Abstract
Patients with large left-hemisphere lesions and post-stroke aphasia often remain nonfluent. Melodic Intonation Therapy (MIT) may be an effective alternative to traditional speech therapy for facilitating recovery of fluency in those patients. In an open-label, proof-of-concept study, 14 subjects with nonfluent aphasia with large left-hemisphere lesions (171±76 cc) underwent two speech/language assessments before, one at midpoint, and two after the end of 75 sessions (1.5 hrs/session) of MIT. Functional MR imaging was done before and after therapy asking subjects to vocalize the same set of 10 bi-syllabic words. We found significant improvements in speech output after a period of intensive MIT (75 sessions for a total of 112.5 hrs) compared to two pre-therapy assessments. Therapy-induced gains were maintained four weeks post-treatment. Imaging changes were seen in a right-hemisphere network that included the posterior superior temporal and inferior frontal gyri, inferior pre- and postcentral gyri, pre-supplementary motor area, and supramarginal gyrus. Functional changes in the posterior right inferior frontal gyri significantly correlated with changes in a measure of fluency. Intense training of intonation-supported auditory-motor coupling and engaging feedforward/feedback control regions in the unaffected hemisphere improves speech-motor functions in subjects with nonfluent aphasia and large left-hemisphere lesions.
Keywords: aphasia, melodic intonation therapy, MRI, neuroplasticity, neurorehabilitation, stroke recovery
Graphical Abstract
Patients with left-hemisphere lesions and post-stroke aphasia often remain nonfluent. Melodic Intonation Therapy (MIT) may be an effective alternative to traditional speech therapy. Subjects with nonfluent aphasia and large left-hemisphere lesions demonstrated long-lasting, significant improvements in speech output and changes in a right-hemisphere network after a period of intensive MIT. Intonation-supported changes in auditory-motor coupling improves speech-motor functions in subjects with nonfluent aphasia and large left-hemisphere lesions.
INTRODUCTION
Stroke survivors with moderate to severe nonfluent aphasia often receive poor prognoses for natural recovery and may not improve with traditional speech therapy2–6. To date, there are no universally-accepted interventions for nonfluent aphasia, nor is there agreement in the literature on criteria for treatment efficacy, though Thompson7 proposed that a treatment should be considered effective when improvements in speech output generalize to untrained language structures or contexts.
In the past two decades, neuroimaging research has done much to increase our knowledge of post-stroke language reorganization by examining both natural recovery and the effects of traditional speech therapy, however the results have been mixed. Some studies have emphasized the role of preserved left-hemisphere language function 8–10, other studies have suggested that improvement occurs when right-hemisphere regions compensate for damaged left-hemisphere structures 11–15, while a third group of studies reported evidence for either bi-hemispheric language processing or transient right-hemisphere activation after a stroke 16–18. Only a few studies have aimed to identify neural correlates of treatment effects by contrasting post- versus pre-therapy functional imaging assessments 9, 19–22. Despite the heterogeneity in patient groups and findings, the general consensus among these studies is that there are two possible routes to recovering language functions. While patients with small left-hemisphere lesions and milder aphasia may recruit perilesional cortex on the left with varying degrees of right-hemispheric involvement 4, 8, 23, 24, the only path available to patients with large left-hemisphere lesions may be through recruitment of right-hemisphere regions that might have the ability to subserve speech-motor functions 4, 8, 25–30. For such patients, therapies that engage those right-hemisphere regions may hold particular promise for increasing speech output beyond the expectations of natural recovery 25, 27, 28, 31–33.
One such treatment, Melodic Intonation Therapy (MIT) 34, 35, was designed specifically to facilitate recovery from severe nonfluent aphasia. Developed in response to the clinical observation that severely aphasic patients can often produce well-articulated, linguistically accurate words while singing, but not while speaking 25, 36–40, the original MIT description uses intoned (sung) patterns to emphasize the natural rise and fall of prosodic speech by translating it into simple, two-pitch “melodies” (higher pitch for accented syllables). We are aware that there are other approaches out there that have used more than two pitches or entire melodies. Our aim was to adhere to the original description and to make the intonation as simple as possible. The two primary elements that distinguish MIT from non-intonation-based therapies are melodic intonation with its inherent continuous voicing, and rhythmic tapping of the patient’s left hand as phrases are intoned and repeated (for details, see 41). The sung repetition or unison singing as well as the left-hand tapping have the same rhythmic component to it. An early MIT study by Belin and colleagues 42 contrasted patients’ production of intoned vs. non-intoned words only after treatment was done with an adapted form of the therapy. More recent imaging studies have examined MIT’s effects in single case studies33, 43, 44, small case series30, 45, and small heterogeneous groups42, 46, 47, as well as pilot randomized trials 48, 49, mostly using a wait-list design. However, MIT’s efficacy is still questionable and the neural mechanism of the therapy-induced effects have not been thoroughly investigated. Thus, the main aim of this study was to examine behavioral outcomes of an intense MIT regimen (more than 100 hours of treatment) as originally suggested by the developers with a pre-post design while also controlling for test-retest effects at baseline and maintenance effects after the intervention in outcome variables. Furthermore, imaging was used as an exploratory aim to examine the neural correlates of the treatment effects. Our hypotheses were that significant effects in a pragmatic speech fluency outcome would be seen and that imaging would show functional changes in a right hemisphere homotop vocal-motor network.
METHODS
Participants
Fourteen chronic stroke patients (13 males, one female, mean ± standard deviation age (54.0±14.2) with persistent, moderate to severe nonfluent aphasia (e.g., mean CIUs/min was 1.9) and relatively unimpaired comprehension were enrolled in our study (see Table 1). The protocol was approved by the local institutional review board, and all patients gave written informed consent. In order to determine eligibility, classify aphasic syndrome, and assess baseline levels of fluency and comprehension, patients were interviewed and then underwent selected subtests of the Boston Diagnostic Aphasia Examination (BDAE 2nd edition; 50). All participants met the following inclusion criteria: (1) age: 21 – 80 years, (2) at least 6 months post-onset of a single, ischemic, left-hemisphere stroke when MIT commenced, (3) moderately to severely restricted verbal output, and (4) moderately well-preserved auditory comprehension (i.e., at minimum, able to understand one-step commands and repeat at least 10 two-syllable words). Exclusion criteria included: (1) cognitive and/or auditory comprehension deficits severe enough to impair patients’ ability to competently participate in the study (i.e., if participant performed <50% correct on Raven’s Coloured Progressive Matrices, a measure of nonverbal IQ 51 and if participant performed <20% correct on Auditory Comprehension subtests of the BDAE 34), (2) an active medical condition that would prevent participation in intensive treatment or follow-up assessments, (3) MRI risk factors, (4) right-hemispheric, bi-hemispheric, or brainstem stroke, (5) fluent aphasia, (6) evidence of neurological disease or psychiatric condition other than a stroke, (7) having received a course of formal MIT prior to screening for this study. Having relatively preserved comprehension, at least for simple commands, ensured that patients were able to participate in the therapy sessions and understand what was asked of them. For participants’ demographic and lesion data as well as their behavioral outcomes, see Table 1.
Table 1.
Demographic, Lesion Characteristics and Baseline Data on non-verb. IQ, Comprehension, Repetition, Fluency, and Naming
| Demographics | Lesion Characteristics | Non-Verbal IQ | Auditory Comprehension | Repetition | Fluency [pre1] | Naming [pre1] | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age @ treat | Timepost-stroket | Lesion-Volume | raw AF-LL | Raven’s CPM | Word Discrimination | Commands | Words | Words per minute | CIUs/min | BNT (max=15) | |
| [yrs] | [mo] | [cc] | [cc] | [%] | [%] | [%] | [%] | [#] | [#] | [#] | |
| 1 | 29 | 120 | 191.7 | 9.7 | 88.9 | -** | - | - | 2.1 | 0.88 | 0 |
| 2 | 47 | 13 | 169.2 | 4.4 | 91.7 | 93.8 | 80 | 50.0 | 17.9 | 3.79 | 12 |
| 3 | 74 | 6 | 123.8 | 4.3 | 66.7 | - | 66.7 | 22.2 | 11.6 | 0.85 | 1 |
| 4 | 80 | 9 | 70.7 | 2.5 | 55.6 | 64.3 | 100 | 30.0 | 1.6 | 0.22 | 1 |
| 5 | 55 | 61 | 198.3 | 9.1 | 83.3 | 54.6 | 45.5 | 30.0 | 4.8 | 0.57 | 1 |
| 6 | 58 | 55 | 123.2 | 7.1 | 77.8 | - | 83.3 | 63.6 | 3.3 | 0.28 | 4 |
| 7 | 62 | 30 | 42.1 | 4.3 | 88.9 | 60.0 | 60.0 | 40.0 | 3.5 | 1.47 | 9 |
| 8 | 56 | 29 | 152.9 | 9.5 | 94.4 | 72.7 | 75 | 20.0 | 8.5 | 0.64 | 4 |
| 9 | 71 | 14 | 73.4 | 3.3 | 80.6 | 100 | 86.7 | 10.0 | 8.2 | 2.25 | 11 |
| 10 | 35 | 9 | 198.8 | 5.2 | 97.2 | 94 | 100 | 12.5 | 11.0 | 0.63 | 4 |
| 11 * | 48 | 12 | 268.8 | 8.3 | 83.3 | 60 | 58.3 | 10.0 | 2.5 | 0.38 | 0 |
| 12 | 44 | 44 | 259.5 | 10.7 | 97.2 | 83.8 | 87.7 | 35.0 | 24.5 | 9.00 | 14 |
| 13 | 49 | 32 | 238.5 | 9.4 | 75.0 | 73.0 | 75.0 | 40.0 | 16.7 | 3.67 | 4 |
| 14 | 55 | 8 | 249.8 | 10.8 | 86.1 | 91.9 | 26.7 | 30.0 | 7.3 | 2.02 | 5 |
| Mean ± SD | 54.5 ± 14.3 | 35.6 ± 31.2 | 168.6 ± 73.9 | 7.0 ± 2.9 | 83.3 ± 11.8 | 77.1 ± 16.3 | 72.7 ± 21.1 | 30.3 ± 15.9 | 8.8 ± 6.9 | 1.90 ± 2.36 | 5.0 ± 4.7 |
: only female patient, all others were male;
: missing values;
: absolute number;
: percent of correct responses;
AF-LL = Arcuate Fasciculus Lesion Load; overlap of lesion on a canonical Arcuate Fasciculus tract derived from an elderly healthy control group;
Time from stroke to baseline assessment (time post-stroke) is expressed in months.
Language assessments and therapy
Our primary outcome measure is a measure of spontaneous speech which can also be regarded as a measure of speech fluency: The number of Correct Information Units (CIUs) (defined as words that are not only intelligible, but also accurate, relevant, and informative in the context of the stimulus) 52 uttered over a minute during the production of spontaneous speech fulfill the requirement that a treatment’s efficacy is assessed by examining its generalizability to untrained language structures or contexts7. For this investigation we used two different tasks to obtain the spontaneous speech samples from which the CIUs/min were extracted: (1) Conversational Interviews 53 comprised of questions that elicited information related to patients’ biographical data, medical history, daily activities, asnd stroke recovery, and (2) Picture Descriptions for which patients were shown complex pictures (e.g., the BDAE 52 “Cookie Theft” picture and Western Aphasia Battery (WAB)54“Picnic” picture), and asked to describe what was happening in the picture. Video recordings were made of all patient assessments and used offline for the transcription, timing, and scoring of patients’ speech samples which was done by a single experienced coder blind to the timepoint of assessment. Furthermore, a blinded reliability study was also conducted on both outcome measures for all patients at two of the five time points (pre1 and post75). For the reliability study, video clips of patients’ pre- and post-treatment speech samples were made, assigned a code number, then randomized with regard to time point and given to the blinded coders for transcription and scoring. The intra- and inter-observer reliabilities for CIUs were ≥0.9.
As a secondary outcome measure, we assessed performance on confrontation naming using the Boston Naming Test (BNT) 55. We used a standard scoring (one point for each item named correctly without cueing).
To ensure baseline stability in this group of chronic aphasic patients, patients were tested and scanned twice, separated by two to four weeks (pre1 and pre2) before any therapy was done. The same tests were then administered after 40 (post40) and 75 treatment sessions (post75), and again at four weeks post-treatment (post75+4) to assess maintenance effects after cessation of daily treatment with MIT. All patients underwent 75 treatment sessions (1.5hrs/day; 5 days/week; total treatment time: 112.5hrs), administered with an intensity similar to that of previous case series 34, 35, 41, 56, 57. We acknowledge that the time intervals between pre1 and pre2 as well as between pre2 and post75 and then between post75 and post75+4 are not equal, but this was a pragmatic, real-world clinical trial. We did not want to loose adherence to this trial by asking for excessively long no-treatment periods and not having too long of a maintenance period. This would allow subjects to pursue other standard or experimental interventions after an intervention period that lasted four to five months.
Experimental stimuli and fMRI paradigm
Based on words that patients were able to produce at baseline, a set of 16 bi-syllabic words/phrases for all subjects was recorded for the fMRI experimental task by a native English speaker using Adobe Audition v1.5 (Adobe, San Jose, CA, USA). From this set of 16 bi-syllabic words/phrases, 10 were chosen for each individual subject’s fMRI studies. The same list of words/phrases used at baseline was used for all follow-up imaging sessions. In the “Overt Speaking” condition, patients were instructed to repeat the words and phrases exactly as they heard them (rate = 1 syllable/sec) after an auditory cue. In the “Silence Control” condition, patients were instructed to take a deep breath after the ding, but otherwise not to vocalize (see Ozdemir and colleagues58 for further details on this and other fMRI tasks done, which cannot all be reported here due to space restrictions).
MR data acquisition
Functional magnetic resonance imaging (fMRI) was performed on a 3.0 Tesla GE Discovery MR 750 scanner (GE Medical Systems, Wilwaukee, WI, USA). A gradient-echo EPI-sequence with an effective repetition time (TR) of 15s, echo time (TE) of 25ms, acquisition time (TA) of 1.75s, and matrix of 64ˣ64 was used for functional imaging. Using a midsagittal scout image, a total of 28 axial slices with a voxel size of 3.75ˣ3.75ˣ5mm were acquired over a period of 1.75 s after each trial. Each acquisition was followed by 13.25s of MR acquisition silence (part of the sparse-temporal fMRI design) during which stimuli were presented and subjects’ responses could be clearly heard and recorded prior to the next acquisition. Initiation of the first image in the set of 28 slices was synchronized with stimulus onset using Presentation software version 7.2.6 (Neurobehavioral Systems, Albany, CA, USA). The fMRI experiment consisted of eight runs, 20 trials per run (four trials for each of five conditions) plus two dummy scans at the beginning of each run. To verify that patients responded to each trial, responses were recorded.
fMRI data analysis
fMRI data were analyzed using the SPM8 software package (Wellcome Trust Centre for Neuroimaging, University College, London, UK) including realignment, spatial normalization, and smoothing using an isotropic Gaussian kernel of 8mm full-width at half-maximum. Condition and subject effects were estimated according to the general linear model 59. Because of the sparse temporal sampling design, there was no temporal auto correlation between the images. Therefore, we did not convolve our data with the hemodynamic response function but used the flexible finite impulse response which averages the blood-oxygen-level-dependent (BOLD) response at each post-stimulus jitter time point. See our previous publications for further details on the analysis of sparse temporal data sets 58, 60.
A design matrix was modeled by combining all jittered image acquisitions for each subject in order to look at condition effects for each subject individually. The two fMRI conditions presented here are “Overt Speaking” (i.e., the active condition) and “Silence Control” (i.e., the silence control condition). All subjects’ pre1 and post75 timepoints were entered into a fixed effects group analysis using the group lesion map (see Figure 1) as an explicit mask. We then created an Overt Speaking > Silence Control pre- and post-therapy contrasts (both family wise error [FWE] p<0.05 corrected) as well as a Overt Speaking post>pre therapy contrast (false discovery rate [FDR] p<0.05). In addition to the fixed effects model, we entered the contrasts from all timepoints (pre1, pre2, post40, post75) into a random effects full factorial design. The fixed-effects analysis takes into account the degree of variability in lesion characteristics and patient outcomes at baseline and allows us only to make inferences about the group of subjects studied. Fixed effects analyses might be appropriate when the inference is about some aspect of a response in the group studies 61. Random-effects analysis allows for broader, more generalizable inferences to be made with regard to the underlying population as well as when making comparisons between groups, such as a patient group and a control group 61, 62. For the full-factorial model, we used a mask to restrict the search volume to intact and unaffected regions of the left and right hemisphere in order to reduce variability caused by variations in left-hemisphere lesion volumes that could create spurious activation effects. The mask image was generated by adding temporal, parietal, and frontal lobes from the WFU PickAtlas toolbox (version 1.04) and multiplying this binary mask with a gray matter mask. The resulting mask was then inserted as an explicit mask into the full factorial model. We focused on differences between the two baseline scans (pre2>pre1), and post therapy effects (post75 vs. pre1). Analyses of other fMRI tasks will be reported elsewhere.
FIGURE 1.
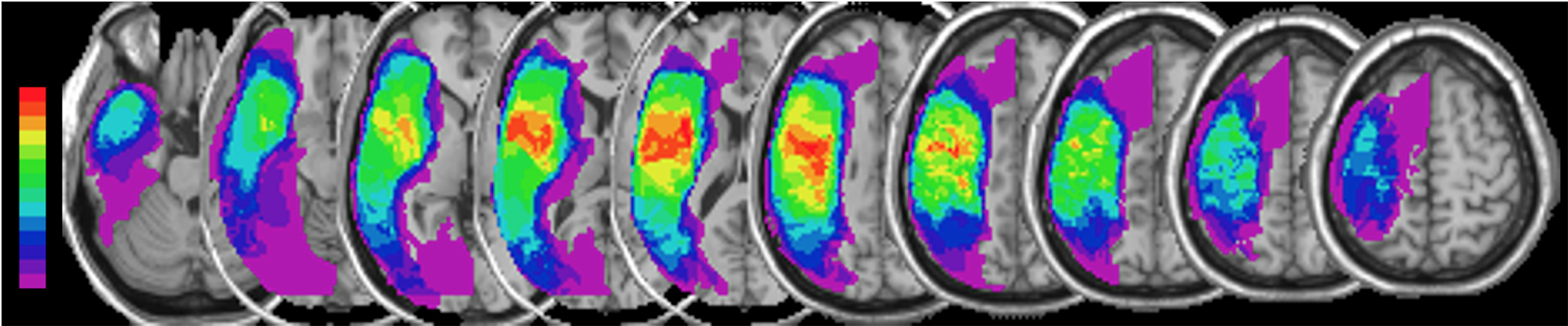
Composite lesion map. After spatial normalization to MNI space, the individual lesion maps of all 14 patients were overlaid and superimposed on a canonical T1-weighted image. The color spectrum bar indicates the number of patients with lesioned tissue in a given voxel or region (e.g., purple represents the lesioned voxels/regions of a single patient; bright red indicates that all 14 patients have lesioned tissue in that voxel or region).
Using MarsBaR 63, we extracted mean regional beta-values of active (i.e., Overt Speaking) vs. control (i.e., Silence Control) conditions using the center coordinates of all clusters in both the fixed effects post75>pre1 contrast (FDR-corrected) and random effects post75>pre1 contrast (uncorrected p<0.05) and created a sphere of 10mm diameter around the center coordinates diameter. The regional beta-values representing the strength of the activation were then correlated with the CIUs/min change scores.
Lesion Map analysis
To include a measure of left-hemisphere lesion severity 64, lesion masks were first drawn manually on normalized T1-weighted images by an experienced investigator who was blinded to any other aspects of each subject’s assessment (see Figure 1 for lesion overlays). Then, for each spatially normalized lesion, an overlap volume was calculate by overlaying the lesion map onto a probabilistic, canonical fiber tract of the Arcuate Fasciculus (AF; see Table 1 for lesion size and lesion load). This caonical, probabilistic, left hemisphere tract was reconstructed fro high-resolution diffusion tenor images of 12 right-handed healthy elderly subjects 65.
RESULTS
Effects of MIT on measures of spontaneous speech and picture naming outcomes
A repeated measures ANOVA revealed significant overall differences (see Figure 2) in our primary outcome measure, CIUs/min, across the five timepoints (pre1, pre2, post40, post75, and post75+4weeks) (Greenhouse-Geisser corrected F(1.913, 24.87) = 17.428, p<0.001). Post-hoc pairwise comparisons (Bonferroni corrected), showed no difference between the two pre-therapy assessments (1.9 vs. 2.23, p=1.0) suggesting stability of the impairment prior to an intervention, but significant differences were found between the first baseline assessment and all other timepoints (post40 1.9 vs. 3.64, p=0.042, post75 1.9 vs. 5.05, p=0.006, post75+4wks 1.9 vs. 4.86, p=0.002). Assessments done at post75 and post75+4 did not show a significant difference (5.05 vs. 4.86, p=1.0) (Figure 2a). Across the 14 patients, the percent change in CIUs/min was 91.5% after 40 sessions of MIT, 165.9% after 75 sessions, and 155.8% at 4 weeks post-treatment (Figure 2b).
FIGURE 2.
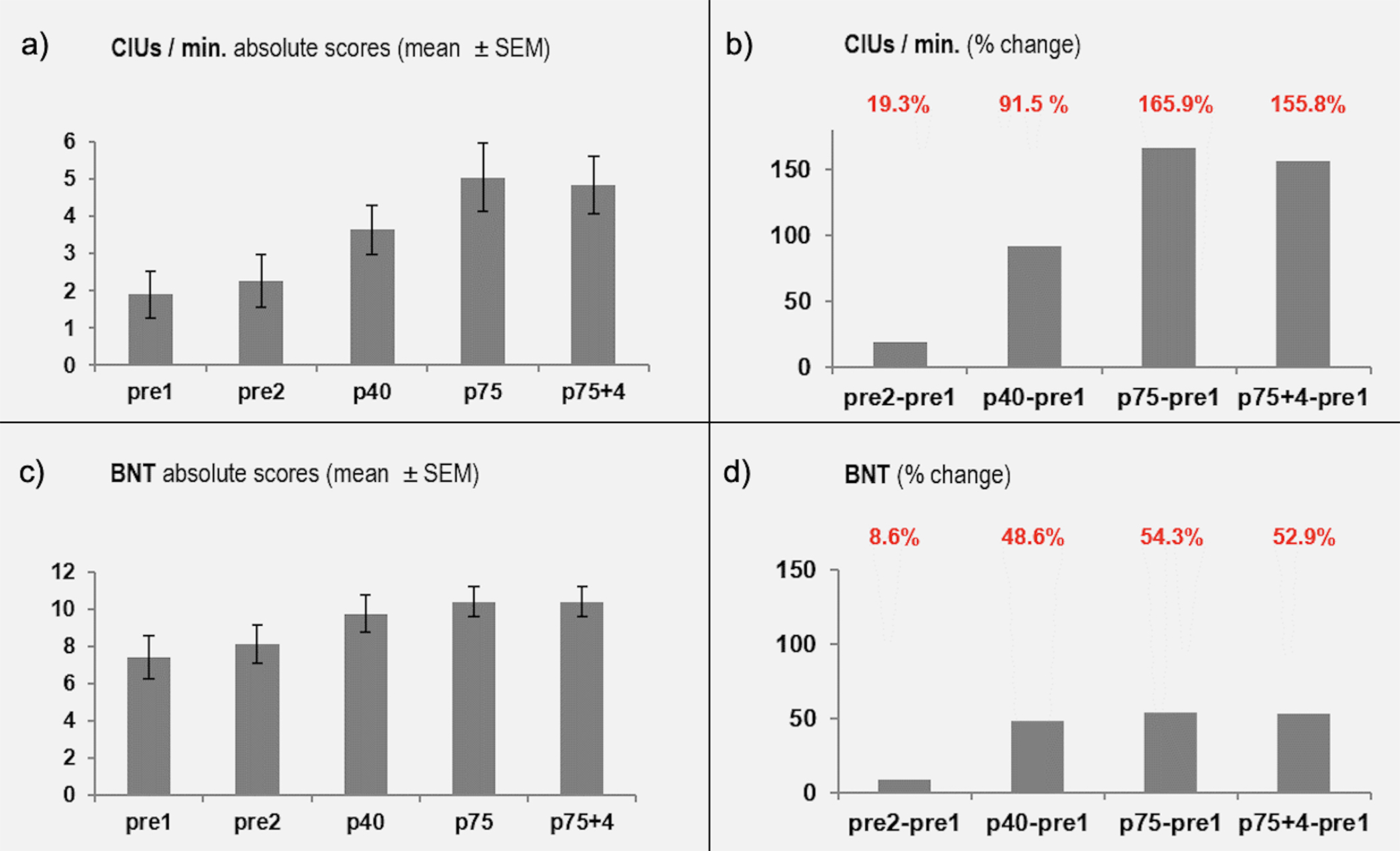
Behavioral outcomes over time. Each pair of graphs shows the absolute performance (Figure 2a and 2c) and percent change (Figure 2b and 2d) in Correct Information Units (CIUs/min) and the Boston Naming Test (BNT). Percent change is always compared to the first baseline (pre1). Assessments were done after 40 and 75 treatment sessions as well as four weeks after the last treatment sessions to assess maintenance effects.
Our secondary outcome measure was the change in picture naming performance using the BNT. We report here the results of the conservative rating of the BNT (i.e., subjects received one point for naming the picture correctly. The repeated measures ANOVA was overall significant F(4, 52) = 8.283, p<001). The comparison between the two pre-therapy baselines was not significant (5.0 vs. 5.43, p=0.451). There was a significant difference between the baseline (pre1) and all remaining timepoints (post40 5.0 vs. 7.43, p=0.006; post75 5.0 vs. 7.71, p<0.001; post75+4 5.0 vs.7.64, p=0.006), but no significant difference between post75 and post75+4 (7.71 vs. 7.64, p=0.922) suggesting maintenance of the effect after the end of the therapy (Figure 2c). The percent change in naming across all patients was 48.6% after 40 sessions of MIT, 54.2% after 75 sessions, and 52.9% four weeks post-treatment (Figure 2d).
Effects of MIT on neural activation patterns for speaking.
Activation pattern before and after therapy
Significant activations (FWE p<0.05 whole brain, extent threshold 10 voxels) were found in the “Overt Speaking” vs. “Silence Control” contrast prior to MIT treatment (pre1) in a right-hemispheric network that included the pre- and postcentral gyrus (MNI coordinates: 52 −6 30), posterior superior temporal gyrus (STG) expanding into the superior temporal sulcus (STS) (60 −4 −4), pre-supplementary and supplementary motor areas (pre-SMA and SMA) (0 −2 64), and the caudate nucleus (−16 22 0). Activated left-hemisphere regions included the cerebellum (−2 −76 16; −6 −46 32), pre-SMA and SMA (0 −2 64), superior parietal lobule (−32 −60 54) and the caudate nucleus (−16 22 0) (Figure 3a).
FIGURE 3.
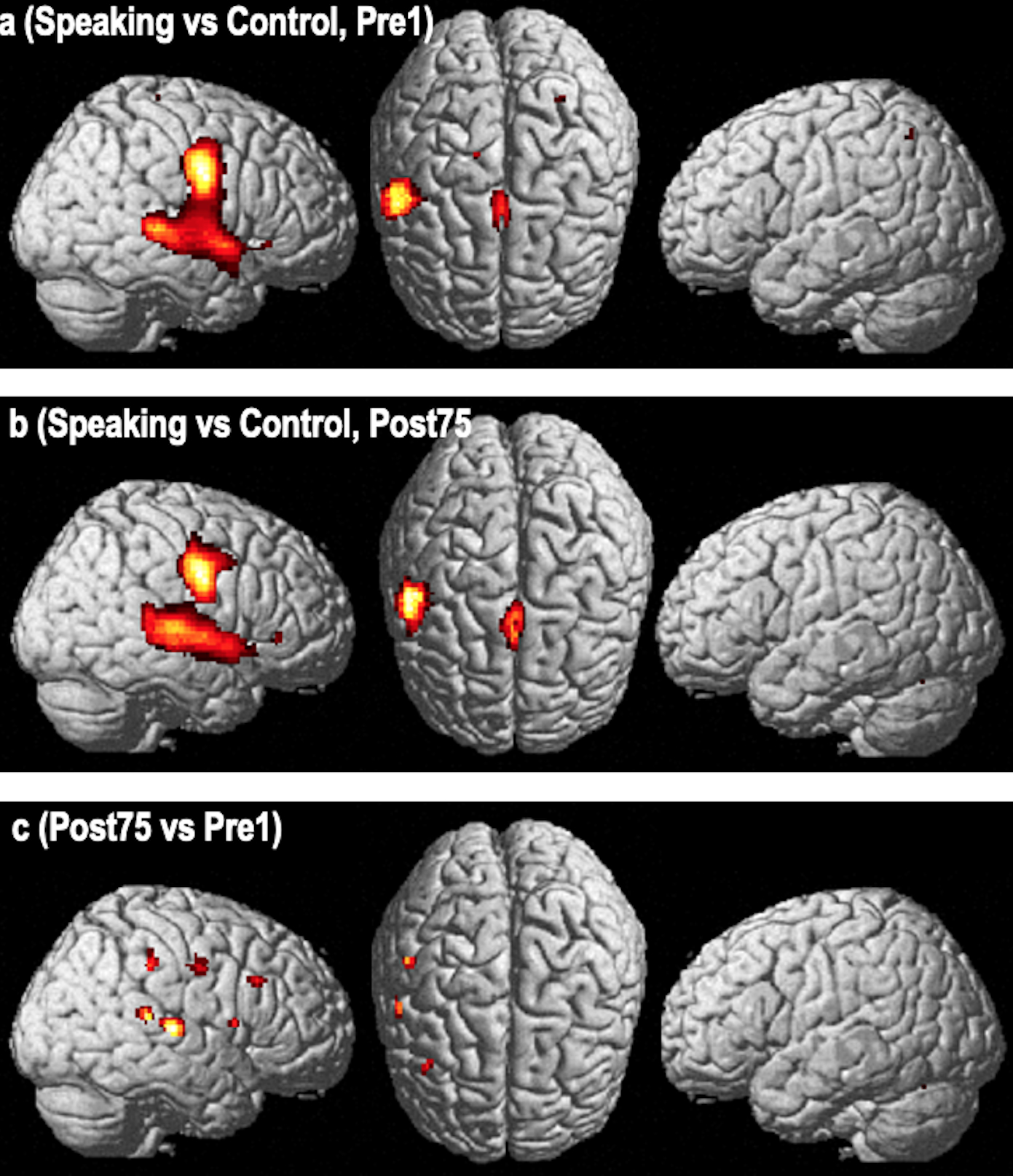
Functional imaging changes using a fixed effects analysis. Activation pattern of the “Over Speaking vs Silence Control Condition” contrast in a group of 14 nonfluent aphasia patients pre- (Fig. 3a) and post-treatment (Fig. 3b) with Melodic Intonation Therapy (Figure 3a and 3b are p<0.05, FWE whole brain corrected, voxel extent threshold=10). The third row (Fig. 3c) is the direct voxel-by-voxel contrast of the pre- and post-therapy studies revealing a pattern of activation changes involving the middle to posterior superior temporal, inferior parietal lobule (supramarginal gyrus), inferior precentral gyrus, and the inferior frontal gyrus (p<0.05, FDR corrected, voxel extent threshold=10).
The post-treatment “Overt Speaking” vs. “Silence Control” contrast (FWE p<0.05) showed an activation pattern that included largely the same right-hemisphere regions (pre- and postcentral gyrus [58 −8 38], STG/STS [62 −20 6], pars triangularis of the inferior frontal gyrus (IFGtri) (50 32 0), pre-SMA and SMA (0 0 60), cerebellum (8 −76 −16), middle cingulate cortex (10 20 30), and caudate nucleus (12 −4 18). Activated structures in the left hemisphere included pre-SMA and SMA (0 0 60), thalamus (−2 −28 6) and the cerebellum (−24 −64 −26) (Figure 3b).
Functional changes over time
Functional changes on a voxel-by-voxel basis were assessed in two ways, first in a fixed-effects analysis and then in a random-effects analysis.
Functional changes over time (fixed effects analysis)
The post- vs. pre-treatment contrasts (FDR p<0.05 whole brain, extent threshold 10 voxels) revealed changes in a right perisylvian network that included the posterior superior temporal gyrus (STG) (62 −22 8), inferior pre- and postcentral gyri (58 −8 40), supramarginal gyrus (SMG) (50 −32 40) and pars opercularis in the IFG (IFGop) (54 10 10), as well as the caudate nucleus (16 −2 24) and thalamus (10 −32 16). The only left-hemisphere structure showing a significant change over time was a small region within the calcarine sulcus (−8 −72 6) (Figure 3c)
Functional changes over time (random effects analysis)
The comparison of the two baseline scans (pre2>pre1) did not reveal any significant voxel-by-voxel changes (Figure 4a). However, in the post>pre-treatment comparison, significant activation changes were seen, but only when results were not corrected for whole brain analysis (p<0.05, uncorrected for whole brain). These changes were in the same overall regions as those seen in the fixed-effects analysis: (1) the posterior IFG (44 22 34), (2) the upper portion of the SMG (52 −34 46), and (3) the right pre-SMA (4 16 52). Smaller clusters were seen in the right STG (46 −34 12), middle temporal gyrus (48 −70 −2), temporal pole (38 4 −24), and precentral gyrus (48 −10 56) (Figure 4b; Table 2).
FIGURE 4.
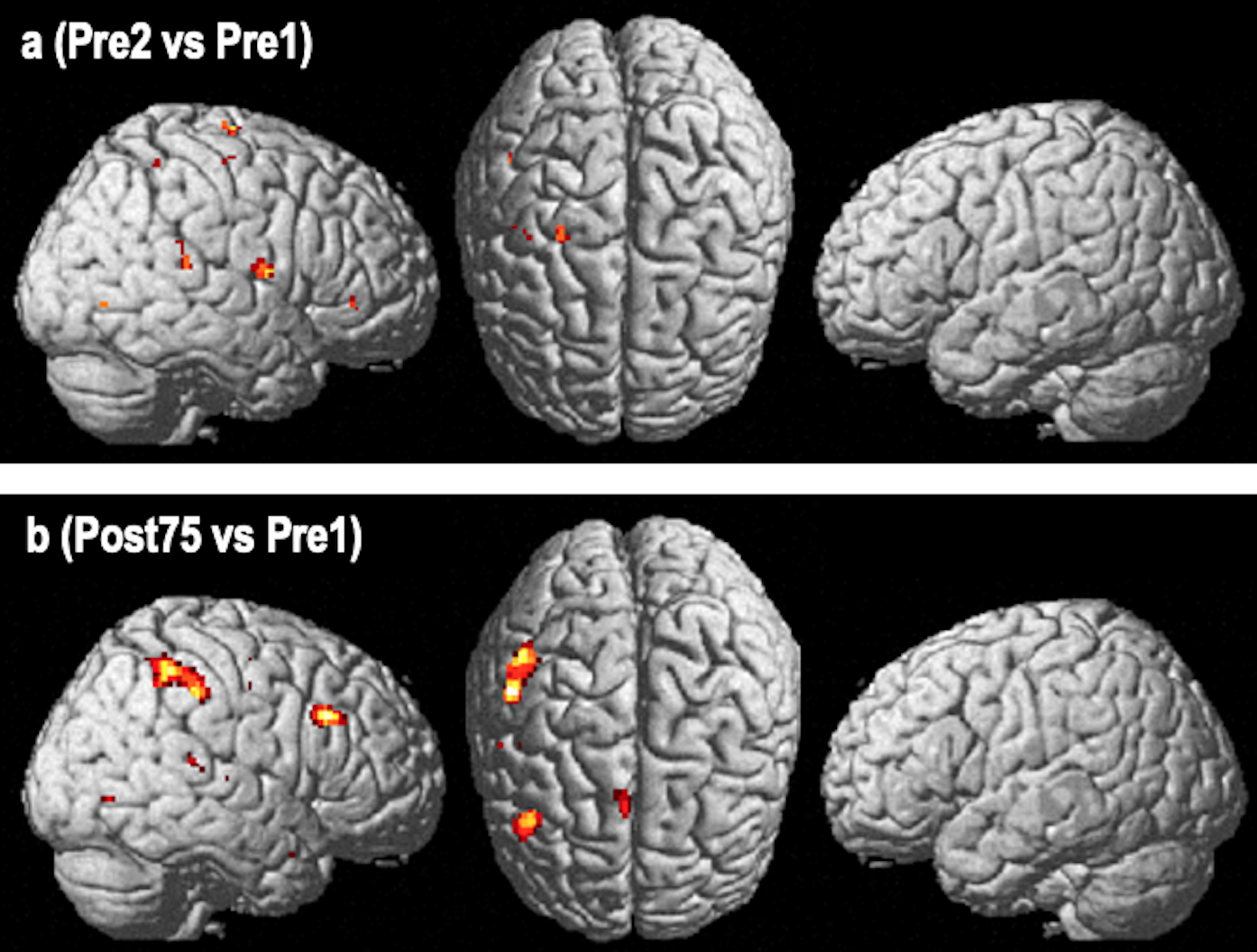
Functional imaging changes using a random effects analysis. Results of image analysis are derived from a random effects analysis using a full factorial model. The Post>Pre-therapy contrast of the Overt Speaking vs Silence Control condition. Fig. 4a – shows prominent changes in a right hemisphere network involving the inferior parietal region (supramarginal gyrus) and the inferior frontal region (Broca’s homotop) with smaller changes in the pre-SMA and the posterior superior temporal gyrus region (p<0.05, uncorr.). The comparison of both baselines (pre2>pre1). Fig. 4b - reveals a scatter of small changes in various regions, but most importantly, no overlap with the post>pre-therapy comparison (p<0.05, uncorrected).
Table 2:
Clusters for Overt Speaking-vs-Silence Control, Post75>Pre1
| cluster | cluster | cluster | voxel | voxel | voxel | voxel | voxel | Anatomical | |
|---|---|---|---|---|---|---|---|---|---|
| p(cor) | equivk | p(unc) | p(FWE-cor) | p(FDR-cor) | T | equivZ | p(unc) | x,y,z {mm} | Region |
| 1.000 | 104 | 0.486 | 0.956 | 0.903 | 2.73 | 2.63 | 0.004 | 44 22 34 | R IFG |
| 1.000 | 0.903 | 1.95 | 1.91 | 0.028 | 38 18 26 | ||||
| 0.995 | 202 | 0.325 | 0.965 | 0.903 | 2.69 | 2.59 | 0.005 | 52 −34 46 | R SMG |
| 0.998 | 0.903 | 2.37 | 2.30 | 0.011 | 46 −44 52 | ||||
| 1.000 | 48 | 0.650 | 0.965 | 0.903 | 2.69 | 2.59 | 0.005 | 38 4 −24 | R ant. MTG |
| 0.982 | 0.903 | 2.60 | 2.51 | 0.006 | 32 8 −20 | ||||
| 1.000 | 18 | 0.799 | 0.990 | 0.903 | 2.53 | 2.44 | 0.007 | 46 −34 12 | R post. STG |
| 1.000 | 4 | 0.921 | 0.995 | 0.903 | 2.44 | 2.36 | 0.009 | 48 −70 −2 | R inf.Temp-Occ |
| 1.000 | 38 | 0.691 | 1.000 | 0.903 | 1.98 | 1.94 | 0.026 | 4 16 52 | R pre-SMA |
| 1.000 | 1 | 0.968 | 1.000 | 0.903 | 1.76 | 1.74 | 0.041 | 46 −36 8 | |
| 1.000 | 2 | 0.950 | 1.000 | 0.903 | 1.75 | 1.72 | 0.042 | 56 −10 46 | R inf. M1 |
| 1.000 | 1 | 0.968 | 1.000 | 0.903 | 1.72 | 1.70 | 0.045 | 44 8 −26 | |
| 1.000 | 1 | 0.968 | 1.000 | 0.903 | 1.69 | 1.67 | 0.047 | 48 −10 56 | |
| 1.000 | 1 | 0.968 | 1.000 | 0.903 | 1.68 | 1.66 | 0.049 | 50 −20 6 |
Legend: Table shows 3 local maxima more than 8.00 mm apart; Height threshold: T=1.67, p=0.049 (1.000) {p<0.05 (unc.)}; Extent threshold: k=0 voxels, p=1.000 (1.000); Expected voxels per cluster, <k>=225.420; Expected number of clusters, <c>=16.11; Expected false discovery rate, <=0.90; Degrees of freedom=[1.0. 52.0]; FWHM=12.8 × 11.9 × 12.7mm; 6.4 × 5.9 × 6.4 (voxels); Volume: 82320=10290 voxels=22.3 resels; Voxel size: 2.0 × 2.0 × 2.0mm (resel=241.79 voxels)
Linking behavioral improvements with imaging changes
In order to assess whether the post75>pre1 activity changes correlated with behavioral improvements, we extracted mean beta values (as a measure of change in activation strength) in the pars opercularis of the right posterior IFG (which showed task-induced changes in the fixed and random-effects analysis) and correlated them with post>pre-treatment speech-fluency changes. We found a significant positive correlation between improvement in patients’ fluency (as measured by CIUs/min) and an activation’s increase (r=0.849, p<0.001) (Figure 5).
FIGURE 5.
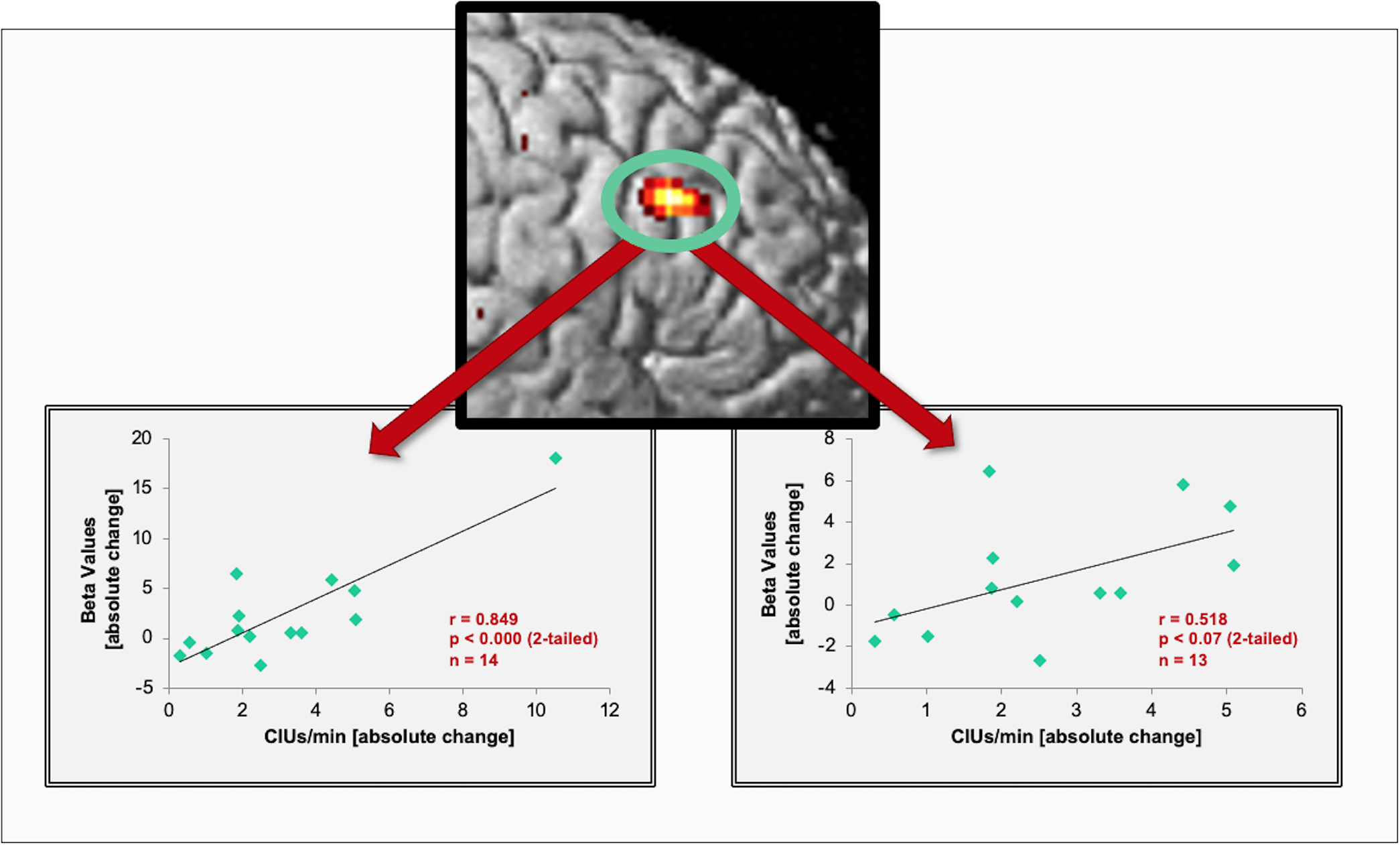
Correlations between speech output improvements and imaging changes. The scatter plot (left) shows a significant correlation between the strength of activation changes (beta-values) in the posterior IFG and improvements in patients’ propositional speech as measured by CIUs/min. The graph on the right shows the resulting non-significant, but similarly positive trend after excluding the subject with the highest correlation score.
DISCUSSION
In this proof-of-concept study, we found significant improvements in speech output and fluency in response to intensive treatment with MIT, as well as functional imaging changes in a right-hemisphere network that included the posterior IFG, SMG, pre-SMA, and STG. Furthermore, changes seen in the right posterior IFG correlated significantly with improvements in speech fluency.
Our imaging data revealed changes in a right hemisphere network of brain regions that has been shown in general to play a critical role in (1) the mapping of sounds to articulatory actions (e.g., posterior IFG 66, (2) the feedforward/feedback control of vocal output subserved by a network of fronto-temporal regions reciprocally connected by a white matter fiber tract called the arcuate fasciculus (AF) 67–70, and (3) the initiation of vocal output via the anterior frontomedial region 71, 72 and its connection with the posterior inferior frontal gyrus through the Frontal Aslant Tract (FAT) 73, 74. This speech-motor network has been shown to be bi-hemispheric in healthy individuals when the rate of production is reduced and controlled 58. Thus, it appears that there is some degree of built-in redundancy in both hemispheres for vocal output 33, 75 that might allow the right hemisphere to facilitate language and speech-motor recovery in patients with large left-hemisphere lesions15. However, our findings also suggest that the speech-motor network in the undamaged right hemisphere must be both engaged and trained intensely over a longer period of time in order to perform the speech-motor functions typically handled by the left hemisphere. The strong behavioral effects seen in our group of patients might be directly related to the intense treatment received by our study patients and might also be one of the explanations why other well-designed waitlist or cross-over studies testing the efficacy of MIT have found smaller or transient effects or have seen effects mainly in trained items, but no generalization effects 46, 48, 49, 76. There are, however, studies that have found positive effects in communication skill assessments using a subject’s own assessment 46, 49, 76. Zumbansen and colleagues compared the effects of rhythm with a combination of rhythm and pitch such as in MIT and found stronger effects in the MIT intervention compared to the rhythm intervention in three patients who received these two interventions as well as speech therapy 47.
The right posterior IFG appears to play a particularly important role in language and speech-motor recovery as it is the only region that showed a significant correlation between imaging signal changes over time and behavioral measures of speech output and fluency. The IFG has been implicated in numerous speech-related functions such as the mapping of sounds to speech-motor actions and the sequencing of those actions 6, 66, 77–79. Patients with severe nonfluent aphasia are often unable to make appropriate sounds for target words and find it difficult to either segment (deconstruct) longer words and phrases or string together multiple syllables to form words and phrases. Engaging the right IFG (which is often the only viable alternative for patients with large left-hemisphere lesions), re-mapping sounds to articulatory motor actions, and increasing patients’ awareness of their auditory feedback are not only critical aspects of recovery from nonfluent aphasia that are specifically addressed by MIT, but are likely of key importance for improving speech output. In a large meta-analysis, Turkeltaub and colleagues 26 found that activation in the right IFG was reliably observed in language production tasks in patients with aphasia and that the location of that activation was homologous to the left-hemisphere site activated in healthy individuals while performing the same tasks.
In contrast to the right IFG, the right SMG facilitates both short-term storage and retrieval of phonological information/material 80, 81 as well as the sensorimotor integration of auditory information that is important for speech motor learning 82, 83. The SMG is also a nodal point of the AF, connecting the temporal and frontal regions either directly or indirectly through a relay in the SMG 84, 85.
Changes in the frontomedial region were mainly observed in the borderzone region of the posterior pre-SMA and SMA-proper 86, 87 — a region which is involved in motor planning and preparation 86 as well as maintaining readiness for action 88. Furthermore, the pre-SMA/SMA-proper has been shown to be involved in a wide variety of simple and complex overt language production tasks 71 and has connections to the inferior frontal gyrus through a known fiber tract (i.e., FAT)73, 74. Some studies have shown that lesions to the SMA/pre-SMA often lead to a deficit in volitional and/or spontaneous action and speech fluency 89, 90.
At first glance, our imaging findings appear inconsistent with those of Belin and colleagues 42 whose PET study reported significant post-therapy activation of the left prefrontal cortex (anterior to Broca’s region) after an intoned words vs. non-intoned words contrast in a group of patients with widely varying amounts of MIT. While our study differs from theirs in that we used a strict pre-vs-post-therapy design, their post-therapy only image analysis did show that the results of their repeating words vs. hearing words contrast were associated with right fronto-central activation. This particular imaging contrast — which is somewhat closer to the main contrast used in our study — also suggests an involvement of right-hemisphere structures during expressive language tasks in MIT-treated patients with severe nonfluent aphasia.
While strong right-lateralized imaging changes may initially seem surprising, the four possible mechanisms by which MIT achieves its therapeutic effect (for details, see 25 actually all have a tendency to engage the right hemisphere more than the left. The first three mechanisms, (i) reduction of speed to approximately one syllable/second, (ii) syllable lengthening that isolates/emphasizes individual phonemes even as they remain part of the continuously-voiced words/phrases, and (iii) “chunking” that combines prosodic information with meaningful content to facilitate production of longer phrases, have all been shown to lead to more right- than left-hemisphere activation in healthy subjects 58, 91, 92. Furthermore, since studies have found that patients with right-hemisphere lesions have greater difficulty with global processing tasks (e.g., melody and contour processing) than those with left-hemisphere lesions 93, 94, it is likely that the melodic element of MIT does indeed engage the right hemisphere, particularly the right temporal lobe, more than therapies that do not include the use of melodic contour. The fourth (iv) mechanism— Left hand-tapping (one tap/syllable, one syllable/sec.)—engages a right-hemispheric, sensorimotor network capable of providing an impulse for verbal production in much the same way that a metronome has been shown to serve as a “pacemaker” when rhythmic motor activities prime and/or entrain sensorimotor networks 95, 96. In addition, research suggesting that hand movements and articulatory movements may share neural correlates 97–100 further supports the notion that hand-tapping is critically important for facilitating the coupling of sounds to orofacial and articulatory actions 66. Since concurrent speech and hand movements occur in daily life, and gestures are frequently used to emphasize/accompany important and/or elusive concepts in speech, rhythmic hand movements synchronized with articulatory movements may have similarly beneficial effects on speech production. Although there is data from a cross-sectional study suggesting that the rhythmic and melodic aspects of singing may elicit different responses in individual aphasic patients 101, it is also possible that the combination of melodic intonation with left-hand tapping creates an additive effect.
As with every study, this, too, has its limitations, the most obvious being the results of the random effects functional imaging analysis which was not significant when whole-brain level corrections were applied. While the finding itself appears somewhat surprising given the strength of the behavioral changes, there are some possible explanations for this— the main one being the sample size. Despite the fact that this is, to our knowledge, the largest homogeneous group of moderate to severely nonfluent aphasic patients to be treated with intensive MIT and imaged before and after treatment, our group of just 14 subjects might be too small for a random effects analysis. Another possibility is that activation tends to be more variable as each patient’s brain must reorganize depending on the lesion size and location, and such variability on the first level makes a second level analysis much more challenging. This is why some have argued that a fixed effects analysis might be more appropriate for a patient data set with variable lesion effects when the inference is about an induced response in the group studied; while a random effects analysis allows one to make more general inferences and might be appropriate when two groups (patient group vs. control group) are compared with each other 61, 62.
In summary, our study showed that MIT may indeed, have significant therapeutic potential and lead to changes in a right-hemispheric speech-motor network; however, further investigation of its efficacy by comparing MIT to a control intervention in a randomized controlled trial are already underway in our group. An optimal control might control for the intensity of the intervention, the 1:1 interaction with the therapist, the presentation and repetition of phrases as well as isolate unique components of MIT such as the melodic intonation and left hand rhythmic hand tapping. We have referred to such a control intervention as Speech-Repetition Therapy (SRT) in other publications30, 102. Optimizing the intervention, combining it with other treatment modalities to enhance its effect 43, 103, and determining optimal dosage and optimal timing when to administer this treatment post-stroke are still needed for this severely nonfluent patient population. The remarkable ability of the injured brain to re-route speech-motor function to homotopic right-hemisphere regions/networks when left-hemisphere pathways are no longer viable, triggered by interventions that might specifically engage these right hemisphere regions, reveals more options for facilitating recovery after an injury75.
ACKNOWLEDGMENTS
The authors acknowledge support from NIH (1RO1 DC008796, 3R01DC008796-02S1, R01 DC009823-01), the Grammy Foundation, the Richard and Rosalyn Slifka Family Fund, the Tom and Suzanne McManmon Family Fund, and the Matina R. Proctor Foundation. The authors are grateful for the contributions of Nancy Helm-Estabrooks, Marjorie Nicholas, and Cynthia K. Thompson whose knowledge, insight, expertise, and guidance were critically important in the design and pilot phases of the study; our speech language pathologists (Lauryn Zipse, Rebecca Baars, Jennifer Batore, Allison Landers, and Karen Chenausky) whose contributions and hard work has made it possible for us to do this study; Sam Fang, Hui (Charles) Li, and Karen Chenausky for their help with the analysis and transcriptions of video recordings; and most especially, our patients (and their supportive families) for participating in this study.
Footnotes
COMPETING INTERESTS
None
LITERATURE:
- 1.Kertesz A, Harlock W, & Coates R (1979). Computer tomographic localization, lesion size, and prognosis in aphasia and nonverbal impairment. Brain Lang, 8(1), 34–50. [DOI] [PubMed] [Google Scholar]
- 2.Pedersen PM, Vinter K, & Olsen TS (2004). Aphasia after stroke: type, severity and prognosis. The Copenhagen aphasia study. Cerebrovasc Dis, 17(1), 35–43. [DOI] [PubMed] [Google Scholar]
- 3.Pedersen PM, Jorgensen HS, Nakayama H, Raaschou HO, & Olsen TS (1995). Aphasia in acute stroke: incidence, determinants, and recovery. Ann Neurol, 38, 659–666. [DOI] [PubMed] [Google Scholar]
- 4.Rosen HJ, Petersen SE, Linenweber MR, Snyder AZ, White DA, Chapman L, Dromerick AW, Fiez JA, & Corbetta MD (2000). Neural correlates of recovery from aphasia after damage to left inferior frontal cortex. Neurology, 55(12), 1883–1894. [DOI] [PubMed] [Google Scholar]
- 5.Wade DT, Hewer RL, David RM, & Enderby PM (1986). Aphasia after stroke: natural history and associated deficits. J Neurol Neurosurg Psychiatry, 49(1), 11–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schlaug G, Norton A, Marchina S, Zipse L, & Wan CY (2010). From singing to speaking: facilitating recovery from nonfluent aphasia. Future Neurol, 5(5), 657–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thompson CK, & Shapiro LP (2007). Complexity in treatment of syntactic deficits. Am J Speech Lang Pathol, 16(1), 30–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heiss WD, & Thiel A (2006). A proposed regional hierarchy in recovery of post-stroke aphasia. Brain Lang, 98(1), 118–123. [DOI] [PubMed] [Google Scholar]
- 9.Meinzer M, Flaisch T, Breitenstein C, Wienbruch C, Elbert T, & Rockstroh B (2008). Functional re-recruitment of dysfunctional brain areas predicts language recovery in chronic aphasia. Neuroimage, 39(4), 2038–2046. [DOI] [PubMed] [Google Scholar]
- 10.Fridriksson J (2010). Preservation and modulation of specific left hemisphere regions is vital for treated recovery from anomia in stroke. J Neurosci, 30(35), 11558–11564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Musso M, Weiller C, Kiebel S, Muller SP, Bulau P, & Rijntjes M (1999). Training-induced brain plasticity in aphasia. Brain, 122 (Pt 9), 1781–1790. [DOI] [PubMed] [Google Scholar]
- 12.Crosson B, Moore AB, Gopinath K, White KD, Wierenga CE, Gaiefsky ME, Fabrizio KS, Peck KK, Soltysik D, Milsted C, Briggs RW, Conway TW, & Gonzalez Rothi LJ (2005). Role of the right and left hemispheres in recovery of function during treatment of intention in aphasia. J Cogn Neurosci, 17(3), 392–406. [DOI] [PubMed] [Google Scholar]
- 13.Richter M, Miltner WH, & Straube T (2008). Association between therapy outcome and right-hemispheric activation in chronic aphasia. Brain, 131(Pt 5), 1391–1401. [DOI] [PubMed] [Google Scholar]
- 14.Raboyeau G, De Boissezon X, Marie N, Balduyck S, Puel M, Bezy C, Demonet JF, & Cardebat D (2008). Right hemisphere activation in recovery from aphasia: lesion effect or function recruitment? Neurology, 70(4), 290–298. [DOI] [PubMed] [Google Scholar]
- 15.Pani E, Zheng X, Wang J, Norton A, & Schlaug G (2016a). Right hemisphere structures predict poststroke speech fluency. Neurology, 86, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Menke R, Meinzer M, Kugel H, Deppe M, Baumgartner A, Schiffbauer H, Thomas M, Kramer K, Lohmann H, Floel A, Knecht S, & Breitenstein C (2009). Imaging short- and long-term training success in chronic aphasia. BMC Neurosci, 10, 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saur D, Lange R, Baumgaertner A, Schraknepper V, Willmes K, Rijntjes M, & Weiller C (2006). Dynamics of language reorganization after stroke. Brain, 129(Pt 6), 1371–1384. [DOI] [PubMed] [Google Scholar]
- 18.Pulvermuller F, Hauk O, Zohsel K, Neininger B, & Mohr B (2005). Therapy-related reorganization of language in both hemispheres of patients with chronic aphasia. Neuroimage, 28(2), 481–489. [DOI] [PubMed] [Google Scholar]
- 19.Cherney LR, & Small SL (2006). Task-dependent changes in brain activation following therapy for nonfluent aphasia: discussion of two individual cases. J Int Neuropsychol Soc, 12(6), 828–842. [DOI] [PubMed] [Google Scholar]
- 20.Vitali P, Abutalebi J, Tettamanti M, Danna M, Ansaldo AI, Perani D, Joanette Y, & Cappa SF (2007). Training-induced brain remapping in chronic aphasia: a pilot study. Neurorehabil Neural Repair, 21(2), 152–160. [DOI] [PubMed] [Google Scholar]
- 21.Meinzer M, Elbert T, Djundja D, Taub E, & Rockstroh B (2007). Extending the Constraint-Induced Movement Therapy (CIMT) approach to cognitive functions: Constraint-Induced Aphasia Therapy (CIAT) of chronic aphasia. NeuroRehabilitation, 22(4), 311–318. [PubMed] [Google Scholar]
- 22.Fridriksson J, Hubbard HI, Hudspeth SG, Holland AL, Bonilha L, Fromm D, & Rorden C (2012). Speech entrainment enables patients with Broca’s aphasia to produce fluent speech. Brain, 135(Pt 12), 3815–3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hillis AE (2007). Aphasia: progress in the last quarter of a century. Neurology, 69(2), 200–213. [DOI] [PubMed] [Google Scholar]
- 24.Heiss WD, Kessler J, Thiel A, Ghaemi M, & Karbe H (1999). Differential capacity of left and right hemispheric areas for compensation of poststroke aphasia. Ann Neurol, 45(4), 430–438. [DOI] [PubMed] [Google Scholar]
- 25.Schlaug G, Marchina S, & Norton A (2008). From singing to speaking: Why patients with Broca’s aphasia can sing and how that may lead to recovery of expressive language functions. Music Perception, 25, 315–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Turkeltaub PE, Messing S, Norise C, & Hamilton RH (2011). Are networks for residual language function and recovery consistent across aphasic patients? Neurology, 76(20), 1726–1734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xing S, Lacey EH, Skipper-Kallal LM, Jiang X, Harris-Love ML, Zeng J, & Turkeltaub PE (2016). Right hemisphere grey matter structure and language outcomes in chronic left hemisphere stroke. Brain, 139(Pt 1), 227–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pani E, Zheng X, Wang J, Norton A, & Schlaug G (2016b). Right hemisphere structures predict poststroke speech fluency. Neurology, 86(17), 1574–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Geranmayeh F, Brownsett SL, & Wise RJ (2014). Task-induced brain activity in aphasic stroke patients: what is driving recovery? Brain, 137(Pt 10), 2632–2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schlaug G, Marchina S, & Norton A (2008). From Singing to Speaking: Why Singing May Lead to Recovery of Expressive Language Function in Patients with Broca’s Aphasia. Music Percept, 25(4), 315–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schlaug G, Marchina S, & Norton A (2009a). Evidence for plasticity in white-matter tracts of patients with chronic Broca’s aphasia underoing intense intonation-based speech therapy. Annals of New York Academy of Sciences, 1169, 385–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vines BW, Norton AC, & Schlaug G (2009). Stimulating Music: Combining Melodic Intonation Therapy with Transcranial DC Stimulation to Facilitate Speech Recovery after Stroke. In S. Shioda, Homma, I., Kato, N. (Ed.), Transmitters and Modulators in Health and Disease. New Frontiers in Neuroscience. (pp. 103–114). Tokyo, Berlin, Heidelberg, New York: Springer. [Google Scholar]
- 33.Zipse L, Norton A, Marchina S, & Schlaug G (2012). When right is all that is left: plasticity of right-hemisphere tracts in a young aphasic patient. Ann N Y Acad Sci, 1252, 237–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Albert ML, Sparks RW, & Helm NA (1973). Melodic intonation therapy for aphasia. Arch Neurol, 29(2), 130–131. [DOI] [PubMed] [Google Scholar]
- 35.Sparks R, Helm N, & Albert M (1974). Aphasia rehabilitation resulting from melodic intonation therapy. Cortex, 10(4), 303–316. [DOI] [PubMed] [Google Scholar]
- 36.Gerstman HL (1964). A Case of Aphasia. J Speech Hear Disord, 29, 89–91. [DOI] [PubMed] [Google Scholar]
- 37.Geschwind N (1971). Current concepts: aphasia. N Engl J Med, 284(12), 654–656. [DOI] [PubMed] [Google Scholar]
- 38.Hebert S, Racette A, Gagnon L, & Peretz I (2003). Revisiting the dissociation between singing and speaking in expressive aphasia. Brain, 126(Pt 8), 1838–1850. [DOI] [PubMed] [Google Scholar]
- 39.Keith RL, & Aronson AE (1975). Singing as therapy for apraxia of speech and aphasia: report of a case. Brain Lang, 2(4), 483–488. [DOI] [PubMed] [Google Scholar]
- 40.Kinsella G, Prior MR, & Murray G (1988). Singing ability after right and left sided brain damage. A research note. Cortex, 24(1), 165–169. [DOI] [PubMed] [Google Scholar]
- 41.Norton A, Zipse L, Marchina S, & Schlaug G (2009). Melodic intonation therapy: shared insights on how it is done and why it might help. Ann N Y Acad Sci, 1169, 431–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Belin P, Van Eeckhout P, Zilbovicius M, Remy P, Francois C, Guillaume S, Chain F, Rancurel G, & Samson Y (1996). Recovery from nonfluent aphasia after melodic intonation therapy: a PET study. Neurology, 47(6), 1504–1511. [DOI] [PubMed] [Google Scholar]
- 43.Al-Janabi S, Nickels LA, Sowman PF, Burianova H, Merrett DL, & Thompson WF (2014). Augmenting melodic intonation therapy with non-invasive brain stimulation to treat impaired left-hemisphere function: two case studies. Front Psychol, 5, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Breier JI, Randle S, Maher LM, & Papanicolaou AC (2010). Changes in maps of language activity activation following melodic intonation therapy using magnetoencephalography: two case studies. J Clin Exp Neuropsychol, 32(3), 309–314. [DOI] [PubMed] [Google Scholar]
- 45.Bonakdarpour B, Eftekharzadeh A, & Ashayeri H (2000). Preliminary report on the effects of melodic intonation therapy in the rehabilitation of Persion aphasic patients. Iranian Journal of Medical Sciences, 25, 156–160. [Google Scholar]
- 46.van der Meulen I, van de Sandt-Koenderman WM, Heijenbrok-Kal MH, Visch-Brink EG, & Ribbers GM (2014). The Efficacy and Timing of Melodic Intonation Therapy in Subacute Aphasia. Neurorehabil Neural Repair, 28(6), 536–544. [DOI] [PubMed] [Google Scholar]
- 47.Zumbansen A, Peretz I, & Hebert S (2014). The Combination of Rhythm and Pitch Can Account for the Beneficial Effect of Melodic Intonation Therapy on Connected Speech Improvements in Broca’s Aphasia. Front Hum Neurosci, 8, 592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van Der Meulen I, Van De Sandt-Koenderman MW, Heijenbrok MH, Visch-Brink E, & Ribbers GM (2016). Melodic Intonation Therapy in Chronic Aphasia: Evidence from a Pilot Randomized Controlled Trial. Front Hum Neurosci, 10, 533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Haro-Martinez AM, Lubrini G, Madero-Jarabo R, Diez-Tejedor E, & Fuentes B (2019). Melodic intonation therapy in post-stroke nonfluent aphasia: a randomized pilot trial. Clin Rehabil, 33(1), 44–53. [DOI] [PubMed] [Google Scholar]
- 50.Goodglass H, & Kaplan E (1983). Boston Diagnostic Aphasia Examination (2nd ed.). Philadelphia: Lea & Febiger. [Google Scholar]
- 51.Raven JC (1995). Coloured Progressive Matrices. Oxford, U.K.: Oxford Psychologists Press. [Google Scholar]
- 52.Nicholas LE, & Brookshire RH (1993). A system for quantifying the informativeness and efficiency of the connected speech of adults with aphasia. Journal of Speech and Hearing Research, 36, 338–350. [DOI] [PubMed] [Google Scholar]
- 53.Borovsky A, Saygin AP, Bates E, & Dronkers N (2007). Lesion correlates of conversational speech production deficits. Neuropsychologia, 45(11), 2525–2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kertesz A (2007). Western Aphasia Battery-Revised. San Antonio: The Psychological Corporation. [Google Scholar]
- 55.Kaplan E, Goodglass H, & Weintraub S (2001). The Boston Naming Test. In. Philadelphia, PA: Lippincott, Williams & Wilkins. [Google Scholar]
- 56.Helm-Estabrooks N, Nicholas M, Morgan A (1989). Melodic intonation therapy. Austin: Pro-Ed. [Google Scholar]
- 57.Helm-Estabrooks N, Albert ML (1991). Manual of aphasia therapy. Austin: Pro-Ed. [Google Scholar]
- 58.Ozdemir E, Norton A, & Schlaug G (2006). Shared and distinct neural correlates of singing and speaking. Neuroimage, 33(2), 628–635. [DOI] [PubMed] [Google Scholar]
- 59.Friston KJ, Holmes AP, Poline J-B, Grasby PJ, Williams SCR, Frackowiak RSJ, & Turner R (1995). Analysis of fMRI time-series revisited. Neuroimage, 2, 45–53. [DOI] [PubMed] [Google Scholar]
- 60.Gaab N, Gaser C, Zaehle T, Jancke L, & Schlaug G (2003). Functional anatomy of pitch memory--an fMRI study with sparse temporal sampling. Neuroimage, 19(4), 1417–1426. [DOI] [PubMed] [Google Scholar]
- 61.Price CJ, Crinion J, & Friston KJ (2006). Design and analysis of fMRI studies with neurologically impaired patients. J Magn Reson Imaging, 23(6), 816–826. [DOI] [PubMed] [Google Scholar]
- 62.Friston KJ, Holmes AP, & Worsley KJ (1999). How many subjects constitute a study? Neuroimage, 10(1), 1–5. [DOI] [PubMed] [Google Scholar]
- 63.Brett M, Anton J-L, Valabreque R, & Poline J-B (2002). Region of interest analysis using an SPM toolbox. Presented at the 8th International Conference on Functional Mapping of the Human Brain, June 2–6, 2002, Sendai, Japan. 2002;Available on CD-ROM in NeuroImage, Vol. 16, No 2. [Google Scholar]
- 64.Marchina S, Zhu LL, Norton A, Zipse L, Wan CY, & Schlaug G (2011). Impairment of speech production predicted by lesion load of the left arcuate fasciculus. Stroke, 42(8), 2251–2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang J, Marchina S, Norton AC, Wan CY, & Schlaug G (2013). Predicting speech fluency and naming abilities in aphasic patients. Front Hum Neurosci, 7, 831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lahav A, Saltzman E, & Schlaug G (2007). Action representation of sound: audiomotor recognition network while listening to newly acquired actions. J Neurosci, 27(2), 308–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Catani M, & Mesulam M (2008). The arcuate fasciculus and the disconnection theme in language and aphasia: history and current state. Cortex, 44(8), 953–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Glasser MF, & Rilling JK (2008). DTI tractography of the human brain’s language pathways. Cereb Cortex, 18(11), 2471–2482. [DOI] [PubMed] [Google Scholar]
- 69.Guenther FH, Ghosh SS, & Tourville JA (2006). Neural modeling and imaging of the cortical interactions underlying syllable production. Brain Lang, 96(3), 280–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schlaug G, Marchina S, & Norton A (2009b). Evidence for plasticity in white-matter tracts of patients with chronic Broca’s aphasia undergoing intense intonation-based speech therapy. Ann N Y Acad Sci, 1169, 385–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tremblay P, & Gracco VL (2009). Contribution of the pre-SMA to the production of words and non-speech oral motor gestures, as revealed by repetitive transcranial magnetic stimulation (rTMS). Brain Res, 1268, 112–124. [DOI] [PubMed] [Google Scholar]
- 72.Deblieck C, Pesenti G, Scifo P, Fazio F, Bricolo E, Lo Russo G, Scialfa G, Cossu M, Bottini G, & Paulesu E (2003). Preserved functional competence of perilesional areas in drug-resistant epilepsy with lesion in supplementary motor cortex: fMRI and neuropsychological observations. Neuroimage, 20(4), 2225–2234. [DOI] [PubMed] [Google Scholar]
- 73.Catani M, Mesulam MM, Jakobsen E, Malik F, Martersteck A, Wieneke C, Thompson CK, Thiebaut de Schotten M, Dell’Acqua F, Weintraub S, & Rogalski E (2013). A novel frontal pathway underlies verbal fluency in primary progressive aphasia. Brain, 136(Pt 8), 2619–2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mandelli ML, Caverzasi E, Binney RJ, Henry ML, Lobach I, Block N, Amirbekian B, Dronkers N, Miller BL, Henry RG, & Gorno-Tempini ML (2014). Frontal white matter tracts sustaining speech production in primary progressive aphasia. J Neurosci, 34(29), 9754–9767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schlaug G (2018). Even when right is all that’s left: There are still more options for recovery from aphasia. Ann Neurol, 83(4), 661–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Haro-Martinez A, Perez-Araujo CM, Sanchez-Caro JM, Fuentes B, & Diez-Tejedor E (2021). Melodic Intonation Therapy for Post-stroke Non-fluent Aphasia: Systematic Review and Meta-Analysis. Front Neurol, 12, 700115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bangert M, & Altenmuller EO (2003). Mapping perception to action in piano practice: a longitudinal DC-EEG study. BMC Neurosci, 4, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bangert M, Peschel T, Schlaug G, Rotte M, Drescher D, Hinrichs H, Heinze HJ, & Altenmuller E (2006). Shared networks for auditory and motor processing in professional pianists: evidence from fMRI conjunction. Neuroimage, 30(3), 917–926. [DOI] [PubMed] [Google Scholar]
- 79.Bohland JW, & Guenther FH (2006). An fMRI investigation of syllable sequence production. Neuroimage, 32(2), 821–841. [DOI] [PubMed] [Google Scholar]
- 80.Jonides J, Schumacher EH, Smith EE, Koeppe RA, Awh E, Reuter-Lorenz PA, Marshuetz C, & Willis CR (1998). The role of parietal cortex in verbal working memory. J Neurosci, 18(13), 5026–5034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ravizza SM, Delgado MR, Chein JM, Becker JT, & Fiez JA (2004). Functional dissociations within the inferior parietal cortex in verbal working memory. Neuroimage, 22(2), 562–573. [DOI] [PubMed] [Google Scholar]
- 82.Shum M, Shiller DM, Baum SR, & Gracco VL (2011). Sensorimotor integration for speech motor learning involves the inferior parietal cortex. Eur J Neurosci, 34(11), 1817–1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rauschecker JP (2011). An expanded role for the dorsal auditory pathway in sensorimotor control and integration. Hear Res, 271(1–2), 16–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Catani M, Jones DK, & ffytche DH (2005). Perisylvian language networks of the human brain. Ann Neurol, 57(1), 8–16. [DOI] [PubMed] [Google Scholar]
- 85.Bernal B, & Ardila A (2009). The role of the arcuate fasciculus in conduction aphasia. Brain, 132(Pt 9), 2309–2316. [DOI] [PubMed] [Google Scholar]
- 86.Picard N, & Strick PL (1996). Motor areas of the medial wall: a review of their location and functional activation. Cereb Cortex, 6(3), 342–353. [DOI] [PubMed] [Google Scholar]
- 87.Rizzolatti G, Luppino G, & Matelli M (1996). The classic supplementary motor area is formed by two independent areas. Adv Neurol, 70, 45–56. [PubMed] [Google Scholar]
- 88.Cunnington R, Windischberger C, & Moser E (2005). Premovement activity of the pre-supplementary motor area and the readiness for action: studies of time-resolved event-related functional MRI. Hum Mov Sci, 24(5–6), 644–656. [DOI] [PubMed] [Google Scholar]
- 89.Mendez MF (2004). Aphemia-like syndrome from a right supplementary motor area lesion. Clin Neurol Neurosurg, 106(4), 337–339. [DOI] [PubMed] [Google Scholar]
- 90.Chainay H, Francois-Xaxier A, Alexandre K, Hugues D, Laurent C, Emmanuelle V, & Stephane L (2009). Motor and language deficits before and after surgical resection of mesial frontal tumour. Clin Neurol Neurosurg, 111(1), 39–46. [DOI] [PubMed] [Google Scholar]
- 91.Zatorre RJ, & Belin P (2001). Spectral and temporal processing in human auditory cortex. Cereb Cortex, 11(10), 946–953. [DOI] [PubMed] [Google Scholar]
- 92.Meyer M, Alter K, Friederici AD, Lohmann G, & von Cramon DY (2002). FMRI reveals brain regions mediating slow prosodic modulations in spoken sentences. Hum Brain Mapp, 17(2), 73–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Peretz I (1990). Processing of local and global musical information by unilateral brain-damaged patients. Brain, 113 (Pt 4), 1185–1205. [DOI] [PubMed] [Google Scholar]
- 94.Schuppert M, Munte TF, Wieringa BM, & Altenmuller E (2000). Receptive amusia: evidence for cross-hemispheric neural networks underlying music processing strategies. Brain, 123 Pt 3, 546–559. [DOI] [PubMed] [Google Scholar]
- 95.Thaut MH, Kenyon GP, Schauer ML, & McIntosh GC (1999). The connection between rhythmicity and brain function. IEEE Eng Med Biol Mag, 18(2), 101–108. [DOI] [PubMed] [Google Scholar]
- 96.Thaut MH, & Abiru M (2010). Rhythmic auditory stimulation in rehabilitation of movement disoders: A review of current research. Music Perception, 27, 263–269. [Google Scholar]
- 97.Tokimura H, Tokimura Y, Oliviero A, Asakura T, & Rothwell JC (1996). Speech-induced changes in corticospinal excitability. Ann Neurol, 40(4), 628–634. [DOI] [PubMed] [Google Scholar]
- 98.Gentilucci M, Benuzzi F, Bertolani L, Daprati E, & Gangitano M (2000). Language and motor control. Exp Brain Res, 133(4), 468–490. [DOI] [PubMed] [Google Scholar]
- 99.Meister IG, Boroojerdi B, Foltys H, Sparing R, Huber W, & Topper R (2003). Motor cortex hand area and speech: implications for the development of language. Neuropsychologia, 41(4), 401–406. [DOI] [PubMed] [Google Scholar]
- 100.Uozumi T, Tamagawa A, Hashimoto T, & Tsuji S (2004). Motor hand representation in cortical area 44. Neurology, 62(5), 757–761. [DOI] [PubMed] [Google Scholar]
- 101.Stahl B, Kotz SA, Henseler I, Turner R, & Geyer S (2011). Rhythm in disguise: why singing may not hold the key to recovery from aphasia. Brain, 134(Pt 10), 3083–3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chenausky KV, Norton AC, Tager-Flusberg H, & Schlaug G (2022). Auditory-motor mapping training: Testing an intonation-based spoken language treatment for minimally verbal children with autism spectrum disorder. Ann N Y Acad Sci, 1515(1), 266–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Vines BW, Norton AC, & Schlaug G (2011). Non-invasive brain stimulation enhances the effects of melodic intonation therapy. Frontiers in Auditory Cognitive Neuroscience, 2, 230. [DOI] [PMC free article] [PubMed] [Google Scholar]


