Abstract
Obesity and metabolic syndrome (MetS) are commonly observed in patients with epilepsy (PWE). Obesity and MetS are not only affecting the physical fitness and quality of life of these patients, rather antiepileptic drugs (AEDs) compliance and seizure control have also been affected. The objective of this review is to search the published literature regarding the prevalence of obesity and MetS in PWE and their relation to the response to AEDs. A comprehensive search using PubMed, Cochrane Databases, and Google Scholar was performed. A supplementary citation search was also conducted by analyzing the reference lists of identified sources. The initial search revealed 364 articles of potential relevance. The studies were analyzed in detail to obtain clinical information relevant to the objectives of the review. Many observational, case control studies, randomized control trials and few review articles were included for critical appraisal and review writing. Epilepsy is associated with MetS and obesity in all age groups. AEDs and lack of exercise are the chief causes while metabolic disturbances such as adiponectin, mitochondrial dysfunction, valproic acid (VPA)-associated insulin resistance, leptin deficiency, and endocrine dysfunction are also addressable factors. Although the risk of drug-resistant epilepsy (DRE) is also higher among obese PWE, the interaction between, MetS, and its components with DRE remain to be fully investigated. Further research is required to elucidate their interplay. Appropriate and careful selection of AEDs without compromising therapeutic efficacy supplemented by lifestyle counseling for exercise and diet should be practiced to avoid weight gain and potential DRE.
Keywords: Antiepileptic drugs, drug-resistant epilepsy, epilepsy, metabolic syndrome
Résumé
L’obésité et le syndrome métabolique (MetS) sont couramment observés chez les patients épileptiques (PWE). L’obésité et le MetS n’affectent pas seulement la condition physique et la qualité de vie de ces patients, mais plutôt l’observance des médicaments antiépileptiques (DEA) et le contrôle des crises ont également été affectés. L’objectif de cette revue est de rechercher la littérature publiée concernant la prévalence de l’obésité et du MetS chez les PWE et leur relation avec la réponse aux DEA. Une recherche exhaustive à l’aide des bases de données PubMed, Cochrane et Google Scholar a été effectuée. Une recherche de citation supplémentaire a également été effectuée en analysant les listes de référence des sources identifiées. La recherche initiale a révélé 364 articles potentiellement pertinents. Les études ont été analysées en détail afin d’obtenir des informations cliniques pertinentes aux objectifs de la revue. De nombreuses études observationnelles, des essais contrôlés randomisés et des articles de synthèse ont été inclus pour l’évaluation critique et la rédaction de la synthèse. L’épilepsie est associée au MetS et à l’obésité dans tous les groupes d’âge. Les antiépileptiques et le manque d’exercice sont les principales causes, tandis que les troubles métaboliques tels que l’adiponectine, le dysfonctionnement mitochondrial, la résistance à l’insuline associée à l’acide valproïque (VPA), la carence en leptine et le dysfonctionnement endocrinien sont également des facteurs adressables. Bien que le risque d’épilepsie résistante aux médicaments (ERD) soit également plus élevé chez les PWE obèses, l’interaction entre le MetS et ses composants avec l’ERD reste à étudier en profondeur. Des recherches supplémentaires sont nécessaires pour élucider leur interaction. Une sélection appropriée et minutieuse des antiépileptiques sans compromettre l’efficacité thérapeutique, complétée par des conseils sur le mode de vie pour l’exercice et le régime alimentaire, doit être pratiquée afin d’éviter la prise de poids et un toucher rectal potentiel.
Mots clés: syndrome métabolique, épilepsie, médicaments antiépileptiques, épilepsie résistante aux médicaments
INTRODUCTION
Obesity, which is now regarded as a common public health problem throughout the world, is defined as “A chronic, multifactorial disease involving excessive body fat with complex psychological, environmental (social and cultural), genetic, physiologic, metabolic, and behavioral causes and consequences.”[1] While Metabolic syndrome (MetS) is a term used when a few specific interrelated cardiovascular risk factors cluster in an individual that results in a two-fold increased risk of cardiovascular disease.[2]
Obesity-induced metabolic dysfunction, inflammation, and dyslipidemias are attributable factors for the development of certain neurological disorders including Alzheimer's and Parkinson's diseases. Scientific evidence suggests that these obesity-related changes not only cause central nervous system damage but the peripheral nervous system is also directly and indirectly impacted by obesity.[3] Obesity and MetS are also commonly observed in people with epilepsy.[4,5]
The relationship between obesity and MetS in patients with epilepsy (PWE) has been addressed in the current published literature.[6,7] There are several possible mechanisms and contributory factors responsible for increased prevalence of overweight and obesity in epilepsy. These factors are behavioral, social, environmental, and iatrogenic factors.[8,9] Some of the antiepileptic drugs (AED) can also cause weight gain and subsequent metabolic disturbances and considerable changes in plasma lipids and hormonal levels.[10,11,12] Obesity and MetS are not only affecting the physical fitness and quality of life of these patients but medication compliance and seizure control have also been affected. Few studies have suggested that a patient's body mass index (BMI) may also predict AED response in PWE and drug-resistant epilepsy (DRE).[12,13,14] PWE receiving polytherapy regimen and few particular AEDs such as valproic acid (VPA) and pregabalin are more prone to have high BMI, obesity, and DRE.
This review aims to search the published literature for the prevalence of obesity and MetS in PWE and their effect on the response to AEDs. The aim is to supplement the existing published literature on the subject, which is very limited. We also hope that this review will help in finding the role of factors that affect response to AEDs in epilepsy patients, thus guiding future studies regarding novel biomarkers of AED response and prognosis.
METHODS AND ANALYSIS
Search strategy
Data sources
A comprehensive search of the published literature was conducted using the search terms “MetS, obesity, and epilepsy” then “obesity and epilepsy control.” The review was limited to articles written in the English language. The databases searched included PubMed, Cochrane Databases, and Google Scholar. A supplementary citation search was also conducted by analyzing the reference lists of identified sources.
Article screening and criteria
The author first categorized as relevant or irrelevant by screening the abstract. Articles were included the studies focused on epilepsy, MetS, obesity, AED proposed mechanisms and associations, and epilepsy control.
Study selection
The initial search revealed 364 articles of potential relevance.
Data extraction
The author in details analyzed the studies, to obtain the clinical information relevant to meeting the objectives of the review.
Data synthesis
The author grouped articles according to topic and types of studies within each group.
RESULTS
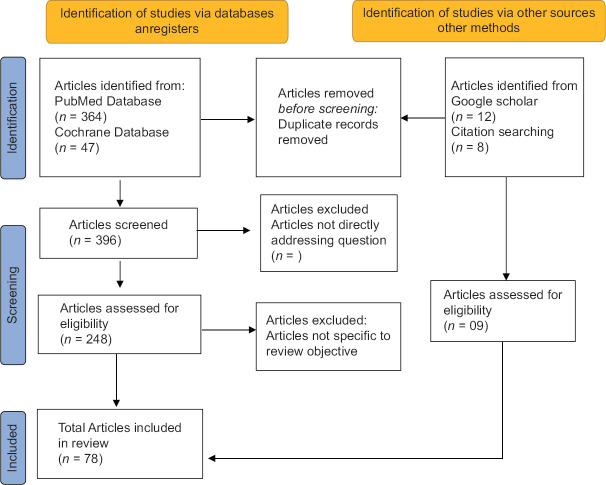
Initially, 364 articles were identified on PubMed [Figure 1]. For greater specificity, only those articles referencing MetS, obesity, and epilepsy control were selected. Articles related to any hormonal or molecular mechanisms associated with these conditions were also included. An electronic search in Cochrane databases resulted in 47 articles. Additional searches on Google Scholar were also performed. Duplicates were excluded. Articles not addressing the question were excluded. This yielded 248 articles. Finally, a total 78 selected articles, specific to the study objectives were selected for this review writing.these selected articles were comprised of observational studies, randomomized control trials and few review articles.
Figure 1.
Flow diagram showing search strategy followed in this review
Table 1 shows the selected studies addressing the prevalence of obesity and MetS, in PWE. The prevalence in different age groups was extracted through large population-based surveys or cross-sectional and case–control studies. Obesity and MetS were reported in PWE of all age groups. Few prospective studies, reviews, and meta-analysis discussed the possible genetic, molecular, and metabolic mechanisms and causes of weight gain leading to MetS and obesity in PWE. Many studies related to the role of AEDs causing weight gain and subsequent metabolic disturbances were identified especially VPA-associated MetS in different age groups, its possible mechanism and comparisons with other AED were elaborated in different prospective, comparative, and randomized control trials.
Table 1.
Metabolic syndrome, obesity- and epilepsy-related studies
| Reference | Study type | Number of patients (n) | Patient population | Study objective | Key results (%) | Outcomes and comments |
|---|---|---|---|---|---|---|
| FX Ndayambaje et al., 2021[18] | Cross- sectional, observational | 1076 | Adult PWE | To assess prevalence of MetS in Rwandan Patients | MetS=30.6 The main risk factors were VPA (P=0.007) and Sedentary lifestyle (P=0.025) |
High prevalence of MetS in epileptic patients |
| Vooturi S et al., 2020[17] | Cross- sectional, observational | 173 | Adult PWE | To assess association of MetS variables with patients QoL | MetS=52.6 Obesity=88.4 More number of women had MetS (47.6 vs. 62.6; P=0.049) | More than half of epileptic patients across all age groups had MetS The number of AEDs and QoL are not associated with MetS |
| Kadima NT et al., 2013[6] | Health interview survey | 26,659 | Adult PWE | To assess the prevalence of nonpsychiatric comorbidities in PWE | Obesity=27.5 Over weight=34.5 | Prevalence of cardiovascular and metabolic disorders in epileptic patients Obesity is higher in PWE than patients without epilepsy |
| Claire Hinnel et al., 2010[15] | Community health survey | 400,055 | Adult population | To assess health-related behaviors and health status between PWE, migraine or diabetes | Obesity in epileptic patients=19.1 Obesity in migrainers=18.0 Obesity in diabetic patients=37.1 Obesity in general population=15.4 | Obesity and comorbidities were more likely in all chronic conditions studied compared to the general population |
| Daniels Z et al., 2009[19] | Case–control | 848 Cases: 251 Controls: 597 | Children and adolescents patients Healthy control | To assess the frequency and factors associated with obesity with newly diagnosed untreated epilepsy | Obesity and overweight=38.6 Adolescents had higher adjusted BMI Z-scores than younger patients | Obesity is common in children with newly diagnosed untreated epilepsy and correlates with increasing age, idiopathic etiology |
| Rosemarie Kobauet et al., 2004[16] | Population bases health interview survey | 8057 | Adult population | To assess behavioral risk factors and comorbidities in adults with epilepsy | Obesity in epileptic patients=34.1 Obesity in adults without epilepsy=23.7 | Adults with epilepsy had high rates of obesity and other behavioral risks and complications associated with other chronic disorders |
MetS=Metabolic syndrome, VPA=Valproic acid, QoL=Quality of life, BMI=Body mass index, PWE=Patients with epilepsy, AEDs=Antiepileptic drugs
We found few studies addressing the influence of obesity and MetS on epilepsy control and how they increase the risk of developing DRE [Table 2]. Most of these studies were either cross-sectional or case–control. Large-scale randomized control studies are lacking.
Table 2.
Studies addressing the metabolic syndrome, obesity and epilepsy control
| Reference | Study type | Number of patients | Study population | Study objective | Key results | Outcome and comments |
|---|---|---|---|---|---|---|
| Man Chen et al., 2021[71] | Case control study | n=1617 Drug responsive epilepsy=1272 Drug resistance epilepsy=345 | Adult PWE | To assess effect of overweight and obesity on DRE | Obesity and increased risk of DRE (adjusted OR, 2.339; 95% CI, 1.724–3.171) Obesity and increased risk of DRE in patients on VPA (adjusted OR, 1.79; 95% CI, 1.15–2.80) | No significant increase in the risk of DRE was found to be associated with overweight But Obesity potentially plays a role in DRE |
| R. Arya et al., 2016[13] | Case control | n=2525 Case=446 Control 2079 | Children with childhood absence epilepsy and healthy controls | To assess the role of obesity and overweight as predictors of short term drug responsiveness | Obesity and overweight in cases=33.8% Obesity and overweight in control=25.3% Obese children with Increase BMI Z score on VPA and ETX, in had higher odds of achieving FFF (OR 2.75, 95% CI 1.01–7.46, P=0.047) SF (OR 4.89, 95% CI 1.08–22.11, P=0.040) | Overweight/obesity status or increasing BMI Z score was associated with improved seizure outcomes in those receiving ETX and VPA and worse outcomes in patients on LTG compared to weight-appropriate children with CAE |
| Rochelly de Azevedo Fernandez et al., 2015[69] | Cross- sectional, observational | n=72 | Adult PWE | To assess the nutritional status of epileptic patients | Overweight/obesity=66.7%BMI kg/m2 in Men with controlled seizures: 27.0±3.3 Men with uncontrolled seizures, 28.5±5.2 (P=0.376) Women with controlled seizures=29.2±5.9 Women with uncontrolled seizures=28.4±7.7 (P=0.737) | PWE are having tendency to be overweight and obese There was significant differences between the BMI values of men and women with uncontrolled and controlled epilepsy Seizure control did not appear to be related to nutritional status and intake |
| Ladino LD, et al., 2014[70] | Cross- sectional, observational | n=100 | Adult PWE | To assess the prevalence of obesity and its association with drug-resistant epilepsy | Obesity=38% DRE in obese=55% DRE in nonobese=55% | There is an association between obesity, idiopathic generalised epilepsy, and family history of epilepsy. But No association was found between drug-resistant epilepsy and obesity |
| Janousek J et al., 2013[12] | Cross- sectional, observational | n=554 | Adult PWE | To assess the frequency of obesity in a patient population with epilepsy | Obesity and Overweight=55.2% in sample Overweight in patients with refractory epilepsy (60.4% vs. 49.2%) Obesity in patients with refractory epilepsy (36.9% vs. 24.6%) Obesity in patients treated with polytherapy (37.7% vs. 25%) | Overweight/obesity and obesity rates were higher in patients with refractory than nonrefractory epilepsy Obesity was more common in patients treated with polytherapy than monotherapy |
DRE=Drug-resistant epilepsy, BMI=Body mass index, VPA=Valproic acid, ETX=Ethosuximide, FFF=Freedom from failure, SF=Seizure freedom, CI=Confidence interval, OR=Odd ratio, CAE=Childhood absence epilepsy, PWE=Patients with epilepsy, LTG=Lamotrigine
DISCUSSION
Metabolic syndrome, obesity, and epilepsy in different populations and age groups
Different epidemiological studies have identified the prevalence of obesity in the form of weight gain and metabolic disturbances as MetS, in PWE.[15,16] However, the long-term conducted studies addressing the relationship between obesity, MetS, and epilepsy are lacking. Obesity and MetS are reported in PWE of all age groups. Vooturi and Jayalakshmi identified MetS in more than half of PWE in a cross-sectional study.[17] Ndayambaje et al. found that MetS is highly prevalent among PWE in Rwandan.[18] A Brazilian cross-sectional study showed that elderly patients with new-onset epilepsy had concomitant abdominal obesity.[7] In one large US study, 34.1% of adult patients with any type of epilepsy were more obese than patients without having epilepsy 27.5%.[6] in one pediatric age group study, older children with idiopathic epilepsy were found more obese than those without epilepsy and concomitant medications did not affect obesity in this cohort.[19] Similar findings were also observed in a study by Arya et al. the study showed the children with newly diagnosed childhood absence epilepsy (CAE) were overweight and obese, despite being on lower carbohydrate intake and caloric restriction.[13]
Possible mechanisms of metabolic syndrome and obesity in patients with epilepsy
There are several possible mechanisms and causes of weight gain leading to MetS and obesity in PWE [Figure 2]. The main contributory causes are metabolic and AEDs related, simultaneous behavioral, social, and environmental factors are considered as further contributing elements. The best-known genetic epilepsy syndrome related to weight gain is the Prader–Willi syndrome. Preliminary studies with experimental models concluded that recurrent seizure activity itself could create a state of chronic inflammation.[20]
Figure 2.
MetS and obesity in epileptic patients, possible mechanisms. MetS = Metabolic syndrome, MnSOD = Manganese superoxide dismutase
Superoxide dismutase 2 (SOD2) is an enzyme related to antioxidant and anti-inflammatory pathways. While a single gene which encodes manganese superoxide dismutase (MnSOD), is located in chromosome 6q25. Single-nucleotide polymorphisms (Ala16Val (rs4880) of the SOD2 encoding gene have shown to be associated with inflammatory pathways and many metabolic disorders, such as obesity and dyslipidemia.[21] Recently, Kegler et al. have investigated the relationship between SOD Ala16Val SNP with epilepsy. An increased proportion of Ala16Val MnSOD polymorphism (VV genotype) was observed in PWE with simultaneous increased inflammatory markers.[22] Furthermore, it has been observed that adiponectin deficiency and mitochondrial dysfunction are common to epilepsy and type two diabetes mellitus (DM), which predispose people with epilepsy to have both obesity and DM.[23,24] Few other important parameters including insulin resistance (IR) and high leptin levels have also been observed in PWE. These parameters are linked to weight gain, lipid abnormalities, and subsequent atherosclerosis in these patients.[25] Among the social and environmental factors, lack of exercise and AEDs are important and addressable factors. Anxiety, depression, and perceived stigma of epilepsy are the factors leading to a lack of exercise and limited physical activities in these patients.[26] In one of the large surveys related to the health status and behavioral patterns of PWE, it was observed that (34% compared to 23.7%) of PWE were obese, they were found to have low levels of exercise despite good seizure control.[16]
Role of antiepileptic drugs in causing metabolic syndrome, obesity
Weight-related issues are observed with many AED, weight loss is seen in patients taking topiramate, (TPX), lamotrigine (LMG) Felbamate, and zonisamide.[27,28,29]
On the other hand, some of the AEDs can cause weight gain and subsequent metabolic disturbances, associated with significant health risks. Sometimes, cosmetic concerns leading to treatment compliance are also observed. Many studies quantify the risk of weight changes with different AEDs. Carbamazepine (CBZ), phenytoin, and VPA may also directly cause considerable changes in plasma lipid levels, which is a major risk factor for MetS.[12,30]
Antiepileptics associated with weight gain and obesity
Valproic acid
VPA is well known for its highest rates of obesity among all AEDs due to several possible mechanisms. VA-related weight gain and metabolic disturbances have been observed in both pediatric and adult age groups. Egger and Brett[30] reported bodyweight gain in 44% of children with epilepsy treated with VA at a daily dosage of 30–50 mg/kg, however, they did not find any relationship between this weight gain with the type of epilepsy or epilepsy control. In a double-blind study comparing LMG and VA, weight changes in patients on monotherapy were assessed.[31] After 32 weeks of treatment, >10% weight gain was reported in 62% of patients on monotherapy with VPA (12.8 ± 9.3 lb) than LMG-treated patients in whom weight remained stable (1.3 ± 11.9 lb). Weight increased in those treated with VPA by week 10 of this study and continued to increase at the end of the study. In this study, clinical variables such as gender, age, dose, and pretreatment body weight failed to identify those patients at a higher risk for weight gain.[32] In a 1-year study of new-onset seizures comparing CBZ, TPX, and VPA. Patients receiving VPA increased their weight by an average of 2.0–5.0 kg. CBZ was weight neutral.[33]
Valproic acid-induced metabolic syndrome and insulin resistance
VPA-induced metabolic disturbances include lipid abnormalities, hyperinsulinemia, centripetal obesity, disturbed hormonal metabolism leading to hyperandrogenism, and polycystic ovaries.[34] MetS has also been frequently observed in a patient taking VPA [Figure 3]. MetS is characterized by a higher frequency of abdominal obesity and hypertriglyceridemia than diabetes in PWE.[35,36]
Figure 3.
Valproic acid-induced MetS and obesity, possible mechanisms. MetS = Metabolic syndrome
It has also been observed that obese PWE treated with VPA are at higher risk of MetS than individuals who are “simply obese” but otherwise well.[37] However, one study from Estonia, comparing the risk of MetS among VA-treated PWA with the general population, did not find a difference in risk.[38]
Studies in both men and women revealed hyperinsulinemia and weight gain in those PWE who were treated with VPA, while CBZ and oxcarbazine (OXC) do not seem to have any significant effects on serum insulin or lipid levels in men with epilepsy.[10,11,27,39] VPA induces obesity, which could be related to the serum values of leptin and adiponectin.
Few studies showed significantly increased leptin level, hyperinsulinemia with oxidative stress, and a rise in BMI, in PWE who were treated with VPA.[40,41]
Further studies have confirmed that significant weight gain is related to high leptin and insulin levels.[42] The role of serum ghrelin and neuropeptide Y (NPY) level in patients taking VPA has been studied because of its link with obesity and weight gain as well. Ghrelin stimulates food intake and regulates energy homeostasis through activating the expression of the NPY and agouti-related protein in hypothalamic neurons, which plays a key role in obesity pathogenesis.[43] Evidence suggests that antioxidant enzymes are key regulators of inflammation. In one study, decreased concentration of lipid-soluble antioxidants α-tocopherol and α- and β-carotene were observed in PWE who gained weight after 1 year of VPA treatment.[44] VPA-treated patients were also found to have higher concentrations of triglycerides, serum cholesterol, uric acid, and lower levels of high-density lipoprotein cholesterol in a few studies.[42,45,46] While VPA-related metabolic disturbances leading to nonalcoholic fatty liver disease have also been reported in certain studies.[47,48]
Valproic acid and endocrine dysfunction
VPA also affects the metabolism of other hormones. Obesity, acne, hirsutism, and frequent anovulatory cycles are observed due to disturbed steroid hormone metabolism, leading to elevates androgens. It was observed in studies that patients who were receiving were found to have polycystic ovary syndrome with menstrual abnormalities and subsequent weight gain and obesity.[49,50]
Carbamazepine
CBZ therapy-induced weight gain is pointed out in a few studies. In one study up to 25% of patients, receiving CBZ had weight gain.[51,52] Excessive fat deposition; an increase in appetite leading to excessive food intake could be the contributory cause of weight gain. The dietary restriction did not put any effect in one study.[53] The role of leptin and insulin was not associated with CBZ-associated weight gain.[54]
Vigabatrin
Bodyweight gain was also pointed to a consistent finding in most preliminary clinical trials of vigabatrin.[55,56] The same was also reported in European and Canadian studies.[57,58]
Gabapentin
Weight gain with has also been reported with gabapentin,[59,60] the body weight gain observed with the use of gabapentin seems to be related to the prescribed dose.[61,62]
Pregabalin
In one study of PWE, receiving pregabalin mean bodyweight increase was 2.5–4.0 kg (standard deviation ± 4.1 kg).[63] Almost the same findings were observed in studies related to patients, who were being treated with pregabalin for neuropathic pain.[64]
Thus, if epilepsy per se does not cause weight gain or loss, then the three most important factors for weight changes are metabolic disturbances, lifestyle, and AEDs.
Metabolic syndrome, obesity, and epilepsy control
Overweight and obesity are recognized as important modifiers of severity, treatment response, natural history, and prognosis for several chronic diseases.[65,66]
Overweight and obesity being the common comorbidities in PWE also have been assessed in relation to seizure control. Satisfactory seizure control still remains the main concern in PWA as about one-third of PWA have seizures refractory to pharmacotherapy, defined as DRE.[67]
Satisfactory seizure control is considered when the patients being treated for epilepsy had more than 50% reduction in seizure frequency, pre- and post-intervention. Unsatisfactory seizure control is considered for patients who were treated for epilepsy, and received well-tolerated, appropriately chosen, and used, AED schedules (as monotherapies or in combination) yet they did not achieve more than 50% reduction of seizure frequency pre-and post-intervention. This included drug-refractory cases or DRE which were those patients who could not achieve sustained seizure freedom despite receiving at least two AED schedules.[68]
We found few studies addressing the influence of obesity and epilepsy control, and how they increase the risk for developing DRE in these patients [Table 2]. One study, addressing the nutritional status of PWE through their anthropometric profile, found no difference between the BMI values of patients with controlled and uncontrolled epilepsy, concluding that nutritional status is not related to seizure control in PWA.[69] In another study Ladino et al. appreciated the link between obesity, idiopathic generalized epilepsy, and family history of epilepsy, however, they did not find any association between obesity and DRE (55% vs. 55%; P = 0.05).[70] Previously Janousek et al. showed that overweight and obesity rates were higher in patients with DRE than in non-DRE (60.4% vs. 49.2% overweight, 36.9% vs. 24.6% obesity).[12]
Similar to Janousek et al.,[12] one recent case–control study by Chen et al. showed that obesity, but not overweight, was associated with DRE, and the risk of DRE was higher among PWA with obesity than among adults without obesity.[71]
Janousek et al. also observed that obesity was more common in patients treated with polytherapy than those treated with monotherapy (37.7% vs. 25.0%). This means indirectly their seizure was difficult to control.[12]
In a study by Arya, et al., weight gain with better seizure control in the pediatric population, is associated with improved seizure outcomes with nonconventional AED when compared to weight-appropriate children with CAE, authors also concluded about the importance of baseline BMI that may also predict AED response, unrelated to pharmacokinetic differences.[13]
The association of better seizure control with stabilization of BMI/weight loss has been a proposed mechanism for seizure treatment and is thought to be the basis of a ketogenic diet for seizure control.[72] The ketogenic diet is a well-established mode of treatment of epilepsy in children. However, there is less documentation on adults.[73]
Strength and limitations
When interpreting the findings yielded by this review, it is essential to consider its strength and limitations. In this review we tried to address an important medical and social issue, that is being faced by PWE, We identified an ample amount of literature and data pointing towards the strong association of obesity and epilepsy, metabolic changes associated with certain old AEDs were also addressed, however, we could not much address about new AEDs in this regard. We tried to give new insights to the clinician about the relationship between obesity MetS and seizure control but found very few studies in this regard.
CONCLUSIONS
Epilepsy is associated with MetS and obesity in all age groups. Different AEDs and lack of exercise are the chief causes while metabolic disturbances such as adiponectin, mitochondrial dysfunction, VPA-associated IR, leptin deficiency, and endocrine dysfunction are also addressed. Although the risk of DRE is also higher among obese PWA, the interaction between MetS and its components with DRE seizure control remains to be fully investigated. Further research is required to elucidate their interplay. Appropriate and careful selection of AEDs without compromising therapeutic efficacy supplemented by lifestyle counseling for exercise and diet should be practiced to avoid weight gain and protection against DRE.
Clinical points
A higher prevalence of MetS and obesity is observed in all age groups of PWE
The risk of DRE is also higher among obese PWA. However, the interaction between, MetS, and its components with DRE seizure control remains to be fully investigated
When managing PWE, the risk of MetS and obesity should be taken into account along with behavioral, social, and environmental factors.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
This study made use of the computational resources and technical services of the Scientific and High-Performance Computing Center at Imam Abdulrahman Bin Faisal University.
REFERENCES
- 1.Health Care Guideline. Prevention and Management of Obesity for Adults. 3rd ed. USA: Institute for Clinical Systems Improvement; 2013. [Google Scholar]
- 2.Nair SS, Harikrishnan S, Sarma PS, Thomas SV. Metabolic syndrome in young adults with epilepsy. Seizure. 2016;37:61–4. doi: 10.1016/j.seizure.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 3.O’Brien PD, Hinder LM, Callaghan BC, Feldman EL. Neurological consequences of obesity. Lancet Neurol. 2017;16:465–77. doi: 10.1016/S1474-4422(17)30084-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ben-Menachem E. Weight issues for people with epilepsy – A review. Epilepsia. 2007;48(Suppl 9):42–5. doi: 10.1111/j.1528-1167.2007.01402.x. [DOI] [PubMed] [Google Scholar]
- 5.Hamed SA. Antiepileptic drugs influences on body weight in people with epilepsy. Expert Rev Clin Pharmacol. 2015;8:103–14. doi: 10.1586/17512433.2015.991716. [DOI] [PubMed] [Google Scholar]
- 6.Kadima NT, Kobau R, Zack MM, Helmers S. Comorbidity in adults with epilepsy.United States, 2010. MMWR Morb Mortal Wkly Rep. 2013;62:849. [PMC free article] [PubMed] [Google Scholar]
- 7.Tedrus GM, Srebernich SM, Santos TB. Correlation between clinical and cognitive aspects and nutritional indicators of elderly patients with new-onset epilepsy. Epilepsy Behav. 2018;85:105–9. doi: 10.1016/j.yebeh.2018.05.041. [DOI] [PubMed] [Google Scholar]
- 8.Williams KW, Elmquist JK. Lighting up the hypothalamus: Coordinated control of feeding behavior. Nat Neurosci. 2011;14:277–8. doi: 10.1038/nn0311-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wong J, Wirrell E. Physical activity in children/teens with epilepsy compared with that in their siblings without epilepsy. Epilepsia. 2006;47:631–9. doi: 10.1111/j.1528-1167.2006.00478.x. [DOI] [PubMed] [Google Scholar]
- 10.Pylvänen V, Knip M, Pakarinen AJ, Turkka J, Kotila M, Rättyä J, et al. Fasting serum insulin and lipid levels in men with epilepsy. Neurology. 2003;60:571–4. doi: 10.1212/01.wnl.0000048209.07526.86. [DOI] [PubMed] [Google Scholar]
- 11.Isojärvi JI, Laatikainen TJ, Knip M, Pakarinen AJ, Juntunen KT, Myllylä VV. Obesity and endocrine disorders in women taking valproate for epilepsy. Ann Neurol. 1996;39:579–84. doi: 10.1002/ana.410390506. [DOI] [PubMed] [Google Scholar]
- 12.Janousek J, Barber A, Goldman L, Klein P. Obesity in adults with epilepsy. Epilepsy Behav. 2013;28:391–4. doi: 10.1016/j.yebeh.2013.05.012. [DOI] [PubMed] [Google Scholar]
- 13.Arya R, Gillespie CW, Cnaan A, Devarajan M, Clark P, Shinnar S, et al. Obesity and overweight as CAE comorbidities and differential drug response modifiers. Neurology. 2016;86:1613–21. doi: 10.1212/WNL.0000000000002611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao S, Juhaeri J, Dai WS. The incidence rate of seizures in relation to BMI in UK adults. Obesity (Silver Spring) 2008;16:2126–32. doi: 10.1038/oby.2008.310. [DOI] [PubMed] [Google Scholar]
- 15.Hinnell C, Williams J, Metcalfe A, Patten SB, Parker R, Wiebe S, et al. Health status and health-related behaviors in epilepsy compared to other chronic conditions – A national population-based study. Epilepsia. 2010;51:853–61. doi: 10.1111/j.1528-1167.2009.02477.x. [DOI] [PubMed] [Google Scholar]
- 16.Kobau R, DiIorio CA, Price PH, Thurman DJ, Martin LM, Ridings DL, et al. Prevalence of epilepsy and health status of adults with epilepsy in Georgia and Tennessee: Behavioral risk factor surveillance system, 2002. Epilepsy Behav. 2004;5:358–66. doi: 10.1016/j.yebeh.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 17.Vooturi S, Jayalakshmi S. Metabolic syndrome in people with epilepsy. Epilepsy Behav. 2020;106:106992. doi: 10.1016/j.yebeh.2020.106992. [DOI] [PubMed] [Google Scholar]
- 18.FX NDaybaje, JB Gahutu, S peter, Bernad Natukunda. prevelence and risk factors among patients with epielpsy attending a neuropsychiatric hospital in kigali,Rawanda. IJMRHS. 2021;04(5):p339–345. [Google Scholar]
- 19.Daniels ZS, Nick TG, Liu C, Cassedy A, Glauser TA. Obesity is a common comorbidity for pediatric patients with untreated, newly diagnosed epilepsy. Neurology. 2009;73:658–64. doi: 10.1212/WNL.0b013e3181ab2b11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vezzani A, French J, Bartfai T, Baram TZ. The role of inflammation in epilepsy. Nat Rev Neurol. 2011;7:31–40. doi: 10.1038/nrneurol.2010.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li C, Zhou HM. The role of manganese superoxide dismutase in inflammation defense. Enzyme Res 2011. 2011:387176. doi: 10.4061/2011/387176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kegler A, Cardoso AS, Caprara AL, Pascotini ET, Arend J, Gabbi P, et al. Involvement of MnSOD Ala16Val polymorphism in epilepsy: A relationship with seizure type, inflammation, and metabolic syndrome. Gene. 2019;711:143924. doi: 10.1016/j.gene.2019.06.014. [DOI] [PubMed] [Google Scholar]
- 23.Aly RH, Amr NH, Saad WE, Megahed AA. Insulin resistance in patients on valproic acid: Relation to adiponectin. Acta Neurol Scand. 2015;131:169–75. doi: 10.1111/ane.12313. [DOI] [PubMed] [Google Scholar]
- 24.Sidhu HS, Srinivas R, Sadhotra A. Evaluate the effects of long-term valproic acid treatment on metabolic profiles in newly diagnosed or untreated female epileptic patients: A prospective study. Seizure. 2017;48:15–21. doi: 10.1016/j.seizure.2017.03.007. [DOI] [PubMed] [Google Scholar]
- 25.Hamed SA. Leptin and insulin homeostasis in epilepsy: Relation to weight adverse conditions. Epilepsy Res. 2007;75:1–9. doi: 10.1016/j.eplepsyres.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 26.Vancampfort D, Ward PB. Physical activity correlates across the lifespan in people with epilepsy: A systematic review. Disabil Rehabil. 2021;43:1359–66. doi: 10.1080/09638288.2019.1665113. [DOI] [PubMed] [Google Scholar]
- 27.Ben-Menachem E, Axelsen M, Johanson EH, Stagge A, Smith U. Predictors of weight loss in adults with topiramate-treated epilepsy. Obes Res. 2003;11:556–62. doi: 10.1038/oby.2003.78. [DOI] [PubMed] [Google Scholar]
- 28.Reife R, Pledger G, Wu SC. Topiramate as add-on therapy: Pooled analysis of randomized controlled trials in adults. Epilepsia. 2000;41:66–71. doi: 10.1111/j.1528-1157.2000.tb02175.x. [DOI] [PubMed] [Google Scholar]
- 29.Lagae L, Meshram C, Giorgi L, Patten A. Effects of adjunctive zonisamide treatment on weight and body mass index in children with partial epilepsy. Acta Neurol Scand. 2015;131:341–6. doi: 10.1111/ane.12373. [DOI] [PubMed] [Google Scholar]
- 30.Egger J, Brett EM. Effects of sodium valproate in 100 children with special reference to weight. Br Med J (Clin Res Ed) 1981;283:577–81. doi: 10.1136/bmj.283.6291.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Biton V, Mirza W, Montouris G, Vuong A, Hammer AE, Barrett PS. Weight change associated with valproate and lamotrigine monotherapy in patients with epilepsy. Neurology. 2001;56:172–7. doi: 10.1212/wnl.56.2.172. [DOI] [PubMed] [Google Scholar]
- 32.Biton V, Levisohn P, Hoyler S, Vuong A, Hammer AE. Lamotrigine versus valproate monotherapy-associated weight change in adolescents with epilepsy: Results from a post hoc analysis of a randomized, double-blind clinical trial. J Child Neurol. 2003;18:133–9. doi: 10.1177/08830738030180021701. [DOI] [PubMed] [Google Scholar]
- 33.Privitera MD, Brodie MJ, Mattson RH, Chadwick DW, Neto W, Wang S, et al. Topiramate, carbamazepine and valproate monotherapy: Double-blind comparison in newly diagnosed epilepsy. Acta Neurol Scand. 2003;107:165–75. doi: 10.1034/j.1600-0404.2003.00093.x. [DOI] [PubMed] [Google Scholar]
- 34.Isojärvi JI, Rättyä J, Myllylä VV, Knip M, Koivunen R, Pakarinen AJ, et al. Valproate, lamotrigine, and insulin-mediated risks in women with epilepsy. Ann Neurol. 1998;43:446–51. doi: 10.1002/ana.410430406. [DOI] [PubMed] [Google Scholar]
- 35.Carmona-Vazquez CR, Ruiz-Garcia M, Pena-Landin DM, Diaz-Garcia L, Greenawalt SR. The prevalence of obesity and metabolic syndrome in paediatric patients with epilepsy treated in monotherapy with valproic acid. Rev Neurol. 2015;61:193–201. [PubMed] [Google Scholar]
- 36.Verrotti A, Manco R, Agostinelli S, Coppola G, Chiarelli F. The metabolic syndrome in overweight epileptic patients treated with valproic acid. Epilepsia. 2010;51:268–73. doi: 10.1111/j.1528-1167.2009.02206.x. [DOI] [PubMed] [Google Scholar]
- 37.Fang J, Chen S, Tong N, Chen L, An D, Mu J, et al. Metabolic syndrome among Chinese obese patients with epilepsy on sodium valproate. Seizure. 2012;21:578–82. doi: 10.1016/j.seizure.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 38.Rakitin A, Eglit T, Kõks S, Lember M, Haldre S. Comparison of the metabolic syndrome risk in valproate-treated patients with epilepsy and the general population in Estonia. PLoS One. 2014;9:e103856. doi: 10.1371/journal.pone.0103856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sharma S, Aneja S. Metabolic syndrome in children with epielpsy on valproate and phenytoin therapy: Across sectional study. Journal of Neurological sciences. 2013;10-15(333):e29–e29. [Google Scholar]
- 40.Nisha Y, Bobby Z, Wadwekar V. Biochemical derangements related to metabolic syndrome in epileptic patients on treatment with valproic acid. Seizure. 2018;60:57–60. doi: 10.1016/j.seizure.2018.06.003. [DOI] [PubMed] [Google Scholar]
- 41.Rehman T, Sachan D, Chitkara A. Serum insulin and leptin levels in children with epilepsy on valproate-associated obesity. J Pediatr Neurosci. 2017;12:135–7. doi: 10.4103/jpn.JPN_152_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pylvanen V, Knip M, Pakarinen A, Kotila M, Turkka J, Isojarvi JI. Serum insulin and leptin levels in valproate associated obesity. Epilepsia. 2002;43:514–7. doi: 10.1046/j.1528-1157.2002.31501.x. [DOI] [PubMed] [Google Scholar]
- 43.Guzel A, Karasalihoglu S, Kucukugurluoglu Y, Sayar E, Kunduracilar H. Evaluation of serum ghrelin and neuropeptide Y levels in epileptic children under valproate treatment. Univ Tip Fak Derg. 2009;26:18–23. [Google Scholar]
- 44.Verrotti A, Greco R, Latini G, De Simone M, Chiarelli F. Obesity and plasma concentrations of alpha-tocopherol and beta-carotene in epileptic girls treated with valproate. Neuroendocrinology. 2004;79:157–62. doi: 10.1159/000077274. [DOI] [PubMed] [Google Scholar]
- 45.Pylvänen V, Pakarinen A, Knip M, Isojärvi J. Insulin-related metabolic changes during treatment with valproate in patients with epilepsy. Epilepsy Behav. 2006;8:643–8. doi: 10.1016/j.yebeh.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 46.Dhir A, Sharma S, Jain P, Bhakhri BK, Aneja S. Parameters of metabolic syndrome in Indian children with epilepsy on valproate or phenytoin monotherapy. J Pediatr Neurosci. 2015;10:222–6. doi: 10.4103/1817-1745.165661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Verrotti A, Agostinelli S, Parisi P, Chiarelli F, Coppola G. Nonalcoholic fatty liver disease in adolescents receiving valproic acid. Epilepsy Behav. 2011;20:382–5. doi: 10.1016/j.yebeh.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 48.Luef G, Rauchenzauner M, Waldmann M, Sturm W, Sandhofer A, Seppi K, et al. Non-alcoholic fatty liver disease (NAFLD), insulin resistance and lipid profile in antiepileptic drug treatment. Epilepsy Res. 2009;86:42–7. doi: 10.1016/j.eplepsyres.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 49.Sahota P, Prabhakar S, Kharbanda PS, Bhansali A, Jain V, Das CP, et al. Seizure type, antiepileptic drugs, and reproductive endocrine dysfunction in Indian women with epilepsy: A cross-sectional study. Epilepsia. 2008;49:2069–77. doi: 10.1111/j.1528-1167.2008.01676.x. [DOI] [PubMed] [Google Scholar]
- 50.Sidhu HS, Srinivasa R, Sadhotra A. Evaluate the effects of antiepileptic drugs on reproductive endocrine system in newly diagnosed female epileptic patients receiving either valproate or lamotrigine monotherapy: A prospective study. Epilepsy Res. 2018;139:20–7. doi: 10.1016/j.eplepsyres.2017.10.016. [DOI] [PubMed] [Google Scholar]
- 51.Richens A, Davidson DL, Cartlidge NE, Easter DJ. A multicentre comparative trial of sodium valproate and carbamazepine in adult onset epilepsy.Adult EPITEG Collaborative Group. J Neurol Neurosurg Psychiatry. 1994;57:682–7. doi: 10.1136/jnnp.57.6.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hogan RE, Bertrand ME, Deaton RL, Sommerville KW. Total percentage body weight changes during add-on therapy with tiagabine, carbamazepine and phenytoin. Epilepsy Res. 2000;41:23–8. doi: 10.1016/s0920-1211(00)00125-x. [DOI] [PubMed] [Google Scholar]
- 53.Lampl Y, Eshel Y, Rapaport A, Sarova-Pinhas I. Weight gain, increased appetite, and excessive food intake induced by carbamazepine. Clin Neuropharmacol. 1991;14:251–5. doi: 10.1097/00002826-199106000-00009. [DOI] [PubMed] [Google Scholar]
- 54.Uludag IF, Kulu U, Sener U, Kose S, Zorlu Y. The effect of carbamazepine treatment on serum leptin levels. Epilepsy Res. 2009;86:48–53. doi: 10.1016/j.eplepsyres.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 55.Remy C, Beaumont D. Efficacy and safety of vigabatrin in the long-term treatment of refractory epilepsy. Br J Clin Pharmacol. 1989;27(Suppl 1):125S–129S. doi: 10.1111/j.1365-2125.1989.tb03473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tartara A, Manni R, Galimberti CA, Morini R, Mumford JP, Iudice A, et al. Six-year follow-up study on the efficacy and safety of vigabatrin in patients with epilepsy. Acta Neurol Scand. 1992;86:247–51. doi: 10.1111/j.1600-0404.1992.tb05079.x. [DOI] [PubMed] [Google Scholar]
- 57.Chadwick D. Safety and efficacy of vigabatrin and carbamazepine in newly diagnosed epilepsy: A multicentre randomised double-blind study.Vigabatrin European Monotherapy Study Group. Lancet. 1999;354:13–9. doi: 10.1016/s0140-6736(98)10531-7. [DOI] [PubMed] [Google Scholar]
- 58.Guberman A, Bruni J. Long-term open multicentre, add-on trial of vigabatrin in adult resistant partial epilepsy.The Canadian Vigabatrin Study Group. Seizure. 2000;9:112–8. doi: 10.1053/seiz.2000.0382. [DOI] [PubMed] [Google Scholar]
- 59.Chadwick DW, Anhut H, Greiner MJ, Alexander J, Murray GH, Garofalo EA, et al. A double-blind trial of gabapentin monotherapy for newly diagnosed partial seizures.International Gabapentin Monotherapy Study Group 945-77. Neurology. 1998;51:1282–8. doi: 10.1212/wnl.51.5.1282. [DOI] [PubMed] [Google Scholar]
- 60.Baulac M, Cavalcanti D, Semah F, Arzimanoglou A, Portal JJ. Gabapentin add-on therapy with adaptable dosages in 610 patients with partial epilepsy: An open, observational study.The French Gabapentin Collaborative Group. Seizure. 1998;7:55–62. doi: 10.1016/s1059-1311(98)90009-7. [DOI] [PubMed] [Google Scholar]
- 61.DeToledo JC, Toledo C, DeCerce J, Ramsay RE. Changes in body weight with chronic, high-dose gabapentin therapy. Ther Drug Monit. 1997;19:394–6. doi: 10.1097/00007691-199708000-00006. [DOI] [PubMed] [Google Scholar]
- 62.Beydoun A, Fischer J, Labar DR, Harden C, Cantrell D, Uthman BM, et al. Gabapentin monotherapy: II. A 26-week, double-blind, dose-controlled, multicenter study of conversion from polytherapy in outpatients with refractory complex partial or secondarily generalized seizures. The US Gabapentin Study Group 82/83. Neurology. 1997;49:746–52. doi: 10.1212/wnl.49.3.746. [DOI] [PubMed] [Google Scholar]
- 63.Hoppe C, Rademacher M, Hoffmann JM, Schmidt D, Elger CE. Bodyweight gain under pregabalin therapy in epilepsy: Mitigation by counseling patients? Seizure. 2008;17:327–32. doi: 10.1016/j.seizure.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 64.Siddall PJ, Cousins MJ, Otte A, Griesing T, Chambers R, Murphy TK. Pregabalin in central neuropathic pain associated with spinal cord injury: A placebo-controlled trial. Neurology. 2006;67:1792–800. doi: 10.1212/01.wnl.0000244422.45278.ff. [DOI] [PubMed] [Google Scholar]
- 65.Kramer H, Dugas L, Shoham D. Obesity as an effect modifier of the risk of death in chronic kidney disease. Nephrol Dial Transplant. 2013;28(Suppl 4):v65–72. doi: 10.1093/ndt/gft242. [DOI] [PubMed] [Google Scholar]
- 66.Zen V, Fuchs FD, Wainstein MV, Gonçalves SC, Biavatti K, Riedner CE, et al. Neck circumference and central obesity are independent predictors of coronary artery disease in patients undergoing coronary angiography. Am J Cardiovasc Dis. 2012;2:323–30. [PMC free article] [PubMed] [Google Scholar]
- 67.Löscher W, Potschka H, Sisodiya SM, Vezzani A. Drug resistance in epilepsy: Clinical impact, potential mechanisms, and new innovative treatment options. Pharmacol Rev. 2020;72:606–38. doi: 10.1124/pr.120.019539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kwan P, Arzimanoglou A, Berg AT, Brodie MJ, Allen Hauser W, Mathern G, et al. Definition of drug resistant epilepsy: Consensus proposal by the ad hoc task force of the ILAE commission on therapeutic strategies. Epilepsia. 2010;51:1069–77. doi: 10.1111/j.1528-1167.2009.02397.x. [DOI] [PubMed] [Google Scholar]
- 69.de Azevedo Fernandez R, Corrêa C, Muxfeld Bianchim M, Schweigert Perry ID. Anthropometric profile and nutritional intake in patients with epilepsy. Nutr Hosp. 2015;32:817–22. doi: 10.3305/nh.2015.32.2.9205. [DOI] [PubMed] [Google Scholar]
- 70.Ladino LD, Hernández-Ronquillo L, Téllez-Zenteno JF. Obesity and its association with generalised epilepsy, idiopathic syndrome, and family history of epilepsy. Epileptic Disord. 2014;16:343–53. doi: 10.1684/epd.2014.0677. [DOI] [PubMed] [Google Scholar]
- 71.Chen M, Wu X, Zhang B, Shen S, He L, Zhou D. Associations of overweight and obesity with drug-resistant epilepsy. Seizure. 2021;92:94–9. doi: 10.1016/j.seizure.2021.07.019. [DOI] [PubMed] [Google Scholar]
- 72.Huffman J, Kossoff EH. State of the ketogenic diet(s) in epilepsy. Curr Neurol Neurosci Rep. 2006;6:332–40. doi: 10.1007/s11910-006-0027-6. [DOI] [PubMed] [Google Scholar]
- 73.Kverneland M, Selmer KK, Nakken KO, Iversen PO, Taubøll E. A prospective study of the modified Atkins diet for adults with idiopathic generalized epilepsy. Epilepsy Behav. 2015;53:197–201. doi: 10.1016/j.yebeh.2015.10.021. [DOI] [PubMed] [Google Scholar]