control group at the 6-month follow-up.
Significant differences among the groups were found for endurance of the levator ani muscle contraction as measured using the EMG (F2,78 = 3.073, p = 0.05). Pairwise comparisons and post hoc tests indicated increased endurance (using the PERFECT scale) in the PFMT group compared with the control group (p = 0.05; mean 1.44; 95% CI: 0.01, 2.87) and decreased endurance of the PFM (using EMG) in the PFMT group compared with the control group (p = 0.03; mean −7.05; 95% CI: −13.61, −0.48). The PFMT group also showed increased power of the PFM than the abdominal muscle training + PFMT and control groups at 6-month follow-up (Table 4).
Table 4 .
Significant Findings of Post Hoc Between-Groups Comparisons for Primary and Secondary Ordinal Outcomes (Mann–Whitney U-Test)
| Measure | Group, mean rank
|
Mann-Whitney U-test | Z score | p-value* | ||
|---|---|---|---|---|---|---|
| 1 (n = 24) | 2 (n = 28) | 3 (n = 29) | ||||
| Urinary frequency, 3 mo | ||||||
| Groups 2 and 3 | – | 34.39 | 23.79 | 255.000 | −2.540 | 0.01 |
| Groups 1 and 3 | 31.63 | – | 23.17 | 237.000 | −2.075 | 0.04 |
| Bulging symptoms, 6 mo | – | 32.80 | 25.33 | 299.500 | −2.473 | 0.01 |
| Discomfort, 6 mo | – | 32.71 | 25.41 | 302.000 | −2.618 | 0.01 |
| Levator ani, power, 6 mo | ||||||
| Groups 1 and 2 | 33.04 | 20.89 | – | 179.000 | −3.114 | 0.00 |
| Groups 1 and 3 | 36.98 | – | 18.47 | 108.500 | −4.743 | 0.00 |
| Sahrmann scale, 6 mo | ||||||
| Groups 1 and 2 | 19.50 | 32.50 | – | 168.000 | −3.246 | 0.00 |
| Groups 2 and 3 | – | 38.43 | 19.90 | 142.000 | −4.507 | 0.00 |
| Groups 1 and 3 | 32.27 | – | 22.64 | 221.000 | −2.380 | 0.02 |
Note: Dashes indicate not applicable.
All ps are statistically significant at p < 0.05.
Group 1 = pelvic floor muscle training group; Group 2 = abdominal muscle training + pelvic floor muscle training group; Group 3 = control group.
Abdominal muscle function: Comparison between groups
Only abdominal training had a significant effect on the PBU values compared with the other interventions at 3 months (p = 0.04; mean 2.171; 95% CI: 0.085, 4.257). At 6 months, both the abdominal training + PFMT and PFMT groups showed significant effects on PBU values compared with the control group (p = 0.00; mean 5.654; 95% CI: 4.101, 7.206, and p = 0.00; mean 4.440; 95% CI: 2.823, 6.057, respectively). The abdominal muscle training + PFMT group showed increased stability with the Sahrmann scale at 6 months compared with both other groups (see Table 4).
Quality of life and pelvic organ prolapse symptoms: Between-groups comparison
No significant differences were found among the groups regarding the QOL domains, but all domains showed a significant improvement (i.e., decreased score) from baseline to 6 months, as shown in the next section (p < 0.00).
The Kruskal–Wallis test, however, showed that there were significant differences (p < 0.05) among the groups in urinary frequency at the 3-month follow-up, in symptoms of bulging and discomfort at the 6-month follow-up, and for pelvic pain at the 6-month follow-up (Table 5). Follow-up analysis with the Mann–Whitney U-test did not indicate any differences between the PFMT and abdominal muscle training + PFMT groups. Compared with the control group, the PFMT group showed increased urinary frequency at the 3-month follow-up. The abdominal muscle training + PFMT group, however, showed increased symptoms in all three categories compared with the control group (see Table 4).
Table 5 .
Significant Main Between-Groups Effects for Primary and Secondary Ordinal Outcomes (Kruskal–Wallis Test)
| Measure | Group, mean rank
|
Kruskal-Wallis H | df | Significance* | ||
|---|---|---|---|---|---|---|
| 1 (n = 24) | 2 (n = 28) | 3 (n = 29) | ||||
| Urinary frequency, 3 mo | 46.04 | 46.04 | 31.97 | 7.264 | 2 | 0.03 |
| Bulging symptoms, 6 mo | 40.48 | 46.57 | 36.05 | 6.257 | 2 | 0.04 |
| Discomfort, 6 mo | 39.81 | 46.86 | 36.33 | 7.719 | 2 | 0.02 |
| Levator ani, power, 6 mo | 57.52 | 37.05 | 31.14 | 21.505 | 2 | 0.00 |
| Sahrmann scale, 6 mo | 39.27 | 56.43 | 27.53 | 23.495 | 2 | 0.00 |
All ps statistically significant at p < 0.05.
Group 1 = pelvic floor muscle training group; Group 2 = abdominal muscle training + pelvic floor muscle training group; Group 3 = control group.
Change in outcome measures over time per group
Table 6 indicates the following results regarding change over time per group and outcome measure. The significant increase in right puborectalis muscle thickness was seen from baseline to 3 months in all three groups, but only the control group continued this trend during the last 3 months. The increased movement of the puborectalis muscle in the PFMT group was most significant from 3 to 6 months, whereas the control group showed decreased movement during that time period. The abdominal muscle training + PFMT group showed a significant effect of the number of repetitions and fast contractions from baseline to 3- and 6-month follow-ups, whereas the PFMT group demonstrated a significant increase in the number of fast contractions during the first 3 months after surgery. Finally, PFMT had a significant effect on the PBU values in the last 3 months, whereas abdominal training + PFMT had a significant effect during the first 3 months after surgery as well. Only the abdominal training + PFMT and control groups seemed to improve on the personal domain of the P-QOL over time (see Table 6).
Table 6 .
Simple Main Effects (Pairwise Comparisons) for Each Group, per Measure, between Time Intervals (N = 81)
| Measure and group | Time*
|
A – B, mean difference | SE | p-value | 95% CI | |
|---|---|---|---|---|---|---|
| A | B | |||||
| Thickness of puborectalis (right), mm | ||||||
| PFMT | 1 | 2 | −0.912† | 0.375 | 0.02 | −1.659, −0.164 |
| 3 | −0.287 | 0.371 | 0.44 | −1.026, 0.451 | ||
| 2 | 1 | 0.912† | 0.375 | 0.02 | 0.164, 1.659 | |
| 3 | 0.625† | 0.286 | 0.03 | 0.056, 1.193 | ||
| 3 | 1 | 0.287 | 0.371 | 0.44 | −0.451, 1.026 | |
| 2 | −0.625† | 0.286 | 0.03 | −1.193, −0.056 | ||
| Abdominal muscle training + PFMT | 1 | 2 | −1.161† | 0.348 | 0.00 | −1.853, −0.470 |
| 3 | −0.609 | 0.343 | 0.08 | −1.293, 0.075 | ||
| 2 | 1 | 1.161† | 0.348 | 0.00 | 0.470, 1.853 | |
| 3 | 0.553† | 0.264 | 0.04 | 0.026, 1.079 | ||
| 3 | 1 | 0.609 | 0.343 | 0.08 | −0.075, 1.293 | |
| 2 | −0.553† | 0.264 | 0.04 | −1.079, −0.026 | ||
| Control | 1 | 2 | −0.536 | 0.341 | 0.12 | −1.215, 0.144 |
| 3 | −1.191† | 0.337 | 0.00 | −1.863, −0.519 | ||
| 2 | 1 | 0.536 | 0.341 | 0.12 | −0.144, 1.215 | |
| 3 | −0.655† | 0.260 | 0.01 | −1.172, −0.138 | ||
| 3 | 1 | 1.191† | 0.337 | 0.00 | 0.519, 1.863 | |
| 2 | 0.655† | 0.260 | 0.01 | 0.138, 1.172 | ||
| Movement of puborectalis, mm | ||||||
| PFMT | 1 | 2 | 0.942 | 1.579 | 0.55 | −2.202, 4.085 |
| 3 | −2.246 | 1.489 | 0.14 | −5.211, 0.719 | ||
| 2 | 1 | −0.942 | 1.579 | 0.55 | −4.085, 2.202 | |
| 3 | −3.187† | 1.457 | 0.03 | −6.088, −0.287 | ||
| 3 | 1 | 2.246 | 1.489 | 0.14 | −0.719, 5.211 | |
| 2 | 3.188† | 1.457 | 0.03 | 0.287, 6.088 | ||
| Abdominal muscle training + PFMT | 1 | 2 | −2.100 | 1.462 | 0.16 | −5.010, 0.811 |
| 3 | −0.537 | 1.379 | 0.70 | −3.282, 2.208 | ||
| 2 | 1 | 2.100 | 1.462 | 0.16 | −0.811, 5.010 | |
| 3 | 1.563 | 1.349 | 0.25 | −1.123, 4.248 | ||
| 3 | 1 | 0.537 | 1.379 | 0.70 | −2.208, 3.282 | |
| 2 | −1.563 | 1.349 | 0.25 | −4.248, 1.123 | ||
| Control | 1 | 2 | −0.989 | 1.437 | 0.49 | −3.849, 1.871 |
| 3 | 4.556† | 1.355 | 0.00 | 1.859, 7.254 | ||
| 2 | 1 | 0.989 | 1.437 | 0.49 | −1.871, 3.849 | |
| 3 | 5.545† | 1.325 | 0.00 | 2.906, 8.183 | ||
| 3 | 1 | −4.556†’ | 1.355 | 0.00 | −7.254, −1.859 | |
| 2 | −5.545† | 1.325 | 0.00 | −8.183, −2.906 | ||
| Levator ani: Repetitions | ||||||
| PFMT | 1 | 2 | −0.458 | 0.669 | 0.50 | −1.790, 0.874 |
| 3 | −1.396† | 0.593 | 0.02 | −2.576, −0.216 | ||
| 2 | 1 | 0.458 | 0.669 | 0.50 | −0.874, 1.790 | |
| 3 | −0.937 | 0.558 | 0.10 | −2.049, 0.174 | ||
| 3 | 1 | 1.396† | 0.593 | 0.02 | 0.216, 2.576 | |
| 2 | 0.938 | 0.558 | 0.10 | −0.174, 2.049 | ||
| Abdominal muscle training + PFMT | 1 | 2 | −2.964† | 0.619 | 0.00 | −4.198, −1.731 |
| 3 | −2.393† | 0.549 | 0.00 | −3.485, −1.300 | ||
| 2 | 1 | 2.964† | 0.619 | 0.00 | 1.731, 4.198 | |
| 3 | 0.571 | 0.517 | 0.27 | −0.458, 1.601 | ||
| 3 | 1 | 2.393† | 0.549 | 0.00 | 1.300, 3.485 | |
| 2 | −0.571 | 0.517 | 0.27 | −1.601, 0.458 | ||
| Control | 1 | 2 | −0.483 | 0.609 | 0.43 | −1.695, 0.729 |
| 3 | −1.103† | 0.539 | 0.04 | −2.177, −0.030 | ||
| 2 | 1 | 0.483 | 0.609 | 0.43 | −0.729, 1.695 | |
| 3 | −0.621 | 0.508 | 0.23 | −1.632, 0.391 | ||
| 3 | 1 | 1.103† | 0.539 | 0.04 | 0.030, 2.177 | |
| 2 | 0.621 | 0.508 | 0.23 | −0.391, 1.632 | ||
| Levator ani: Fast contractions | ||||||
| PFMT | 1 | 2 | −2.042† | 0.797 | 0.01 | −3.628, −0.455 |
| 3 | −2.917† | 0.769 | 0.00 | −4.447, −1.386 | ||
| 2 | 1 | 2.042† | 0.797 | 0.01 | 0.455, 3.628 | |
| 3 | −0.875 | 0.651 | 0.18 | −2.170, 0.420 | ||
| 3 | 1 | 2.917† | 0.769 | 0.00 | 1.386, 4.447 | |
| 2 | 0.875 | 0.651 | 0.18 | −0.420, 2.170 | ||
| Abdominal muscle training + PFMT | 1 | 2 | −3.000† | 0.738 | 0.00 | −4.469, −1.531 |
| 3 | −4.214† | 0.712 | 0.00 | −5.632, −2.797 | ||
| 2 | 1 | 3.000† | 0.738 | 0.00 | 1.531, 4.469 | |
| 3 | −1.214† | 0.602 | 0.05 | −2.414, −0.015 | ||
| 3 | 1 | 4.214† | 0.712 | 0.00 | 2.797, 5.632 | |
| 2 | 1.214† | 0.602 | 0.05 | 0.015, 2.414 | ||
| Control | 1 | 2 | −0.828 | 0.725 | 0.26 | −2.271, 0.616 |
| 3 | 0.190 | 0.699 | 0.79 | −1.203, 1.582 | ||
| 2 | 1 | 0.828 | 0.725 | 0.26 | −0.616, 2.271 | |
| 3 | 1.017 | 0.592 | 0.09 | −0.161, 2.196 | ||
| 3 | 1 | −0.190 | 0.699 | 0.79 | −1.582, 1.203 | |
| 2 | −1.017 | 0.592 | 0.09 | −2.196, 0.161 | ||
| Abdominal muscle, PBU, mmHg | ||||||
| PFMT | 1 | 2 | 0.083 | 0.967 | 0.93 | −1.843, 2.009 |
| 3 | −2.000† | 0.908 | 0.03 | −3.807, −0.193 | ||
| 2 | 1 | −0.083 | 0.967 | 0.93 | −2.009, 1.843 | |
| 3 | −2.083† | 0.877 | 0.02 | −3.829, −0.338 | ||
| 3 | 1 | 2.000† | 0.908 | 0.03 | 0.193, 3.807 | |
| 2 | 2.083† | 0.877 | 0.02 | 0.338, 3.829 | ||
| Abdominal muscle training + PFMT | 1 | 2 | −3.071† | 0.896 | 0.00 | −4.855, −1.288 |
| 3 | −4.571† | 0.840 | 0.00 | −6.244, −2.899 | ||
| 2 | 1 | 3.071† | 0.896 | 0.00 | 1.288, 4.855 | |
| 3 | −1.500 | 0.812 | 0.07 | −3.116, 0.116 | ||
| 3 | 1 | 4.571† | 0.840 | 0.00 | 2.899, 6.244 | |
| 2 | 1.500 | 0.812 | 0.07 | −0.116, 3.116 | ||
| Control | 1 | 2 | 0.431 | 0.880 | 0.63 | −1.321, 2.183 |
| 3 | 2.414† | 0.826 | 0.01 | 0.770, 4.057 | ||
| 2 | 1 | −0.431 | 0.880 | 0.63 | −2.183, 1.321 | |
| 3 | 1.983† | 0.798 | 0.02 | 0.395, 3.571 | ||
| 3 | 1 | −2.414† | 0.826 | 0.01 | −4.057, −0.770 | |
| 2 | −1.983† | 0.798 | 0.02 | −3.571, −0.395 | ||
| Personal domain (P-QOL) | ||||||
| PFMT | 1 | 2 | −30.278 | 16.381 | 0.07 | −63.568, 3.012 |
| 3 | 2.976 | 16.583 | 0.86 | −30.725, 36.677 | ||
| 2 | 1 | 30.278 | 16.381 | 0.07 | −3.012, 63.568 | |
| 3 | 33.254† | 13.905 | 0.02 | 4.997, 61.511 | ||
| 3 | 1 | −2.976 | 16.583 | 0.86 | −36.677, 30.725 | |
| 2 | −33.254† | 13.905 | 0.02 | −61.511, −4.997 | ||
| Abdominal muscle training + PFMT | 1 | 2 | 15.139 | 11.423 | 0.19 | −8.075, 38.352 |
| 3 | 8.631 | 11.564 | 0.46 | −14.869, 32.131 | ||
| 2 | 1 | −15.139 | 11.423 | 0.19 | −38.352, 8.075 | |
| 3 | −6.508 | 9.696 | 0.51 | −26.212, 13.196 | ||
| 3 | 1 | −8.631 | 11.564 | 0.46 | −32.131, 14.869 | |
| 2 | 6.508 | 9.696 | 0.51 | −13.196, 26.212 | ||
| Control | 1 | 2 | 7.778 | 10.211 | 0.45 | −12.974, 28.530 |
| 3 | 11.905 | 10.338 | 0.26 | −9.104, 32.913 | ||
| 2 | 1 | −7.778 | 10.211 | 0.45 | −28.530, 12.974 | |
| 3 | 4.127 | 8.668 | 0.64 | −13.488, 21.742 | ||
| 3 | 1 | −11.905 | 10.338 | 0.26 | −32.913, 9.104 | |
| 2 | −4.127 | 8.668 | 0.64 | −21.742, 13.488 | ||
Time 1 = baseline; Time 2 = 3 months; Time 3 = 6 months.
Significant at the 0.05 level.
PFMT = pelvic floor muscle training; PBU = pressure biofeedback unit; P-QOL = Prolapse-specific Quality of Life questionnaire.
There was also a significant difference across the three time points for endurance as measured with the PERFECT scale (F2,156 = 13.564, p = 0.00) and for all domains of the P-QOL (p < 0.00; online Table S1).
Exercise compliance
Both intervention groups had a median of four follow-up sessions over 6 months. Exercise compliance rates at 3 months were more than 60%; at 6 months, they were more than 55% (online Table S2). The subgroups revealed no significant changes in compliance from 3 to 6 months (PFMT group, p = 0.32, Z = −1.00, n = 13; abdominal muscle training + PFMT group, p = 1.00, Z = 0.00, n = 22), although the control group showed a tendency toward less compliance at 6 months (p = 0.06, Z = −1.890, n = 16). Friedman’s test indicated no significant differences in compliance among the groups at 3 months (p = 0.22, c22 = 3.038) or at 6 months (p = 0.14, c22 = 4).
Discussion
The results of the current study do not indicate a benefit of combining PFMT or abdominal training with reconstructive surgery to improve the symptoms of POP and QOL. QOL increased in all three groups over 6 months, which might indicate that the improved QOL was due to the surgical repair rather than the interventions. Both intervention groups showed increased symptoms of urinary frequency in the short term (3 mo) compared with the control group. This phenomenon was not present at the 6-month follow-up. Because PFMT is the common variable in the two intervention groups, one might need to consider the influence that the sudden and frequent change in PFM tension had on the detrusor inhibitory reflex immediately after surgery.
Bulging and discomfort increased in the abdominal muscle training + PFMT group compared with the control group over 6 months. This may raise the question of whether these exercises might have exacerbated these symptoms as a result of increased intra-abdominal pressure. However, both intervention groups showed a significantly improved effect on abdominal muscle contraction as measured with the PBU and Sahrmann scale compared with the control group. One may therefore ask why the PFMT group did not show increased symptoms if it also showed increased abdominal muscle strength. The explanation might be that the PFMT group showed a significantly improved effect on the power and endurance of the PFM as measured with the PERFECT scale and amount of movement when compared with the control group.
The latter findings are clinically important because they might indicate that the PFMT counteracted any negative effects of abdominal muscle contraction by contributing to the pelvic organ support mechanism. They might also support the evidence that there is a synergy between abdominal and PFM contraction because the PFMT group also improved on the abdominal muscle measures. Similarly, the abdominal training + PFMT group improved significantly on the PFM measures, such as the number of repetitions and fast contractions, compared with both the control and the PFMT groups.37
Comparison of the findings from this study with the outcomes of similar studies was limited because of differences in populations, length of follow-up, exercise protocols, and outcome measures. No previous studies on perioperative management with reconstructive surgery have included assessing or training the abdominal musculature or ultrasound assessment of the PFM (movement, thickness of perineal body and puborectalis, levator hiatus length).
The findings of this study regarding the effect on QOL and symptoms are similar to those of most previous studies.13,14,38–40 The heterogeneity among the studies might support an increased generalisability of the evidence for the effect of perioperative management on the symptoms of POP and QOL. There seem to be similar limited benefits in populations in high- and low-resource settings, with the presence of POP with or without urinary incontinence, in groups aged 18–81 years, with different numbers of follow-up visits, and with different PFMT programmes in terms of duration, intensity, and progression and when measured with a variety of outcome measures.
McClurg and colleagues were the only researchers who found perioperative PFMT to have an improved effect on symptoms after 12 months.41 Like Barber and colleagues39 and the current study, they included individualized PFM exercises instead of a standard protocol. Recent literature has emphasized that studies of PFMT should investigate exercise protocols based on scientific exercise guidelines and the principles of specificity and overload because they will have a substantial effect on the outcomes.37,41 Unfortunately the findings of McClurg and colleagues were based on a small sample (N = 57), which might indicate caution when interpreting their results.41
Similar to the studies of Jarvis and colleagues, Pauls and colleagues, and McClurg and colleagues,13,38,41 this study showed the benefit of PFMT with surgery to improve PFM function. However, the variables we used – namely, power, increased number of fast contractions, and endurance – on which the PFMT group showed improvement were not similar to the measures investigated in those studies and are therefore not comparable. The PFMT group in our study demonstrated a beneficial effect on strength, but not on EMG measures. This differs from the findings of Frawley and colleagues, Barber and colleagues, and Duarte and colleagues,14,39,40 who did not find improvement in PFM strength or EMG measures. The lack of individualized progression and reporting of adherence might be a reason for the limited benefit of PFMT on the postoperative PFM function found in those studies.14,38,39 It is therefore important to note that the intervention groups in this study improved in the strength of the PFM and abdominal muscles even with the limited number (four) of postoperative sessions that participants received, mostly because of lack of transport to the clinics. This contradicts the results of studies with a high number of postoperative follow-up sessions,39,40 which have demonstrated a limited benefit of PFMT on strength measures.
The participants in this study had a good adherence to exercise. Together with the individualized programme, this might have been responsible for the positive outcome, which can be of significant clinical value in a resource-restricted setting, although the study setting may limit the generalisability of the results to resource-restricted health systems.
Other limitations include that the results at 6 months should be interpreted with caution as a result of missing data for participants who were unable to physically attend the clinic. However, the rate of adherence to exercises was still higher than 50% at 6 months, which may be of clinical value in areas in which accessibility to health care is restricted and patients need to depend on home exercise programmes. Longer-term follow-up, larger sample sizes, and the use of three- or four-dimensional ultrasound might have demonstrated more accurately whether the improved PFM and abdominal function observed in the short term, and a continued home-exercise programme in the long term, had any additional beneficial effects on the QOL, symptoms, and surgery success rate.
Conclusion
The results of this study support evidence that postoperative physiotherapy does not significantly improve POP symptoms or QOL over 6 months compared with a control group receiving standard care. The PFMT and abdominal muscle training + PFMT groups demonstrated increased symptoms of urinary frequency, and only the abdominal muscle training + PFMT group showed increased symptoms of bulging and discomfort. These symptoms may be a cause for concern with respect to the effects they may have on the pelvic organ support mechanisms after surgery. However, significant beneficial effects were seen in the PFMT group on PFM function, which may again lead to improved pelvic organ support. Introducing different methods of PFM assessment and variables may provide deeper and new insight into the effect of postoperative physiotherapy.
Key Messages
What is already known on this topic
Physiotherapy has proved to be beneficial for the conservative management of pelvic organ prolapse; however, the effects of postoperative physiotherapy and inclusion of abdominal muscle training still remain unclear, and there is a paucity of studies on this topic in the literature.
What this study adds
This study indicates that postoperative pelvic floor muscle training can have a beneficial effect on pelvic floor muscle function and therefore pelvic organ support, specifically in resource-poor settings in which patients need to depend on home exercise programmes. However, adding abdominal muscle training led to increased symptoms of bulging and discomfort.
Supplementary Material
References
- 1.Svihrova V, Svihra J, Luptak J, et al. Disability-adjusted life years (DALYs) in general population with pelvic organ prolapse: a study based on the Prolapse Quality-of-Life questionnaire (P-QOL). Eur J Obstet Gynecol Reprod Biol. 2014;182:22–6. 10.1016/j.ejogrb.2014.08.024. Medline:25216448 [DOI] [PubMed] [Google Scholar]
- 2.Olsen AL, Smith VJ, Bergstrom JO, et al. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol. 1997;89(4):501–6. 10.1016/S0029-7844(97)00058-6. Medline:9083302 [DOI] [PubMed] [Google Scholar]
- 3.Henn EW, Van Rensburg JA, Cronje HS. Management of anterior vaginal prolapse in South Africa: national survey. SAMJ. 2009;99(4):229–30. Medline:19588773. [PubMed] [Google Scholar]
- 4.Haya N, Feiner B, Baessler K, et al. Perioperative interventions in pelvic organ prolapse surgery. Cochrane Database Syst Rev. 2018;8:CD013105. 10.1002/14651858.CD013105. Medline:30121957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maher C, Feiner B, Baessler K, et al. Surgical management of pelvic organ prolapse in women. Cochrane Database Syst Rev. 2013;4:CD004014. 10.1002/14651858.CD004014.pub5. Medline:23633316 [DOI] [PubMed] [Google Scholar]
- 6.Bo K, Berghmans B, Morkved S, et al. Physical therapy for the pelvic floor: bridging science and clinical practice. 2nd ed. Edinburgh: Churchill Livingstone; 2015. [Google Scholar]
- 7.Hagen S, Stark D, Glazener C, et al. A randomised controlled trial of pelvic floor muscle training for stages I and II pelvic organ prolapse. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20(1):45–51. 10.1007/s00192-008-0726-4. Medline:18806910 [DOI] [PubMed] [Google Scholar]
- 8.Comerford MJ, Mottram SL. Functional stability re-training: principles and strategies for managing mechanical dysfunction. Man Ther. 2001;6(1):3–14. 10.1054/math.2000.0389. Medline:11243904 [DOI] [PubMed] [Google Scholar]
- 9.Doaee M, Moradi-Lakeh M, Nourmohammadi A, et al. Management of pelvic organ prolapse and quality of life: a systematic review and meta-analysis. Int Urogynecol J. 2014;25(2):153–63. 10.1007/s00192-013-2141-8. Medline:23783578 [DOI] [PubMed] [Google Scholar]
- 10.Shafik A, Doss S, Asaad S. Etiology of the resting myoelectric activity of the levator ani muscle: physioanatomic study with a new theory. World J Surg. 2003;27(3):309–14. 10.1007/s00268-002-6584-1. Medline:12607057 [DOI] [PubMed] [Google Scholar]
- 11.Thompson JA, O’Sullivan PB, Briffa NK, et al. Altered muscle activation patterns in symptomatic women during pelvic floor muscle contraction and Valsalva manoeuvre. Neurourol Urodyn. 2006;25(3):268–76. 10.1002/nau.20183. Medline:16496395 [DOI] [PubMed] [Google Scholar]
- 12.Bo K, Sherburn M, Allen T. Transabdominal ultrasound measurement of pelvic floor muscle activity when activated directly or via a transversus abdominis muscle contraction. Neurourol Urodyn. 2003;22(6):582–8. 10.1002/nau.10139. Medline:12951667 [DOI] [PubMed] [Google Scholar]
- 13.Jarvis SK, Hallam TK, Lujic S, et al. Peri-operative physiotherapy improves outcomes for women undergoing incontinence and or prolapse surgery: results of a randomised controlled trial. Aust NZ J Obstet Gynecol. 2005;45(4):300–3. Medline:16029296 [DOI] [PubMed] [Google Scholar]
- 14.Frawley HC, Phillips BA, Bo K, et al. Physiotherapy as an adjunct to prolapse surgery: an assessor-blinded randomized controlled trial. Neurourol Urodyn. 2010;29(5):719–25. 10.1002/nau.20828.Medline:19816918 [DOI] [PubMed] [Google Scholar]
- 15.Lakeman MME, Schraffordt Koops SE, Berghmans BC, et al. Peri-operative physiotherapy to prevent recurrent symptoms and treatment following prolapse surgery: supported by evidence or not? Int Urogynecol J. 2013;24(3):371–5. 10.1007/s00192-012-1973-y. Medline:23152045 [DOI] [PubMed] [Google Scholar]
- 16.Faul F, Erdfelder E, Lang A-G, et al. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39(2):175–91. 10.3758/bf03193146. Medline:17695343 [DOI] [PubMed] [Google Scholar]
- 17.Hagen S, Stark D, Maher C, et al. Conservative management of pelvic organ prolapse in women. Cochrane Database Syst Rev. 2009;4:CD003882. 10.1002/14651858.CD003882.pub3. Medline:17054190 [DOI] [PubMed] [Google Scholar]
- 18.Robert M, Ross S. Conservative management of urinary incontinence. J Obstet Gynaecol Can. 2006;28(12):1113–18. 10.1016/S1701-2163(16)32326-X. Medline:17169236 [DOI] [PubMed] [Google Scholar]
- 19.Sapsford RR. Rehabilitation of pelvic floor muscles utilizing trunk stabilization. Man Ther. 2004;9(1):3–12. 10.1016/S1356-689X(03)00131-0. Medline:14723856 [DOI] [PubMed] [Google Scholar]
- 20.Laycock J, Standley A, Crothers E, et al. Clinical guidelines for the physiotherapy management of females aged 16–65 with stress urinary incontinence. London: Chartered Society of Physiotherapy; 2001. [Google Scholar]
- 21.Mills JD, Taunton JE, Mills WA. The effect of a 10-week training regimen on lumbo-pelvic stability and athletic performance in female athletes: a randomized-controlled trial. Phys Ther Sport. 2005;6(2):60–6. 10.1016/j.ptsp.2005.02.006. [DOI] [Google Scholar]
- 22.Comerford MJ, Mottram SL. Kinetic control: the management of uncontrolled movement. Sydney: Churchill Livingstone Australia; 2012. [Google Scholar]
- 23.MacKenzie JF, Grimshaw PN, Jones CDS, et al. Muscle activity during lifting: examining the effect of core conditioning of multifidus and transversus abdominis. Work. 2014;47(4):453–62. 10.3233/WOR-131706. Medline:24004762 [DOI] [PubMed] [Google Scholar]
- 24.Bump RC, Mattiasson A, Bo K, et al. The standardization of terminology of female pelvic organ prolapse and pelvic floor dysfunction. Am J Obstet Gynecol. 1996;175(1):10–17. 10.1016/S0002-9378(96)70243-0. Medline:8694033 [DOI] [PubMed] [Google Scholar]
- 25.Digesu GA, Khullar V, Cardozo L, et al. P-QOL: a validated questionnaire to assess the symptoms and quality of life of women with urogenital prolapse. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16(3):176–81. 10.1007/s00192-004-1225-x. Medline:15875234 [DOI] [PubMed] [Google Scholar]
- 26.Brandt C, Van Rooyen C, Cronje HS. Validation of the Prolapse Quality of Life questionnaire (P-QoL): an Afrikaans version in a South African population. S Afr J Obstet Gynecol. 2016;22(2):38–41. 10.7196/SAJOG.2016.v22i2.1077. [DOI] [Google Scholar]
- 27.Brandt C, Janse van Vuuren EC. An International Classification of Function, Disability and Health (ICF)-based investigation of movement impairment in women with pelvic organ prolapse. S Afr J Physiother. 2019;75(1):a472. 10.4102/sajp.v75i1.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dietz HP. Ultrasound imaging of the pelvic floor. Part I: two-dimensional aspects. Ultrasound Obstet Gynecol. 2004;23(1):80–92. 10.1002/uog.939. Medline:14971006 [DOI] [PubMed] [Google Scholar]
- 29.Dietz HP, Shek C, Clarke B. Biometry of the pubovisceral muscle and levator hiatus by three-dimensional pelvic floor ultrasound. Ultrasound Obstet Gynecol. 2005;25(6):580–5. 10.1002/uog.1899.Medline:15883982 [DOI] [PubMed] [Google Scholar]
- 30.Devreese A, Staes F, De Weerdt W, et al. Clinical evaluation of pelvic floor muscle function in continent and incontinent women. Neurourol Neurodyn. 2004;23(3):190–7. 10.1002/nau.20018.Medline:15098213 [DOI] [PubMed] [Google Scholar]
- 31.Laycock J, Jerwood D. Pelvic floor muscle assessment: the PERFECT scheme. Physiotherapy. 2001;87(12):631–42. 10.1016/S0031-9406(05)61108-X. [DOI] [Google Scholar]
- 32.Auchincloss C, McLean L. Does the presence of a vaginal probe alter pelvic floor muscle activation in young, continent women? J Electromyogr Kinesiol. 2012;22(6):1003–9. 10.1016/j.jelekin.2012.06.006. Medline:22892546 [DOI] [PubMed] [Google Scholar]
- 33.Thompson JA, O’Sullivan PB, Briffa K, et al. Assessment of pelvic floor movement using transabdominal and transperineal ultrasound. Int Urogynecol J. 2005;16(4):285–92. 10.1007/s00192-005-1308-3. Medline:15782286 [DOI] [PubMed] [Google Scholar]
- 34.Sluijs EM, Kok GJ, Van der Zee J. Correlates of exercise compliance in physical therapy. Phys Ther. 1993;73(11):771–82. 10.1093/ptj/73.11.771. Medline:8234458 [DOI] [PubMed] [Google Scholar]
- 35.Heit M, Culligan P, Rosenquist C, et al. Is pelvic organ prolapse a cause of pelvic or low back pain? Obstet Gynecol. 2002;99(1):23–8. 10.1016/s0029-7844(01)01626-x. Medline:11777505 [DOI] [PubMed] [Google Scholar]
- 36.Du Prel J-B, Hommel G, Rohrig B, et al. Confidence intervals or P-values? Dtsch Arztebl Int. 2009;106(19):335–9. 10.3238/arztebl.2009.0335. Medline:19547734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ferla L, Darski C, Paiva LL, et al. Synergism between abdominal and pelvic floor muscles in healthy women: a systematic review of observational studies. Fisioter Mov. 2016;29(2):399–410. 10.1590/0103-5150.029.002.AO19. [DOI] [Google Scholar]
- 38.Pauls RN, Crisp CC, Novicki K, et al. Impact of physical therapy on quality of life and function after vaginal reconstructive surgery. Female Pelvic Med Reconstr Surg. 2013;19(6):271–7. 10.1097/SPV.0000000000000090. Medline:23982575 [DOI] [PubMed] [Google Scholar]
- 39.Barber MD, Brubaker L, Burgio KL, et al. Comparison of 2 transvaginal surgical approaches and perioperative behavioral therapy for apical vaginal prolapse. The optimal randomized trial. JAMA. 2014;311(10):1023–34. 10.1001/jama.2014.1719. Medline:24618964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Duarte TB, Bø K, Brito LGO, et al. Perioperative pelvic floor muscle training did not improve outcomes in women undergoing pelvic organ prolapse surgery: a randomised trial. Int J Physiother. 2020;66(1):27–32. 10.1016/j.jphys.2019.11.013. Medline:31843420 [DOI] [PubMed] [Google Scholar]
- 41.McClurg D, Hilton P, Dolan L, et al. Pelvic floor muscle training as an adjunct to prolapse surgery: a randomised feasibility study. Int Urogynecol J. 2014;25(7):883–91. 10.1007/s00192-013-2301-x. Medline:24500453 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


