Abstract
Dysregulated elevation of interleukin-6 (IL-6) signaling is implicated in the pathogenesis of multiple pathophysiological states, and the functional neutralization of the IL-6 pathway with monoclonal antibodies has been proven an effective therapeutic method in treating various diseases with abnormally enhanced IL-6 signaling, and its clinical indications are expanding. Here, we report that by using the conventional hybridoma technology and humanization mutation method, we develop a novel humanized anti-IL-6 receptor (IL-6R) antibody—namely, HZ0412a. In our study, we found that HZ0412a exhibits higher binding affinity to soluble recombinant human IL-6R than tocilizumab. Importantly, in contrast to tocilizumab—a humanized anti-IL-6R antibody approved by the US Food and Drug Administration for the treatment of rheumatoid arthritis, juvenile idiopathic arthritis, giant cell arteritis and Castleman’s disease—HZ0412a does not significantly affect the binding of IL-6 to IL-6R. Further analysis revealed that HZ0412a prevents IL-6R from binding to gp130 in vitro, while tocilizumab has a minimal effect under the same condition. Using various cell-based assays, we demonstrate that HZ0412a is noninferior to tocilizumab in inhibiting IL-6 signaling. Finally, we showed that HZ0412a is well tolerated in cynomolgus monkeys after a single subcutaneous injection at a dose of 1 or 5 mg/kg. Taken together, our results indicated that HZ0412a targets an epitope on human IL-6R that is different from that of tocilizumab, and the epitope region is essential for the interaction between IL-6R and gp130. This distinctive mode of action plus its high affinity to IL-6R led to the high potency of HZ0412a in suppressing in vitro IL-6 signaling.
Keywords: antibody, interleukin-6 (IL-6), high affinity
Statement of Significance: We developed a new humanized anti-IL-6R antibody, HZ0412a, that disrupts the interaction between IL-6R and gp130. This distinctive mode of action plus its high affinity to IL-6R led to the high potency of HZ0412a in suppressing in vitro IL-6 signaling.
INTRODUCTION
Interleukin-6 (IL-6) is a pleiotropic cytokine with prominent proinflammatory effects. The upregulation of IL-6 expression and/or the imbalance of its biological functions have been implicated in numerous pathological conditions, such as tumorigenesis, rheumatic diseases, autoimmune diseases, infections and inflammation [1, 2]. More recently, elevated IL-6 levels have been found in patients with COVID-19, and the role of IL-6-mediated proinflammatory responses in the pathogenesis of severe acute respiratory syndrome has been elucidated [3, 4]. To exert its biological activities, IL-6 first binds to IL-6 receptor α, which is either membrane-bound (IL-6R) or in an extracellular soluble form (sIL-6R). The IL-6/IL-6R or IL-6/sIL-6R heterodimer interacts with another membrane-bound glycoprotein (gp130, also known as IL-6 receptor β) to form a larger complex, which then activates Janus kinases/signal transducers and activators of the transcription 3 (JAK/STAT3) pathway, among others [1]. In contrast to the ubiquitous expression of gp130, membrane-bound IL-6R is only expressed in a limited number of cell types, e.g. hepatocytes and leukocytes, so IL-6 signaling via membrane-bound IL-6R (classic signaling) only takes place in a handful of cells. To the contrary, trans-signaling of IL-6/sIL-6R/gp130 occurs in nearly all human tissues and is underlying most of the proinflammatory effects of IL-6 [1].
Tocilizumab is a humanized anti-IL-6R antibody first reported in 1993 [5] and one of the humanized anti-IL-6R antibodies to date that have received the US Food and Drug Administration (FDA) approval for treating diseases like rheumatoid arthritis, giant cell arteritis and cytokine release syndrome, where elevated IL-6/IL-6R signaling is involved [6, 7]. In June 2021, the FDA issued an emergency use authorization (EUA) for tocilizumab to treat COVID-19 in certain hospitalized patients. It is noteworthy that more indications for IL-6/IL-6R antibody treatment are being tested or have been proposed [8–10].
In this study, we report the development of a novel humanized antibody to human IL-6R HZ0412a, which selectively binds to recombinant human IL-6R with high affinity. Importantly, HZ0412a and tocilizumab target different epitopes on IL-6R, which renders HZ0412a’s unique features. Furthermore, HZ0412a is non-inferior to tocilizumab in inhibiting IL-6 signaling, as demonstrated in three in vitro functional tests. Finally, HZ0412a is well tolerated in cynomolgus monkeys after a single subcutaneous injection of doses as high as 5 mg/kg. Thus, our studies laid the foundation for future in vivo studies to test the efficacy and safety of HZ0412a.
MATERIALS AND METHODS
Cell lines and reagents
DS-1 cells (B-lymphoma, ATCC #CRL-11102) and DLD-1 cells (colorectal adenocarcinoma, ATCC #CCL-221) were cultured in RPMI1640 (Hyclone SH30809.01). Hep G2 cells (hepatocarcinoma, ATCC #HB-8065) were cultured in EMEM (ATCC #30–2003). HEK293F cells (Kairui Biotech) were cultured in DMEM (Gibco 11995500CP). All culture media were supplemented with 10% fetal bovine serum (FBS) (Hyclone #SH30071.03) and 100 U/ml penicillin/streptomycin (GIBCO #15140), and cell cultures were maintained at 37°C with 5% CO2. In the DS-1 cell proliferation assay, the number of cells was determined with the Cell Counting Kit-8 (CCK-8, Sigma) following the manufacturer’s instructions. Serum amyloid A (SAA) secreted by Hep G2 cells was measured using an enzyme-linked immunosorbent assay (ELISA) kit from R&D Systems (DY3019–05).
Tocilizumab, except in mass photometry (MP) experiments, was purchased from Roche. Tocilizumab (Cat#: A2012) used in MP experiments was from Selleckchem. Human gp130 protein (Cat#: 230–30084) was ordered from RayBiotech. IL-11R (Cat#: 10252-H08H), LIFR (Cat#: 10628-H08H), OSMR (Cat#: 11226-H08H), CNTFR (Cat#: 11012 -H08H), G-CSFR (Cat#: 10218 -H08H), IL-4R (Cat#: 10402- H08H), TSLPR (Cat#: 9749-H08H), cynomolgus IL6R (Cat#: 90197-CNAE) and rat IL6R (Cat#: 80076-RNAE) were purchased from Sino Biological. IL-12Rβ1 (Cat#: TP321974), IL-23R (Cat#: TP762223), IL-27Rα (Cat#: TP307012), IL-31Rα (Cat#: TP720674) and IL-17R (Cat#: TP302390) were purchased from Origene. IL-12Rβ2 (Cat#: ab158762) was purchased from Abcam. sIL-6R-Fc (Cat#: ILR-H5259) used in MP experiments was ordered from ACROBiosystems.
Animals
BALB/c mice (female, 6–8 weeks age) were purchased from Beijing Vital River Laboratory Animal Technology Co. Ltd. Male and female cynomolgus monkeys (3–6 years old, weighing 2–4 kg) were purchased from Beijing Prima BioTech Inc. The monkeys were housed in cages and maintained on a 12-h light/12-h dark cycle. All animal experiments were carried out according to the procedures approved by the Animal Care and Use Committee of IPHASE Therapeutic Ltd and were in compliance with all relevant ethical regulations and guidelines set forth by the National Institutes of Health (NIH).
Plasmid construction
The coding sequences of human IL-6 (accession number: NP_000591) and the extracellular segment of human IL-6R (i.e. sIL-6R, residues 1–372, accession number: NP_000556) were each cloned into the pcDNA3.1 expression vectors. 6 × His, the mouse IgG1 Fc region or the human IgG1 Fc region had been engineered into the pcDNA3.1 vectors so that rIL-6 or sIL-6R with appropriate C-terminal tags could be produced. To construct the chimeric antibody vector, total RNA from a hybridoma clone was extracted and reverse-transcribed. The sequences of the variable regions from both the light and heavy chains were amplified and sequenced by PCR using degenerate primers and eventually cloned into the chimeric antibody vector that contains the coding sequences for the signal peptide, human IgG1 heavy-chain and kappa light-chain constant regions.
Protein expression and purification
To express rIL-6 or sIL-6R fusion proteins, HEK293F cells were transiently transfected with pcDNA3.1 vectors (see above) using the transfection reagent from Kairui Biotech (Cat# K20001). The cell culture supernatant was collected later, and secreted fusion proteins were purified with either nickel columns (Bestchrom) or protein A columns (GE Healthcare Bio-Sciences). The purity of all protein products was assessed by SDS-PAGE, and protein quantification was conducted with a BCA kit (Cat#: PC0020, Solarbio). Purified proteins were stored at −20°C.
Generation and humanization of murine monoclonal antibodies
HZ0412a and its variants are genetically engineered antibodies, humanized from murine anti-human IL-6R monoclonal antibodies (mAbs) using the complementarity-determining region (CDR) grafting method as described [11]. In brief, purified fusion protein sIL-6R-mFc was used to immunize BALB/c mice. Antibody titers in murine blood were assessed, and the spleen cells from animals with high blood antibody titers were collected and fused with SP2/0 cells to make hybridomas using a standard protocol [12]. Hybridomas were selected and the supernatants from the resulting clones were screened using ELISA. IL-6R antibodies were further assessed for their ability to inhibit IL-6-induced STAT3 phosphorylation in DLD-1 cells using western blot. After positive clones were identified, antibody variable regions from these hybridomas were cloned into a human full-length IgG framework and subsequently humanized and engineered to minimize interactions with the immune system. Humanization was performed by grafting the CDRs into the closest human variable (V) regions of the light- and heavy-chain framework as previously described [13]. The antigen-binding assay and the inhibition of STAT3 phosphorylation were used to assess their bioactivities.
Antigen-binding assay
Antigen-binding by anti-IL-6R antibodies was measured by using an ELISA kit from R&D Systems (Cat# DY227). Briefly, 96-well plates were coated with sIL-6R-mFc fusion protein (100 μg/ml, 100 μl per well) in phosphate-buffered saline (PBS) overnight at 4°C. After blocking for 1 h with 0.4% BSA in PBS at room temperature, increasing concentrations of humanized IL-6R antibodies and tocilizumab were added into the plates at room temperature for 2 h. For IL-6 and IL-6R interaction blocking assays, 96-well plates were coated with 2.0 μg/ml of rIL-6-His fusion protein (100 μL/well) in PBS for 16 h at 4°C. sIL-6R-mFc fusion protein (2.0 μg/ml, 50 μl per well) was added either in the absence or presence of increasing concentrations of antibodies at room temperature for 1 h. For IL-6/IL-6R and the gp130 interaction blocking assay, 96-well plates were coated with 4.0 μg/ml of gp130-His fusion protein (100 μl per well) in PBS overnight at 4°C. rhIL-6-hFc (8 μg/ml, 25 μl per well) and sIL-6R-mFc fusion (8 μg/ml, 25 μl per well) were added either in the absence or presence of increasing-concentration antibodies at 37°C for 1 h. Plates were subsequently washed three times and incubated with an appropriate horseradish peroxidase (HRP)–conjugated secondary antibody for 1 h at room temperature. After washing, plates were developed with TMB. The absorbance was measured at 450 nM. Each sample was tested in triplicates. The values of EC50 were calculated based on the four-parameter logistic equation using SPSS software.
Binding affinity analysis
The binding affinity of humanized anti-IL-6R antibodies to sIL-6R on a single-molecule level was measured by MP as previously described [14]. Briefly, microscope coverslips (24 × 50 mm, Fisher Scientific) were rinsed consecutively in isopropanol and H2O and blown dry in a stream of clean nitrogen. A solution containing an antibody (Ab) (30 nM) and an antigen (Ag) at various concentrations was prepared with 1 × PBS at room temperature and incubated at room temperature for 5 min, which was long enough for Ab-Ag binding to reach equilibrium. A 10-microliter solution was applied to the microscope coverslips and mounted on a OneMP instrument (Refeyn, UK) and the measurement was carried out at room temperature. [Ab], [Ag] and [Ab-Ag] were deduced from the mass distribution plots of Ab (30 nM)/Ag (30 nM) reactions, and the dissociation constant, KD, was calculated according to the equation
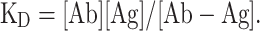 |
As a positive control, the KD of tocilizumab was measured at the same time.
Western blot
IL-6-induced protein phosphorylation is detected by western blot as previously described [11]. Briefly, the cells were lysed in buffer containing 20 mM Tris pH 7.5, 140 mM NaCl, 1 mM EDTA, 10% glycerol, 1% Triton X-100, 1 mM DTT, 50 mM NaF, protease inhibitor cocktail (Sigma P8340) and phosphatase inhibitor cocktail (Sigma P2850). Supernatants were collected after centrifugation at 13,000 rpm for 5 min at 4°C. The proteins were separated on SDS-PAGE and immunoblotted with an antibody recognizing p-STAT3 at Tyrosine 705 (R&D SYSTEMS).
Pharmacokinetic analysis in cynomolgus monkeys
All animals were monitored twice daily for mortality and moribundity. Body weights were measured at least once pre-study and weekly thereafter starting on day 7. Blood was collected by venipuncture into tubes with no anticoagulant at different time points (Fig. 3). The serum level of HZ0412a was measured by ELISA as described above using sIL-6R-mFc fusion protein as the coating reagent, followed by detection with an HRP-conjugated anti-human IgG secondary antibody. The observed serum concentration–time profiles in monkeys were analyzed using a non-compartmental model with an iterative non-linear regression program (Phoenix NLME, Certara, NJ). The following pharmacokinetic (PK) parameters were obtained: area under the serum drug concentration–time curve between time 0 and time last (AUClast); dose-normalized AUClast (AUClast/dose); highest observed blood/plasma drug concentration (Cmax); dose-normalized Cmax (Cmax/dose); time to highest observed drug concentration (tmax); apparent terminal half-life (t1/2); apparent volume of distribution of parent drug (Vz/F); and apparent clearance (CL/F).
Figure 3.
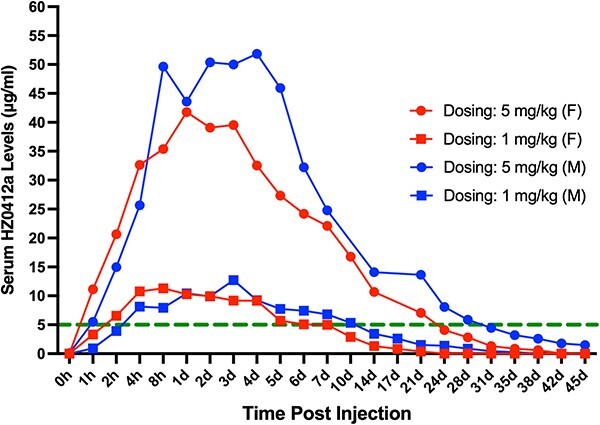
Pharmacokinetic analysis of HZ0412a in cynomolgus monkeys. The administration of HZ0412a (1 or 5 mg/kg) was achieved by subcutaneous injection. One male (M) and one female (F) animals were injected at each dose. At indicated time points, blood was collected by venipuncture into tubes without an anticoagulant. The serum HZ0412a levels were measured by ELISA as described in section Materials and Methods. The green dash line indicates the serum level of 5 μg/ml. Based on our previous in vitro data, serum HZ0412a levels over 5 μg/ml are expected to be able to inhibit most of the IL-6 effects.
Statistical analysis
All data are presented as mean ± SD (the bars in figures). The replication of individual experiments is indicated in relevant figures. Statistical significance between the results of two groups was determined by an unpaired, two-tailed t-test, and P < 0.05 is considered significant.
RESULTS
Generation of potent murine monoclonal antibodies against human sIL-6R
After immunizing BALB/c mice with sIL-6R-mFc fusion protein and selecting hybridoma clones, we identified several murine mAbs exhibiting strong binding to sIL-6R by ELISA (data not shown) and potent inhibition of the phosphorylation of STAT3 at Tyrosine 705 in DLD-1 cells (Fig. S1A). We used tocilizumab, under the brand name of Actemra, as a positive control. Using the universal antibody primers, we successfully cloned heavy- and light-chain variable regions (VH and VL) and determined the amino acid sequences of seven VL (1 K–7 K) and five VH (1H–5H). We then cloned the variable sequences into the plasmid pcDNA3.1, which contained the code sequences for human constant regions (CL-IgK and CH-IgG1). The resulting recombinant antibodies with various VL/VH combinations were tested for antigen binding and the ability to inhibit STAT3 phosphorylation. Our data showed that the combination of VL-1 K and VH-1H had the highest antigen-binding affinity (data not shown) as well as neutralizing activity against rIL-6 (Fig. S1B).
Humanization and characterization of HZ0412a antibody
To humanize the antibody, we modified the variable regions of light-chain 1 K and heavy-chain 1H using framework regions (FRs) of the closest human germline sequences as a template and assembled the resulting variants VL-1 K (1 K-1, 1 K-2 and 1 K-3) and VH-1H (1H-1, 1H-2 and 1H-3) into the pcDNA3.1 plasmids, respectively, as described above. After co-transfecting the paired plasmids into HEK293F cells, humanized anti-IL-6R antibodies in the supernatant were collected and purified using protein A columns. Western blot results shown in Fig. S1C demonstrate the bioactivity of three humanized antibodies in inhibiting the phosphorylation of STAT3. Notably, their inhibitory effects appear stronger than tocilizumab at the same concentrations. The antibodies 1 K1/1H3, 1 K2/1H3 and 1 K3/1H3 are hereafter designated as HZ0412a, HZ0412b and HZ0412c, respectively. To assess the antigen-binding activity of these humanized antibodies, we performed ELISA using the sIL-6R-mFc coated plates as described in section Materials and Methods. HZ0412a and its variants, as well as tocilizumab, bind to sIL-6R-mFc in a dose-dependent manner (Fig. 1A), and the half-binding concentrations EC50 are 0.015 μg/ml for HZ0412a and 0.031 μg/ml for tocilizumab. The protein–protein interaction between HZ0412a and sIL-6R was studied by MP [14]. As shown in Fig. 1B, HZ0412a manifested a strong specific association with sIL-6R, with the KD being measured at 14.5 nM (SD = ±2.3, n = 2). Under the same condition, tocilizumab also showed strong affinity to its antigen and the KD is 38.8 nM (SD = ±4.8, n = 3), while an irrelevant antibody showed no binding to sIL-6R at all (data not shown). Taken together, all these results demonstrate that the binding affinity of HZ0412a to sIL-6R is higher than tocilizumab.
Figure 1.
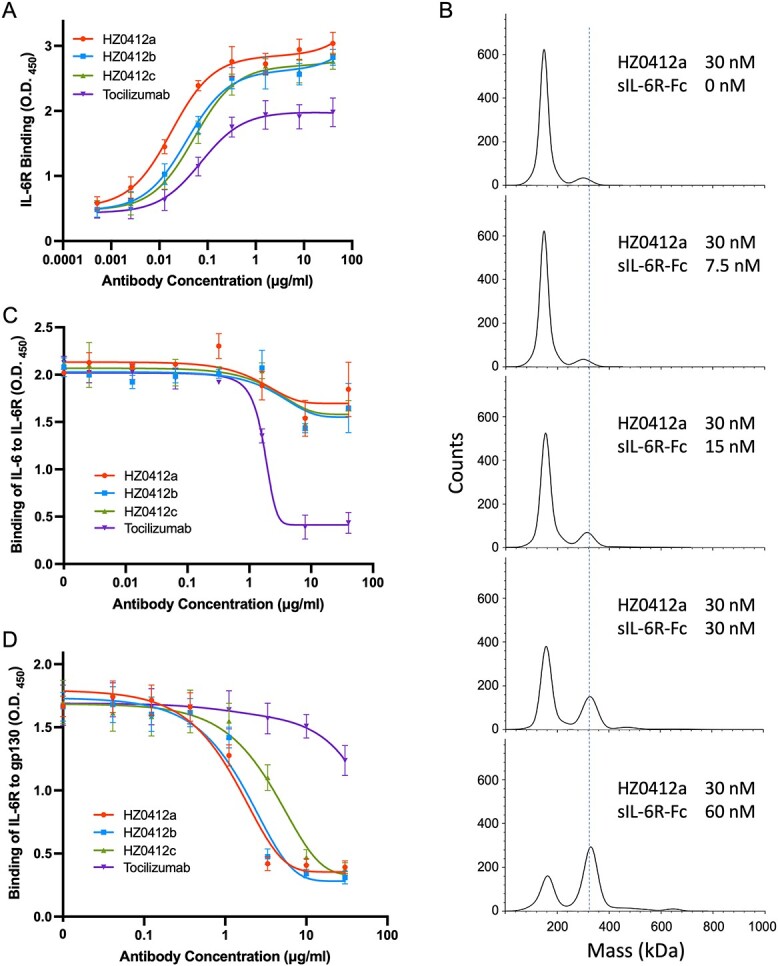
Characterization of humanized antibody HZ0412a and its variants. (A) Concentration-dependent binding of IL-6R antibodies to rhIL-6. Antibodies at different concentrations were incubated with rhIL-6-mFc fusion proteins that had been coated in 96-well plates. After extensive washing, the amount of antibodies retained in the wells was measured by ELISA. (B) Measurement of HZ0412a-sIL-6R interaction by MP. Typical mass distribution plots of 30 nM HZ0412a with sIL-6R-Fc, at different concentrations (0, 7.5, 15, 30 and 60 nM) are shown. KD was calculated according to the mass distribution plot of Ab (30 nM)/Ag (30 nM) reaction. (C) HZ0412a and its variants do not significantly affect the binding of rhIL-6 to sIL-6R. sIL-6R-mFc fusion proteins were incubated with surface-coated rhIL-6-His fusion proteins in 96-well plates in the presence of different concentrations of antibodies. After extensive washing, the amount of sIL-6R-mFc fusion proteins retained in the wells were measured by ELISA. (D) HZ0412a and its variants block the binding of IL-6R to gp130 in a concentration-dependent manner. 96-well plates were first coated with purified recombinant gp130-His fusion proteins. Then, rhIL-6-hFc, sIL-6R-mFc and antibodies at various concentrations were added to the wells. After incubation and strict wash, the amount of sIL-6R-mFc fusion proteins retained in the wells were measured by ELISA. All ELISA results are expressed as the absorbance at 450 nm. Tocilizumab was used as a positive control and included in all experiments, which were repeated at least three times. Mean absorbance at 450 nm and SD values are presented.
Using the same ELISA method, we found that HZ0412a binds to IL-6 receptors from both cynomolgus monkey and rat origins, but it took place at higher concentrations (Fig. S2A and B). On the other hand, tocilizumab binds to cynomolgus monkey IL-6R with an EC50 similar to human IL-6R, and it does not recognize rat IL-6R at all (Fig. S2A and B). We further tested the cross-reactivity of HZ0412a to other cytokine receptors. ELISA results demonstrate that HZ0412a does not have any specific binding to the receptors of other human IL-6 family cytokines such as IL-11, oncostatin M (OSM) and the leukemia inhibitory factor (LIF). The IL-6 receptor β, gp130, does not directly interact with HZ0412a either (Fig. S2C). Together, our results demonstrate that HZ0412a is highly specific for human IL-6R.
Tocilizumab has been shown to prevent IL-6R from binding to its ligand [5]. We then examine the effect of HZ0412a on the interaction between IL-6 and IL-6R. We coated 96-well plates with sIL-6R-His fusion protein and measured the binding of rIL-6-mFc under various conditions. As shown in Fig. 1C, IL-6 binds to sIL-6R very well in the absence of humanized antibodies, and as expected, tocilizumab blocks the binding in a dose-dependent manner. However, the effect of HZ0412a and its variant antibodies are marginal, if any (Fig. 1C). Our results suggest that HZ0412a targets a different epitope on human IL-6R compared to tocilizumab, and the epitope is outside of the region critical for IL-6 binding.
HZ0412a and its variants inhibit IL-6-induced phosphorylation of STAT3 (Fig. S1C), yet they do not block IL-6/IL-6R binding. A feasible explanation of those seemingly contradictory data is that HZ0412a may disrupt the interaction between IL-6R and gp130, the signal transducer of IL-6 signaling. To test this possibility, we coated 96-well plates with gp130-His fusion protein and then added an equal amount of rIL-6-hFc and sIL-6R-mFc fusion proteins. After incubation and extensive wash, a significant amount of sIL-6R-mFc could be detected in the wells by ELISA (Fig. 1D). However, in the presence of our humanized antibodies, the amount of IL-6R retained in wells decreased dramatically in a dose-dependent manner. When used at 6.7 μg/ml, HZ0412a almost completely blocked the binding of IL-6R to gp130. In contrast, tocilizumab at the same concentration had almost no effect (Fig. 1D). Taken together, these results suggest that HZ0412a and tocilizumab have different work mechanisms, though they both bind to human IL-6R.
To investigate the neutralizing activity of HZ0412a, we utilized three cell-based assays. First, we measured the effects of the antibodies on IL-6-induced phosphorylation of STAT3 in DLD-1 cells. As shown in Fig. 2A, both HZ0412a and tocilizumab inhibit the phosphorylation of STAT3 in a dose-dependent manner. From the dose–effect curves in Fig. 2B, the IC50 of HZ0412a is calculated at 6.56 ng/ml and that of tocilizumab is 0.27 μg/ml. These results demonstrate that HZ0412a and its variants are more potent blockers of IL-6-induced JAK/STAT3 signaling than tocilizumab.
Figure 2.
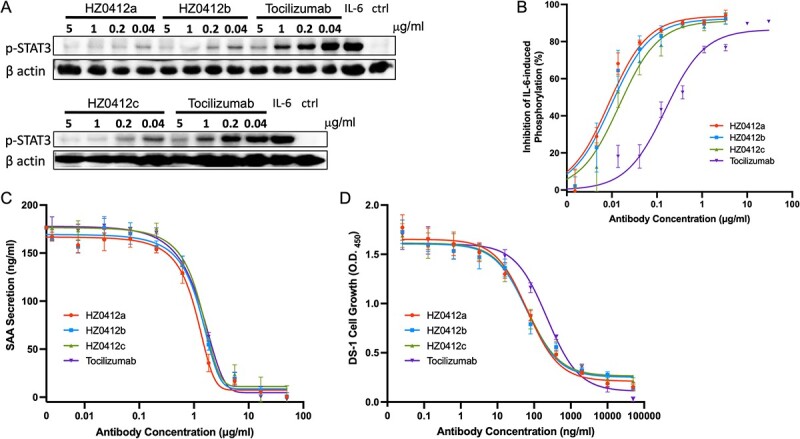
IL-6 signaling is blocked by HZ0412a and its variants in vitro. (A) All humanized IL-6R antibodies inhibited IL-6-induced phosphorylation of STAT3 in DLD-1 cells in a concentration-dependent manner. Cells were stimulated with rhIL-6 and the phosphorylation of STAT3 at Tyr705 was detected by a phosphorylation site–specific antibody as described in section Materials and Methods. A representative western blot result is shown. β actin was chosen to monitor equal loading of samples. (B) Summary of phosphorylation inhibition experiments. The specific phosphorylation signals on western blot were quantified and normalized with corresponding β actin signals. The normalized signals in the absence of IL-6R antibodies are used as a reference (100%), and the degrees of signal reduction are plotted against the concentrations of antibodies. (C) The inhibition of IL-6/IL-1β-induced SAA secretion by IL-6R antibodies in Hep G2 cells is concentration-dependent. Hep G2 cells (2 × 105) were stimulated with IL-6 and IL-1β with or without antibodies as described in section Materials and Methods. The supernatant was collected for the measurement of SAA by ELISA. (D) IL-6-induced DS-1 cell growth can be suppressed by IL-6R antibodies in a concentration-dependent manner. To quantify the DS-1 cell proliferation, the Cell Counting Kit-8 (CCK8) was used, and the results are expressed as mean absorbance at 450 nm with SD values. Tocilizumab was included as a positive control. All experiments were repeated at least three times.
HZ0412a suppresses IL-6-induced SAA secretion and cell proliferation
SAA is an acute-phase protein and a precursor of amyloid A (AA) protein in AA (secondary) amyloidosis, which is a serious complication of chronic inflammatory diseases including RA [15]. SAA can be expressed and secreted by the hepatocellular carcinoma cell line Hep G2 after stimulation with a cocktail of IL-1β (final concentration 25 ng/ml)/rIL-6-his (100 ng/ml)/sIL-6R (200 ng/ml). Using this model, we further examined the ability of HZ0412a to inhibit IL-6 signaling. In the absence of HZ0412a, SAA secretion could be detected in the supernatant at high levels (Fig. 2C). After pre-incubating the cocktail with HZ0412a at concentrations higher than 0.21 μg/ml, the secretion of SAA starts to decline, and the inhibition is concentration-dependent (Fig. 2C). Tocilizumab is found to have a similar inhibitory effect. The IC50 of HZ0412a in this assay is 0.85 μg/ml, whereas tocilizumab is 1.43 μg/ml.
DS-1 is a B-lymphoma cell line, and its proliferation is supported by endogenous IL-6 and can be enhanced by the addition of exogenous IL-6. In this study, we found that the growth effect of IL-6 (20 ng/ml) was abolished by HZ-0412 and tocilizumab in a concentration-dependent manner (Fig. 2D). The IC50 of HZ-0412 in this test is 57.84 ng/ml, whereas tocilizumab is 242.9 ng/ml. Taken together, our data demonstrate the constant high potency of HZ0412a in inhibiting IL-6 signaling.
Single dosing of HZ0412a is well tolerated in non-human primate and PK studies
HZ0412a was administrated to cynomolgus monkeys as a single subcutaneous injection at a dose of 1 or 5 mg/kg. One male and one female were used for each dosing. All animals were evaluated for changes in clinical signs, food consumption, body weights and clinical pathology parameters. No treatment-related effects were observed. To study the PK of HZ0412a, we collected blood samples from those monkeys by venipuncture using tubes with no anticoagulant before dosing and at different time points after dosing. The blood levels of HZ0412a were measured as described in section Materials and Methods. The serum concentration–time profiles following a single subcutaneous injection dose of 1 or 5 mg/kg in monkeys are shown in Fig. 3. The results are based on a non-compartmental model and summarized in Table 1. Following a single subcutaneous dose of 1–5 mg/kg, Tmax was highly variable ranging from 8 to 96 h and Cmax appeared to be dose proportional. AUC was dose proportional. The apparent CL and apparent Vz after 1 and 5 mg/kg SC were ~ 400 ml/h/kg and ~ 60,000–96,000 ml/kg, respectively. Collectively, these data clearly showed that HZ0412a could be safely administrated at doses that are able to achieve potentially therapeutic serum levels.
Table 1.
PK parameters of HZ0412a after single subcutaneous administration in cynomolgus monkeys.
| 1 mg/kg (n = 2,1F/1M) | 5 mg/kg (n = 2,1F/1M) | ||||||
|---|---|---|---|---|---|---|---|
| Parameters | Units | Mean | ± | SD | Mean | ± | SD |
| AUC(0-t) | mg/ml*h | 2.55 | ± | 0.774 | 12.8 | ± | 3.62 |
| AUC(0-∞) | mg/ml*h | 2.57 | ± | 0.810 | 13.1 | ± | 3.87 |
| MRT(0-t) | d | 7.36 | ± | 2.44 | 8.84 | ± | 0.348 |
| MRT(0-∞) | d | 7.66 | ± | 2.82 | 9.31 | ± | 0.491 |
| VRT(0-t) | d^2 | 39.4 | ± | 24.1 | 58.4 | ± | 2.79 |
| VRT(0-∞) | d^2 | 49.5 | ± | 37.1 | 74.7 | ± | 1.55 |
| t 1/2z | d | 4.08 | ± | 2.21 | 5.32 | ± | 0.455 |
| T max | d | 1.67 | ± | 1.89 | 2.00 | ± | 1.41 |
| CLz/F | ml/h/kg | 0.417 | ± | 0.118 | 1.04 | ± | 0.766 |
| V z/F | L/kg | 0.0525 | ± | 0.0134 | 0.0905 | ± | 0.00919 |
| C max | mg/L | 12.0 | ± | 0.992 | 46.8 | ± | 7.120 |
DISCUSSION
Our characterization of HZ0412a demonstrated a favorable anti-IL-6R antibody profile, including effectively binding to IL-6R and successfully antagonizing the interaction of IL-6R and gp130. HZ0412a binds with high affinity to the human IL-6 receptor, with a binding affinity (KD) of 14.5 nM. This binding profile is almost three folds stronger than tocilizumab that has been approved in anti-IL-6 therapies.
HZ0412a strongly inhibited IL-6-induced acute phase protein secretion and cell proliferation, but it did not block the binding of IL-6 to its receptor. Mechanistically, HZ0412a disrupts the interaction between IL-6R and gp130, the downstream signal transducer of IL-6 signaling—a very different mechanism from that of tocilizumab. However, it is reminiscent of a humanized anti-IL-6 antibody, olokizumab, whose binding to IL-6 epitope site 3 does not interrupt the interaction between IL-6 and IL-6R. Instead, it blocks the interaction with gp130 [16]. The phase 3 clinical trial of olokizumab in the treatment of rheumatoid arthritis has shown favorable results [17]. Whether there are any extra benefits from targeting IL-6R with HZ0412a would be an interesting question and needs further studies. Yet, there is potential advantage if the antibody only interrupts the interaction between IL-6R and gp130. Previous studies showed that antibodies that block IL-6 and IL-6R interaction may trigger complex feedback, increase IL-6 levels in vivo over time [18] and eventually dampen their own inhibitory effect on IL-6 signaling.
Pharmacokinetic evaluation of HZ0412a in single-dose study in cynomolgus monkeys indicated that HZ0412a was well tolerated with a dose-proportional and typical mAb pharmacokinetic profile. However, it is worth noting that due to HZ0412a’s low affinity to cynomolgus IL-6R, cynomolgus monkeys may not be a good animal model to carry out toxicity studies. Taken together, these data demonstrate that HZ0412a is a potent IL-6 receptor antagonist, with properties that support its clinical investigation in patients with cancers and autoimmune diseases.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank Dr. Yihong Ye at NIDDK (NIH) for extremely important discussion and support. We also thank Dr. Yi Zhang at Hackensack Meridian Health Center for Discovery and Innovation for his insightful comments and discussion and Steven Schneible at Coriell Institute for assistance with manuscript editing.
Contributor Information
Jianzhong Han, Coriell Institute for Medical Research, Camden, NJ 08103, USA.
Xiaolei Liu, Department of Medicine, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, PA 19104, USA.
Yue Xu, Laboratory of Molecular Biology, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, MD 20892, USA.
Qian Wang, IPHASE Therapeutic Ltd., Philadelphia, PA 19454, USA.
Li Li, IPHASE Therapeutic Ltd., Philadelphia, PA 19454, USA.
Kehe Du, IPHASE Therapeutic Ltd., Philadelphia, PA 19454, USA.
Chenchen Li, Department of Medicine, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, PA 19104, USA.
Hongjun Liu, IPHASE Therapeutic Ltd., Philadelphia, PA 19454, USA.
Yu Chen, Department of Pulmonary and Critical Care Medicine, Xuanwu Hospital Capital Medical University, Beijing 100053, China.
Jian Huang, Coriell Institute for Medical Research, Camden, NJ 08103, USA; Center for Metabolic Disease Research, Temple University Lewis Katz School of Medicine, Philadelphia, PA 19140, USA; Cooper Medical School of Rowan University, Camden, NJ 08103, USA.
FUNDING
This work was supported by the funding from IPHASE Therapeutic and a seed grant to J.H. from Coriell Institute for Medical Research.
CONFLICTS OF INTEREST STATEMENT
H.L. is the founder of IPHASE Therapeutic. Q.W., L.L. and K.D. are employed by IPHASE Therapeutic. No potential conflicts of interest were disclosed by the other authors.
AUTHORS’ CONTRIBUTIONS
H.L., Y.C. and J.H. contributed to the conception and design of the study. J.Z.H., X.L., Q.W. and L.L. performed the experiments and statistical analysis. J.Z.H. and X.L. wrote the first draft of the manuscript. L.L. wrote sections of the manuscript. All authors contributed to manuscript revision, read and approved the submitted version.
DATA AVAILABILITY STATEMENT
The data underlying this article are available in the article and in its online supplementary material.
ANIMAL RESEARCH
The animal study was reviewed and approved by the Institutional Animal Care and Use Committee that belongs to iPhase Pharma Services, Ltd., China.
ETHICS AND CONSENT STATEMENT
No patients were involved in this study, so the consent was not required.
References
- 1. Wolf, J, Rose-John, S, Garbers, C. Interleukin-6 and its receptors: a highly regulated and dynamic system. Cytokine 2014; 70: 11–20. [DOI] [PubMed] [Google Scholar]
- 2. Kang, S, Tanaka, T, Narazaki, Met al. Targeting Interleukin-6 Signaling in clinic. Immunity 2019; 50: 1007–23. [DOI] [PubMed] [Google Scholar]
- 3. Han, H, Ma, Q, Li, Cet al. Profiling serum cytokines in COVID-19 patients reveals IL-6 and IL-10 are disease severity predictors. Emerg Microbes Infect 2020; 9: 1123–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rubin, EJ, Longo, DL, Baden, LR. Interleukin-6 receptor inhibition in Covid-19 - cooling the inflammatory soup. N Engl J Med 2021; 384: 1564–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sato, K, Tsuchiya, M, Saldanha, Jet al. Reshaping a human antibody to inhibit the interleukin 6-dependent tumor cell growth. Cancer Res 1993; 53: 851–6. [PubMed] [Google Scholar]
- 6. Venkiteshwaran, A. Tocilizumab. MAbs 2009; 1: 432–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Burmester, GR, Lin, Y, Patel, Ret al. Efficacy and safety of sarilumab monotherapy versus adalimumab monotherapy for the treatment of patients with active rheumatoid arthritis (MONARCH): a randomised, double-blind, parallel-group phase III trial. Ann Rheum Dis 2017; 76: 840–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Choy, EH, de Benedetti, F, Takeuchi, Tet al. Translating IL-6 biology into effective treatments. Nat Rev Rheumatol 2020; 16: 335–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Heo, TH, Wahler, J, Suh, N. Potential therapeutic implications of IL-6/IL-6R/gp130-targeting agents in breast cancer. Oncotarget 2016; 7: 15460–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Masjedi, A, Hashemi, V, Hojjat-Farsangi, Met al. The significant role of interleukin-6 and its signaling pathway in the immunopathogenesis and treatment of breast cancer. Biomed Pharmacother 2018; 108: 1415–24. [DOI] [PubMed] [Google Scholar]
- 11. Liu, X, Li, L, Wang, Qet al. A novel humanized anti-Interleukin-6 antibody HZ0408b with anti-rheumatoid arthritis therapeutic potential. Front Immunol 2022; 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Holzlohner, P, Hanack, K. Generation of murine monoclonal antibodies by Hybridoma technology. J Vis Exp 2017; 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Almagro, JC, Fransson, J. Humanization of antibodies. Front Biosci 2008; 13: 1619–33. [DOI] [PubMed] [Google Scholar]
- 14. Wu, D, Piszczek, G. Measuring the affinity of protein-protein interactions on a single-molecule level by mass photometry. Anal Biochem 2020; 592: 113575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Uchiyama, Y, Yorozu, K, Hashizume, Met al. Tocilizumab, a humanized anti-interleukin-6 receptor antibody, ameliorates joint swelling in established monkey collagen-induced arthritis. Biol Pharm Bull 2008; 31: 1159–63. [DOI] [PubMed] [Google Scholar]
- 16. Shaw, S, Bourne, T, Meier, Cet al. Discovery and characterization of olokizumab: a humanized antibody targeting interleukin-6 and neutralizing gp130-signaling. MAbs 2014; 6: 774–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Smolen, JS, Feist, E, Fatenejad, Set al. Olokizumab versus placebo or Adalimumab in rheumatoid arthritis. N Engl J Med 2022; 387: 715–26. [DOI] [PubMed] [Google Scholar]
- 18. Rossi, JF, Lu, ZY, Jourdan, Met al. Interleukin-6 as a therapeutic target. Clin Cancer Res 2015; 21: 1248–57. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the article and in its online supplementary material.


